Open Access Article
Open Access ArticleAdvances in multifunctional diagnostic hydrogels for complex chronic wound healing and monitoring
Kun
Lei
 *abc,
Junjun
Fang
a,
Guosheng
Wang
c and
Xinchang
Pang
*abc,
Junjun
Fang
a,
Guosheng
Wang
c and
Xinchang
Pang
 a
a
aSchool of Medical Technology and Engineering, Henan University of Science and Technology, 263 Kaiyuan Road, Luolong District, Luoyang 471023, China. E-mail: leikun@haust.edu.cn
bState Key Laboratory of Polymer Materials Engineering, Sichuan University, No. 24, South 1st Section, 1st Ring Road, Chengdu 610065, China
cHenan Tuoren Medical Device Research Institute Co. LTD, South of Weiqi Road, Changyuan, Xinxiang 453424, China
First published on 30th May 2025
Abstract
The multidimensional pathological manifestations induced by chronic wounds present a significant challenge to the medical field, for which accelerated and comprehensive therapeutic strategies remain elusive. Metabolic imbalances in the chronic wound micro-ecosystem have been identified as a potential trigger for a complex array of clinical symptoms, underscoring the urgent and critical need for accelerated and deeper comprehensive therapies for chronic wounds. Despite the development of numerous strategies and materials, considerable challenges remain in the search for an efficacious and widely applicable treatment for chronic wounds. The treatment of chronic wounds remains a bottleneck. Bioactive hydrogels as promising wound dressings are widely used to promote wound healing and treatment due to their excellent comprehensive physicochemical performances. Besides, performing efficient wound management is essential for complex chronic wounds. Conductive hydrogel bioelectronics have been recognized as one of the promising solutions for wound management, which could be employed as a diagnostic wound dressing to record and monitor the electrophysiological and non-electrophysiological signals of the wounds. In this review, we systematically outline the recent advancements in diagnostic hydrogel research, encompassing adhesive and hemostasis, antimicrobial, antioxidative, immunoregulatory, and stimulus-responsive hydrogels for wound healing, as well as hydrogel-based sensors for wound monitoring. The wound healing and monitoring mechanisms of multifunctional diagnostic hydrogels are emphatically evaluated and elucidated. Finally, the research prospect and production of multifunctional diagnostic hydrogel wound dressings in the physio/chemo-therapy of chronic wounds is envisaged.
1. Introduction
Skin has two main layers: the epidermis and the dermis. The epidermis consists of several layers of structures, and the uppermost layer is composed of dead cells which are periodically shed and gradually replaced by cells from the basal layer.1 The role of the dermis is to connect the epidermis to the subcutaneous tissue and to provide firmness and elasticity to the epidermis through the presence of collagen and elastic fibers. The subcutaneous tissue is located deep within the dermis and is the connective tissue that connects the dermis to the underlying structures. In addition, the subcutaneous tissue contains adipose tissue, which serves to store fat and protect the deeper tissues. The skin is a part of the body which is constantly exposed to the external environment and is therefore particularly vulnerable to a variety of injuries, such as burns, cuts, scars, and calluses from healing wounds. These injuries are usually caused by sharp objects, overheating of the skin or excessive pressure and friction. Skin injuries trigger a series of repetitive healing processes. Numerous cellular entities, including but not limited to fibroblasts and macrophages, are integral to the process of wound repair, particularly when the extent of the injury is substantial.2–6 The structural and functional characteristics of the skin enable it to initiate a series of complex healing processes after injury. However, in chronic wounds, these normal physiological processes are often disturbed, causing the wound to fall into a persistent inflammatory state and fail to smoothly enter the proliferative and remodeling phases. For example, hyperglycaemia-induced metabolic disturbances in diabetic wounds impair vascular function, reducing the supply of nutrients and oxygen, while suppressing the activity of immune cells, increasing the risk of infection. The complexity of these pathological mechanisms calls for the development of novel wound dressings with versatility, such as hydrogel dressings capable of regulating the wound microenvironment, promoting angiogenesis, and enhancing antioxidant capacity and antimicrobial capacity, and the development of novel wound dressings that are more versatile than conventional wound dressings, to meet the clinical needs of chronic wound treatment.Injuries are categorized into acute and chronic wounds based on their causes and consequences.7,8 Acute wounds generally undergo an appropriate process of organized repair, resulting in permanent restoration of anatomical and functional integrity. On the other hand, wounds in which the normal organized and timely repair process fails to restore anatomical and functional integrity are termed as chronic wounds. Chronic wounds can be categorized as vascular ulcers (e.g., venous and arterial ulcers), diabetic ulcers, and pressure ulcers.4 Persistent or excessive inflammation, chronic infection, biofilm formation by drug-resistant microorganisms, and impaired response of skin and/or epidermal cells to reparative stimuli are among the common characteristics shared by these wounds. Instead of going through the three stages of normal healing, the wound enters a state of persistent inflammation, and this chronic wound is usually a complication of an underlying condition like diabetes.9 If a wound does not heal properly, it results in a chronic wound that places a burden on both the patient and the healthcare system.
Dressings not only cover the wound but also serve as a catalyst for the reorganization of skin cells and promote the subsequent infiltration and integration of host tissues, thus exerting a profound effect on the wound healing process. For centuries, dressings have played a key role in protecting wounds and actively stimulating the healing process. Historical practices involved the use of natural materials such as leaves, fabrics, or herbs to wrap wounds to relieve pain, prevent infection and accelerate the healing process.10 Up to now, a multitude of wound dressings, such as gauze and foam, have been developed and implemented in clinical practice.11–15 However, traditional wound dressings encounter several challenges that impede optimal wound management. These challenges include: (i) due to inactivation of fibrin hydrolase, adhesion between the wound and the dressings can occur, leading to potential pain and injury during dressing changes; (ii) limited capacity of traditional dressings to adequately moisturize and repair wounds, potentially hindering the processes of wound healing and cellular regeneration; (iii) insufficient absorptive capability of traditional dressings to effectively manage wound secretions, resulting in prolonged moisture and impeding the progress of the healing process; (iv) conventional dressings often only offer partial support in wound healing and repair, lacking the ability to provide real-time monitoring of the wound.11
Hydrogel is a three-dimensional network of hydrophilic polymers with a large amount of water in the matrix connected by points or junctions, renowned for its exceptional traits, including remarkable permeability, biocompatibility, and the capacity to foster a humid wound healing.16–18 These attributes surpass the constraints of conventional dressings, positioning hydrogels as the quintessential choice for wound management. In recent years, many scholars have also shown a growing trend in the study of hydrogels as wound dressings, including natural polymers, such as hyaluronic acid and chitosan,19,20 as well as synthetic hydrophilic polymers (e.g., polyvinyl alcohol (PVA) and polyvinylpyrrolidone (PVP).21,22 Incorporation of ionic conductors into hydrogel matrices is a thoroughly compelling approach to obtain conductive hydrogels. This method not only gives hydrogels a wide sensing range, but also high transparency and good biocompatibility, making these materials a leading technology in the field of biomedical engineering and sensor technology.23 This category of hydrogel serves as a sensitive material for real-time monitoring of wound healing progress, detecting variations in pH, temperature, and humidity levels. Moreover, it facilitates drug delivery through mechanical response, NIR activation, or UV responsiveness.24,25 This comprehensive review delineates prevalent therapeutic strategies for chronic wounds, commencing with an in-depth analysis of wound healing mechanisms and the etiology of chronic wound development, methodically investigating the salutary effects of multifunctional hydrogels on the amelioration of chronic wounds, including adhesive and hemostasis, antimicrobial, antioxidative, immunoregulatory, stimulus-responsive, and wound monitoring hydrogels (Fig. 1). Concurrently, it integrates precise, real-time wound surveillance with the therapeutic efficacy of electrically conductive hydrogels, thereby offering substantive insights to clinicians.
2. Acute wound
2.1. Formation of acute wound
Skin covers the surface of the body and is the largest organ of the human body, with protection, sensation, secretion, excretion, respiration, and other functions. From an anatomical point of view, the skin can be divided into three main layers: the epidermis, the dermis, and the subcutaneous tissue, with a clear distinction between each layer in terms of structure and function.26 The epidermis, which consists of stratified squamous epithelium, is responsible for forming the skin's protective barrier against external microorganisms and chemicals, as well as for minimizing water loss.27 In addition, the epidermis is involved in the immune response, mediated through specialized cells such as Langerhans cells. The dermis, situated beneath the epidermis, is composed of dense connective tissue, and is subdivided into the papillary and reticular layers, between which there is no distinct boundary. Within this anatomically complex region, an extensive array of vascular, neural, follicular, and glandular structures is present, comprising blood vessels, nerves, hair follicles, and sweat glands. The papillary layer, which interfaces with the epidermis's basal layer, is characterized by an abundance of capillaries, lymphatic vessels, nerve endings, and sensory receptor, facilitating sensory functions. The reticular layer, contiguous with the subcutaneous tissue, is enriched with collagen, elastin, and reticular fibers, which are intricately interwoven to confer substantial elasticity and resilience upon the skin. In addition, this layer is likewise covered with an extensive network of blood vessels, lymphatic vessels, and nerve endings. The epidermis and dermis are underlain by subcutaneous tissue, which is a loose connective tissue with many fat cells. Comprehending the fundamental architecture of the skin enables a more profound understanding of the wound-healing mechanism.The development of acute wounds is a complex process that involves various physiological mechanisms interacting with each other. The most apparent characteristic is the loss of skin integrity, particularly when it extends to the dermis and/or deeper layers, which is caused by external forces resulting from various physical or chemical factors. In cases of physical trauma, wounds can manifest as lacerations, punctures, pressure wounds, or abrasions, each of which can cause varying degrees of damage to the epidermis and/or dermis. On the other hand, chemical injuries typically result from exposure to corrosive substances, such as strong acids or alkaline substances.
2.2. Acute wound healing
Wound healing usually undergoes an organized and appropriate repair process leading to sustained restoration of anatomical and functional integrity. Traditionally, the procedure of wound repair is categorized into four stages (Fig. 2): hemostasis, inflammation, proliferation, and tissue remodeling.28The process starts with vasoconstriction, a reflexive narrowing of the blood vessels that reduces blood flow to the injured area. At the same time, platelets stick to the exposed endothelial surface of the damaged vessel, aggregating to form a temporary platelet plug. This plug acts as a scaffold for the deposition of fibrin, a protein that cross-links to form a stable meshwork, encapsulating the platelets to create a pale thrombus.30 This thrombus effectively seals the breach in the vessel wall, halting further blood loss.
In addition, the formation of the thrombus is essential for the subsequent wound healing phase. This temporary matrix not only helps to reduce blood loss but also facilitates the infiltration of essential cells such as fibroblasts and macrophages. These cells are crucial to the repair process and the restoration of tissue integrity. The body efficiently manages to contain the damage and initiate the healing process following vascular injury through intricate cellular and molecular mechanisms. This process highlights the remarkable capacity of the human body to rapidly respond to and recover from injury, underscoring the sophisticated nature of hemostasis as a critical component of the innate immune response.31
3. Chronic wound
Chronic wounds emerge from a complex interaction where injuries stagnate beyond the expected healing time, often exceeding four weeks, in stark contrast to the predictable healing of acute wounds through hemostasis, inflammation, proliferation and remodeling (Fig. 2). Persistent infections, impaired blood supply, and systemic conditions such as diabetes or vascular disease perpetuate inflammation cycles, disrupting normal healing. This leads to delayed recovery, increased risk of infection, and significant clinical management challenges. These characteristics distinctly separate chronic from acute wounds.Each wound possesses the potential to transition into a chronic state. They may be systematically categorized into four distinct groups based on their etiology, anatomical location, penetration depth, and morphological characteristics: arterial, diabetic, pressure, and venous ulcers.37,38 The evaluation and intervention concerning chronic wounds necessitate a comprehensive strategy that encompasses the myriad factors responsible for their persistence beyond conventional healing durations. Chronic wounds are thus categorized when they do not advance through the expected, orderly, and prompt stages of repair, or when the healing process is incapable of reinstating anatomical and functional integrity within a span of three to six months following adequate care. Frequently, chronic wounds may remain in a protracted inflammatory state, affected by a variety of elements, including compromised venous return, mechanical stress, and underlying medical conditions that induce a pro-inflammatory milieu, rendering the wound milieu unstable and recalcitrant to healing.
Instead of the orderly and timely progression seen in acute wound healing, chronic wounds enter a detrimental cycle of impaired healing. The inflammatory phase in chronic wounds is prolonged, and they exhibit an aberrant inflammatory response.39 Proper granulation tissue formation, which is essential for healing, is not achieved. This tissue typically supports new vessel growth and wound closure, but in chronic wounds, this process is disrupted. One of the reasons for this impairment is the presence of elevated levels of matrix metalloproteases (MMPs), which degrade the extracellular matrix and prevent proper tissue repair.40 The proteases are usually regulated by their inhibitors, but in chronic wounds, the balance is disrupted, resulting in protease levels exceeding those of their inhibitors. This proteolytic activity hinders the wound from progressing into the proliferative phase of healing, thus maintaining a cycle of inflammation and impeding repair. Additionally, chronic wounds often have a hypoxic environment due to restricted blood flow, further hindering the healing process.41
Moreover, the microbiota's complexity in chronic wounds, which includes both planktonic and biofilm-associated bacteria, significantly contributes to the prolonged inflammatory state and, as a result, the wound's chronicity.42,43 Advances in molecular techniques, such as meta transcriptomics and metabolomics, have improved our understanding of the relationship between the wound microbiota and biofilm formation. The transfer of microbiota from a genetically modified mouse with an impaired innate immune response to a wild-type mouse has demonstrated the acquisition of an impaired healing phenotype. This highlights the influence of the microbiota on wound outcomes.44 Although certain microorganisms, such as Staphylococcus aureus and Pseudomonas aeruginosa, are commonly found in chronic wounds, others, such as Staphylococcus epidermidis, may promote healing through the production of antimicrobial peptides and activation of skin-resident T cells.45–47 It is important to note that this information is based on objective evaluations and scientific evidence. Moreover, current evidence indicates that the diversity of the microbiota in the wound may be a more dependable indicator of wound chronicity. A lower diversity is related to slower rates of healing.48,49
Diabetes-associated hyperglycemia causes vascular damage, resulting in reduced capillarogenesis and functionality. These factors are crucial for supplying nutrients and oxygen to wound sites.50,51 Research suggests that diabetic wounds suppress key angiogenic factors, such as VEGF-A, impairing vascular responses.52–54 These findings highlight the potential significance of targeted therapeutic interventions that aim to enhance angiogenic responses to improve wound healing outcomes in diabetic patients.55,56 Interestingly, the impact of diabetes on angiogenesis varies depending on the pathological context, resulting in tissue or organ-specific angiogenic responses. Diabetic retinopathy, for example, is characterized by excessive angiogenesis, which leads to microaneurysms, hemorrhages, and vascular edema.57 In contrast, diabetic wounds exhibit significantly reduced angiogenesis, resulting in decreased vascularity, capillary density, and delayed wound closure. Furthermore, diabetes impairs immune responses, as elevated glucose levels compromise neutrophil function, reducing the body's ability to fight infections. The production of cytokines, which are essential for coordinating immune responses, is decreased in diabetic patients. Meanwhile, macrophage functionality, which is critical for wound debridement and initiation of healing, is impaired.58 This compromised immune function significantly increases the risk and persistence of chronic wounds, complicating and prolonging the wound healing process.
