Open Access Article
Open Access ArticleAnion-endowed high-dielectric water-deficient interface towards ultrastable Zn metal batteries†
Xiangjie
Liu
a,
Xiaoxin
Nie
a,
Yujiao
Yang
a,
Meng
Yao
 *b,
Jiaxian
Zheng
c,
Hanfeng
Liang
*b,
Jiaxian
Zheng
c,
Hanfeng
Liang
 *c,
Mi
Zhou
d,
Jin
Zhao
e,
Yingqian
Chen
f and
Du
Yuan
*c,
Mi
Zhou
d,
Jin
Zhao
e,
Yingqian
Chen
f and
Du
Yuan
 *a
*a
aCollege of Materials Science and Engineering, Changsha University of Science and Technology, 960, 2nd Section, Wanjiali RD (S), Changsha, Hunan 410004, China. E-mail: aduyuandu@outlook.com; yuandu@csust.edu.cn
bCollege of Materials Science and Engineering, Sichuan University, Chengdu, Sichuan 610065, China. E-mail: yaomeng@scu.edu.cn
cState Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China. E-mail: hfliang@xmu.edu.cn
dNingbo Merak Advanced Materials Technology Co., Ltd, Lane 189, Canghai Road, Ningbo High-tech Zone, Zhejiang 315100, China
eState Key Laboratory of Organic Electronics and Information Displays & Institute of Advanced Materials (IAM), Nanjing University of Posts & Telecommunications, 9 Wenyuan Road, Nanjing 210023, China
fDepartment of Chemistry, National University of Singapore, Block S8, 3 Science Drive 3, Singapore 117543, Singapore
First published on 13th March 2025
Abstract
To achieve reversible metallic Zn anodes for aqueous rechargeable zinc batteries, regulating the electrolyte–Zn interface is the key to addressing the side reactions on Zn. Beyond water-deficiency, design rules for constructing the highly efficient electrochemical interface are still vague. Anions, as primary electrolyte constituents, not only play a role in solvation structure, but also influence the electrolyte–Zn interface. Here, the characteristics of representative anions in current aqueous zinc electrolytes are surveyed. A candidate combining polarizability, H-bond tuning ability and high solubility is proposed to construct a high-dielectric water-deficient electrolyte–Zn interface to regulate the interfacial chemistry on Zn. The anion-dominated electrochemical interface promotes the Zn deposition kinetics and achieves uniform Zn deposition with high stability, which further enables the in situ formation of an SEI for highly stable Zn stripping/plating, e.g., at 20 mA cm−2 and 20 mA h cm−2. Furthermore, this built-in interface exhibits an effect in stabilizing the V2O5 cathode, endowing the V2O5/Zn cell with ultra-stable long-term cycling, e.g., 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 with a high retention rate of 89.7%. Our design offers insight into guidelines for the development of novel electrolytes towards rationally designed electrochemical interfaces.
000 cycles at 10 A g−1 with a high retention rate of 89.7%. Our design offers insight into guidelines for the development of novel electrolytes towards rationally designed electrochemical interfaces.
Introduction
Aqueous rechargeable batteries are attracting attention as next-generation energy storage alternatives. Benefitting from their metallic Zn anode, zinc metal batteries (ZMBs) have the merits of natural abundance, promising electrochemical performance, and environmental friendliness.1–3 However, uncontrolled detrimental water-induced side reactions lead to deterioration in the lifespan of the Zn anode.4–7 Water molecules can be reduced on Zn, where the hydrogen evolution reaction (HER) leads to an increase in local pH. This causes the uncontrollable formation of surface by-products, and the resulting uneven surface aggravates the nonuniform distribution of the electric field, accelerating dendrite growth and the HER. The above entangled factors significantly reduce the Zn utilization efficiency. To regulate the interfacial reactions on Zn anodes, a water-deficient inner Helmholtz plane (IHP) in an electric double layer (EDL) is typically proposed, which depresses water activity by altering the hydrogen bond (H-bond) characteristics via zinc salt concentration, additive, gelation, etc.;8–23 however, these approaches have been insufficient to achieve the stable high utilization of Zn.In the emerging zinc salts for ZMBs,24–29 organic anions play an indispensable role in reshaping solvation structure from the conventional [Zn(H2O)6]2+ configuration and H-bond network, based on their metal chelating characteristics and hydrophilicity/hydrophobicity. Unlike the solvent-dominant EDL in conventional nonaqueous electrolytes, anions can present strong affinity to the metal surface, causing water solvent molecules to be repelled. Hence, it is imperative to provide a comprehensive insight into the contributions of anions to constructing IHPs via the tuning of zincophilic interactions and H-bond characteristics. Additionally, the dielectric constant (i.e., relative permittivity) represents the response of a material to an external electric field, where the regulated migration of charged species generates a collective electric dipole moment. The importance of dielectric properties is being underscored for rechargeable batteries,30e.g., dielectric solvent protocols to manipulate interphase characteristics in liquid state electrolytes,31,32 lithium salt dissociation in solid state polymer electrolytes,33 and built-in field promoted ion transport in 3D gradient hosts.34 In addition to influencing the solvation structure, the dielectric properties inherent to the molecular species in electrolytes could fine-tune the EDL region via intermolecular fields, facilitate charge transfer, diminish the ion concentration gradient and circumvent ion depletion to suppress dendrite growth. However, the impact of the dielectric contribution from anions has rarely been explored in aqueous electrolytes. Further, the formation of an in situ solid-electrolyte interface (SEI) is a feasible approach to address the deterioration of the interphase due to water-induced reactions; the constituents of the EDL and their electrochemical stabilities determine the SEI characteristics. Among the current novel approaches towards the formation of in situ SEIs on Zn, such as electrolyte additives,35,36 aqueous/non-aqueous hybrid solvents,29 and water-in-salt electrolytes,37 the ability to form anion-derived SEIs is highly attractive for constructing stable electrolyte–Zn interfaces,38 where the relatively positive reduction potential of Zn0 (−0.76 V vs. SHE) compared with that of Li0 makes the decomposition of anions in the electrolyte system become contentious.39–41 Hence, the effects of anion-dominant IHPs on interfacial reactions on Zn warrants scrutinization.
Herein, the characteristics of representative anions in aqueous zinc electrolytes are first surveyed. Based on the correlated intrinsic properties, anions combining polarizability, H-bond tuning ability and aqueous solubility for the construction of a high-dielectric water-deficient electrolyte–Zn interface are proposed (Fig. 1a). We then demonstrate the tuning of the EDL, which impacts the interfacial chemistry on Zn, via a saturated fluorine-free hydrophilic eco-friendly anion without the use of additives or nonaqueous co-solvents. The anion-dominated high-dielectric interface can effectively repel water molecules and favor the desolvation process as verified by MD, thus promoting Zn deposition kinetics and achieving uniform Zn deposition. It further enables the in situ formation of an SEI for highly stable Zn stripping/plating, e.g. at 20 mA cm−2 and 20 mA h cm−2. Further, the V2O5/Zn ZMB cell exhibits ultra-stable long-term cycling, e.g., 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1 with a high retention rate of 89.7%, and the role of the electrochemical interface in stabilizing the V2O5 cathode is revealed. We further demonstrate the extension of the high-dielectric interface via a gel electrolyte with the same type of anion.
000 cycles at 10 A g−1 with a high retention rate of 89.7%, and the role of the electrochemical interface in stabilizing the V2O5 cathode is revealed. We further demonstrate the extension of the high-dielectric interface via a gel electrolyte with the same type of anion.
