Open Access Article
Open Access ArticleHighly efficient and selective Sr2+ capture using robust two-dimensional MOF nanosheets decorated with cage-like cavities†
Xiao-Ran Jiaa,
Zi-Xuan Gaoa,
Hai-Ming Feic,
Li-Juan Lana,
Cai-Xia Yu*a,
Yong Qian*b and
Lei-Lei Liu *a
*a
aSchool of Environmental and Material Engineering, Yantai University, Yantai 264005, P. R. China. E-mail: liuleileimail@163.com
bState Key Laboratory of Nuclear Resources and Environment, East China University of Technology, Nanchang, Jiangxi 330013, P. R. China
cCollege of Chemical Engineering, Huaqiao University, Xiamen 361021, P. R. China
First published on 1st July 2025
Abstract
The urgent need for efficient extraction of radio-strontium (Sr) from complex aquatic environments arises from its extreme radiotoxicity to both ecosystems and human health, which remains a significant challenge. In this study, we developed an ultrathin 2D Cu-MOF-COOH nanosheet with cage-like cavities for strontium separation. Incorporating the permanent cavity structures on the MOF nanosheet can fully utilize its structural characteristics of a largely exposed surface area and accessible adsorption sites in pollutant removal, and the comprehensive interactions between pollutants with the active sites and cavities on the exposed surfaces can achieve highly selective and efficient capture. Consequently, the Cu-MOF-COOH nanosheet exhibited superior capture performances, in terms of removal kinetics, selectivity, and uptake capacity, which are obviously better than its 3D counterpart. Moreover, it demonstrated ultra-high selectivity and anti-interference ability, enabling efficient Sr2+ removal even in the presence of large excesses of Ca2+, Ba2+, and other alkali and alkaline earth metal ions. Remarkable anti-interference performances were further validated by its practical applications in diverse real-world samples, including lake water, simulated groundwater, and radioactive wastewater, with Sr2+ removal efficiencies exceeding 91%. These exceptional extraction performances can be attributed to the synergistic interactions between the accessible active sites (carboxylate groups) and cage-like cavities with Sr2+, which were clarified through a series of characterization studies and theoretical calculations. This study presents a highly promising material for the separation of radioactive Sr2+ from aqueous solution and, more importantly, offers a novel strategy for the rational design of ultrathin MOF nanosheets with cavity structures, which holds great potential for expanding the applications of MOF nanosheets.
Introduction
With the growing concerns over the energy crisis and climate change, nuclear power emerged as a promising alternative to fossil fuels.1 The safe management of radioactive wastes produced by nuclear power plants entails considerable difficulties. Among these, strontium-90 (90Sr) stands out as a particularly problematic radioisotope due to its relatively long half-life (t1/2 = 28.80 years) and high-energy β-emission (0.546 MeV).2,3 Moreover, the large inventory, high environmental mobility, and extremely high radiotoxicity made 90Sr one of the primary environmental concerns.4 Detailed radioecological investigations on the Chernobyl accident revealed the ultra-high mobility of radio-strontium in water bodies, where it exists in the form of Sr2+.5,6 Besides, due to its similar chemical characteristics to Ca2+, Sr2+ can be readily combined with bones, ultimately leading to bone cancers and leukemia.7,8 As a consequence, it is urgent to develop an efficient and targeted method for the separation of 90Sr from aqueous solutions.Metal–organic frameworks (MOFs) are a class of crystalline hybrid materials formed through the intricate coordination of metal ions or clusters with organic ligands.9–12 Due to their rationally designed and systematically adjustable structures and chemical functionalities, MOFs can be tailored to trap target pollutants within their pre-organized pores, with specific capture sites.13–17 Therefore, MOFs have emerged as advanced solid materials for efficient remediation of various environmental pollutants, including small-molecule organic pollutants,18,19 dyes,20 heavy metal ions,21,22 and radioisotopes.23–26 To date, numerous MOFs have been developed for Sr2+ separation, with several exhibiting high uptake capacities and selectivity. Yuan and Wang et al. synthesized a novel MOF-18Cr6, which held regular cavities and demonstrated selective separation of Sr2+ with a maximum uptake capacity of 84.93 mg g−1.27 Xiao and co-workers incorporated 18-crown-6 and 24-crown-8 into a Zr-MOF, resulting in a material that exhibited rapid adsorption kinetics and a high adsorption capacity for Sr2+ (149 mg g−1).8 Recently, Shi and Mei et al. designed and synthesized acyl-anchored metal–organic cages with interior cryptand-like recognition sites, which achieved highly selective removal of trace Sr2+.28 Park et al. constructed an anionic MOF and adopted the ion-exchange method for selective capture of Sr2+ from wastewater, achieving an adsorption capacity of 41.1 mg g−1.3 Despite the tunable nature and periodic structure, which conferred efficient extraction performance in Sr2+ removal, the limited diffusion rate and accessibility to the active sites embedded within the bulky framework significantly impaired the adsorption performances of most 3D MOFs, including their adsorption kinetics, selectivity, and uptake capacities. In particular, selectivity, along with the corresponding anti-interference capability, is a crucial factor for the application of MOF materials in complex practical water samples. Although MOFs can be purposefully modified with specific functional groups to enhance the selectivity for target removal, and this approach does yield some positive results,29,30 the indirect interaction between the binding sites in bulk MOFs with pollutants, primarily driven by electrostatic forces that initially attract the pollutants into the pores, significantly reduces the capture selectivity.31 And it still remains a significant challenge to separate Sr2+ from complicated systems that contain alkali and alkaline earth metal ions with similar electronic structures and chemical properties.32
To address the limitation of the restricted access to the internal sites within three-dimensional (3D) MOFs, 2D MOF nanosheets, characterized by their adequately exposed surfaces and active sites, have been successfully synthesized.33 The highly open structure of MOF nanosheets allows for the exposure of more accessible active sites, which would facilitate intimate contact and adequate interactions with pollutant molecules on the exposed surfaces, leading to enhanced selectivity and uptake capacities.34–37 Despite the numerous structural advantages in pollutant capture, the exfoliated nanosheets lack the necessary containers or cavities to accommodate pollutants, which is beneficial for the stable capture of pollutants.38,39 To fully utilize the unique structural advantages of 2D MOF nanosheets in pollutant removal, functionalizing pores or cavities onto their surfaces could be an effective strategy, which would not only maintain the accessibility of active sites but also provide the necessary space for pre-enriching and accommodating pollutant molecules, thereby enhancing the overall performance of the material in pollutant removal.
