Open Access Article
Open Access ArticleRecent progress in fluorescence-based chemosensing of pesticides
Giriraj
Kalaiarasi
 *ab,
Ananthu
Shanmughan
*ab,
Ananthu
Shanmughan
 a,
Yohaeswari
Jegadeesan
a,
Mannanthara Kunhumon
Noushija
a,
Yohaeswari
Jegadeesan
a,
Mannanthara Kunhumon
Noushija
 a,
Alenthwar Vamshi
Krishna
a,
Alenthwar Vamshi
Krishna
 a,
Harsha
Gangadharan
a,
Deivasigamani
Umadevi
*a and
Sankarasekaran
Shanmugaraju
a,
Harsha
Gangadharan
a,
Deivasigamani
Umadevi
*a and
Sankarasekaran
Shanmugaraju
 *a
*a
aDepartment of Chemistry, Indian Institute of Technology Palakkad, Palakkad-678623, Kerala, India. E-mail: umadevi@iitpkd.ac.in; shanmugam@iitpkd.ac.in
bDepartment of Chemistry, Karpagam Academy of Higher Education, Coimbatore-641021, Tamil Nadu, India. E-mail: kalaiarasichemist@gmail.com
First published on 3rd April 2025
Abstract
Poisoning of agricultural products through the use of pesticides has created a high risk to the environment and human health. In recent years, substantial research has been devoted to replacing harmful chemical pesticides with naturally derived organic compounds and the safer detection of pernicious pesticide residues by selective and sensitive methods using suitable sensor systems has also been given equal priority. Among various sensing methods that are currently available, fluorescence-based sensing has acquired widespread acceptance and become a feasible technique for the trace analysis of pesticide residues due to several practical advantages. In this review article, we provide a systematic overview of the recent progress made in using fluorescence-based chemosensing of different classes of pesticides and their success in real-world applications. Various fluorescence chemosensors highlighted in this article are categorized based on their sensing propensity for a particular class of pesticides. In the initial section of the article, we have highlighted a detailed discussion on the classification of pesticides, and various methods available for pesticide detection, and the later sections report various chemosensors reported to date for sensing different classes of pesticides. Finally, we put forward a short discussion on the advantages and existing practical difficulties in employing fluorescent chemosensors for pesticide detection and also state the future perspective of this field toward developing practically useful sensing systems.
1. Introduction
A class of chemicals known as pesticides can eradicate, discourage, or manage pests that negatively impact plant growth.1 Pesticides can be synthetic or natural and used to prevent weed growth while crops are produced and stored.2 These pests include nematodes, plant pathogens, insects, mollusks, birds, rodents, fish, mammals, and other microorganisms.1,2 All insecticides, herbicides, rodenticides, and fungicides that affect certain pests are called pesticides.3 According to a study by Savary et al., pests have caused production losses of rice (25–41%), potatoes (8–21%), wheat (10–28%), maize (20–41%), and soybeans (11–32%) on a global scale.4 Other substances classified as pesticides include growth regulators, chemical disinfectants, plant defoliants, and chemicals used in swimming pools.5 The usage of pesticides dates back to the dawn of human civilization. An efficient method to remove pests was essential since civilizations have had to contend with the difficulty of growing and conserving food supplies while dealing with pest interference. Originally used as a fungicide, elemental sulfur was eventually replaced by sulfides, arsenic (As2O3), lead hydrogen arsenate (PbHAsO4), and mercury to eradicate pests.6,7 In recent years, pesticide use has globally increased by 15–20 times to improve agricultural productivity and profitability.8 Despite their widespread usage in agriculture, pesticides are also used in non-agricultural settings, such as sports grounds, grass management, pet care, vegetation control in industries (roadways, railroads), and managing pests in buildings.9,10 Continuous pesticide exposure and ingestion of pesticide-contaminated food have long-term negative effects on the ecosystem and human health. Pesticides have a variety of functions for each species and have caused harm by reacting with creatures that are not their intended target.11–13 Indirect harm to non-target creatures can occur through changes to interactions between individual and bulk populations, or direct harm can occur through effects on gene expression, reproduction, behaviour, and life cycle.14When pesticides enter the body of a person or an animal, they are digested, stored, eliminated, and built up as fat.15 They cause neurological, endocrine, reproductive, respiratory, gastrointestinal, dermatological, and carcinogenic conditions.11,16 In some circumstances, the extended exposure might be fatal.17 While 80% of the pesticides penetrate the environment, just 0.1% are exposed to target organisms and carry out their actions.18 Pesticide residues are usually present in water bodies, cooked foods, fruit juices, and animal feeds and cannot be eliminated by simple washing.19–21 Concerns over the health consequences on children have grown after the finding that human breast milk contains chemical pesticide residues.22,23 Dichlorodiphenyltrichloroethane, or DDT, was the most widely used pesticide in the 1940s until it was discovered to be carcinogenic and to have negative effects on the associated biota. As a result, it was outlawed globally.24–32 Environmental impact quotient (EIQ) and environmental risk index measurements are typically used to assess pesticide toxicity.33 Pesticides have multiple effects on soil, such as lowering microbial biomass, soil respiration, and natural nitrogen fixation.34 By disrupting the natural exchange of information between leguminous plants and symbiotic microbes, pesticides can harm soil fertility and lower crop output.35 The food chain is the source of the detrimental effects on human health. When pesticides reach the human body, they mimic and oppose hormones, resulting in hormonal imbalance, reproductive issues, a decline in immunity and IQ, and cancer development.36,37 Nearly one million people have been afflicted with acute pesticide poisoning, with fatality rates ranging from 0.4% to 1.9%, according to the World Health Organization (WHO).38 Additionally, prolonged use slows the development of pesticide resistance.
Discriminative sensing of pesticides is crucial and essential due to their harmful effects. Pesticides have been detected using different methods, including capillary electrophoresis, enzyme-linked immune absorbent tests, and chromatographic methods combined with mass spectrometry.39–43 Traditional detection methods use capillary electrophoresis, enzyme-linked immune absorbent tests, and extremely complex chromatographic procedures in conjunction with mass spectrometry. The drawbacks of these instrumental procedures include their high cost, intricate methodology, and need for expert labor.9 Therefore, a straightforward, efficient, and user-friendly approach must be created. Sensing technology is a sophisticated technique that allows on-site detection and is very practical and economical. This technique depends on the combined impact of the modifications seen in electrochemistry, fluorescence spectroscopy, Raman spectroscopy, and ultraviolet-visible spectroscopy.5,44,45 However, this requires significant time and resources to monitor common pesticide exposure, which may restrict their ability to perform high-throughput experiments on sizable populations. Among them, fluorescence-based sensors are superior because they provide great selectivity, outstanding signal-to-noise ratios, and real-time sample analysis.
Given this, research in the area of fluorescence-based chemosensing has received a lot of interest.46–48 In general, the use of ‘turn-on’ (enhancement) fluorescence sensors is more advantageous for sensing applications because the interactions between sensors and analytes can easily be visualized by the naked eye due to the significant enhancement in fluorescence emission intensities.49 However, the straightforward design of ‘turn-on’ fluorescent sensors is complicated and it often demands a careful design strategy. Further, the real-time applications of fluorescence ‘turn-on’ sensors are insufficient due to the lack of reversibility, selectivity, and small Stokes shift.50 In contrast, the design and sensor applications of ‘turn-off’ fluorescence sensors are explored to a greater extent. In particular, the use of ‘turn-off’ fluorescent sensors for the detection of various classes of pesticides is well studied.51 Fluorescence-based chemosensing of pesticides has been achieved thus far through employing luminescent organic & hybrid polymers, metal-containing scaffolds, nanoparticles, and discrete macrocycles.52 Mechanistically, the detection occurs via many pathways,53–55 and the fluorescence “turn-off” response (quenching), which indicates the pesticide-induced decrease of fluorescence intensity, is commonly observed. Energy transfer between the pesticide and the sensor system may be the reason for the quenching of fluorescence emission intensity. Förster resonance energy transfer (FRET), photoinduced electron transfer (PET), electron exchange (EE), and inner filter effect (IFE) are the four mechanisms responsible for this energy transfer.56
In FRET, the donor (fluorophore) and acceptor (analyte) molecules are placed at a Förster distance of 15–60 Å. When an excited fluorophore relaxes to the ground state, it transfers excited energy to the acceptor molecule that promotes it to the excited state. The reabsorption of excited energy by the acceptor molecule causes notable fluorescence quenching. The energy transfer is possible only when the fluorophore's emission spectrum overlaps with the analyte's absorption spectrum. The interaction between the fluorophore and analyte is greater when they are at a shorter distance.56 In PET, when the photoexcited fluorophore relaxes to the ground state, the excited state energy of the fluorophore is transferred to the acceptor (analyte) molecule, which causes fluorescence emission quenching. For the enhanced energy transfer to occur, the frontier molecular orbitals of the fluorophore and analyte must be similar and the lowest-unoccupied molecular orbital (LUMO) of the analyte must be lower than the LUMO of the fluorophore.56 The EE process involves the transfer of an electron from an excited fluorophore to the excited state of the acceptor molecule, leading to the fluorescence quenching of the fluorophore, while simultaneously an electron transfer from the ground state of the acceptor to the ground state of the fluorophore occurs. The electron transfer can be between two systems or parts of the same system, and EE is a short-range electron transfer process. In IFE, the reabsorption of emitted photons from the excited fluorophore by the other molecules in the samples causes fluorescence quenching. Fluorescence quenching is also possible if the sample absorbs the excitation light before it excites the fluorophore.
The pesticide classification according to distinct domains, a thorough explanation of the fluorescence-based chemosensing of various pesticide classes, the detection process, the detection limit, and their applications in real-world samples are all covered in detail in the following section of this article.
2. Classification of pesticides
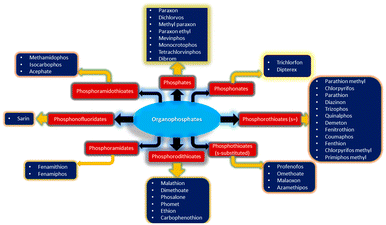
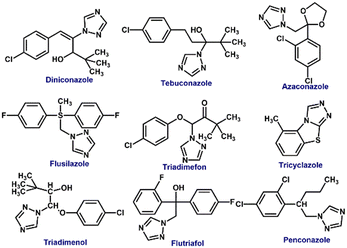
Pesticides are different from one another in terms of their identity based on physical and chemical properties. In addition, each pesticide is formulated for a specific activity against a target group of pests. Without knowing its chemical structure and variation in physiochemical properties, pesticide detection is tedious. Therefore, pesticides must be systematically classified and grouped for designing suitable detection methods. Pesticides can be categorized based on chemical composition, target organism, mode of entrance & action, and source of origin. Pesticides can be of natural origin (biopesticides derived from plants, animals, and microorganisms) or synthetically made (i.e. chemical pesticides). Biopesticides offer high specificity to target pests, low toxicity, and are environment friendly. Biopesticides are subdivided into microbial pesticides, plant-incorporated protectants, and biochemical pesticides. Chemical pesticides are environment-polluting, non-specific, and found to act upon several non-targeted organisms.36,57 Depending on the mode of entry, they are classified as systemic (mediated through plants and ability to transfer to untreated tissues, e.g. glyphosate), contact (entry only through physical contact with the pests, e.g. paraquat), stomach poisons (mouth as a mode of entry, e.g. malathion), fumigants (vapor producing pesticides, e.g. aluminum phosphide) and repellents (repel pests from treated areas, e.g. methiocarb).36 Chemical composition-based classification of pesticides has been considered the most practical and relevant approach. This type of classification includes all classes of pesticides (insecticides, herbicides, rodenticides, and fungicides) and acts as evidence for their observed efficacy, and physical and chemical properties. Accordingly, there are five main categories of pesticides as described herein.58Organochlorines are chlorinated hydrocarbons (chloroalkanes and chloroarenes) mostly containing five or more than five chlorine atoms (see Fig. 1 for structures). They are the early examples of pesticides used in agriculture.6 Most of these chemicals have been applied as insecticides and structurally fall into five types – a) DDT and its structural analogues, b) hexachlorocyclohexane (HCH), c) cyclodienes, d) toxaphene, and e) mirex and chlordecone.57 Owing to their high chemical stability, organochlorine pesticides accumulate in high concentrations in soil and severely affect human health through the food chain. For instance, the half-life period of DDT is about 15 years, and it remains in the body for more than 50 years.59 It has been proven to accumulate in the adipose tissue and affect the central nervous system.60 First, the nerve membranes get affected which then cause changes in the flow of K+ and Na+ ions through nerve cells.57 This will lead to convulsions, acute poisoning, seizures, and paralysis in the later stage.2
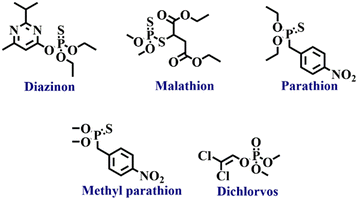
Organophosphate pesticides are phosphoric acid esters (see Fig. 2 for structures) that exhibit action against several pests by exerting multiple functions. The use of organophosphate pesticides has increased steadily in recent years because of the impact on agricultural productivity and increased crop yields.61,62 These biodegradable pesticides are comparatively less harmful environmental pollutants. However, in humans, they inhibit acetylcholine, an enzyme that disturbs acetylcholinesterase neurotransmitters, which results in the failure of nerve impulses across the synapse, which could end up in paralysis.63 Symptoms include headache, nausea, convulsions, cramps, loss of reactions, coma, and eventually death.64,65 Another concern about using organophosphate is phosphate poisoning, which is in general treated mainly by intake of atropine, an anticholinergic drug.66
Another type of pesticide is carbamates, which are organic acid esters derived from carbamic acid (see Fig. 3 for structures). They are used as insecticides, herbicides, nematicides, and fungicides. Carbamates are short-lived and their toxicity generally coincides with the organophosphate's toxicity. Carbamates also disturb the activity of acetylcholinesterase and produce acute symptoms like cough, mitosis, cardiovascular and gastrointestinal effects, and illness associated with the central nervous system.67,68 They pose minimal environmental pollution and can be degraded easily.2,6
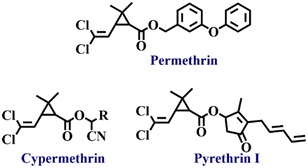
Pyrethroids are naturally derived organic pesticides and due to their high demand, their derivatives can also be synthetically produced. Pyrethroids are generally used as insecticides (see Fig. 4 for structures).2 Active components of these pesticides are pyrethrin I and pyrethrin II with small amounts of jasmolins and cinerins. Pyrethroids are less toxic to mammals and animals but comparatively toxic to insects and fish. Although insect pests absorb pyrethroids, their penetration power into soil and solubility in water are not very effective in affecting underground pests. Many commercial products like pet sprays, pet shampoos, mosquito repellents, and human head lice drugs contain pyrethroids.69,70
Another type of classification of pesticides is based on their target organism. Table 1 summarizes different pesticides classified according to their target pests and notable examples.
| Pesticides | Target pests | Example |
|---|---|---|
| Insecticides | Insects, arthropods | Aldicarb |
| Fungicides | Fungi | Azoxystrobin |
| Bactericides | Bacteria | Cu-complexes |
| Herbicides | Weeds, unwanted plants | Atrazine |
| Larvicides | Larva | Methoprene |
| Molluscicides | Molluscs | Metaldehyde |
| Nematicides | Nematodes | Aldicarb |
| Ovicides | Insect and mite eggs | Benzoxazine |
| Piscicides | Fish | Rotenone |
| Rodenticides | Rodents | Warfarin |
| Silvicides | Wood vegetation | Tebuthiuron |
| Termiticides | Termites | Fipronil |
| Virucides | Virus | Scytovirin |
| Avicides | Birds | Avitrol |
| Acaricides | Mites | Bifenazate |
| Algaecides | Algae | Copper sulfate |
| Desiccant | Plant tissues | Boric acid |
| Lampricides | Lamprey larva | Trifluoromethyl |
| Predacides | Mammal predators | Strychnine |
| Mothballs | Moth larvae, molds | Dichlorobenzene |
| Attractant | Wide range of pests | Pheromones |
| Insect growth regulator | Insects | Diflubenzuron |
| Defoliant | Plant foliage | Tribufos |
| Bait | Variety of organisms | Anticoagulants |
| Fumigant | Variety of organisms | Aluminum phosphide |
| Repellents | Range of pests | Methiocarb |
3. Various approaches for pesticide detection
The conventional detection of pesticides involves the utilization of different techniques including gas chromatography (GC), high-performance liquid chromatography (HPLC), surface-enhanced Raman spectroscopy (SERS), accelerator mass spectrometry (AMS), and capillary electrophoresis (CE). However, the requirement of skilled manpower, sophisticated instrumental setup, and non-portability make these techniques not feasible for practical on-site sensing applications.57,71,72 To tackle these difficulties, alternate detection methods were sought for relatively low cost, time-saving, and operational simplicity. As alluded to above, the use of fluorescent-based chemosensors for the selective detection of pesticides has taken a front lead and is of several categories.Colorimetric sensors offer naked-eye determination of specific chemical compounds in pesticide residues. They provide an opportunity for on-site detection of real-time samples owing to their practical applicability and simplicity.73–78 The colorimetric sensors work by inducing changes in the color and intensity of the absorption bands before and after pesticide addition.79 Qualitative analysis of pesticide concentrations is possible by observing changes in the color, whereas recording UV-visible spectra of the resultant compounds permits quantitative analysis of pesticide residues.80,81 Recent years have witnessed a flourishment of colorimetric sensors based on nanoparticles (NPs) for the detection of various classes of pesticides. In particular, NPs as sensors have been greatly seen in agricultural food analysis.74–78,82,83 In certain cases, the application of NPs has provided a higher level of sensitivity than a chemical treatment method. For example, silver NPs wrapped with graphene oxide allowed for the detection of carbaryl pesticide at a lower limit of detection (LoD) between 0.1 and 50 ppm;84 this level of sensitivity was found to be superior to that observed from an azo coupling reaction-based colorimetric probe.85 Demonstration of a dual technology (smartphone-assisted and spectroscopic) has been reported with a nanoenzyme for the on-site detection of organophosphates.86 Ghoto and his group have developed colorimetric probes for pesticide detection based on Cu (coated with cetyltrimethyl ammonium bromide) and Ag NPs (coated with sodium dodecyl sulfate) with LoD values of 97.9 and 9.1 ng ml−1.87,88 By employing an origami paper sensor, Bordbar et al. showed selective detection of organophosphate and carbamate pesticides in water, rice, and apple juice, and also reported detection in the vapor phase.89,90
Paper sensor-based detection methods have evolved from enzyme activity inhibition, mostly acetylcholinesterase (AChE) and butyrylcholinesterase (BChE). Inhibition of enzyme activity may be brought about by preventing acetylthiocholine, acetylcholine, and butyrylcholine from being converted to thiocholine, choline, and acetic acid by these enzymes. This interrupts the signaling and transmission of neuronal synapses. It is well-known that organophosphates effectively inhibit AChE. Consequently, this enzyme can be used as an indicator for organophosphate detection.91,92 Organophosphate and carbamate contents in vegetables and fruits have been determined on-site by employing a double film screening card constructed from AChE and indoxyl acetate. The film detected phoxim in apples, lettuce, and cabbage between 5–20 μg mL−1.92 For the same class of pesticides, a biosensor based on AChE has been developed by the Badawy team, which worked based on the change in yellow coloration.93 A similar probe was also developed and used for malathion detection with a LoD of 2.5 ppm within 5 min of incubation.94
The detection of pesticides has also been accomplished by the electrochemical method and is dependent on the working electrode (transducer). They work by producing an electrical signal due to the interaction of the target pesticides with the transducer. Sensitivity and selectivity can be improved by fabrication, surface modification, and enzyme immobilization.95 For instance, polymers like poly(3,4-ethylenedioxythiophene) and carbon nanotubes were used to develop NP-modified electrodes, able to detect mancozeb (a fungicide) in water using cyclic voltammetry analysis (LoD = 10 μmol L−1).96 A similar approach with carbon nanosphere for the selective detection of carbofuran, carbaryl, fenobucarb, and isoprocarb in corn and wheat samples has been reported.97 MXene-carbon nanohorns, silicon carbide, and CuO nanocomposite-derived electrodes have been designed to serve this purpose.98–102 Fabrication methods for electrochemical sensors include thin film and thick film technologies. Thin films are relatively costly due to the application of lithographic technology but show high reproducibility. Thick films use cost-effective techniques like inkjet printing, 3D printing, and screen printing. Printed electrodes have different electroanalytical performances and possess varying catalytic abilities, composition, and specificity for samples being analyzed.103–108
Fluorescence-based sensing is one of the widely used methods for pesticide detection. They display their action by decreasing or increasing the sensor emission intensity after mixing pesticides and the method depends on the sample concentration.109 Many reports have shown that the pesticides act as fluorescent quenchers.110 Small molecule pesticide samples could be analyzed with conjugated polymers as sensors. Recently, the use of conjugated polymers in solution has been reported for targeted two-photonic excitation, and these polymers have been incorporated within carbon nanotubes.111,112 NPs derived from luminescent polymers combined with Au NPs were found to detect paraoxon by quenching the initial fluorescence intensity. This combination has practical application in analyzing real-time lake water and cabbage extracts.113 A europium coordination polymer was used to detect fipronil in European eggs in solution and a paper strip-based sensor by the fluorescence quenching mechanism. Interestingly, this sensor was so selective that other pesticide samples with structurally similar analogues induced no change in the emission intensity.114,115 Also, discrete macrocycles with different photophysical properties have been employed to detect pesticides. Fluorescent macrocycles can be built through supramolecular architecture by attaching themselves to a non-fluorescent macrocycle116 or by the covalent binding of two or more macrocycles to a fluorescent linker. Dithianon has been detected by a covalent linker bound with two cyclodextrins.117 The sensor ability was mainly due to the interaction between the signalling element of the macrocycle and the pesticide sample, which can fit well enough into the macrocycle cavity.118 In addition, quantum dots (e.g. carbon quantum dots),119,120 nanocrystals (e.g. cadmium sulfide nanocrystals),121,122 and metal–organic frameworks (MOFs) have been found effective as fluorescent sensors.123,124
After reviewing the previously described methods, environmental and analytical chemists have created effective fluorescence sensing approaches for pesticide detection. Among the various detection strategies adopted for pesticide detection in environmental samples, agriculture, food safety, and quality control, the fluorescence-based detection method plays a vital role due to its low cost, appropriateness for quick pesticide detection screening procedures, and target molecule specificity and sensitivity. Moreover, this method makes it feasible to obtain high-quality image data. Importantly, this method exhibits good pesticide residue sensing performance and its simplicity makes fluorescence-based sensing a well-suited method for pesticide detection.
4. Fluorescence-based chemosensing of pesticides
The design and development of fluorescent-based chemosensors for detecting environmental pollutants and hazardous substances have significantly increased in recent years. In light of this, several fluorescent chemosensors have been reported for pesticide detection. In this section of the review, we have exemplified in detail a variety of fluorescent chemosensors utilized for the selective detection of various classes of pesticides. Besides, the synthesis and properties of sensors, pesticide detection mechanisms, and sensitivity have also been mentioned.4.1 Fluorescence sensing of organophosphate (OPP) pesticides
Organophosphate (OPP) pesticides have been used extensively to increase agricultural output. However, higher concentrations of OPPs are detrimental to the environment, human health, and other organisms. Numerous ways, such as percutaneous absorption, inhalation, and ingestion from air, water, sediment, and crops, introduce OPPs to living things. OPPs can easily damage soil, aquatic life, and other living things because of their high adsorption capacity and increased water solubility.125 An estimated 100 different OPPs have been created for commercial usage. The first OPP that was created with agricultural insecticidal properties was hexaethyl tetraphosphate.126,127 Various categories of OPPs are given in Fig. 5. While OPPs undeniably contribute to substantial increases in agricultural productivity, their adverse effects on human health and the environment have emerged as an enormous challenge to society and the natural world. As a result of the serious contamination by OPPs, it has become crucial to develop sensors capable of detecting OPPs in soil, air, fruits, vegetables, beverages, and more.128–130 Due to the extensive literature reports on the detection of OPPs, we aimed herein to compile only the fluorescent-based chemosensors used for various types of organophosphate detection. Table 2 summarizes the sensing properties of different fluorescence sensors employed for detection of various OPPs.| Sensors | Analytes | LoD | Ref. |
|---|---|---|---|
| Graphene quantum dots (GQDs) | Paraoxon | 2 nM | 130 |
| Mg,N-CD-PAM | Paraoxon | 0.87 nM | 131 |
| AIE nanoparticles (PTDNPs) | Paraoxon | 0.38 ng ml−1 | 113 |
| {(Ru(bpy)32+-ZIF-90)} and MnO2 NSs | Methyl parathion | 0.037 ng mL−1 | 132 |
| L-tyrosine methyl ester functionalized carbon dots (Tyr-CDs) | Methyl parathion | 4.8 × 10−11 M | 133 |
| CdTe-QDs/CTAB | Methyl parathion | 18 ng mL−1 | 134 |
| Carbon dots | Paraoxon-ethyl | 0.22 μM | 135 |
| NS-Cdots | Dichlorvos | 5.0 × 10−10 M | 136 |
| CdTe quantum dots (QDs) | Chlorpyrifos | ∼0.1 nM | 137 |
| Gold-based nanobeacon | Isocarbophos, profenofos, phorate and omethoate | 0.35 μM | 138 |
| Cu(II) complexes of 8-((E)-((thiophen-2-yl)methylimino)methyl)-7-hydroxy-4-methyl-2H-chromen-2-one | Azamethiphos | 55 nM | 139 |
| Supramolecular structure-based fluorescent sensor Eu-IRMOF-3-EBA | Prothiofos and profenofos | 0.0018 ppb | 140 |
| Up-conversion fluorescent nanoparticles-gold nanoparticles | Malathion | 1.42 nM | 141 |
| Eu(III)-complex of bathophenanthroline | Azinphos, ethyl malathion, and heptachlor | 0.68 μM (azinphos), 0.92 μM (ethyl malathion) and 0.35 μM (heptachlor) | 142 |
| AgNPs-β-cyclodextrins hybrid material @ 2,3-dihydro-5-oxo-5H-thiazolo[3,2-a]pyridine-7-carboxylic acid | Malathion | 0.01 μg mL−1 | 143 |
| Rhodamine B (RB) functionalized AuNPs | Dimethoate | 0.004 ppm | 144 |
| AgNPs/oxMWCNTs | Dimethoate | 0.003 μg mL−1 | 145 |
| Dithizone (DZ)-CdTe QDs | Dimethoate | 0.005 μg mL−1 | 146 |
| Molecularly imprinted polymer-CDs | Dimethoate | 1.83 × 10−11 mol L−1 | 147 |
| RB-Ag NPs | Fenamithion | 10.000 nM | 148 |
| 1,8-Naphthalimide dye, quaternary ammonium salt with a boronate group | Trichlorfon, methyl parathion, and acephate | 4.72 × 10−9 g L−1, 3.36 × 10−10 g L−1 and 1.16 × 10−9 g L−1 | 149 |
| 2-Amino terephthalic acid co-coordinated Co-MOF complex | Bis(p-nitrophenyl) phosphate (BNPP) and nitrophenyl phosphate (PNPP) | 352 nM (PNPP) | 150 |
| CdSe@SiO2@MIP | Parathion-methyl | 0.004 mg kg−1 | 151 |
| NaYF4:Yb, Er up-conversion NPs combined with Au Nps | Parathion-methyl, monocrotophos, and dimethoate | 0.67 ng L−1 | 152 |
| Surface molecularly imprinted CdTe nanoparticles | Parathion | 0.218 μmol L−1 | 153 |
| N-doped carbon dots (NCD) | Methyl parathion | 0.338 μmol L−1 | 154 |
| CuInS2-QDs and Pb(II) | Methyl parathion | 0.06 μmol L−1 | 155 |
| Eu(III) complexes containing 4-hydroxy benzylidene imidazolinone with nitrogen-containing heterocyclic 1,10-phenanthroline | Methyl parathion | 95 nM | 156 |
| ZnPO-MOF containing 1,2,4,5-tetrakis(4-carboxyphenyl) benzene | Methyl parathion | 0.456 nM | 157 |
| Zirconium MOF appended 1,2,4,5-tetrakis(4-carboxyphenyl)benzene | Methyl parathion | 0.438 nM | 158 |
| Europium(III) complexes containing amino-substituted β-cyclodextrin | Fenitrothion | 1 × 10−12 M | 159 |
| Europium-8-allyl-3-carboxy coumarin (Eu(III)-ACC) | Chlorpyrifos, crotoxphos, and endosulfan | 6.53 μmol L−1 for chlorpyrifos, 0.004 μmol L−1 for endosulfan and 3.72 μmol L−1 for crotoxyphos | 160 |
| Europium-doped titanium oxide nano-powder | Chlorpyrifos | 3.2 × 10−11 mol L−1 | 161 |
| Mn(II)-doped ZnS quantum dots coated with an acrylamide-based MIP | Chlorpyrifos | 0.89 μM | 162 |
| Plant-based green carbon dots | Diazinon, glyphosate, and amicarbazone | 0.25, 0.5, and 2 ng mL−1 | 163 |
| L-cysteine capped CdS-QDs/DF20 | Diazinon | 0.13 nM | 164 |
| Tb(III)-complex of 3-ally-salicylohydrazide | Dichlorvos | 1.183 μM | 165 |
| Hg(II) complex of novel cholesterol derivative, 4-chloro-7-nitro-1,2,3-benzoxadiazole (CTN) using triazole as a linker | Dichlorvos, glyphosate, chlorpyrifos, diazinon, and phoxim | 0.015, 0.018, 0.087, 0.098, and 0.113 μg mL−1 | 166 |
| Biginelli derivatives of cobalt complexes | Malathion, azamethiphos | 9.2 nM and 11 nM | 167 |
| Picolyl-functionalized rhodamine derivative | Glyphosate | 4.1 nM | 168 |
| p-tert-butylcalix[4]arene | Glyphosate | 7.91 × 10−7 M | 169 |
| Carbon dots + Fe(III) | Glyphosate | 8.75 ppb | 170 |
| CDs with AgNPs (CDs/AgNPs) | Glyphosate. | 12 ng mL−1 | 171 |
| Zr-MOF, Fe3O4@SiO2@UiO-67 | Glyphosate | 0.093 mg L−1 | 172 |
| CdTe quantum dots capped with thioglycolic acid (TGA-CdTe-QDs) and gold nanoparticles stabilized with cysteamine (CS-AuNPs) | Glyphosate | 9.8 ng kg−1 | 173 |
| 2D MOF nanosheets with calix[4]arenes | Glyphosate | 2.25 μM | 174 |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O. Different types of fluorescence sensors were designed and used for phosphate detection. For example, graphene quantum dots (G-QDs) in conjunction with the AChE enzyme were employed to detect paraoxon with a good sensitivity for detection.130 As an effective AChE inhibitor, paraoxon induced a notable fluorescence quenching by forming aggregates. The half-maximum inhibitor concentration (IC50) was determined to be 19.86 nM and the LoD was 2 nM. In another study, Peng and coworkers reported the microwave-assisted synthesis of magnesium and nitrogen co-doped carbon dots, Mg,N-CDs.131 Mg,N-CDs displayed blue emission and good solution processability. The fluorescent sensing probe was designed by mixing Mg,N-CDs with pralidoxime (PAM, a medicine used to treat phosphate poisoning) as a linker. Mg,N-CDs in the presence of PAM exhibited good fluorescence ‘turn-off’ sensing responses for paraoxon pesticides (Fig. 6). In this method, PAM enabled the electron transfer mechanism from Mg, N-CDs to paraoxon resulting in fluorescence emission quenching. The LoD value was reported to be 0.87 nM and Mg,N-CDs displayed selective sensing responses even in the presence of interfering analytes. The sensing studies were further demonstrated using real-water samples.
O. Different types of fluorescence sensors were designed and used for phosphate detection. For example, graphene quantum dots (G-QDs) in conjunction with the AChE enzyme were employed to detect paraoxon with a good sensitivity for detection.130 As an effective AChE inhibitor, paraoxon induced a notable fluorescence quenching by forming aggregates. The half-maximum inhibitor concentration (IC50) was determined to be 19.86 nM and the LoD was 2 nM. In another study, Peng and coworkers reported the microwave-assisted synthesis of magnesium and nitrogen co-doped carbon dots, Mg,N-CDs.131 Mg,N-CDs displayed blue emission and good solution processability. The fluorescent sensing probe was designed by mixing Mg,N-CDs with pralidoxime (PAM, a medicine used to treat phosphate poisoning) as a linker. Mg,N-CDs in the presence of PAM exhibited good fluorescence ‘turn-off’ sensing responses for paraoxon pesticides (Fig. 6). In this method, PAM enabled the electron transfer mechanism from Mg, N-CDs to paraoxon resulting in fluorescence emission quenching. The LoD value was reported to be 0.87 nM and Mg,N-CDs displayed selective sensing responses even in the presence of interfering analytes. The sensing studies were further demonstrated using real-water samples.
 | ||
| Fig. 6 Fluorescence ‘turn-off’ sensing of paraoxon using the Mg,N-CDs probe through electron transfer from PAM to paraoxon. Reprinted with permission from ref. 131. Copyright 2018 Springer Nature. | ||
A luminescent nanoparticle aggregate was developed from an amphiphilic luminescent polymer in an aqueous buffer medium and it was combined with gold nanoparticles to produce the aggregate-based sensing probe (PTDNPs), which was used for paraoxon detection through the analyte-induced dis-aggregation mechanism.113 Interestingly, the luminescent nanoparticle aggregates could identify paraoxon even in polluted lake water and cabbage extracts with a recovery efficiency of up to 93%. The LoD value was calculated to be 0.38 ng mL−1, a comparable level to various other sensors exemplified herein. Another group reported an interesting fluorescence probe (P5C10) based on a coumarin fluorophore which was directly attached to a pillar[5]arene core to sense methyl parathion with high affinity (2.38 × 10−4 M−1).175 It was demonstrated that P5C10 forms a selective 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex with methyl paraoxon through π–π stacking interactions, while other analytes displayed poor host–guest complexation with P5C10. The association constant was determined to be 2.38 × 104 L mol−1. In another study, Li et al. developed a novel MOF-based sensing platform that consists of a Ru(bpy)32+-ZIF-90-MnO2 to detect methyl parathion with a wide concentration range of 0.050–60 ng mL−1 and with a LoD of 0.037 ng mL−1.132 The visual color changes also indicated the high selectivity of Ru(bpy)32+−ZIF-90−MnO2 for methyl parathion. The team led by Ai and Mang reported a simple, sensitive fluorescence probe Tyr-CDs for methyl parathion detection.133 The probe Tyr-CDs are carbon dots functionalized with L-tyrosine methyl ester (Tyr-CDs) and the tyrosinase system. The fluorescence emission of carbon dots was quenched by the quinone produced by the oxidation of tyrosine methyl ester by tyrosinase enzyme. However, the presence of methyl parathion inhibited the enzyme activity and thus reduced the rate of fluorescence quenching. It was found that the enzyme inhibitory activity was linearly related to the concentrations of methyl parathion and the LoD value was determined to be 4.8 × 10−11 M. The selective sensing was further successfully explored in cabbage, milk, and fruit juice samples.
