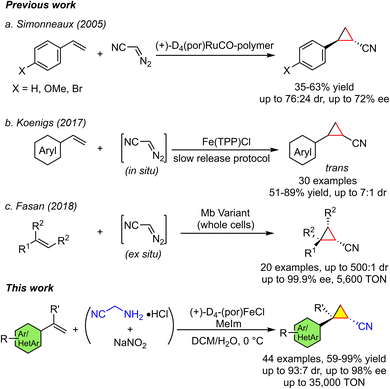
Open Access Article
Open Access ArticleChiral iron porphyrin (+)-D4-(por)FeCl catalyzes highly enantioselective cyclopropanation of alkenes using in situ generated diazoacetonitrile with up to 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/h2_char_2009.gif) 000 product turnover†
000 product turnover†
Hao-Chong
Tan
a,
Ka-Pan
Shing
bd,
Hua-Hua
Wang
a,
Yungen
Liu
 a and
Chi-Ming
Che
a and
Chi-Ming
Che
 *bcd
*bcd
aDepartment of Chemistry, Southern University of Science and Technology, Shenzhen 518055, Guangdong, P. R. China
bState Key Laboratory of Synthetic Chemistry, Department of Chemistry, The University of Hong Kong, Pokfulam Road, Hong Kong, P. R. China. E-mail: cmche@hku.hk
cHKU Shenzhen Institute of Research and Innovation, Shenzhen, Guangdong 518057, P. R. China
dLaboratory for Synthetic Chemistry and Chemical Biology Limited, Units 1503-1511, 15/F, Building 17W, Hong Kong Science Park, New Territories, Hong Kong, P. R. China
First published on 11th April 2025
Abstract
Transition metal-catalyzed asymmetric cyclopropanation of alkenes is an important strategy to construct chiral cyclopropane skeletons of pharmaceutical interest, but highly enantioselective and practical carbene transfer reactions based on Earth abundant and bio-compatible metals are still a difficult challenge. In this work, we use a chiral iron porphyrin (+)-D4-(por)FeCl catalyst and in situ generated α-diazoacetonitrile for highly enantioselective cyclopropanation of arylalkene. This reaction is applicable to a wide range of arylalkenes (44 examples) with yield up to 99%, diastereomeric ratio (dr) up to 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7, and enantiomeric excess (ee) values up to 98%. Importantly, for the cyclopropanation reaction of 3,4-difluorostyrene (1.40 g, 10.0 mmol) with α-diazoacetonitrile in the presence of 0.002 mol% of (+)-D4-(por)FeCl as a catalyst, the turnover number and enantioselectivity of the cyclopropyl nitrile product reached 31
7, and enantiomeric excess (ee) values up to 98%. Importantly, for the cyclopropanation reaction of 3,4-difluorostyrene (1.40 g, 10.0 mmol) with α-diazoacetonitrile in the presence of 0.002 mol% of (+)-D4-(por)FeCl as a catalyst, the turnover number and enantioselectivity of the cyclopropyl nitrile product reached 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 88% ee, respectively. Using cyclopropyl nitriles as a starting material, downstream functionalization derivatives including cyclopropyl carboxylic acids, cyclopropylamines, and cyclopropylmethanamines can be produced as key intermediates for the preparation of a series of bioactive or drug-like molecules. In addition, the chiral Fe(II)porphyrin–cyanocarbene intermediate [(−)-D4-(por)FeII(:CHCN)], which is directly responsible for the carbene transfer reaction, has been characterized by 1H NMR, HR ESI-MS, UV-vis and ATR-FTIR spectroscopy.
000 and 88% ee, respectively. Using cyclopropyl nitriles as a starting material, downstream functionalization derivatives including cyclopropyl carboxylic acids, cyclopropylamines, and cyclopropylmethanamines can be produced as key intermediates for the preparation of a series of bioactive or drug-like molecules. In addition, the chiral Fe(II)porphyrin–cyanocarbene intermediate [(−)-D4-(por)FeII(:CHCN)], which is directly responsible for the carbene transfer reaction, has been characterized by 1H NMR, HR ESI-MS, UV-vis and ATR-FTIR spectroscopy.
Introduction
Cyclopropane scaffolds are commonly found in many pharmaceuticals and bioactive natural products.1,2 Typically, cyclopropane skeletons3–5 are prepared by carbene transfer reactions using diazo compounds as precursors, especially in an asymmetric manner,6 and noble metal catalysts of rhodium, ruthenium and iridium are often used in asymmetric carbene transfer reactions. In the context of developing sustainable catalysis, there has been great interest in developing iron catalysis for organic synthesis7–10 due to iron's Earth abundance and biocompatibility.11 Although iron carbene complexes have been reported for a long time,12 the development of practical iron-catalyzed carbene transfer reactions has lagged behind.13 Furthermore, there are few studies on the detection and reactivity of reactive iron–carbene intermediates involved in the catalytic cycle. In the literature, Woo,14 Che,15 Gross16 and Carreira17,18 reported iron macrocyclic complexes (porphyrin and corrole) as catalysts for carbene transfer reactions (cyclopropanation of alkenes). In most cases, high product yields and high stereoselectivities (mainly trans-configuration) are obtained. Asymmetric cyclopropanation of alkenes using synthetic chiral iron porphyrin catalysts has only been reported sporadically by Gross,19 Woo,20 Simonneaux,21,22 Wong and Che,23 Zhang,24,25 and Gallo.26,27 Although good to high product yields (>80%) and high diastereoselectivities (dr > 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, mainly trans-configuration) were obtained, the enantioselectivities were moderate (typically <80% ee, excluding Zhang's recent report25). When chiral non-heme iron complexes are used as catalysts, lower enantioselectivities are obtained20,28,29 except for the intramolecular cyclopropanation of α-diazoesters or indoles catalyzed by iron bisoxazoline complexes.30,31 So far, for iron-catalyzed carbene transfer reactions, only engineered iron enzymes (such as P450, P411 variants and myoglobins with iron porphyrins as co-factors) have been reported to achieve excellent enantioselectivity and high product turnover numbers (TONs) (Fig. 1).32–35 In the literature, a rhodium-catalyzed styrene cyclopropanation using a donor/acceptor diazo-compound (trichloroethyl p-bromophenyl diazoacetate) resulted in a product TON and enantioselectivity of 100
1, mainly trans-configuration) were obtained, the enantioselectivities were moderate (typically <80% ee, excluding Zhang's recent report25). When chiral non-heme iron complexes are used as catalysts, lower enantioselectivities are obtained20,28,29 except for the intramolecular cyclopropanation of α-diazoesters or indoles catalyzed by iron bisoxazoline complexes.30,31 So far, for iron-catalyzed carbene transfer reactions, only engineered iron enzymes (such as P450, P411 variants and myoglobins with iron porphyrins as co-factors) have been reported to achieve excellent enantioselectivity and high product turnover numbers (TONs) (Fig. 1).32–35 In the literature, a rhodium-catalyzed styrene cyclopropanation using a donor/acceptor diazo-compound (trichloroethyl p-bromophenyl diazoacetate) resulted in a product TON and enantioselectivity of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 94% ee, respectively. However, when the catalyst loading was reduced, the enantioselectivity decreased (400
000 and 94% ee, respectively. However, when the catalyst loading was reduced, the enantioselectivity decreased (400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 with 90% ee, Fig. 1).36 In this context, metal porphyrin complexes may be a promising catalyst choice due to their excellent activity and stability. Taking the asymmetric cyclopropanation of styrene with ethyl diazoacetate (EDA) as an example, chiral ruthenium porphyrin catalyst (−)D4-(por)Ru(IMe)2 gave the trans-cyclopropane product with a TON of 23
000 with 90% ee, Fig. 1).36 In this context, metal porphyrin complexes may be a promising catalyst choice due to their excellent activity and stability. Taking the asymmetric cyclopropanation of styrene with ethyl diazoacetate (EDA) as an example, chiral ruthenium porphyrin catalyst (−)D4-(por)Ru(IMe)2 gave the trans-cyclopropane product with a TON of 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 750 and an ee of 95%;37 The artificial metalloenzyme Ir(Me)-PIX (with iridium porphyrin as a co-factor) afforded a cis-cyclopropane product of 10
750 and an ee of 95%;37 The artificial metalloenzyme Ir(Me)-PIX (with iridium porphyrin as a co-factor) afforded a cis-cyclopropane product of 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 TON and 98% ee (Fig. 1).38 Engineered iron enzymes (with iron porphyrins as cofactors) produced even more exciting results: Mb (H64V, V68A) (myoglobin variant) gave a trans-product TON of 46
000 TON and 98% ee (Fig. 1).38 Engineered iron enzymes (with iron porphyrins as cofactors) produced even more exciting results: Mb (H64V, V68A) (myoglobin variant) gave a trans-product TON of 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 with 99.9% ee,39 and the P411BM3-CIS variant achieved a cis-product TON of 67
800 with 99.9% ee,39 and the P411BM3-CIS variant achieved a cis-product TON of 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 with 99% ee (Fig. 1).40
800 with 99% ee (Fig. 1).40
Among various carbene precursors, diazo compounds are the most commonly used.41 However, diazo compounds have inherent disadvantages in preparation and storage due to their potentially explosive properties. It is more applicable when diazo compounds can be generated and used in situ,42,43 simplifying the reaction process and broadening the application scope of such reactions, especially when the reactions are carried out on a large scale.17,18 In this context, α-diazoacetonitrile, which can be generated in situ through the reaction of commercially available aminoacetonitrile hydrochloride with sodium nitrite,44 was studied (Scheme 1). Simonneaux and co-workers reported the asymmetric cyclopropanation of styrene catalyzed by (+)-D4-(por)RuCO or polymer-supported (+)-D4-(por)RuCO using α-diazoacetonitrile as a carbene precursor; cyclopropane products were obtained with ee up to 90% and dr up to 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7.45 Using in situ generated α-diazoacetonitrile as the carbene precursor, Koenigs and co-workers46,47 reported the catalytic cyclopropanation of styrene by Fe(TPP)Cl in a slow-release protocol, providing the product in up to 89% yields and up to 7
7.45 Using in situ generated α-diazoacetonitrile as the carbene precursor, Koenigs and co-workers46,47 reported the catalytic cyclopropanation of styrene by Fe(TPP)Cl in a slow-release protocol, providing the product in up to 89% yields and up to 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr.48 Using in situ generated α-diazoacetonitrile as the carbene precursor, Fasan and co-workers reported that Mb variants (in whole cells) catalyzed the asymmetric cyclopropanation of alkenes to provide up to 99.9% ee and up to 500
1 dr.48 Using in situ generated α-diazoacetonitrile as the carbene precursor, Fasan and co-workers reported that Mb variants (in whole cells) catalyzed the asymmetric cyclopropanation of alkenes to provide up to 99.9% ee and up to 500![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr of cyclopropane product.49 In this work, we used in situ generated α-diazoacetonitrile as the carbene precursor and chiral iron porphyrin (+)-D4-(por)FeCl as the catalyst for the asymmetric cyclopropanation of aryl alkenes. Good product yields (up to 99%), high diastereoselectivities (up to >93
1 dr of cyclopropane product.49 In this work, we used in situ generated α-diazoacetonitrile as the carbene precursor and chiral iron porphyrin (+)-D4-(por)FeCl as the catalyst for the asymmetric cyclopropanation of aryl alkenes. Good product yields (up to 99%), high diastereoselectivities (up to >93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr), high enantioselectivities (up to 99% ee) and high product TONs (up to 35
7 dr), high enantioselectivities (up to 99% ee) and high product TONs (up to 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 with 85% ee) were achieved (Scheme 1). This catalytic reaction has a broad substrate scope (44 examples) and can be used to prepare core scaffolds for the synthesis of bioactive or drug-like molecules. In addition, the chiral Fe(II)–porphyrin–cyanocarbene intermediate [FeII(por)(:CHCN)], which is directly responsible for the carbene transfer reaction, has been characterized by 1H NMR, HR ESI-MS, UV-vis and ATR-FTIR spectroscopy.
000 with 85% ee) were achieved (Scheme 1). This catalytic reaction has a broad substrate scope (44 examples) and can be used to prepare core scaffolds for the synthesis of bioactive or drug-like molecules. In addition, the chiral Fe(II)–porphyrin–cyanocarbene intermediate [FeII(por)(:CHCN)], which is directly responsible for the carbene transfer reaction, has been characterized by 1H NMR, HR ESI-MS, UV-vis and ATR-FTIR spectroscopy.
Results and discussion
Similar to Koenigs's strategy, we used aminoacetonitrile hydrochloride to prepare α-diazoacetonitrile in situ.48,50 Using (+)-D4-(por)FeCl as a catalyst, it was found that 93% ee and 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 dr were obtained at room temperature using a slow-addition protocol48 (Table 1, entry 1). For comparison, 84% ee and 81
15 dr were obtained at room temperature using a slow-addition protocol48 (Table 1, entry 1). For comparison, 84% ee and 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 dr were obtained via the one-pot addition method (Table 1, entry 2). There was a slight decrease in enantioselectivity when using other biphasic solutions (toluene/H2O or PhCF3/H2O) (Table 1, entries 3 and 4). Higher enantioselectivities (88% ee) and diastereoselectivities (88
19 dr were obtained via the one-pot addition method (Table 1, entry 2). There was a slight decrease in enantioselectivity when using other biphasic solutions (toluene/H2O or PhCF3/H2O) (Table 1, entries 3 and 4). Higher enantioselectivities (88% ee) and diastereoselectivities (88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 dr) were obtained when the one-pot reaction was performed at 0 °C (Table 1, entry 5). The best results were obtained with N-methyl imidazole (MeIm) as an additive (Table 1, entry 6, 91% yield, 97% ee and 90
12 dr) were obtained when the one-pot reaction was performed at 0 °C (Table 1, entry 5). The best results were obtained with N-methyl imidazole (MeIm) as an additive (Table 1, entry 6, 91% yield, 97% ee and 90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10 dr).23,51 Using (−)-D4-(por)FeCl as a catalyst, the enantiomer of 2a was obtained in 92% yield and −95% ee (Table 1, entry 7 vs. entry 6). Under optimized conditions, using other chiral iron porphyrins such as (S)-tBu-chenporFeCl, (S,R)-cmcporFeCl, (S,S)-cmcporFeCl, β-(+)-D4-porFeCl and β-(S)-chiral-porFeCl,52 cyclopropanation products were obtained with an ee of 10–81% (Table 1, entries 8–12). With FeSalen(S,S)(3,5-tBu)X (Salen(S,S)(3,5-tBu) = (S,S)-(+)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine, X = Cl or OTf) as a catalyst, no reaction was observed (Table 1, entry 13). Under optimal conditions (as those in Table 1, entry 6), we examined the scope of styrene derivatives (Scheme 2). In general, good enantioselectivities (up to 98% ee) and high diastereoselectivities (up to 93
10 dr).23,51 Using (−)-D4-(por)FeCl as a catalyst, the enantiomer of 2a was obtained in 92% yield and −95% ee (Table 1, entry 7 vs. entry 6). Under optimized conditions, using other chiral iron porphyrins such as (S)-tBu-chenporFeCl, (S,R)-cmcporFeCl, (S,S)-cmcporFeCl, β-(+)-D4-porFeCl and β-(S)-chiral-porFeCl,52 cyclopropanation products were obtained with an ee of 10–81% (Table 1, entries 8–12). With FeSalen(S,S)(3,5-tBu)X (Salen(S,S)(3,5-tBu) = (S,S)-(+)-N,N′-bis(3,5-di-tert-butylsalicylidene)-1,2-cyclohexanediamine, X = Cl or OTf) as a catalyst, no reaction was observed (Table 1, entry 13). Under optimal conditions (as those in Table 1, entry 6), we examined the scope of styrene derivatives (Scheme 2). In general, good enantioselectivities (up to 98% ee) and high diastereoselectivities (up to 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 dr) and high product yields (up to 97%) were obtained. For para-substituted styrene derivatives, strong electron-donating groups such as MeO (2f, 87% ee), Me2N (2h, 87% ee) and strong electron-withdrawing groups such as CN (2k, 85% ee), NO2 (2l, 85% ee), CF3 (2m, 83% ee), CO2Me (2n, 63% ee) and steric bulky groups (2i, tBu, 63% ee) were found to slightly reduce the enantioselectivity of the product (Scheme 2). Lowering the temperature to −15 °C improved the enantioselectivity (to 90% ee for 2n). When the substituent is located in the meta-position (2o–2s) or ortho-position (2t–2x), the enantioselectivity of the resulting cyclopropanation products decreases slightly (84–90% ee). This reaction is sensitive to substituents on disubstituted alkenes (Scheme 2). For α-substituted styrene derivatives (2aa–2ae), both diastereoselectivity and enantioselectivity decrease significantly with increasing α-substituent size. On the other hand, neither cis- nor trans-β-methylstyrene could be converted into the corresponding cyclopropanation products (Scheme 2). The trans-configurations of compounds 2g, 2l, 2z, 2aa and 2ad were unambiguously determined by X-ray crystallography.
7 dr) and high product yields (up to 97%) were obtained. For para-substituted styrene derivatives, strong electron-donating groups such as MeO (2f, 87% ee), Me2N (2h, 87% ee) and strong electron-withdrawing groups such as CN (2k, 85% ee), NO2 (2l, 85% ee), CF3 (2m, 83% ee), CO2Me (2n, 63% ee) and steric bulky groups (2i, tBu, 63% ee) were found to slightly reduce the enantioselectivity of the product (Scheme 2). Lowering the temperature to −15 °C improved the enantioselectivity (to 90% ee for 2n). When the substituent is located in the meta-position (2o–2s) or ortho-position (2t–2x), the enantioselectivity of the resulting cyclopropanation products decreases slightly (84–90% ee). This reaction is sensitive to substituents on disubstituted alkenes (Scheme 2). For α-substituted styrene derivatives (2aa–2ae), both diastereoselectivity and enantioselectivity decrease significantly with increasing α-substituent size. On the other hand, neither cis- nor trans-β-methylstyrene could be converted into the corresponding cyclopropanation products (Scheme 2). The trans-configurations of compounds 2g, 2l, 2z, 2aa and 2ad were unambiguously determined by X-ray crystallography.
| Entry | Additive | Solvent | T (°C) | Time (h) | Yieldb (%) | drb | eec (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol) and catalyst (1 mol%) in 0.5 mL of DCM, diazo was generated in situ by one-pot reaction of amino-hydrochloride salt (0.4 mmol) with NaNO2 (0.6 mmol) in 2 mL of degassed water. b Determined by 1H NMR analysis of crude products. c Determined by HPLC. d Aminoacetonitrile hydrochloride (0.4 mmol, in 1 mL of degassed water) and sodium nitrite (0.6 mmol, in 1 mL of degassed water) were added dropwise using a syringe pump for 10 h and further reacted for 4 h. e (−)-D4-(por)FeCl (1 mol%) was used. f (S)-tBu-chenporFeCl (1 mol%) was used. g (S,S)-cmcporFeCl (1 mol%) was used. h (S,R)-cmcporFeCl (1 mol%) was used. i β-(+)-D4-porFeCl (1 mol%) was used. j β-(S)-chiral-porFeCl (1 mol%) was used. k FeSalen(S,S)(3,5-tBu)X (X = Cl or OTf) (10 mol%) was used. MeIm, methyl imidazole. NR, no reaction. | |||||||
| 1d | None | DCM/H2O | r. t. | 10 + 4d | 82 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
93 |
| 2 | None | DCM/H2O | r. t. | 14 | 85 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
84 |
| 3 | None | Toluene/H2O | r. t. | 14 | 83 | 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15 15 |
78 |
| 4 | None | PhCF3/H2O | r. t. | 14 | 81 | 81![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19 19 |
79 |
| 5 | None | DCM/H2O | 0 | 14 | 87 | 88![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 12 |
88 |
| 6 | MeIm (0.01 eq.) | DCM/H 2 O | 0 | 14 | 91 |
90![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 10 10
|
97 |
| 7e | MeIm (0.01 eq.) | DCM/H2O | 0 | 14 | 92 | 89![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 11 |
−95 |
| 8f | MeIm (0.01 eq.) | DCM/H2O | 0 | 14 | 90 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
65 |
| 9g | MeIm (0.01 eq.) | DCM/H2O | 0 | 14 | 93 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
81 |
| 10h | MeIm (0.01 eq.) | DCM/H2O | 0 | 14 | 93 | 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20 20 |
15 |
| 11i | MeIm (0.01 eq.) | DCM/H2O | 0 | 14 | 86 | 77![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 23 23 |
20 |
| 12j | MeIm (0.01 eq.) | DCM/H2O | 0 | 14 | 77 | 82![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 18 18 |
10 |
| 13k | None | DCM/H2O | 0 | 14 | NR | — | — |

|
|||||||
For alkenes tethered to aromatic heterocycles or benzo-heterocycles (Scheme 3, 3a–3i), the yields of cyclopropanation products are 58–99%, ee is 76–91%, and dr is 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20–92
20–92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. N-Vinyl indoles also provided the corresponding cyclopropanation products (3j, 3k) with 87% ee and 90% ee, respectively. The cyclopropanation product 4 was obtained from 4-phenyl-buta-1,3-diene, with a dr of 64
8. N-Vinyl indoles also provided the corresponding cyclopropanation products (3j, 3k) with 87% ee and 90% ee, respectively. The cyclopropanation product 4 was obtained from 4-phenyl-buta-1,3-diene, with a dr of 64![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 36 and a yield of 79%; the ee values of the trans-isomer and cis-isomer are 65% and 8%, respectively (Scheme 3). No cyclopropanation reaction was observed using allylic benzene as a substrate.
36 and a yield of 79%; the ee values of the trans-isomer and cis-isomer are 65% and 8%, respectively (Scheme 3). No cyclopropanation reaction was observed using allylic benzene as a substrate.
Our catalytic cyclopropanation reaction is scalable and user-friendly (Scheme 4). In the presence of 0.02 mol% of (+)-D4-(por)FeCl, 1.40 g of 3,4-difluorostyrene (5) was converted into 1.58 g of product 6 (88% yield, 88% ee, TON of 4400). For a batch reaction of 14.01 g of 5 in the presence of 0.002 mol% of (+)-D4-(por)FeCl as a catalyst, a product TON of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 (86% ee) was obtained. We attribute the slight erosion in enantioselectivity to inefficient mechanical stirring. Finally, the optimized conditions were: 1.40 g of 5 was reacted in the presence of 0.002 mol% of (+)-D4-(por)FeCl to produce a cyclopropane product with a TON of up to 31
000 (86% ee) was obtained. We attribute the slight erosion in enantioselectivity to inefficient mechanical stirring. Finally, the optimized conditions were: 1.40 g of 5 was reacted in the presence of 0.002 mol% of (+)-D4-(por)FeCl to produce a cyclopropane product with a TON of up to 31![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 and 88% ee (Scheme 4). Under similar reaction conditions, styrene was used as the substrate (1.04 g scale) to give the corresponding cyclopropanation product with a TON of 35
000 and 88% ee (Scheme 4). Under similar reaction conditions, styrene was used as the substrate (1.04 g scale) to give the corresponding cyclopropanation product with a TON of 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000, an ee of 85% ee, and a dr of 92
000, an ee of 85% ee, and a dr of 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8. Starting from 6, Ticagrelor (AZD6140, antiplatelet agent)53 and 7 (LSD1 inhibitor)54 can be prepared. In the literature, for the cyclopropanation reaction of styrene/styrene derivatives and α-diazoacetonitrile, when Fe(F20TPP)Cl is used as the catalyst, the obtained product TON is up to 2133 (using styrene as a substrate).48 Using engineered myoglobin as the catalyst and p-chlorostyrene as the substrate, the product TON is reported to be as high as 5600.49
8. Starting from 6, Ticagrelor (AZD6140, antiplatelet agent)53 and 7 (LSD1 inhibitor)54 can be prepared. In the literature, for the cyclopropanation reaction of styrene/styrene derivatives and α-diazoacetonitrile, when Fe(F20TPP)Cl is used as the catalyst, the obtained product TON is up to 2133 (using styrene as a substrate).48 Using engineered myoglobin as the catalyst and p-chlorostyrene as the substrate, the product TON is reported to be as high as 5600.49
 | ||
| Scheme 4 Gram-scale reactions catalyzed by chiral iron porphyrins and examples that can be prepared from 6. | ||
Chiral cyclopropyl nitrile can be easily converted into a variety of structurally diverse cyclopropane scaffolds including cyclopropylmethanamine, cyclopropylcarboxylic acid and cyclopropylamine, as valuable chiral building blocks for the preparation of bioactive or drug-like molecules.55 For examples: (1) alcoholysis of 3a gives the corresponding cyclopropyl carboxylic ester 8 with a yield of 98%, from which 9 (selective sodium hydrogen exchanger isoform-1 inhibitor) can be prepared (Scheme 5a; for the synthetic route,56 see the ESI†). (−)-Tasimelteon (a melatonin receptor agonist)57 can be synthesized from the enantiomer of 3a (3a can be prepared by using (−)-D4-(por)FeCl as catalyst) according to literature methods (Scheme 5b).58 This strategy is more step-economical59 than similar approaches starting from EDA,60 or via chiral epoxide intermediates.61,62 Since our cyclopropanation reaction proceeds in an asymmetric manner, optical resolution of racemic amine 10 is unnecessary.58
 | ||
| Scheme 5 (a) Preparation of 9 from 3a. (b) Preparation of (−)-Tasimelteon from the enantiomer of 3a. | ||
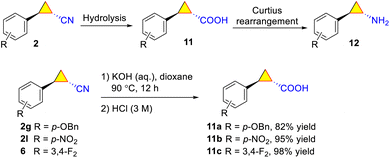
(2) Hydrolysis of aryl cyclopropyl nitrile 2 under alkaline conditions gave the corresponding aryl cyclopropyl carboxylic acid (11) in high yield (Scheme 6).49 Using acid 11 as the starting material, aryl cyclopropyl amines (12) were prepared in high yields via Curtius rearrangement.63–66 A series of bio-active compounds containing a chiral cyclopropyl moiety such as ST-161 (antiviral reagent against Lassa virus),67VU0359595 (selective PLD1 inhibitor),68 and S 17092 (human proline endopeptidase inhibitor)69 (Fig. 2) can be prepared from 2a or its enantiomer. Our method is simpler70 than the cyclopropanation of α,β-unsaturated carboxylic acid derivative of Oppolzer's chiral sultam (bornane[10,2]sultam, auxiliary) using diazomethane (used to construct the stereochemistry of the cyclopropyl moiety).71
(3) Phenylcyclopropylamine (tranylcypromine, TCP) and its derivatives are key and versatile intermediates in the preparation of bioactive or drug-like molecules (Fig. 2).72,73 For examples, Diprovocim-1 (toll-like receptor agonist)74 can be prepared from 2a. RN-1 (LSD1 inhibitor)75 and ORY-2001 (Vafidemstat, LSD1 inhibitor)76 can be prepared starting from 2g. MC2580 derivative (LSD1 inhibitor)77 can be prepared starting from 2l. Compound 13 (dipeptidyl peptidase IV inhibitor)78 can be prepared starting from 2p.
We performed time course experiments to gain a deeper understanding of the reaction mechanism (Fig. 3). There is a clear induction period before the reaction proceeds rapidly (Fig. 3a). Furthermore, MeIm shortened the induction period, possibly due to the trans effect via axial ligation to iron porphyrin. Under similar reaction conditions in the presence of MeIm, one-pot addition of diazoacetonitrile drastically shortened the induction period (<3 min) and the catalytic reaction was finished within 10 min (Fig. 3b). The reaction was found to be sensitive to O2 (Fig. 3b). When carried out under O2, the reaction slowed down significantly, and the product yield was only 29% in 60 min. In the presence of N-tert-butyl-2-phenylnitrone (PBN) or 2,2,6,6-tetramethyl-1-piperinedinyloxy (TEMPO), the cyclopropanation product was obtained in yield within 5% (Fig. 3c). However, the decrease in product yield in the presence of radical scavengers cannot exclude the interaction between free radical scavengers and metal catalysts, which can also lead to the loss of catalyst activity.79 Butylated hydroxytoluene (BHT), which has weak interaction with metal catalysts, was used as a free radical scavenger, and the cyclopropanation product was obtained with a yield of 70%. The finding may differ from Zhang's recent report,25 in which Fe(III)-based metalloradical species (α-Fe(IV)-alkyl and γ-Fe(IV)-alkyl radicals) were proposed. On the other hand, non-radical pathways in iron porphyrin-catalyzed carbene transfer reactions have also been proposed and supported by both experimental and computational results.80
We performed a series of experiments to characterize the reactive Fe(II) porphyrin cyanocarbene [(por)Fe(:CHCN)] intermediate (Scheme 7). [(−)-D4-(por)Fe(THF)2] was prepared by stirring (−)-D4-(por)FeCl with Zn dust in degassed and anhydrous tetrahydrofuran (THF) overnight. [(−)-D4-(por)Fe(THF)2] can be characterized by 1H NMR. All sharp resonance peaks appear within δ −1.8 to 12.5 ppm. It was found that [(−)-D4-(por)Fe(THF)2] reacted with an equimolar diazoacetonitrile (0.07 M solution in toluene) to immediately produce [(−)-D4-(por)Fe(:CHCN)], as shown by using UV-vis (in benzene) and 1H NMR spectroscopy. In a non-coordinating solvent (benzene), [(−)-D4-(por)Fe(:CHCN)] has UV-vis absorption peaks at 417 and 509 nm (Fig. 4). Studies have found that [(−)-D4-(por)Fe(:CHCN)] is unstable in benzene under aerobic conditions, producing a species with UV-vis absorption spectrum similar to Fe(III) porphyrin (spectral changes in benzene solution show isosbestic points at 387, 512 and 554 nm, see Fig. S2, ESI†). Comparing the UV-vis absorption spectra of [(−)-D4-(por)Fe(THF)2] and [(−)-D4-(por)Fe(:CHCN)], the Soret and Q bands blue shift from 430 and 547 nm to 422 and 538 nm (solvent: THF), respectively (see Fig. S1, ESI†).
 | ||
| Fig. 4 UV-vis absorption spectrum of [(−)-D4-(por)Fe(THF)2], [(−)-D4-(por)FeCl] and [(−)-D4-(por)Fe(:CHCN)] in benzene-d6. | ||
The 1H NMR spectrum (Fig. 5) of [(−)-D4-(por)Fe(:CHCN)] in C6D6 shows characteristic peaks at δ 17.8 (s, 1H, [:CHCN]), 8.77–8.82 (d× 2, 8H, splitting via desymmetrization of the chiral Fe–carbene species) and 7.30 (s, 4H) ppm, consistent with diamagnetic Fe(II) carbene porphyrins.81 The 1H NMR chemical shifts fall into a similar range for typical terminal metal-carbene porphyrins containing [:CH(CN)] fragment such as [Fe(TTP)(:CHY)] (Y = mesityl, 19.71 ppm in C6D6),82 [Ru(TTP)(:CHY)] (Y = CO2Et, 13.43 ppm in C6D6),83 [Ru(TMP)(:CHY)] (Y = CO2Et, 13.79 ppm in C6D6)84 and [Ru(TTPPP)(H2O)(:CHY)] (Y = CO2Et, 13.07 ppm in CDCl3).85 The highly de-shielded protons may originate from multiple bonding features between Fe and Ccarbene, bringing the coordinated carbene group closer to the de-shielded zone around Fe porphyrin. The [(−)-D4-(por)Fe(:CHCN)] intermediate was also characterized by HR-MS (Fig. 5). The experimental mass value (C86H77FeN5, 1235.5517 m/z) and isotopic pattern of the spectrum match well with the theoretical values (1235.5523 m/z).
[(−)-D4-(por)FeII(:CHCN)] was characterized based on the absorbance band of ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) using ATR-FTIR spectroscopy (Fig. 6). The ν(C
N) using ATR-FTIR spectroscopy (Fig. 6). The ν(C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) of [(−)-D4-(Por)FeII(:CHCN)] is observed at 2178 cm−1, which is different from the organic species NCCH
N) of [(−)-D4-(Por)FeII(:CHCN)] is observed at 2178 cm−1, which is different from the organic species NCCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCN (trans) (2235 cm−1) and CH(CN)N2 (2100 cm−1). This excludes the possibility that Fe(II) porphyrin is mixed with organic species after treatment with diazoacetonitrile and the possible dimeric byproduct NCCH
CHCN (trans) (2235 cm−1) and CH(CN)N2 (2100 cm−1). This excludes the possibility that Fe(II) porphyrin is mixed with organic species after treatment with diazoacetonitrile and the possible dimeric byproduct NCCH![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CHCN coordinated with the Fe(II)porphyrin.
CHCN coordinated with the Fe(II)porphyrin.
Shortly after adding styrene (20 equiv.) to a solution of [(−)-D4-(por)FeII(:CHCN)] in C6D6 at room temperature, the cyclopropanation product was obtained in 98% yield. This enantioselectivity (−82% ee) is in good agreement with the value determined under catalytic conditions (84% ee by (+)-D4-(por)FeCl, Table 1, entry 2), showing that [(−)-D4-(por)FeII(:CHCN)] is an active reaction intermediate directly involved in the cyclopropanation reaction.
In the literature, Mansuy,86 Che15 and Hu87 reported the crystal structures of some Fe(porphyrin)–monocarbene complexes containing carbene moieties, such as [:CCl2], [:CPh2] and [:C(Ph)CO(morpholine)] (Fig. 7). On the other hand, the Fe(porphyrin)–carbene complex containing the [:CHR] carbene moiety was only characterized by 1H-NMR.82,88,89 In this work, we captured and characterized a highly reactive iron carbene containing a chiral porphyrin ligand and further performed the stoichiometric reaction of this chiral iron carbene intermediate with styrene. The enantioselectivity of the chiral cyclopropanation product was found to be comparable to that obtained under catalytic conditions, indicating that the chiral iron carbene intermediate is directly involved in and responsible for the asymmetric cyclopropanation reaction.
Based on the above experimental results, a plausible mechanism for the asymmetric cyclopropanation of alkenes catalyzed by (+)-D4-(por)FeCl was proposed (Scheme 8). Under the reaction conditions, (+)-D4-(por)Fe(III)Cl is reduced in situ to give [(+)-D4-(por)Fe(II)(L)] (L = H2O or MeIm) (Int-1). After further reaction with diazoacetonitrile, the Fe(II)–carbene intermediate [(+)-D4-(por)FeII(:CHCN)(MeIm)] (Int-2) was generated and detected by HR ESI-MS (cal. 1317.6058 m/z, found 1317.6054 m/z, Fig. S6†). The sterically bulky D4-porphyrin ligand provides a chiral environment, resulting in enantioselectivity of the carbene transfer reaction. Styrene attacks the iron–carbene intermediate in different directions, producing two transition states, one of which is energetically unfavorable (TS-avs.TS-b). The favorable conformation (TS-a) dominates during the reaction and produces the (1S,2S)-isomer as the major product.
Conclusions
In summary, by using chiral iron porphyrin (+)-D4-(por)FeCl as the catalyst and diazoacetonitrile (in situ generated) as the carbene precursor, we have achieved the iron-catalyzed arylalkene cyclopropanation reaction with a broad substrate scope, excellent diastereoselectivity, high enantioselectivity, and good product yield. Reactive chiral Fe–carbene intermediates have been characterized by 1H NMR, HR ESI-MS, UV-vis and ATR-FTIR spectroscopy. The scalable reaction and product TONs up to 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 demonstrate the versatility of our approach in preparing key intermediates for the synthesis of bioactive or drug-like molecules using chiral iron porphyrin catalysts.
000 demonstrate the versatility of our approach in preparing key intermediates for the synthesis of bioactive or drug-like molecules using chiral iron porphyrin catalysts.
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data have been deposited at the CCDC under 2349759 (2g), 2349760 (2l), 2349761 (2z), 2349764 (2aa), and 2351894 (2ad).Author contributions
The manuscript was written through contributions of all authors. All authors have given approval to the final version of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the Southern University of Science and Technology for financial support. This work was supported by the General Research Fund from the Hong Kong Research Grants Council (17315122), the research grant from the Innovation and Technology Commission (HKSAR, China) to the State Key Laboratory of Synthetic Chemistry, and the Laboratory for Synthetic Chemistry and Chemical Biology under the Health@InnoHK Program launched by the Innovation and Technology Commission, Hong Kong, P. R. China.References
- T. T. Talele, The “Cyclopropyl Fragment” is a Versatile Player that Frequently Appears in Preclinical/Clinical Drug Molecules, J. Med. Chem., 2016, 59, 8712–8756 CrossRef CAS PubMed.
- Z. Časar, Synthetic Approaches to Contemporary Drugs that Contain the Cyclopropyl Moiety, Synthesis, 2020, 52, 1315–1345 CrossRef.
- C. Ebner and E. M. Carreira, Cyclopropanation Strategies in Recent Total Syntheses, Chem. Rev., 2017, 117, 11651–11679 CrossRef CAS PubMed.
- W. Wu, Z. Lin and H. Jiang, Recent advances in the synthesis of cyclopropanes, Org. Biomol. Chem., 2018, 16, 7315–7329 RSC.
- G. L. Beutner and D. T. George, Opportunities for the Application and Advancement of the Corey–Chaykovsky Cyclopropanation, Org. Process Res. Dev., 2023, 27, 10–41 CrossRef CAS.
- D. Intrieri, D. M. Carminati and E. Gallo, The ligand influence in stereoselective carbene transfer reactions promoted by chiral metal porphyrin catalysts, Dalton Trans., 2016, 45, 15746–15761 RSC.
- M. J. Weissenborn and R. M. Koenigs, Iron-porphyrin Catalyzed Carbene Transfer Reactions – an Evolution from Biomimetic Catalysis towards Chemistry-inspired Non-natural Reactivities of Enzymes, ChemCatChem, 2020, 12, 2171–2179 CrossRef CAS.
- C. Damiano, P. Sonzini and E. Gallo, Iron catalysts with N-ligands for carbene transfer of diazo reagents, Chem. Soc. Rev., 2020, 49, 4867–4905 RSC.
- V. F. Batista, D. C. G. A. Pinto and A. M. S. Silva, Iron: A Worthy Contender in Metal Carbene Chemistry, ACS Catal., 2020, 10, 10096–10116 CrossRef CAS.
- P. Kaur and V. Tyagi, Recent Advances in Iron-Catalyzed Chemical and Enzymatic Carbene-Transfer Reactions, Adv. Synth. Catal., 2021, 363, 877–905 CrossRef CAS.
- S. Rana, J. P. Biswas, S. Paul, A. Paik and D. Maiti, Organic synthesis with the most abundant transition metal-iron: from rust to multitasking catalysts, Chem. Soc. Rev., 2021, 50, 243–472 RSC.
- M. Brookhart and G. O. Nelson, Isolation of stable secondary cationic iron carbene complexes dicarbonyl(η5-cyclopentadienyl)phenylcarbeneiron hexafluorophosphate and carbonyl(η5-cyclopentadienyl)triphenylphosphine(phenylcarbene)iron hexafluorophosphate, J. Am. Chem. Soc., 1977, 99, 6099–6101 CrossRef CAS.
- P. Helquist, in Advances in Metal–Organic Chemistry, ed. L. S. Liebeskind, JAI, 1991, vol. 2, pp. 143–194 Search PubMed.
- J. R. Wolf, C. G. Hamaker, J.-P. Djukic, T. Kodadek and L. K. Woo, Shape and stereoselective cyclopropanation of alkenes catalyzed by iron porphyrins, J. Am. Chem. Soc., 1995, 117, 9194–9199 CrossRef CAS.
- Y. Li, J.-S. Huang, Z.-Y. Zhou, C.-M. Che and X.-Z. You, Remarkably Stable Iron Porphyrins Bearing Nonheteroatom-Stabilized Carbene or (Alkoxycarbonyl)carbenes: Isolation, X-ray Crystal Structures, and Carbon Atom Transfer Reactions with Hydrocarbons, J. Am. Chem. Soc., 2002, 124, 13185–13193 CrossRef CAS PubMed.
- I. Aviv and Z. Gross, Corrole-based applications, Chem. Commun., 2007, 1987–1999 RSC.
- B. Morandi and E. M. Carreira, Iron-Catalyzed Cyclopropanation with Trifluoroethylamine Hydrochloride and Olefins in Aqueous Media: In Situ Generation of Trifluoromethyl Diazomethane, Angew. Chem., Int. Ed., 2010, 49, 938–941 CrossRef CAS PubMed.
- B. Morandi and E. M. Carreira, Iron-Catalyzed Cyclopropanation in 6 M KOH with in Situ Generation of Diazomethane, Science, 2012, 335, 1471–1474 CrossRef CAS PubMed.
- Z. Gross, N. Galili and L. Simkhovich, Metalloporphyrin catalyzed asymmetric cyclopropanation of olefins, Tetrahedron Lett., 1999, 40, 1571–1574 CrossRef CAS.
- G. Du, B. Andrioletti, E. Rose and L. K. Woo, Asymmetric Cyclopropanation of Styrene Catalyzed by Chiral Macrocyclic Iron(II) Complexes, Organometallics, 2002, 21, 4490–4495 CrossRef CAS.
- P. Le Maux, S. Juillard and G. Simonneaux, Asymmetric Synthesis of Trifluoromethylphenyl Cyclopropanes Catalyzed by Chiral Metalloporphyrins, Synthesis, 2006, 1701–1704 CAS.
- I. Nicolas, T. Roisnel, P. L. Maux and G. Simonneaux, Asymmetric intermolecular cyclopropanation of alkenes by diazoketones catalyzed by Halterman iron porphyrins, Tetrahedron Lett., 2009, 50, 5149–5151 CrossRef CAS.
- T.-S. Lai, F.-Y. Chan, P.-K. So, D.-L. Ma, K.-Y. Wong and C.-M. Che, Alkene cyclopropanation catalyzed by Halterman iron porphyrin: participation of organic bases as axial ligands, Dalton Trans., 2006, 4845–4851 RSC.
- Y. Chen and X. P. Zhang, Asymmetric Cyclopropanation of Styrenes Catalyzed by Metal Complexes of D2-Symmetrical Chiral Porphyrin: Superiority of Cobalt over Iron, J. Org. Chem., 2007, 72, 5931–5934 CrossRef CAS PubMed.
- W.-C. C. Lee, D.-S. Wang, Y. Zhu and X. P. Zhang, Iron(III)-based metalloradical catalysis for asymmetric cyclopropanation via a stepwise radical mechanism, Nat. Chem., 2023, 15, 1569–1580 CrossRef CAS PubMed.
- D. Intrieri, S. Le Gac, A. Caselli, E. Rose, B. Boitrel and E. Gallo, Highly diastereoselective cyclopropanation of alpha-methylstyrene catalysed by a C2-symmetrical chiral iron porphyrin complex, Chem. Commun., 2014, 50, 1811–1813 RSC.
- D. M. Carminati, D. Intrieri, A. Caselli, S. Le Gac, B. Boitrel, L. Toma, L. Legnani and E. Gallo, Designing ‘Totem’ C2-Symmetrical Iron Porphyrin Catalysts for Stereoselective Cyclopropanations, Chem.–Eur. J., 2016, 22, 13599–13612 CrossRef CAS PubMed.
- C.-T. Yeung, K.-C. Sham, W.-S. Lee, W.-T. Wong, W.-Y. Wong and H.-L. Kwong, Cobalt and iron complexes of chiral C1- and C2-terpyridines: Synthesis, characterization and use in catalytic asymmetric cyclopropanation of styrenes, Inorg. Chim. Acta, 2009, 362, 3267–3273 CrossRef CAS.
- B. Wang, I. G. Howard, J. W. Pope, E. D. Conte and Y. Deng, Bis(imino)pyridine iron complexes for catalytic carbene transfer reactions, Chem. Sci., 2019, 10, 7958–7963 RSC.
- J. J. Shen, S. F. Zhu, Y. Cai, H. Xu, X. L. Xie and Q. L. Zhou, Enantioselective Iron-Catalyzed Intramolecular Cyclopropanation Reactions, Angew. Chem., Int. Ed., 2014, 53, 13188–13191 CrossRef CAS PubMed.
- H. Xu, Y. P. Li, Y. Cai, G. P. Wang, S. F. Zhu and Q. L. Zhou, Highly Enantioselective Copper- and Iron-Catalyzed Intramolecular Cyclopropanation of Indoles, J. Am. Chem. Soc., 2017, 139, 7697–7700 CrossRef CAS PubMed.
- J. G. Gober and E. M. Brustad, Non-natural carbenoid and nitrenoid insertion reactions catalyzed by heme proteins, Curr. Opin. Chem. Biol., 2016, 35, 124–132 CrossRef CAS PubMed.
- O. F. Brandenberg, R. Fasan and F. H. Arnold, Exploiting and engineering hemoproteins for abiological carbene and nitrene transfer reactions, Curr. Opin. Biotechnol., 2017, 47, 102–111 CrossRef CAS PubMed.
- Y. Yang and F. H. Arnold, Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer, Acc. Chem. Res., 2021, 54, 1209–1225 CrossRef CAS PubMed.
- R. Fasan and M. G. Siriboe, Engineered Myoglobin Catalysts for Asymmetric Intermolecular Cyclopropanation Reactions, Bull. Jpn. Soc. Coord. Chem., 2022, 80, 4–13 CrossRef PubMed.
- B. Wei, J. C. Sharland, P. Lin, S. M. Wilkerson-Hill, F. A. Fullilove, S. McKinnon, D. G. Blackmond and H. M. L. Davies, In Situ Kinetic Studies of Rh(II)-Catalyzed Asymmetric Cyclopropanation with Low Catalyst Loadings, ACS Catal., 2020, 10, 1161–1170 CrossRef CAS.
- K.-H. Chan, X. Guan, V. K.-Y. Lo and C.-M. Che, Elevated Catalytic Activity of Ruthenium(II)–Porphyrin-Catalyzed Carbene/Nitrene Transfer and Insertion Reactions with N-Heterocyclic Carbene Ligands, Angew. Chem., Int. Ed., 2014, 53, 2982–2987 CrossRef CAS PubMed.
- H. M. Key, P. Dydio, Z. Liu, J. Y. E. Rha, A. Nazarenko, V. Seyedkazemi, D. S. Clark and J. F. Hartwig, Beyond Iron: Iridium-Containing P450 Enzymes for Selective Cyclopropanations of Structurally Diverse Alkenes, ACS Cent. Sci., 2017, 3, 302–308 CrossRef CAS PubMed.
- M. Bordeaux, V. Tyagi and R. Fasan, Highly Diastereoselective and Enantioselective Olefin Cyclopropanation Using Engineered Myoglobin-Based Catalysts, Angew. Chem., Int. Ed., 2015, 54, 1744–1748 CrossRef CAS PubMed.
- P. S. Coelho, Z. J. Wang, M. E. Ener, S. A. Baril, A. Kannan, F. H. Arnold and E. M. Brustad, A serine-substituted P450 catalyzes highly efficient carbene transfer to olefins in vivo, Nat. Chem. Biol., 2013, 9, 485–487 CrossRef CAS PubMed.
- A. Ford, H. Miel, A. Ring, C. N. Slattery, A. R. Maguire and M. A. McKervey, Modern Organic Synthesis with alpha-Diazocarbonyl Compounds, Chem. Rev., 2015, 115, 9981–10080 CrossRef CAS PubMed.
- V. K. Aggarwal, J. de Vicente and R. V. Bonnert, Catalytic Cyclopropanation of Alkenes Using Diazo Compounds Generated in Situ. A Novel Route to 2-Arylcyclopropylamines, Org. Lett., 2001, 3, 2785–2788 CrossRef CAS PubMed.
- Q. Xiao, Y. Zhang and J. Wang, Diazo Compounds and N-Tosylhydrazones: Novel Cross-Coupling Partners in Transition-Metal-Catalyzed Reactions, Acc. Chem. Res., 2013, 46, 236–247 CrossRef CAS PubMed.
- P. K. Mykhailiuk, New Life for Diazoacetonitrile (N2CHCN): in situ Generation and Practical Synthesis of CN-Pyrazoles, Eur. J. Org Chem., 2015, 2015, 7235–7239 CrossRef CAS.
- Y. Ferrand, P. Le Maux and G. Simonneaux, Macroporous chiral ruthenium porphyrin polymers: a new solid-phase material used as a device for catalytic asymmetric carbene transfer, Tetrahedron: Asymmetry, 2005, 16, 3829–3836 CrossRef CAS.
- C. Empel, K. J. Hock and R. M. Koenigs, Dealkylative intercepted rearrangement reactions of sulfur ylides, Chem. Commun., 2019, 55, 338–341 RSC.
- K. J. Hock, A. Knorrscheidt, R. Hommelsheim, J. Ho, M. J. Weissenborn and R. M. Koenigs, Tryptamine Synthesis by Iron Porphyrin Catalyzed C−H Functionalization of Indoles with Diazoacetonitrile, Angew. Chem., Int. Ed., 2019, 58, 3630–3634 CrossRef CAS PubMed.
- K. J. Hock, R. Spitzner and R. M. Koenigs, Towards nitrile-substituted cyclopropanes – a slow-release protocol for safe and scalable applications of diazo acetonitrile, Green Chem., 2017, 19, 2118–2122 RSC.
- A. L. Chandgude and R. Fasan, Highly Diastereo- and Enantioselective Synthesis of Nitrile-Substituted Cyclopropanes by Myoglobin-Mediated Carbene Transfer Catalysis, Angew. Chem., Int. Ed., 2018, 57, 15852–15856 CrossRef CAS PubMed.
- P. K. Mykhailiuk and R. M. Koenigs, Diazoacetonitrile (N2CHCN): A Long Forgotten but Valuable Reagent for Organic Synthesis, Chem.–Eur. J., 2020, 26, 89–101 CrossRef CAS PubMed.
- V. K.-Y. Lo, K.-P. Shing and C.-M. Che, in Advances in Organometallic Chemistry, ed. P. J. Pérez, Academic Press, 2023, vol. 79, pp. 195–259 Search PubMed.
- H.-H. Wang, H. Shao, G. Huang, J. Fan, W.-P. To, L. Dang, Y. Liu and C.-M. Che, Chiral Iron Porphyrins Catalyze Enantioselective Intramolecular C(sp3)−H Bond Amination Upon Visible-Light Irradiation, Angew. Chem., Int. Ed., 2023, 62, e202218577 CrossRef CAS PubMed.
- B. Springthorpe, A. Bailey, P. Barton, T. N. Birkinshaw, R. V. Bonnert, R. C. Brown, D. Chapman, J. Dixon, S. D. Guile, R. G. Humphries, S. F. Hunt, F. Ince, A. H. Ingall, I. P. Kirk, P. D. Leeson, P. Leff, R. J. Lewis, B. P. Martin, D. F. McGinnity, M. P. Mortimore, S. W. Paine, G. Pairaudeau, A. Patel, A. J. Rigby, R. J. Riley, B. J. Teobald, W. Tomlinson, P. J. H. Webborn and P. A. Willis, From ATP to AZD6140: The discovery of an orally active reversible P2Y12 receptor antagonist for the prevention of thrombosis, Bioorg. Med. Chem. Lett., 2007, 17, 6013–6018 CrossRef CAS PubMed.
- M.-J. Huang, J.-W. Guo, Y.-D. Fu, Y.-Z. You, W.-Y. Xu, T.-Y. Song, R. Li, Z.-T. Chen, L.-H. Huang and H.-M. Liu, Discovery of new tranylcypromine derivatives as highly potent LSD1 inhibitors, Bioorg. Med. Chem. Lett., 2021, 41, 127993 CrossRef CAS PubMed.
- Y. Ota and T. Suzuki, Drug Design Concepts for LSD1-Selective Inhibitors, Chem. Rec., 2018, 18, 1782–1791 CrossRef CAS PubMed.
- S. Ahmad, L. M. Doweyko, S. Dugar, N. Grazier, K. Ngu, S. C. Wu, K. J. Yost, B.-C. Chen, J. Z. Gougoutas, J. D. DiMarco, S.-J. Lan, B. J. Gavin, A. Y. Chen, C. R. Dorso, R. Serafino, M. Kirby and K. S. Atwal, Arylcyclopropanecarboxyl Guanidines as Novel, Potent, and Selective Inhibitors of the Sodium Hydrogen Exchanger Isoform-1, J. Med. Chem., 2001, 44, 3302–3310 CrossRef CAS PubMed.
- S. Dhillon and M. Clarke, Tasimelteon: First Global Approval, Drugs, 2014, 74, 505–511 CrossRef CAS PubMed.
- S. Mi, X. Sun, C. Wu and X. Zhang, A Facile and Practical Synthesis of (-)-tasimelteon, J. Chem. Res., 2016, 40, 667–669 CrossRef CAS.
- X.-A. Li, L. Yue, J. Zhu, H. Ren, H. Zhang, D.-y. Hu, G. Han, J. Feng and Z.-d. Nan, Total synthesis of Tasimelteon, Tetrahedron Lett., 2019, 60, 1986–1988 CrossRef CAS.
- P. Bajaj, G. Sreenilayam, V. Tyagi and R. Fasan, Gram-Scale Synthesis of Chiral Cyclopropane-Containing Drugs and Drug Precursors with Engineered Myoglobin Catalysts Featuring Complementary Stereoselectivity, Angew. Chem., Int. Ed., 2016, 55, 16110–16114 CrossRef CAS PubMed.
- A. K. Singh, M. N. Rao, J. H. Simpson, W.-S. Li, J. E. Thornton, D. E. Kuehner and D. J. Kacsur, Development of a Practical, Safe, and High-Yielding Process for the Preparation of Enantiomerically Pure trans-Cyclopropane Carboxylic Acid, Org. Process Res. Dev., 2002, 6, 618–620 CrossRef CAS.
- W. Wang, X. Meng, J. Zhu and X. Zhang, An efficient and practical asymmetric synthesis of (−)-tasimelteon, Synth. Commun., 2019, 49, 129–135 CrossRef CAS.
- J. Weinstock, Notes- A Modified Curtious Reaction, J. Org. Chem., 1961, 26, 3511 CrossRef CAS.
- R. Csuk, M. J. Schabel and Y. v. Scholz, Synthesis of the enantiomer of the antidepressant tranylcypromine, Tetrahedron: Asymmetry, 1996, 7, 3505–3512 CrossRef CAS.
- M. Pannala, S. Kher, N. Wilson, J. Gaudette, I. Sircar, S.-H. Zhang, A. Bakhirev, G. Yang, P. Yuen, F. Gorcsan, N. Sakurai, M. Barbosa and J.-F. Cheng, Synthesis and structure–activity relationship of 4-(2-aryl-cyclopropylamino)-quinoline-3-carbonitriles as EGFR tyrosine kinase inhibitors, Bioorg. Med. Chem. Lett., 2007, 17, 5978–5982 CrossRef CAS PubMed.
- R. Yu, S. Z. Cai, C. Li and X. Fang, Nickel-Catalyzed Asymmetric Hydroaryloxy- and Hydroalkoxycarbonylation of Cyclopropenes, Angew. Chem., Int. Ed., 2022, 61, e202200733 CrossRef CAS PubMed.
- J. R. Burgeson, D. N. Gharaibeh, A. L. Moore, R. A. Larson, S. M. Amberg, T. C. Bolken, D. E. Hruby and D. Dai, Lead optimization of an acylhydrazone scaffold possessing antiviral activity against Lassa virus, Bioorg. Med. Chem. Lett., 2013, 23, 5840–5843 CrossRef CAS PubMed.
- J. A. Lewis, S. A. Scott, R. Lavieri, J. R. Buck, P. E. Selvy, S. L. Stoops, M. D. Armstrong, H. A. Brown and C. W. Lindsley, Design and synthesis of isoform-selective phospholipase D (PLD) inhibitors. Part I: Impact of alternative halogenated privileged structures for PLD1 specificity, Bioorg. Med. Chem. Lett., 2009, 19, 1916–1920 CrossRef CAS PubMed.
- B. Portevin, A. Benoist, G. Rémond, Y. Hervé, M. Vincent, J. Lepagnol and G. De Nanteuil, New Prolyl Endopeptidase Inhibitors: In Vitro and in Vivo Activities of Azabicyclo[2.2.2]octane, Azabicyclo[2.2.1]heptane, and Perhydroindole Derivatives, J. Med. Chem., 1996, 39, 2379–2391 CrossRef CAS PubMed.
- H. Wang, X. Zhou and Y. Mao, Asymmetric Synthesis of Cyclopropylamine Derivatives, Heterocycles, 2014, 89, 1767–1800 CrossRef CAS.
- J. Vallgårda and U. Hacksell, Stereoselective palladium-catalyzed cyclopropanation of α,β-unsaturated carboxylic acids derivatized with oppolzer's sultam, Tetrahedron Lett., 1991, 32, 5625–5628 CrossRef.
- S. Miyamura, K. Itami and J. Yamaguchi, Syntheses of Biologically Active 2-Arylcyclopropylamines, Synthesis, 2017, 49, 1131–1149 CrossRef CAS.
- X.-J. Dai, Y. Liu, X.-P. Xiong, L.-P. Xue, Y.-C. Zheng and H.-M. Liu, Tranylcypromine Based Lysine-Specific Demethylase 1 Inhibitor: Summary and Perspective, J. Med. Chem., 2020, 63, 14197–14215 CrossRef CAS PubMed.
- M. D. Morin, Y. Wang, B. T. Jones, Y. Mifune, L. Su, H. Shi, E. M. Y. Moresco, H. Zhang, B. Beutler and D. L. Boger, Diprovocims: A New and Exceptionally Potent Class of Toll-like Receptor Agonists, J. Am. Chem. Soc., 2018, 140, 14440–14454 CrossRef CAS PubMed.
- R. Neelamegam, E. L. Ricq, M. Malvaez, D. Patnaik, S. Norton, S. M. Carlin, I. T. Hill, M. A. Wood, S. J. Haggarty and J. M. Hooker, Brain-Penetrant LSD1 Inhibitors Can Block Memory Consolidation, ACS Chem. Neurosci., 2012, 3, 120–128 CrossRef CAS PubMed.
- T. Maes, C. Mascaró, D. Rotllant, M. M. P. Lufino, A. Estiarte, N. Guibourt, F. Cavalcanti, C. Griñan-Ferré, M. Pallàs, R. Nadal, A. Armario, I. Ferrer, A. Ortega, N. Valls, M. Fyfe, M. Martinell, J. C. Castro Palomino and C. Buesa Arjol, Modulation of KDM1A with vafidemstat rescues memory deficit and behavioral alterations, PLoS One, 2020, 15, e0233468 CrossRef CAS PubMed.
- Y.-C. Zheng, B. Yu, Z.-S. Chen, Y. Liu and H.-M. Liu, TCPs: privileged scaffolds for identifying potent LSD1 inhibitors for cancer therapy, Epigenomics, 2016, 8, 651–666 CrossRef CAS PubMed.
- T.-Y. Tsai, T. Hsu, C.-T. Chen, J.-H. Cheng, T.-K. Yeh, X. Chen, C.-Y. Huang, C.-N. Chang, K.-C. Yeh, S.-H. Hsieh, C.-H. Chien, Y.-W. Chang, C.-H. Huang, Y.-W. Huang, C.-L. Huang, S.-H. Wu, M.-H. Wang, C.-T. Lu, Y.-S. Chao and W.-T. Jiaang, Novel trans-2-aryl-cyclopropylamine analogues as potent and selective dipeptidyl peptidase IV inhibitors, Bioorg. Med. Chem., 2009, 17, 2388–2399 CrossRef CAS PubMed.
- T. R. Porter and J. M. Mayer, Radical reactivity of the Fe(III)/(II) tetramesitylporphyrin couple: hydrogen atom transfer, oxyl radical dissociation, and catalytic disproportionation of a hydroxylamine, Chem. Sci., 2014, 5, 372–380 RSC.
- Y. Wei, A. Tinoco, V. Steck, R. Fasan and Y. Zhang, Cyclopropanations via Heme Carbenes: Basic Mechanism and Effects of Carbene Substituent, Protein Axial Ligand, and Porphyrin Substitution, J. Am. Chem. Soc., 2018, 140, 1649–1662 CrossRef CAS PubMed.
- H. X. Wang, Q. Wan, K. H. Low, C. Y. Zhou, J. S. Huang, J. L. Zhang and C. M. Che, Stable group 8 metal porphyrin mono- and bis(dialkylcarbene) complexes: synthesis, characterization, and catalytic activity, Chem. Sci., 2020, 11, 2243–2259 RSC.
- C. G. Hamaker, G. A. Mirafzal and L. K. Woo, Catalytic Cyclopropanation with Iron(II) Complexes, Organometallics, 2001, 20, 5171–5176 CrossRef CAS.
- J. P. Collman, P. J. Brothers, L. McElwee-White, E. Rose and L. J. Wright, Cleavage of ruthenium and osmium porphyrin dimers: formation of organometallic ruthenium porphyrin complexes and highly reduced metalloporphyrin species, J. Am. Chem. Soc., 1985, 107, 4570–4571 CrossRef CAS.
- J. P. Collman, E. Rose and G. D. Venburg, Reactivity of ruthenium 5,10,15,20-tetramesitylporphyrin towards diazoesters: formation of olefins, J. Chem. Soc., Chem. Commun., 1993, 934–935 RSC.
- Q. H. Deng, J. Chen, J. S. Huang, S. S. Y. Chui, N. Zhu, G. Y. Li and C. M. Che, Trapping Reactive Metal–Carbene Complexes by a Bis-Pocket Porphyrin: X-ray Crystal Structures of Ru=CHCO2Et and trans-[Ru(CHR)(CO)] Species and Highly Selective Carbenoid Transfer Reactions, Chem.–Eur. J., 2009, 15, 10707–10712 CrossRef CAS PubMed.
- D. Mansuy, M. Lange, J. C. Chottard, J. F. Bartoli, B. Chevrier and R. Weiss, Dichlorocarbene Complexes of Iron(II)-Porphyrins–Crystal and Molecular Structure of Fe(TPP)(CCl2)(H2O), Angew. Chem., Int. Ed., 1978, 17, 781–782 CrossRef.
- C. Ma, S. Wang, Y. Sheng, X.-L. Zhao, D. Xing and W. Hu, Synthesis and Characterization of Donor–Acceptor Iron Porphyrin Carbenes and Their Reactivities in N–H Insertion and Related Three-Component Reaction, J. Am. Chem. Soc., 2023, 145, 4934–4939 CrossRef CAS PubMed.
- J. P. Battioni, J. C. Chottard and D. Mansuy, A new route to thiocarbonyliron complexes: preparation of FeII[porphyrin][C(Cl)SR] carbene complexes and their conversion into FeII[porphyrin][CS] complexes, Inorg. Chem., 1982, 21, 2056–2062 CrossRef CAS.
- I. Artaud, N. Gregoire, P. Leduc and D. Mansuy, Formation and fate of iron–carbene complexes in reactions between a diazoalkane and iron-porphyrins: relevance to the mechanism of formation of N-substituted hemes in cytochrome P-450-dependent oxidation of sydnones, J. Am. Chem. Soc., 1990, 112, 6899–6905 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and copies of 1H NMR and 13C NMR spectra. CCDC 2349759 (2g), 2349760 (2l), 2349761 (2z), 2349764 (2aa), and 2351894 (2ad). For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5sc00461f |
| This journal is © The Royal Society of Chemistry 2025 |