Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Three-state molecular switch based on terarylene photo- or redox-induced reversible isomerisation†
Nicolò
Baggi‡
 *a,
Anne
Léaustic
*a,
Anne
Léaustic
 a,
Jean-Noël
Rebilly
a,
Jean-Noël
Rebilly
 a,
François
Mavré
a,
François
Mavré
 b,
Eric
Rivière
b,
Eric
Rivière
 a,
Christian
Herrero
a,
Christian
Herrero
 a,
François
Maurel
a,
François
Maurel
 b and
Pei
Yu
b and
Pei
Yu
 *a
*a
aUniversité Paris-Saclay, CNRS, Institut de Chimie Moléculaire et des Matériaux d’Orsay, 19 Avenue des Sciences, Orsay, 91400, France. E-mail: nicolo.baggi@upc.edu; pei.yu@universite-paris-saclay.fr
bUniversité de Paris Cité, ITODYS, CNRS, Paris, F-75013, France
First published on 10th June 2025
Abstract
Multi-addressable molecular photoswitches whose isomerisation can be triggered not only by light, but also by other stimuli are appealing for the development of novel smart materials as well as for broadening the areas for their potential application. Diarylethenes (DAEs) are among the most studied switches for this purpose, since tailored functionalisation can make them responsive not only to UV or visible light, but also to other inputs, such as an electrochemical one. In this work, we synthesised five terarylene-based switches and investigated their photochemical and redox properties. Unlike their DAEs analogues, whose cyclisation upon an oxidation-reduction sequence is well-established, our systems undergo a similar oxidative ring-closing of the neutral open form to a doubly charged closed form while the subsequent reduction leads to ring-opening to the neutral open form. Moreover, the neutral closed form can also be re-opened by a catalytic amount of oxidant. With the support of theoretical modelling and cyclic voltammetry simulations, a general mechanism is proposed to rationalise this original bidirectional dual-responsive behaviour.
Introduction
Molecular photochromic systems capable of responding to multiple stimuli to interconvert between at least two states are appealing for the development of smart materials.1–9 Among different photoswitches investigated for this purpose, diarylethenes (DAEs) are popular thanks to their excellent modulable properties in both isomeric states: the generally colourless open form (OF) and the more conjugated, coloured closed form (CF).10–12 Moreover, they can be designed to respond to other external inputs besides light, thus leading to a multi-responsive behaviour (Fig. 1).13 For example, several groups have focused on the acid-induced isomerisation of diarylethenes.14–18Another appealing stimulus is the electrochemical one and redox-responsive dithienylethenes (DTEs) undergoing oxidative19–23 or reductive24–27 ring-closing or oxidative ring-opening reactions have been reported.28,29 However, this input is generally not viable to achieve bidirectional isomerisation in solution, except for a few cases.30–32 Focusing on the oxidative cyclisation, thiophenes are generally needed to achieve such redox-active behaviour and their replacement with other electron-poor heterocycles such as thiazoles hinders it.
Only one example in the literature has demonstrated its occurrence in thiazole-containing systems, thanks to the presence of very strong electron-donating substituents.33
The investigation towards dual photo- and redox-induced isomerisation has been also performed on terarylenes,34 a sub-class of diarylethenes with good photochromic properties (e.g. photon-quantitative cyclisation quantum yields),35,36 in which a third (hetero)aryl moiety is used in place of the standard central ethene bridging unit (e.g. cyclopentene, perfluorocyclopentene, etc.). Oxidative cycloreversion of terphenylthiazoles has been extensively investigated by Kawai's group, showing that the OF could be regained much more effectively through the electrochemical route than through the photochemical one, thanks to an oxidative cascade propagation started by the ring-opening of a closed form radical species.37,38 Reductive cyclisation of terarylenes bearing N-methylpyridinium groups has also been reported.39,40 Moreover, bidirectional electrochemical isomerisation has been achieved with terarylenes designed to combine reductive ring-closing and oxidative ring-opening reactions.39 Nonetheless, no oxidative cyclisation has been described for this sub-class of derivatives, to our knowledge.
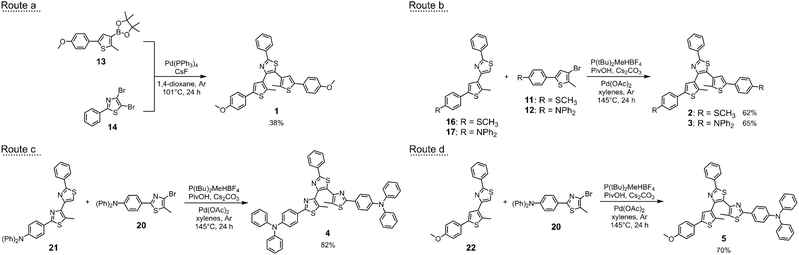
Inspired by the DTEs characterised by this behaviour described in the literature, we designed terarylenes 1, 2 and 3 depicted in Fig. 2 to develop dual photo- and redox-responsive terarylenes. They bear a central phenylthiazole bridge and two electron-rich thienyl-based arms. Additionally, we prepared terphenylthiazole 4 and the mixed system 5 to investigate if any oxidative cyclisation could be observed in absence or partial replacement of the thiophene rings. Different electron-donating groups were selected and their impact on the electrochemical properties of the switches was evaluated through cyclic voltammetry (CV) and spectroelectrochemistry. While the cyclic voltammograms of the open form isomers of 1, 2 and 3 suggest that an oxidation–reduction sequence affords the neutral closed forms as in the dithienylethene analogues, a different outcome is observed during the bulk electrolysis, with the recovery of the starting neutral open form, thus implying the occurrence of a reversible redox-induced isomerisation between this species and the doubly charged closed form. Moreover, a catalytic and oxidative ring-opening of their neutral closed forms is also achieved. Such unprecedented behaviour is analysed and the rationale behind explained with the support of stopped-flow and EPR measurements, theoretical modelling, and CV simulations. Finally, the observed redox responses of terphenylthiazole 4 and the mixed system 5 clearly indicate that the presence of at least one thiophene-based arm is required to observe the targeted oxidative cyclisation by cyclic voltammetry in these systems.
Results and discussion
Synthetic procedures
Switches 1, 2 and 3 were obtained by synthesising the thienyl-based arms upon reacting 3,5-dibromo-2-methyl-thiophene (6, Scheme S1†) with the needed boronic acids via Suzuki–Miyaura coupling reactions. These intermediates were then used in two different routes (Scheme 1, routes a and b) to afford the target photochromes. Route a involves the synthesis of the boronic acid pinacol ester (13) which is then reacted with 2-phenyl-4,5-di-bromo-thiazole (14) in a double cross-coupling reaction. On the other hand, route b envisages the C–H arylation of intermediates 16 and 17 with the brominated intermediates 11 and 12. Overall, even if both routes are effective for the synthesis of these derivatives, route b is recommendable since it allows to obtain the photochromes in higher yields and it offers higher flexibility as the intermediates 16 and 17 can be potentially used in the synthesis of dissymmetric derivatives, bearing differently functionalised arms.In the case of photoswitches 4 and 5 (Scheme 1, routes c and d), 2-(4-(diphenylamino)phenyl)-4-bromo-5-methyl-thiazole (20) was used in the direct arylation of intermediates 21 and 22 to afford the desired terphenylthiazole 4 and the dissymmetric terarylene 5. Full details of the synthetic procedures (Scheme S1 and S2) are provided in the ESI,† with the characterisations of all new products.
Photochromic properties
The photochromic behaviour of 1 was investigated in acetonitrile at room temperature using steady-state absorption spectroscopy, while 2, 3 and 5 were studied in acetonitrile + 2% v/v dichloromethane to guarantee complete solubility. Dichloromethane was used for terphenylthiazole 4 for solubility reasons.The solutions were not degassed before irradiation. The spectral evolution of terarylene 1 in pure acetonitrile under UV irradiation at 320 nm is provided in Fig. 3, where the open form (1o) spectrum is indicated with a black solid line and the achieved photostationary state (α1c = 94%) with a blue solid line. A red dashed line is used to show the spectrum recorded after having kept the irradiated solution in the dark at room temperature for 85 minutes and suggests that closed isomer 1c is thermally stable under those conditions.
The open form of terarylene 1 is characterised by an intense absorption band in the UV region, with λmax = 300 nm. Under irradiation at 320 nm, a rapid conversion to the closed form is observed, as confirmed by the growth of a broad absorption band peaking at 636 nm and a sharp band at 400 nm, that is accompanied by a shoulder. This reaction is reversible and 1o can be restored upon light irradiation at 600 nm (Fig. S1†).
The spectra for the other photochromes 2–5 are provided in Fig. S2–S5† and show that the OFs absorb in the UV region too, with λmax between 300 and 375 nm, and their respective CFs are characterised by broad absorption bands peaking between 600 and 700 nm after UV light irradiation. As in the case of 1c, terarylenes 2c–5c are thermally stable and can be irradiated in the visible to induce the cycloreversion to the corresponding open form isomers.
Moreover, for switches 4 and 5 bearing a 2-(4-(diphenylamino)phenyl)-4-bromo-5-methyl-thiazole arm (20) which is strongly fluorescent (emission spectrum in Fig. S6†) with a quantum yield of 67% in dichloromethane (λmax = 462 nm), an emission band at 445 nm was observed for both compounds (emission spectra in Fig. S7 and S8†). The fluorescence quantum yields in CH2Cl2 for 4 and 5 are 7% and 11%, respectively.
Electrochemical properties
The electrochemical properties of the five photochromes were investigated by cyclic voltammetry (CV) at room temperature in acetonitrile or dichloromethane/tetrabutylammonium hexafluorophosphate (TBAPF6) 0.1 M with a scan rate (ν) of 100 mV s−1. Dichloromethane was used for 2–5 since no total dissolution could be achieved in acetonitrile.For all the five switches, an irreversible two-electron oxidation wave was detected for the open forms (1o–5o), as it can be observed in Fig. 4. This oxidation occurred at lower potential for 3o when compared to 1o and 2o, as expected from the stronger electron-donating diphenylamino group (Fig. 4c).
Focusing on the cyclic voltammogram of 1, it can be observed that the neutral open form isomer (1o) is irreversibly oxidised at 1.12 V vs. SCE. On the back scan, two one-electron cathodic waves appear at E1/2 = 0.28 V and 0.42 V vs. SCE, where E1/2 is the average potential between anodic and cathodic peak potentials. By comparing these features with those observed in the CV recorded for the photogenerated 1c after irradiation of 1o at 365 nm (Fig. 4a, grey dashed line), it can be concluded that such waves correspond to the two consecutive single-electron reductions of the doubly oxidised closed form (1c2+), produced by the oxidation of 1o during the forward scan, leading eventually to the neutral closed form species (1c). This behaviour suggests that redox-active 1 undergoes a relatively fast chemical ring-closing reaction following the electrochemical process, as previously proposed by Feringa and co-workers for similarly functionalised dithienylethenes.21
Whether the ring closure occurs only at the radical state (through an ECE mechanism) or at the dicationic state (through an EEC mechanism) is discussed later.
A similar electrochemical behaviour was observed for 2 and 3 (Fig. 4b and c). However, the redox waves of the closed form isomers appeared to be more separated in the case of 2, while almost completely merged for 3. The larger the separation, the more stable the radical species (or the less thermodynamically favourable its dismutation) is. This might be in part explained by the use of dichloromethane as solvent.21
A different behaviour was observed for 4o (Fig. 4d). After a two-electron oxidation at around 1 V vs. SCE, the back scan shows an irreversible reduction wave at 0.40 V vs. SCE which cannot be unequivocally ascribed to the reduction of the dicationic closed form 4c2+ since the photogenerated 4c also displays a two-electron redox process at the same potential, but in a reversible manner. Moreover, it is worth noting that this behaviour can be repeated over 25 cycles with no current loss (Fig. S9†). A possible explanation is the impossibility to electrochemically induce the isomerisation at the dicationic state for 4, the electrochemical response corresponding thus only to the reversible, but slow two-electron process. Additionally, multiple-cycle CVs were recorded for 4 after irradiation and showed that 4c could be oxidised to 4c2+ and back reversibly (Fig. S10†) while no irreversible wave at 0.40 V vs. SCE was detected.
On the contrary, the behaviour of the open form isomer of 5 is similar to 1–3 since it can be irreversibly oxidised at 1.02 V vs. SCE leading to two one-electron cathodic waves at E1/2 = 0.35 V and 0.51 V vs. SCE on the back scan, suggesting the reduction of 5c2+ to 5c, as confirmed by the CV on the solution irradiated at 365 nm, where the same two redox waves are observed (Fig. 4e). These results prove that the presence of at least one electron-rich thienyl-based arm is necessary for the oxidative cyclisation to occur in the investigated switches. Additionally, as this derivative and its precursor are fluorescent, a potential application in electrofluorochromism can be envisioned (Fig. S11† for a proof-of-concept experiment on precursor 20).41–45
The main electrochemical characteristics are summarised in Table 1.
Interestingly, unlike their dithienylethene counterparts,21,22 our thiophene-containing systems showed an atypical behaviour upon recording multiple-scan CVs with the irradiated solutions. The case of terarylene 1 is depicted in Fig. 5, while the voltammograms for 2, 3 and 5 are provided in Fig. S12–S14.† A loss of the closed form isomer at the vicinity of the electrode surface is found over multiple oxidation–reduction cycles in the potential window of the redox processes of 1c since the peak current is decreasing at each iteration (Fig. 5a).
Besides, when the CV is recorded over the whole potential window (Fig. 5b), an increase of the peak current corresponding to 1o oxidation is observed, meaning its local concentration also increases. The two above observations suggest the occurrence of an electro-activated cycloreversion from 1c to 1o close to the electrode. This behaviour, if confirmed, would be the first experimental observation of coexisting oxidative ring-closing and ring-opening reactions.
This unanticipated feature was further investigated by recording CVs for 1o and 1c at different scan rates and the obtained voltammograms are presented in Fig. S15.† Concerning 1o (Fig. S15,† left), the two-electron oxidation remained unambiguously irreversible while increasing the scan rate from 25 mV s−1 to 4 V s−1 (limit of our equipment), suggesting a relatively large rate constant for the chemical step leading to ring closure. In the case of the DTE counterpart of 1, Feringa and co-workers reported that the irreversible signature was observed up to 1000 V s−1, corresponding to a ring-closure kinetic constant greater than 104 s−1. While it would be reasonable to assume comparable rates for our investigated thienyl-based terarylenes, the lower limit for ring closure would rather be 102 s−1 if ring closure occurs at the dicationic state (EEC mechanism, vide infra and in the ESI†).
In contrast, a significant impact could be observed on the two waves related to the closed form isomer at faster scan rates, suggesting an improved reversibility of the corresponding electrochemical processes. This indicates that at higher scan rates (i.e. short measurement times), the above mentioned electro-activated cycloreversion from 1c to 1o barely has time to occur. This is confirmed with CVs after partial conversion to the photogenerated closed form (Fig. S15,† right), where the disappearance of the CF species in the diffusion layer was significant at a slow scan rate such as 25 mV s−1, while it was almost prevented at ν ≥ 1 V s−1.
Spectroelectrochemical studies
To better elucidate the redox-triggered processes of 1 and possibly identifying the transient species involved in its isomerisation under an electrochemical stimulus, spectroelectrochemical experiments at room temperature were carried out in acetonitrile/TBAPF6 0.1 M. A solution of 1o was initially electrolysed at 1.30 V vs. SCE. The induced spectral evolution was followed by UV-vis spectroscopy (Fig. 6a).Because of the oxidation, the bands at 253 nm and 302 nm decreased while three new bands grew at 400 nm, 470 nm, and 600 nm and remained quite stable after the end of the oxidation. These new spectral features are attributed to the dicationic species 1c2+. The formation of this species after the oxidation is so fast that neither the monocationic radicals (either in the open or the closed form) nor 1o2+ are observed as intermediate species during the oxidation.
Then, a second electrolysis was carried out at 0.50 V, that is the potential at which the wave for the one-electron reduction 1c2+ → 1c+˙ starts, thus attempting to obtain an optical signature of the radical species. Instead, a return to the open form is observed (Fig. 6b).
Similar spectral evolutions were observed also for 2, 3 and 5 and the corresponding spectroelectrochemical experiments are provided in Fig. S17–S26.† This result suggests a possible ring-opening reaction at the radical stage. If happening, 1o+˙ generated from cycloreversion would be rapidly reduced to 1o while maintaining the electrode potential at 0.50 V during the electrolysis, since it is electrochemically generated from 1o at much higher potential (at least 1.12 V). In addition, a full return to 1o can be achieved by further lowering the potential to 0.20 V (Fig. S16†), indicating that 1o can be switched to 1c2+ and then reobtained through an oxidation–reduction sequence.
Possible mechanism ruling the bidirectional dual-responsive isomerisation
Terarylene 1 was chosen to investigate the underlying mechanism of such bidirectional behaviour. Additionally, given their qualitatively similar redox behaviour, the presented mechanism should be valid also for 2, 3 and 5.Since the photo-generated neutral 1c is thermally stable at room temperature, the ring-opening reaction might occur either at the dicationic redox state,46,47 or at the radical one.
First, the thermal stability of the dicationic species 1c2+ was qualitatively evaluated by oxidising a solution of 1o in acetonitrile with two equivalents of tris(4-bromophenyl)ammoniumyl hexachloroantimonate (also known as “magic blue”,  vs. Fc in acetonitrile).48 The obtained spectrum is shown in Fig. S27.† The solution was kept in the dark at room temperature for 1 h and, as only a slight spectral variation was detected, 1c2+ can be considered relatively stable. Consequently, we postulate that the thermal ring-opening is more probably occurring at the radical redox state (see also the “Theoretical modelling” section below), according to the following chemical equilibrium (eqn (1)):
vs. Fc in acetonitrile).48 The obtained spectrum is shown in Fig. S27.† The solution was kept in the dark at room temperature for 1 h and, as only a slight spectral variation was detected, 1c2+ can be considered relatively stable. Consequently, we postulate that the thermal ring-opening is more probably occurring at the radical redox state (see also the “Theoretical modelling” section below), according to the following chemical equilibrium (eqn (1)):
| 1c+˙ ⇆ 1o+˙ | (1) |
Stopped-flow experiments were carried out to investigate this cycloreversion at the radical state.
Upon reducing a solution of 1c2+ (previously prepared by electrolysing a solution of 1o at 1.2 V vs. SCE, details in the “Stopped-flow measurements and chemical red/ox experiments” section of the ESI, Fig. S28†) with 1 eq of decamethylferrocene (Me10Fc,  vs. SCE in acetonitrile),48 no bands of the dicationic species could be observed after the mixing time (10 ms), but a band at 422 nm and one at 763 nm were detected (Fig. 7) and attributed to 1c+˙.
vs. SCE in acetonitrile),48 no bands of the dicationic species could be observed after the mixing time (10 ms), but a band at 422 nm and one at 763 nm were detected (Fig. 7) and attributed to 1c+˙.
 | ||
| Fig. 7 Stopped-flow absorption spectral evolution upon reduction of a 1c2+ solution (2.27 × 10−5 M) with a Me10Fc solution (2.27 × 10−5 M) in CH3CN. Mixing time: 10 ms. Optical path: 1 cm. | ||
The disappearance of the radical bands was accompanied by a concurrent growth of the bands of 1c2+ with isosbestic points at 453 nm, 497 nm and 651 nm. This is ascribed to the cross-dismutation between the newly formed 1o+˙and a neighbour 1c+˙ molecule, as in eqn (2), which leads to a recovery of 50% of the initial concentration of 1c2+.
| 1o+˙ + 1c+˙ → 1o + 1c2+ | (2) |
The same spectral evolution was observed when such chemical reduction conditions were applied in stationary UV-vis experiments, confirming the formation of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1o/1c2+ solution after the reduction of 1c2+ with 1 eq of Me10Fc (Fig. S30†).
1 1o/1c2+ solution after the reduction of 1c2+ with 1 eq of Me10Fc (Fig. S30†).
Next, stopped-flow monitoring of the reduction of 1c2+ with 1.8 eq of decamethylferrocene was performed. Upon using such a larger amount of chemical reductant, the formation of 1c in addition to 1c+˙ could be detected after the mixing time (Fig. 8a). The presence of the neutral closed form isomer allowed the occurrence of the cascade mechanism proposed by Kawai's group37 (eqn (3)), where 1c+˙ acts as a “catalyst” of the ring-opening of 1c towards 1o. This is suggested by the decrease of the band of 1c between 600 nm and 700 nm, while the band of 1c+˙ at 763 nm remained stationary until the total consumption of the neutral closed form (ca. 10 s, Fig. 8b) before disappearing with an apparent kinetic constant of 0.18 s−1 (Fig. S31b†).
| 1o+˙ + 1c → 1o + 1c+˙ | (3) |
The occurrence of this bimolecular reaction was further confirmed by stationary UV-vis spectroscopy (Fig. S32†). A fast return to 1o was observed upon addition of a catalytic amount (0.10 eq) of ferrocenium tetrafluoroborate (freshly prepared solution in acetonitrile, under Ar;  vs. SCE in acetonitrile)48 to a non-degassed solution of 1 at the photostationary state (α1c = 94%) in acetonitrile at room temperature. One spectrum every 0.4 min was recorded after the addition of such sub-stoichiometric amount of oxidant and ≥90% of 1c was reopened to 1o in 2 min ca. (orange dashed-dotted line in Fig. S31†).
vs. SCE in acetonitrile)48 to a non-degassed solution of 1 at the photostationary state (α1c = 94%) in acetonitrile at room temperature. One spectrum every 0.4 min was recorded after the addition of such sub-stoichiometric amount of oxidant and ≥90% of 1c was reopened to 1o in 2 min ca. (orange dashed-dotted line in Fig. S31†).
Two radical species being involved, the disproportionation of 1c+˙ (eqn (4)) and 1o+˙ (eqn (5), where 1o2+ would then spontaneously cyclise to afford 1c2+) should be also considered to give a more complete overview of all the possible reactions that could occur.47
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1c+˙ ⇆ 1c + 1c2+ 1c+˙ ⇆ 1c + 1c2+ | (4) |
2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1o+˙ ⇆ 1o + 1o2+ 1o+˙ ⇆ 1o + 1o2+ | (5) |
Note that the disproportionation of 1c+˙ (eqn (4)) is not thermodynamically favourable as the electrochemical behaviour of 1c shows two successive single-electron processes (as discussed above), but the reverse reaction (i.e. comproportionation) is, and this has an impact on the CV response (see “Cyclic voltammetry simulation” section in the ESI†).
The dismutation of 1o+˙ (eqn (5)) is likely an unfavourable competitive reaction of the cross-dismutation (eqn (2)) or the oxidative cascade ring-opening of 1c (eqn (3)) in most experimental conditions described here and therefore it will not be explicitly considered in the next sections since 1o+˙ is poorly accumulated, unlike 1c+˙, thus making this reaction statistically less probable.
Based on the different results obtained, the overall bidirectional dual-responsive behaviour of 1o is summarised in Fig. 9 and highlights the versatile nature of such a molecular switch which has reversible access to two different closed form isomers depending on the chosen stimulus. The neutral open form can reversibly isomerise towards the neutral closed form (1c) upon light irradiation and switch to the dicationic closed form (1c2+) upon oxidation-induced cyclisation. The generation of the intermediate radical species 1c+˙ allows to restore 1o through cycloreversion at the radical state, meaning that 1o and 1c2+ can thus be interconverted by reversible isomerisation through an oxidation–reduction sequence and that 1o can also be restored upon one-electron oxidation of 1c.
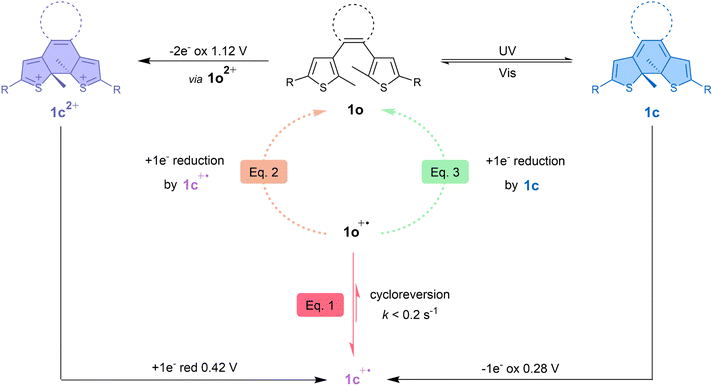 | ||
| Fig. 9 Mechanism summarising the bidirectional photochemical and electrochemical reactions of terarylene 1 involving its open form (1o), closed form (1c) and dicationic closed form (1c2+). The cycloreversion at the radical state (eqn (1)) is indicated with red arrows. The bimolecular reaction between 1o+˙ and 1c+˙ (eqn (2)) is graphically shown with an orange dotted arrow and the cascade reaction proposed by Kawai's group37 between 1o+˙ and 1c (eqn (3)) is depicted with a light green dotted arrow. R = p-OMe-Ph. | ||
To further support the proposed mechanism, coupled electrolysis-EPR spectroscopy of 1o (2.5 mM) in CH2Cl2/TBAPF6 0.24 M was carried out with an in house three-electrode setup placed inside an EPR tube (details in the ESI† and its “Coupled electrolysis-EPR spectroscopy” section). Once determined the potentials to use through cyclic voltammetry (Fig. S33†), EPR spectra were recorded before electrolysis (i.e. for 1o), during an oxidation at 1.3 V (i.e. for 1c2+) and during a reduction at 0.5 V (Fig. S34†). While no signal was observed for 1o and 1c2+, a non-persistent organic radical (g = 2.0037) was detected on the first scan during the electrolysis at 0.5 V, thus providing evidence of the generation of a radical species (i.e.1c+˙). The fact that the radical could not be accumulated is in agreement with the occurrence of the ring-opening reaction at the radical state (eqn (1)) and the ensuing consumption of 1o+˙ during the experiment.
Having experimentally determined the apparent kinetic constant for the ring-opening of 1c+˙, cyclic voltammetry simulations as well as simulations of the time traces of the stopped-flow experiments were carried out to support the proposed mechanism (details in the ESI,† “Cyclic voltammetry simulations” and “Time traces simulations” sections, respectively) and estimate the equilibrium constant of such cycloreversion at the radical state (eqn (1)) and the rate constants of the bimolecular reactions (eqn (2) and eqn (3)).
The disproportionation of 1c+˙ (eqn (4)) was included in both the time traces and CV models. As mentioned above, the thermodynamic equilibrium constant for this reaction is imposed by the ΔE between the two consecutive one-electron transfers involving the closed form and it is equal to 0.0043. The rate constants for disproportionation and comproportionation (eqn (4)) were estimated by cyclic voltammetry simulations to be at least greater than 103 s−1 M−1 and 2.32 × 105 s−1 M−1, respectively, while keeping their ratio fixed to 0.0043. Note that only a lower possible value of the rate constants may be estimated since above a certain value the contribution to the CV simulation is negligible, probably due to the fact that the reaction becomes too fast so that the process is limited by the diffusion rate of 1c and 1c2+. These values were used in the time traces simulations (vide infra and the corresponding sections in the ESI†).
The simulations of the CVs of 1 at ν = 100 mV s−1 were performed with DigiElch8 (“Cyclic voltammetry simulations” section in the ESI†). Concerning the simulation of the cyclic voltammogram of 1o (Table S1†), the formation of 1c2+ was ascribed to two concerted one-electron oxidations of 1o leading to spontaneous ring-closing reaction at the dicationic state with a kinetic constant k of at least 100 s−1 in accordance with the literature.21 Attempts to model this electrochemical reaction through an ECE mechanism (i.e. one-electron oxidation of 1o to 1o+˙ which spontaneously cyclises to 1c+˙ and then is further oxidised to 1c2+) showed that improved reversibility should have been observed at faster scan rates (which is not the case experimentally), thus supporting the proposition of two simultaneous single-electron transfers (EEC mechanism).33 Regarding the ring-opening of 1c+˙ leading to 1o+˙, a kinetic constant (kb) equal to 0.1 s−1 was used. For the bimolecular reactions between 1o+˙ and either 1c+˙ (eqn (2), vide supra) or 1c (eqn (3), vide supra), irreversibility was assumed and rate constants of at least 5 × 106 s−1 M−1 and 9 × 106 s−1 M−1, respectively, were considered. A larger constant for the reaction between 1o+˙ and 1c (eqn (3)) compared to that for the reaction between 1o+˙ and 1c+˙ (eqn (2)) is coherent with the neutral closed form species 1c being a better reductant than the closed form radical 1c+˙.
The simulated CV is depicted in Fig. S36,† where Keq for 1o+˙ ⇆ 1c+˙ is set to 20, and the inclusion of the disproportionation of 1c+˙ (eqn (4), vide supra, and light orange row in Tables S1 and S2†) is needed to obtain a satisfying fit. This is even more relevant in the case of the modelling of the CVs for 1c (Table S2 and Fig. S37†) since there is coexistence of 1c and 1c2+ during the experiment. Indeed, once 1c2+ diffuses towards the solution after being formed upon oxidation at the vicinity of the electrode, it can react with 1c diffusing from the bulk of the solution towards the electrode. This comproportionation reaction can lead to an accumulation of 1c+˙ far from the electrode, which then reopens into 1o+˙, itself being reduced either by 1c (most probably) or by another 1c+˙ (cross-dismutation reaction) eventually affording 1o, thus contributing significantly to the electrochemically-induced cycloreversion.
A further refinement of the modelling for 1o by including a parasitic process consuming 1c2+ due to degradation of redox-active DAEs under the electrochemical conditions21,33 is discussed in the ESI.†
Regarding the CVs of the photo-generated 1c at ν = 100 mV s−1 (Fig. S37†), the disappearance of the waves over multiple cycles could be modelled with the above-mentioned values, further confirming the validity of the rate constants for the processes involved and the overall mechanism.
Finally, CVs were also modelled for different scan rates and the experimental trends were reproduced (Fig. S38 and S39†).
The simulation (Fig. S40†) of the time trace for the decay of the band at 763 nm obtained upon reduction of 1c2+ with 1 eq of Me10Fc (Fig. S29†) was carried out using Kintek Explorer.49,50 It was assumed that the ring-opening at the radical state (eqn (1)), the cross-dismutation between 1o+˙ and 1c+˙ (eqn (2)), the oxidative ring-opening of 1c (eqn (3)) and the disproportionation of 1c+˙ (eqn (4)) were involved. The shape of the trace was best reproduced with k = 0.122 s−1 for 1c+˙→1o+˙, k = 2.19 s−1 for 1o+˙→1c+˙, k = 5.68 × 106 s−1 M−1 for 1o+˙ + 1c+˙ → 1o + 1c2+, and 8.09 × 107 s−1 M−1 for 1o+˙ + 1c → 1o + 1c+˙ which are values in good accordance with the CV simulations.
Next, the time traces for the evolution of the bands at 600 nm and 763 nm observed when 1c2+ was reduced with 1.8 eq of Me10Fc (Fig. S31†) were simulated (Fig. S41 and S42†). Satisfyingly, the same rate constants led to an acceptable match between simulated and experimental time traces at such wavelengths, further validating their estimation.
Theoretical modelling
To better rationalise and reinforce the proposed mechanism, DFT calculations were carried out on the five investigated terarylenes (computational details provided in the ESI†). The geometries of the open and closed forms of 1–5 were fully optimised at the ωB97XD/6-311G(d,p) level of calculations in acetonitrile in the different redox states 0, +1 and +2. Unless otherwise stated, the antiparallel open form is considered since that is the conformer that can undergo ring closure reaction upon irradiation according to the Woodward–Hoffmann rules.51 The relative energies of the CFs with respect to OFs are presented in Table 2.| Redox state | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| a The antiparallel geometries of 1 and 2 in the dicationic open form state couldn't be obtained since the optimisation converged to the corresponding dicationic closed forms. The relative energies of the closed forms were then calculated from the parallel conformation of the open forms. | |||||
| +0 | |||||
| ΔECF–OF | 78.8 | 80.8 | 78.9 | 70.5 | 73.3 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| +1 | |||||
| ΔECF–OF | −22.1 | −15.1 | 5.7 | −29.6 | 0.5 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||||
| +2 | |||||
| ΔECF–OF | −100.2a | −170.7a | −130.7 | −127.5 | −140.3 |
As expected, all the five antiparallel open forms in neutral state are more stable than the corresponding closed forms, and the energy differences between the two isomers are not significantly affected by the different electron-donating nature of the substituents in this redox state.
The computed energy difference is in the range of 70.5 kJ mol−1 for 4 to 80.8 kJ mol−1 for compound 2. This is well in line with the aromaticity change of thienyl or thiazolyl arms from OF and CF isomers as previously proposed by Nakamura.52 The destabilisation due to the loss of aromaticity is calculated to be between 60 and 65 kJ mol−1 (Table S3†). In stark contrast, the relative stability of the two forms is dramatically reversed in the dicationic redox state, with the CF being stabilised by more than 100 kJ mol−1 for all the members of the series.
An intermediate situation is found in the radical redox state, with much smaller energy differences between the two isomers. These computational results are in good agreement with the observed experimental data, i.e. the two-electron oxidation-induced cyclisation of 1o–3o and 5o, the thermal stability of the corresponding CF2+, and are also in favour of a ring-opening mechanism of the CF at the radical redox state rather than at the dicationic one.
Additionally, the same calculations were also carried out on the DTE analogue of 1 investigated by Feringa and co-workers (“Additional calculation data” section in the ESI†). As expected, the neutral closed form isomer of the DTE is more stable than 1c (Table S4,† 37.3 kJ mol−1 for DTEcvs. 78.8 kJ mol−1) and the dicationic closed form isomers have similar relative stabilities (Table S4,† −109.6 kJ mol−1 for DTEc2+vs. −100.2 kJ mol−1), in accordance with the oxidative cyclisation that both systems show. However, in the case of the radical state, 1c+˙ is considerably less stable than its DTE radical counterpart (Table S4,† −22.1 kJ mol−1vs. −46.9 kJ mol−1 for DTEc+˙), suggesting that the ring-opening is more favourable in our systems and thus supporting the proposed mechanism.
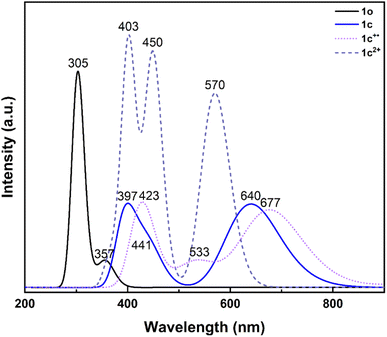
TD-DFT calculations were also performed to simulate the optical properties of 1o, 1c, 1c+˙ and 1c2+ (Fig. 10). The calculated spectra for the neutral open form and closed form of 1 are in very good agreement with the experimental findings. For 1o, a weak transition at 357 nm (S0 → S1) that appears as a shoulder in the experimental spectrum and two strong transitions at 305 nm (S0 → S3) and 301 nm (S0 → S4) were predicted. Interestingly, these two transitions promote an electron towards the LUMO and LUMO+1 orbitals, respectively. Careful analyses indicate that these virtual orbitals exhibit a significant density on the two reactive carbon atoms and a bonding character for the to-be-formed C–C bond (Fig. S43†).
 | ||
| Fig. 10 Calculated absorption spectra of 1o (black solid line), 1c (blue solid line), 1c+˙ (light violet dotted line) and 1c2+ (purple dashed line). | ||
The LUMO+1 being strongly bonding between the reactive carbons can be labelled as “photochromic” since it triggers the ring closure reaction.53,54 The analysis of the HOMO−1 orbital of 1o is also interesting since it can be anticipated that this orbital becomes the HOMO for 1o2+. HOMO−1 shows a strong bonding character between the reactive carbon atoms and exhibits “thermochromic” topology for the dicationic state. Indeed, this can explain a spontaneous ring closure of the open form in the dicationic state and why no antiparallel form could be optimised. Similar behaviour for HOMO−1 is observed for 2o2+ while less strong bonding character is observed for 3o2+, 4o2+ and 5o2+ (Fig. S44†).
As for 1c, the calculations predict two strong transitions at 640 nm (S0 → S1) and 397 nm (S0 → S3), which compare well with the 636 nm and 400 nm experimental bands. As observed experimentally, a shoulder is also calculated at 441 nm (S0 → S2).
In the calculated UV-vis spectrum of 1c+˙, the energy of the lowest-energy visible band is overestimated (677 nm vs. 763 nm, absolute error of 0.21 eV, S0 → S2), but overall, compares well with the experimental spectrum obtained during the stopped-flow measurements by also predicting a transition at 423 nm (S0 → S10, λexp: 423 nm) and one at 533 nm (S0 → S4, λexp: 557 nm), shoring up the attribution of such spectrum to the formation of 1c+˙.
Last, in the case of 1c2+, the theoretical spectrum is dominated by three electronic transitions at 570 nm, 450 nm, and 403 nm in good agreement with the experimental values (λexp: 600, 470 nm and 400 nm), thus supporting the formation of such species upon two-electron oxidation of 1o.
Conclusions
In summary, three terarylenes bearing electron-rich thienyl-based arms, one terphenylthiazole and one mixed dissymmetric system have been synthesised and their photo- and redox-active behaviours investigated. These terarylenes (except for terphenylthiazole-based 4) were found to display both oxidative ring-closing of their open forms and oxidative catalytic ring-opening of their closed forms, besides the expected photoswitching. These redox-active processes have been reported for dithienylethenes, but not their co-occurrence within the same molecule. Moreover, despite sharing similar CV features with their known DTE analogues, oxidation–reduction electrolysis sequences on 1o, 2o, 3o and 5o do not afford the corresponding neutral closed forms. A return to the neutral open forms is observed instead. In other words, this family of switches can be reversibly operated between two different closed form isomers depending on the chosen stimulus. With the support of CV, stopped-flow and EPR experiments as well as by DFT modelling, kinetics and cyclic voltammetry simulations, the observed bidirectional photo- and redox-induced multi-state isomerisation is rationalised. This unprecedented behaviour is ascribed to the combination of the features compatible with the largely thermodynamically-driven oxidative cyclisation like in analogous DTEs (i.e. electron-rich thiophene), and a comparatively less stabilised closed form radical giving rise to an equilibrium with its corresponding open form counterpart. Albeit thermodynamically unfavoured, the ring-opening at the radical state is strongly accelerated by the thermodynamically favourable and fast consumption of the open form radical in bimolecular redox reactions either (1) with a neighbouring closed form radical or (2) with a neutral closed form molecule. Such rich redox-switching behaviour, along with easier structural modifications show the potential of terarylenes for the development of advanced photo- and redox-responsive molecular switches.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
N. B. and P. Y. performed the synthesis of the investigated switches. N. B. carried out the UV-vis spectroscopy and electrochemistry experiments. A. L. assisted N. B. in the spectroelectrochemical measurements. J.-N. R. carried out the stopped flow experiments and the time traces simulations. F. Mav. supported N. B. in conducting the cyclic voltammetry simulations. E. R. and N. B. realised the electrofluorochromism experiment. C. H. carried out the coupled electrolysis-EPR experiment. F. Mau. performed all the theoretical modelling. N. B., A. L., J.-N. R., F. Mav. and P. Y. discussed together to rationalise the redox-active behaviour of the investigated switches. A. L. and P. Y. supervised N. B. during the whole project. N. B., A. L. and P. Y. designed the project. P. Y. and F. Mau. were responsible for funding acquisition. N. B. prepared the original draft. All the authors contributed to the review and editing of the original draft.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Funding from the Agence Nationale de la Recherche (ANR-17-CE07-0056, coordinated by Dr Rémi Métivier) is acknowledged by the authors. Theoretical modelling was performed by using P3MB facility (Modelling Platform for Molecules, Materials and Biomolecules – P3MB, ITODYS, Université Paris Cité, CNRS UMR 7086). The authors are also grateful to ANR (Agence Nationale de la Recherche) and CGI (Commissariat à l’Investissement d’Avenir) for their financial support through Labex SEAM (Science and Engineering for Advanced Materials and devices), ANR-10-LABX-096 and ANR-18-IDEX-0001.Notes and references
- J. Andréasson, U. Pischel, S. D. Straight, T. A. Moore, A. L. Moore and D. Gust, J. Am. Chem. Soc., 2011, 133, 11641–11648 CrossRef PubMed.
- S. Pu, Z. Tong, G. Liu and R. Wang, J. Mater. Chem. C, 2013, 1, 4726–4739 RSC.
- D. Roldan, S. Cobo, F. Lafolet, N. Vilà, C. Bochot, C. Bucher, E. Saint-Aman, M. Boggio-Pasqua, M. Garavelli and G. Royal, Chem.–Eur. J., 2015, 21, 455–467 CrossRef CAS PubMed.
- F. Zhao, L. Grubert, S. Hecht and D. Bléger, Chem. Commun., 2017, 53, 3323–3326 Search PubMed.
- A. H. Heindl, J. Becker and H. A. Wegner, Chem. Sci., 2019, 10, 7418–7425 RSC.
- H. Nie, J. L. Self, A. S. Kuenstler, R. C. Hayward and J. Read de Alaniz, Adv. Opt. Mater., 2019, 7, 1900224 Search PubMed.
- F. Stricker, D. M. Sanchez, U. Raucci, N. D. Dolinski, M. S. Zayas, J. Meisner, C. J. Hawker, T. J. Martínez and J. R. de Alaniz, Nat. Chem., 2022, 14, 942–948 Search PubMed.
- M. Zitzmann, F. Hampel and H. Dube, Chem. Sci., 2023, 14, 5734–5742 RSC.
- Y. Lv, H. Ye and L. You, Chem. Sci., 2024, 15, 3290–3299 Search PubMed.
- M. Irie, Chem. Rev., 2000, 100, 1685–1716 CrossRef CAS PubMed.
- M. Irie, T. Fukaminato, K. Matsuda and S. Kobatake, Chem. Rev., 2014, 114, 12174–12277 CrossRef CAS PubMed.
- M. Irie, Diarylethene Molecular Photoswitches: Concepts and Functionalities, Wiley-VCH, Weinheim, 2021 Search PubMed.
- Q. Ai, K. Lan, L. Li, Z. Liu and X. Hu, Adv. Sci., 2024, 2410524 CrossRef CAS PubMed.
- X. Deng and L. S. Liebeskind, J. Am. Chem. Soc., 2001, 123, 7703–7704 Search PubMed.
- S. Kobatake and Y. Terakawa, Chem. Commun., 2007, 1698–1700 RSC.
- T. Nakashima, K. Miyamura, T. Sakai and T. Kawai, Chem.–Eur. J., 2009, 15, 1977–1984 Search PubMed.
- Y. Kutsunugi, C. Coudret, J. C. Micheau and T. Kawai, Dyes Pigm., 2012, 92, 838–846 CrossRef CAS.
- L. Chocron, N. Baggi, E. Ribeiro, V. Goetz, P. Yu, K. Nakatani and R. Métivier, Chem. Sci., 2024, 15, 16034–16039 RSC.
- A. Peters and N. R. Branda, Chem. Commun., 2003, 954–955 Search PubMed.
- G. Guirado, C. Coudret, M. Hliwa and J. P. Launay, J. Phys. Chem. B, 2005, 109, 17445–17459 Search PubMed.
- W. R. Browne, J. J. D. De Jong, T. Kudernac, M. Walko, L. N. Lucas, K. Uchida, J. H. Van Esch and B. L. Feringa, Chem.–Eur. J., 2005, 11, 6414–6429 CrossRef CAS PubMed.
- W. R. Browne, J. J. D. De Jong, T. Kudernac, M. Walko, L. N. Lucas, K. Uchida, J. H. Van Esch and B. L. Feringa, Chem.–Eur. J., 2005, 11, 6430–6441 CrossRef CAS PubMed.
- B. He and O. S. Wenger, J. Am. Chem. Soc., 2011, 133, 17027–17036 CrossRef CAS PubMed.
- B. Gorodetsky, H. D. Samachetty, R. L. Donkers, M. S. Workentin and N. R. Branda, Angew. Chem., Int. Ed., 2004, 43, 2812–2815 CrossRef CAS PubMed.
- A. Léaustic, E. Anxolabéhère-Mallart, F. Maurel, S. Midelton, R. Guillot, R. Métivier, K. Nakatani and P. Yu, Chem.–Eur. J., 2011, 17, 2246–2255 CrossRef PubMed.
- M. Kleinwächter, E. Teichmann, L. Grubert, M. Herder and S. Hecht, Beilstein J. Org. Chem., 2018, 14, 2812–2821 CrossRef PubMed.
- L. Sicard, F. Lafolet, M. Boggio-Pasqua, C. Bucher, G. Royal and S. Cobo, ChemPhysChem, 2022, 23, e202200004 CrossRef CAS PubMed.
- A. Peters and N. R. Branda, J. Am. Chem. Soc., 2003, 125, 3404–3405 CrossRef CAS PubMed.
- Y. Moriyama, K. Matsuda, N. Tanifuji, S. Irie and M. Irie, Org. Lett., 2005, 7, 3315–3318 CrossRef CAS PubMed.
- B. Gorodetsky and N. R. Branda, Adv. Funct. Mater., 2007, 17, 786–796 CrossRef CAS.
- I. Gallardo, G. Guirado, G. Prats and M. Takeshita, Phys. Chem. Chem. Phys., 2009, 11, 1502–1507 RSC.
- I. Gallardo, G. Guirado, M. Moreno, G. Prats and M. Takeshita, Chem.–Eur. J., 2012, 18, 9807–9812 Search PubMed.
- M. Herder, M. Utecht, N. Manicke, L. Grubert, M. Pätzel, P. Saalfrank and S. Hecht, Chem. Sci., 2013, 4, 1028–1040 RSC.
- T. Nakashima, K. Atsumi, S. Kawai, T. Nakagawa, Y. Hasegawa and T. Kawai, Eur. J. Org Chem., 2007, 3212–3218 CrossRef CAS.
- S. Fukumoto, T. Nakashima and T. Kawai, Angew. Chem., Int. Ed., 2011, 50, 1565–1568 CrossRef CAS PubMed.
- R. Li, T. Nakashima, O. Galangau, S. Iijima, R. Kanazawa and T. Kawai, Chem.–Asian J., 2015, 10, 1725–1730 CrossRef CAS PubMed.
- T. Nakashima, Y. Kajiki, S. Fukumoto, M. Taguchi, S. Nagao, S. Hirota and T. Kawai, J. Am. Chem. Soc., 2012, 134, 19877–19883 CrossRef CAS PubMed.
- J. P. Calupitan, T. Nakashima, Y. Hashimoto and T. Kawai, Chem.–Eur. J., 2016, 22, 10002–10008 Search PubMed.
- N. Baggi, A. Léaustic, S. Groni, E. Anxolabéhère-Mallart, R. Guillot, R. Métivier, F. Maurel and P. Yu, Chem.–Eur. J., 2021, 27, 12866–12876 Search PubMed.
- E. Chatir, M. Boggio-Pasqua, F. Loiseau, C. Philouze, G. Royal and S. Cobo, Chem.–Eur. J., 2022, 28, e202103755 CrossRef CAS PubMed.
- P. Audebert and F. Miomandre, Chem. Sci., 2013, 4, 575–584 RSC.
- C. Quinton, V. Alain-Rizzo, C. Dumas-Verdes, F. Miomandre, G. Clavier and P. Audebert, RSC Adv., 2014, 4, 34332–34342 RSC.
- H.-T. Lin, C.-L. Huang and G.-S. Liou, ACS Appl. Mater. Interfaces, 2019, 11, 11684–11690 CrossRef CAS PubMed.
- B. Sk, M. Sarkar, K. Singh, A. Sengupta and A. Patra, Chem. Commun., 2021, 57, 13590–13593 RSC.
- G. A. Corrente, D. A. González, E. Aktas, A. L. Capodilupo, F. Ruighi, G. Accorsi, D. Imbardelli, C. Rodriguez-Seco, E. Martinez-Ferrero, E. Palomares and A. Beneduci, J. Mater. Chem. C, 2023, 11, 17115–17127 RSC.
- J. Areephong, W. R. Browne, N. Katsonis and B. L. Feringa, Chem. Commun., 2006, 3930–3932 RSC.
- H. Logtenberg and W. R. Browne, Org. Biomol. Chem., 2013, 11, 233–243 RSC.
- N. G. Connelly and W. E. Geiger, Chem. Rev., 1996, 96, 877–910 CrossRef CAS PubMed.
- K. A. Johnson, Z. B. Simpson and T. Blom, Anal. Biochem., 2009, 387, 20–29 CrossRef CAS PubMed.
- K. A. Johnson, Z. B. Simpson and T. Blom, Anal. Biochem., 2009, 387, 30–41 CrossRef CAS PubMed.
- R. B. Woodward and R. Hoffmann, Angew. Chem., Int. Ed., 1969, 8, 781–853 CrossRef CAS.
- S. Nakamura and M. Irie, J. Org. Chem., 1988, 53, 6136–6138 CrossRef CAS.
- A. Perrier, F. Maurel and J. Aubard, J. Photochem. Photobiol., A, 2007, 189, 167–176 CrossRef CAS.
- A. D. Laurent, J.-M. André, E. A. Perpète and D. Jacquemin, J. Photochem. Photobiol., A, 2007, 192, 211–219 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc02845k |
| ‡ Current address: Department of Chemical Engineering Universitat Politècnica de Catalunya, EEBE, Eduard Maristany 10-14, Barcelona, 08019, Spain. |
| This journal is © The Royal Society of Chemistry 2025 |