Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A new type of C2H2 binding site in a cis-bridging hexafluorosilicate ultramicroporous material that offers trace C2H2 capture†
Bai-Qiao
Song‡
 *a,
Mei-Yan
Gao‡
b,
Lisa
Mercene van Wyk
c,
Cheng-Hua
Deng
*a,
Mei-Yan
Gao‡
b,
Lisa
Mercene van Wyk
c,
Cheng-Hua
Deng
 b,
Alan C.
Eaby
b,
Alan C.
Eaby
 b,
Shi-Qiang
Wang
b,
Shi-Qiang
Wang
 b,
Shaza
Darwish
b,
Shaza
Darwish
 b,
Dan
Li
a,
Shao-Jie
Qin
a,
Yun-Lei
Peng
*d,
Qing-Yuan
Yang
b,
Dan
Li
a,
Shao-Jie
Qin
a,
Yun-Lei
Peng
*d,
Qing-Yuan
Yang
 e,
Leonard J.
Barbour
e,
Leonard J.
Barbour
 c and
Michael J.
Zaworotko
c and
Michael J.
Zaworotko
 *b
*b
aCollege of Materials and Chemistry & Chemical Engineering, Chengdu University of Technology, Chengdu 610059, China. E-mail: bqsong@cdut.edu.cn
bDepartment of Chemical Sciences and Bernal Institute, University of Limerick, Limerick V94 T9PX, Republic of Ireland. E-mail: xtal@ul.ie
cDepartment of Chemistry and Polymer Science, University of Stellenbosch, Matieland 7602, South Africa
dDepartment of Applied Chemistry, College of Science, China University of Petroleum-Beijing, Beijing 102249, China. E-mail: ylpeng@cup.edu.cn
eSchool of Chemical Engineering and Technology, Xi'an Jiaotong University, Xi'an 710049, China
First published on 23rd April 2025
Abstract
Hybrid ultramicroporous materials (HUMs) comprising hexafluorosilicate (SiF62−, SIFSIX) and their variants are promising physisorbents for trace acetylene (C2H2) capture and separation, where the inorganic anions serve as trans-bridging pillars. Herein, for the first time, we report a strategy of fluorine binding engineering in these HUMs via switching the coordination mode of SIFSIX from traditional trans to rarely explored cis. The first example of a rigid HUM involving cis-bridging SIFSIX, SIFSIX-bidmb-Cu (bidmb = 1,4-bis(1-imidazolyl)-2,5-dimethylbenzene), is reported. The resulting self-interpenetrated network is found to be water stable and exhibits strong binding to C2H2 but weak binding to C2H4 and CO2, affording a high Qst of 55.7 kJ mol−1 for C2H2, a high C2H2 uptake of 1.86 mmol g−1 at 0.01 bar and high ΔQst values. Breakthrough experiments comprehensively demonstrate that SIFSIX-bidmb-Cu can efficiently capture and recover C2H2 from 50/50 or 1/99 C2H2/CO2 and C2H2/C2H4 binary mixtures. In situ single crystal X-ray diffraction (SCXRD) combined with dispersion-corrected density functional theory (DFT-D) calculations reveals that the C2H2 binding site involves two cis-SiF62− anions in close proximity (F⋯F distance of 7.16 Å), creating a new type of molecular trap that affords six uncoordinated fluoro moieties to chelate each C2H2via sixfold C–H⋯F hydrogen bonds. This work therefore provides a new strategy for binding site engineering with selective C2H2 affinity to enable trace C2H2 capture.
Introduction
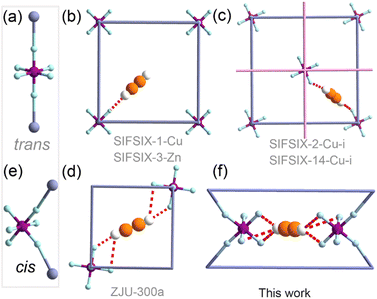
Acetylene (C2H2) is a high volume feedstock used for the production of various commodity chemicals.1 The production of C2H2 involves partial combustion of methane or thermal cracking of hydrocarbons, inevitably generating impurities, for example, carbon dioxide (CO2).2 As a result, purification of C2H2 is a needed prerequisite for its downstream use.3 Capture of trace C2H2 impurities from gas mixtures is also of importance: recovery of trace C2H2 is desirable in a circular economy and for safety, given that C2H2 is a flammable, explosive and toxic chemical;4 removal of trace C2H2 is a necessary procedure to produce polymer-grade ethylene (C2H4).5 Traditional purification technologies, such as organic solvent extraction (e.g., N,N-dimethylformamide or acetone) or catalytic partial hydrogenation using noble metal catalysts,6 have high energy footprints and costs for waste solvent disposal. New approaches for trace C2H2 capture and separation are therefore desirable.7Physisorption using porous materials is generally recognized as an efficient and energy-saving approach to C2H2 capture and separation.4,8 In this context, metal–organic materials such as metal–organic frameworks (MOFs)9 or coordination polymers (CPs)10 offer potential utility, thanks to their high surface area and modularity that allow for fine-tuning of the pore size, shape and chemistry.11–22 In this context, hybrid ultramicroporous materials (HUMs) composed of inorganic (e.g. SiF62− – SIFSIX) and organic linker ligands have been reported to show benchmark trace C2H2 capture performance, thanks to their sub-nanometer pore size (<0.7 nm). In these HUMs, the C2H2 molecules are bound to the pore surface via strong hydrogen bonds because of the relatively protic nature of the CH moieties of acetylene. For example, the strong C–H⋯F hydrogen bonds between C2H2 and the fluoro atom(s) of SiF62− (SIFSIX) anions23–25 exemplify how molecular recognition can drive selectivity towards C2H2.26–28
In such HUM materials, SIFSIX anions invariably adopt a trans-bridging coordination mode (Fig. 1a, abbreviated hereafter as trans-SIFSIX).29–32 C2H2 binding in C2H2 selective trans-SIFSIX HUMs can be classified into one of the three modes (Fig. 1b–d):33 a single C–H⋯F interaction (e.g., SIFSIX-1-Cu and SIFSIX-3);23 dual C–H⋯F interactions from two SIFSIX anions (e.g. SIFSIX-2-Cu-i and SIFSIX-14-Cu-i);24 quadruple C–H⋯F interactions from two SIFSIX anions involving bifurcated hydrogen bonds (e.g. ZJU-300a).33 The trans-bridging coordination geometry of the SIFSIX anion in fact limits the free (uncoordinated) fluoro sites accessible for each C2H2 molecule (up to two per anion, as shown in Fig. 1d).
