Open Access Article
Open Access ArticlePhotocatalytic synthesis of 2-oxabicyclo[2.1.1]hexanes: cobalt-enhanced efficiency†
Si-Yuan
Tang‡
a,
Zhan-Jie
Wang‡
a,
Jin-Jiao
Wu
a,
Zhi-Xi
Xing
a,
Ze-Yi
Du
b and
Huan-Ming
Huang
 *a
*a
aSchool of Physical Science and Technology, ShanghaiTech University, Shanghai 201210, China. E-mail: huanghm@shanghaitech.edu.cn
bDepartment of Medicinal Chemistry, School of Pharmacy, Fudan University, No. 826 Zhangheng Road, Shanghai 201203, China
First published on 26th May 2025
Abstract
Development of new synthetic strategies to prepare C(sp3)-rich arene bioisosteres, especially their heteroatom incorporating analogs, is less explored, but highly in demand. Here we report a photocatalytic [2π + 2σ] cycloaddition reaction between bicyclo[1.1.0]butanes and aldehydes enabled by cobalt under visible light irradiation. The key step is that bicyclo[1.1.0]butanes could be oxidized to generate radical cation intermediates which could be promoted by cobalt, facilitating a nucleophilic addition to the aldehydes. This unprecedented strategy exhibits broad functional group tolerance and efficiently constructs complex molecular architectures and derivatives of natural products with good to excellent yields. Detailed mechanistic studies and product manipulation have demonstrated the viability of this open-shell approach. The desired 2-oxa-BCH motif demonstrated excellent acidity tolerance and significantly enhanced lipophilicity potentially leading to enhanced metabolic properties and in vitro bioactivities compared to its parent phenyl-type bioisostere.
Introduction
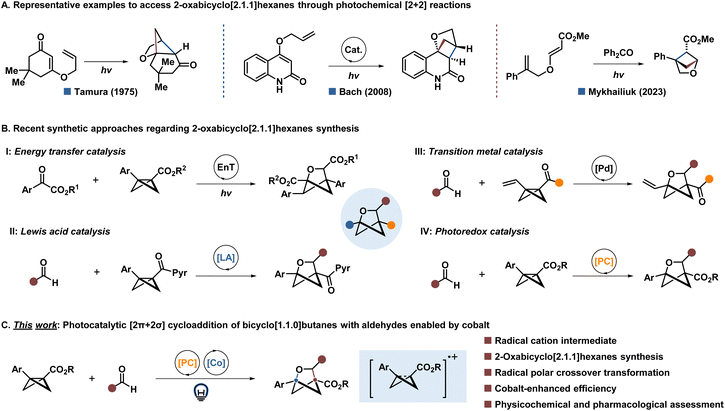
The phenyl ring is a prevalent functional group in pharmaceuticals and materials science.1 In recent years, the concept of “escaping from flatland” has gained traction among medicinal and synthetic chemists,2 prompting the exploration of novel synthetic methods to create conformationally rigid structures characterized by a higher content of sp3-hybridized carbon atoms compared to traditional arenes.3 Notable examples of such structures include bicyclo[1.1.1]pentanes (BCPs),4 bicyclo[2.1.1]hexanes (BCHs),5 and bicyclo[3.1.1]heptanes (BCHeps).6 In recent developments, Mykhailiuk and colleagues demonstrated that C(sp3)-rich arene bioisosteres incorporating heteroatoms could potentially replace medicinally relevant heteroaromatic rings while offering enhanced properties such as better aqueous solubility, improved metabolic stability, and reduced lipophilicity7 However, synthetic approaches for such bioisosteres have been less thoroughly explored. Traditionally, 2-oxabicyclo[2.1.1]hexanes (2-oxa-BCHs) are synthesized using classical photochemical [2 + 2] reactions (Fig. 1A), yet these methods often come with limitations in terms of substrate scope and functional group compatibility.8Recently, strain-release cycloaddition between bicyclo[1.1.0]butanes (BCBs) and aldehydes has advanced as a promising approach to construct 2-oxabicyclo[2.1.1]hexanes (2-oxa-BCHs) (Fig. 1B).9 Notably, Glorius and co-workers developed two elegant synthetic approaches for 2-oxa-BCHs using energy transfer catalysis (Fig. 1B, I)10a or Lewis acid catalysis (Fig. 1B, II),10b respectively. Moreover, recent advancements by researchers such as Zi,11a Zheng,11b and Yang11c have demonstrated the use of palladium catalysis to synthesize 2-oxa-BCHs from vinylbicyclo[1.1.0]butanes (VBCBs) and carbonyl compounds (Fig. 1B, III). Glorius and co-workers developed dearomative cycloaddition reactions12a and [2π + 2σ] cycloaddition12b to afford various BCHs through oxidative activation of ester-substituted BCBs. During the investigation for our research, we found that Walker and his team demonstrated an elegant example of formal [2π + 2σ] cycloaddition between BCBs and alkenes or aldehydes using a simple photoredox catalyst under visible light, although they faced challenges related to relatively low yields and limited substrate scope for oxa-BCHs (Fig. 1B, IV).13 Additionally, Leitch,14a Glorius,14b–e Mykhailiuk,7c Feng,14f–i Li,14j Wang & Li,14k Zheng,14l Deng,14m Aggarwal,14n Zhou,14o Studer,14p and many others9 introduced several synthetic strategies towards the synthesis of C(sp3)-rich arene bioisosteres incorporating heteroatoms successfully.
Inspired by these elegant examples and our recent achievements15 in the area of radical-polar chemistry,16 we disclose a rare example of the photocatalytic synthesis of 2-oxa-BCHs by coupling bicyclo[2.1.1]hexenes (BCBs) with aldehydes, promoted by cobalt under visible light conditions (Fig. 1C). Our approach involves the oxidation of BCBs by strongly oxidative photoredox catalysis to generate radical cation intermediates under visible light conditions. These intermediates could be promoted by cobalt, which facilitates nucleophilic addition to both aromatic and aliphatic aldehydes.
Results and discussion
Reaction design and optimization
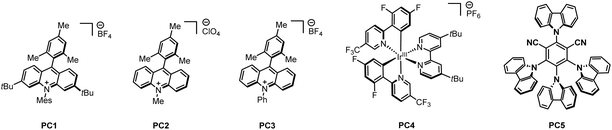
With this in mind, we investigated the coupling reaction between BCBs 1a and aliphatic aldehyde 2a, and we successfully formed the desired 2-oxa-BCHs 3 with a 90% isolated yield using 1 mol% of photocatalyst PC1 and 7.5 mol% CoCl2 in dichloroethane (DCE), under the irradiation of 450 nm LEDs for 12 hours (Table 1, entry 1). We screened several photocatalysts and found that a highly oxidative photocatalyst is crucial for achieving high yields (entries 2–5). Increasing the amounts of either the photocatalyst or the cobalt catalyst resulted in lower yields (entries 6–10). We also explored the impact of the DCE concentration (entries 11 and 12), the addition of various additives (entries 13 and 14), changing solvents (entries 15–17), and varying reaction times (entries 18 and 19), all of which led to decreased yields. Control experiments demonstrated the necessity of visible light, the photocatalyst, and the cobalt catalyst to achieve excellent yields (entries 20–24). Notably, the absence of the cobalt catalyst resulted in only a 56% isolated yield, highlighting its importance for optimal yield (entry 23).| Entry | Variation from the “optimized conditions” | Isolated yield [%] |
|---|---|---|
| a Reaction conditions: CoCl2 (7.5 mol%), Mes2Acr-tBu2BF4 (PC1, 1 mol%), freshly prepared BCB 1a (0.2 mmol, 2 equiv.) and aldehyde 2a (0.1 mmol, 1 equiv.) were dissolved in DCE (0.5 mL, 0.2 M). The mixture was irradiated with 30 W 450 nm blue LEDs with a cooling fan for 12 h under N2. | ||
| 1 | None | 90 |
| 2 | PC2 instead of PC1 | ND |
| 3 | PC3 instead of PC1 | 50 |
| 4 | PC4 instead of PC1 | ND |
| 5 | PC5 instead of PC1 | ND |
| 6 | 2 mol% PC1 | 84 |
| 7 | 10 mol% CoCl2 | 84 |
| 8 | 1 eq. of CoCl2 | 75 |
| 9 | CoI2 instead of CoCl2 | ND |
| 10 | CoCl2·6H2O instead of CoCl2 | 75 |
| 11 | Concentration: 0.4 M | 75 |
| 12 | Concentration: 0.1 M | 73 |
| 13 | 1 eq. of K2CO, as an additive | ND |
| 14 | 1 eq. of collidine as an additive | ND |
| 15 | DCM instead of DCE | 87 |
| 16 | MeCN instead of DCE | ND |
| 17 | THF instead of DCE | ND |
| 18 | 18 h instead of 12 h | 87 |
| 19 | 6 h instead of 12 h | 78 |
| 20 | No PC1, CoCl2 (7.5 mol%) | ND |
| 21 | No PC1, CoCl2 (1 eq.) | 6 |
| 22 | No light at 40 °C | ND |
| 23 | No CoCl2 | 56 |
| 24 | No PC1 and CoCl2 | ND |
|
||
Substrate scope
With the optimized conditions established, we began to explore the substrate scope using various aliphatic aldehydes (Fig. 2). The reaction conditions were found to be compatible with a range of functional groups on the aromatic ring, including fluoro (4), chloro (5), methyl (6), tert-butyl (7), and methoxy (8). The absence of a cobalt catalyst resulted in significantly reduced or trace yields of the desired products (4–5). We further investigated a variety of aliphatic aldehydes, such as ethyl (9), benzyl (10 and 16), cyclopropyl (11), cyclobutyl (12), cyclopentyl (13), cyclohexyl (14–15), isopropyl (16 and 20), protected alcohols (18 and 21), and esters (19 and 24–25), as well as chloro (22). Our method was then successfully applied to the synthesis of drug-related 2-oxa-BCHs, derived from various aliphatic aldehydes, such as those in fenbufen (26), flurbiprofen (27), aspirin (28), ketoprofen (29), ibuprofen (30), isoxepac (31), and valproate (32).We then proceeded to explore various aromatic aldehydes and BCHs (Fig. 3). The newly developed catalytic system successfully tolerated simple phenyl aldehyde, yielding the desired 2-oxa-BCH 33 with an 85% isolated yield, as confirmed by X-ray analysis (CCDC 2389698). A range of functional groups on the aromatic ring were also examined, including methyl (34), phenyl (35), fluoro (36, 46–47), chloro (37), bromo (38), trifluoromethyl (39), phenoxy (40), tert-butyl (41), methoxy (42–45), benzofuran (48), and bicyclic aromatic rings (49), all of which were tolerated, resulting in isolated yields of 58–79%. Various BCBs were screened as well, including those with substituted aromatic rings, such as methyl (50–52), fluoro (53), chloro (54), and naphthyl (55). The desired 2-oxa-BCHs were obtained in moderate to excellent yields. Additionally, different ester groups were investigated, including complex motifs such as L-menthol (58) and pregnenolone (59). The desired 2-oxa-BCHs (56–59) were obtained in good yields. Notably, the reactions involving BCBs with ester groups resulted in very low yields (30% NMR yield) when catalysed by Lewis acid,10b highlighting the unique efficacy of cobalt.
