Open Access Article
Open Access ArticleCrown ether–cycloparaphenylene hybrid multimacrocycles: insights into supramolecular gas sensing and biological potential†
Yaning
Hu
a,
Tong
Li
bc,
Taotao
Su
a,
Wudi
Shi
a,
Yabing
Yu
a,
Beibei
Li
a,
Meng-Hua
Li
 a,
Sheng
Zhang
a,
Sheng
Zhang
 a,
Yuan-Qing
Xu
a,
Qi
Liu
a,
Yuan-Qing
Xu
a,
Qi
Liu
 *a,
Di
Wu
*a,
Di
Wu
 *b and
Youzhi
Xu
*b and
Youzhi
Xu
 *a
*a
aCollege of Chemistry and Molecular Sciences, Henan University, Kaifeng 475004, P. R. China. E-mail: qiliu@henu.edu.cn; youzhixu@henu.edu.cn
bCollege of Public Health, Zhengzhou University, Zhengzhou 450001, P. R. China. E-mail: wudi22@zzu.edu.cn
cCollege of Chemistry, Zhengzhou University, Zhengzhou 450001, P. R. China
First published on 18th June 2025
Abstract
The topologically intriguing multimacrocyclic architecture is endowed with distinct physical and chemical properties. The synthesis of hybrid macrocycles combining crown ethers and cycloparaphenylenes (CPPs) presents a promising strategy for developing multifunctional supramolecular systems. Herein, we first report the precise construction of a series of crown ether–CPP hybrid multimacrocycles with enhanced photophysical properties and diverse host–guest interactions. Notably, the trimacrocyclic hybrid adopts a molecular tweezer-like conformation, resulting in a significantly higher fullerene binding affinity compared to the bismacrocycle. The fullerene complex showed improved conductivity and sensitivity with a limit of detection (LOD) of 19 ppb for NO2 with excellent cyclic stability and reliability. Additionally, the bismacrocycle exhibits significant cytotoxicity against cancer cell lines at low concentrations and enables fluorescence-based detection of inflammatory responses, highlighting its potential for biosensing applications. These findings underscore the versatility of crown ether–CPP hybrid macrocycles in supramolecular sensing and biochemistry, offering new avenues for the design of functional nanomaterials.
Introduction
In the realm of supramolecular chemistry, the synthesis and exploration of innovative macrocycles play a pivotal role in advancing materials suitable for applications in drug delivery, catalysis, sensing, and various other fields.1 Cycloparaphenylenes (CPPs) represent a unique class of π-conjugated macrocycles, distinguished by their strained structure and size-dependent chemical2 and photophysical properties.3 These intriguing molecules have attracted considerable attention from researchers in the fields of chemistry and materials science.4 Recent studies have highlighted the fascinating properties of topologically unique multi-macrocycles, which exhibit behavior distinct from that of monocyclic structures (Fig. 1a).5 For instance, Du et al. reported CPP-based bismacrocycles that exhibit dual-emission behavior, characterized by tunable aggregation-induced emission and enhanced chiroptical properties.6 Similarly, Yam and Jiang have independently synthesized rigid and heterotopic bismacrocycles combining CPP and pillar[5]arene, showcasing remarkable luminescent and host–guest recognition properties (Fig. 1b).7 | ||
| Fig. 1 (a) Illustration of two types of multimacrocyclic topology. (b) Selected examples of CPP-hybrid multimacrocycles. | ||
Crown ethers are an intriguing class of macrocycles with flexible skeleton, renowned for their ability to selectively bind metal cations through coordination interactions and adeptly fabricate mechanically interlocked molecules (MIMs) via hydrogen bonding.8 This synergy of properties offers exciting opportunities for innovative molecular designs. For example, we recently have reported the synthesis of a rare hetero[3]catenane using a ring-in-ring assembly strategy, incorporating unaltered [12]CPP, 24-crown-8 and a dibenzylammonium macrocycle (Fig. 1b).9 To further explore the potential of the hydrids of CPPs and crown ethers. Herein, we present the synthesis of hybrid bis- and trimacrocycles combining CPPs and crown ethers. We then investigated their supramolecular properties and the application in organic electronic devices. Additionally, we conducted cell imaging and in vitro antitumor activity studies to evaluate their potential for biological applications.
