Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Photostable triphenylmethyl-based diradicals with a degenerate singlet-triplet ground state and strong photoluminescence†
Mona E.
Arnold‡
 a,
Anika
Lebzelter‡
a,
Anika
Lebzelter‡
 a,
Philipp
Thielert
a,
Philipp
Thielert
 b,
Rémi
Blinder
b,
Rémi
Blinder
 c,
Jonas
Schmid
a,
Julia
Zolg
c,
Jonas
Schmid
a,
Julia
Zolg
 ad,
Emanuele
Spatola
e,
Fedor
Jelezko
ad,
Emanuele
Spatola
e,
Fedor
Jelezko
 cd,
Max
von Delius
cd,
Max
von Delius
 de,
Sabine
Richert§
de,
Sabine
Richert§
 *b and
Alexander J. C.
Kuehne
*b and
Alexander J. C.
Kuehne
 *ad
*ad
aInstitute of Macromolecular and Organic Chemistry, Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany. E-mail: alexander.kuehne@uni-ulm.de
bInstitute of Physical Chemistry, University of Freiburg, Albertstraße 21, 79104 Freiburg, Germany. E-mail: sabine.richert@physchem.uni-freiburg.de
cInstitute for Quantum Optics, Ulm University, Albert Einstein Allee 11, 89081 Ulm, Germany
dCenter for Integrated Quantum Science and Technology (IQST), Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany
eInstitute of Organic Chemistry, Ulm University, Albert-Einstein-Allee 11, 89081 Ulm, Germany
First published on 7th July 2025
Abstract
We present a new class of luminescent diradicals based on tris(trichlorophenyl)methyl (TTM) cores symmetrically bridged by indolocarbazole donors. These diradicals exhibit pure diradical character y0 and unprecedented photoluminescence quantum yields ϕ of up to 18%, addressing key challenges in the development of stable, emissive organic diradicals. Light emitting diradicals represent a formidable challenge for synthetic chemists; for applications as molecular color centers in quantum sensing and as emitters in optoelectronics. Unlike conventional approaches that require the conversion of closed-shell precursors, we directly couple brominated TTM radicals via Buchwald–Hartwig coupling. The magnetic and optical properties of the resulting molecules are comprehensively characterized by electron paramagnetic resonance EPR, UV-vis absorption, and photoluminescence spectroscopy. This work unites the robust photophysics of discrete TTM radicals with the electronic versatility of donor-bridged multi-spin systems, offering a promising design strategy for functional open-shell emitters.
Introduction
Quantum sensing offers unparalleled sensitivity compared to conventional sensors, enabling more precise detection of motion, as well as electric- and magnetic fields.1–4 This breakthrough promises transformative improvements in how we measure, navigate, observe, and interact with the world around us. Among quantum sensors, nitrogen-vacancy (NV) color centers in diamond stand out due to their luminescent spin-triplet manifold, which allows spin state initialization and readout using light.5–7 However, challenges such as precise positioning, scalability, and purity of NV color centers hinder their widespread application. By contrast, molecular color centers have been realized in metal complexes offering precise structural reproducibility and scalability through synthetic chemistry.8 Also organic molecules, such as triarylmethyl (trityl) radicals may be employed as viable spin carrying alternatives as they exhibit an unpaired radical electron. Chlorinated trityl radicals are exceptionally stable, owing to electronic effects and steric protection of the radical electron in their p-orbital.9,10 Functionalization with electron-donating groups – for example carbazole (Cz) – can render these monoradicals (with spin S = 1/2) highly fluorescent, achieving photoluminescence quantum yields ϕ as high as ≈ 90% (see Fig. 1, TTM-Cz).11–14 However, organic color centers can only be realized in molecules with spin S > 1/2. Trityl radicals can be linked to form diradicals, wherein two unpaired electrons in degenerate orbitals couple through dipolar and exchange interactions.15–21 These diradicals are categorized as Kekulé or non-Kekulé hydrocarbons. Formally, Kekulé diradicals exhibit an equilibrium between a closed-shell quinoidal and an open-shell diradical structure (with y0 representing the diradical character) with a singlet ground state (GS) and overall S = 0 (see Fig. 1, TTM–TTM, PTM–PTM).15,20,21 By contrast, non-Kekulé diradicals, such as Schlenk–Brauns radicals, exhibit a triplet ground state (S = 1), due to meta-positioning of the methine groups with their unpaired electrons via a central phenyl ring (see Fig. 1, m-PTH).22,23 Diradicals with a triplet ground state mimic the electronic structure of NV centers, making them promising candidates for quantum sensing and related technologies.24 Although trityl-based diradicals with accessible triplet states can generate ground-state polarization after optical excitation – an essential step toward enabling optically detected magnetic sensing – these molecules currently lack sufficient photoluminescence.15,16,25,26 This limitation restricts their application to ensemble measurements, precluding single-molecule readout, which is crucial for achieving the highest sensitivity and resolution. Developing fluorescent diradicals could bridge this gap, offering the reproducibility and scalability of synthetic chemistry alongside the functionality of molecular color centers.Here, we report the synthesis and characterization of a new type of fluorescent diradical with high ϕ of 18%. The diradicals are derived from the tris(trichlorophenyl)methyl radical (TTM) motif but they differ from the typical Kekulé or Schlenk–Brauns geometry. Instead, we employ indolocarbazoles (ICz) as electron donating bridges, to which we attach two TTM radicals yielding stable diradicals with y0 ≈ 1. We study the influence of the relative orientation of the TTM radicals (with respect to the donor and each other) on the charge transfer (CT) character of the excited state (ES) and the ϕ. Moreover, the molecular arrangement of these diradicals influences the coupling between the radical electrons. The new donor-bridged diradicals introduced here represent a new approach and a successful realization of light emitting diradicals.
Results and discussion
Synthesis
We synthesize three different indolocarbazole-bridged TTM diradicals by making use of the Buchwald–Hartwig cross-coupling reaction (see Fig. 2). Because the individual donor strength has crucial impact on how easily the methine group of the trityl moiety can be deprotonated and oxidized, the conversion of the closed-shell precursor is often challenging in donor-functionalized trityl radicals.2,27,28 Therefore, we first convert the triarylmethane precursor to the radical and couple the radicals directly to the indolocarbazole donors. The radicals remain intact during the Pd-catalyzed Buchwald–Hartwig cross-coupling reaction (vide infra EPR discussion).29 To improve the selectivity, we employ a novel para-bromine-functionalized TTM (Br-TTM) derivative (see Fig. 2). The closed-shell Br-HTTM precursor is obtained after a three-step synthesis in an overall high yield.30,31 The Br-TTM radical is obtained by following the most widely established protocol for TTM radical conversion, via deprotonation using KOtBu, followed by mild oxidation using p-chloranil. The three diradicals are obtained after Buchwald–Hartwig coupling reactions of the Br-TTM to the different indolocarbazoles. While the yields are moderate, the diradicals are obtained pure and without the otherwise required deprotonation and oxidation steps in several repetitions.15,26,32 | ||
Fig. 2 (a) Synthesis of indolocarbazole-bridged diradicals. Bromine-functionalized TTM is attached to the different indolocarbazoles by Buchwald–Hartwig coupling. The radical character is retained during the cross-coupling reaction. (b) Solid state structure of 5,7-ICz-TTM2, as determined by single-crystal XRay crystallography (diffusion of nhexane into toluene, crystal system: triclinic, space group: P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) ) (c) DFT-calculated structures of 5,7-ICz-TTM2, 5,8-ICz-TTM2, and 5,11-ICz-TTM2 indicating the inter-spin distance and the relative orientation of the methine groups (level of theory: M06-L-GD3/def2-SVP with a CPCM solvation model to simulate a cyclohexane environment.33–36). ) (c) DFT-calculated structures of 5,7-ICz-TTM2, 5,8-ICz-TTM2, and 5,11-ICz-TTM2 indicating the inter-spin distance and the relative orientation of the methine groups (level of theory: M06-L-GD3/def2-SVP with a CPCM solvation model to simulate a cyclohexane environment.33–36). | ||
The diradicals are characterized by electron paramagnetic resonance (EPR) spectroscopy. Since the radical electrons typically deshield the nuclear spins in the molecules, we cannot perform NMR-spectroscopy on the open-shell molecules. That is why we also synthesize the closed-shell compounds, on which we perform 1H-NMR spectroscopy (see Experimental section and Fig. S39–S51 in the ESI†). The successful synthesis of the six molecules is also confirmed by mass spectrometry (see Fig. S33–S38 in the ESI†).
Molecular geometry and inter-spin distance
Diradical 5,7-ICz-TTM2 has been successfully crystallized by solvent diffusion of nhexane into a solution of 5,7-ICz-TTM2 in toluene. Single crystal XRay diffraction of 5,7-ICz-TTM2 allows its categorization into the centrosymmetric P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group (see Fig. 2). The central triphenylmethyl-carbon atom exhibits sp2 hybridization with a bond length of 1.43 to 1.44 Å to the three connected di- or trichlorophenyl rings, substantiating the pure diradical character (y0 ≈ 1) of 5,7-ICz-TTM2 (see Table 1). Moreover, from the crystal structure, an inter-spin distance of d = 7.99 Å is obtained. Interestingly, the two p-orbitals of the TTM radicals are oriented in an almost perpendicular fashion, which will minimize magnetic dipole coupling between the two radical electrons in the crystal.
space group (see Fig. 2). The central triphenylmethyl-carbon atom exhibits sp2 hybridization with a bond length of 1.43 to 1.44 Å to the three connected di- or trichlorophenyl rings, substantiating the pure diradical character (y0 ≈ 1) of 5,7-ICz-TTM2 (see Table 1). Moreover, from the crystal structure, an inter-spin distance of d = 7.99 Å is obtained. Interestingly, the two p-orbitals of the TTM radicals are oriented in an almost perpendicular fashion, which will minimize magnetic dipole coupling between the two radical electrons in the crystal.