Results and discussion
Correlation among the intrinsic properties of anions in aqueous zinc electrolytes
Via the Clausius–Mossotti relation, the macroscopic dielectric constant (εr) is correlated with microscopic polarizability (α).42 To explore the contribution of anions to the dielectric properties, an investigation of the molecular polarizabilities of representative anions was first conducted using density functional theory (DFT) (Fig. 1b); their molecular polarizabilities followed the order PS− (phenolsulfonate) > OTf− (trifluorosulfonate) > SA− (sulfamate) > SO42− (sulfate) > OAc− (acetate). The ESP values of anions represent their electronegativities,43 and the calculated ESP values followed the order OTf− > SA− > PS− > OAc− > SO42− (Fig. 1c). A more negative electrostatic potential (ESP) value indicates stronger interaction between opposite charges, suggesting high rigidity of the charge distribution and consequently low α. Except for PS−, the decreasing α values with anions show increasing ESP values. The largest ESP value of SO42− can be attributed to its divalent nature, and the discrepancy for PS− might be ascribed to the delocalization of electrons on the benzene ring.The dielectric properties of the corresponding aqueous zinc electrolytes for ZMB were then measured (Fig. 1d) at standardized molar concentrations and at their saturation concentrations. Among the saturated electrolytes, 4 M Zn(SA)2 presents the highest dielectric constant; the dielectric constants of the saturated zinc electrolytes follow the order 4 M Zn(SA)2 > 3 M Zn(OTf)2 > 3 M ZnSO4 > 2 M Zn(OAc)2 > 1.6 M Zn(PS)2. Note that the trend of the measured dielectric constants of the aqueous zinc electrolytes does not follow that of their anion polarizabilities exactly. Additionally, for a given zinc salt, increasing concentration leads to an enhanced dielectric constant. The above suggests that the dielectric contribution from the solvent, i.e., water molecules, cannot be overlooked. Thus, the aqueous solubilities of the zinc salts were considered. To dissolve an ionic solid, its lattice energy must be overcome by the solvent. A large εr value for an anion favors the dissociation of the metallic cation and anion and leads to strong ion–dipolar interactions with solvent molecules, promoting salt dissolution. However, this intuitive model is a simplified one, since the solubility of a salt is complex, involving hydrophobicity, ion-pair interactions, deep eutectic interactions, etc. As a result, the empirical saturation concentrations of the zinc salts were determined (Fig. 1e) and expressed explicitly as the molar ratios of zinc ions to water molecules. Among the candidates, Zn(SA)2 presents the highest solubility, i.e., the molar ratio of n(Zn2+)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) n(H2O) of 1
n(H2O) of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5.5, which could be ascribed to the large εr and H-bond donor/acceptor characteristics of SA−. The solubility trend of the aqueous zinc electrolytes coincides with that of their dielectric constants. This implies that anions with high polarizability and solubility are desirable in order to endow the electrolyte with promising dielectric properties.
5.5, which could be ascribed to the large εr and H-bond donor/acceptor characteristics of SA−. The solubility trend of the aqueous zinc electrolytes coincides with that of their dielectric constants. This implies that anions with high polarizability and solubility are desirable in order to endow the electrolyte with promising dielectric properties.
Further, the ability of anion to alter the water states as well as the H-bond network was evaluated by deconvoluting the –OH stretching in the Fourier-transform infrared (FTIR) spectra of the zinc electrolytes (Fig. 1f and S1†). Three types of water states can be differentiated,44,45 namely, weakly bonded isolated water (∼3550 cm−1), cluster water with an ice-like liquid state (∼3400 cm−1), and bulk water with an ice-like state (∼3240 cm−1); the relative proportion of free water was chosen to reflect the change in the water state. Among the candidates, 4 M Zn(SA)2 significantly reduced the relative proportion of free water to ∼6.0%. The ability of the anions to alter water state was further tested by fixing the molar ratios of the anion (SA−, OTf−, OAc−, SO42−) relative to the water molecules (Fig. S2†), and the promising ability of SA− was associated with it having both an H-bond acceptor and donor.
Based on the intercorrelated characteristics/intrinsic properties of the anions, the fluorine-free and eco-friendly anion SA− was selected to demonstrate the impact of a high-dielectric water-deficient electrolyte–Zn interface on regulating the interfacial chemistry.
Physicochemical properties of Zn(SA)2 electrolytes
The inherited vibrational characteristics of the aqueous Zn(SA)2 electrolytes from the corresponding zinc salt can be clearly observed in Fig. 2a (Fig. S3 and Table S1†), i.e., deformation of –NH2 at 1557 cm−1, symmetric and asymmetric stretching vibrations of –SO3− at 1245 and 1048 cm−1, and –SO3− deformation at 562 cm−1. Additionally, the concentration-dependent tuning of the water states in the Zn(SA)2 electrolytes shows that isolated and cluster water transform to bulk water as the electrolyte approaches saturation (Fig. S4†), i.e., the relative percentage of bulk water increases from 80.6% to 87.5%, while those of isolated and cluster water drop from 10.3% to 6.0% and 9.1% to 6.5%, respectively (Fig. 2b and c), which indicates the effective adjustment of water activity and reshaping of the H-bond network. The changes in H-bond induced by Zn(SA)2 were then verified via the deshielding effect as revealed in the 1H NMR spectra; the significant broadening was attributed to complex interactions surrounding the H2O molecules (Fig. S5†). To explore the solvation structure of Zn2+ in Zn(SA)2 electrolytes, molecular dynamics (MD) simulations of the radial distribution functions (RDF) and coordination number (CN) distribution functions were conducted. The relatively shorter Zn–O length for O in SA− (∼1.8 Å) than that for water molecules (∼1.9 Å) indicates the incorporation of SA− into the zinc solvation sheath (Fig. 2d). As the salt concentration increases, CNSA− drastically increases from 0.8 (2 M) to 1.6 (4 M), indicating the significant replacement of water molecules in the first solvation sheath with CNH2O decreasing from 4.7 to 3.5. DFT calculations revealed the much stronger binding energy of Zn2+–SA (−18.36 eV) compared to that of Zn2+–H2O (−4.73 eV) (Fig. 2e), indicating that Zn2+ prefers to bind with SA− rather than H2O. ESP mapping further confirmed the affinity of –SO3− to Zn2+, with the NH3+ group being exposed outwards (Fig. 2e). Thus, the MD snapshots in Fig. 2f display the solvation structure of 4 M Zn(SA)2 (see also Fig. S6†), where Zn2+ is coordinated with three H2O molecules and two SA− in 4 M Zn(SA)2.The physicochemical properties of Zn(SA)2 electrolytes were studied. The decreased water activity with increasing Zn(SA)2 concentration leads to a higher onset potential of the oxygen evolution reaction, increasing from 2.26 V to 2.67 V vs. Zn/Zn2+ for 4 M Zn(SA)2 with depressed current density compared to its counterpart (Fig. 2g), thus widening the electrochemical window from 2.32 V to 2.72 V vs. Zn/Zn2+ for 4 M Zn(SA)2. Note that the lower intensity of the redox peak could be attributed to slower ion transport as discussed below. Additionally, better anti-corrosion properties can be obtained by tuning the Zn(SA)2 concentration, with a positive shift of the corrosion voltage (from −0.94 V to −0.89 V vs. Ag/AgCl) and lower corrosion current density (from 5.90 to 5.28 mA cm−2) being observed with increasing the concentration to saturation (Fig. 2h). The ion transport behavior of the Zn(SA)2 electrolytes was also studied. The ion conductivity of the Zn(SA)2 electrolytes decreases from 73.92 to 34.85 mS cm−1 with increasing concentration, which could be related to its large solvation structure (Fig. 2i). The transference number (tZn2+) was also measured to understand the contribution of the cations to ion transport (Fig. 2i and S7†). The significant enhancement of tZn2+ from 0.24 (2 M) to 0.76 (4 M) suggests the hindrance of anion motion, which could be ascribed to the greater incorporation of anions in the solvation sheath in 4 M Zn(SA)2.