Recently, the first cis-bridging coordination mode of SIFSIX anions was observed in a flexible HUM (Fig. 1e).34–36 This cis-bridging exposes four identically oriented fluoro moieties to the pore walls28 and promotes the possibility of formation of sixfold C–H⋯F interactions stemming from trifurcated hydrogen bonds (Fig. 1e and f). Herein, we report the first rigid HUM composed of cis-SIFSIX anions, [CuSiF6(bidmb)2]n (SIFSIX-bidmb-Cu, where bidmb = 1,4-bis(1-imidazol-yl)-2,5-dimethyl benzene), and its sorption performance for C2H2 and related sorbates. As detailed herein, SIFSIX-bidmb-Cu is water stable with a binding site involving six fluoro moieties that offer outstanding separation performance for 50/50 or 1/99 mixtures of C2H2/CO2 and C2H2/C2H4.
Results and discussion
Synthesis and crystal structures
Slow diffusion of a methanol solution of bidmb into a water solution of CuSiF6·6H2O after one month generated SIFSIX-bidmb-Cu as purple block crystals suitable for single-crystal X-ray diffraction (SCXRD). Microcrystalline powders can be produced by a direct mixing method (Fig. S1†). The purity of bulk samples was confirmed by powder X-ray diffraction (PXRD) (Fig. S2†).SCXRD analysis revealed that SIFSIX-bidmb-Cu crystallized in the triclinic space group P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (Table S1†). All Cu2+ cations adopt the same octahedral coordination geometry with four imidazolyl nitrogen atoms from four bidmb ligands at the equatorial positions and two fluorine atoms from SIFSIX at the axial positions (Fig. S3†). Cu2+ and bidmb generate the expected square lattice (sql) topology networks of formula Cu(bidmb)2 (Fig. 2a and b and S4†). These sql layers exhibit inclined interpenetration subtended by an angle of ca. 50° (Fig. 2c and d). SIFSIX anions crosslink the resulting networks through a cis-bridging mode to form a 3D self-catenated network with the simplified topological point symbol of 48.52.65 (Fig. 2e and f), a topology that was first observed in SIFSIX materials (Fig. S5†). In SIFSIX-bidmb-Cu, the cis-SIFSIX anions and Cu centers generate an undulating CuSIFSIX chain (Fig. S6a†). Hydrogen bonds between fluorine atoms and hydrogen atoms of bidmb (dF⋯H = 2.3–3.4 Å) are present (Fig. S7 and S8†).
(Table S1†). All Cu2+ cations adopt the same octahedral coordination geometry with four imidazolyl nitrogen atoms from four bidmb ligands at the equatorial positions and two fluorine atoms from SIFSIX at the axial positions (Fig. S3†). Cu2+ and bidmb generate the expected square lattice (sql) topology networks of formula Cu(bidmb)2 (Fig. 2a and b and S4†). These sql layers exhibit inclined interpenetration subtended by an angle of ca. 50° (Fig. 2c and d). SIFSIX anions crosslink the resulting networks through a cis-bridging mode to form a 3D self-catenated network with the simplified topological point symbol of 48.52.65 (Fig. 2e and f), a topology that was first observed in SIFSIX materials (Fig. S5†). In SIFSIX-bidmb-Cu, the cis-SIFSIX anions and Cu centers generate an undulating CuSIFSIX chain (Fig. S6a†). Hydrogen bonds between fluorine atoms and hydrogen atoms of bidmb (dF⋯H = 2.3–3.4 Å) are present (Fig. S7 and S8†).
The structure contains one dimensional (1D) channels parallel to the crystallographic b-axis with a void space of 23.1% as determined by PLATON (Fig. 2g–i)37 occupied by disordered MeOH and water molecules (Fig. S9†). The diameter of the 1D channel is ca. 4.6 Å (Fig. S10†) and is inclined relative to the CuSiF6 chain at an angle of 62° (Fig. S6b and c†). The connectivity and pore structure are therefore distinct from pcu topology SIFSIX materials in which 1D channels align parallel to MSIFSIX chains. The inclined structure herein results in SIFSIX anions exposed to the channel with F⋯F distances of 7.16 Å (Fig. S11†). The electrostatic potential (ESP) of SIFSIX-bidmb-Cu and other benchmark SIFSIX materials was calculated to investigate the difference in pore chemistry arising from the coordination mode of SIFSIX anions (trans vs. cis). The ESP was mapped onto the Connolly surface with a probe radius of 1.7 Å, and the same color gradation scale was used for comparison (Fig. 2j–n). Thanks to the strong electronegativity of fluorine atoms, the most negative electrostatic potential (ESP) is distributed in the region close to the SIFSIX anions in all SIFSIX materials. However, in SIFSIX-bidmb-Cu, the ESP is more negative than that of other SIFSIX materials.
In the first flexible cis-SIFSIX HUM, the sql layers are also composed of bis-imidazolyl ligands, but Cu centers are parallel to each other and pillared by cis-SIFSIX anions to form a pcu topology network.34–36 In SIFSIX-bidmb-Cu, sql layers are inclined to interpenetrate, reducing the pore size and changing the pore chemistry. HUMs formed by angular inorganic anions that pillar interpenetrated sql nets are unusual, but as exemplified by mmo nets containing tetrahedral anions (XO42−, where X = W and Mo)38 that bridge three sets of mutually perpendicular sql nets (Fig. S5†), exceptional sorption performance is possible.
