 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Generation and UV-photolysis of N-methyleneformamide†
Viktor
Paczelt
 ,
Vladimir D.
Drabkin
,
Vladimir D.
Drabkin
 ,
Daniel
Kühn
,
Daniel
Kühn
 and
André K.
Eckhardt
and
André K.
Eckhardt
 *
*
Lehrstuhl für Organische Chemie II, Ruhr-Universität Bochum, 44801 Bochum, Germany. E-mail: Andre.Eckhardt@ruhr-uni-bochum.de
First published on 15th May 2025
Abstract
Simple imines have been detected in space and are considered as building blocks to larger heteroaromatic, cyclic and biologically relevant compounds. Herein, we revisited the formation of the simplest acylimine, namely N-methyleneformamide, by high-vacuum flash pyrolysis (HVFP) as well as its spectroscopic characterization by cryogenic matrix isolation infrared (IR) and UV/Vis spectroscopy. N-Methyleneformamide prefers a gauche over an s-trans conformation in contrast to the parent 1,3-butadiene. In UV-photolysis experiments we identified formaldehyde:HCN and formaldimine:CO complexes as the major decomposition products. As further photolysis products we observed HCN:CO and HNC:CO complexes. All experimental findings are supported by deuterium labeling experiments and high-level ab initio coupled cluster calculations. N-Methyleneformamide should be considered as a candidate for an interstellar search. The sequence R2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–RC
N–RC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O also occurs in cytosine making the title compound highly relevant for prebiotic chemistry and the search for the molecular origins of life.
O also occurs in cytosine making the title compound highly relevant for prebiotic chemistry and the search for the molecular origins of life.
Introduction
Imines are fundamental building blocks in prebiotic chemistry, e.g., in Strecker's amino acid synthesis or in HCN dimerization and the adenine forming Ferris Orgel reaction.1–3 Simple imines are continuously discovered in space, including formaldimine (H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH, 1),4 cyanomethanimine (NCHC
NH, 1),4 cyanomethanimine (NCHC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NH, 2)5 and others (Scheme 1).6–9 Only last year the high energy isomer of the HCN dimer 2, namely N-cyanomethanimine (H2C
NH, 2)5 and others (Scheme 1).6–9 Only last year the high energy isomer of the HCN dimer 2, namely N-cyanomethanimine (H2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) NCN, 3) including the prebiotically relevant NCN backbone, was discovered in space.10 This provides evidence that other differently substituted methanimines might also be present in space including N-methyleneformamide (4). Computational studies predict the formation of 4 from H2CO and HCN,11 as well as HCNO and CH2 under plausible interstellar conditions.12
NCN, 3) including the prebiotically relevant NCN backbone, was discovered in space.10 This provides evidence that other differently substituted methanimines might also be present in space including N-methyleneformamide (4). Computational studies predict the formation of 4 from H2CO and HCN,11 as well as HCNO and CH2 under plausible interstellar conditions.12
 | ||
| Scheme 1 Formaldimine (1) and the HCN dimers cyanoformaldimine (2) and N-cyanomethanimine (3) have been detected in the interstellar medium (ISM) while N-methyleneformamide (4) is not yet detected. | ||
N-Methyleneformamide is the simplest N-acylimine and was already part of theoretical13–19 and experimental investigations.20 It is noteworthy that the sequence R2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–RC
N–RC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O also occurs in the nucleobase cytosine, making 4 highly relevant for the field of prebiotic chemistry. N-Methyleneformamide was previously generated from a cyclopentadiene based precursor in a retro-aza-Diels–Alder reaction at 450 °C in the gas phase under high vacuum and characterized using low-temperature infrared spectroscopy.20 However, only the two most prominent vibrations, namely the CO and CN stretching vibrations at 1735 and 1695 cm−1, were observed in a solid film. Even in the condensed phase and in the absence of a solvent, 4 showed propensity to polymerize at −196 °C. Due to the high reactivity, a low temperature nuclear magnetic resonance (NMR) spectrum could not be reported in contrast to other reactive N-acylmethanimines.20 In a mass spectrometric and microwave spectroscopic study only trans-nitrosoethylene (H2CHCNO) could be identified as the elusive C2H3NO isomer.21 To the best of our knowledge the microwave spectrum of 4 has not yet been reported, despite its high dipole moment of 3.0 D (gauche, B3LYP/6-311+G**).18
O also occurs in the nucleobase cytosine, making 4 highly relevant for the field of prebiotic chemistry. N-Methyleneformamide was previously generated from a cyclopentadiene based precursor in a retro-aza-Diels–Alder reaction at 450 °C in the gas phase under high vacuum and characterized using low-temperature infrared spectroscopy.20 However, only the two most prominent vibrations, namely the CO and CN stretching vibrations at 1735 and 1695 cm−1, were observed in a solid film. Even in the condensed phase and in the absence of a solvent, 4 showed propensity to polymerize at −196 °C. Due to the high reactivity, a low temperature nuclear magnetic resonance (NMR) spectrum could not be reported in contrast to other reactive N-acylmethanimines.20 In a mass spectrometric and microwave spectroscopic study only trans-nitrosoethylene (H2CHCNO) could be identified as the elusive C2H3NO isomer.21 To the best of our knowledge the microwave spectrum of 4 has not yet been reported, despite its high dipole moment of 3.0 D (gauche, B3LYP/6-311+G**).18
N-Methyleneformamide is a 1,3-butadiene heteroanalog, preferring a gauche over a planar s-trans conformation according to density functional theory (DFT) calculations (cf. Computational analysis, Fig. 5).18,19 The presence of both an imino and a carbonyl functional group gives 4 a rotational profile around the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C
N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O dihedral angle, which is at odds with other (hetero)butadiene analogs.22 The energetic preference for the gauche conformer was rationalized with electronic repulsion effects.11 The repulsive interactions are greater than the conjugation effect in the two planar conformations of 4.18,19 Only recently, the non-planar gauche preference for the parent 1,3-butadiene was demonstrated experimentally in a Fourier-transform microwave (FTMW) spectroscopy study.23 The lower energy s-trans conformer does not possess a dipole moment and is invisible to radio astronomy. However, the cyano tagged butadiene analog, namely s-trans-E-1-cyano-1,3-butadiene, was observed in TMC-1 in 2023 suggesting that more heteroatom substituted butadienes might be present in space.24
O dihedral angle, which is at odds with other (hetero)butadiene analogs.22 The energetic preference for the gauche conformer was rationalized with electronic repulsion effects.11 The repulsive interactions are greater than the conjugation effect in the two planar conformations of 4.18,19 Only recently, the non-planar gauche preference for the parent 1,3-butadiene was demonstrated experimentally in a Fourier-transform microwave (FTMW) spectroscopy study.23 The lower energy s-trans conformer does not possess a dipole moment and is invisible to radio astronomy. However, the cyano tagged butadiene analog, namely s-trans-E-1-cyano-1,3-butadiene, was observed in TMC-1 in 2023 suggesting that more heteroatom substituted butadienes might be present in space.24
Panda et al. explored various C2H3NO isomers in the context of interstellar chemistry. Methylisocyanate (H3CNCO), glycolonitrile (HOCH2CN), 4 and 2-iminoacetaldehyde (HC(O)CHNH) were identified as the lowest energy C2H3NO isomers.11 We reported recently the spectroscopic characterization of interstellar relevant imines by UV photolysis of the corresponding azide precursors.25–27 In the UV photolysis of 2-azidoacetaldehyde we identified 2-iminoacetaldehyde and two unassigned bands at 993.7 and 1469.9 cm−1 that did not show any photochemistry. As 4 was suggested as a viable photoproduct but IR spectroscopically only characterized by two bands, we revisited the preparation of 4 and investigated its photochemistry experimentally under matrix isolation conditions.
Results and discussion
Infrared spectroscopy
For the generation of 4 we synthesized N-formyl-2-azabicyclo[2.2.1]hept-5-ene (5) as described by Lasne et al. including the 3,3-deuterium isotopically labelled compound (Scheme S1†).20 We performed high-vacuum flash pyrolysis (HVFP) experiments with 5 at 450 °C and directly trapped all pyrolysis products with an excess of argon on a cold matrix window at 3.4 K (Scheme 2). Besides the well-known signals of matrix isolated cyclopentadiene (8) in argon,28 we identified another set of signals at 2956.8, 2908.0, 2873.7, 1719.4, 1664.0, 1473.1, 1372.6, 1196.1, 1057.0, 1030.5, 992.6, and 786.6 cm−1 (Tables 1 and S1† (extended version)), which we assigned to the fundamentals of gauche-4 based on our harmonic frequency calculations of gauche-4 at the CCSD(T)/cc-pVTZ29–33 and anharmonic frequency calculations at the B2PLYP/6-311++G(2d,2p)34–36 level of theory (Fig. S1, Table 1, S1†). It is worth noting that our experimental results are in very good agreement with the anharmonic calculations of the B2PLYP double hybrid functional as already pointed out in benchmark studies.37 There is no spectroscopic evidence for s-trans-4 in our pyrolysis spectrum. Cyclopentadiene and gauche-4 are the retro-aza-Diels–Alder reaction products of 5. We irradiated the matrix with UV light (λ = 254 nm, 40 min) and observed a 40% decomposition of gauche-4 along with the reported disrotatory photoinduced intramolecular cycloaddition of 8 forming bicyclo[2.1.0]pent-2-ene (BCP) as observed by its two most intense bands at 774 and 720 cm−1(Fig. S2†).28 We identified complexed formaldimine38 and carbon monoxide as well as the reported HCN:formaldehyde complex39 as the primary photolysis products of gauche-4 (Fig. S2†).| Mode | Approx. description | B2PLYP/6-311++G(2d,2p) | Experiment | ||
|---|---|---|---|---|---|
| ṽ anh./cm−1 | I anh./km mol−1 | ṽ exp./cm−1 | I rel | ||
| a rel. experimental intensities (w = weak, m = medium, s = strong, vs = very strong); n.o.: not observed. | |||||
| 3 | δ(NCO), τ(HCNC) | 502.9 | 7.0 | n.o | — |
| 4 | τ(HCNC) | 790.5 | 8.6 | 786.6 | w |
| 5 | v(CN), δ(NCO), τ(NCHO) | 985.0 | 64.0 | 992.6 | s |
| 6 | τ(NCHO) | 1040.6 | 46.1 | 1030.5 | m |
| 7 | τ(NCHH) | 1078.5 | 17.3 | 1057.0 | m |
| 8 | δ(HCN) | 1207.1 | 18.6 | 1196.1 | m |
| 9 | δ(HCO) | 1376.8 | 7.7 | 1372.6 | w |
| 10 | δ(HCH) | 1488.9 | 15.6 | 1473.1 | m |
| 11 | v(CN) | 1665.6 | 127.8 | 1664.0 | s |
| 12 | v(CO) | 1712.1 | 163.3 | 1719.4 | vs |
| 13 | v(CH) | 2858.0 | 63.0 | 2874.7 | m |
| 14 | v sym(CH2) | 2912.4 | 21.5 | 2908.0 | w |
| 15 | v as(CH2) | 3045.6 | 15.5 | 3029.9 | w |
As we observed critical overlaps in the IR spectrum of gauche-4 with 8 and its photochemistry, we investigated another precursor for the generation of gauche-4. In the [4 + 2] retro-aza-Diels–Alder reaction of 5, cyclopentadiene acts as the diene and 4 as the dienophile. We synthesized 5,6-dihydro-4H-1,3-oxazine (6) as an alternative precursor (Scheme S2†). In the corresponding [4 + 2] retro-aza-Diels–Alder reaction ethylene and 4 should be the reaction products as the dienophile and diene, respectively. The oxazine heterocycle is highly sensitive and polymerizes as a neat liquid even at −26 °C in the dark within three days. We conducted HVFP experiments with 6 in the 450–750 °C temperature range (Scheme 2). The compound is more stable than 5 and starts decomposing at around 650 °C. At 750 °C we identified the same set of signals for gauche-4 as described in the pyrolysis of 5 as the major pyrolysis product along with the characteristic sets of signals for ethylene,40 HCN, formaldehyde, formaldimine, CO and undecomposed starting material (Fig. S3†). In contrast to 8, ethylene and the other pyrolysis side products do not show any photochemistry after UV irradiation (λ > 254 nm), and we observed a difference spectrum after photolysis of gauche-4 only (Fig. 1). The tiny signals in Fig. 1, which are pointing downwards and are marked with an asterisk (*) at 3419.0, 2984.4, 2111.6, and 1978.3 cm−1, are determined to be the corresponding overtone signals of the fundamentals of gauche-4 at 1719.4, 1473.1, 1057.0, and 992.6 cm−1. The characteristic signal in the carbonyl region at 1779.2 cm−1 is a combinational band of the fundamentals at 992.6 and 786.6 cm−1. After prolonged photolysis we also identified the HCN:CO and HNC:CO complexes as further photolysis products.41 We assume that IR inactive dihydrogen forms together with these complexes.
Deuterium labelling experiment
To provide further spectroscopic evidence for the generation of gauche-4, we synthesized 3,3-deuterium labelled N-formyl-2-azabicyclo[2.2.1]hept-5-ene (5-d2). The compound can easily be prepared by reducing Vince lactam42 (2-azabicyclo[2.2.1]hept-5-en-3-one, 9) with lithium aluminum deuteride (LiAlD4) and subsequent formylation (Scheme S1†).20 We carried out pyrolysis experiments with the deuterium labeled compound in a similar fashion and assigned a new set of signals at 2281.0, 2159.8, 1711.9, 1623.3, 1121.4, 1039.1, 979.3, 926.6, 847.6, and 707.8 cm−1 to deuterium labeled gauche-4-d2 (D2CNHCO) based on our CCSD(T)/cc-pVTZ frequency calculations (Tables 2 and S2† (extended version), Fig. 2). Irradiation of the matrix with 254 nm results in the disappearance of the described signals and the formation of complexed formaldimine-d2 (ref. 38) (D2CNH) and CO as well as a DCN:formaldehyde-d1 complex. The tiny signal at 3415.8 cm−1, which is marked with an asterisk, is assigned to the overtone signal of the fundamental of gauche-4-d2 at 1717.6 cm−1. The characteristic marked signal in the carbonyl region at 1692.9 cm−1 is a combinational band of the fundamentals at 707.8 and 979.3 cm−1.| Mode | Approx. description | B2PLYP/6-311++G(2d,2p) | Experiment | ||
|---|---|---|---|---|---|
| ṽ anh./cm−1 | I anh./km mol−1 | ṽ exp./cm−1 | I rel | ||
| a rel. experimental intensities (w = weak, m = medium, s = strong); ° = overlap. | |||||
| 4 | τ(DCNC), δ(NCO) | 709.7 | 10.7 | 707.8 | w |
| 5 | τ(NCDD) | 865.0 | 5.5 | 847.6 | w |
| 6 | δ(DCD), δ(HCO) | 934.9 | 33.7 | 926.6 | w |
| 7 | δ(NCD), δ(DCD) | 982.1 | 76.9 | 979.3 | m |
| 8 | τ(NCHO) | 1044.8 | 16.3 | 1039.1 | w |
| 9 | δ(DCD) | 1129.0 | 32.3 | 1121.4 | m |
| 10 | δ(HCO) | 1366.5 | 4.0 | 1368.7° | w |
| 11 | v(CN) | 1623.5 | 71.9 | 1623.3 | m |
| 12 | v(CO) | 1707.2 | 193.9 | 1717.6 | s |
| 13 | v sym(CD2) | 2161.7 | 29.9 | 2159.8 | w |
| 14 | v as(CD2) | 2289.0 | 12.9 | Ov.°, (CO2) | w |
| 15 | v(CH) | 2861.8 | 63.5 | 2871.4 | w |
UV/Vis spectroscopy
We also repeated the pyrolysis experiments with 6 and conducted UV/Vis spectroscopy experiments with the trapped pyrolysis products. As the UV/Vis absorptions of 8 show critical overlaps in the UV region with the calculated transitions of gauche-4, precursor 6 is more suitable for UV/Vis experiments than 5. The major pyrolysis side product, ethylene, shows absorption below 200 nm,43 as well as the other minor by-products formaldehyde,44 HCN,45 and CO.46 Only methylene imine has a transition at around 250 nm;47 however, in the pyrolysis IR spectrum it is observed only in traces (Fig. S3†). Precursor 6 has a calculated weak transition at λ = 206 nm (oscillator strength f = 0.007) at the TD-ωB97XD/6-311++G(2d,2p)34,35,48 level of theory that is in very good agreement with our experimental UV/Vis spectrum in argon (λmax = 211 nm, Fig. S9†). For gauche-4 we calculated transitions at the same level of theory at λ = 283 nm (f = 0.005), λ = 237 nm (f = 0.001), and λ = 196 nm (f = 0.028) (Fig. 3). After 15 min pyrolysis of 6, we recorded the black spectrum depicted in Fig. 3 with three absorption maxima at λmax = 288 nm, λmax = 251 nm, and λmax = 208 nm. The two absorption bands at λmax = 288 and λmax = 251 nm are in good agreement with the calculated transition while the absorption maximum at λmax = 208 nm overlaps with the absorption of undecomposed precursor 6 (Fig. S9†). After irradiation of the matrix with λ = 308 nm for 150 min all bands decreased while no new signals appeared (vide supra) as depicted in the red curve in Fig. 3. According to our TD-DFT calculations the electronic transition at 283 nm can be associated with a HOMO (Highest Occupied Molecular Orbital)–LUMO (Lowest Unoccupied Molecular Orbital) transition (89%). The HOMO represents a non-bonding (n) electron pair of oxygen, while the LUMO is the π* orbital of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond based on the depicted natural bond orbitals in Fig. 3. Hence, the HOMO–LUMO transition can be attributed to an n–π* transition. The calculated transitions at 237 and 208 nm are more mixed. The major contribution for both transitions comes from the HOMO−1 to LUMO transition (n–π*, 41% and 53%) while there are minor contributions from the HOMO–LUMO+1 (n–π*, 14% and 15%) and the HOMO–LUMO+2 (n–σ*, 18% and 12%) transitions.
N double bond based on the depicted natural bond orbitals in Fig. 3. Hence, the HOMO–LUMO transition can be attributed to an n–π* transition. The calculated transitions at 237 and 208 nm are more mixed. The major contribution for both transitions comes from the HOMO−1 to LUMO transition (n–π*, 41% and 53%) while there are minor contributions from the HOMO–LUMO+1 (n–π*, 14% and 15%) and the HOMO–LUMO+2 (n–σ*, 18% and 12%) transitions.
Computational analysis
We calculated the closed-shell thermal decomposition pathways starting from 5 (Fig. S30†) and 6 (Fig. 4) that lead to the formation of 4 at the DLPNO-CCSD(T)/CBS//B3LYP-D3(BJ)/6-311++G(2d,2p)49–56 level of theory including zero-point vibrational energy (ZPVE) corrections (ΔH0) and Gibbs free energy corrections at 750 °C (ΔG(750 °C), blue in Fig. 4). The Gibbs barrier for the decomposition of 6 at 750 °C in the retro-aza-Diels–Alder reaction is 46.0 kcal mol−1 (TS1) and is associated with the formation of 4 and ethylene. We located another transition state TS2 that is much higher in energy (89.9 kcal mol−1) and connects precursor 6 directly to 7, CO and ethylene. The imaginary frequency of TS2 resembles a ring breathing vibration in 6. However, a stepwise decomposition of 6via gauche4 and TS5 (65.7 kcal mol−1) is more favorable at the high pyrolysis temperatures. We located another transition state TS4 (70.5 kcal mol−1) that is associated with the dissociation of 4 into 1 and CO. All dissociation products were also identified in our pyrolysis spectrum at elevated temperatures. For 5 the Gibbs barrier for the retro-aza-Diels–Alder reaction at 450 °C is 41.0 kcal mol−1 (TS6, Fig. S29†). This is in good agreement with our experimental results, as 5 already decomposed at much lower pyrolysis temperatures (vide supra).Our matrix isolation experiments were conducted at 3.4 K. At this low temperature thermal contributions to the Gibbs free energy are negligible and our experiments are well represented by ZPVE corrected electronic energies (ΔH0). There are two conformers for 4, namely gauche and s-trans. The planar s-trans conformer is 1.4 kcal mol−1 higher in energy, while the planar s-cis conformation represents a transition state (0.4 kcal mol−1), which connects two gauche minima of 4 in a double valley. We performed a relaxed scan of the electronic energy of the τ(CNCO) dihedral angle in 4 at the DLPNO-CCSD(T)/CBS//B3LYP-D3(BJ)/6-311++G(2d,2p) level of theory. The obtained rotational profile is shown in Fig. 5. The dihedral angle τ(CNCO) in gauche4 is (−)54.2° calculated at the CCSD(T)/cc-pVTZ level of theory (Fig. 4). However, at the chosen DFT level the τ(CNCO) dihedral angle for the optimized geometry deviates by almost 10° and is (−)64.6°, which is a bit larger than with the B2PLYP functional ((−)61.2). The gauche and s-trans conformers of 4 are connected viaTS3. With augmented coupled cluster energies and ZPVE corrections we obtain a barrierless transition in Fig. 4. This demonstrates the dependency of the augmented coupled cluster energy on the molecular geometry and ZPVE corrections (Fig. 5). The small barrier and the high energy nature of s-trans4 (1.4 kcal mol−1) are a reasonable explanation of why we only observed the gauche conformer in our low temperature matrix isolation experiments.
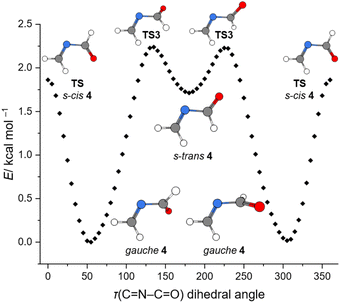 | ||
Fig. 5 Rotational profile around the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–C N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O dihedral angle of N-methyleneformamide (4) calculated at the DLPNO-CCSD(T)/CBS//B3LYP-D3(BJ)/6-311++G(2d,2p) level of theory. O dihedral angle of N-methyleneformamide (4) calculated at the DLPNO-CCSD(T)/CBS//B3LYP-D3(BJ)/6-311++G(2d,2p) level of theory. | ||
Conclusions
In summary, we report the full matrix isolation IR and UV/Vis spectroscopic characterization of gauche N-methyleneformamide and its photochemical UV light induced fragmentation into formaldehyde:HCN and formaldimine:CO complexes. As further decomposition products we observed.HCN:CO and HNC:CO complexes. Theoretical explorations with multiconfigurational methods on such photochemical decompositions could shed some more light on the detailed reaction mechanisms in the future.
In contrast to the parent 1,3-butadiene N-methyleneformamide prefers a gauche over an s-trans conformation. N-Methyleneformamide is a promising candidate for interstellar detection. To facilitate an interstellar search, the molecule should be characterized by rotational spectroscopy to provide the necessary laboratory rotational transitions. If this compound is formed in laboratory astrochemistry experiments or is directly observed in space, it would suggest a possible connection between simple acylimines and larger biologically relevant molecules, such as cytosine as a nucleobase in which the sequence R2C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–RC
N–RC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O appears. This discovery could potentially increase the complexity of prebiotic molecules of interest to astrobiology and the search for the molecular origins of life.
O appears. This discovery could potentially increase the complexity of prebiotic molecules of interest to astrobiology and the search for the molecular origins of life.
Data availability
The data that support the findings of this study are available in the ESI.† These include selected IR and UV/Vis spectra and data, synthetic procedures, NMR spectra, Cartesian Coordinates and energies of calculated structures. All evaluated spectra are available free of charge electronically at https://doi.org/10.17877/RESOLV-2025-M724WNW4.Author contributions
V. P. and D. K. synthesized the precursors. V. P. and V. D. D. conducted all matrix isolation experiments. V. P. and A. K. E. performed the computational studies, analyzed the data and co-wrote the manuscript. A. K. E. conceived the idea and supervised the project.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Fonds der Chemischen Industrie (Liebig Fellowship A. K. E. and V. P.) and funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) as part of the Emmy-Noether program (DFG project 530220027) and under Germany's Excellence Strategy – EXC-2033-390677874 – RESOLV. Continuous and generous support by all organic chemistry professors at RUB is acknowledged.Notes and references
- A. Strecker, Adv. Cycloaddit., 1850, 75, 27–45 Search PubMed.
- A. Strecker, Adv. Cycloaddit., 1854, 91, 349–351 Search PubMed.
- J. P. Ferris and L. E. Orgel, J. Am. Chem. Soc., 1966, 88, 3829–3831 CrossRef CAS PubMed.
- P. D. Godfrey, R. D. Brown, B. J. Robinson and M. W. Sinclair, Astrophys. Lett., 1973, 13, 119 CAS.
- D. P. Zaleski, N. A. Seifert, A. L. Steber, M. T. Muckle, R. A. Loomis, J. F. Corby, O. Martinez, K. N. Crabtree, P. R. Jewell, J. M. Hollis, F. J. Lovas, D. Vasquez, J. Nyiramahirwe, N. Sciortino, K. Johnson, M. C. McCarthy, A. J. Remijan and B. H. Pate, Astrophys. J. Lett., 2013, 765, L10 CrossRef.
- R. A. Loomis, D. P. Zaleski, A. L. Steber, J. L. Neill, M. T. Muckle, B. J. Harris, J. M. Hollis, P. R. Jewell, V. Lattanzi, F. J. Lovas, O. M. Jr, M. C. McCarthy, A. J. Remijan and B. H. Pate, Astrophys. J., 2013, 765, L9 CrossRef.
- P. D. Gensheimer, Astrophys. Space Sci., 1997, 251, 199–202 CrossRef CAS.
- F. J. Lovas, J. M. Hollis, A. J. Remijan and P. R. Jewell, Astrophys. J., 2006, 645, L137 CrossRef CAS.
- L. Bizzocchi, D. Prudenzano, V. M. Rivilla, A. Pietropolli-Charmet, B. M. Giuliano, P. Caselli, J. Martín-Pintado, I. Jiménez-Serra, S. Martín, M. A. Requena-Torres, F. Rico-Villas, S. Zeng and J.-C. Guillemin, Astron. Astrophys., 2020, 640, A98 CrossRef CAS.
- D. S. Andrés, V. M. Rivilla, L. Colzi, I. Jiménez-Serra, J. Martín-Pintado, A. Megías, Á. López-Gallifa, A. Martínez-Henares, S. Massalkhi, S. Zeng, M. Sanz-Novo, B. Tercero, P. de Vicente, S. Martín, M. A. Requena-Torres, G. Molpeceres and J. G. de la Concepción, Astrophys. J., 2024, 967, 39 CrossRef.
- S. Panda, A. Chiranjibi, D. Awasthi, S. Ghosal and A. Anoop, ACS Earth Space Chem., 2023, 7, 1739–1752 CrossRef CAS.
- H. Ji, A. Rágyanszki and R. A. Fournier, ChemRxiv, 2023, preprint, DOI:10.26434/chemrxiv-2023-kpm5r.
- K. B. Wiberg, P. R. Rablen and J. H. Baraban, J. Org. Chem., 2018, 83, 8473–8482 CrossRef CAS PubMed.
- R. Allmann, R. Kupfer, M. Nagel and E.-U. Würthwein, Chem. Ber., 1984, 117, 1597–1605 CrossRef CAS.
- V. A. Petukhov, N. E. Agafonov and I. A. Abronin, Bull. Acad. Sci. USSR, 1984, 33, 414–417 CrossRef.
- W. Förner, J. Ladik, P. Otto and J. ížek, Chem. Phys., 1985, 97, 251–262 CrossRef.
- M. A. McAllister and T. T. Tidwell, J. Chem. Soc., Perkin Trans. 2, 1994, 2239 RSC.
- H. M. Badawi, J. Mol. Struct., 2002, 617, 9–15 Search PubMed.
- D. Yu. Afanas’yev and A. V. Prosyanik, J. Mol. Struct., 2005, 730, 45–49 Search PubMed.
- M. C. Lasne, J. L. Ripoll and A. Thuillier, J. Chem. Res., Synop., 1982, 8, 214–215 Search PubMed.
- T. Sakaizumi, M. Nishikawa, A. Usami, H. Satoh and O. Ohashi, J. Anal. Appl. Pyrolysis, 1995, 34, 219–227 CrossRef CAS.
- K. B. Wiberg, P. R. Rablen and M. Marquez, J. Am. Chem. Soc., 1992, 114, 8654–8668 Search PubMed.
- J. H. Baraban, M.-A. Martin-Drumel, P. B. Changala, S. Eibenberger, M. Nava, D. Patterson, J. F. Stanton, G. B. Ellison and M. C. McCarthy, Angew. Chem., Int. Ed., 2018, 57, 1821–1825 CrossRef CAS PubMed.
- I. R. Cooke, C. Xue, P. B. Changala, H. T. Shay, A. N. Byrne, Q. Y. Tang, Z. T. P. Fried, K. L. Kelvin Lee, R. A. Loomis, T. Lamberts, A. Remijan, A. M. Burkhardt, E. Herbst, M. C. McCarthy and B. A. McGuire, Astrophys. J., 2023, 948, 133 CrossRef CAS.
- A. K. Eckhardt, Chem. Commun., 2022, 58, 8484–8487 Search PubMed.
- V. Paczelt, R. C. Wende, P. R. Schreiner and A. K. Eckhardt, Angew. Chem., Int. Ed., 2023, 62, e202218548 CrossRef CAS PubMed.
- V. D. Drabkin, V. Paczelt and A. K. Eckhardt, Chem. Commun., 2023, 59, 12715–12718 RSC.
- J. Miyazaki and Y. Yamada, J. Mol. Struct., 2004, 692, 145–153 CrossRef CAS.
- J. Čížek, J. Chem. Phys., 1966, 45, 4256–4266 CrossRef.
- K. Raghavachari, G. W. Trucks, J. A. Pople and M. Head-Gordon, Chem. Phys. Lett., 1989, 157, 479–483 CrossRef CAS.
- R. J. Bartlett, J. D. Watts, S. A. Kucharski and J. Noga, Chem. Phys. Lett., 1990, 165, 513–522 CrossRef CAS.
- J. F. Stanton, Chem. Phys. Lett., 1997, 281, 130–134 CrossRef CAS.
- T. H. Dunning Jr, J. Chem. Phys., 1989, 90, 1007–1023 CrossRef.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650–654 CrossRef CAS.
- T. Clark, J. Chandrasekhar, G. W. Spitznagel and P. V. R. Schleyer, J. Comput. Chem., 1983, 4, 294–301 CrossRef CAS.
- S. Grimme, J. Chem. Phys., 2006, 124, 034108 CrossRef PubMed.
- V. Barone, G. Ceselin, M. Fusè and N. Tasinato, Front. Chem., 2020, 8, 584203 CrossRef CAS PubMed.
- M. E. Jacox and D. E. Milligan, J. Mol. Spectrosc., 1975, 56, 333–356 CrossRef CAS.
- S. B. H. Bach and B. S. Ault, J. Phys. Chem., 1984, 88, 3600–3604 CrossRef CAS.
- E. Rytter and D. M. Gruen, Spectrochim. Acta Part A Mol. Spectrosc., 1979, 35, 199–207 CrossRef.
- S. V. Kameneva, D. A. Tyurin and V. I. Feldman, Phys. Chem. Chem. Phys., 2017, 19, 24348–24356 RSC.
- R. Singh and R. Vince, Chem. Rev., 2012, 112, 4642–4686 Search PubMed.
- J. R. Platt, H. B. Klevens and W. C. Price, J. Chem. Phys., 1949, 17, 466–469 Search PubMed.
- D. C. Moule and A. D. Walsh, Chem. Rev., 1975, 75, 67–84 CrossRef CAS.
- G. Herzberg and K. K. Innes, Can. J. Phys., 1957, 35, 842–879 CrossRef CAS.
- H. Lefebvre-Brion, C. Moser and R. K. Nesbet, J. Chem. Phys., 1961, 35, 1702–1707 Search PubMed.
- A. Teslja, B. Nizamov and P. J. Dagdigian, J. Phys. Chem. A, 2004, 108, 4433–4439 CrossRef CAS.
- J.-D. Chai and M. Head-Gordon, Phys. Chem. Chem. Phys., 2008, 10, 6615–6620 RSC.
- Y. Guo, C. Riplinger, U. Becker, D. G. Liakos, Y. Minenkov, L. Cavallo and F. Neese, J. Chem. Phys., 2018, 148, 011101 Search PubMed.
- T. Helgaker, W. Klopper, H. Koch and J. Noga, J. Chem. Phys., 1997, 106, 9639–9646 CrossRef CAS.
- A. Halkier, T. Helgaker, P. Jørgensen, W. Klopper, H. Koch, J. Olsen and A. K. Wilson, Chem. Phys. Lett., 1998, 286, 243–252 CrossRef CAS.
- A. D. Becke, Phys. Rev. A, 1988, 38, 3098–3100 CrossRef CAS PubMed.
- C. Lee, W. Yang and R. G. Parr, Phys. Rev. B:Condens. Matter Mater. Phys., 1988, 37, 785–789 Search PubMed.
- A. D. Becke, J. Chem. Phys., 1993, 98, 5648–5652 CrossRef CAS.
- T. H. Dunning Jr, J. Chem. Phys., 1989, 90, 1007–1023 CrossRef.
- S. Grimme, S. Ehrlich and L. Goerigk, J. Comput. Chem., 2011, 32, 1456–1465 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc02777b |
| This journal is © The Royal Society of Chemistry 2025 |





