 Open Access Article
Open Access ArticleRigid cationic ligands enable high-efficiency NIR-II photoluminescence in copper(I) iodide hybrid semiconductors
Jingwen
Chen
ab,
Xueqian
Wu
a,
Minghui
Zhang
a,
Simon J.
Teat
 c,
Guozhong
Xu
*b,
Jingbai
Li
*a,
Xiuze
Hei
c,
Guozhong
Xu
*b,
Jingbai
Li
*a,
Xiuze
Hei
 *a and
Jing
Li
*a and
Jing
Li
 *dae
*dae
aHoffman Institute of Advanced Materials, Shenzhen Polytechnic University, Shenzhen, 518055, China. E-mail: lijingbai@szpu.edu.cn; xiuzehei@szpu.edu.cn
bCollege of Chemical Engineering, University of Science and Technology Liaoning, Anshan 114051, China. E-mail: gz_xu@ustl.edu.cn
cAdvanced Light Source, Lawrence Berkeley National Laboratory, Berkeley, CA 94720, USA
dDepartment of Chemistry and Chemical Biology, Rutgers University, Piscataway, NJ 08854, USA. E-mail: jingli@rutgers.edu
eDepartment of Information Display, Kyung Hee University, Seoul 02447, Republic of Korea
First published on 26th August 2025
Abstract
Near-infrared (NIR) luminescent materials are pivotal for advanced optoelectronic and biomedical applications, yet attaining efficient emission in the NIR-II region (950–1400 nm) remains challenging. Here, we introduce a ligand cationization strategy for designing copper(I) iodide-organic hybrid materials that emit in the NIR-II region (920–1120 nm) with PLQYs up to 8.58%. By incorporating rigid cationic ligands with CuI modules, we synergistically achieve bandgap narrowing (to 1.51 eV) and structural rigidification via ionic-dative bonding, effectively suppressing non-radiative decay while extending emission beyond 1100 nm. Coupled with solution processability—enabled by the successful synthesis of nanometer-sized nanoparticles in various shapes—and excellent thermal stability (≥210 °C), this work establishes ligand cationization as a universal approach for designing efficient NIR-II emitters.
Introduction
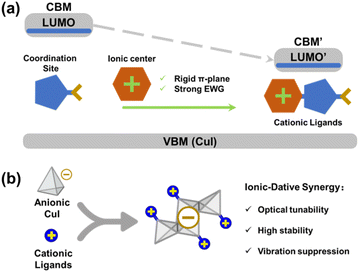
Near-infrared (NIR) luminescent materials (700–1400 nm) have emerged as essential components in advanced optoelectronic technologies, including bioimaging, night vision, and optical communication, due to their superior tissue penetration and minimized background interference.1–4 While small organic molecules, perovskites, and quantum dots have successfully achieved emission in the NIR-I region (700–950 nm),4 their extension to the longer-wavelength NIR-II range (950–1700 nm) remains challenging: organic systems are often limited by complex synthesis and low photoluminescence quantum yields (PLQYs),5,6 whereas inorganic counterparts struggle with stability and bandgap tunability.7,8 Copper(I) iodide-based hybrid materials (CuI-L), which integrate the structural modularity of organic ligands with the chemostability of inorganic motifs, present a compelling alternative due to their structural diversity, high stability, and unique photophysical properties.9–11 However, existing CuI-L systems emit predominantly in the visible or short-wavelength NIR-I regions, with limited exploration of their NIR-II potential.12–14 This limitation arises primarily from the lack of efficient strategies for bandgap narrowing and the suppression of non-radiative decay pathways.The photoluminescence of many CuI-L hybrids arises from the excited states based on metal halide-to-ligand charge transfer process [(M + X)LCT], which is heavily influenced by ligand electronic states and structural rigidity.10,15 Current efforts to red-shift emission wavelengths rely on extending ligand conjugation or introducing electron-withdrawing groups (EWGs) to lower the energies of the lowest unoccupied molecular orbitals (LUMOs) of ligands; however, these approaches often compromise PLQYs due to exacerbated quenching as the results of extended molecular vibrations and rotations, or fail to sufficiently narrow the bandgap.14,16,17 This underscores the need for a unified design strategy that simultaneously tailors electronic structures and suppresses non-radiative losses effectively.
Herein, we propose a “rigid cationic ligands” strategy to engineer AIO-type18,19 copper(I) iodide-organic hybrids (AIO-CuI-L) for tunable NIR-II photoluminescence. By incorporating a rigid, conjugated cationic center to ligands with low-lying LUMO energies, we tackle the two key challenges: bandgap narrowing and exciton stabilization (Scheme 1). This is achieved through (1) bandgap reduction via the enhanced electron-withdrawing effects of the cationic centers and (2) suppression of vibrational quenching as a result of rigidified inorganic–organic interfaces enabled by synergistic ionic-dative bonding.20
 | ||
| Scheme 1 Schematic illustration of “rigid cationic ligand” strategy for (a) band gap and (b) structure engineering of AIO-type CuI-L hybrid materials. | ||
Using methylpyridinium-functionalized benzothiadiazole/benzoselenadiazole ligands, we succeed in synthesizing four different AIO-CuI-L hybrid materials with emissions in the NIR-II region (920–1120 nm) and PLQYs up to ∼8.6%, surpassing previous CuI-L systems by over 5-fold. These emitters exhibit high stability and solution processability, enabling the fabrication of uniform nanoparticles. Assisted by comprehensive optical characterizations coupled with density functional theory (DFT) analysis, this work establishes a universal design principle for functional hybrids, bridging molecular-level engineering with macroscopic optoelectronic performance.
