Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Bimodal sensor employing a novel approach for simultaneous selective detection of Ni2+ and biomolecules via turn-on fluorescence supported by DFT and molecular docking†
Hazeena
Shinziya
a,
Avijit Kumar
Das
 *a,
Malavika S
Kumar
a,
Anish
Nag
b and
Malay
Dolai
*a,
Malavika S
Kumar
a,
Anish
Nag
b and
Malay
Dolai
 c
c
aDepartment of Chemistry, Christ University, Hosur Road, Bangalore, Karnataka 560029, India. E-mail: avijitkumar.das@christuniversity.in
bDepartment of Life Science, Christ University, Hosur Road, Bangalore, Karnataka 560029, India
cDepartment of Chemistry, Prabhat Kumar College, Purba Medinipur, Contai, West Bengal 721404, India
First published on 13th May 2025
Abstract
A bimodal sensor, (E)-2-(4-(diphenylamino)styryl)-1-methylquinolin-1-ium (DSM), was designed and synthesized for the simultaneous fluorescence turn-on detection of Ni2+ ion and biomolecules such as ct-DNA, BSA, and ovalbumin. Due to its distinct size and steric properties, DSM exhibits different binding modes when interacting with Ni2+ and DNA/proteins. The probe DSM possesses dual functionalities, allowing it to selectively detect Ni2+ at one binding site while interacting with ct-DNA, BSA, and ovalbumin at another. Thus, interactions of DSM with Ni2+ result in fluorescence enhancement at 377 nm and 400 nm, with a detection limit of 1.53 μM and binding constant of 1.2 × 106 M−1. Moreover, the binding of DSM with Ni2+ has been demonstrated via UV-vis, mass spectra, Jobs plots and DFT analysis. Conversely, binding of DSM with ct-DNA, ovalbumin and BSA led to an increase in the fluorescence at 425 nm and 435 nm, respectively, with the detection limit at micromolar (ct-DNA) and nanomolar (BSA and ovalbumin) levels. These interactions have been validated through UV-vis spectroscopy, fluorescence studies, and molecular docking analysis. Thus, this study underscores the potential of DSM as a versatile tool for simultaneous detection of both metal ions and biomolecules with a unique bimodal approach.
1. Introduction
The development of various chemosensors to detect different metal ions has garnered notable attention due to their extensive relevance in medical, biological, and industrial applications.1–3 To date, many researchers have investigated various analytical techniques for detecting different metal ions and anions, including atomic absorption spectroscopy, surface-enhanced Raman scattering, ion-selective electrodes, and inductively coupled plasma mass spectrometry.4 However, these techniques often involve complex procedures, demand skilled operators, and incur significant costs.5 Therefore, among the various analytical techniques, colorimetric and fluorescent assays are highly favoured as optical methods, as they provide numerous benefits, such as rapid response and high selectivity and sensitivity. Consequently, for the purpose of detecting different metal ions, a multitude of colorimetric and fluorescent sensors have been created.6–10Across various metal ions, nickel is a vital metal nutrient essential for sustaining human life through different functions involving several nickel-containing enzymes and coenzymes, such as urease, Ni–Fe hydrogenases, and F430.11 Meanwhile, excess nickel accumulation can adversely affect the respiratory and immune systems, although the exact mechanism of nickel imbalance has not yet been identified. Selective fluorescent probes can be used as cell-imaging reagents to detect trace levels of Ni2+ in living systems.12,13 Selective detection of Ni2+ is extremely important, but only a few Ni2+-selective peptide,14,15 protein,16 polymer17,18 and fluorescent sensors have been developed so far.19–22 The development of chemosensors for selective detection of Ni2+ is challenging.