Calix[n]arenes (n = 4, 6, and 8), with a special cup-shaped structure and easily modified rims, can be an ideal ligand for the construction of MOF nanosheets with cavity structures.40–42 Herein, the calix[4]arene of 5,11,17,23-tetra-tert-butyl-25,26,27,28-tetrakis[(carboxyl)methoxy]calix[4]arene (H4L; Scheme S1†), functionalized with carboxyl groups and tert-butyl groups, was selected for the fabrication of MOFs for Sr2+ separation. In the design, the ligand H4L was modified with four carboxyl groups on the lower rims, which would be facile for the construction of cage-like cavities by the connection with metal ions, and the left uncoordinated carboxyl groups in the cavity can serve as Sr2+ capture sites (Scheme 1); the upper rims of H4L were modified with four tert-butyl groups, which generally do not get involved in MOF construction, thus their space hindered effect is conducive to obtaining a layered structure. The large spatial dimensions of the tert-butyl groups are anticipated to endow the resulting MOFs with large interlayer distances, facilitating their efficient exfoliation into ultrathin nanosheets.43 Additionally, the tert-butyl groups on the 2D surfaces can prevent the nanosheets from stacking, which indirectly increases the available interaction area for Sr2+ extraction. Moreover, the excellent hydrophobic properties of these tert-butyl groups, which were decorated on the exfoliated MOF nanosheets, would endow MOF nanosheets with high stability in aqueous solution, even under harsh conditions of strong acids or bases. Thus, the solvothermal reaction of CuCl2·2H2O with H4L generated a MOF with a layered structure, {[Cu(H2L)(H2O)]·0.5H2O}n (Cu-MOF-COOH), which can be readily delaminated into an ultrathin nanosheet with cavity structures by a straightforward ultrasonic method (Scheme 1). As anticipated, the synergistic interaction between the extensively accessible adsorption sites (carboxylate groups) and the cage-like cavities with Sr2+ on the exposed surfaces resulted in exceptional capture performance for Sr2+, with high removal efficiencies (>91%) from various water samples.
Results and discussion
Characterization of Cu-MOF-COOH
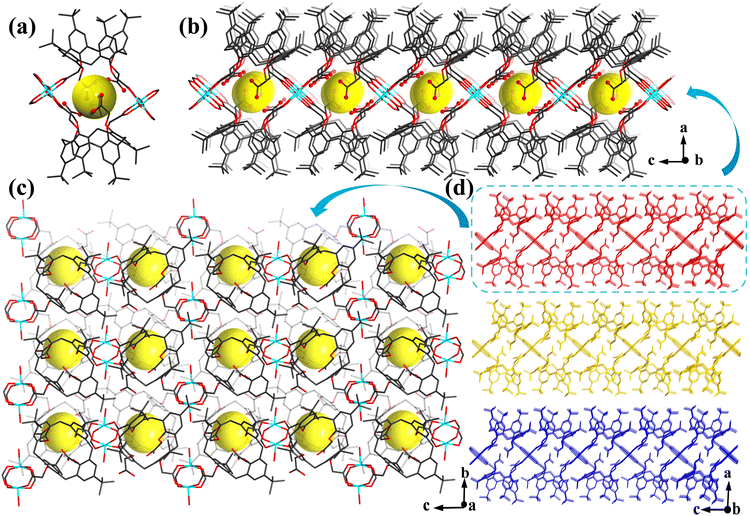
Single-crystal X-ray crystallographic analysis reveals the crystallization of Cu-MOF-COOH in the monoclinic space group P21/c. Fig. S1† illustrates that two Cu(H2O) subunits are connected by four carboxylate groups to form a paddle-wheel unit, [Cu2(COO)4(H2O)2]. Two paddle-wheel units are then linked through two H2L2− ligands, resulting in cage-like secondary building units (Fig. 1a). These units are interconnected to create a 2D layered structure (Fig. 1b and c), which are further stacked together to form a 3D structure (Fig. 1d). Notably, the upper rims of the H4L ligand are modified with four tert-butyl groups, with large spatial dimensions, which impart the resulting layered MOFs with substantial interlayer distances (Fig. 1d), thereby facilitating their efficient exfoliation into ultrathin nanosheets. The cage-like cavities on the largely exposed surface are filled with extensive and accessible uncoordinated carboxylate O atoms (Fig. 1a and b), allowing for easy interaction with Sr2+ that facilitates efficient and selective separation. Notably, within the cavities, the oxygen atoms of carboxylate groups at ortho-positions are capable of coordinating with Sr2+ through a bidentate chelating mode. The oxygen–oxygen distances are measured to be 5.210 Å and 5.213 Å (Fig. S2†), which are comparable to that of a previous study,28 where an oxygen–oxygen distance of approximately 5.18 Å is optimal for Sr2+ capture. These distances are well-suited for Sr2+ capture, due to the size-matching effect.28 The cage-like cavities would play a pivotal role in the interactions between Sr2+ and the O sites, and the synergistic interactions between the accessible active sites and cage-like cavities with Sr2+ would ensure stable capture of Sr2+, overcoming the problem of conventional 2D materials' easy desorption.Exfoliation of Cu-MOF-COOH
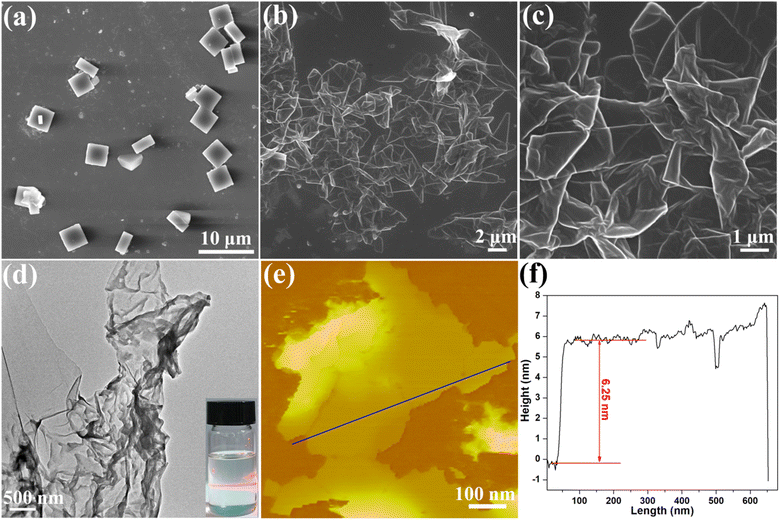
Given the large interlayer spacing and weak interactions between the layers in Cu-MOF-COOH, a liquid-based ultrasonication approach was utilized to exfoliate the bulk MOF. By immersing it in MeOH/isopropanol solution and subjecting it to ultrasonication for 6 h, a colloidal suspension was formed. Upon illumination with a red laser, a noticeable Tyndall effect can be observed (inset in Fig. 2d), indicating the successful exfoliation into 2D Cu-MOF-COOH nanosheets, which was further validated by the particle size distribution analysis of dynamic light scattering. As shown in Fig. S3,† the obtained nanosheets exhibit an average dimension of 187 nm after exfoliation. A series of measurements, encompassing scanning electron microscopy (SEM), transmission electron microscopy (TEM), and atomic force microscopy (AFM), were conducted to scrutinize the morphology of the resultant MOF nanosheets. SEM images clearly showed the transformation of the square-shaped bulk Cu-MOF-COOH (Fig. 2a) into flake-like 2D Cu-MOF-COOH nanosheets (Fig. 2b and c). Notably, some Cu-MOF-COOH nanosheets exhibited curling, due to their ultrathin nature, which was further confirmed by the TEM image shown in Fig. 2d. AFM measurements provided detailed information on the thickness of Cu-MOF-COOH nanosheets, revealing an approximate thickness of 6.25 nm (Fig. 2e and f), which consisted of about 3 layers. The aforementioned findings verified that Cu-MOF-COOH was successfully exfoliated into ultrathin nanosheets. Moreover, the element mappings of Cu-MOF-COOH nanosheets (Fig. S4†) revealed a uniform distribution of Cu, C, and O elements throughout the nanosheets.To further confirm the successful exfoliation of 2D nanosheets, the powder X-ray diffraction (PXRD) measurements were performed. Fig. S5† shows the PXRD patterns of 3D Cu-MOF-COOH and Cu-MOF-COOH nanosheets. It is obvious that the diffraction pattern of 3D Cu-MOF-COOH exhibited a strong diffraction peak at 3.90°, corresponding to the (100) facet, which indicated that periodic stacking formed along the a axis in 3D Cu-MOF-COOH. After exfoliation, the peak at 3.90° (100) clearly displayed a slight shift to 3.80°, indicating the expansion of the interlayer spacing. Moreover, the peak at 3.80° (100) was broadened, and its intensity was significantly reduced compared to that of its 3D bulk counterpart (inset in Fig. S5†), which further confirmed that the nanosheets were exfoliated along the a-axis direction.44,45 Fourier transform infrared (FT-IR) spectroscopy and thermogravimetric analysis (TGA) were conducted to investigate the integrity of the framework after exfoliation. The curves of these measurements for Cu-MOF-COOH nanosheets were found to be almost identical to those of their 3D counterparts (Fig. S6 and S7†), confirming that the framework structure remained intact after exfoliation. The clear diffraction spots in the selected area electron diffraction (SAED) image (Fig. S8†) further indicated that the 2D nanosheets retained their crystallinity after exfoliation.
The water stability of Cu-MOF-COOH nanosheets was also investigated by immersing them in aqueous solutions with varied pH values. After immersing in aqueous solution at pH of 1.0, 2.0, 3.0, 5.0, 7.0, 9.0, 10.0, 12.0 and 13.0 for 12 hours, only 2.16%, 1.31%, 0.27%, 0.13%, 0.01%, 0.09% 0.06%, 0.73% and 3.31% Cu2+ leaching was determined (Table S1†), respectively. Moreover, the acquired PXRD patterns matched those of the original ones well (Fig. S9†), demonstrating the remarkable stability of Cu-MOF-COOH nanosheets, even under extremely acidic and alkaline conditions. This robustness can be attributed to the abundant hydrophobic tert-butyl functional groups decorated on the MOFs, which impart a high degree of hydrophobicity to Cu-MOF-COOH nanosheets, as evidenced by their high contact angle (130.4°; Fig. S10†). This hydrophobicity can prevent water molecules from attacking the central metal ions, enhancing the chemical stability of the MOF nanosheets. These results demonstrate the high water stability of Cu-MOF-COOH nanosheets, which is essential for their application in Sr2+ separation from actual water samples.
Sr2+ capture studies
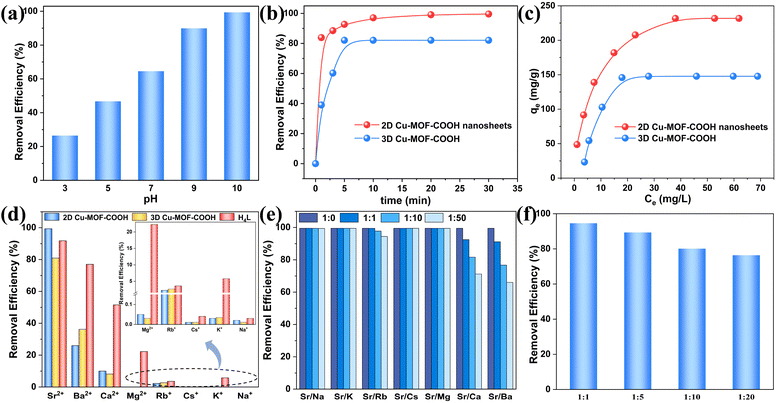
The pH value is a crucial element controlling the uptake of an adsorbent by affecting the surface charge of adsorbents and the type of metal ions in the solution. Thus, it is essential to investigate the influence of pH on Sr2+ extraction. Given the formation of Sr2+ hydroxide precipitates under alkaline conditions, we investigated Sr2+ extraction behavior within a pH range from 3.0 to 10.0. As shown in Fig. 3a, at a pH of 3.0, the extraction efficiency of Sr2+ was only 26.46%, which can be attributed to the competition between Sr2+ with the excess H+ in the solution. As pH increased, the removal efficiency for Sr2+ improved significantly, reaching a peak value of 99.34% at pH 10.0. This enhancement can be attributed to the deprotonation of carboxyl groups in Cu-MOF-COOH nanosheets under weak alkaline conditions, favouring Sr2+ removal through electrostatic interactions, and it was supported by zeta potential analyses of Cu-MOF-COOH nanosheets at different pH levels (Fig. S11†). As pH increases from 3.0 to 10.0, the increase in surface charge enhances the electrostatic attraction with Sr2+, thereby improving Sr2+ removal. Thus, a pH of 10.0 was selected as the optimal pH for further investigation.The rapid sequestration of radioactive elements serves as an effective strategy to mitigate radiation risks. To this end, the kinetic properties of Cu-MOF-COOH nanosheets were evaluated in Sr2+ solution at a concentration of 5 ppm. As illustrated in Fig. 3b, Cu-MOF-COOH nanosheets exhibited a relatively rapid kinetics for Sr2+, achieving an extraction efficiency of 83.88% within the first minute. The extraction gradually approached equilibrium, ultimately reaching a removal efficiency of 99.56%. Under the same experimental conditions, 3D Cu-MOF-COOH removed only 39.05% of Sr2+ within the initial minute and ultimately achieved a removal efficiency of 82.09% (Fig. 3b). The significant difference in the removal rates between 2D and 3D MOFs during the initial stage is primarily attributed to the accessibility of the contaminants to the adsorption sites on extractants. The abundant active sites on the surface of Cu-MOF-COOH nanosheets, which are readily accessible, provide numerous opportunities for Sr2+ to interact with the binding sites, ultimately leading to a significantly higher removal efficiency within a short period. For 3D Cu-MOF-COOH, the high density of active sites confined within the framework resulted in diminished Sr2+ capture performance. To examine the rate-controlling mechanisms of these extraction processes, pseudo-first-order and pseudo-second-order kinetic models were employed to fit the data for both Cu-MOF-COOH nanosheets and 3D Cu-MOF-COOH (Fig. S12†). As illustrated in Table S2,† the pseudo-second-order kinetic model with higher R2 values (0.999 and 0.998) can better fit the data of both 2D and 3D Cu-MOF-COOH, demonstrating that the extraction process is primarily governed by chemical interactions.
To examine the maximum capture capacities for Sr2+, the extraction experiments were conducted in Sr2+ solutions with different initial concentrations. As illustrated in Fig. 3c, the trapping capacities of Cu-MOF-COOH nanosheets increased with rising Sr2+ concentrations, ultimately reaching a maximum value of 231.72 mg g−1, which was much higher than that of most materials (Table S3†). To gain further insights into the extraction process, the experimental data were fitted with both the Langmuir and Freundlich models (Fig. S13†). The fitting results, presented in Table S4,† revealed that the Langmuir model, with a higher correlation coefficient (R2 = 0.999), was more suitable for describing the isotherm data of the MOF nanosheets. The calculated maximum value, 257.07 mg g−1, was close to the experimentally determined value (231.72 mg g−1). As a comparison, the extraction behavior of 3D bulk Cu-MOF-COOH was also investigated, and the equilibrium extraction capacity was determined to be 147.79 mg g−1, significantly lower than that of 2D Cu-MOF-COOH nanosheets. This observation is in line with our predictions, as the bulk Cu-MOF-COOH encapsulates a substantial quantity of capture sites within its internal structure, which poses a considerable hindrance to the effective interaction with Sr2+. These findings highlighted the significant improvement in contaminant accessibility to the active sites within MOF cavities through exfoliation, and the contaminant molecules can be stably captured by sufficient interaction with these active sites.
Wastewaters generally contain various alkali and alkaline earth metal ions such as Na+, K+, Rb+, Cs+, Mg2+, Ca2+, Ba2+, etc., with similar electronic structures and chemical properties to Sr2+, which may compete for binding sites with Sr2+.46 Therefore, it is necessary to evaluate the selectivity and anti-interference performance of Cu-MOF-COOH nanosheets in the presence of these metal ions. Selective extraction experiments were conducted by adding the extractants to single metal ion solutions (Na+, K+, Rb+, Cs+, Mg2+, Ca2+, Ba2+ and Sr2+), with a concentration of 10 ppm. As shown in Fig. 3d, the MOF nanosheets exhibited distinct selectivity for Sr2+, with a removal ratio of up to 99.34%, which far exceeded that of other ions. For Ca2+ and Ba2+, which have quite similar physicochemical properties to Sr2+, their removal efficiencies were only 9.98% and 25.97%, respectively. And for other alkali and alkaline earth metal ions, their removal efficiencies were notably lower, falling below 2.23%. The above results demonstrated the ultrahigh selectivity of Cu-MOF-COOH nanosheets, which can be attributed to the synergistic interaction between the accessible active sites and the cage-like cavities with Sr2+ on the exposed surfaces. To verify our conjecture, the selective extraction performances for bulk Cu-MOF-COOH and H4L ligands were further investigated under the same experimental conditions. As shown in Fig. 3d, bulk Cu-MOF-COOH, with a large number of carboxylate groups and cavity structure that were buried in the framework, showed a much lower selectivity and removal rate (80.92%) for Sr2+ compared to that of Cu-MOF-COOH nanosheets (99.34%). The H4L ligand, with sufficient –COOH groups but without a suitable cavity structure, demonstrated a certain trapping ability for most metal ions except Na+ and Cs+, resulting in much lower selectivity for Sr2+. Therefore, the unique structure of Cu-MOF-COOH nanosheets, which features numerous cage-like cavities on their exposed surfaces and each cavity contains several active sites, can be responsible for the remarkable selectivity for Sr2+.
Anti-interference ability is vital for the practical application of an extractant, and it was systematically evaluated in this study. The anti-interference performance of Cu-MOF-COOH nanosheets was first evaluated against these monovalent metal ions (Na+, K+, Rb+, and Cs+) by adding different metal ions into Sr2+ solution (5 ppm) with different ratios. The results shown in Fig. 3e demonstrated that the presence of single monovalent metal ions, with the same concentrations as Sr2+, posed almost no effect on Sr2+ extraction, and the removal ratios for Sr2+ reached up to 99.45%, which is virtually equal to the removal ability for Sr2+ in the absence of interfering metal ions. Even when the concentrations of Na+, K+, and Cs+ increased to 50 times that of Sr2+, the extraction efficiency for Sr2+ remained almost unchanged, with a removal rate still exceeding 99%. For Rb+, even with an elevated concentration of 250 ppm, 94.44% of Sr2+ can still be effectively removed.
The anti-interference performances toward divalent metal ions, specifically Mg2+, Ca2+ and Ba2+, were further investigated. Notably, the presence of Mg2+ exhibited negligible influence on Sr2+ removal, even at a high Mg2+/Sr2+ ratio of 50, and Cu-MOF-COOH nanosheets demonstrated an ultra-high Sr2+ removal rate of 99.46% (Fig. 3e). For Ca2+ or Ba2+, when their concentrations are equivalent to that of Sr2+, the MOF nanosheets still retained effective removal, with Sr2+ removal efficiencies up to 92.48% and 91.16%, respectively (Fig. 3e). Even when the concentrations of Ca2+ and Ba2+ increased to 250 ppm, the removal efficiencies for Sr2+ remained at 71.18% and 66.00%, respectively. It should be noted that, although the MOF nanosheets exhibited a certain removal for Ba2+ (25.97%), the impact of Ba2+ on Sr2+ removal was not as substantial. In contrast, numerous studies have demonstrated that the presence of Ca2+ or Ba2+ significantly impeded Sr2+ removal. For instance, when Ca2+ was introduced into Sr2+ solution at the concentration that was 1 time (1-fold) of Sr2+, the removal efficiency for Sr2+ by potassium phosphatoantimonate (K2SbPO6) dropped drastically from the original 98.85% to 70.05%; when Ca2+ was introduced into Sr2+ solution at the concentration that was 50 times (50-fold) of Sr2+, the removal efficiency for Sr2+ drops drastically to 25.94%.47 Similarly, Ba2+ has also been shown to profoundly affect Sr2+ removal, as evidenced by a study on crown ether-based amino-modified mesoporous silica, where the removal efficiency for Sr2+ decreased significantly as the Ba2+/Sr2+ molar ratio increased from 1 to 100.48 The interference of Ca2+ and Ba2+ on Sr2+ removal is understandable because of their high proximity to Sr2+ in the periodic table and their shared chemical properties, which are known to be challenging to separate from Sr2+ in many circumstances.49 Especially for Ca2+, the high similarity to Sr2+ enables it to readily enter the body and substitute for calcium in bones, potentially leading to bone cancer.
In practical applications, Sr2+ often coexists with various competing metal ions. It is imperative to evaluate the anti-interference performance of Cu-MOF-COOH nanosheets in more complex environments. To this end, Sr2+ extraction performance was further assessed in the presence of mixed ions (Na+, K+, Rb+, Cs+, Mg2+, Ca2+ and Ba2+) with concentrations ranging from 5 to 100 ppm. The results shown in Fig. 3f demonstrated exceptional extraction performance for Sr2+. Specifically, at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 concentration ratio of the mixed metal ions to Sr2+, the removal rate for Sr2+ reached up to 94.53%. Even in the presence of 100 ppm mixed ions (the concentration ratio of mixed metal ions to Sr2+ is 20
1 concentration ratio of the mixed metal ions to Sr2+, the removal rate for Sr2+ reached up to 94.53%. Even in the presence of 100 ppm mixed ions (the concentration ratio of mixed metal ions to Sr2+ is 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), the MOF nanosheets retained a removal ratio for Sr2+ of more than 76.35%, highlighting their remarkable anti-interference ability in the complex aqueous systems. In a similar simulated system, comprising five metal ions (Na+, Mg2+, K+, Ca2+ and Ba2+), where the concentration of these cations was 10 times higher than that of Sr2+, the adsorbents of ZrP and CA@ZrP exhibited removal rates for Sr2+ of only 28% and 40.8%, respectively.50 Meanwhile, the concentrations of competing ions in actual radioactive wastewater typically do not reach such high levels. Data from the elemental analysis report of a nuclear power plant's low-level radioactive wastes showed that the concentrations of the major elements (>1 ppm) of Na+, Ca2+, Mg2+, and K+, are 145 ± 5.8, 16.5 ± 2.4, 13.5 ± 3.1, and 92.3 ± 55.4 ppm, respectively.51 These results suggested that the targeted Sr2+ capture by Cu-MOF-COOH nanosheets can be little affected by the presence of competing ions in practical scenarios.
1), the MOF nanosheets retained a removal ratio for Sr2+ of more than 76.35%, highlighting their remarkable anti-interference ability in the complex aqueous systems. In a similar simulated system, comprising five metal ions (Na+, Mg2+, K+, Ca2+ and Ba2+), where the concentration of these cations was 10 times higher than that of Sr2+, the adsorbents of ZrP and CA@ZrP exhibited removal rates for Sr2+ of only 28% and 40.8%, respectively.50 Meanwhile, the concentrations of competing ions in actual radioactive wastewater typically do not reach such high levels. Data from the elemental analysis report of a nuclear power plant's low-level radioactive wastes showed that the concentrations of the major elements (>1 ppm) of Na+, Ca2+, Mg2+, and K+, are 145 ± 5.8, 16.5 ± 2.4, 13.5 ± 3.1, and 92.3 ± 55.4 ppm, respectively.51 These results suggested that the targeted Sr2+ capture by Cu-MOF-COOH nanosheets can be little affected by the presence of competing ions in practical scenarios.
The good selectivity and robust anti-interference abilities of Cu-MOF-COOH nanosheets motivated us to delve deeper into their performance in removing Sr2+ from actual water samples. Given the variability in the constituents and their concentration across diverse natural water bodies, the feasibility of employing MOF nanosheets for Sr2+ removal was evaluated in different water systems, including tap water, lake water, and simulated groundwater (Table 1 and Fig. S14†). The results shown in Table 1 demonstrated that the removal rate for Sr2+ reached an impressive value of 97.19% in simulated groundwater. Although the removal efficiency was slightly lower in tap water, MOF nanosheets still managed to remove 95.44% of Sr2+. This observation may be attributed to the higher concentration of Ca2+ in tap water (33.45 ppm) compared to that in simulated groundwater (24.59 ppm), as outlined in Table 1. It is noteworthy that, even in lake water, which contains extensive competing ions and organic matrices, the removal rate for Sr2+ by MOF nanosheets still remained high, at 93.42%. To further assess the performance of MOF nanosheets under more challenging conditions, Sr2+ removal was evaluated in simulated radioactive wastewater, which includes extensive metal ions (Table 1). Remarkably, even in this highly complex system, the removal rate for Sr2+ still reached 91.04%. These results demonstrated the superior selectivity and anti-interference capabilities of the Cu-MOF-COOH nanosheet, making it a promising candidate for efficient Sr2+ separation in practical applications.
| Water samples | Coexisting ion concentrations (ppm) | Sr2+ initial concentration (ppm) | Sr2+ removal rate |
|---|---|---|---|
| Contaminated tap water | Na+ (7.12) K+ (6.87) | 5.14 | 95.44% |
| Mg2+ (9.19) Ca2+ (33.45) | |||
| Contaminated lake water | Na+ (6.91) K+ (6.66) | 5.25 | 93.42% |
| Mg2+ (8.08) Ca2+ (29.57) | |||
| Contaminated simulated groundwater | Na+ (125.35) K+ (6.25) | 5.34 | 97.19% |
| Mg2+ (9.79) Ca2+ (24.59) | |||
| Simulated low-level radioactive wastewater | Al3+ (0.01) Mo3+ (4.85) Cu2+ (0.04) | 4.45 | 91.04% |
| Mg2+ (1.10) Pb2+ (0.07) Zn2+ (0.06) | |||
| Cs+ (6.61) Mn2+ (3.40) Fe2+ (3.21) | |||
| Ca2+ (2.01) Rb+ (3.57) |
Extraction mechanism studies
To gain insights into the outstanding performance in Sr2+ separation, a series of characterization studies including PXRD, FT-IR, and X-ray photoelectron spectroscopy (XPS) were performed. As shown in Fig. S15,† the consistent PXRD patterns before and after Sr2+ extraction provided evidence for the robust structural stability of Cu-MOF-COOH nanosheets. In the FT-IR spectrum (Fig. S16†), a new peak that appeared at 740 cm−1 after extraction is attributed to the stretching vibration of the Sr–O bond, confirming the successful loading of Sr2+ onto the nanosheets.52 After Sr2+ extraction, the peaks of the asymmetric and symmetric vibrational modes of COO− shifted from 1616 and 1413 cm−1 to 1613 and 1419 cm−1, respectively, demonstrating the coordination interactions between Sr2+ and carboxylate groups.53,54 XPS spectra were further employed to provide more information on the interactions between MOF nanosheets and Sr2+. For Cu-MOF-COOH nanosheets before and after Sr2+ extraction, all the characteristic peaks of C 1s, O 1s, and Cu 2p can be observed in their XPS survey spectra (Fig. 4a). A unique peak of Sr 3d only appeared in the spectrum of Cu-MOF-COOH nanosheets after extraction, which directly confirmed Sr2+ extraction by MOF nanosheets (Fig. 4a). The high-resolution spectrum of Sr 3d can be divided into peaks at 135.69 eV and 133.87 eV (Fig. 4b), corresponding to the characteristic peaks of Sr 3d3/2 and Sr 3d5/2, respectively.55 These peaks showed an obvious shift towards lower binding energies in comparison to those of original Sr(NO3)2, which were located at 136.18 eV (for Sr 3d3/2) and 134.48 eV (for Sr 3d5/2),50 indicating the interactions between Sr2+ with the MOF nanosheets. Following Sr2+ extraction, the peaks of C–O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O in the high-resolution spectrum of O 1s (Fig. 4c) exhibited a shift from 533.53 and 532.62 eV to 533.66 and 532.84 eV, respectively, which can be stemmed from the interactions between Sr2+ and the oxygen-containing functional groups that induce the alteration in the electronic environment of the oxygen atoms.8 The novel peak appeared at 532.06 eV after Sr2+ extraction is attributed to Sr–O binding, providing further evidence for the interactions between Sr2+ and the oxygen element. Combined with FT-IR analysis, these obvious changes of binding energies in XPS spectra demonstrated a strong affinity between Sr2+ and the carboxylate groups in MOF nanosheets.
O in the high-resolution spectrum of O 1s (Fig. 4c) exhibited a shift from 533.53 and 532.62 eV to 533.66 and 532.84 eV, respectively, which can be stemmed from the interactions between Sr2+ and the oxygen-containing functional groups that induce the alteration in the electronic environment of the oxygen atoms.8 The novel peak appeared at 532.06 eV after Sr2+ extraction is attributed to Sr–O binding, providing further evidence for the interactions between Sr2+ and the oxygen element. Combined with FT-IR analysis, these obvious changes of binding energies in XPS spectra demonstrated a strong affinity between Sr2+ and the carboxylate groups in MOF nanosheets.