Results and discussion
Structural description
We selected benzothiadiazole and benzoselenadiazole as core ligands as they have appropriate LUMO energy levels and strong coordination capability with CuI.13 To further reduce the bandgap of the resulting hybrid semiconductors and enable NIR-II emission, an additional approach is necessary to further engineer these ligands. DFT calculations reveal that conventional modifications with either neutral EWGs or π-plane expansion fail to sufficiently lower the LUMO energies of benzothiadiazole and benzoselenadiazole (ΔELUMO < 0.5 eV). In sharp contrast, introducing the cationic methylpyridinium group substantially reduces their LUMO levels by ∼1.5 eV (Fig. S1 and S2), leading to narrow energy gaps of the resultant CuI hybrid materials conducive to NIR-II emission. This pronounced reduction of LUMO stems primarily from the stronger electrostatic induction from the cationic center of the methylpyridinium group, which redistributes the electron density on the benzothiadiazole and benzoselenadiazole more effectively than that from the partial charges on the EWGs or the electron delocalization over the extended conjugation (Fig. S3). Furthermore, the N atoms of the cationized ligands could retain their coordination ability, forming synergistic ionic-dative bonds with CuI modules. This bonding strategy has been proven to enhance structural rigidity and stability.12,20To verify the proposed strategy, we synthesized the above rigid cationic ligands by a two-step process, and their purity was confirmed with 1H NMR (Fig. S4 and S5). Reactions of these ligands with CuI under different conditions yielded high-quality single crystals of the four title compounds. Single-crystal X-ray analysis revealed that their structures range from molecular 0D clusters to layered 2D networks with the formulas 0D-Cu2I4(LS)2 (1), 0D-Cu2I4(LSe)2 (2), 1D-Cu6I8(LS)2 (3), and 2D-Cu6I8(LSe)2 (4), as depicted in Fig. 1a–d and S8. The crystallographic data are summarized in Table S1. Notably, all compounds maintain charge neutrality through balanced coordination between cationic ligands and anionic CumIm+22− modules, as observed in other reported AIO compounds.18,20
Compounds 1 and 2 share the same structure type and are isostructural, where 0D-Cu2I42− dimeric clusters coordinate to two ligands via Cu–N bonds. Compound 3 consists of 1D anionic (Cu6I8)2− chains that are coordinated and charge-balanced by terminal ligands. Compound 4 is a layered structure made of 1D anionic (Cu6I8)2− chains interconnected via bidentate ligands. The Cu–N bond lengths in all compounds are found to be 2.03 to 2.07 Å, similar to other reported hybrid CuX-L compounds consist of Cu–N bonds.21,22 In all compounds, Cu atoms are tetrahedrally coordinated to either three I atoms and one N atom from the ligand, or four I atoms. The coordination numbers of I atoms range from 1 to 3.
Critical to emission performance, π–π interactions between adjacent ligands were observed across all title compounds, with vertical distances (d) of 3.32–4.14 Å and displacement angles (θ) of 14.97–34.02°. Interestingly, two different kinds of π–π interactions were found in all compounds, as shown in Fig. 1e and S9. Among them, compound 3 demonstrates the most aligned π–π stacking, followed by compound 2. The subtle geometric variations, arising from the ligand composition (S vs. Se) and structural dimensionality changes of CuI motifs, are anticipated to profoundly influence exciton dynamics—a hypothesis validated in subsequent photophysical studies.
High-quality single crystals and powder samples of all compounds were used for various characterizations, with their phase purity verified by powder X-ray diffraction (PXRD) analysis (Fig. S10). FT-IR spectroscopic study further validated the coordination-induced structural rigidification. As shown in Fig. 1f, taking compounds 2 and 4 as examples, the signals of the coordinated ligands remain characteristic of free ligands while showing marked attenuation of selenadiazole-related vibrations, including the N–Se–N scissoring at 720 cm−1, and the β(C–H) of the phenyl-ring at 1020 and 1230 cm−1. Concurrently, intensified peaks observed at 810 cm−1, 1340 cm−1 (pyridine ring β(C–H)), and 3000–3100 cm−1 (methyl v(C–H)). These spectral evolution trends, which are also consistently observed in compounds 1 and 3 (Fig. S11), arise from distinct bonding environmental changes during the Cu–N bond formation: Free ligands exhibit vibrational constraints from coulombic interactions between I− anions and pyridinium cations, whereas coordinated ligands experience enhanced rigidity through ionic-dative synergy—Cu–N bonds restrict selenadiazole motions, while charge redistribution stabilizes conjugated backbones. These results demonstrate that ligand cationization not only modulates electronic structures but also imposes steric constraints to mitigate non-radiative decay pathways.