1 host–guest complex with methyl paraoxon through π–π stacking interactions, while other analytes displayed poor host–guest complexation with P5C10. The association constant was determined to be 2.38 × 104 L mol−1. In another study, Li et al. developed a novel MOF-based sensing platform that consists of a Ru(bpy)32+-ZIF-90-MnO2 to detect methyl parathion with a wide concentration range of 0.050–60 ng mL−1 and with a LoD of 0.037 ng mL−1.132 The visual color changes also indicated the high selectivity of Ru(bpy)32+−ZIF-90−MnO2 for methyl parathion. The team led by Ai and Mang reported a simple, sensitive fluorescence probe Tyr-CDs for methyl parathion detection.133 The probe Tyr-CDs are carbon dots functionalized with L-tyrosine methyl ester (Tyr-CDs) and the tyrosinase system. The fluorescence emission of carbon dots was quenched by the quinone produced by the oxidation of tyrosine methyl ester by tyrosinase enzyme. However, the presence of methyl parathion inhibited the enzyme activity and thus reduced the rate of fluorescence quenching. It was found that the enzyme inhibitory activity was linearly related to the concentrations of methyl parathion and the LoD value was determined to be 4.8 × 10−11 M. The selective sensing was further successfully explored in cabbage, milk, and fruit juice samples.
Meanwhile, that year (2015) Yan et al. developed a sensitive fluorescence probe, mercaptopropionic acid (MPA)-capped CdTe-QDs for detecting methyl parathion showing a detection limit of 18 ng mL−1. In this study, the probe works based on the electron transfer phenomenon that takes place between (MPA)-capped CdTe-QDs and p-nitrophenol (a product formed from hydrolysis of methyl parathion by OPH) in cetyltrimethylammonium bromide (CTAB) and the electron-deficient p-nitrophenol gets absorbed on the electronegative MPA-capped CdTe-QDs through strong hydrophobic interactions. As a result, the fluorescence of the probe was quenched.134 By combining double QDs with nanoporphyrin (QDs-nanoporphyrin), a paper-based fluorescence visualization sensor was developed and used to detect dichlorvos, demeton, and dimethoate by using it through a “turn-off-on” detection mode.176 In 2017, Chang et al. reported the synthesis of fluorescence carbon dots through simple acid carbonization of sucrose in-house.135 This has been used to detect paraoxon-ethyl with a LoD of 0.220 ± 0.020 μM. Additionally, Hu et al. created an effective fluorescent probe that emits blue fluorescence, such as nitrogen and sulfur co-doped CDs (NS-Cdots), to detect OPs-dichlorvos (DDVP) in Chinese cabbage samples (Fig. 7 for details).136 The observed LoD was 5.0 × 10−10 M.
 | ||
| Fig. 7 Schematic representation of fluorescence turn-off-on based sensing of OPs-dichlorvos (DDVP). Reprinted with permission from ref. 136. Copyright 2019 Springer Nature. | ||
 | ||
| Fig. 8 Hydrolysis of chlorpyrifos (CP) to diethylphosphorothioate (DEP) and trichloro-2-pyridinol (TCP) in a basic medium (above). The fluorescence of CdTe QDs was quenched by the coordination of dithizone on the surface of CdTe QDs. The fluorescence emission of CdTe QDs was subsequently restored due to the replacement of dithizone by the DEP ligand (below). Reprinted with permission from ref. 137. Copyright 2010 American Chemical Society. | ||
In 2015, Dou et al. developed a fluorescence assay based on a gold-based nanobeacon probe to detect various OPPs such as isocarbophos, profenofos, phorate, and omethoate.138 Under optimized conditions, this method was fast and highly responsive for the concentration limit of 0.035 μM, 0.134 μM, 0.384 μM, and 2.35 μM for isocarbophos, profenofos, phorate, and omethoate, respectively. Additionally, this technique can detect trace amounts of organophosphorus pesticides in real substances. The detection of azamethiphos has been done by Bhasin et al. using a MOF-based fluorescent chemosensor such as Cu(II) complexes of 8-((E)-((thiophen-2-yl)methylimino)methyl)-7- hydroxy-4-methyl-2H-chromen-2-one in aqueous medium.139 Abdelhameed and team reported the sensing nature of the supramolecular structure-based fluorescent probe Eu-IRMOF-3-EBA, which was synthesized by modifying IRMOF-3 with ethylbenzoylacetate and then coordinating it with Eu(III) ions. The supramolecular structure exhibited robust fluorescence emission in the near-infrared range, which significantly diminished upon prothiofos and profenofos exposures.140
Hsu et al. synthesized a novel turn-on fluorescent sensor based on silver-nanoparticles-modified oxidized multiwalled carbon nanotubes (AgNPs/oxMWCNTs) which has a peroxidase-like activity to detect dimethoate in lake water and fruits.145 The presence of dimethoate inhibits the catalytic activity of AgNPs/oxMWCNTs because of the contact between dimethoate and AgNPs. This interaction caused a decrease in fluorescence, and the LoD was determined to be 0.003 μg mL−1. Sheng et al. developed the dithizone (DZ)-CdTe QDs fluorescent quenching system for FRET-based detection of dimethoate.146 After mixing dimethoate (DMT), the dithizone molecule was removed from the surface of the quantum dots (QDs), which restored the fluorescence in the CdTe QDs. The minimum detectable concentration using this technique was 0.005 μg mL−1. Another interesting sensor based on a molecularly imprinted polymer was developed for dimethoate detection via the FRET mechanism.147 A doped molecular templated polymer was obtained by electropolymerization. The fluorescence signal from the sensor was amplified by the FRET process between the sensor and the doped molecularly imprinted polymer. This sensor was used to detect dimethoate in real samples with a good recovery (varying from 95% to 106%), and its LoD under ideal conditions was 1.83 × 10−11 mol L−1.
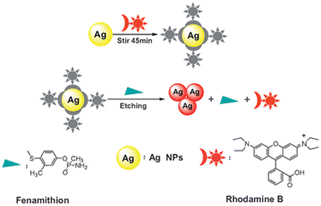 | ||
| Fig. 9 The proposed representation of fenamithion sensing based on RB-Ag NPs. Reprinted with permission from ref. 148. Copyright 2011 Royal Society of Chemistry. | ||
 | ||
| Fig. 10 The proposed sensing strategy to detect organophosphorus pesticides using boronate-1,8-naphthalimide fluorescent dye. Reprinted with permission from ref. 149. Copyright 2016 Royal Society of Chemistry. | ||
 | ||
| Fig. 11 Schematic representation of the UCNPs-AuNPs fluorescence assay for the detection of pesticides. Reprinted with permission from ref. 152. Copyright 2015 Elsevier. | ||
Using fluorescence quenching as a basis, Tang et al. synthesized molecularly imprinted CdTe nanoparticles with molecular recognition ability for organophosphorus detection.153 When compared to diazinon, chlorpyrifos, and pyrimithate, the synthesized compound exhibited a notable selectivity and strong binding affinity towards parathion, allowing for high recoveries (97.72% to 100.59%) of parathion detection in water samples. In 2017, the Song group developed an N-doped carbon dots (NCD) fluorescent probe for analyzing methyl parathion, and the LoD was found to be 0.338 μmol L−1.154 Of note, the present method was applied to detect methyl parathion in agricultural and real-world samples. Yan and team created a fluorescent probe based on CuInS2-QDs and Pb(II) for methyl parathion detection.155 Using nitrogen-containing heterocyclic 1,10-phenanthroline and 4-hydroxybenzylidene imidazolinone, Hu and colleagues produced Eu(III) complexes that showed fluorescence emission due to energy transfer. When methyl parathion was added, fluorescence quenching occurred at λ = 617 nm, and a LoD of 95 nM was noted.156
To monitor methyl parathion, Xu et al. created a highly luminous ZnPO-MOF with 1,2,4,5-tetrakis(4-carboxyphenyl)benzene as a luminescent chemosensor.157 ZnPO-MOF exhibited high selectivity for methyl parathion through a fluorescence turn-off mechanism (Fig. 12). The probe could detect methyl parathion at as low as 0.456 nM concentrations. This method can be applied to parathion-methyl detection in irrigation water. He and his team developed a water-stable luminescent zirconium MOF (Zr-MOF) from the reaction of Zr(IV) and 1,2,4,5-tetrakis(4-carboxyphenyl)benzene for the detection of methyl parathion in spiked food and environmental samples with low LoD values.158 In another example, Eu(III) complexes containing amino-substituted β-cyclodextrin detect the presence of fenitrothion and the detection limit was as low as 1 × 10−12 M. The observed result was due to the encapsulation of fenitrothion in the inside cavity of the per-6-amino-β-cyclodextrin:Eu(III) complex which is the consequence of the absorption energy transfer emission (AETE) process.159
 | ||
| Fig. 12 Schematic representation for the synthesis of ZnPO-MOF and its application in organophosphorus detection. The strong blue emission of ZnPO-MOF was quenched in the presence of pesticides. Reprinted with permission from ref. 157. Copyright 2018 Elsevier. | ||
Using a time-resolved approach, Azab et al. developed a new fluorescence sensor called europium-allyl-3-carboxycoumarin (Eu(III)-ACC) to detect endosulfan, crotoxphos, and chlorpyrifos in water.160 The probe was fluorescent but the fluorescence of the probe was quenched in the presence of chlorpyrifos and crotoxphos whereas the presence of endosulfan in the target sample increased the fluorescence of the probe. The LoD was observed to be 6.53 μmol L−1 for chlorpyrifos, 3.72 μmol L−1 for crotoxphos, and 0.004 μmol L−1 for endosulfan. They have prepared europium-doped titanium oxide nanopowder by the sol–gel method and used it to detect chlorpyrifos with significant fluorescence quenching.161 The existence of an electron transport mechanism was discovered through experimental and computational investigations. To detect the insecticide chlorpyrifos (CPF), Ren's team created a fluorescent probe, such as Mn(II)-doped ZnS quantum dots coated with an acrylamide-based molecularly imprinted polymer (MIP-coated QDs). The LoD was determined to be 17 nmol L−1. Chlorpyrifos could be found in actual samples using this simple, safe, and affordable approach.162
The development of a plant-based carbon dot fluorescence sensor has been reported by Tafreshi et al. for the qualitative and quantitative analysis of pesticides (diazinon, glyphosate, and amicarbazone) in water and in plant nutritional products.163 The LoD was found to be 0.25, 0.5, and 2 ng ml−1 for diazinon, amicarbazone, and glyphosate, respectively, and the developed sensor can be applied for the detection of pesticides in real-world samples. In 2019, Arvand et al. reported an efficient fluorescent sensor based on L-cysteine capped CdS-QDs/DF20 to analyze diazinon in environmental and agriculture samples based on the FRET mechanism, and the LoD was found to be 0.13 nM (Fig. 13).164 Ibrahim and coworkers synthesized a fluorescent probe, Tb(III) complex of 3-ally-salicylohydrazide, for detecting dichlorvos.165 The probe possessed good luminescence properties and the fluorescence at λ = 546 nm was enhanced with the addition of dichlorvos. Lu et al. have synthesized a Hg(II) complex of novel cholesterol derivative, 4-chloro-7-nitro-1,2,3-benzoxadiazole (CTN) using triazole as a linker, that showed a fluorescent emission at λ = 561 nm.166 The sensor detected five major organophosphorus pesticides, glyphosate, dichlorvos, chlorpyrifos, diazinon, and phoxim, with detection limits of 0.015, 0.018, 0.087, 0.098, and 0.113 μg mL−1 and the intensity of fluorescence emission gradually raised with the blue shift from λ = 561 to 527 nm in the presence of any of these pesticides. Kaur et al. developed two distinct Biginelli derivatives of cobalt complexes to enable the detection of malathion and azamethiphos using the fluorescence turn-on method. The LoD values for malathion and azamethiphos were 9.2 nM and 11 nM in aqueous medium, respectively.167
 | ||
| Fig. 13 The principle of the fluorescence “turn off–on” aptasensor for diazinon detection based on QDs-aptamer and GO. Reprinted with permission from ref. 164. Copyright 2019 Elsevier. | ||
 | ||
| Fig. 14 Schematic illustration of glyphosate sensing based on the Cu(II)-indicator displacement strategy. Reprinted with permission from ref. 168. Copyright 2021 Elsevier. | ||
Yang et al. prepared a metal–organic framework-based novel sensor Zr-MOF, Fe3O4@SiO2@UiO-67 via a versatile layer-by-layer assembly strategy for the detection of glyphosate.172 The fluorescence was enhanced due to the interaction of Zr-MOF with glyphosate and the LoD was 0.093 μg L−1. Guo et al. designed a turn-on fluorescence probe for the detection of glyphosate, based on the FRET mechanism between CdTe QDs capped with thioglycolic acid (TGA-CdTe-QDs) and gold nanoparticles stabilized with cysteamine (CS-AuNPs). This technique has been applied to successfully and satisfactorily identify glyphosate in apples and the LoD was found to be 9.8 ng kg−1.173 Yu et al. developed a sensor by combining two supramolecular systems such as two-dimensional MOF nanosheets with calix[4]arenes for glyphosate detection. A substantial increase in fluorescence emission was observed in the presence of pesticides based on electron transfer.174 The fluorescence sensing properties of various sensors highlighted in this article for organophosphate pesticide detection are given in Table 2.
4.2 Fluorescence sensing of carbamate pesticides
Carbamates are the derivatives of carbamic acid with ester and thioester functionalities.178 They are comparably safer to use than organophosphates, organochlorines, and pyrethroid pesticides.179 Despite having low bioaccumulation, they have been found to disrupt endocrine.180 Acetylcholinesterase enzyme required for the hydrolysis of acetylcholine is the main target of these compounds, where they induce carbamylation at neuromuscular junctions and neuronal synapses and inhibit acetylcholine breakdown.179–181 Few carbamates have been commercialized as drugs like rivastigmine (for treating Alzheimer's and Parkinson's disease), urethane (for chemotherapy), meprobamate (for anxiety), darunavir (an antiretroviral drug) and carbachol (for ophthalmic irregularities).182 Nevertheless, they are majorly applied as pesticides in agriculture and their maximum residue limit (MRL) can vary between 0.01 and 100 mg kg−1.178–183 Remaining levels of oxamyl, carbofuran, aldicarb, methomyl, benomyl, methiocarb, carbendazim, and pirimicarb have been detected in agricultural goods.178,180,181 Monitoring environmental samples for carbamate pollutants has become crucial due to the effects that prolonged use of carbamates has had on non-target organisms.184–186 Common carbamate pesticides are highlighted in Fig. 15.The application of colorimetric and fluorescence methods for carbamate detection has been the subject of several reports. The probes used range from basic organic molecules to quantum dots and nanoparticles. The majority of fluorescent probes for carbaryl detection that have been reported were chosen based on how well they worked with the enzyme carboxylesterase, which has an ester group. Under experimental conditions, the probe works by hydrolyzing the ester into acid (see Fig. 16). When there is an increase in the quantity of carbaryl, the carboxylesterase inhibition activity is enhanced, thereby the emission of the probe gets suppressed.187,188 Carbaryl detection has been accomplished by nanoparticles as probes, in which the inhibition of acetylcholinesterase activity was applied as a strategy. For instance, a rhodamine B-covered gold nanoparticle with a dual method of detection (fluorescence and colorimetric) reported by Liu et al. (see Fig. 17)189 and carbon quantum dots coupled with nanoparticles reported by Chen et al. demonstrated substantial quenching of the fluorescence intensity upon the addition of carbaryl pesticide.190,191
 | ||
| Fig. 16 The mechanism of fluorescence off–on sensing of carboxylesterase and carbaryl pesticide. Reprinted with permission from ref. 187. Copyright 2021 Elsevier. | ||
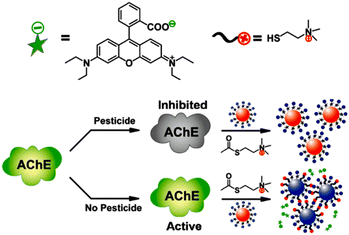 | ||
| Fig. 17 The schematic illustration of the design of dual mode of colorimetric and fluorometric assays for the detection of pesticides. Reprinted with permission from ref. 189. Copyright 2012 American Chemical Society. | ||
A colorimetric sensor array has been fabricated with five hydrogen peroxide and thiocholine-sensitive probes for carbamate detection (carbaryl, metolcarb, methomyl, isoprocarb, fenobucarb). The prevention of thiocholine production has been used as a base for this detection.192,193 Cadmium telluride (CdTe) quantum dots have been formulated as sensors for the detection of carbaryl in rice, Chinese cabbage (LoD = 0.147 μM), and Iranian apple (see Fig. 18).194 Carbendazim, because of the presence of benzimidazole, possesses significant chemical stability and exists for a longer period in the environment. Cucurbits have been used for this purpose, which mainly function by complex formation with carbendazim. This newly formed interaction enhanced the fluorescence intensity of the pesticide residue and allowed its detection even with low concentrations.195,196
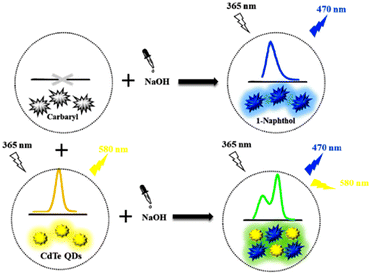 | ||
| Fig. 18 The proposed ratiometric fluorescence nanoprobe using CdTe QDs for the detection of carbaryl pesticide. Reprinted with permission from ref. 194. Copyright 2021 Elsevier. | ||
Yang et al. have demonstrated the detection of carbendazim (with a LoD of 0.002 μM) using a combined probe established from N,P-doped carbon quantum dots and AuNPs based on the FRET mechanism (Fig. 19).197 Similarly, S-doped graphene QDs were used as sensors for the detection of carbofuran and thiram with a LoD of 0.45 ppb and 1.6 ppb, respectively (Fig. 20),198 and vitamin B12 coated carbon QDs with dual fluorescence emission for detecting carbofuran were developed, which involves the charge transfer complex formation by carbofuran on the surface of QDs.109
 | ||
| Fig. 19 The formation of N,P-doped carbon quantum dots and their use to detect carbendazim pesticide via the FRET mechanism. Reprinted with permission from ref. 197. Copyright 2018 Elsevier. | ||
 | ||
| Fig. 20 (a) The microwave-assisted sonochemical synthesis of S-GQD, and (b) the fabrication of S-GQD-based fluorescent (PVA/S-GQD) films for the sensitive detection of carbofuran. Reprinted with permission from ref. 198. Copyright 2020 Elsevier. | ||
Core–shell QDs have been used to selectively detect trace levels of carbamate insecticides (carbofuran, aldicarb, and methomyl) found in medicinal plants. Acetylcholinesterase was effectively inhibited by carbamates in this fluorescent sensing method. Carbofuran had the strongest inhibitory activity among the carbamates that were evaluated.199 Zhang et al. have developed a “turn-on” sensor for thiram by using Mn-doped ZnS QDs combined with Ag(I) ion. Inhibition of the quenching process takes place due to the formation of a stable thiram–Ag(I) complex (Fig. 21) with a LoD of 25 nM in fresh fruits.200 Recently, a fluorescent probe based on multi-color nitrogen dots for sensing thiram and chlorpyrifos in pear, lettuce, lychee, orange, and cucumber samples was reported by Tang et al., in combination with copper and iron ions.201
 | ||
| Fig. 21 (A) The sensing mechanism for the detection of thiram. (B) UV-vis absorption spectra of Ag(I), thiram, and Ag–thiram complex (inset: colloidal suspension of the complex). (C) The Raman spectra of thiram and Ag–thiram complex. Reprinted with permission from ref. 200. Copyright 2017 Elsevier. | ||
A sensitive and selective fluorescent probe has been constructed from an MOF functionalized with Zr(IV) and Tb(III) for detecting thiabendazole in oranges. This system can sense thiabendazole in about 35 minutes, with a LoD of 0.271 μM. This is because energy transferred from the MOF to thiabendazole causes the fluorescence intensity to be quenched.202 The complex formation of thiabendazole with cucurbit[6]uril and cucurbit[7]uril, and cartap with cucurbit[7]uril has been formulated as a strategy for their detection. When the complexation was initiated, the fluorescence of thiabendazole became enhanced in neutral aqueous medium with a detection limit in the range of 5.51 to 8.85 × 10−9 mol L−1,203 and quenching in the case of cartap with a LoD of 0.0029 μg mL−1.204 A hybrid chemosensor developed from NaYF4:Yb, Ho/Au nanocomposites has been used for the detection of cartap in farm and water samples. The presence of cartap caused aggregation of nanocomposites, which in turn enhanced the FRET process between NaYF4:Yb, Ho, and Au nanocomposites.205 Zeng et al. reported the detection of metolcarb by using a naphthol appended calix[4]arene (NOC4) combined with a micro-structured gold surface. Because of the new complex formation, the fluorescent emission was found to be enhanced with a detection limit of 0.1 μM.206 It was reported that the initial fluorescence intensity of rhodamine B functionalized AuNPs was restored in the presence of thiodicarb.207
Disruption of aggregation produced by pyrene appended β-cyclodextrin in aqueous solution was observed by the introduction of pirimicarb. The same probe was employed for the detection of common aromatic trinitro explosives like trinitrotoluene, picric acid, and trinitrobenzene.116 Highly luminescent CdTe QDs coated with 5,11,17,23-tetra-tert-butyl-25,27-diethoxy-26,28 dihydroxycalix[4]arene (C[4]/SiO2/CdTe) allowed the sensitive detection of methomyl via significant enhancement in fluorescence, which exhibited a linear relationship with methomyl concentration and showed a LoD of 0.08 μM (Fig. 23).208 It has been reported that cyclodextrins can be used for fluorescence-based herbicide detection, in which binding to cyclodextrin increases the emission intensity of weakly fluorescent carbamate pesticides.209,210 In the presence of either β- or γ-cyclodextrin, bendiocarb and promecarb showed 1.74 and 3.8-fold increases in fluorescence emission, respectively (Fig. 22).
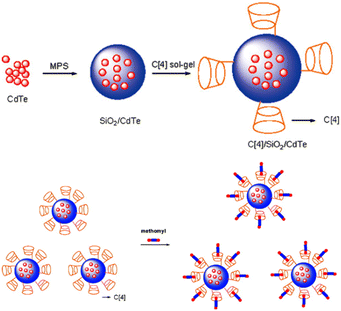 | ||
| Fig. 22 The fabrication of the C[4]/SiO2/CdTe luminescent probe and its pesticide sensing through host–guest complexation. Reprinted with permission from ref. 208. Copyright 2007 American Chemical Society. | ||
A turn-off fluorescence sensor for the identification and detection of cartap and methyl thiophanate has been published by Fan et al. This work involved the approach of simultaneously using water-soluble cadmium QDs (ZnCdSe and CdSe QDs) for the detection of the analyte. In real-time samples, the LoD was found to be 2 × 10−8 mol L−1.211Table 3 summarizes the sensing properties of various fluorescence probes discussed in this article for selective detection of carbamates.
| Probe | Analyte | LOD | Ref. |
|---|---|---|---|
| Rhodamine B covered gold nanoparticles | Carbaryl, diazinon, malathion, phorate | Carbaryl 0.1 μg L−1, diazinon 0.1 μg L−1, malathion 0.3 μg L−1, phorate 1 μg L−1 | 189 |
| CdTe QDs | Carbaryl | 0.147 μM | 193 |
| CdTe QDs | Carbaryl | 0.12 ng mL−1 | 194 |
| Cucurbit[7]uril | Carbendazim | 0.10 mg kg−1 | 196 |
| N,P-doped carbon quantum dots and AuNPs | Carbendazim | 0.002 μM | 197 |
| S-doped graphene QD | Carbofuran | 0.45 ppb | 198 |
| Thiram | 1.6 ppb | ||
| Vitamin B12 coated carbon QD | Carbofuran | 12.2 μM | 109 |
| Mn-doped ZnS QD- Ag+ ion | Thiram | 25 nM | 200 |
| Nitrogen dots combined with copper and iron ions | Thiram | 0.1 μg mL−1 | 201 |
| Chlorpyrifos | 0.01–0.50 μg mL−1 | ||
| Tb3+ functionalized Zr-MOF | Thiabendazole | 0.271 μM | 202 |
| Cucurbituril | Thiabendazole, cartap | 5.51–8.85 × 10−9 mol L−1 | 203 |
| 0.0029 μg mL−1 | 204 | ||
| NaYF4:Yb, Ho/Au nanocomposites | Cartap | 0.0029 μg m L−1 | 205 |
| Naphthol appended calix[4]arene@gold surface | Metolcarb | 0.1 μM | 206 |
| Rhodamine B functionalized Au NPs | Thiodicarb | 0.08 ppm | 207 |
| β-Cyclodextrin | Pirimicarb | 60 nM | 116 |
| C[4]/SiO2/CdTe | Methomyl | 0.08 μM | 208 |
| Cyclodextrin | Bendiocarb | 0.57 ± 0.02 μg mL−1 | 209, 210 |
| Promecarb | 0.091 ± 0.002 μg mL−1 | ||
| ZnCdSe-CdSe QD | Methyl thiophanate | 2 × 10−8 mol L−1 | 211 |
4.3 Fluorescence sensing of pyrethroid pesticides
Pyrethroids are a class of pesticides derived from pyrethrins, which are commonly used as insecticides. They are moderately toxic and possess moderate biodegradability. Nearly 70 pyrethroid derivatives have now been applied in agriculture, and some of their structures have been shown in Fig. 24.212 However, because of their high absorbability, they have an impact on animal development and raise the risk of cancer with extended exposure. Pyrethroids can sometimes be detrimental to plant growth.213–215 and it has been suggested that sperm count is affected in post-pyrethroid exposure.216,217 There are only limited reports available for the identification and detection of pyrethroids, which include sensors, surface-enhanced Raman scattering, and surface plasmon resonance. | ||
| Fig. 24 (A) Schematic illustration of the synthesis of SiO2@FITC-APTS@MIPs fluorescence sensor and (B) the extent of fluorescence quenching by different kinds of pyrethroid pesticides. Reprinted with permission from ref. 218. Copyright 2015 American Chemical Society. | ||
Fluorescent MIPs have been identified as a method for pyrethroid detection. MIP microspheres are made by precipitation polymerization with allyl fluorescein as a monomer and the cyhalothrin analyte as a template. This template was utilized to identify the analyte in actual honey samples and demonstrated great sensitivity and selectivity for cyhalothrin.219 Another report was made by Liu and co-workers, who developed supramolecular architectures containing an MIP based on a luminescent Eu complex and Si nanospheres. The resultant template used analyte-induced fluorescence quenching to detect λ-cyhalothrin with great selectivity.220 To create a composite pesticide sensor, Wei et al. combined luminescent CdTe quantum dots with MIPs synthesized in the presence of a bifenthrin template. This resulted in a marked decrease in fluorescence emission due to bifenthrin binding, with very low detection limits and high levels of selectivity reported. The synthetic method induces polymerization on the CdTe quantum dot surfaces using a biphasic solvent solution.221 Silica-based MIPs have been used for detecting cyhalothrin with the help of silica nanospheres embedded in CdSe QDs and SiO2.218,222,223 These methods eliminated the interfering materials in the sample and improved the LoD value (see Fig. 24).
Wei et al. created a fluorescent technique to detect λ-cyhalothrin by transferring aqueous CdTe QDs using octadecyl-4-vinylbenzyl-dimethyl-ammonium chloride (OVDAC) as a surfactant (Fig. 25).224 The Ren team created MIPs and employed them to produce a composite material coated with MIPs, using QDs. This composite material was designed to selectively recognize cyphenothrin.225 A new, eco-friendly MIP-QD nanosensor has been developed to specifically extinguish cyfluthrin's fluorescence. This nanosensor was created using an enhanced reverse microemulsion process and is based on FeSe-QDs. The ionic and hydrogen bonding interactions prevented charge transfer from FeSe-QDs to cyfluthrin and produced excellent linearity, selectivity, and sensitivity.226
 | ||
| Fig. 25 Schematic illustration for the preparation of MIPs-OVDAC/CdTe QDs and their fluorescence-quenching-based sensing of λ-cyhalothrin antibiotics. Reprinted with permission from ref. 224. Copyright 2016 Elsevier. | ||
A fluorescent MIP sensor was developed specifically for detecting pyrethroid pesticides by using SiO2/ZnO QDs. This sensor has a very low (LoD) of 0.13 μM. The sensor demonstrated a quick and selective detection of cyhalothrin in practical river-water samples within a 15-minute timeframe.227 Atom transfer radical polymerization has been used to construct a sensor composed of a MIP.228 Cyhalothrin can be detected using this sensor. Within the range of 2–80 nM, the fluorescence intensity of SiO2-MPS@FMIP, which is composed of a fluorescent MIP and a SiO2 core modified with MPS (3-(methacryloxyl) propyl trimethoxysilane), showed a linear relationship with the cyhalothrin concentration. Cyhalothrin's LoD was found to be 0.0037 nM.229
Using CD functionalized core–shell nanospheres, dual emission determination of λ-cyhalothrin has been established by tracking the transition from green to blue fluorescence.230 Sulfur-doped carbon dots coated with MIPs utilizing acrylamide and 1-vinyl-3-butylimidazolium tetrafluoroborate [VBIm][BF4] were used to detect LC, a pesticide residue that shows a LoD of 0.5 μg kg−1.231 A ZnO-based MIP containing cyhalothrin recognition sites exhibited a linear relationship between the concentration of cyhalothrin and the fluorescence intensity obtained in the concentration range of 0 to 80 μmol L−1.232 To identify deltamethrin in fruit and vegetable samples, water-soluble CdTe QDs and fluorescent SiO2 molecularly imprinted nanospheres embedded in CdTe QDs functioned as a fluorescence nanosensor.233 CD-encapsulated covalent organic frameworks grafted with poly(N-isopropyl acrylamide) were developed for the detection of pyrethroids, which are temperature-responsive and have a detection limit of 0.69 μg L−1 (Fig. 26).229 Two molecularly imprinted polymeric microspheres and two fluorescent tracers for benzimidazoles and pyrethroids were fabricated and used for the simultaneous determination of benzimidazoles and pyrethroids with a LoD ranging from 5.2 to 17 ng mL−1.234
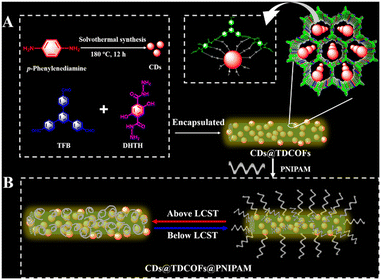 | ||
| Fig. 26 Schematic representation of (A) the fabrication of the CDs@TDCOFs@PNIPAM fluorescence sensor for (B) on/off detection of pyrethroids. Reprinted with permission from ref. 229. Copyright 2022 American Chemical Society. | ||
A novel host–guest supramolecular probe with an albumin host and flavonoid guest was successfully synthesized for the ratiometric determination of cyfluthrin with a fast detection response of 10 s and a LoD of 70 ppb along with a distinct orange to green emissive color change. The smartphone-assisted on-site analysis enabled the signal outputs to be captured and analyzed.235 A fluorophore synthesized from the interaction between two boron derivatives with the nickel complex as a catalyst and triphenylphosphine as an additive exhibited a dual emissive phenomenon. The intensity ratio of these two emissions served as a ratiometric method for the detection of pyrethroids and recorded a LoD of 1.5 μg L−1.236Table 4 summarizes the selective detection of pyrethroids using fluorescent probes.
| Probe | Analyte | LoD | Ref. |
|---|---|---|---|
| Molecularly imprinted polymers microspheres | Cyhalothrin | 0.004 nm | 219 |
| m-SiO2-Eu(TTA)3Bpc@MIPs | λ-Cyhalothrin | 220 | |
| MIPs (PS)-OVDAC/CdTe QDs | Bifenthrin | 0.08 μmol L−1 | 221 |
| CdSe QDs-SiO2-MIPs | λ-Cyhalothrin | 3.6 μg L−1 | 222 |
| Molecularly imprinted fluorescent hollow nanoparticles | λ-Cyhalothrin | 10.26 nM | 223 |
| SiO2@FITC-APTS@MIPs | λ-Cyhalothrin | 9.17 nM L−1 | 218 |
| MIPs-octadecyl-4-vinylbenzyl-dimethyl-ammonium chloride (OVDAC)-CdTeQDs | λ-Cyhalothrin | 0.03 μmol L−1 | 224 |
| QDs-based MIPs-coated composite (ZnS-Mn2+) | Cyphenothrin | 9.0 nmol L−1 | 225 |
| MIP-FeSe-QDs | Cyfluthrin in fish | 1.0 μg kg−1 | 226 |
| SiO2-MPS@FMIP | Cyhalothrin | 0.0037 nM | 228 |
| CDs-SiO2 | λ-Cyhalothrin | 0.048 μg L−1 | 230 |
| Sulfur-doped carbon dots - MIPs | λ-Cyhalothrin | 0.5 μg kg−1 | 231 |
| SiO2-MIPs-CdTe QDs | Deltamethrin | 0.16 μg ml−1 | 233 |
| Host–guest supramolecular probe with an albumin host and flavonoid guest | Cyfluthrin | 70 ppb | 235 |
| Boron-based non-covalent ratiometric fluorophore | Pyrethroids | 1.5 μg L−1 | 236 |
4.4 Fluorescence sensing of triazine and triazole pesticides
Triazine and triazole moieties find widespread use as pesticides in crop production like sugar cane, grapes, corn, rice, and pulses. Their water solubility enables them to stay in the soil for a long time, and this stability may have ill effects on water and food.237 HPLC, GC, MS, and GC-MS were among the methods utilized to identify and quantify their contamination. However, since these techniques fail to identify traces of remaining pesticides, an effective detection technique needs to be created.238,239 Examples of triazine and triazole pesticides are given in Fig. 27 and 29. | ||
| Fig. 28 The schematic representation of the synthesis of nitrogen-doped carbon quantum dots (above) and their turn-on fluorescent sensing of the herbicide atrazine through multiple hydrogen-bonding interactions (below). Reprinted with permission from ref. 242. Copyright 2018 Elsevier. | ||
Liu et al. developed a novel MIP-Fe3O4-chitosan-based sensor for the detection of atrazine and the method involved the direct competition between atrazine and its fluorescent analogue (5-(4,6-dichlorotriazinyl)amino fluorescein (5-DTAF)). Increased fluorescence was observed, with a LoD of 0.86 μM and a linear relationship with the log(atrazine) concentration in the range of 2.32 to 185.4 μM.246 Halder and his colleagues conducted extensive computational research on luminous MOFs employed for pesticide detection. They examined a copper MOF and a cadmium MOF that both contained 4,4′-bipyridine ligands and succinate dianions as bridging components.247 According to the study, succinate's oxygen atoms were necessary for coordinating with the NH and OH groups of the pesticides, particularly atrazine and dicofol. They also had aromatic π–π stacking between the bipyridine and aromatic pesticides. The second case involved the utilization of a magnetic covalent organic framework (COF) to identify chlorpyrifos, atrazine, and diquat dibromide in polluted water solutions.248 The procedure entailed the pesticides adhering to the COF and then removing them using magnetic solid-phase extraction. Following the regeneration of the COF, this cycle was carried out up to five times.
