Open Access Article
Open Access ArticleP![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e002.gif) B and As
B and As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/h2_char_e002.gif) B triple bonds in the linear PB2O− and AsB2O− species†
B triple bonds in the linear PB2O− and AsB2O− species†
Han-Wen
Gao
 ,
Jie
Hui
,
Jie
Hui
 and
Lai-Sheng
Wang
and
Lai-Sheng
Wang
 *
*
Department of Chemistry, Brown University, Providence, RI 02912, USA. E-mail: lai-sheng_wang@brown.edu
First published on 17th March 2025
Abstract
Due to its electron deficiency, boron triple bonds are relatively scarce. We use high-resolution photoelectron imaging to investigate the structures and bonding of the EB2O− (E = P, As) type of clusters, which are found to have [E![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–B
B–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O]− closed-shell linear structures with E
O]− closed-shell linear structures with E![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bonds. The B atoms in the linear EB2O− species undergo sp hybridization, while the E atoms also undergo sp hybridization to form a σ bond with the sp orbital of B along with two π bonds formed by the px and py orbitals. The high-resolution photoelectron imaging data reveal detachment transitions from the EB2O− (1Σ+) anions to the EB2O (2Π) neutrals. The electron affinities of PB2O and AsB2O are measured to be 3.592(1) eV and 3.432(1) eV, respectively; the vibrational frequencies for the E–B, B–B, and B–O stretching modes are measured for both species. The spin–orbit splitting of the 2Π state to 2Π3/2 and 2Π1/2 is measured to be 153 cm−1 and 758 cm−1 for PB2O and AsB2O, respectively.
B triple bonds. The B atoms in the linear EB2O− species undergo sp hybridization, while the E atoms also undergo sp hybridization to form a σ bond with the sp orbital of B along with two π bonds formed by the px and py orbitals. The high-resolution photoelectron imaging data reveal detachment transitions from the EB2O− (1Σ+) anions to the EB2O (2Π) neutrals. The electron affinities of PB2O and AsB2O are measured to be 3.592(1) eV and 3.432(1) eV, respectively; the vibrational frequencies for the E–B, B–B, and B–O stretching modes are measured for both species. The spin–orbit splitting of the 2Π state to 2Π3/2 and 2Π1/2 is measured to be 153 cm−1 and 758 cm−1 for PB2O and AsB2O, respectively.
1. Introduction
Borylene (:BR) is the boron analogue of carbene (:CR2).1 The HOMO of borylene is an sp hybridized σ-type orbital, while the LUMO and LUMO+1 are the two empty p (px and py) orbitals. Compared with carbenes, borylenes are more reactive due to their two vacant p orbitals. This also makes borylenes better π-acceptors and they can be stabilized by favourable back-donation of d electrons from transition metals. The first borylene complex was synthesized within the ligand sphere of a stable transition metal complex in 1998 with the form of LnMBR.2 Since then, many borylene complexes have been synthesized.3 Owing to their ability to form π bonds by the two vacant p orbitals, borylenes have the potential to form triple bonds with transition metals. Transition metal–boron triple-bond characters were first hinted in the [(OC)5Cr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) BSi(SiMe3)3] complex,3f in which the Cr–B bond length is slightly shorter than a Cr
BSi(SiMe3)3] complex,3f in which the Cr–B bond length is slightly shorter than a Cr![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B double bond. The shortest transition metal–boron bond length was reported in an LnFeBF compound4 with an Fe–B bond length comparable to the Fe
B double bond. The shortest transition metal–boron bond length was reported in an LnFeBF compound4 with an Fe–B bond length comparable to the Fe![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple-bond. Transition metal–boron triple bonds have been observed in the linear ReB2O− and IrB2O− gaseous anions.5 Interestingly, RhB2O− was found to have a bent structure with a short Rh–B bond, leading to the discovery of the first Rh
B triple-bond. Transition metal–boron triple bonds have been observed in the linear ReB2O− and IrB2O− gaseous anions.5 Interestingly, RhB2O− was found to have a bent structure with a short Rh–B bond, leading to the discovery of the first Rh![[quadruple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e003.gif) B quadruple bond.6 These gaseous anions were produced by laser vaporization, in which the stable BO boronyl unit was readily formed due to trace amounts of oxygen contaminants, serving as a convenient monovalent substituent in the simple borylene model of :B–BO.
B quadruple bond.6 These gaseous anions were produced by laser vaporization, in which the stable BO boronyl unit was readily formed due to trace amounts of oxygen contaminants, serving as a convenient monovalent substituent in the simple borylene model of :B–BO.
In contrast to transition metal–boron complexes, main group elements are more challenging to form triple bonds with boron due to the absence of d electrons. However, the B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bond can be formed using donor ligands, such as in [OC → B
B triple bond can be formed using donor ligands, such as in [OC → B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B ← CO] or the first isolated B
B ← CO] or the first isolated B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B stabilized by two bulky carbenes.7 If B acquires an electron, B− is isoelectronic to C and can form B
B stabilized by two bulky carbenes.7 If B acquires an electron, B− is isoelectronic to C and can form B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bonds, such as in the dianion [OB–B
B triple bonds, such as in the dianion [OB–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–BO]2− and [Bi
B–BO]2− and [Bi![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–BO]– or the recent Bi
B–BO]– or the recent Bi![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) BH− species.8–10 Triple bonds between group 15 elements with boron are particularly interesting because they are analogous to C
BH− species.8–10 Triple bonds between group 15 elements with boron are particularly interesting because they are analogous to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C triple bonds. Numerous iminoborane R–N
C triple bonds. Numerous iminoborane R–N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–R′ moieties featuring a N
B–R′ moieties featuring a N![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bond have been synthesized, as evidenced by their linear structures and short N–B bond lengths.11 However, as one moves down the periodic table, R–E
B triple bond have been synthesized, as evidenced by their linear structures and short N–B bond lengths.11 However, as one moves down the periodic table, R–E![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–R′ type compounds start to exhibit bent structures, especially on the R–E side.12 For example, the simplest HPBH molecule, which is isoelectronic with alkynes, is predicted to be bent,13 indicating a P–B bond order lower than three. The bending is a result of less s-orbital overlap as one descends the periodic table.12,14 The reduced s orbital contribution by the E atom in the RE side leads to a shift from sp to sp2 hybridization, transforming the geometry from linear to bent. In sp2 hybridization, only one p orbital remains available to form a π bond. Equivalently, in the MO picture under the bent geometry, the in-plane π bonding orbital has the same symmetry as the antibonding σ orbital. This leads to significant mixing between the two orbitals, weakening the in-plane π bond and making it more like a lone pair, consistent with the valence bond perspective. Thus, the bent geometry inherently reduces the bond order below three. The few synthesized phosphaborene (R–P
B–R′ type compounds start to exhibit bent structures, especially on the R–E side.12 For example, the simplest HPBH molecule, which is isoelectronic with alkynes, is predicted to be bent,13 indicating a P–B bond order lower than three. The bending is a result of less s-orbital overlap as one descends the periodic table.12,14 The reduced s orbital contribution by the E atom in the RE side leads to a shift from sp to sp2 hybridization, transforming the geometry from linear to bent. In sp2 hybridization, only one p orbital remains available to form a π bond. Equivalently, in the MO picture under the bent geometry, the in-plane π bonding orbital has the same symmetry as the antibonding σ orbital. This leads to significant mixing between the two orbitals, weakening the in-plane π bond and making it more like a lone pair, consistent with the valence bond perspective. Thus, the bent geometry inherently reduces the bond order below three. The few synthesized phosphaborene (R–P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B–R′) and arsaborene (R–As
B–R′) and arsaborene (R–As![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B–R′) molecules are found to exhibit bent geometries with P
B–R′) molecules are found to exhibit bent geometries with P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B and As
B and As![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) B double bonds.15
B double bonds.15
Thus, it is challenging to synthesize molecules with P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B or As
B or As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bonds. No compounds with such triple bonds have been isolated in the bulk except a theoretical suggestion of a P
B triple bonds. No compounds with such triple bonds have been isolated in the bulk except a theoretical suggestion of a P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bond protected by bulky groups.16 Alternatively, molecules containing P
B triple bond protected by bulky groups.16 Alternatively, molecules containing P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B or As
B or As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bonds can be prepared in the gas phase or low temperature matrices, where it is possible to form linear molecules with bare P or As atoms unprotected by ligands. The PBH radical was observed in a low temperature matrix, an open-shell species with three π electrons and a P–B bond order of 2.5.17 In this article, we use high-resolution photoelectron imaging (PEI) to study EB2O− (E = P, As) clusters produced by a laser vaporization source. High-resolution photoelectron spectra from photoelectron imaging (PEI) of cryogenically cooled anions and theoretical calculations confirm that the EB2O− species are electron-precise linear molecules, [E
B triple bonds can be prepared in the gas phase or low temperature matrices, where it is possible to form linear molecules with bare P or As atoms unprotected by ligands. The PBH radical was observed in a low temperature matrix, an open-shell species with three π electrons and a P–B bond order of 2.5.17 In this article, we use high-resolution photoelectron imaging (PEI) to study EB2O− (E = P, As) clusters produced by a laser vaporization source. High-resolution photoelectron spectra from photoelectron imaging (PEI) of cryogenically cooled anions and theoretical calculations confirm that the EB2O− species are electron-precise linear molecules, [E![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–B
B–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O]–, containing a terminal E
O]–, containing a terminal E![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bond (E = P, As). The electron affinities (EAs), vibrational frequencies, and spin–orbit (SO) splitting of the neutral EB2O radicals are obtained for the first time.
B triple bond (E = P, As). The electron affinities (EAs), vibrational frequencies, and spin–orbit (SO) splitting of the neutral EB2O radicals are obtained for the first time.
2. Experimental and theoretical methods
2.1. Experimental methods
The experiment was carried out on a high-resolution PEI apparatus newly equipped with a cryogenically cooled 3D Paul trap. The PEI apparatus has been described in detail previously,18 and has been proven to be effective to cool anionic clusters generated from a laser vaporization source.19 In the current work, the PB2O− and AsB2O− clusters were produced by laser vaporization of cold-pressed P/10B and As/10B targets, respectively. The boronyl species were generated due to trace amounts of oxide impurity on the target surface. In fact, the PB2O− and AsB2O− clusters were particularly prominent in the mass spectra, indicating that they are highly stable thermodynamically. A helium carrier gas was used to quench the laser-induced plasma. Clusters formed inside the nozzle were entrained by the helium carrier gas and underwent a supersonic expansion. After passing through a skimmer, the collimated cluster beam travelled directly into the 3D Paul trap cooled down to 4.2 K by a two-stage closed-cycle helium refrigerator. Clusters of interest were selected and intercepted by a detachment laser in the interaction zone of a velocity map imaging (VMI) system. Photodetachment was performed using the frequency-doubled output of a dye laser, as well as the third harmonic output from an Nd:YAG laser. Photoelectrons were projected onto a set of microchannel plates coupled with a phosphor screen and a charge-coupled-device camera. The VMI lens was calibrated using PEI of Bi− at various photon energies. The photoelectron images were analysed by the maximum entropy method (MEVELER).20 The resolution of the VMI system was ∼0.6% for electrons with high kinetic energies and as low as 1.2 cm−1 for very slow photoelectrons.18Photoelectron angular distributions (PADs) can be obtained from photoelectron images, characterized by an anisotropy parameter (β). Under the electric dipole approximation, when an electron with angular momentum l is detached from an atom, the outgoing photoelectron carries an angular momentum of l ± 1. If an electron is detached from an s atomic orbital (l = 0), the outgoing photoelectron will have l = 1 (pure p-wave) with β = 2. If an electron is detached from an atomic p orbital, the outgoing electron will carry s + d partial waves with β = −1.21 While the β value is non-trivial to interpret for electron detachment from molecular orbitals (MOs),22 it provides qualitative information about the symmetries of the MOs.
2.2. Theoretical methods
We employed the ABCluster software to perform the global minimum search.23 Several initial structures were optimized using density functional theory (DFT) at the B3LYP/6-311+G* (ref. 24) level with the Gaussian 09 package.25 Additional calculations were also done at the TPSSh/aug-cc-pVTZ level of theory.26 Frequency calculations were carried out to compare with the experimental data and to ensure that the optimized structures were true minima. To account for SO interactions, we first utilized the state-averaged (SA)-complete active space self-consistent field (CASSCF) method27 followed by multi-reference configuration interaction (MRCI) calculations28 to get the low-lying electronic states. An active space consisting of 17 electrons and 9 orbitals was chosen for both PB2O and AsB2O. Then we carried out SO coupling calculations using the Breit-Pauli Hamiltonian in Molpro.29,30 The aug-cc-pVTZ basis set was applied for the SO coupling calculations. Franck–Condon (FC) factors were computed using the ezFCF program.31 Chemical bonding analyses were done using adaptive natural density partitioning (AdNDP).323. Experimental and theoretical results
3.1. Experimental results
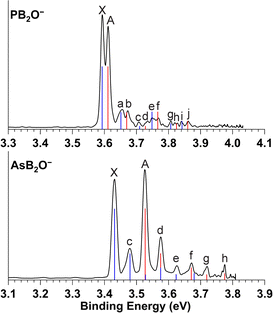
High-resolution PEI data of cryogenically cooled PB2O− at 3.809 eV and 4.023 eV are shown in Fig. 1. The 4.023 eV spectrum (Fig. 1b) reveals two prominent peaks, X and A, which have similar intensities and are followed by numerous weak vibrational features labelled as peaks a–j. In the 3.809 eV spectrum (Fig. 1a), peaks a–f are better resolved, revealing that peaks X and A, as well as the pairs of a and b, c and d, and e and f, all have similar spacings and intensity patterns. It turns out that peaks g and h, and peaks i and j in the 4.023 eV spectrum, are similar pairs. These observations suggest that the pairs of peaks originate from two electronic states with parallel potential energy surfaces, likely corresponding to two SO-split states. Peaks X and A should be the 0–0 transitions to the two SO states of the neutral PB2O molecule. Peak X defines an accurate electron affinity (EA) of 3.592 eV for PB2O, while the spacing between X and A yields a SO splitting energy of 153 cm−1. The presence of SO splitting suggests that PB2O− and PB2O should have linear structures and the HOMO of PB2O− must be a π orbital. | ||
| Fig. 1 Photoelectron images and spectra of cryogenically cooled PB2O− at (a) 3.809 eV and (b) 4.032 eV. The double arrow below the image indicates the laser polarization. | ||
The photoelectron angular distributions provide further insights into the nature of the HOMO, from which an electron is detached. In the 4.023 eV spectrum, all peaks exhibit s + d-type angular distributions, indicating that they are due to electron detachment from a π-type MO. At the lower photon energy of 3.809 eV, peaks that are closer to the detachment threshold, such as e and f, exhibit more isotropic angular distributions, due to the significant suppression of higher-order partial waves near the detachment threshold. The binding energies of all the observed peaks, their shifts relative to peaks X and A, and the assignments are given in Table 1.
| BE (eV)a | Assignment | Shift (cm−1) | ||
|---|---|---|---|---|
| a The numbers in the parentheses represent the uncertainty in the last digit. | ||||
| X | 3.592(1) | 2Π3/2 (000) | 0 | |
| A | 3.611(1) | 2Π1/2 (000) | 153 | 0 |
| a | 3.651(1) | 2Π3/2 (301) | 476 | |
| b | 3.668(1) | 2Π1/2 (301) | 460 | |
| c | 3.714(1) | 2Π3/2 (302) | 984 | |
| d | 3.729(1) | 2Π1/2 (302) | 952 | |
| e | 3.749(1) | 2Π3/2 (201) | 1266 | |
| f | 3.765(1) | 2Π1/2 (201) | 1245 | |
| g | 3.807(1) | 2Π3/2 (301201) | 1734 | |
| h | 3.825(3) | 2Π1/2 (301201) | 1726 | |
| i | 3.840(1) | 2Π3/2 (101) | 2000 | |
| j | 3.862(1) | 2Π1/2 (101) | 2024 | |
High-resolution PEI data of cryogenically cooled AsB2O− at 3.496 eV and 3.809 eV are shown in Fig. 2. The 3.809 eV spectrum (Fig. 2b) reveals two strong peaks X and A, similar to those observed in PB2O−. Peaks c, e and f, and peaks d, g and h form two sets of similar vibrational progressions following peaks X and A. In addition to the better resolved peaks X and c, the 3.496 eV spectrum (Fig. 2a) also reveals two very weak peaks, a and b. The overall similarity between the spectra of AsB2O− and PB2O− suggests that AsB2O− also adopts a linear structure, with peaks X and A being the 0–0 transitions to two SO states. Peak X defines an accurate EA of 3.432 eV for AsB2O, and the spacing between peaks X and A gives a SO splitting of 758 cm−1. As expected from the periodic trend from P to As, the EA of AsB2O decreases and the SO effect increases. All the resolved peaks for AsB2O in the 3.809 eV spectrum display s + d-type angular distributions and become more isotropic in the near-threshold spectrum at 3.496 eV, similar to the observation in the spectra of PB2O. The binding energies of all the observed peaks, their shifts relative to peaks X and A, and the assignments are given in Table 2.
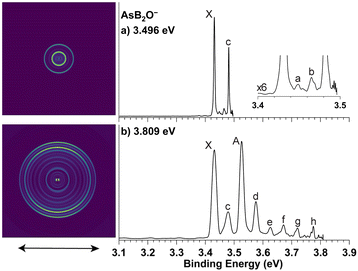 | ||
| Fig. 2 Photoelectron images and spectra of cryogenically cooled AsB2O− at (a) 3.496 eV and (b) 3.809 eV. The double arrow below the image indicates the laser polarization. | ||
| BE (eV)a | Assignment | Shift (cm−1) | ||
|---|---|---|---|---|
| a The numbers in the parentheses represent the uncertainty in the last digit. | ||||
| X | 3.432(1) | 2Π3/2 (000) | 0 | |
| a | 3.449(1) | 2Π3/2 (401) | 137 | |
| b | 3.465(1) | 2Π3/2 (402) | 266 | |
| c | 3.482(1) | 2Π3/2 (301) | 403 | |
| A | 3.526(1) | 2Π1/2 (000) | 758 | 0 |
| 2Π3/2 (302) | 758 | |||
| d | 3.576(1) | 2Π1/2 (301) | 403 | |
| 2Π3/2 (201) | 11615 | |||
| e | 3.626(1) | 2Π1/2 (302) | 807 | |
| 2Π3/2 (301201) | 1565 | |||
| f | 3.672(1) | 2Π1/2 (201) | 1178 | |
| 2Π3/2 (101) | 1936 | |||
| g | 3.719(1) | 2Π1/2 (301201) | 1557 | |
| h | 3.775(1) | 2Π1/2 (101) | 2008 | |
3.2. Theoretical results
The global minimum (GM) structures and low-lying isomers of PB2O− and AsB2O− are presented in Fig. S1.† The GM structures of EB2O− (E = P, As) are found to have closed-shell linear structures with C∞v symmetry and a 1σ22σ23σ24σ21π45σ22π4 (1Σ+) electronic configuration. All other isomers are much higher in energy. The optimized geometries at the B3LYP level for EB2O− and neutral EB2O are shown in Fig. 3. The calculated vibrational frequencies of the neutral radicals are compared with the experimental values in Table 3. The results calculated at the TPSSh level agree well with those at the B3LYP level, as compared in Fig. S1 and S2 and Table S1.† The MOs for EB2O− are given in Fig. S3.† The HOMOs of EB2O− are π bonding orbitals essentially localized on the E–B bond. Detachment of an electron from the HOMO yields a 2Π state for the neutral molecule with an electron configuration of …1π45σ22π3. The 2Π state splits into 2Π3/2 and 2Π1/2 due to SO coupling, with the 2Π3/2 SO state being lower in energy. The calculated EAs to the 2Π neutral ground state for both molecules at the B3LYP/6-311+G* level, in which SO coupling is not considered explicitly, are compared with the experimental values in Table 3. We computed the 2Π state and an additional low-lying 2Σ+ state using the SA3-CASSCF(17,9) and MRCI methods, and these states were included in the calculations of SO splitting between 2Π1/2 and 2Π3/2. The resulting SO splitting is computed to be 145 and 740 cm−1 for PB2O and AsB2O, respectively, as summarized in Table 3.| EA (eV)a | SO splitting (cm−1) | Frequency (cm−1)b | ||||
|---|---|---|---|---|---|---|
| Exp. | Cal. | Exp. | Cal. | Exp.c | Cal. | |
| a Spin–orbital coupling is not considered in the computed EAs. b Calculated frequencies are scaled by a factor of 0.97. ν1 = B–O stretching, ν2 = B–B stretching, ν3 = E–B stretching, ν4 = cis-bending. The scaling factor is based on the NIST computational chemistry comparison and benchmark database (https://cccbdb.nist.gov/vibscalejustx.asp). c The experimental uncertainty is estimated to be ±10 cm−1. | ||||||
| PB2O | 3.592 | 3.52 | 153 | 145 | 2010 (ν1) | 2010 |
| 1260 (ν2) | 1254 | |||||
| 470 (ν3) | 472 | |||||
| AsB2O | 3.432 | 3.39 | 758 | 740 | 2000 (ν1) | 2003 |
| 1165 (ν2) | 1161 | |||||
| 400 (ν3) | 389 | |||||
| 135 (ν4) | 143 | |||||
4. Discussion
4.1. Comparison between experiment and theory
The calculated EAs for the linear structures, 3.52 eV for PB2O and 3.39 eV for AsB2O at the B3LYP level, are in good agreement with the experimental data of 3.592 eV and 3.432 eV, respectively. The computed SO splitting for the 2Π state, 145 cm−1 for PB2O and 740 cm−1 for AsB2O, also agrees well with the spacing between peaks X and A: 153 cm−1 for PB2O and 758 cm−1 for AsB2O. The excellent agreement between theory and experiment confirms that peaks X and A indeed correspond to the two SO states and validates the linear structures of EB2O− (E = P, As).To understand the observed vibrational structures, we computed the FC factors using the optimized geometries and vibrational frequencies at the B3LYP/6-311+G* level, as shown in Fig. 4, where the blue and red bars represent FC factors for transitions to the 2Π3/2 and 2Π1/2 states, respectively. The overall FC profiles agree well with the experimental spectra, further validating the linear structures. For detachment transitions from the linear anions to linear neutrals, FC-active modes can only involve stretching vibrations. In the case of PB2O, we observed all three stretching modes, which are essentially the P–B, B–B, and B–O vibrations, designated as modes ν3, ν2, and ν1 (Fig. S4†). Using the FC simulations, we can readily assign the observed vibrational peaks for PB2O (Table 1). Peaks a and b correspond to ν3 of the two SO states, while peaks c and d correspond to the overtone of ν3 of the two SO states. Peaks e and f are from ν2 of the two SO states. Peaks g and h represent the combination modes of ν3 and ν2 for the two SO states. Peaks i and j correspond to ν1 of the two SO states.
In the spectra of AsB2O− (Table 2), peak c corresponds to excitation to the ν3 mode of the 2Π3/2 state, while its overtone overlaps with the 0–0 transition of the 2Π1/2 state, resulting in peak A appearing slightly more intense than peak X. The ν2 excitation of the 2Π3/2 state also overlaps with the excitation of the ν3 mode of the 2Π1/2 state, causing peak d to appear slightly more intense than peak c. Peak e is the overlap of the overtone of ν1 of the 2Π1/2 state and the combination of ν3 and ν2 of the 2Π3/2 state. Peak f is dominated by excitation to ν2 of the 2Π1/2 state with minor contribution from ν1 of the 2Π3/2 state. Peaks g and h are from the combination of ν1 and ν2 of the 2Π1/2 state and ν1 of the 2Π1/2 state, respectively.
In the 3.496 eV spectrum of AsB2O− (Fig. 2a), two very weak peaks a and b are observed. These peaks do not correspond to any FC-active modes. Instead, their frequencies agree with the calculated fundamental and overtone of the cis-bending mode ν4 (Fig. S4†). For linear molecules, overtones of bending modes are symmetry-allowed, while the fundamental of the bending mode is forbidden. In a previous PES study on BiBH−,10 which has a similar linear structure, we also found a weak bending vibrational feature attributed to vibronic coupling between the 2Π state and a higher-lying 1Σ+ excited state. The observation of the weak 401 bending feature in the spectrum of AsB2O− could also come from vibronic coupling.
4.2. Chemical bonding in PB2O− and AsB2O−
We analysed the chemical bonding in EB2O− using the AdNDP method (Fig. 5) and the MOs (Fig. S3†). The AdNDP results for EB2O− (E = P, As) are nearly identical. We can identify two lone pairs on E and O, a triple bond between E and B, a B–B single bond and another triple bond between B and O. Thus, the two EB2O− species are electron-precise molecules [E![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–B
B–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O]–. The triple bonds can also be readily seen from the HOMO and HOMO-1 (Fig. S3†), representing the E–B π and σ bonds, respectively. We calculated the Wiberg bond index matrix on the natural atomic orbital (NAO) basis. The Wiberg bond orders for the P
O]–. The triple bonds can also be readily seen from the HOMO and HOMO-1 (Fig. S3†), representing the E–B π and σ bonds, respectively. We calculated the Wiberg bond index matrix on the natural atomic orbital (NAO) basis. The Wiberg bond orders for the P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B and As
B and As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B bonds in PB2O− and AsB2O− are 2.84 and 2.83, respectively, consistent with the AdNDP and MO analyses. PB2O− and AsB2O− are the first molecules observed to contain P
B bonds in PB2O− and AsB2O− are 2.84 and 2.83, respectively, consistent with the AdNDP and MO analyses. PB2O− and AsB2O− are the first molecules observed to contain P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B and As
B and As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bonds. In the valence bond model, the B atom undergoes sp hybridization, where one of the sp hybridized orbitals forms a σ bond with BO and the other sp orbital forms another σ bond with P or As. The P and As atoms also undergo sp hybridization, so that the s orbital from P or As makes considerable contribution to the σ bond. One of the sp orbitals forms a σ bond with the B atom, and the other sp orbital gives rise to the lone pair on P/As. The calculated atomic orbital composition shows that the s orbital contributes 34% to the P sp hybridized orbitals, while it contributes 31% to the As sp hybridized orbitals. The 2px and 2py orbitals of B form two π bonds with the corresponding p orbitals of P or As, giving rise to the P
B triple bonds. In the valence bond model, the B atom undergoes sp hybridization, where one of the sp hybridized orbitals forms a σ bond with BO and the other sp orbital forms another σ bond with P or As. The P and As atoms also undergo sp hybridization, so that the s orbital from P or As makes considerable contribution to the σ bond. One of the sp orbitals forms a σ bond with the B atom, and the other sp orbital gives rise to the lone pair on P/As. The calculated atomic orbital composition shows that the s orbital contributes 34% to the P sp hybridized orbitals, while it contributes 31% to the As sp hybridized orbitals. The 2px and 2py orbitals of B form two π bonds with the corresponding p orbitals of P or As, giving rise to the P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B and As
B and As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bond. The contribution of the valence s orbital to the σ bond in P
B triple bond. The contribution of the valence s orbital to the σ bond in P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B and As
B and As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B is quite different from the weak triple bond in Bi
B is quite different from the weak triple bond in Bi![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B, where the 6s orbital is basically a lone pair.9,10 The strong relativistic effects significantly stabilize the 6s orbital relative to the 6p orbitals, making it unfavourable for sp hybridization.
B, where the 6s orbital is basically a lone pair.9,10 The strong relativistic effects significantly stabilize the 6s orbital relative to the 6p orbitals, making it unfavourable for sp hybridization.
The P–B and As–B bond lengths in PB2O− and AsB2O− are also consistent with their triple bond nature. According to Pyykko's additive covalent radii,33 the P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B and As
B and As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bond lengths are 1.67 Å and 1.79 Å, respectively, in excellent agreement with the calculated P–B and As–B bond lengths of 1.68 Å and 1.78 Å in PB2O− and AsB2O− (Fig. 3). In the PB2O and AsB2O neutrals, removal of an electron from the π bonding orbital (HOMO in Fig. S3†) weakens the P–B and As–B bonds, resulting in a bond order of 2.5. Consequently, the P–B and As–B bond lengths in the neutral species increase to 1.72 Å and 1.83 Å, respectively, indicating the significant strength of the π bond in PB2O− and AsB2O−. It should be noted that the P–B bond length in PB2O is consistent with that in the linear PBH (1.72 Å) species observed in a low temperature matrix, where the P–B bond order is also 2.5.17 It is interesting to point out that the sp lone pair on the terminal P or As atoms in PB2O− and AsB2O− may be able to coordinate to a bulky Lewis acid to allow the isolation of the first P
B triple bond lengths are 1.67 Å and 1.79 Å, respectively, in excellent agreement with the calculated P–B and As–B bond lengths of 1.68 Å and 1.78 Å in PB2O− and AsB2O− (Fig. 3). In the PB2O and AsB2O neutrals, removal of an electron from the π bonding orbital (HOMO in Fig. S3†) weakens the P–B and As–B bonds, resulting in a bond order of 2.5. Consequently, the P–B and As–B bond lengths in the neutral species increase to 1.72 Å and 1.83 Å, respectively, indicating the significant strength of the π bond in PB2O− and AsB2O−. It should be noted that the P–B bond length in PB2O is consistent with that in the linear PBH (1.72 Å) species observed in a low temperature matrix, where the P–B bond order is also 2.5.17 It is interesting to point out that the sp lone pair on the terminal P or As atoms in PB2O− and AsB2O− may be able to coordinate to a bulky Lewis acid to allow the isolation of the first P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B and As
B and As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bonds.
B triple bonds.
5. Conclusion
In conclusion, we report a high-resolution photoelectron imaging investigation of PB2O− and AsB2O−. The electron affinities of neutral PB2O and AsB2O are measured to be 3.592 eV and 3.432 eV, respectively. Vibrationally resolved photoelectron imaging, combined with computational chemistry, confirms that PB2O− and AsB2O− have linear structures, [P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–B
B–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O] – and [As
O] – and [As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B–B
B–B![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) O]–, with P
O]–, with P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B and As
B and As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bonds. Both PB2O− and AsB2O− are closed-shell with a 1Σ+ electronic state, whereas their neutrals are open shell with a 2Π3/2 ground state. The spin–orbit split 2Π1/2 excited state is determined to be 153 cm−1 and 758 cm−1 higher in energy for PB2O and AsB2O, respectively. The PB2O− and AsB2O− species represent the first molecules containing P
B triple bonds. Both PB2O− and AsB2O− are closed-shell with a 1Σ+ electronic state, whereas their neutrals are open shell with a 2Π3/2 ground state. The spin–orbit split 2Π1/2 excited state is determined to be 153 cm−1 and 758 cm−1 higher in energy for PB2O and AsB2O, respectively. The PB2O− and AsB2O− species represent the first molecules containing P![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B and As
B and As![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) B triple bonds, which can serve as benchmarks for studying P–B and As–B multiple bonds and may be viable for bulk syntheses.
B triple bonds, which can serve as benchmarks for studying P–B and As–B multiple bonds and may be viable for bulk syntheses.
Data availability
The data that supports the findings of this study is available from the corresponding author upon request.Author contributions
H. W. G. and J. H. did the experiment. H. W. G. analysed the data and wrote the first draft of the manuscript. J. H. did the calculations. L. S. W. guided and advised the project, and revised and finalized the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the U.S. National Science Foundation (Grant No. CHE-2403841). The calculations were performed using resources at the Centre for Computation and Visualization (CCV) of Brown University.References
- (a) A. W. Ehlers, E. V. Barends, F. M. Bickelhaupt and U. Radius, Chem.–Eur. J., 1998, 4, 210 CAS; (b) D. Vidovic, G. A. Pierce and S. Aldridge, Chem. Commun., 2009, 1157 Search PubMed; (c) H. Braunschweig, R. D. Dewhurst and V. H. Gessner, Chem. Soc. Rev., 2013, 42, 3197 Search PubMed; (d) M. Soleilhavoup and G. Bertrand, Angew. Chem., Int. Ed., 2017, 56, 10282 CAS.
- (a) H. Braunschweig, C. Kollann and U. Englert, Angew. Chem., Int. Ed., 1998, 37, 3179 CAS; (b) A. H. Cowley, V. Lomelf and A. Voigt, J. Am. Chem. Soc., 1998, 120, 6401 CAS.
- (a) H. Braunschweig, M. Colling, C. Kollann, H. G. Stammler and B. Neumann, Angew. Chem., Int. Ed., 2001, 40, 2298 CAS; (b) D. L. Coombs, S. Aldridge, C. Jones and D. J. Willock, J. Am. Chem. Soc., 2003, 125, 6356 CAS; (c) D. L. Coombs, S. Aldridge, A. Rossin, C. Jones and D. J. Willock, Organometallics, 2004, 23, 2911 CAS; (d) H. Braunschweig, M. Burzler, T. Kupfer, K. Radacki and F. Seeler, Angew. Chem., Int. Ed., 2007, 46, 7785 Search PubMed; (e) H. Braunschweig, K. Radacki and K. Uttinger, Angew. Chem., Int. Ed., 2007, 46, 3979 CAS; (f) B. Blank, M. Colling-Hendelkens, C. Kollann, K. Radacki, D. Rais, K. Uttinger, G. R. Whittell and H. Braunschweig, Chem.–Eur. J., 2007, 13, 4770 CrossRef CAS; (g) H. Braunschweig, M. Forster, T. Kupfer and F. Seeler, Angew. Chem., Int. Ed., 2008, 47, 5981 CrossRef CAS PubMed; (h) G. Alcaraz, U. Helmstedt, E. Clot, L. Vendier and S. Sabo-Etienne, J. Am. Chem. Soc., 2008, 130, 12878 CrossRef CAS PubMed; (i) S. Bertsch, H. Braunschweig, B. Christ, M. Forster, K. Schwab and K. Radacki, Angew. Chem., Int. Ed., 2010, 49, 9517 CrossRef CAS.
- M. J. Drance, J. D. Sears, A. M. Mrse, C. E. Moore, A. L. Rheingold, M. L. Neidig and J. S. Figueroa, Science, 2019, 363, 1203 CrossRef CAS PubMed.
- T. Chen, L. F. Cheung, W. J. Chen, J. Cavanagh and L. S. Wang, Angew. Chem., Int. Ed., 2020, 59, 15260 CrossRef CAS PubMed.
- L. F. Cheung, T. T. Chen, G. S. Kocheril, W. J. Chen, J. Czekner and L. S. Wang, J. Phys. Chem. Lett., 2020, 11, 659 CrossRef CAS PubMed.
- (a) M. Zhou, N. Tsumori, Z. Li, K. Fan, L. Andrews and Q. Xu, J. Am. Chem. Soc., 2002, 124, 12936 CrossRef CAS PubMed; (b) H. Brauschweig, R. D. Dewhurst, K. Hammond, J. Mies, K. Radacki and A. Vargas, Science, 2012, 336, 1420 CrossRef PubMed.
- S. D. Li, H. J. Zhai and L. S. Wang, J. Am. Chem. Soc., 2008, 130, 2573 CrossRef CAS PubMed.
- T. Jian, L. F. Cheung, T. T. Chen and L. S. Wang, Angew. Chem., Int. Ed., 2017, 56, 9551 CrossRef CAS PubMed.
- H. W. Gao, J. Hui and L. S. Wang, Chem. Commun., 2023, 59, 12431 Search PubMed.
- (a) P. Paetzold, A. Richter, T. Thijssen and S. Würtenberg, Chem. Ber., 1979, 112, 3811 CrossRef CAS; (b) J. Wang, P. Jia, W. Sun, Y. Wei, Z. Lin and Q. Ye, Inorg. Chem., 2022, 61, 8879 CrossRef CAS PubMed.
- R. C. Fischer and P. P. Power, Chem. Rev., 2010, 110, 3877 CrossRef CAS PubMed.
- J. D. Watts and L. C. V. Zant, Chem. Phys. Lett., 1996, 251, 119 CrossRef CAS.
- P. P. Power, Nature, 2010, 463, 171–177 CAS.
- (a) E. Rivard, W. A. Merrill, J. C. Fettinger, R. Wolf, G. H. Spikes and P. P. Power, Inorg. Chem., 2007, 46, 2971 CAS; (b) J. Li, Z. Lu and L. L. Liu, J. Am. Chem. Soc., 2022, 144, 23691 Search PubMed; (c) A. Koner, B. Morgenstern and D. M. Andrada, Angew. Chem., Int. Ed., 2022, 61, e202203345 CAS; (d) E. Rivard, W. A. Merrill, J. C. Fettinger and P. P. Power, Chem. Commun., 2006, 36, 3800 Search PubMed; (e) M. Wen, R. Medel, P. V. Zasimov, C. Müller and S. Riedel, Chem. Sci., 2024, 15, 8045 RSC; (f) E. A. LaPierre, B. O. Patrick and I. Manners, J. Am. Chem. Soc., 2023, 145, 7107 CrossRef CAS PubMed.
- J. S. Lu, M. C. Yang and M. D. Su, ACS Omega, 2018, 3, 76 CrossRef CAS PubMed.
- L. Wang, X. Jiang, G. Wang, X. Zeng and M. Zhou, Chem.–Eur. J., 2023, 29, e202203704 CrossRef CAS PubMed.
- I. León, Z. Yang, H. T. Liu and L. S. Wang, Rev. Sci. Instrum., 2014, 85, 083106 Search PubMed.
- (a) G. S. Kocheril, H. W. Gao, D. F. Yuan and L. S. Wang, J. Chem. Phys., 2022, 157, 171101 CrossRef CAS PubMed; (b) G. S. Kocheril, H. W. Gao and L. S. Wang, Mol. Phys., 2024, 122, e2182610 Search PubMed; (c) G. S. Kocheril, H. W. Gao and L. S. Wang, J. Chem. Phys., 2023, 158, 236101 CrossRef CAS PubMed; (d) H. W. Gao, H. W. Choi, J. Hui, W. J. Chen, G. S. Kocheril and L. S. Wang, J. Chem. Phys., 2023, 159, 114301 CrossRef CAS PubMed; (e) H. W. Gao, H. W. Choi, J. Hui and L. S. Wang, J. Phys. Chem. A, 2024, 128, 3579 CrossRef CAS PubMed.
- B. Dick, Phys. Chem. Chem. Phys., 2019, 21, 19499 RSC.
- J. Cooper and R. N. Zare, J. Chem. Phys., 1968, 48, 942 CrossRef CAS.
- A. Sanov and R. Mabbs, Int. Rev. Phys. Chem., 2008, 27, 53 Search PubMed.
- J. Zhang and M. Dolg, Phys. Chem. Chem. Phys., 2015, 17, 24173 Search PubMed.
- C. T. Lee, W. T. Yang and R. G. Parr, Phys. Rev. B:Condens. Matter Mater. Phys., 1988, 37, 785 CAS.
- M. J. Frisch et al. , Gaussian 09, Revision A.1, Gaussian, Inc., Wallingford, CT, 2009 Search PubMed.
- J. Tao, J. P. Perdew, V. N. Staroverov and G. E. Scuseria, Phys. Rev. Lett., 2003, 91, 146401 Search PubMed.
- H. J. Werner and P. J. Knowles, J. Chem. Phys., 1985, 82, 5053 Search PubMed.
- H. J. Werner and P. J. Knowles, J. Chem. Phys., 1988, 89, 5803 CAS.
- A. Berning, M. Schweizer, H. J. Werner, P. J. Knowles and P. Palmieri, Mol. Phys., 2000, 98, 1823 CAS.
- H. J. Werner et al., MOLPRO, a package of ab initio programs, see http://www.molpro.net.
- S. Gozem and A. I. Krylov, WIREs Comp. Mol. Sci., 2021, 12, e1546 Search PubMed.
- D. Y. Zubarev and A. I. Boldyrev, Phys. Chem. Chem. Phys., 2008, 10, 5207 RSC.
- P. Pyykko, J. Phys. Chem. A, 2015, 119, 2326 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc00812c |
| This journal is © The Royal Society of Chemistry 2025 |