Additionally, immune cells, including neutrophils, produce more reactive oxygen species (ROS) in diabetes. In human cells, many reactive oxygen anion radicals, such as the superoxide anion radical (O2−), are produced when oxygen is converted to water. Numerous studies have shown that low concentrations of ROS promote normal wound healing by stimulating cell migration and angiogenesis, while excessive ROS can inhibit or even impair wound healing, particularly in chronic wounds. Oxidative stress occurs when there is an imbalance between an excess of reactive oxygen species and a deficiency of antioxidants.59–62
Meanwhile, in diabetic wounds, the altered angiogenic response is compounded by macrophage dysfunction. Macrophages shift from a pro-inflammatory to a reparative phenotype in normal wounds, however, in a high-sugar wound environment, macrophage switching from a pro-inflammatory to a reparative phenotype is blocked, resulting in the accumulation of pro-inflammatory macrophages in the wound and the formation of an inflammatory microenvironment characterized by oxidative stress and cellular damage.58 The db/db mouse model is characterized by obesity, diabetes, and dyslipidemia due to a mutation in the leptin receptor gene. This model has shown significant delays in wound healing, demonstrating the critical role of macrophages as a source of VEGF-A and other pro-angiogenic mediators in wounds.63,64 Despite the deep understanding of the pathological mechanism of chronic wounds, the current treatment methods still have significant limitations and cannot fully meet the clinical needs. For example, although traditional wound dressings can provide a certain degree of protection and a humid environment, they lack the ability to dynamically regulate the wound microenvironment, making it difficult to effectively deal with the complex pathological characteristics of chronic wounds, such as persistent inflammation, biofilm formation and angiogenesis disorders. In addition, the existing dressings have limited ability to monitor the wound healing process and cannot reflect the change of the wound state in time, resulting in poor treatment effect and prolonged healing time, which brings a heavy burden to patients.
4. Management of chronic wound
Managing and treating chronic wounds is a complex challenge in modern medicine. In our previous manuscript, we discussed the various factors that make these wounds difficult to heal using conventional methods. Chronic inflammation, microbial invasions, tissue ischemia, and the patient's concurrent pathologies can significantly impede natural reparative mechanisms, rendering wounds obstinate and recalcitrant to resolution. The spectrum of strategies for addressing chronic wounds is extensive in this context. Standard protocols involve thorough cleansing and debridement to reduce the risk of infection, the use of hydrophilic dressings to create an optimal wound environment, and the application of compressive dressings or elasticized bandages to reduce swelling and improve blood flow.65 For wounds with a prolonged healing process, various advanced treatments can be used, such as negative pressure wound therapy, localized administration of cellular therapies and regenerative tissue engineering techniques.Infection control, based on debridement, is a critical factor in the management of chronic wounds. The level of bioburden in a wound is a vital determinant in determining whether a wound will heal or not. Reduction of the bioburden of each wound is a key component of wound bed preparation and individualized management of each wound.66,67 Properly healing chronic wounds relies on the notion that treatments, such as topical antimicrobials, can effectively eliminate biofilm bacteria without harming the necessary cells for wound healing (like fibroblasts, keratinocytes, and blood vessel cells). This concept is illustrated by the therapeutic index (TI), a ratio that measures the safety of a drug and is used to assess the balance between a drug's toxicity to human cells and its ability to kill bacteria or fungi. The therapeutic index is classically defined as the ratio between the dose of a drug that determines the incidence and/or severity of side effects inconsistent with the target indication (e.g. 50% of subjects at the toxic dose; TD50) and the dose that determines the expected pharmacological effect (e.g. 50% of subjects at the effective dose; ED50). The ratio between the dose that produces the expected pharmacological effect (e.g., ED50) and the toxic dose (e.g., TD50) is crucial for assessing the safety of a drug.68
Wound dressings, as a crucial element in the management and modulation of wounds, encompass a plethora of functions and responsibilities.69 Adequate wound dressings establish an optimum setting for healing, thwart secondary infections, and foster tissue repair and regeneration. Their capacity to absorb wound exudate, maintain wound moisture, and safeguard the adjacent tissues not only mitigates the risk of infection and inflammation but also accelerates the wound healing process. Currently, the standard of care for chronic wounds is through the application of wound dressings that need to be changed regularly and continuous monitoring of wounds by healthcare professionals. Other biomedical engineering approaches are also dedicated to developing an ideal dressing design, in addition to maintaining moisture balance. The essential criteria for dressings include (i) biocompatibility; (ii) protection of the wound from debridement and mechanical trauma; (iii) facilitation of wound environment and gas exchange; (iv) efficient absorption of wound exudate; (v) easy removal without disrupting the underlying tissue; (vi) protection against infections and bacterial ingress; (vii) sterile, non-toxic, and non-allergic. Currently, wound dressings can be classified into four main groups based on the treatment they provide: passive, interactive, advanced, and bioactive wound dressings.
Passive dressings, consisting of gauze, cotton, strips, and other low-adhesion dressings, are dry and their primary function is to isolate the wound from the external environment, which may provide a degree of protection against contamination of the wound. Some sterile gauze dressings are capable of absorbing exudate and fluid from open wounds through the dressing's fibers. Passive dressings often become moist and adhere to the wound, making removal painful and requiring frequent changes, due to copious exudate from the wound. Semi-permeable films and foams, as well as amorphous hydrogels, are types of interactive dressings that allow for the exchange of water vapor and oxygen while providing a barrier against bacteria and other microorganisms from the external environment. Amorphous hydrogels are available in the form of gels, elastic solid sheets, or films, and they help to maintain humidity and facilitate wound debridement by rehydrating non-viable tissue.70 However, the accumulation of fluid within a single hydrogel dressing can lead to skin maceration and bacterial proliferation. Advanced and bioactive dressings are pivotal for the repair and management of chronic wounds. As these systems are applicable to specific types of wounds, the cost and manufacturing technology may be prohibitive. Moreover, there is still a great deal of room for development for the modulation of drug release and the monitoring of various characteristics of the wound surface, and thus the research effort remains heavy.
5. Advanced hydrogel dressings
In recent years, hydrogel has emerged as a highly anticipated material for wound treatment, attributed to its elevated water content, hydrophilic nature, and porous structure.68–71 These characteristics facilitate the absorption of wound exudate, while maintaining optimal wettability and permeability of the wound bed. Furthermore, hydrogels possess flexible and tunable physicochemical properties, enabling them to be endowed with multifunctionality for dynamically responding to and regulating the chronic wound's microenvironment. Hydrogel-based dressings have a performance advantage over other chronic wound treatment strategies due to their ease of modification and customization. For instance, hydrogel dressings can integrate cells, antimicrobials, antivirals, antifungals, growth factors, and biologically active molecules, giving them functional properties that expedite wound contraction and healing processes. Meanwhile, hydrogels with sensing capabilities allow for real-time monitoring of wound status, providing timely feedback to healthcare professionals to optimize therapeutic strategies. This section discusses research advances in hydrogel wound dressings from the perspective of functional modification and provides an overview of their relevant content.5.1. Hydrogel dressings in chronic wound healing
Chronic wounds pose profound challenges stemming from their inherent complexity and protracted nature. The healing process of such wounds is often delineated by a confluence of factors, encompassing malnutrition, circulatory dysfunction, infections, and peri-wound pressure. These factors can culminate in a dearth of nutritional and oxygen supply, heightening the susceptibility to bacterial assault. Furthermore, excessive exudate may obstruct the natural drying and crusting mechanisms of the wound, thereby further procrastinating the healing process. In summary, the paramount aim in managing chronic wounds, irrespective of the therapeutic modality employed, is to disrupt the deleterious cycle of impaired healing and foster a salutary healing trajectory. Bacterial infections pose a significant challenge to wound healing, with far-reaching and complex implications. During the wound healing process, bacterial infections severely impede wound healing by disrupting the integrity and function of the wound tissue and delaying the formation of neovascularization and granulation tissue. To add insult to injury, certain bacteria are resistant to commonly used antibiotic treatments, making the treatment of bacterial infections even more complex and difficult. Bacterial infection leads to inflammatory response, while the persistent inflammatory response leads to a significant accumulation of ROS that exceeds the cells' antioxidant capacity. This also prevents the wound from transitioning from the inflammatory phase to the proliferative phase. Furthermore, due to their typically large size, chronic wounds pose significant challenges in terms of achieving adequate apposition with conventional dressings. Additionally, these dressings often fail to maintain a stable and moist healing environment for the wound surface, thus compromising their effectiveness in the treatment of chronic wounds. As mentioned above, given the many barriers that interfere with the wound healing process, this section outlines the construction strategies for different functional hydrogels based on these barriers and describes the unique features of these strategies.Protocatechuic aldehyde (PA), derived from a natural compound, possesses properties that include commendable anti-inflammatory and antibacterial actions, and its distinctive phenolic structure facilitates the formation of reversible Schiff bases with amino groups.77,78 Fu et al. have engineered a novel, entirely natural hydrogel system designated as FGMA/FG/PA, and within their study, they explored the potential advanced applications of this hydrogel in the context of chronic wound dressings for diabetic patients.79 The FGMA/FG/PA hydrogel is an entirely natural hydrogel matrix, composed of fish gelatin (FG), protocatechuic aldehyde (PA), and photo-sensitive methacrylated gelatin (FGMA). These bio-based constituents exhibit favorable biocompatibility and biological activity. Researchers posit that their interaction with macrophages may modulate the polarization state of these cells. Investigators conducted a comprehensive evaluation of the hydrogel's capacity to eliminate reactive oxygen species (ROS), utilizing the 1,1-diphenyl-2-picrylhydrazyl (DPPH) radical, hydroxyl radical (·OH), and superoxide anion radical (·O2−) to assess its ROS-scavenging efficacy. The experimental outcomes demonstrated that as the proportion of PA within the hydrogel increased, there was a progressive lightening of the solution's color, indicative of the FGMA/FG/PA hydrogel's significant free radical scavenging activity (Fig. 3a and b). As depicted in Fig. 3c, the application of the FGMA/FG/PA hydrogel to wound areas resulted in a significant reduction in the proportion of the pro-inflammatory marker iNOS (characteristic of M1 macrophages) and a concurrent significant increase in the anti-inflammatory marker CD206 (associated with M2 macrophages). This indicates that the FGMA/FG/PA hydrogel promotes the transition of M1 macrophages to the M2 phenotype. Additionally, the observed changes in inflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10 expression further support these findings (Fig. 3d–f). In addition, hydrogels with inherent immunomodulatory capacity can also safely transition from M1 macrophages to M2 macrophages. Qi et al. designed a GGG hydrogel consisting of a glycyrrhizic acid-induced (GA) self-assembly network and a photocrosslinked gelatin methacrylate matrix. The gallium ion (Ga3+) in this hydrogel, a novel antibacterial agent, binds to outer membrane receptors on the surface of bacterial cells by mimicking ferric ions (Fe3+), leading to iron depletion in bacteria and affecting iron-dependent metabolic pathways, thus inhibiting the growth of bacteria and the production of reactive oxygen species.80 GA can directly eliminate ROS, reduce oxidative stress and protect cells from oxidative damage. GGG hydrogels may also enhance the antioxidant capacity of cells by up-regulating the expression of antioxidant enzymes such as superoxide dismutase (SOD) and glutathione peroxidase (GPX), thereby eliminating excessive ROS.
 | ||
| Fig. 3 (a and b) Scavenging activity against some free radicals. (c) Immunofluorescence of iNOS and CD206. (d) Immunofluorescence staining for proinflammatory cytokine IL-6 and anti-inflammatory cytokine IL-10. (e and f) The IL-6+ and IL-10+ areas were counted on the 7th day. Reproduced with permission from ref. 79. Copyright 2023, Wiley-VCH GmbH. | ||
Hydrogels also have unique properties that can be used as drug carriers, and they can also demonstrate a critical role in the regulation of immune responses. Some plant essential oils are widely used as antimicrobial agents and immunomodulators because of their unique properties.81–83 Hydrogels are often hydrophilic, while essential oils are hydrophobic, which makes it difficult to combine them with hydrophilic hydrogel dressings. In a 2024 study by Wang et al., the surfactant Tween-20 (T-20) was utilized to stabilize Pickering emulsions by complexing with attapulgite (ATP) nanoclay through hydrogen bonding, thereby modulating ATP's hydrophilicity and enhancing its efficacy in stabilizing oil–water interfaces.84 The resultant Pickering emulsion, integrated with both T-20 and ATP, was employed to effectively encapsulate wormwood essential oil (WEO) within a hydrogel matrix, and then a multifunctional hydrogel dressing (HD-WEO) was synthesized. This encapsulation improved the essential oil's loading capacity and aqueous stability and concurrently augmented the hydrogel's antimicrobial and immunomodulatory properties. WEO contains many bioactive components, such as 1,8-cineole, β-caryophyllene, camphor, α-terpineol, eugenol and germacrene D, which can act synergistically, through a variety of ways to play the role of anti-bacterial and anti-inflammatory agents. At the same time, WEO in HD-WEO may reduce the protein and mRNA expression levels of inflammatory factors by inhibiting the JAK STAT signaling pathway and scavenging ROS, which helps reduce proinflammatory cytokine production by type M1 macrophages. In vitro, HD-WEO-treated macrophages showed significantly up-regulated expression of M2-type markers such as CD206, IL-10 and Arg-1 by immunofluorescence staining and RT-PCR, while the expression of M1 markers such as CD86, IL-1β and iNOS was significantly down-regulated. In vivo, a significant increase in the presence of M2 type macrophages (CD206) and a decrease in the protein level of M1 type cytokine (IL-1β) were observed in an infectious diabetic wound model treated with HD-WEO, and the level of IL-10 was increased. These results confirm the ability of HD-WEO to promote M2 polarization of macrophages in vivo. By regulating the immunological microenvironment, these hydrogels have illuminated a path for the treatment of diabetic wounds, highlighting their significant potential in diabetes wound care.