Characterization of SIFSIX-bidmb-Cu
TGA analysis revealed that SIFSIX-bidmb-Cu is thermally stable up to 230 °C with guest molecules released by ca. 120 °C (Fig. S12†). Variable-temperature PXRD (VT-PXRD) data are consistent with SIFSIX-bidmb-Cu being a rigid sorbent (Fig. S13†). SCXRD analysis of the fully activated form, SIFSIX-bidmb-Cu′, also suggests rigidity (Table S1†).The permanent porosity of SIFSIX-bidmb-Cu′ was tested by N2 (77 K) and CO2 (195 K) sorption experiments. Both gases exhibited type-I sorption isotherms with saturated uptake values of 5.79 and 4.51 mmol g−1, respectively (Fig. 3a). Based on the 77 K N2 adsorption isotherm, the Langmuir and Brunauer–Emmett–Teller surface areas were calculated to be 543.9 and 480 m2 g−1, respectively (Fig. S14 and S15†). The calculated pore volume, 0.16 cm3 g−1, is close to the theoretical value (0.18 cm3 g−1) on the basis of SCXRD. To our knowledge, SIFSIX-bidmb-Cu is the first rigid HUM (and only the second SIFSIX material) comprising cis-SIFSIX anions.34–36
Single-component adsorption isotherms
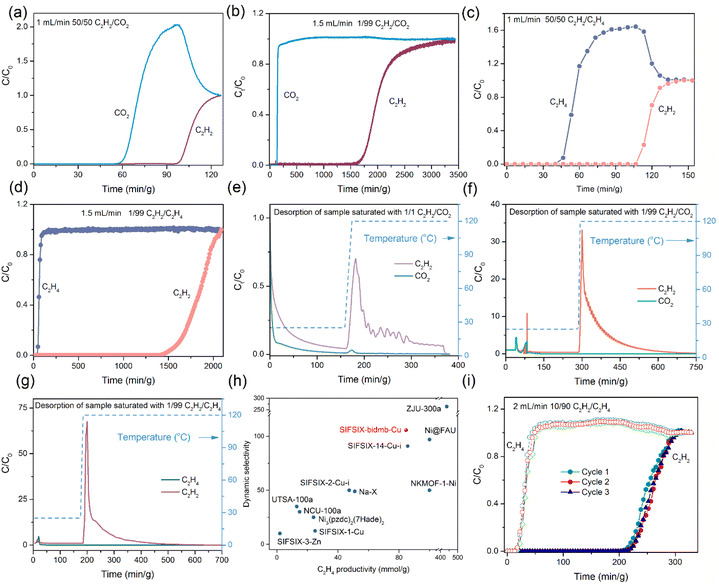
The single-component sorption isotherms of SIFSIX-bidmb-Cu′ for C2H2/CO2/C2H4 were collected at 298 and 273 K. As shown in Fig. 3b, each gas exhibits a type-I sorption isotherm at 298 K. The saturated uptake values of C2H2, CO2 and C2H4 at 1 bar were observed to be 2.65, 2.68, and 2.04 mmol g−1, respectively. Notably, SIFSIX-bidmb-Cu showed much steeper C2H2 uptake than CO2 and C2H4 at lower pressures (Fig. 3c). Substantially higher C2H2 uptake was exhibited at 0.01 bar (1.86 mmol g−1) than for CO2 (0.64 mmol g−1) and C2H4 (0.33 mmol g−1). The uptake of C2H2 at 0.01 bar, an indicator of the trace C2H2 capture ability of a sorbent, is higher than most SIFSIX materials (Fig. 3d and S16†), such as SIFSIX-1-Cu (0.45 mmol g−1),23 SIFSIX-3-Zn (0.85 mmol g−1),23 and SIFSIX-2-Cu-i (1.38 mmol g−1),23 comparable to the benchmark SIFSIX-14-Cu-i (1.83 mmol g−1),24 but is lower than ZJU-300a (3.23 mmol g−1).33 The PXRD patterns of SIFSIX-bidmb-Cu′ collected at 273 and 298 K at 1 bar C2H2 are consistent with that calculated for SIFSIX-bidmb-Cu (Fig. S17†), further suggesting a rigid structure. Consecutive C2H2 adsorption–desorption experiments conducted at 298 K indicated that adsorbed C2H2 was not fully removed under vacuum at room temperature (about 50% uptake remained), requiring heating to 120 °C (Fig. S18†). This provides evidence of the strong binding affinity for C2H2 and also suggests a method to capture and recover C2H2via temperature control. Interestingly, the C2H2-saturated sample was regenerated under vacuum at room temperature after exposure to air for one year (Fig. S19–S21†). In contrast, multiple cycles of sorption experiments on CO2 and C2H4 displayed negligible changes even if regeneration was implemented under high vacuum at room temperature (Fig. S22 and S23†), implying weaker interactions with SIFSIX-bidmb-Cu′. The time-dependent adsorption kinetics profiles of SIFSIX-bidmb-Cu′ were also investigated at 298 K (Fig. S24†). SIFSIX-bidmb-Cu′ exhibited a notably faster initial rate for C2H2 adsorption than CO2, reaching 41.7% and 11.3% of 1 bar uptake in 40 seconds for C2H2 and CO2, respectively. C2H2 adsorption reached equilibrium within 25 min, but CO2 adsorption only reached ca. 40% of loading by this time.The coverage-dependent isosteric heat of adsorption (Qst) of SIFSIX-bidmb-Cu′ for each gas derived from sorption isotherms indicated Qst values at near-zero coverage as follows: C2H2 (55.7 kJ mol−1) > C2H4 (38.1 kJ mol−1) > CO2 (20.1 kJ mol−1) (Fig. S25–S28†). Notably, the Qst value of C2H2 at a low loading (55.7 kJ mol−1) surpasses those of most prominent SIFSIX materials (Fig. S29†), including ZNU-9 (33.1 kJ mol−1),39 SIFSIX-21-Ni (37.9 kJ mol−1),29 SIFSIX-Cu-TPA (39 kJ mol−1),40 SIFSIX-14-Cu-i (40.0 kJ mol−1),24 SIFSIX-2-Cu-i (41.8 kJ mol−1)23 and SIFSIX-17-Ni (44.2 kJ mol−1),41 and is comparable to those of UTSA-300a (57.6 kJ mol−1),30 NCU-100a (60.5 kJ mol−1)42 and ZJU-300a (61.1 kJ mol−1).33 Conversely, the Qst(CO2) value at a low loading (20.1 kJ mol−1) is low, being marginally higher than that of SIFSIX-21-Ni (19.8 kJ mol−1).29 Consequently, the difference in Qst values between C2H2 and CO2 (ΔQst,C2H2–CO2), 35.6 kJ mol−1, is to our knowledge the highest reported value for SIFSIX materials (SIFSIX-2-Cu-i = 9.9 kJ mol−1,43 SIFSIX-22-Zn = 11.5 kJ mol−1,44 SIFSIX-Cu-TPA = 13.4 kJ mol−1,40 and SIFSIX-21-Ni = 18.1 kJ mol−1) (Fig. S30 and S31†).29 Furthermore, this ΔQst value is higher than the values of other top-performing sorbents like SOFOUR-1-Zn (24 kJ mol−1),44 SOFOUR-TEPE-Zn (26.3 kJ mol−1),45 Cu(bpy)NP (26.2 kJ mol−1)46 and SNNU-98-Mn (31 kJ mol−1),47 comparable to that of Ni(4-DPDS)2CrO4 (38.4 kJ mol−1),48 but inferior to those of sorbents with open metal centres such as ATC-Cu (43.6 kJ mol−1)49 and CuI@UiO-66-(COOH)2 (45.6 kJ mol−1).50 The difference between Qst(C2H2) and Qst(C2H4), ΔQst,C2H2–C2H4, of 17.6 kJ mol−1 is also comparable to that of the benchmark SIFSIX material ZJU-300a (21.1 kJ mol−1)33 and superior to those of SIFSIX-3-Ni (10.2 kJ mol−1),51 SIFSIX-2-Cu-i (11.2 kJ mol−1)43 and SIFSIX-14-Cu-i (13 kJ mol−1) (Fig. S32†).24 These Qst and ΔQst values are indicative of the relative binding strength of SIFSIX-bidmb-Cu′ for these sorbates.
Separation properties
To explore the separation properties of SIFSIX-bidmb-Cu′, selectivities for C2H2/CO2 and C2H2/C2H4 gas mixtures in different compositions at 100 kPa and 298 K were calculated from the ideal adsorbed solution theory (IAST) (Fig. S33–S42†). For both C2H2/CO2 and C2H2/C2H4, the selectivity values indicate potential for trace removal of C2H2. For 50/50 (v/v) C2H2/CO2 and 1/99 C2H2/C2H4, the two gas ratios most commonly investigated, the selectivity values were determined to be 20.3 and 140.2, respectively (Fig. S36†). The equimolar C2H2/CO2 selectivity of SIFSIX-bidmb-Cu′ is higher than most SIFSIX materials (Fig. S43†), e.g. SIFSIX-Cu-TPA (5.3),40 SIFSIX-21-Ni (10),29 ZNU-9 (10.3),39 SIFSIX-17-Ni (11.7)41 and ZJU-280a (18.1),52 and is only lower than those sorbents that exhibit molecular sieving, including UTSA-300a (743)30 and NCU-100a (1786.6).42 The 1/99 C2H2/C2H4 selectivity of SIFSIX-bidmb-Cu′ is also superior to that of most SIFSIX materials (Fig. S44†), including SIFSIX-3-Ni (5.03),51 SIFSIX-2-Cu (6),23 SIFSIX-1-Cu (10.63),23 ZNU-9 (11.64),39 SIFSIX-2-Cu-i (44.5),43 ZJU-280a (44.5),52 and sql-SIFSIX-bpe-Zn (53.1),53 but inferior to that of the molecular sieve SIFSIX-14-Cu-i (6320).24 Notably, SIFSIX-bidmb-Cu′ ranks second among sorbents that simultaneously exhibit 50/50 C2H2/CO2 and 1/99 C2H2/C2H4 selectivities, only being inferior to SIFSIX-TEPE-Cu (Fig. S45†).54Since 2016, when SIFSIX HUMs were found to offer superior acetylene sorption/separation, different strategies have been tried to improve the sorption/separation performance, including pore size control via interpenetration (e.g. SIFSIX-2-Cu vs. SIFSIX-2-Cu-i),23,24 organic linker functionalization41 and anion replacement to fine-tune pore chemistry (e.g. SiF62− to TiF62−).51 In contrast, C2H2 capture performance improvement via trans-to-cis bridging coordination mode change of SIFSIX anions has not been explored. This research work fills the gap and shows that cis-SIFSIX anions can also be favorable for the construction of C2H2-selective sorbents with cis-SIFSIX anions, offering more fluoro binding sites than trans analogs. The selectivity for 1/99 C2H2/C2H4 has been improved from 44.5 (SIFSIX-2-Cu-i) to 140.2 (SIFSIX-bidmb-Cu), while the selectivity for 50/50 C2H2/CO2 has been increased to 20.3 (SIFSIX-bidmb-Cu) from 6.5 (TIFSIX-2-Cu-i). The cis-bridging mode of SIFSIX anions resembles that of the tetrahedral anions XO42− (X = W, Mo, S etc..), which are increasingly used in HUMs.38,44,45
Dynamic breakthrough experiments
The experimental separation performance of SIFSIX-bidmb-Cu′ for C2H2/CO2 and C2H2/C2H4 mixtures was tested by transient column breakthrough experiments performed at 298 K and 100 kPa (Fig. 4a–d). SIFSIX-bidmb-Cu′ showed excellent C2H2 separation performance for both 50/50 and 1/99 binary mixtures. For C2H2/CO2 mixtures, CO2 breakthrough occurred first with retention times of 59.7 and 128.1 min g−1 for the 50/50 and 1/99 mixtures, respectively (Fig. 4a and b). In contrast, the corresponding C2H2 breakthrough occurred after retention times of 98.6 and 1591.2 min g−1, respectively. The breakthrough times for C2H2 and CO2 were 38.9 and 1463.1 min g−1 for 50/50 and 1/99 gas mixtures, respectively. The calculated C2H2 uptake values were 2.48 and 1.38 mmol g−1 for 50/50 and 1/99 mixtures, respectively. The former is comparable to that of the benchmark materials, including JCM-1 (2.2 mmol g−1),55 NKMOF-1-Ni (2.48 mmol g−1),56 CuI@UiO-66-(COOH)2 (2.89 mmol g−1)50 and Ni(4-DPDS)2CrO4 (2.96 mmol g−1).48 Captured C2H2 can be recovered with high purity (>99% purity) through heating the saturated sample at 120 °C after removal of co-adsorbed CO2via a period of helium flush at room temperature (the longer the flushing time, the purer the concentration of C2H2) (Fig. 4e and f). The temperature-controlled recovery of C2H2 is in good agreement with the sorption experiments where heating at 120 °C was needed to fully remove the strongly adsorbed C2H2 molecules. To our knowledge, SIFSIX-bidmb-Cu is just the second sorbent that yields high purity C2H2 (>99% purity) from a 1/99 C2H2/CO2 stream on desorption,44 which is relevant for recycling of C2H2, safety and environmental protection. The equimolar C2H2/CO2 separation factor for SIFSIX-bidmb-Cu, a criterion for evaluating the separation potential of sorbents, was calculated to be 7.8, higher than those of SIFSIX-Cu-TPA (1.97),40 FJUT-1 (5.17),57 CuI@UiO-66-(COOH)2 (3.4),50 JCM-1 (4.4),55 FeNi-M'MOF (1.7),58 and Ni(4-DPDS)2CrO4 (6.7).48For 50/50 and 1/99 C2H2/C2H4 binary mixtures, the C2H4 gas broke through the adsorption bed with high-purity at 39.7 and 41.5 min g−1, while the breakthrough time of C2H2 was longer at 106.2 and 1452.4 min g−1, corresponding to breakthrough intervals of 66.5 and 1410.9 min g−1, respectively (Fig. 4c and d). The calculated C2H2 capture capacities were 2.68 and 1.22 mmol g−1 for 50/50 and 1/99 mixtures, respectively. The productivity of 99.999% pure C2H4 was calculated to be 84.6 mmol g−1, which is comparable to the benchmark SIFSIX-14-Cu-i (84.6 mmol g−1) and higher than those of SIFSIX-3-Zn (1.94 mmol g−1), MUF-17 (8.57 mmol g−1),59 SIFSIX-1-Cu (12.4 mmol g−1),23 SIFSIX-dps-Cu (14.9 mmol g−1),43 ZNU-9 (48.57 mmol g−1)39 and Cu(bpy)NP (20.57 mmol g−1).46 According to the breakthrough curves, the C2H2/C2H4 dynamic selectivity (1/99) of SIFSIX-bidmb-Cu was calculated to be 105.7 (Fig. 4h), notably higher than previously studied sorbents, including SIFSIX-3-Zn (9), SIFSIX-1-Cu (11), and SIFSIX-2-Cu-i (45),23 comparable to Ni@FAU (97)8 and SIFSIX-14-Cu-i (91),24 but lower than ZJU-300a (264).33 Similarly, captured C2H2 can be recovered with high purity (>99% purity) via heating the sample at 120 °C after a helium flush at room temperature (Fig. 4g), thus providing a facile approach to obtain pure C2H2 and C2H4. Additionally, the breakthrough performance for the 10/90 C2H2/C2H4 mixture was investigated at 298 K and 100 kPa with a total gas flow rate of 2 mL min−1 (Fig. 4i), resulting in retention times of 15.8 and 216.3 min g−1 for C2H4 and C2H2, respectively, and a breakthrough time lag of 200.5 min g−1. Consecutive breakthrough experiments for 10/90 C2H2/C2H4 on SIFSIX-bidmb-Cu′ revealed no performance loss, suggesting recyclability which was supported by PXRD data (Fig. S46†). The breakthrough experiments for 50/50 C2H2/CO2 and 1/99 C2H2/C2H4 mixtures conducted at higher gas flow rates (5 mL min−1) were in good agreement with those at lower flow rates, demonstrating that the separation performance was unaffected by gas flow rates and highlighting the fast sorption kinetics of C2H2 (Fig. S47†).
Binding site identification via SCXRD and DFT-D calculations
In order to gain insight into the difference in binding affinity between the three sorbates and the framework, the binding sites of SIFSIX-bidmb-Cu′ were explored via SCXRD on gas-loaded single crystals in combination with dispersion-corrected density functional theory (DFT-D) calculations (Fig. 5). In situ SCXRD studies of SIFSIX-bidmb-Cu′ under 1 bar gas pressure at 298 K using an environmental gas cell were conducted first. In all cases, the gas molecules could not be modeled crystallographically (most likely owing to their high thermal motion at 298 K); however, the most probable locations of the guest molecules within the host framework can be inferred from difference electron density maps (Fig. S48–S52†). In order to further visualize the binding sites, SCXRD studies at 100 K were performed on crystals loaded with C2H2 and CO2 at 298 K. C2H2@SIFSIX-bidmb-Cu′ was found to exhibit two types of binding sites (site I and site II) for C2H2 molecules, each located between two adjacent SiF62− anions (Fig. 5a–d and g). The C2H2 molecule at each site is chelated by two SiF62− anions in an end-on fashion through C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H⋯F hydrogen bonding (dC
C–H⋯F hydrogen bonding (dC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C–H⋯F: 2.16–3.16 Å), and there are van der Waals (vdW) interactions between C2H2 and bidmb ligands (dC–H⋯C
C–H⋯F: 2.16–3.16 Å), and there are van der Waals (vdW) interactions between C2H2 and bidmb ligands (dC–H⋯C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C: 3.19–3.63 Å) (Fig. 5d and g), affording a zig-zag SiF62−···C2H2(I)⋯SiF62−···C2H2(II)⋯SiF62− chain along the channel direction (b-axis) (Fig. 5a–c). At site I, two parallel and centrosymmetric C2H2 molecules are simultaneously chelated by two SIFSIX anions, wherein each is unsymmetrically bound to four terminal fluorine atoms from two SiF62− anions (Fig. 5d and g). At site II, the C2H2 molecule is located at an inversion center and is symmetrically bound to six terminal fluoro atoms from two SIFSIX anions (Fig. S48†).60 For the two binding sites, the DFT-D calculated static binding energies (ΔE) are ∼67.5 and ∼72.4 kJ mol−1, respectively, which are higher than the strongest calculated C2H2 binding strength in SIFSIX materials (∼52.9 kJ mol−1 in SIFSIX-2-Cu-i,43 ∼56.0 kJ mol−1 in SIFSIX-14-Cu-i24 and ∼51.7/56.8 kJ mol−1 in UTSA-300a30).
C: 3.19–3.63 Å) (Fig. 5d and g), affording a zig-zag SiF62−···C2H2(I)⋯SiF62−···C2H2(II)⋯SiF62− chain along the channel direction (b-axis) (Fig. 5a–c). At site I, two parallel and centrosymmetric C2H2 molecules are simultaneously chelated by two SIFSIX anions, wherein each is unsymmetrically bound to four terminal fluorine atoms from two SiF62− anions (Fig. 5d and g). At site II, the C2H2 molecule is located at an inversion center and is symmetrically bound to six terminal fluoro atoms from two SIFSIX anions (Fig. S48†).60 For the two binding sites, the DFT-D calculated static binding energies (ΔE) are ∼67.5 and ∼72.4 kJ mol−1, respectively, which are higher than the strongest calculated C2H2 binding strength in SIFSIX materials (∼52.9 kJ mol−1 in SIFSIX-2-Cu-i,43 ∼56.0 kJ mol−1 in SIFSIX-14-Cu-i24 and ∼51.7/56.8 kJ mol−1 in UTSA-300a30).
For CO2@SIFSIX-bidmb-Cu′, the CO2 molecules could not be modeled adequately, consistent with the low Qst of CO2 adsorption. The difference electron density maps obtained from SCXRD studies at 298 K and 100 K on CO2-loaded crystals reveal similar guest distribution regions (Fig. S50†). We performed DFT-D calculations to determine the binding sites of CO2 and C2H4. Three CO2 binding sites (site I, site II and site III) were identified (Fig. 5e and h). CO2 molecules at sites I and II lie in similar positions but with different orientations than that of C2H2. Each CO2 molecule is bound to one (site II and site III) or two (site I) terminal fluorine atoms from a SiF62− anion in a side-on mode through O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C⋯F electrostatic interactions (dO
C⋯F electrostatic interactions (dO![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C⋯F: 2.86–3.18 Å), accompanied by vdW interactions between CO2 and bidmb ligands (dC–H⋯O
C⋯F: 2.86–3.18 Å), accompanied by vdW interactions between CO2 and bidmb ligands (dC–H⋯O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C: 2.50–3.70 Å). The CO2 molecules on site I and site II should be half-occupied, as they are too close to the inversion centers of the structure (Fig. S53†). The DFT-D calculated static binding energies (ΔE) for the three CO2 binding sites were determined to be ∼48.0, ∼45.1 and ∼52.8 kJ mol−1, respectively. We attribute this to the lack of synergetic fluorine–CO2 binding interactions.
C: 2.50–3.70 Å). The CO2 molecules on site I and site II should be half-occupied, as they are too close to the inversion centers of the structure (Fig. S53†). The DFT-D calculated static binding energies (ΔE) for the three CO2 binding sites were determined to be ∼48.0, ∼45.1 and ∼52.8 kJ mol−1, respectively. We attribute this to the lack of synergetic fluorine–CO2 binding interactions.
For C2H4, two binding sites were identified (site I and site II), displaying similar positions and orientations to those of C2H2 (Fig. 5f and i). On each site, C2H4 is chelated by SIFSIX pairs in an end-on mode through ![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H⋯F hydrogen bonding (d
C–H⋯F hydrogen bonding (d![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–H⋯F: 2.29–3.45 Å), which is accompanied by vdW interactions between C2H4 and bidmb ligands (dC–H⋯C
C–H⋯F: 2.29–3.45 Å), which is accompanied by vdW interactions between C2H4 and bidmb ligands (dC–H⋯C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C: 3.15–3.61 Å). Each SiF62− anion at site I and site II provides two and one free fluoro atoms that bind to one hydrogen atom of C2H4, respectively. Similarly, the C2H4 molecule at site II should be half-occupied, as it resides close to the inversion center (Fig. S54†). For the two C2H4 binding sites, the DFT-D calculated static binding energy (ΔE) was determined to be ∼58.0 and ∼58.4 kJ mol−1, respectively. We attribute these lower energies to weaker C–H⋯F hydrogen bonds (C2H2 is more acidic than C2H4). The C2H2 binding sites from SCXRD data collected at 100 K and those of CO2 and C2H4 calculated from DFT-D are in good agreement with the guest locations inferred from the difference electron density maps of in situ SCXRD at 298 K (Fig. S49–S52†). The DFT-D calculated static binding energy (ΔE) is in the sequence of C2H2 > C2H4 > CO2 and matches well with the order of Qst values from sorption isotherms.
C: 3.15–3.61 Å). Each SiF62− anion at site I and site II provides two and one free fluoro atoms that bind to one hydrogen atom of C2H4, respectively. Similarly, the C2H4 molecule at site II should be half-occupied, as it resides close to the inversion center (Fig. S54†). For the two C2H4 binding sites, the DFT-D calculated static binding energy (ΔE) was determined to be ∼58.0 and ∼58.4 kJ mol−1, respectively. We attribute these lower energies to weaker C–H⋯F hydrogen bonds (C2H2 is more acidic than C2H4). The C2H2 binding sites from SCXRD data collected at 100 K and those of CO2 and C2H4 calculated from DFT-D are in good agreement with the guest locations inferred from the difference electron density maps of in situ SCXRD at 298 K (Fig. S49–S52†). The DFT-D calculated static binding energy (ΔE) is in the sequence of C2H2 > C2H4 > CO2 and matches well with the order of Qst values from sorption isotherms.
In trans-SIFSIX HUMs, due to the symmetric coordination mode of the SiF62− anion, the four free (uncoordinated) F sites are equally distributed around the coordination axis. The free rotation of trans-SIFSIX anions around the coordination axis will modulate the number of free F sites located on the pore surface at a binding site. For example, in SIFSIX-1-Cu,23 only one free F site orients toward the channel, and thus a single C–H⋯F hydrogen bonding was observed (the pore size is large too). When the pore size was reduced by, for instance, interpenetration, the distance between two anions is less (e.g., SIFSIX-2-Cu-i and SIFSIX-14-Cu-i),23,24 and dual C–H⋯F hydrogen bonds can occur. When the rotation of trans anions results in two free F sites located on the pore surface, each anion can provide two free F sites for one C2H2, and totally four C–H⋯F hydrogen bonds can be formed (e.g. ZJU-300a).33 Due to geometry limitations, at most, two of the four F sites of a trans-SiF62− anion can be accessible for one C2H2 molecule. Symmetric bridging of trans-SIFSIX anions can result in strong CO2 trapping (e.g. SIFSIX-3-Ni).16 However, for cis-SIFSIX anions, the four free F sites are located on the same side of the coordination axis and one C2H2 molecule can simultaneously engage with three F sites of each anion. We attribute this feature to the highly selective binding of C2H2vs. both CO2 and C2H4 observed herein.
Water stability tests
SIFSIX materials sustained by trans-SIFSIX anions (e.g. SIFSIX-14-Cu-i) have been reported to undergo degradation or phase transformation when exposed to humidity.61 Conversely, SIFSIX-bidmb-Cu bulk samples of SIFSIX-bidmb-Cu retained crystallinity after one year of exposure to both liquid water and ambient humidity, as confirmed by PXRD (Fig. 6a) and VT-PXRD (Fig. S55†) experiments. The adsorption isotherms of CO2 (195 K), N2 (77 K), C2H2 (298 K), CO2 (298 K) and C2H4 (298 K) of samples exposed to air or water revealed negligible differences vs. pristine materials (Fig. 6b, S56 and S57†). Water stability was also demonstrated by consecutive water adsorption–desorption experiments that revealed no uptake loss even after ten cycles and no structural alteration according to PXRD (Fig. S58 and S59†). The high hydrolytic stability of SIFSIX-bidmb-Cu is consistent with that of the first cis-SIFSIX sorbent.34 The use of imidazole linkers could also be a factor62 along with self-catenation.63Conclusions
This work reveals that cis-bridging SIFSIX anions can offer different sorbate binding sites relative to trans variants, in this case with high C2H2 affinity and low CO2 affinity. In addition, this is the first example of a cis-bridging SIFSIX rigid HUM, and it exhibits C2H2 molecular traps enabled by multiple fluoro binding sites that exhibit relatively weak CO2 and C2H4 binding. Overall, the combination of high C2H2 uptake at 0.01 bar, high Qst for C2H2, large difference in Qst between C2H2 and CO2, and high C2H2/CO2 and C2H2/C2H4 selectivities make SIFSIX-bidmb-Cu′ a leading sorbent for trace C2H2 capture. This work opens up a new avenue for the design and construction of SIFSIX materials with distinct structural features and pore chemistry. Other fluorinated linkers will be targeted in order to fine-tune C2H2 binding affinity.Data availability
The data supporting this article have been included as part of the ESI.† The crystallographic information can be found in the ESI† associated with this work and at the Cambridge Crystallographic Data Center under deposition numbers 2350654–2350657, 2361231–2361233, viahttps://www.ccdc.cam.ac.uk/data_request/cif, or by emailing E-mail: data_request@ccdc.cam.ac.uk, or by contacting The Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44 1223 336033.Author contributions
B. Q.-Song and M. Y. Gao contributed equally to this work and carried out the synthesis, characterization analysis, sorption and separation measurements, and writing – original draft; C.-H. Deng and Q.-Y. Yang assisted with the gas sorption measurement and data analysis; S.-Q. Wang and S. Darwish assisted with the characterization analysis; L. M. van Wyk, A. C. Eaby and L. J. Barbour conducted the in situ single crystal X-ray diffraction measurements; D. Li and S.-J. Qin performed computational simulations; B.-Q. Song, Y. -L. Peng and M. J. Zaworotko carried out the methodology, supervision, and writing – review & editing; all authors contributed to preparing the manuscript.Conflicts of interest
The authors declare no competing financial interests.Acknowledgements
The authors acknowledge the support of National Natural Science Foundation of China (Nos. 22201025 and 22371221), start-up funding from Chengdu University of Technology (10912 KYQD2022-09754) and the European Research Council (ADG 885695).Notes and references
- H. Schobert, Chem. Rev., 2014, 114, 1743–1760 CrossRef CAS PubMed.
- A. Granada, S. B. Karra and S. M. Senkan, Ind. Eng. Chem. Res., 1987, 26, 1901–1905 CrossRef CAS.
- D. S. Sholl and R. P. Lively, Nature, 2016, 532, 435–437 CrossRef PubMed.
- X. Han and S. Yang, Angew. Chem., Int. Ed., 2023, 62, e202218274 CrossRef CAS PubMed.
- T. Ren, M. Patel and K. Blok, Energy, 2006, 31, 425–451 CrossRef CAS.
- H. Molero, B. F. Bartlett and W. T. Tysoe, J. Catal., 1999, 181, 49–56 CrossRef CAS.
- J.-R. Li, R. J. Kuppler and H.-C. Zhou, Chem. Soc. Rev., 2009, 38, 1477–1504 RSC.
- Y. Chai, X. Han, W. Li, S. Liu, S. Yao, C. Wang, W. Shi, I. da-Silva, P. Manuel, Y. Cheng, L. D. Daemen, A. J. Ramirez-Cuesta, C. C. Tang, L. Jiang, S. Yang, N. Guan and L. Li, Science, 2020, 368, 1002–1006 CrossRef CAS PubMed.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- S. Kitagawa, R. Kitaura and S.-i. Noro, Angew. Chem., Int. Ed., 2004, 43, 2334–2375 CrossRef CAS PubMed.
- F. Xiang, H. Zhang, Y. Yang, L. Li, Z. Que, L. Chen, Z. Yuan, S. Chen, Z. Yao, J. Fu, S. Xiang, B. Chen and Z. Zhang, Angew. Chem., Int. Ed., 2023, 62, e202300638 CrossRef CAS PubMed.
- R. E. Sikma, N. Katyal, S.-K. Lee, J. W. Fryer, C. G. Romero, S. K. Emslie, E. L. Taylor, V. M. Lynch, J.-S. Chang, G. Henkelman and S. M. Humphrey, J. Am. Chem. Soc., 2021, 143, 13710–13720 CrossRef CAS PubMed.
- E. D. Bloch, W. L. Queen, R. Krishna, J. M. Zadrozny, C. M. Brown and J. R. Long, Science, 2012, 335, 1606–1610 CrossRef CAS PubMed.
- M. Bonneau, C. Lavenn, J.-J. Zheng, A. Legrand, T. Ogawa, K. Sugimoto, F.-X. Coudert, R. Reau, S. Sakaki, K.-i. Otake and S. Kitagawa, Nat. Chem., 2022, 14, 816–822 CrossRef CAS PubMed.
- J. Tian, Q. Chen, F. Jiang, D. Yuan and M. Hong, Angew. Chem., Int. Ed., 2023, 62, e202215253 CrossRef CAS PubMed.
- K.-J. Chen, D. G. Madden, S. Mukherjee, T. Pham, K. A. Forrest, A. Kumar, B. Space, J. Kong, Q.-Y. Zhang and M. J. Zaworotko, Science, 2019, 366, 241–246 CrossRef CAS PubMed.
- L. K. Macreadie, K. B. Idrees, C. S. Smoljan and O. K. Farha, Angew. Chem., Int. Ed., 2023, 62, e202304094 CrossRef CAS PubMed.
- W. Gong, H. Cui, Y. Xie, Y. Li, X. Tang, Y. Liu, Y. Cui and B. Chen, J. Am. Chem. Soc., 2021, 143, 14869–14876 CrossRef CAS PubMed.
- W.-G. Cui, T.-L. Hu and X.-H. Bu, Adv. Mater., 2020, 32, 1806445 CrossRef CAS PubMed.
- H. Wang, Y. Liu and J. Li, Adv. Mater., 2020, 32, 2002603 CrossRef CAS PubMed.
- S.-M. Wang, M. Shivanna, S.-T. Zheng, T. Pham, K. A. Forrest, Q.-Y. Yang, Q. Guan, B. Space, S. Kitagawa and M. J. Zaworotko, J. Am. Chem. Soc., 2024, 146, 4153–4161 CrossRef CAS PubMed.
- Y. Zhang, J. Hu, R. Krishna, L. Wang, L. Yang, X. Cui, S. Duttwyler and H. Xing, Angew. Chem., Int. Ed., 2020, 59, 17664–17669 CrossRef CAS PubMed.
- X. Cui, K. Chen, H. Xing, Q. Yang, R. Krishna, Z. Bao, H. Wu, W. Zhou, X. Dong, Y. Han, B. Li, Q. Ren, M. J. Zaworotko and B. Chen, Science, 2016, 353, 141–144 CrossRef CAS PubMed.
- B. Li, X. Cui, D. O'Nolan, H.-M. Wen, M. Jiang, R. Krishna, H. Wu, R.-B. Lin, Y.-S. Chen, D. Yuan, H. Xing, W. Zhou, Q. Ren, G. Qian, M. J. Zaworotko and B. Chen, Adv. Mater., 2017, 29, 1704210 CrossRef PubMed.
- K. Adil, Y. Belmabkhout, R. S. Pillai, A. Cadiau, P. M. Bhatt, A. H. Assen, G. Maurin and M. Eddaoudi, Chem. Soc. Rev., 2017, 46, 3402–3430 RSC.
- T. Wang, E. Lin, Y.-L. Peng, Y. Chen, P. Cheng and Z. Zhang, Coord. Chem. Rev., 2020, 423, 213485 CrossRef CAS.
- S. Mukherjee and M. J. Zaworotko, Trends Chem., 2020, 2, 506–518 CrossRef CAS.
- A. Ebadi Amooghin, H. Sanaeepur, R. Luque, H. Garcia and B. Chen, Chem. Soc. Rev., 2022, 51, 7427–7508 RSC.
- N. Kumar, S. Mukherjee, N. C. Harvey-Reid, A. A. Bezrukov, K. Tan, V. Martins, M. Vandichel, T. Pham, L. M. van Wyk, K. Oyekan, A. Kumar, K. A. Forrest, K. M. Patil, L. J. Barbour, B. Space, Y. Huang, P. E. Kruger and M. J. Zaworotko, Chem, 2021, 7, 3085–3098 CAS.
- R.-B. Lin, L. Li, H. Wu, H. Arman, B. Li, R.-G. Lin, W. Zhou and B. Chen, J. Am. Chem. Soc., 2017, 139, 8022–8028 CrossRef CAS PubMed.
- D. Li, M.-Y. Gao, C.-H. Deng, G.-B. Li, S.-J. Qin, Q.-Y. Yang and B.-Q. Song, Small, 2024, 20, 2402523 CrossRef CAS PubMed.
- Y. Huang, J. Wan, T. Pan, K. Ge, Y. Guo, J. Duan, J. Bai, W. Jin and S. Kitagawa, J. Am. Chem. Soc., 2023, 145, 24425–24432 CrossRef CAS PubMed.
- X.-W. Gu, E. Wu, J.-X. Wang, H.-M. Wen, B. Chen, B. Li and G. Qian, Sci. Adv., 2023, 9, eadh0135 CrossRef CAS PubMed.
- B.-Q. Song, Q.-Y. Yang, S.-Q. Wang, M. Vandichel, A. Kumar, C. Crowley, N. Kumar, C.-H. Deng, V. GasconPerez and M. Lusi, J. Am. Chem. Soc., 2020, 142, 6896–6901 CrossRef CAS PubMed.
- B.-Q. Song, M. Shivanna, M.-Y. Gao, S.-Q. Wang, C.-H. Deng, Q.-Y. Yang, S. J. Nikkhah, M. Vandichel, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2023, 62, e202309985 CrossRef CAS PubMed.
- Q. Dong, X. Zhang, S. Liu, R. B. Lin, Y. Guo, Y. Ma, A. Yonezu, R. Krishna, G. Liu and J. Duan, Angew. Chem., Int. Ed., 2020, 59, 22756–22762 CrossRef CAS PubMed.
- A. Spek, J. Appl. Crystallogr., 2003, 36, 7–13 CrossRef CAS.
- M. H. Mohamed, S. K. Elsaidi, L. Wojtas, T. Pham, K. A. Forrest, B. Tudor, B. Space and M. J. Zaworotko, J. Am. Chem. Soc., 2012, 134, 19556–19559 CrossRef CAS PubMed.
- Y. Zhang, W. Sun, B. Luan, J. Li, D. Luo, Y. Jiang, L. Wang and B. Chen, Angew. Chem., Int. Ed., 2023, 62, e202309925 CrossRef CAS PubMed.
- H. Li, C. Liu, C. Chen, Z. Di, D. Yuan, J. Pang, W. Wei, M. Wu and M. Hong, Angew. Chem., Int. Ed., 2021, 60, 7547–7552 CrossRef CAS PubMed.
- S. Mukherjee, N. Kumar, A. A. Bezrukov, K. Tan, T. Pham, K. A. Forrest, K. A. Oyekan, O. T. Qazvini, D. G. Madden, B. Space and M. J. Zaworotko, Angew. Chem., Int. Ed., 2021, 60, 10902–10909 CrossRef CAS PubMed.
- J. Wang, Y. Zhang, P. Zhang, J. Hu, R.-B. Lin, Q. Deng, Z. Zeng, H. Xing, S. Deng and B. Chen, J. Am. Chem. Soc., 2020, 142, 9744–9751 CrossRef CAS PubMed.
- J. Wang, Y. Zhang, Y. Su, X. Liu, P. Zhang, R.-B. Lin, S. Chen, Q. Deng, Z. Zeng, S. Deng and B. Chen, Nat. Commun., 2022, 13, 200 CrossRef CAS PubMed.
- D. Sensharma, D. J. O'Hearn, A. Koochaki, A. A. Bezrukov, N. Kumar, B. H. Wilson, M. Vandichel and M. J. Zaworotko, Angew. Chem., Int. Ed., 2022, 61, e202116145 CrossRef CAS PubMed.
- X. Liu, P. Zhang, H. Xiong, Y. Zhang, K. Wu, J. Liu, R. Krishna, J. Chen, S. Chen, Z. Zeng, S. Deng and J. Wang, Adv. Mater., 2023, 35, 2210415 CrossRef CAS PubMed.
- Y. Liu, J. Liu, H. Xiong, J. Chen, S. Chen, Z. Zeng, S. Deng and J. Wang, Nat. Commun., 2022, 13, 5515 CrossRef CAS PubMed.
- J.-W. Wang, S.-C. Fan, H.-P. Li, X. Bu, Y.-Y. Xue and Q.-G. Zhai, Angew. Chem., Int. Ed., 2023, 62, e202217839 CrossRef CAS PubMed.
- F. Zheng, R. Chen, Z. Ding, Y. Liu, Z. Zhang, Q. Yang, Y. Yang, Q. Ren and Z. Bao, J. Am. Chem. Soc., 2023, 145, 19903–19911 CrossRef CAS PubMed.
- Z. Niu, X. Cui, T. Pham, G. Verma, P. C. Lan, C. Shan, H. Xing, K. A. Forrest, S. Suepaul, B. Space, A. Nafady, A. M. Al-Enizi and S. Ma, Angew. Chem., Int. Ed., 2021, 60, 5283–5288 CrossRef CAS PubMed.
- L. Zhang, K. Jiang, L. Yang, L. Li, E. Hu, L. Yang, K. Shao, H. Xing, Y. Cui, Y. Yang, B. Li, B. Chen and G. Qian, Angew. Chem., Int. Ed., 2021, 60, 15995–16002 CrossRef CAS PubMed.
- K.-J. Chen, H. S. Scott, D. G. Madden, T. Pham, A. Kumar, A. Bajpai, M. Lusi, K. A. Forrest, B. Space and J. J. Perry IV, Chem, 2016, 1, 753–765 CAS.
- Q.-L. Qian, X.-W. Gu, J. Pei, H.-M. Wen, H. Wu, W. Zhou, B. Li and G. Qian, J. Mater. Chem. A, 2021, 9, 9248–9255 RSC.
- M. Shivanna, K.-i. Otake, B.-Q. Song, L. M. van Wyk, Q.-Y. Yang, N. Kumar, W. K. Feldmann, T. Pham, S. Suepaul, B. Space, L. J. Barbour, S. Kitagawa and M. J. Zaworotko, Angew. Chem., Int. Ed., 2021, 60, 20383–20390 CrossRef CAS PubMed.
- L. Wang, Y. Zhang, P. Zhang, X. Liu, H. Xiong, R. Krishna, J. Liu, H. Shuai, P. Wang, Z. Zhou, J. Chen, S. Chen, S. Deng and J. Wang, AIChE J., 2024, 70, e18396 CrossRef CAS.
- J. Lee, C. Y. Chuah, J. Kim, Y. Kim, N. Ko, Y. Seo, K. Kim, T. H. Bae and E. Lee, Angew. Chem., Int. Ed., 2018, 57, 7869–7873 CrossRef CAS PubMed.
- Y.-L. Peng, T. Pham, P. Li, T. Wang, Y. Chen, K.-J. Chen, K. A. Forrest, B. Space, P. Cheng, M. J. Zaworotko and Z. Zhang, Angew. Chem., Int. Ed., 2018, 57, 10971–10975 CrossRef CAS PubMed.
- L. Zhang, T. Xiao, X. Zeng, J. You, Z. He, C.-X. Chen, Q. Wang, A. Nafady, A. M. Al-Enizi and S. Ma, J. Am. Chem. Soc., 2024, 146, 7341–7351 CrossRef CAS PubMed.
- J. Gao, X. Qian, R.-B. Lin, R. Krishna, H. Wu, W. Zhou and B. Chen, Angew. Chem., Int. Ed., 2020, 59, 4396–4400 CrossRef CAS PubMed.
- O. T. Qazvini, R. Babarao and S. G. Telfer, Chem. Mater., 2019, 31, 4919–4926 CrossRef CAS.
- T. Jacobs, G. O. Lloyd, J.-A. Gertenbach, K. K. Müller-Nedebock, C. Esterhuysen and L. J. Barbour, Angew. Chem., Int. Ed., 2012, 51, 4913–4916 CrossRef CAS.
- D. O'Nolan, A. Kumar and M. J. Zaworotko, J. Am. Chem. Soc., 2017, 139, 8508–8513 CrossRef PubMed.
- J.-P. Zhang, Y.-B. Zhang, J.-B. Lin and X.-M. Chen, Chem. Rev., 2011, 112, 1001–1033 CrossRef PubMed.
- N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575–10612 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2350654−2350657, 2361231–2361233. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc00697j |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2025 |