| Compound | λ abs/nm | ε (GS-ES)/103 M−1 cm−1 | λ em/nm | ϕ/% | τ/ns | k r/106 s−1 | k nr/106 s−1 | y 0 (P, M)a | |ΔEST|/kJ mol−1 | dipax/MHz | d/Å (DFT) | d/Å (EPR) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 5,7-ICz-TTM2 | 641 | 6.00 | 680 | 18 | 15 | 12 | 55 | 0.98 | 0.052 | 83.4 | 8.02 | 8.5 |
| 5,8-ICz-TTM2 | 661 | 8.49 | 716 | 2 | 3 | 8 | 357 | 0.99 | — | 22.3 | 13.47 | 13.3 |
| 5,11-ICz-TTM2 | 674 | 4.13 | 720 | 2 | 3 | 8 | 357 | 0.97 | — | 17.5 | 15.59 | 14.4 |
Unfortunately, we were unable to crystallize the other two diradicals 5,8-ICz-TTM2 and 5,11-ICz-TTM2, which is why we perform DFT calculations for the three diradicals to investigate their structure and inter-spin distances. We optimize the molecules in their GS geometry at the M06-L-GD3/def2-SVP level of theory with a CPCM solvation model to simulate a cyclohexane environment.33–36 Single-point calculations are performed on the optimized structures employing PBE0-GD3/def2-TZVP.34–37 All three molecules exhibit a diradical character of y0 ≈ 1, indicating that the torsion around the TTM–N bond, effectively breaks conjugation and therefore making it irrelevant whether the nitrogens in the different ICz isomers are oriented towards each other in para- or meta-position (cf.Table 1 and Fig. 2). This is in stark contrast to Kekulé or Schlenk–Brauns diradicals, where the respective connectivity determines the singlet or triplet character of the GS. The calculated inter-spin distances increase from d = 8.02 Å for 5,7-ICz-TTM2 to 13.47 Å for 5,8-ICz-TTM2 and 15.59 Å for diradical 5,11-ICz-TTM2 (see Table 1). The calculated d of 5,7-ICz-TTM2 is in excellent agreement with the inter-spin distance observed experimentally in the crystal, whereas the perpendicular orientation of the radical p-orbitals is not reproduced in the DFT calculations, indicating that in solution the trityl units may have some degree of rotational freedom with respect to the ICz donor unit (see Fig. 2).
Within the crystal, the TTM propellers adapt a P,M-configuration with opposite helical chirality of the two TTM groups. We calculate the P,P- and P,M-configurations for 5,7-ICz-TTM2 as the diastereomers may have different physical properties. We do not calculate the molecule in the M,M- and M,P-configurations as they will behave analogously to their respective enantiomers. For small distances between the TTM moieties as in 5,7-ICz-TTM2, their relative orientation can induce strain and affect the total energy of the molecular system. Indeed, the P,M-configuration is stabilized by 7.6 kJ mol−1 with respect to the P,P-diastereomer. However, this small energy difference renders both isomers accessible in solution at room temperature.
Electron paramagnetic resonance spectroscopy
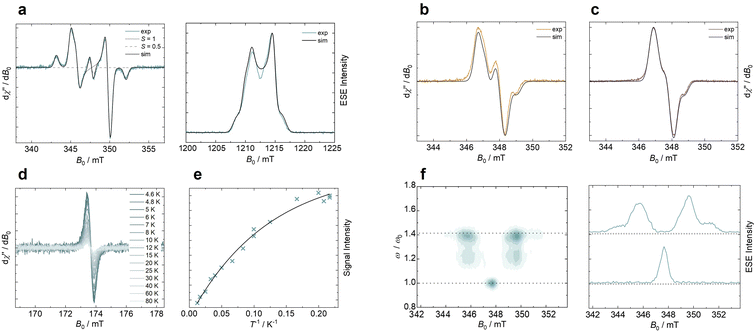
To characterize their magnetic properties, we perform electron paramagnetic resonance (EPR) spectroscopy of the three diradicals at the X- and Q-band in frozen toluene solution at T = 80 K (see Fig. 3a–c). Details on the setup and experimental parameters are given in the ESI.† A field-swept phase-inverted echo-amplitude detected nutation (PEANUT) experiment is carried out for 5,7-ICz-TTM2 (see Fig. 3f).38 The Fourier transform yields the nutation frequency of the spin species with the applied microwave pulse. A nutation frequency of is expected for a triplet spin species, with ω0 being a pure spin 1/2 (see Fig. 3f). The observed minor S = 1/2 contribution in 5,7-ICz-TTM2 at 347.7 mT is attributed to residual monoradical impurity (ca. 2.5% according to the simulated EPR spectrum), which we use as a reference (vide infra cw-EPR discussion). By this evaluation, the main signal of 5,7-ICz-TTM2 can be clearly attributed to a triplet spin species. The obtained spectra of 5,7-ICz-TTM2 are thus simulated as a spin triplet (S = 1), yielding an anisotropic g-tensor (g = 2.0026, 2.0042, 2.0037) and zero-field splitting parameters ǀDǀ = 125.2 MHz and ǀEǀ = 5.5 MHz.
is expected for a triplet spin species, with ω0 being a pure spin 1/2 (see Fig. 3f). The observed minor S = 1/2 contribution in 5,7-ICz-TTM2 at 347.7 mT is attributed to residual monoradical impurity (ca. 2.5% according to the simulated EPR spectrum), which we use as a reference (vide infra cw-EPR discussion). By this evaluation, the main signal of 5,7-ICz-TTM2 can be clearly attributed to a triplet spin species. The obtained spectra of 5,7-ICz-TTM2 are thus simulated as a spin triplet (S = 1), yielding an anisotropic g-tensor (g = 2.0026, 2.0042, 2.0037) and zero-field splitting parameters ǀDǀ = 125.2 MHz and ǀEǀ = 5.5 MHz.
To estimate the coupling strength for the three diradical compounds, the experimental spectra are simulated using a coupled spin system of two spin S = 1/2 (see ESI Fig. S29 and Table S3†). The axial dipolar coupling dipax increases from 5,11-ICz-TTM2 over 5,8-ICz-TTM2 to 5,7-ICz-TTM2, due to the decreased inter-spin distance (see Table 1). The dipolar coupling analysis can be used to obtain an experimental estimate of the inter-spin distance in the diradicals (see ESI† for details). These inter-spin distances closely match the distances between the radical centers determined using DFT, supporting the validity of the results and the suitability of our quantum chemical methods (see Table 1).