Photophysical study
The optical absorption spectra of all compounds were collected at room temperature using diffuse reflectance spectroscopy (Fig. 2a). Their optical band gaps range from 1.51 to 1.62 eV, estimated from the absorption edges using the Tauc method. The narrow band gaps are also reflected by the black colors of the samples, indicating strong visible light absorption. Room temperature photoluminescence spectroscopy reveals that upon excitation under 450 nm irradiation, all compounds demonstrate broadband low energy emissions in the NIR-II region. The emission maxima are 970, 1020, 920 and 1120 nm for compounds 1–4, respectively, well in trend with their estimated band gaps. Remarkably, this represents the first set of CuX-based materials that emit in the NIR-II region, surpassing the previously reported 1D-(mqx)2Cu2I4 which exhibits the longest emission wavelength peaked at 875 nm.13 The emission peaks and spectral shapes of all four compounds remain unchanged with varying excitation energies (Fig. 2b, c and S12), indicating a single excitation process governs their photoluminescence.23 The full-width at half-maximum (FWHM) values of all four compounds are ∼250 meV, implying the charge transfer characteristics of their excited states, similar to other reported hybrid NIR emitters.13,24–26The photoluminescence quantum yields (PLQYs) of all four compounds were determined at room temperature (Fig. S13 and S14) under 450 nm excitation, as listed in Table 1. Notably, compound 4 achieved an impressive PLQY of 8.58%, with an emission peak centered at 1120 nm—setting a new record for metal-halide hybrids with simultaneously the longest emission wavelength and highest efficiency reported to date in the NIR-II region (Fig. 2d and Table S11). This dual optimization clearly outperforms conventional CuX systems (e.g., PLQY of 1.7% at 875 nm),13 underscoring the effectiveness of the “rigid cationic ligand” strategy.
| Compound | B. G. (eV) | λ em (nm) | PLQYa (%) | Lifetimeb (μs) | k r (×104 s−1) | k nr (×104 s−1) | T d (°C) |
|---|---|---|---|---|---|---|---|
| a λ ex = 450 nm, data obtained at RT. b Data obtained at 278 K. c T d: decomposition temperature. | |||||||
| 1 | 1.60 | 970 | 6.45 ± 0.15 | 9.6 | 2.0 | 29.2 | 210 |
| 2 | 1.56 | 1020 | 4.01 ± 0.79 | 2.7 | 2.7 | 64.0 | 265 |
| 3 | 1.62 | 920 | 1.61 ± 0.46 | 1.7 | 1.2 | 70.3 | 255 |
| 4 | 1.51 | 1120 | 8.58 ± 0.96 | 2.9 | 3.3 | 35.1 | 275 |
To quantitatively assess their emission efficiencies, total radiative rates (kr) and non-radiative decay rates (knr) were estimated based on eqn (1) and (2),27,28 where ηPL is the PLQY value and τPL is the average PL lifetime.
| ηPL = kr/(kr + knr) | (1) |
| τPL = 1/(kr + knr) | (2) |
Note that the temperature used for PLQY and PL lifetime measurements are slightly different (RT and 278 K, respectively). Hence, the kr are expected to be slightly underestimated and the knr are slightly overestimated. As demonstrated in Fig. 2e and Table 1, despite minor variations in kr (1.2–3.3 × 104 s−1), their knr differ significantly (29.2–70.3 × 104 s−1), primarily governing PLQY differences. Such non-radiative deactivation of the photogenerated excitons can be attributed to the short-range Dexter energy-transfer process mediated by the overlap of two adjacent ligand wavefunctions, which is dominant in many NIR luminescent materials.29–32 The energy transfer rates of Dexter coupling modes, kD, can be estimated based on eqn (3) and are listed in Table S2, where d (vertical π–π distance), θ (displacement angle), and ω (dihedral angle) define the intermolecular coupling geometry between two π planes.33,34
| kD ∝ cos(θ)·cos(ω)·e−d | (3) |
Fig. 2e correlates kD with the kr and knr values of all four compounds. The simultaneous increase of kD and knr from compounds 1 to 3 (made of LS) validates our above analysis that the enhanced π–π interactions within the structures can lead to severe non-radiative relaxations via Dexter energy transfer, thus reducing the PLQYs of the compounds.35,36 For the same reason, the kD and knr decrease from compounds 2 to 4 (made of LSe). Notably, compound 1 demonstrates a kD value comparable to 2 and higher than 4, yet its knr remains lower than both 2 and 4. This ligand-dependent divergence may be attributed to the van der Waals radius differences of S and Se atoms (1.80 Å and 1.90 Å, respectively),37 as well as the significantly larger spin–orbit coupling (SOC) of Se atom, which causes apparent underestimated kD for LSe system relative to LS system.
Temperature-dependent PL spectroscopy and lifetime measurements were carried out at various temperatures for all title compounds, as shown in Fig. 3 and S15–S17. All title compounds demonstrate little to no emission peak shifts and FWHM changes across a temperature range of 78 K to 287 K, indicative of suppressed electron-phonon coupling. Quantitative analysis through temperature-dependent FWHM evolution (eq. (4)) yielded Huang–Rhys factor (S) and photon frequency (ħωphoton),38 while the exciton binding energies (Eb) were derived from the Arrhenius plots of the PL thermal quenching (eqn (5)),39 as depicted in Fig. 3c and S15–S17.
 | (4) |
 | (5) |
Owing to the unique coordination chemistry featuring strongly bonded cation–anion pairs, the title compounds demonstrate enhanced structural rigidity and strong exciton localization effects compared to many other CuX based hybrid materials,40,41 enabled by the rigid ionic-dative frameworks. The relatively smaller Eb values of compounds 2 (27.1 meV) and 3 (79.5 meV) may be attributed to their pronounced π–π interactions as described above.
All title compounds exhibit PL lifetimes at microsecond-scale across the studied temperature range (78–278 K), confirming the phosphorescence nature of their NIR emissions. Biexponential decay fittings resolved two distinct radiative pathways with amplitude ratios show little temperature-dependence (Fig. 3b, S15–S17 and Tables S3–S6). In conjunction with the observed subtle thermochromism of their emissions, these results suggest the coexistence of two weakly coupled triplet excited states (T1 and T2) with near-degenerate energies, which have been seen in previous studies on d10 metal complexes.42,43 This phenomenon potentially arises from the different charge transfer dynamics of the differently overlapped frontier orbitals, induced by the two distinct π–π interactions observed within the structures.44,45
Phosphorescence mechanism
To gain insights into the role of structural features in regulating the photoluminescence mechanisms, we performed DFT calculations to study the phosphorescence mechanism of title compounds. The projected density of states (PDOS) computed with PBE0 hybrid functional show dominant contributions of Cu 3d and I 5p orbitals in the valence band maximum (VBM) and ligand orbitals (e.g., p orbitals of C, N, S, Se) in the conduction band minimum (CBM), as depicted in Fig. 3d and S15. We computed the singlet and triplet excited states with time-dependent DFT (TDDFT) calculations including SOC for all title compounds, with detailed data summarized in Tables S7–S10. Fig. 3e and S16–S19 illustrate the frontier crystal orbitals participating in the electronic transitions. While the highest occupied crystal orbital (HOCO) comes from inorganic CuI modules, and the lowest unoccupied crystal orbital (LUCO) centers on the organic ligands. These results confirm that both absorption and NIR emissions of all title compounds are associated with the excited states generated from the metal halide-to-ligand charge transfer processes [(M + X)LCT], which enables facile optical tuning through ligand modifications.19,46,47In compound 1, the two distinct π–π stacking configurations in the crystal structures (Fig. 1e and S9) lead to closely spaced low-lying singlet (1.30 and 1.31 eV) and triplet (1.24 and 1.25 eV) states. The narrow energy gap between S2 and S1 facilitates ultrafast internal conversion to populate S1 after photoexcitation. The S1–T1 and S1–T2 energy gaps are 0.05 and 0.06 eV, respectively. Analysis of the SOC matrix element (SOCME) reveals significantly stronger S1–T1 coupling (52.94 cm−1) compared to S1–T2 (12.46 cm−1), suggesting a faster intersystem crossing (ISC) from S1 to T1 than T2. In contrast, the S0–T1 and S0–T2 couplings (100.10 and 129.41 cm−1, respectively) are comparable, which could lead to similar non-radiative rate constants. The singlet triplet SOC introduces two low-lying spin states dominated by T1 and T2, responsible for two phosphorescence decay pathways. The oscillator strengths are 2.1 × 10−4 and 2.6 × 10−4 for the T1 and T2 featured spin states, respectively, implying comparable radiative decay rate constants from T1 and T2 to S0. Since the phosphorescence decay is a product of both singlet-to-triplet ISC and triplet radiative transitions, the stronger SOC of T1 with S1 accelerates the overall phosphorescence decay relative to T2. These computational findings, consistent across all title compounds, provide a possible explanation for the experimentally observed biexponential phosphorescence decay.