Zn deposition behavior on the Zn(SA)2–Zn interface
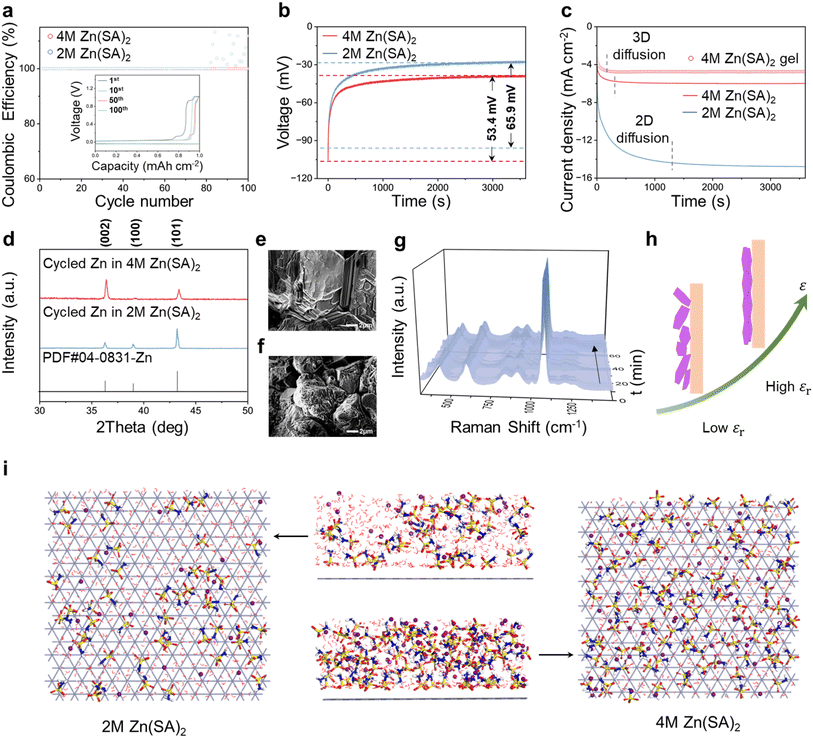
The impact of the Zn(SA)2–Zn interface on the Zn deposition behavior was then investigated. Coulombic efficiency (CE) was first evaluated by assembling Cu|Zn asymmetric cells under a constant current/capacity density of 1 mA cm−2 and 1 mA h cm−2. During cycling, the Cu|Zn cell with 4 M Zn(SA)2 exhibits a high CE of 99.9% from the first cycle, with the overpotential stabilizing from 76 mV (1st cycle) to 68 mV (10th cycle), 62 mV (50th cycle), and gradually to 60 mV (100th cycle) (Fig. 3a). In contrast, the cell with 2 M Zn(SA)2 presents fluctuating polarization after ∼82 cycles (Fig. S8†).Using chronopotentiometry, the nucleation overpotentials for the Cu|Zn cells with 4 M and 2 M Zn(SA)2 cells were determined to be 56.4 mV and 65.9 mV (Fig. 3b), respectively. The lower nucleation overpotential of the cell with 4 M Zn(SA)2 indicates its favorable nucleation kinetics, leading to more uniform Zn deposition. The deposition behaviors for the Zn(SA)2 electrolytes were further studied using chronoamperometry (CA) under a constant overpotential of −150 mV (Fig. 3c), which can sensitively reflect the changes in the active surface area during nucleation.46,47 The cell with 4 M Zn(SA)2 shows quick stabilization of the current density at ∼472 s compared to ∼1500 s for its 2 M counterpart, indicating a switch from a 2D mode (lateral nucleation/growth) to a 3D mode (vertical deposition). The fast switch to the 3D mode implies the saturation of the exposed surface area for Zn deposition, suggesting that 4 M Zn(SA)2 is more conducive to uniform Zn deposition. Scharifker–Hills analysis48 indicates that the CA of the 4 M Zn(SA)2 electrolyte closely approaches the curve predicted by the instantaneous nucleation theory (Fig. S9†). X-ray diffraction (XRD) spectra was performed for Zn electroplated on Cu (Fig. 3d). At an applied current/capacity density of 10 mA cm−2 and 10 mA h cm−2, the intensity ratio of (002)/(101) is 2.02 for the deposited Zn using 4 M Zn(SA)2, which is much higher than that of only 0.28 obtained using 2 M Zn(SA)2. Clearly, using 4 M Zn(SA)2 is more conducive to exposure of the Zn(002) crystal plane (see also Fig. S10† for Zn deposition at 1 mA cm−2 and 1 mA h cm−2). The exposure of the Zn(002) crystal planes using 4 M Zn(SA)2 can be clearly verified by SEM (Fig. 3e and S11†), in contrast to the diversely oriented crystal planes obtained using its 2 M counterpart with a much rougher surface morphology (Fig. 3f). Additionally, the promotion of uniform Zn (002) deposition is exemplified at 1 mA cm−2 and 1 mA h cm−2 (Fig. S12†).
The regulated deposition behavior reflects the importance of a high-dielectric interface. In situ Raman spectroscopy was then applied to monitor the vibrational species on the interface. Compared to the spectrum of the bulk electrolyte, the significantly blue-shifted O–S–O, degenerate –SO3− deformation, and symmetric –SO3− stretching at the electrolyte–Zn interface suggest the reshaping of the EDL by SA− (Fig. S13†). These interfacial vibrational features were well retained during Zn deposition (Fig. 3g), reflecting the stabilization of the EDL, which will be further explored. MD simulation was applied to visualize the anion-dominated interface for 4 M Zn(SA)2, in which water molecules are largely repelled from the interface (Fig. 3i and S14†). Accordingly, the merits of high-dielectric electrolyte–Zn have been depicted in Fig. 3h. The anion-induced high dielectric interface can effectively reshape the IHP, which presents a tendency to retain anion species.49 The reshaped IHP can regulate the interfacial electric field and homogenize ion flux,50 favoring interfacial ion transport and desolvation kinetics. This leads to an accelerated switch to the vertical growth mode and promotes uniform deposition with a Zn (002) texture, which can significantly suppress the growth of Zn dendrites,51–56 in contrast to the nonuniform and disoriented deposits obtained using its low dielectric counterpart.
Electrochemical performance of the Zn(SA)2–Zn interface
The rate performance of Zn stripping/plating in Zn|Zn symmetric cells was evaluated under a series of current densities from 0.1 to 20 mA cm−2 (Fig. 4a). The long-term cycling stability of the Zn|Zn cells using Zn(SA)2 electrolytes was compared at 10 mA cm−2 and 10 mA h cm−2 (Fig. 4b and S15†). The polarization of the symmetric cell cycled in 4 M Zn(SA)2 remained nearly constant (∼143.8 mV at 10th cycle) after 2000 h (∼112.2 mV), demonstrating its promising lifespan (see also Fig. S16† for comparison of 1 mA cm−2 and 1 mA h cm−2). The cell with 4 M Zn(SA)2 presents steady polarization even under a high current/capacity density of 20 mA cm−2 and 20 mA h cm−2 (DOD of ∼34%) (Fig. S17†), in contrast to the rapid shorting with 2 M Zn(SA)2; the symmetric cell with 2 M Zn(SA)2 shorts after only ∼100 h. In electrochemical impedance spectroscopy (EIS), the cell with 4 M Zn(SA)2 shows a lower charge transfer resistance (RCT) of 7.6 Ω than its 2 M Zn(SA)2 counterpart (17.7 Ω) before cycling (Fig. 4c, S18 and Table S2†). The situation is maintained after cycling, with a lower RCT (37.7 Ω) for 4 M Zn(SA)2 than its 2 M counterpart (56.4 Ω). This indicates the beneficial interfacial charge transfer by 4 M Zn(SA)2, favoring the reaction kinetics. This is further supported by the favored desolvation process in 4 M Zn(SA)2 during each dehydration step (Fig. S19†). Additionally, EIS analysis suggests the possible presence of an SEI on Zn after cycling in Zn(SA)2 electrolytes (RSEI of 13.9 Ω, Fig. S18†). After cycling in 4 M Zn(SA)2, the Zn anode is uniformly covered by a dark surface layer (Fig. 4d). An ex situ SEM study reveals that this surface layer consists of ∼2–5 μm plate-like structures (Fig. 4e) with a uniform distribution of N, O, S, and Zn observed through EDX mapping (Fig. 4f), and is ∼18 μm thick (Fig. 4g).To analyze the electrochemical interface, alternating current voltammetry was applied to determine the concentration-dependence of the reshaping of the EDL in Zn(SA)2 electrolytes (Fig. 4h). 4 M Zn(SA)2 results in a positive shift in the potential of zero charge (PZC, defined as the minimum capacitance) and a lower capacitance. These characteristics verify the design of a dielectric electrochemical interface via highly polarizable anions. A recent study presented a comprehensive understanding of SEI formation by sulfamate anions on Zn anodes, based on the hypothesized reduction of preferentially adsorbed SA−.19 The stability of the SA− anion was first studied to examine the details of the electrolyte–Zn interface. The frontier molecular orbitals, i.e., the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) of SA−, were calculated using DFT (Fig. S20†). Its LUMO was located at 0.52 eV, suggesting that SA− cannot be easily reduced.57–59 The reduction stability of SA− when coupled with metallic Zn was further investigated. The obtained reduction potential of SA− (−2.94 V vs. Zn/Zn2+) is well below that of Zn0 reduction (Fig. 4h), as evidenced by a symmetric redox pair at 1.18/0.88 V vs. Zn/Zn2+ uncovered in cyclic voltammetry (CV), which could be ascribed to the adsorption/desorption of SA− (Fig. S21†).60–62
