Open Access Article
Open Access ArticleComprehensive studies to improve ultrasensitive detection of HIV-1 p24 antigen†
Evan
Reboli
a,
Ajoke
Williams
a,
Ankan
Biswas
a,
Tianwei
Jia
b,
Ying
Luo
c,
Mukesh
Kumar
d and
Suri
Iyer
 *a
*a
aDepartment of Chemistry, Kennedy College of Science, University of Massachusetts Lowell, 520 Olney Science Center, Lowell, Massachusetts 01854, USA. E-mail: Sure_iyer@uml.edu
bDepartment of Surgery, Beth Israel Deaconess Medical Center, Harvard Medical School, National Center for Functional Glycomics, CLS 11087-3 Blackfan Circle, Boston, Massachusetts 02115, USA
cDepartment of Chemistry, Center for Diagnostics and Therapeutics, Georgia State University, 788 Petit Science Center, Atlanta, Georgia 30302, USA
dDepartment of Biology, Center for Diagnostics and Therapeutics, Georgia State University, 622 Petit Science Center, Atlanta, Georgia 30302, USA
First published on 28th April 2025
Abstract
Early and accurate detection of HIV-1 p24 antigen is crucial for timely diagnosis and treatment, particularly in resource-limited settings where traditional methods often lack the necessary sensitivity for early-stage detection or is expensive. Here, we developed a layer-by-layer signal amplification platform employing fluorescent silica nanoparticles functionalized via bioorthogonal TCO/TZ chemistry. We evaluated nanoparticles of different sizes (25, 50, and 100 nm) and two dye-doped nanoparticle formulations to optimize signal intensity, detection limits, and nonspecific binding. The 25 nm RITC-doped nanoparticles demonstrated superior performance, achieving an ultra-low detection limit of 7 fg mL−1 with a broad linear range up to 1 ng mL−1. Compared to FITC-doped nanoparticles, RITC-doped nanoparticles provided enhanced brightness and signal strength. Further optimization revealed that using 50 μg of 25 nm nanoparticles yielded the best sensitivity while minimizing nonspecific binding. This nanoparticle-based assay significantly outperformed commercial ELISA kits, offering a broad dynamic range and improved sensitivity. Our platform presents a highly sensitive and adaptable approach for HIV-1 p24 antigen detection, with broad potential applications in point-of-care diagnostics and detection of other low-abundance biomarkers, ultimately enhancing early disease detection and treatment accessibility.
Introduction
Human immunodeficiency virus (HIV) remains a global health challenge, with 40 million people living with the virus, including 1.3 million newly infected individuals, according to United Nations Program on HIV/AIDS reports (UNAIDS 2023).1,2 With millions of individual infected and unaware of their status, HIV testing is essential for diagnosing new infections and for monitoring the viral loads.3 Therefore, it is very important to develop a detection strategy for early diagnosis and clinical treatment.4Detection of HIV mainly relies on HIV antibodies in the blood, but in early stages these antibodies may not be present making this method less effective.5 Therefore nucleic acid amplification testing (NAAT) is used to detect presence of HIV RNA.6 Although advancements in mutualization and nucleic acid amplifications have been made these tests are still cost prohibitive for resource-poor areas.6,7 CRISPR-based methods, while highly promising, offer rapid and sensitive HIV RNA detection but require expensive instruments, trained personnel and is cost prohibitive.8 Early detection enables the prompt start of antiretroviral therapy (ART), which is crucial for controlling the viral concentration, and preventing the progression to acquired immunodeficiency syndrome (AIDS).9 Children born to HIV-positive mothers can contract the virus through breast milk, making regular testing essential for the early detection of infection. Early diagnosis could help prevent this by providing timely information.10 Monitoring the viral load in children with HIV is crucial for tracking disease progression and determining when to adjust antiretroviral therapies.11 Fingerpick blood sampling is the most used biochip in point-of-care biomarker testing due to its minimally invasive nature, making it a practical alternative for HIV detection, particularly in low-resource settings where conducting full blood tests and utilizing hematology analyzers is not feasible.12,13
HIV p24 antigen is a well-conserved structural protein within HIV and is used to monitor viral load.14 p24 antigen can be detected using fourth-generation point-of-care (POC) lateral flow immunoassays approximately 15 days after HIV infection.15,16 However, it remains a challenge early in infection and detecting lower concentrations. Recently, we reported an ultrasensitive p24 assay with 46 fg mL−1 (1.84 fM) limit of detection (LOD) and a very broad linear range spanning 8 orders of magnitude, 46 fg mL−1 to 10 ng mL−1, utilizing a layer-by-layer fluorescent silica nanoparticles and bioorthogonal chemistries.17 The signal enhancement strategy shown in Fig. 1. First, anti-p24 antibodies are coated on the plate and blocked. Next varying concentrations of p24 are added and subsequently washed to remove unbound antigen. A secondary antibody modified with tetrazine (Ab2-TZ) is added, creating a sandwich of the antigen between two antibodies. After washing to remove excess Ab2-TZ, silica nanoparticles doped with either fluorescein isothiocyanate (FITC) or rhodamine B isothiocyanate (RITC) and functionalized with trans-cyclooctene (TCO) (FITC-SiO2-PEG5k-TCO & RITC-SiO2-PEG5k-TCO) are added to the microwell. The FITC-SiO2-PEG5k-TCO reacts with the tetrazine (TZ) conjugated to the antibody creating the first layer. Excess particles are washed leaving the first layer of bound particles, with unreacted TCO on their surfaces. The second layer is formed by the addition of the dye-doped silica nanoparticles functionalized with TZ (FITC-SiO2-PEG5k-TZ & RITC-SiO2-PEG5k-TZ), which readily reacts with unbound TCO forming the second layer with bound TZ particles. Similar to the first layer, particles functionalized with TCO can be added to further enhance the signal.
