 Open Access Article
Open Access ArticleWater-soluble BODIPY dyes: a novel approach for their sustainable chemistry and applied photonics†
Christopher
Schad
a,
Cesar
Ray
a,
Carolina
Díaz-Norambuena
ab,
Sergio
Serrano-Buitrago
a,
Florencio
Moreno
 a,
Beatriz L.
Maroto
a,
Beatriz L.
Maroto
 a,
Inmaculada
García-Moreno
c,
Mónica
Muñoz-Úbeda
de,
Iván
López-Montero
a,
Inmaculada
García-Moreno
c,
Mónica
Muñoz-Úbeda
de,
Iván
López-Montero
 def,
Jorge
Bañuelos
def,
Jorge
Bañuelos
 *b and
Santiago de la
Moya
*b and
Santiago de la
Moya
 *a
*a
aDepartamento de Química Orgánica, Facultad de Ciencias Químicas, Universidad Complutense de Madrid, Ciudad Universitaria s/n, Madrid 28040, Spain. E-mail: santmoya@ucm.es
bDepartamento de Química-Física, Facultad de Ciencia y Tecnología, Universidad del País Vasco-EHU, Bilbao 48080, Spain. E-mail: jorge.banuelos@ehu.eus
cDepartamento de Química-Física de Materiales, Instituto de Química-Física Blas Cabrera, Consejo Superior de Investigaciones Científicas (CSIC), Serrano 119, Madrid 28006, Spain
dDepartamento de Química Física, Facultad de Ciencias Químicas, Universidad Complutense de Madrid, Ciudad Universitaria s/n, 28040, Madrid, Spain
eInstituto de Investigación Biomédica Hospital Doce de Octubre (imas12), Avda. de Córdoba s/n, 28041, Madrid, Spain
fInstituto Pluridisciplinar, Universidad Complutense de Madrid, P° Juan XXIII 1, 28040, Madrid, Spain
First published on 2nd April 2025
Abstract
The BODIPY family of organic dyes has emerged as a cornerstone in photonics research development, driving innovation and advancement in various fields of high socio-economic interest. However, the majority of BODIPY dyes exhibit hydrophobic characteristics, resulting in poor solubility in water and other hydrophilic solvents. This solubility is paramount for their optimal utilization in a myriad of photonic applications, particularly in the realms of biology and medicine. Furthermore, it facilitates safer and more sustainable manipulation and chemical modification of these expansive dyes. Nevertheless, bestowing BODIPYs with water solubility while preserving their other essential properties, notably their photophysical signatures, poses a significant challenge. In this context, we present a straightforward general chemical modification aimed at converting conventional hydrophobic BODIPYs into highly hydrophilic variants, thus enabling their efficient solubilization in water and other hydrophilic solvents with minimal disruption to the dye's inherent photophysics. The efficacy of this methodology is demonstrated through the synthesis of a number of water-soluble BODIPY dyes featuring diverse substitution patterns. Furthermore, we showcase their utility in a spectrum of photonics-related applications, including in-water BODIPY chemistry and dye-laser technology, and fluorescence microscopy.
Introduction
Water stands out as the most sustainable medium for reactions and processes due to its abundance, affordability, non-toxicity, and safe handling characteristics.1 Nature itself has chosen water as the primary medium for biological chemistry, where all organic reactions and life-sustaining interactions occur.2 Additionally, the growing environmental awareness in recent years has heightened the importance of utilizing water as the preferred medium over organic solvents.3 Unlike organic solvents, which con-tribute to smog, air pollution, ozone production, and ultimately climate change, water offers a cleaner and more environmentally friendly alternative.1,3Photonics has played a pivotal role in spearheading numerous innovations that have reshaped our lifestyles in recent years, with applications spanning a broad spectrum of fields, ranging from energy to healthcare. Many indispensable photonic applications thrive in aqueous environments, extending far beyond fluorescence bioimaging4 and photodynamic therapy (PDT).5 These include optical sensing of analytes in aqueous environments,6 photocatalytic water purification7 and hydrogen production,8 artificial photosynthesis,9 underwater wireless optical communications,10 and optogenetics,11 among others.
In most of these applications, particularly in biology and medicine, molecular organic dyes play a fundamental role owing to their organic nature and compact size, which offer key advantages such as high biocompatibility and membrane permeability, or facile adaptation through organic chemistry.12 Among these dyes, BODIPYs (boron dipyrromethenes; see Fig. 1) stand out as a noteworthy family of highly-fluorescent and photophysically adaptable organic dyes.13
 | ||
| Fig. 1 Selected examples of useful BODIPY dyes (dipyrrin ligand in red).13a–d | ||
However, the hydrophobic nature of the BODIPYs poses a significant limitation in their optimal use in photonic applications that require a proper solubility in pure water or in highly hydrophilic media. In this context, several approaches have been developed to date to impart water solubility to BODIPYs.14 Unfortunately, most of them are not straightforward and involve the covalent attachment of hydrophilic moieties to the dye's π-conjugated core (dipyrrin ligand). This often leads to significant distortions in the photophysics of the original dye, as the BODIPY chromophore resides within such a π-conjugated core.
In this regard, a few recent approaches have focused on introducing the hydrophilic moieties at the BODIPY's boron centre, which does not participate in the chromophoric system, thus preventing photophysical distortion. This is usually achieved by creating B–O bonds through well-known boron fluorine substitution reactions using O-nucleophiles to construct water-soluble 4,4-dioxygenated BODIPYs (O-BODIPYs; e.g., see Fig. 2).14e,j,o,q Unfortunately, these methods are far from being efficient, general and/or straightforward, and the obtained BODIPY derivatives may suffer from significant chemical instability due to the change from the strongly electron-withdrawing difluoroboron moiety in common BODIPY dyes (F-BODIPYs) to the less electron-withdrawing dioxygenated boron in the O-BODIPYs.
 | ||
| Fig. 2 Examples of hydrophilic O-BODIPY dyes based on highly-hydrophilic PEG chains.14e,q | ||
In connection with this interest, we have recently established a general procedure that allows for the effective transformation of BODIPY into 4,4-diacyloxyl derivatives (aka COO-BODIPYs) under mild reaction conditions in a single synthetic step, and using carboxylic acids as readily-available O-nucleophilic reagents (e.g., see Scheme 1).15a Notably, COO-BODIPYs are very robust and exhibit photophysical behaviours even superior to those of the corresponding F-BODIPYs,15 with their chemical stability under harsh acidic conditions being significantly greater than that of related O-BODIPYs. Since this soft synthetic method is compatible with a large number of functional groups, it should be easily extended to carboxylic acids functionalized with highly hydrophilic moieties, thus promoting water solubility. In this work, we demonstrate this possibility by reporting a specific methodology based on the preferential use of commercial γ-butyrobetaine hydrochloride as the solubilizing reagent, enabling the effective transformation of a wide range of F-BODIPYs (standard BODIPYs) into the corresponding water-soluble COO-BODIPYs,16 and showcasing its effectiveness and utility in a number of applications, particularly in aqueous green BODIPY chemistry and dye-laser technology, and fluorescence microscopy.
 | ||
| Scheme 1 Synthesis of COO-BODIPYs 6–10 with hydrophilic moieties of different nature pending at boron. Bn: benzyl; Cbz: (benzyloxy)carbonyl. See the ESI† for experimental details. | ||
Results and discussion
Setting the method
The ideal chemical methodology for converting hydrophobic BODIPYs into hydrophilic BODIPYs should be straightforward and cost-effective, use inexpensive and readily available reagents, and efficiently enhance solubility while preserving the photophysical properties of the original dye. Accordingly, we first selected five marketed, cheap carboxylic acid derivatives possessing hydrophilic moieties, or specific functions acting as precursors of these moieties. Particularly, [2-(2-methoxyethoxy)ethoxy]acetic acid (1), isonicotinic acid (2), 3-(pyridin-4-yl)propanoic acid (3), (3-carboxypropyl)trimethylammonium chloride (γ-butyrobetaine hydrochloride, 4), and N-[(benzyloxy)carbonyl]-L-glutamic acid 1-benzyl ester (5) (Scheme 1). These materials were proved to transform two marketed hydrophobic BODIPYs, particularly the well-known laser dyes PM546 (1,3,5,7,8-pentamethyl-F-BODIPY) and PM567 (2,6-diethyl-1,3,5,7,8-pentamethyl-F-BODIPY), in the COO-BODIPYs derivatives 6–10, all of them, therefore, possessing hydrophilic moieties in their at-boron pending acyloxy moieties (Scheme 1).In all cases, at-boron acyloxylation was conducted following our methodology for synthesizing COO-BODIPYs.15a Thus, the starting F-BODIPY was treated with BCl3 in dichloromethane (DCM) solution at room temperature and then, with Et3N and the selected carboxylic acid, to obtain the corresponding COO-BODIPY in yields ranging from 62% for 6, to 95% for pre-8. This remarks the generality of our synthetic methodology, even when highly hydrophilic moieties are involved in the selected carboxylic acid reagent.
For the synthesis of 7, 8 and 10, a second step was needed to construct the hydrophilic group: nitrogen quarternisation in the case of 7 and 8, or N-Cbz and O-Bn deprotections by catalytic hydrogenation in the case of 10. These steps proceeded smoothly in almost quantitative yields (see Scheme 1).
To assess the increase in water solubility resulting from the introduction of the selected acyloxyl groups into the corresponding starting hydrophobic F-BODIPY, we computationally calculated log P values (i.e., the decimal logarithm of the equilibrium partition coefficient between 1-octanol and water) at 25 °C for starting and final dyes. For this purpose, we used the popular Percepta software.17 The obtained values (see Table 1) show that all the selected hydrophilic acyloxyl moieties contribute to increasing water solubility. Thus, all the log P values for the new COO-BODIPYs 6–10 (ranging from +2.80 for 6 to −4.05 for 9) are lower than those for parent F-BODIPYs PM567 (+3.64) and PM546 (+2.89).
| a It refers to the corresponding neutral carboxyl and amino groups. |
|---|

|
Among these acyloxyl moieties, the PEGylated in 6 induces the lowest increase of the water solubility (from log P = +2.89 to log P = +2.80), still being 1-octanol the preferred solvent against water. This should be probably improved by using longer polyether chains in the starting carboxylic acid reagent.14i,q The amino acid moiety in 10 also increases water solubility, being the estimated partition between 1-octanol and water close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (log P = +0.19). However, it must be noted here that the used computational program did not allow the calculation of log P for the zwitterionic species, but just for the molecule with neutral amino and carboxyl groups. Therefore, the log P value estimated for 10 is not reliable, and the solubility in water is probably higher than the one herein calculated. Noticeably, in the case of 7–9, having ionic acyoxyl moieties, log P is estimated to be negative (see Table 1), which indicates that water is the prefer-red solvent against 1-octanol. In fact, log P fully reverses when transforming F-BODIPY PM567 (log P = +3.64) into γ-butyrobetaine-based COO-BODIPY 9 (log P = −4.05).
1 (log P = +0.19). However, it must be noted here that the used computational program did not allow the calculation of log P for the zwitterionic species, but just for the molecule with neutral amino and carboxyl groups. Therefore, the log P value estimated for 10 is not reliable, and the solubility in water is probably higher than the one herein calculated. Noticeably, in the case of 7–9, having ionic acyoxyl moieties, log P is estimated to be negative (see Table 1), which indicates that water is the prefer-red solvent against 1-octanol. In fact, log P fully reverses when transforming F-BODIPY PM567 (log P = +3.64) into γ-butyrobetaine-based COO-BODIPY 9 (log P = −4.05).
With the aim of confirming that the substitution of fluorines by the selected, different hydrophilic acyloxyl moieties increases the solubility of the BODIPY dye in water while maintaining the dye photophysics, we measured the fluorescence signatures of COO-BODIPYs 6–10 from diluted solutions (ca. 2 × 10−6 M) in methanol and in water, and compared them with those exhibited by corresponding parent F-BODIPYs PM546 and PM567, in almost similar experimental conditions (Table 2).
| Dye | λ ab (nm) | ε max (M−1 cm−1) | λ fl (nm) | ϕ | τ (ns) |
|---|---|---|---|---|---|
| a Insoluble in pure water. Addition of a minimum amount of MeOH (ca. 0.5% v/v) was required to enable complete dissolution in water, albeit the dyes are prone to self-associate into non-fluorescent aggregates. | |||||
| PM546 | 492.5 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
505.5 | 0.81 | 5.58 |
| 461.0 |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 000
|
501.5 | — | — | |
| PM567 | 516.0 | 74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
534.0 | 0.81 | 6.10 |
| 482.5 |
25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 000
|
535.0 | — | — | |
| 6 | 496.5 | 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
506.5 | 0.91 | 6.23 |
| 493.0 |
23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 000
|
501.5 | 0.73 | 6.20 | |
| 7 | 517.0 | 65![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
536.5 | 0.67 | 6.29 |
| 517.5 |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 000
|
538.0 | 0.41 | 6.14 | |
| 8 | 520.0 | 56![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
537.0 | 0.61 | 5.44 |
| 518.0 |
50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 000
|
536.0 | 0.63 | 5.55 | |
| 9 | 519.5 | 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
533.5 | 0.74 | 6.87 |
| 516.5 |
44![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 000
|
535.0 | 0.71 | 6.89 | |
| 10 | 519.0 | 43![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
537.0 | 0.83 | 6.67 |
| 516.0 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/i_char_2009.gif) 000 000
|
534.0 | 0.80 | 6.96 | |
Due to their low solubility in water, hydrophobic F-BODIPYs PM546 and PM567 required the addition of a minimum amount of methanol (ca. 0.5% v/v) to allow accurate measurements in water. In spite of this, they underwent an effective aggregation in water, even at low dye concentration ca. 10−6 M, causing a dramatic loss of the fluorescence signal, which does not occur in methanol (see Table 2). This fluorescence-quenching aggregation (H-aggregates18) in water also led to noticeable flattening and broadening of the absorption profile, owing to the growth of a new and prominent band at shorter wavelengths, which is more significant for more hydrophobic peralkylated PM567 (see Fig. S2†). Contrarily, their COO-BODIPY derivatives 6–10 showed no aggregation signs in water, even at concentrations higher than 10−3 M for some cases (e.g., see Fig. S3†), and showed a photophysical behaviour comparable to that for them in methanol. It must be noted here that non-ionic COO-BODIPY 6 required the addition of a minimum amount of methanol to allow its photophysical characterization in water. Noteworthy, the shapes and positions of the absorption and emission bands of hydrophilic COO-BODIPYs 6–10 in water are similar to those for the corresponding parent hydrophobic F-BODIPYs in methanol (see Table 2, and cf. Fig. S2 and S3†), and their fluorescence quantum yields in water solution are higher than 0.60 with the exception of 7.
Thanks to the significantly increased water solubility (Table 1), synthetic simplicity and low-cost, and maintained photophysical properties (Table 2), the one-step conversion of F-BODIPYs into ionic γ-butyrobetaine-based COO-BODIPYs (see Scheme 1) offers a practical method to impart water solubility to typically hydrophobic F-BODIPYs, with minimal impact on their photophysics.
Exploring the method's scope
To explore the scope of the proposed chemical methodology, we selected a small battery of hydrophobic F-BODIPYs with specific instability and/or reactivity features (see PM505, PM597, PM605, 11 and 12 in Table 3).Thus, marketed PM505 (1,3,5,7-tetramethyl-F-BODIPY), without substitution at the BODIPY meso position, is prone to undergo at-meso nucleophilic additions to the π-conjugated BODIPY-chromophore system.19 On the other hand, commercial, crowdedly-peralkylated PM597 (2,6-di-tert-butyl-1,3,5,7,8-pentamethyl-F-BODIPY) easily undergoes strain-releasing de-tert-butylation,20 whiles commercial PM605 (8-(acetoxymethyl)-2,6-diethyl-1,3,5,7-tetramethyl-F-BODIPY) and synthetically-accessible chlorinated 11 (ref. 21) are specially featured to undergo nucleophilic substitution of acetoxyl group and aromatic nucleophilic substitution of halogen, respectively.22 Finally, readily-accessible propargylated F-BODIPY 12 (ref. 23) was selected to test the possibility of competing at-boron substitution by the involved acidic terminal-alkyne group (formation of ethynyl C-BODIPYs).24
Satisfactorily, all these additional F-BODIPYs were straightforwardly converted into the corresponding γ-butyrobetaine-based COO-BODIPYs 13–17 (see Table 3) following the established methodology (i.e., (a) BCl3, (b) Et3N, (c) γ-butyrobetaine hydrochloride (4), at room temperature; see Scheme 1). In all these cases, the chemical yield in isolated COO-BODIPY exceeded 70%, with the exception of highly unstable PM597, whose transformation in COO-BODIPY 14 took place with 54% yield (see Table 3, and the ESI† for experimental details).
Noteworthy, the calculated log P values for compounds 13–17 range from −2.72 for highly alkylated 14, to −5.38 for less substituted 13 (see Table S1†), supporting a significant enhancement in water solubility due to the conducted γ-butyrobetaine derivatization. Moreover, we confirmed that this derivatization did not significantly alter the photophysical behaviour of the corresponding parent F-BODIPY in methanol, as the absorption and emission wavelengths, as well as the fluorescence quantum yields, remained comparable.
Most importantly, the conducted derivatization enabled similar dye photophysics in pure water (see Table S2†). The only exceptions to this rule are the absorption capabilities of γ-butyrobetaine-based COO-BODIPYs 14 and 15, which were significantly lower to those recorded for their corresponding parent F-BODIPYs (PM597 and PM605, respectively) in methanol, particularly for 14 in water (see Table S2†). This behaviour can be attributed to the previously mentioned high reactivity of the involved BODIPY cores. Indeed, the photonic performance of compound 14 resulted time-sensitive, as the dye solution in both solvents gradually bleached over time.
As a result, the obtained new set of γ-butyrobetaine-based COO-BODIPYs (Table 3) sustains the versatility and success of the developed method to solubilize hydrophobic F-BODIPYs in water, and, together with the previous ones (Table 2), constitute a valuable palette of selectable highly-bright water-soluble fluorophores with spectral bands spanning from the green to the red edge of the visible spectrum (Fig. S4†).
Showcasing some applications
The synthetic accessibility, chemical robustness, photophysical behaviour, and water solubility of the γ-butyrobetaine-based COO-BODIPYs make them ideal candidates to optimize and broaden the use of the commonly hydrophobic F-BODIPY dyes in applications where water is essential or even critical. To demonstrate their potential, we have selected three key applications: green BODIPY chemistry in aqueous media, in-water BODIPY lasing, and fluorescent biolabeling using BODIPY-dye solutions in pure water.Green BODIPY chemistry in aqueous media
It is obvious that water is the most inexpensive and environmentally friendly solvent. In this context, facilitating chemical processes of hydrophobic substances in water is pivotal to advance the implementation of more sustainable (greener) chemistry.1,3 On the other hand, in vivo organic chemistry is gaining attention in both biology and medicine, particularly in that concerning the metal-catalyzed formation of C–C bonds involving chromophoric systems,25 and its employment in advancing the study and applications of optogenetics.11 In this context, facilitating in-water BODIPY chemistry is essential to advance a greener BODIPY chemistry and processing, but also to harness the excellent photophysical behaviour of the tunable BODIPY chromophore in advanced biophotonic applications requiring previous chemical transformations of the dyed material in cells.In order to explore the potential of the water-soluble γ-butyrobetaine-based COO-BODIPYs to facilitate in-water BODIPY chemistry, we selected three common, useful chemical transformations of the BODIPY dyes: nucleophilic aromatic substitution of halogen in 3-haloBODIPYs,22a–d,f,g Suzuki–Miyaura coupling of haloBODIPYs,26 and “click” reaction of alkyne-based BODIPYs with azides.27 For this purpose we choose chlorinated COO-BODIPY 16 and propargylated COO-BODIPY 17 (Scheme 2).
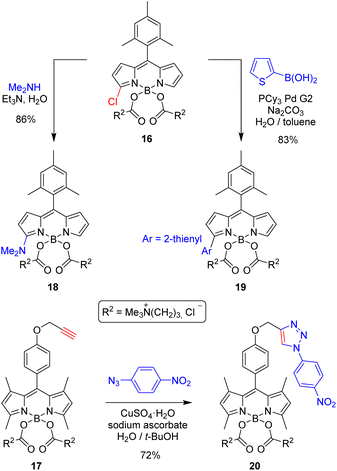 | ||
| Scheme 2 Efficient chemical derivatizations of γ-butyrobetaine-based COO-BODIPYs 16 and 17 in aqueous media (see the ESI† for experimental details). | ||
As shown in Scheme 2, 3-chloro-substituted COO-BODIPY 16 was able to react satisfactorily with dimethylamine in water at 50 °C, using triethylamine as proton scavenger. The expected substitution product (18) was obtained in just 90 min in 86% yield. Likewise, the Suzuki–Miyaura coupling of 16 with 2-thienylboronic acid in aqueous media, using chloro[(tricyclohexylphosphine)-2-(2′-aminobiphenyl)]palladium(II) (PCy3 Pd G2) as precatalyst and sodium carbonate as base, led to the expected C–C coupling product (19) in 83% yield.
On the other hand, propargylated COO-BODIPY 17 could be straightforwardly “clicked” with 1-azido-4-nitrobenzene in highly-hydrophilic aqueous tert-butanol, using CuSO4/sodium ascorbate, to generate 20 in 72% yield. Noteworthy, the log P values calculated for the resulting final dyes (18–20) were negative in all cases (below −3.8; see Table S1†), remarking the capability of at-boron pending γ-butyrobetaine moieties to enhance the solubility of the BODIPY dyes in water.
BODIPY-laser operation in pure water
The thermo-optic properties of the gain-medium solvent are key in dye lasing, which along with the dye-flow rate largely determine the maximum repetition rate at which a dye laser can be operated.28 Indeed, it has confirmed the superior thermo-optic properties of water in comparison to alcohols for laser operation.29 However, the use of water as solvent for high-power dye lasers has been significantly restricted by the fact that several of the commonly used laser dyes are highly hydrophobic and, therefore, not easily soluble in water at the typical concentrations necessary for laser operation. This insolubility in water promotes the formation of non-fluorescent dye dimers and higher aggregates, which induces an absorptive loss of pump power in dye lasers. Conversely, aqueous gain media result in lower laser efficiency than alcoholic media in these cases.30 This is especially true for the valuable but highly hydrophobic F-BODIPY laser dyes.31 Consequently, the development of aqueous active media for lasing is a topic of interest in dye laser technology.29b,32To explore the capability of the newly developed, highly-hydrophilic COO-BODIPYs to support dye-laser operation in pure water, we selected compounds 7–9, 15 and 19 (see Tables 1 and 3, and Scheme 2). This selection allows us for assessing potential differential behaviours influenced by the characteristics of the BODIPY chromophore (e.g., extension of the π-conjugation or different capability for light absorption/emission) and the hydrophilic acyloxyl moieties at the boron position (pyridinium cation for 7 and 8, and γ-butyrobetaine for 9, 15, and 19).
For comparison, laser behaviour in alcohol (methanol) was also measured under nearly identical experimental conditions (Table 4). It must be noted here that COO-BODIPYs 7–9, and 15 are hydrophilic derivatives of the well-known marketed F-BODIPY laser dyes PM567 (green-emitting) and PM605 (red-emitting), respectively.
Regardless of the solvent (methanol or pure water), under the selected operational conditions (transversal pumping and strong focusing of the incoming pump radiation), high dye concentrations (in the mM range) were needed to assure total absorption of the excitation radiation within the first millimetres of the optical cell. This is required to achieve a near circular cross-section of the emitted beam, which is essential to induce highly efficient laser action, and to allow the accurate determination of the dependence of the laser action (laser efficiency, EffL, and laser-peak wavelength, λL) on the dye concentration. In this regard, it should be noted here that less water-soluble 7 and 8, both based on pyridinium iodide, needed to be sonicated under mild conditions to achieve their total solubilization in pure water at concentrations higher than 1.5 mM.
The recorded laser action (Table 4) is guided by the photophysical behaviour of the corresponding dye (see Tables 2, and S2†). Thus, the higher the fluorescent quantum yield and the redder the fluorescent emission, the more efficient and redder becomes the laser action. At the dye concentration that optimizes the laser action (ca. 1.5 mM for 7–9 and 15, and ca. 2.5 mM for 19), the selected dyes reach high laser efficiencies in highly hydrophilic methanol, ranging from 47% for 15, to 58% for 9, with the laser emissions peaked in the 550–590 nm spectral region. Further increasing of the dye concentration results in a decrease of the laser efficiency, owing to the activation of reabsorption/reemission processes with remarkable deleterious effect in the laser action (see Table 4).
| Dye | Laser actiona | Dye concentration (mM) | ||||
|---|---|---|---|---|---|---|
| 0.6 | 1.0 | 1.5 | 2.5 | 3.5 | ||
| a EffL = energy conversion efficiency; λL: laser-peak wavelength. | ||||||
| 7 | EffL (%) | 33 | 43 | 48 | 34 | 19 |
| 27 | 37 | 43 | 29 | 10 | ||
| λ L (nm) | 550 | 553 | 554 | 558 | 561 | |
| 8 | EffL (%) | 37 | 49 | 51 | 39 | 27 |
| 31 | 46 | 48 | 32 | 21 | ||
| λ L (nm) | 548 | 552 | 556 | 558 | 562 | |
| 9 | EffL (%) | 38 | 53 | 58 | 41 | 26 |
| 33 | 50 | 55 | 38 | 19 | ||
| λ L (nm) | 550 | 552 | 555 | 559 | 563 | |
| 15 | EffL (%) | 37 | 44 | 47 | 37 | 26 |
| 35 | 41 | 45 | 34 | 22 | ||
| λ L (nm) | 567 | 572 | 577 | 580 | 583 | |
| 19 | EffL (%) | 22 | 35 | 46 | 55 | 49 |
| 19 | 30 | 43 | 50 | 43 | ||
| λ L (nm) | 575 | 580 | 585 | 587 | 589 | |
Noteworthy, this efficient laser action in methanol is retained in pure water (Table 4). Moreover, increasing the dye concentration in water red-shifts the laser-peak wavelength, but this displacement induces neither a laser-band broadening nor a shoulder growing (Fig. 3). These outstanding behaviours are due to the high solubility of the studied COO-BODIPY dyes in the selected hydrophilic media, including water, which allows reaching the required optimum operational dye concentration in the hydrophilic solvent without inducing dye aggregation, even in water at dye concentrations as high as 4 mM (Table 4 and Fig. 3).
 | ||
| Fig. 3 Dependence of the laser spectra of COO-BODIPYs 9 and 19 on the dye concentration in pure-water solution. | ||
Since dye photostability under severe, long-term laser pumping conditions is an important parameter for any advanced photonic and biophotonic application based on organic dye (e.g., fluorescence imaging), the lasing photostability of the selected COO-BODIPYs dyes in water was assessed by following the evolution of their laser-induced fluorescence emission as a function of the number of pumping laser pulses (see the ESI† for experimental details). Surprisingly, the new dyes resulted highly photostable even in water, since their laser emission intensities decrease a merely 10% after 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 pump pulses under the selected, drastic pumping conditions. Indeed, the studied COO-BODIPYs are, to the best of our knowledge, the first BODIPY dyes enabling highly efficient and photostable laser emission in pure water. It should be noted here that the replacement of the organic solvent by water in the gain medium does not entail modifying any other experimental variable (dye concentration, pump fluence, cavity configuration, temperature, etc.) to record highly efficient and stable laser emission.
000 pump pulses under the selected, drastic pumping conditions. Indeed, the studied COO-BODIPYs are, to the best of our knowledge, the first BODIPY dyes enabling highly efficient and photostable laser emission in pure water. It should be noted here that the replacement of the organic solvent by water in the gain medium does not entail modifying any other experimental variable (dye concentration, pump fluence, cavity configuration, temperature, etc.) to record highly efficient and stable laser emission.
Fluorescent live-cell imaging using BODIPY solutions in pure water
Fluorescence bio-imaging is pivotal for advancing biology and medicine.33 It relies on the use of specific fluorescent markers, particularly small molecular fluorophores known as molecular bioprobes or simply molecular probes. F-BODIPYs are especially effective as molecular probes due to their excellent fluorescence properties, chemical and photophysical adaptability, biocompatibility, and cell membrane permeability.13e,f,q,s However, a major limitation of the non-ionic molecular probes, including most BODIPY bioprobes, is their high hydrophobicity, resulting in a poor solubility in water, including the aqueous cell cultures. This often leads to undesired issues limiting biolabeling efficacy, such as dye aggregation during the staining operation, either in the aqueous dye staining solution or the cell culture.34To mitigate these problems, small amounts of organic solvents like dimethylsulfoxide (DMSO) or ethanol are typically added to the staining solutions. However, the associated cytotoxicity of these solvents constrains their use, limiting also the percentage of solvent that can be added to the cell culture.35 In this context, the rapid conversion of hydrophobic F-BODIPYs probes into highly water-soluble γ-butyrobetaine-based COO-BODIPYs could offer an effective solution for improving their use as molecular probes. Nevertheless, the involved at-boron chemical modification could potentially impact key properties such as biocompatibility, cell membrane permeability, and staining specificity.
To assess to what extent these properties might be affected, we have selected polyalkylated F-BODIPYs PM505, a known molecular probe,36 the structurally similar but more hydrophobic PM567, and their corresponding γ-butyrobetaine-based COO-BODIPYs (13 and 9, respectively; see Table 1), and investigate their differential capability to fluorescently label live cells using pure-water solutions. For this purpose, wild-type mouse embrionary fibroblasts, MEFs, were selected.
As expected, 1 mM stock solutions of 9 and 13 in pure water could be easily obtained without detecting dye aggregation to the naked eye (see Fig. S5†). However, this is not the case for F-BODIPY precursors PM567 and PM505, where significant aggregation remains even upon intensive vortex agitation plus sonication (Fig. S5†). Satisfactorily, 9 and 13 in pure water stained straightforwardly living MEFs without using any additional organic solvent in the cell culture, allowing the fluorescence bioimaging of live cell systems with notable efficiency (Fig. 4; see the ESI† for experimental details).
 | ||
| Fig. 4 BioLiving MEFs incubated with γ-butyrobetaine-based COO-BODIPYs (9 and 13) in water solution at ca. 120 nM after 24 h incubation. Scale bars are 10 μm. | ||
These results demonstrate that the capability of PM567 and PM505 for cell uptake is retained in their water-soluble γ-butyrobetaineated derivatives. Indeed, the bioimaging patterns obtained by using PM567, PM505, 9 and 13 under similar staining conditions are almost comparable, all of them staining different subcellular structures, particularly lysosomes, as demonstrated by co-localization with LysoTracker Red (see Fig. S6†).
Nonetheless, some different biospecificity should be expected upon the γ-butyrobetaine derivatization of the selected F-BODIPY dyes (PM567 and PM505), taking into account the lack of groups for specific biorecognition in their molecular structures. Thus, in cases of F-BODIPY probes involving these biolabeling groups, the biospecificity should be strongly ruled by them, the influence of the different at-boron pending groups (fluorines vs. γ-butyrobetaine-based acyloxyls) being minimal.
Noteworthy, the study of the cell viability by the AlamarBlue reduction assay (see the ESI† for experimental details) demonstrates the conducted solubilizing BODIPY modification does not distort the high biocompatibility of the starting F-BODIPY dye. Thus, the γ-butyrobetaine COO-BODIPYs 9 and 13 exhibited very low cytotoxicities, which are similar to those exhibited by the corresponding parent F-BODIPYs PM567 and PM505, even at dye concentrations as high as 500 nM in cell culture (see Fig. S7†). Moreover, computational prediction of the pharmacokinetic parameters of the selected paired compounds using the free web tool SwissADME37 revealed similar values for γ-butyrobetaine COO-BODIPYs 9 and 13 and the corresponding F-BODIPYs PM567 and PM505, except for the skin permeation parameter (Kp), which was higher for F-BODIPYs in agreement with their greater lipophilicity (e.g., Kp = 10−5 cm s−1 for PM567vs. Kp = 10−6.7 cm s−1 for 9).
Conclusions
A straightforward chemical modification to easily convert hydrophobic BODIPYs into highly-hydrophilic BODIPYs has been developed. The transformation involves the at-boron substitution of fluorine atoms in F-BODIPYs by hydrophilic acyloxyl chains based on γ-butyrobetaine, yielding highly hydrophilic COO-BODIPYs. This chemical transformation is done in just one step using mild reaction conditions and a low-cost commercial reagent. It has been demonstrated that it can be applied to different starting F-BODIPYs, even to highly reactive ones, generating the corresponding COO-BODIPYs in moderate to high yields, which supports the generality of the methodology. The obtained chemically-modified BODIPY dyes exhibit a drastic enhancement of water solubility, as supported by their calculated log P values and, importantly, they retain the main photophysical signatures of the starting F-BODIPYs, such as position of the spectral absorption and emission bands or fluorescence quantum yield.The potential of this chemical methodology to impart water solubility in common F-BODIPY dyes has been exemplified by using some of the obtained γ-butyrobetaine-based COO-BODIPY dyes in selected applications requiring aqueous media, such as in-water BODIPY chemistry, BODIPY-laser operation in pure water, and fluorescence biolabeling using BODIPY-dye solutions in pure water. The success in all these applications demonstrate the feasibility of the described methodology to impart water solubility to the typically highly-hydrophobic F-BODIPY dyes, which should expand the optimal use of these tunable dyes in specific applications requiring water or highly-hydrophilic media, particularly in the biophotonics field.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
S. d. l. M., conceptualization; S. d. l. M. and J. B. supervision; B. L. M., investigation (molecular design); C. S. and C. R., investigation (synthetic development); F. M. J, investigation (structural characterization); C. D.-N., investigation ((photo)physical behaviour); S. S.-B., investigation (in-water chemistry validation); I. G.-M., investigation (laser operation validation); M. M.-U. and I. L.-M., investigation (live-cell imaging validation); S. d. l. M., J. B.; B. L. M., I. G.-M and I. L.-M., writing (original draft); S. d. l. M., writing (review and editing).Conflicts of interest
There are no conflicts to declare.Acknowledgements
S. d. l. M. acknowledges support from Spanish MICINN, (AEI/10.13039/501100011033; research grants PID2020-114755GB-C32 and PID2024-157648-NB-C21). S. S.-B. acknowledges UCM for a pre-doctoral contract (CT58/21-CT59/21). J. B. acknowledges support from Spanish MICINN (AEI/10.13039/501100011033; research grants PID2020-114755GB-C33 and PID2024-157648-NB-C22) and Basque Government (research grant IT1639-22). C. D.-N. acknowledges Spanish MICINN for a pre-doctoral contract (PRE2018-086808). I. G.-M. acknowledges support from Spanish MICINN (AEI/10.13039/501100011033; research grant PID2023-149991NB-I00).Notes and references
- F. Zhou, Z. Hearne and C.-J. Li, Curr. Opin. Green Sustainable Chem., 2019, 18, 118 Search PubMed.
- Water and life: the unique properties of H2O, ed. R. M. Lynden-Bell, S. C. Morris, J. D. Barrow, J. L. Finney and C. Harper, CRC Press, 2010 Search PubMed.
- (a) M. Cortes-Clerget, J. Yu, J. R. A. Kincaid, P. Walde, F. Gallou and B. H. Lipshutz, Chem. Sci., 2021, 12, 4237 RSC; (b) R. N. Butler and A. G. Coyne, Chem. Rev., 2010, 110, 6302 CrossRef CAS PubMed.
- Y. Li, Q. Chen, X. Pan, W. Lu and J. Zhang, Top. Curr. Chem., 2022, 380, 22 CrossRef CAS PubMed.
- J. H. Correia, J. A. Rodrigues, S. Pimenta, T. Dong and Z. Yang, Pharmaceutics, 2021, 13, 1332 CrossRef CAS.
- J. Krämer, R. Kang, L. M. Grimm, L. De Cola, P. Picchetti and F. Biedermann, Chem. Rev., 2022, 122, 3459 CrossRef.
- M. N. Chong, B. Jin, C. W. K. Chow and C. Saint, Water Res., 2010, 44, 2997 CrossRef CAS.
- F. Xu and B. Weng, J. Mater. Chem. A, 2023, 11, 4473 RSC.
- Z. Wang, Y. Hu, S. Zhang and Y. Sun, Chem. Soc. Rev., 2022, 51, 6704 RSC.
- C. W. Chow, J. Lightwave Technol., 2024, 42, 3972 Search PubMed.
- V. Emiliani, E. Entcheva, R. Hedrich, P. Hegemann, K. R. Konrad, C. Lüscher, M. Mahn, Z.-H. Pan, R. R. Sims, J. Vierock and O. Yizhar, Nat. Rev. Methods Primers, 2022, 2, 55 CrossRef CAS PubMed.
- W. Cheng, H. Chen, C. Liu, C. Ji, G. Ma and M. Yin, View, 2020, 1, 20200055 CrossRef.
- (a) J. Bañuelos Prieto, F. López Arbeloa, V. Martínez Martínez, T. Arbeloa López and I. López Arbeloa, Phys. Chem. Chem. Phys., 2004, 6, 4247 RSC; (b) F. López Arbeloa, J. Bañuelos, V. Martínez, T. Arbeloa and I. López Arbeloa, Int. Rev. Phys. Chem., 2005, 24, 339 Search PubMed; (c) Probes for Organelles in Molecular Probes™ Handbook. A Guide to Fluorescent Probes and Labeling Technologies, ed. J. Johnson and M. T. Z. Spence, Life Technologies, 2010 Search PubMed; (d) Y. Cakmak, S. Kolemen, S. Duman, Y. Dede, Y. Dolen, B. Kilic, Z. Kostereli, L. T. Yildirim, A. L. Dogan, D. Guc and E. U. Akkaya, Angew. Chem., Int. Ed., 2011, 50, 11937 CrossRef CAS PubMed; (e) J. Bañuelos, Chem. Rec., 2016, 16, 335 CrossRef; (f) P. Kaur and K. Singh, J. Mater. Chem. C, 2019, 7, 11361 RSC; (g) J. Piskorz, W. Porolnik, M. Kucinska, J. Dlugaszewska, M. Murias and J. Mielcarek, ChemMedChem, 2021, 16, 399 CrossRef CAS PubMed; (h) B. M. Squeo and M. Pasini, Supramol. Chem., 2020, 32, 56 CrossRef CAS; (i) B. M. Squeo, L. Ganzer, T. Virgili and M. Pasini, Molecules, 2020, 26, 153 CrossRef; (j) L. Wang, H. Ding, X. Ran, H. Tang and D. Cao, Dyes Pigm., 2020, 172, 107857 CrossRef CAS; (k) T. Mikulchyk, S. Karuthedath, C. S. P. De Castro, A. A. Buglak, A. Sheehan, A. Wieder, F. Laquai, I. Naydenova and M. A. Filatov, J. Mater. Chem. C, 2022, 10, 11588 RSC; (l) L. Yuan, Y. Su, H. Cong, B. Yu and Y. Shen, Dyes Pigm., 2023, 208, 110851 CrossRef; (m) H.-B. Cheng, X. Cao, S. Zhang, K. Zhang, Y. Cheng, J. Wang, J. Zhao, L. Zhou, X.-J. Liang and J. Yoon, Adv. Mater., 2023, 35, 2207546 CrossRef CAS; (n) S. Das, S. Dey, S. Patra, A. Bera, T. Ghosh, B. Prasad, K. D. Sayala, K. Maji, A. Bedi and S. Debnath, Biomolecules, 2023, 13, 1723 CrossRef CAS PubMed; (o) D. Wang, X. Wang, S. Zhou, P. Gu, X. Zhu, C. Wang and Q. Zhang, Coord. Chem. Rev., 2023, 482, 215074 CrossRef CAS; (p) I. S. Yadav and R. Misra, J. Mater. Chem. C, 2023, 11, 8688 Search PubMed; (q) N. A. Bumagina and E. V. Antina, Coord. Chem. Rev., 2024, 505, 215688 Search PubMed; (r) X. Hu, Z. Fang, C. Zhu, Y. Yang, Z. Yang and W. Huang, Adv. Funct. Mater., 2024, 34, 2401325 Search PubMed; (s) D. Zhang, L. Liu, X. Zhang, J. Lu and X.-D. Jiang, RCM, 2024, 3, 103 Search PubMed; (t) W. Porolnik, T. Koczorowski, E. Wieczorek-Szweda, W. Szczolko, M. Falkowski and J. Piskorz, Spectrochim. Acta, Part A, 2024, 314, 124188 CrossRef CAS PubMed; (u) H. Ahmad, S. Muhammad, M. Mazhar, A. Farhan, M. S. Iqbal, H. Hiria, C. Yu, Y. Zhang and B. Guo, Coord. Chem. Rev., 2025, 526, 216383 CrossRef CAS.
- (a) L. Li, J. Han, B. Nguyen and K. Burgess, J. Org. Chem., 2008, 73, 1963 Search PubMed; (b) Ö. Dilek and S. L. Bane, Bioorg. Med. Chem. Lett., 2009, 19, 6911 CrossRef PubMed; (c) S.-L. Niu, G. Ulrich, P. Retailleau, J. Harrowfield and R. Ziessel, Tetrahedron Lett., 2009, 50, 3840 CAS; (d) S. L. Niu, G. Ulrich, R. Ziessel, A. Kiss, P.-Y. Renard and A. Romieu, Org. Lett., 2009, 11, 2049 CAS; (e) Y. Tokoro, A. Nagai and Y. Chujo, Tetrahedron Lett., 2010, 51, 3451 CrossRef CAS; (f) S. Zhu, J. Zhang, G. Vegesna, F.-T. Luo, S. A. Green and H. Liu, Org. Lett., 2011, 13, 438 CrossRef CAS PubMed; (g) S.-L. Niu, C. Massif, G. Ulrich, P.-Y. Renard, A. Romieu and R. Ziessel, Chem.–Eur. J., 2012, 18, 7229 CrossRef CAS; (h) A. Romieu, C. Massif, S. Rihn, G. Ulrich, R. Ziessel and P.-Y. Renard, New J. Chem., 2013, 37, 1016 RSC; (i) A. B. Nepomnyashchii, A. J. Pistner, A. J. Bard and J. Rosenthal, J. Phys. Chem. C, 2013, 117, 5599 CrossRef CAS PubMed; (j) B. Brizet, C. Bernhard, Y. Volkova, Y. Rousseling, P. D. Harvey, C. Goze and F. Denat, Org. Biomol. Chem., 2013, 11, 7729 RSC; (k) G. Fan, L. Yang and Z. Chen, Front. Chem. Sci. Eng., 2014, 8, 405 CrossRef CAS; (l) J. Xu, Q. Li, Y. Yue, Y. Guo and S. Shao, Biosens. Bioelectron., 2014, 56, 58 CrossRef CAS; (m) B. Wu, L. Xu, S. Wang, Y. Wang and W. Zhang, Polym. Chem., 2015, 6, 4279 RSC; (n) K. M. Bardon, S. Selfridge, D. S. Adams, R. A. Minns, R. Pawle, T. C. Adams and L. Takiff, ACS Omega, 2018, 3, 13195 CrossRef CAS PubMed; (o) I. S. Turan, G. Gunaydin and E. G. Akkaya, Nat. Commun., 2018, 9, 805 CrossRef PubMed; (p) M. Işık, I. Simsek Turan and S. Dartar, Tetrahedron Lett., 2019, 60, 1421 CrossRef; (q) A. Blázquez-Moraleja, D. Álvarez-Fernández, R. Prieto Montero, I. García-Moreno, V. Martínez-Martínez, J. Bañuelos, I. Sáenz-de-Santa-María, M. D. Chiara and J. L. Chiara, Dyes Pigm., 2019, 170, 107545 CrossRef; (r) D. Khuong Mai, B. Kang, T. Pegarro Vales, I. W. Badon, S. Cho, J. Lee, E. Kim and H.-J. Kim, Molecules, 2020, 25, 3340 CrossRef PubMed; (s) D. Kand, P. Liu, M. X. Navarro, L. J. Fischer, L. Rousso-Noori, D. Friedmann-Morvinski, A. H. Winter, E. W. Miller and R. Weinstain, J. Am. Chem. Soc., 2020, 142, 4970 CrossRef CAS PubMed; (t) L. J. Patalag, S. Ahadi, O. Lashchuk, P. G. Jones, S. Ebbinghaus and D. B. Werz, Angew. Chem., Int. Ed., 2021, 60, 8766 CrossRef CAS; (u) H. Yanai, S. Hoshikawa, Y. Moriiwa, A. Shoji, A. Yanagida and T. Matsumoto, Angew. Chem., Int. Ed., 2021, 60, 5168 CrossRef CAS PubMed; (v) A. M. Gómez, C. Uriel, A. Oliden-Sánchez, J. Bañuelos, I. García-Moreno and J. C. López, J. Org. Chem., 2021, 86, 9181 CrossRef PubMed; (w) J. Jiménez, C. Díaz-Norambuena, S. Serrano, S. C. Ma, F. Moreno, B. L. Maroto, J. Bañuelos, G. Muller and S. de la Moya, Chem. Commun., 2021, 57, 5750 RSC; (x) C. S. Mahanta, V. Ravichandiran and S. P. Swain, ACS Appl. Bio Mater., 2023, 6, 2995 CrossRef CAS PubMed.
- (a) C. Ray, C. Schad, F. Moreno, B. L. Maroto, J. Bañuelos, T. Arbeloa, I. García-Moreno, C. Villafuerte, G. Muller and S. de la Moya, J. Org. Chem., 2020, 85, 4594 CAS; (b) C. Schad, E. Avellanal-Zaballa, E. Rebollar, C. Ray, E. Duque-Redondo, F. Moreno, B. L. Maroto, J. Bañuelos, I. García-Moreno and S. de la Moya, Phys. Chem. Chem. Phys., 2021, 24, 27441 RSC.
- S. de la Moya Cerero, F. Moreno Jiménez, B. Lora Maroto, C. Ray Leiva, C. Schad Alburquerque, J. Bañuelos and C. Díaz Norambuena, Nuevos colorantes BODIPY solubles en agua mediante funcionalización sencilla en boro. ES 2923998A1, 2022 Search PubMed.
- Percepta software, version 2028.1.1, ACD/labs, Toronto, Canada, 2018 Search PubMed.
- A. B. Descalzo, P. Ashokkumar, Z. Shen and K. Rurack, ChemPhotoChem, 2020, 4, 120 CAS.
- V. P. Yakubovskyi, N. O. Didukh, Y. V. Zatsikha and Y. P. Kovtun, ChemistrySelect, 2016, 1, 1462 CAS.
- G. Durán-Sampedro, A. R. Agarrabeitia, L. Cerdan, M. E. Perez-Ojeda, A. Costela, I. García-Moreno, I. Esnal, J. Bañuelos, I. L. Arbeloa and M. J. Ortiz, Adv. Funct. Mater., 2013, 23, 4195 CrossRef.
- X. Zhou, C. Yu, Z. Feng, Y. Yu, J. Wang, E. Hao, Y. Wei, X. Mu and L. Jiao, Org. Lett., 2015, 17, 4632 CAS.
- (a) T. Rohand, M. Baruah, W. Qin, N. Boens and W. Dehaen, Chem. Commun., 2006, 266, 266 Search PubMed; (b) Y. A. Volkova, B. Brizet, P. D. Harvey, A. D. Averin, C. Goze and F. Denat, Eur. J. Org Chem., 2013, 2013, 4270 CrossRef CAS; (c) X.-X. Chen, L.-Y. Niu, N. Shao and Q.-Z. Yang, Anal. Chem., 2019, 91, 4301–4306 CrossRef CAS; (d) Q. Gong, Q. Wu, X. Guo, H. Li, W. Li, C. Yu, E. Hao and L. Jiao, Org. Lett., 2021, 23, 7661 CrossRef CAS; (e) A. Blázquez-Moraleja, L. Maierhofer, E. Mann, R. Prieto-Montero, A. Oliden-Sánchez, L. Celada, V. Martínez-Martínez, M.-D. Chiara and J. L. Chiara, Org. Chem. Front., 2022, 9, 5774 RSC; (f) C. Díaz-Norambuena, C. Ray, T. Arbeloa, A. Oliden-Sánchez, F. Moreno, B. L. Maroto, J. Bañuelos and S. de la Moya, Dyes Pigm., 2024, 222, 111907 CrossRef; (g) L. Wang, C. Cheng, Z.-Y. Li, X. Guo, Q. Wu, E. Hao and L. Jiao, Org. Lett., 2024, 26, 3026 CrossRef CAS PubMed.
- J. Park, D. Feng and H.-C. Zhou, J. Am. Chem. Soc., 2015, 137, 1663 CrossRef CAS PubMed.
- (a) G. Ulrich, C. Goze, S. Goeb, P. Retailleau and R. Ziessel, New J. Chem., 2006, 30, 982 RSC; (b) A. Harriman, G. Izzet and R. Ziessel, J. Am. Chem. Soc., 2006, 128, 10868 CrossRef CAS PubMed; (c) C. Goze, G. Ulrich and R. Ziessel, J. Org. Chem., 2007, 72, 313 CrossRef CAS PubMed; (d) G. Ulrich, S. Goeb, A. De Nicola, P. Retailleau and R. Ziessel, Synlett, 2007, 1517, 1517–1520 Search PubMed; (e) R. Ziessel, C. Goze and G. Ulrich, Synthesis, 2007, 936, 936 CrossRef; (f) A. Harriman, L. Mallon and R. Ziessel, Chem.–Eur. J., 2008, 14, 11461 CrossRef CAS PubMed; (g) A. Harriman, L. J. Mallon, S. Goeb, G. Ulrich and R. Ziessel, Chem.–Eur. J., 2009, 15, 4553 CAS; (h) G. Durán-Sampedro, I. Esnal, A. R. Agarrabeitia, J. Bañuelos Prieto, L. Cerdán, I. García-Moreno, A. Costela, I. López-Arbeloa and M. J. Ortiz, Chem.–Eur. J., 2014, 20, 2646 CrossRef PubMed.
- (a) M. Martínez-Calvo and J. L. Mascareñas, Coord. Chem. Rev., 2018, 359, 57 CrossRef; (b) S. L. Scinto, D. A. Bilodeau, R. Hincapie, W. Lee, S. S. Nguyen, M. Xu, C. W. am Ende, M. G. Finn, K. Lang, Q. Lin, J. P. Pezacki, J. A. Prescher, M. S. Robillard and J. M. Fox, Nat. Rev. Methods Primers, 2021, 1, 30 CrossRef CAS PubMed; (c) D. Schauenburg and T. Weil, Adv. Sci., 2024, 11, 2303396 CrossRef CAS PubMed.
- (a) T. Rohand, W. Qin, N. Boens and W. Dehaen, Eur. J. Org Chem., 2006, 2006, 4658 CrossRef; (b) Y. Hayashi, S. Yamaguchi, W. Y. Cha, D. Kim and H. Shinokubo, Org. Lett., 2011, 13, 2992 CrossRef CAS PubMed; (c) Y. Hayashi, N. Obata, M. Tamaru, S. Yamaguchi, Y. Matsuo, A. Saeki, S. Seki, Y. Kureishi, S. Saito, S. Yamaguchi and S. Shinokubo, Org. Lett., 2012, 14, 866 CrossRef CAS PubMed; (d) Z. Liu, S. G. Thacker, S. Fernandez-Castillejo, E. B. Neufeld, A. T. Remaley and R. Bittman, ChemBioChem, 2014, 15, 2087 CrossRef CAS PubMed; (e) G. Li, Y. Otsuka, T. Matsumiya, T. Suzuki, J. Li, M. Takahashi and K. Yamada, Materials, 2018, 11, 1297 CrossRef PubMed; (f) W. Miao, Y. Feng, Q. Wu, W. Sheng, M. Li, Q. Liu, E. Hao and L. Jiao, J. Org. Chem., 2019, 84, 9693 CrossRef CAS PubMed; (g) L. He, Y. Li, Y. Zhao, S. Gao, Z. Wang, X. Li, Y. Yang and W. Jiang, J. Org. Chem., 2024, 89, 17643 CrossRef CAS PubMed; (h) A. Kondo, H. Takano and H. Shinokubo, Chem. Lett., 2025, 54, upae218 CrossRef.
- (a) A. N. Kursunlu and E. Güler, Supramol. Chem., 2013, 25, 512 CrossRef CAS; (b) R. S. Yalagala, S. A. Mazinani, L. A. Maddalena, J. A. Stuart, F. Yan and H. Yan, Carbohydr. Res., 2016, 424, 15 CrossRef CAS PubMed; (c) N. Stuhr-Hansen, C.-D. Vagianou and O. Blixt, Chem.–Eur. J., 2017, 23, 9472 CrossRef CAS PubMed; (d) M. M. Haghdoost, E. Sauvageau, P. Oguadinma, H.-V. Tran, S. Lefrancois and A. Castonguay, J. Inorg. Biochem., 2020, 210, 111105 CrossRef CAS PubMed; (e) T. M. Williams, N. E. M. Kaufman, Z. Zhou, S. S. Singh, S. D. Jois and M. d. G. H. Vicente, Molecules, 2021, 26, 593 CrossRef CAS PubMed; (f) S. Cetindere, S. T. Clausing, M. Anjass, Y. Luo, S. Kupfer, B. Dietzek and C. Streb, Chem.–Eur. J., 2021, 27, 17181 CrossRef CAS PubMed; (g) M. Peřina, R. Börzsei, H. Ágoston, T. Hlogyik, M. Poór, R. Rigó, C. Özvegy-Laczka, G. Batta, C. Hetényi, V. Vojáčková and R. Jorda, Eur. J. Pharm. Sci., 2024, 199, 106813 CrossRef PubMed; (h) B. Lu, X. Lu, M. Mu, S. Meng, Y. Feng and Y. Zhang, Heliyon, 2024, 10, e26907 CrossRef CAS PubMed.
- (a) B. Wellegehausen, L. Laepple and H. Welling, Appl. Phys., 1975, 6, 335 CrossRef CAS; (b) H. El-Kashef, Physica B, 2000, 279, 295 CrossRef CAS; (c) H. El-Kashef, Physica B, 2002, 311, 376 CrossRef CAS.
- (a) S. Sinha, A. Ray and K. Dasgupta, J. Appl. Phys., 2000, 87, 3222 CrossRef CAS; (b) S. Sinha, A. K. Ray, S. Kundu, S. Sasikumar and K. Dasgupta, Appl. Phys. B, 2002, 75, 85 CrossRef CAS.
- (a) O. G. Peterson, S. A. Tuccio and B. B. Snavely, Appl. Phys. Lett., 1970, 17, 245–247 CrossRef CAS; (b) A. Sen, A. K. Mora, S. K. Agarwalla, G. Sridhar, S. Kundu and S. Nath, Spectrochim. Acta, Part A, 2022, 282, 121642 CrossRef CAS PubMed.
- Laser & Fluorescent Dyes Exciton, https://exciton.luxottica.com/laser-dyes.html, accessed 2024-11-23 Search PubMed.
- (a) M. Okada and K. Takizawa, IEEE J. Quantum Electron., 1980, 16, 770 CrossRef; (b) A. K. Ray, S. Sinha, S. Kundu, S. Kumar, S. K. Nair, T. Pal and K. Dasgupta, Appl. Opt., 2002, 41, 1704 CrossRef CAS PubMed.
- (a) J. V. Frangioni, Curr. Opin. Chem. Biol., 2003, 7, 626–634 CrossRef CAS PubMed; (b) R. Weissleder, Science, 2006, 312, 1168 CrossRef CAS PubMed; (c) J. Rao, A. Dragulescu-Andrasi and H. Yao, Curr. Opin. Biotechnol., 2007, 18, 17 CrossRef CAS PubMed; (d) M. Schäferling, Angew. Chem., Int. Ed., 2012, 51, 3532 CrossRef PubMed; (e) M. L. James and S. S. Gambhir, Physiol. Rev., 2012, 92, 897 CrossRef CAS PubMed; (f) Z. Guo, S. Park, J. Yoon and I. Shin, Chem. Soc. Rev., 2014, 43, 16 RSC; (g) C. Li, G. Chen, Y. Zhang, F. Wu and Q. Wang, J. Am. Chem. Soc., 2020, 142, 14789 CrossRef CAS PubMed; (h) S. Wang, W. X. Ren, J.-T. Hou, M. Won, J. An, X. Chen, J. Shu and J. S. Kim, Chem. Soc. Rev., 2021, 50, 8887 CAS; (i) Y. Fu, X. Zhang, L. Wu, M. Wu, T. D. James and R. Zhang, Chem. Soc. Rev., 2025, 54, 201 Search PubMed.
- (a) M. Sun, K. Müllen and M. Yin, Chem. Soc. Rev., 2016, 45, 1513 Search PubMed; (b) S. Kolemen and E. U. Akkaya, Coord. Chem. Rev., 2018, 354, 121 CAS; (c) M. Liu, S. Ma, M. She, J. Chen, Z. Wang, P. Liu, S. Zhang and J. Li, Chin. Chem. Lett., 2019, 30, 1815 CrossRef CAS; (d) G. Jiang, H. Liu, H. Liu, G. Ke, T.-B. Ren, B. Xiong, X.-B. Zhang and L. Yuan, Angew. Chem., Int. Ed., 2024, 63, e202315217 Search PubMed.
- (a) N. C. Santos, J. Figueira-Coelho, J. Martins-Silva and C. Saldanha, Biochem. Pharmacol., 2003, 65, 1035 CAS; (b) J. L. Hanslick, K. Lau, K. K. Noguchi, J. W. Olney, C. F. Zorumski, S. Mennerick and N. B. Farber, Neurobiol. Dis., 2009, 34, 1 Search PubMed; (c) J. Galvao, B. Davis, M. Tilley, E. Normando, M. R. Duchen and M. F. Cordeiro, Faseb. J., 2014, 28, 1317 CrossRef CAS PubMed; (d) M. Awan, I. Buriak, R. Fleck, B. Fuller, A. Goltsev, J. Kerby, M. Lowdell, P. Mericka, A. Petrenko, Y. Pretenko, O. Rogulska, A. Stolzing and G. N. Stacey, Regener. Med., 2020, 15, 1463 CAS.
- Y. Zhao, Y. Gu, F. Qi, A. Li, X. Tang, D. Li, X. Wu and J. Liu, Mater. Des., 2023, 228, 111860 CrossRef CAS.
- A. Daina, O. Michielin and V. Zoete, Sci. Rep., 2017, 7, 42717 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: General methods and equipment, synthetic procedures and characterization, NMR spectra of new compounds, as well as additional figures concerning (photo)physical and (bio)photonical behaviors. See DOI: https://doi.org/10.1039/d5sc01295c |
| This journal is © The Royal Society of Chemistry 2025 |