Results and discussion
Synthesis and characterization
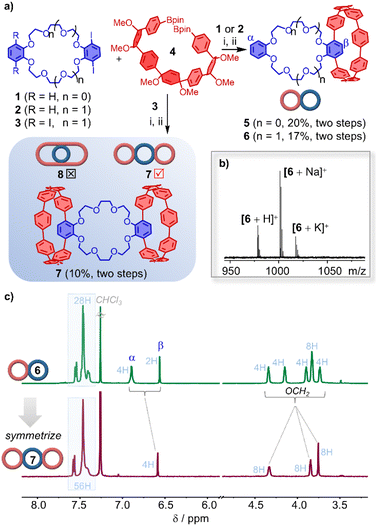
The pivotal intermediates for the synthesis of bis- and trimacrocycles are outlined in Fig. 2a. The hoop-shaped multimacrocycles 5–7, varying in diameter, were successfully synthesized via Suzuki–Miyaura cross-coupling between iodinated dibenzo-crown ethers 1–2 and diboronate 4, followed by reductive aromatization. The [8]CPP-based bismacrocycles 5 and 6, incorporating 18-crown-6 and 24-crown-8, were obtained in two steps with yields of 20% and 17%, respectively. Notably, the reaction between iodinated dibenzo-crown ethers 3 and diboronate 4 is theoretically expected to yield both the [8]CPP-based trimacrocycle 7 and the [16]CPP-based alternative topological multimacrocycle 8. However, after separation and purification, combined with NMR and emission spectroscopy analysis, only the 24-crown-8-bridged trimacrocycle 7 was successfully synthesized, with a two-step yield of 10%. This outcome is presumably due to the mismatch between the torsional angle of substrate 4 and the size of 3, which hinders the formation of the alternative multimacrocycle.10These multimacrocycles were fully characterized by NMR spectroscopy and high-resolution mass spectrometry (HRMS) after standard workup and purification. The crown ether units within the macrocyclic backbone facilitated clear detection of sodium and potassium ion association in the mass spectra (Fig. 2b). Compared to bismacrocycle 6, trimacrocycle 7 features two identical [8]CPP units flanking the dibenzo-24-crown-8 core, enhancing molecular symmetry, as confirmed by its 1H NMR spectrum (Fig. 2c, and S1–S4†).
As shown in Fig. 3 (left), the structure of 6 can be recognized as an integration of the two classic macrocycles, dibenzo-24-crown-8 and [8]CPP. The two independent cavities do not overlap with each other, the distance between the two phenyl rings of dibenzo-24-crown-8 and the diameter of [8]CPP are calculated to be 10.5 Å and 10.4 Å, respectively. Then, we dissolved equimolar amounts of compounds 6 and KPF6 in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 CHCl3–MeOH solution and obtained single crystals of the metal complex 6⊃K+. Analyzing the X-ray crystal structure of complex 6⊃K+, we can see that the 24-crown-8 ring tightly encapsulates the potassium ion by folding, primarily via multiple electrostatic interactions, the average distance of O⋯K is 2.5 Å (Fig. 3, right). Due to the introduction of the 24-crown-8 ring, [8]CPP no longer presents ordered columnar packing,11 but a random packing with no apparent supramolecular interactions between the complex molecules was found in the solid state (Fig. S5 and S6†). This also leads to 6 having better solubility in organic solvents.
1 CHCl3–MeOH solution and obtained single crystals of the metal complex 6⊃K+. Analyzing the X-ray crystal structure of complex 6⊃K+, we can see that the 24-crown-8 ring tightly encapsulates the potassium ion by folding, primarily via multiple electrostatic interactions, the average distance of O⋯K is 2.5 Å (Fig. 3, right). Due to the introduction of the 24-crown-8 ring, [8]CPP no longer presents ordered columnar packing,11 but a random packing with no apparent supramolecular interactions between the complex molecules was found in the solid state (Fig. S5 and S6†). This also leads to 6 having better solubility in organic solvents.
Photophysical properties
The absorption spectra of multimacrocycles 5, 6 and 7 closely resemble that of [8]CPP, exhibiting a primary absorption maximum around 335 nm and a shoulder near 400 nm, attributed to the HOMO → LUMO transition. The molar extinction coefficients (ε) at 335 nm for 5 (7.8 × 104 M−1 cm−1) and 6 (8.5 × 104 M−1 cm−1) are comparable to that of [8]CPP, whereas 7 (1.3 × 105 M−1 cm−1) exhibits approximately 1.5 times higher absorption. This increase suggests that the two CPP units in 7 behave as independent chromophores, consistent with Beer's Law, although possible excitonic coupling effects cannot be ruled out.In dichloromethane at 298 K, 5, 6 and 7 display fluorescence emission bands centered at 530 nm, 527 nm and 530 nm, respectively, upon excitation at 340 nm (Fig. 4a and b). Compared to [8]CPP, these multimacrocycles exhibit a slight blue shift, which may arise from subtle structural distortions induced by the crown ether ring. Such distortions could slightly reduce effective π-conjugation, thereby increasing the energy gap between the excited and ground states.
More notably, their fluorescence quantum yields (ΦF = 0.55 for 5, 0.58 for 6, 0.60 for 7) are significantly higher than that of [8]CPP (ΦF = 0.10),3 indicating a strong enhancement in radiative emission efficiency. The fluorescence enhancement can be attributed to multiple factors. First, orbital symmetry breaking in the CPP backbone may increase the transition dipole moment, thereby enhancing radiative decay rates (Fig. 4c).12 Second, the introduction of crown ether moieties may impose structural rigidity, reducing non-radiative relaxation pathways such as intramolecular rotations or vibrations. Time-resolved fluorescence measurements reveal that the fluorescence lifetimes of 5 (4.2 ns), 6 (2.8 ns) and 7 (4.3 ns) are comparable to that of [8]CPP, suggesting that the increased fluorescence quantum yield is primarily due to suppression of non-radiative decay rather than an intrinsic increase in excited-state lifetime. In the solid state, 5, 6 and 7 maintain ΦF values of 16%, 14% and 18%, respectively, despite the potential for aggregation-caused quenching (ACQ). This suggests that the presence of crown ether substituents helps mitigate intermolecular π–π stacking, likely by introducing steric hindrance and restricting molecular aggregation.
Guest recognition and gas sensing
Analysis of the X-ray crystal structure of 6 revealed that the flexible crown ether backbone adopts a folded conformation. This structural feature led us to hypothesize that trimacrocycle 7 might serve as an effective host for fullerene recognition (Fig. S7 and Table S3†).13 While [10]CPP is well known for encapsulating C60via strong π–π interactions, the smaller size of [8]CPP generally results in weaker binding. Given the structural modifications introduced in 7, we sought to evaluate its binding properties toward C60 through fluorescence quenching titrations in toluene.The binding study revealed that 6⊃C60 forms with an association constant (KA = (1.5 ± 0.05) ×105 M−1, Fig. S10†), which is significantly lower than [10]CPP⊃C60 (3.6 × 10−6 M−1)14 but higher than unmodified [8]CPP, likely due to additional dispersion interactions introduced by the crown ether side chains. In contrast, titration experiments between 7 and C60 exhibited a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (host
1 (host![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) guest) binding equilibrium, with the a much higher association constant (K1:1 = (2.4 ± 0.5)×106 M−1, Fig. 5a) compared to 6⊃C60. This suggests that 7 adopts a molecular tweezer-like conformation,15 where its two CPP units cooperatively encapsulate the fullerene, significantly enhancing its binding affinity. DFT-optimized structures confirm that C60 sits symmetrically within the cavity of 7, stabilized primarily by dispersion forces between the π-conjugated CPP units and the fullerene surface (Fig. S11 and Table S4†). Independent Gradient Model (IGM) analysis further highlights strong non-covalent interactions in the 7⊃C60 complex, reinforcing the enhanced binding affinity observed experimentally (Fig. 5b).
guest) binding equilibrium, with the a much higher association constant (K1:1 = (2.4 ± 0.5)×106 M−1, Fig. 5a) compared to 6⊃C60. This suggests that 7 adopts a molecular tweezer-like conformation,15 where its two CPP units cooperatively encapsulate the fullerene, significantly enhancing its binding affinity. DFT-optimized structures confirm that C60 sits symmetrically within the cavity of 7, stabilized primarily by dispersion forces between the π-conjugated CPP units and the fullerene surface (Fig. S11 and Table S4†). Independent Gradient Model (IGM) analysis further highlights strong non-covalent interactions in the 7⊃C60 complex, reinforcing the enhanced binding affinity observed experimentally (Fig. 5b).
The interactions and photophysical properties of CPP and C60 have been extensively studied. However, their potential applications in semiconductor devices remain underexplored. Therefore, we further investigated the use of CPP and its fullerene complexes in organic electronic devices. Thin film electrodes were fabricated on interdigital electrodes (IDEs) using the quasi-Langmuir–Shäfer (QLS) technique, which were then employed as chemiresistive gas sensors (CGS) for detecting hazardous gases (Fig. S12†).16 The conductivity characteristics of 7 and the 7⊃C60 complex were initially assessed through I–V curves (Fig. S13†). Due to the incorporation of an electron transport mediator and the push–pull electronic effects between C60 and 7, the 7⊃C60 complex demonstrated significantly enhanced conductivity (6.37 × 10−7 S cm−1) compared to 7 alone, making it more suitable for CGS applications.17
We applied the 7⊃C60-based chemiresistive sensor for detecting a variety of potential interfering gases. As shown in Fig. 5c, the 7⊃C60 complex exhibited a markedly higher response to NO2 than to other test gases, highlighting its exceptional specificity toward NO2. Subsequently, we examined the sensitivity of both 7⊃C60 and 7 to NO2 in the concentration range of 0.1–1.5 ppm. As illustrated in Fig. 5d and S14,† the 7⊃C60 complex displayed superior response and recovery characteristics to NO2 in this concentration range, attributable to its enhanced conductivity and the presence of the donor–acceptor (D–A) structure. Furthermore, as depicted in Fig. 5e, the 7⊃C60 complex exhibited a strong linear relationship with NO2 concentrations from 0.1 to 1.5 ppm, with a sensitivity of 18.29% ppm−1. Based on this, the limit of detection (LOD) for NO2 was calculated to be 19 ppb, indicating the sensor's potential for practical applications. Additionally, the cyclic stability of the 7⊃C60 complex at 1 ppm NO2 was evaluated, with results showing a coefficient of variation not exceeding 1.2%, demonstrating the excellent cyclic stability of the 7⊃C60-based sensor (Fig. S15†).
To further understand the significantly enhanced gas-sensing response of the 7⊃C60 complex compared to 7, we first tested the transient photocurrent of both devices in air (Fig. 6a). The results revealed that the 7⊃C60 complex exhibited a higher current intensity under simulated sunlight compared to 7, indicating that photo-generated charge carriers in the 7⊃C60 complex are more easily separated and directed for transport. This provides further evidence of a distinct D–A effect in the 7⊃C60 complex.18 As shown in Fig. 6b, during the NO2 detection process, 7 effectively captures and concentrates NO2 molecules. Due to the D–A structure, the charge transfer barrier between the 7 and NO2 is disrupted by C60. As an electron transport mediator, C60 facilitates efficient electron transfer to NO2, leading to a significant decrease in the electron density on the 7⊃C60 complex. This, in turn, increases the resistance and enhances the gas-sensing response. Upon removal of NO2 from the 7⊃C60 complex, the electrons released by NO2 return to the complex, restoring the sensor's resistance to its baseline state. Moreover, the D–A structure ensures the directional transport of electrons, thereby enabling stable responses to NO2 detection.
 | ||
| Fig. 6 (a) Photocurrent of the 7⊃C60 complex and 7 at 5 V. (b) The proposed sensing mechanism of 7⊃C60 for NO2. | ||
Biological application evaluation
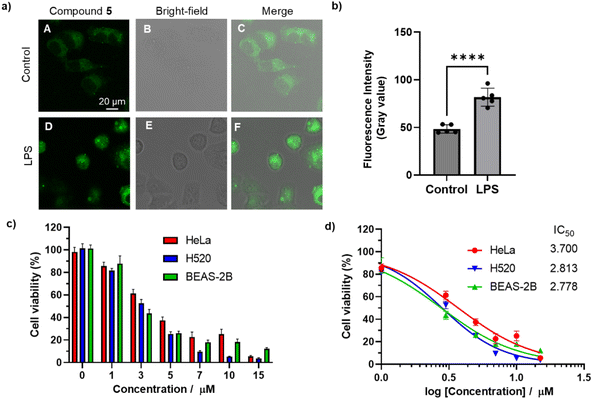
Incorporating polar side-chain functionalization into the CPP ring may enhance the macrocycles' utility in biosensing and bioimaging applications.19 Since compounds 5 and 6 exhibited similar properties, but 5 was synthesized more efficiently, and compound 7 showed poor solubility in polar solvents, bismacrocycle 5 was selected for cell imaging and in vitro antitumor activity studies to evaluate its biological potential. Fluorescence imaging with the HeLa cervical cancer cell line was conducted to assess the bismacrocycle's cellular uptake. After 90 minutes of incubation, weak green fluorescence was observed within the cells, indicating successful penetration of the cell membrane (Fig. 7a(A–C)). Lipopolysaccharide (LPS), a well-known inflammatory stimulus, induces reactive oxygen species (ROS) production in cells, increasing cytoplasmic viscosity and promoting inflammation.20 When cells were pretreated with LPS (1 μg mL−1) for 30 minutes before exposure to the bismacrocycle 5, a marked increase in fluorescence intensity was observed (Fig. 7a(D and E)). This enhancement was significantly greater than that in the control group (Fig. 7b), suggesting the bismacrocycle's potential for identifying and distinguishing inflammatory cells. The observed increase in fluorescence after LPS pretreatment may be attributed to changes in cellular viscosity, which could facilitate the radiative transition of the compound in inflammatory cells.21 This finding is important, as it highlights the bismacrocycle's ability to detect and monitor inflammatory responses within cells.Next, the cytotoxicity of the compound was evaluated in HeLa, H520 (lung cancer), and BEAS-2B (normal lung epithelial) cells using the MTT assay. As shown in Fig. 7c, the bismacrocycle 5 exhibited significant cytotoxicity across all cell lines at a minimal concentration of 3 μM after 24 hours of incubation. The half-maximal inhibitory concentration (IC50) values for HeLa, H520, and BEAS-2B cells were determined to be 3.700, 2.813, and 2.778 μM, respectively (Fig. 7d). These relatively low IC50 values underscore the bismacrocycle's potent antitumor activity, which has not been previously reported in the literature.
The bismacrocycle's potent cytotoxicity at low concentrations, with IC50 values notably lower than those of comparable nanohoops, suggests a superior therapeutic index and emphasizes its potential as an effective anticancer agent. This is particularly significant given the growing demand for drugs with high potency. Collectively, these findings lay a strong foundation for advancing the compound as a novel imaging agent and anticancer therapeutic, with promising applications in the imaging and treatment of various cancer types. These results also demonstrate that compound 5 possesses remarkable bioactivity compared to other crown ether derivatives, which is attributed to the incorporation of cycloparaphenylene.22
Conclusion