Hence, we focused our efforts on studying the effect of (1) nanoparticles of varying sizes (25 nm, 50 nm, and 100 nm) to potentially optimize their packing density around Ab2-TZ and (2) increasing the “brightness” of the particles by encapsulating them with a brighter dye (RITC).
Functionalized FITC-doped nanoparticles have been extensively employed in various in vivo applications due to their exceptional photostability. Building on their success in other imaging techniques, we initially investigated their potential as signal enhancers in a novel layer-by-layer assay.18,19 To further enhance signal intensity, we aimed to identify a dye with superior brightness.
The molecular brightness of a fluorophore is a critical factor in fluorescence-based applications, it is determined by the product of its molar absorptivity (ε) and fluorescence quantum yield (Φ), which collectively dictate the total light absorbed and the efficiency of fluorescence emission.20 Consequently, we replaced FITC with rhodamine B isothiocyanate (RITC), a rhodamine derivative with a molar absorptivity of 106![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 and a fluorescence quantum yield of 1.06, resulting in a molecular brightness of 112
000 M−1 cm−1 and a fluorescence quantum yield of 1.06, resulting in a molecular brightness of 112![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 360 M−1 cm−1.21,22 This represents an approximate 20% increase in brightness compared to fluorescein derivatives, which have a molar absorptivity of 78
360 M−1 cm−1.21,22 This represents an approximate 20% increase in brightness compared to fluorescein derivatives, which have a molar absorptivity of 78![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 M−1 cm−1 and a quantum yield of 0.92, yielding a molecular brightness of 71
000 M−1 cm−1 and a quantum yield of 0.92, yielding a molecular brightness of 71![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 M−1 cm−1.23,24
760 M−1 cm−1.23,24
In addition to enhanced brightness, rhodamine derivatives offer several advantages over fluorescein, including longer excitation and emission wavelengths, higher quantum efficiency, and improved water solubility. Furthermore, their distinct color shifts and elevated relative fluorescence units (RFU) make them particularly valuable for fluorescence-based biosensing applications.25
Experimental
Materials and equipment
Tetraethyl orthosilicate (TEOS), 3-aminopropyl triethoxysilane (APTES), fluorescein isothiocyanate (FITC, isomer I), dimethylformamide (DMF), triethylamine (TEA), ammonium hydroxide (NH4OH), N-hydroxysuccinimide (NHS), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC), hydrochloric acid (HCl), sodium hydroxide (NaOH), phosphate-buffered saline (PBS), bovine serum albumin (BSA), sulfo-NHS, Tween 20, and ethanol were purchased from Sigma-Aldrich. PEG-bis-CH2CO2H, MW 5000, (COOH-5k-PEG-COOH), methyltetrazine-PEG4-amine HCl salt , and TCO-PEG6-amine (TCO-PEG6-NH2) were purchased from BroadPharma. The mouse anti-HIV-1 p24 paired antibody and recombinant HIV-1 p24 protein were purchased from Prospec Protein Specialists, USA. Rhodamine B isothiocyanate (RITC), and absolute ethanol (EtOH) were purchased from Fisher Scientific. All chemicals were used as received without further purification. Ultrapure water obtained from a Millipore water purification system (18.2 MΩ cm−1, Milli-Q, Merck Millipore, Darmstadt, Germany) was used in all experiments.
, and TCO-PEG6-amine (TCO-PEG6-NH2) were purchased from BroadPharma. The mouse anti-HIV-1 p24 paired antibody and recombinant HIV-1 p24 protein were purchased from Prospec Protein Specialists, USA. Rhodamine B isothiocyanate (RITC), and absolute ethanol (EtOH) were purchased from Fisher Scientific. All chemicals were used as received without further purification. Ultrapure water obtained from a Millipore water purification system (18.2 MΩ cm−1, Milli-Q, Merck Millipore, Darmstadt, Germany) was used in all experiments.