The high loading capacity of hydrogels enables the selection of antibacterial materials for the preparation of inherent antibacterial hydrogels. Inherent antibacterial hydrogel dressings are prepared from materials with inherent antimicrobial properties, eliminating the need to introduce additional antibacterial agents. Its antimicrobial mechanism relies mainly on the interaction of cationic groups with the negative charges on the bacterial surface, thus exhibiting an effective antimicrobial effect. Inherent antibacterial hydrogels are made from a wide range of materials, including natural and synthetic materials such as chitosan and its derivatives, hyaluronic acid, and natural polysaccharides. These materials are often described as simple and having good biocompatibility. In addition, since inherent antibacterial hydrogels do not involve the use of antibiotics, the problem of bacterial resistance can be avoided, which is particularly important in the context of long-term use of antimicrobial materials. Given the characteristics of inherent antibacterial hydrogel dressings, their antimicrobial effectiveness appears to be relatively subdued in comparison to the approach of antibiotics and antimicrobials, which directly dismantle bacterial cells. Instead of resorting to direct cellular destruction, the antimicrobial mechanism exhibited by these hydrogels relies predominantly on their distinctive physical or chemical attributes to constrain bacterial proliferation and reproduction. Consequently, inherent antibacterial hydrogel dressings, in their inherent form, may not attain an antimicrobial potency commensurate with that of antibiotics and antimicrobial agents. Li et al. reviewed the structure of antimicrobial hydrogels, mechanism of action, drug loading effects, and hydrogels with intrinsic antimicrobial activity and synergistic effect.69 Yao and colleagues examined hundreds of typical studies on different compositions, preparation methods, antimicrobial mechanisms, and internal antimicrobial factors to summarize the application of natural antimicrobial agents to adhesive hydrogels for wound dressings.70 Building on this, Jia's group discussed different cross-linking methods, prevalent natural and synthetic polymer-based hydrogels, and the combination and impact of antimicrobial hydrogel wound dressings.71 Chitosan, a derivative of the natural polysaccharide chitin resulting from the partial elimination of acetyl groups, exhibits noteworthy physicochemical characteristics and biocompatibility. Its inherent antimicrobial attributes render it potent in suppressing the proliferation of pathogenic microorganisms. Furthermore, chitosan enhances wound healing processes, mitigates the likelihood of infection, and possesses structural and functional analogies to human tissue, thereby greatly reducing the risk of rejection by the human body.85–89 Carvalho et al. pioneered the development of innovative biocompatible 3D-scaffolds, formulated from a composite of chitosan and gelatin, with the aim of recapitulating the majority of the physicochemical characteristics exhibited by natural skin tissue.88 The methacrylamide anhydride was reacted with chitosan, resulting in the synthesis of the methacrylamide chitosan (ChMA) polymer. Subsequently, the ChMA polymer underwent a photocrosslinking process, preceded by freeze-drying, to facilitate the formation of the ChMA hydrogel. Furthermore, GelChMA was prepared through the meticulous mixing of a gelatin solution with the ChMA solution. Gelatin, as a natural protein, has good biocompatibility and biodegradability. Combining gelatin with chitosan can further improve the biocompatibility of chitosan-based hydrogels, while gelatin contains a variety of cell-recognition sites that can promote cell adhesion and proliferation, which can help accelerate tissue regeneration and repair processes. To assess the degradation characteristics of ChMA and GelChMA hydrogels, an in vitro degradation weight methodology was employed. The degradation indices of ChMA and GelChMA were approximately 40% and 90%, respectively (Fig. 4a). This discrepancy arose due to the GelChMA hydrogel matrix exhibiting significantly reduced cross-linking and enlarged pore sizes in comparison to the ChMA scaffold. Within the scope of this investigation, the incorporation of gelatin into ChMA yielded a three-dimensional scaffold possessing a high degree of hybrid hydrogel degradability. Additionally, the presence of methacrylate gelatin influenced hydrogel stability. These observations are in alignment with the swelling behavior depicted in Fig. 4b. This swelling behavior implies that the hydrogel scaffold possesses an outstanding three-dimensional porous structure, exhibiting a high level of interconnectivity and hydrophilic properties inherent to the biopolymer network. These properties significantly enhance the hydrogel's water-holding capacity. Furthermore, the structure is capable of binding active molecules, such as enzymes and antioxidants, thereby mitigating pain and expediting the healing process of chronic wounds. Meanwhile, the ChMA and GelChMA hydrogels showed excellent cytocompatibility with a cell viability response of more than 95% (Fig. 4c), exhibiting the same cytocompatibility as HEK293T cells. This indicates that the high biocompatibility of chitosan and gelatin polymers facilitates cellular metabolic mitochondrial activity.
 | ||
| Fig. 4 Antimicrobial and biofilm elimination capacity of different types of antibacterial hydrogels: (a) histogram of swelling behavior of ChMA (blank) and GelChMA (dashed) in PBS at 37 °C, after immersion for 1, 4 and 24 h; (b) histogram of degradation behavior of ChMA (blank) and GelChMA (dashed) in PBS at 37 °C, after immersion for 1, 4 and 24 h; (c) histogram of cell viability of embryonic cell lines (HEK293T) towards ChMA and GelChMA hydrogel scaffolds using MTT assay. Reproduced with permission from ref. 88. Copyright 2017, Elsevier. (d and e) Release profiles of vancomycin-loaded hydrogels in different media for the same 17.5% concentration and in PBS for the 17.5% and 8% hydrogels in PBS only; (f) antibacterial assay measurement of CFU after 20 h incubation with bacteria. (g) Bacterial blockage. (h) Bacterial growth curve showing long-lasting antibacterial activity. Reproduced with permission from ref. 90. Copyright 2024, Wiley-VCH GmbH. (i) Antibacterial activity of Ag@ZnO NCs was investigated against S. aureus. (j) Antibiofilm activity of NCs was evaluated against S. aureus. (k) XTT assay was performed to investigate the metabolic activity of S. aureus. Antibacterial activity of PVP/PVA (PP) hydrogel prepared with different concentrations (0, 25, 50, 100, 200 and 300 μg mL−1) of Ag@ZnO NCs for (l) S. aureus, (m) MRSA, and (n) P. aeruginosa. Reproduced with permission from ref. 99. Copyright 2021, Elsevier. | ||
The development of antibacterial hydrogels has witnessed significant progress through dynamic-responsive smart materials. As demonstrated in a recent study, Cherri et al. engineered a disulfide-bridged hyperbranched polyglycerol hydrogel system (SS-hPG/PEG-SH) via thiol-Michael addition click chemistry, achieving redox-triggered vancomycin release modulation.90 The hydrogel exhibited broad-spectrum antibacterial efficacy against Escherichia coli (K-12 strain), achieving >99% bacterial inhibition through direct contact-killing mechanisms. Notably, vancomycin-loaded hydrogels demonstrated sustained drug release profiles, with less than 20% passive diffusion in phosphate-buffered saline (PBS) and accelerated release under 10 mM DTT conditions. Rheological analysis revealed concentration-dependent mechanical properties, with 17.5% hydrogels displaying an elastic modulus of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 Pa and mesh sizes inversely correlated with crosslinking density. Long-term antibacterial assays confirmed persistent inhibition of bacterial growth over 20 hours, outperforming free vancomycin controls. Cytocompatibility evaluations using L929 fibroblasts showed >90% cell viability, while in vivo studies in a murine infected wound model demonstrated accelerated healing with complete hydrogel degradation within 7 days. This redox-responsive system combines tunable drug release with inherent bacterial penetration blocking capabilities, offering a promising strategy for managing multidrug-resistant infections (Fig. 4d–h).
000 Pa and mesh sizes inversely correlated with crosslinking density. Long-term antibacterial assays confirmed persistent inhibition of bacterial growth over 20 hours, outperforming free vancomycin controls. Cytocompatibility evaluations using L929 fibroblasts showed >90% cell viability, while in vivo studies in a murine infected wound model demonstrated accelerated healing with complete hydrogel degradation within 7 days. This redox-responsive system combines tunable drug release with inherent bacterial penetration blocking capabilities, offering a promising strategy for managing multidrug-resistant infections (Fig. 4d–h).
Apart from the previously mentioned chitosan-based hydrogels, inorganic metal-composite hydrogel dressings exhibit a diverse array of antimicrobial attributes. The underlying mechanism primarily stems from the antimicrobial characteristics of the metal ions, coupled with the hydrogel's wetting milieu: this milieu effectively sustains the moisture levels of the wound surface, thereby fostering optimal conditions for cellular proliferation and migration. Furthermore, when metal ions are integrated into the hydrogel matrix, they are capable of being gradually released and subsequently interact with the wound surface, potently suppressing bacterial growth and mitigating the likelihood of infection. Nanoparticles (NPs) represent a nascent technological advancement, characterized by their distinctive antimicrobial mechanisms. In a study conducted by Wang et al., NPs were recognized as a promising substitute for antibiotics, exhibiting considerable potential in the management of bacterial infections.91 Nevertheless, a significant constraint in the current research pertaining to the antibacterial mechanisms of NPs lies in the absence of harmonized standards.92 Lee et al. conducted a comprehensive review of contemporary research, evaluating the antimicrobial potency of a diverse array of nanoparticles. These NPs demonstrated in vitro antimicrobial activity against multidrug-resistant organisms (MDROs), encompassing notable ESKAPE pathogens such as Enterococci, Staphylococcus aureus, Klebsiella pneumoniae, Pseudomonas aeruginosa, and various Enterobacteriaceae species.93 On the foundation of prior research, Khan and his research team successfully synthesized the Au@ZnO core–shell nanocomposite.94 This innovative material addresses the inherent toxicity limitation of zinc oxide NPs towards healthy cells and tissues, while simultaneously exhibiting enhanced antibacterial and anti-biofilm activities.95–98 However, the high cost of the material limits the possibility of its development as a therapeutic drug. In their 2021 report, the researchers detailed the synthesis of Ag@ZnO NCs impregnated hydrogels, employing hibiscus leaf extract as a biomimetic reducing agent to synthesize biologically compatible Ag NPs.99 The article provides an exhaustive discussion on the antimicrobial activity of hydrogels and the antimicrobial film activity of Ag@ZnO NCs, respectively.
First and foremost, they delve into the thorough examination of the antimicrobial capabilities exhibited by Ag@ZnO NCs in our investigation. To illustrate this point, they utilize the antimicrobial activity against S. aureus as a representative case in this research, offering a comprehensive understanding of the antimicrobial potential demonstrated by these nanocomposites. The findings reveal a noteworthy decrement in the MIC values of the synthetic NCs as the concentration of NCs increased, thus demonstrating their potent inhibitory effect on the growth of the pathogens detected. Under the experimental conditions where 50 μg mL−1 of the agent was applied, a notable reduction in the growth of S. aureus cells was evident. Specifically, after a treatment duration of 12 hours, a reduction of approximately 6 log units in growth was observed. Furthermore, upon extending the incubation period to 24 hours, it was observed that the growth of S. aureus cells was completely abrogated, indicating the effective inhibitory action of the agent under the given conditions (Fig. 4i). The antimicrobial membrane activity of S. aureus, then, was investigated to test the ability of Ag@ZnO NCs to eliminate mature biofilms. Increased concentrations of NCs, ranging from 25 to 200 μg mL−1, yielded comparable outcomes. Notably, under these conditions, the biofilm exhibited significant disruption, resulting in a marked reduction of the biomass to below 10% (Fig. 4j). Similarly, XTT assays were performed on S. aureus treated with Ag@ZnO NCs, and for all concentrations used in this study, the metabolic activity of the treated cells was significantly reduced (Fig. 4k). This comprehensive set of results underscores the remarkable antimicrobial properties demonstrated by these nanoparticles. In addition, for the synthesized hydrogels, the research team also showed clear inhibition zones showing different concentrations of NCs through inhibition zone tests (Fig. 4l–n).
Consequently, the development of hydrogels with hemostatic properties holds significant importance in wound management. These hydrogels can rapidly conform to and fill irregular wound geometries, establishing nanofiber barriers while simultaneously absorbing exudates. Research has demonstrated that the hemostatic efficacy of hydrogels is not solely reliant on physical sealing mechanisms; rather, it is also enhanced to concentrate coagulation factors. Furthermore, the hemostatic hydrogel can effectively seal the wound by leveraging the adhesive properties of tissue adhesive hydrogels.106,107 This adhesive capability allows for prolonged and seamless adherence to the wound site, thereby mitigating the potential risk of infection associated with exposure to the external environment.
The process of adhesion can be explained by different mechanisms, which can be divided into physical, chemical effects and other mechanisms. The bioadhesive model of mussels and geckos gives researchers great insight into physical interaction induced adhesion. The gecko's foot structure has millions of nano-sized bristles that can be attached by Johannes Diderik van der Waals forces, and the gecko can easily detach.108 By mimicking the gecko's reversible adhesion mechanism, the researchers developed materials that could control adhesion strength in different directions, allowing for strong adhesion and easy debonding. Inspired by the principle of gecko adhesion, researchers developed a bionic medical bandage using biocompatible and biodegradable materials, showing a high adhesive force of bandage (4.8 N cm−2) even on the fresh intestinal wall of animals. In order to further verify the reliability of the bandage adhesion, the bandage was pasted on the abdomen of living mice, and the adhesive force was still 0.8 N cm−2. The researchers hope that the bandage could one day be used as a special suture in wound care and surgery. However, gecko mimics lose their adhesion when immersed in water. Huber et al. found that the adhesive force of gecko increases with the increase of relative humidity, and even if the relative humidity reaches 88%, the thickness of water film adsorbed on the substrate is only about 0.2 nm, while the thickness of the water film is approximately the thickness of the monolayer.109 Peng and Chen established a theoretical model to analyze the effects of relative humidity and water droplets on the adhesion of biomimetic nano-films with finite size.110 They found that the capillary force produced by a droplet acts as a repulsive force, and that its absolute value increases with the volume of the liquid, while van der Waals force decreases with the volume of the droplet. The interaction of capillary force and Johannes Diderik van der Waals force makes the total adhesion force decrease gradually with the increase of droplet volume, and when the droplet volume increases to a certain value, the total adhesion force decreases to zero.111
Mussels are able to adhere strongly to water, and their secretions contain 1,2-dihydroxybenzene groups that can form hydrogen bonds, cation–π interactions, ion complexes, and so on; these are some of the key factors for achieving stable underwater/wet adhesion.112–114 Inspired by the remarkable adhesion capabilities of mussels to various surfaces in aquatic environments, researchers have investigated the unique adhesive properties of dopamine (DA) for potential wound healing applications. Consequently, DA is grafted onto hyaluronic acid (HA) through chemical crosslinking using 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS), thereby endowing HA with superior tissue adhesion and hemostatic properties. The incorporation of recombinant human type III collagen (rhCol) results in an HA–DA@rhCol complex that demonstrates exceptional performance in chronic wound healing.115–117 Building on this foundation, Wang et al. synthesized the HA–DA@rhCol hydrogel using a H2O2/HRP catalytic system to facilitate the oxidative coupling of catechol groups.118 This approach effectively integrates the advantages of HA–DA and rhCol, resulting in a hydrogel that exhibits exceptional performance, including remarkable adhesive properties and efficient hemostatic capabilities. The adhesive strength of the 1.5% HA–DA@rhCol hydrogel was the highest at 7.35 ± 1.45 kPa, which was better than that of conventional commercial dressings (about 5 kPa). In the experimental model of hepatic hemorrhage in rats, the 1.5% HA–DA@rhCol group had significantly less bleeding than the control group, showing an excellent hemostatic effect. In vitro and in vivo, HA–DA@rhCol hydrogels have a hemolytic rate of less than 3% and do not destroy blood components or cause platelet coagulation or aggregation. In addition, co-culture experiments showed that the hydrogel had no cytotoxicity to NIH-3T3 cells and could promote cell proliferation to some extent. At the same time, DA endows the hydrogel with an excellent photothermal effect and antibacterial activity in vitro, which can effectively inhibit the growth of bacteria and reduce the risk of wound infection in diabetes mellitus, which is of great significance for the treatment of DFUs.