Current research is also focusing on the interactions of ligands with proteins and nucleic acids, as these molecules serve as a model for drug design and cancer treatment.23 Thus, suitable dye molecules that bind to DNA and protein can be used for the detection of nucleic acids or proteins.24 The binding interactions of the dye with nucleic acid can be easily understood by the change in the absorption or emission spectra. The relatively low demands and the high sensitivity of the equipment aid in promoting the fluorometric detection of biomacromolecules as the key method. In this regard, a variety of fluorescent probes are particularly helpful (“light-up probes”) since their emission intensity dramatically increases when they form complexes with proteins or DNA.25
In this respect, various fluorescent probes whose emission intensity increases significantly upon complex formation with DNA or proteins are especially useful (“light-up probes”). This light up fluorescence technique is commonly applied in biology, biochemistry and also in medicine.26 Along with the biomacromolecules, several small molecules turn out to be essential within cells, and their concentration in the cellular medium determine function or malfunction of the physiological processes. Thus, we herein developed a multifunctional styryl dye, (E)-2-(4-(diphenylamino)styryl)-1-methylquinolin-1-ium (DSM), for selective detection of the metal ion Ni2+ and biomacromolecules like ct-DNA, BSA and ovalbumin. In this scenario, it has been demonstrated that styryl dyes with a cationic hetarene unit with donor-acceptor sites exhibit favourable properties for DNA sensing.27–29 Additionally, we have shown that donor (triphenyl amine)-substituted- with acceptor (quinolinium) derivatives DSM exhibit favorable photophysical properties, making them suitable as versatile building blocks for fluorescent light-up probes for DNA or protein.30,31 Although there are reports for the detection of Ni2+ and DNA/proteins by developing individual sensors, the simultaneous detection of Ni2+ and DNA/proteins by designing a single multifunctional chemosensor like DSM is rare (Table S1†). Therefore, here we have combined two types of sensing phenomena in DSM that associates with DNA/proteins through the quaternary quinolinium motif and Ni2+ through the triphenyl amine part (Scheme 1).
Herein, we have developed a multifunctional sensor (E)-2-(4-(diphenylamino)styryl)-1-methylquinolin-1-ium (DSM) using Knoevenagel-condensation32 of 1,2-dimethylquinolin-1-ium (1) with 4-(diphenylamino)benzaldehyde in 66% yield (Scheme 2). The chemical structure of DSM was confirmed using 1H-NMR, 13C-NMR, and mass spectrometry (Fig. S9–S11, ESI†).
 | ||
| Scheme 2 (a) Methyl iodide and ethanol refluxed at 50 °C, 48 h. (b) Piperidine and ethanol refluxed at 70 °C, overnight. | ||
2. Experimental
2.1 Materials and instrumentation
2.2 Synthesis and characterization of DSM
Synthesis of 1,2-dimethyl quinolinium iodide was carried out using the reported literature procedure.33 2-Methyl quinaldine (0.725 g, 4.5 mmol) was dissolved in ethanol and methyl iodide (9.3 mmol) was added to it. The reaction mixture was then heated to reflux for 48 hours at 80 °C. A yellow-colored precipitate was formed and the precipitate was filtered and dried for the next step without further purification. 1,2-Dimethyl quinolinium iodide (0.208 g, 0.7 mmol) and 4-diphenyl amino benzaldehyde (0.2 g, 7.31 mmol) were dissolved in ethanol (10 ml) and a catalytic amount of piperidine was added to the reaction mixture, which was further refluxed for 24 hours. Reaction progress was monitored by TLC and a red precipitate was formed by the addition of diethyl ether to the reaction mixture. The red precipitate was further purified via column chromatography using 2–3% methanol in chloroform as the eluent to obtain a pure red product.Yield: 200 mg, 66%. Mp-135–140 °C. 1H NMR (DMSO-d6, 400 MHz): δ (ppm): 8.51 (d, 1H, J = 9.2 Hz), 8.33 (d, 2H, J = 8 Hz), 8.21 (d, 1H, J = 15.6 Hz), 8.16 (m, 2H, J = 15.6 Hz), 7.89 (m, 3H), 7.71 (d, 1H, J = 15.6 Hz), 7.42 (t, 4H, J = 7.6 Hz), 7.2 (q, 6H), 6.96 (d, 2H, J = 8.4 Hz), 4.51 (s, 3H). 13C NMR (DMSO-d6, 100 MHz): δ (ppm): 156.6, 150.9, 147.6, 146.3, 143.6, 139.6, 135.1, 131.6, 130.4, 129.1, 127.9, 127.8, 126.3, 125.5, 121.2, 120.2, 119.6, 116.3. Mass (MS): M+ calculated for C30H25N2+ is 413.201; found: 413.250.
3. Results and discussion
3.1 Binding study with Ni2+
The emission properties of DSM were studied by adding different interfering metal cations like Cu2+, Hg2+, Al3+, Ni2+, Mn2+, Zn2+, Co2+, Cd2+, Cr3+, Fe2+, Fe3+, and Pb2+ (c = 2 × 10−4 M) in CH3CN-HEPES buffer (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v, pH = 7.4). Initially, DSM exhibits two absorption peaks at 331 nm and 497 nm, and results a gradual decrease of absorbance with a moderate shift of wavelength with the incremental addition of Ni2+ (Fig. 1a). For DSM and DSM–Ni2+, the molar absorption coefficients at 497 nm were determined to be 5.3 × 104 liter mol−1 cm−1 and 2.5 × 104 liter mol−1 cm−1, respectively.
3, v/v, pH = 7.4). Initially, DSM exhibits two absorption peaks at 331 nm and 497 nm, and results a gradual decrease of absorbance with a moderate shift of wavelength with the incremental addition of Ni2+ (Fig. 1a). For DSM and DSM–Ni2+, the molar absorption coefficients at 497 nm were determined to be 5.3 × 104 liter mol−1 cm−1 and 2.5 × 104 liter mol−1 cm−1, respectively.
In the emission experiment, the ligand DSM itself exhibits a weak emission signal at 400 nm (λex = 331 nm) with a quantum yield (Φ = 0.19). With further increase in the concentration of Ni2+, the emission intensity of DSM was notably enhanced by 6-fold by the generation of another blue shifted emission peak at 377 nm (Δλ = 23 nm) along with the ligand signal at 400 nm with a high quantum yield (Φ = 0.76) exhibiting naked eye fluorescence colour change from light orange to blue (Fig. 1c). The decrease of the absorbance and increase of the emission intensity of DSM varied up to the saturation level with increasing concentrations of Ni2+ (Fig. 1b and d).
3.2 Interference study
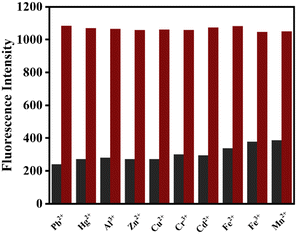
Fluorescence titration experiments were performed to evaluate the selective binding affinity of DSM towards Ni2+ in the presence of various metal ions such as Cu2+, Hg2+, Al3+, Ni2+, Mn2+, Co2+, Zn2+, Cd2+, Cr3+, Fe2+, Fe3+, and Pb2+ in CH3CN–aqueous HEPES buffer (7/3, v/v, pH = 7.4). It is noted that the presence of Ni2+ only led to a detectable increase in the fluorescence intensity and no other significant equivalent emission enhancement was observed in the presence of other interfering metal ions (Fig. 2a and b). The bar diagram represents the fluorescence behaviour of DSM towards various metal ions with a remarkable selectivity towards Ni2+. The blue bar with the highest intensity indicates the fluorescence enhancement of DSM in the presence of Ni2+ and orange bars represent no considerable binding of DSM with other metal ions (Fig. 2c).3.3 Competition study
Fluorometric analysis was used to investigate the effect of competing metal ions on DSM binding to Ni2+ to better analyse the selectivity of DSM for Ni2+, where 10 equivalents of Ni2+ and other metal ions were used in a cross-contamination study. Notably, the fluorescence enhancement for Ni2+ binding remained unperturbed (red bars), and the other interfering metal ions did not affect the sensing of Ni2+ (black bars). This indicates that the receptor DSM is highly selective and sensitive for Ni2+ (Fig. 3).The detection limit of DSM for Ni2+ was calculated as 1.53 μM based on the fluorescence titration studies using the formula DL = K × Sb1/S, where K = 3, Sb1 is the standard deviation of the blank solution, and S is the slope of the calibration curve (Fig. S4†).34 The binding stoichiometry was demonstrated through Job's plot analysis showing 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding of DSM with Ni2+ (Fig. S8†) and the association constant (Ka) of DSM with Ni2+ was calculated as 1.2 × 106 M−1 (error <10%) from the fluorescence titration experiment (Fig. S2†).35
1 binding of DSM with Ni2+ (Fig. S8†) and the association constant (Ka) of DSM with Ni2+ was calculated as 1.2 × 106 M−1 (error <10%) from the fluorescence titration experiment (Fig. S2†).35
3.4 Reversibility test
To further explore the binding nature of DSM with Ni2+, a fluorescence titration experiment was performed. By adding excess Na2EDTA (0–2.0 equivalents) to the DSM–Ni2+ solution, the fluorescence intensity was gradually decreased due to the removal of Ni2+ from the metal binding chamber of DSM by the strong binding of Na2EDTA with Ni2+ (Fig. 4). The experiment was repeated for several cycles with alternate additions of Ni2+ and Na2EDTA (Fig. S14†). A systematic switching on–off pattern was observed between DSM and DSM with Ni2+ followed by the addition of EDTA. Thus, the reversible binding pattern of Ni2+ with DSM has been demonstrated. | ||
| Fig. 4 Fluorescence changes of DSM + Ni 2+ complex solution (c = 2 × 10−5 M) upon the addition EDTA (c = 2 × 10−4 M). | ||
3.5 Dipstick method
To investigate the practical application of sensor DSM towards the sensing of Ni2+, test strips were prepared by immersing the TLC plates into the receptor solution (c = 2 × 10−5 M) in CH3CN–H2O (7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3, v/v). When DSM–Ni2+ and DSM-coated test strips were exposed to UV light, there was no significant fluorescence observed for the DSM-coated test strip (Fig. S13,† left), but the DSM–Ni2+-treated test kit exhibited a noticeable turn-on blue fluorescence, which was also observed in the solution phase (Fig. S13,† right). This demonstrates that the test strips, also referred to as dipsticks, selectively detect Ni2+ by enhancement of fluorescence which can also be detectable with the naked eye in solid phase. Such dipsticks provide instant qualitative results, eliminating the need for complex instrumental analysis.
3, v/v). When DSM–Ni2+ and DSM-coated test strips were exposed to UV light, there was no significant fluorescence observed for the DSM-coated test strip (Fig. S13,† left), but the DSM–Ni2+-treated test kit exhibited a noticeable turn-on blue fluorescence, which was also observed in the solution phase (Fig. S13,† right). This demonstrates that the test strips, also referred to as dipsticks, selectively detect Ni2+ by enhancement of fluorescence which can also be detectable with the naked eye in solid phase. Such dipsticks provide instant qualitative results, eliminating the need for complex instrumental analysis.
3.6 Probable binding mode in solution phase
Initially, DSM alone exhibited weak fluorescence, but a notable fluorescence enhancement was observed at 400 nm in the presence of Ni2+. Since DSM is a donor–acceptor type of molecule, there is no significant fluorescence in the absence of Ni2+ due to the highly efficient PET process from the donor triphenyl amine moiety to acceptor quinolinium backbone (Scheme 3) (off-state). Significantly, the lone pair of electrons on the nitrogen of the triphenyl amine moiety coordinates with Ni2+, which consequently suppresses the PET process and operates through the chelation enhanced fluorescence (CHEF) effect, which results in notable fluorescence enhancement (on-state).36 The emission intensity of DSM is notably weak (Φ = 0.01), which is likely due to radiationless deactivation of the excited state caused by the isomerization process due to free rotation around the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, a general phenomenon commonly observed in styryl dyes. Due to co-ordination of Ni2+ with DSM, C
C bond, a general phenomenon commonly observed in styryl dyes. Due to co-ordination of Ni2+ with DSM, C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond isomerization was inhibited owing to steric hindrance, which resulted in increased fluorescence intensity of the ligand.37 This observation can be verified by blocking C
C bond isomerization was inhibited owing to steric hindrance, which resulted in increased fluorescence intensity of the ligand.37 This observation can be verified by blocking C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond rotation by increasing the viscosity of the solution due to the inhibition of rotational relaxation of the excited molecule (Fig. S1, ESI†).38 Notably, in highly viscous media with a high proportion of glycerol in methanol, a noticeable rise in fluorescence intensity was observed at 461 nm. Due to the suppression of twisting intramolecular charge transfer (TICT) brought on by conformational changes from rotation around the C
C bond rotation by increasing the viscosity of the solution due to the inhibition of rotational relaxation of the excited molecule (Fig. S1, ESI†).38 Notably, in highly viscous media with a high proportion of glycerol in methanol, a noticeable rise in fluorescence intensity was observed at 461 nm. Due to the suppression of twisting intramolecular charge transfer (TICT) brought on by conformational changes from rotation around the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond, geometrical rearrangement in the excited state may slow down under high viscosity conditions, leading to enhanced fluorescence. The formation of a complex between DSM and Ni2+ has been verified through the emergence of a mass peak at m/z = 582.950, attributed to [DSM + Ni2+ + 2Cl− + CH3CN] (Fig. S12†), which has also been justified by a 1
C bond, geometrical rearrangement in the excited state may slow down under high viscosity conditions, leading to enhanced fluorescence. The formation of a complex between DSM and Ni2+ has been verified through the emergence of a mass peak at m/z = 582.950, attributed to [DSM + Ni2+ + 2Cl− + CH3CN] (Fig. S12†), which has also been justified by a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometric ratio in the Jobs plot analysis and theoretical calculation.
1 binding stoichiometric ratio in the Jobs plot analysis and theoretical calculation.
3.7 Theoretical calculation for Ni2+ binding study
The theoretical calculation has been demonstrated through optimization of the DSM and DSM–Ni2+ structures through DFT and TD-DFT calculations. In the optimized structure of the DSM–Ni2+ complex, Ni2+ shows tetrahedral coordination with the charged ligand DSM employing four co-ordinations, consisting of one nitrogen from the triphenyl amine group of DSM, two from counter anions Cl− and one from the nitrogen of the acetonitrile solvent. From the optimized structure of the DSM–Ni2+ complex, the calculated Ni–N (triphenyl amine and acetonitrile nitrogen) bond distances are 1.916 Å and 1.839 Å, respectively, and the Ni–Cl bond distance is 2.171–2.191 Å. Meanwhile, the bond angles of nitrogen and chlorine with Ni are estimated as 176.86° and 175.23°, respectively. Additionally, the HOMO–LUMO energy gap is found to be ΔE = 6.82 eV and 4.97 eV for DSM and the DSM–Ni2+ complex, respectively, indicating their contribution to the stabilization of complex formation (Fig. 5c). Based on the DFT-optimized structures, the total energies of DSM alone and the DSM–Ni2+ complex are 136.55 kcal mol−1 and 225.59 kcal mol−1, respectively. Therefore, the energy difference between the DSM–Ni2+ complex and DSM alone is calculated to be 89.04 kcal mol−1. On the other hand, the changes in the absorbance of DSM occur upon binding with Ni2+ at 330 nm and 490 nm. From the theoretical study, the corresponding estimated absorption bands have been calculated at 329 nm (E = 4.760, f = 0.0079 nm) and 498 nm (E = 3.489, f = 0.1609). For the transition from S0 → S15 and S0 → S2, the experimental data are in good agreement with the theoretical computations; hence, the experimental data align well with the theoretical calculations. | ||
| Fig. 5 Geometrically optimized molecular structures of (a) DSM and (b) DSM–Ni2+ complex. (c) Frontier molecular orbital with the energy difference of DSM and the DSM–Ni2+ complex. | ||
3.8 Biological applications
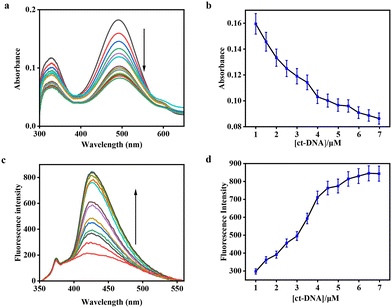
Herein, the EB replacement assay has been performed by the fluorescence experiment in Tris-HCl buffer, pH = 7.2. Initially, high fluorescence intensity has been observed for EB bound ct-DNA at 627 nm, which was significantly quenched with the increase in concentrations of the compound DSM. This fluorescence quenching is mainly due to the replacement of EB from EB bound ct-DNA complex by DSM. This confirms the intercalation binding mode of DSM with ct-DNA.41
By using the linear Stern–Volmer equation, the quenching of fluorescence in ct-DNA bound EB can be well understood, in which DSM is the quencher:42
| I0/I = 1 + KsvQ |
Initially, DSM exhibits strong absorption signals at 330 nm and 490 nm. However, increasing concentrations of BSA and ovalbumin results in significant decreases in the corresponding absorption signals (Fig. 8a and 9a). In the fluorescence experiment, DSM exhibited weak emission signal in the absence of BSA and ovalbumin at 425 nm with very low quantum yield (Φ0 = 0.01). However, incremental increases in the concentration of BSA and ovalbumin to the DSM solution resulted in the enhancement of fluorescence intensities by 3-fold at 435 nm and 425 nm with comparatively high fluorescence quantum yields at Φ = 0.02 and Φ = 0.13, respectively (Fig. 8c and 9c). The detection limits of DSM for BSA and ovalbumin calculated from UV-vis and fluorescence measurements are 7 nM, 5 nM and 17 nM, 5 nM, respectively (Fig. S6 and S7†). The binding constants of DSM with BSA and ovalbumin obtained from non-linear fitting curves from spectrofluorometric titrations are 1.2 × 104 M−1 and 5.4 × 105 M−1, respectively (Fig. S3b and c†).
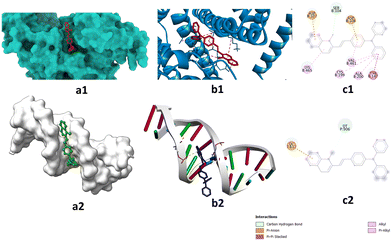
The same mechanism that explains the fluorescence light-up effect in the presence of proteins also explains the association of DSM with nucleic acids. This mechanism involves a combination of restricted conformational freedom of the molecule and suppressed formation of an ICT or CS excited state within the binding site. The variation of absorbance and the fluorescence response of DSM on binding with DNA and proteins is due to the different polarity of the binding cavities and the CS or ICT state is stabilized more proficiently inside the more polar DNA binding cavity compared with the less polar proteins (BSA and ovalbumin) binding pocket.43,44 The comparatively significant light-up effect in the presence of proteins resulted in a decrease of the CS or ICT state within the binding site.
4. Conclusion
The multifunctional sensor DSM was successfully designed and synthesized for the simultaneous fluorescence turn-on detection of metal ion Ni2+ and biomolecules such as ct-DNA, BSA, and ovalbumin. The unique structural characteristics of DSM allow it to exhibit different binding modes due to its distinct size and steric demands. The bimodal functionalities of DSM enable selective detection of Ni2+ at one binding site while interacting with ct-DNA, BSA, and ovalbumin at another. The interaction of DSM with Ni2+ results in fluorescence enhancement, with a specific detection limit and binding constant. Similarly, binding investigations with ct-DNA, BSA, and ovalbumin also show increased fluorescence with the detection limit at the micromolar (ct-DNA) and nanomolar (BSA and ovalbumin) levels. The binding of DSM with Ni2+ was confirmed through mass spectrometry, Job's plots, and DFT analysis, while the binding interactions with ct-DNA, BSA, and ovalbumin were demonstrated using UV-vis, fluorescence, and docking studies. This study highlights the potential of DSM as a versatile probe for detecting both metal ions and biomolecules.Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts of interest to declare.Acknowledgements
The authors would like to thank Christ University, Bengaluru, for the research facilities and Centre for Research, Christ University, for the seed money grant (grant approval number CU-ORS-SM-24/09). Avijit Kumar Das specially acknowledges State University Research Excellence (SERB-SURE) of the Science and Engineering Research Board (SERB) (File Number: SUR/2022/002461) under the Department of Science and Technology, Government of India, for financial support by the research grant and research fellowship for Hazeena Shinziya.References
- V. Raju, R. SelvaKumar, S. K. AshokKumar, Y. Tharakeswar and S. K. Sahoo, Inorg. Chem. Commun., 2019, 101, 74–80 CrossRef CAS.
- M. Yang, L. Ma, J. Li and L. Kang, RSC Adv., 2019, 9, 16812–16818 RSC.
- W. Sik Na, P. Raj, N. Singh and D. O. Jang, Tetrahedron Lett., 2019, 39, 151075 CrossRef.
- C. Gao, X. Liu, X. Jin, J. Wu, Y. Xie, W. Liu, X. Yao and Y. Tang, Sens. Actuators, B, 2013, 185, 125–131 CrossRef CAS.
- H. H. Hammud, S. E. Shazly, G. Sonji, N. Sonji and K. H. Bouhadir, Spectrochim. Acta, Part A, 2015, 150, 94–103 CrossRef CAS PubMed.
- (a) D. Maity, D. Karthigeyan, T. K. Kundu and T. Govindaraju, Sens. Actuators, B, 2013, 176, 831–837 CrossRef CAS; (b) A. K. Das and S. Goswami, Sens. Actuators, B, 2017, 245, 1062–1125 CrossRef CAS.
- (a) W. Du, R. J. Liu, J. Fang, H. Gao, Y. W. Wang and Y. Peng, Tetrahedron, 2019, 75, 130477 CrossRef CAS; (b) S. Goswami, S. Maity, A. C. Maity, A. k. Das, B. Pakhira, K. Khanra, N. Bhattacharyya and S. Sarkar, RSC Adv., 2015, 5, 5735–5740 RSC; (c) M. S. Kumar, A. K. Das, Y. Bylappa and A. Nag, RSC Adv., 2025, 15, 6708–6717 RSC.
- (a) T. B. Wei, B. R. Yong, L. R. Dang, Y. M. Zhang, H. Yao and Q. Lin, Dyes Pigm., 2019, 171, 107707 CrossRef CAS; (b) S. Vishnu, A. K. Das, Y. Bylappa, A. Nag and M. Dolai, Anal. Methods, 2024, 16, 8164–8178 RSC.
- Z. Gu, H. Cheng, X. Shen, T. He, K. Jiang, H. Qiu, Q. Zhang and S. Yin, Spectrochim. Acta, Part A, 2018, 203, 315–323 CrossRef CAS PubMed.