Zeta-potential were measured by the Horiba SZ-100 Dynamic Light Scattering (DLS) Instrument, plates were read using the molecular devices spectra Max M3 (Plate Reader), and transmission electron microscopy (TEM) images were generated via the Philip CM12 transmission electron microscope.
Synthesis of FITC-SiO2-OH 100 nm and fabrication of FITC-SiO2-NH2, FITC-SiO2-PEG5k-COOH, FITC-SiO2-PEG5k-TZ, FITC-SiO2-PEG5k-TCO 100 nm
All reactions were performed under inert atmosphere as described in our previously reported paper.17Synthesis of FITC-SiO2-OH 50 nm nanoparticles
FITC-SiO2-OH was prepared according to reported procedures with modifications.26 In a 100 mL round-bottomed flask, FITC (10 mg) was mixed with EtOH (5 mL). APTES (20 μL, 0.085 mmol) was added under inert conditions. The mixture was stirred for 24 h at rt to yield the FITC–APTES adduct. Next absolute EtOH (50 mL), TEOS (0.75 mL, 3.36 mmol), NH4OH (30%, 2.1 mL) were added. The reaction mixture was stirred for 24 h at rt. The yellow dispersion was washed with absolute EtOH (10 mL × 3) through cycles of centrifugation (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 20 min)/sonication/redispersion. Finally, the yellow nanomaterial was redispersed in absolute EtOH (10 mL). RITC-SiO2-OH was prepared in a similar manner.
000g, 20 min)/sonication/redispersion. Finally, the yellow nanomaterial was redispersed in absolute EtOH (10 mL). RITC-SiO2-OH was prepared in a similar manner.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 20 min) and washed with EtOH 3×. The EtOH was removed, and the material was dried in vacuo for 2 h.
000 × g, 20 min) and washed with EtOH 3×. The EtOH was removed, and the material was dried in vacuo for 2 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 20 min) and washed with DMF 3× and EtOH 3×. The EtOH was removed, and the material was dried in vacuo for 2 h.
000 × g, 20 min) and washed with DMF 3× and EtOH 3×. The EtOH was removed, and the material was dried in vacuo for 2 h.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 × g, 20 min), washed with EtOH (1 mL) 3×, and PBS (1 mL) 3×. The final FITC-SiO2-PEG5k-TCO nanoparticles were resuspended in PBS (5 mg mL−1). The resulting stock solution was stored at 4 °C for further experimentation.
000 × g, 20 min), washed with EtOH (1 mL) 3×, and PBS (1 mL) 3×. The final FITC-SiO2-PEG5k-TCO nanoparticles were resuspended in PBS (5 mg mL−1). The resulting stock solution was stored at 4 °C for further experimentation.
Synthesis of FITC-SiO2-OH 25 nm nanoparticles
Initially FITC (10 mg) was mixed with EtOH absolute (1 mL) and APTES (140 μL, 0.6 mmol) in a round bottom flask under inert conditions. The mixture was stirred for 18 h forming a FITC–APTES adduct. To a flask containing EtOH (30 mL), TEOS (1.2 mL, 5.8 mmol), and NH4OH (30% aq solution, 1.2 mL) FITC–APTES adduct quickly was added (100 μL). This reaction was stirred vigorously for 24 h at rt, under inert conditions. After 24 h, TEOS (240 μL, 1.2 mmol) was added to the reaction mixture at rt under inert conditions and stirred vigorously for a further 24 h. The yellow dispersion was washed with absolute ethanol 3× (10 mL) through cycles of centrifugation (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g, 25 min)/sonication/redispersion. Finally, the nano material was redispersed in EtOH (5 mL).
000g, 25 min)/sonication/redispersion. Finally, the nano material was redispersed in EtOH (5 mL).
Results and discussion
Characterization of dye doped nanoparticles
First, we generated all materials and characterized them meticulously. FITC and RITC dye were used to form fluorescent silica nanoparticles ranging from 100 nm, 50 nm, and 25 nm as described.26,28 TEM images (Fig. 2) unequivocally confirmed the uniformity of nanoparticle size, which was further quantified using ImageJ to generate size distribution histograms (Fig. 3), demonstrating the expected frequency of the target diameters. The particles surface of the nanoparticles was then modified with a polyethylene glycol spacer terminated with a carboxylic group to reduce nonspecific binding. Next, TCO or TZ was conjugated to the fluorescent silica nanoparticles by first activating the surface with EDC/NHS, followed by the addition of NH2-PEG-TCO or NH2-PEG-TZ. This process resulted in the formation of FITC-SiO2-PEG5k-TCO or FITC-SiO2-PEG5k-TZ, respectively. | ||
| Fig. 2 TEM images of (A) 100 nm FITC-SiO2-OH, (B) 50 nm FITC-SiO2-OH, (C) 25 nm FITC-SiO2-OH, (D) 100 nm RITC-SiO2-OH, (E) 50 nm RITC-SiO2-OH, (F) 25 nm RITC-SiO2-OH. | ||
 | ||
| Fig. 3 Size distribution of (A) 100 nm FITC-SiO2-OH, (B) 50 nm FITC-SiO2-OH, (C) 25 nm FITC-SiO2-OH, (D) 100 nm RITC-SiO2-OH, (E) 50 nm RITC-SiO2-OH, (F) 25 nm RITC-SiO2-OH. | ||
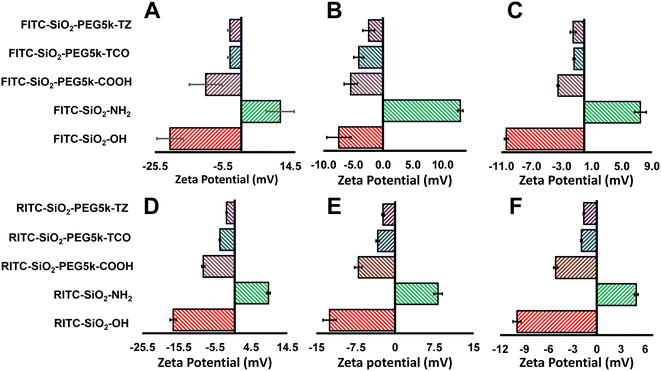
Zeta potential measurements were used throughout the surface modification process of the fluorescent silica nanoparticles to confirm the success of each modification step and assess surface charge, a technique commonly applied in related studies such as fluorescence immunoassays on paper and bio responsive quantum dot-enzyme platforms.29,30 Different surface chemistries exhibit varying surface potential charges, with the zeta potential of the dye-doped SiO2-OH particles initially ranging from −25 mV to −9 mV, attributed to the presence of hydroxyl groups on the surface, as shown in Fig. 4. Upon coating the surface with amine functional groups, the zeta potential shifted to a positive range of +9 to +15 mV, clearly indicating the successful addition of the amine group. Next, bis-carboxylic PEG linker was conjugated to the surface, where the zeta potential ranged from −9 to −3 mV. Finally, the addition of TZ or TCO functionalities caused a dip in the zeta potential rendering it slightly negative which reflects the final modification.
Functional and photostability
We evaluated the performance of each dye doped fluorescent silica nanoparticles at the same weight concentration. Next the excitation and emission spectra of equivalent weighted particles was measured and compared (Fig. S1 and S2†) for RITC and FITC. The results showed that nanoparticles with a size of 100 nm exhibited the highest intensity, where 25 nm particles were the lowest intensity, all things being equal (Fig. S3†).To ensure robustness we measured photostability and the functional stability of the TCO/TZ modified particles. Photostability studies show the particle fluorescence intensity remains stable over a month (Fig. S2†). Functional stability studies were conducted to assess the stability of TCO- and TZ-functionalized particles. Three concentrations of p24 antigen—control, 100 fg mL−1, and 1 ng mL−1 were analyzed weekly to determine any changes in performance trends. While a nonspecific binding increase was observed over the experiment, the overall trend in detection remained for three weeks (Fig. S3†).
Concentration optimization studies
To evaluate the optimal concentration of 50 nm fluorescent-doped nanoparticles for assay sensitivity, we tested three concentrations: 25 μg, 50 μg, and 75 μg of FITC-SiO2-TZ/TCO particles. At 25 μg (Fig. 4A), the assay achieved a limit of detection (LOD) of 150 fg mL−1 and a limit of quantification (LOQ) of 4 pg mL−1, with a linear range of 150 fg mL−1 to 1 ng mL−1. This concentration resulted in low non-specific binding and reduced variability but exhibited weaker signal intensity due to the limited number of particles. Increasing the concentration to 50 μg of particles (Fig. 4B) enhanced the LOD to 66 fg mL−1 and the LOQ to 1 pg mL−1, with a linear range of 66 fg mL−1 to 1 ng mL−1. This concentration provided an optimal balance between signal strength and non-specific binding, despite moderate levels of the latter. Further increasing the concentration to 75 μg (Fig. 4C) resulted in an excess of particles, raising the LOD significantly to 500 pg mL−1 and diminishing assay sensitivity. Therefore, 50 μg was identified as the optimal concentration, offering the best trade-off between signal strength and non-specific binding.Packing optimization studies
We hypothesized that smaller nanoparticles would improve packing efficiency, as illustrated in Fig. 5. To estimate packing density, we calculated the binding capacity of a 96-well plate (Table 1), assuming 400–500 ng of IgG antibody can bind per cm2 of the plate's surface area. For a 24 kDa p24 antibody (Ab1), this corresponds to approximately 1.0 × 1013 to 1.25 × 1013 antibodies per cm2, or 3.2 × 1012 antibodies per well, based on a binding density of 400 ng cm−2. The concentration range of p24 antigen in each well (200 μL) spans from 0.1 fg mL−1 to 10 μg mL−1, translating to approximately 5.0 × 102 to 5.0 × 1016 antigens per well.| Entry | 100 nm | 50 nm | 25 nm |
|---|---|---|---|
| Area covered by 4 particles (A1) | 1.156 × 10−9 cm2 | 5.76 × 10−10 cm2 | 3.61 × 10−10 cm2 |
| Surface area of well (A2) | 0.32 cm2 | 0.32 cm2 | 0.32 cm2 |
| Average length of PEG5k | 35 nm | 35 nm | 35 nm |
| Max NPs fit in 1st layer {N1 = 4(A2/A1)} | 1.10 × 109 | 2.22 × 109 | 3.55 × 109 |
| No of NPs in 1 μg (N2) | 1.5 × 107 | 6.7 × 107 | 1.65 × 108 |
| NPs required (μg) to cover the well surface (N1/N2) | 73 μg | 33 μg | 21 μg |
Using TEM analysis, we estimated approximately 6.7 × 107 dye-doped 50 nm particles per 1 μg. With an estimated particle binding area of 5.67 × 10−10 cm2 and a total well surface area of 0.32 cm2, the maximum particle binding capacity in a single layer was calculated to be 2.22 × 109 particles, equivalent to 33 μg of particles. These calculations underscore the importance of selecting appropriately sized nanoparticles and their concentrations to maximize assay sensitivity and minimize non-specific interactions. Layer-by-layer attachment of the particles are shown in confocal images (Fig. S5†). Increasing brightness of the particles are clearly visible in 2nd and 3rd layer.
Size comparison studies
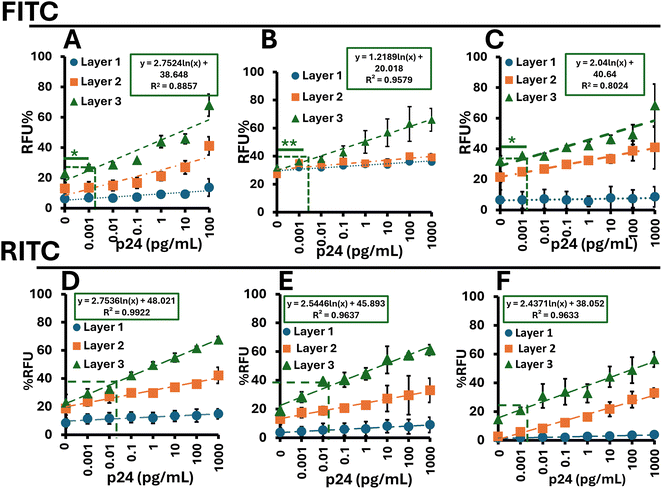
Size comparison studies were performed, as shown in Fig. 6, with the hypothesis that smaller particles would yield a lower limit of detection (LOD) due to improved packing of the layers. Starting with 100 nm FITC-doped particles (Fig. 7A), we reduced the particle size to 50 nm (Fig. 7B), and finally to 25 nm (Fig. 7C). The LOD decreased from 163 fg mL−1 for the 100 nm particles to 66 fg mL−1 for the 50 nm particles, and further to 13 fg mL−1 for the 25 nm particles. | ||
| Fig. 6 Theoretical packing density calculation of different sized (100 nm, 50 nm, and 25 nm) nanoparticles and number of antigen and antibody per well of 96 well plate during the assay. | ||
Similarly, the RITC-doped particles (Fig. 7D–F) exhibited a comparable trend, where particle size inversely affected the LOD. The 100 nm RITC-doped particles showed an initial LOD of 50 fg mL−1, which decreased to 42 fg mL−1 with the 50 nm particles, and 7 fg mL−1 with the 25 nm particles. These results, along with the extended linear range, are summarized in Table 2. Notably, the linear range extended from 7 fg mL−1 to 1 ng mL−1 with the 25 nm RITC-doped particles.
| Nanoparticle type | LOD (fg ml−1) | Linear range | Linearity (R2) |
|---|---|---|---|
| 100 nm FITC | 163 | 0.163–1 ng mL−1 | 0.86 |
| 50 nm FITC | 66 | 0.0066–1 ng mL−1 | 0.98 |
| 25 nm FITC | 13 | 0.0013–1 ng mL−1 | 0.81 |
| 100 nm RITC | 50 | 0.0504–1 ng mL−1 | 0.99 |
| 50 nm RITC | 42 | 0.0420–1 ng mL−1 | 0.96 |
| 25 nm RITC | 7 | 0.0072–1 ng mL−1 | 0.94 |
Dye comparison studies
When comparing dyes to dyes regardless of particle size we see that RITC-doped particles vastly outperform their FITC counterparts, this is likely due to the increase “brightness” of the fluorophore. We see this trend repeated in 100 nm FITC and 100 nm doped RITC particles in Fig. 7A and D respectively, 50 nm FITC and 50 nm doped RITC particles in Fig. 7B and E respectively, and for the 25 doped particles in Fig. 7C and F.Comparison with standard ELISA
To benchmark the performance of our novel assay, we conducted a direct comparison with a commercially available ELISA kit (human, mouse, & rat HIV-1 Gag p24 ELISA Kit – Quantikine, R&D Systems), which was enhanced with Amplex™ Red and Amplex™ UltraRed fluorescent substrates to align with the fluorescence-based nature of our method. The commercial ELISA demonstrated a linear range of 7.8–500 pg mL−1 and a limit of detection (LOD) of 3.35 pg mL−1 as seen in Fig. 8A. In contrast, our layer-by-layer amplification assay, incorporating 25 nm RITC-doped nanoparticles, exhibited a markedly superior performance with an LOD of 7 fg mL−1 and an extended linear range of 0.0072–1 ng mL−1 in layer 3 (Fig. 8B). Our assay offers over eight orders of magnitude greater sensitivity and a broader dynamic range than the commercial ELISA. Furthermore, as shown in Table 3, a direct comparison of our assay with other bioanalytical sensors for p24 demonstrates that our platform achieves superior sensitivity and an unmatched linear range.| Detection methods | Strategy | LOD | Detection range | Reference | Year |
|---|---|---|---|---|---|
| Fluorescence | Streptavidin-conjugated AuNCs | 5.0 pg mL−1 | Up to 1000 pg mL−1 | 31 | 2018 |
| Fluorescence and visual | TdT, CuNPs | 0.025 fg mL−1 | 0.025–1000 fg mL−1 | 32 | 2022 |
| LFIA-naked eye | PtNCs, CN/DAB | 0.8 pg mL−1 | 0.8–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pg mL−1 000 pg mL−1 |
33 | 2018 |
| Fluorescence | Streptavidin labeled FSN | 8.2 pg mL−1 | 8.2–1000 pg mL−1 | 34 | 2017 |
| Fluorescence | β-Sheets bind with Congo red | 0.61 pg mL−1 (3F-based) | 0.61–150 pg mL−1 | 35 | 2020 |
| 2.44 pg mL−1 (2F-based) | 2.44–150 pg mL−1 | ||||
| PEC | ALP-encapsulated liposomes | 0.63 pg mL−1 | 0.63–50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pg mL−1 000 pg mL−1 |
36 | 2018 |
| Electrochemical | Fe3O4@SiO2Ab1/AuNPs/EV-p24 Ab2 | 0.5 pg mL−1 | 0.5–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pg mL−1 000 pg mL−1 |
37 | 2013 |
| Fluorescence | Layer-by-layer signal amplification | 0.017 pg mL−1 (PBS) | 0.017–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pg mL−1 000 pg mL−1 |
17 | 2024 |
| 0.046 pg mL−1 (serum) | 0.046–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pg mL−1 000 pg mL−1 |
||||
| Fluorescence | Layer-by-layer signal amplification | 0.007 pg mL −1 |
0.007–10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 000 pg mL
−1 000 pg mL
−1
|
This work | 2024 |
Conclusions
We successfully synthesized and characterized FITC- and RITC-doped silica nanoparticles ranging in size from 25 nm, 50 nm, and 100 nm. Particle sizes were confirmed via TEM, while ImageJ analyses and zeta potential measurements validated surface modifications, including the addition of polyethylene glycol spacers and TCO or TZ functionalities. These nanoparticles were employed in an extensive comparative study to enhance the ultrasensitive detection of the HIV-1 p24 antigen. By leveraging bioorthogonal chemistries and advanced signal amplification techniques, we optimized their detection capabilities for improved sensitivity in point-of-care applications. Our bioorthogonal layer-by-layer approach differs from conventional multivalent binding (e.g., streptavidin–biotin) by reducing steric hindrance and enhancing binding kinetics, improving target accessibility and signal amplification. This is because we are using two small molecules that are much smaller in size compared to avidin, which is a protein. The advancement of multifunctional nanoparticles in diagnostics can significantly benefit from the use of mutually orthogonal combinations.38RITC-doped nanoparticles consistently outperformed FITC-doped counterparts, offering superior signal enhancement, due to improved molecular brightness. Our findings further validated that smaller nanoparticles enhance packing density, significantly lowering the limits of detection (LOD). Notably, the 3rd layer of 25 nm RITC-doped nanoparticles demonstrated a LOD of 7 fg mL−1 and an extended linear range from 7 fg mL−1 to 1 ng mL−1. —eliminating the need for sample dilution, even with highly concentrated specimens. This broad linear range spanning seven orders of magnitude simplifies sample preparation, reduces the risk of dilution errors, and makes the assay more user-friendly and efficient. Beyond HIV-1 p24 antigen detection, these developments demonstrate the platform's potential for diverse diagnostic applications requiring sensitive detection of low analyte concentrations. The method offers a broad linear range that eliminates the need for sample dilution when detecting multiple biomarkers. However, challenges remain, including limited nanoparticle stability (3 weeks at rt and the requirement of multiple wash steps) which can increase assay complexicity and time. We are actively working to improve nanoparticle stability and streamline the process for point-of-care diagnostics.
Data availability
The data supporting this article have been included as part of the ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for to the National Institute of Allergy and Infectious Diseases (grant no. 5R61AI140475). We would also like to express our gratitude to the UMass Lowell Core Research Facilities for their invaluable support throughout this project. Special thanks to Wendy Gavin for her expert assistance with the Dynamic Light Scattering Instrument (Horiba SZ-100) and to Anna Maria Routsi for her guidance and help with the TEM imaging. Their expertise greatly contributed to the success of our work.References
- UNAIDS, Global HIV & AIDS statistics — Fact sheet, 2024 Search PubMed.
- S. Payagala and A. Pozniak, Clin. Dermatol., 2024, 42, 119–127 CrossRef.
- M. Wu, B. Yang, L. Shi, Q. Tang, J. Wang, W. Liu, B. Li and Y. Jin, Anal. Chim. Acta, 2024, 1304, 342553 CrossRef CAS PubMed.
- E. M. Girardi, C. A. P. Sabin, A. d'Arminio and M. Monforte, JAIDS, J. Acquired Immune Defic. Syndr., 2007, 46, S3 CrossRef PubMed.
- G. Pantaleo, B. Correia, C. Fenwick, V. S. Joo and L. Perez, Nat. Rev. Drug Discovery, 2022, 21, 676–696 CrossRef CAS.
- P. Patel, D. Mackellar, P. Simmons, A. Uniyal, K. Gallagher, B. Bennett, T. J. Sullivan, A. Kowalski, M. M. Parker, M. LaLota, P. Kerndt, P. S. Sullivan and Centers for Disease Control and Prevention Acute HIV Infection Study Group, Arch. Intern. Med., 2010, 170, 66–74 CrossRef CAS PubMed.
- S. Tian, L. Huang, Y. Gao, Z. Yu and D. Tang, Sens. Diagn., 2023, 2, 707–713 RSC.
- N. Uno, Z. Li, L. Avery, M. M. Sfeir and C. Liu, Anal. Chim. Acta, 2023, 1262, 341258 CrossRef CAS.
- T. Liu, A. J. Politza, A. Kshirsagar, Y. Zhu and W. Guan, ACS Sens., 2023, 8, 4716–4727 CrossRef CAS PubMed.
- L. Abuogi, L. Noble and C. Smith, Pediatrics, 2024, 153, e2024066843 CrossRef PubMed.
- K. A. Veldsman, B. Laughton, A. Janse van Rensburg, P. Zuidewind, E. Dobbels, S. Barnabas, S. Fry, M. F. Cotton and G. U. van Zyl, AIDS, 2021, 35, 1247 CrossRef CAS.
- M. M. Bond and R. R. Richards-Kortum, Am. J. Clin. Pathol., 2015, 144, 885–894 CrossRef CAS.
- E. Cheah, D. P. Tran, M. T. Amen, R. D. Arrua, E. F. Hilder and B. Thierry, Anal. Chem., 2022, 94, 1256–1263 CrossRef CAS.
- S. Tang, J. Zhao, A. Wang, R. Viswanath, H. Harma, R. F. Little, R. Yarchoan, S. L. Stramer, P. N. Nyambi, S. Lee, O. Wood, E. Y. Wong, X. Wang and I. K. Hewlett, Clin. Vaccine Immunol., 2010, 17, 1244–1251 CrossRef CAS.
- N. E. Rosenberg, C. D. Pilcher, M. P. Busch and M. S. Cohen, Curr. Opin. HIV AIDS, 2015, 10, 61 CrossRef CAS PubMed.
- E. R. Gray, R. Bain, O. Varsaneux, R. W. Peeling, M. M. Stevens and R. A. McKendry, AIDS, 2018, 32, 2089–2102 CrossRef CAS PubMed.
- T. Jia, V. Saikam, Y. Luo, X. Sheng, J. Fang, M. Kumar and S. S. Iyer, ACS Omega, 2024, 9, 14604–14612 CrossRef CAS.
- K. Wang, X. He, X. Yang and H. Shi, Acc. Chem. Res., 2013, 46, 1367–1376 CrossRef CAS.
- C. Xie, D. Yin, J. Li, L. Zhang, B. Liu and M. Wu, Nano Biomed. Eng., 2009, 1, 27–31 Search PubMed.
- A. H. Ashoka, I. O. Aparin, A. Reisch and A. S. Klymchenko, Chem. Soc. Rev., 2023, 52, 4525–4548 RSC.
- L. D. Lavis and R. T. Raines, ACS Chem. Biol., 2008, 3, 142–155 CrossRef CAS PubMed.
- J. B. Grimm and L. D. Lavis, Nat. Methods, 2022, 19, 149–158 CrossRef CAS.
- J. B. Grimm, B. P. English, J. Chen, J. P. Slaughter, Z. Zhang, A. Revyakin, R. Patel, J. J. Macklin, D. Normanno and R. H. Singer, Nat. Methods, 2015, 12, 244–250 CrossRef CAS PubMed.
- I. McKay, D. Forman and R. White, Immunology, 1981, 43, 591 CAS.
- H. N. Kim, M. H. Lee, H. J. Kim, J. S. Kim and J. Yoon, Chem. Soc. Rev., 2008, 37, 1465–1472 RSC.
- M. L. Vera, A. Cánneva, C. Huck-Iriart, F. G. Requejo, M. C. Gonzalez, M. L. Dell'Arciprete and A. Calvo, J. Colloid Interface Sci., 2017, 496, 456–464 CrossRef CAS PubMed.
- S.-W. Lin, C.-F. Shen, C.-C. Liu and C.-M. Cheng, Front. Bioeng. Biotechnol., 2021, 9, 752681 CrossRef PubMed.
- G. Durgun, K. Ocakoglu and S. Ozcelik, J. Phys. Chem. C, 2011, 115, 16322–16332 CrossRef CAS.
- S. Lv, Y. Tang, K. Zhang and D. Tang, Anal. Chem., 2018, 90, 14121–14125 CrossRef CAS PubMed.
- Z. Qiu, J. Shu and D. Tang, Anal. Chem., 2017, 89, 5152–5160 CrossRef CAS PubMed.
- A. D. Kurdekar, L. A. Avinash Chunduri, C. S. Manohar, M. K. Haleyurgirisetty, I. K. Hewlett and K. Venkataramaniah, Sci. Adv., 2018, 4, eaar6280 CrossRef CAS.
- P. Chen, Z. Tang, K. Huang, Y. Wei, D. Li, Y. He, M. Li, D. Tang, Y. Bai, Y. Xie, J. Huang, C. Tao and B. Ying, Sens. Actuators, B, 2022, 354, 131209 CrossRef CAS.
- C. N. Loynachan, M. R. Thomas, E. R. Gray, D. A. Richards, J. Kim, B. S. Miller, J. C. Brookes, S. Agarwal, V. Chudasama, R. A. McKendry and M. M. Stevens, ACS Nano, 2018, 12, 279–288 CrossRef CAS.
- A. D. Kurdekar, L. A. A. Chunduri, S. M. Chelli, M. K. Haleyurgirisetty, E. P. Bulagonda, J. Zheng, I. K. Hewlett and V. Kamisetti, RSC Adv., 2017, 7, 19863–19877 RSC.
- H. Cao, Y. Liu, H. Sun, Z. Li, Y. Gao, X. Deng, Y. Shao, Y. Cong and X. Jiang, Anal. Chem., 2020, 92, 11089–11094 CrossRef CAS.
- J. Zhuang, B. Han, W. Liu, J. Zhou, K. Liu, D. Yang and D. Tang, Biosens. Bioelectron., 2018, 99, 230–236 CrossRef CAS.
- N. Gan, X. Du, Y. Cao, F. Hu, T. Li and Q. Jiang, Materials, 2013, 6, 1255–1269 CrossRef CAS.
- W. R. Algar, D. E. Prasuhn, M. H. Stewart, T. L. Jennings, J. B. Blanco-Canosa, P. E. Dawson and I. L. Medintz, Bioconjugate Chem., 2011, 22, 825–858 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sd00011d |
| This journal is © The Royal Society of Chemistry 2025 |