For 5,7-ICz-TTM2, we observe the formally forbidden half-field transition of the triplet spin state, confirming our assignment and the accessibility of the triplet state (see Fig. 3d). We further conduct variable-temperature (VT) EPR experiments between 4.6 and 80 K and plot the doubly-integrated signal intensities of the half-field transition against the temperature (see Fig. 3d and e). The Bleaney–Bowers fit reveals anti-ferromagnetic coupling indicating a slight stabilization of the singlet state versus the triplet state (see Section 6.3 in the ESI† for details).39–43 However, the energetic separation of singlet and triplet states |ΔEST| is as low as 0.052 kJ mol−1 (0.012 kcal mol−1), indicating near-degeneracy of the two states. Due to the increased distance, the inter-spin coupling is reduced for 5,8-ICz-TTM2 and 5,11-ICz-TTM2, and |ΔEST| is expected to be even smaller (see Table 1). For 5,8-ICz-TTM2, the intensity of the half-field transition is found to be reduced considerably as compared to 5,7-ICz-TTM2, while no half-field transition is observed for 5,11-ICz-TTM2, indicating that the two unpaired spins behave as isolated doublets rather than a coupled triplet state. These findings correlate well with the spin density maps, where we see that the unpaired electrons are mainly situated at the central methine carbon and slightly delocalized across the attached phenyl rings (see Fig. S27 in the ESI†).
Optical characterization
The new diradicals are also investigated using UV-vis absorption and photoluminescence spectroscopy. We employ cyclohexane as a non-polar solvent to prevent strong solvent–solute interactions. We observe absorption features in the UV region that can be related to the indolocarbazole donors in addition to an intense absorption peak in the UV and a rather weak absorption band in the visible region, as is typical for TTM-derived radicals (see Fig. 4).11,14,44,45TD-DFT calculations on the previously optimized structures employing PBE0-GD3/def2-TZVP with a SMD solvent model yield calculated absorption spectra that are in excellent agreement with the experiment (see Fig. S24–S26 and Table S3 in the ESI†).46 We observe similar excitation energies for both triplet and broken-symmetry singlet states (see Fig. S24 & S26 in the ESI†). The P,M-configuration of 5,7-ICz-TTM2 is closer to the experiment than the P,P-configuration, which is in line with the slightly smaller energy of the former, favoring the formation of the mixed chirality diastereomer. Presumably, the experimental spectrum results from a mixture of both diastereomers. When we dissolve the crystals of diastereomerically pure 5,7-ICz-TTM2 in cyclohexane, we observed the same optical behavior as prior to crystallization, indicating that the TTM propellers can invert quickly at room temperature in solution (see Fig. S11†).
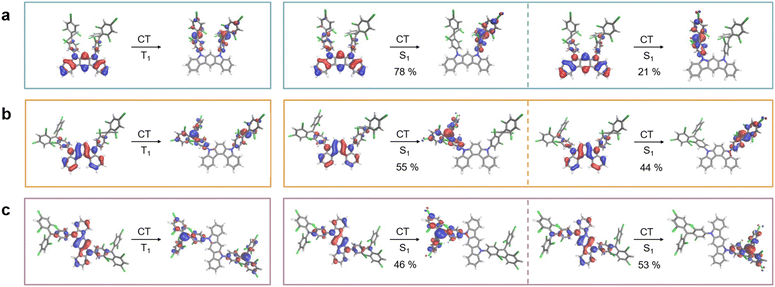
The natural transition orbitals (NTOs) confirm the nature of the electron donating ICz, yielding a clear CT excited state with the hole residing on the ICz moiety, whereas the electron is residing on the trityl units for both (singlet and triplet) spin states (see Fig. 5).
For the triplet state, electron density migrates from the ICz site to both singly occupied molecular orbitals (SOMOs) located on the TTM units (see Fig. 5). For the singlet state, the lowest ES is reflected by a linear combination of configurations resulting from charge migration from the highest doubly occupied molecular orbital (HDMO) to a single SOMO. Thus, the CT character of this mixed configurational exciton for the singlet is the same as observed for the triplet state (see Fig. 5). This behavior is similar for all three diradicals and in agreement with experimental and theoretical observations for the ES in related monoradicals.11,14,47 In other words, in the GS, the HDMO is located on the ICz, while the SOMOs reside on the TTM units. Thus, the excitation energy is determined by the electron donating capability of the ICz donor. We compare the energies of the highest occupied molecular orbital (HOMO) of the isolated ICz groups as a measure for their donor strength. When we calculate the isolated donors, we find a HOMO energy, which is 0.1 eV lower for 5,7-ICz than for 5,8-ICz and 5,11-ICz (see ESI†). Thus, their higher HOMO energy renders 5,8- and 5,11-ICz stronger electron donors than 5,7-ICz. As such, the ICz bridged TTM diradicals clearly behave similar to the TTM-Cz monoradicals, with their strong and solvent-dependent photoluminescence.11,48 Interestingly, the CT related bands in the visible spectrum appear at lower energy for 5,8-ICz-TTM2 and 5,11-ICz-TTM2, compared to 5,7-ICz-TTM2 (see Fig. 4 and Table 1). These characteristics correlate well with the above-described TD-DFT results and calculated HOMO energies and absorption spectra. The larger energy gap of diradical 5,7-ICz-TTM2 is also reflected in the photoluminescence spectra, where 5,7-ICz-TTM2 exhibits a maximum at 680 nm, whereas 5,8-ICz-TTM2 and 5,11-ICz-TTM2 show maxima in the NIR spectrum at 716 and 720 nm, respectively (see Fig. 4 and Table 1).
We determine ϕ for all three diradicals in cyclohexane solutions. Because of the high donor strength of 5,8-ICz and 5,11-ICz the quantum yield of the respective diradicals is ϕ = 2%, similar to monoradicals functionalized with donors of similar strength (see Table 1).14 By contrast, the emission of 5,7-ICz-TTM2 is considerably enhanced with a quantum yield of ϕ = 18%. We attribute this increased ϕ to the clear CT state upon excitation evoked by the 5,7-ICz of lower and therefore more appropriate donor strength than 5,8-ICz and 5,11-ICz in 5,8-ICz-TTM2 and 5,11-ICz-TTM2. When changing the solvent from cyclohexane to the slightly more polar toluene, all the three diradicals are rendered dark, further substantiating our conclusion that the ES exhibits a pronounced CT character.
Excited state dynamics and photostability
The excited state kinetics of the diradicals are investigated to rationalize their different emission quantum yields. We employ photoluminescence lifetime studies to determine the rate constants for radiative (kr) and non-radiative (knr) relaxation (see Table 1), using the following well-known relations:We find kr's of the same order of magnitude for all three compounds, while the radiative rate constant is slightly higher for 5,7-ICz-TTM2. By contrast, knr is almost one order of magnitude smaller for 5,7-ICz-TTM2 compared to 5,8-ICz-TTM2 and 5,11-ICz-TTM2. Thus, the suppression of non-radiative relaxation is identified as the main reason for the improved ϕ. For related monoradicals, we have demonstrated that low-lying CT excited states can be deactivated efficiently through conical intersections with the potential energy surface of the GS.13 Therefore, ϕ drops in a series of carbazole-functionalized radicals with increased CT character of the ES. Simultaneously, the energy of the ES and thus the emission correlates with ϕ. Low-energy emission, as observed for 5,8-ICz-TTM2 and 5,11-ICz-TTM2 is typically connected to poor emission efficiency.13 Thus, we conclude that the difference in the ES energy between 5,7-ICz-TTM2, as compared to 5,8-ICz-TTM2 and 5,11-ICz-TTM2, results in its superior value of ϕ.
To investigate the photostability of the diradicals, we irradiate cyclohexane solutions with UV-light (λ = 395 nm, see Section 4 in the ESI† for details) and use TTM and TTM-Cz as references. Since the luminescent monoradical is a possible degradation product of our diradicals, we refrain from determining the photostability by photoluminescence and we use UV-vis spectroscopy instead (see Section 4 in the ESI† for details). Both reference compounds degrade completely within 3 seconds and 1 minute, respectively. By contrast, the diradicals have superior photostability. For 5,7-ICz-TTM2 we extract a half-life t1/2 = 19 min (5,8-ICz-TTM2: t1/2 = 34 min and 5,11-ICz-TTM2: t1/2 > 60 min), which indicates that the photostability is increased by more than two (to three) orders of magnitude compared to TTM-Cz and TTM monoradicals, respectively (see Section 4.4 of the ESI†). This highly improved photostability is likely related to the pronounced CT character of the ES. Superior stability has previously been reported for TTM-Cz derived radicals with red-shifted absorption and emission.44
Conclusions
We have introduced a novel class of TTM-based diradicals employing indolocarbazole as a linker. In the series of investigated compounds, the inter-spin distance is systematically increased from 8 to 16 Å by choosing three different constitutional isomers bridging the trityl moieties. We observe a weak dipolar interaction between the unpaired electron spins leading to a degenerate singlet-triplet GS. For 5,7-ICz-TTM2 with its short radical–radical distance and clear CT excited state, the diradical exhibits an emission peaking at 680 nm with ϕ = 18%, which is one of the highest reported values for TTM-derived diradicals (see Table S2 in ESI†). When the TTM groups are attached to 5,8-ICz and 5,11-ICz of higher donor strength, the emission is shifted to the near-infrared region. We find the 5,7-ICz derivative to be at least 2–3 orders of magnitude more photostable, than related monoradicals. These findings on emission wavelength, quantum yield, and photostability are essential for the design of future fluorescent diradicals. While the spin–spin interaction in the investigated diradicals is too weak to lift the degeneracy of the GS, the N-donor-bridged diradicals serve as model systems to understand the emission properties of future related compounds. A triplet ground state might be achieved by further decreasing the inter-spin distance while retaining the optical properties, whereas higher ϕ may be achieved when using bridges with slightly decreased donor strength. In the future, such structures could be developed to serve as molecular qubits with optical read-out of the spin-state.Data availability
Crystallographic data for compound 1 has been deposited at the CCDC under 2410919 and can be obtained from https://www.ccdc.cam.ac.uk/data_request/cif. DFT data for this article, including TD-DFT results are available at Zenodo at https://doi.org/10.5281/zenodo.15488688.Author contributions
A. L. carried out the synthesis and characterization of the compounds. A. J. C. K. and S. R. conceptualized the project. M. E. A. supervised the project. R. B., F. J., S. R., and P. T. conducted EPR experiments and analysis. J. Z. and J. S. performed (TD)-DFT calculations. E. S. and M. v. D. crystallized the compounds and conducted XRay crystallography. F. J., M. v. D., S. R., and A. J. C. K. provided supervision, infrastructure, and secured funding. M. E. A., A. L., and A. J. C. K. wrote the first draft and all authors revised and edited the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank Dr Ashley Redman (University of Freiburg) for acquiring the EPR PEANUT data. J. Z. acknowledges IQST for a PhD program within the IQST Graduate School @QuantumBW supported by the Baden-Württemberg Ministry of Science, Research, and Arts. F. J. acknowledges ERC grant HyperQ (no. 856432), the EU (projects FLORIN, QuMicro, CQuENS and QCIRCLE). The authors acknowledge support by the state of Baden-Württemberg through bwHPC and the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) – Project numbers INST 40/575-1 FUGG (JUSTUS 2 cluster), 500226157, 445471845, 445471097, 445470598, and 417643975.Notes and references
- M. H. Abobeih, J. Randall, C. E. Bradley, H. P. Bartling, M. A. Bakker, M. J. Degen, M. Markham, D. J. Twitchen and T. H. Taminiau, Atomic-scale imaging of a 27-nuclear-spin cluster using a quantum sensor, Nature, 2019, 576, 411–415 CrossRef CAS.
- R. Matsuoka, S. Kimura, T. Miura, T. Ikoma and T. Kusamoto, Single-molecule Magnetoluminescence from a Spatially Confined Persistent Diradical Emitter, J. Am. Chem. Soc., 2023, 145, 13615–13622 CrossRef CAS.
- C. J. Yu, S. Von Kugelgen, D. W. Laorenza and D. E. Freedman, A Molecular Approach to Quantum Sensing, ACS Cent. Sci., 2021, 7, 712–723 CrossRef CAS PubMed.
- C. L. Degen, F. Reinhard and P. Cappellaro, Quantum sensing, Rev. Mod. Phys., 2017, 89, 035002 CrossRef.
- J. M. Taylor, P. Cappellaro, L. Childress, L. Jiang, D. Budker, P. R. Hemmer, A. Yacoby, R. Walsworth and M. D. Lukin, High-sensitivity diamond magnetometer with nanoscale resolution, Nat. Phys., 2008, 4, 810–816 Search PubMed.
- I. Lovchinsky, A. O. Sushkov, E. Urbach, N. P. de Leon, S. Choi, K. De Greve, R. Evans, R. Gertner, E. Bersin, C. Müller, L. McGuinness, F. Jelezko, R. L. Walsworth, H. Park and M. D. Lukin, Nuclear magnetic resonance detection and spectroscopy of single proteins using quantum logic, Science, 2016, 351, 836–841 CrossRef CAS.
- M. Pfender, P. Wang, H. Sumiya, S. Onoda, W. Yang, D. B. R. Dasari, P. Neumann, X. Y. Pan, J. Isoya, R. B. Liu and J. Wrachtrup, High-resolution spectroscopy of single nuclear spins via sequential weak measurements, Nat. Commun., 2019, 10, 594 CrossRef CAS PubMed.
- D. W. Laorenza, A. Kairalapova, S. L. Bayliss, T. Goldzak, S. M. Greene, L. R. Weiss, P. Deb, P. J. Mintun, K. A. Collins, D. D. Awschalom, T. C. Berkelbach and D. E. Freedman, Tunable Cr4+Molecular Color Centers, J. Am. Chem. Soc., 2021, 143, 21350–21363 CrossRef CAS PubMed.
- O. Armet, J. Veciana, C. Rovira, J. Riera, J. Castañer, E. Molins, J. Rius, C. Miravitlles, S. Olivella and J. Brichfeus, Inert carbon free radicals. 8. Polychlorotriphenylmethyl radicals. Synthesis, structure, and spin-density distribution, J. Phys. Chem., 1987, 91, 5608–5616 CrossRef CAS.
- M. Ballester, Inert Free Radicals (IFR): A Unique Trivalent Carbon Species, Acc. Chem. Res., 1985, 18, 380–387 CrossRef CAS.
- V. Gamero, D. Velasco, S. Latorre, F. López-Calahorra, E. Brillas and L. Juliá, [4-(N-Carbazolyl)-2,6-dichlorophenyl]bis(2,4,6-trichlorophenyl)methyl radical an efficient red light-emitting paramagnetic molecule, Tetrahedron Lett., 2006, 47, 2305–2309 CrossRef CAS.
- A. Abdurahman, T. J. H. Hele, Q. Gu, J. Zhang, Q. Peng, M. Zhang, R. H. Friend, F. Li and E. W. Evans, Understanding the luminescent nature of organic radicals for efficient doublet emitters and pure-red light-emitting diodes, Nat. Mater., 2020, 19, 1224–1229 CrossRef CAS PubMed.
- C. Lu, E. Cho, Z. Cui, Y. Gao, W. Cao, J. L. Brédas, V. Coropceanu and F. Li, Towards Efficient and Stable Donor-Acceptor Luminescent Radicals, Adv. Mater., 2023, 35, 2208190 CrossRef CAS.
- M. E. Arnold, L. Roß, P. Thielert, F. Bartley, J. Zolg, F. Bartsch, L. A. Kibler, S. Richert, C. Bannwarth and A. J. C. Kuehne, On the Effect of Donor Strength on the Photoluminescence Performance in Mono-Substituted N-Donor Triarylmethyl Radicals, Adv. Opt. Mater., 2024, 12, 2400697 CrossRef CAS.