 | ||
| Fig. 30 The schematic representation of a dual-emission mesoporous structured MIP sensor encapsulated with CdTe/CdS QDs for the ratiometric detection of diniconazole. Reprinted with permission from ref. 249. Copyright 2017 Elsevier. | ||
To identify non-fluorescent triazoles such as azaconazole, flusilazole, tricyclazole, triadimefon, tebuconazole, penconazole, flutriafol, and triadimenol isomer A in aqueous solution, the host–guest complex of thioflavin T (ThT) and twisted cucurbit[14]uril (tQ[14]) was used as a fluorescent probe. Flusilazole caused a particular reaction in the ThT@tQ[14] probe, which led to a significant decrease in fluorescence intensity. The probe detected flusilazole at a minimum concentration of 1.27 × 10−8 mol L−1.250 In one example, researchers reported a thiazole-twisted cucurbit[14]uril (tQ [14]) (Li et al., 2016), for fluorescence-based triazole-containing pesticide detection. Triazoles significantly reduced fluorescence when they were introduced. It was discovered that two distinct supramolecular mechanisms were at work: one in which the triazole and thiazole competed for the same binding site, ultimately leading to fluorescence quenching, and another in which the triazole bound to a different section of tQ [14] than thiazole, resulting in cooperative effects that facilitated the decreases in fluorescence intensity.251
4.5 Fluorescence sensing of organochlorine pesticides
Presently, the growing population necessitates the rapid production of vegetables, cereals, and fruits, resulting in the overutilization of pesticides, particularly organochlorine pesticides (OCPs) in crop agriculture and animal husbandry. Most of the OCPs are degraded slowly under standard environmental conditions. OCPs can enter the human body through the skin and lungs. They have the potential to impact the central nervous system, resulting in convulsions, hyperreflexia, ataxia, and tremors. Prolonged exposure to OCPs at low concentrations can lead to immunosuppression, reproductive abnormalities, hypersensitivity reactions, and potentially even cancer. Some of the organochlorine structures are given in Fig. 1. In this part of the review, the detection of organochlorines using fluorescence-based chemosensors has been discussed. Glutathione-coated CdS (GSH-CdS) QDs were reported by Walia and Acharya for the selective detection of dicofol, in the presence of other pesticides namely dimethoate, chlorpyrifos, and imidacloprid.252 The chloride groups of dicofol interacted with the –NH2 and –COOH groups of glutathione, increasing the fluorescence of GSH-CdS QDs to allow for “turn-on” detection, and the probe was able to detect dicofol down to 55 ± 11 ppb.In another example, polar OCPs were detected using methylammonium lead halide perovskite QDs (MAPB-QDs) based on the observation that the fluorescence spectra of MAPB-QDs were blue-shifted when polar OCPs were present.253 Wang et al. and Yang et al. have used Mn-doped ZnS QDs imprinted with an MIP for the detection of pentachlorophenol (PCP).254,255 This probe can detect the spiked PCP in river water, tap water, and spring water with good recoveries. In another example, Liu et al. prepared the probe from the combination of graphene quantum dots GQDs with CdS QDs to form GQDs-CdS nanocrystals to detect the presence of pentachlorophenol in water samples.256,257 To detect 2,4-dichlorophenoxyacetic acid (2,4-D), mesoporous structured imprinting microspheres were attached to quantum dots (QDs) to produce a novel fluorescence sensor.258 An electron-transfer-induced fluorescence quenching process makes this detection possible. The sensor demonstrated a detection limit of 2.1 nM and was effectively utilized to detect 2,4-D in bean sprout samples. The recoveries achieved ranged from 95.0% to 110.1%, indicating great accuracy and precision.
In another contribution, Zhang et al. developed a novel paper@QDs@MIPs fluorescence sensor to detect 2,4-D based on electron-transfer-induced fluorescence quenching.258 This sensor is inexpensive with a lower recognition rate and detection limit of 0.12 μM. Wang et al. and Xu et al. constructed a novel “signal-on” type MIP-based ratiometric fluorescence sensor which detects 2,4-D by using nitrobenzoxadiazole (NBD) and QD@SiO2 as a core.259–261 The proposed sensor showed a high sensitivity for 2,4-D with a low LoD (0.13 μM) (Fig. 31). By using electron transfer to cause the fluorescence quenching, Xu et al. have created a novel MOF-based probe, such as the Mg(II) complex containing 4,4′-(4-aminopyridine-3,5-diyl)dibenzoic acid, that can detect electron-deficient 2,6-dichloro-4-nitroaniline (DCN).261 The probe showed good selectivity towards DCN in the presence of other pesticides and the LoD was found to be 150 ppb. Xu et al. have developed a Cd-LMOF complex by reacting a rigid conjugated tricarboxylic acid ligand 4,4′-(9-(4′-carboxy-[1,1′-biphenyl]-4-yl)-9H-carbazole-3,6-diyl)dibenzoic acid (H3CBCD) with Cd(II), which showed a strong blue fluorescent emission.262,263 The synthesized complex was used to detect DCN via electron transfer. Besides PET, the resonance energy transfer contributes to the observed fluorescent quenching.
 | ||
| Fig. 31 (a) Fluorescence ‘turn-on’ optosensing of herbicide in pure milk using a ratiometric fluorescent microsphere. (b) Fluorescent spectra and fluorescence colors of the polymerizable NBD monomer (in CH3CN, b1) and red CdTe QDs (in H2O, b2) as well as those of the grafted dual fluorescent 2,4-D-MIP (b3) and its mixture with 2,4-D (b4) in pure bovine milk. Reprinted with permission from ref. 261. Copyright 2020 Elsevier. | ||
Guo et al. have synthesized a 2D Zn-based MOF containing 4-(tetrazol-5-yl)phenyl-4,2′:6′,4′′-terpyridine and terephthalic acid as the ligand with blue fluorescence for the detection of DCN and the LoD was found to be 1.90 μM.263,264 Tao et al. have reported a Zn-based luminescent MOF appended (E)-1,2-diphenyl-1,2-bis(4-(pyridin-4-yl)phenyl)ethene to detect DCN with AIE properties.264,265 In 2019, Wang et al. developed a novel MOF Zn(II) complex of 3,5-di(2′,4′-dicarboxylphenyl)benzoic acid and 1,2-di(4-pyridyl)ethylene by the solvothermal method to detect the presence of 2,6-dichloro-4-nitroaniline with low detection limits in aqueous solution.266 In another study, Chi et al. reported carboxylic acid substituent appended Zn-based coordination polymers for 2,6-dichloro-4-nitroaniline detection.267 A copper-based MOF containing the 4,4′-bipyridine and succinate dianion has been developed and studied using theoretical density functional theory analysis. This research shows that the MOF may selectively detect pesticides similar to atrazine and dicofol.247 The Feng group developed a novel two-dimensional (2D) cadmium-based MOF for the detection of DCN due to PET and FRET.268
Sharma and coworkers synthesized a fluorescent supramolecular assembly based on anthracene/perylene bisamide (PBI) derivatives to detect the presence of organophosphate (CPF) and organochlorine (DCN) pesticides in aqueous media by the inner filter effect for the “on–off” detection of DCN.269 In addition, researchers have investigated the practical uses of supramolecular assemblies for detecting CPF and DCN in contaminated water and agricultural products, including grapes and apples. To detect dicofol, Wang et al. developed a fluorometric chemosensor based on mercaptoethanol and boron dipyrromethene (BODIPY) (Fig. 32). A ‘turn-off’ fluorescence behavior was noticed upon reaction with dicofol and a detection limit of 200 ppb.270 Two ternary Cd(II) coordination polymers{[Cd(tptc)0.5(bpz)(H2O)]·0.5H2O}n (CP1) and [Cd(tptc)0.5(bpy)]n (CP2) were designed through the mixed ligand strategy.271 Two Cd(II) coordination polymers (CPs) exhibit remarkable chemical stability and luminescence properties. These CPs demonstrate efficient multi-functional fluorescent responses towards dichloro-4-nitroaniline in aqueous media. The detection limits for CP1 and CP2 are 112 ppb and 638 ppb, respectively. Table 5 summarizes the fluorescent sensing properties of several sensors for organochlorine pesticide detection.
 | ||
| Fig. 32 Proposed reaction mechanism of mercaptoethanol with dicofol and BODIPY. Reprinted with permission from ref. 270. Copyright 2022 Elsevier. | ||
| Probe | Analyte | LoD | Ref. |
|---|---|---|---|
| Glutathione coated CdS (GSH-CdS) QDs | Dicofol | 55 ± 11 ppb | 253 |
| Methylammonium lead halide perovskite quantum dots (MAPB-QDs) | pentachlorophenol | 0.02 μM | 254 |
| Mn-doped ZnS QDs-MIPs | pentachlorophenol | 86 nM | 255 |
| Mn-doped ZnS QDs-Fe3O4 nanoparticles | pentachlorophenol | 0.5 μmol L−1 | 256 |
| GQDs-CdS nanocrystals | pentachlorophenol | 3 pg m L−1 | 257 |
| Mesoporous structured imprinting microspheres on the surfaces of quantum dots (QDs) | 2,4-D | 2.1 nM | 258 |
| Paper@QDs@MIPs | 2,4-D | 90 nM | 259 |
| Nitrobenzoxadiazole (NBD) and QD@SiO2 | 2,4-D | 0.14 μM | 261 |
| Mg(II) complex containing 4,4′-(4-aminopyridine-3,5-diyl)dibenzoic acid | 2,6-Dichloro-4-nitroaniline | 150 ppb | 262 |
| [Cd3(CBCD)2(DMA)4(H2O)2]·10DMA (Cd-CBCD). Cd-CBCD | 2,6-Dichloro-4-nitroaniline | 145 ppb | 263 |
| Zn-based MOF containing 4-(tetrazol-5-yl)phenyl-4,2′:6′,4′′-terpyridine and terephthalic acid | 2,6-Dichloro-4-nitroaniline | 1.90 μM | 265 |
| Zn(II) complex of (E)-1,2-diphenyl-1,2-bis(4-(pyridin-4-yl)phenyl)ethene | 2,6-Dichloro-4-nitroaniline | 0.13 ppm | 264 |
| Zn(II) complex of 3,5-di(2′,4′-dicarboxylphenyl)benzoic acid and 1,2-di(4-pyridyl)ethylene | 2,6-Dichloro-4-nitroaniline | ∼166 ppb | 266 |
| Zn-based coordination polymers | 2,6-Dichloro-4-nitroaniline | 6.7 × 10−5 M−1 (CP1) & 2.8 × 10−5 M−1 (CP2) | 267 |
| Cd-based metal–organic framework | 2,6-Dichloro-4-nitroaniline | 0.221 ppm | 268 |
| Anthracene/perylene bisamide (PBI) derivatives | Chlorpyrifos & DCN | 0.10 × 10−7 M−1 & 0.18 nM | 269 |
| Mercaptoethanol and boron dipyrromethene | Dicofol | 200 ppb | 270 |
| Cd(II) coordination polymers | 2,6-Dichloro-4-nitroaniline | 112 ppb (CP1) & 638 ppb (CP2) | 271 |
4.6 Fluorescence sensing of neonicotinoid pesticides
The discovery of neonicotinamides, a new class of insecticides, marks a significant advancement in pesticide research in recent years. They specifically affect the central nervous system of insects by acting as an antagonist of their molecular target site, the post-synaptic nicotinic receptors (nAChRs). Neonicotinamide pesticides are an emerging contaminant, hence it is crucial to identify and eliminate them. A new fluorescent probe, ZnS:Mn-aptamer, has been developed using quantum dots (QDs) for acetamiprid visual detection.The fluorescence of the sensor was quenched by the FRET between the multi-walled carbon nanotubes (MWCNTs) and ZnS:Mn-aptamer. When acetamiprid was added, preferential binding with ZnS:Mn-aptamer took place which caused a reduction in FRET and thereby turning on the fluorescence.272,273 Hu et al. reported a new nanosensor based on aptamers for the detection of acetamiprid utilizing FRET between NH2-NaYF4:Yb, holmium silica dioxide (Ho@SiO2) up-conversion NPs (UCNPs) and AuNPs.274 A colorimetric and fluorometric approach involving an acetamiprid-binding aptamer (ABA), AuNPs, and UCNPs was employed for the ultrasensitive and selective detection of acetamiprid. The ABA underwent a structural switch from a DNA duplex to an aptamer–acetamiprid complex and also dissociated from the AuNPs. The dual approach used the principles of salt-induced AuNP aggregation, analyte-triggered structural switch of aptamers, and UCNP signal amplification.275
An AuNPs-QDs system caused a fluorescent quenching of RF-QDs induced by combining ratiometric fluorescent QDs (RF-QDs) with AuNPs due to IFE (Fig. 33). The interaction of acetamiprid with AuNPs exhibited a change of color from purple to dark blue due to aggregation.250 An imprinted fluorescent nanoprobe based on SiO2-coated NaYF4:Yb, Er UCNPs encapsulated with an MIP has been fabricated for acetamiprid detection. The fluorescence of UCNP@MIP was suppressed when combined with acetamiprid, due to photo-induced electron transfer. The detection limit for this combination was 8.3 ng mL−1. The approach was effectively adopted acetamiprid detection in apple and strawberry samples, yielding recoveries ranging from 89.6% to 97.9%.276 A MIP based on silane-doped carbon dots (Si–CDs) has been developed as a probe for acetamiprid (ACT) detection with high selectivity. The fluorescence signal of MIP@Si–CDs displayed a detection limit of 2 nM and utilized ACT detection in real samples.277 Qu et al. utilized the ability of calixarene to bind fenamithion and acetamiprid to sufficiently remove these pesticides from the proximity of the QDs and restore their fluorescence (Fig. 34).278 It has been found that imidacloprid significantly quenches the fluorescence of poly-(2,6-dimethoxynaphthalene).279 Scientists combined MOFs with luminescent indicators to identify and degrade nitenpyram (NIT). Fluorescent probes for MOFs were created using porphyrin fluorophores. The probe experienced electron transfer under the influence of NIT, leading to the suppression of fluorescence.280 Liu et al. developed a test strip-based fluorescent sensor for thiacloprid based on polydopamine (PDA) MIPs and N-GQDs. Thiacloprid was selectively captured via PDA-MIP, increasing the fluorescence intensity of N-GQDs. This increase in intensity was directly proportional to the concentration of thiacloprid within the range of 0.1–10 mg L−1. The limit of detection for thiacloprid was found to be 0.03 mg L−1, which is rather low.281Table 6 summarizes the fluorescence sensing properties of various fluorescent sensors for neonicotinoid pesticide detection.
 | ||
| Fig. 33 The fluorescent detection of acetamiprid through the inner-filter effect of gold nanoparticles on QDs. Reprinted with permission from ref. 276. Copyright 2014 Elsevier. | ||
 | ||
| Fig. 34 Host–guest complexation of acetamiprid enhances the fluorescence of CdTe QDs in the presence of p-sulfonatocalix[4]arene. Reprinted with permission from ref. 278. Copyright 2009 Elsevier. | ||
| Probe | Analyte | LoD | Ref. |
|---|---|---|---|
| ZnS:Mn aptamer quantum dots | Acetamiprid | 0.7 nM | 272 |
| CdTe quantum dots-AuNPs | Acetamiprid | 7.29 nM | 273 |
| NH2-NaYF4:Yb, holmium silica dioxide (Ho@SiO2) UCNPs and AuNPs | Acetamiprid | 3.2 nM | 274 |
| AuNPs-upconversion nanoparticles | Acetamiprid | 0.36 nm | 275 |
| RF quantum dots-AuNPs | Acetamiprid | 16.8 μg L−1 | 250 |
| SiO2-coated NaYF4:Yb, Er upconversion nanoparticles (UCNP)-IPs | Acetamiprid | 8.3 ng mL−1 | 276 |
| MIP@Si–CDs | Acetamiprid | 2 nM | 277 |
| CdTe (QDs)-p-sulfonatocalix[4]arene | Fenamithion and acetamiprid | 1.2 × 10−8 M (fenamithion) and 3.4 × 10−8 M (acetamiprid) | 278 |
| Poly(2,6-dimethoxynaphthalene) based probe | Imidacloprid | 3.093 ng mL−1 | 279 |
| Bifunctional nanoscale porphyrinic MOF probe | Nitenpyram | 0.03 μg mL−1 | 280 |
| Nitrogen-doped graphene QDs (GQDs) | Thiacloprid | 0.03 mg L−1 | 281 |
5. Conclusions and outlook
In summary, we have exemplified in detail the chemosensing applications of fluorescence materials reported so far to detect various classes of structurally assorted pesticides. We have systematically categorized pesticides into several groups depending upon their structures and functions and each section highlights the fluorescence sensing properties of different sensors for the detection of a particular class of pesticides including their mechanism of fluorescence sensing, selectivity, and sensitivity for detection (see Tables 1–6 for further details). At the beginning of this review, we have discussed the classification of pesticides, different detection methods currently available for pesticide detection, and fluorescence-based sensing mechanisms proposed for pesticide detection. In the later part, a detailed report on various fluorescence-based sensing materials for different types of pesticides is provided. The evolution of fluorescence-based sensing materials is a topical area of research and is at the forefront of the materials sciences. The fluorescence-based sensing methods have different ways of finding innovative materials for efficiently sensing various analytes. The selectivity of materials can be tailored to specifically target particular analytes in complex sample matrices, and sensing capability can be improved by linking individual binding units into the polymeric network that supplies a large number of binding sites for detection and thus, significant enhancement in sensitivity of materials. The real-time utility of fluorescence-based sensors for pesticide detection is, however, limited by several issues and difficulties that need to be fixed to realize their useful applications, even with the notable advancement.Most of the sensor systems/molecules discussed herein detect pesticides through a fluorescence quenching mechanism, except in a few cases where the binding of pesticides enhances the fluorescence intensity of sensors. In general, it is proclaimed that fluorescence turn-on sensing would be more beneficial because the naked eye can easily visualize the sensing. The other challenge is that the current methodologies have been established by detecting a single analyte, but numerous real-time samples contain a mixture of pesticides. Therefore, the design of suitable sensors with discriminating capability would be appealing. Moreover, on-site analysis can resolve the environmental impact of these pesticides. Another notable issue with the reported sensors is their poor stability in real-time sensing media such as stability under aqueous conditions and under varied pH ranges. Hence, a collective detection of pesticide residues that can enable on-site determination has to be demonstrated. An alternative strategy could be thought from the use of remote-operated sensors. Moreover, systematic studies are necessary for better understanding the interactions between sensors and pesticides because there is a substantial absence of knowledge on the precise mechanism of fluorescence sensing for several of the sensors covered in this review. According to the review's findings, many areas require more study to create and build workable fluorescence-based sensor systems for real-time pesticide detection. Given the information above, we encourage researchers to develop a sustainable method of detecting pesticides that overcomes present obstacles and relies on fluorescence sensing to improve the environment.
This review article provides a detailed report on fluorescence chemosensors reported to date for structurally assorted classes of pesticide detection. Particular emphasis has been dedicated to the sensing mechanism, sensitivity, mode of binding, and efficient sensing of pesticides in real samples. We also highlighted the current challenges with the existing sensors and the perspective on addressing these challenges to develop practically useful sensor systems for pesticide detection. We strongly believe that this review will inspire researchers working in the related areas to find suitable fluorescent-based sensors for real-time monitoring of pesticides.
Data availability
No primary research results, software, or code have been included and no new data were generated or analyzed as part of this review.Author contributions
G. Kalaiarasi: conceptualization, software, resources, visualization, funding acquisition, writing – original draft; Y. Jegadeesan: software, resources, visualization, writing – original draft; A. Shanmughan: software, resources, visualization, writing – original draft; M. K. Noushija: software, resources, visualization, writing – review & editing; A. V. Krishna: software, resources, visualization, writing – review & editing; H. Gangadharan: software, resources, visualization, writing – review & editing; D. Umadevi: conceptualization, software, resources, visualization, supervision, investigation, project administration, funding acquisition, writing – review & editing; S. Shanmugaraju: conceptualization, software, resources, visualization, project administration, supervision, investigation, funding acquisition, writing – original draft, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank the Indian Institute of Technology Palakkad (ERG research grant 2023-168-CHY-SHS-ERG-SP to SS) and the Science and Engineering Research Board (EMEQ research grant EEQ/2023/000386 to S.S.), India, for financial support. D. U. thanks DST India for funding (DST/WOS-A/CS-68/2021). G. K. sincerely thanks the Tamil Nadu State Council for Science and Technology (TNSCST) for the Young Scientist Fellowship.References
- R. Repetto and S. Baliga, Trends and patterns of pesticide use: in Pesticides and the immune system; Public Health Risks, World Resources Institute, Washington, D.C., 1996, pp. 3–8 Search PubMed.
- J. T. Zacharia, Pestic. Mod. world-trends Pestic. Anal., 2011, pp. 1–18 Search PubMed.
- I. C. Yadav, N. L. Devi, J. H. Syed, Z. Cheng, J. Li, G. Zhang and K. C. Jones, Sci. Total Environ., 2015, 511, 123–137 CrossRef CAS PubMed.
- S. Savary, L. Willocquet, S. J. Pethybridge, P. Esker, N. McRoberts and A. Nelson, Nat. Ecol. Evol., 2019, 3, 430–439 CrossRef PubMed.
- S. A. Nsibande and P. B. C. Forbes, Anal. Chim. Acta, 2016, 945, 9–22 CrossRef CAS PubMed.
- Y. Abubakar, H. Tijjani, C. Egbuna, C. O. Adetunji, S. Kala, T. L. Kryeziu, J. C. Ifemeje and K. C. Patrick-Iwuanyanwu, in Natural remedies for pest, disease and weed control, Elsevier, 2020, pp. 29–42 Search PubMed.
- M. A. Rodrigo, N. Oturan and M. A. Oturan, Chem. Rev., 2014, 114, 8720–8745 CrossRef CAS PubMed.
- E.-C. Oerke, J. Agric. Sci., 2006, 144, 31–43 CrossRef.
- A. Samsidar, S. Siddiquee and S. M. Shaarani, Trends Food Sci. Technol., 2018, 71, 188–201 CrossRef CAS.
- Canadian Cancer Society, Cosmetic Pesticides. Information Brief, 2013.
- W. Mnif, A. I. H. Hassine, A. Bouaziz, A. Bartegi, O. Thomas and B. Roig, Int. J. Environ. Res. Public Health, 2011, 8, 2265–2303 CrossRef CAS PubMed.
- D. Goulson, Nature, 2014, 511, 295–296 CrossRef CAS PubMed.
- S. Zheng, B. Chen, X. Qiu, M. Chen, Z. Ma and X. Yu, Chemosphere, 2016, 144, 1177–1192 CrossRef CAS PubMed.
- J. Römbke, R. M. Schmelz and C. Pelosi, Front. Environ. Sci., 2017, 5, 20 CrossRef.
- M. Pirsaheb, M. Limoee, F. Namdari and R. Khamutian, Med. J. Islam. Repub. Iran, 2015, 29, 228 Search PubMed.
- G.-E. Séralini, E. Clair, R. Mesnage, S. Gress, N. Defarge, M. Malatesta, D. Hennequin and J. S. de Vendômois, Environ. Sci. Eur., 2014, 26, 1–17 CrossRef.
- D. S. Thakur, R. Khot, P. P. Joshi, M. Pandharipande and K. Nagpure, Toxicol. Int., 2014, 21, 328 CrossRef PubMed.
- J. K. Sathish Kumar, S. S. Monica, B. Vinothkumar, A. Suganthi and M. Paramasivam, Agricultural Reviews, 2024, 45, R-2325 Search PubMed.
- E. Reiler, E. Jørs, J. Bælum, O. Huici, M. M. A. Caero and N. Cedergreen, Sci. Total Environ., 2015, 527, 262–269 CrossRef PubMed.
- A. Witczak and H. Abdel-Gawad, J. Environ. Sci. Health, Part B, 2014, 49, 917–928 CrossRef CAS PubMed.
- S. Chourasiya, P. S. Khillare and D. S. Jyethi, Environ. Sci. Pollut. Res., 2015, 22, 5793–5806 CrossRef CAS PubMed.
- C. Buscail, C. Chevrier, T. Serrano, F. Pelé, C. Monfort, S. Cordier and J.-F. Viel, Occup. Environ. Med., 2015, 72, 837–844 CrossRef PubMed.
- D. Lu, D. Wang, R. Ni, Y. Lin, C. Feng, Q. Xu, X. Jia, G. Wang and Z. Zhou, Environ. Sci. Pollut. Res., 2015, 22, 9293–9306 CrossRef CAS PubMed.
- E. Homburg and E. Vaupel, Hazardous Chemicals: Agents of Risk and Change, 1800–2000, Berghahn Books, 2019, vol. 17 Search PubMed.
- N. C. Deziel, J. L. Warren, H. Huang, H. Zhou, A. Sjodin and Y. Zhang, Environ. Res., 2021, 192, 110333 CrossRef CAS PubMed.
- P. Booij, I. Holoubek, J. Klánová, J. Kohoutek, A. Dvorská, K. Magulová, S. Al-Zadjali and P. Čupr, Sci. Total Environ., 2016, 550, 231–240 CrossRef CAS PubMed.
- G. L. Daly, Y. D. Lei, C. Teixeira, D. C. G. Muir, L. E. Castillo, L. M. M. Jantunen and F. Wania, Environ. Sci. Technol., 2007, 41, 1124–1130 CrossRef CAS PubMed.
- M. J. Adkesson, J. M. Levengood, J. W. Scott, D. J. Schaeffer, J. N. Langan, S. Cárdenas-Alayza, S. De La Puente, P. Majluf and S. Yi, J. Wildl. Dis., 2018, 54, 304–314 CrossRef CAS PubMed.
- L. Hunt, C. Bonetto, V. H. Resh, D. F. Buss, S. Fanelli, N. Marrochi and M. J. Lydy, Sci. Total Environ., 2016, 547, 114–124 CrossRef CAS PubMed.
- P. E. Davies, L. S. J. Cook and J. L. Barton, Aust. J. Mar. Freshwater Res., 1994, 45, 209–226 CrossRef CAS.
- E. De Gerónimo, V. C. Aparicio, S. Bárbaro, R. Portocarrero, S. Jaime and J. L. Costa, Chemosphere, 2014, 107, 423–431 CrossRef PubMed.
- F. P. Carvalho, J.-P. Villeneuve, C. Cattini, J. Rendón and J. M. de Oliveira, Chemosphere, 2009, 74, 988–995 CrossRef CAS PubMed.
- H. De Bon, J. Huat, L. Parrot, A. Sinzogan, T. Martin, E. Malezieux and J.-F. Vayssières, Agron. Sustainable Dev., 2014, 34, 723–736 CrossRef CAS.
- B. K. Singh and A. Walker, FEMS Microbiol. Rev., 2006, 30, 428–471 CrossRef CAS PubMed.
- C. Potera, Agriculture: Pesticides disrupt nitrogen fixation, Environ. Health Perspect., 2007, 115, A579 CrossRef PubMed.
- I. C. Yadav and N. L. Devi, Environ. Sci. Eng., 2017, 6, 140–158 Search PubMed.
- M. C. R. Alavanja, C. Samanic, M. Dosemeci, J. Lubin, R. Tarone, C. F. Lynch, C. Knott, K. Thomas, J. A. Hoppin and J. Barker, Am. J. Epidemiol., 2003, 157, 800–814 CrossRef PubMed.
- Z.-Q. Jia, Y.-C. Zhang, Q.-T. Huang, A. K. Jones, Z.-J. Han and C.-Q. Zhao, J. Hazard. Mater., 2020, 394, 122521 CrossRef CAS PubMed.
- H. Guan, W. E. Brewer, S. T. Garris and S. L. Morgan, J. Chromatogr. A, 2010, 1217, 1867–1874 CrossRef CAS PubMed.
- Y. Fu, X. Dou, L. Zhang, J. Qin, M. Yang and J. Luo, J. Chromatogr., B, 2019, 1125, 121730 CrossRef CAS PubMed.
- R. Mondal, A. Mukherjee, S. Biswas and R. K. Kole, Chemosphere, 2018, 206, 217–230 CrossRef CAS PubMed.
- A. Stachniuk, A. Szmagara, R. Czeczko and E. Fornal, J. Environ. Sci. Health, Part B, 2017, 52, 446–457 CrossRef CAS PubMed.
- W. Gui, C. Tian, Q. Sun, S. Li, W. Zhang, J. Tang and G. Zhu, Water Res., 2016, 95, 185–194 CrossRef CAS PubMed.
- F. Arduini, S. Cinti, V. Caratelli, L. Amendola, G. Palleschi and D. Moscone, Biosens. Bioelectron., 2019, 126, 346–354 CrossRef CAS PubMed.
- Y. Li, Q. Luo, R. Hu, Z. Chen and P. Qiu, Chin. Chem. Lett., 2018, 29, 1845–1848 CrossRef CAS.
- S. Chen, Y.-L. Yu and J.-H. Wang, Anal. Chim. Acta, 2018, 999, 13–26 CrossRef CAS PubMed.
- A. Bigdeli, F. Ghasemi, S. Abbasi-Moayed, M. Shahrajabian, N. Fahimi-Kashani, S. Jafarinejad, M. A. F. Nejad and M. R. Hormozi-Nezhad, Anal. Chim. Acta, 2019, 1079, 30–58 CrossRef CAS PubMed.
- S. Shanmugaraju and P. S. Mukherjee, Chem. Commun., 2015, 51, 16014–16032 RSC; X. Yan, H. Li and X. Su, TrAC, Trends Anal. Chem., 2018, 103, 1–20 CrossRef CAS.
- R. Singh, P. Thakur, A. Thakur, H. Kumar, P. Chawla, J. V. Rohit, R. Kaushik and N. Kumar, Int. J. Environ. Anal. Chem., 2021, 101, 3006–3022 CrossRef CAS.
- G. K. Mishra, V. Sharma and R. K. Mishra, Biosensors, 2018, 8, 28 CrossRef PubMed.
- N. Serio, J. Roque, A. Badwal and M. Levine, Analyst, 2015, 140, 7503–7507 RSC.
- S. Farooq, J. Nie, Y. Cheng, Z. Yan, J. Li, S. A. S. Bacha, A. Mushtaq and H. Zhang, Analyst, 2018, 143, 3971–3989 RSC.
- S. K. Panigrahi and A. K. Mishra, J. Photochem. Photobiol., C, 2019, 41, 100318 CrossRef.
- D. Cao, L. Zhu, Z. Liu and W. Lin, J. Photochem. Photobiol., C, 2020, 44, 100371 CrossRef CAS.
- L. Wu, C. Huang, B. P. Emery, A. C. Sedgwick, S. D. Bull, X.-P. He, H. Tian, J. Yoon, J. L. Sessler and T. D. James, Chem. Soc. Rev., 2020, 49, 5110–5139 RSC.
- M. Levine, Front. Chem., 2021, 9, 616815 CrossRef CAS PubMed; T. Skorjanc, D. Shetty and M. Valant, ACS Sens., 2021, 6, 1461–1481 CrossRef PubMed; H. Bhambri, S. Khullar, Sakshi and S. K. Mandal, Mater. Adv., 2022, 3, 19–124 RSC.
- M. A. Hassaan and A. El Nemr, Egypt. J. Aquat. Res., 2020, 46, 207–220 CrossRef.
- K. H. Büchel, Chemistry of pesticides, Wiley New York, New York, 1983, p. 518 Search PubMed.
- E. J. Mrema, F. M. Rubino, G. Brambilla, A. Moretto, A. M. Tsatsakis and C. Colosio, Toxicology, 2013, 307, 74–88 CrossRef CAS PubMed.
- S. Ragab, A. El Sikaily and A. El Nemr, Egypt. J. Aquat. Res., 2016, 42, 365–374 CrossRef.
- L. Gámiz-Gracia, A. M. García-Campaña, J. J. Soto-Chinchilla, J. F. Huertas-Pérez and A. González-Casado, TrAC, Trends Anal. Chem., 2005, 24, 927–942 CrossRef.
- C. Bolognesi, Mutat. Res., Rev. Mutat. Res., 2003, 543, 251–272 CrossRef CAS PubMed.
- A. Vale and M. Lotti, Handb. Clin. Neurol., 2015, 131, 149–168 Search PubMed.
- A. El Nemr and M. M. El-Sadaawy, Int. J. Sediment Res., 2016, 31, 44–52 CrossRef.
- A. El Nemr, G. F. El-Said and A. Khaled, Water Environ. Res., 2016, 88, 325–337 CrossRef CAS PubMed.
- E. L. Robb, A. C. Regina and M. B. Baker, Organophosphate Toxicity, StatPearls Publishing, Treasure Island, Florida, 2024 Search PubMed.
- F. P. Garcia, S. Y. C. Ascencio, J. C. G. Oyarzún, A. C. Hernandez and P. V. Alavarado, Journal of Research in Environmental Science and Technology, 2012, 1, 279–293 Search PubMed.
- J. R. Reigart, Recognition and management of pesticide poisonings, DIANE Publishing, 2009 Search PubMed.
- R. C. Gupta and J. W. Crissman, in Haschek and Rousseaux's Handbook of Toxicologic Pathology, Elsevier, 2013, pp. 1349–1372 Search PubMed.
- S. Saari, A. Näreaho and S. Nikander, Canine parasites and parasitic diseases, Academic Press, 2018 Search PubMed.
- F. M. Fishel and J. A. Ferrell, Managing Pesticide Drift, https://edis.ifas.ufl.edu/publication/PI232.
- D. Li, M. He, B. Chen and B. Hu, J. Chromatogr. A, 2019, 1583, 19–27 CrossRef CAS PubMed.
- R. Umapathi, S. Sonwal, M. J. Lee, G. M. Rani, E.-S. Lee, T.-J. Jeon, S.-M. Kang, M.-H. Oh and Y. S. Huh, Coord. Chem. Rev., 2021, 446, 214061 CrossRef CAS.
- R. Singh, N. Kumar, R. Mehra, H. Kumar and V. P. Singh, Trends Environ. Anal. Chem., 2020, 26, e00086 CrossRef CAS.
- P. Chawla, R. Kaushik, V. J. S. Swaraj and N. Kumar, Environ. Nanotechnol., Monit. Manage., 2018, 10, 292–307 Search PubMed.
- H. Patel, D. Rawtani and Y. K. Agrawal, Trends Food Sci. Technol., 2019, 85, 78–91 CrossRef CAS.
- M. Liu, A. Khan, Z. Wang, Y. Liu, G. Yang, Y. Deng and N. He, Biosens. Bioelectron., 2019, 130, 174–184 CrossRef CAS PubMed.
- I. S. Che Sulaiman, B. W. Chieng, M. J. Osman, K. K. Ong, J. I. A. Rashid, W. M. Z. Wan Yunus, S. A. M. Noor, N. A. M. Kasim, N. A. Halim and A. Mohamad, Microchim. Acta, 2020, 187, 1–22 CrossRef PubMed.
- G. M. Fernandes, W. R. Silva, D. N. Barreto, R. S. Lamarca, P. C. F. L. Gomes, J. F. da S. Petruci and A. D. Batista, Anal. Chim. Acta, 2020, 1135, 187–203 CrossRef CAS PubMed; Z.-L. Li, X.-R. Qi, Y.-H. Xu, J.-Q. Zhao, G.-F. Zuo and M.-M. Wang, Food Chem., 2025, 472, 142904 CrossRef PubMed.
- M. Bhattu, M. Verma and D. Kathuria, Anal. Methods, 2021, 13, 4390–4428 RSC.
- P. Shah, X. Zhu and C. Li, Expert Rev. Mol. Diagn., 2013, 13, 83–91 CrossRef CAS PubMed.
- F. Li, M. You, S. Li, J. Hu, C. Liu, Y. Gong, H. Yang and F. Xu, Biotechnol. Adv., 2020, 39, 107442 CrossRef CAS PubMed.
- R. Jin, L. Zhao, X. Yan, X. Han, M. Liu, Y. Chen, Q. Li, D. Su, F. Liu and P. Sun, Biosens. Bioelectron., 2020, 167, 112457 CrossRef CAS PubMed.
- P. N. Minh, V.-T. Hoang, N. X. Dinh, O. Van Hoang, N. Van Cuong, D. T. B. Hop, T. Q. Tuan, N. T. Khi, T. Q. Huy and A.-T. Le, New J. Chem., 2020, 44, 7611–7620 RSC.
- M.-G. Lee, V. Patil, Y.-C. Na, D. S. Lee, S. H. Lim and G.-R. Yi, Sens. Actuators, B, 2018, 261, 489–496 CrossRef CAS.
- J. Wei, L. Yang, M. Luo, Y. Wang and P. Li, Ecotoxicol. Environ. Saf., 2019, 179, 17–23 CrossRef CAS PubMed.
- S. A. Ghoto, M. Y. Khuhawar, T. M. Jahangir and J. ul D. Mangi, J. Nanostruct. Chem., 2019, 9, 77–93 CrossRef CAS.
- S. A. Ghoto, M. Y. Khuhawar and T. M. Jahangir, Anal. Sci., 2019, 35, 631–637 CrossRef CAS PubMed.
- M. M. Bordbar, T. A. Nguyen, F. Arduini and H. Bagheri, Microchim. Acta, 2020, 187, 1–13 CrossRef PubMed.
- M. M. Bordbar, T.-A. Nguyen, A. Q. Tran and H. Bagheri, Sci. Rep., 2020, 10, 17302 CrossRef CAS PubMed.
- J. Kaur and P. K. Singh, Phys. Chem. Chem. Phys., 2020, 22, 15105–15119 RSC.
- Z. Sun, L. Tian, M. Guo, X. Xu, Q. Li and H. Weng, Food Control, 2017, 81, 23–29 CrossRef CAS.
- M. E. I. Badawy and A. F. El-Aswad, Int. J. Anal. Chem., 2014, 536823 Search PubMed.
- M. Kavruk, V. C. Özalp and H. A. Öktem, J. Anal. Methods Chem., 2013, 932946 Search PubMed.
- S. Uniyal and R. K. Sharma, Biosens. Bioelectron., 2018, 116, 37–50 CrossRef CAS PubMed.
- R. Zamora, F. Masís-Meléndez, H. Phillips, L. A. Alvarado-Marchena and R. Starbird, Int. J. Electrochem. Sci., 2018, 13, 1931–1944 CrossRef CAS.
- F. Della Pelle, C. Angelini, M. Sergi, M. Del Carlo, A. Pepe and D. Compagnone, Talanta, 2018, 186, 389–396 CrossRef CAS PubMed.
- X. Tu, F. Gao, X. Ma, J. Zou, Y. Yu, M. Li, F. Qu, X. Huang and L. Lu, J. Hazard. Mater., 2020, 396, 122776 CrossRef CAS PubMed.
- C. Gu, Q. Wang, L. Zhang, P. Yang, Y. Xie and J. Fei, Sens. Actuators, B, 2020, 305, 127478 CrossRef CAS.
- Y. Bakytkarim, S. Tursynbolat, Q. Zeng, J. Huang and L. Wang, J. Electroanal. Chem., 2019, 841, 45–50 CrossRef CAS.
- J. P. Giraldo, H. Wu, G. M. Newkirk and S. Kruss, Nat. Nanotechnol., 2019, 14, 541–553 CrossRef CAS PubMed.
- F. Zhao, J. He, X. Li, Y. Bai, Y. Ying and J. Ping, Biosens. Bioelectron., 2020, 170, 112636 CrossRef CAS PubMed.
- Z. Usmani, M. Sharma, A. K. Awasthi, G. D. Sharma, D. Cysneiros, S. C. Nayak, V. K. Thakur, R. Naidu, A. Pandey and V. K. Gupta, J. Hazard. Mater., 2021, 416, 126154 CrossRef CAS PubMed.
- H. Teymourian, M. Parrilla, J. R. Sempionatto, N. F. Montiel, A. Barfidokht, R. Van Echelpoel, K. De Wael and J. Wang, ACS Sens., 2020, 5, 2679–2700 CrossRef CAS PubMed.
- X. Yang and H. Cheng, Micromachines, 2020, 11, 243 CrossRef PubMed.
- D. Zhang, J. Jiang, J. Chen, Q. Zhang, Y. Lu, Y. Yao, S. Li, G. L. Liu and Q. Liu, Biosens. Bioelectron., 2015, 70, 81–88 CrossRef CAS PubMed.
- K. Xu, Q. Chen, Y. Zhao, C. Ge, S. Lin and J. Liao, Sens. Actuators, B, 2020, 319, 128221 CrossRef CAS.
- D. Ji, Z. Shi, Z. Liu, S. S. Low, J. Zhu, T. Zhang, Z. Chen, X. Yu, Y. Lu and D. Lu, Smart Mater. Med., 2020, 1, 1–9 Search PubMed.
- B. B. Campos, R. Contreras-Cáceres, T. J. Bandosz, J. Jiménez-Jiménez, E. Rodríguez-Castellón, J. C. G. E. da Silva and M. Algarra, Sens. Actuators, B, 2017, 239, 553–561 CrossRef CAS; N. Zhou, M. Cai, S. Zheng, H. Guo and F. Yang, Sens. Actuators, B, 2017, 417, 136051 CrossRef.
- Y. Hao, Q. Yin, Y. Zhang, M. Xu and S. Chen, Molecules, 2019, 24, 3716 CrossRef CAS PubMed; P. He, Y. Chen, L. Lin, H. Guo and F. Yang, Talanta, 2024, 276, 126269 CrossRef PubMed.
- S. Goswami, S. Cekli, E. Alarousu, R. W. Winkel, M. Younus, O. F. Mohammed and K. S. Schanze, Macromolecules, 2020, 53, 6279–6287 CrossRef CAS.
- T. T. Vu, T. N. N. Dau, C. T. Ly, D. C. Pham, T. T. N. Nguyen and V. T. Pham, J. Mater. Sci., 2020, 55, 9070–9081 CrossRef CAS.
- J. Chen, X. Chen, Q. Huang, W. Li, Q. Yu, L. Zhu, T. Zhu, S. Liu and Z. Chi, ACS Appl. Mater. Interfaces, 2019, 11, 32689–32696 CrossRef CAS PubMed.
- X. Li, W. Ma, H. Li, Q. Zhang and Z. Ma, Trends Food Sci. Technol., 2020, 97, 185–195 CrossRef CAS.
- S.-L. Yang, J.-N. Lu, S.-J. Zhang, C.-X. Zhang and Q.-L. Wang, Analyst, 2018, 143, 4901–4906 RSC.
- P.-L. Champagne, R. Kumar and C.-C. Ling, Sens. Actuators, B, 2019, 281, 229–238 CrossRef CAS.
- T. Tian, F. Qiu, K. Dong and D. Yang, Toxicol. Environ. Chem., 2012, 94, 1034–1042 CrossRef CAS.
- C. Jiang, Z. Song, L. Yu, S. Ye and H. He, TrAC, Trends Anal. Chem., 2020, 133, 116086 CrossRef CAS.
- M. Ganesan and P. Nagaraaj, Anal. Methods, 2020, 12, 4254–4275 RSC.
- A. Mehta, A. Mishra, S. Basu, N. P. Shetti, K. R. Reddy, T. A. Saleh and T. M. Aminabhavi, J. Environ. Manage., 2019, 250, 109486 CrossRef CAS PubMed.
- P. C. Dey and R. Das, Spectrochim. Acta, Part A, 2019, 207, 156–163 CrossRef CAS PubMed.
- M. Lasagna, M. S. Hielpos, C. Ventura, M. N. Mardirosian, G. Martín, N. Miret, A. Randi, M. Núñez and C. Cocca, Ecotoxicol. Environ. Saf., 2020, 205, 111312 CrossRef CAS PubMed.
- M. A. Ghasemzadeh, B. Mirhosseini-Eshkevari, M. Tavakoli and F. Zamani, Green Chem., 2020, 22, 7265–7300 RSC.
- D. Y. Heo, H. H. Do, S. H. Ahn and S. Y. Kim, Polymers, 2020, 12, 2061 CrossRef CAS PubMed.
- J. Kaushal, M. Khatri and S. K. Arya, Ecotoxicol. Environ. Saf., 2021, 207, 111483 CrossRef CAS PubMed.
- T. C. Kwong, Ther. Drug Monit., 2002, 24, 144–149 CrossRef CAS PubMed.
- R. Cochran, in Hayes' Handbook of Pesticide Toxicology, Elsevier, 2010, pp. 337–355 Search PubMed.
- K. Vikrant, D. C. W. Tsang, N. Raza, B. S. Giri, D. Kukkar and K.-H. Kim, ACS Appl. Mater. Interfaces, 2018, 10, 8797–8817 CrossRef CAS PubMed.
- J. Cao, M. Wang, H. Yu, Y. She, Z. Cao, J. Ye, A. M. Abd El-Aty, A. Hacımüftüoğlu, J. Wang and S. Lao, J. Agric. Food Chem., 2020, 68, 7298–7315 CrossRef CAS PubMed.
- N. Li, X. Wang, J. Chen, L. Sun and P. Chen, 2D Mater., 2015, 2, 34018 CrossRef.
- J. Peng, W. Yin, J. Shi, X. Jin and G. Ni, Microchim. Acta, 2019, 186, 1–9 CrossRef PubMed.
- J. Li, Y. Weng, C. Shen, J. Luo, D. Yu and Z. Cao, Anal. Methods, 2021, 13, 2981–2988 RSC.
- J. Hou, J. Dong, H. Zhu, X. Teng, S. Ai and M. Mang, Biosens. Bioelectron., 2015, 68, 20–26 CrossRef CAS PubMed.
- X. Yan, H. Li, X. Wang and X. Su, Talanta, 2015, 131, 88–94 CrossRef CAS PubMed.
- M. M. F. Chang, I. R. Ginjom and S. M. Ng, Sens. Actuators, B, 2017, 242, 1050–1056 CrossRef CAS.
- Y. Hu, J. Li and X. Li, Anal. Bioanal. Chem., 2019, 411, 7879–7887 CrossRef CAS PubMed.
- K. Zhang, Q. Mei, G. Guan, B. Liu, S. Wang and Z. Zhang, Anal. Chem., 2010, 82, 9579–9586 CrossRef CAS PubMed.
- X. Dou, X. Chu, W. Kong, J. Luo and M. Yang, Anal. Chim. Acta, 2015, 891, 291–297 CrossRef CAS PubMed.
- A. K. K. Bhasin, P. Raj, P. Chauhan, S. K. Mandal, S. Chaudhary, N. Singh and N. Kaur, New J. Chem., 2020, 44, 3341–3349 RSC.
- R. M. Abdelhameed, M. El-Naggar, M. Taha, S. Nabil, M. A. Youssef, N. S. Awwad and M. T. El Sayed, J. Mol. Struct., 2020, 1199, 127000 CrossRef CAS.
- Q. Chen, R. Sheng, P. Wang, Q. Ouyang, A. Wang, S. Ali, M. Zareef and M. M. Hassan, Spectrochim. Acta, Part A, 2020, 241, 118654 CrossRef CAS PubMed.
- H. A. Azab, A. S. Orabi and A. M. Abbas, J. Lumin., 2015, 160, 181–187 CrossRef CAS.
- M. Wang, K. Su, J. Cao, Y. She, A. M. Abd El-Aty, A. Hacımüftüoğlu, J. Wang, M. Yan, S. Hong and S. Lao, Talanta, 2019, 192, 295–300 CrossRef CAS PubMed.
- S.-H. Hung, J.-Y. Lee, C.-C. Hu and T.-C. Chiu, Food Chem., 2018, 260, 61–65 CrossRef CAS PubMed.
- C.-W. Hsu, Z.-Y. Lin, T.-Y. Chan, T.-C. Chiu and C.-C. Hu, Food Chem., 2017, 224, 353–358 CrossRef CAS PubMed.
- Z. Sheng, J. Zhang, C. Li and L. Chen, Anal. Methods, 2016, 8, 8506–8513 RSC.
- S. Li, J. Luo, G. Yin, Z. Xu, Y. Le, X. Wu, N. Wu and Q. Zhang, Sens. Actuators, B, 2015, 206, 14–21 CrossRef CAS.
- Z. Cui, C. Han and H. Li, Analyst, 2011, 136, 1351–1356 RSC.
- Y. Shen, F. Yan, X. Huang, X. Zhang, Y. Zhang, C. Zhang, J. Jin, H. Li and S. Yao, RSC Adv., 2016, 6, 88096–88103 RSC.
- W. Bai, G. Qin, J. Wang, L. Li and Y. Ni, Dyes Pigm., 2021, 193, 109473 CrossRef CAS.
- Q. Sun, Q. Yao, Z. Sun, T. Zhou, D. Nie, G. Shi and L. Jin, Chin. J. Chem., 2011, 29, 2134–2140 CrossRef CAS.
- Q. Long, H. Li, Y. Zhang and S. Yao, Biosens. Bioelectron., 2015, 68, 168–174 CrossRef CAS PubMed.
- J. Tang and L. Xiang, Pol. J. Environ. Stud., 2016, 25, 787–793 CrossRef CAS PubMed.
- W. Song, H.-J. Zhang, Y.-H. Liu, C.-L. Ren and H.-L. Chen, Chin. Chem. Lett., 2017, 28, 1675–1680 CrossRef CAS.
- X. Yan, H. Li, Y. Yan and X. Su, Food Chem., 2015, 173, 179–184 CrossRef CAS PubMed.
- X. Hu, F. Wang, Q. Peng, J. Hu, H. Peng, L. Li, B. Zheng, J. Du and D. Xiao, RSC Adv., 2019, 9, 13048–13053 RSC.
- X. Xu, Y. Guo, X. Wang, W. Li, P. Qi, Z. Wang, X. Wang, S. Gunasekaran and Q. Wang, Sens. Actuators, B, 2018, 260, 339–345 CrossRef CAS.
- K. He, Z. Li, L. Wang, Y. Fu, H. Quan, Y. Li, X. Wang, S. Gunasekaran and X. Xu, ACS Appl. Mater. Interfaces, 2019, 11, 26250–26260 CrossRef CAS PubMed.
- K. Kanagaraj, A. Affrose, S. Sivakolunthu and K. Pitchumani, Biosens. Bioelectron., 2012, 35, 452–455 CrossRef CAS PubMed.
- H. A. Azab, G. M. Khairy and R. M. Kamel, Spectrochim. Acta, Part A, 2015, 148, 114–124 CrossRef CAS PubMed.
- F. Yao, Y. Sun, C. Tan, S. Wei, X. Zhang, X. Hu and J. Fan, J. Korean Chem. Soc., 2011, 55, 932–935 CrossRef CAS.
- X. Ren, H. Liu and L. Chen, Microchim. Acta, 2015, 182, 193–200 CrossRef CAS.
- F. Ashrafi Tafreshi, Z. Fatahi, S. F. Ghasemi, A. Taherian and N. Esfandiari, PLoS One, 2020, 15, e0230646 CrossRef CAS PubMed.
- M. Arvand and A. A. Mirroshandel, Food Chem., 2019, 280, 115–122 CrossRef CAS PubMed.
- I. A. Ibrahim, A. M. Abbas and H. M. Darwish, Luminescence, 2017, 32, 1541–1546 CrossRef CAS PubMed.
- Y. Lü, Q. Sun, B. Hu, X. Chen, R. Miao and Y. Fang, New J. Chem., 2016, 40, 1817–1824 RSC.
- G. Kaur, A. Singh, A. Singh, N. Kaur and N. Singh, Dalton Trans., 2018, 47, 5595–5606 RSC.
- J. Guan, J. Yang, Y. Zhang, X. Zhang, H. Deng, J. Xu, J. Wang and M.-S. Yuan, Talanta, 2021, 224, 121834 CrossRef CAS PubMed.
- B. C. M. A. Ashwin, C. Saravanan, T. Stalin, P. Muthu Mareeswaran and S. Rajagopal, ChemPhysChem, 2018, 19, 2768–2775 CrossRef CAS PubMed.
- J. Hou, X. Wang, S. Lan, C. Zhang, C. Hou, Q. He and D. Huo, Anal. Methods, 2020, 12, 4130–4138 RSC.
- L. Wang, Y. Bi, J. Hou, H. Li, Y. Xu, B. Wang, H. Ding and L. Ding, Talanta, 2016, 160, 268–275 CrossRef CAS PubMed.
- Q. Yang, J. Wang, X. Chen, W. Yang, H. Pei, N. Hu, Z. Li, Y. Suo, T. Li and J. Wang, J. Mater. Chem. A, 2018, 6, 2184–2192 RSC.
- J. Guo, Y. Zhang, Y. Luo, F. Shen and C. Sun, Talanta, 2014, 125, 385–392 CrossRef CAS PubMed.
- C.-X. Yu, F.-L. Hu, J.-G. Song, J.-L. Zhang, S.-S. Liu, B.-X. Wang, H. Meng, L.-L. Liu and L.-F. Ma, Sens. Actuators, B, 2020, 310, 127819 CrossRef CAS.
- F. Zhang, X. Cao, D. Tian and H. Li, Chin. J. Chem., 2015, 33, 368–372 CrossRef CAS.
- Q. Wang, Q. Yin, Y. Fan, L. Zhang, Y. Xu, O. Hu, X. Guo, Q. Shi, H. Fu and Y. She, Talanta, 2019, 199, 46–53 CrossRef CAS PubMed.
- M. Roth, R. H. Richards, D. P. Dobson and G. H. Rae, Aquaculture, 1996, 140, 217–239 CrossRef CAS.
- V. Scognamiglio, G. Rea, F. Arduini and G. Palleschi, Biosensors for sustainable food-new opportunities and technical challenges, Elsevier, 2016 Search PubMed.
- S. Patel and S. Sangeeta, Environ. Sci. Pollut. Res., 2019, 26, 91–100 CrossRef CAS PubMed.
- T. M. B. F. Oliveira, M. F. Barroso, S. Morais, P. de Lima-Neto, A. N. Correia, M. B. P. P. Oliveira and C. Delerue-Matos, Talanta, 2013, 106, 137–143 CrossRef CAS PubMed.
- B. Tiwari, S. Kharwar and D. N. Tiwari, Cyanobacteria, Elsevier, 2019, pp. 303–325 Search PubMed.
- A. K. Ghosh and M. Brindisi, J. Med. Chem., 2015, 58, 2895–2940 CrossRef CAS PubMed.
- M. Kundu, P. Krishnan, R. K. Kotnala and G. Sumana, Trends Food Sci. Technol., 2019, 88, 157–178 CrossRef CAS.
- N. I. M. Fauzi, Y. W. Fen, N. A. S. Omar and H. S. Hashim, Sensors, 2021, 21, 3856 CrossRef CAS PubMed.
- T. M. B. F. Oliveira, F. W. P. Ribeiro, C. P. Sousa, G. R. Salazar-Banda, P. de Lima-Neto, A. N. Correia and S. Morais, TrAC, Trends Anal. Chem., 2020, 124, 115779 CrossRef CAS.
- N. L. Mdeni, A. O. Adeniji, A. I. Okoh and O. O. Okoh, Molecules, 2022, 27, 618 CrossRef CAS PubMed.
- J. Dai, Y. Zhao, Y. Hou, G. Zhong, R. Gao, J. Wu, B. Shen and X. Zhang, Dyes Pigm., 2021, 192, 109444 CrossRef CAS.
- C. Ma, J. Wu, W. Sun, Y. Hou, G. Zhong, R. Gao, B. Shen and H. Huang, Sens. Actuators, B, 2020, 325, 128798 CrossRef CAS.
- D. Liu, W. Chen, J. Wei, X. Li, Z. Wang and X. Jiang, Anal. Chem., 2012, 84, 4185–4191 CrossRef CAS PubMed.
- S. Qian and H. Lin, Anal. Chem., 2015, 87, 5395–5400 CrossRef CAS PubMed.
- Y. Chen, X. Qin, C. Yuan, R. Shi and Y. Wang, Dyes Pigm., 2020, 181, 108529 CrossRef CAS.
- D. Zhao, C. Chen, J. Sun and X. Yang, Analyst, 2016, 141, 3280–3288 RSC.
- C. Zhang, H. Cui, J. Cai, Y. Duan and Y. Liu, J. Agric. Food Chem., 2015, 63, 4966–4972 CrossRef CAS PubMed.
- F. Shahdost-Fard, N. Fahimi-Kashani and M. R. Hormozi-Nezhad, Talanta, 2021, 221, 121467 CrossRef CAS PubMed.
- N. Saleh and N. A. F. Al-Rawashdeh, J. Fluoresc., 2006, 16, 487–493 CrossRef CAS PubMed.
- M. Del Pozo, L. Hernández and C. Quintana, Talanta, 2010, 81, 1542–1546 CrossRef CAS PubMed.
- Y. Yang, D. Huo, H. Wu, X. Wang, J. Yang, M. Bian, Y. Ma and C. Hou, Sens. Actuators, B, 2018, 274, 296–303 CrossRef CAS.
- R. V. Nair, R. T. Thomas, A. P. Mohamed and S. Pillai, Microchem. J., 2020, 157, 104971 CrossRef CAS.
- J.-C. Wei, B. Wei, W. Yang, C.-W. He, H.-X. Su, J.-B. Wan, P. Li and Y.-T. Wang, Food Chem. Toxicol., 2018, 119, 430–437 CrossRef CAS PubMed.
- C. Zhang, K. Zhang, T. Zhao, B. Liu, Z. Wang and Z. Zhang, Sens. Actuators, B, 2017, 252, 1083–1088 CrossRef CAS.
- Z. Tang, Z. Chen, G. Li and Y. Hu, Anal. Chim. Acta, 2020, 1136, 72–81 CrossRef CAS PubMed.
- X.-X. Peng, G.-M. Bao, Y.-F. Zhong, L. Zhang, K.-B. Zeng, J.-X. He, W. Xiao, Y.-F. Xia, Q. Fan and H.-Q. Yuan, Food Chem., 2021, 343, 128504 CrossRef CAS PubMed.
- Y. Huang, J. Wang, S.-F. Xue, Z. Tao, Q.-J. Zhu and Q. Tang, J. Inclusion Phenom. Macrocyclic Chem., 2012, 72, 397–404 CrossRef CAS.
- Z. Wang, L. Wu, B. Shen and Z. Jiang, Talanta, 2013, 114, 124–130 CrossRef CAS PubMed.
- J. Xu, L. DU, W. U. Hao, W. WU and Y. CHANG, J. Integr. Agric., 2012, 11, 1861–1870 CrossRef.
- X. Zeng, J. Ma, L. Luo, L. Yang, X. Cao, D. Tian and H. Li, Org. Lett., 2015, 17, 2976–2979 CrossRef CAS PubMed.
- M.-H. Tseng, C.-C. Hu and T.-C. Chiu, Dyes Pigm., 2019, 171, 107674 CrossRef CAS.
- H. Li and F. Qu, Chem. Mater., 2007, 19, 4148–4154 CrossRef CAS.
- N. L. Pacioni and A. V. Veglia, Anal. Chim. Acta, 2007, 583, 63–71 CrossRef CAS PubMed.
- T. Ogoshi and A. Harada, Sensors, 2008, 8, 4961–4982 CrossRef CAS PubMed.
- Y. Fan, L. Liu, D. Sun, H. Lan, H. Fu, T. Yang, Y. She and C. Ni, Anal. Chim. Acta, 2016, 916, 84–91 CrossRef CAS PubMed; X. Kang, J. Yuan, X. Gu, X. Li, T. Wen, M. He, Y. He, X. Yang and Y. Li, ACS Appl. Nano Mater., 2025, 8, 1663–1672 Search PubMed.
- Y. Chen, J. Lai, M. Zhang, T. Zhao, S. Wang, J. Li and S. Liu, Food Sci., 2022, 43, 285–292 Search PubMed.
- X. Liu, Q. Zhang, S. Li, P. Mi, D. Chen, X. Zhao and X. Feng, Chemosphere, 2018, 199, 16–25 CrossRef CAS PubMed.
- I. Bragança, P. C. Lemos, P. Barros, C. Delerue-Matos and V. F. Domingues, Sci. Total Environ., 2018, 619, 685–691 CrossRef PubMed.
- I. Bragança, A. P. Mucha, M. P. Tomasino, F. Santos, P. C. Lemos, C. Delerue-Matos and V. F. Domingues, Chemosphere, 2019, 220, 1179–1186 CrossRef PubMed.
- X. Zhang, T. Zhang, X. Ren, X. Chen, S. Wang and C. Qin, Front. Endocrinol., 2021, 12, 656106 CrossRef PubMed.
- E. T. Knapke, D. de P. Magalhaes, M. A. Dalvie, D. Mandrioli and M. J. Perry, Toxicology, 2022, 465, 153017 CrossRef CAS PubMed.
- J. Wang, L. Gao, D. Han, J. Pan, H. Qiu, H. Li, X. Wei, J. Dai, J. Yang and H. Yao, J. Agric. Food Chem., 2015, 63, 2392–2399 CrossRef CAS PubMed.
- L. Gao, X. Li, Q. Zhang, J. Dai, X. Wei, Z. Song, Y. Yan and C. Li, Food Chem., 2014, 156, 1–6 CrossRef CAS PubMed.
- C. Liu, Z. Song, J. Pan, X. Wei, L. Gao, Y. Yan, L. Li, J. Wang, R. Chen and J. Dai, J. Phys. Chem. C, 2013, 117, 10445–10453 CrossRef CAS.
- X. Wei, T. Hao, Y. Xu, K. Lu, H. Li, Y. Yan and Z. Zhou, RSC Adv., 2015, 5, 79511–79518 RSC.
- H. Li, Y. Li and J. Cheng, Chem. Mater., 2010, 22, 2451–2457 CrossRef CAS.
- J. Wang, H. Qiu, H. Shen, J. Pan, X. Dai, Y. Yan, G. Pan and B. Sellergren, Biosens. Bioelectron., 2016, 85, 387–394 CrossRef CAS PubMed.
- X. Wei, T. Hao, Y. Xu, K. Lu, H. Li, Y. Yan and Z. Zhou, Sens. Actuators, B, 2016, 224, 315–324 CrossRef CAS.
- X. Ren and L. Chen, Biosens. Bioelectron., 2015, 64, 182–188 CrossRef CAS PubMed.
- X. Li, H.-F. Jiao, X.-Z. Shi, A. Sun, X. Wang, J. Chai, D.-X. Li and J. Chen, Biosens. Bioelectron., 2018, 99, 268–273 CrossRef CAS PubMed.
- H. Li, X. Wei, Y. Xu, K. Lu, Y. Zhang, Y. Yan and C. Li, J. Alloys Compd., 2017, 705, 524–532 CrossRef CAS.
- L. Gao, W. Han, Y. Yan, X. Li, C. Li and B. Hu, Anal. Methods, 2016, 8, 2434–2440 RSC.
- Y. Zhang, X. Zhu, M. Li, H. Liu and B. Sun, J. Agric. Food Chem., 2022, 70, 6059–6071 CrossRef CAS PubMed.
- X. Zhu, L. Han, H. Liu and B. Sun, Food Chem., 2022, 379, 132154 CrossRef CAS PubMed.
- D. Zhang, J. Tang and H. Liu, Anal. Bioanal. Chem., 2019, 411, 5309–5316 CrossRef CAS PubMed.
- Q. Wang, J. Jiang, W. Sui, X. Lin and B. Liu, Anal. Lett., 2017, 50, 1139–1149 CrossRef CAS.
- S. Ge, J. Lu, L. Ge, M. Yan and J. Yu, Spectrochim. Acta, Part A, 2011, 79, 1704–1709 CrossRef CAS PubMed.
- Y. Cai, X. He, P. L. Cui, W. Z. Yuan, J. P. Wang and J. Liu, Food Chem., 2020, 319, 126539 CrossRef CAS PubMed.
- T. Qin, X. Zhao, C. Song, T. Lv, S. Chen, Z. Xun, Z. Xu, Z. Zhang, H. Xu and C. Zhao, Chem. Eng. J., 2023, 451, 139022 CrossRef CAS.
- F. Fang, Q. Zhao, R. Fan, H. Wang, J. Zhu and X. Wang, Microchem. J., 2022, 172, 106954 CrossRef CAS.
- J. Shah, M. Rasul Jan, B. Ara and F.-N. Shehzad, Environ. Monit. Assess., 2011, 178, 111–119 CrossRef CAS PubMed.
- M. Majd and S. Nojavan, Microchim. Acta, 2021, 188, 1–12 CrossRef PubMed.
- A. Amiri, M. Baghayeri and M. Sedighi, Microchim. Acta, 2018, 185, 1–9 CrossRef PubMed.
- S. Wang, Y. She, S. Hong, X. Du, M. Yan, Y. Wang, Y. Qi, M. Wang, W. Jiang and J. Wang, J. Hazard. Mater., 2019, 367, 686–693 CrossRef CAS PubMed.
- S. Zoughi, F. Faridbod, A. Amiri and M. R. Ganjali, Food Chem., 2021, 350, 129197 CrossRef CAS PubMed.
- S. Mohapatra, M. K. Bera and R. K. Das, Sens. Actuators, B, 2018, 263, 459–468 CrossRef CAS.
- D. Sahoo, A. Mandal, T. Mitra, K. Chakraborty, M. Bardhan and A. K. Dasgupta, J. Agric. Food Chem., 2018, 66, 414–423 CrossRef CAS PubMed.
- S. A. Nsibande and P. B. C. Forbes, Luminescence, 2019, 34, 480–488 CrossRef CAS PubMed.
- H. Su, L. Chen, B. Sun and S. Ai, Sens. Actuators, B, 2012, 174, 458–464 CrossRef CAS.
- G. Liu, T. Li, X. Yang, Y. She, M. Wang, J. Wang, M. Zhang, S. Wang, F. Jin and M. Jin, Carbohydr. Polym., 2016, 137, 75–81 CrossRef CAS PubMed.
- S. Halder, A. Barma, C. Rizzoli, P. Ghosh and P. Roy, ACS Appl. Nano Mater., 2019, 2, 5469–5474 CrossRef CAS.
- V. Romero, S. P. S. Fernandes, P. Kovář, M. Pšenička, Y. V. Kolen'ko, L. M. Salonen and B. Espiña, Microporous Mesoporous Mater., 2020, 307, 110523 CrossRef CAS.
- M. Amjadi and R. Jalili, Biosens. Bioelectron., 2017, 96, 121–126 CrossRef CAS PubMed.
- Q. Yu, C. He, Q. Li, Y. Zhou, N. Duan and S. Wu, Microchim. Acta, 2020, 187, 1–10 CrossRef PubMed.
- Y. Fan, R.-H. Gao, Y. Huang, B. Bian, Z. Tao and X. Xiao, Front. Chem., 2019, 7, 154 CrossRef CAS PubMed.
- Q. Li, S.-C. Qiu, J. Zhang, K. Chen, Y. Huang, X. Xiao, Y. Zhang, F. Li, Y.-Q. Zhang and S.-F. Xue, Org. Lett., 2016, 18, 4020–4023 CrossRef CAS PubMed.
- S. Walia and A. Acharya, J. Nanopart. Res., 2014, 16, 1–10 CrossRef CAS.
- Y. Yang, A. Han, S. Hao, X. Li, X. Luo, G. Fang, J. Liu and S. Wang, Analyst, 2020, 145, 6683–6690 RSC.
- H.-F. Wang, Y. He, T.-R. Ji and X.-P. Yan, Anal. Chem., 2009, 81, 1615–1621 CrossRef CAS PubMed.
- M. Yang, A. Han, J. Duan, Z. Li, Y. Lai and J. Zhan, J. Hazard. Mater., 2012, 237, 63–70 CrossRef PubMed.
- Q. Liu, K. Wang, J. Huan, G. Zhu, J. Qian, H. Mao and J. Cai, Analyst, 2014, 139, 2912–2918 Search PubMed.
- M. Jia, Z. Zhang, J. Li, H. Shao, L. Chen and X. Yang, Sens. Actuators, B, 2017, 252, 934–943 CrossRef CAS.
- Z. Zhang, X. Ma, B. Li, J. Zhao, J. Qi, G. Hao, R. Jianhui and X. Yang, Analyst, 2020, 145, 963–974 Search PubMed.
- X. Wang, J. Yu, X. Wu, J. Fu, Q. Kang, D. Shen, J. Li and L. Chen, Biosens. Bioelectron., 2016, 81, 438–444 CrossRef CAS PubMed.
- S. Xu, Y. Zou and H. Zhang, Talanta, 2020, 211, 120711 Search PubMed.
- N. Xu, Q. Zhang, B. Hou, Q. Cheng and G. Zhang, Inorg. Chem., 2018, 57, 13330–13340 Search PubMed.
- N. Xu, Q. Zhang and G. Zhang, Dalton Trans., 2019, 48, 2683–2691 Search PubMed.
- X.-Y. Guo, Z.-P. Dong, F. Zhao, Z.-L. Liu and Y.-Q. Wang, New J. Chem., 2019, 43, 2353–2361 Search PubMed.
- C.-L. Tao, B. Chen, X.-G. Liu, L.-J. Zhou, X.-L. Zhu, J. Cao, Z.-G. Gu, Z. Zhao, L. Shen and B. Z. Tang, Chem. Commun., 2017, 53, 9975–9978 RSC.
- X.-Q. Wang, D.-D. Feng, J. Tang, Y.-D. Zhao, J. Li, J. Yang, C. K. Kim and F. Su, Dalton Trans., 2019, 48, 16776–16785 RSC.
- J. Chi, B. Zhong, Y. Li, P. Shao, G. Liu, Q. Gao and B. Chen, Z. Anorg. Allg. Chem., 2021, 647, 1284–1293 CrossRef CAS.
- D. Feng, J. Tang, J. Yang, X. Ma, C. Fan and X. Wang, J. Mol. Struct., 2020, 1221, 128841 CrossRef CAS.
- P. Sharma, M. Kumar and V. Bhalla, ACS Omega, 2020, 5, 19654–19660 CrossRef CAS PubMed.
- F. Wang, X. Zhang, C. Huangfu, M. Zhu, C. Li and L. Feng, Food Chem., 2022, 370, 131033 CrossRef CAS PubMed.
- L. Fan, F. Wang, D. Zhao, X. Sun, H. Chen, H. Wang and X. Zhang, Spectrochim. Acta, Part A, 2020, 239, 118467 CrossRef CAS PubMed.
- B. Lin, Y. Yu, R. Li, Y. Cao and M. Guo, Sens. Actuators, B, 2016, 229, 100–109 CrossRef CAS.
- J. Guo, Y. Li, L. Wang, J. Xu, Y. Huang, Y. Luo, F. Shen, C. Sun and R. Meng, Anal. Bioanal. Chem., 2016, 408, 557–566 CrossRef CAS PubMed.
- W. Hu, Q. Chen, H. Li, Q. Ouyang and J. Zhao, Biosens. Bioelectron., 2016, 80, 398–404 CrossRef CAS PubMed.
- L. Yang, H. Sun, X. Wang, W. Yao, W. Zhang and L. Jiang, Microchim. Acta, 2019, 186, 1–11 CrossRef CAS PubMed.
- X. Yan, H. Li, Y. Li and X. Su, Anal. Chim. Acta, 2014, 852, 189–195 CrossRef CAS PubMed.
- M. P. Shirani, B. Rezaei and A. A. Ensafi, Spectrochim. Acta, Part A, 2019, 210, 36–43 Search PubMed.
- F. Qu, X. Zhou, J. Xu, H. Li and G. Xie, Talanta, 2009, 78, 1359–1363 CrossRef CAS PubMed.
- W. Lei, Z. Gu, W. Si, F. Wang and Q. Hao, J. Electrochem. Soc., 2013, 160, H502 CrossRef CAS.
- J. Liu, W. H. Xiong, L. Y. Ye, W. S. Zhang and H. Yang, J. Agric. Food Chem., 2020, 68, 5572–5578 Search PubMed.
- Y. Liu, N. Cao, W. Gui and Q. Ma, Talanta, 2018, 183, 339–344 Search PubMed.
| This journal is © The Royal Society of Chemistry 2025 |