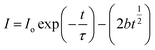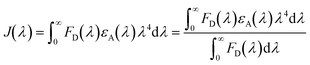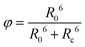DOI:
10.1039/D4SD00277F
(Paper)
Sens. Diagn., 2025,
4, 63-74
Biocompatible luminescent Ce3+-sensitized SrF2:Tb3+ for anticounterfeiting and forensic fingerprint detection
Received
19th August 2024
, Accepted 24th October 2024
First published on 8th November 2024
Abstract
Ce3+-sensitized Tb3+-activated SrF2 nanophosphors (NPs) were synthesized using the hydrothermal method. Crystallographic characterization using X-ray diffraction confirms the formation of cubic SrF2 with space group Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m for all samples. The FESEM image indicates spherical-shaped particles. Surface functionalization renders that the NPs are dispersible in water. The strong green emission of SrF2:5% Tb3+, 5% Ce3+ at 541 nm increases by 50-fold than that of SrF2:5% Tb3+. The resonance energy transfer between Ce3+ and Tb3+via multipolar interactions is observed. The energy transfer efficiency and spectral overlap integral are calculated. The absolute quantum yield of SrF2:5% Tb3+, 5% Ce3+ is observed to be ∼12%. The NPs show excellent biocompatibility towards the HeLa cell line with 70% cell viability. Intercellular uptake of the SrF2:5% Tb3+, 5% Ce3+ nanophosphor is fair, and its potential for anti-counterfeiting and forensic fingerprint applications is observed.
m for all samples. The FESEM image indicates spherical-shaped particles. Surface functionalization renders that the NPs are dispersible in water. The strong green emission of SrF2:5% Tb3+, 5% Ce3+ at 541 nm increases by 50-fold than that of SrF2:5% Tb3+. The resonance energy transfer between Ce3+ and Tb3+via multipolar interactions is observed. The energy transfer efficiency and spectral overlap integral are calculated. The absolute quantum yield of SrF2:5% Tb3+, 5% Ce3+ is observed to be ∼12%. The NPs show excellent biocompatibility towards the HeLa cell line with 70% cell viability. Intercellular uptake of the SrF2:5% Tb3+, 5% Ce3+ nanophosphor is fair, and its potential for anti-counterfeiting and forensic fingerprint applications is observed.
1. Introduction
Recently, rare earth-doped luminescent inorganic materials have become promising in various applications, such as solid-state lighting, displays, photovoltaic devices, sensors, dental applications, biological labeling, security ink, etc.1–7 The presence of well-shielded intra 4f–4f transitions in rare earth ions are responsible for their unique luminescence properties, such as sharp band emissions, long luminescence lifetime, and excellent stability, making them popular activators.8–11 However, they encounter weak luminescence efficacy due to Laporte's forbidden transitions, limiting their applications. Increasing the luminescence efficacy by adopting methods like sensitization, charge compensation, core–shell formation, etc. has been reported by many researchers. Luo et al. reported enhanced luminescence due to efficient energy transfer from Ce3+ to Tb3+ in Ce3+ and Tb3+ co-doped glasses and glass ceramics containing SrF2 nanocrystals.12 Likewise, charge compensation and efficient energy transfer from Ce3+ to Tb3+ or Mn2+ in Ca9ZnLi(PO4)7 have also been reported.13,14 Similarly, the enhanced luminescence in the white region by the core–shell formation of the lanthanide-doped SrF2 core and CaF2 as the shell has been reported.15 Amongst them, sensitization by choosing an appropriate sensitizer is an easier way to improve luminescence. For sensitization, Ce3+ ions are commonly used as a sensitizer for other rare-earth ions because they have emissions in the range of 300–360 nm, which overlaps with the excitation spectrum of the other rare-earth ions.16,17 Tb3+ ions, which have an excitation wavelength in the range of 280–450 nm, correspond to the emissions of Ce3+ ions.18 Thus, good energy transfer can occur easily from Ce3+ to Tb3+ ions, resulting in the enhancement of emission intensity, i.e., Ce3+ ions can act as a sensitizer.
Alkaline earth fluorides of cubic type, especially CaF2, SrF2, and BaF2, are excellent host materials for rare earth metal ions. This is due to their outstanding luminescence properties, such as low phonon energies, large band gaps, high stabilities, high ionicities, and ionic conductivities.15–22 Many researchers have extensively studied fluoride phosphors because of their ease of synthesis and good luminescence. Zhang's group reported the mesoporous lanthanide doped SrF2 for luminescence properties and field emission displays. They also reported the synthesis of monodisperse CaF2:Ce3+/Tb3+ hollow spheres as smart drug carriers.23 Ansari et al. reported the synthesis of a highly stable water dispersion of CaF2:Ce/Tb nanocrystals for optical band gap and photoluminescence properties.24 Besides, many research groups have reported the structural, spectroscopic, and cytotoxicity studies of upconverting SrF2 nanoparticles.25–27 Yan et al. also reported the single phase SrF2:Tb3+, Eu3+ phosphors using the chemical co-precipitation method. They studied the high energy efficiency and multi-color emissions, which have potential applications in white light-emitting diodes.28 Another author also reported the luminescent enhancement of Ce3+ and Tb3+ doped CaF2 and they analyzed the effect of Li+ ion co-doped in the nanoparticles and hyperthermia application of Ce3+ doped CaF2/Fe3O4 nanocomposites.20 Several applications, such as security inks and fingerprint detection of the upconverted nanophosphors, have been reported by many researchers.29,30 Jin reported the Eu3+ doped BaF2 and SrF2 nanocrystals for luminescence properties and telecommunication.31 An eco-friendly synthesis of SrF2 nanoparticles and studies on the in vitro biocompatibility and antibacterial properties have been reported.32 Eu-doped BaF2 nanoparticles were used for bioimaging applications.33 However, reports on multifunctional applications such as biocompatibility, security inks, and fingerprint detection of Ce3+ and Tb3+ co-doped SrF2 are still rare.
In this present work, we have chosen SrF2 as a host matrix, Tb3+ as the activator, and Ce3+ as the sensitizer for preparing single-phase multifunctional biocompatible luminescent NPs. SrF2:Tb3+, Ce3+ nanophosphors have been synthesized using trisodium citrate as a capping agent by a hydrothermal method. The crystal structure, morphology, luminescence properties, quantum yield and energy transfer mechanism were studied. Because of the potential biological applications of synthesized NPs, their cytotoxicity was assessed. The biocompatibility of the as-synthesized nanophosphors was performed on the HeLa cell line. Moreover, intracellular uptakes of our NPs were also analyzed using fluorescence microscopy. Furthermore, luminescence anti-counterfeiting and forensic fingerprint detection were also investigated.
2. Experimental sections
2.1. Reagents and materials
Strontium nitrate (Sr(NO)3, Sisco-chem, 98.50%), purified ammonium fluoride (NH4F, Merck), trisodium citrate dihydrate (C6H5Na3O7·2H2O, Himedia, 99.00%), terbium(III) nitrate pentahydrate (Tb(NO)3·5H2O, Sigma-Aldrich, 99.90%), cerium(III) carbonate hydrate (Ce2(CO3)3·xH2O, Sigma-Aldrich, 99.90%), ethylene glycol and deionized water were used in the synthesis of the materials. The regents were analytically pure and used without further purification.
2.2. Synthesis of Sr0.90F2:5% Tb3+, 5% Ce3+ (SrF2:5% Tb3+, 5% Ce3+) samples
In a typical synthesis of SrF2:5% Tb3+, 5% Ce3+ samples, first, a stoichiometric proportion of Ce2(CO3)3·xH2O (0.048 g) was dissolved in concentrated HNO3 to obtain its nitrate, and the excess acid was removed by the addition of water, followed by evaporation to dryness. Then, it was dissolved together with a stoichiometric proportion of Sr(NO)3 (0.676 g) and in 20 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethylene glycol and water solution. 20 mL of 1
1 ethylene glycol and water solution. 20 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethylene glycol and aqueous solution of 0.595 g NH4F were added and stirred vigorously for 30 min. Thereafter, 20 mL of 1
1 ethylene glycol and aqueous solution of 0.595 g NH4F were added and stirred vigorously for 30 min. Thereafter, 20 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethylene glycol, an aqueous solution of 0.089 g Tb(NO)3·5H2O, 20 mL of 1
1 ethylene glycol, an aqueous solution of 0.089 g Tb(NO)3·5H2O, 20 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethylene glycol and an aqueous solution of 1.176 g C6H5Na3O7·2H2O were added. The whole mixture was stirred for another 30 minutes and then transferred to a Teflon-lined autoclave. The vessel was kept in a preheated oven at 140 °C for 2 hours, after which it was removed and cooled to room temperature. The product was collected by centrifugation and washed with deionized water and acetone. Finally, the product was dried at 40 °C for further analysis.
1 ethylene glycol and an aqueous solution of 1.176 g C6H5Na3O7·2H2O were added. The whole mixture was stirred for another 30 minutes and then transferred to a Teflon-lined autoclave. The vessel was kept in a preheated oven at 140 °C for 2 hours, after which it was removed and cooled to room temperature. The product was collected by centrifugation and washed with deionized water and acetone. Finally, the product was dried at 40 °C for further analysis.
2.3. Cell viability studies: MTT assay
The National Centre for Cell Science (NCCS) in Pune provided the HeLa cancer cell line. The cells were grown in DMEM (Dulbecco's modified Eagle medium) medium containing 10% FBS and 0.1% penicillin–streptomycin at 37 °C in a humidified atmosphere containing 5% CO2. The biocompatibility and cytotoxicity of nanocomposites were assessed using the MTT {3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide} reduction assay.34 After 48 h of treatment, 15 μL of MTT solution (5 mg mL−1) was added to each well (96 well plate) and incubated for 3 h. During this time, the developed formazan crystals were dissolved by adding 100 μL of DMSO to each well, and absorbance was measured at 570 nm using a Varioskan™ LUX multimode microplate reader.35 Cell viability was calculated as: [A570(treated cells) − background]/[A570(untreated cells) − background] × 100. Studies were performed in triplicate for each data point.
2.4. Intracellular uptake studies
HeLa cells (5 × 104) were seeded on coverslips placed in the wells of a 6-well plate and treated with different concentrations of NPs for 24 h. The cells were washed with PBS twice, permeabilized with 0.1% Triton X100 for 5 min, and washed with PBS twice. The cells were fixed with 4% paraformaldehyde for 10 min and washed with PBS twice. The fixed cells were stained with DAPI (4,6-diamidino-2-phenylindole) for 5 min in the dark and washed with PBS twice.36 The intracellular uptake imaging of the NPs in HeLa cell lines was performed using fluorescence microscopy.
2.5. Characterization
Powder XRD was carried out on the samples using a PANalytical X-ray diffractometer (X'Pert Pro) Cu (Kα) radiation (λ = 1.5405 Å), 2θ range of 15–90° to determine the identification and purity of the phase. The morphology and size of the synthesized phosphors were observed by FE-SEM (JEOL, JSM 6390LV). An energy dispersive X-ray spectroscopy (EDS/EDX) attached to a Quanta – 250 SEM was used to analyze the elemental composition. The absorption spectra were recorded using a UV-vis spectrophotometer (UV-2600, Shimadzu). Shimadzu IRAffinity 1S was used to record the Fourier transform infrared (FT-IR) spectra. The photoluminescence spectra and their decay as well as quantum yield (QY) were measured with a Horiba make Fluoromax-4CP spectrofluorometer equipped with an arc xenon lamp 150 W and 5 W microsecond pulse lamp. The QY was measured by integrating the sphere K-sphere ‘petite’ (Photon Technology International). All the measurements were done in the emission mode. The absolute QY was calculated using the relation,  where Iemission = luminescence emission intensity of the sample, Isample holder = the intensity of excitation light only the quartz slide and Isample = the intensity of the light used for exciting the sample placed on the quartz slide. The difference in the denominator gives the photons absorbed by the sample. All the PL measurements were carried out on the glass (for PL and decay lifetime) and quartz slides (for QY) at room temperature. The luminescence decay lifetime for Ce3+ emission was measured by the TCSPC technique with Horiba make DeltaFlex having pulse LED laser 295 ± 10 nm as source (pulse width ≤1.2 ns).
where Iemission = luminescence emission intensity of the sample, Isample holder = the intensity of excitation light only the quartz slide and Isample = the intensity of the light used for exciting the sample placed on the quartz slide. The difference in the denominator gives the photons absorbed by the sample. All the PL measurements were carried out on the glass (for PL and decay lifetime) and quartz slides (for QY) at room temperature. The luminescence decay lifetime for Ce3+ emission was measured by the TCSPC technique with Horiba make DeltaFlex having pulse LED laser 295 ± 10 nm as source (pulse width ≤1.2 ns).
3. Results and discussions
3.1. XRD studies
Fig. 1 shows the X-ray diffraction patterns of SrF2, SrF2:5% Tb3+, SrF2:5% Tb3+, 5% Ce3+ nanophosphors. The diffraction patterns can be indexed to the standard diffraction data 00-006-0262 for the cubic phase of SrF2 possessing the Fm![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. No additional phases, such as Tb2O3, Ce2O3, Ce3O4, etc., are observed upon adding Tb3+ and Ce3+ ions into the SrF2 lattice. The calculated lattice parameters, crystallite sizes, and unit cell volumes are shown in Table 1. The crystallite sizes were calculated using the Scherrer formula: d = kλ/β
m space group. No additional phases, such as Tb2O3, Ce2O3, Ce3O4, etc., are observed upon adding Tb3+ and Ce3+ ions into the SrF2 lattice. The calculated lattice parameters, crystallite sizes, and unit cell volumes are shown in Table 1. The crystallite sizes were calculated using the Scherrer formula: d = kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ. The strongest peak corresponding to the (111) plane was used to calculate the average crystallite size of the samples. Again, using the Williamson–Hall model, the crystallite sizes were also calculated, which were almost similar to those calculated using the Scherrer formula. From Table 1, it is observed that the unit cell volume is increased on Tb3+ substitution, whereas the ionic radius of the Sr2+ ion (1.32 Å) is larger than that of Tb3+ (0.92 Å) at coordination number 6.37 Our result is contradictory to the Vegard's law; the unit cell volume must be slightly decreased as compared to that of pure SrF2. This is due to the occupancy of the excess fluoride ions in the interstitial sites, which compensate for the excess positive charge due to the substitution of trivalent lanthanide ions at Sr2+ sites. The balanced chemical equation can be represented as 2Sr2+ → Ln3+ + F−. It may also be due to the formation of tetragonal (C4v) or trigonal (C3v) and electronic repulsions between F− ions, which leads to the expansion of unit cell volume.38,39 However, a small increase in the unit cell volume is observed for Ce3+ and Tb3+ co-doped SrF2 NPs, which is due to the ionic radius of Ce3+ (1.01 Å) ion being greater than that of Tb3+ ion (0.92 Å) at coordination number 6.37,40
θ. The strongest peak corresponding to the (111) plane was used to calculate the average crystallite size of the samples. Again, using the Williamson–Hall model, the crystallite sizes were also calculated, which were almost similar to those calculated using the Scherrer formula. From Table 1, it is observed that the unit cell volume is increased on Tb3+ substitution, whereas the ionic radius of the Sr2+ ion (1.32 Å) is larger than that of Tb3+ (0.92 Å) at coordination number 6.37 Our result is contradictory to the Vegard's law; the unit cell volume must be slightly decreased as compared to that of pure SrF2. This is due to the occupancy of the excess fluoride ions in the interstitial sites, which compensate for the excess positive charge due to the substitution of trivalent lanthanide ions at Sr2+ sites. The balanced chemical equation can be represented as 2Sr2+ → Ln3+ + F−. It may also be due to the formation of tetragonal (C4v) or trigonal (C3v) and electronic repulsions between F− ions, which leads to the expansion of unit cell volume.38,39 However, a small increase in the unit cell volume is observed for Ce3+ and Tb3+ co-doped SrF2 NPs, which is due to the ionic radius of Ce3+ (1.01 Å) ion being greater than that of Tb3+ ion (0.92 Å) at coordination number 6.37,40
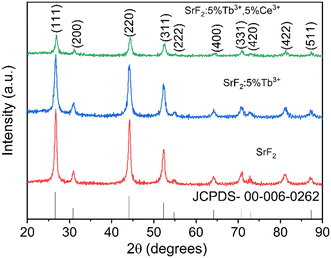 |
| | Fig. 1 XRD pattern of SrF2, SrF2:5% Tb3+, and SrF2:5% Tb3+, 5% Ce3+ nanophosphors. | |
Table 1 Lattice parameters and crystallite sizes of SrF2, SrF2:5% Tb3+, and SrF2:5% Tb3+, 5% Ce3+ nanophosphors
| Sample |
Lattice parameter a (Å) |
Unit cell volume (Å3) |
Crystallite size (nm) |
| Debye |
Williamson |
| Reference JCPDS 00-006-0262 SrF2 |
5.80 |
195.11 |
|
|
| SrF2 |
5.76 |
191.76 |
10 |
12 |
| SrF2:5% Tb3+ |
5.77 |
192.19 |
8 |
9 |
| SrF2:5% Tb3+, 5% Ce3+ |
5.78 |
193.58 |
7 |
8 |
3.2. FESEM and EDS studies
The morphologies of the SrF2:5% Tb3+, 5% Ce3+ nanophosphors were studied by FESEM imaging, as shown in Fig. 2(a). The FESEM image shows the formation of spherical-shaped nanoparticles with a particle size range of 50–60 nm. Fig. 2(b) shows the typical histogram of the particle diameter (size) for the sample SrF2:5% Tb3+, 5% Ce3+. The energy dispersive spectra of SrF2, SrF2:5% Tb3+, SrF2:5% Ce3+, and SrF2:5% Tb3+, 5% Ce3+ nanophosphors, respectively, are shown in Fig. 2(c–f). The Sr, F, Ce, and Tb elements are detected from the respective nanophosphor samples, indicating the successful preparation of Tb3+ and Ce3+ co-doped SrF2 NP.
 |
| | Fig. 2 (a) FESEM image, (b) histogram of the particle diameters, and (c)–(f) energy dispersive spectrum (EDS) of SrF2, SrF2:5% Tb3+, SrF2:5% Ce3+ and SrF2:5% Tb3+, 5% Ce3+ nanophosphors. | |
3.3. FT-IR studies
Fig. 3 shows the FT-IR spectrum of the as-prepared SrF2:5% Tb3+, 5% Ce3+ nanophosphors. The strong single broad peak at around 3600–3300 cm−1 corresponds to the O–H bond stretching vibration of the ethylene glycol/H2O used as a solvent. The peaks at 1069–1252 cm−1 correspond to the CH2 bending from ethylene glycol.41 Other bands at 1401 and 1575 cm−1 arise from the symmetric and asymmetric vibrations of COO− from the capping agent added during the synthesis, which gets adsorbed on the surface of the particles.42 The band at 460 cm−1 is assigned to the Sr–F stretching vibration. The presence of ethylene glycol and trisodium citrate dihydrate, along with the NPs, will help in the incorporation/dispersion of NPs in polar media and thin films.
 |
| | Fig. 3 FT-IR spectrum of SrF2:5% Tb3+, 5% Ce3+ nanophosphors. | |
3.4. Optical studies
The absorption spectra of the as-synthesized SrF2, SrF2:5% Tb3+, and SrF2:5% Tb3+, 5% Ce3+ nanophosphors are shown in Fig. 4. The NPs show a strong absorption in the UV spectral region of 200–400 nm. It is observed that there is a peak at around 235 to 256 nm, with the maximum at 245 nm in Tb3+ doped SrF2 NPs. On the other hand, for Ce3+ co-doped Tb3+ doped SrF2 NPs, in addition to the above peak, there is another peak at 281 to 306 nm with the maximum at 293 nm. Since the optical bandgap of SrF2 is ∼11 eV,43 the absorption band lies in the vacuum UV region, which is beyond our spectrophotometer detection capability. Therefore, the corresponding absorption bands observed in the region above may be related to the defect states localized in the forbidden region of SrF2. Researchers have already reported similar studies on unactivated CaF2, SrF2, BaF2 and lanthanide-activated CaF2.44,45 As Mukherjee et al. have reported in the lanthanide-activated CaF2,44 these defect states may arise from the creation of vacancies to maintain charge neutrality during the substitution of Sr2+ sites by Ce3+ and/or Tb3+. Also, F-interstitial sites may act as defect sites during lanthanide substitution for charge compensation (as the F− source was taken 2 times its molar requirement).
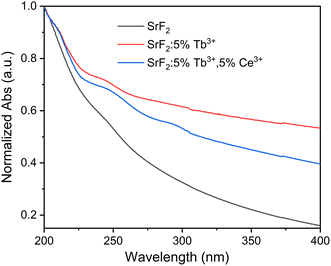 |
| | Fig. 4 UV-vis spectra of SrF2, SrF2:5% Tb3+, and SrF2:5% Tb3+, 5% Ce3+ nanophosphors. | |
3.5. Photoluminescence (PL) studies
PL excitation spectra of SrF2:5% Ce3+, SrF2:5% Tb3+ and SrF2:5% Tb3+, 5% Ce3+ nanophosphors monitored at the emission wavelengths at 330 nm (for SrF2:5% Ce3+) and 541 nm for the remaining 2 (two) are displayed in Fig. 5(a). The excitation spectrum of SrF2:5% Ce3+ consists of two broad excitation bands in the range of 230 to 320 nm, with the maximum peaks at 260 and 297 nm, corresponding to the spin-allowed 4f–5d transitions of Ce3+ ions. These are originated from the transitions of 2F5/2 and 2F7/2 ground states to the 2D3/2 and 2D5/2 excited states of 5d orbitals.46 Though, the excitation spectrum of SrF2:5% Tb3+ shows a broad peak at 250–260 nm, which corresponds to the 4f–5d and Tb–O charge transfer.8 In addition, the excitation peaks observed in the longer wavelength region at 349 (7F6 → 5D1), 355 (7F6 → 5D1), 363 (7F6 → 5D1) and 370 (7F6 → 5D1) nm are associated with the 4f–4f transitions of Tb3+ ions from the 7F6 ground state to the different higher excited states. Meanwhile, the SrF2:5% Tb3+, 5% Ce3+ sample shows two main absorption peaks at 260 and 297 nm. These peaks are related to the spin-allowed 4f–5d transitions of Ce3+ (as discussed above). Here, the 4f–5d transition and/or Tb–O charge transfer band of Tb3+ is not seen, as it might have merged with the 260 nm excitation peak due to Ce3+. The excitation peaks are also observed in the longer wavelength region due to absorption within the Tb3+ ions (as discussed above). The strong excitation peak at 260 and 297 nm related to Ce3+ indicates the strong energy transfer from Ce3+ to the excited states of Tb3+. Fig. 5(b) shows the normalized excitation spectra of SrF2:5% Ce3+, SrF2:5% Tb3+ and SrF2:5% Tb3+, 5% Ce3+ at different emission peaks related to their characteristic emissions, i.e., 330 nm for Ce3+ and 488 (5D4 → 7F6), 541 (5D4 → 7F5), 583 (5D4 → 7F4) and 620 (5D4 → 7F3) nm of Tb3+ to check any additional peaks, which can be correlated to the Ce3+ (sensitizer/donor) emission. Here, it is observed that excitation bands related to the Ce3+ emission are observed only in SrF2:5% Ce3+ and SrF2:5% Tb3+, 5% Ce3+. Such a band is not observed in SrF2:5% Tb3+.
 |
| | Fig. 5 (a) Excitation spectra of SrF2:5% Tb3+, SrF2:5% Ce3+ and SrF2:5% Tb3+, 5% Ce3+ (b) normalized excitation spectra of SrF2:5% Tb3+, SrF2:5% Ce3+ and SrF2:5% Tb3+, 5% Ce3+ samples at all the emission peaks. | |
The PL emission spectra of SrF2:5% Ce3+, SrF2:5% Tb3+ and SrF2:5% Tb3+, 5% Ce3+ nanophosphors under different excitation wavelengths are shown in Fig. 6(a). For SrF2:5% Ce3+, the excitation wavelengths are 260 and 297 nm. Both spectra show the characteristic emission of Ce3+ in the range of 300–400 nm with a maximum at 330 nm. This is due to the transition from the excited d-level to the f-level ground state of Ce3+. On the other hand, excitation wavelengths for SrF2:5% Tb3+ are related to the Tb3+ absorption at 255 and 370 nm. The characteristic emission peaks of Tb3+ are seen at 488 (5D4 → 7F6), 541 (5D4 → 7F5), 583 (5D4 → 7F4) and 620 (5D4 → 7F3) nm. Meanwhile, the emission spectra for SrF2:5% Tb3+, 5% Ce3+ were recorded under the excitation of 260, 297, and 370 nm. Here, all the emissions related to Ce3+ and Tb3+ are observed. However, the Ce3+ emission intensity is drastically reduced compared to SrF2:5% Ce3+, while the Tb3+-related emission peaks are enhanced when compared to SrF2:5% Tb3+. Thus, it suggests the efficient energy transfer from the Ce3+ excited states to the excited states of Tb3+ ions (discussed later).47 The emission peak corresponding to 5D4 → 7F5 at 541 nm is dominant over others; therefore, the overall emission color is green. The emission intensity of SrF2:5% Tb3+, 5% Ce3+ is found to be enhanced ∼50 times than that of SrF2:5% Tb3+ under similar radiation exposure.
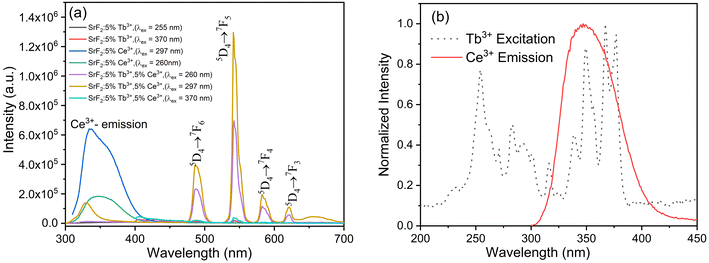 |
| | Fig. 6 (a) Emission spectra of SrF2:5% Tb3+, SrF2:5% Ce3+ and SrF2:5% Tb3+, 5% Ce3+ nanophosphors and (b) normalized spectral overlap of SrF2:5% Ce3+ emission and SrF2:5% Tb3+ excitation. | |
According to Dexter's theory,48 the spectral overlap between the excitation spectrum of the activator and the emission spectrum of the sensitizer is required to support the energy transfer from sensitizer to the activator. Therefore, we demonstrated the normalized spectral overlap between the SrF2:5% Tb3+ excitation and SrF2:5% Ce3+ emission, as shown in Fig. 6(b). A broad spectral overlap between these two spectra in the wavelength ranges from 300 to 450 nm, indicating the occurrence of efficient energy transfer from Ce3+ to Tb3+ ions in SrF2:5% Tb3+, 5 Ce3+.49,50 The probable energy transfer mechanism is shown in Fig. 7. The sensitization of Tb3+ by Ce3+ in SrF2 may also occur via a charge trap-mediated process. Such an energy transfer process was illustrated by Mukherjee et al. in Tb3+/Eu3+ co-doped CaF2.44 Here, the sensitization of Tb3+ by Ce3+ in SrF2 may also occur via a charge trap-mediated process where the photogenerated electron from the initially excited Ce3+ energy level recombines with a photogenerated hole at the Tb3+ center and thus populates the Tb3+ luminescent energy level 5D4. Later, the radiative transition occurs from the excited state 5D4 to the ground states with enhanced emission.
 |
| | Fig. 7 Schematic energy transfer pathways from Ce3+ to Tb3+. | |
The PL decay lifetime curves of the SrF2:5% Ce3+ and SrF2:5% Tb3+, 5% Ce3+ samples at 295 ± 10 nm excitation when the emission is monitored at 330 nm along with the instrument response function (IRF) are shown in Fig. 8(a). The decay lifetime values of SrF2:5% Ce3+, SrF2:5% Tb3+, 5% Ce3+ samples were calculated using eqn (1)
| | I(t) = I0![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ) exp(−t/τ) | (1) |
The decay lifetimes are found to be ∼15.35 and 7.82 ns, respectively, whereas the value of IRF is ∼1.7 ns. The typical curve fitting using
eqn (1) is shown in
Fig. 8(b). The PL decay lifetime of Ce
3+ is decreased in the presence of Tb
3+. This is due to the non-radiative excited electron transfer from the excited states of Ce
3+ to the excited states of Tb
3+ at the expense of radiative transition to the ground state within Ce
3+. Thus, this establishes the occurrence of energy transfer from Ce
3+ to Tb
3+. Further, this corroborates the existence of energy transfer discussed in steady-state PL studies. The energy transfer efficiency (
φ) was calculated from the lifetime values using
51| |  | (2) |
where
τS and
τSO are the lifetimes of Ce
3+ in the presence and absence of Tb
3+, respectively. The
φ is found to be 49%.
Fig. 9(a and b) shows the PL decay curves of the
5D
4 level of Tb
3+ for the SrF
2:5% Tb
3+ and SrF
2:5% Tb
3+, 5% Ce
3+ at 370 nm excitation when the emission is monitored at 541 nm.
Fig. 9(c) shows the decay curve of the SrF
2:5% Tb
3+, 5% Ce
3+ sample at 297 nm excitation when the emission is monitored at 541 nm. The following decay equation was used to calculate the PL decay lifetime:
51| | 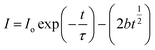 | (3) |
Here,
I and
Io represent the emission intensities at a given time, ‘
t’ and
t = 0, respectively, and ‘
b’ is the quencher concentration dependent on diffusion co-efficient, while the power of ‘
t’ is taken ½ as the energy transfer is assumed to be resonance type. ‘
τ’ is the PL decay lifetime. The PL decay lifetime values of the SrF
2:5% Tb
3+ and SrF
2:5% Tb
3+, 5% Ce
3+ samples at 370 nm excitation when the emission is monitored at 541 nm were found to be 4.10 and 4.56 ms, respectively. The lifetime values of the SrF
2:5% Tb
3+, 5% Ce
3+ samples at 297 nm excitation when the emission is monitored at 541 nm was found to be ∼6 ms.
 |
| | Fig. 8 (a) Decay profile of IRF, SrF2:5% Ce3+ and SrF2:5% Tb3+, 5% Ce3+ samples excited at 295 ± 10 nm and emission is monitored at 330 nm, (b) decay profile of SrF2:5% Ce3+ nanophosphors excited at 295 ± 10 nm and emission is monitored at 330 nm. | |
 |
| | Fig. 9 (a and b) Decay profile of SrF2:5% Tb3+ and SrF2:5% Tb3+, 5% Ce3+ nanophosphors excited at 370 nm and emission is monitored at 541 nm and (c) decay profile of SrF2:5% Tb3+, 5% Ce3+ samples excited at 297 nm and emission is monitored at 541 nm. | |
Generally, the energy transfer between Ce3+ and Tb3+ is possible via two mechanisms: exchange interaction and multipolar interaction. The distance between the donor and the acceptor plays an important role in determining the probable type of energy transfer. This can be checked by calculating the critical distance (Rc) between Tb3+ and Ce3+ ions, using the following equation,52,53
| |  | (4) |
where
V represents the unit cell volume,
Xc is the concentration and
N is the number of the cations at the center of the unit cell in the host. In the SrF
2 host lattice, the unit cell volume is 193.58 Å
3, the total concentration is 10% and the value of
N is 4. The calculated value of
Rc using
eqn (4) is found to be 9.7 Å, which is larger than 5 Å. This indicates the energy transfer mechanism from Ce
3+ to the excited state of Tb
3+ in the SrF
2 is dominated by multipolar interactions; thereby, the exchange interaction type of energy transfer can be ignored.
53
To further elucidate the resonance energy transfer, the overlap spectral integral (J(λ)), which is the expression of the degree of spectral overlap between the donor emission and the acceptor absorption, can be calculated as51
| | 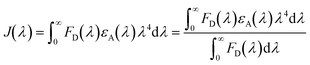 | (5) |
FD(
λ) is the luminescence intensity of the donor and is dimensionlesss.
εA(
λ) is the molar extinction coefficient of the acceptor in M
−1 cm
−1, and
λ is expressed in nanometre. According to Förster's resonance energy transfer theory, the energy transfer efficiency (
φ) is dependent on the distance between the donor and acceptor (
Rc),
51| | 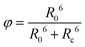 | (6) |
where
R0 is the Förster critical distance, which is defined as the distance for
φ to be 50%. Using
eqn (6) and with the obtained
φ = 49% and
Rc = 9.7 Å, the
R0 is calculated to be 9.8 Å. Again,
R0 can be further defined as follows:
51| | | R06 = 8.79 × 10−5 (κ2n−4ηDJ(λ)) | (7) |
where ‘
n’ is the refractive index of the medium (here, SrF
2),
κ2 is the relative dipole orientation in the space of the transition dipoles of the donor and acceptor and is usually assumed to be ⅔.
ηD is the quantum yield of the donor in the absence of the acceptor. The spectral integral (
J(
λ)) is expressed in M
−1 cm
−1 nm
4. Using
eqn (7) and taking ‘
n’ = 1.48 and
ηD = 22% (given in the next paragraph),
J(
λ) is calculated to 1.05 × 10
10 M
−1 cm
−1 nm
4. It is also stated that if 0.5
R0 <
Rc < 2
R0, efficient energy transfer occurs.
53b
3.6. CIE chromaticity coordinates and quantum yield efficiency
The calculated CIE coordinates diagram of SrF2:5% Tb3+ and SrF2:5% Tb3+, 5% Ce3+ nanophosphors excited at 255 nm are shown in Fig. 10(a). The corresponding chromaticity coordinates for SrF2:5% Tb3+ and SrF2:5% Tb3+, 5% Ce3+ nanophosphors, respectively, are (x = 0.34, y = 0.55) and (x = 0.36, y = 0.56). It can be seen that the emission of Tb3+ ions and Ce3+ ions co-doped SrF2 nanophosphors corresponds to the green color. Moreover, as the Ce3+ ions co-doped into the Tb3+ ions doped SrF2 nano-phosphor, the intensity of emission change is observed while the luminescence color is hardly changed. Fig. 10(b and c) shows the powder photographic image of the SrF2:5% Tb3+, 3% Ce3+ phosphor before and after 254 nm UV irradiation, suggesting the bright green color as suggested from the CIE diagram. We also prepared a thin film of the SrF2:5% Tb3+, 3% Ce3+ phosphor by using 5 mg of 10% PVA and borax solution. The physical photographic image of a thin film is shown in Fig. 10(d and e). The colour correlated temperature (CCT) was calculated by using the following equation:54
| CCT = −437n3 + 3601n2 − 6861n + 5517 |
and| | | n = (x − 0.3320)/(0.1858 − y) | (8) |
where x and y are the color coordinates.
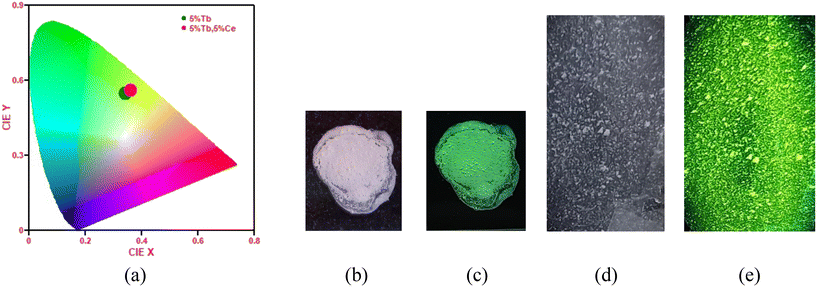 |
| | Fig. 10 (a) CIE chromaticity diagram of SrF2:5% Tb3+ and SrF2:5% Tb3+, 5% Ce3+ nanophosphors excited at 255 nm. (b) and (d) Before exposure and (c) and (e) after exposure of powder and a thin film of the SrF2:5% Tb3+, 5% Ce3+ nanophosphors, respectively. | |
The CCT values are found to be 5368 and 5023 for the SrF2:5% Tb3+ and SrF2:5% Tb3+, 5% Ce3+ nanophosphors, and the values lie in the cold white region. Therefore, the as-prepared samples have a potential application in WLEDs. The absolute method was used to measure the quantum yield efficiency of the SrF2:5% Tb3+, 5% Ce3+ and SrF2:5% Ce3+ phosphor using an integrating sphere. The value is found to be ∼12% and 22%, respectively. Fig. 11(a and b) shows the quantum yield values of SrF2:5% Tb3+, 5% Ce3+ and SrF2:5% Ce3+ nanophosphors from the instrument, respectively.
 |
| | Fig. 11 Calculation of quantum yield for (a) SrF2:5% Tb3+, 5% Ce3+ and (b) SrF2:5% Ce3+ nanophosphors using FluorEssence™ software in Horiba Fluoromax-4CP spectrofluorometer. | |
3.7. Cell viability studies: MTT assay
The cell viability of the SrF2:5% Tb3+, 5% Ce3+ nano-phosphor was carried out using HeLa cells by the MTT assay method as shown in Fig. 12. Different concentrations (6–200 μg mL−1) of the nanophosphors were added to the cells and incubated for 24 h. At lower concentrations (6–25 μg mL−1), >70% of cells were viable, while at higher concentrations (50–200 μg mL−1), >65% viability was observed. These data suggest the as-prepared nanophosphors displayed good cell viability over a wide concentration range.
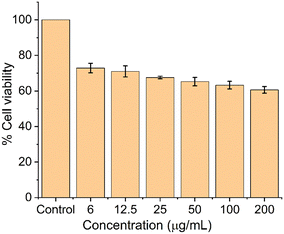 |
| | Fig. 12 Cell viability studies using HeLa cells at various SrF2:5% Tb3+, 5% Ce3+ nano-phosphor concentrations. | |
3.8. Intracellular uptake studies in HeLa cells by fluorescence microscopy
Since SrF2:5% Tb3+, 5% Ce3+ nano-phosphor showed good biocompatibility towards HeLa cells, we decided to investigate the cellular uptake studies of the as-prepared nano-phosphor. Fig. 13 shows the intracellular uptake of the samples (100, 200, and 300 μg mL−1 concentrations) in HeLa cells after a 24-hour incubation period. Fluorescence microscopy was used to track the cellular uptake and internalization of the phosphor samples. The nuclei of the cells were counterstained with the DAPI dye (blue color) to help mark the locations of the cells under fluorescence microscopy. The green fluorescence from the sample-treated cell indicated the incorporation of the samples into HeLa cells. We also note that the blue and green colors are co-localized, which clearly shows that most of the phosphor samples internalize into the nuclei of the cells and not the cytosol. The intensity of the green fluorescence increased with increasing concentration of the phosphor samples, indicating a concentration-dependent nuclear accumulation. This study demonstrated that cultured cells easily internalize phosphor samples, predominantly into the nuclei.
 |
| | Fig. 13 Fluorescence microscopy imaging of HeLa cells after a 24 h incubation with varying concentrations of phosphor samples. (a) Untreated cells, (b) 100 μg mL−1, (c) 200 μg mL−1, and (d) 300 μg mL−1 of SrF2:5% Tb3+, 5% Ce3+ nano-phosphor. The nuclei are stained with DAPI (blue), while the phosphor samples exhibited green color. | |
3.9. Applications for fingerprint detection and security ink
In addition to a high intracellular image, practical use of the visible fingerprint image of the SrF2:5% Tb3+, 5% Ce3+ phosphor was also carried out. Nowadays, fingerprint detection is the most important evidence in any criminal investigation. In this paper, we use the SrF2:5% Tb3+, 5% Ce3+ nano-phosphor for fingerprint detection on a glass slide, which is shown in Fig. 14(a and b). The powder sample was sprinkled on the surface of the glass slide and wiped out softly. The image was recorded under the 254 nm UV light. The fingerprint image of the sample before and after the irradiation of 254 nm UV light can be easily recognized by the naked eye, suggesting the as-prepared phosphor can have a potential application in forensic science.
 |
| | Fig. 14 Demonstration of fingerprint detection on (a and b) glass slide and (c and d) security ink for anti-counterfeiting application using SrF2:5% Tb3+, 5% Ce3+ nanophosphors before and after 254 nm UV irradiation. | |
Moreover, the as-prepared phosphor was also demonstrated in the security ink for an anti-counterfeiting application. The rare-earth-doped particles show visible emissions, thus, they have received attention for anti-counterfeiting applications. The invisible ink was prepared by dispersing 10 mg of the as-prepared phosphor in 2 mL of 10% PVA solution. The mixed solution was sonicated in an ultrasonic bath for 30 minutes to obtain a homogeneous dispersion. Then, a few words like MU were written with this ink, as shown in Fig. 14(c and d). Under daylight, the sample containing ink was invisible (Fig. 14c). But the green-colored MU patterns (Fig. 14d) can be observed under the irradiation of 254 nm UV light, indicating the as-prepared phosphor is highly compatible with anti-counterfeiting performance.
4. Conclusions
In conclusion, we have reported the synthesis and characterization, including biocompatibility studies of SrF2:Tb3+, Ce3+ nanophosphors. XRD patterns show the formation of the cubic phase of SrF2 NPs. A small increase in the unit cell volume is observed with Ce3+ and Tb3+ co-doping due to excess fluoride ions in the interstitial sites of the host matrix. FESEM micrographs show the formation of spherical-shaped NPs. From FTIR, it is concluded that the capping agent, i.e., trisodium citrate, is present in the as-prepared NPs. A defect-generated broad absorption is observed with good dispersibility in the optical studies. The phosphors show a strong green emission at 541 nm upon excitation at 297 nm, which is due to efficient energy transfer from Ce3+ (5D5/2) to Tb3+ (5D2) ions. With Ce3+ co-doping, Tb3+ lifetime increases to 6 ms while Ce3+ lifetime decreases from 15.35 and 7.82 ns. The energy transfer efficiency and the spectral overlap integral calculated were found to be 49% and 1.05 × 1010 M−1 cm−1 nm4. The synthesized phosphors have excellent biocompatibility towards the HeLa cell line and their cellular uptake was studied. Using the 254 nm wavelength excitation, the applicability of synthesized NPs towards security inks as anti-counterfeiting patterns and forensic fingerprint detection were designed and successfully performed. Thus, the synthesized nanophosphor will have potential applications in different areas, such as security ink, forensic fingerprint detection and in vitro bioimaging.
Data availability
All the data are available from the corresponding authors upon request.
Conflicts of interest
There are no conflicts to declare.
Acknowledgements
Authors are grateful to Ranjoy Wangkhem, Nagaland University, Nagaland, for assisting the Photoluminescence facility; Rameshwari Heisnam, Institute of Bioresources and Sustainable Development, Imphal, Manipur; Sophisticated Analytical Instrumentation Centre, Tezpur University for providing facilities for data acquisition.
References
- R. Grasser, A. Scharmann and K. R. Strack, On the intrinsic nature of the blue luminescence in CaWO4, J. Lumin., 1982, 27, 263–272 CrossRef CAS.
- M. Nikl, P. Bohacek, E. Mihokova, A. Vedda, M. Martini, G. P. Pazzi, P. Fabeni, M. Kobayashi and Y. Usuki, Enhanced efficiency of doubly doped PbWO4 scintillator, Radiat. Eff. Defects Solids, 2002, 157, 937–941 CrossRef CAS.
- H. Q. Wang, M. Batentschuk, A. Osvet, L. Pinna and C. J. Brabec, Rare-Earth Ion Doped Up-Conversion Materials for Photovoltaic Applications, Adv. Mater., 2011, 23, 2675–2680 CrossRef CAS PubMed.
- S. Sarkar, M. Chatti, V. N. K. B. Adusumalli and V. Mahalingam, Highly Selective and Sensitive Detection of Cu2+ Ions Using Ce(III)/Tb(III)-Doped SrF2 Nanocrystals as Fluorescent Probe, ACS Appl. Mater. Interfaces, 2015, 7, 25702–25708 CrossRef CAS PubMed.
- M. Azami, S. Jalilifiroozinezhad, M. Mozafari and M. Rabiee, Synthesis and solubility of calcium fluoride/hydroxy-fluorapatite nanocrystals for dental applications, Ceram. Int., 2011, 37, 2007–2014 CrossRef CAS.
- R. Joshi, R. S. Perala, S. B. Shelar, A. Ballal, B. P. Singh and R. S. Ningthoujam, Super Bright Red Upconversion in NaErF4:0.5%Tm@NaYF4:20%Yb Nanoparticles for Anti-counterfeit and Bioimaging Applications, ACS Appl. Mater. Interfaces, 2021, 13, 3481–3490 CrossRef CAS.
- W. Cross, T. Blumenthal, J. Kellar, P. S. May, J. Meruga and Q. Luu, Rare-Earth Doped Nanoparticles in Security Printing Applications, MRS Proc., 2012, 1471, 1270 Search PubMed.
- A. K. Parchur, A. I. Prasad, A. A. Ansari, S. B. Raia and R. S. Ningthoujam, Luminescence properties of Tb3+-doped CaMoO4 nanoparticles: annealing effect, polar medium dispersible, polymer film and core-shell formation, Dalton Trans., 2012, 41, 11032 RSC.
- G. Phaomei, R. S. Ningthoujam, W. R. Singh, R. S. Loitongbam, N. S. Singh, A. Rath, R. R. Juluri and R. K. Vatsa, Luminescence switching behavior through redox reaction in Ce3+ co-doped LaPO4:Tb3+ nanorods: Re-dispersible and polymer film, Dalton Trans., 2011, 40, 11571–11580 RSC.
- N. S. Singh, N. K. Sahu and D. Bahadur, Multicolor tuning and white light emission from lanthanide doped YPVO4 nanorods: energy transfer studies, J. Mater. Chem. C, 2014, 2, 548–555 RSC.
- N. Yaiphaba, Effect of Ce3+ Co-doping on GdPO4:Tb3+ Nanoparticles: Photoluminescence and Energy Transfer Studies, Asian J. Chem., 2021, 33, 903–908 CAS.
- Q. Luo, X. Qiao, X. Fan and X. Zhang, Preparation and luminescence properties of Ce3+ and Tb3+ co-doped glasses and glass ceramics containing SrF2 nanocrystals, J. Non-Cryst. Solids, 2010, 356, 2875–2879 CrossRef CAS.
- Q. Luo, X. Qiao, X. Fan and X. Zhang, Photoluminescence and efficient energy transfer from Ce3+ to Tb3+ or Mn2+ in Ca9ZnLi(PO4)7 host, Appl. Phys. A: Mater. Sci. Process., 2012, 108, 569–576 CrossRef.
- L. Wang, Y. Xu, D. Wang, R. Zhou, N. Ding, M. Shi, Y. Chen, Y. Jiang and Y. Wang, Deep red phosphors SrAl12O19:Mn4+, M (M = Li+, Na+, K+, Mg2+) for high colour rendering white LEDs, Phys. Status Solidi A, 2013, 210, 1433–1437 CrossRef CAS.
- W. Tang, S. Ding, Y. Yuan and G. Xie, Effect of Ce3+ and Tb3+ codoping on luminescence properties of Na3SrMg11(PO4)9:Eu2+ phosphors, Opt. Mater., 2020, 106, 110003 CrossRef CAS.
- D. Jia, X. J. Wang, W. Jia and W. M. Yen, Persistent energy transfer in CaAl2O4:Tb3+, Ce3+, J. Appl. Phys., 2003, 93, 148–152 CrossRef CAS.
- W. He, H. Du, J. Fu, F. Luan, D. Li, L. Sun and D. Guo, Efficient energy transfer from Ce3+ to Tb3+ in BaF2: green-emitting phosphors for potential applications in the detection of Cu2+ ions, New J. Chem., 2021, 45, 1446–1455 RSC.
- N. Kumam, N. P. Singh, L. P. Singh and S. K. Srivastava, Enhancement of luminescence in white emitting strontium fluoride core @ calcium fluoride shell nanoparticles, Nanoscale Res. Lett., 2015, 10, 6–13 CrossRef.
- B. Xu, H. He, Z. Gu, S. Jin, Y. Ma and T. Zhai, Improving 800 nm Triggered Upconversion Emission for Lanthanide-Doped CaF2 Nanoparticles through Sodium Ion Doping, J. Phys. Chem. C, 2017, 121, 18280–18287 CrossRef CAS.
- N. P. Singh, N. Kumam, L. P. Singh, N. R. Singh and S. K. Srivastava, Luminescent enhancement study of 3Tb,5Ce,5Li:CaF2: effect of Li+ ion concentration and hyperthermia applications of 3Tb:CaF2/Fe3O4 nanocomposite, New J. Chem., 2019, 43, 1328–1339 RSC.
- R. B. Hughes-Currie, K. V. Ivanovskikh, J. P. R. Wells, M. F. Reid, R. A. Gordon, L. Seijo and Z. Barandiarán, X-ray Excitation Triggers Ytterbium Anomalous Emission in CaF2:Yb but Not in SrF2:Yb, J. Phys. Chem. Lett., 2017, 8, 1175–1178 CrossRef CAS PubMed.
- R. N. Grass and W. J. Stark, Flame synthesis of calcium-, strontium-, barium fluoride nanoparticles and sodium chloride, Chem. Commun., 2005, 1767–1769 RSC.
- C. Zhang, Z. Hou, R. Chai, Z. Cheng, Z. Xu, C. Li, L. Huang and J. Lin, Mesoporous SrF2 and SrF2:Ln3+ (Ln = Ce, Tb, Yb, Er) Hierarchical Microspheres: Hydrothermal Synthesis, Growing Mechanism, and Luminescent Properties, J. Phys. Chem. C, 2010, 114, 6928–6936 CrossRef CAS.
- A. A. Ansari, A. K. Parchur, B. Kumar and S. B. Rai, Highly aqueous soluble CaF2:Ce/Tb nanocrystals: effect of surface functionalization on structural, optical band gap, and photoluminescence properties, J. Mater. Sci.: Mater. Med., 2016, 27, 1–11 CrossRef CAS PubMed.
- D. Przybylska, A. Ekner-Grzyb, B. F. Grześkowiak and T. Grzyb, Upconverting SrF2 nanoparticles doped with Yb3+/Ho3+, Yb3+/Er3+ and Yb3+/Tm3+ ions – optimisation of synthesis method, structural, spectroscopic and cytotoxicity studies, Sci. Rep., 2019, 9, 1–12 CrossRef.
- M. Quintanilla, I. X. Cantarelli, M. Pedroni, A. Speghini and F. Vetrone, Intense ultraviolet upconversion in water dispersible SrF2:Tm3+,Yb3+ nanoparticles: the effect of the environment on light emissions, J. Mater. Chem. C, 2015, 3, 3108–3113 RSC.
- S. Balabhadra, Upconverting Nanoparticles Working As Primary Thermometers In Different Media, J. Phys. Chem. C, 2017, 121, 13962–13968 CrossRef CAS.
- Y. Yan, Y. Tan, D. Li, F. Luan and D. Guo, Efficient energy transfer, multi-colour emitting and temperature sensing behavior of single-phase Tb3+, Eu3+ co-doped strontium fluoride phosphors, J. Lumin., 2019, 211, 209–217 CrossRef CAS.
- K. Shwetabh, M. M. Upadhyay and K. Kumar, Synthesis and upconversion emission studies of CaYF5:Ho3+/Yb3+ phosphor and its applications in optical thermometry, fingerprint detection, and security ink, RSC Adv., 2023, 13, 9377–9386 RSC.
- J. Liang, T. Fan, J. T. Lü, T. Guan, T. T. Deng and B. Xiong, Dual-mode luminescence anti-counterfeiting and white light emission of NaGdF4:Ce,Eu,Tb/carbon dot hydrophilic nanocomposite ink, RSC Adv., 2023, 13, 25681–25690 RSC.
- Y. Jin, Hydrothermal Synthesis and Luminescent Properties of (Sr, Ba)F2:Eu3+ Nanostructures, J. Nanosci. Nanotechnol., 2016, 16, 9856–9861 CrossRef CAS.
- M. Karimi, S. Hesaraki and N. Nezafati,
In vitro biodegradability–bioactivity-biocompatibility and antibacterial properties of SrF2 nanoparticles synthesized by one-pot and eco-friendly method based on ternary strontium chloride-choline chloride-water deep eutectic system, Ceram. Int., 2018, 44, 12877–12885 CrossRef CAS.
- R. K. Sharma, S. Nigam, Y. N. Chouryal, S. Nema, S. P. Bera, Y. Bhargava and P. Ghosh, Eu-Doped BaF2 Nanoparticles for Bioimaging Applications, ACS Appl. Nano Mater., 2019, 2, 927–936 CrossRef CAS.
- M. B. Hansen, S. E. Nielsen and K. Berg, Re-examination and further development of a precise and rapid dye, J. Immunol. Methods, 1989, 119, 203–210 CrossRef CAS PubMed.
- T. Mosmann, Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
- B. Chazotte, Labeling Nuclear DNA Using DAPI, Cold Spring Harb. Protoc., 2011, 80–82 Search PubMed.
- R. D. Shannon, Revised Effective Ionic Radii and Systematic Studies of Interatomic Distances in Halides and Chalcogenides, Acta Crystallogr. Sect. A, 1976, 32, 751–767 CrossRef.
- L. Van Pieterson, M. F. Reid, R. T. Wegh, S. Soverna and A. Meijerink, 4fn→4fn-1 5d transitions of the light lanthanides: Experiment and theory, Phys. Rev. B: Condens. Matter Mater. Phys., 2002, 65, 1–16 Search PubMed.
- L. R. K. T. Jacob, Shubhra Raj, Vegard's law: a fundamental relation or an approximation?, Int. J. Mater. Res., 2007, 98, 776–779 CrossRef.
- B. V. Ratnam, M. Jayasimhadri, J. Yoon, K. Jang, H. S. Lee, S. S. Yl, S. H. Kim and J. H. Jeong, Luminescent Properties of Tb3+-Doped NaCaPO4 Phosphor, J. Korean Phys. Soc., 2009, 55, 2383–2387 CrossRef CAS.
-
W. Kemp, Organic Spectroscopy, Macmillan Educational Ltd, Houldmills, Hampshire, 1987 Search PubMed.
- A. K. Thottoli and A. K. A. Unni, Effect of trisodium citrate concentration on the particle growth of ZnS nanoparticles, J. Nanostructure Chem., 2013, 3, 1 Search PubMed.
- T. Demkiv, M. Chylii, V. Vistovskyy, A. Zhyshkovych, N. Gloskovska, P. Rodnyi, A. Vasil'ev, A. Gektin and A. Voloshinovskii, Intrinsic luminescence of SrF2 nanoparticles, J. Lumin., 2017, 190, 10–15 CrossRef CAS.
- N. Bhunia, M. Bhar and P. Mukherjee, Maximizing Terbium-Europium Electronic Interaction: Insight from Variation of Excitation Energy, J. Phys. Chem. C, 2023, 127, 6425–6438 CrossRef CAS.
- M. S. Bhadane, K. H. Gavhane, V. S. Ghemud, S. S. Dahiwale, P. S. Patil and S. D. Dhole, A post annealing effect on SrF2 nanoparticles: Structural, morphological, functional and dosimetric properties, J. Alloys Compd., 2020, 846, 156343 CrossRef CAS.
- D. D. Yengkhom, G. S. Ningombam, R. Heisnam, N. Sharma, R. S. Nongmaithem and F. A. S. Chipem, Luminescence enhancement and tunable color emission in Eu/Dy/Sm codoped CaW1-xMoxO4 phosphor, Inorg. Chem. Commun., 2022, 141, 1–10 CrossRef.
- N. K. Sahu, N. S. Singh, L. Pradhan and D. Bahadur, Ce3+ sensitized GdPO4:Tb3+ with iron oxide nanoparticles: a potential biphasic system for cancer theranostics, Dalton Trans., 2014, 43, 11728–11738 RSC.
- T. C. Dexter, A Theory of Sensitized Luminescence in Solids, J. Chem. Phys., 1953, 21, 836–850 CrossRef.
- S. Wang, B. Devakumar, Q. Sun, J. Liang, L. Sun and X. Huang, Highly efficient near-UV-excitable Ca2YHf2Al3O12:Ce3+,Tb3+ green emitting garnet phosphors with potential application in high-color rendering warm-white LEDs, J. Mater. Chem. C, 2020, 8, 4408–4420 RSC.
- L. Sun, B. Devakumar, J. Liang, S. Wang, Q. Sun and X. Huang, Highly efficient Ce3+→Tb3+ energy transfer induced bright narrowband green emissions from garnet-type Ca2YZr2(AlO4)3:Ce3+,Tb3+ phosphors for white LEDs withhigh color rendering index, J. Mater. Chem. C, 2019, 7, 10471–10480 RSC.
-
J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, Singapore, 3rd edn, 2006 Search PubMed.
- G. Blasse, Energy transfer in oxidic phosphors, Phys. Lett. A, 1968, 28(6), 444–445 CrossRef CAS.
-
(a) O. S. Singh, R. Wangkhem, N. Yaiphaba, T. D. Singh and N. S. Singh, Bi3+ sensitized Gd2O3:Eu3+: A potential red phosphor for UV LED pumped white light emission, J. Alloys Compd., 2022, 902, 163831 CrossRef CAS;
(b) T. S. Singh and S. Mitra, Interaction of cinnamic acid derivatives with serum albumins: A fluorescence spectroscopic study, Spectrochim. Acta A, 2011, 78, 942–948 CrossRef PubMed.
- D. Durmus, Correlated color temperature: Use and limitations, Light. Res. Technol., 2022, 54, 363–375 CrossRef.
|
| This journal is © The Royal Society of Chemistry 2025 |
Click here to see how this site uses Cookies. View our privacy policy here.  Open Access Article
Open Access Article *b and
Ningombam
Yaiphaba
*b and
Ningombam
Yaiphaba
 *a
*a
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m for all samples. The FESEM image indicates spherical-shaped particles. Surface functionalization renders that the NPs are dispersible in water. The strong green emission of SrF2:5% Tb3+, 5% Ce3+ at 541 nm increases by 50-fold than that of SrF2:5% Tb3+. The resonance energy transfer between Ce3+ and Tb3+via multipolar interactions is observed. The energy transfer efficiency and spectral overlap integral are calculated. The absolute quantum yield of SrF2:5% Tb3+, 5% Ce3+ is observed to be ∼12%. The NPs show excellent biocompatibility towards the HeLa cell line with 70% cell viability. Intercellular uptake of the SrF2:5% Tb3+, 5% Ce3+ nanophosphor is fair, and its potential for anti-counterfeiting and forensic fingerprint applications is observed.
m for all samples. The FESEM image indicates spherical-shaped particles. Surface functionalization renders that the NPs are dispersible in water. The strong green emission of SrF2:5% Tb3+, 5% Ce3+ at 541 nm increases by 50-fold than that of SrF2:5% Tb3+. The resonance energy transfer between Ce3+ and Tb3+via multipolar interactions is observed. The energy transfer efficiency and spectral overlap integral are calculated. The absolute quantum yield of SrF2:5% Tb3+, 5% Ce3+ is observed to be ∼12%. The NPs show excellent biocompatibility towards the HeLa cell line with 70% cell viability. Intercellular uptake of the SrF2:5% Tb3+, 5% Ce3+ nanophosphor is fair, and its potential for anti-counterfeiting and forensic fingerprint applications is observed.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethylene glycol and water solution. 20 mL of 1
1 ethylene glycol and water solution. 20 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethylene glycol and aqueous solution of 0.595 g NH4F were added and stirred vigorously for 30 min. Thereafter, 20 mL of 1
1 ethylene glycol and aqueous solution of 0.595 g NH4F were added and stirred vigorously for 30 min. Thereafter, 20 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethylene glycol, an aqueous solution of 0.089 g Tb(NO)3·5H2O, 20 mL of 1
1 ethylene glycol, an aqueous solution of 0.089 g Tb(NO)3·5H2O, 20 mL of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ethylene glycol and an aqueous solution of 1.176 g C6H5Na3O7·2H2O were added. The whole mixture was stirred for another 30 minutes and then transferred to a Teflon-lined autoclave. The vessel was kept in a preheated oven at 140 °C for 2 hours, after which it was removed and cooled to room temperature. The product was collected by centrifugation and washed with deionized water and acetone. Finally, the product was dried at 40 °C for further analysis.
1 ethylene glycol and an aqueous solution of 1.176 g C6H5Na3O7·2H2O were added. The whole mixture was stirred for another 30 minutes and then transferred to a Teflon-lined autoclave. The vessel was kept in a preheated oven at 140 °C for 2 hours, after which it was removed and cooled to room temperature. The product was collected by centrifugation and washed with deionized water and acetone. Finally, the product was dried at 40 °C for further analysis.
 where Iemission = luminescence emission intensity of the sample, Isample holder = the intensity of excitation light only the quartz slide and Isample = the intensity of the light used for exciting the sample placed on the quartz slide. The difference in the denominator gives the photons absorbed by the sample. All the PL measurements were carried out on the glass (for PL and decay lifetime) and quartz slides (for QY) at room temperature. The luminescence decay lifetime for Ce3+ emission was measured by the TCSPC technique with Horiba make DeltaFlex having pulse LED laser 295 ± 10 nm as source (pulse width ≤1.2 ns).
where Iemission = luminescence emission intensity of the sample, Isample holder = the intensity of excitation light only the quartz slide and Isample = the intensity of the light used for exciting the sample placed on the quartz slide. The difference in the denominator gives the photons absorbed by the sample. All the PL measurements were carried out on the glass (for PL and decay lifetime) and quartz slides (for QY) at room temperature. The luminescence decay lifetime for Ce3+ emission was measured by the TCSPC technique with Horiba make DeltaFlex having pulse LED laser 295 ± 10 nm as source (pulse width ≤1.2 ns).
![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) m space group. No additional phases, such as Tb2O3, Ce2O3, Ce3O4, etc., are observed upon adding Tb3+ and Ce3+ ions into the SrF2 lattice. The calculated lattice parameters, crystallite sizes, and unit cell volumes are shown in Table 1. The crystallite sizes were calculated using the Scherrer formula: d = kλ/β
m space group. No additional phases, such as Tb2O3, Ce2O3, Ce3O4, etc., are observed upon adding Tb3+ and Ce3+ ions into the SrF2 lattice. The calculated lattice parameters, crystallite sizes, and unit cell volumes are shown in Table 1. The crystallite sizes were calculated using the Scherrer formula: d = kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos
cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ. The strongest peak corresponding to the (111) plane was used to calculate the average crystallite size of the samples. Again, using the Williamson–Hall model, the crystallite sizes were also calculated, which were almost similar to those calculated using the Scherrer formula. From Table 1, it is observed that the unit cell volume is increased on Tb3+ substitution, whereas the ionic radius of the Sr2+ ion (1.32 Å) is larger than that of Tb3+ (0.92 Å) at coordination number 6.37 Our result is contradictory to the Vegard's law; the unit cell volume must be slightly decreased as compared to that of pure SrF2. This is due to the occupancy of the excess fluoride ions in the interstitial sites, which compensate for the excess positive charge due to the substitution of trivalent lanthanide ions at Sr2+ sites. The balanced chemical equation can be represented as 2Sr2+ → Ln3+ + F−. It may also be due to the formation of tetragonal (C4v) or trigonal (C3v) and electronic repulsions between F− ions, which leads to the expansion of unit cell volume.38,39 However, a small increase in the unit cell volume is observed for Ce3+ and Tb3+ co-doped SrF2 NPs, which is due to the ionic radius of Ce3+ (1.01 Å) ion being greater than that of Tb3+ ion (0.92 Å) at coordination number 6.37,40
θ. The strongest peak corresponding to the (111) plane was used to calculate the average crystallite size of the samples. Again, using the Williamson–Hall model, the crystallite sizes were also calculated, which were almost similar to those calculated using the Scherrer formula. From Table 1, it is observed that the unit cell volume is increased on Tb3+ substitution, whereas the ionic radius of the Sr2+ ion (1.32 Å) is larger than that of Tb3+ (0.92 Å) at coordination number 6.37 Our result is contradictory to the Vegard's law; the unit cell volume must be slightly decreased as compared to that of pure SrF2. This is due to the occupancy of the excess fluoride ions in the interstitial sites, which compensate for the excess positive charge due to the substitution of trivalent lanthanide ions at Sr2+ sites. The balanced chemical equation can be represented as 2Sr2+ → Ln3+ + F−. It may also be due to the formation of tetragonal (C4v) or trigonal (C3v) and electronic repulsions between F− ions, which leads to the expansion of unit cell volume.38,39 However, a small increase in the unit cell volume is observed for Ce3+ and Tb3+ co-doped SrF2 NPs, which is due to the ionic radius of Ce3+ (1.01 Å) ion being greater than that of Tb3+ ion (0.92 Å) at coordination number 6.37,40


![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) exp(−t/τ)
exp(−t/τ)