The adhesive properties of mussel foot proteins are predominantly attributed to the incorporation of L-3,4-dihydroxyphenylalanine (L-DOPA), an amino acid that features a catechol moiety.114 The introduction of DOPA functional groups via diverse synthetic approaches and binding mechanisms has led to the development of an array of bio-adhesive formulations.119 Hwang et al. utilized Escherichia coli to engineer a bioadhesive derived from MAP and explored the cross-linking reactions mediated by metal ions and quinones.120 Within this catechol–quinone equilibrium system, concurrent oxidation and reduction processes occur at equimolar rates, thereby sustaining a relatively stable concentration of both catechol and quinone species. Nevertheless, the prolonged use of hydrogels in intricate biological settings may be susceptible to perturbations by exogenous factors, such as oxidizing agents, which could disrupt the established balance. o-Phthalaldehyde (OPA) is the oxidation product of 1,2-dihydroxybenzene, in which two hydroxyl groups are oxidized to aldehyde groups. Although OPA itself does not have adhesive properties, Chen's team first reported condensation reactions between OPA and N-nucleophilic reagents (primary amines, hydrazides, and aminoxides) to form hydrogels. The method has a super-fast gelling rate, high modulus and low critical gelling concentration (CGC), and no catalyst is needed.121 Based on the above studies, Ren used the OPA/N-nucleophile condensation reaction to construct a hydrogel adhesive, in which hydrazide-modified hyaluronic acid (HA) and OPA-capped four-arm polyethylene glycol (4-arm-PEG-OPA) were used as the building units.122 Firstly, the OPA component within hydrogels is capable of reacting with amino groups present on tissue surfaces, such as the primary amines found in extracellular matrix proteins, to form stable phthalimide bonds (Fig. 5a). The formation of these chemical bonds constitutes a primary driving force for the adhesion between hydrogels and tissues, thereby facilitating robust adhesion. Concurrently, the reaction between OPA and the acyl hydrazide group results in the formation of reversible hydrazone bonds. This dynamic crosslinking network imparts a certain degree of energy dissipation capability to the hydrogels, thereby preventing the destruction of the adhesive interface and enhancing the adhesive stability of the hydrogels. In addition, hydrogels may form hydrogen bonds with tissue surfaces and exhibit potential for π–π stacking between aromatic ring structures in hydrogels, such as the benzene ring in OPA and aromatic amino acid residues on tissue surfaces; these physical interactions further enhanced the adhesive properties of hydrogels. The adhesive strength of the hydrogel with pigskin was 27.6 ± 3.9 kPa, which was significantly higher than that of the commercial hydrogel with fibrin glue (12.1 ± 3.5 kPa) and benzaldehyde/hydrazide crosslinking (0.9 ± 0.8 kPa). In addition, the hydrogel can also be stretched on the pigskin and undergo bending, twisting and water washing operations without falling off, showing good adhesion stability. In vitro blood coagulation experiments demonstrate that the hydrogel significantly accelerates blood coagulation, with coagulation times markedly reduced compared to the control group. In a rat model of hepatic hemorrhage, the hydrogel achieves hemostasis within 10 seconds, substantially decreasing blood loss (Fig. 5b and c). The hemostasis time and blood loss are both significantly lower than those observed in the fibrin glue group. Additionally, in a rabbit model of femoral vein and artery bleeding, the hydrogel effectively seals vascular incisions within 30 seconds, preventing further bleeding.
 | ||
| Fig. 5 (a) Mechanisms of dynamic cross-linking in bulk hydrogel and firm hydrogel-tissue adhesion, respectively. (b and c) Hemostatic effect of 7% (w/v) HA-PEG hydrogel on the rat liver in massive bleeding models. Expression of p-p65/p43 (β-actin) in RS1 cells. Reproduced with permission from ref. 122. Copyright 2023, American Association for the Advancement of Science. (d) Comprehensive process of the application of Biopatch. (e–g) Comparison of hemostatic time and blood loss in rabbit liver injury models. Reproduced with permission from ref. 123. Copyright 2024, Wiley-VCH GmbH. | ||
For a hemostatic seal that does not rely on blood clotting, the inadequate strength of the adhesive wound may result in sealing failure for high-pressure and extensive bleeding wounds. Additionally, the simple wound-sealing hemostatic behavior does not leverage the body's inherent and effective protective mechanisms of blood clotting agents. To address this challenge, Zeng et al. developed a double-layer Biopatch, consisting of a superabsorbent adhesive hydrogel layer and a high-strength antibacterial polyurethane–cetyltrimethylammonium bromide (PU–CTAB) layer.123 The hydrogel layer is formulated from chitosan (CS), polyacrylic acid grafted with N-hydroxysuccinimide ester (PAAN), and polyethylene glycol methacrylate (PEGDMA). The PU–CTAB layer is fabricated by blending cetyltrimethylammonium bromide (CTAB), a cationic surfactant, with polyurethane emulsion and uniformly coating it onto the hydrogel surface (Fig. 5d). Biopatch exhibited excellent adhesion to various biological tissues and engineered solid surfaces. The shear strength of Biopatch to blood-dyed pigskin was 51.8 ± 11.2 kPa, and the interfacial toughness was 317 ± 20.6 J m−2, which was much higher than that of other adhesive materials. Its adhesion strength and toughness on different blood-stained wounds are also excellent, and can effectively seal bleeding wounds. At the same time, from the in vitro blood clotting test, the blood clotting time from the control group was decreased from 12 minutes to 3 minutes. In vivo, the hemostatic time of Biopatch was less than 30 seconds, and the hemostatic effect of Biopatch was significant in rabbit ear artery, femoral artery and liver injury (Fig. 5e–g) models. Besides, for some special adhesive applications, such as postoperative abdominal adhesion, a unique asymmetric adhesion property (namely Janus adhesion) is urgently needed to address postoperative tissue adhesion. Recently, Tan et al. designed a Janus patch with asymmetric adhesion performance to stop postoperative abdominal adhesion, including two functional layers: an adhesive poly(lactic acid-co-ethylethylene phosphate) copolymer layer to obtain tissue adhesion via hydrogen bonding, hydrophilicity, and electrostatic interactions, and a non-adhesive electrospun PLA membrane layer.124 Compared with the non-adhesive PLA layer, the adhesive Janus patch layer indicated excellent adhesive abilities on various tissues.
A comprehensive discussion of the mechanisms of oxidative stress and diabetic wound healing is provided by Zhang et al.126 They summarized the antioxidant therapies used in diabetic wound healing over the last five years. The NF-κB and Nrf2/Keap1 pathways are considered to be key pathways in oxidative stress.131–136 ROS are thought to be a key factor in the induction of the NF-κB pathway. In recent years, photothermal therapy (PTT) reliant on photothermal reactions has gained significant popularity as an antimicrobial modality. Concurrently, near-infrared (NIR) light possesses ROS-scavenging capabilities, as agents exhibiting robust light absorption in the NIR region can effectively transduce light energy into heat, thereby eliciting localized thermal therapy. Ding and his colleagues designed a NIR-triggered Au nanocage to quantify ROS levels by measuring 2,7-dichlorofluorescein (DCF) fluorescence intensity in an in vitro antimicrobial performance assay and found little to no ROS expression in bacteria in the NIR-irradiated group.134 Cai's investigative collective has devised an innovative, multifunctional hydrogel dressing for wounds (designated HPZ8) that incorporates PDA–ZIF8 nanoparticles.128 These nanoparticles exhibit robust antioxidant capabilities and photothermal antimicrobial properties. The dressing effectively scavenges reactive oxygen species (ROS) and harnesses near-infrared (NIR) irradiation for an enhanced photothermal therapy (PTT), thus contributing significantly to wound management and healing. Qi and his research team reported on the synthesis of physically cross-linked polyphenol/polysaccharide hydrogels achieved through the incorporation of minute tannic acid (TAMP) particles into a cationic guar gum (CG) matrix.136 By harnessing the antioxidant and photothermal attributes of TAMP, along with the mechanical reinforcement provided by injectable CG, TAMP/CG demonstrates the potential to safeguard cells from ROS-induced oxidative stress. Furthermore, localized photothermal heating, inducible by near-infrared light and reaching temperatures of 42 °C, offers additional protection against oxidative damage (Fig. 6a); this is because TAMP@CG/NIR effectively inhibited the expression of phosphorylated NF-κB (Fig. 6b and c). Under standard conditions, transcription factors are located within the cytoplasm, with translocation occurring subsequent to CG treatment. However, upon heat-assisted TAMP@CG administration, the translocation process was observed to be suppressed (Fig. 6d). This attenuation was attributed to the augmented release and internalization of TAMP, thereby preventing the translocation of NF-κB into the nucleus. Ultimately, NIR-assisted TAMP@CG achieved a dual effect, namely the scavenging of ROS and the suppression of NF-κB, resulting in a marked decrease in both ROS levels and proinflammatory factors.
 | ||
| Fig. 6 (a) Wound healing mechanism of TAMP/CG. (b) Western blot analysis of p-p65 in RS1 cells. (c) Expression of p-p65/p43 (β-actin) in RS1 cells. (d) Fluorescence images of intracellular NF-κB in RS1 cells. Reproduced with permission from ref. 136. Copyright 2022, American Chemical Society. | ||
 | ||
| Fig. 7 The design strategy of glucose and MMP-9 dual-responsive shape self-adaptive hydrogels for treating chronic diabetic wound. Reproduced with permission from ref. 144. Copyright 2022, Elsevier & KeAi. | ||
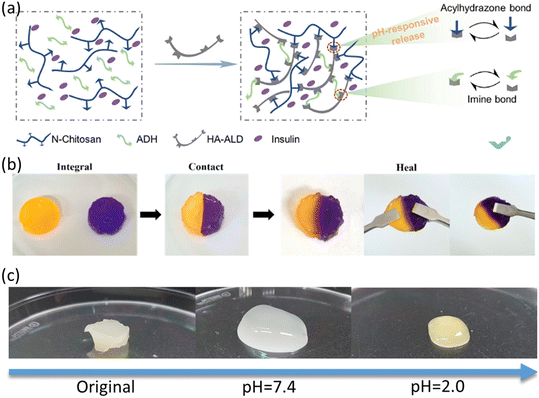
The unique pathophysiological context of diabetic wounds is marked not only by hyperglycemia, but also by an acidic pH, both characteristics playing a pivotal role.145–147 In specific pH conditions, chemical bonds such as hydrazone and imine bonds exhibit phenomena of rupture or rearrangement, thereby modifying the network architecture and porosity of the hydrogel. In the context of pH-responsive hydrogels loaded with insulin, the insulin molecules are typically encapsulated within the three-dimensional network structure of the hydrogel. A change in the external pH environment results in a corresponding alteration of the pH-sensitive bonds within the hydrogel, leading to a shift in their structure and potentially leading to their breakage or rearrangement. This transition in the network structure of the hydrogel facilitates the liberation of insulin molecules that would otherwise remain entrapped. For example, Prabaharan et al. designed folate-conjugated unimolecular micelles based on amphiphilic hyperbranched block copolymer, Boltorn H40-poly(L-aspartate-doxorubicin)-b-poly(ethylene glycol)/FA-conjugated poly(ethylene glycol)(H40-P(LA-DOX)-b-PEG-OH/FA), the anti-cancer drug DOX is covalently coupled to hydrophobic fragments of amphiphilic block copolymer arms via pH-sensitive linkage, and the release profile of DOX in micelles is strongly dependent on environmental pH.146 Consequently, in an acidic environment, such as an infected wound site, the hydrazone bond remains unperturbed, preserving the structural integrity of the hydrogel and minimizing the rate of insulin release. However, as the pH of the environment increases, either naturally during the healing process or due to external interventions, the hydrazone bonds begin to rupture, loosening the network structure of the hydrogel, thereby enhancing insulin release.
Similarly, a hydrogel has been formulated with the addition of glycine insulin,148 which employs N-carboxyethyl chitosan (N-chitosan), hyaluronic acid–aldehyde (HA–ALD), and adipic dihydrazone (ADH) to form an arylhydrazone and imine bond. The dynamic arylhydrazone bond displays remarkable stability under neutral environmental conditions. However, in slightly acidic media, it undergoes a transformation into a reversible chemical reaction mode, a property that exerts a profound influence on the hydrogel network structure (Fig. 8a). The origin of this pH sensitivity can be attributed to the specific response of the hydrogel matrix to acidic pH conditions, as evidenced by a change in the volume swelling of the matrix or an increase in the concomitant hydrolysis process. This phenomenon directly reflects the reduced stability of the arylhydrazone bond in acidic environments. The utilization of passive delivery systems for antimicrobials, particularly those involving direct encapsulation within hydrogels, represents a pivotal administration route for addressing infected wounds. However, these mechanisms inherently pose a risk of precipitating the abrupt discharge of antimicrobial agents. This unchecked release paradigm encompasses a myriad of detrimental outcomes, spanning from perturbations in the delicate equilibrium of the cutaneous microbiota to systemic toxicological effects, thereby hindering the intricate and dynamic biological machinery underpinning wound healing.149 Such perturbations exhibit marked incompatibility with the optimal trajectory of the healing process. Carboxymethyl chitosan-based composites as coagulants in drug delivery systems have been intensively investigated by the scientific community due to their unique pH-responsive properties.150 Jeong et al. explored a novel self-healing hydrogel system composed of oxidized hydroxybutyryl glucan (OHbG) and quaternized carboxymethyl chitosan (QCMCS), designed for pH-responsive drug delivery. The hydrogel demonstrated impressive self-healing properties, with the storage modulus rapidly recovering after being subjected to 500% strain (Fig. 8b). This recovery is primarily due to the dynamic reorganization of Schiff base bonds within the hydrogel network. For drug release characteristics, using 5-fluorouracil (5-FU) as a model drug, the study revealed a high drug release rate of 96.57% at pH 2.0, which significantly decreased to 63.22% at pH 7.4 (Fig. 8c). This pH-responsive drug release feature positions the hydrogels as promising candidates for targeted drug delivery in the gastrointestinal and intestinal systems.151
5.2. Hydrogel dressings in wound monitoring
Looking at the development of wound dressings, from single functional wound dressings to multifunctional wound accessories in recent years, almost all the focus has been on a simple one-time strategy, such as immunomodulatory-, antimicrobial-, adhesive and hemostatic-, stimuli-responsive hydrogel dressings. Although these measures can significantly improve the microenvironment of chronic wounds and the healing process, wound healing is a complicated process and some parameters near the wounds are also varying continually, meaning that more strategies are required to continuously conduct wound management and monitoring to help adjust the treatment strategies. Developing hydrogel dressing systems that embody intelligent monitoring capabilities for pivotal microenvironmental factors such as the pH level, temperature, blood glucose concentration, and pressure holds significant importance in wound management. The recent advent of flexible electronics and the concomitant exploration of novel biomaterials has given rise to a plethora of advanced wound dressing modalities.152–157 These modalities are capable of quantifying the physicochemical properties of both acute and chronic wounds, thereby facilitating precision in wound assessment and management.158–162Zhang et al. designed a three-layer structure based on a nanofibrous membrane-microenvironmental sensor-ultraviolet cross-linking hydrogel,156 in which, through hypoxia-inducible factor-1α (HIF-1α), the expression of VEGF can be effectively assisted to promote the formation of neovascularization and wound healing (Fig. 9A and B). Nanofibers assembled layer-by-layer (LBL) from chitosan/collagen in this three-layer structure play a role in promoting skin regeneration, and the membrane promotes cell migration by upregulating the secretion of ECM proteins and triggering the integrin/FAK signaling pathway. Meanwhile, the GelMA + β-cd UV-crosslinked hydrogel group was evaluated for its capacity to facilitate vascular repair. It was observed that the hydrogel group demonstrated a progressive enhancement in vascular network formation from day 3 onwards, which persisted until day 7. In comparison, the control group did not exhibit any evidence of vascular network formation at day 3 (Fig. 9C). The intermediate sensing layer incorporates integrated temperature and humidity sensors, which helps to predict wound infections. Regarding temperature monitoring, the smart dressing demonstrates satisfactory accuracy, with an average deviation of less than 0.3 degrees Celsius (Fig. 9D). Additionally, it exhibits a brief response time (less than 30 seconds) and commendable long-term stability (Fig. 9E). A two-week monitoring period was conducted to assess the temperature of the wounds. The initial four days of monitoring indicated a slight elevation in temperature, suggestive of the progression towards the inflammatory stage of wound healing. The subsequent decline in temperature after the fourth day also indicated the onset of proliferation and tissue remodeling (Fig. 9F). However, although this smart dressing has demonstrated its potential in chronic wound treatment, it still faces some challenges in clinical application. For example, how to further improve the accuracy and reliability of the monitoring system, and how to achieve real-time data transmission and processing.
 | ||
| Fig. 9 (A) Schematic diagram of the three-layer structure of the smart dressing. (a) Integrated smart dressing includes a sensor, nanofibre membrane and GelMA hydrogel. (b) Diagram of the prepolymer and hydrogel. (c) Diagram of the microenvironment sensor. (d) Photo and diagram of the nanofiber membrane. (B) Integrated smart dressings facilitate the real-time monitoring of the wound microenvironment via mobile software. (C) Comparison of hydrogel and control groups forming a neovascular network and gradually maturing. (D) The temperature sensing of smart dressing with accurate monitoring in the scope of 33–41 °C, the deviation between the recorded average temperature and the corresponding set temperature. (E) Time–temperature curves were recorded continuously for 15 min at different temperatures. (F) Local temperature changes during wound healing. Reproduced with permission from. 156. Copyright 2021, Frontiers. | ||
During the healing process of chronic wounds, there are many key indicators that can be used to assess the state of the wound and the healing process, such as pH, temperature, humidity, and inflammatory markers. Among these indicators, pH has a special place because of its ease of measurement and sensitivity to the state of the wound. Changes in pH may serve as an early warning sign of wound infection or healing arrest. For example, when pH is elevated, it may indicate the presence of bacterial infection or biofilm formation in the wound, as bacterial metabolic activity can lead to a rise in pH. When a skin injury occurs, the pH rises slightly as the skin undergoes hemostasis and inflammation, but due to several factors such as the effects of diabetes, the pH in the alkaline environment of a chronic wound can increase significantly to 7–8.157 Mirani et al. engineered a bifunctional smart hydrogel dressing (GelDerm) that innovatively combines spatially decoupled therapeutic and diagnostic modules within a unified platform (Fig. 10a).163 The design incorporates 3D-printed pH and glucose sensor arrays embedded in an alginate hydrogel matrix, enabling continuous monitoring of wound microenvironment parameters. The pH detection system employs bromocresol purple as a chromogenic indicator, demonstrating linear chromatic transitions across pH 4–9 through smartphone-based image quantification via a custom-developed iDerm application (Fig. 10b–d). This module exhibits temperature-independent stability (34–40 °C), rapid response (<35 min), and sustained functionality over 30 day storage (Fig. 10e and f). Complementarily, the glucose-sensing component utilizes a glucose oxidase–peroxidase cascade coupled with potassium iodide chromogenesis, achieving visual quantification of physiological glucose levels (0–12 mM) through red-channel intensity analysis (R2 = 0.98). Notably, in vivo evaluation using diabetic murine models revealed exceptional biocompatibility and therapeutic efficacy. The hydrogel demonstrated 98% reduction in wound bacterial colonization (CFU counts) compared to untreated controls, while co-delivery of basic fibroblast growth factor (bFGF) and vascular endothelial growth factor (VEGF) synergistically enhanced wound closure rates to 81.17 ± 3.45% by day 7 post-treatment. This dual-modality architecture, which physically separates diagnostic and therapeutic functions while maintaining system integration, establishes a novel paradigm for multidimensional chronic wound management through simultaneous real-time biomarker tracking and controlled regenerative factor release. Hou et al. reported an adhesive and self-healing flexible diagnostic wound dressing to observe diabetic wound healing physical signs, such as pH, temperature, and glucose level, and monitor electrophysiological signals, including the electromyographic (EMG) signal, electrocardiogram (ECG) signal, and electroencephalogram (EEG).162 The diagnostic hydrogel wound dressing was obtained by the polymerization of acrylic acid (AA), 2-(diethylamino)ethyl acrylate (DMAEA), poly(ethylene glycol)methyl ether acrylate (PEGA), and 2-(3-(6-methyl-4-oxo-1,4-dihydropyrimidin-2-yl)-ureido)ethyl methacrylate (UPyMA). Due to dynamic multiple hydrogen bonds and ionic interactions, the hydrogel dressing shows good flexibility, skin compatibility, self-healing and adhesive properties.
 | ||
| Fig. 10 (a) Schematic of GelDerm showing its integrated arrays of drug-releasing and sensing modules. (b–d) Variation of the gray intensity of the pH sensor with time at temperatures from 34 to 37 °C in a buffer solution environment with a pH range of 4 to 9. The changes of gray intensity (e) and response time (f) at different pH values from 34 °C to 37 °C were studied. Reproduced with permission from ref. 163. Copyright 2023, Wiley-VCH GmbH. | ||
As a flexible bioelectronic device, the diagnostic hydrogel can monitor the glucose level (1–30 mM), body temperature (18.8–40 °C), and pH values (4–7) near the infected diabetic wounds.
In the field of chronic wound management, although current diagnostic hydrogels can provide abundant data, their complexity also brings a heavy learning burden to physicians. A lot of time is needed to master the interpretation and application of these data, which limits the wide application of smart hydrogel dressings to a certain extent. Zhang et al. used a similar but more vivid approach to synthesize boron-based (BT) probes using borax (B) and tannic acid (TA) to construct guar gum/polyvinyl alcohol/BT (GPBT) hydrogels,164 which provide intuitive visual monitoring of chronic wounds through color change and remote diagnosis via a smartphone. The boronic acid group is capable of forming borate ester bonds, which are highly sensitive to changes in pH, with the hydroxyl groups present in polymers, and hydrogels comprising borate ester bonds often display pH-responsive behavior. Under 365 nm excitation, borax has no fluorescence effect and TA emits a light blue fluorescence, while the BT probe exhibits a clear green fluorescence, a phenomenon that researchers have attributed to the fact that the multiple aromatic rings and hydroxyl groups of the TA molecule absorb energy that excites the molecule to undergo a leapfrog, which produces the fluorescence (Fig. 11a and b). Moreover, the images were captured using a mobile phone and analyzed for their RGB signals (Fig. 11c and e). During this process, the collected RGB data were processed using MATLAB to establish the relationship between pH and RGB, with a special normalization process (Fig. 11d and f). The study provides an innovative solution for the monitoring and management of chronic wounds by developing this borate probe-based smart hydrogel dressing. The visual monitoring and remote diagnosis capabilities of this dressing not only improve the efficiency of wound care, but also provide a valuable reference for the development of intelligent medical technology in the future.
 | ||
| Fig. 11 (a) Schematic diagram of synthetic GPBT hydrogel dressings. (b) The B, TA and BT probes exhibit fluorescence under UV 365 nm. (c) The RGB images of GPBT2 hydrogel under different pH conditions under visible light irradiation. (d) The relationship between pH value and R, G, B and the fitting curve of hydrogel under visible light. (e) RGB images of GPBT2 hydrogels irradiated by ultraviolet light at different pH. (f) The relationship between pH value and R, G, B, and the fitting curve of hydrogel under UV light. Reproduced with permission from ref. 164. Copyright 2023, Wiley-VCH GmbH. | ||
6. Conclusion and outlook
Therapeutic management of chronic wounds remains an unresolved and formidable challenge within the field of clinical practice. The application of multifunctional hydrogels in the therapeutic intervention for chronic wound healing has ushered in novel methods and opportunities in wound care. In this review, we thoroughly dissected the intricate wound healing process, elucidating the key stages from hemostasis to tissue remodeling, and highlighted the pivotal differences between acute and chronic wounds. Concurrently, we underscore the promising prospects for these hydrogels in chronic wound healing, with the aspiration of offering a comprehensive and structured foundation of knowledge that may guide future investigations.Advancements in multifunctional hydrogel technologies have opened new avenues for addressing chronic wounds. The unique properties of hydrogels, including their high-water content, biocompatibility, and tunable physicochemical properties, make them ideal candidates for wound dressings. Antibacterial hydrogels, for instance, demonstrate promising results in combating bacterial infections, a significant contributor to chronic wounds. Antioxidant hydrogels, on the other hand, effectively regulate reactive oxygen species (ROS) levels, thus mitigating oxidative stress and facilitating wound healing. Stimuli-responsive hydrogels further enhance wound management by precisely releasing therapeutic agents in response to specific microenvironmental cues. Integrating long-term wound monitoring capabilities into multifunctional hydrogels represents a strategic direction for personalized and precise wound care. By continuously monitoring wound microenvironmental factors such as pH, temperature, and blood glucose levels, these smart hydrogel dressings can dynamically respond to changes in the wound milieu, enabling timely interventions and optimized therapeutic strategies. This approach promises to transform wound management, providing a more proactive and effective treatment paradigm.
However, several challenges must be addressed to realize the full potential of these smart hydrogel dressings. First and foremost, improving the monitoring precision of hydrogel dressings for parameters like pH, temperature, and blood glucose is imperative. Achieving higher accuracy in these measurements is crucial for reliable decision-making and intervention. Simultaneously, the real-time acquisition and processing of dynamic wound data poses a time-sensitive challenge, requiring efficient data transmission and analysis systems. The commercialization of hydrogel dressings embedded with wound monitoring and treatment capabilities faces significant hurdles. Many wound monitoring hydrogel dressings are still in the experimental stage, and their transition from the laboratory to the marketplace is a lengthy and complex process. Given that most of these products are disposable, their commercialization journey is fraught with regulatory and cost considerations.
Addressing these challenges mentioned above needs a multifaceted strategic approach. The enhancement of monitoring precision requires the integration of advanced sensor technologies and data-processing algorithms. To optimize data transmission and analytical efficiency, collaborations with experts in wearable technology and mobile health applications ought to be prioritized. Commercialization initiatives must adopt cost-effective manufacturing methodologies while ensuring early engagement with regulatory bodies to expedite market entry. Furthermore, in addition to manufacturing and regulatory considerations, comprehensive investigation into the long-term stability and biocompatibility of hydrogel systems is essential to guarantee their clinical safety and efficacy. Future investigations should prioritize elucidating the mechanisms underlying hydrogel interactions within wound microenvironments, with specific emphasis on their influence on cellular behavior, immune modulation, and tissue regeneration. The development of multifunctional hydrogels, which amalgamate therapeutic, diagnostic, and regenerative functionalities, holds significant potential to facilitate comprehensive wound management strategies. To substantiate these observations, comparative analyses between novel hydrogel-based technologies and established therapeutic modalities are imperative to demonstrate their clinical efficacy and unique therapeutic advantages. Through the systematic resolution of these interrelated challenges and spanning material stability to clinical validation, multifunctional hydrogel dressings may revolutionize chronic wound care, thereby enhancing patient prognoses and healthcare system efficiency.
Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This work was financially supported by the Opening Project of State Key Laboratory of Polymer Materials Engineering (Sichuan University) (Grant No. sklpme2024-1-04) and Postdoctoral Research Project Funding of Henan Province (Grant No. HN2025162).References
- S. Suzuki, K. Matsuda, N. Isshiki, Y. Tamada and Y. Ikada, Experimental study of a newly developed bilayer artificial skin, Biomaterials, 1990, 11, 356 CrossRef CAS PubMed.
- P. Bainbridge, Wound healing and the role of fibroblasts, J. Wound Care, 2013, 22, 145 Search PubMed.
- H. E. DsJardins, D. S. Foster and M. T. Longaker, Fibroblasts and wound healing: an update, Regener. Med., 2018, 13, 491–495 CrossRef PubMed.
- K. Raziyeva, Y. Kim, Z. Zharkinbekov, K. Kassymbek and S. A. Jimi, Immunology of acute and chronic wound healing, Biomolecules, 2021, 11, 700 CrossRef CAS PubMed.
- W. S. Liu, Y. Liu, J. Gao, H. Zheng, Z. M. Lu and M. Li, Biomembrane-based nanostructure-and microstructure-loaded hydrogels for promoting chronic wound healing, Int. J. Nanomed., 2023, 11, 385 CrossRef PubMed.
- H. Brem and M. Tomic, Cellular and molecular basis of wound healing in diabetes, J Clin. Invest., 2007, 117, 1219–1222 CrossRef CAS PubMed.
- E. M. Tottoli, R. Dorati, I. Genta, E. Chiesa, S. Pisani and B. Conti, Skin wound healing process and new emerging technologies for skin wound care and regeneration, Pharmaceutics, 2020, 12, 735 CrossRef CAS PubMed.
- M. B. Witte and A. Barbul, General principles of wound healing, Surg. Clin. North Am., 1997, 77, 509–528 CrossRef CAS PubMed.
- R. Nunan, K. G. Harding and P. Martin, Clinical challenges of chronic wounds: searching for an optimal animal model to recapitulate their complexity, Dis. Models Mech., 2014, 7, 1205–1213 CrossRef CAS PubMed.
- I. G. Broughton, J. E. Janis and C. E. Attinger, A brief history of wound care, Plast. Reconstr. Surg., 2006, 117, 66 Search PubMed.
- Y. Liang, Z. Li, Y. Huang, R. Yu and B. Guo, Functional hydrogels as wound dressings to enhance wound healing, ACS Nano, 2021, 15, 7078–7093 CrossRef CAS PubMed.
- V. Brumberg, T. Astrelina, T. Malivanova and A. Samoilov, Modern wound dressings: hydrogel dressings, Biomedicines, 2021, 9, 1235 CrossRef CAS PubMed.
- Y. Zhao, B. Yi, J. Hu, D. Zhang, G. Li, Y. Lu and Q. Zhou, Double cross-linked biomimetic hyaluronic acid-based hydrogels with thermo-stimulated self-contraction and tissue adhesiveness for accelerating post-wound closure and wound healing, Adv. Funct. Mater., 2023, 33, 2300710 CrossRef CAS.
- H. Ying, J. Zhou, M. Wang, D. Su, Q. Ma, G. Lv and J. Chen, In situ formed collagen-hyaluronic acid hydrogel as biomimetic dressings for promoting spontaneous wound healing, Mater. Sci. Eng., C, 2019, 101, 487–498 CrossRef CAS PubMed.
- J. Zhao, P. Qiu, Y. Wang, Y. Wang, J. Zhou, B. Zhang, L. Zhang and D. Gou, Chitosan-based hydrogel wound dressings: from mechanism to applications, a Review, Int. J. Biol. Macromol., 2023, 244, 125250 CrossRef CAS PubMed.
- S. Liu, D. Li, Y. Wang, G. Zhou, K. Ge and L. Jiang, Adhesive, antibacterial and double crosslinked carboxylated polyvinyl alcohol/chitosan hydrogel to enhance dynamic skin wound healing, Int. J. Biol. Macromol., 2023, 228, 744–753 CrossRef CAS PubMed.
- X. Z. Wan, M. Q. Liu, F. L. Zhang, L. P. Xu and S. T. Wang, Interfacial chemistry in functional hydrogel coatings, Angew. Chem., Int. Ed., 2025, 64, e202425552 CrossRef CAS PubMed.
- W. C. Liang, K. Lei, J. K. Zhang, M. Yang, S. Wang, S. S. Yan, F. Lin, J. G. Yu, G. G. Liu, X. P. Wan and Y. Xie, A gelatin-based bioadhesive featuring mechanically induced glue-to-gel transition, Adv. Funct. Mater., 2025, 2501016 CrossRef.
- K. D. Yang, J. F. Yang, R. N. Chen, Q. Dong and Y. S. Zhou, Fast self-healing hyaluronic acid hydrogel with a double-dynamic network for skin wound repair, ACS Appl. Mater. Interfaces, 2024, 16, 37569–37580 CrossRef CAS PubMed.
- X. Y. Zhang, Y. P. Liang, S. F. Huang and B. L. Guo, Chitosan-based self-healing hydrogel dressing for wound healing, Adv. Colloid Interface Sci., 2024, 332, 103267 CrossRef CAS PubMed.
- S. Sabrin, S. H. Hong, K. C. S. Kumar, J. S. Oh, A. L. K. Derrick-Roberts, D. K. Karmokar, H. Habibullah, R. D. Short, B. Ghimire, R. Fitridge and E. J. Szili, Electrochemically enhanced antimicrobial action of plasma-activated Poly(Vinyl Alcohol) hydrogel dressings, Adv. Funct. Mater., 2024, 34, 2314345 CrossRef CAS.
- K. C. Aye, T. Rojanarata, T. Ngawhirunpat, P. Opanasopit, C. Pornpitchanarong and P. Patrojanasophon, Development and characterization of curcumin nanosuspension-embedded genipin-crosslinked chitosan/polyvinylpyrrolidone hydrogel patch for effective wound healing, Int. J. Biol. Macromol., 2024, 274, 133519 CrossRef PubMed.
- M. Y. Shan, X. Chen, X. Y. Zhang, S. K. Zhang, L. L. Zhang, J. X. Chen, X. H. Wang and X. Y. Liu, Injectable conductive hydrogel with self-healing, motion monitoring, and bacteria theranostics for bioelectronic wound dressing, Adv. Healthcare Mater., 2024, 13, 2303876 CrossRef CAS PubMed.
- K. Fang, R. Wang, H. Zhang, L. Zhou, T. Xu, Y. Xiao, Y. Zhou, G. Gao, J. Chen and D. Liu, Mechano-responsive, tough, and antibacterial zwitterionic hydrogels with controllable drug release for wound healing applications, ACS Appl. Mater. Interfaces, 2020, 12, 52307–52318 CrossRef CAS PubMed.
- C. L. Ren, T. X. Wang, W. Luo, X. L. Pan, B. Hu, G. Y. Li, H. F. Zhou and L. Jin, Near-Infrared-responsive nanofiber hydrogel with gradual drug release properties for wound healing, ACS Appl. Nano Mater., 2024, 7, 15517–15525 CrossRef CAS.
- P. M. Tricarico, D. Mentino, A. De Marco, C. Del Vecchio, S. Garra, G. Cazzato, C. Foti, S. Crovella and G. Calamita, Aquaporins are one of the critical factors in the disruption of the skin barrier in inflammatory skin diseases, Int. J. Mol. Sci., 2022, 23, 4020 CrossRef CAS PubMed.
- I. G. Broughton, J. E. Janis and C. E. Attinger, Wound healing: an overview, Plast. Reconstr. Surg., 2006, 117, 156 Search PubMed.
- A. Zaidi and L. Green, Physiology of haemostasis, Anaesthesiol. Intensivmed., 2022, 23, 111–117 Search PubMed.
- D. S. Minors, Haemostasis, blood platelets and coagulation, Anaesthesiol. Intensivmed., 2004, 6, 189–191 Search PubMed.
- G. Gethin, Understanding the inflammatory process in wound healing, Br. J. Community Nurs., 2022, 12, 17–22 Search PubMed.
- K. Tzavlaki and A. Moustakas, TGF-β signaling, Biomolecules, 2020, 10, 487 CrossRef CAS PubMed.
- K. Kalliopi and A. Moustakas, TGF-β1-a truly transforming growth factor in fibrosis and immunity, Biomolecules, 2021, 11, 521 CrossRef PubMed.
- D. A. Clark and R. Coker, Transforming growth factor-beta (TGF-beta), Int. J. Biochem. Cell Biol., 1998, 30, 293–298 CrossRef CAS PubMed.
- K. R. Ammann, K. J. DeCook, M. Li and M. J. Slepian, Migration versus proliferation as contributor to in vitro wound healing of vascular endothelial and smooth muscle cells, Exp. Cell Res., 2019, 376, 58–66 CrossRef CAS PubMed.
- S. Mathew-Steiner, S. Roy and C. K. Sen, Collagen in wound healing, Bioengineering, 2021, 8, 63 CrossRef CAS PubMed.
- R. Harries, D. Bosanquet and K. Harding, Wound bed preparation: TIME for an update, Int. Wound J., 2016, 13, 8–14 CrossRef PubMed.
- S. Smet, S. Probst, S. Holloway, A. Fourie, H. Beele and D. Beeckman, The measurement properties of assessment tools for chronic wounds: a systematic review, Int. J. Nurs. Stud., 2021, 121, 103998 CrossRef PubMed.
- J. Wyffels and L. Edsberg, Granulation tissue of chronic pressure ulcers as a predictive indicator of wound closure, Adv. Skin Wound Care, 2011, 24, 464–473 CrossRef PubMed.
- H. Cook, P. Stephens, K. J. Davies, D. Thomas and K. Harding, Defective extracellular matrix reorganization by chronic wound fibroblasts is associated with alterations in TIMP-1, TIMP-2, and MMP-2 activity, J. Invest. Dermatol., 2020, 115, 225–233 CrossRef PubMed.
- E. Ugwu, A. Anyanwu and M. Olamoyegun, Ankle brachial index as a surrogate to vascular imaging in evaluation of peripheral artery disease in patients with type 2 diabetes, BMC Cardiovasc. Disord., 2021, 21, 1–6 CrossRef PubMed.
- Y. K. Wu, N. Cheng and C. M. Cheng, Biofilms in chronic wounds: pathogenesis and diagnosis, Trends Biotechnol., 2019, 37, 505–517 CrossRef CAS PubMed.
- A. Clinton and T. Carter, Chronic wound biofilms: pathogenesis and potential therapies, Jianyan Yixue, 2015, 46, 277–284 Search PubMed.
- T. R. Johnson, B. L. Gomez, M. K. McIntyre, M. K. Dubick, R. J. Christy, S. E. Nicholson and D. M. Burmeister, The cutaneous microbiome and wounds: new molecular targets to promote wound healing, Int. J. Mol. Sci., 2018, 19, 2699 CrossRef PubMed.
- R. Pandya, L. Beck and T. Yoshida, Effect of triamcinolone & manuka honey on Staphylococcus aureus growth & hemolytic activity, J. Invest. Dermatol., 2021, 141, 40 CrossRef.
- H. Williams, R. Crompton, H. A. Thomason, L. Campbell, G. Singh, A. J. McBain, S. M. Cruickshank and M. J. Hardman, Cutaneous Nod2 expression regulates the skin microbiome and wound healing in a murine model, J. Invest. Dermatol., 2017, 137, 2427–2436 CrossRef CAS PubMed.
- L. Kalan, M. Loesche, B. P. Hodkinson, K. Heilmann, G. Ruthel, S. E. Gardner and E. A. Grice, Redefining the chronic-wound microbiome: fungal communities are prevalent, dynamic, and associated with delayed healing, J. Invest. Dermatol., 2019, 121, 2501 Search PubMed.
- M. Loesche, S. E. Gardner, L. Kalan, J. Horwinski, Q. Zheng, B. P. Hodkinson, A. S. Tyldsley, C. L. Franciscus and S. L. Hillis, Temporal stability in chronic wound microbiota is associated with poor healing, J. Invest. Dermatol., 2017, 137, 237–244 CrossRef CAS PubMed.
- C. D. Tipton, R. D. Wolcott, N. E. Sanford, C. Miller, G. Pathak, T. K. Silzer, J. Sun, D. Fleming, K. P. Rumbaugh and T. D. Little, Patient genetics is linked to chronic wound microbiome composition and healing, PLoS Pathog., 2020, 16, e1008511 CrossRef CAS PubMed.
- U. A. Okonkwo and L. A. DiPietro, Diabetes and wound angiogenesis, Int. J. Mol. Sci., 2017, 18, 1419 CrossRef PubMed.
- U. A. Okonkwo, L. Chen, D. Ma, V. A. Haywood, M. Barakat, N. Urao and L. A. DiPietro, Compromised angiogenesis and vascular integrity in impaired diabetic wound healing, PLoS One, 2020, 15, e0231962 CrossRef CAS PubMed.
- G. K. Kolluru, S. C. Bir and C. G. Kevil, Endothelial dysfunction and diabetes: effects on angiogenesis, vascular remodeling, and wound healing, Int. J. Vasc. Med., 2012, 2012, 918267 Search PubMed.
- M. Bourhis, J. Palle, I. Galy-Fauroux and M. Terme, Direct and indirect modulation of T cells by VEGF-A counteracted by anti-angiogenic treatment, Front. Immunol., 2021, 12, 616837 CrossRef CAS PubMed.
- T. M. Honnegowda, P. Kumar, E. G. P. Udupa, U. Kumar and P. Rao, Role of angiogenesis and angiogenic factors in acute and chronic wound healing, Plast. Aesthet. Res., 2015, 2, 243–249 CrossRef.
- R. D. Galiano, O. M. Tepper, C. R. Pelo, K. A. Bhatt, M. Callaghan, N. Bastidas, S. Bunting, H. G. Steinmetz and G. C. Gurtner, Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells, Am. J. Pathol., 2004, 164, 1935–1945 CrossRef CAS PubMed.
- U. A. Okonkwo, L. Chen, D. Ma, V. A. Haywood, M. Barakat, N. Urao and L. A. DiPietro, Compromised angiogenesis and vascular integrity in impaired diabetic wound healing, PLoS One, 2020, 15, e0231962 CrossRef CAS PubMed.
- D. A. Antonetti, P. S. Silva and A. W. Stitt, Current understanding of the molecular and cellular pathology of diabetic retinopathy, Nat. Rev. Endocrinol., 2021, 17, 195–206 CrossRef PubMed.
- G. Daryabor, M. R. Atashzar, D. Kabelitz, S. Meri and K. Kalantar, The effects of type 2 diabetes mellitus on organ metabolism and the immune system, Front. Immunol., 2020, 11, 1582 CrossRef CAS PubMed.
- Y. Xiong, Q. Feng, L. Lu, K. Zha, T. Yu, Z. Lin, Y. Hu, A. C. Panayi, V. Chu and X. Xiong, Immunomodulatory hydrogels: advanced regenerative tools for diabetic foot ulcer, Adv. Funct. Mater., 2023, 33, 2213066 CrossRef CAS.
- J. Zeng, Z. Sun, F. Zeng, C. Gu and X. Chen, M2 macrophage-derived exosome-encapsulated microneedles with mild photothermal therapy for accelerated diabetic wound healing, Mater. Today Bio, 2023, 20, 106649 Search PubMed.
- M. Liu, R. Ding, Z. Li, N. Xu, Y. Gong, Y. Huang, J. Jia, H. Du, Y. Yu and G. Luo, Hyaluronidase-responsive bactericidal cryogel for promoting healing of infected wounds: inflammatory attenuation, ROS scavenging, and immune regulation, Adv. Sci., 2024, 11, 2306602 CrossRef CAS PubMed.
- A. Hassanshahi, M. Moradzad, M. Ghalamkari, S. Fadaei, A. J. Cowin and M. Hassanshahi, Macrophage-mediated inflammation in skin wound healing, Cells, 2022, 11, 2953 CrossRef CAS.
