Open Access Article
Open Access ArticleModulation of the electronic structure of nickel selenide via iron doping for energy-saving hydrogen production coupled with sulfion upgradation†
Shuixiang
Xie‡
a,
Xiaojun
Wang‡
a,
Yuhuan
Li
a,
Shijie
Liu
a,
Jiahui
Qian
a,
Yuhan
Zhang
a,
Linling
Jiang
a,
Zhe
Cao
a,
Zhenhao
Yan
a,
Xiaoyu
Wan
a,
Zhaohang
Yang
a,
Longhua
Zou
*b,
Wei
Zhang
*a and
Rui-Qing
Li
 *a
*a
aSchool of Textile and Clothing, Nantong University, Nantong 226019, PR China. E-mail: liruiqing@ntu.edu.cn; zhangwei@ntu.edu.cn
bCollege of Food and Biological Engineering, Chengdu University, Chengdu 610106, China. E-mail: zoulonghua@cdu.edu.cn
First published on 29th May 2025
Abstract
Hybrid water electrolysis is a promising approach for energy-saving hydrogen (H2) generation by replacing the oxygen evolution reaction with the thermodynamically advantageous sulfion oxidation reaction (SOR). Herein, we designed iron-modified nickel selenide nanosheet arrays (Fe-Ni0.85Se) and used them as an electrocatalyst in bifunctional hydrogen evolution reaction (HER) and SOR to simultaneously facilitate H2 production and sulfion conversion into a valuable sulfur product. Fe-Ni0.85Se requires a low overpotential of 114 mV for the HER and a working potential of 0.340 V for the anodic SOR to attain 10 mA cm−2. Moreover, the two-electrode hybrid electrolysis cell employing Fe-Ni0.85Se as the cathode and anode requires a small voltage of 0.439 V at 10 mA cm−2, which greatly reduces the operating voltage by 1.186 V compared with that for overall water splitting, realizing energy-saving H2 production and high-value-added sulfur production. Theoretical calculations prove that Fe modification can accelerate water dissociation, optimize the adsorption behavior of hydrogen adsorption and sulfion, and promote the conversion process of sulfur intermediates. This study offers a simple approach to develop bifunctional catalytic electrodes for economically viable H2 generation and sulfur recovery.
Introduction
Fossil fuel consumption and environmental deterioration have increased the demand for sustainable and clean energy sources.1,2 As a leading clean energy source, hydrogen (H2) possesses the advantages of high energy density and environmental benignness, which are crucial for future energy transitions.3 Traditional H2 production methods face many problems, such as serious environmental pollution, complicated equipment processes and large investment/operational expenses. In comparison, electrocatalytic overall water splitting (OWS) powered by renewable energy is a promising technology to generate high-purity H2 because of its mild operating conditions and simplicity.4,5 However, this technology suffers from high voltages and electricity expenses owing to the presence of the sluggish oxygen evolution reaction (OER) at the anode, which results in increased energy consumption.6,7 At present, precious-metal-based materials including Pt/C, RuO2 and IrO2 are excellent materials used to lower energy expenditure, but their low reserves and high costs hinder their commercialization.8–10 Therefore, it is appealing to exploit high-efficiency electrocatalytic systems and inexpensive catalysts.Currently, researchers have adopted hybrid water electrolysis (HWE) by employing the thermodynamically favorable oxidation reactions of small molecules, including methanol, glycerol, urea, 5-hydroxymethylfurfural (HMF), and hydrazine, as substitutes for the OER at the anode, leading to optimized catalytic systems and reduced energy consumption.11–13 Wang et al. prepared oxygen-vacancy-rich Co3O4 and coupled the catalytic oxidation of HMF with the hydrogen evolution reaction (HER) to produce FDCA and H2 at low voltages.14 Similarly, Duan et al. reported the Au/CoOOH catalyst, which catalytically converted benzyl alcohol into high-value-added products while realizing energy-saving H2 production.15 Among these alternative reactions, the sulfion oxidation reaction (SOR; S2− = S + 2e−, −0.48 V vs. RHE) has the thermodynamic advantage.16,17 Meanwhile, toxic sulfion-containing wastewater is common in many industrial processes and has adverse effects on human health and the ecological environment. Therefore, combining the SOR with the HER can simultaneously achieve low-voltage H2 generation and the degradation/conversion of sulfur-rich sewage to value-added sulfur without adding other oxidants.18,19
As we know, the formation and conversion of polysulfide intermediates are accompanied by a 16-electron transfer process during the SOR, resulting in slow catalytic kinetics.20,21 Meanwhile, sulfur species can easily poison metallic catalysts, greatly reducing their activities and stabilities. To resolve these challenges, researchers have engaged diverse strategies such as heteroatom doping and heterostructure construction to regulate electronic structures and reduce reaction energy barriers for realizing high catalytic performances.22–25 However, most prepared catalysts display monofunctional catalytic performances for either the SOR or the HER, which can lead to the incompatibility and deterioration of catalysts and high preparation costs when pairing them in an electrolytic cell. Therefore, relevant studies on bifunctional catalysts for the HER and SOR to achieve H2 production are of great significance.