Time-dependent ex situ FTIR-ATR and X-ray photoelectron spectroscopy (XPS) were correlated to monitor the SEI growth during subsequent plating/stripping steps. After the first charge, the deposition surface presents broad vibrational features corresponding to NH3+ symmetric stretching and degenerate rocking, and –SO3− symmetric deformation (Fig. 4i and Table S1†). In the corresponding stripped spectrum, the apparent features of NH3+ symmetric stretching, –NH2 stretching, –SO3− asymmetric stretching and symmetric deformation, and S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O appear with distinguishable –OH stretching. This is consistent with the surface vibrational characteristics after long-term cycling, namely, –NH2 stretching vibration (∼3172 cm−1), NH3+ and –NH2 groups (3085–2925 cm−1), symmetric NH3+ stretching (2880 cm−1), degenerate NH3+ deformation (1559 cm−1), asymmetric –SO3− stretching (1301 cm−1) and symmetric –SO3− deformation (1038 cm−1), which suggests the presence of SA− anions on the cycled surface. Accordingly, chemical information was collected for the deposition surface using XPS (Fig. 4j and k). After the first charge, NH2SO3− (170.0 and 168.7 eV, S 2p) and corresponding N–S (397.3 and 399.2 eV, N 1s) peaks are detected, supporting the presence of SA− during Zn deposition. In parallel, the presence of –SO3− (167.8 eV, S 2p) and –NH2 (400.8 eV, N 1s) can be observed in the Zn after the first discharge, and their relative proportions increase from 9.2% to 12.2% and from 24.23% to 37.9% after cycling (at the 100th cycle). Additionally, Zn–OH (Zn 2p) appears on the Zn after the first discharge, and its proportion increases from 33% to 41.4% (Fig. S22 and Table S3†). The XRD spectrum further displays a narrow diffraction peak emerging at ∼9.31°, reflecting the crystallinity of the surface structure (Fig. S23†). Consequently, the surface evolution information supports the formation of an SEI on Zn. Considering the electrochemical stability of SA−, it implies that the structure of the induced SEI could be attributed to the SA− incorporating zinc hydroxide complex Zn(SO3NH2)x(OH)y·nH2O.63 The ionic conductivity of this SEI is estimated to be ∼0.26 mS cm−1, which is comparable to those of currently reported novel surface layers for Zn anode.44,64–69
O appear with distinguishable –OH stretching. This is consistent with the surface vibrational characteristics after long-term cycling, namely, –NH2 stretching vibration (∼3172 cm−1), NH3+ and –NH2 groups (3085–2925 cm−1), symmetric NH3+ stretching (2880 cm−1), degenerate NH3+ deformation (1559 cm−1), asymmetric –SO3− stretching (1301 cm−1) and symmetric –SO3− deformation (1038 cm−1), which suggests the presence of SA− anions on the cycled surface. Accordingly, chemical information was collected for the deposition surface using XPS (Fig. 4j and k). After the first charge, NH2SO3− (170.0 and 168.7 eV, S 2p) and corresponding N–S (397.3 and 399.2 eV, N 1s) peaks are detected, supporting the presence of SA− during Zn deposition. In parallel, the presence of –SO3− (167.8 eV, S 2p) and –NH2 (400.8 eV, N 1s) can be observed in the Zn after the first discharge, and their relative proportions increase from 9.2% to 12.2% and from 24.23% to 37.9% after cycling (at the 100th cycle). Additionally, Zn–OH (Zn 2p) appears on the Zn after the first discharge, and its proportion increases from 33% to 41.4% (Fig. S22 and Table S3†). The XRD spectrum further displays a narrow diffraction peak emerging at ∼9.31°, reflecting the crystallinity of the surface structure (Fig. S23†). Consequently, the surface evolution information supports the formation of an SEI on Zn. Considering the electrochemical stability of SA−, it implies that the structure of the induced SEI could be attributed to the SA− incorporating zinc hydroxide complex Zn(SO3NH2)x(OH)y·nH2O.63 The ionic conductivity of this SEI is estimated to be ∼0.26 mS cm−1, which is comparable to those of currently reported novel surface layers for Zn anode.44,64–69
V2O5/Zn full cell
Further, the influence of anion-derived high-dielectric electrochemical interface on the performance of V2O5/Zn full cells was evaluated. Higher activity was observed for the cell with 4 M Zn(SA)2 than its 2 M counterpart or the benchmark 3 M Zn(OTf)2 (Fig. 5a). The cell with 4 M Zn(SA)2 also exhibits lower polarization gaps of ∼90 mV (peak l and 2) and ∼70 mV (peak 3 and 4) than those for 2 M Zn(SA)2 (∼200 mV and ∼120 mV) and 3 M Zn(OTf)2 (∼120 mV and ∼80 mV), suggesting faster reaction kinetics and better reversibility as supported by the stable CV cycling (Fig. S24†). The advantage of 4 M Zn(SA)2 is also supported by the rate performance (Fig. 5b). When the current density increases to 1 A g−1, the cell with 4 M Zn(SA)2 presents a capacity of 370.0 mA h g−1, which is higher than those of 2 M Zn(SA)2 (261.0 mA h g−1) and 3 M Zn(OTf)2 (336.2 mA h g−1). When the current density returns to 0.2 A g−1, the cell with 4 M Zn(SA)2 exhibits a higher capacity retention of 96.6% than its 2 M counterpart (88.0%) and 3 M Zn(OTf)2 (85.6%). The favorable kinetics in the full cell can be attributed to the reduced charge transfer resistance of the electrochemical interface achieved using 4 M Zn(SA)2 (Fig. S25 and Table S4†). | ||
| Fig. 5 Performance of ZMB cells. (a) CV curves of the V2O5/Zn cells with Zn(SA)2 and Zn(OTf)2 electrolytes at 0.1 mV s−1, in which two redox pairs, e.g., 0.63/0.54 and 1.04/0.97 in 4 M Zn(SA)2, can be attributed to H+/Zn2+ insertion/desertion in mixed valence state V2O5.22,70 (b) Rate performance of the full cells comparing the Zn(SA)2 and Zn(OTf)2 electrolytes. (c) Galvanostatic charge–discharge curves of the cell with 4 M Zn(SA)2 at a series of current densities from 0.1 to 10 A g−1. (d) Long-term cycling of the full cells with zinc aqueous electrolytes at 10 A g−1. (e) Ex situ XRD patterns of the cycled V2O5 cathodes in different electrolytes at 1 A g−1, which show the presence of crystalline phases of V2O5 (○) and ZVO (△). (f) Cycling of the full cell at 1 A g−1 with an N/P ratio of ∼1.25. (g) Pouch cell with the gel electrolyte cycled at 1 Ag−1. | ||
In the galvanostatic charge–discharge (GCD) curve at 0.1 A g−1 (Fig. 5c), the cell with 4 M Zn(SA)2 exhibits two distinct sloping discharge/charge regions at ∼0.5 and 1.2 V, consistent with the redox peaks in the CV curves (Fig. 5a), giving a capacity of 447.7 mA h g−1. The cycling stability of the V2O5/Zn cells was also assessed. At 1 A g−1, the cell with 4 M Zn(SA)2 can retain 88.0% of its maximum capacity after 3000 cycles with nearly 100.0% CE (Fig. S26†). In stark contrast, the cell with 2 M Zn(SA)2 presents only ∼20 mA h g−1 after 1000 cycles with a poor capacity retention of 5.4%, and that with 3 M Zn(OTf)2 also shows a low retention rate of 29.8% after 2000 cycles. Under a high current density of 10 A g−1, the cell with 4 M Zn(SA)2 delivers superior capacity retention of 89.7% even after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, compared to 5.3% and 64.2% for 2 M Zn(SA)2 and 3 M Zn(OTf)2 after only 2000 cycles (Fig. 5d). An ex situ study was conducted to investigate the change in the V2O5 cathode. After long cycling in 4 M Zn(SA)2, the cathode clearly exhibits a diffraction peak at ∼7.36° (corresponding to a slightly reduced (001) interlayer spacing of 12.0 Å compared to that of 14.1 Å calculated from the peak at ∼6.28° for the pristine state) (Fig. 5e), indicating the good retention of the layered structure of V2O5. In contrast, the cathodes in 2 M Zn(SA)2 and 3 M Zn(OTf)2 do not retain their original layered structure, but instead exhibit a phase change to zinc vanadate (ZVO, Zn3V2O7(OH)2·2H2O) (∼12.15° for (001)),71 leading to significant capacity decay. FTIR spectra (Fig. S27 and Table S5†) confirmed that the cathode cycled in 4 M Zn(SA)2 preserves the vibrational characteristics of the VOBV stretching (453 cm−1) and top VOA in-plane stretching (987 cm−1) with reference to the original V2O5.27 Based on the above, the superior cycling stability of V2O5/Zn with 4 M Zn(SA)2 could be attributed to the following. The electrochemical interface constructed by 4 M Zn(SA)2 favors uniform Zn deposition, promotes exposure of the electrochemically stable Zn (002) facets, and depresses the water activity and surface corrosion. Further, the uniform ion-conducting anion-induced SEI facilitates interfacial reactions compared to its OTf− counterpart during cycling. It thereby enhances the reversibility of Zn stripping/plating at high CEs. Together with the mildly acidic environment (pH of 4 M Zn(SA)2: ∼3.4), it shifts the chemical equilibrium of V2O5 + 3H2O ↔ 2VO2(OH)2− + 2H+ toward the stabilization of V2O5,71 where an organic-type surface layer formed on V2O5 with sulfonate groups (Fig. S27 and S28†) could effectively conduct cations, favoring the interfacial kinetics.72