Mechanistic studies and the proposed mechanism
With a relatively broad substrate scope established, we began to explore the reaction mechanism through radical cation trapping experiments (Fig. 4A). Under an oxygen atmosphere using the dual catalytic system, the epoxide derivative 60 was obtained in a 21% isolated yield, suggesting the presence of a radical cation intermediate.17 Additionally, this intermediate was trapped by methanol, yielding the corresponding product 61 in a 30% isolated yield. Further evidence supporting the involvement of radical intermediates came from the detection and isolation of a PBN adduct 62 and a TEMPO adduct 63 (Fig. 4B). We also utilized the aliphatic aldehyde 2x, which contains a three-membered ring, and the desired 2-oxa-BCH 64 was formed smoothly. This outcome suggests that a ketyl radical is not formed in our catalytic system (Fig. 4C). After screening alternative transition metal additives, we determined that CoCl2 remains the most effective for achieving a high yield of 3 (Fig. 4D). Stern–Volmer and UV-vis experiments were conducted, clearly indicating that only the photocatalyst (PC1, Mes2AcrtBu2BF4) absorbs the visible light (Fig. 4F) and that only BCBs 2a quenched the photoexcited PC1 (Fig. 4E). The quantum yield of this dual catalytic system was measured, and the result (ϕ = 0.16) suggests that a radical chain mechanism is unlikely. Based on these mechanistic studies (Fig. 4G), we propose that BCB 2a (+1.79 V vs. Ag/AgCl)12b could be oxidized by PC1, (E [PC*/PC−˙] = 2.0 V vs. SCE)18 to generate the radical cation intermediate II. This intermediate can be reduced to intermediate III and its tautomer IV by the reduced form of PC1,19 which is then trapped by aldehyde 2a to form intermediate V. The final 2-oxa-BCHs 3 is produced after ring closure, completing the photocatalytic cycle. Alternatively, radical cation intermediate II could be promoted by cobalt(I), generating intermediate VI and its tautomer VII. A Reformatsky-type addition to aliphatic aldehyde 2a efficiently forms intermediate V, leading to the final 2-oxa-BCHs 3. The Co(II) could be reduced back to Co(I) by the reduced PC1, allowing both catalytic cycles to turn over efficiently.20,21Synthetic application and pharmacological activity of 2-oxa-BCHs
To explore the application of our reaction and the 2-oxa-BCH motif we synthesized, we first tested the model reaction on a gram scale with an excellent 92% yield of 2-oxa-BCHs 3 and diverse manipulation could be achieved (Fig. 5A). The ester group in 3 can be transferred to the corresponding amide 65. Alternatively, 2-oxa-BCHs 3 can be reduced to alcohol 66, which could then be converted to azide 67. Additionally, the ester group in 3 could be easily hydrolyzed under basic conditions to yield the corresponding carboxylic acid 68 with excellent yield. Different tertiary alcohols such as 69 and 70 could be obtained from 3 through MeLi nucleophilic addition or the Kulinkovich reaction. Finally, two different drug derivatives 71 and 72 could be synthesized from atomoxetine and leelamine via amide coupling and the ammonolysis reaction to further evaluate the physicochemical properties of our 2-oxa-BCH motif on mature drug scaffolds, therefore assessing the preliminary parameters in drug development such as lipophilicity, acidity and stability. | ||
Fig. 5 Synthetic application and physicochemical properties of 2-oxa-BCHs. (A) (a) Morpholine (2 eq.), LiHMDS (2 eq.), toluene, RT, 12 h; (b) BH3·THF (2.5 eq.), THF, 65 °C, 12 h; (c) DPPA (2 eq.), DBU (3 eq.), THF, 65 °C, 48 h; (d) NaOH (3 eq.), THF/MeOH/H2O = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 50 °C, 6 h; (e) MeLi (2 eq.), THF, 40 °C, 12 h; (f) Ti(OiPr)4 (1.4 eq.), EtMgBr (2.8 eq.), THF, RT, 12 h; (g) 68 (1 eq.), atomoxetine (1.2 eq.), EDCI·HCl (1.5 eq.), DMAP (0.2 eq.), DCM, RT, 12 h; (h) 3 (1 eq.), leelamine (5 eq.), LiHMDS (5 eq.), toluene, RT, 12 h. (B) C log P: calculated logarithm of the partition coefficient using ChemDraw Office; tPSA: the calculated topological polar surface area using ChemDraw Office; CpKa: the predicted acid dissociation constant using MolGpKa; plasma stability was predicted using PredPS. Acidity tolerance of 71 and 72 was assessed under indicated acidic conditions at 37 °C for 24 hours by calculating the recovery rate. More details could be found in the ESI.† 1, 50 °C, 6 h; (e) MeLi (2 eq.), THF, 40 °C, 12 h; (f) Ti(OiPr)4 (1.4 eq.), EtMgBr (2.8 eq.), THF, RT, 12 h; (g) 68 (1 eq.), atomoxetine (1.2 eq.), EDCI·HCl (1.5 eq.), DMAP (0.2 eq.), DCM, RT, 12 h; (h) 3 (1 eq.), leelamine (5 eq.), LiHMDS (5 eq.), toluene, RT, 12 h. (B) C log P: calculated logarithm of the partition coefficient using ChemDraw Office; tPSA: the calculated topological polar surface area using ChemDraw Office; CpKa: the predicted acid dissociation constant using MolGpKa; plasma stability was predicted using PredPS. Acidity tolerance of 71 and 72 was assessed under indicated acidic conditions at 37 °C for 24 hours by calculating the recovery rate. More details could be found in the ESI.† | ||