Herein, we developed hierarchical and efficient bimetallic selenide (Fe-Ni0.85Se) nanosheet arrays, which serve as a bifunctional catalyst to catalyze H2 production and sulfion ion oxidation. As expected, Fe-Ni0.85Se displays remarkable catalytic activities for the HER and SOR. The combined Fe-Ni0.85Se–based hybrid water electrolyzer possesses good catalytic activity and durability and needs a low cell voltage of 0.439 V at 10 mA cm−2, achieving energy-efficiency H2 production and sulfion upgradation to valuable sulfur. The outstanding catalytic performances of Fe-Ni0.85Se are attributed to the regulation of the composition and electronic structure, which promotes catalytic intermediate adsorption and decreases the energy barriers of catalytic reactions.
Results and discussion
Synthesis and characterization
The preparation scheme for Fe-Ni0.85Se is displayed in Fig. 1a. Initially, the hydrothermal process was adopted to synthesize NiFe layered double hydroxide (NiFe LDH) on a nickel foam (NF) substrate, and the corresponding phase was confirmed by X-ray diffraction (XRD; Fig. S1†). The scanning electron microscopy (SEM) images show the uniform and interconnected growth of NiFe LDH nanosheets with a relatively smooth surface on the NF (Fig. 1b and c). Subsequently, black Ni0.85Se and Fe-Ni0.85Se products were obtained by a hydrothermal selenization process. The several typical diffraction peaks at 33.1°, 44.6°, 50.5°, 59.9°, 61.7° and 69.6° can be attributed to the (101), (102), (110), (103), (201) and (202) lattice planes of hexagonal Ni0.85Se (JCPDS No. 18-0888), respectively, confirming the synthesis of Ni0.85Se and Fe-Ni0.85Se (Fig. 1d). Although Fe-Ni0.85Se maintains a similar nanosheet-like morphology (Fig. 1e and S2†), careful observation shows that the Fe-Ni0.85Se nanosheets are composed of many nanoparticles with diameters of about 100 nm, which is beneficial for exposing abundant active sites and promoting rapid mass transport. Transmission electron microscopy (TEM) was performed to identify the morphology of Fe-Ni0.85Se, which further confirms its nanosheet structure composed of nanoparticles (Fig. 1f). In the high-resolution TEM (HRTEM) image (Fig. 1g), the interplanar spacing of 0.268 nm is attributed to the (101) crystal plane of Fe-Ni0.85Se. The elemental mapping images (Fig. 1h–k) reveal the presence of Ni, Fe and Se elements, which are uniformly distributed on the surface of Fe-Ni0.85Se nanosheets.X-ray photoelectron spectroscopy (XPS) tests were carried out to detect surface chemical states. In Fig. 2a, the survey XPS spectrum confirms the coexistence of Ni, Fe and Se elements in the Fe-Ni0.85Se sample. Fig. 2b displays the high-resolution Ni 2p spectrum of Fe-Ni0.85Se, and the two peaks located at 852.5 and 869.9 eV belong to the Ni 2p3/2 and Ni 2p1/2 of Ni2+, and the binding energies at 855.5 and 873.1 eV are indexed to the Ni 2p3/2 and Ni 2p1/2 of Ni3+, respectively.26 The remaining two peaks at 861.1 and 879.4 eV are ascribed to satellite peaks. Similarly, the Fe 2p spectrum (Fig. 2c) shows three pairs of 2p3/2/2p1/2 doublet peaks located at 707.8/721.5, 712.0/725.3 and 716.6/729.7 eV.27 For Se species, the Se 3d spectrum of Fe-Ni0.85Se (Fig. 2d) is deconvoluted into two peaks at 53.7 and 54.8 eV, which are attributed to Se 3d5/2 and Se 3d3/2, respectively, indicating the presence of the metal–Se bond. The peak at 58.5 eV is attributed to the Se–O bond, ascribed to the unavoidable slight surface oxidation.28
Electrochemical performances
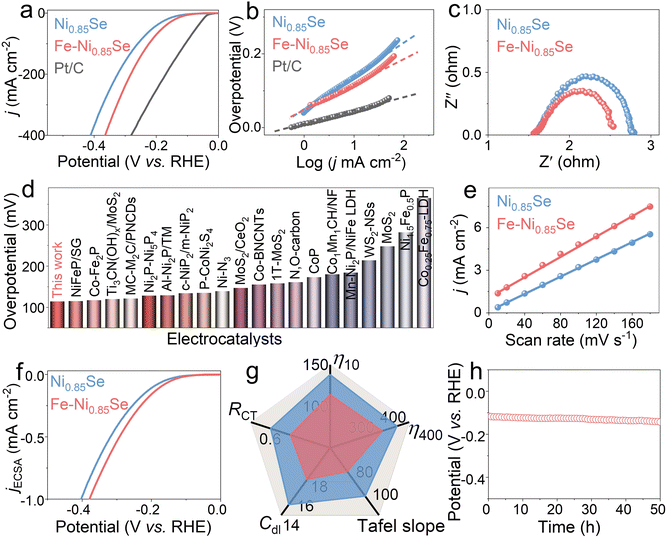
The HER performance of Fe-Ni0.85Se was assessed in a three-electrode system. In Fig. 3a and g, Fe-Ni0.85Se displays excellent HER activity and requires small overpotentials of 114 and 365 mV to deliver current densities of 10 and 400 mA cm−2, lower than those of Ni0.85Se (138 and 411 mV), respectively, confirming the crucial role of Fe introduction in improving catalytic performance of Ni0.85Se. The catalytic activity of Fe-Ni0.85Se also surpasses that of many developed HER catalysts (Fig. 3d and Table S1†).Tafel plots were fitted from the corresponding polarization curves to investigate HER kinetics. Fe-Ni0.85Se possesses a small Tafel slope of 71 mV dec−1 (Fig. 3b and g), smaller than that of Ni0.85Se (93 mV dec−1), implying that the HER process of Fe-Ni0.85Se follows a Volmer–Heyrovsky pathway. The low Tafel slope of Fe-Ni0.85Se suggests that it has quick HER kinetics and outstanding catalytic activities because of the electronic structure optimization of Ni0.85Se after Fe doping.29,30 To deeply understand the origin of the high intrinsic activities of Fe-Ni0.85Se, electrochemical impedance spectroscopy (EIS) was performed. In Fig. 3c and g, the Nyquist plots show that Fe-Ni0.85Se has a smaller charge-transfer resistance (Rct; 0.5 Ω) than Ni0.85Se (0.6 Ω), implying the key role of Fe doping in promoting the charge transfer rate. Furthermore, the number of catalytic sites on Fe-Ni0.85Se can be quantified using the electrochemically active surface area (ECSA) derived from the electrochemical double-layer capacitance (Cdl), which is calculated from cyclic voltammograms (Fig. S3†). In Fig. 3e and g, the calculated Cdl and ECSA values of Fe-Ni0.85Se (17.7 mF cm−2 and 442.5 cm−2) are higher than those of Ni0.85Se (15.1 mF cm−2 and 377.5 cm−2), indicating that Fe-Ni0.85Se has more catalytic sites than Ni0.85Se, in agreement with the remarkable activity of Fe-Ni0.85Se.31 In Fig. 3f, the ECSA-normalized curves further reveal that Fe-Ni0.85Se still has higher catalytic performances than Ni0.85Se, indicating that Fe doping can effectively improve the intrinsic activity of Ni0.85Se and expose more catalytic sites. Furthermore, long-term stability is a key parameter for catalysts. In Fig. 3h, the chronopotentiometry (v–t) curve displays that Fe-Ni0.85Se has outstanding durability with almost-unchanged potentials during a 50-h test. Moreover, the nanosheet-like morphology of Fe-Ni0.85Se does not change greatly (Fig. S4†), and the Ni, Fe and Se elements are homogeneously distributed on the surface of Fe-Ni0.85Se nanosheets (Fig. S5†), indicating their outstanding structural stability.
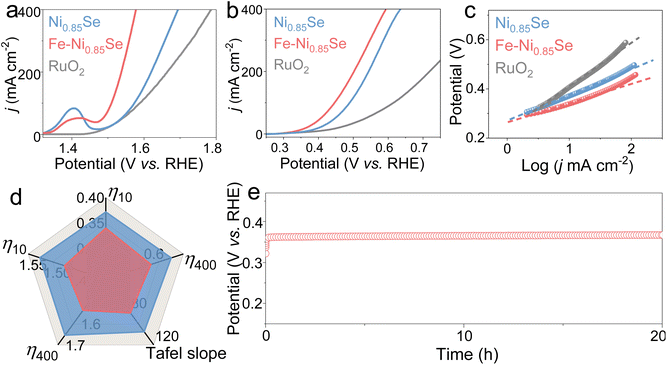
The OER performance of Fe-Ni0.85Se was further measured. In Fig. 4a, Fe-Ni0.85Se displays good OER activities and requires low potentials of 1.485 and 1.577 mV to deliver 50 and 400 mA cm−2, much lower than those required by Ni0.85Se (1.536 and 1.694 mV) and RuO2 (1.538 and 1.786 mV), respectively. Even so, the OER potentials for Fe-Ni0.85Se are still high, leading to high energy consumption when coupled with the HER. Therefore, it is promising to replace the OER with the SOR to lower the anode potential and achieve energy-efficient H2 production. The SOR activities were evaluated in 1 M NaOH containing different concentrations of Na2S (0.5–1.5 M). The SOR activity of Fe-Ni0.85Se rapidly increases when the concentration of Na2S increases to 1 M (Fig. S6†). In 1 M NaOH containing 1 M Na2S, Fe-Ni0.85Se displays splendid SOR activities with low potentials of 0.340 and 0.593 V at 10 and 400 mA cm−2 (Fig. 4b and d), smaller than those of Ni0.85Se (0.372 and 0.635 V), RuO2 (0.406 and 0.893 V) and most previously developed SOR materials (Table S2†), respectively. Moreover, the SOR process on Fe-Ni0.85Se shows greatly reduced potentials compared with the OER, confirming the feasibility of replacing the OER with the SOR to realize low cell voltages. The corresponding Tafel plots (Fig. 4c) manifest that Fe-Ni0.85Se still possesses a smaller Tafel slope value (81 mV dec−1) than Ni0.85Se (104 mV dec−1) and RuO2 (179 mV dec−1), implying that Fe-Ni0.85Se has fast SOR kinetics.32 The stability of Fe-Ni0.85Se was also studied. In Fig. 4e, Fe-Ni0.85Se exhibits almost-constant potentials over a 20-h test, and the corresponding nanosheet-like morphology (Fig. S7†) and homogeneous distribution of Ni, Fe and Se elements are well maintained (Fig. S8†), further illustrating its good durability for the SOR.
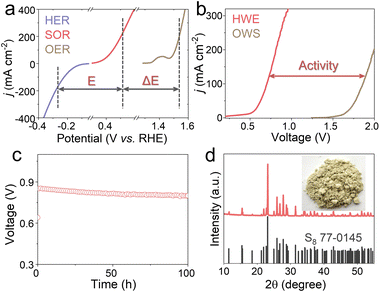
Motivated by the eminent HER and SOR performances of Fe-Ni0.85Se (Fig. 5a), traditional and hybrid two-electrode electrolyzers were assembled. In Fig. 5b, the HWE electrolyzer can output current densities of 10 and 200 mA cm−2 at low cell voltages (V10 and V200) of 0.439 and 0.811 V, respectively, lower than those needed in the conventional OWS system (1.625 and 1.998 V). In Fig. 5c, the stability test curve shows that Fe-Ni0.85Se operates steadily for 100 h with negligible voltage degradation, confirming its outstanding durability. After the durability test, the relevant electrolyte was acidified with sulfuric acid, and yellow powders were obtained, which are verified to be elemental sulfur (S8, PDF#77-0145, Fig. 5d), implying high valuable sulfur recovery. These results indicate that the substitution of the OER by the SOR not only significantly decreases the cell voltages of H2 production but also affords a high-value sulfur product in sulfion-rich wastewater.
Theoretical analysis
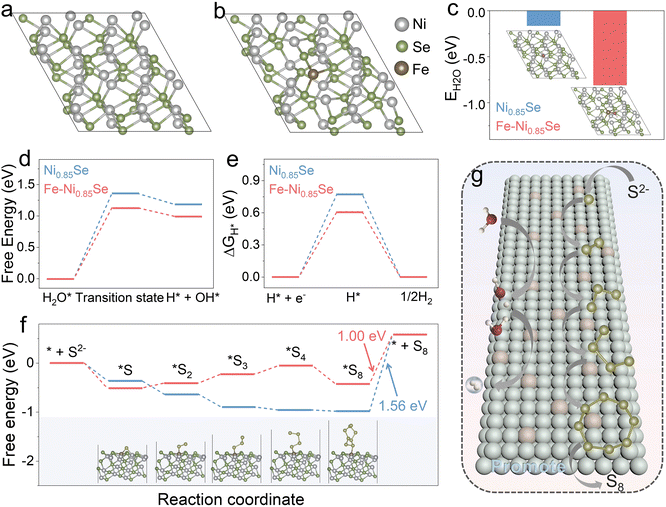
To reveal the origin of the high HER and SOR performances of Fe-Ni0.85Se, we carried out density functional theory (DFT) simulations to illustrate its reaction mechanism. The structure models are displayed in Fig. 6a and S9†. The adsorption and dissociation of H2O and free energy change of H* (ΔGH*) for the HER were analyzed. In Fig. 6b, Fe-Ni0.85Se shows a lower H2O adsorption energy (EH2O; −0.81 eV) than Ni0.85Se (−0.16 eV), indicating that H2O molecules are easily adsorbed on the surface of Fe-Ni0.85Se for the subsequent dissociation process.33 For Ni0.85Se, the H2O dissociation and adsorbed hydrogen (H*) generation energy barriers are calculated to 1.36 and 1.18 eV, respectively. After Fe doping, the corresponding energy barriers of Fe-Ni0.85Se decrease to 1.13 and 0.99 eV, respectively, indicating that the H2O dissociation process of Fe-Ni0.85Se is more thermodynamically favorable to produce H* than Ni0.85Se.34 Meanwhile, the ΔGH* value of Fe-Ni0.85Se is calculated to be 0.61 eV (Fig. 6e), which is close to thermoneutrality compared with that of Ni0.85Se (0.77 eV), indicating the fast H2 production capacity of Fe-Ni0.85Se.35 The structure models of H2O dissociation and H* on Ni0.85Se and Fe-Ni0.85Se are displayed in Fig. S10–12†. Additionally, DFT analysis of the SOR process was carried out, and the intermediate energy changes of the stepwise oxidation of S2− to S8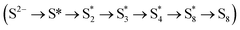 on Ni0.85Se and Fe-Ni0.85Se were estimated on Ni0.85Se and Fe-Ni0.85Se (Fig. 6f and S13†). Notably, Fe-Ni0.85Se has a more negative energy barrier value for S2− adsorption (ΔG*S, −0.51 eV) than Ni0.85Se (−0.36 eV), implying the favorable S2− adsorption for Fe-Ni0.85Se, which is vital for the subsequent desulfurization process.36,37 According to calculated free energy changes (Fig. 6f), the desorption process from *S8 to S8 is identified as the rate-determining step (RDS) for Ni0.85Se, requiring a high energy barrier of 1.56 eV. After Fe doping, Fe-Ni0.85Se has a low free energy barrier of 1.00 eV for desorbing *S8 to S8, which effectively increases SOR performances. These results indicate that Fe introduction not only promotes water dissociation and optimizes the thermodynamic efficiency of H* during the HER but also speeds up the oxidation process of S2− for the SOR, consistent with the above-discussed high HER and SOR performances of Fe-Ni0.85Se.
on Ni0.85Se and Fe-Ni0.85Se were estimated on Ni0.85Se and Fe-Ni0.85Se (Fig. 6f and S13†). Notably, Fe-Ni0.85Se has a more negative energy barrier value for S2− adsorption (ΔG*S, −0.51 eV) than Ni0.85Se (−0.36 eV), implying the favorable S2− adsorption for Fe-Ni0.85Se, which is vital for the subsequent desulfurization process.36,37 According to calculated free energy changes (Fig. 6f), the desorption process from *S8 to S8 is identified as the rate-determining step (RDS) for Ni0.85Se, requiring a high energy barrier of 1.56 eV. After Fe doping, Fe-Ni0.85Se has a low free energy barrier of 1.00 eV for desorbing *S8 to S8, which effectively increases SOR performances. These results indicate that Fe introduction not only promotes water dissociation and optimizes the thermodynamic efficiency of H* during the HER but also speeds up the oxidation process of S2− for the SOR, consistent with the above-discussed high HER and SOR performances of Fe-Ni0.85Se.
Conclusions
In summary, we constructed an Fe-Ni0.85Se catalyst via hydrothermal and selenization strategies, which exhibited splendid catalytic activities towards the HER and SOR in an alkaline electrolyte due to the synergistic effect of Fe incorporation and uniform nanosheet arrays. The resultant Fe-Ni0.85Se realizes the 10 mA cm−2 at a low overpotential of 114 mV for the HER and a potential of 0.340 V for the SOR. Meanwhile, the assembled Fe-Ni0.85Se–based electrolyzer achieves energy-saving H2 generation and sulfion upgradation to a value-added sulfur product, which needs low cell voltages of 0.439 and 0.811 V to reach 10 and 200 mA cm−2. DFT calculations reveal that Fe incorporation plays vital roles in increasing HER and SOR performances, which can enhance the adsorption of catalytic reactants and intermediates and lower energy barriers for the HER and SOR. This study offers a new scheme for the efficient preparation of H2 and sulfur in sulfion-containing wastewater.Data availability
The relevant experimental and characterization data are available in the article and the ESI.†Author contributions
Shuixiang Xie: data curation and formal analysis. Xiaojun Wang: data curation and formal analysis. Yuhuan Li: investigation. Shijie Liu: formal analysis. Jiahui Qian: investigation. Yuhan Zhang: data curation. Linling Jiang: data curation. Zhe Cao: formal analysis. Zhenhao Yan: investigation. Xiaoyu Wan: writing-review and editing. Zhaohang Yang: investigation. Longhua Zou: software. Wei Zhang: conceptualization, writing-review and editing. Rui-Qing Li: conceptualization, writing-review and editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 22302103), the Natural Science Foundation of Jiangsu Province (No. BK20230619), the Natural Science Foundation of the Jiangsu Higher Education Institutions of China (No. 23KJB540003), the Natural Science Foundation of Nantong Municipality (No. JC2023015), the Opening Project of Key Laboratory of Advanced Electrode Materials for Novel Solar Cells for Petroleum and Chemical Industry of China, Suzhou University of Science and Technology (No. 2024A038), the Postgraduate Research & Practice Innovation Program of Jiangsu Province (No. KYCX25_3762), the College Students Innovation and Entrepreneurship Training Program, and the Large Instruments Open Foundation of Nantong University (No. KFJN2457). The authors also thank Ceshigo Research Service (https://www.ceshigo.com) for theoretical calculation and the Nantong University Analysis and Testing Center for technical support.Notes and references
- J. Turner, Science, 2022, 376, 1361 CrossRef.
- P. Shi, J. Li, Y. Song, N. Xu and J. Zhu, Nano Lett., 2024, 24, 5673 CrossRef CAS PubMed.
- X. Zhou, J. Zhang, M. Zhang, X. Du, W. Bao, J. Han, X. Lin, P. Zhang and Z. Luo, Inorg. Chem. Front., 2025, 12 10.1039/D5QI00418G.
- Y. Wu, M. Chen, H. Sun, T. Zhou, X. Chen, G. Na, G. Qiu, D. Li, N. Yang, H. Zheng, Y. Chen, B. Wang, J. Zhao, Y. Zhang, J. Zhang, F. Liu, H. Cui, T. He and Q. Liu, Appl. Catal. B, 2025, 360, 124548 CrossRef CAS.
- X. Deng, X. Zheng, Z. Gong, W. Tan and X. Pei, Chin. J. Rare Met., 2023, 47, 43 Search PubMed.
- R. Li, S. Guo, X. Wang, X. Wan, S. Xie, Y. Liu, C. Wang, G. Zhang, J. Cao, J. Dai, M. Ge and W. Zhang, Chem. Sci., 2024, 15, 10084 RSC.
- C. Walter, P. Menezes and M. Driess, Chem. Sci., 2021, 12, 8603 RSC.
- Y. Wu, M. Chen, D. Liu, H. Sun, T. Zhou, G. Na, G. Qiu, D. Li, Y. Chen, J. Zhao, Y. Zhang, J. Zhang, H. Pan, F. Liu, H. Cui and Q. Liu, J. Mater. Sci. Technol., 2025, 215, 111 CrossRef CAS.
- Y. Wang, S. Wang, Z. Ma, L. Yan, X. Zhao, Y. Xue, J. Huo, X. Yuan, S. Li and Q. Zhai, Adv. Mater., 2022, 34, 2107488 CrossRef CAS PubMed.
- D. Li, M. Chen, D. Liu, C. Shen, H. Sun, Y. Zhang, T. He, Q. Lu, B. Li, T. Zhou, B. Wang, Y. Wu, G. Na, Y. Chen, J. Zhao, Y. Zhang, J. Zhang, F. Liu, H. Cui and Q. Liu, Adv. Energy Mater., 2024, 15, 2404714 CrossRef.
- P. Zhu, M. Shi, Z. Shen, X. Liao and Y. Chen, Chem. Sci., 2024, 15, 4723 RSC.
- P. Zhou, X. Lv, S. Tao, J. Wu, H. Wang, X. Wei, T. Wang, B. Zhou, Y. Lu, T. Frauenheim, X. Fu, S. Wang and Y. Zou, Adv. Mater., 2022, 34, 2204089 CrossRef CAS PubMed.
- R. Li, S. Zeng, B. Sang, C. Xue, K. Qu, Y. Zhang, W. Zhang, G. Zhang, X. Liu, J. Deng, O. Fontaine and Y. Zhu, Nano Res., 2023, 16, 2543 CrossRef CAS.
- Y. Lu, T. Liu, C. Dong, C. Yang, L. Zhou, Y. Huang, Y. Li, B. Zhou, Y. Zou and S. Wang, Adv. Mater., 2022, 34, 2107185 CrossRef CAS PubMed.
- Z. Li, Y. Yan, S. Xu, H. Zhou, M. Xu, L. Ma, M. Shao, X. Kong, B. Wang, L. Zheng and H. Duan, Nat. Commun., 2022, 13, 147 CrossRef CAS PubMed.
- L. Zhang, Z. Wang and J. Qiu, Adv. Mater., 2022, 34, 2109321 CrossRef CAS PubMed.
- W. Wang, Q. Mao, S. Jiang, K. Deng, H. Yu, Z. Wang, Y. Xu, L. Wang and H. Wang, Appl. Catal., B, 2024, 340, 123194 CrossRef CAS.
- R. Li, X. Wang, S. Xie, S. Guo, Z. Cao, Z. Yan, W. Zhang and X. Wan, Chem. Sci., 2025, 16, 809 RSC.
- H. Wang, H. Yao, Q. Guo and H. Xia, J. Nanjing Univ. Nat. Sci. Ed., 2024, 23, 45 Search PubMed.
- L. Yi, Y. Ji, P. Shao, J. Chen, J. Li, H. Li, K. Chen, X. Peng and Z. Wen, Angew. Chem., Int. Ed., 2021, 60, 21550 CrossRef CAS PubMed.
- Y. Pei, D. Li, C. Qiu, L. Yan, Z. Li, Z. Yu, W. Fang, Y. Lu and B. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202411977 CrossRef CAS PubMed.
- R. Li, X. Xu, J. Zeng, X. Zhang, X. Wan, S. Guo, X. Wang, S. Xie, Z. Cao, Y. Zhang, C. Wang, J. Deng, O. Fontaine, M. Ge, J. Dai, G. Zhang, W. Zhang, X. Wang and Y. Zhu, Nano Lett., 2025, 25, 1272 CrossRef CAS PubMed.
- R. Xu and Z. Wang, J. Nantong Univ., Nat. Sci. Ed., 2024, 23, 21 Search PubMed.
- T. Xue, Z. Huang, H. Gu and H. Zhang, Chin. J. Rare Met., 2024, 48, 90 Search PubMed.
- R. Li, H. Su, S. Xie, X. Wan, C. Wang, G. Zhang, M. Ge, J. Dai, C. Xue, C. Li, J. Cao and W. Zhang, Rare Met, 2024, 43, 6426 CrossRef CAS.
- H. Yan, Y. Xie, A. Wu, Z. Cai, L. Wang, C. Tian, X. Zhang and H. Fu, Adv. Mater., 2019, 31, 1901174 CrossRef PubMed.
- C. Lyu, Y. Li, J. Cheng, Y. Yang, K. Wu, J. Wu, H. Wang, W. Lau, Z. Tian, N. Wang and J. Zheng, Small, 2023, 19, 2302055 CrossRef CAS PubMed.
- Y. Chang, P. Zhai, J. Hou, J. Zhao and J. Gao, Adv. Energy Mater., 2022, 12, 2102359 Search PubMed.
- X. Du, J. Zhang, X. Zhou, M. Zhang, N. Wang, X. Lin, P. Zhang and Z. Luo, Green Chem., 2025, 27, 3515 RSC.
- H. Luo, L. Li, F. Lin, Q. Zhang, K. Wang, D. Wang, L. Gu, M. Luo, F. Lv and S. Guo, Adv. Mater., 2024, 36, 2403674 CrossRef CAS PubMed.
- H. Ding, C. Su, J. Wu, H. Lv, Y. Tan, X. Tai, W. Wang, T. Zhou, Y. Lin, W. Chu, X. Wu, Y. Xie and C. Wu, J. Am. Chem. Soc., 2024, 146, 7858 CrossRef CAS PubMed.
- H. Yu, W. Wang, Q. Mao, K. Deng, Y. Xu, Z. Wang, X. Li, H. Wang and L. Wang, J. Mater. Chem. A, 2023, 11, 2218 RSC.
- Y. Hu, L. Shao, Z. Jiang, L. Shi, Q. Li, K. Shu, H. Chen, G. Li, Y. Dong, T. Wang, J. Li, L. Jiao and Y. Deng, Adv. Funct. Mater., 2024, 34, 2411011 CrossRef CAS.
- Q. Zhou, H. Hu, Z. Chen, X. Ren and D. Ma, Chem. Sci., 2025, 16, 1597 RSC.
- T. Wang, L. Miao, S. Zheng, H. Qin, X. Cao, L. Yang and L. Jiao, ACS Catal., 2023, 13, 4091 CrossRef CAS.
- Y. Pei, D. Li, C. Qiu, L. Yan, Z. Li, Z. Yu, W. Fang, Y. Lu and B. Zhang, Angew. Chem., Int. Ed., 2024, 63, e202411977 CrossRef CAS PubMed.
- D. He, P. Yang, K. Yang, J. Qiu and Z. Wang, Adv. Funct. Mater., 2024, 34, 2407601 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc01884f |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |