Open Access Article
Open Access ArticleSquaramide-based ion pair receptors can facilitate transmembrane transport of KCl and zwitterions including highly polar amino acids†
Marta
Zaleskaya-Hernik
 a,
Rayhanus
Salam
a,
Rayhanus
Salam
 b,
Mario J.
González
b,
Mario J.
González
 b,
Marcin
Wilczek
a,
Łukasz
Dobrzycki
b,
Marcin
Wilczek
a,
Łukasz
Dobrzycki
 a,
Nathalie
Busschaert
a,
Nathalie
Busschaert
 *b and
Jan
Romański
*b and
Jan
Romański
 *a
*a
aFaculty of Chemistry, University of Warsaw, Pasteura 1, Warsaw, PL 02-093, Poland. E-mail: jarom@chem.uw.edu.pl
bDepartment of Chemistry, Tulane University, New Orleans, Louisiana 70118, USA. E-mail: nbusschaert@tulane.edu
First published on 18th March 2025
Abstract
Misregulation of transmembrane ion transport in biological systems has been linked to a variety of diseases. As a result, supramolecular chemists have been trying to develop small molecules that facilitate the transmembrane transport of several ionic species. However, ion transport by small molecules is a passive process and needs to be overall charge neutral (i.e., when an ion is transported across a membrane, another ion needs to be transported as well to avoid charge separation). Ion pair receptors could therefore have great potential as transmembrane ion transporters because they can facilitate transport of an overall neutral species. Furthermore, ditopic ion pair receptors also have the potential to transport biologically important zwitterionic species, such as amino acids. In this manuscript, we report the synthesis of a series of ditopic receptors based on squaramides as the anion binding unit and 18-crown-6 as the cation binding unit. UV-vis and NMR titrations revealed that these compounds can bind a variety of chloride salts, especially KCl. Furthermore, liquid–liquid extractions and transport experiments using bulk liquid membranes and liposomes indicate that these ditopic receptors are capable of transporting chloride salts and hydrophilic amino acids. In fact, compound 5 was even able to facilitate the transport of amino acids with charged side chains at physiological pH (arginine and glutamate), making it the first example of a small molecule that can transport these highly polar and charge-dense species. These findings open up the possibility of using these receptors in a wide range of biological applications.
Introduction
Lipid bilayer membranes are essential components of living systems, forming compartments that physically separate the chemical environment on either side of the bilayer. Membrane proteins provide additional functionality including, but not limited to, control of molecular recognition and transmembrane transport of ions and small molecules. Genetically determined dysfunction of natural protein carriers and channels is the cause of many human diseases.1–3 Mutations in the genes encoding chloride transport proteins cause severe diseases such as cystic fibrosis, Barter's syndrome or Dent's disease.4–7 Therefore, over the last several decades, much effort has been put into discovering small-molecule synthetic anion transporters that facilitate ion transport through the phospholipid membranes of cells.8–16 It has been proven that to design a good transporter, the role of its size, lipophilicity, and affinity towards anions should be well balanced. The length and degree of branching of alkyl side chains are also extremely important, as they affect the rate of anion transport.17–22 Nevertheless, supramolecular chemists have developed a large number of effective receptors capable of transporting chloride across biological membranes.23–28 Most of these receptors are monotopic, i.e. they possess only one domain that binds one type of ion. However, few have taken up the challenge of designing an effective transporter capable of simultaneously binding an anion and a cation.29 One of the first ion pair receptors for transmembrane transport was described by Smith et al. in 2003.30 The macrobicyclic receptor was able to coordinate an ion pair within the macrocyclic cavity and was capable of transporting chloride in the presence of an alkali metal. Subsequently, other groups have reported ion pair transporters based on calix[4]pyrrole and oligoethers,31,32 and (aza)crown ethers linked to ureas, thioureas, or squaramides.33–35 The ditopic nature of these ion pair receptors may open up the opportunity for the coordination and transport of zwitterionic species such as amino acids. This is a crucial issue because impaired natural amino acid transporters have also been linked to a variety of diseases, such as diabetes, neurodegenerative disorders, and amino aciduria.36 However, the number of reports on the transport of amino acids in lipid bilayers using synthetic carriers is limited. Transport of such molecules is difficult because they occur as highly polar zwitterions at physiological pH. While several research groups have presented synthetic channels that facilitate the migration of amino acids across lipid membranes,37–39 the use of small-molecule transporters is rare. After some initial reports on the use of ditopic receptors to facilitate amino acid transport across bulk liquid membranes,40–43 a number of groups have recently reported synthetic small molecules capable of amino acid transport across phospholipid bilayers. For example, Sunamoto et al. developed a photoactivatable zwitterionic transporter for phenylalanine,44 Gale and co-workers have shown the transmembrane transport of glycine using the combination of a squaramide and a hydrophobic aldehyde,45 Ballester and colleagues recently presented a monotopic calixpyrrole-based receptor capable of selectively transporting L-proline,46 while Chmielewski and co-workers reported a monotopic anion receptor capable of transporting various amino acids at physiological pH.47 However, the progress in amino acid transport facilitated by small molecules is still lacking due to the highly hydrophilic nature of amino acids. As a result, the above-mentioned transporters have only been shown to transport amino acids with neutral side chains. The transport of amino acids with charged side chains, such as glutamate and arginine, has not yet been achieved with small molecule transporters. To achieve this, an extraordinary synthetic transporter is needed that can compensate for the high charge and highly polar nature of these amino acids, and that can compensate for any charge imbalance that occurs upon the transport of charged amino acids. Such a gap in this field prompted us to take on the challenge and design an effective ditopic transporter for chloride salts and hydrophilic amino acids. Squaramides have been commonly used as monotopic anion transporters, but to our surprise, very few squaramide-based ion pair receptors have been investigated as transmembrane transporters and there are no precedents for their use in the transport of zwitterions.34,48–55 Thus, to fill this niche, we designed a family of squaramide-based anion and ion pair receptors that differ in binding strength and lipophilicity and tested how these factors affect transmembrane ion transport. We developed an ion pair receptor capable of effectively transporting chloride salts, as well as hydrophilic amino acids in their fully charged form (glycine, serine, glutamate and arginine) across lipid bilayers at physiological pH.Results and discussion
Design and synthesis
To investigate the influence of various structural factors on salt binding and transport properties, we designed a family of anion and ion pair receptors 1–5 (Fig. 1). Ditopic receptors 2, 3 and 5 differ in the length and branching of their side arms and in the nature of electron-donating or -withdrawing substituents located on the phenyl ring directly connected to the squaramide unit. We also developed analogous anion receptors 1 and 4, which lack the cation binding domain. The synthesis of receptors 1–3 was carried out using a two-step protocol applying the sequential amidation of dimethyl squarate with appropriately prepared amines in various combinations (3,4-dimethoxyaniline, 3,4-dibutoxyaniline or 4-aminobenzo-18-crown-6) (see ESI† for detailed synthetic information and characterization data). The synthesis of receptors 4 and 5 was accomplished in four stages, starting from 5-nitroisophthalic acid, and was aimed at increasing the lipophilicity of the receptors and changing the nature of the substituents to electron-withdrawing. The generated 5-nitro isophthalic chloride was reacted with two equivalents of dibutylamine to obtain diamide TN possessing a nitro group on the phenyl ring with 70% yield. In the next step, tin chloride dihydrate was used to reduce the nitro function and the obtained amine TA was reacted with dimethyl squarate to obtain a monoamide TM with 65% yield. Finally, compound TM was reacted with 3,4-dimethoxyaniline or 4-aminobenzo-18-crown-6 ether to give receptors 4 and 5 in yields of 70% and 78%, respectively (Scheme 1).Ion pair binding
Ion binding studies for chloride anions (in the form of tetrabutylammonium salts or in situ formed potassium or sodium salts) were carried out using UV-vis titrations in acetonitrile. Association constants were calculated by fitting the anion-induced bathochromic shifts of the absorption maxima to a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding model using the Bindfit program. The results are presented in Table 1. Receptor 2 was excluded from the binding study due to self-association in such conditions, which was confirmed by a dilution test (see ESI Fig. S20†). As listed in Table 1, the values of the calculated association constants are lower for the complexes of chloride with receptors 1 and 3 than with 4 and 5. This is attributed to the presence of electron-donating groups located on the aryl units of the squaramide function of the former (1 and 3) and the presence of two electron-withdrawing groups (amides) in the latter (4 and 5). The titrations carried out in the presence of one equivalent of potassium or sodium cations demonstrated the assumed expectations: only ion pair receptors 3 and 5 could bind chloride anions in an enhanced manner with greater enhancement for potassium than sodium cations. The decrease in the values of the stability constant for complexes of 1 and 4 with chloride anions in the presence of potassium cations suggests the participation of ion pair formation outside the receptor in such conditions.
1 binding model using the Bindfit program. The results are presented in Table 1. Receptor 2 was excluded from the binding study due to self-association in such conditions, which was confirmed by a dilution test (see ESI Fig. S20†). As listed in Table 1, the values of the calculated association constants are lower for the complexes of chloride with receptors 1 and 3 than with 4 and 5. This is attributed to the presence of electron-donating groups located on the aryl units of the squaramide function of the former (1 and 3) and the presence of two electron-withdrawing groups (amides) in the latter (4 and 5). The titrations carried out in the presence of one equivalent of potassium or sodium cations demonstrated the assumed expectations: only ion pair receptors 3 and 5 could bind chloride anions in an enhanced manner with greater enhancement for potassium than sodium cations. The decrease in the values of the stability constant for complexes of 1 and 4 with chloride anions in the presence of potassium cations suggests the participation of ion pair formation outside the receptor in such conditions.
| L | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|
| a UV-vis, solvent CH3CN, temperature 293 K, [1] = 2.0 × 10−5 M, [3] = 2.6 × 10−5 M, [4] = 2.0 × 10−5 M, [5] = 3.0 × 10−5 M; anions added as TBA salts [TBAX] ∼1.5 × 10−3 M; M−1, errors <10%. b Self-association. c Not determined. | |||||
| K TBACl | 1.00 × 105 ± 3.1% | —b | 1.08 × 105 ± 7.5% | 3.05 × 105 ± 2.4% | 3.14 × 105 ± 5.6% |
| K KCl | 0.75 × 105 ± 4.5% | —b | 2.12 × 105 ± 8.3% | 2.23 × 105 ± 1.4% | 5.11 × 105 ± 9.2% |
| K NaCl | —c | —b | —c | —c | 4.19 × 105 ± 3.0% |
| K TBABr | —c | —b | —c | —c | 1.91 × 104 ± 1.9% |
| K KBr | —c | —b | —c | —c | 4.80 × 104 ± 2.5% |
| K TBANO3 | —c | —b | —c | —c | 2.10 × 103 ± 1.5% |
| K KNO3 | —c | —b | —c | —c | 5.53 × 103 ± 1.4% |
| K TBANO2 | —c | —b | —c | —c | 7.36 × 104 ± 2.6% |
| K KNO2 | —c | —b | —c | —c | 1.09 × 105 ± 4.1% |
Titration experiments were extended to other selected anions (Br−, NO3−, NO2−) for receptor 5, and, due to the greater enhancement, for in situ generated potassium salts. In each case, the enhancement of the association constants in the presence of the cation was retained. This confirms the cooperative binding of the salts by receptor 5. Chloride binding was also verified independently by 1H NMR titrations in DMSO-d6. This also opened the possibility of performing titration experiments with receptor 2, which does not aggregate in DMSO. Based on the shifts of signals corresponding to squaramide and aromatic protons, the association constants of the complexes for chloride in the absence and presence of a cation were determined (Fig. 2). For receptor 2 the values were calculated to be KTBACl = 390 ± 1.9% M−1 and KKCl = 580 ± 3.2% M−1, while for receptor 5KTBACl = 560 ± 0.5% M−1 and KKCl = 710 ± 0.3% M−1. The higher association constants for receptor 5 compared to receptor 2 can be attributed to the different nature of the substituents in their structures. In the presence of a potassium cation, chloride was strongly bound to ditopic receptors 2 and 5, confirming the cooperative binding of the ion pair.
Further evidence that the ditopic receptor can function as an ion pair receptor came from single-crystal X-ray diffraction analysis of alkali metal salt complexes. Crystals of receptor 2 complexed with potassium bromide were grown by slow diffusion of diethyl ether into a solution of this complex in acetonitrile obtained after solid–liquid extraction. The single crystal structure of the investigated compound 2·KBr reveals the expected complexation of the salt with the cation captured by the crown ether and the Br− anion bound to the squaramide unit (Fig. 3). The investigated crystal is disordered and contains ca. 11% sodium, which originates from the glassware used during experimental procedures. In the crystal lattice, the salt complexing ligands are forming stacks of dimers, due to the electrostatic interaction between bound K+ and Br− ions.
Ion pair and amino acid extraction
Considering the good solubility of the salt receptors in chloroform, we performed qualitative extraction studies to test whether ion pair receptors can extract salts from an aqueous solution into an organic phase. Specifically, we treated a solution of receptors 2 or 5 in deuterated chloroform with an aqueous solution of potassium and sodium chloride salts. We followed the formation of complexes with the salts in the organic phase by 1H NMR (Fig. 4). It was found that the signals corresponding to the squaramide protons and the aromatic protons shifted downfield when a solution of 5 in wet CDCl3 was contacted with the aqueous salt solution. The chloroform solution of complexes of 5 with KCl or NaCl was then shaken with deionized water and the signals returned to the initial state of the receptor in wet chloroform. These experiments suggest that the receptors are able not only to extract but also to release ions into water, making them promising salt transporters. The same property was proven for receptor 2 which could also extract and release salts (see ESI†).The presence of two heteroditopic binding domains in receptors 2 and 5 prompted us to test the ability of these receptors to interact with zwitterions. We decided to test the interaction of these receptors with α-amino acids under similar interfacial conditions as in the case of ion pairs. We chose a hydrophilic amino acid with a simple 1H NMR spectrum (glycine) and performed liquid–liquid extractions of an aqueous glycine solution with a chloroform solution of receptors 2 or 5. By examining the chloroform solution after extraction by 1H NMR, we found that the ditopic receptors can form complexes with glycine and extract it from the aqueous layer (Fig. 4b). All our attempts to crystallize complexes of 2 or 5 with glycine failed, and to establish the nature of the interaction between the receptors and glycine we therefore analyzed the complexes by 2D NMR. ROESY NMR experiments of the chloroform solution after glycine extraction showed that the ammonium function of the glycine is located near the benzocrown ether (Fig. 5), while the carboxylate anion interacts with the squaramide groups, as indicated by a shift in the signals corresponding to squaramide protons from 9.00 and 8.87 ppm to 9.86 and 9.64 ppm, respectively. The α-protons of glycine show cross peaks with the aromatic protons and the ethylene protons of the crown ether located close to the aromatic ring. Similar observations were noted for receptor 5 (see ESI Fig. S40–S42†). This data suggests that the receptor simultaneously interacts with the amino acid through both anion and cation binding domains, potentially forming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (amino acid
1 complex (amino acid![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) receptor) or complexes of higher stoichiometry. DOSY experiments carried out for receptor 5 showed that the diffusion coefficient changed only slightly from D = 3.99 × 10−9 m2 s−1 to D = 3.91 × 10−9 m2 s−1 suggesting that the formation of complexes with higher stoichiometry upon extraction is unlikely.
receptor) or complexes of higher stoichiometry. DOSY experiments carried out for receptor 5 showed that the diffusion coefficient changed only slightly from D = 3.99 × 10−9 m2 s−1 to D = 3.91 × 10−9 m2 s−1 suggesting that the formation of complexes with higher stoichiometry upon extraction is unlikely.
 | ||
| Fig. 5 Interaction scheme of 2 with the Gly molecule confirmed by ROESY and partial ROESY spectra of interactions (A)–(C). | ||
Transport across bulk liquid membranes
Initially, the ability to transport chloride salts by 5 was verified by applying a bulk liquid membrane (U-tube) using aqueous NaCl or KCl solutions as the source phase, a solution of 5 (5 mM) in chloroform as the membrane, and distilled water as the receiving phase. The chloride concentration in the receiving phase was monitored using conductometry (Fig. 6a). We found that receptor 5 can transport chloride salts across the chloroform membrane with an efficiency calculated after ten days of 84% for KCl and 34% for NaCl. The higher efficiency for potassium chloride transport than sodium chloride transport correlates with the association constants obtained during the UV-vis titrations. Using the same method, the ability of 2 and 5 to act as amino acid transporters was also examined. D2O was used as the receiving phase and the concentration of glycine in the receiving phase was monitored by 1H NMR. Measurements were carried out in coaxial NMR tubes with the insert containing an internal standard (CH3COOK). After each measurement, the analyzed sample was immediately returned to the U-tube and the experiment was continued. For this reason, this experiment should be treated qualitatively. Nevertheless, we observed an increase in Gly concentration in the receiving phase, proving that 2 and 5 are capable of transporting this highly hydrophilic amino acid (Fig. 6b).Transmembrane transport
Because the ditopic receptors were shown to bind and extract KCl as an ion pair, we wanted to investigate whether these receptors can also facilitate K+/Cl− symport across model liposome membranes. To verify this, we prepared 200 nm large unilamellar vesicles (LUVs) consisting of 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 POPC
3 POPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cholesterol lipids, which contained a buffered KCl solution on the inside and a buffered Na2SO4 solution on the outside of the liposome (see ESI† for experimental details). KCl efflux facilitated by the addition of 1–5 was measured using both a chloride selective electrode and a potassium selective electrode (Fig. 7). Sulfate was chosen as the external anion because it has a high charge and high hydration energy, making it difficult to transport. Under these circumstances, K+/Cl− symport is more likely than Cl−/SO42− antiport. As can be seen in Fig. 7, receptors 3, 4 and 5 are able to facilitate both chloride and potassium transport, whereas receptors 1 and 2 are inactive. This is probably due to their effective chloride binding and high lipophilicity, while compound 1 is a weaker chloride binder due to the electron donating groups and receptor 2 lacks long lipophilic alkyl chains. When comparing the magnitude of the Cl− and K+ efflux facilitated by these transporters (Fig. 7C), the ion pairing effect becomes clear. For receptor 3, the chloride efflux and potassium efflux are comparable, as would be expected for a ditopic receptor. For compound 5, the potassium efflux appears more efficient than the chloride efflux. This could be due to a combination of K+/Cl− symport and K+/Na+ antiport, but it must also be noted that the potassium selective electrode gave rise to a pronounced drift due to some interference from the Na+ ions in the external buffer (giving rise to a bigger drift for the blank DMSO run and % efflux values >100%). Nevertheless, the potassium electrode data still provides evidence for K+ transport and compound 5 probably has very comparable K+ and Cl− efflux abilities due to its ion pair binding ability. In contrast, the K+ efflux induced by receptor 4 is significantly lower than the Cl− efflux induced by the same compound, indicating that 4 is better at transporting anions than cations. This is expected given the lack of a crown ether moiety in this receptor.
cholesterol lipids, which contained a buffered KCl solution on the inside and a buffered Na2SO4 solution on the outside of the liposome (see ESI† for experimental details). KCl efflux facilitated by the addition of 1–5 was measured using both a chloride selective electrode and a potassium selective electrode (Fig. 7). Sulfate was chosen as the external anion because it has a high charge and high hydration energy, making it difficult to transport. Under these circumstances, K+/Cl− symport is more likely than Cl−/SO42− antiport. As can be seen in Fig. 7, receptors 3, 4 and 5 are able to facilitate both chloride and potassium transport, whereas receptors 1 and 2 are inactive. This is probably due to their effective chloride binding and high lipophilicity, while compound 1 is a weaker chloride binder due to the electron donating groups and receptor 2 lacks long lipophilic alkyl chains. When comparing the magnitude of the Cl− and K+ efflux facilitated by these transporters (Fig. 7C), the ion pairing effect becomes clear. For receptor 3, the chloride efflux and potassium efflux are comparable, as would be expected for a ditopic receptor. For compound 5, the potassium efflux appears more efficient than the chloride efflux. This could be due to a combination of K+/Cl− symport and K+/Na+ antiport, but it must also be noted that the potassium selective electrode gave rise to a pronounced drift due to some interference from the Na+ ions in the external buffer (giving rise to a bigger drift for the blank DMSO run and % efflux values >100%). Nevertheless, the potassium electrode data still provides evidence for K+ transport and compound 5 probably has very comparable K+ and Cl− efflux abilities due to its ion pair binding ability. In contrast, the K+ efflux induced by receptor 4 is significantly lower than the Cl− efflux induced by the same compound, indicating that 4 is better at transporting anions than cations. This is expected given the lack of a crown ether moiety in this receptor.
Given the ability of 3–5 to facilitate KCl transport across liposomal membranes and their ability to transport glycine across a bulk liquid membrane, we wanted to test the ability of 1–5 to transport amino acids across liposomal membranes as well. A convenient method to assess this is the Cu2+-calcein assay developed by Gale and co-workers.45 Preliminary studies using this assay indicated that compound 5 is able to transport glycine efficiently, followed by 4 and 3, with minimal transport seen for 1 and 2, which is in agreement with the KCl transport ability trend (see ESI†). However, control experiments without amino acids suggested that compound 5 is also able to transport Cu2+ (see ESI†), and the results observed for the Cu2+-calcein assay can therefore not be unequivocally attributed to amino acid transport. To provide more direct evidence for the amino acid transport ability of 5, we decided to use a liposome-based 13C NMR experiment. For this assay, 13C-labeled glycine (Gly-1-13C) was added externally to a suspension of giant POPC multilamellar vesicles, and solutions of receptor 5 or 4 in DMSO (or DMSO for the reference experiment) were added to initiate glycine transport. After 15 min, paramagnetic Mn2+ was added to suppress the 13C NMR signal from extravascular glycine, and then 13C NMR spectra of the vesicle suspension were measured. The results show that in the absence of the receptor only very slow, direct diffusion of the amino acid occurs, while an intense signal from internal Gly-1-13C was observed for the experiment with receptor 5 (10 mol% transporter to lipid) (Fig. 8a). A similar effect on glycine transport was observed for receptor 4 (10 mol% transporter to lipid), but the signal intensity of the intact glycine signal suggests a slower guest transport (see ESI†).
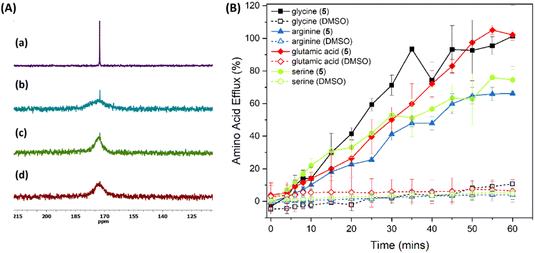
We also wanted to test if compound 5 can transport amino acids other than glycine across phospholipid bilayers. Unfortunately, not many amino acids are available as 13C-labelled analogs at a reasonable price. We therefore used the fluorescamine assay developed by Hou and co-workers (see ESI† for experimental details).38 In this assay, LUVs encapsulating the amino acid of interest are prepared and the unencapsulated amino acid is removed by dialysis. A DMSO solution of the transporter is then added to the dialysis tubing containing the liposomes, and the tubing is subsequently transferred to a fresh external buffer solution (pH 7.4). Aliquots of the external buffer solution are removed at regular intervals to measure the amino acid concentration using the covalent fluorescent probe fluorescamine. We obtained the best results when the original dialysis to remove unencapsulated amino acid was performed overnight. When only 2 hours of dialysis was performed, the negative control without transporter showed significant drift due to the remaining external amino acid. However, amino acids that are less polar than glycine (e.g., alanine and phenylalanine) have sufficient membrane permeability that all internal amino acid is lost at this time. The assay is therefore most useful to measure the transport of highly polar amino acids. The results for the ability of compound 5 to transport glycine, serine, glutamate and arginine is shown in Fig. 8B. It is clear that ditopic receptor 5 is able to facilitate the transmembrane transport of all four of these highly polar amino acids, including glutamate and arginine which have a net negative and positive charge respectively (the experiments are performed at physiological pH where the amine, carboxylic acid and side chain functional groups are fully charged). Although it appears that 5 can facilitate transmembrane transport of these amino acids with similar rates, it must be noted that the amino acid efflux observed is the result of transmembrane transport followed by dialysis. It is therefore quite likely that the rate determining step is dialysis of external amino acid, and not transmembrane transport induced by 5. The assay should thus be treated as qualitative instead of quantitative. Nevertheless, the results clearly show the ability of 5 to transport highly polar amino acids with charged side chains. Furthermore, leakage assays and dynamic light scattering (DLS) experiments showed that 5 does not form large pores or functions as a membrane disruptor (see ESI†), confirming that the results are due to true transmembrane transport. Presumably, the reason why 5 is such a good amino acid transporter is due to its ditopic nature, which allows compensation of any charge gradient induced by the transport of a charged amino acid via the transport of an appropriate anion or cation. Compound 5 thus represents the first small molecule that has been shown to transport highly polar amino acids with charged side chains.
Conclusions
In this manuscript, we report the synthesis and characterization of a small library of squaramide and crown ether containing ditopic receptors. UV-vis and 1H NMR titrations in organic solvents revealed that these compounds can bind to free chloride anions and to chloride salts, especially KCl. Furthermore, liquid–liquid extractions showed that the ditopic receptors are able to extract KCl or NaCl from an aqueous solution to an organic solution and that the salt can be released again upon the addition of distilled water. U-tube experiments, as well as liposome-based assays, confirmed that these compounds are also able to perform transmembrane transport of KCl as an ion pair and of hydrophilic zwitterions such as amino acids. The best results were obtained for compound 5, which contains 18-crown-6 and a squaramide unit appended with highly lipophilic electron-withdrawing amide groups to enhance both binding and transport abilities. This compound was even able to facilitate the transmembrane transport of amino acids with charged side chains, such as glutamate and arginine. We are currently exploring the biological activity of this compound.Data availability
The data supporting this study are available in the ESI.†Author contributions
M. Z.-H. – data curation, formal analysis, conceptualization, methodology, investigation, software, visualization, validation, resources, performed experiments and analyzed the experimental data, writing – original draft & editing; R. S. and M. J. G.– performed the transport experiments and analyzed the experimental data, software, and visualization. M. W. – performed the 2D NMR experiments and analyzed the experimental data, methodology, and visualization. Ł. D. performed the crystal data experiments and analyzed the experimental data, software, methodology, visualization, writing – original draft. N. B. – conceptualization, methodology, funding acquisition, investigation, validation, visualization, writing – review & editing. J. R. – conceptualization, methodology, funding acquisition, investigation, project administration, supervision, validation, visualization, writing – review & editing.Conflicts of interest
There are no conflicts to declare.Acknowledgements
R. S, M. J. G. and N. B. would like to thank Tulane University for start-up funds and the National Science Foundation for funding (NSF CHE-2108699). J. R., M. Z.-H., M. W. would like to thank funds from the National Science Centre, Poland, (Grant No. 2018/30/E/ST5/00841).Notes and references
- B. Alberts, A. Johnson, J. Lewis, M. Raff, K. Roberts and P. Walter, Molecular biology of the cell, 4th edn, Ann. Bot., 2002,(3), 401–450 Search PubMed.
- N. Kucerka, M.-P. Nieh and J. Katsaras, Fluid phase lipid areas and bilayer thicknesses of commonly used phosphatidylcholines as a function of temperature, Biochim. Biophys. Acta, Biomembr., 2011, 1808, 2761–2771 CAS.
- L. E. Bickerton, T. G. Johnson, A. Kerckhoffs and M. J. Langton, Supramolecular chemistry in lipid bilayer membranes, Chem. Sci., 2021, 12, 11252–11274 CAS.
- D. N. Sheppard, D. P. Rich, L. S. Ostedgaard, R. J. Gregory, A. E. Smith and M. J. Welsh, Mutations in CFTR associated with mild-disease-form CI channels with altered pore properties, Nature, 1993, 362, 160–164 CAS.
- D. B. Simon, R. S. Bindra, T. A. Mansfield, C. Nelson-Williams, E. Mendonca, R. Stone, S. Schurman, A. Nayir, H. Alpay, A. Bakkaloglu, J. Rodriguez-Soriano, J. M. Morales, S. A. Sanjad, C. M. Taylor, D. Pilz, A. Brem, H. Trachtman, W. Griswold, G. A. Richard, E. John and R. P. Lifton, Mutations in the chloride channel gene, CLCNKB, cause bartter's syndrome type III, Nat. Genet., 1997, 17, 171–178 CrossRef CAS PubMed.
- D. B. Simon, F. E. Karet, J. M. Hamdan, A. D. Pietro, S. A. Sanjad and R. P. Lifton, Bartter's syndrome, hypokalaemic alkalosis with hypercalciuria, is caused by mutations in the Na–K–2ci cotransporter NKCC2, Nat. Genet., 1996, 13, 183–188 CrossRef CAS PubMed.
- S. E. Lloyd, S. H. Pearce, S. E. Fisher, K. Steinmeyer, B. Schwappach, S. J. Scheinman, B. Harding, A. Bolino, M. Devoto, P. Goodyer, S. P. A. Rigden, O. Wrong, T. J. Jentsch, I. W. Craig and R. V. Thakker, A common molecular basis for three inherited kidney stone diseases, Nature, 1996, 379, 445–449 CrossRef CAS PubMed.
- L. Chen, S. N. Berry, X. Wu, E. N. W. Howe and P. A. Gale, Advances in anion receptor chemistry, Chem, 2020, 6, 61–141 CAS.
- J. T. Davis, P. A. Gale and R. Quesada, Advances in anion transport and supramolecular medicinal chemistry, Chem. Soc. Rev., 2020, 49, 6056–6086 RSC.
- N. Busschaert, C. Caltagirone, W. V. Rossom and P. A. Gale, Applications of supramolecular anion recognition, Chem. Rev., 2015, 115, 8038–8155 CrossRef CAS PubMed.
- C. M. Dias, H. Li, H. Valkenier, L. E. Karagiannidis, P. A. Gale, D. N. Sheppard and A. P. Davis, Anion transport by ortho-phenylene bis-ureas across cell and vesicle membranes, Org. Biomol. Chem., 2018, 16, 1083–1087 RSC.
- M. J. Langton, C. J. Serpell and P. D. Beer, Anion recognition in water: recent advances from a supramolecular and macromolecular perspective, Angew. Chem., Int. Ed., 2016, 55, 1974–1987 CrossRef CAS PubMed.
- N. H. Evans and P. D. Beer, Advances in anion supramolecular chemistry: from recognition to chemical applications, Angew. Chem., Int. Ed., 2014, 53(44), 11716–11754 CAS.
- S. Kubik, Anion recognition in water, Chem. Soc. Rev., 2010, 39, 3648–3663 CAS.
- P. Molina, F. Zapata and A. Coballero, Anion recognition strategies based on combined noncovalent interactions, Chem. Rev., 2017, 117, 9907–9972 CAS.
- Y. Liu, A. Sengupta, K. Raghavachari and A. H. Flood, Anion binding in solution: beyond the electrostatic regime, Chem, 2017, 3, 411–427 CAS.
- S. J. Edwards, I. Marques, C. M. Dias, R. A. Tromans, N. R. Lees, V. Félix, H. Valkenier and A. P. Davis, Tilting and tumbling in transmembrane anion carriers: activity tuning through n-alkyl substitution, Chem.–Eur. J., 2016, 22, 2004–2011 CAS.
- H. Valkenier, L. W. Judd, H. Li, S. Hussain, D. N. Sheppard and A. P. Davis, Preorganized bis-thioureas as powerful anion carriers: chloride transport by single molecules in large unilamellar vesicles, J. Am. Chem. Soc., 2014, 136, 12507–12512 CAS.
- V. Saggiomo, S. Otto, I. Marques, V. Félix, T. Torroba and R. Quesada, The role of lipophilicity in transmembrane anion transport, Chem. Commun., 2012, 48, 5274–5276 CAS.
- H. Valkenier, C. J. E. Haynes, J. Herniman, P. A. Gale and A. P. Davis, Lipophilic balance – a new design principle for transmembrane anion carriers, Chem. Sci., 2014, 5, 1128–1134 CAS.
- N. J. Knight, E. Hernando, C. J. E. Haynes, N. Busschaert, H. J. Clarke, K. Takimoto, M. García-Valverde, J. G. Frey, R. Quesada and P. A. Gale, QSAR analysis of substituent effects on tambjamine anion transporters, Chem. Sci., 2016, 7, 1600–1608 CAS.
- K. M. Bąk, K. Chabuda, H. Montes, R. Quesada and M. J. Chmielewski, 1,8-Diamidocarbazoles: an easily tuneable family of fluorescent anion sensors and transporters, Org. Biomol. Chem., 2018, 16, 5188–5196 Search PubMed.
- Y. Cha, E.-S. Kim and J. Koo, Amino acid transporters and glutamine metabolism in breast cancer, Int. J. Mol. Sci., 2018, 19, 907–924 CrossRef PubMed.
- P. D. Dobson and D. B. Kell, Carrier-mediated cellular uptake of pharmaceutical drugs: an exception or the rule?, Nat. Rev. Drug Discovery, 2008, 7, 205–220 CrossRef CAS PubMed.
- I. Alfonso and R. Quesada, Biological activity of synthetic ionophores: ion transporters as prospective drugs?, Chem. Sci., 2013, 4, 3009–3019 RSC.
- A. I. Share, K. Patel, C. Nativi, E. J. Cho, O. Francesconi, N. Busschaert, P. A. Gale, S. Roelens and J. L. Sessler, Chloride anion transporters inhibit growth of methicillin-resistant Staphylococcus aureus (MRSA) in vitro, Chem. Commun., 2016, 52, 7560–7563 RSC.
- S.-H. Park, E. N. W. Howe, J. Y. Hyun, L.-J. Chen, I. Huang, G. Vargas-Zuñiga, N. Busschaert, P. A. Gale, J. L. Sessler and I. Shin, Determinants of ion-transporter cancer cell death, Chem, 2019, 5, 2079–2098 CAS.
- W. G. Ryder, E. G. Wu, L. Chen, M. Fares, D. A. McNaughton, K. Tran, C. Yu and P. A. Gale, Furazan bis-ureas: a heterocyclic scaffold for anion binding and transport, Org. Chem. Front., 2024, 11, 1290–1298 RSC.
- Q. He, G. I. Vargas-Zúñiga, S. H. Kim, S. K. Kim and J. L. Sessler, Macrocycles as ion pair receptors, Chem. Rev., 2019, 119, 9753–9835 CrossRef CAS PubMed.
- A. V. Koulov, J. M. Mahoney and B. D. Smith, Facilitated transport of sodium or potassium chloride across vesicle membranes using a ditopic salt-binding macrobicycle, Org. Biomol. Chem., 2003, 1, 27–29 RSC.
- C. C. Tong, R. Quesada, J. L. Sessler and P. A. Gale, meso-Octamethylcalix[4]pyrrole: an old yet new transmembrane ion-pair transporter, Chem. Commun., 2008, 6321–6323 RSC.
- I. W. Park, J. Yoo, B. Kim, S. Adhikari, S. K. Kim, Y. Yeon, C. J. E. Haynes, J. L. Sutton, C. C. Tong, V. M. Lynch, J. L. Sessler, P. A. Gale and C. H. Lee, Oligoether-strapped calix[4]pyrrole: an ion-pair receptor displaying cation-dependent chloride anion transport, Chem.–Eur. J., 2012, 18, 2514–2523 CAS.
- J. H. Lee, Y. R. Choi, P. Kang, M. G. Choi and K. S. Jeong, Synthetic K+/Cl--selective symporter across a phospholipid membrane, J. Org. Chem., 2014, 79, 6403–6409 CAS.
- X. H. Yu, X. J. Cai, X. Q. Hong, K. Y. Tam, K. Zhang and W. H. Chen, Synthesis and biological evaluation of aza-crown ether–squaramide conjugates as anion/cation symporters, Future Med. Chem., 2019, 11(10), 1091–1106 CAS.
- Z. Zhao, B. Tang, X. Yan, X. Wu, Z. Li, P. A. Gale and Y.-B. Jiang, Crown ether-thiourea conjugates as ion transporters, Front. Chem. Sci. Eng., 2022, 16, 81–91 CAS.
- P. Kandasamy, G. Gyimesi, Y. Kanai and M. A. Hediger, Amino acid transporters revisited: new views in health and disease, Trends Biochem. Sci., 2018, 43, 752–789 Search PubMed.
- J. Sánchez-Quesada, H. S. Kim and M. R. Ghadiri, A synthetic pore-mediated transmembrane transport of glutamic acid, Angew. Chem., Int. Ed., 2001, 40, 2503–2506 Search PubMed.
- L. Chen, W. Si, L. Zhang, G. Tang, Z.-T. Li and J.-L. Hou, Chiral Selective transmembrane transport of amino acids through artificial channels, J. Am. Chem. Soc., 2013, 135, 2152–2155 CAS.
- Y. Li, J. Dong, W. Gong, X. Tang, Y. Liu, Y. Cui and Y. Liu, Artificial biomolecular channels: enantioselective transmembrane transport of amino acids mediated by homochiral zirconium metal–organic cages, J. Am. Chem. Soc., 2021, 143, 20939–20951 CAS.
- N. Demirel, Y. Bulut and H. Hosgoren, Enantioselective transport and liquid–liquid extraction of amino acids as their potassium and sodium salts by optically active diaza-18-crown-6 ethers, Chirality, 2004, 16, 347–350 CAS.
- H. Tsukube, Active and passive transport of amino-acid and oligopeptide derivatives by artificial ionophore-K+ complexes, J. Chem. Soc. Perkin Trans., 1982, 1, 2359–2363 Search PubMed.
- M. Pietraszkiewicz, M. Kozbial and O. Pietraszkiewicz, Chiral discrimination of amino acids and their potassium or sodium salts by optically active crown ether derived from d-mannose, J. Membr. Sci., 1998, 138, 109–113 CAS.
- P. Breccia, M. V. Gool, R. Perez-Fernandez, S. Martin-Santamaria, F. Gago, P. Prados and C. J. Mendoza, Guanidinium receptors as enantioselective amino acid membrane carriers, J. Am. Chem. Soc., 2003, 125, 8270–8284 Search PubMed.
- J. Sunamoto, K. Iwamoto, Y. Mohri and T. Kominato, Liposomal membranes. 13. Transport of an amino acid across liposomal bilayers as mediated by a photoresponsive, J. Am. Chem. Soc., 1982, 104, 5502–5504 Search PubMed.
- X. Wu, N. Busschaert, N. J. Wells, Y.-B. Jiang and P. A. Gale, Dynamic covalent transport of amino acids across lipid bilayers, J. Am. Chem. Soc., 2015, 137, 1476–1484 CrossRef CAS.
- L. Martinez-Crespo, J. L. Sun-Wang, A. F. Sierra, G. Aragay, E. Errasti-Murugarren, P. Bartoccioni, M. Palacin and P. Ballester, Facilitated diffusion of proline across membranes of liposomes and living cells by a calix[4]pyrrole cavitand, Chem, 2020, 6, 3054–3070 CAS.
- K. Masłowska-Jarzyna, K. M. Bąk, B. Zawada and M. J. Chmielewski, pH-Dependent transport of amino acids across lipid bilayers by simple monotopic anion carriers, Chem. Sci., 2022, 13, 12374–12381 RSC.
- L. A. Marchetti, L. K. Kumawat, N. Mao, J. C. Stephens and R. B. P. Elmes, The versatility of squaramides: from supramolecular chemistry to chemical biology, Chem, 2019, 5, 1398–1485 CAS.
- N. Busschaert, I. L. Kirby, S. Young, S. J. Coles, P. N. Horton, M. E. Light and P. A. Gale, Squaramides as potent transmembrane anion transporters, Angew. Chem., Int. Ed., 2012, 51, 4426–4430 CrossRef CAS PubMed.
- N. Busschaert, S.-H. Park, K.-H. Baek, Y. P. Choi, J. Park, E. N. W. Howe, J. R. Hiscock, L. E. Karagiannidis, I. Marques, V. Félix, W. Namkung, J. L. Sessler, P. A. Gale and I. Shin, A synthetic ion transporter that disrupts autophagy and induces apoptosis by perturbing cellular chloride concentrations, Nat. Chem., 2017, 9, 667–675 Search PubMed.
- X. Bao, X. Wu, S. N. Berry, E. N. W. Howe, Y.-T. Chang and P. A. Gale, Fluorescent squaramides as anion receptors and transmembrane anion transporters, Chem. Commun., 2018, 54, 1363–1366 Search PubMed.
- W. Zhong-Kun, H. Xiao-Qiao, H. Jinhui, X. Yuan-Yuan and C. Wen-Hua, Synthesis and biological activity of squaramido-tethered bisbenzimidazoles as synthetic anion transporters, RSC Adv., 2021, 11, 3972–3980 Search PubMed.
- G. Picci, M. Kubicki, A. Garau, V. Lippolis, R. Mocci, A. Porcheddu, R. Quesada, P. C. Ricci, M. A. Scorciapino and C. Caltagirone, Simple squaramide receptors for highly efficient anion binding in aqueous media and transmembrane transport, Chem. Commun., 2020, 56, 11066–11069 RSC.
- G. Picci, I. Carreira-Barral, D. Alonso-Carrillo, C. Busonera, J. Milia, R. Quesada and C. Caltagirone, C. The role of indolyl substituents in squaramide-based anionophores, Org. Biomol. Chem., 2022, 20, 7981–7986 RSC.
- G. Picci, R. Montis, V. Lippolis and C. Caltagirone, Squaramide-based receptors in anion supramolecular chemistry: insights into anion binding, sensing, transport and extraction, Chem. Soc. Rev., 2024, 53, 3952–3975 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2338837. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc00866b |
| This journal is © The Royal Society of Chemistry 2025 |