000 cycles, compared to 5.3% and 64.2% for 2 M Zn(SA)2 and 3 M Zn(OTf)2 after only 2000 cycles (Fig. 5d). An ex situ study was conducted to investigate the change in the V2O5 cathode. After long cycling in 4 M Zn(SA)2, the cathode clearly exhibits a diffraction peak at ∼7.36° (corresponding to a slightly reduced (001) interlayer spacing of 12.0 Å compared to that of 14.1 Å calculated from the peak at ∼6.28° for the pristine state) (Fig. 5e), indicating the good retention of the layered structure of V2O5. In contrast, the cathodes in 2 M Zn(SA)2 and 3 M Zn(OTf)2 do not retain their original layered structure, but instead exhibit a phase change to zinc vanadate (ZVO, Zn3V2O7(OH)2·2H2O) (∼12.15° for (001)),71 leading to significant capacity decay. FTIR spectra (Fig. S27 and Table S5†) confirmed that the cathode cycled in 4 M Zn(SA)2 preserves the vibrational characteristics of the VOBV stretching (453 cm−1) and top VOA in-plane stretching (987 cm−1) with reference to the original V2O5.27 Based on the above, the superior cycling stability of V2O5/Zn with 4 M Zn(SA)2 could be attributed to the following. The electrochemical interface constructed by 4 M Zn(SA)2 favors uniform Zn deposition, promotes exposure of the electrochemically stable Zn (002) facets, and depresses the water activity and surface corrosion. Further, the uniform ion-conducting anion-induced SEI facilitates interfacial reactions compared to its OTf− counterpart during cycling. It thereby enhances the reversibility of Zn stripping/plating at high CEs. Together with the mildly acidic environment (pH of 4 M Zn(SA)2: ∼3.4), it shifts the chemical equilibrium of V2O5 + 3H2O ↔ 2VO2(OH)2− + 2H+ toward the stabilization of V2O5,71 where an organic-type surface layer formed on V2O5 with sulfonate groups (Fig. S27 and S28†) could effectively conduct cations, favoring the interfacial kinetics.72
Based on the above merits, a full cell with an N/P ratio of ∼1.25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (i.e., DODZn of 80%) was assembled using the Zn deposited by the Zn(SA)2 electrolyte, and showed steady cycling at 1 A g−1 with a retention rate of ∼100% (Fig. 5f). To further extend the proposed electrochemical interface in ZMBs, 4 M Zn(SA)2@polyacrylamide (PAAm) gel was adopted as an electrolyte via in situ polymerization, and greater enhancement of the dielectric properties was observed for the Zn(SA)2@PAAm gel (Fig. 1d). In terms of Zn deposition, the switch to the 3D mode can be further sped up to 152 s (Fig. 3c). The Zn(SA)2@PAAm gel provides a DOD of ∼85% at 50 mA cm−2 and 50 mA h cm−2, with a lifespan of over 400 h (Fig. S29†). A pouch cell for the gel electrolyte was then fabricated, which showed a promising retention rate of 96.2% at 1 A g−1 (Fig. 5g). The scalability and cost effectiveness were further estimated to demonstrate its potential application (Table S6†). The above demonstration verifies the importance of constructing a high-dielectric water-deficient interface for regulating the interface reactions in aqueous rechargeable batteries.
1 (i.e., DODZn of 80%) was assembled using the Zn deposited by the Zn(SA)2 electrolyte, and showed steady cycling at 1 A g−1 with a retention rate of ∼100% (Fig. 5f). To further extend the proposed electrochemical interface in ZMBs, 4 M Zn(SA)2@polyacrylamide (PAAm) gel was adopted as an electrolyte via in situ polymerization, and greater enhancement of the dielectric properties was observed for the Zn(SA)2@PAAm gel (Fig. 1d). In terms of Zn deposition, the switch to the 3D mode can be further sped up to 152 s (Fig. 3c). The Zn(SA)2@PAAm gel provides a DOD of ∼85% at 50 mA cm−2 and 50 mA h cm−2, with a lifespan of over 400 h (Fig. S29†). A pouch cell for the gel electrolyte was then fabricated, which showed a promising retention rate of 96.2% at 1 A g−1 (Fig. 5g). The scalability and cost effectiveness were further estimated to demonstrate its potential application (Table S6†). The above demonstration verifies the importance of constructing a high-dielectric water-deficient interface for regulating the interface reactions in aqueous rechargeable batteries.
Conclusion
Based on the intercorrelated intrinsic properties of anions for aqueous zinc electrolytes, a high-dielectric water-deficient electrolyte–Zn interface was constructed using an anion that combines polarizability and H-bond tuning ability; specifically, the representative anion sulfamate was selected. The concentration dependence of the physicochemical properties of Zn(SA)2 aqueous electrolytes was correlated with the solvation structure, which effectively tunes the H-bond characteristics as well as the transport behavior. The SA− anion-derived interface promotes a fast switch from the 2D to the 3D nucleation mode and achieves uniform Zn deposition with favorable (002) texture formation, whose stability was verified using in situ Raman spectroscopy. The constructed electrochemical interface facilitates highly stable Zn stripping/plating, e.g., at 10 mA cm−2 and 10 mA h cm−2, and the in situ formation of an anion-involved SEI was unveiled based on electrochemically stable SA− on the interface. Furthermore, the electrochemical interface enables the construction of a V2O5/Zn cell with ultra-stable long-term cycling, e.g., 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles at 10 A g−1, and its role in stabilizing the V2O5 cathode is revealed. The concept of a high-dielectric interface was extended to a gel electrolyte, the use of which achieved high utilization of Zn in a symmetric cell (DOD ∼85%) and a promising cycling stability of 96.2% at 1 A g−1 in a pouch cell. Hence, the proposed high-dielectric water-deficient interface provides a regulation platform for the promising use of metallic Zn anodes, which also provides guidelines for novel electrolyte design in emerging battery systems.
000 cycles at 10 A g−1, and its role in stabilizing the V2O5 cathode is revealed. The concept of a high-dielectric interface was extended to a gel electrolyte, the use of which achieved high utilization of Zn in a symmetric cell (DOD ∼85%) and a promising cycling stability of 96.2% at 1 A g−1 in a pouch cell. Hence, the proposed high-dielectric water-deficient interface provides a regulation platform for the promising use of metallic Zn anodes, which also provides guidelines for novel electrolyte design in emerging battery systems.
Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
X. Liu: conduct experiment, data analysis, draft the manuscript; X. Nie, Y. Yang, J. Zheng: conduct experiment, assist data discussion and analysis; M. Zhou: discussion on salt synthesis; J. Zhao: discussion on interface chemistry; M. Yao, H. Liang: conceptualization, data discussion, revise the manuscript; Y. Chen: calculation, data discussion; D. Yuan: conceptualization, experimental design, data analysis, revise and finalize the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
D. Yuan would like to acknowledge the funding support from the 100 Talented Team of Hunan Province (XiangZu [2016] 91), and the Natural Science Foundation of Hunan Province (2022JJ30613). M. Yao would like to acknowledge the National Natural Science Foundation of China (22408239) and Sichuan Science and Technology Program (2024NSFSC0987). H. Liang would like to acknowledge Fujian Provincial Science and Technology Program for External Cooperation, 2024I0001.References
- L. Tang, H. Peng, J. Kang, H. Chen, M. Zhang, Y. Liu, D. H. Kim, Y. Liu and Z. Lin, Zn-based batteries for sustainable energy storage: strategies and mechanisms, Chem. Soc. Rev., 2024, 53, 4877–4925 RSC.
- Z. Wu, Y. Wang and C. Zhi, Zinc-anode reversibility and capacity inflection as an evaluation criterion, Joule, 2024, 8, 2442–2448 CrossRef.
- J. Zhu, Z. Tie, S. Bi and Z. Niu, Towards More Sustainable Aqueous Zinc-Ion Batteries, Angew Chem. Int. Ed. Engl., 2024, 63, e202403712 CrossRef CAS PubMed.
- K. Roy, A. Rana, J. N. Heil, B. M. Tackett and J. E. Dick, For Zinc Metal Batteries, How Many Electrons go to Hydrogen Evolution? An Electrochemical Mass Spectrometry Study, Angew Chem. Int. Ed. Engl., 2024, 63, e202319010 CrossRef CAS PubMed.
- M. Li, Z. Li, X. Wang, J. Meng, X. Liu, B. Wu, C. Han and L. Mai, Comprehensive understanding of the roles of water molecules in aqueous Zn-ion batteries: from electrolytes to electrode materials, Energy Environ. Sci., 2021, 14, 3796–3839 RSC.
- J. Wei, P. Zhang, J. Sun, Y. Liu, F. Li, H. Xu, R. Ye, Z. Tie, L. Sun and Z. Jin, Advanced electrolytes for high-performance aqueous zinc-ion batteries, Chem. Soc. Rev., 2024, 53, 10335–10369 RSC.
- L. Ren, Z. Hu, C. Peng, L. Zhang, N. Wang, F. Wang, Y. Xia, S. Zhang, E. Hu and J. Luo, Suppressing metal corrosion through identification of optimal crystallographic plane for Zn batteries, Proc. Natl. Acad. Sci. U. S. A., 2024, 121, e2309981121 CrossRef CAS PubMed.
- F. Bu, Z. Sun, W. Zhou, Y. Zhang, Y. Chen, B. Ma, X. Liu, P. Liang, C. Zhong, R. Zhao, H. Li, L. Wang, T. Zhang, B. Wang, Z. Zhao, J. Zhang, W. Li, Y. S. Ibrahim, Y. Hassan, A. Elzatahry, D. Chao and D. Zhao, Reviving Zn0 Dendrites to Electroactive Zn2+ by Mesoporous MXene with Active Edge Sites, J. Am. Chem. Soc., 2023, 145, 24284–24293 CrossRef CAS PubMed.
- Z. Ju, T. Zheng, B. Zhang and G. Yu, Interfacial chemistry in multivalent aqueous batteries: fundamentals, challenges, and advances, Chem. Soc. Rev., 2024, 53, 8980–9028 RSC.
- W. Zhong, Z. Shen, J. Mao, S. Zhang, H. Cheng, Y. Kim and Y. Lu, Mitigating cathodic dissolution through interfacial water masking to enhance the longevity of aqueous zinc–ion batteries, Energy Environ. Sci., 2024, 17, 2059–2068 RSC.
- Y. Liang, M. Qiu, P. Sun and W. Mai, Comprehensive Review of Electrolyte Modification Strategies for Stabilizing Zn Metal Anodes, Adv. Funct. Mater., 2023, 33, 2304878 CrossRef CAS.
- G. Gao, X. Huo, B. Li, J. Bi, Z. Zhou, Z. Du, W. Ai and W. Huang, Customizing the water-scarce, zinc ion-rich Helmholtz plane of a zinc anode for Ah-scale Zn metal batteries, Energy Environ. Sci., 2024, 17, 7850–7859 RSC.
- Y. Pan, Z. Zuo, Y. Jiao and P. Wu, Constructing Lysozyme Protective Layer via Conformational Transition for Aqueous Zn Batteries, Adv. Mater., 2024, 36, 2314144 CrossRef CAS PubMed.
- S. Chen, Y. Xia, R. Zeng, Z. Luo, X. Wu, X. Hu, J. Lu, E. Gazit, H. Pan, Z. Hong, M. Yan, K. Tao and Y. Jiang, Ordered planar plating/stripping enables deep cycling zinc metal batteries, Sci. Adv., 2024, 10, eadn2265 CrossRef CAS PubMed.
- Z. Sun, F. Bu, Y. Zhang, W. Zhou, X. Li, X. Liu, H. Jin, S. Ding, T. Zhang, L. Wang, H. Li, W. Li, C. Zhang, D. Zhao, Y. Wang and D. Chao, Electron-Donating Conjugation Effect Modulated Zn2+ Reduction Reaction for Separator-Free Aqueous Zinc Batteries, Angew Chem. Int. Ed. Engl., 2024, 63, e202402987 CrossRef CAS PubMed.
- X. Cai, X. Wang, Z. Bie, Z. Jiao, Y. Li, W. Yan, H. J. Fan and W. Song, A Layer-by-Layer Self-Assembled Bio-Macromolecule Film for Stable Zinc Anode, Adv. Mater., 2024, 36, 2306734 CrossRef CAS PubMed.
- Y. Li, H. Yao, X. Liu, X. Yang and D. Yuan, Roles of electrolyte additive in Zn chemistry, Nano Res., 2023, 16, 9179–9194 CrossRef CAS.
- C. Yuan, L. Yin, P. Du, Y. Yu, K. Zhang, X. Ren, X. Zhan and S. Gao, Microgroove-patterned Zn metal anode enables ultra-stable and low-overpotential Zn deposition for long-cycling aqueous batteries, Chem. Eng. J., 2022, 442, 136231 CrossRef CAS.
- C. Yuan, J. Xiao, C. Liu and X. Zhan, Elucidating synergistic mechanisms of an anion–cation electrolyte additive for ultra-stable zinc metal anodes, J. Mater. Chem. A, 2024, 12, 19060–19068 RSC.
- H. Yao, Y. Li, Z. Chen, J. Chen, C. F. Du, Y. Chen, J. Chen, M. W. Wong, J. Zhao and D. Yuan, Anion Chemistry towards On-Site Construction of Solid-Electrolyte Interface for Highly Stable Metallic Zn Anode, Angew Chem. Int. Ed. Engl., 2024, 63, e202411056 CrossRef CAS PubMed.
- J.-L. Yang, L. Liu, Z. Yu, P. Chen, J. Li, P. A. Dananjaya, E. K. Koh, W. S. Lew, K. Liu, P. Yang and H. J. Fan, Dielectric–Metallic Double-Gradient Composition Design for Stable Zn Metal Anodes, ACS Energy Lett., 2023, 8, 2042–2050 CrossRef CAS.
- J. Wang, Y. Yu, R. Chen, H. Yang, W. Zhang, Y. Miao, T. Liu, J. Huang and G. He, Induced Anionic Functional Group Orientation-Assisted Stable Electrode-Electrolyte Interphases for Highly Reversible Zinc Anodes, Adv. Sci., 2024, 11, e2402821 CrossRef PubMed.
- P. Sun, L. Ma, W. Zhou, M. Qiu, Z. Wang, D. Chao and W. Mai, Simultaneous Regulation on Solvation Shell and Electrode Interface for Dendrite-Free Zn Ion Batteries Achieved by a Low-Cost Glucose Additive, Angew Chem. Int. Ed. Engl., 2021, 60, 18247–18255 CrossRef CAS PubMed.
- N. Zhang, F. Cheng, Y. Liu, Q. Zhao, K. Lei, C. Chen, X. Liu and J. Chen, Cation-Deficient Spinel ZnMn2O4 Cathode in Zn(CF3SO3)2 Electrolyte for Rechargeable Aqueous Zn-Ion Battery, J. Am. Chem. Soc., 2016, 138, 12894–12901 CrossRef CAS PubMed.
- X. Xu, H. Su, J. Zhang, Y. Zhong, Y. Xu, Z. Qiu, H. B. Wu, X. Wang, C. Gu and J. Tu, Sulfamate-Derived Solid Electrolyte Interphase for Reversible Aqueous Zinc Battery, ACS Energy Lett., 2022, 7, 4459–4468 CrossRef CAS.
- S. Chen, D. Ji, Q. Chen, J. Ma, S. Hou and J. Zhang, Coordination modulation of hydrated zinc ions to enhance redox reversibility of zinc batteries, Nat. Commun., 2023, 14, 3526 CrossRef CAS PubMed.
- D. Yuan, X. Li, H. Yao, Y. Li, X. Zhu, J. Zhao, H. Zhang, Y. Zhang, E. T. J. Jie, Y. Cai and M. Srinivasan, A Liquid Crystal Ionomer-Type Electrolyte toward Ordering-Induced Regulation for Highly Reversible Zinc Ion Battery, Adv. Sci., 2023, 10, 2206469 CrossRef CAS PubMed.
- H. Du, Y. Dong, Q.-J. Li, R. Zhao, X. Qi, W.-H. Kan, L. Suo, L. Qie, J. Li and Y. Huang, A New Zinc Salt Chemistry for Aqueous Zinc-Metal Batteries, Adv. Mater., 2023, 35, 2210055 CrossRef CAS PubMed.
- K. Zhou, G. Liu, X. Yu, Z. Li and Y. Wang, Carbonate Ester-Based Electrolyte Enabling Rechargeable Zn Battery to Achieve High Voltage and High Zn Utilization, J. Am. Chem. Soc., 2024, 146, 9455–9464 CrossRef CAS PubMed.
- J. Xu, H. Li, Y. Jin, D. Zhou, B. Sun, M. Armand and G. Wang, Understanding the Electrical Mechanisms in Aqueous Zinc Metal Batteries: From Electrostatic Interactions to Electric Field Regulation, Adv. Mater., 2024, 36, 2309726 CrossRef CAS PubMed.
- N. Yao, X. Chen, X. Shen, R. Zhang, Z.-H. Fu, X.-X. Ma, X.-Q. Zhang, B.-Q. Li and Q. Zhang, An Atomic Insight into the Chemical Origin and Variation of the Dielectric Constant in Liquid Electrolytes, Angew Chem. Int. Ed. Engl., 2021, 60, 21473–21478 CrossRef CAS PubMed.
- S. Zhang, R. Li, T. Deng, Q. Ma, X. Hong, H. Zhang, R. Zhang, S. Ding, Y. Wu, H. Zhu, M. Li, H. Zhang, D. Lu, B. Ma, L. Lv, Y. Li, L. Chen, Y. Shen, R. Guo and X. Fan, Oscillatory solvation chemistry for a 500 Wh kg−1 Li-metal pouch cell, Nat. Energy, 2024, 9, 1285–1296 CrossRef CAS.
- X. Guo, Z. Ju, X. Qian, Y. Liu, X. Xu and G. Yu, A Stable Solid Polymer Electrolyte for Lithium Metal Battery with Electronically Conductive Fillers, Angew Chem. Int. Ed. Engl., 2023, 62, e202217538 CrossRef CAS PubMed.
- Y. Zhang, M. Yao, T. Wang, H. Wu and Y. Zhang, A 3D Hierarchical Host with Gradient-Distributed Dielectric Properties toward Dendrite-free Lithium Metal Anode, Angew Chem. Int. Ed. Engl., 2024, 63, e202403399 CrossRef CAS PubMed.
- D. Xie, Y. Sang, D.-H. Wang, W.-Y. Diao, F.-Y. Tao, C. Liu, J.-W. Wang, H.-Z. Sun, J.-P. Zhang and X.-L. Wu, ZnF2-Riched Inorganic/Organic Hybrid SEI: in situ-Chemical Construction and Performance-Improving Mechanism for Aqueous Zinc-ion Batteries, Angew Chem. Int. Ed. Engl., 2023, 62, e202216934 CrossRef CAS PubMed.
- Q. Nian, X. Luo, D. Ruan, Y. Li, B.-Q. Xiong, Z. Cui, Z. Wang, Q. Dong, J. Fan, J. Jiang, J. Ma, Z. Ma, D. Wang and X. Ren, Highly reversible zinc metal anode enabled by strong Brønsted acid and hydrophobic interfacial chemistry, Nat. Commun., 2024, 15, 4303 CrossRef CAS PubMed.
- F. Wang, O. Borodin, T. Gao, X. Fan, W. Sun, F. Han, A. Faraone, J. A. Dura, K. Xu and C. Wang, Highly reversible zinc metal anode for aqueous batteries, Nat. Mater., 2018, 17, 543–549 CrossRef CAS PubMed.
- H. Yao, Y. Li, Z. Chen, J. Chen, C.-F. Du, Y. Chen, J. Chen, M. W. Wong, J. Zhao and D. Yuan, Anion Chemistry towards On-Site Construction of Solid-Electrolyte Interface for Highly Stable Metallic Zn Anode, Angew Chem. Int. Ed. Engl., 2024, 63, e202411056 CrossRef CAS PubMed.
- Y. Dong, L. Miao, G. Ma, S. Di, Y. Wang, L. Wang, J. Xu and N. Zhang, Non-concentrated aqueous electrolytes with organic solvent additives for stable zinc batteries, Chem. Sci., 2021, 12, 5843–5852 RSC.
- Y. Dai, J. Li, C. Zhang, R. Lu, X. Tao, K. A. Owusu, G. He, Y. Zhou and J. Lu, Fluorinated Interphase Enables Reversible Zn2+ Storage in Aqueous ZnSO4 Electrolytes, ACS Energy Lett., 2023, 8, 4762–4767 CrossRef CAS.
- X. Zeng, J. Mao, J. Hao, J. Liu, S. Liu, Z. Wang, Y. Wang, S. Zhang, T. Zheng, J. Liu, P. Rao and Z. Guo, Electrolyte Design for In Situ Construction of Highly Zn2+-Conductive Solid Electrolyte Interphase to Enable High-Performance Aqueous Zn-Ion Batteries under Practical Conditions, Adv. Mater., 2021, 33, 2007416 CrossRef CAS PubMed.
- C. Kittel, Introduction to Solid State Physics, John Wiley & Sons, 8th edn, 2004 Search PubMed.
- Q. Zhang, K. Xia, Y. Ma, Y. Lu, L. Li, J. Liang, S. Chou and J. Chen, Chaotropic Anion and Fast-Kinetics Cathode Enabling Low-Temperature Aqueous Zn Batteries, ACS Energy Lett., 2021, 6, 2704–2712 CrossRef CAS.
- W. Yuan, G. Ma, X. Nie, Y. Wang, S. Di, L. Wang, J. Wang, S. Shen and N. Zhang, In-situ construction of a hydroxide-based solid electrolyte interphase for robust zinc anodes, Chem. Eng. J., 2022, 431, 134076 CrossRef CAS.
- T. Petit, L. Puskar, T. Dolenko, S. Choudhury, E. Ritter, S. Burikov, K. Laptinskiy, Q. Brzustowski, U. Schade, H. Yuzawa, M. Nagasaka, N. Kosugi, M. Kurzyp, A. Venerosy, H. Girard, J.-C. Arnault, E. Osawa, N. Nunn, O. Shenderova and E. F. Aziz, Unusual Water Hydrogen Bond Network around Hydrogenated Nanodiamonds, J. Phys. Chem. C, 2017, 121, 5185–5194 CrossRef CAS.
- B. Scharifker and G. Hills, Theoretical and experimental studies of multiple nucleation, Electrochim. Acta, 1983, 28, 879–889 CrossRef CAS.
- R. Zhao, H. Wang, H. Du, Y. Yang, Z. Gao, L. Qie and Y. Huang, Lanthanum nitrate as aqueous electrolyte additive for favourable zinc metal electrodeposition, Nat. Commun., 2022, 13, 3252 CrossRef CAS PubMed.
- L. Benea and E. Danaila, Nucleation and Growth Mechanism of Ni/TiO2 Nanoparticles Electro-Codeposition, J. Electrochem. Soc., 2016, 163, D655 CrossRef CAS.
- S. Zhang, R. Li, T. Deng, Q. Ma, X. Hong, H. Zhang, R. Zhang, S. Ding, Y. Wu, H. Zhu, M. Li, H. Zhang, D. Lu, B. Ma, L. Lv, Y. Li, L. Chen, Y. Shen, R. Guo and X. Fan, Oscillatory solvation chemistry for a 500 Wh kg−1 Li-metal pouch cell, Nat. Energy, 2024, 9, 1285–1296 CrossRef CAS.
- Z. Ju, T. Zheng, B. Zhang and G. Yu, Interfacial chemistry in multivalent aqueous batteries: fundamentals, challenges, and advances, Chem. Soc. Rev., 2024, 53, 8980–9028 RSC.
- Z. Chen, Y. Wang, Q. Wu, C. Wang, Q. He, T. Hu, X. Han, J. Chen, Y. Zhang, J. Chen, L. Yang, X. Wang, Y. Ma and J. Zhao, Grain Boundary Filling Empowers (002)-Textured Zn Metal Anodes with Superior Stability, Adv. Mater., 2024, 2411004 CrossRef CAS PubMed.
- Z. Chen, Q. Wu, X. Han, C. Wang, J. Chen, T. Hu, Q. He, X. Zhu, D. Yuan, J. Chen, Y. Zhang, L. Yang, Y. Ma and J. Zhao, Converting Commercial Zn Foils into Single (002)-Textured Zn with Millimeter-Sized Grains for Highly Reversible Aqueous Zinc Batteries, Angew Chem. Int. Ed. Engl., 2024, 63, e202401507 CrossRef CAS PubMed.
- J. Zhang, W. Huang, L. Li, C. Chang, K. Yang, L. Gao and X. Pu, Nonepitaxial Electrodeposition of (002)-Textured Zn Anode on Textureless Substrates for Dendrite-Free and Hydrogen Evolution-Suppressed Zn Batteries, Adv. Mater., 2023, 35, 2300073 CrossRef CAS PubMed.
- Z. Hao, Y. Zhang, Z. Hao, G. Li, Y. Lu, S. Jin, G. Yang, S. Zhang, Z. Yan, Q. Zhao and J. Chen, Metal Anodes with Ultrahigh Reversibility Enabled by the Closest Packing Crystallography for Sustainable Batteries, Adv. Mater., 2023, 35, 2209985 CrossRef CAS PubMed.
- D. Yuan, J. Zhao, H. Ren, Y. Chen, R. Chua, E. T. J. Jie, Y. Cai, E. Edison, W. Manalastas Jr, M. W. Wong and M. Srinivasan, Anion Texturing Towards Dendrite-Free Zn Anode for Aqueous Rechargeable Batteries, Angew Chem. Int. Ed. Engl., 2021, 60, 7213–7219 CrossRef CAS PubMed.
- J. Zheng, Q. Zhao, T. Tang, J. Yin, C. D. Quilty, G. D. Renderos, X. Liu, Y. Deng, L. Wang, D. C. Bock, C. Jaye, D. Zhang, E. S. Takeuchi, K. J. Takeuchi, A. C. Marschilok and L. A. Archer, Reversible epitaxial electrodeposition of metals in battery anodes, Science, 2019, 366, 645–648 CrossRef CAS PubMed.
- X.-B. Cheng, R. Zhang, C.-Z. Zhao and Q. Zhang, Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review, Chem. Rev., 2017, 117, 10403–10473 CrossRef CAS PubMed.
- P. Peljo and H. H. Girault, Electrochemical potential window of battery electrolytes: the HOMO–LUMO misconception, Energy Environ. Sci., 2018, 11, 2306–2309 RSC.
- J. Lopez, D. G. Mackanic, Y. Cui and Z. Bao, Designing polymers for advanced battery chemistries, Nat. Rev. Mater., 2019, 4, 312–330 CrossRef CAS.
- C. Yan, H.-R. Li, X. Chen, X.-Q. Zhang, X.-B. Cheng, R. Xu, J.-Q. Huang and Q. Zhang, Regulating the Inner Helmholtz Plane for Stable Solid Electrolyte Interphase on Lithium Metal Anodes, J. Am. Chem. Soc., 2019, 141, 9422–9429 CrossRef CAS PubMed.
- X. Wang, M. Salari, D.-E. Jiang, J. Chapman Varela, B. Anasori, D. J. Wesolowski, S. Dai, M. W. Grinstaff and Y. Gogotsi, Electrode material–ionic liquid coupling for electrochemical energy storage, Nat. Rev. Mater., 2020, 5, 787–808 CrossRef CAS.
- R. Subbaraman, D. Strmcnik, V. Stamenkovic and N. M. Markovic, Three Phase Interfaces at Electrified Metal−Solid Electrolyte Systems 1. Study of the Pt(hkl)−Nafion Interface, J. Phys. Chem. C, 2010, 114, 8414–8422 CrossRef CAS.
- N. V. Zubkova, I. V. Pekov, N. V. Chukanov, D. A. Ksenofontov, V. O. Yapaskurt, S. N. Britvin and D. Y. Pushcharovsky, A New Sulfamate Cu3(OH)5[SO3(NH2)]: A Product of the Anthropogenic Alteration of Copper Sulfides, Moscow Univ. Geol. Bull., 2022, 77, 617–622 CrossRef.
- X. Guo, J. Lu, M. Wang, A. Chen, H. Hong, Q. Li, J. Zhu, Y. Wang, S. Yang, Z. Huang, Y. Wang, Z. Pei and C. Zhi, Solid-electrolyte interphase governs zinc ion transfer kinetics in high-rate and stable zinc metal batteries, Chem, 2024, 10, 3607–3612 CAS.
- P. Xiong, Y. Kang, N. Yao, X. Chen, H. Mao, W.-S. Jang, D. M. Halat, Z.-H. Fu, M.-H. Jung, H. Y. Jeong, Y.-M. Kim, J. A. Reimer, Q. Zhang and H. S. Park, Zn-Ion Transporting, In Situ Formed Robust Solid Electrolyte Interphase for Stable Zinc Metal Anodes over a Wide Temperature Range, ACS Energy Lett., 2023, 8, 1613–1625 CrossRef CAS.
- Y. Zhou, G. Li, S. Feng, H. Qin, Q. Wang, F. Shen, P. Liu, Y. Huang and H. He, Regulating Zn Ion Desolvation and Deposition Chemistry Toward Durable and Fast Rechargeable Zn Metal Batteries, Adv. Sci., 2023, 10, 2205874 CrossRef CAS PubMed.
- D. Han, C. Cui, K. Zhang, Z. Wang, J. Gao, Y. Guo, Z. Zhang, S. Wu, L. Yin, Z. Weng, F. Kang and Q.-H. Yang, A non-flammable hydrous organic electrolyte for sustainable zinc batteries, Nat. Sustainability, 2021, 5, 205–213 CrossRef.
- M. Yao, R. Pan, Y. Ren, Y. Fu, Y. Qin, C. Mao, Z. Zhang, X. Guo and G. Li, Regulating solvation shells and interfacial chemistry in zinc-ion batteries using glutaronitrile based electrolyte, J. Mater. Chem. A, 2022, 10, 14345–14354 RSC.
- J. Hao, B. Li, X. Li, X. Zeng, S. Zhang, F. Yang, S. Liu, D. Li, C. Wu and Z. Guo, An In-Depth Study of Zn Metal Surface Chemistry for Advanced Aqueous Zn-Ion Batteries, Adv. Mater., 2020, 32, e2003021 CrossRef PubMed.
- J. Zhao, H. Ren, Q. Liang, D. Yuan, S. Xi, C. Wu, W. Manalastas, J. Ma, W. Fang, Y. Zheng, C.-F. Du, M. Srinivasan and Q. Yan, High-performance flexible quasi-solid-state zinc-ion batteries with layer-expanded vanadium oxide cathode and zinc/stainless steel mesh composite anode, Nano Energy, 2019, 62, 94–102 CrossRef CAS.
- Y. Kim, Y. Park, M. Kim, J. Lee, K. J. Kim and J. W. Choi, Corrosion as the origin of limited lifetime of vanadium oxide-based aqueous zinc ion batteries, Nat. Commun., 2022, 13, 2371 CrossRef CAS PubMed.
- Y. Dai, C. Zhang, J. Li, X. Gao, P. Hu, C. Ye, H. He, J. Zhu, W. Zhang, R. Chen, W. Zong, F. Guo, I. P. Parkin, D. J. L. Brett, P. R. Shearing, L. Mai and G. He, Inhibition of Vanadium Cathodes Dissolution in Aqueous Zn-Ion Batteries, Adv. Mater., 2024, 36, 2310645 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc00364d |
| This journal is © The Royal Society of Chemistry 2025 |