As shown in Fig. 5B, after introducing the 2-oxa-BCH motif, the lipophilicity of the modified substrates 71 and 72 significantly increased with higher C log P and tPSA values. The predicted pKa values for the parent compound atomoxetine and leelamine, as well as their derivatives 71 and 72 also illustrated significant changes.22 These variations in physicochemical properties are likely to significantly influence the ADME properties and this hypothesis agreed with the later result when we predicted the stability of four substrates in human plasma by using a published attention-based graph neural network program PredPS.23 The lower values of 71 and 72 compared to the parent atomoxetine and leelamine suggested improved stability in human plasma. In addition, we practically measure the stability of both 71 and 72 under acidic conditions at 37 °C that simulate the in vivo gastric conditions. Under even more acidic conditions than those found in the stomach,24 both 71 and 72 remained stable after 24 hours in 0.2 M and 2 M HCl, demonstrating the remarkable acidity tolerance of the 2-oxa-BCH motif. Interestingly, the two substrates exhibited different behaviors in concentrated HCl as most of the 71 was hydrolyzed, whereas a significant portion of 72 was still recoverable. This difference was potentially due to that the tertiary amide in 71 was easier to be protonated therefore leading to an acid-promoted hydrolysis. All in all, our 2-oxa-BCH motif could provide different physicochemical properties and illustrate excellent acid tolerance; therefore, we further synthesized 73 and 74 to compare the pharmacological behaviour between our 2-oxa-BCH motif and its parent phenyl bioisostere.
As shown in Fig. 6A, our 2-oxa-BCH motif (in cream stick representation) possessed a similar shape compared to its bioisostere (in cyan stick representation) with a comparable bond length and angle. More importantly, the 2-oxa-BCH motif contained oxygen as a hydrogen bond acceptor and possessed a more lipophilic and stereo scaffold, therefore potentially contributing to van der Waals force during binding to the target enzymes and therefore enhancing the pharmacological activities. By measuring the binding affinity of 73 and 74 towards the KOR (Kappa Opioid Receptor), we found that our 2-oxa-BCH motif supplied almost 10 times higher potency compared to the parent bioisostere. To reveal the plausible reasons behind this binding affinity enhancement, we further did the docking analysis of 73 and 74 (Fig. 6B). Both 73 (in cream stick representation, Fig. 6B) and 74 (in cyan stick representation, Fig. 6B) were located at the typical binding pocket of the KOR with similar binding orientations (gray ribbon, Fig. 6B) and conserved interactions between the morphinan of 73 and 74 and the KOR agreed with the previous report.25 To be specific, 73 (in cream stick representation, Fig. 6B) and 74 (in cyan stick representation, Fig. 6B) could form a salt bridge towards the KOR between the basic tertiary amine and D1383.32 (in gray stick representation, Fig. 6B) whereas a hydrogen bond (in the red line, Fig. 6B) was observed through the methoxy group of the substrates to Y1393.33 (in gray stick representation, Fig. 6B). Lipophilic interactions such as van der Waals forces mainly existed between the phenanthrene and M1423.36, V2305.42 and I2906.51 (in green stick representation, Fig. 6B), as well as between the cyclopropylmethyl group and W2876.48 and Y3207.43 (in purple stick representation, Fig. 6B). In addition, some unique interactions belonging to 73 were observed that could potentially explain its higher potency compared to the parent 74. Predicted hydrogen bonds between the amide linkage and residues S211EL2 and Y3127.24 could be observed (in red lines, Fig. 6B), as well as potential π–π stacking interactions between the phenyl group and Y3127.24 could further enhance the binding affinity. Moreover, the terminal phenyl group was located in the lipophilic “sandwich”-shaped pocket (constructed by N1223.16, S1233.17, I208EL2, E209EL2, and C210EL2) and such plausible van der Waals forces could also contribute to the KOR binding affinity.
 | ||
| Fig. 6 Pharmacological activity and docking analysis of 2-oxa-BCHs. (A) Structures of compounds 73 and 74, and their terminal motif comparison (in cream and cyan stick representation respectively) and KOR binding affinity of 73 and 74. The bond length and angle of the benzene bioisostere were referenced from CCDC 127167. (B) A putative binding mode of compound 73 (in cream stick representation) and 74 (in cyan stick representation) in a complex with the KOR (PDB entry: 6B73). Possible interactions between 73, 74 and KOR residues; the hydrogen bond (in red lines) and binding surface (in blue surface) were simulated using software Chimera 1.13.1. | ||
Conclusions
In summary, we have developed a photocatalytic [2π + 2σ] cycloaddition reaction between bicyclo[1.1.0]butanes and aldehydes, promoted by cobalt under visible light irradiation. This method enables the formation of a diverse library of 2-oxa-BCHs in good to excellent yields, offering broad substrate scope and functional group tolerance under mild conditions. Key steps in this process include the formation of a radical cation intermediate and enhancement of efficiency using cobalt. The 2-oxa-BCH motif we developed in this work displays not only excellent acidity tolerance but also enhanced substrate–drug target interactions compared to its phenyl-type bioisostere due to its unique chemical features. We anticipate that this new synthetic approach will find further applications in the synthesis of C(sp3)-rich arene bioisosteres incorporating different heteroatoms and the 2-oxa-BCH motif holds great potential as a bioisostere for the phenyl motif in future drug design.Data availability
Materials and methods, detailed optimization studies, experimental procedures, mechanistic studies and NMR spectra are available in the ESI† and from the corresponding authors upon request.Author contributions
H.-M. H. conceived and directed the project; S.-Y. T., Z.-J. W. and Z.-X. X. performed all the experiments and analysed all the data. In vitro assays were performed by Z.-Y. D. and molecular modelling studies were conducted by S.-Y. T. and Z.-Y. D. H.-M. H. and S.-Y. T. wrote the manuscript with contributions from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We are grateful for financial support from the National Natural Science Foundation of China (22201179 & 22471168 to H.-M. H.), the startup funding from ShanghaiTech University (H.-M. H.), the Postdoctoral Fellowship Program of CPSF (No. GZC20231674 to S.-Y. T.) and the Double First-Class Initiative Fund of ShanghaiTech University (S.-Y. T.). We sincerely thank Prof. Chaodan Pu, Zhuo Zhao, Shu-Ya Wen and Ying Zhang (all at ShanghaiTech University), Wei Li (Fudan University), and Yujun Wang (Shanghai Institute of Materia Medica) for help with substrate synthesis, mechanistic study, X-ray analysis and drug activity tests.Notes and references
- R. D. Taylor, M. Maccoss and A. D. G. Lawson, J. Med. Chem., 2014, 57, 5845–5859 CrossRef CAS PubMed.
- F. Lovering, J. Bikker and C. Humblet, J. Med. Chem., 2009, 52, 6752–6756 CrossRef CAS PubMed.
- (a) M. A. M. Subbaiah and N. A. Meanwell, J. Med. Chem., 2021, 64, 14046–14128 Search PubMed; (b) P. K. Mykhailiuk, Org. Biomol. Chem., 2019, 17, 2839–2849 RSC; (c) J. Tsien, C. Hu, R. R. Merchant and T. Qin, Nat. Rev. Chem., 2024, 8, 605–627 CrossRef CAS PubMed.
- Selected examples, see: (a) B. R. Shire and E. A. Anderson, JACS Au, 2023, 3, 1539–1553 CrossRef CAS PubMed; (b) J. M. Anderson, N. D. Measom, J. A. Murphy and D. L. Poole, Angew. Chem., Int. Ed., 2021, 60, 24754–24769 Search PubMed; (c) M. M. D. Pramanik, H. Qian, W. J. Xiao and J. R. Chen, Org. Chem. Front., 2020, 7, 2531–2537 RSC; (d) R. Gianatassio, J. M. Lopchuk, J. Wang, C. Pan, L. R. Malins, L. Prieto, T. A. Brandt, M. R. Collins, G. M. Gallego, N. W. Sach, J. E. Spangler, H. Zhu, J. Zhu and P. S. Baran, Science, 2016, 351, 241–246 CrossRef CAS PubMed; (e) X. Zhang, R. T. Smith, C. Le, S. J. McCarver, B. T. Shireman, N. I. Carruthers and D. W. C. MacMillan, Nature, 2020, 580, 220–226 CrossRef CAS PubMed; (f) Y. Yang, J. Tsien, J. M. E. Hughes, B. K. Peters, R. R. Merchant and T. Qin, Nat. Chem., 2021, 13, 950–955 CrossRef CAS PubMed; (g) J. H. Kim, A. Ruffoni, Y. S. S. Al-Faiyz, N. S. Sheikh and D. Leonori, Angew. Chem., Int. Ed., 2020, 59, 8225–8231 CrossRef CAS.
- Selected examples, see: (a) A. Denisenko, P. Garbuz, S. V. Shishkina, N. M. Voloshchuk and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2020, 59, 20515–20521 CrossRef CAS PubMed; (b) R. Kleinmans, T. Pinkert, S. Dutta, T. O. Paulisch, H. Keum, C. G. Daniliuc and F. Glorius, Nature, 2022, 605, 477–482 CrossRef CAS PubMed; (c) S. Agasti, F. Beltran, E. Pye, N. Kaltsoyannis, G. E. M. Crisenza and D. J. Procter, Nat. Chem., 2023, 15, 535–541 CrossRef CAS PubMed; (d) T. Rigotti and T. Bach, Org. Lett., 2022, 24, 8821–8825 CrossRef CAS PubMed; (e) S. Paul, D. Adelfinsky, C. Salome, T. Fessard and M. K. Brown, Chem. Sci., 2023, 14, 8070–8075 RSC; (f) A. Denisenko, P. Garbuz, Y. Makovetska, O. Shablykin, D. Lesyk, G. Al-Maali, R. Korzh, I. V. Sadkova and P. K. Mykhailiuk, Chem. Sci., 2023, 14, 14092–14099 RSC; (g) M. Reinhold, J. Steinebach, C. Golz and J. C. L. Walker, Chem. Sci., 2023, 14, 9885–9891 Search PubMed; (h) J. M. Posz, N. Sharma, P. A. Royalty, Y. Liu, C. Salome, T. C. Fessard and M. K. Brown, J. Am. Chem. Soc., 2024, 146, 10142–10149 CrossRef CAS PubMed; (i) S. Hu, Y. Pan, D. Ni and L. Deng, Nat. Commun., 2024, 15, 6128 CrossRef CAS; (j) R. Guo, Y.-C. Chang, L. Herter, C. Salome, S. E. Braley, T. C. Fessard and M. K. Brown, J. Am. Chem. Soc., 2022, 144, 7988–7994 CrossRef CAS PubMed; (k) Y. Liu, S. Lin, Y. Li, J.-H. Xue, Q. Li and H. Wang, ACS Catal., 2023, 13, 5096–5103 Search PubMed; (l) M. Xu, Z. Wang, Z. Sun, Y. Ouyang, Z. Ding, T. Yu, L. Xu and P. Li, Angew. Chem., Int. Ed., 2022, 61, e202214507 CrossRef CAS PubMed; (m) N. Radhoff, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2023, 62, e202304771 Search PubMed.
- Selected examples, see: (a) A. S. Harmata, T. E. Spiller, M. J. Sowden and C. R. J. Stephenson, J. Am. Chem. Soc., 2021, 143, 21223–21228 CrossRef CAS PubMed; (b) T. Iida, J. Kanazawa, T. Matsunaga, K. Miyamoto, K. Hirano and M. Uchiyama, J. Am. Chem. Soc., 2022, 144, 21848–21852 Search PubMed; (c) N. Frank, J. Nugent, B. R. Shire, H. D. Pickford, P. Rabe, A. J. Sterling, T. Zarganes-Tzitzikas, T. Grimes, A. L. Thompson, R. C. Smith, C. J. Schofield, P. E. Brennan, F. Duarte and E. A. Anderson, Nature, 2022, 611, 721–726 CrossRef CAS; (d) T. V. T. Nguyen, A. Bossonnet, M. D. Wodrich and J. Waser, J. Am. Chem. Soc., 2023, 145, 25411–25421 Search PubMed; (e) Y. Zheng, W. Huang, R. K. Dhungana, A. Granados, S. Keess, M. Makvandi and G. A. Molander, J. Am. Chem. Soc., 2022, 144, 23685–23690 CrossRef CAS PubMed.
- Selected examples, see: (a) V. V. Levterov, Y. Panasyuk, V. O. Pivnytska and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2020, 59, 7161–7167 Search PubMed; (b) D. Dibchak, M. Snisarenko, A. Mishuk, O. Shablykin, L. Bortnichuk, O. Klymenko-Ulianov, Y. Kheylik, I. V. Sadkova, H. S. Rzepa and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2023, 62, e202304246 CrossRef CAS PubMed; (c) V. V. Levterov, Y. Panasiuk, O. Shablykin, O. Stashkevych, K. Sahun, A. Rassokhin, I. Sadkova, D. Lesyk, A. Anisiforova, Y. Holota, P. Borysko, I. Bodenchuk, N. M. Voloshchuk and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2024, 63, e202319831 CrossRef CAS PubMed.
- Selected examples, see: (a) Y. Tamura, H. Ishibashi, M. Hirai, Y. Kita and M. Ikeda, J. Org. Chem., 1975, 40, 2702–2710 CrossRef CAS; (b) S. Breitenlechner and T. Bach, Angew. Chem., Int. Ed., 2008, 47, 7957–7959 CrossRef CAS; (c) D. Albrecht, F. Vogt and T. Bach, Chem.–Eur. J., 2010, 16, 4284–4296 CrossRef CAS; (d) A. Denisenko, P. Garbuz, N. M. Voloshchuk, Y. Holota, G. Al-Maali, P. Borysko and P. K. Mykhailiuk, Nat. Chem., 2023, 15, 1155–1163 CrossRef CAS; (e) D. M. Whalley, L. Carlino, O. D. Putra, N. A. Anderson, S. C. Coote and O. Lorthioir, Chem. Sci., 2024, 15, 19564–19570 Search PubMed.
- Selected reviews, see: (a) P. Bellotti and F. Glorius, J. Am. Chem. Soc., 2023, 145, 20716–20732 Search PubMed; (b) J. Turkowska, J. Durka and D. Gryko, Chem. Commun., 2020, 56, 5718–5734 RSC; (c) Q.-B. Zhang, F. Li, B. Pan, S. Zhang, X.-G. Yue and Q. Liu, Green Chem., 2024, 26, 11083–11105 RSC; (d) A. Fawcett, Pure Appl. Chem., 2020, 92, 751–765 CrossRef CAS; (e) C. B. Kelly, J. A. Milligan, L. J. Tilley and T. M. Sodano, Chem. Sci., 2022, 13, 11721–11737 RSC; (f) J. L. Tyler and V. K. Aggarwal, Chem.–Eur. J., 2023, 29, e202300008 CrossRef CAS PubMed; (g) M. Golfmann and J. C. L. Walker, Commun. Chem., 2023, 6, 9 CrossRef CAS PubMed; (h) Q. Q. Hu, J. Chen, Y. Yang, H. Yang and L. Zhou, Tetrahedron Chem, 2024, 9, 100070 CrossRef CAS; (i) S. J. Sujansky and X. Ma, Asian J. Org. Chem., 2024, 13, 1–13 CrossRef.
- (a) Y. Liang, R. Kleinmans, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2022, 144, 20207–20213 CrossRef CAS PubMed; (b) Y. Liang, F. Paulus, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2023, 62, e202305043 CrossRef CAS PubMed.
- (a) T. Qin, M. He and W. Zi, Nat. Synth., 2024, 4, 124–133 CrossRef; (b) T. Li, Y. Wang, Y. Xu, H. Ren, Z. Lin, Z. Li and J. Zheng, ACS Catal., 2024, 14, 18799–18809 CrossRef CAS; (c) W. Wang, J.-A. Xiao, L. Zheng, W.-J. Liang, L. Yang, X.-X. Huang, C. Lin, K. Chen, W. Su and H. Yang, Org. Lett., 2024, 26, 10645–10650 CrossRef CAS PubMed.
- (a) S. Dutta, D. Lee, K. Ozols, C. G. Daniliuc, R. Shintani and F. Glorius, J. Am. Chem. Soc., 2024, 146, 2789–2797 CrossRef CAS PubMed; (b) J. L. Tyler, F. Schäfer, H. Shao, C. Stein, A. Wong, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2024, 146, 16237–16247 CrossRef CAS PubMed.
- M. Golfmann, M. Reinhold, J. D. Steen, M. S. Deike, B. Rodemann, C. Golz, S. Crespi and J. C. L. Walker, ACS Catal., 2024, 14, 13987–13998 CrossRef CAS.
- Selected examples, see: (a) K. Dhake, K. J. Woelk, J. Becica, A. Un, S. E. Jenny and D. C. Leitch, Angew. Chem., Int. Ed., 2022, 61, e202204719 CrossRef CAS PubMed; (b) H. Wang, H. Shao, A. Das, S. Dutta, H. T. Chan, C. Daniliuc, K. N. Houk and F. Glorius, Science, 2023, 381, 75–81 CrossRef CAS PubMed; (c) S. Dutta, Y.-L. Lu, J. E. Erchinger, H. Shao, E. Studer, F. Schäfer, H. Wang, D. Rana, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2024, 146, 5232–5241 CrossRef CAS PubMed; (d) C. C. Chintawar, R. Laskar, D. Rana, F. Schäfer, N. Van Wyngaerden, S. Dutta, C. G. Daniliuc and F. Glorius, Nat. Catal., 2024, 7, 1232–1242 CrossRef CAS; (e) F. Zhang, S. Dutta, A. Petti, D. Rana, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2025, e202418239 CAS; (f) J.-L. Zhou, Y. Xiao, L. He, X.-Y. Gao, X.-C. Yang, W.-B. Wu, G. Wang, J. Zhang and J.-J. Feng, J. Am. Chem. Soc., 2024, 146, 19621–19628 CrossRef CAS PubMed; (g) W.-B. Wu, B. Xu, X.-C. Yang, F. Wu, H.-X. He, X. Zhang and J.-J. Feng, Nat. Commun., 2024, 15, 8005 CrossRef CAS PubMed; (h) F. Wu, W. Wu, Y. Xiao, Z. Li, L. Tang, H. He, X. Yang, J. Wang, Y. Cai, T. Xu, J. Tao, G. Wang and J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202406548 CrossRef CAS PubMed; (i) H. He, F. Wu, X. Zhang and J. Feng, Angew. Chem., Int. Ed., 2025, 64, e202416741 CrossRef CAS PubMed; (j) X. Wang, R. Gao and X. Li, J. Am. Chem. Soc., 2024, 146, 21069–21077 CrossRef CAS PubMed; (k) Y. Liu, S. Lin, Z. Ding, Y. Li, Y. Tang, J. Xue, Q. Li, P. Li and H. Wang, Chem, 2024, 10, 3699–3708 CrossRef CAS; (l) Z. Lin, H. Ren, X. Lin, X. Yu and J. Zheng, J. Am. Chem. Soc., 2024, 146, 18565–18575 CrossRef CAS PubMed; (m) J. Zhang, J. Y. Su, H. Zheng, H. Li and W. P. Deng, Angew. Chem., Int. Ed., 2024, 63, e202318476 CrossRef CAS PubMed; (n) M. Zanini, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2024, 63, e202410207 CrossRef CAS PubMed; (o) X.-G. Zhang, Z.-Y. Zhou, J.-X. Li, J.-J. Chen and Q.-L. Zhou, J. Am. Chem. Soc., 2024, 146, 27274–27281 CrossRef CAS PubMed; (p) S. Dutta, C. G. Daniliuc, C. Mück-Lichtenfeld and A. Studer, J. Am. Chem. Soc., 2024, 146, 27204–27212 CrossRef CAS PubMed.
- (a) Y. Zhang, S. S. Chen, K. D. Li and H.-M. Huang, Angew. Chem., Int. Ed., 2024, e202401671 CAS; (b) S.-Y. Tang, Z.-J. Wang, Y. Ao, N. Wang and H.-M. Huang, Nat. Commun., 2025, 16, 1354 CrossRef CAS PubMed; (c) Y. Ao, N. Wang, S.-Y. Tang, Z.-J. Wang, L.-H. Zou and H.-M. Huang, ACS Catal., 2025, 15, 2212–2221 CrossRef CAS.
- Selected reviews, see: (a) L. Pitzer, J. L. Schwarz and F. Glorius, Chem. Sci., 2019, 10, 8285–8291 RSC; (b) H. Huang, P. Bellotti and F. Glorius, Acc. Chem. Res., 2022, 55, 1135–1147 CrossRef CAS PubMed; (c) M. Kojima and S. Matsunaga, Trends Chem., 2020, 2, 410–426 CrossRef CAS; (d) K. Ram Bajya and S. Selvakumar, Eur. J. Org Chem., 2022, 2022, e202200229 CrossRef CAS; (e) D. Kalsi, S. Dutta, N. Barsu, M. Rueping and B. Sundararaju, ACS Catal., 2018, 8, 8115–8120 CrossRef CAS.
- K. Gollnick and M. Weber, Tetrahedron Lett., 1990, 31, 4585–4588 CrossRef CAS.
- (a) N. A. Romero, K. A. Margrey, N. E. Tay and D. A. Nicewicz, Science, 2015, 349, 1326–1330 CrossRef CAS PubMed; (b) B. Chen, Y. Du and W. Shu, Angew. Chem., Int. Ed., 2022, 61, e202200773 CrossRef CAS PubMed; (c) L. Pitzer, F. Sandfort, F. Strieth-Kalthoff and F. Glorius, Angew. Chem., Int. Ed., 2018, 57, 16219–16223 CrossRef CAS PubMed.
- J. B. McManus, N. P. R. Onuska and D. A. Nicewicz, J. Am. Chem. Soc., 2018, 140, 9056–9060 CrossRef CAS PubMed.
- Selected reviews, see: (a) A. Y. Chan, I. B. Perry, N. B. Bissonnette, B. F. Buksh, G. A. Edwards, L. I. Frye, O. L. Garry, M. N. Lavagnino, B. X. Li, Y. Liang, E. Mao, A. Millet, J. V Oakley, N. L. Reed, H. A. Sakai, C. P. Seath and D. W. C. MacMillan, Chem. Rev., 2022, 122, 1485–1542 CrossRef CAS PubMed; (b) K. L. Skubi, T. R. Blum and T. P. Yoon, Chem. Rev., 2016, 116, 10035–10074 CrossRef CAS PubMed; (c) F.-D. Lu, J. Chen, X. Jiang, J.-R. Chen, L.-Q. Lu and W.-J. Xiao, Chem. Soc. Rev., 2021, 50, 12808–12827 RSC; (d) J. A. Milligan, J. P. Phelan, S. O. Badir and G. A. Molander, Angew. Chem., Int. Ed., 2019, 58, 6152–6163 CrossRef CAS PubMed.
- (a) Y.-L. Li, S.-Q. Zhang, J. Chen and J.-B. Xia, J. Am. Chem. Soc., 2021, 143, 7306–7313 CrossRef CAS PubMed; (b) X. Jiang, H. Jiang, Q. Yang, Y. Cheng, L.-Q. Lu, J. A. Tunge and W.-J. Xiao, J. Am. Chem. Soc., 2022, 144, 8347–8354 CrossRef CAS PubMed; (c) H. Jiang, X.-K. He, X. Jiang, W. Zhao, L.-Q. Lu, Y. Cheng and W.-J. Xiao, J. Am. Chem. Soc., 2023, 145, 6944–6952 CrossRef CAS PubMed; (d) T. Liang, Y. Wu, J. Sun, M. Li, H. Zhao, J. Zhang, G. Zheng and Q. Zhang, Chin. J. Chem., 2023, 41, 3253–3260 CrossRef CAS; (e) Y.-P. Shao and Y.-M. Liang, ACS Catal., 2025, 1147–1157 CrossRef CAS; (f) J. Hou, A. Ee, W. Feng, J.-H. Xu, Y. Zhao and J. Wu, J. Am. Chem. Soc., 2018, 140, 5257–5263 CrossRef CAS PubMed.
- X. Pan, H. Wang, C. Li, J. Z. H. Zhang and C. Ji, J. Chem. Inf. Model., 2021, 61, 3159–3165 CrossRef CAS PubMed.
- W. D. Jang, J. Jang, J. S. Song, S. Ahn and K. S. Oh, Comput. Struct. Biotechnol. J., 2023, 21, 3532–3539 CrossRef CAS PubMed.
- W. J. G. Hugh MacLean, J. Physiol., 1928, 65, 63–76 CrossRef PubMed.
- L. Kong, X. Shu, S. Tang, R. Ye, H. Sun, S. Jiang, Z. Li, J. Chai, Y. Fang, Y. Lan, L. Yu, Q. Xie, W. Fu, Y. Wang, W. Li, Z. Qiu, J. Liu and L. Shao, J. Med. Chem., 2022, 65, 10377–10392 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2389698. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc02836a |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |