 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Light and protonation-controlled complex formation between sulfate ions and a stiff-stilbene based bis(cyclopeptide)†
Stefan
Mommer‡
 a,
Benedict
Wyrwol
b,
Jasper E.
Bos
a,
Stefan
Kubik
a,
Benedict
Wyrwol
b,
Jasper E.
Bos
a,
Stefan
Kubik
 *b and
Sander J.
Wezenberg
*b and
Sander J.
Wezenberg
 *a
*a
aLeiden Institute of Chemistry, Leiden University, Einsteinweg 55, 2333 CC Leiden, The Netherlands. E-mail: s.j.wezenberg@lic.leidenuniv.nl
bFachbereich Chemie–Organische Chemie, RPTU Kaiserslautern-Landau, Erwin-Schrödinger-Str. 54, 67663 Kaiserslautern, Germany. E-mail: stefan.kubik@rptu.de
First published on 24th March 2025
Abstract
Anion–ligand coordination has been used to generate a number of supramolecular structures. Of particular interest is the transformation between different types of complexes using various stimuli. While there are multiple examples where this has been achieved with metal–ligand coordination complexes through incorporation of molecular photoswitches, the same has not yet been realized with anion–ligand coordination-driven assemblies. In this study, a sulfate-binding bis(cyclopeptide) with a photoswitchable stiff-stilbene linker is presented. Its (E)- and (Z)-isomers, and the different degrees of protonation of the anion (HSO4−vs. SO42−), give rise to different assembly states. The accessible products have 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1
1, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 host–guest stoichiometries and can be interconverted by light irradiation and acid/base addition, resulting in a highly controllable responsive system that demonstrates the potential of sulfate coordination-driven supramolecular assembly.
2 host–guest stoichiometries and can be interconverted by light irradiation and acid/base addition, resulting in a highly controllable responsive system that demonstrates the potential of sulfate coordination-driven supramolecular assembly.
Introduction
Coordination chemistry has been used to assemble a wide range of nanostructures, including supramolecular boxes, cages, and helicates.1 Recent efforts have been directed at inducing reversible transformations between different types of metal–ligand complexes, as a means to control their properties and functions.2 Among the stimuli that have been applied to achieve this, light has proven to be highly promising as it can be delivered with high spatiotemporal precision without the build-up of waste products.3 The main strategy here is to incorporate molecular photoswitches into the ligands.4 However, the heavy metal atoms often used to assemble these nanostructures can be toxic, limiting biological application.Anions are ubiquitous in biological and chemical processes. In recent years, they have gradually become important motifs in coordination driven self-assembly.5,6 They exhibit a rich variety of geometries and coordination sites and hence, in combination with suitable hydrogen bond-donating ligands, various supramolecular architectures can be accessed.5,6 While these developments pave the way to a new generation of self-assembled structures and materials with new functions, the incorporation of photoswitchable units has not yet been realized.
Inspired by these developments and motivated to gain control over anion–ligand complex formation using light, we designed the photoswitchable bis(cyclopeptides) (E)-bisCP and (Z)-bisCP containing a rigid stiff-stilbene linker (Scheme 1). Analogous bis(cyclopeptides) with flexible linkers have previously been shown to form complexes with anions such as sulfate and halides, in which the anion resides in a cavity between the two cyclopeptide rings and interacts with six converging peptide NH groups.7 Anion binding was studied mainly in aqueous solvent mixtures and in water, where complex formation was largely driven by the desolvation of the anion binding site and the hydrophobic proline residues when the two receptor halves came together, but studies have also been performed in acetonitrile and DMSO.8 In the latter solvent, iodide binding is negligible since it does not benefit from solvophobic effects, while binding to the strongly coordinating sulfate anion is substantial.
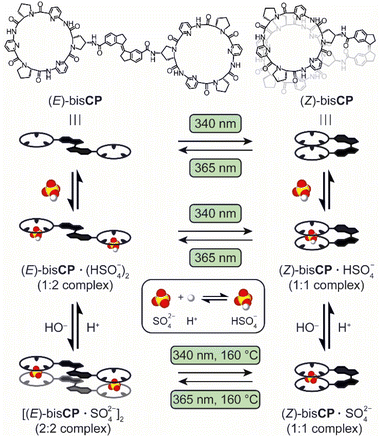 | ||
| Scheme 1 Light- and protonation-controlled switching between stiff-stilbene-bridged bisCP in different isomeric and HSO4−/SO42− anion-complexed states. | ||
The stiff-stilbene photoswitch was used previously as a backbone of tweezer-type molecular anion receptors, which exhibited large differences in binding affinity between the (E)- and (Z)-isomers.9 Its use as a photoswitchable linker is advantageous owing to its large geometrical change upon isomerization and its high thermal stability.10 While a significant number of photo-switchable anion receptors have been developed over the past decade,11,12 it should be noted that the photocontrolled binding of sulfate is largely unexplored.13 In the present case, we anticipated that incorporation of stiff-stilbene between the cyclopeptide anion-binding moieties would afford a receptor that in the (Z)-form is able to bind the sulfate anion by simultaneous interaction with these moieties (see Scheme 1). In contrast, in the (E)-form the cyclopeptide residues would be too far apart from each other to give a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex and higher-order species could therefore be formed. In addition, the use of DMSO as the solvent would allow the protonation of the sulfate dianion (SO42−) and the deprotonation of the corresponding hydrogen sulfate (HSO4−) anion to be used as independent stimuli to control complex formation (the pKa of HSO4− is 2.0 in water and 14.5 in DMSO),14 as was previously demonstrated for another system by Chmielewski and co-workers.15
1 host–guest complex and higher-order species could therefore be formed. In addition, the use of DMSO as the solvent would allow the protonation of the sulfate dianion (SO42−) and the deprotonation of the corresponding hydrogen sulfate (HSO4−) anion to be used as independent stimuli to control complex formation (the pKa of HSO4− is 2.0 in water and 14.5 in DMSO),14 as was previously demonstrated for another system by Chmielewski and co-workers.15
Here, we show that reversible photoisomerisation of the rigid stilbene linker in bisCP can be achieved by irradiation with light of different wavelengths. The (Z)-isomer binds HSO4− and SO42− with different affinities, but 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexes are formed in both cases. In contrast, the (E)-isomer forms a 1
1 complexes are formed in both cases. In contrast, the (E)-isomer forms a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex with HSO4−, while a distinct 2
2 complex with HSO4−, while a distinct 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 sandwich-type complex is formed in the presence of dianionic SO42− (see Scheme 1). Thus, the mode of sulfate binding not only depends on the configuration of the linker but also on the degree of protonation of the anion. The multiple parameters controlling the supramolecular assembly make this system highly dynamic, and bisCP thus represents a unique example of a switchable receptor whose binding mode can be reversibly controlled by two orthogonal stimuli.
2 sandwich-type complex is formed in the presence of dianionic SO42− (see Scheme 1). Thus, the mode of sulfate binding not only depends on the configuration of the linker but also on the degree of protonation of the anion. The multiple parameters controlling the supramolecular assembly make this system highly dynamic, and bisCP thus represents a unique example of a switchable receptor whose binding mode can be reversibly controlled by two orthogonal stimuli.
Results and discussion
Synthesis and photoswitching behaviour
The configurational isomers (E)- and (Z)-bisCP were synthesized independently by a TBTU-mediated coupling of the respective stiff-stilbene bis(carboxylic acid) with a known cyclopeptide containing one (2S,4S)-4-aminoproline subunit (Fig. S1 in the ESI†).8,16 The (E)-isomer of the stiff-stilbene bis(carboxylic acid) was prepared using an adapted protocol from Akbulatov et al.,17 while the (Z)-isomer was obtained using a procedure that some of us reported previously.12g Upon completion of the amide coupling reactions, the products were precipitated and purified by preparative HPLC. They were obtained in analytically pure form in yields of 51% for (E)-bisCP and 32% for (Z)-bisCP (Fig. S2–S7 in the ESI†).The photoswitching properties of the bis(cyclopeptides) were studied first by UV-vis spectroscopy. The absorbance spectrum of (E)-bisCP in 0.5 vol% H2O/DMSO18 (c = 10 μM) showed three maxima at λ = 290, 331 and 347 nm (Fig. 1A). When the solution was irradiated with 340 nm light, the maxima at λ = 331 and 347 nm decreased and slightly shifted bathochromically.19 Furthermore, an absorption shoulder band emerged around λ = 365 nm. These UV-vis spectral changes were indicative of the transformation of the stiff-stilbene (E)-isomer to the (Z)-isomer.9,10 The sample was irradiated until no further spectral changes were observed, i.e. the photostationary state (PSS) was reached. The reverse spectral changes were observed upon irradiation with 365 nm light to regenerate the (E)-bisCP isomer. In both cases, a clear isosbestic point was maintained at λ = 355 nm, indicating unimolecular conversion (Fig. S26 and S27 in the ESI†).
The photoisomerization process was additionally investigated by 1H NMR spectroscopy. Upon exposure of a solution of (E)-bisCP in 0.5 vol% H2O/DMSO-d6 (c = 0.5 mM) to 340 nm light, an additional set of proton signals became present, which could be assigned to (Z)-bisCP (Fig. 1B and S28 in the ESI†). For example, alongside the aromatic proton signals (doublet and singlet at δ = 8.47 and 7.94 ppm, red) characteristic of the stiff-stilbene (E)-isomer, two new aromatic proton signals (at δ = 8.40 and 8.23 ppm, blue) appeared. Integration of the 1H NMR signals at the point where PSS had been reached gave an isomer ratio of 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50. When the same sample was subsequently irradiated with 365 nm light, the proton signals of (Z)-bisCP disappeared and the original 1H NMR spectrum of (E)-bisCP was recovered, which is in line with what was observed by UV-vis spectroscopy.
50. When the same sample was subsequently irradiated with 365 nm light, the proton signals of (Z)-bisCP disappeared and the original 1H NMR spectrum of (E)-bisCP was recovered, which is in line with what was observed by UV-vis spectroscopy.
Sulfate complexation studies
The interaction between the bis(cyclopeptide) isomers and SO42− was initially studied by 1H NMR titrations in 0.5 vol% H2O/DMSO-d6.18 In the 1H NMR spectrum of (E)-bisCP, the most conclusive signals for monitoring sulfate complexation are the broad singlets of the NH protons (δ = 9.88–9.64 ppm, Fig. 2A), due to their active involvement in anion–hydrogen bonding. In addition, the H(α) protons of the L-proline unit, which give three broad triplets (δ = 5.75–5.52 ppm) in the uncomplexed form, are oriented close to the anion binding site and will be substantially deshielded upon interaction with sulfate.7a,8Following the stepwise addition of (TBA)2SO4 (TBA = tetrabutylammonium) to (E)-bisCP until 5 equiv., these two sets of signals gradually shifted downfield (to δ = 10.45–10.30 and to 6.21–6.00 ppm, respectively, see Fig. 2B and S8 in the ESI†), which suggests the formation of a complex with fast binding kinetics. Unexpectedly, at around 0.5 equiv. of SO42−, an additional set of signals became visible, including three sharp singlets for the NH protons, which were shifted even further downfield (δ = 12.19, 12.25 and 12.40 ppm), whereas the signals for the cyclopeptide H(α) protons were also further downfield (δ = 7.04–6.54 ppm). The chemical shift of these signals was almost unaffected by the (TBA)2SO4 concentration, indicating the formation of a species in slow exchange on the NMR timescale. Another (presumably more stable) complex thus additionally formed in the mixture. We hypothesized that the former shifting of signals could be due to complexation with HSO4− anions, likely formed by proton transfer from water molecules to (TBA)2SO4 due to the basicity of SO42− in DMSO.14 We ascribed the latter, more downfield shifted signal set to an SO42− complex, since the double negative charge should cause more pronounced proton deshielding and more efficient interactions with the bis(cyclopeptide). These assignments were supported by the fact that the use of TBA(HSO4) instead of (TBA)2SO4 caused the relative amount of the presumed SO42− complex at the end of the titration to decrease from 35% to 28% (Fig. S9 in the ESI†). Furthermore, this relative amount slightly dropped beyond the addition of 1 equiv., which could be an indication that the SO42− and HSO4− complexes of (E)-bisCP have distinct stoichiometry (Fig. S10 in the ESI†). Finally, modified Job plot analysis using the gradually shifted signals hinted at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (E)-bisCP/HSO4− complex stoichiometry (see Fig. S10 in the ESI†).
2 (E)-bisCP/HSO4− complex stoichiometry (see Fig. S10 in the ESI†).
An analogous observation was made when the non-substituted parent cyclopeptide (CP) as a model compound was titrated with TBA(HSO4) to also afford a mixture of fast- and slow-exchanging complexes of which the relative ratio changed throughout the titration. In this case, modified Job plot analysis using the gradually shifted signals was in closer agreement with the monotopic cyclopeptide's expected 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexation (Fig. S20 and S21 in the ESI†). Notably, when either (E)-bisCP or the parent CP was titrated with an exclusively mono-anionic guest, i.e. bromide, only gradual downfield shifting of the 1H NMR signals was observed (Fig. S16 and S22,† respectively). Moreover, these chemical shift changes were very similar to those allocated to HSO4− complex formation. Fitting of this titration data to 1
1 complexation (Fig. S20 and S21 in the ESI†). Notably, when either (E)-bisCP or the parent CP was titrated with an exclusively mono-anionic guest, i.e. bromide, only gradual downfield shifting of the 1H NMR signals was observed (Fig. S16 and S22,† respectively). Moreover, these chemical shift changes were very similar to those allocated to HSO4− complex formation. Fitting of this titration data to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 models for (E)-bisCP and parent CP, respectively, afforded a log
1 models for (E)-bisCP and parent CP, respectively, afforded a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka of ∼3 for each bromide binding event (Fig. S17 and S23†).
Ka of ∼3 for each bromide binding event (Fig. S17 and S23†).
Further evidence for the existence of protonation-dependent complexes was obtained by performing acid–base titrations. To this end, an aliquot (0.5 mL) of a solution of (E)-bisCP in the presence of 5 equiv. of (TBA)2SO4 was taken and titrated with minute quantities of H2SO4 (50 mM) (Fig. 2C and S11 in the ESI†). This titration eventually led to exclusive formation of the presumed HSO4− complex at the expense of the SO42− complex. In a separate experiment, small amounts of N,N-diisopropyl-ethylamine (DIPEA) were added to the (E)-bisCP/(TBA)2SO4 mixture, which caused the disappearance of all peaks related to the HSO4− complex in favour of the peaks belonging to the SO42− complex (Fig. 2D and S12 in the ESI†). In addition, when the entire 1H NMR titration of (E)-bisCP with (TBA)2SO4 was performed in the presence of 0.05 vol% of DIPEA, only the signals of the slow-exchanging species, assigned to the SO42− complex, were observed (Fig. S13 in the ESI†). Importantly, this titration saturated at a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of (E)-bisCP and the SO42− dianion.
1 stoichiometry of (E)-bisCP and the SO42− dianion.
1H NMR titrations in which TBAHSO4 or TBA2SO4 was added to a solution of (Z)-bisCP were also performed (as previously, the titration with TBA2SO4 contained additional 0.05 vol% DIPEA to ensure complete deprotonation of SO42−). In both titrations, the broad singlet signals of the NH protons shifted downfield (from δ = 9.75–9.50 ppm to δ = 12.38–12.08) and appeared as three sharp singlets (Fig. S14 and S15 in the ESI†). Analogously, the broad signals of the H(α) proton of the L-proline unit shifted downfield (from δ = 5.75–5.50 ppm to δ = 6.75–6.55 ppm). These chemical shifts confirm the complexation of both anions. Furthermore, the titrations saturated at 1 equiv. of anion and the exchange was slow on the NMR timescale, suggesting efficient 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding due to the good preorganization of the receptor.
1 binding due to the good preorganization of the receptor.
To determine what type of complex formed, ESI mass spectra of solutions of (E)-bisCP and (Z)-bisCP in 5 vol% H2O/CH3OH containing 1 equiv. of Na2SO4 were recorded.19 The spectrum of (Z)-bisCP in the negative mode featured mainly one signal whose m/z ratio and isotopic pattern were consistent with the expected doubly charged 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (Z)-bisCP·SO42− (m/z calcd 856.79, exp. 856.79, see Fig. S24 in the ESI†). In addition, a weak signal was visible at m/z = 1735.41, which could be attributed to the singly charged sodium adduct of the 1
1 complex (Z)-bisCP·SO42− (m/z calcd 856.79, exp. 856.79, see Fig. S24 in the ESI†). In addition, a weak signal was visible at m/z = 1735.41, which could be attributed to the singly charged sodium adduct of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex (m/z calcd 1735.56, exp. 1735.41). The ESI mass spectrum of (E)-bisCP also contained a major signal at m/z = 856.79. However, in contrast to the spectrum of (Z)-bisCP, the individual lines were separated by only 0.25 m/z units, indicating that this signal corresponded to a fourfold negatively charged complex with the composition [(E)-bisCP·SO42−]2 (Fig. S25 in the ESI†). A smaller signal in the spectrum appeared at the m/z ratio of the corresponding triply charged sodium adduct.
1 complex (m/z calcd 1735.56, exp. 1735.41). The ESI mass spectrum of (E)-bisCP also contained a major signal at m/z = 856.79. However, in contrast to the spectrum of (Z)-bisCP, the individual lines were separated by only 0.25 m/z units, indicating that this signal corresponded to a fourfold negatively charged complex with the composition [(E)-bisCP·SO42−]2 (Fig. S25 in the ESI†). A smaller signal in the spectrum appeared at the m/z ratio of the corresponding triply charged sodium adduct.
These mass spectra confirm that the convergent arrangement of the two cyclopeptide rings in (Z)-bisCP allowed them to engage simultaneously in anion binding, as in other bis(cyclopeptides) containing flexible linkers.8,20 Unexpectedly, however, the divergent arrangement in (E)-bisCP induced by the rigid stiff-stilbene linker did not prevent the cyclopeptides from forming sandwich complexes, but gave rise to a previously unobserved binding mode in which two (E)-bisCP units bridged two anions.
DFT calculations were performed to visualize the arrangements of the bis(cyclopeptides) in these complexes. These calculations were based on the crystal structures of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 sandwich complex between iodide and CP as well as the 1
2 sandwich complex between iodide and CP as well as the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sulfate complex of a bis(cyclopeptide) with a flexible linker.7b,21 As can be seen in Fig. 3, the stiff-stilbene linker in (Z)-bisCP induces an orientation of the two cyclopeptide rings that allows them to simultaneously bind the sulfate anion in an apparently strain-free manner, resulting in a complex structure that is closely related to that observed for another bis(cyclopeptide).21 In (E)-bisCP, on the other hand, the formation of the characteristic sandwich-type complexes requires two stiff-stilbene linked bis(cyclopeptide) molecules to come together. In the corresponding 2
1 sulfate complex of a bis(cyclopeptide) with a flexible linker.7b,21 As can be seen in Fig. 3, the stiff-stilbene linker in (Z)-bisCP induces an orientation of the two cyclopeptide rings that allows them to simultaneously bind the sulfate anion in an apparently strain-free manner, resulting in a complex structure that is closely related to that observed for another bis(cyclopeptide).21 In (E)-bisCP, on the other hand, the formation of the characteristic sandwich-type complexes requires two stiff-stilbene linked bis(cyclopeptide) molecules to come together. In the corresponding 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex, the two stiff-stilbene units are aligned almost perfectly. Aromatic stacking interactions, reinforced by the desolvation of the hydrophobic surfaces, are therefore likely to contribute to the stability of the 2
2 complex, the two stiff-stilbene units are aligned almost perfectly. Aromatic stacking interactions, reinforced by the desolvation of the hydrophobic surfaces, are therefore likely to contribute to the stability of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex.
2 complex.
Since the complexation kinetics of both these SO42− complexes were slow on the NMR timescale, we resorted to isothermal titration calorimetry (ITC) to quantify their stability. Therefore, a solution of TBAHSO4 in 0.5 vol% H2O/0.05 vol% DIPEA/DMSO was titrated to a solution of the bis(cyclopeptides) in the same solvent mixture. As described for the 1H NMR experiments, the presence of the base ensures that only the SO42− complexes can form in solution. In the case of (E)-bisCP, exothermic heat pulses were observed, and a steep binding isotherm suggested strong and highly cooperative binding. Fitting this isotherm to the mathematical model of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex gave an inflection point n of 0.8, close to the inflection point of 1 expected for a 2
1 complex gave an inflection point n of 0.8, close to the inflection point of 1 expected for a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex (Fig. S39 in the ESI†). A reference measurement using the monotopic unsubstituted cyclopeptide as host resulted in a similar isotherm, but this time with an inflection point at 0.5, indicative of the formation of a sandwich-type 2
2 complex (Fig. S39 in the ESI†). A reference measurement using the monotopic unsubstituted cyclopeptide as host resulted in a similar isotherm, but this time with an inflection point at 0.5, indicative of the formation of a sandwich-type 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 host–guest complex. Due to the high steepness of the binding isotherms and, in the case of (E)-bisCP, the complexity of the 2
1 host–guest complex. Due to the high steepness of the binding isotherms and, in the case of (E)-bisCP, the complexity of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 binding model, the stability constants could not be derived. Moreover, competitive titrations were not performed because isotherms afforded by titrations of other anions (halides and nitrate) could not be fitted. Nonetheless, the isotherms for the titration with SO42− still provide evidence that the interaction with (E)-bisCP is very efficient (log
2 binding model, the stability constants could not be derived. Moreover, competitive titrations were not performed because isotherms afforded by titrations of other anions (halides and nitrate) could not be fitted. Nonetheless, the isotherms for the titration with SO42− still provide evidence that the interaction with (E)-bisCP is very efficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka > 7).
Ka > 7).
The titration with (Z)-bisCP under similar conditions also gave a steep binding isotherm with an inflection point of 0.8. This time, however, we were able to first determine the stability of the Br− complex of the bis(cyclopeptide) by using TBABr as guest salt (Fig. S40 in the ESI†). The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka of 4.1 hence obtained by ITC was in excellent agreement with that determined independently by an NMR titration (Fig. S18 and S19 in the ESI†). This stability constant allowed us to perform a competitive ITC titration by adding TBA2SO4 to the Br− complex of (Z)-bisCP (Fig. S40 in the ESI†). The resulting isotherm was fitted to the one-site binding model, yielding a log
Ka of 4.1 hence obtained by ITC was in excellent agreement with that determined independently by an NMR titration (Fig. S18 and S19 in the ESI†). This stability constant allowed us to perform a competitive ITC titration by adding TBA2SO4 to the Br− complex of (Z)-bisCP (Fig. S40 in the ESI†). The resulting isotherm was fitted to the one-site binding model, yielding a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka of 9.0 for the SO42− complex of (Z)-bisCP, which was slightly larger than the log
Ka of 9.0 for the SO42− complex of (Z)-bisCP, which was slightly larger than the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ka observed for a doubly-linked bis(cyclopeptide) in 67 vol% acetonitrile/water.22
Ka observed for a doubly-linked bis(cyclopeptide) in 67 vol% acetonitrile/water.22
We also attempted to quantify the stability of the HSO4− complexes by titrating a solution of TBAHSO4 in 0.5 vol% H2O/DMSO into solutions of (E)- and (Z)-bisCP in the same solvent mixture. For both isomers, weak heat pulses were observed, which in the case of (E)-bisCP resulted in a flat binding isotherm that could not be reliably fitted (Fig. S41 in the ESI†). In the case of (Z)-bisCP, the binding isotherm was discontinuous. The difficulties encountered in these measurements might be due to the additional acid–base equilibrium between SO42− and HSO4−. Nonetheless, the significantly different shapes of the observed binding isotherms provided strong evidence that the HSO4− complexes of both bis(cyclopeptides) were significantly less stable than the SO42− complexes. This lower stability is reasonable, since HSO4− exhibits a lower charge and repulsive interactions can occur between NH groups and the proton on the anion.
Light-controlled complexation
As shown above, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexation modes of (E)-bisCP can be accessed by addition of acid and base, respectively, while (Z)-bisCP is always a 1
2 complexation modes of (E)-bisCP can be accessed by addition of acid and base, respectively, while (Z)-bisCP is always a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complexed species, regardless of the sulfate protonation state. We additionally sought to isomerize the bis(cyclopeptide) in the presence of HSO4− and SO42− such that the 1
1 complexed species, regardless of the sulfate protonation state. We additionally sought to isomerize the bis(cyclopeptide) in the presence of HSO4− and SO42− such that the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes would interconvert with the 1
2 complexes would interconvert with the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex in situ (see Scheme 1). Hence, the effect of irradiation on the NH and proline H(α) proton resonances, which are most sensitive to the configuration of the bis(cyclopeptide) and their interaction with anions, was studied by 1H NMR spectroscopy. As described above, the signals of these protons appear between δ = 10.5 and 10.3 ppm (NH) and between δ = 6.3 and 6.0 ppm [H(α)] in the spectrum of a solution of (E)-bisCP in 0.5 vol% H2O/DMSO-d6 (0.5 mM) containing 5 equiv. of TBAHSO4 (Fig. 4A). Under these conditions, the 1
1 complex in situ (see Scheme 1). Hence, the effect of irradiation on the NH and proline H(α) proton resonances, which are most sensitive to the configuration of the bis(cyclopeptide) and their interaction with anions, was studied by 1H NMR spectroscopy. As described above, the signals of these protons appear between δ = 10.5 and 10.3 ppm (NH) and between δ = 6.3 and 6.0 ppm [H(α)] in the spectrum of a solution of (E)-bisCP in 0.5 vol% H2O/DMSO-d6 (0.5 mM) containing 5 equiv. of TBAHSO4 (Fig. 4A). Under these conditions, the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [(E)-bisCP·(HSO4−)2] complex was predominantly present. When this solution was irradiated with 340 nm light, a reduction in peak intensity of these characteristic signals was observed as the bisCP switched from the (E)- to the (Z)-configuration. At the same time, three new sharp singlets appeared at δ = 12.31, 12.18, and 12.16 ppm, which correspond to the NH proton signals of (Z)-bisCP·HSO4− (Fig. 4B and S29 in the ESI†). Integration of the different 1H NMR signal sets gave a PSS340 (E/Z) ratio of 46
2 [(E)-bisCP·(HSO4−)2] complex was predominantly present. When this solution was irradiated with 340 nm light, a reduction in peak intensity of these characteristic signals was observed as the bisCP switched from the (E)- to the (Z)-configuration. At the same time, three new sharp singlets appeared at δ = 12.31, 12.18, and 12.16 ppm, which correspond to the NH proton signals of (Z)-bisCP·HSO4− (Fig. 4B and S29 in the ESI†). Integration of the different 1H NMR signal sets gave a PSS340 (E/Z) ratio of 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 54, which is thus minimally affected by the presence of the anion [i.e. PSS340 (E/Z) = 50
54, which is thus minimally affected by the presence of the anion [i.e. PSS340 (E/Z) = 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 in the absence of HSO4−, see above]. Next, the solution was irradiated at 365 nm to reverse the configurational change (Fig. 4C). Upon this sequential irradiation, the NH signals of the 1
50 in the absence of HSO4−, see above]. Next, the solution was irradiated at 365 nm to reverse the configurational change (Fig. 4C). Upon this sequential irradiation, the NH signals of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [(Z)-bisCP·HSO4−] complex disappeared almost fully, while the NH proton signals of the 1
1 [(Z)-bisCP·HSO4−] complex disappeared almost fully, while the NH proton signals of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [(E)-bisCP·(HSO4−)2] complex regained their original intensity. These results show that 1
2 [(E)-bisCP·(HSO4−)2] complex regained their original intensity. These results show that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 HSO4− complexes of (E)-bisCP and (Z)-bisCP, respectively, can be successfully interconverted by light.
1 HSO4− complexes of (E)-bisCP and (Z)-bisCP, respectively, can be successfully interconverted by light.
Next, we investigated the effect of irradiation on the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [(E)-bisCP2·(SO42−)2] complex. To this end, a solution of (E)-bisCP in a mixture of 0.5% vol% H2O/0.05% vol% DIPEA/DMSO-d6 (0.5 mM) in the presence of 5 equiv. of TBA2SO4 was prepared. When the sample was irradiated with 340 nm light at 20 °C, little E-to-Z isomerization was observed. Instead, new signals appeared in the 1H NMR spectrum (Fig. S30 in the ESI†), which became more pronounced when the irradiation wavelength was changed to 365 nm. The same signals appeared when a sample of the 1
2 [(E)-bisCP2·(SO42−)2] complex. To this end, a solution of (E)-bisCP in a mixture of 0.5% vol% H2O/0.05% vol% DIPEA/DMSO-d6 (0.5 mM) in the presence of 5 equiv. of TBA2SO4 was prepared. When the sample was irradiated with 340 nm light at 20 °C, little E-to-Z isomerization was observed. Instead, new signals appeared in the 1H NMR spectrum (Fig. S30 in the ESI†), which became more pronounced when the irradiation wavelength was changed to 365 nm. The same signals appeared when a sample of the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [(Z)-bisCP·(SO42−)] complex was irradiated at 365 nm. Here, irradiation was continued until the 1H NMR spectrum indicated complete conversion (Fig. S35 in the ESI†). A mass spectrum (positive mode) was then recorded in which a peak at m/z = 825.31 indicated the presence of monotopic peptides containing an appended indanone residue (Fig. S36 and S37 in the ESI†). This residue is known to be formed during photo-oxidation of stiff-stilbene,23 and the bis(cyclopeptide) was therefore apparently cleaved at the double bond upon irradiation.
1 [(Z)-bisCP·(SO42−)] complex was irradiated at 365 nm. Here, irradiation was continued until the 1H NMR spectrum indicated complete conversion (Fig. S35 in the ESI†). A mass spectrum (positive mode) was then recorded in which a peak at m/z = 825.31 indicated the presence of monotopic peptides containing an appended indanone residue (Fig. S36 and S37 in the ESI†). This residue is known to be formed during photo-oxidation of stiff-stilbene,23 and the bis(cyclopeptide) was therefore apparently cleaved at the double bond upon irradiation.
We hypothesized that isomerization of the SO42− complexes is impeded because of their high stabilities. Hence, to weaken the binding interactions, irradiation was performed additionally at 160 °C (Fig. 5 and S32 in the ESI†). When the solution of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [(E)-bisCP2·(SO42−)2] complex was irradiated with 340 nm light, the respective NH proton signals shifted from δ = 12.40, 12.26, and 12.20 ppm to δ = 12.30, 12.18, and 12.16 ppm as a sign of conversion to the 1
2 [(E)-bisCP2·(SO42−)2] complex was irradiated with 340 nm light, the respective NH proton signals shifted from δ = 12.40, 12.26, and 12.20 ppm to δ = 12.30, 12.18, and 12.16 ppm as a sign of conversion to the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 [(Z)-bisCP·SO42−] complex (Fig. 5A and B). Eventually, a PSS was reached with a ratio of 38
1 [(Z)-bisCP·SO42−] complex (Fig. 5A and B). Eventually, a PSS was reached with a ratio of 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 62 (E/Z), which is significantly higher than the 50
62 (E/Z), which is significantly higher than the 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 ratio obtained for the uncomplexed bis(cyclopeptide). Consecutive irradiation of the PSS340 mixture with 365 nm light resulted in recovery of the 2
50 ratio obtained for the uncomplexed bis(cyclopeptide). Consecutive irradiation of the PSS340 mixture with 365 nm light resulted in recovery of the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 [(E)-bisCP2·(SO42−)2] complex, although partial decomposition was observed (∼4%, signals at δ = 12.55, 12.29, and 12.09 ppm, Fig. 5C). It should be noted that in the presence of a weaker binding anion such as bromide, seamless photoswitching was observed (Fig. S31 in the ESI†). The observed decomposition at lower temperature to the indanone product (Fig. S30, S33 and S34†) thus indeed seems to be due to the tight binding of SO42−.
2 [(E)-bisCP2·(SO42−)2] complex, although partial decomposition was observed (∼4%, signals at δ = 12.55, 12.29, and 12.09 ppm, Fig. 5C). It should be noted that in the presence of a weaker binding anion such as bromide, seamless photoswitching was observed (Fig. S31 in the ESI†). The observed decomposition at lower temperature to the indanone product (Fig. S30, S33 and S34†) thus indeed seems to be due to the tight binding of SO42−.
Overall, the above findings confirm that switching between the 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 sandwich complex and the 1
2 sandwich complex and the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex is feasible, even though traces of the indanone product were detected when (Z)-bisCP·SO42− was irradiated back to [(E)-bisCP·SO42−]2 at 160 °C. Advantageously, photoisomerization not only becomes viable by raising the temperature, but also by protonation of the SO42− dianion since conversion in the presence of the HSO4− anion was shown to be clean. Accordingly, interconversion between the [(E)-bisCP·SO42−]2 and (Z)-bisCP·SO42− complex is possible by first protonating the anion, followed by irradiation at 20 °C and subsequent addition of base to deprotonate the anion. The different switching pathways are summarized in Scheme 1.
1 complex is feasible, even though traces of the indanone product were detected when (Z)-bisCP·SO42− was irradiated back to [(E)-bisCP·SO42−]2 at 160 °C. Advantageously, photoisomerization not only becomes viable by raising the temperature, but also by protonation of the SO42− dianion since conversion in the presence of the HSO4− anion was shown to be clean. Accordingly, interconversion between the [(E)-bisCP·SO42−]2 and (Z)-bisCP·SO42− complex is possible by first protonating the anion, followed by irradiation at 20 °C and subsequent addition of base to deprotonate the anion. The different switching pathways are summarized in Scheme 1.
Conclusion
A bis(cyclopeptide) containing a rigid stiff-stilbene linker can be isomerized by light between its (E)- and (Z)-configuration. In the (Z)-form, this compound forms 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 sandwich-type complexes with hydrogen sulfate (HSO4−) as well as the sulfate dianion (SO42−) via simultaneous interaction with both cyclopeptide anion-binding moieties. In the (E)-form, in which the cyclo-peptides are further away from each other, 1
1 sandwich-type complexes with hydrogen sulfate (HSO4−) as well as the sulfate dianion (SO42−) via simultaneous interaction with both cyclopeptide anion-binding moieties. In the (E)-form, in which the cyclo-peptides are further away from each other, 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 receptor/HSO4− complexation was observed, while with SO42− a 2
2 receptor/HSO4− complexation was observed, while with SO42− a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complex was found. Photoisomerization allows interconversion between the respective 1
2 complex was found. Photoisomerization allows interconversion between the respective 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/1
1/1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 1
2 and 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1/2
1/2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexes for HSO4− and SO42−. Protonation of the SO42− dianion, which weakens the complex, provides an attractive orthogonal stimulus to switch between the 1
2 complexes for HSO4− and SO42−. Protonation of the SO42− dianion, which weakens the complex, provides an attractive orthogonal stimulus to switch between the 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 and 2
2 and 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 complexed states. Our results demonstrate how the structure and composition of anion–ligand complexes can be controlled by two types of stimuli. We predict that in the future different (and more sophisticated) types of nanostructures can be accessed by introducing other anions. The presented dual-stimulus control would then allow, for example, the encapsulation and release of cargo molecules or activation of catalysis on demand. Work in this direction is currently ongoing in our laboratories.
2 complexed states. Our results demonstrate how the structure and composition of anion–ligand complexes can be controlled by two types of stimuli. We predict that in the future different (and more sophisticated) types of nanostructures can be accessed by introducing other anions. The presented dual-stimulus control would then allow, for example, the encapsulation and release of cargo molecules or activation of catalysis on demand. Work in this direction is currently ongoing in our laboratories.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
S. M., B. W., S. K., and S. J. W. conceived the project. S. M. and B. W. synthesized the materials. S. M. carried out the UV-vis and 1H NMR photoisomerization studies. S. M. and J. E. B. conducted the 1H NMR titrations. B. W. prepared the ESI mass spectra in the aqueous solvent mixture and performed the ITC experiments. S. K. performed the DFT calculations. S. M., S. K. and S. J. W. wrote the manuscript. The project was guided by S. K. and S. J. W.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from the European Research Council (Starting Grant no. 802830 to S. J. W.) and the Dutch Research Council (NWO-ENW, Vidi Grant no. VI.Vidi.192.049 to S. J. W.) is gratefully acknowledged. We thank M. Luczak in the group of G. Niedner-Schatteburg at RPTU for help with the ESI-MS spectra.Notes and references
- (a) M. Yoshizawa, J. K. Klosterman and M. Fujita, Angew. Chem., Int. Ed., 2009, 48, 3418–3438 CrossRef CAS; (b) R. Chakrabarty, P. S. Mukherjee and P. J. Stang, Chem. Rev., 2011, 111, 6810–6918 CAS; (c) M. M. J. Smulders, I. A. Riddell, C. Browne and J. R. Nitschke, Chem. Soc. Rev., 2013, 42, 1728–1754 CAS.
- (a) A. J. McConnell, C. S. Wood, P. P. Neelakandan and J. R. Nitschke, Chem. Rev., 2015, 115, 7729–7793 Search PubMed; (b) W. Wang, Y.-X. Wang and H.-B. Yang, Chem. Soc. Rev., 2016, 45, 2656–2693 Search PubMed.
- (a) S. J. Wezenberg, Chem. Lett., 2020, 49, 609–615 Search PubMed; (b) E. Benchimol, J. Tessarolo and G. H. Clever, Nat. Chem., 2024, 16, 13–21 Search PubMed.
- (a) S.-S. Sun, J. A. Anspach and A. J. Lees, Inorg. Chem., 2002, 41, 1862–1869 CrossRef CAS PubMed; (b) S. Chen, L.-J. Chen, H.-B. Yang, H. Tian and W. Zhu, J. Am. Chem. Soc., 2012, 134, 13596–13599 Search PubMed; (c) M. Han, R. Michel, B. He, Y.-S. Chen, D. Stalke, M. John and G. H. Clever, Angew. Chem., Int. Ed., 2013, 52, 1319–1323 Search PubMed; (d) X. Yan, J.-F. Xu, T. R. Cook, F. Huang, Q.-Z. Yang, C.-H. Tung and P. J. Stang, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 8717–8722 Search PubMed; (e) M. Han, Y. Luo, B. Damaschke, L. Gómez, X. Ribas, A. Jose, P. Peretzki, M. Seibt and G. H. Clever, Angew. Chem., Int. Ed., 2016, 55, 445–449 CAS; (f) C. Stuckhardt, D. Roke, W. Danowski, E. Otten, S. J. Wezenberg and B. L. Feringa, Beilstein J. Org. Chem., 2019, 15, 2767–2773 CAS; (g) A. D. W. Kennedy, R. G. DiNardi, L. L. Fillbrook, W. A. Donald and J. E. Beves, Chem.–Eur. J., 2022, 28, e202104461 Search PubMed; (h) R. G. DiNardi, A. O. Douglas, R. Tian, J. R. Price, M. Tajik, W. A. Donald and J. E. Beves, Angew. Chem., Int. Ed., 2022, 61, e202205701 Search PubMed; (i) D. Hugenbusch, M. Lehr, J.-S. von Glasenapp, A. J. McConnell and R. Herges, Angew. Chem., Int. Ed., 2023, 62, e202212571 CAS; (j) J. Zhu, X. Chen, X. Jin and Q. Wang, Chin. Chem. Lett., 2023, 34, 108002 CrossRef CAS; (k) R. G. DiNardi, S. Rasheed, S. S. Capomolla, M. Him Chack, I. A. Middleton, L. K. Macreadie, J. P. Violi, W. A. Donald, P. J. Lusby and J. E. Beves, J. Am. Chem. Soc., 2024, 146, 21196–21202 CrossRef PubMed.
- For review articles, see: (a) N. Busschaert, C. Caltagirone, W. Van Rossom and P. A. Gale, Chem., Rev., 2015, 115, 8038–8155 CAS; (b) D. Yang, J. Zhao, X.-J. Yang and B. Wu, Org. Chem. Front., 2018, 5, 662–690 CAS; (c) J. Zhao, D. Yang, X.-J. Yang and B. Wu, Coord. Chem. Rev., 2019, 378, 415–444 Search PubMed; (d) S. A. Boer, E. M. Foyle, C. M. Thomas and N. G. White, Chem. Soc. Rev., 2019, 48, 2596–2614 Search PubMed; (e) W. Zhang, J. Zhao and D. Yang, ChemPlusChem, 2022, 87, e202200294 CAS.
- For selected examples, see: (a) J. Sánchez-Quesada, C. Seel, P. Prados, J. de Mendoza, I. Dalcol and E. Giralt, J. Am. Chem. Soc., 1996, 118, 277–278 Search PubMed; (b) J. Keegan, P. E. Kruger, M. Nieuwenhuyzen, J. O'Brien and N. Martin, Chem. Commun., 2001, 2192–2193 RSC; (c) S. Li, C. Jia, B. Wu, Q. Luo, X. Huang, Z. Yang, Q.-S. Li and X.-J. Yang, Angew. Chem., Int. Ed., 2011, 50, 5721–5724 CAS; (d) B. Wu, F. Cui, Y. Lei, S. Li, N. de Sousa Amadeu, C. Janiak, Y.-J. Lin, L.-H. Weng, Y.-Y. Wang and X.-J. Yang, Angew. Chem., Int. Ed., 2013, 52, 5096–5100 CAS; (e) J. Zhao, D. Yang, Y. Zhao, X.-J. Yang, Y.-Y. Wang and B. Wu, Angew. Chem., Int. Ed., 2014, 53, 6632–6636 CrossRef CAS; (f) H.-G. Jeon, H. B. Jang, P. Kang, Y. R. Choi, J. Kim, J. H. Lee, M.-G. Choi and K.-S. Jeong, Org. Lett., 2016, 18, 4404–4407 CrossRef CAS PubMed; (g) W. Zhang, D. Yang, J. Zhao, L. Hou, J. L. Sessler, X.-J. Yang and B. Wu, J. Am. Chem. Soc., 2018, 140, 5248–5256 CAS; (h) X. Bai, C. Jia, Y. Zhao, D. Yang, S.-C. Wang, A. Li, Y.-T. Chan, Y.-Y. Wang, X.-J. Yang and B. Wu, Angew. Chem., Int. Ed., 2018, 57, 1851–1855 CAS; (i) E. M. Fatila, M. Pink, E. B. Twum, J. A. Karty and A. H. Flood, Chem. Sci., 2018, 9, 2863–2872 CAS; (j) T. S. C. MacDonald, B. L. Feringa, W. S. Price, S. J. Wezenberg and J. E. Beves, J. Am. Chem. Soc., 2020, 142, 20014–20020 Search PubMed.
- (a) S. Kubik, R. Kirchner, D. Nolting and J. Seidel, J. Am. Chem. Soc., 2002, 124, 12752–12760 Search PubMed; (b) S. Kubik, Acc. Chem. Res., 2017, 50, 2870–2878 CAS.
- F. Sommer, Y. Marcus and S. Kubik, ACS Omega, 2017, 2, 3669–3680 Search PubMed.
- (a) T. Shimasaki, S.-i. Kato, K. Ideta, K. Goto and T. Shinmyozu, J. Org. Chem., 2007, 72, 1073–1087 Search PubMed; (b) S. J. Wezenberg and B. L. Feringa, Org. Lett., 2017, 19, 324–327 CAS; (c) S. J. Wezenberg, K.-J. Chen, J. E. Bos, B. L. Feringa, E. N. W. Howe, X. Wu, M. A. Siegler and P. A. Gale, J. Am. Chem. Soc., 2022, 144, 331–338 CrossRef CAS; (d) S. J. Wezenberg, Chem. Commun., 2022, 58, 11045–11058 RSC.
- (a) D. Villarón and S. J. Wezenberg, Angew. Chem., Int. Ed., 2020, 59, 13192–13202 CrossRef; (b) R. T. O'Neill, R. Boulatov and M. Photoswitches, Chemistry, Properties, and Applications, ed. Z. L. Pianowski, Wiley-VCH, Weinheim, 2022, pp. 253–281 Search PubMed.
- For literature reviews, see: (a) A. Lutolli and A. H. Flood, Molecular Photoswitches: Chemistry, Properties, and Applications, ed. Z. L. Pianowski, Wiley-VCH, Weinheim, 2022, pp. 542–563 Search PubMed; (b) J. de Jong, J. E. Bos and S. J. Wezenberg, Chem. Rev., 2023, 123, 8530–8574 CrossRef CAS.
- For selected examples, see: (a) Y. Hua and A. H. Flood, J. Am. Chem. Soc., 2010, 132, 12838–12840 CrossRef CAS PubMed; (b) S. J. Wezenberg, M. Vlatković, J. C. M. Kistemaker and B. L. Feringa, J. Am. Chem. Soc., 2014, 136, 16784–16787 CAS; (c) K. Dąbrowa, P. Niedbała and J. Jurczak, Chem. Commun., 2014, 50, 15748–15751 Search PubMed; (d) S. T. J. Ryan, J. del Barrio, R. Suardíaz, D. F. Ryan, E. Rosta and O. A. Scherman, Angew. Chem., Int. Ed., 2016, 55, 16096–16100 CAS; (e) Z. Kokan and M. J. Chmielewski, J. Am. Chem. Soc., 2018, 140, 16010–16014 CAS; (f) X. Chi, W. Cen, J. A. Queenan, L. Long, V. M. Lynch, N. M. Khashab and J. L. Sessler, J. Am. Chem. Soc., 2019, 141, 6468–6472 CrossRef CAS PubMed; (g) D. Villarón, M. A. Siegler and S. J. Wezenberg, Chem. Sci., 2021, 12, 3188–3193 RSC; (h) A. Kerckhoffs, I. Moss and M. J. Langton, Chem. Commun., 2023, 59, 51–54 RSC; (i) B. Shao, H. Fu and I. Aprahamian, Science, 2024, 385, 544–549 CrossRef CAS PubMed; (j) J. de Jong, M. A. Siegler and S. J. Wezenberg, Angew. Chem., Int. Ed., 2024, 63, e202316628 CrossRef CAS PubMed; (k) J. de Jong, M. A. Siegler and S. J. Wezenberg, Chem. Commun., 2025, 61, 2548–2551 Search PubMed.
- S. Xiong and Q. He, Chem. Commun., 2021, 57, 13514–13517 Search PubMed.
- I. M. Kolthoff and M. K. Chantooni Jr, J. Am. Chem. Soc., 1968, 90, 5961–5964 CAS.
- K. M. Bąk and M. J. Chmielewski, Eur. J. Org Chem., 2015, 4077–4080 Search PubMed.
- J. Bartl and S. Kubik, ChemPlusChem, 2020, 85, 963–969 CAS.
- S. Akbulatov, Y. Tian and R. Boulatov, J. Am. Chem. Soc., 2012, 134, 7620–7623 CAS.
- All spectroscopic investigations, binding studies, and photo-switching experiments were performed in DMSO containing 0.5 vol% H2O to ensure a constant water content of the solutions. This solvent mixture has been frequently used in anion binding studies, see references 5a and 9..
- While the bis(cyclopeptides) and their complexes were stable in H2O/CH3OH, photoisomerisation experiments in this solvent mixture showed signs of degradation..
- C. Reyheller, B. P. Hay and S. Kubik, New J. Chem., 2007, 31, 2095–2102 CAS.
- Z. Rodriguez-Docampo, S. I. Pascu, S. Kubik and S. Otto, J. Am. Chem. Soc., 2006, 128, 11206–11210 CAS.
- Z. Rodriguez-Docampo, E. Eugenieva-Ilieva, C. Reyheller, A. M. Belenguer, S. Kubik and S. Otto, Chem. Commun., 2011, 47, 9798–9800 CAS.
- M. P. O'Hagan, S. Haldar, M. Duchi, T. A. A. Oliver, A. J. Mulholland, J. C. Morales and M. C. Galan, Angew. Chem., Int. Ed., 2019, 58, 4334–4338 Search PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc00766f |
| ‡ Present address: Macromolecular Engineering Laboratory, ETH Zurich, Sonneggstrasse 3, 8092, Zurich, Switzerland |
| This journal is © The Royal Society of Chemistry 2025 |