- S. C. Lee and C. Kim, Anal. Sci., 2019, 35, 1189–1193 CrossRef CAS PubMed.
- A. Sigel, H. Sigel and R. K. O. Sigel, Nickel and Its Surprising Impact in Nature, John Wiley & Sons, 2007, vol. 2 Search PubMed.
- D. W. Domaille, E. L. Que and C. J. Chang, Nat. Chem. Biol., 2008, 4, 168–175 CrossRef CAS PubMed.
- E. L. Que, D. W. Domaille and C. J. Chang, Chem. Rev., 2008, 108, 1517–1549 CrossRef CAS PubMed.
- D. A. Pearce, G. K. Walkup and B. Imperiali, Bioorg. Med. Chem. Lett., 1998, 15, 1963–1968 CrossRef PubMed.
- A. Torrado, G. K. Walkup and B. Imperiali, J. Am. Chem. Soc., 1998, 3, 609–610 CrossRef.
- L. L. Salins, E. S. Goldsmith, M. C. Ensor and S. Daunert, Anal. Bioanal. Chem., 2002, 372, 174–180 CrossRef CAS PubMed.
- B. Wang, Y. Hu and Z. Su, React. Funct. Polym., 2008, 7, 1137–1143 CrossRef.
- (a) B. Y. Wang, X. Y. Liu, Y. L. Hu and Z. X. Su, Polym. Int., 2009, 6, 703–709 CrossRef; (b) S. Goswami, S. Chakraborty, A. K. Das, A. Manna, A. Bhattacharyya, C. K. Quah and H.-K. Fun, RSC Adv., 2014, 4, 20922–20926 RSC.
- (a) L. Fabbrizzi, M. Licchelli, P. Pallavicini, A. Perotti, A. Taglietti and D. Sacchi, Chem. – Eur. J., 1996, 21, 75–82 CrossRef; (b) R. K. Pathak, J. Dessingou and C. P. Rao, Anal. Chem., 2012, 84, 8294–8300 CrossRef CAS PubMed.
- C. Bargossi, M. C. Fiorini, M. Montalti, L. Prodi and N. Zaccheroni, Coord. Chem. Rev., 2000, 208, 17–32 CrossRef CAS.
- (a) L. J. Jiang, Q. H. Luo, Z. L. Wang, D. J. Liu, Z. Zhang and H. W. Hu, Polyhedron, 2001, 20, 2807–2812 CrossRef CAS; (b) R. Joseph, B. Ramanujam, H. Pal and C. P. Rao, Tetrahedron Lett., 2008, 49, 6257–6261 CrossRef CAS.
- H. Wang, D. Wang, Q. Wang, X. Li and C. A. Schalley, Org. Biomol. Chem., 2010, 8, 1017–1026 RSC.
- Molecular Aspects of Anticancer Drug–DNA Interactions, ed. S. Neidle and M. Waring, CRC, Boca Raton, FL, 1993 Search PubMed.
- A. Granzhan, H. Ihmels and K. Jäger, Chem. Commun., 2009, 1249–1251 RSC.
- (a) G. Cosa, K. S. Focsaneanu, J. R. N. McLean, J. P. McNamee and J. C. Scaiano, Photochem. Photobiol., 2001, 73, 585–599 CrossRef CAS PubMed; (b) H. Shinziya, R. S. Menon and A. K. Das, RSC Adv., 2024, 14, 30631–30646 RSC.
- D. Monchaud and M. P. Teulade-Fichou, Org. Biomol. Chem., 2008, 4, 627–636 RSC.
- (a) R. W. Dirks and H. J. Tanke, Chem. Biol., 2006, 13, 559–561 CrossRef CAS PubMed; (b) C. V. Kumar, R. S. Turner and E. H. Asuncion, J. Photochem. Photobiol., A, 1993, 74, 231–238 CrossRef CAS; (c) A. K. Das, S. I. Druzhinin, H. Ihmels, M. Müller and H. Schönherr, Chem. – Eur. J., 2019, 25, 12703–12707 CrossRef CAS PubMed.
- (a) V. B. Kovalska, D. V. Kryvorotenko, A. O. Balanda, M. Y. Losytskyy, V. P. Tokar and S. M. Yarmoluk, Dyes Pigm., 2005, 67, 47–54 CrossRef CAS; (b) J.-S. Lee, Y. K. Kim, M. Vendrel and Y.-T. Chang, Mol. BioSyst., 2009, 5, 411–421 RSC; (c) D. V. Berdnikova, O. A. Fedorova, E. V. Tulyakova, H. Li, S. Kölsch and H. Ihmels, Photochem. Photobiol., 2015, 91, 723–731 CrossRef CAS PubMed.
- (a) Q. Li, Y. Kim, J. Namm, A. Kulkarni, G. R. Rosania, Y. H. Ahn and Y. T. Chang, Chem. Biol., 2006, 13, 615–623 CrossRef CAS PubMed; (b) M. Q. Wang, S. Liu, C. P. Tang, A. Raza, S. Li, L. X. Gao, J. Sun and S. P. Guo, Dyes Pigm., 2017, 136, 78–84 CrossRef CAS; (c) A. Manna and S. Chakravorti, J. Phys. Chem. B, 2012, 116, 5226–5233 CrossRef CAS PubMed.
- (a) R. W. Sinkeldam, N. J. Greco and Y. Tor, Chem. Rev., 2010, 110, 2579–2619 CrossRef CAS PubMed; (b) T. Deligeorgiev, A. Vasilev, S. Kaloyanova and J. J. Vaquero, Color. Technol., 2010, 126, 55–80 CrossRef CAS; (c) S. Vishnu, A. Nag and A. K. Das, Anal. Methods, 2024, 16, 5263–5271 RSC.
- (a) H. Özhalici-Ünal, C. L. Pow, S. A. Marks, L. D. Jesper, G. L. Silva, N. I. Shank, E. W. Jones, J. M. Burnette, P. B. Berget and B. A. Armitage, J. Am. Chem. Soc., 2008, 130, 12620–12621 CrossRef PubMed; (b) A. K. Das, H. Ihmels and S. Kölsch, Photochem. Photobiol. Sci., 2019, 18, 1373 CrossRef CAS PubMed.
- L. F. Tietze and U. Beifuss, Angew. Chem., Int. Ed. Engl., 1993, 32, 131–163 CrossRef.
- V. F. Traven, A. V. Manaev, A. Yu. Bochkov, T. A. Chibisova and I. V. Ivanov, Russ. Chem. Bull., 2012, 61, 1342–1362 CrossRef CAS.
- M. Shortreed, R. Kopelman, M. Kuhn and B. Hoyland, Anal. Chem., 1996, 8, 1414–1418 CrossRef PubMed.
- (a) H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 1949, 71, 2703–2707 CrossRef CAS; (b) Y. Shiraishi, Y. Kohno and T. Hirai, Ind. Eng. Chem. Res., 2005, 44, 847–851 CrossRef CAS.
- H. Niu, J. Liu, H. M. O'Connor, T. Gunnlaugsson, T. D. James and H. Zhang, Chem. Soc. Rev., 2023, 52, 2322–2357 RSC.
- R. N. Dsouza, U. Pischel and W. M. Nau, Chem. Rev., 2011, 111, 7941–7980 CrossRef CAS PubMed.
- M. A. Martin, M. Ballesteros and B. D. Castillo, Anal. Chim. Acta, 1985, 170, 95–100 CrossRef CAS.
- W. Sbliwa, G. Matusiak and B. Bachowska, Croat. Chem. Acta, 2006, 79, 513 Search PubMed.
- (a) J. H. Zhou, S. Q. Xia, J. R. Chen, X. S. Wang, B. W. Zhang, H. J. Zhang, P. Zou, X. Cheng Ai and J. P. Zhang, J. Photochem. Photobiol., A, 2004, 165, 143–147 CrossRef CAS; (b) Q. Zhang, F. Zhang, W. Wang and X. Wang, J. Inorg. Biochem., 2006, 100, 1344–1352 CrossRef CAS PubMed; (c) Y. F. Song and P. Yang, Polyhedron, 2001, 20, 501–506 CrossRef CAS.
- D. J. Patel, Acc. Chem. Res., 1979, 12, 118–125 CrossRef CAS.
- Y. Chen, M. Cai, Y. Zhang and W. Zheng, Study on The Mechanism of Chitosan Complex Formation with PEGFPC∼ 3 DNA, Pharmaceutical Biotechnology Beijing, 2005, vol. 5, p. 291 Search PubMed.
- A. Cuervo, P. D. Dans, J. L. Carrascosa and L. Fumagalli, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 3624–3630 Search PubMed.
- J. Eden, P. R. C. Gascoyne and R. Pethig, J. Chem. Soc., Faraday Trans., 1980, 76, 426–434 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sd00028a |
| This journal is © The Royal Society of Chemistry 2025 |