In this study, we have successfully designed and synthesized a series of crown ether–CPP hybrid multimacrocycles and explored their supramolecular properties, photophysical behavior, and biological applications. The integration of crown ethers into the CPP framework not only improves solubility and enhances fluorescence quantum yield but also introduces new binding sites that facilitate selective host–guest interactions. Notably, the trimacrocyclic hybrid adopts a molecular tweezer-like conformation, leading to a ca. 15-fold increase in fullerene binding affinity compared to its bismacrocyclic counterpart. This enhanced host–guest recognition behavior, supported by fluorescence quenching titrations and density functional theory (DFT) calculations, provides new insights into the design of high-affinity supramolecular receptors. Additionally, the fullerene complex was explored as a chemiresistive gas sensor for NO2 detection. It showed improved conductivity and sensitivity with a LOD of 19 ppb, thanks to its D–A structure and fullerene's role as an electron transport mediator. The sensor also demonstrated excellent cyclic stability and reliable, directional electron transport for NO2 detection.The biological evaluation of these hybrid macrocycles further underscores their potential for biomedical applications. The bismacrocycle exhibits significant cytotoxicity against cancer cell lines at micromolar concentrations, with IC50 values lower than those of comparable nanohoops, highlighting its promise as a novel anticancer agent. Moreover, its fluorescence-based detection of inflammatory responses suggests potential applications in biosensing and imaging. These findings collectively demonstrate that crown ether–CPP hybrids represent a new class of multifunctional macrocycles with broad applicability in supramolecular sensing23 and biochemistry.24
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Y. Xu proposed the concept. Y. Hu, T. Su and W. Shi carried out the synthesis of the compounds, conducted the characterizations, and analyzed the data. B. Li and M.-H. Li contributed to the analysis of single-crystal structures. Q. Liu and Y. Yu prepared the electronic devices and performed the sensing experiments. T. Li and D. Wu carried out cell imaging and in vitro antitumor activity studies. Y. Xu, Q. Liu and D. Wu wrote the manuscript. Y.-Q. Xu and S. Zhang contributed to the discussions and revisions of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Y. Xu acknowledges funding from the National Natural Science Foundation of China (22471060 and 22201064) and The Joint Fund of Henan Provincial Science and Technology Research and Development Plan (235200810074). D. Wu acknowledges the Key Scientific Research Project of Colleges and Universities in Henan Province (24A330005).References
- (a) T. Ogoshi, T. A. Yamagishi and Y. Nakamoto, Chem. Rev., 2016, 116, 7937–8002 CrossRef CAS PubMed; (b) X. Chang, Y. Xu and M. von Delius, Chem. Soc. Rev., 2024, 53, 47–83 RSC; (c) Y.-J. Long, X.-N. Han, Y. Han and C.-F. Chen, Chin. Chem. Lett., 2025, 36, 110600 CrossRef CAS; (d) Y. Yu, Y. Hu, C. Ning, W. Shi, A. Yang, Y. Zhao, Z.-Y. Cao, Y. Xu and P. Du, Angew. Chem., Int. Ed., 2024, 63, e202407034 CrossRef CAS.
- (a) T. D. Clayton, J. M. Fehr, T. W. Price, L. N. Zakharov and R. Jasti, J. Am. Chem. Soc., 2024, 146, 30607–30614 CrossRef CAS PubMed; (b) Y. Xu, M. -Yi Leung, L. Yan, Z. Chen, P. Li, Y.-H. Cheng, M. H.-Y. Chan and V. W.-W. Yam, J. Am. Chem. Soc., 2024, 146, 13226–13235 CrossRef CAS PubMed; (c) W. Shi, Y. Zhao and Y. Xu, Trends Chem., 2025, 7, 1–2 CrossRef CAS.
- E. R. Darzi and R. Jasti, Chem. Soc. Rev., 2015, 44, 6401–6410 RSC.
- (a) Y. Li, H. Kono, T. Maekawa, Y. Segawa, A. Yagi and K. Itami, Acc. Mater. Res., 2021, 2, 681–691 CrossRef CAS; (b) J. Malinčík, S. Gaikwad, J. P. Mora-Fuentes, M.-A. Boillat, A. Prescimone, D. Häussinger, A. G. Campaña and T. Šolomek, Angew. Chem., Int. Ed., 2022, 61, e202208591 CrossRef; (c) M. Hermann, D. Wassy and B. Esser, Angew. Chem., Int. Ed., 2021, 60, 15743–15766 CrossRef CAS; (d) A. Stergiou, J. Rio, J. H. Griwatz, D. Arcon, H. A. Wegner, C. Ewels and N. Tagmatarchis, Angew. Chem., Int. Ed., 2019, 58, 17745–17750 CrossRef CAS.
- (a) X.-L. Chen, S.-Q. Yu, X.-H. Huang and H.-Y. Gong, Molecules, 2023, 28, 6043 CrossRef CAS PubMed; (b) Y. Zhang, D. Yang, S. H. Pun, H. Chen and Q. Miao, Precis. Chem., 2023, 1, 107–111 CrossRef CAS; (c) J. He, M.-H. Yu, Z. Lian, Y.-Q. Fan, S.-Z. Guo, X.-N. Li, Y. Wang, W.-G. Wang, Z.-Y. Cheng and H. Jiang, Chem. Sci., 2023, 14, 4426–4433 RSC; (d) X. Zhang, Y. Xu and P. Du, Acc. Mater. Res., 2025, 6, 399–410 CrossRef CAS; (e) G. Li, L.-L. Mao, J.-N. Gao, X. Shi, Z.-Y. Huo, J. Yang, W. Zhou, H. Li, H.-B. Yang, C.-H. Tung, L.-Z. Wu and H. Cong, Angew. Chem., Int. Ed., 2025, 64, e202419435 CrossRef CAS; (f) Y. Yang, O. Blacque, S. Sato and M. Juríček, Angew. Chem., Int. Ed., 2021, 60, 13529–13535 CrossRef CAS PubMed; (g) L. Ren, Y. Han, X. Hou, Y. Zou, T. Jiao and J. Wu, CCS Chem., 2024, 6, 2758–2769 CrossRef CAS; (h) X.-W. Chen, Q.-S. Deng, B.-H. Zheng, J.-F. Xing, H.-R. Pan, X.-J. Zhao and Y.-Z. Tan, J. Am. Chem. Soc., 2024, 146, 31665–31670 CrossRef CAS; (i) K. Li, S. Yoshida, R. Yakushiji, X. Liu, C. Ge, Z. Xu, Y. Ni, X. Ma, J. Wu, S. Sato and Z. Sun, Chem. Sci., 2024, 15, 18832–18839 RSC; (j) Y. Liu, P. Lei, Y. Feng, S. Fu, X. Liu, S. Zhang, B. Tu, C. Chen, Y. Li, L. Wang and Q.-D. Zeng, Chin. Chem. Lett., 2024, 35, 109571 CrossRef CAS; (k) K. Senthilkumar, M. Kondratowicz, T. Lis, P. J. Chmielewski, J. Cybinska, J. L. Zafra, J. Casado, T. Vives, J. Crassous, L. Favereau and M. Stępień, J. Am. Chem. Soc., 2019, 141, 7421–7427 CrossRef CAS PubMed.
- X. Zhang, H. Liu, G. Zhuang, S. Yang and P. Du, Nat. Commun., 2022, 13, 3543 CrossRef CAS.
- (a) Y. Xu, F. Steudel, M. Leung, B. Xia, M. von Delius and V. W-W Yam, Angew. Chem., Int. Ed., 2023, 62, e202302978 CrossRef CAS; (b) Y. Fan, S. Fan, L. Liu, S. Guo, J. He, X. Li, Z. Lian, W. Guo, X. Chen, Y. Wang and H. Jiang, Chem. Sci., 2023, 14, 11121–11130 RSC; (c) Y. Zhou, G. Zhuang and P. Du, Chin. Chem. Lett., 2024, 35, 108593 CrossRef CAS.
- (a) J. E. M. Lewis, P. D. Beer, S. J. Loeb and S. M. Goldup, Chem. Soc. Rev., 2017, 46, 2577–2591 RSC; (b) L. Chen, X. Sheng, G. Li and F. Huang, Chem. Soc. Rev., 2022, 51, 7046–7065 RSC; (c) N. H. Evans and P. D. Beer, Chem. Soc. Rev., 2014, 43, 4658–4683 RSC; (d) A. Goswami, S. Saha, P. K. Biswas and M. Schmittel, Chem. Rev., 2019, 120, 125–199 CrossRef.
- W. Shi, Y. Hu, L. Leanza, Y. Shchukin, P. A. Hoffmann, M. Li, C. Ning, Z.-Y. Cao, Y.-Q. Xu, P. Du, M. von Delius, G. M. Pavan and Y. Xu, Angew. Chem., Int. Ed., 2025, 64, e202421459 CrossRef CAS.
- H. Zhong, K. Lan, J. Ming, D. Zhang and C. Cheng, Org. Lett., 2025, 27, 4349–4354 CrossRef CAS.
- J. Xia, J. W. Bacon and R. Jasti, Chem. Sci., 2012, 3, 3018–3021 RSC.
- T. C. Lovell, C. E. Colwell, L. N. Zakharov and R. Jasti, Chem. Sci., 2019, 10, 3786–3790 RSC.
- (a) Y. Xu and M. von Delius, Angew. Chem., Int. Ed., 2020, 59, 559–573 CrossRef CAS; (b) C. García-Simón, M. Costas and X. Ribas, Chem. Soc. Rev., 2016, 45, 40–62 RSC.
- T. Iwamoto, Y. Watanabe, T. Sadahiro, T. Haino and S. Yamago, Angew. Chem., Int. Ed., 2011, 50, 8342–8344 CrossRef CAS PubMed.
- (a) A. Sacristan-Martin, H. Barbero, S. Ferrero, D. Miguel, R. Garcia-Rodriguez and C. M. Alvarez, Chem. Commun., 2021, 57, 11013–11016 RSC; (b) B. Scholz, A. S. Oshchepkov, O. Papaianina, C. Ruppenstein, V. A. Akhmetov, D. I. Sharapa, K. Y. Amsharov and M. E. Pérez-Ojeda, Chem.–Eur. J., 2023, 29, e202302778 CrossRef CAS; (c) M. S. Mirzaei, S. Mirzaei, V. M. E. Castro, C. Lawrence and R. H. Sánchez, Chem. Commun., 2024, 60, 14236–14239 RSC.
- Y. Zhang, Q. Liu, Q. Sun, H. Li, J. Shen, H. Liu, W. Chen, Y. Zhang and Y. Chen, ACS Sens., 2023, 8, 4353–4363 CrossRef CAS.
- Q. Liu, Q. Li, Y. Li, T. Su, B. Hou, Y. Zhao and Y. Xu, Angew. Chem., Int. Ed., 2025, 64, e202502536 CrossRef CAS.
- (a) Q. Liu, Q. Sun, H. Li, Y. Zhang, L. Feng, W. Chen and Y. Chen, Sens. Actuators, B, 2025, 428, 137250 CrossRef CAS; (b) K. Ding, K. Zhang, X. Liu, Q. Xu, X. Cheng, M. Yao, W. Wang, Y. Zeng, T. Ye, Y. He, W. Lin, R. Zhou, Ya. Chen and S. Li, ACS Nano, 2025, 19, 8238–8254 CrossRef CAS PubMed.
- (a) K. Günther, H. Kono, H. Shudo, D. Shimizu, R. Isoda, M. Nakamura, A. Yagi, K. Amaike and K. Itami, Angew. Chem., Int. Ed., 2025, 64, e202414645 CrossRef; (b) B. M. White, Y. Zhao, T. E. Kawashima, B. P. Branchaud, M. D. Pluth and R. Jasti, ACS Cent. Sci., 2018, 4, 1173–1178 CrossRef CAS PubMed.
- (a) Y. Zhang, Z. Li, W. Hu and Z. Liu, Anal. Chem., 2019, 91, 10302–10309 CrossRef CAS PubMed; (b) Y.-Y. Li, J.-L. Hu, J.-R. Wu, Y.-R. Wang, A.-H. Zhang, Y.-W. Tan, Y.-J. Shang, T. Liang, M. Li, Y.-L. Meng and Y.-F. Kang, Biosens. Bioelectron., 2024, 254, 116233 CrossRef CAS PubMed.
- (a) S. Feng, S. Gong, Z. Zheng and G. Feng, Sens. Actuators, B, 2022, 351, 130940 CrossRef CAS; (b) W.-N. Wu, Y.-F. Song, X.-L. Zhao, Y. Wang, Y.-C. Fan, Z.-H. Xu and T. D. James, Chem. Eng. J., 2023, 464, 142553 CrossRef CAS.
- H. Zhang, R. Ye, Y. Mu, T. Li and H. Zeng, Nano Lett., 2021, 21, 1384–1391 CrossRef CAS PubMed.
- (a) Q. Chen and K. Zhu, Chem. Soc. Rev., 2024, 53, 5677–5703 RSC; (b) Z. Zhang, J. Zhao and X. Yan, Acc. Chem. Res., 2024, 57, 992–1006 CrossRef CAS PubMed; (c) C. Wang, L. Xu, Z. Jia and T.-P. Loh, Chin. Chem. Lett., 2024, 35, 109075 CrossRef CAS; (d) M. Regehly, Y. Garmshausen, M. Reuter, N. F. König, E. Israel, D. P. Kelly, C.-Y. Chou, K. Koch, B. Asfari and S. Hecht, Nature, 2020, 588, 620–624 CrossRef CAS; (e) Z.-B. Tang, L. Bian, X. Miao, H. Gao, L. Liu, Q. Jiang, D. Shen, L. Xu, A. C.-H. Sue, X. Zheng and Z. Liu, Nat. Synth., 2025 DOI:10.1038/s44160-025-00791-x.
- K. Chinner, N. Grabicki, R. Hamaguchi, M. Ikeguchi, K. Kinbara, S. Toyoda, K. Sato and O. Dumele, Chem. Sci., 2024, 15, 16367–16376 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2393489 and 2393490. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc03476k |
| This journal is © The Royal Society of Chemistry 2025 |