Thermal stability and processability
Thermal stability of all title compounds was assessed by thermogravimetric analysis (TGA). As shown in Fig. S20, all compounds exhibit remarkable structure robustness and remain stable up to 210 °C, with compound 4 demonstrating the highest decomposition temperature of 275 °C. In general, compounds containing Se-based ligands are more stable than those with S-based ligands, attributed to their more electron-rich coordination sites. Building extended networks to increase structural rigidity enhances stability as well, consistent with previous findings on related coordination systems.9,48,49 Notably, the title compounds demonstrate significantly improved thermal stability compared to CuI-L hybrid structures made of charge-neutral benzothiadiazole/benzoselenadiazole ligands (Td ≤ 150 °C).14 This substantial enhancement underscores the synergistic stabilization effect of ionic-dative bonding nature.To meet the practical demands for NIR related applications, we carried out a comprehensive study to evaluate the solution processability and to develop nano-sized synthesis strategy for the title NIR emitters. The compounds demonstrate intriguing solubility in DMSO, despite their structural rigidity, evidenced by the 1H NMR spectra of dissolved compounds (Fig. S6 and S7). Consistent with previous studies, the dissolved species were identified as small fragments of anionic CuI clusters remaining coordinated to cationic ligands,28,50 and the reversibility of this dissolution process was confirmed by recrystallizations. Besides this solution processability, we further synthesized nano-sized samples with high dispersibility. Taking compound 2 as an example: Uniformly shaped nanoparticles were formed upon rapid injection of ligand solutions into a CuI/KI/PVP colloidal mixture at 70 °C. Scanning electron microscopy (SEM) revealed spherical nanoparticles (∼210 nm in diameter, Fig. 4) with smooth surfaces, while TEM-EDS elemental mapping further confirmed the homogeneous spatial distribution of constituent elements (Cu, I, N, Se) within individual nanoparticles. The phase purity was validated by well-matched experimental and simulated PXRD patterns, retained FT-IR vibrational signatures, and consistent UV-Vis absorption edges, as shown in Fig. 4 and S21. Interestingly, progressive structural transformations from initial nanospheres (∼210 nm) to elongated nanorods (∼3 μm), followed by centrally constricted intermediates (∼5 μm), and ultimately hierarchical nanoflowers (15–20 μm) were observed through timed reaction processes (Reaction time: 1 s, 5 s, 15 s and 1 min, respectively). Similar size/morphology progression was also observed for compound 1 (Fig. S22), enabling customizable light–matter interactions for targeted applications, via nanostructure tuning.
Conclusions
In this work, we have designed and synthesized a series of four narrow bandgap CuI-based hybrid semiconductor materials made of anionic CunIn+22− and cationic ligands. All compounds emit in NIR-II region (920–1120 nm) with PLQY up to 8.58%. The cationic centers induce a strong electron-withdrawing effect to the coordination core, thus lowering the LUMO energy of the ligands and reducing the bandgap effectively, while the rigid ionic-dative bonding network suppresses non-radiative decay by restricting molecular vibrations. This dual functionality renders those materials efficient photoluminescence in NIR-II region. Coupled with processability for nano structural control (210 nm nanoparticles to 20 μm nanoflowers), and thermal resilience (≥210 °C), this cationization-driven bandgap engineering provides a new possibility for high-performance NIR-II materials.Author contributions
J. W. Chen: formal analysis, investigation, writing – original draft; X. Q. Wu: investigation, formal analysis; M. H. Zhan: investigation, formal analysis; S. J. Teat: investigation, formal analysis, resources; G. Z. Xu: validation, supervision; J. B. Li: formal analysis, validation, writing – review & editing; X. Z. Hei: conceptualization, funding acquisition, writing – review & editing, supervision, project administration; J. Li: conceptualization, writing – review & editing, supervision, project administration.Conflicts of interest
There are no conflicts to declare.Data availability
Supplementary Information: Methods, 1H NMR spectroscopy, Crystal data and structural plots, photophysical properties, DFT calculations, thermal stability and processibility. The data supporting this article can be found in the SI. See DOI: https://doi.org/10.1039/d5sc04588f.Acknowledgements
The authors acknowledge the financial support from Shenzhen Science and Technology Program (grant no. 20231124150717002 and No. RCBS20231211090634054), Guangdong Basic and Applied Basic Research Foundation (grant no. 2023A1515110006) and National Science Foundation of China grants (22303053). This research used the Advanced Light Source (ALS), which is a DOE Office of Science User Facility under contract no. DE-AC02-05CH11231.References
- Y. Gu, Z. Guo, W. Yuan, M. Kong, Y. Liu, Y. Liu, Y. Gao, W. Feng, F. Wang, J. Zhou, D. Jin and F. Li, High-sensitivity imaging of time-domain near-infrared light transducer, Nat. Photonics, 2019, 13, 525–531 CrossRef CAS.
- Y.-C. Wei, S. F. Wang, Y. Hu, L.-S. Liao, D.-G. Chen, K.-H. Chang, C.-W. Wang, S.-H. Liu, W.-H. Chan, J.-L. Liao, W.-Y. Hung, T.-H. Wang, P.-T. Chen, H.-F. Hsu, Y. Chi and P.-T. Chou, Overcoming the energy gap law in near-infrared OLEDs by exciton–vibration decoupling, Nat. Photonics, 2020, 14, 570–577 CrossRef CAS.
- A. Minotto, I. Bulut, A. G. Rapidis, G. Carnicella, M. Patrini, E. Lunedei, H. L. Anderson and F. Cacialli, Towards efficient near-infrared fluorescent organic light-emitting diodes, Light: Sci. Appl., 2021, 10, 18 CrossRef CAS.
- M. Vasilopoulou, A. Fakharuddin, F. P. García de Arquer, D. G. Georgiadou, H. Kim, A. R. b. Mohd Yusoff, F. Gao, M. K. Nazeeruddin, H. J. Bolink and E. H. Sargent, Advances in solution-processed near-infrared light-emitting diodes, Nat. Photonics, 2021, 15, 656–669 CrossRef.
- H. Guo, Q. Peng, X.-K. Chen, Q. Gu, S. Dong, E. W. Evans, A. J. Gillett, X. Ai, M. Zhang, D. Credgington, V. Coropceanu, R. H. Friend, J.-L. Brédas and F. Li, High stability and luminescence efficiency in donor–acceptor neutral radicals not following the Aufbau principle, Nat. Mater., 2019, 18, 977–984 CrossRef PubMed.
- Q. Zhang, R. Wang, B. Feng, X. Zhong and K. Ostrikov, Photoluminescence mechanism of carbon dots: triggering high-color-purity red fluorescence emission through edge amino protonation, Nat. Commun., 2021, 12, 6856 CrossRef PubMed.
- Y.-H. Jia, S. Neutzner, Y. Zhou, M. Yang, J. M. F. Tapia, N. Li, H. Yu, J. Cao, J.-P. Wang, A. Petrozza, C.-P. Wong and N. Zhao, Role of Excess FAI in Formation of High-Efficiency FAPbI3-Based Light-Emitting Diodes, Adv. Funct. Mater., 2020, 30, 1906875 CrossRef.
- Y. Liu, F. Di Stasio, C. Bi, J. Zhang, Z. Xia, Z. Shi and L. Manna, Near-Infrared Light Emitting Metal Halides: Materials, Mechanisms, and Applications, Adv. Mater., 2024, 36, 2312482 CrossRef PubMed.
- X. Hei and J. Li, All-in-one: a new approach toward robust and solution-processable copper halide hybrid semiconductors by integrating covalent, coordinate and ionic bonds in their structures, Chem. Sci., 2021, 12, 3805–3817 RSC.
- J. Troyano, F. Zamora and S. Delgado, Copper(i)–iodide cluster structures as functional and processable platform materials, Chem. Soc. Rev., 2021, 50, 4606–4628 RSC.
- V. W.-W. Yam, V. K.-M. Au and S. Y.-L. Leung, Light-Emitting Self-Assembled Materials Based on d8 and d10 Transition Metal Complexes, Chem. Rev., 2015, 115, 7589–7728 CrossRef PubMed.
- W. Liu, Y. Fang and J. Li, Copper Iodide Based Hybrid Phosphors for Energy-Efficient General Lighting Technologies, Adv. Funct. Mater., 2018, 28, 1705593 CrossRef.
- K. Zhu, G. M. Carignan, S. J. Teat, S. Rangan, X. Hei, L. H. Nguyen and J. Li, Narrow Band Gap Hybrid Copper(I)Iodides: Designer Materials for Optoelectronic Applications, Chem. Mater., 2024, 36, 11139–11149 CrossRef.
- Y. Fang, K. Zhu, S. J. Teat, O. G. Reid, X. Hei, K. Zhu, X. Fang, M. Li, C. A. Sojdak, M. Cotlet and J. Li, Robust and Highly Conductive Water-Stable Copper Iodide-Based Hybrid Single Crystals, Chem. Mater., 2022, 34, 10040–10049 CrossRef CAS.
- D. A. Popy and B. Saparov, “This or that” – light emission from hybrid organic–inorganic vs. coordination Cu(i) halides, J. Mater. Chem. C, 2025, 13, 521–560 RSC.
- H. Miao, Y. Zhou, P. Wang, Z. Huang, W. Zhaxi, L. Liu, F. Duan, J. Wang, X. Ma, S. Jiang, W. Huang, Q. Zhang and D. Wu, High-temperature negative thermal quenching phosphors from molecular-based materials, Chem. Commun., 2023, 59, 1229–1232 RSC.
- R. Kobayashi, H. Kihara, T. Kusukawa, H. Imoto and K. Naka, Dinuclear Rhombic Copper(I) Iodide Complexes with Rigid Bidentate Arsenic Ligands, Chem. Lett., 2021, 50, 382–385 CrossRef CAS.
- W. Liu, K. Zhu, S. J. Teat, G. Dey, Z. Shen, L. Wang, D. M. O'Carroll and J. Li, All-in-One: Achieving Robust, Strongly Luminescent and Highly Dispersible Hybrid Materials by Combining Ionic and Coordinate Bonds in Molecular Crystals, J. Am. Chem. Soc., 2017, 139, 9281–9290 CrossRef CAS PubMed.
- X. Hei, W. Liu, K. Zhu, S. J. Teat, S. Jensen, M. Li, D. M. O'Carroll, K. Wei, K. Tan, M. Cotlet, T. Thonhauser and J. Li, Blending Ionic and Coordinate Bonds in Hybrid Semiconductor Materials: A General Approach toward Robust and Solution-Processable Covalent/Coordinate Network Structures, J. Am. Chem. Soc., 2020, 142, 4242–4253 CrossRef CAS.
- X. Hei and J. Li, Making coordination networks ionic: a unique strategy to achieve solution-processable hybrid semiconductors, Mater. Chem. Front., 2023, 7, 4598–4604 RSC.
- W. Ki, X. Hei, H. T. Yi, W. Liu, S. J. Teat, M. Li, Y. Fang, V. Podzorov, E. Garfunkel and J. Li, Two-Dimensional Copper Iodide-Based Inorganic–Organic Hybrid Semiconductors: Synthesis, Structures, and Optical and Transport Properties, Chem. Mater., 2021, 33, 5317–5325 CrossRef CAS.
- X. Hei, K. Zhu, G. Carignan, S. J. Teat, M. Li, G. Zhang, M. Bonite and J. Li, Solution-processable copper(I) iodide-based inorganic-organic hybrid semiconductors composed of both coordinate and ionic bonds, J. Solid State Chem., 2022, 314, 123427 CrossRef CAS.
- M. Xie, C. Han, Q. Liang, J. Zhang, G. Xie and H. Xu, Highly efficient sky blue electroluminescence from ligand-activated copper iodide clusters: Overcoming the limitations of cluster light-emitting diodes, Sci. Adv., 2019, 5, eaav9857 CrossRef CAS PubMed.
- B. Su, S. Geng, Z. Xiao and Z. Xia, Highly Distorted Antimony(III) Chloride [Sb2Cl8]2− Dimers for Near-Infrared Luminescence up to 1070 nm, Angew. Chem., Int. Ed., 2022, 61, e202208881 CrossRef CAS PubMed.
- V. Morad, Y. Shynkarenko, S. Yakunin, A. Brumberg, R. D. Schaller and M. V. Kovalenko, Disphenoidal Zero-Dimensional Lead, Tin, and Germanium Halides: Highly Emissive Singlet and Triplet Self-Trapped Excitons and X-ray Scintillation, J. Am. Chem. Soc., 2019, 141, 9764–9768 CrossRef CAS PubMed.
- B. Li, J. Jin, M. Yin, X. Zhang, M. S. Molokeev, Z. Xia and Y. Xu, Sequential and Reversible Phase Transformations in Zero-Dimensional Organic-Inorganic Hybrid Sb-based Halides towards Multiple Emissions, Angew. Chem., Int. Ed., 2022, 61, e202212741 CrossRef CAS PubMed.
- T. M. Kirse, I. Maisuls, L. Spierling, A. Hepp, J. Kösters and C. A. Strassert, One Dianionic Luminophore with Three Coordination Modes Binding Four Different Metals: Toward Unexpectedly Phosphorescent Transition Metal Complexes, Adv. Sci., 2024, 11, 2306801 CrossRef CAS.
- J. Chen, K. Zhou, J. Li, G. Xu, X. Hei and J. Li, Strongly photoluminescent and radioluminescent copper(i) iodide hybrid materials made of coordinated ionic chains, Chem. Sci., 2025, 16, 1106–1114 RSC.
- F. Witte, P. Rietsch, N. Nirmalananthan-Budau, F. Weigert, J. P. Götze, U. Resch-Genger, S. Eigler and B. Paulus, Aggregation-induced emission leading to two distinct emissive species in the solid-state structure of high-dipole organic chromophores, Phys. Chem. Chem. Phys., 2021, 23, 17521–17529 RSC.
- M. Zhao, M. Li, W. Li, S. Du, Z. Chen, M. Luo, Y. Qiu, X. Lu, S. Yang, Z. Wang, J. Zhang, S.-J. Su and Z. Ge, Highly Efficient Near-Infrared Thermally Activated Delayed Fluorescent Emitters in Non-Doped Electroluminescent Devices, Angew. Chem., Int. Ed., 2022, 61, e202210687 CrossRef CAS PubMed.
- C. Ou, L. An, Z. Zhao, F. Gao, L. Zheng, C. Xu, K. Zhang, J. Shao, L. Xie and X. Dong, Promoting near-infrared II fluorescence efficiency by blocking long-range energy migration, Aggregate, 2023, 4, e290 CrossRef CAS.
- X.-Y. Dai, M. Huo and Y. Liu, Phosphorescence resonance energy transfer from purely organic supramolecular assembly, Nat. Rev. Chem., 2023, 7, 854–874 CrossRef CAS.
- P. Srujana, P. Sudhakar and T. P. Radhakrishnan, Enhancement of fluorescence efficiency from molecules to materials and the critical role of molecular assembly, J. Mater. Chem. C, 2018, 6, 9314–9329 RSC.
- P. Srujana and T. P. Radhakrishnan, Establishing the Critical Role of Oriented Aggregation in Molecular Solid State Fluorescence Enhancement, Chem.–Eur. J., 2018, 24, 1784–1788 CrossRef CAS PubMed.
- A. Cravcenco, C. Ye, J. Gräfenstein and K. Börjesson, Interplay between Förster and Dexter Energy Transfer Rates in Isomeric Donor–Bridge–Acceptor Systems, J. Phys. Chem. A, 2020, 124, 7219–7227 CrossRef CAS PubMed.
- G. Li, D. Jiang, G. Shan, W. Song, J. Tong, D. Kang, B. Hou, Y. Mu, K. Shao, Y. Geng, X. Wang and Z. Su, Organic Supramolecular Zippers with Ultralong Organic Phosphorescence by a Dexter Energy Transfer Mechanism, Angew. Chem., Int. Ed., 2022, 61, e202113425 CrossRef PubMed.