- X. Song, Z. Zhu, X. Qian, X. Liu, S. Chen and H. Tang, Multi-omics characterization of type 2 diabetes mellitus-induced cognitive impairment in the db/db mouse model, Molecules, 2022, 27, 1904 CrossRef CAS PubMed.
- M. P. Cohen, E. Hud, E. Shea and C. W. Shearman, Vitreous fluid of db/db mice exhibits alterations in angiogenic and metabolic factors consistent with early diabetic retinopathy, Ophthalmic Res., 2007, 40, 5–9 CrossRef.
- E. Eriksson, P. Y. Liu, G. S. Schultz, M. M. Martins-Green, R. Tanaka, D. Weir, L. J. Gould, D. G. Armstrong, G. W. Gibbons and R. Wolcott, Chronic wounds: treatment consensus, Wound Repair Regen., 2022, 30, 156–171 CrossRef PubMed.
- S. E. Gardner and R. A. Frantz, Wound bioburden and infection-related complications in diabetic foot ulcers, Biol. Res. Nurs., 2008, 10, 44–53 CrossRef PubMed.
- M. C. Nolff, S. Reese, M. Fehr, R. Dening and A. Meyer-Lindenberg, Assessment of wound bio-burden and prevalence of multi-drug resistant bacteria during open wound management, J. Small Anim. Pract., 2016, 57, 255–259 CrossRef CAS PubMed.
- P. Y. Muller and M. N. Milton, The determination and interpretation of the therapeutic index in drug development, Nat. Rev. Drug Discovery, 2012, 11, 751–761 CrossRef CAS PubMed.
- P. Y. Muller and M. N. Milton, Natural polymer-based antimicrobial hydrogels without synthetic antibiotics as wound dressings, Biomacromolecules, 2020, 21, 2983 CrossRef PubMed.
- S. Li, S. Dong, W. Xu, S. Tu, L. Yan, C. Zhao, J. Ding and X. Chen, Antibacterial hydrogels, Adv. Sci., 2018, 5, 1700527 CrossRef PubMed.
- H. Yao, M. Wu, L. Wu, Z. Wu, M. Bae, S. Park, S. Wang, W. Zhang, J. Gao and D. Wang, Design strategies for adhesive hydrogels with natural antibacterial agents as wound dressings: status and trends, Mater. Today Bio, 2022, 16, 100429 CrossRef CAS PubMed.
- B. Jia, G. Li, E. Cao, J. Luo, X. Zhao and H. Huang, Recent progress of antibacterial hydrogels in wound dressings, Mater. Today Bio, 2023, 19, 100582 CrossRef CAS PubMed.
- B. A. Lipsky, E. Senneville, Z. G. Abbas, J. Aragon-Sanchez, M. Diggle, J. M. Embil, S. Kono, L. A. Lavery, M. Malone and S. A. Van Asten, Guidelines on the diagnosis and treatment of foot infection in persons with diabetes (IWGDF 2019 update), Diabetes Care, 2020, 36, e3280 Search PubMed.
- H. Elimam, A. M. Abdulla and I. M. Taha, Inflammatory markers and control of type 2 diabetes mellitus, Diabetes/Metab. Res. Rev., 2019, 13, 800–804 Search PubMed.
- X. Wang, W. Bao, J. Liu, Y. Y. OuYang, D. Wang, S. Rong, X. Xiao, Z. L. Shan, Y. Zhang and P. Yao, Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis, Diabetes Metab. Syndr., 2013, 36, 166–175 CAS.
- Y. Xiong, L. Chen, P. Liu, T. Yu, C. Lin, Y. Yan, C. Hu, W. Zhou, Y. Sun and A. C. Panayi, All-in-one: multifunctional hydrogel accelerates oxidative diabetic wound healing through timed-release of exosome and fibroblast growth factor, Small, 2022, 18, 2104229 CrossRef CAS PubMed.
- W. W. Zhang, H. W. Chen, J. K. Zhao, P. F. Chai, G. L. Ma, X. F. Shi, Y. H. Dong, Y. Q. Jiang, Q. Zhang, Z. G. Hu and Q. C. Wei, A guanosine/konjac glucomannan supramolecular hydrogel with antioxidant, antibacterial and immunoregulatory properties for cutaneous wound treatment, Carbohydr. Polym., 2024, 326, 121580 CrossRef CAS PubMed.
- J. Liang, K. Zhang, J. Li, J. Su, F. Guan and J. Li, Injectable protocatechuic acid based composite hydrogel with hemostatic and antioxidant properties for skin regeneration, Mater. Des., 2022, 222, 111109 CrossRef CAS.
- G. Wei, Y. Guan, Y. Yin, J. Duan, D. Zhou, Y. Zhu, W. Quan, M. Xi and A. Wen, Anti-inflammatory effect of protocatechuic aldehyde on myocardial ischemia/reperfusion injury in vivo and in vitro, Inflammation, 2013, 36, 592–602 CrossRef CAS PubMed.
- Y. J. Fu, Y. F. Shi, L. Y. Wang, Y. F. Zhao, R. K. Wang, K. Li, S. T. Zhang, X. J. Zha, W. Wang and X. Zhao, All-natural immunomodulatory bioadhesive hydrogel promotes angiogenesis and diabetic wound healing by regulating macrophage heterogeneity, Adv. Sci., 2023, 10, 2206771 CrossRef CAS PubMed.
- X. Qi, Y. Shi, C. Zhang, E. Cai, G. Xiang, Y. Li, B. Zeng and J. Shen, A hybrid hydrogel with intrinsic immunomodulatory functionality for treating multidrug-resistant Pseudomonas aeruginosa infected diabetic foot ulcers, ACS Mater. Lett., 2024, 6, 2533–2547 CrossRef CAS.
- E. Kamelnia, R. Mohebbati, R. Kamelnia, H. R. El-Seedi and M. H. Boskabady, Anti-inflammatory, immunomodulatory and anti-oxidant effects of Ocimum basilicum L. and its main constituents: a review, Iran. J. Basic Med. Sci., 2023, 26, 617 Search PubMed.
- N. S. Dosoky, L. N. Kirpotina, I. A. Schepetkin, A. I. Khlebnikov, B. L. Lisonbee, J. L. Black, H. Woolf, T. L. Thurgood, B. L. Graf, P. Satyal and S. Giordano, Volatile composition, antimicrobial activity, and in vitro innate immunomodulatory activity of Echinacea purpurea (L.) Moench essential oils, Molecules, 2023, 28, 7330 CrossRef CAS PubMed.
- T. A. El-Bassossy, A. A. Abdelgawad, M. A. Abo-Zaid, A. H. Amin, S. A. El-Agamy, K. M. Elazab and A. H. Ismail, Evaluation of the immunomodulatory, antioxidant, and histopathological effects of Cymbopogon schoenanthus essential oil extract on kidney and spleen in BALB/C mice, J. Umm Al-Qura Univ. Appl. Sci., 2023, 9, 411–422 CrossRef.
- F. Wang, Q. Sun, Y. Li, R. Xu, R. Li, D. Wu, R. Huang, Y. Yang and Y. Li, Hydrogel encapsulating wormwood essential oil with broad-spectrum antibacterial and immunomodulatory properties for infected diabetic wound healing, Adv. Sci., 2024, 11, 2305078 CrossRef CAS PubMed.
- F. Liu, Y. L. Guan, D. Z. Yang, Z. Li and K. D. Yao, Antibacterial action of chitosan and carboxymethylated chitosan, J. Appl. Polym. Sci., 2001, 79, 1324–1335 CrossRef.
- V. K. Mourya and N. N. Inamdar, Chitosan-modifications and applications: opportunities galore, React. Funct. Polym., 2008, 68, 1013–1051 CrossRef CAS.
- J. Fu, F. Yang and Z. Guo, The chitosan hydrogels: from structure to function, New J. Chem., 2018, 42, 17162–17180 RSC.
- I. C. Carvalho and H. S. Mansur, Engineered 3D-scaffolds of photocrosslinked chitosan-gelatin hydrogel hybrids for chronic wound dressings and regeneration, Mater. Sci. Eng., C, 2017, 78, 690–705 CrossRef CAS PubMed.
- M. Ishihara, K. Obara, S. Nakamura, M. Fujita, K. Masuoka, Y. Kanatani, B. Takase, H. Hattori, Y. Morimoto and M. Ishihara, Chitosan hydrogel as a drug delivery carrier to control angiogenesis, J. Artif. Organs, 2006, 9, 8–16 CrossRef CAS PubMed.
- M. Cherri, P. S. Stergiou, Z. Ahmadian, T. L. Povolotsky, B. Thongrom, X. Fan, E. Mohammadifar and R. Haag, Redox-responsive hydrogels loaded with an antibacterial peptide as controlled drug delivery for healing infectious wounds, Adv. Healthcare Mater., 2024, 13, 2401289 CrossRef CAS PubMed.
- L. Wang, C. Hu and L. Shao, The antimicrobial activity of nanoparticles: present situation and prospects for the future, Int. J. Nanomed., 2017, 14, 1227–1249 CrossRef PubMed.
- M. P. Ribeiro, A. Espiga, D. Silva, P. Baptista, J. Henriques, C. Ferreira, J. C. Silva, J. P. Borges, E. Pires and P. Chaves, Development of a new chitosan hydrogel for wound dressing, Wound Repair Regen., 2009, 17, 817–824 CrossRef PubMed.
- N. Y. Lee, W. C. Ko and P. R. Hsueh, Nanoparticles in the treatment of infections caused by multidrug-resistant organisms, Front. Pharmacol., 2019, 10, 1153 CrossRef CAS PubMed.
- M. I. Khan, S. K. Behera, P. Paul, B. Das, M. Suar, R. Jayabalan, D. Fawcett, G. E. J. Poinern and S. K. Tripathy, Biogenic Au@ZnO core-shell nanocomposites kill Staphylococcus aureus without provoking nuclear damage and cytotoxicity in mouse fibroblasts cells under hyperglycemic condition with enhanced wound healing proficiency, Med. Microbiol. Immunol., 2019, 208, 609–629 CrossRef CAS PubMed.
- L. E. Shi, S. K. Behera, P. Paul and M. Suar, Synthesis, antibacterial activity, antibacterial mechanism and food applications of ZnO nanoparticles: a review, Food Addit. Contam.,: Part A, 2014, 31, 173–186 CrossRef CAS PubMed.
- B. Abebe, E. A. Zereffa, A. Tadesse and H. C. A. Murthy, A review on enhancing the antibacterial activity of ZnO: mechanisms and microscopic investigation, Nanoscale Res. Lett., 2020, 15, 1–19 CrossRef PubMed.
- K. Tam, A. B. Djurisic, C. M. N. Chan, Y. Y. Xi, C. K. Tse, Y. H. Leung, W. K. Chan, F. C. C. Leung and D. W. T. Au, Antibacterial activity of ZnO nanorods prepared by a hydrothermal method, Thin Solid Films, 2008, 516, 6167–6174 CrossRef CAS.
- R. J. Vandebriel and W. H. De Jong, A review of mammalian toxicity of ZnO nanoparticles, Nanotechnol., Sci. Appl., 2012, 5, 61–71 CrossRef CAS PubMed.
- M. I. Khan, P. Paul, S. K. Behera, B. Jena, S. K. Tripathy, C. S. Lundborg and A. Mishra, To decipher the antibacterial mechanism and promotion of wound healing activity by hydrogels embedded with biogenic Ag@ZnO core-shell nanocomposites, Chem. Eng. J., 2021, 417, 128025 CrossRef CAS.
- L. I. Moura, A. M. Dias, E. Carvalho and H. C. De Sousa, Recent advances on the development of wound dressings for diabetic foot ulcer treatment-a review, Acta Biomater., 2013, 9, 7093–7114 CrossRef CAS PubMed.
- S. M. Opal, Interactions between coagulation and inflammation, Scand. J. Infect. Dis., 2003, 35, 545–554 CrossRef CAS PubMed.
- M. Levi, Inflammation and coagulation, Inflammation, 2017, 833–860 CAS.
- R. Lassila and R. A. Campbell, Management of coagulation disorders in severe inflammation, HemaSphere, 2019, 3, 95–98 CrossRef PubMed.
- S. Margetic, Inflammation and hemostasis, Mol. Genet. Metab., 2012, 22, 49–62 CAS.
- V. W. M. van Hinsbergh, Endothelium-Role in regulation of coagulation and inflammation, Semin. Thromb. Hemostasis, 2012, 34, 93–106 CAS.
- G. Singh, A. Nayal, S. Malhotra and V. Koul, Dual functionalized chitosan based composite hydrogel for haemostatic efficacy and adhesive property, Carbohydr. Polym., 2020, 247, 116757 CrossRef CAS PubMed.
- S. Chen and Z. Peng, Progress in the bionic study on gecko's micro-adhesion mechanism, Mech. Eng., 2007, 29, 9–17 Search PubMed.
- A. Mahdavi, L. Ferreira, C. Sundback, J. W. Nichol, E. P. Chan, D. J. D. Carter, C. J. Bettinger, S. Patanavanich, L. Chignozha and E. Ben-Joseph, A biodegradable and biocompatible gecko-inspired tissue adhesive, Proc. Natl. Acad. Sci. U. S. A., 2008, 105, 2307–2312 CrossRef CAS PubMed.
- G. Huber, H. Mantz, R. Spolenak, K. Mecke, K. Jacobs, S. N. Gorb and E. Arzt, Evidence for capillarity contributions to gecko adhesion from single spatula nanomechanical measurements, Proc. Natl. Acad. Sci. U. S. A., 2005, 102, 16293–16296 CrossRef CAS PubMed.
- Z. Peng and S. Chen, Effects of the relative humidity and water droplet on adhesion of a bio-inspired nano-film, Colloids Surf., B, 2011, 85, 717–721 CrossRef PubMed.
- J. Chen, Q. Peng, J. Liu and H. Zeng, Mussel-inspired cation-π interactions: Wet adhesion and biomimetic materials, Langmuir, 2023, 39, 17600–17610 CrossRef CAS PubMed.
- L. Li and H. Zeng, Marine mussel adhesion and bio-inspired wet adhesives, Biotribology, 2016, 5, 44–51 CrossRef.
- H. G. Silverman and F. F. Roberto, Understanding marine mussel adhesion, Mar. Biotechnol., 2007, 9, 661–681 CrossRef CAS PubMed.
- Y. Li and Y. Cao, The molecular mechanisms underlying mussel adhesion, Nanoscale Adv., 2019, 1, 4246–4257 RSC.
- V. M. Quan, K. M. Le, H. T. Dat, P.-T. H. Van, M.-A. T. Le, K. T.-T. Nguyen, H. N. Doan and T.-H. Nguyen, Design of a non-oxidative adhesive dopamine-grafted hyaluronic acid/NOCC hydrogel for enhanced cell spheroid formation and soft tissue regeneration, React. Funct. Polym., 2025, 206, 106108 CrossRef CAS.
- H. Shu, Z. Xia, X. Qin, X. Wang, W. Lu, Q. Luo, Z. Zhang and X. Xiong, The clinical efficacy of collagen dressing on chronic wounds: A meta-analysis of 11 randomized controlled trials, Front. Surg., 2022, 9, 978407 CrossRef PubMed.
- C. Fu, S. Shi, N. Wei, Y. Fan, H. Gu, P. Liu and J. Xiao, Biocompatible triple-helical recombinant collagen dressings for accelerated wound healing in microneedle-injured and photodamaged skin, Cosmetics, 2023, 10, 31 CrossRef CAS.
- Y. Wang, Y. Zhang, Y.-P. Yang, M.-Y. Jin, S. Huang, Z.-M. Zhuang, T. Zhang, L.-L. Cao, X.-Y. Lin and J. Chen, Versatile dopamine-functionalized hyaluronic acid-recombinant human collagen hydrogel promoting diabetic wound healing via inflammation control and vascularization tissue regeneration, Bioact. Mater., 2024, 35, 330–345 CAS.
- W. Zhu, Y. J. Chuah and D.-A. Wang, Bioadhesives for internal medical applications: A review, Acta Biomater., 2018, 74, 1–16 CrossRef CAS PubMed.
- D. S. Hwang, Y. Gim, D. G. Kang, Y. K. Kim and H. J. Cha, Recombinant mussel adhesive protein Mgfp-5 as cell adhesion biomaterial, J. Biotechnol., 2007, 127, 727–735 CrossRef CAS PubMed.
- Z. Zhang, C. He, Y. Rong, H. Ren, T. Wang, Z. Zou and X. Chen, A fast and versatile cross-linking strategy via o-phthalaldehyde condensation for mechanically strengthened and functional hydrogels, Natl. Sci. Rev., 2021, 8, nwaa128 CrossRef CAS PubMed.
- H. Ren, Z. Zhang, X. Cheng, Z. Zou and X. Chen, Injectable, self-healing hydrogel adhesives with firm tissue adhesion and on-demand biodegradation for sutureless wound closure, Sci. Adv., 2023, 9, eadh4327 CrossRef CAS PubMed.
- Z. Zeng, J. Zhang, Y. Gao, Y. Song, L. Liu, M. Zhu, W. Ma, J. Fu, D. Miao and C. Huang, Bioadhesive first-aid patch with rapid hemostasis and high toughness designed for sutureless sealing of acute bleeding wounds, Adv. Healthcare Mater., 2024, 2403412 Search PubMed.
- X. F. Tan, X. Y. Hu, X. Y. Pan, W. J. Xie, D. Y. Li, Y. Y. Yuan and J. Wang, An integrated Janus patch with asymmetric tissue adhesion for enhanced postoperative adhesion prevention, Polym. Sci. Technol., 2025, 1, 120–131 CrossRef CAS.
- M. Schäfer and S. Werner, Oxidative stress in normal and impaired wound repair, Pharmacol. Res., 2008, 58, 165–171 CrossRef PubMed.
- W. Zhang, L. Chen, Y. Xiong, A. C. Panayi, A. Abududilibaier, Y. Hu, C. Yu, W. Zhou, Y. Sun and M. Liu, Antioxidant therapy and antioxidant-related bionanomaterials in diabetic wound healing, Front. Bioeng. Biotechnol., 2021, 9, 707479 CrossRef PubMed.
- I. Bellezza, I. Giambanco, A. Minelli and R. Donato, Nrf2-Keap1 signaling in oxidative and reductive stress, Biochim. Biophys. Acta, Mol. Cell Res., 2018, 1865, 721–733 CrossRef CAS PubMed.
- J. Cai, S. Liu, Q. Zhong, Y. Shang, Z. Chen, Y. Yao, B. Zhou, F. Yin, J. Zhao and L. Zheng, Multifunctional PDA/ZIF8-based hydrogel dressing modulates the microenvironment to accelerate chronic wound healing by ROS scavenging and macrophage polarization, Chem. Eng. J., 2024, 487, 150632 CrossRef CAS.
- J. W. Kaspar, S. K. Niture and A. K. Jaiswal, Nrf2:INrf2 (Keap1) signaling in oxidative stress, Free Radical Biol. Med., 2009, 47, 1304–1309 CrossRef CAS PubMed.
- J. Janda, V. Nfonsam, F. Calienes, J. E. Sligh and J. Jandova, Modulation of ROS levels in fibroblasts by altering mitochondria regulates the process of wound healing, Arch. Dermatol. Res., 2016, 308, 239–248 CrossRef CAS PubMed.
- J. K. Kundu and Y.-J. Surh, Nrf2-Keap1 signaling as a potential target for chemoprevention of inflammation-associated carcinogenesis, Pharm. Res., 2010, 27, 999–1013 CrossRef CAS PubMed.
- H. J. Kim and N. D. Vaziri, Contribution of impaired Nrf2-Keap1 pathway to oxidative stress and inflammation in chronic renal failure, Am. J. Physiol., 2010, 298, F662–F671 CAS.
- W. Tu, H. Wang, S. Li, Q. Liu and H. Sha, The anti-inflammatory and anti-oxidant mechanisms of the Keap1/Nrf2/ARE signaling pathway in chronic diseases, Aging Dis., 2019, 10, 637–651 CrossRef PubMed.
- J. Ding, B. Gao, Z. Chen and X. Mei, An NIR-triggered Au nanocage used for photo-thermo therapy of chronic wound in diabetic rats through bacterial membrane destruction and skin cell mitochondrial protection, Front. Pharmacol., 2021, 12, 779944 CrossRef CAS PubMed.
- H. Zhao, Y. Wang, Y. Liu, K. Yin, D. Wang, B. Yu and H. Xing, ROS-induced hepatotoxicity under cypermethrin: Involvement of the crosstalk between Nrf2/Keap1 and NF-κB/IκB-α pathways regulated by proteasome, Environ. Sci. Technol., 2021, 55, 6171–6183 CrossRef CAS PubMed.
- X. Qi, X. Tong, S. You, R. Mao, E. Cai, W. Pan, C. Zhang, R. Hu and J. Shen, Mild hyperthermia-assisted ROS scavenging hydrogels achieve diabetic wound healing, ACS Macro Lett., 2022, 11, 861–867 CrossRef CAS PubMed.
- Y. K. Sung and S. W. Kim, Recent advances in polymeric drug delivery systems, Biomater. Res., 2020, 24, 12 CrossRef CAS PubMed.
- M. Gregoritza and F. P. Brandl, The Diels-Alder reaction: A powerful tool for the design of drug delivery systems and biomaterials, Eur. J. Pharm. Biopharm., 2015, 97, 438–453 CrossRef CAS PubMed.
- J. N. Cambre and B. S. Sumerlin, Biomedical applications of boronic acid polymers, Polymer, 2011, 52, 4631–4643 CrossRef CAS.
- S. Fuchs, A. U. Ernst, L. H. Wang, K. Shariati, X. Wang, Q. Liu and M. Ma, Hydrogels in emerging technologies for type 1 diabetes, Chem. Rev., 2020, 121, 11458–11526 CrossRef PubMed.
- E. Manzo, A. Schiano Moriello, F. Tinto, R. Verde, M. Allara, L. Petrocellis, E. Pagano, A. A. Izzo, V. Di Marzo and S. Petrosino, A glucuronic acid-palmitoylethanolamide conjugate (GLUPEA) is an innovative drug delivery system and a potential bioregulator, Cell, 2021, 10, 450 CrossRef CAS PubMed.
- W. Zhao, J. Hu and W. Gao, Glucose oxidase-polymer nanogels for synergistic cancer-starving and oxidation therapy, ACS Appl. Mater. Interfaces, 2017, 9, 23528–23535 CrossRef CAS PubMed.
- R. Narayan, S. Gadag, R. J. Mudakavi, S. Garg, A. M. Raichur, Y. Nayak and U. K. Nayak, Mesoporous silica nanoparticles capped with chitosan-glucuronic acid conjugate for pH-responsive targeted delivery of 5-fluorouracil, J. Drug Delivery Sci. Technol., 2021, 63, 102472 CrossRef CAS.
- W. Zhou, Z. Duan, J. Zhao, R. Fu, C. Zhu and D. Fan, Glucose and MMP-9 dual-responsive hydrogel with temperature sensitive self-adaptive shape and controlled drug release accelerates diabetic wound healing, Bioact. Mater., 2022, 17, 1–17 CAS.
- L. A. Wallace, L. Gwynne and T. Jenkins, Challenges and opportunities of pH in chronic wounds, Ther. Delivery, 2019, 10, 719–735 CrossRef CAS PubMed.
- M. Prabaharan, J. J. Grailer, S. Pilla, D. A. Steeber and S. Gong, Amphiphilic multi-arm-block copolymer conjugated with doxorubicin via pH-sensitive hydrazone bond for tumor-targeted drug delivery, Biomaterials, 2009, 30, 5757–5766 CrossRef CAS PubMed.
- S. Saghazadeh, C. Rinoldi, M. Schot, S. S. Kashaf, F. Jalilian, K. Nuutila, G. Giatsidis, P. Mostafalu and H. Derakhshandeh, Drug delivery systems and materials for wound healing applications, Adv. Drug Delivery Rev., 2018, 127, 138–166 CrossRef CAS PubMed.
- Z. Li, Y. Zhao, H. Liu, M. Ren, Z. Wang, X. Wang, H. Liu, Y. Feng, Q. Lin and C. Wang, pH-responsive hydrogel loaded with insulin as a bioactive dressing for enhancing diabetic wound healing, Mater. Des., 2021, 210, 110104 CrossRef CAS.
- H. Derakhshandeh, S. S. Kashaf, F. Aghabaglou, I. O. Ghanavati and A. Tamayol, Smart bandages: The future of wound care, Trends Biotechnol., 2018, 36, 1259–1274 CrossRef CAS PubMed.
- M. Cheng, Y. Cui, Y. Guo, P. Zhao, J. Wang, R. Zhang and X. Wang, Design of carboxymethyl chitosan-reinforced pH-responsive hydrogels for on-demand release of carvacrol and simulation of release kinetics, Food Chem., 2023, 405, 134856 CrossRef CAS PubMed.
- J. P. Jeong, K. Kim, E. Oh, S. Park and S. Jung, Self-healing hydrogels with intrinsic antioxidant and antibacterial properties based on oxidized hydroxybutanoyl glycan and quaternized carboxymethyl chitosan for pH-responsive drug delivery, Gels, 2025, 11, 169 CrossRef CAS PubMed.
- S. T. Zhang, W. X. He, J. W. Dong, Y. K. Chan, S. Q. Lai and Y. Deng, Tailoring versatile nanoheterojunction-incorporated hydrogel dressing for wound bacterial biofilm infection theranostics, ACS Nano, 2025, 19, 10922–10942 CrossRef CAS PubMed.
- Y. T. Yang, J. X. Wang, S. F. Huang, M. Li, J. Y. Chen, D. D. Pei, Z. Tang and B. L. Guo, Bacteria-responsive programmed self-activating antibacterial hydrogel to remodel regeneration microenvironment for infected wound healing, Natl. Sci. Rev., 2024, 11, nwae044 CrossRef CAS PubMed.
- Y. Cao, N. Li, M. Y. Chen, H. F. Cao, C. Zhou, Z. Q. Dong, J. Liang, Q. G. Wang, Y. J. Fan and X. D. Zhang, A cationic hydrogel for visual diagnosis and treatment of bacterial-infected wounds via mild, on-demand dual-phototherapy, Chem. Eng. J., 2025, 508, 160984 CrossRef CAS.
- Y. Wang, K. Liu, W. Y. Wei and H. L. Dai, A multifunctional hydrogel with photothermal antibacterial and antiOxidant activity for smart monitoring and promotion of diabetic wound healing, Adv. Funct. Mater., 2024, 34, 2402531 CrossRef CAS.
- Y. Zhang, T. Li, C. Zhao, J. Li, R. Huang, Q. Zhang, Y. Li and X. Li, An integrated smart sensor dressing for real-time wound microenvironment monitoring and promoting angiogenesis and wound healing, Front. Cell Dev. Biol., 2021, 9, 701525 CrossRef PubMed.
- Y. Yu, L. Zhang, B. Hu, Z. Wang, Q. Gu, W. Wang, C. Zhu and S. Wang, Borate bonds-containing pH-responsive chitosan hydrogel for postoperative tumor recurrence and wound infection prevention, Carbohydr. Polym., 2024, 339, 122262 CrossRef CAS PubMed.
- A. Roy, S. Zenker, S. Jain, R. Afshari, Y. Oz, Y. T. Zheng and N. Annabi, A highly stretchable, conductive, and transparent bioadhesive hydrogel as a flexible sensor for enhanced real-time human health monitoring, Adv. Mater., 2024, 36, 2404225 CrossRef CAS PubMed.
- X. Y. Chen, W. L. Song, H. W. Zhang, Y. W. Xu, B. C. Yi and Q. H. Zhou, Recent progress in stimulus-responsive hydrogel-based sensors for inflammation-associated diagnosis and surveillance, Chem. Eng. J., 2025, 506, 159756 CrossRef CAS.
- W. Wang, H. L. Zhou, Z. S. Xu, Z. H. Li, L. Q. Zhang and P. B. Wan, Flexible conformally bioadhesive MXene hydrogel electronics for machine learning-facilitated human-interactive sensing, Adv. Mater., 2024, 36, 2401035 CrossRef CAS PubMed.
- K. Q. Chang, C. Zhang and T. X. Liu, A comprehensive review on fabrication and structural design of polymer composites for wearable pressure sensors, Polym. Sci. Technol., 2025, 1, 3–24 CrossRef CAS.
- Z. S. Hou, T. J. Wang, L. Wang, J. J. Wang, Y. Zhang, Q. Zhou, Z. H. Zhang, P. Li and W. Huang, Skin-adhesive and self-healing diagnostic wound dressings for diabetic wounds healing recording and electrophysiological signal monitoring, Mater. Horiz., 2024, 11, 1997–2009 RSC.
- B. Mirani, Z. Hadisi, E. Pagan, S. Mohammad, H. Dabiri, A. Rijt, L. Almutairi, I. Noshadi, D. Armstrong and M. Akbari, Smart dual-sensor wound dressing for monitoring cutaneous wounds, Adv. Healthcare Mater., 2023, 12, 2203233 CrossRef CAS PubMed.
- H. Zhang, W. X. Li, S. Tang, Y. Chen, L. M. Lan, S. Li, M. Xiong, X. Hu, H. Y. Sun and J. Liu, A boron-based probe driven theranostic hydrogel dressing for visual monitoring and matching chronic wound healing, Adv. Funct. Mater., 2023, 33, 2305580 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |