 Open Access Article
Open Access Article“Three functions in one”: multifunctional rare-earth cyamelurates with magnetism, luminescence, and giant optical nonlinearity†
Jing
Zhang
 a,
Yuxiao
Liu
b,
Fangyan
Wang
a,
Yuxiao
Liu
b,
Fangyan
Wang
 b,
Pifu
Gong
b,
Pifu
Gong
 c,
Zhaoyi
Li
d,
Xinyuan
Zhang
c,
Zhaoyi
Li
d,
Xinyuan
Zhang
 *a,
Fei
Liang
*a,
Fei
Liang
 *b,
Shu
Guo
*b,
Shu
Guo
 d,
Zhanggui
Hu
a and
Yicheng
Wu
ab
d,
Zhanggui
Hu
a and
Yicheng
Wu
ab
aState Key Laboratory of Crystal Materials, Tianjin Key Laboratory of Functional Crystal Materials, Institute of Functional Crystals, Tianjin University of Technology, Tianjin 300384, China. E-mail: xyzhang@email.tjut.edu.cn
bState Key Laboratory of Crystal Materials and Institute of Crystal Materials, Shandong University, Jinan 250100, China. E-mail: liangfei@sdu.edu.cn
cFunctional Crystals Lab, Technical Institute of Physics and Chemistry, Chinese Academy of Sciences, Beijing 100190, China
dShenzhen Institute for Quantum Science and Engineering, Southern University of Science and Technology, Shenzhen 518055, China
First published on 20th May 2025
Abstract
A highly-integrated optical device requires multifunctional crystals, which respond strongly to external stimuli, such as photoluminescence, nonlinear polarization, and magnetoelectric coupling. However, it is usually difficult for these functions to coexist in a single-phase crystal, and can even be incompatible. Here, we propose a ‘unit assembly’ strategy in rare-earth cyamelurates, which combines active rare-earth ions (Gd3+, Y3+, Lu3+) and π-conjugated [HxC6N7O3](3−x)– units into one crystal with an aligned arrangement. For the first time, we synthesized three new rare-earth cyamelurate crystals RE(H1.5C6N7O3)2·10H2O (RE = Y, Gd, Lu) by a facile solution method. Benefitting from the strong polarizability and delocalized electrons of the π-conjugated units, all three crystals display giant second-order optical nonlinearity (12–15.3 × KDP), broadband photoluminescence (300–500 nm), and strong anisotropic birefringence (Δn > 0.22) at 1064 nm. Moreover, the Gd-analogue exhibits paramagnetic behaviour in a wide temperature range. These results highlight rare-earth cyamelurates as promising multifunctional optical crystals with three concurrent functional responses, indicating potential applications in next-generation photonics and optoelectronics.
Introduction
The integration of multiple functionalities into a single crystal remains a long-standing challenge in the field of inorganic functional materials. Currently, the composition and structure of functional units are key factors affecting the overall performance of functional materials. Rational design of these building units can effectively improve the optical response of the materials under an external field.1 In the past thirty years, many nonlinear optical (NLO) crystals with excellent performances have been discovered,2–9 and several anionic groups have been identified as effective fundamental building blocks for enhancing nonlinear responses.10–16 These include (SeO3)2− and (IO3)−, which possess stereochemically active lone pairs; (NbO6)7− and (VO6)7−, which exhibit Jahn–Teller effects; and (BO3)3−, (CO3)2−, and (B3O6)3−, which feature planar π-conjugated groups. Among these various kinds of anions, π-conjugated groups with large optical anisotropy and microscopic second-order susceptibility are regarded as some of the most successful. This has led to the discovery of a series of benchmark crystals, e.g. KBe2BO3F2 (KBBF), β-BaB2O4 (BBO), LiB3O5 (LBO), etc.17–19 However, these small π-conjugated units typically exhibit an ultrawide bandgap, thus hindering their fluorescent emission and magnetic responses. Therefore, it remains a great challenge to design multifunctional crystals within an inorganic π-conjugated system.Recently, metal cyanurates with large π-conjugated (C3N3O3)3− unit have been developed as effective nonlinear optical materials,20–23 including KLi(HC3N3O3)·2H2O, Cs3Na(H2C3N3O3)4·3H2O, and RE5(C3N3O3)(OH)12 (RE = Y, Yb, and Lu). More interestingly, owing to the reduced forbidden gap of the (C3N3O3)3− unit, several cyanurates display a strong photoluminescence response, such as Pb(H2C3N3O3)F with blue emission around 482 nm.24 Besides, C4N3H6SO3NH2 with a π-conjugated [C4N3H6] group also shows blue-violet and green fluorescence near 360 and 520 nm,25 thus indicating that the introduction of a large π-conjugated unit could be a feasible strategy to design multifunctional crystals with concurrent optical nonlinearity and photoluminescence.
Following this design principle, we focused on a large π-conjugated group, (C6N7O3)3−, as a building unit for multifunctional optical crystals. Compared to previously studied π-conjugated units, such as (BO3)3− (conjugated electronic configuration π34), (CO3)2− (π44), (B3O6)3− (π99), and (C3N3O3)3− (π99), the (C6N7O3)3− unit possesses the ultra-large π-conjugated electron configuration π1716, indicating its strong pπ–pπ conjugated interaction and delocalized electron distribution on the planar ring. In 2022, we synthesized the first SHG active alkali cyamelurate, K3C6N7O3·2H2O, which exhibited excellent nonlinear optical properties (SHG ∼4 × KDP, Δncal > 0.446).26 Subsequently, iso-cyamelurates (HxC6N7O3)x−3 (x = 1, 2) were also explored as a promising candidate with both enhanced SHG efficiency and birefringence, including A0.5H2C6N7O3·4H2O (A = Ca2+, Sr2+, Ba2+) (SHG > 5 × KDP, Eg ∼4.0 eV), Cd(H2C6N7O3)2·4H2O (SHG ∼1.4 × KDP, Eg ∼4.0 eV) Sr[H2C6N7O3]2·4H2O, and Er(C6N7O3)·5H2O.27–31 However, the integration of strong optical nonlinearity, broadband photoluminescence, and magnetic response has never been realized in the cyamelurate system.
Here, we propose a ‘unit assembly’ strategy to design multifunctional crystals, which combines the active metal ion and π-conjugated [HxC6N7O3](3−x)– unit into rare-earth cyamelurates. The rare-earth ions are considered to enrich the optical response of cyamelurate materials in the following ways: (i) Closed-shell configurations and half-filled 4f orbitals of rare-earth cations (Y3+, La3+, and Lu3+) can inhibit d–d and f–f electronic transitions, expanding the cutoff edge from the visible to the ultraviolet (UV) region and thus broadening the band gap. (ii) Rare-earth cations usually exhibit strong coordination properties, favoring the alignment of ligand anions in non-centrosymmetric arrangements. (iii) Rare-earth ions may impart intriguing magnetic behavior originating from the 4f electronic configuration. In this work, Y3+, Gd3+, and Lu3+ are regarded as key elements to fabricate new SHG-active cyamelurate crystals. Fortunately, three new isostructural rare earth cyamelurates Y(H1.5C6N7O3)2·10H2O (I), Gd(H1.5C6N7O3)2·10H2O (II), and Lu(H1.5C6N7O3)2·10H2O (III) were successfully designed and synthesized by a feasible aqueous solution-cooling method, with strong SHG intensities (>12 × KDP), wide fluorescence, sufficient birefringence (Δn ∼0.22 at 1064 nm), and paramagnetic behaviour over a wide temperature range in II. The structure–property relationships were investigated through theoretical calculations.
Results and discussion
Crystal structure
Colourless plate-like crystals of the three compounds were obtained, and XRD diffraction (Fig. S1†) was used to confirm the purity of the sample powder. The three RE(H1.5C6N7O3)2·10H2O (RE = Y, Gd, Lu) compounds are isostructural, and all the detailed structural information, additional bond lengths, and bond angle information are listed in Tables 1 and S1–S12.† Herein, only compound II is illustrated as an example. Gd(H1.5C6N7O3)2·10H2O (II) crystallizes in the acentric orthorhombic Fdd2 space group (No. 43). As shown in Fig. 1a, the Gd3+ cation is coordinated with eight O atoms, of which the Gd–O distances range from 2.34–2.48 Å. Among the eight coordinated O atoms of the Gd–O polyhedron, the two contributed planar iso-cyamelurate [H2C6N7O3]− and [HC6N7O3]2− anion groups are at the angle of ∼75.74°, and the dihedral angle is slightly different in I and III with values of 76.02° and 76.25°, respectively. The stacking pattern of the Gd3+ cation is shown in Fig. 1b, and the distances between Gd⋯Gd interactions are measured as 6.15 Å and 6.58 Å, respectively. Fig. 1c displays the packing diagram with coordinated Gd polyhedra, and the vertical distance between the Gd⋯Gd plane along the c-axis is 5.82 Å, while the nearest anion layers are at a distance of 3.09 Å. Interestingly, as the ligand anions are connected on the two sides of the Gd3+ cations like the wings of a butterfly, the crystal structure is like an orderly arrangement of butterfly teams (Fig. 1d).| Y(H1.5C6N7O3)2·10H2O | Gd(H1.5C6N7O3)2·10H2O | Lu(H1.5C6N7O3)2·10H2O | |
|---|---|---|---|
| a R 1 = ∑‖Fo| − |Fc‖/∑|Fo|, ωR2 = [∑ω(Fo2 − Fc2)2/∑ω(Fo2)2]1/2. | |||
| Formula weight | 708.35 | 776.69 | 794.41 |
| Crystal system | Orthorhombic | Orthorhombic | Orthorhombic |
| Space group | Fdd2 | Fdd2 | Fdd2 |
| a (Å) | 67.056(8) | 67.4247(9) | 66.982(3) |
| b (Å) | 6.1193(7) | 6.14740(10) | 6.1537(2) |
| c (Å) | 11.4807(13) | 11.6319(3) | 11.6511(4) |
| α (deg) | 90 | 90 | 90 |
| β (deg) | 90 | 90 | 90 |
| γ (deg) | 90 | 90 | 90 |
| V/Å3 | 4710.9(9) | 4821.27(18) | 4802.4(3) |
| Z | 8 | 8 | 8 |
| ρ calc (g cm−3) | 1.997 | 2.140 | 2.197 |
| μ (mm−1) | 2.589 | 2.862 | 4.222 |
| F (000) | 2880 | 3080 | 3136 |
| Flack parameter | 0.01(2) | 0.04(4) | 0.02(3) |
| Goodness-of-fit on F2 | 1.093 | 1.133 | 1.146 |
| Reflections collected | 27![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 752 752 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 858 858 |
4777 |
| Final R indexes [I ≥ 2σ(I)] a | R 1 = 0.0728, ωR2 = 0.2150 | R 1 = 0.0566, ωR2 = 0.1731 | R 1 = 0.0492, ωR2 = 0.1496 |
| Final R indexes [all data] a | R 1 = 0.0801, ωR2 = 0.2251 | R 1 = 0.0645, ωR2 = 0.1808 | R 1 = 0.0532, ωR2 = 0.1538 |
| CCDC no. | 2385148 | 2385149 | 2385150 |
Chemical phase and thermal stability
Fig. S2† displays the results of thermogravimetry (TG) and differential scanning calorimetry (DSC) analysis of compounds I, II, and III. There are generally two weight loss steps in the process of heating, which correspond to the loss of crystalline water starting from 109 °C and sample decomposition occurring around 230 °C. Besides, the powder XRD diffraction confirmed that the sample can match the calculated diffraction peaks after six months in a dryer, indicating the stability of the three hydrates (Fig. S3†).Linear and nonlinear optical properties
The IR spectrum (Fig. S4†) indicates the presence of the cyamelurate group, in which the absorption peaks at 1654, 1516, 1406, and 1149 cm−1 can be attributed to aromatic C-N heterocycles, while bending the vibrational mode of s-triazine units can be assigned to peaks at 809 cm−1.32 The UV-vis transmission spectrum of compound II was measured with a transparent crystal plate (Fig. 2a), and the cutoff edge is estimated as 300 nm, corresponding to the band gap of 4.1 eV (Fig. 2b). The diffuse reflection spectra of the three compounds are nearly the same, also indicating the enhanced band gaps ∼ 4.0 eV (Fig. S5†), which are far larger than previously reported alkali cyamelurate of K3(C6N7O3) and K3C6N7O3·2H2O, Eg ∼3.0 eV.33 The SHG response of the three compounds was measured by the Kurtz–Perry method34 under 1064 nm incident light. Samples of I, II, and III respectively display very strong SHG efficiencies of 13.9, 15.3, and 12 × KDP with phase-matching behavior (Fig. 2c), which is the largest among reported metal cyamelurates, such as Ba(H2C6N7O3)2·8H2O (12 × KDP) and A0.5H2C6N7O3·4H2O (A = Ca2+, Sr2+) (>5 × KDP). Moreover, all three compounds show a broadband UV photoluminescence around 350 nm under the excitation wavelength of 288 nm (Fig. 2d and S6†). The emission spectra exhibit that the full width at half maximum (FWHM) is > 100 nm for compounds I and II, which is apparently wider than that previously reported in A3(C6N7O3) (A = Li, Na, K, Rb, Cs) (FWHM ∼50 nm), K3C6N7O3·2H2O (∼60 nm), Ca0.5H2C6N7O3·4H2O (∼82 nm), and K0.5In0.5(H2C6N7O3)2·9H2O (∼88 nm).35,36 Such wide photoluminescence could be attributed to the reduced bandgap of the cyamelurate unit and the possible electron-phonon coupling effect between rare-earth ions and their surrounding lattices.Magnetic properties
The temperature-dependent magnetic susceptibility (χ) and its inverse (1/χ) for compound II are presented in Fig. 2e. The magnetic susceptibility data of compound II is fitted to a modified Curie–Weiss (C–W) law: χ = C/(T − Θ), where C is the Curie constant and Θ is the Weiss temperature. As shown, compound II obeys the C–W law and is paramagnetic. The effective magnetic moment μeff is 6.97 μB for the high-temperature region (100–280 K), which matches the theoretical value for Gd3+. For the low-temperature range (2–10 K), the derived value of Θ for compound II is −0.54 K, which suggests weak magnetic exchange interactions among the adjacent Gd3+ cations.37–39 The magnetization saturates around 5 T when the temperature is 2 K in compound II (Fig. 2f). Combining the strong optical nonlinearity, ultraviolet fluorescence, and paramagnetic behaviour, the Gd(H1.5C6N7O3)2·10H2O (II) crystal is expected to be a promising multi-functional material in optoelectronic applications.Theoretical calculations
To explore the origin of the second-order nonlinear optics, the electronic structure of compound II was calculated. As shown in Fig. 3a, it is a direct-gap semiconductor with a spin-up gap of 2.71 eV and a spin-down gap of 1.67 eV. Furthermore, the spin-up density of states (DOS) and partial density of states (PDOS) of II are analyzed. Fig. 3b depicts that the contribution from crystalline water molecules is negligible, and the valence band maximum and the conduction band minimum were primarily occupied by N 2p orbitals together with O 2p and C 2p orbitals, indicating the contribution of conjugated π-bonds. Interestingly, not only unoccupied C p, N p, and O p orbitals but also Gd d and f orbitals contribute to the bottom of the conduction band, corresponding to the clear participation of the rare earth Gd in the construction of energy band structures. Therefore, it should be the synergistic effect of the rare-earth ion and the large π-conjugated group that determines the optical response of Gd(H1.5C6N7O3)2·10H2O. For the Y and Lu-analogues, their band structure and PDOS were plotted in Fig. S7 and S8.†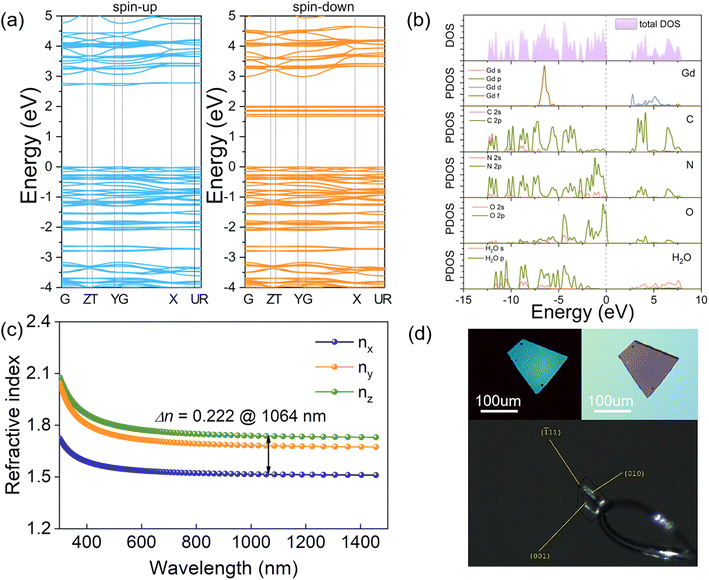 | ||
| Fig. 3 (a) Calculated band structure of II and (b) DOS and partial DOS of II. (c) The calculated birefringence of II. (d) The original and the extinction state of the crystal plane (001) of II. | ||
Furthermore, the calculated optical anisotropy of compound II exhibits a large variation from the visible to near-IR region with nz > ny > nx. As shown in Fig. 3c, the calculated birefringence nz – nx is 0.22 at 1.06 μm, which is comparable to the commercialized birefringent crystal α-BBO (Δn ∼0.12) and calcite (Δn ∼0.17).40,41 The calculated refractive index of I and III was given in Fig. S9† and the Δncal is around 0.2. In addition, the in-plane birefringence of single crystals I, II, and III was estimated experimentally by using orthogonal polarization microscopy with a halogen lamp.42 The measured values of I, II, and III are all around 0.20 (Fig. 3d and S10†), which also agree well with the calculated values. This suitable optical anisotropy could be due to the large π-conjugated interaction of the cyamelurate (C6N7O3)3− groups.
Moreover, Fig. 4 displays the reported SHG intensities of the compounds containing a single kind of FBUs of large π-conjugated (B3O6)3−,43–47 (C3N6)6−,48–50 (C3N3O3)3−,51–55 and (C4N2O3)2− groups,56,57 and large π-conjugated groups of [C6N7(NCN)3]3−,58 [C6N7(NH)2],59 and (C6N7O3)3−.26–31 The commercialized NLO crystal BBO was plotted as a benchmark crystal with a strong SHG response of 5.6 × KDP. As classical (B3O6)-type units, the contribution of the FBUs could favor the SHG response reaching up to 5.6 × KDP for recently discovered borate of CsYB3O6F, 5.3 × KDP for (iso)-cyanurates of KLi(HC3N3O3)·2H2O/RbNa(HC3N3O3)·2H2O, and 10.8 × KDP for the barbiturate of Li2(H2C4N2O3)·2H2O. In contrast, the compounds with large π-conjugated FBUs, such as [C6N7(NCN)3]3−, [C6N7(NH)2] and (C6N7O3)3−, generally exhibit larger SHG effects with a greater possibility than metal borates/cyanurates (>4 × KDP).
 | ||
| Fig. 4 SHG response of the compounds containing six-member ring B3O6-type π-conjugated groups and large C6N7-type π-conjugated groups. | ||
Among the large π-conjugated FBUs constructed compounds, only Cd(H2C6N7O3)2·4H2O displays small SHG signals < 2 × KDP, while A3[C6N7(NCN)3]·8H2O (A = Rb, Cs)/Ba(H2C6N7O3)2·8H2O can even reach the SHG intensities around 10 × KDP. Remarkably, benefiting from the synergistic effects of the large π–conjugated (C6N7O3)3− group and rare earth metals, compounds I, II, and III exhibit extraordinary second-order harmonic generation (SHG) properties (13.9, 15.3, 12 × KDP, respectively) far beyond those reported previously for π-conjugated NLO compounds. To the best of our knowledge, this represents the strongest SHG effect among all π-conjugated NLO crystals, indicating the great contribution of (HxC6N7O3)3−x units for strengthening the nonlinear optical response.
Conclusions
Three new cyamelurate crystals of RE(H1.5C6N7O3)2·10H2O (RE = Y, Gd, Lu) containing large π-conjugated orbitals were successfully synthesized. The three compounds displayed very strong SHG activity, sufficient birefringence, paramagnetic behaviour in the Gd-analogue, and broadband ultraviolet photoluminescence concurrently, suggesting that this series of compounds could be promising multi-functional optical materials. We believe this work provides a feasible approach for designing novel high-performance (iso)-cyamelurates and enriching new multi-functional integrated optoelectronic crystals, pointing to applications such as self-frequency doubling, magneto-optical switches, magnetoelectric coupling, and broadband fluorescent response.Data availability
All data are available in the article and the ESl† or can be made available from the corresponding authors upon reasonable request. CCDC deposition numbers 2385148–2385150 (for compounds I, II, and III) contain the supplementary crystallographic data for this paper. By emailing E-mail: data_request@ccdc.cam.ac.uk, or by contacting the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK: fax: +44 1223 336033.Author contributions
Jing Zhang: Investigation, writing – original draft; Yuxiao Liu: Investigation; Fangyan Wang: Investigation; Pifu Gong: Investigation; Zhaoyi Li: Investigation; Xinyuan Zhang: Investigation, writing-review & editing; Fei Liang: Theoretical calculations, writing-review & editing; Shu Guo: Investigation, formal analysis; Zhanggui Hu: Supervision; Yicheng Wu: Supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 52272007, 52422201, 52372010, 22275201, and 22205091), National Key Research and Development Program of China (2021YFA0717800), and Natural Science Foundation of Shandong Province (ZR2023ZD53).Notes and references
- C. T. Chen, Y. C. Wu and R. K. Li, Int. Rev. Phys. Chem., 1989, 8, 65–91 Search PubMed.
- L. Wu, C. Lin, H. Tian, Y. Zhou, H. Fan, S. Yang, N. Ye and M. Luo, Angew. Chem., Int. Ed., 2023, 63, e202315647 CrossRef.
- M. Mutailipu, J. Li and S. Pan, Adv. Funct. Mater., 2024, 2419204 Search PubMed.
- M. Mutailipu, J. Han, Z. Li, F. Li, J. Li, F. Zhang, X. Long, Z. Yang and S. Pan, Nat. Photonics, 2023, 17, 694–701 CrossRef CAS.
- Y. Chu, H. S. Wang, Q. Chen, X. Su, Z. Chen, Z. Yang, J. Li and S. Pan, Adv. Funct. Mater., 2023, 34, 2314933 CrossRef.
- R. L. Tang, D. X. Yang, L. Ma, Y. L. Lv, W. Liu and S. P. Guo, Adv. Opt. Mater., 2024, 2403044 Search PubMed.
- L. Qi, X. Jiang, K. Duanmu, C. Wu, Z. Lin, Z. Huang, M. G. Humphrey and C. Zhang, J. Am. Chem. Soc., 2024, 146, 9975–9983 CrossRef CAS PubMed.
- Y. Kang, C. Yang, J. Gou, Y. Zhu, Q. Zhu, W. Xu and Q. Wu, Angew. Chem., Int. Ed., 2024, 63, e202402086 CrossRef CAS PubMed.
- D. Dou, Q. Shi, H. Li, B. Zhang, D. Yang and Y. Wang, Adv. Sci., 2024, 11, 2401325 CrossRef CAS PubMed.
- J. Chen, C. L. Hu, X. H. Zhang, B. X. Li, B. P. Yang and J. G. Mao, Angew. Chem., Int. Ed., 2020, 59, 5381–5384 CrossRef CAS PubMed.
- X. Liu, L. Kang, P. Gong and Z. Lin, Angew. Chem., Int. Ed., 2021, 60, 13574–13578 CrossRef CAS.
- M. Mutailipu, F. Li, C. Jin, Z. Yang, K. R. Poeppelmeier and S. Pan, Angew. Chem., Int. Ed., 2022, 61, e202202096 CrossRef CAS PubMed.
- C. Wu, G. Wei, X. Jiang, Q. Xu, Z. Lin, Z. Huang, M. G. Humphrey and C. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202208514 CrossRef CAS.
- K. Chen, C. Lin, J. Chen, G. Yang, H. Tian, M. Luo, T. Yan, Z. Hu, J. Wang, Y. Wu, N. Ye and G. Peng, Angew. Chem., Int. Ed., 2023, 62, e202217039 CrossRef CAS PubMed.
- P.-F. Li, C.-L. Hu, J.-G. Mao and F. Kong, Mater. Horiz., 2024, 11, 1704–1709 RSC.
- Q. Zhang, R. An, X. Long, Z. Yang, S. Pan and Y. Yang, Angew. Chem., Int. Ed., 2024, e202415066 Search PubMed.
- C. T. Chen, G. L. Wang, X. Y. Wang and Z. Y. Xu, Appl. Phys. B, 2009, 97, 9–25 CrossRef CAS.
- C. T. Chen, B. C. Wu, A. D. Jiang and G. M. You, Sci. China B, 1985, 28, 235–243 Search PubMed.
- C. T. Chen, Y. C. Wu, A. D. Jiang, B. C. Wu, G. M. You, R. K. Li and S. J. Lin, J. Opt. Soc. A. B, 1989, 6, 616–621 CrossRef CAS.
- D. Lin, M. Luo, C. Lin, F. Xu and N. Ye, J. Am. Chem. Soc., 2019, 141, 3390–3394 CrossRef CAS PubMed.
- X. Meng, F. Liang, J. Tang, K. Kang, Q. Huang, W. Yin, Z. Lin and M. Xia, Eur. J. Inorg. Chem., 2019, 23, 2791–2795 CrossRef.
- X. Meng, X. Zhang, Q. Liu, Z. Zhou, X. Jiang, Y. Wang, Z. Lin and M. Xia, Angew. Chem., Int. Ed., 2022, 62, e202214848 Search PubMed.
- F. Liang, L. Kang, X. Zhang, M.-H. Lee, Z. Lin and Y. Wu, Cryst. Growth Des., 2017, 17, 4015–4020 Search PubMed.
- Y. Chen, C. Hu, Z. Fang, Y. Li and J. Mao, Inorg. Chem., 2022, 61, 1778–1786 CrossRef CAS PubMed.
- D. Dou, Q. Shi, H. Li, B. Zhang, D. Yang and Y. Wang, Adv. Sci., 2024, 11, 2401325 CrossRef CAS PubMed.
- X. Zhang, X. Du, J. Wang, F. Wang, F. Liang, Z. Hu, Z. Lin and Y. Wu, ACS Appl. Mater. Interfaces, 2022, 14, 53074–53080 CrossRef CAS.
- X. Du, F. Wang, F. Liang, Z. Hu, Y. Wu and X. Zhang, Inorg. Chem. Front., 2023, 10, 5979–5985 RSC.
- Y. Li, W. Huang, Y. Zhou, X. Song, J. Zheng, H. Wang, Y. Song, M. Li, J. Luo and S. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202215145 CrossRef CAS PubMed.
- Y. Li, X. Zhang, J. Zheng, Y. Zhou, W. Huang, Y. Song, H. Wang, X. Song, J. Luo and S. Zhao, Angew. Chem., Int. Ed., 2023, 62, e202304498 CrossRef CAS.
- N. E. Braml and W. Schnick, Z. Anorg. Allg. Chem., 2012, 639, 275–279 CrossRef.
- A. S. Isbjakowa, V. V. Chernyshev, V. A. Tafeenko and L. A. Aslanov, Struct. Chem., 2022, 33, 607–615 CrossRef CAS.
- M. Essalhi, M. Mohan, G. Marineau-Plante, A. Schlachter, T. Maris, P. D. Harvey and A. Duong, Dalton Trans., 2022, 51, 15005–15016 RSC.
- W. Wang, Z. Chen, X. Yang, P. Audebert, S. Sahoo, J. Chen, Y. Liu, S. Pamir Alpay, L. Xie and G. Wei, Appl. Catal. A Gen., 2022, 641, 118669 CrossRef CAS.
- S. K. Kurtz and T. T. Perry, J. Appl. Phys., 1968, 39, 3798–3813 CrossRef CAS.
- E. Horvath-Bordon, E. Kroke, I. Svoboda, H. Fueß, R. Riedel, S. Neeraj and A. K. Cheetham, Dalton Trans., 2004, 22, 3900–3908 RSC.
- L. Zhang, F. Wang, X. Zhang, F. Liang, Z. Hu and Y. Wu, Cryst. Growth Des., 2024, 24, 627–631 CrossRef CAS.
- W. Yin, K. Feng, W. Wang, Y. Shi, W. Hao, J. Yao and Y. Wu, Inorg. Chem., 2012, 51, 6860–6867 CrossRef CAS PubMed.
- M. Ashtar, Y. Bai, L. Xu, Z. Wan, Z. Wei, Y. Liu, M. A. Marwat and Z. Tian, Inorg. Chem., 2021, 60, 3626–3634 CrossRef CAS.
- T. Wang, R. Guo, Q. Liu, Q. Wu, X. Meng, Z. Zhou, S. Guo and M. Xia, Inorg. Chem., 2024, 63, 13171–13175 CrossRef CAS.
- V. P. Solntsev, E. G. Tsvetkov and V. A. Gets, et al. , J. Cryst. Growth, 2002, 236, 290–296 CrossRef CAS.
- G. Ghosh, Opt. Commun., 1999, 163, 95–102 CrossRef CAS.
- B. E. Sorensen, Eur. J. Mineral., 2013, 25, 5–10 CrossRef.
- H. Liu, B. Zhang, L. Li and Y. Wang, ACS Appl. Mater. Interfaces, 2021, 13, 30853–30860 CrossRef CAS PubMed.
- Z. Fang, X. Jiang, M. Duan, Z. Hou, C. Tang, M. Xia, L. Liu, Z. Lin, F. Fan, L. Bai and C. Chen, Chem.–Eur. J., 2018, 24, 7856–7860 CrossRef CAS PubMed.
- H. Wu, Z. Wei, Z. Hu, J. Wang, Y. Wu and H. Yu, Angew. Chem., Int. Ed., 2024, 63, e202406318 CrossRef CAS.
- H. Liu, Y. Wang, B. Zhang, Z. Yang and S. Pan, Chem. Sci., 2020, 11, 694–698 RSC.
- H. Qiu, F. Li, Z. Li, Z. Yang, S. Pan and M. Mutailipu, J. Am. Chem. Soc., 2023, 145, 24401–24407 CrossRef CAS PubMed.
- Y. Li, Q. Wu, Z. Lin, Y. Liu, Y. Zhou, X. Chen, M. Li, M. Hong, J. Luo and S. Zhao, Fund. Res., 2023, 3, 974–978 CAS.
- L. Liu, Z. Bai, L. Hu, D. Wei, Z. Lin and L. Zhang, J. Mater. Chem. C, 2021, 9, 7452–7457 RSC.
- Z. Bai, J. Lee, H. Kim, C. L. Hu and K. M. Ok, Small, 2023, 19, 2301756 CrossRef CAS.
- J. Lu, Y.-K. Lian, L. Xiong, Q.-R. Wu, M. Zhao, K.-X. Shi, L. Chen and L.-M. Wu, J. Am. Chem. Soc., 2019, 141, 16151–16159 CrossRef CAS.
- X. Meng, F. Liang, K. Kang, J. Tang, T. Zeng, Z. Lin and M. Xia, Inorg. Chem., 2019, 58, 11289–11293 CrossRef CAS.
- Y. Song, D. Lin, M. Luo, C. Lin, Q. Chen and N. Ye, Inorg. Chem. Front., 2020, 7, 150–156 RSC.
- Y. Chen, C. Hu, Z. Fang and J. Mao, Inorg. Chem. Front., 2021, 8, 3547–3555 RSC.
- M. Aibibula, L. Wang and S. Huang, ACS Omega, 2019, 4, 22197–22202 CrossRef CAS PubMed.
- D. Lin, M. Luo, C. Lin, L. Cao and N. Ye, Cryst. Growth Des., 2020, 20, 4904–4908 CrossRef CAS.
- Y. Xu, C. Lin, D. Lin, M. Luo, D. Zhao, L. Cao and N. Ye, Inorg. Chem., 2020, 59, 15962–15968 CrossRef CAS.
- D. Dou, B. Zhang, D. Yang and Y. Wang, Chem. Sci., 2024, 15, 19496–19503 RSC.
- R. Wei, H. Huang, D. Yang, Y. Wang and B. Zhang, Adv. Opt. Mater., 2024, 12, 2401814 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental section, crystallographic data, PXRD patterns, thermal analysis, IR spectrum, UV–visible–NIR diffuse reflectance spectrum, and band structures. CCDC 2385148–2385150. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc01230a |
| This journal is © The Royal Society of Chemistry 2025 |