- A. Bondi, van der Waals Volumes and Radii, J. Phys. Chem., 1964, 68, 441–451 CrossRef.
- W. Stadler, D. M. Hofmann, H. C. Alt, T. Muschik, B. K. Meyer, E. Weigel, G. Müller-Vogt, M. Salk, E. Rupp and K. W. Benz, Optical investigations of defects in Cd1-xZnxTe, Phys. Rev. B, 1995, 51, 10619–10630 CrossRef PubMed.
- J.-H. Wei, J.-F. Liao, L. Zhou, J.-B. Luo, X.-D. Wang and D.-B. Kuang, Indium-antimony-halide single crystals for high-efficiency white-light emission and anti-counterfeiting, Sci. Adv., 2021, 7, eabg3989 CrossRef PubMed.
- T. Xu, Y. Li, M. Nikl, R. Kucerkova, Z. Zhou, J. Chen, Y.-Y. Sun, G. Niu, J. Tang, Q. Wang, G. Ren and Y. Wu, Lead-Free Zero-Dimensional Organic-Copper(I) Halides as Stable and Sensitive X-ray Scintillators, ACS Appl. Mater. Interfaces, 2022, 14, 14157–14164 CrossRef.
- S. Fang, B. Zhou, H. Li, H. Hu, H. Zhong, H. Li and Y. Shi, Highly Reversible Moisture-Induced Bright Self-Trapped Exciton Emissions in a Copper-Based Organic–Inorganic Hybrid Metal Halide, Adv. Opt. Mater., 2022, 10, 2200605 CrossRef.
- R. Hamze, J. L. Peltier, D. Sylvinson, M. Jung, J. Cardenas, R. Haiges, M. Soleilhavoup, R. Jazzar, P. I. Djurovich, G. Bertrand and M. E. Thompson, Eliminating nonradiative decay in Cu(I) emitters: >99% quantum efficiency and microsecond lifetime, Science, 2019, 363, 601–606 CrossRef PubMed.
- X. Hei, S. J. Teat, M. Li, M. Bonite and J. Li, Highly soluble copper(i) iodide-based hybrid luminescent semiconductors containing molecular and one-dimensional coordinated anionic inorganic motifs, J. Mater. Chem. C, 2023, 11, 3086–3094 RSC.
- M.-Y. Leung, M.-C. Tang, W.-L. Cheung, S.-L. Lai, M. Ng, M.-Y. Chan and V. Wing-Wah Yam, Thermally Stimulated Delayed Phosphorescence (TSDP)-Based Gold(III) Complexes of Tridentate Pyrazine-Containing Pincer Ligand with Wide Emission Color Tunability and Their Application in Organic Light-Emitting Devices, J. Am. Chem. Soc., 2020, 142, 2448–2459 CrossRef.
- H. Wang, H. Ma, N. Gan, K. Qin, Z. Song, A. Lv, K. Wang, W. Ye, X. Yao, C. Zhou, X. Wang, Z. Zhou, S. Yang, L. Yang, C. Bo, H. Shi, F. Huo, G. Li, W. Huang and Z. An, Abnormal thermally-stimulated dynamic organic phosphorescence, Nat. Commun., 2024, 15, 2134 CrossRef.
- X. Hei, S. J. Teat, M. Li, M. Bonite and J. Li, Solution-Processable Copper Halide Based Hybrid Materials Consisting of Cationic Ligands with Different Coordination Modes, Inorg. Chem., 2023, 62, 3660–3668 CrossRef CAS PubMed.
- X. Hei, S. J. Teat, W. Liu and J. Li, Eco-friendly, solution-processable and efficient low-energy lighting phosphors: copper halide based hybrid semiconductors Cu4X6(L)2 (X = Br, I) composed of covalent, ionic and coordinate bonds, J. Mater. Chem. C, 2020, 8, 16790–16797 RSC.
- Y. Fang, W. Liu, S. J. Teat, G. Dey, Z. Shen, L. An, D. Yu, L. Wang, D. M. O'Carroll and J. Li, A Systematic Approach to Achieving High Performance Hybrid Lighting Phosphors with Excellent Thermal- and Photostability, Adv. Funct. Mater., 2017, 27, 1603444 CrossRef.
- W. Liu, Y. Fang, G. Z. Wei, S. J. Teat, K. Xiong, Z. Hu, W. P. Lustig and J. Li, A Family of Highly Efficient CuI-Based Lighting Phosphors Prepared by a Systematic, Bottom-up Synthetic Approach, J. Am. Chem. Soc., 2015, 137, 9400–9408 CrossRef CAS.
- A. V. Artem'ev, E. A. Pritchina, M. I. Rakhmanova, N. P. Gritsan, I. Y. Bagryanskaya, S. F. Malysheva and N. A. Belogorlova, Alkyl-dependent self-assembly of the first red-emitting zwitterionic {Cu4I6} clusters from [alkyl-P(2-Py)3]+ salts and CuI: when size matters, Dalton Trans., 2019, 48, 2328–2337 RSC.
| This journal is © The Royal Society of Chemistry 2025 |


![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C) around 1610 cm−1.
C) around 1610 cm−1.