- X. Chang, M. E. Arnold, R. Blinder, J. Zolg, J. Wischnat, J. van Slageren, F. Jelezko, A. J. C. Kuehne and M. von Delius, A Stable Chichibabin Diradicaloid with Near-Infrared Emission, Angew. Chem., Int. Ed., 2024, 63, e202404853 CrossRef CAS PubMed.
- A. Abdurahman, J. Wang, Y. Zhao, P. Li, L. Shen and Q. Peng, A Highly Stable Organic Luminescent Diradical, Angew. Chem., Int. Ed., 2023, 135, e202300772 CrossRef.
- D. Mesto, M. Orza, B. Bardi, A. Punzi, I. Ratera, J. Veciana, G. Farinola, A. Painelli, F. Terenziani, D. Blasi and F. Negri, Luminescent Trityl-based Diradicaloids: A Theoretical and Experimental Assessment of Charge-Resonance in Low-Lying Excited States, Chem.–Eur. J., 2025, 31, e202500749 CrossRef CAS PubMed.
- A. Punzi, Y. Dai, C. N. Dibenedetto, E. Mesto, E. Schingaro, T. Ullrich, M. Striccoli, D. M. Guldi, F. Negri, G. M. Farinola and D. Blasi, Dark State of the Thiele Hydrocarbon: Efficient Solvatochromic Emission from a Nonpolar Centrosymmetric Singlet Diradicaloid, J. Am. Chem. Soc., 2023, 145, 20229–20241 CrossRef CAS.
- D. Schäfter, J. Wischnat, L. Tesi, J. A. De Sousa, E. Little, J. McGuire, M. Mas-Torrent, C. Rovira, J. Veciana, F. Tuna, N. Crivillers and J. van Slageren, Molecular One- and Two-Qubit Systems with Very Long Coherence Times, Adv. Mater., 2023, 35, 2302114 CrossRef.
- M. Ballester, J. Castañer, J. Riera and A. Ibáñez, Two new inert carbon biradicals of the triphenylmethyl series. Synthesis, conformation and exchange, Tetrahedron Lett., 1980, 21, 2435–2458 CrossRef CAS.
- M. Ballester, I. Pascual, J. Riera and J. Castañer, ‘Spin-Charge Exchange’ in Allodial Radical Ions, a Novel Intramolecular Single Electron Transfer Equilibrium, J. Org. Chem., 1991, 56, 217–225 CrossRef CAS.
- J. Veciana, C. Rovira, N. Ventosa, M. Isabel Crespo, F. Palacio and J. Veciana, et al., Stable polyradicals with high-spin ground states. 2. Synthesis and characterization of a complete series of polyradicals derived from 2, 4, 6-trichloro-. alpha.,. alpha.,. alpha.’’,. alpha.’,. alpha.’’,. alpha.’’-hexakis (pentachlorophenyl) mesitylene with S= 1/2, 1, and 3/2 ground states, J. Am. Chem. Soc., 1993, 115, 57–64 CrossRef CAS.
- J. Veciana, C. Rovira, O. Armet, V. M. Domingo, M. I. Crespo and F. Palacio, Stable Triplets and Quartets from Carbon Centered Polyradicals, Mol. Cryst. Liq. Cryst. Incorporating Nonlinear Opt., 1989, 176, 77–84 CrossRef CAS.
- Y. R. Poh, D. Morozov, N. P. Kazmierczak, R. G. Hadt, G. Groenhof and J. Yuen-Zhou, Alternant Hydrocarbon Diradicals as Optically Addressable Molecular Qubits, J. Am. Chem. Soc., 2024, 146, 15549–15561 CrossRef CAS PubMed.
- C. P. Yu, R. Chowdhury, Y. Fu, P. Ghosh, W. Zeng, T. B. E Mustafa, J. Grüne, L. E. Walker, D. G. Congrave, X. Wei Chua, P. Murto, A. Rao, H. Sirringhaus, F. Plasser, C. P. Grey, R. H. Friend and H. Bronstein, Near-infrared luminescent open-shell π-conjugated systems with a bright lowest-energy zwitterionic singlet excited state, Sci. Adv., 2024, 10, 3476 CrossRef.
- S. M. Kopp, S. Nakamura, B. T. Phelan, Y. R. Poh, S. B. Tyndall, P. J. Brown, Y. Huang, J. Yuen-Zhou, M. D. Krzyaniak and M. R. Wasielewski, Luminescent Organic Triplet Diradicals as Optically Addressable Molecular Qubits, J. Am. Chem. Soc., 2024, 146, 27935–27945 CrossRef CAS PubMed.
- A. Mizuno, R. Matsuoka, S. Kimura, K. Ochiai and T. Kusamoto, Spin-Correlated Luminescence of a Carbazole-Containing Diradical Emitter: Single-Molecule Magnetoluminescence and Thermally Activated Emission, J. Am. Chem. Soc., 2024, 146, 18470–18483 CrossRef CAS PubMed.
- R. Xiaotian, W. Ota, T. Sato, M. Furukori, Y. Nakayama, T. Hosokai, E. Hisamura, K. Nakamura, K. Matsuda, K. Nakao, A. P. Monkman and K. Albrecht, Carbazole-Dendronized Luminescent Radicals, Angew. Chem., Int. Ed., 2023, 62, e202302550 CrossRef CAS.
- M. Gross, F. Zhang, M. E. Arnold, P. Ravat and A. J. C. Kuehne, Aza[7]helicene Functionalized Triphenylmethyl Radicals with Circularly Polarized Doublet Emission, Adv. Opt. Mater., 2024, 12, 2301707 CrossRef CAS.
- M. Ballester, J. Riera, J. Castañer, C. Rovira and O. Armet, An Easy, High-yield Synthesis of Highly Chlorinated Mono-, Di- and Triarylmethanes, Synthesis, 1986, 1986, 64–66 CrossRef.
- M. E. Arnold and A. J. C. Kuehne, (2,6-Dichloro-4-iodophenyl)bis(2,4,6-trichlorophenyl)methane as a precursor in efficient cross-coupling reactions for donor and acceptor functionalized triphenylmethyl radicals, Dyes Pigm., 2023, 208, 110863 CrossRef.
- V. C. Wakchaure, X. Chang, J. Zolg, R. Blinder, M. Arnold, F. Jelezko, A. Kuehne and M. von Delius, Carbazole-Functionalized Chichibabin Diradicaloids with Redshifted Absorption and Enhanced Photoluminescence, Chem. Commun., 2025, 61, 8855–8858 RSC.
- Y. Zhao and D. G. Truhlar, The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements: Two new functionals and systematic testing of four M06-class functionals and 12 other functionals, Theor. Chem. Acc., 2008, 120, 215–241 Search PubMed.
- F. Weigend and R. Ahlrichs, Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy, Phys. Chem. Chem. Phys., 2005, 7, 3297–3305 RSC.
- F. Weigend, Accurate Coulomb-fitting basis sets for H to Rn, Phys. Chem. Chem. Phys., 2006, 8, 1057–1065 RSC.
- S. Grimme, J. Antony, S. Ehrlich and H. Krieg, A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu, J. Chem. Phys., 2010, 132, 154104 CrossRef.
- M. Ernzerhof and G. E. Scuseria, Assessment of the Perdew-Burke-Ernzerhof exchange-correlation functional, J. Chem. Phys., 1999, 110, 5029–5036 CrossRef CAS.
- S. Stoll, G. Jeschke, M. Willer and A. Schweiger, Nutation-Frequency Correlated EPR Spectroscopy: The PEANUT Experiment, J. Magn. Reson., 1998, 130(1), 86–96 CrossRef CAS.
- J. A. Weil and J. R. Bolton, Electron paramagnetic resonance: elementary theory and practical applications, John Wiley & Sons, 2007 Search PubMed.
- B. Figgis and R. Martin, Magnetic studies with copper(II) salts. Part I. Anomalous paramagnetism and δ-bonding in anhydrous and hydrated copper(II) acetates, J. Chem. Soc., 1956, 746, 3837–3846 RSC.
- B. Bleaney and K. D. Bowers, The spherical shell nuclear model Anomalous paramagnetism of copper acetate, Proc. R. Soc. London, Ser. A, 1952, 214, 451–465 CAS.
- S. K. Hoffmann, W. Hilczer and J. Goslar, Applied Magnetic Resonance Weak Long-Distance Superexchange Interaction and Its Temperature Variations in Copper(II) Compounds Studied by Single Crystal EPR, Appl. Magn. Reson., 1994, 7, 289–321 CrossRef CAS.
- P. F. Richardson and R. W. Kreilick, Copper Complexes with Free-Radical Ligands, J. Am. Chem. Soc., 1977, 99, 8183–8187 CrossRef CAS.
- K. Matsuda, R. Xiaotian, K. Nakamura, M. Furukori, T. Hosokai, K. Anraku, K. Nakao and K. Albrecht, Photostability of luminescent tris(2,4,6-trichlorophenyl)methyl radical enhanced by terminal modification of carbazole donor, Chem. Commun., 2022, 58, 13443–13446 RSC.
- K. Nakamura, K. Matsuda, R. Xiaotian, M. Furukori, S. Miyata, T. Hosokai, K. Anraku, K. Nakao and K. Albrecht, Effects of halogen atom substitution on luminescent radicals: a case study on tris(2,4,6-trichlorophenyl)methyl radical-carbazole dyads, Faraday Discuss., 2023, 250, 192–201 RSC.
- A. V. Marenich, C. J. Cramer and D. G. Truhlar, Universal solvation model based on solute electron density and on a continuum model of the solvent defined by the bulk dielectric constant and atomic surface tensions, J. Phys. Chem. B, 2009, 113, 6378–6396 CrossRef CAS.
- L. Fajarí, R. Papoular, M. Reig, E. Brillas, J. L. Jorda, O. Vallcorba, J. Rius, D. Velasco and L. Juliá, Charge transfer states in stable neutral and oxidized radical adducts from carbazole derivatives, J. Org. Chem., 2014, 79, 1771–1777 CrossRef.
- M. López, D. Velasco, F. López-Calahorra and L. Juliá, Light-emitting persistent radicals for efficient sensor devices of solvent polarity, Tetrahedron Lett., 2008, 49, 5196–5199 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2410919. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc03673a |
| ‡ These authors contributed equally to this work. |
| § Current address: Institute of Physical and Theoretical Chemistry, Goethe University Frankfurt, Max-von-Laue-Straße 7, 60438 Frankfurt, Germany. |
| This journal is © The Royal Society of Chemistry 2025 |