Theoretical model calculations were performed to gain a deep understanding of the interactions between Cu-MOF-COOH nanosheets and Sr2+ at the molecular level. Based on the crystal structure of Cu-MOF-COOH and analysis of FT-IR and XPS studies, we selected the cage-like cavity unit (Fig. S2†) as a model for theoretical calculations, and the uncoordinated carboxylate oxygen atoms within the cavities as potential binding sites. Typically, Sr2+ adopts 8- to 9-coordinate geometries in aqueous or framework environments. Thus, the constructed theoretical model would account not only for the potential binding of carboxylate sites but also for the inclusion of terminal water ligands to achieve the typical 8-coordinate geometry for Sr2+ in aqueous environments. Initially, a simple model was constructed, in which each Sr2+ was mono-coordinated with an oxygen atom from a carboxylate group and seven-coordinated with seven water molecules (Fig. 5a). The binding energy (Eb) for this model was calculated to be −1.68 eV (model I). To identify more energetically favored extraction modes, more complex coordination modes with carboxylate groups were constructed (Fig. 5b–f), including bridging mode (model II) and chelation mode (model III). In model II, Sr2+ was coordinated by two oxygen atoms from two different carboxylate groups and six oxygen atoms from six water molecules (model II-a and model II-b; Fig. 5b and c). Compared to model I, these models in model II didn't show a significant decrease in the binding energies (−1.50 and −1.82 eV), suggesting that bridging coordination isn't the optimal coordination model. In chelation modes, Sr2+ formed a single chelate ring by coordinating with two oxygen atoms from one carboxylate group (model III-a, Eb = −2.17 eV; Fig. 5d), and it can also be chelated by two carboxylate groups at ortho-positions (model III-b and III-c, Eb = −2.69 and −2.95 eV; Fig. 5e and f), forming a dual-ring structure. By comparing these binding energies, the chelation models (model III-a, III-b and III-c; Fig. 5d–f) exhibit much lower values than those of other models. In particular, the double carboxylate chelation in model III-c provides the most energetically favorable and stable configuration among all the constructed models, highlighting the critical role of carboxylate groups in the selective capture of Sr2+.
The H4L ligand, characterized by four carboxyl groups, holds intriguing potential for Sr2+ extraction. To assess its binding ability for Sr2+ capture, theoretical calculations were utilized to model the extraction process and calculate the molecular-level binding energy of the ligand towards Sr2+ (Fig. S17†). In the mono-coordinated mode, Sr2+ interacted with one oxygen atom within a carboxylate group and seven water molecules, yielding a binding energy of −0.56 eV (Fig. S17a†). In the bridging interaction mode, Sr2+ coordinated with two carboxylate groups and six water molecules, resulting in a binding energy of −1.07 eV (Fig. S17b†). In the chelate mode, Sr2+ could form either a single chelate ring (−1.18 eV) with one carboxylate group or a dual-ring structure (−1.95 eV) by chelating two carboxylate groups (Fig. S17c and d†). By comparing these binding energies, the double carboxylate chelation model emerged as the most energetically favorable configuration among all the constructed models. This result is in line with our expectations and consistent with the previous observations for Cu-MOF-COOH nanosheets. These models of the H4L ligand exhibit much higher binding energies compared to those observed in Cu-MOF-COOH nanosheets (−2.95 eV), which suggests that the binding affinity with Sr2+ is inferior to that of MOF nanosheets. These theoretical results are consistent with the experimental observations that the extraction selectivity of the H4L ligand is obviously lower than that of Cu-MOF-COOH nanosheets (Fig. 3d).
The good selectivity of MOF nanosheets toward Sr2+ can stem from the synergistic interaction between the carboxylate groups and the cage-like cavities with Sr2+. To elucidate the role of the cage-like cavities in Sr2+ extraction, we constructed another model (Fig. S18†) that was similar to the optimal mode (model III-c; Fig. 5f) but without the cage-like cavities. The calculated Eb value for this model (−1.34 eV) is significantly higher than the Eb observed in Cu-MOF-COOH nanosheets (−2.95 eV), which indicates that the absence of the cage-like cavities is unfavorable for the synergistic interaction with Sr2+, resulting in a substantial reduction in the interaction strength. These results demonstrated that the presence of the cage-like cavity is crucial for enhancing the binding strength and selectivity toward Sr2+. In particular, the synergistic interaction between the cage-like cavity and the carboxylate groups with Sr2+ provides an optimized spatial confinement and a specific coordination environment for Sr2+, thereby playing a pivotal role in the selective capture of Sr2+.
Furthermore, to confirm the high selectivity of MOF nanosheets toward Sr2+, we calculated the binding energies for various metal ions. As shown in Fig. S19,† MOF nanosheets exhibited the lowest binding energy for Sr2+ (−2.95 eV), followed by Ba2+ (−2.49 eV), Ca2+ (−1.66 eV) and Mg2+ (−1.59 eV), while the binding energies towards alkali metal ions are significantly higher, indicating the strongest interactions with Sr2+. This result aligns with previous experimental results, providing robust evidence for the exceptional affinity and selectivity of Cu-MOF-COOH nanosheets for Sr2+.
Reusability of Cu-MOF-COOH nanosheets
The regeneration and recyclability of MOF nanosheets are crucial for assessing their economic viability in wastewater treatment. A detailed investigation into the reusability of MOF nanosheets was conducted by using 0.01 M Na2HPO4 solution as an eluent. The results shown in Fig. S20† demonstrated fair regeneration ability of Cu-MOF-COOH nanosheets. Specifically, the extraction rate experienced a minimal decline of 0.43% in the initial cycle, and the MOF nanosheets still retained a high removal rate of 87.95% even after five cycles. The gradual decrement in extraction capacity can be attributed to the occupation of binding sites by the residual Sr2+. Furthermore, the concentrations of Cu2+ in the toluene phase and aqueous phase were determined after Sr2+ extraction to calculate the Cu2+ leaching rate. Through the inductively coupled plasma mass spectrometry measurement, only 0.24% and 0.13% of Cu2+ were determined in the toluene phase and aqueous phase (Table S1†), respectively, which demonstrated the good chemical stability of Cu-MOF-COOH nanosheets. The PXRD result further evidenced the retained crystallinity and structural stability of the MOF nanosheets after regeneration tests (Fig. S21†), indicating their fair reproducibility.Conclusions
In conclusion, an ultrathin 2D Cu-MOF-COOH nanosheet with a cavity structure was elaborately fabricated. The as-synthesized MOF nanosheets showed a high uptake capacity (231.72 mg g−1) for Sr2+, which is much higher than that of most other materials, including 3D Cu-MOF-COOH (147.79 mg g−1). The MOF nanosheets exhibited exceptional selectivity for Sr2+ and a robust resistance to interference, even when exposed to high concentrations of competing ions such as Ca2+, Ba2+, and other alkali and alkaline-earth metals. The outstanding performance was further verified by their ability to efficiently remove over 91% of Sr2+ from a range of water samples, including lake water, simulated groundwater, and radioactive wastewater. The synergistic interactions between the carboxylate groups and the cage-like cavities with Sr2+ can account for the exceptional extraction performance for Sr2+, which has been systematically investigated and clarified by detailed characterization studies and theoretical calculations. Compared to their 3D counterparts, Cu-MOF-COOH nanosheets exhibited obviously better extraction performance due to their highly open structure, which effectively exposes their surface active sites, facilitating intimate contact and comprehensive interactions with Sr2+ in the cage-like cavities.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
X.-R. Jia: investigation, methodology, conceptualization, writing-original draft. Z.-X. Gao: investigation, validation. H.-M. Fei: writing-original draft. L.-J. Lan: resources. C.-X. Yu: conceptualization, funding acquisition, methodology, writing-original draft. Y. Qian: resources, supervision, funding acquisition. L.-L. Liu: conceptualization, project administration, supervision, funding acquisition, writing-review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 42407657 and 22466002), the Natural Science Foundation of Shandong Province (No. ZR2021MB106 and ZR2021QB090), the Training Program for Academic and Technical Leaders of Major Disciplines of Jiangxi Province (No. 20225BCJ22008) and the Natural Science Foundation of Jiangxi Province (No. 20242BAB26061).References
- W. Wu, D. Chen, M. Zhang, X. Zhao, R. Zhao, C. Geng, J. Jia and G. Zhu, J. Am. Chem. Soc., 2025, 147, 2228–2236 CrossRef CAS PubMed.
- Y.-M. Zhao, L. Cheng, K.-Y. Wang, X. Hao, J. Wang, J.-Y. Zhu, M. Sun and C. Wang, Adv. Funct. Mater., 2022, 32, 2112717 CrossRef CAS.
- Y. Kim, K. Jin, I.-H. Park, S. Lee, J. Park and J. Park, Chem. Eng. J., 2024, 484, 149321 CrossRef CAS.
- H.-Y. Sun, B. Hu, T.-T. Lv, Y.-L. Guo, Y.-X. Yao, L. Yang, M.-L. Feng and X.-Y. Huang, Small, 2023, 19, 2208212 CrossRef CAS PubMed.
- N. Yang, X. Guo, J. Yu, Q. Liu, J. Liu, J. Zhu, R. Chen and J. Wang, J. Hazard. Mater., 2025, 485, 136955 CrossRef CAS PubMed.
- S. G. Lim, C. Y. Oh, S. H. Kim, K. Ra, M. Cha and J.-H. Yoon, Environ. Sci. Technol., 2024, 58, 6170–6180 CrossRef CAS PubMed.
- X. Du, H. Xie, T. Qin, Y. Yuan and N. Wang, Nat. Commun., 2024, 15, 6530 CrossRef CAS PubMed.
- L. Li, K. Kang, T.-S. Chee, Z. Tian, Q. Sun and C. Xiao, Adv. Sci., 2024, 11, 2308663 CrossRef CAS PubMed.
- H. Taketomi, N. Hosono and T. Uemura, J. Am. Chem. Soc., 2024, 146, 16369–16374 CrossRef CAS PubMed.
- K. Geng, Y. Sun, Y. Zhao, Z. Shao, Y. Wei, J. Huang, Y. Cui, X. Xu and H. Hou, J. Am. Chem. Soc., 2025, 147, 9844 CrossRef CAS PubMed.
- L. Xiao, C. Cheng, Z. Li, C. Zheng, J. Du, M. Song, Y. Wan, S. Li, G. Jun and M. Zhao, Nano Res., 2023, 16, 11334–11341 CrossRef CAS.
- S. Yang, Y. Sun, K. Geng, Y. Cui, J. Huang, X. Meng and H. Hou, Adv. Opt. Mater., 2024, 12, 2400475 CrossRef CAS.
- S.-H. Zhou, R.-D. Wang, Y. Yang, H.-Y. Li, L.-J. Luo and F.-Z. Jiang, J. Clean. Prod., 2024, 476, 143807 Search PubMed.
- J. Zhang, L. Chen, X. Dai, L. Zhu, C. Xiao, L. Xu, Z. Zhang, E. V. Alekseev, Y. Wang, C. Zhang, H. Zhang, Y. Wang, J. Diwu, Z. Chai and S. Wang, Chem, 2019, 5, 977–994 CAS.
- Y. Qin, J. Du, Q. Zhang, C. Cheng, Z. Dong, Q. Zhang, S. Li, J. Guo, Z. Tang and M. Zhao, Adv. Mater., 2025, 37, 2419515 CrossRef CAS PubMed.
- D. Guo, C. Yan, B. Huang, T. Jin, Z. Liu and Y. Qian, Inorg. Chem., 2025, 64, 1777–1787 CrossRef CAS PubMed.
- S. Deng, X. Kong, X. Fu, Z.-W. Huang, Z.-H. Zhou, L. Mei, J.-P. Yu, L.-Y. Yuan, Y.-Q. Zhu, N.-N. Wang, K.-Q. Hu and W.-Q. Shi, Inorg. Chem., 2025, 64, 224–231 CrossRef CAS PubMed.
- L. González, R. Gil-San-Millán, J. A. R. Navarro, C. R. Maldonado, E. Barea and F. J. Carmona, J. Mater. Chem. A, 2022, 10, 19606–19611 Search PubMed.
- X. Zhao, X. Gao, R. Ding, H. Huang, X. Gao and B. Liu, J. Colloid Interface Sci., 2023, 639, 59–67 Search PubMed.
- Y. Wang, D. Ren, J. Ye, Q. Li, D. Yang, D. Wu, J. Zhao and Y. Zou, Sep. Purif. Technol., 2024, 329, 125149 CrossRef CAS.
- Y. Qing, W. Gao, Y. Long, Y. Kang and C. Xu, Inorg. Chem., 2023, 62, 6909–6919 CrossRef CAS PubMed.
- M.-R. Yao, H. Wu, C.-X. Yu, J. Ding, Y.-L. Zhou and L.-L. Liu, Sep. Purif. Technol., 2025, 360, 131048 CrossRef CAS.
- C.-X. Yu, W. Jiang, M. Lei, M.-R. Yao, X.-Q. Sun, Y. Wang, W. Liu and L.-L. Liu, Small, 2024, 20, 2308910 CrossRef CAS PubMed.
- K. Jin, B. Lee and J. Park, Coord. Chem. Rev., 2021, 427, 213473 CrossRef CAS.
- R.-J. Cao, H.-Y. Zhou, Q.-Y. Wu, Z. Xiao, T.-Y. Xiu, J. Li, H.-B. Tang, L.-Y. Yuan, W.-S. Wu and W.-Q. Shi, Adv. Mater., 2025, 37, 2414659 CrossRef CAS PubMed.
- Q. Peng, B. Huang, L. Peng, D. Guo, T. Jin, Z. Liu and Y. Qian, Sep. Purif. Technol., 2024, 337, 126391 CrossRef CAS.
- L. Feng, X. Chen, M. Cao, S. Zhao, H. Wang, D. Chen, Y. Ma, T. Liu, N. Wang and Y. Yuan, Angew. Chem., Int. Ed., 2023, 62, e202312894 CrossRef CAS PubMed.
- W. Jin, Q. Wu, Y. Lou, Z. Huang, F. Liu, B. Hu, J. Yu, K. Hu, L. Yuan, W. Shi and L. Mei, Sci. Bull., 2025, 70, 683–693 CrossRef CAS PubMed.
- G. Lin, B. Zeng, X. Liu, J. Li, B. Zhang and L. Zhang, J. Clean. Prod., 2022, 381, 134766 CrossRef CAS.
- B. Han and A. Chakraborty, Desalination, 2022, 541, 116045 CrossRef CAS.
- X. Chen, W. Wang, Y. Song, Y. Zhou, H. Li and J. Pan, J. Hazard. Mater., 2022, 438, 129522 CrossRef CAS PubMed.
- R. Liu, Q. Zhao, Z. Wang, R. Jiang, C. Lin, L. Wu, G. Chen, P. He, L. Zhu, J. Chen and T. Duan, J. Mater. Chem. A, 2024, 12, 14608–14618 RSC.
- M.-R. Yao, Z.-Y. Wang, A.-C. Geng, K.-Y. Wu, M.-J. Luo, Z.-H. Li, Y.-Q. Sun, M.-J. Gao, C.-X. Yu and L.-L. Liu, Sep. Purif. Technol., 2024, 341, 126892 CrossRef CAS.
- H. Sun, K.-Z. Wang, M.-R. Yao, C.-X. Yu, Y.-H. Song, J. Ding, Y.-L. Zhou, D. Liu and L.-L. Liu, Inorg. Chem. Front., 2023, 10, 6566–6577 RSC.
- J. Yu, J. Wang, H. Zhang, Q. Liu, J. Liu, J. Zhu, J. Yu and R. Chen, Carbohydr. Polym., 2024, 323, 121426 CrossRef CAS PubMed.
- J.-Y. Tian, W.-C. Lv, A.-S. Shen, Y. Ma, M. Wang, S. Zhang, X.-L. Liu, Z. Zhang and M. Du, Sep. Purif. Technol., 2023, 327, 124903 CrossRef CAS.
- T. Prakasam, S. K. Sharma, F. Ravaux, F. Benyettou, M. Lusi, V. Sabu, P. Bazin, T. Delclos, R. Jagannathan, J. Whelan, M. El-Roz, M. A. Olson, M. Abdellatief, O. S. Mudraj, F. Gándara and A. Trabolsi, Chem, 2025, 11, 102307 CAS.
- L.-L. Liu, J. Chen, Y. Zhang, C.-X. Yu, W. Du, X.-Q. Sun, J.-L. Zhang, F.-L. Hu, Y. Mi and L.-F. Ma, J. Mater. Chem. A, 2021, 9, 546–555 RSC.
- C.-X. Yu, W. Jiang, C.-W. Zhang, H. Fang, L.-Z. Wang, M.-J. Gao, Y.-L. Zhou, Y. Qian and L.-L. Liu, Inorg. Chem., 2024, 63, 15105–15114 CrossRef CAS PubMed.
- B. Garai, D. Shetty, T. Skorjanc, F. Gándara, N. Naleem, S. Varghese, S. K. Sharma, M. Baias, R. Jagannathan, M. A. Olson, S. Kirmizialtin and A. Trabolsi, J. Am. Chem. Soc., 2021, 143, 3407–3415 CrossRef CAS PubMed.
- T. Skorjanc, D. Shetty, F. Gándara, S. Pascal, N. Naleem, S. Abubakar, L. Ali, A. K. Mohammed, J. Raya, S. Kirmizialtin, O. Siri and A. Trabolsi, ACS Appl. Mater. Interfaces, 2022, 14, 39293–39298 CrossRef CAS PubMed.
- L.-D. Yu, Y.-J. Tong, N. Li, Y. Yang, P. Ye, G. Ouyang and F. Zhu, Chem. Commun., 2022, 58, 11697–11700 RSC.
- P. Yang, J. Jiang, J.-P. Ma, B. Zheng, Y. Yan, J. Wang, Y. Zou, Q.-K. Liu and Y. Chen, Inorg. Chem., 2022, 61, 1521–1529 CrossRef CAS PubMed.
- Z. Li, K. Li, Y. Li, Y. Yu, J. Lv, X. Liu, K. Guan, W. Lei, S. Zhang and H. Zhang, Adv. Funct. Mater., 2024, 34, 2310371 CrossRef CAS.
- L.-J. Ji, T.-Y. Yang, G.-Q. Feng, S. Li, W. Li and X.-H. Bu, Adv. Mater., 2024, 36, 2404756 CrossRef CAS PubMed.
- Y. Wang, W. Zhang, X. Zeng, T. Deng and J. Wang, Sep. Purif. Technol., 2021, 278, 119640 CrossRef.
- Y.-L. Guo, H.-Y. Sun, X. Zeng, T.-T. Lv, Y.-X. Yao, T.-H. Zhuang, M.-L. Feng and X.-Y. Huang, Chem. Eng. J., 2023, 460, 141697 CrossRef CAS.
- R. I. Ripon, Z. A. Begum, B. Ahmmad, F. Hirose, Y. Takagai and I. M. M. Rahman, J. Environ. Chem. Eng., 2024, 12, 113984 CrossRef CAS.
- X. Zhang and Y. Liu, J. Hazard. Mater., 2020, 398, 122907 CrossRef CAS PubMed.
- R. Liu, G. Chen, Z. Wang, Q. Zhao, L. Wu, Q. Li, R. Tian, X. Chen, X. Li, Z. Chen, L. Zhu, J. Chen and T. Duan, Inorg. Chem., 2023, 62, 5799–5809 CrossRef CAS PubMed.
- I. B. Rae, S. Pap, D. Svobodova and S. W. Gibb, Sci. Total Environ., 2019, 650, 2411–2422 CrossRef CAS PubMed.
- Q. Li, X. Wang, L. Song, L. He, Q. Yu, X. Xiao and S. Ding, J. Environ. Chem. Eng., 2023, 11, 110495 CrossRef CAS.
- R. Paz, H. Viltres, N. K. Gupta, C. Leyva, R. P. Dhavale, H.-H. Park, A. Romero-Galarza, A. R. Rajabzadeh and S. Srinivasan, J. Environ. Chem. Eng., 2023, 11, 110084 CrossRef CAS.
- R. Paz, N. K. Gupta, H. Viltres, C. Leyva, A. Romero-Galarza, S. Srinivasan and A. R. Rajabzadeh, Sep. Purif. Technol., 2022, 298, 121606 CrossRef CAS.
- J. Diao, J. Zu, G. Han, Y. Xue, X. Pan, M. Jin, S. Liu and Q. Tang, Sep. Purif. Technol., 2023, 325, 124644 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Materials and physical measurements, extraction experiments, calculation method, particle size distribution, elemental mapping images, PXRD patterns, FT-IR spectra, TGA curves, SAED patterns, contact angle, zeta potentials, and additional figures, tables and an X-ray crystallographic file in CIF format. CCDC 2427696. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc02612a |
| This journal is © The Royal Society of Chemistry 2025 |