Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
A new post-synthetic route to graft amino groups in porous organic polymers for CO2 capture†
Qihaoyue Wang‡
,
Lin Lin‡,
Li Jiang,
Zihao Wang,
Yina Zhang,
Qiance Han,
Xin Huang,
Changyan Zhu,
Jiangtao Jia *,
Zheng Bian
*,
Zheng Bian * and
Guangshan Zhu
* and
Guangshan Zhu *
*
Key Laboratory of Polyoxometalate and Reticular Material Chemistry of Ministry of Education, Faculty of Chemistry, Northeast Normal University, Changchun, Jilin 130024, China. E-mail: jiangtaojia@nenu.edu.cn; bianz070@nenu.edu.cn; zhugs@nenu.edu.cn
First published on 1st July 2025
Abstract
Herein, we report the development of a post-synthetic modification approach to introduce a high loading of formyl groups onto porous aromatic framework (PAF)-5 via Friedel–Crafts alkylation followed by hydrolysis. Rigorous characterization by NMR, X-ray photoelectron spectroscopy, and Fourier-transform infrared spectroscopy authenticated the successful integration of aldehyde moieties into PAF-5, affording PAF-5-CHO. Subsequent functionalization of PAF-5-CHO with various amines produced three amine-functionalized PAF derivatives. Notably, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA exhibited a 78% enhancement in carbon dioxide (CO2) adsorption capacity, reaching 3.78 mmol g−1 at 1 bar and 298 K relative to PAF-5-CHO. Breakthrough experiments demonstrated that PAF-5-C
N-EDA exhibited a 78% enhancement in carbon dioxide (CO2) adsorption capacity, reaching 3.78 mmol g−1 at 1 bar and 298 K relative to PAF-5-CHO. Breakthrough experiments demonstrated that PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA could effectively separate CO2 from simulated flue gas (CO2/N2 = 15
N-EDA could effectively separate CO2 from simulated flue gas (CO2/N2 = 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85, v/v; 10 mL min−1). In situ infrared spectroscopy, density functional theory calculations and temperature-programmed desorption studies provided insights into the CO2 adsorption mechanism.
85, v/v; 10 mL min−1). In situ infrared spectroscopy, density functional theory calculations and temperature-programmed desorption studies provided insights into the CO2 adsorption mechanism.
Introduction
Since the Industrial Revolution in the 18th century, the excessive exploitation and combustion of carbon-based fossil fuels such as coal, oil, and natural gas have driven a remarkable increase in carbon dioxide (CO2) emissions across the globe.1 As a result, severe environmental issues have emerged, including the greenhouse effect, ocean acidification, sea-level rise, and climate change, all of which pose significant threats to human well-being. Replacing traditional fossil fuels with clean energy sources such as solar, hydrogen, wind, or nuclear energy represents a promising strategy for mitigating CO2 emissions. However, the current exploitation and implementation of clean energy fall short of meeting the established emission reduction targets.2 Therefore, CO2 capture and storage (CCS), a process that separates CO2 from the flue gas of coal-fired power plants and stores it underground, remains a key strategy for reducing global CO2 emissions during the transition to clean energy.3There are five principal methods for capturing CO2: membrane separation, solvent-based absorption, physical adsorption, cryogenic separation, and chemical looping.4 Among these, aqueous amine solutions, which capture CO2 via the formation of carbamates or bicarbonates, remain the most mature and widely applied technology. However, aqueous amine systems suffer from significant drawbacks, including volatility, oxidative degradation, equipment corrosion, and high operational costs.5 Due to these limitations, several new CCS methods have emerged in recent years, among which solid porous materials, such as activated carbons, metal–organic frameworks (MOFs), porous organic polymers (POPs), silica, and zeolites, have received considerable attention and extensive research.6–11 These materials offer advantages like high adsorption efficiency, low energy consumption, stability, and ease of regeneration.
Post-synthetic modification (PSM) is a vital approach for developing and enhancing solid materials such as MOFs and POPs, offering extensive functionality and broad applicability.12–19 Incorporating CO2-philic functional groups into porous materials by PSM has been proven to enhance strong interactions between CO2 and materials, significantly improving their selectivity for CO2 capture.20–24 Porous aromatic frameworks (PAFs), a subset of POPs, possess high specific surface areas and porosities, which significantly enhance gas contact efficiency. PAFs are typically constructed through C–C covalent bonds, and their robust skeletons confer high chemical stability, enabling them to withstand harsh conditions during the PSM process. In previous studies, PSM has been used to introduce functional groups such as –SH, –NH2, –SO3H, and –Cl into PAF frameworks, enabling their applications in areas such as seawater desalination, gas adsorption, and catalysis.25–36 In addition, PAFs are ideal candidates for the incorporation of CO2-philic functional groups, which can strongly interact with CO2 and thus facilitate efficient capture.
In this study, we propose a new PSM strategy to enhance the CO2 capture efficiency of PAFs. This method allows for the simple and efficient introduction of aldehyde groups into the PAF framework by way of the Friedel–Crafts reaction, resulting in the synthesis of PAF-5-CHO. Notably, direct PSM of –CHO groups in the frameworks has not yet been reported. The aldehyde group has the potential to be transformed into different functional groups.37,38 Accordingly, PAF-5-CHO was further functionalized with amino groups via Schiff base reactions with organic amines aiming to enhance CO2 capture performance. The resulting series of amino-functionalized PAFs exhibit excellent CO2 capture and separation properties.
Results and discussion
Synthesis and characterization of PAF-5-CHO
PAF-5 was synthesized as reported39 and the detailed synthesis method of PAF-5-CHO is described in the Experimental section. The dichloromethylation of PAF-5 in chloroform under the activation of anhydrous AlCl3 and subsequent hydrolysis afforded PAF-5-CHO (Fig. 1a). In the solid-state carbon 13C NMR spectrum of PAF-5 (Fig. 1b), the peaks at 135 ppm and 120 ppm correspond to the substituted and unsubstituted carbons on phenyl rings, respectively.39 Compared to PAF-5, the spectrum of PAF-5-CHO (Fig. 1c) showed similar peaks at 133 ppm and 120 ppm for the substituted and unsubstituted carbons on phenyl rings, but with a different signal at 183 ppm. According to the literature,40 this peak was attributed to the carbon of the aldehyde group, confirming the successful introduction of aldehyde functional groups. The signals at 40 ppm and 67 ppm were attributed to the carbon of –CHCl– and –CHCl2 groups,41 respectively, indicating that partial –CHCl2 groups and –CHCl– groups remained unhydrolyzed due to the steric effect or dead-end pore (Fig. S1†). Additionally, in the Fourier transform infrared (FTIR) spectrum (Fig. 1d), the peak at 1700 cm−1 was characteristic of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond of aldehyde groups,23 while the peak at 783 cm−1 was attributed to the unhydrolyzed C–Cl bond.27 The formation mechanism of aldehyde groups in PAF-5-CHO by way of Friedel–Crafts alkylation and acidic hydrolysis is illustrated in Fig. 1e. Notably, the method to introduce –CHCl2 groups by Friedel–Crafts alkylation is limited to the rigid organic framework. In the rigid aromatic porous material, these –CHCl2 groups can't further react with other aromatic units, making them completely stay on the framework.
O bond of aldehyde groups,23 while the peak at 783 cm−1 was attributed to the unhydrolyzed C–Cl bond.27 The formation mechanism of aldehyde groups in PAF-5-CHO by way of Friedel–Crafts alkylation and acidic hydrolysis is illustrated in Fig. 1e. Notably, the method to introduce –CHCl2 groups by Friedel–Crafts alkylation is limited to the rigid organic framework. In the rigid aromatic porous material, these –CHCl2 groups can't further react with other aromatic units, making them completely stay on the framework.
Additionally, the high-resolution C 1s XPS spectrum of PAF-5 (Fig. S2†) shows only a single peak corresponding to C–C bonds. In contrast, the C 1s spectrum of PAF-5-CHO displays new peaks at 286.5 eV and 288.7 eV, corresponding to C–Cl and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds, respectively.42 Elemental oxygen analysis of PAF-5-CHO (Table S1†) reveals an oxygen content of 7.475 wt%, indicating the introduction of approximately 1.5 aldehyde groups per structural unit of PAF-5. However, thermogravimetric analysis (TGA) results (Fig. S3a†) show a weak thermal stability for PAF-5-CHO compared to PAF-5 due to the lower pyrolysis temperature of –CHO groups than aromatic groups. Furthermore, the scanning electron microscope (SEM) images (Fig. S4a–c†) exhibit no marked changes after the modification process. Powder X-ray diffraction (PXRD) patterns (Fig. S5†) indicate that PAF-5-CHO remains an amorphous material.
O bonds, respectively.42 Elemental oxygen analysis of PAF-5-CHO (Table S1†) reveals an oxygen content of 7.475 wt%, indicating the introduction of approximately 1.5 aldehyde groups per structural unit of PAF-5. However, thermogravimetric analysis (TGA) results (Fig. S3a†) show a weak thermal stability for PAF-5-CHO compared to PAF-5 due to the lower pyrolysis temperature of –CHO groups than aromatic groups. Furthermore, the scanning electron microscope (SEM) images (Fig. S4a–c†) exhibit no marked changes after the modification process. Powder X-ray diffraction (PXRD) patterns (Fig. S5†) indicate that PAF-5-CHO remains an amorphous material.
Synthesis of organic amine-modified PAF-5 materials
To introduce CO2-philic functional groups, we selected three organic amines with different chain lengths and successfully synthesized a series of amine-functionalized PAF-5 materials via Schiff base reactions (Fig. 2a). In the FTIR spectra of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA, PAF-5-C
N-EDA, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-DETA, and PAF-5-C
N-DETA, and PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-TETA (Fig. 2b), the emergence of –NH2 vibration at 3300 cm−1 and –C
N-TETA (Fig. 2b), the emergence of –NH2 vibration at 3300 cm−1 and –C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N vibration at 1640 cm−1 confirms the successful occurrence of the Schiff base reaction.23 In the XPS total spectrum (Fig. 2c), the presence of the N element can be clearly evident. Additionally, in the high-resolution N 1s XPS spectra of PAF-5-C
N vibration at 1640 cm−1 confirms the successful occurrence of the Schiff base reaction.23 In the XPS total spectrum (Fig. 2c), the presence of the N element can be clearly evident. Additionally, in the high-resolution N 1s XPS spectra of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA, PAF-5-C
N-EDA, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-DETA, and PAF-5-C
N-DETA, and PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-TETA (Fig. S6†), the peaks at 398.6 eV, 398.3 eV, and 398.2 eV are assigned to C
N-TETA (Fig. S6†), the peaks at 398.6 eV, 398.3 eV, and 398.2 eV are assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bonds, while the peaks at 399.4 eV, 399.1 eV, and 399 eV correspond to –NH2 and –NH– species.43–45 Elemental analysis (Table S2†) further supports the formation of imine bonds, showing a significant increase in nitrogen content for the three amine-functionalized materials compared to PAF-5-CHO. Among them, PAF-5-C
N bonds, while the peaks at 399.4 eV, 399.1 eV, and 399 eV correspond to –NH2 and –NH– species.43–45 Elemental analysis (Table S2†) further supports the formation of imine bonds, showing a significant increase in nitrogen content for the three amine-functionalized materials compared to PAF-5-CHO. Among them, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-TETA exhibits the highest nitrogen content at 10.04 wt%, equivalent to approximately 0.8 -NH2 groups per triphenyl benzene unit of PAF-5. Based on these data, it can be inferred that around 50% of the aldehyde groups participate in the reaction. Moreover, TGA analysis (Fig. S3b†) demonstrates that the amine-functionalized PAF-5-CHO materials retain stability in air up to 235 °C. The weight loss observed between 235 °C and 520 °C is attributed to the decomposition of the amine chains.20 SEM images (Fig. S4d–f†) reveal no significant morphological changes in the materials after amine modification.
N-TETA exhibits the highest nitrogen content at 10.04 wt%, equivalent to approximately 0.8 -NH2 groups per triphenyl benzene unit of PAF-5. Based on these data, it can be inferred that around 50% of the aldehyde groups participate in the reaction. Moreover, TGA analysis (Fig. S3b†) demonstrates that the amine-functionalized PAF-5-CHO materials retain stability in air up to 235 °C. The weight loss observed between 235 °C and 520 °C is attributed to the decomposition of the amine chains.20 SEM images (Fig. S4d–f†) reveal no significant morphological changes in the materials after amine modification.
Gas adsorption and desorption
The adsorption isotherms of PAF-5, PAF-5-CHO, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA, PAF-5-C
N-EDA, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-DETA, and PAF-5-C
N-DETA, and PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-TETA at 77 K are shown in Fig. 3a. Their corresponding Brunauer–Emmett–Teller (BET) surface areas are 1660, 1510, 1423, 1224, and 1101 m2 g−1, respectively. The reduction in the BET surface area for PAF-5-CHO is likely due to the aldehyde groups occupying the pore channels, as evidenced by the decrease in pore width from 1.75 nm to 1.03 nm (Fig. S7†). PAF-5-C
N-TETA at 77 K are shown in Fig. 3a. Their corresponding Brunauer–Emmett–Teller (BET) surface areas are 1660, 1510, 1423, 1224, and 1101 m2 g−1, respectively. The reduction in the BET surface area for PAF-5-CHO is likely due to the aldehyde groups occupying the pore channels, as evidenced by the decrease in pore width from 1.75 nm to 1.03 nm (Fig. S7†). PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA, PAF-5-C
N-EDA, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-DETA, and PAF-5-C
N-DETA, and PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-TETA exhibit further reductions in both BET surface area and pore volume relative to PAF-5-CHO. When the transformation of aldehyde groups into long-chains happens, the materials become heavier and the larger amine molecules occupy more pore space. Fig. 3b and S8† illustrate the CO2 adsorption isotherms of PAF-5-CHO and the three amine-functionalized PAF-5 materials at 298 K and 273 K. Across the entire experimental pressure range (0–100 kPa), the CO2 adsorption capacity of PAF-5-C
N-TETA exhibit further reductions in both BET surface area and pore volume relative to PAF-5-CHO. When the transformation of aldehyde groups into long-chains happens, the materials become heavier and the larger amine molecules occupy more pore space. Fig. 3b and S8† illustrate the CO2 adsorption isotherms of PAF-5-CHO and the three amine-functionalized PAF-5 materials at 298 K and 273 K. Across the entire experimental pressure range (0–100 kPa), the CO2 adsorption capacity of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA, PAF-5-C
N-EDA, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-DETA, and PAF-5-C
N-DETA, and PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-TETA shows a remarkable improvement compared to PAF-5-CHO. This demonstrates that organic amine modification significantly enhances CO2 adsorption performance. Among the three materials, PAF-5-C
N-TETA shows a remarkable improvement compared to PAF-5-CHO. This demonstrates that organic amine modification significantly enhances CO2 adsorption performance. Among the three materials, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA shows the greatest improvement in CO2 adsorption capacity, primarily attributed to its high specific surface area and the high loading of amine. Specifically, at 298 K, the CO2 adsorption capacity of PAF-5-C
N-EDA shows the greatest improvement in CO2 adsorption capacity, primarily attributed to its high specific surface area and the high loading of amine. Specifically, at 298 K, the CO2 adsorption capacity of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA is 3.78 mmol g−1, which represents a 78% increase compared to PAF-5-CHO, and the adsorption capacity remains nearly unchanged after six cycles (Fig. S9†).
N-EDA is 3.78 mmol g−1, which represents a 78% increase compared to PAF-5-CHO, and the adsorption capacity remains nearly unchanged after six cycles (Fig. S9†).
 | ||
| Fig. 3 (a) N2 uptake at 77 K; (b) CO2 uptake of PAF-5-CHO and its derivations at 298 K; (c) CO2 −Qst of PAF-5-CHO and its derivation; (d) comparison of CO2 uptake with other amine-functionalized POPs at 15 kPa and 100 kPa.20,46–57 | ||
Flue gas emitted from coal-fired power plants contains approximately 15% CO2 at 100 kPa, thus the CO2 adsorption capacity at 15 kPa is highly relevant for practical applications. At 298 K and 15 kPa, among the three amine-functionalized materials, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-TETA exhibits the highest CO2 adsorption capacity (Fig. S10†). Despite having the lowest BET surface area, PAF-5-C
N-TETA exhibits the highest CO2 adsorption capacity (Fig. S10†). Despite having the lowest BET surface area, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-TETA shows the highest nitrogen content—10.004% (Table S2†). This indicates that at low pressure, CO2 adsorption is more strongly influenced by the amine group content rather than the BET surface area. Specifically, at 298 K and 15 kPa, the CO2 adsorption capacity of PAF-5-CHO is 0.41 mmol g−1, while that of PAF-5-C
N-TETA shows the highest nitrogen content—10.004% (Table S2†). This indicates that at low pressure, CO2 adsorption is more strongly influenced by the amine group content rather than the BET surface area. Specifically, at 298 K and 15 kPa, the CO2 adsorption capacity of PAF-5-CHO is 0.41 mmol g−1, while that of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-TETA reaches 1.18 mmol g−1, representing a twofold increase compared to PAF-5-CHO.
N-TETA reaches 1.18 mmol g−1, representing a twofold increase compared to PAF-5-CHO.
Furthermore, the isosteric enthalpy of adsorption (Qst) plot (Fig. 3c) determined from adsorption isotherm calculations at 273 K and 298 K shows that all three materials obtained after organic amine modification are significantly improved compared to PAF-5-CHO. The −Qst of PAF-5-CHO is 25 kJ mol−1, whereas PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-TETA, PAF-5-C
N-TETA, PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-DETA, and PAF-5-C
N-DETA, and PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA exhibit higher −Qst values of 34, 33, and 32 kJ mol−1, respectively. Moreover, the CO2 desorption isothermal curves of these three materials do not exhibit significant hysteresis loops, indicating their excellent cycling potential. Compared to other amine-functionalized POPs (Fig. 3d), the three materials presented in this study exhibit higher adsorption capacities at both 15 kPa and 100 kPa. Thus, these materials demonstrate considerable promise for utilization in CO2 capture and recovery applications.
N-EDA exhibit higher −Qst values of 34, 33, and 32 kJ mol−1, respectively. Moreover, the CO2 desorption isothermal curves of these three materials do not exhibit significant hysteresis loops, indicating their excellent cycling potential. Compared to other amine-functionalized POPs (Fig. 3d), the three materials presented in this study exhibit higher adsorption capacities at both 15 kPa and 100 kPa. Thus, these materials demonstrate considerable promise for utilization in CO2 capture and recovery applications.
Separation ability of PAF-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/h3_char_e001.gif) N-EDA
N-EDA
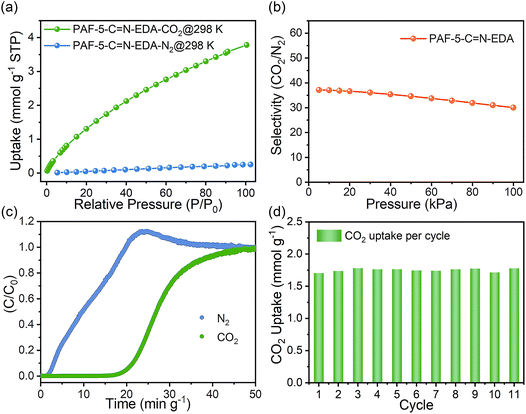
Based on a comprehensive evaluation of the CO2 adsorption performance and the cost of the adsorbents, we selected PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA for subsequent CO2/N2 separation experiments. Using the CO2 and N2 adsorption isotherms of PAF-5-C
N-EDA for subsequent CO2/N2 separation experiments. Using the CO2 and N2 adsorption isotherms of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA at 298 K (Fig. 4a), the CO2/N2 selectivity was calculated using the Ideal Adsorbed Solution Theory (IAST) method. Fig. 4b shows that the CO2/N2 selectivity of PAF-5-C
N-EDA at 298 K (Fig. 4a), the CO2/N2 selectivity was calculated using the Ideal Adsorbed Solution Theory (IAST) method. Fig. 4b shows that the CO2/N2 selectivity of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA is approximately 37. To evaluate its actual separation performance, breakthrough experiments were conducted using 0.075 g of PAF-5-C
N-EDA is approximately 37. To evaluate its actual separation performance, breakthrough experiments were conducted using 0.075 g of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA powder. At 298 K and 1 bar, a CO2/N2 (15
N-EDA powder. At 298 K and 1 bar, a CO2/N2 (15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85, v/v) gas mixture was passed through a tightly packed fixed-bed column containing PAF-5-C
85, v/v) gas mixture was passed through a tightly packed fixed-bed column containing PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA at a flow rate of 10 mL min−1. As shown in Fig. 4c, N2 breakthrough occurred almost immediately, while CO2 breakthrough was delayed for a retention time (15 min g−1), demonstrating excellent CO2 separation performance. The CO2 capture capacity calculated from a single breakthrough experiment was 1.00 mmol g−1, consistent with the results from the CO2 adsorption isotherm. After eleven consecutive breakthrough cycles (Fig. S11 and S12†), the material retained good separation performance, with an average CO2 adsorption capacity of 1.75 mmol g−1 during the breakthrough process.
N-EDA at a flow rate of 10 mL min−1. As shown in Fig. 4c, N2 breakthrough occurred almost immediately, while CO2 breakthrough was delayed for a retention time (15 min g−1), demonstrating excellent CO2 separation performance. The CO2 capture capacity calculated from a single breakthrough experiment was 1.00 mmol g−1, consistent with the results from the CO2 adsorption isotherm. After eleven consecutive breakthrough cycles (Fig. S11 and S12†), the material retained good separation performance, with an average CO2 adsorption capacity of 1.75 mmol g−1 during the breakthrough process.
CO2 adsorption mechanism
To analyze the CO2 adsorption mechanism, we first performed density functional theory (DFT) simulations to investigate the potential interactions between the PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA framework and CO2 molecules. Five potential CO2 adsorption configurations were constructed and optimized (Fig. S13†). The calculated adsorption energies reveal that the interaction between the amine group and the C atom of CO2 represents the dominant contribution, exhibiting the lowest adsorption energy (−0.31 eV). This suggests that the amine groups serve as the most favorable active sites for CO2 adsorption. In this configuration, the N–C distance measures 2.76 Å, which is shorter than their sum of van der Waals radius. Additionally, the O–C–O angle of the adsorbed *CO2 deviates slightly from linearity, changing from 180° to 175.0°. Charge transfer analysis indicates electron donation from PAF-5-C
N-EDA framework and CO2 molecules. Five potential CO2 adsorption configurations were constructed and optimized (Fig. S13†). The calculated adsorption energies reveal that the interaction between the amine group and the C atom of CO2 represents the dominant contribution, exhibiting the lowest adsorption energy (−0.31 eV). This suggests that the amine groups serve as the most favorable active sites for CO2 adsorption. In this configuration, the N–C distance measures 2.76 Å, which is shorter than their sum of van der Waals radius. Additionally, the O–C–O angle of the adsorbed *CO2 deviates slightly from linearity, changing from 180° to 175.0°. Charge transfer analysis indicates electron donation from PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA to the adsorbed *CO2, with a Bader charge of 0.14|e| localized on *CO2. These results demonstrate that the adsorbed *CO2 undergoes activation, consistent with the observed negative adsorption energy. To further assess the diffusion rate of CO2 molecules on the PAF-5-C
N-EDA to the adsorbed *CO2, with a Bader charge of 0.14|e| localized on *CO2. These results demonstrate that the adsorbed *CO2 undergoes activation, consistent with the observed negative adsorption energy. To further assess the diffusion rate of CO2 molecules on the PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA model, we examined the diffusion pathway and the corresponding energy barrier of a single CO2 molecule moving between two adjacent amine active sites (Fig. S14†). The computed diffusion barrier is remarkably low (0.09 eV), suggesting that CO2 molecules can diffuse rapidly through the porous structure of the material.
N-EDA model, we examined the diffusion pathway and the corresponding energy barrier of a single CO2 molecule moving between two adjacent amine active sites (Fig. S14†). The computed diffusion barrier is remarkably low (0.09 eV), suggesting that CO2 molecules can diffuse rapidly through the porous structure of the material.
We conducted in situ IR spectroscopy to monitor the adsorption process of CO2 on PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA. During the experiment, CO2 gas flowed through the PAF-5-C
N-EDA. During the experiment, CO2 gas flowed through the PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA adsorbent. The absorption spectra shown in Fig. S15† were obtained by collecting the PAF-5-C
N-EDA adsorbent. The absorption spectra shown in Fig. S15† were obtained by collecting the PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA absorption spectra as a background, and the band produced by absorbed CO2 at 2360 cm−1 can be observed.58 In the IR spectra of PAF-5-C
N-EDA absorption spectra as a background, and the band produced by absorbed CO2 at 2360 cm−1 can be observed.58 In the IR spectra of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA with adsorbed CO2 (Fig. 5b), the following features were identified: (i) the O
N-EDA with adsorbed CO2 (Fig. 5b), the following features were identified: (i) the O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O− band of carbamate NHCOO− at 1532 cm−1 and (ii) the +N–H band of NH3+ at 1656 cm−1. The adsorption mechanism inferred from the in situ IR analysis is illustrated in Fig. 5a, and the process occurs in two stages. To further confirm the adsorption mechanism, temperature-programmed desorption (TPD) of the post-breakthrough sample was performed (Fig. 5c). The first desorption peak corresponds to weakly adsorbed CO2 on the adsorbent and the column walls, while the second peak, appearing at temperatures above 100 °C, corresponds to the intermediate species described as Stage I in the mechanism diagram. A third peak represents the desorption of CO2 species associated with Stage II of the mechanism temperatures above 100 °C, corresponding to the intermediate species described as Stage I in the mechanism diagram. A third peak represents the desorption of CO2 species associated with Stage II of the mechanism.
O− band of carbamate NHCOO− at 1532 cm−1 and (ii) the +N–H band of NH3+ at 1656 cm−1. The adsorption mechanism inferred from the in situ IR analysis is illustrated in Fig. 5a, and the process occurs in two stages. To further confirm the adsorption mechanism, temperature-programmed desorption (TPD) of the post-breakthrough sample was performed (Fig. 5c). The first desorption peak corresponds to weakly adsorbed CO2 on the adsorbent and the column walls, while the second peak, appearing at temperatures above 100 °C, corresponds to the intermediate species described as Stage I in the mechanism diagram. A third peak represents the desorption of CO2 species associated with Stage II of the mechanism temperatures above 100 °C, corresponding to the intermediate species described as Stage I in the mechanism diagram. A third peak represents the desorption of CO2 species associated with Stage II of the mechanism.
Conclusion
We reported a post-modification method for PAFs that can introduce aldehyde groups into the framework of PAF-5 in a simple and efficient manner. Materials modified with aldehyde groups can be subjected to the subsequent reactions, and in this paper, we prepared a series of amino-modified PAFs by using the aldehyde group for the Schiff base reaction. Such materials were characterized using CO2 adsorption–desorption isotherms and CO2/N2 dynamic breakthroughs, and we observed that PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA exhibits excellent performance in capturing post-combustion CO2. Through the investigation of the CO2 adsorption mechanism, it was found that there are two distinct adsorption sites involved in the process. This study highlights the potential of this class of materials in post-combustion CO2 capture technology.
N-EDA exhibits excellent performance in capturing post-combustion CO2. Through the investigation of the CO2 adsorption mechanism, it was found that there are two distinct adsorption sites involved in the process. This study highlights the potential of this class of materials in post-combustion CO2 capture technology.
Experimental
Materials
All starting materials and solvents, unless otherwise specified, were obtained from Innochem Co. and used.Synthesis of PAF-5 as reported
In a glovebox, 1,5-cyclooctadiene (cod) (0.93 mL, 7.92 mmol), bis(1,5-cyclooctadiene) nickel (0) (Ni(cod)2) (2.18 g, 7.93 mmol), 2,2′-bipyridyl (1.24 g, 7.94 mmol), and anhydrous N,N′-dimethylformamide (DMF) (40 mL) were added to a 250 mL double-neck flask. A solution of 1,3,5-tris(4-bromophenyl) benzene (TBB) in DMF (80 mL) was then slowly added to the mixture at 80 °C. The resultant system was further kept at 80 °C for 48 h. After cooling to room temperature, 6 M HCl was added to the mixture, and the mixture was stirred for 1 hour until it turned green with a snowflake-like solid formation, washed and dried to obtain the product.Synthesis of PAF-5-CHO
PAF-5 (100 mg), anhydrous AlCl3 (200 mg, 1.5 mmol) and CHCl3 (40 mL) were added to a 100 mL flask. The mixture was stirred vigorously, heated to 80 °C and the reaction continued for one day. After quenching with 50 mL of methanol, the solid was filtered, and washed using 1 M hydrochloric acid and water successively. After drying under vacuum, a brownish yellow solid was obtained.General procedure for the amine-functionalized materials
Take the synthesis of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA as an example: PAF-5-C
N-EDA as an example: PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA (100 mg) and ethylenediamine (EDA, 10 mL) were added to a sealed flask. The mixture was kept at 80 °C for 3 days. The solid was filtered, washed with water and methanol, and then dried in vacuo to produce PAF-5-C
N-EDA (100 mg) and ethylenediamine (EDA, 10 mL) were added to a sealed flask. The mixture was kept at 80 °C for 3 days. The solid was filtered, washed with water and methanol, and then dried in vacuo to produce PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA as a brown powder in quantitative yield.
N-EDA as a brown powder in quantitative yield.
Materials and physical measurements
Fourier transform infrared spectroscopy (FTIR) spectra were recorded using a Nicolet IS50 Fourier transform infrared spectrometer. Solid-state 13C cross-polarization magic angle spinning nuclear magnetic resonance (NMR) spectra were acquired with a Bruker Avance III 400 MHz NMR spectrometer at a MAS rate of 5 kHz. X-ray photoelectron spectroscopy (XPS) spectra were obtained on a KRATOS Axis Ultra DLD system, equipped with an Al Kα ray source (hv = 1486.6 eV) under a high vacuum of 9.8 × 10−10 torr. N2 isotherms at 77 K and vapor isotherms were measured using an Autosorb adsorptometer (JW-TB 400 and JW-ZQ 100). Thermal gravimetric analysis (TGA) was performed on a METTLER-TOLEDO TGA/DSC 3+ analyzer at a heating rate of 10 °C min−1 under an air flow of 60 mL min−1. Scanning electron microscope (SEM) images were captured using a Hitachi SU-70 microscope. Powder X-ray diffraction (PXRD) was performed on a Rigaku Smartlab X-ray diffractometer with Cu-Kα radiation at 40 kV and 200 mA with a step size of 0.01. Elemental analysis was carried out with a Eurovector EA3000 Analyzer. Elemental oxygen analysis was carried out with a Vario EL cube.Breakthrough experiments
Breakthrough experiments were performed using a dynamic gas breakthrough device, mixSorb S (3P Instruments). The sample was heated under a vacuum at 100 °C for 8 h. A 0.075 g sample of PAF-5-C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N-EDA was loaded onto a stainless-steel column (1 mL volume, 0.45 cm inner diameter). A CO2/N2 (15:
N-EDA was loaded onto a stainless-steel column (1 mL volume, 0.45 cm inner diameter). A CO2/N2 (15:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 85, v/v) gas mixture was allowed to flow into the column. A mass spectrometer (Master 400) was used to monitor the gas flow from the column.
85, v/v) gas mixture was allowed to flow into the column. A mass spectrometer (Master 400) was used to monitor the gas flow from the column.
Data availability
The data are available in the ESI.†Author contributions
J. J., G. Z., and Z. B. conceptualized and supervised. Q. W. planned research and synthesis; Q. W. and L. L. analysed the data. Q. W. and L. L. wrote the manuscript. L. J., Z. W., and Y. Z. synthesized and analysed for the discussion. Q. H. performed SEM characterization, and J. J. and G. Z. performed the revision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors are grateful for financial support from the National Key R&D Program of China (2022YFB3805900 and 2022YFB3805902), the National Natural Science Foundation of China (grant no. 22131004 and U21A20330), the Fundamental Research Funds for the Central Universities (2412024QD012; 2412024QD019) and the “111” project (No. B18012).Notes and references
- T. M. McDonald, W. R. Lee, J. A. Mason, B. M. Wiers, C. S. Hong and J. R. Long, J. Am. Chem. Soc., 2012, 134, 7056–7065 CrossRef CAS PubMed.
- K. Menyah and Y. Wolde-Rufael, Energy Policy, 2010, 38, 2911–2915 CrossRef CAS.
- M. Bui, C. S. Adjiman, A. Bardow, E. J. Anthony, A. Boston, S. Brown, P. S. Fennell, S. Fuss, A. Galindo, L. A. Hackett, J. P. Hallett, H. J. Herzog, G. Jackson, J. Kemper, S. Krevor, G. C. Maitland, M. Matuszewski, I. S. Metcalfe, C. Petit, G. Puxty, J. Reimer, D. M. Reiner, E. S. Rubin, S. A. Scott, N. Shah, B. Smit, J. P. M. Trusler, P. Webley, J. Wilcox and N. Mac Dowell, Energy Environ. Sci., 2018, 11, 1062–1176 RSC.
- P. Madejski, K. Chmiel, N. Subramanian and T. Kuś, Energies, 2022, 15, 887 CrossRef CAS.
- R. L. Siegelman, E. J. Kim and J. R. Long, Nat. Mater., 2021, 20, 1060–1072 CrossRef CAS PubMed.
- H. Lyu, O. I.-F. Chen, N. Hanikel, M. I. Hossain, R. W. Flaig, X. Pei, A. Amin, M. D. Doherty, R. K. Impastato, T. G. Glover, D. R. Moore and O. M. Yaghi, J. Am. Chem. Soc., 2022, 144, 2387–2396 CrossRef CAS PubMed.
- Z. Zhou, T. Ma, H. Zhang, S. Chheda, H. Li, K. Wang, S. Ehrling, R. Giovine, C. Li, A. H. Alawadhi, M. M. Abduljawad, M. O. Alawad, L. Gagliardi, J. Sauer and O. M. Yaghi, Nature, 2024, 635, 96–101 CrossRef CAS PubMed.
- J. A. Microporous and Mesoporous MaterialsMason, T. M. McDonald, T.-H. Bae, J. E. Bachman, K. Sumida, J. J. Dutton, S. S. Kaye and J. R. Long, J. Am. Chem. Soc., 2015, 137, 4787–4803 CrossRef PubMed.
- B. Dutcher, M. Fan and A. G. Russell, ACS Appl. Mater. Interfaces, 2015, 7, 2137–2148 CrossRef CAS PubMed.
- O. Cheung and N. Hedin, RSC Adv., 2014, 4, 14480–14494 RSC.
- O. I.-F. Chen, C.-H. Liu, K. Wang, E. Borrego-Marin, H. Li, A. H. Alawadhi, J. A. R. Navarro and O. M. Yaghi, J. Am. Chem. Soc., 2024, 146, 2835–2844 CrossRef CAS PubMed.
- R. Uy and F. Wold, Science, 1977, 198, 890–896 CrossRef CAS PubMed.
- J. S. Seo, D. Whang, H. Lee, S. I. Jun, J. Oh, Y. J. Jeon and K. Kim, Nature, 2000, 404, 982–986 CrossRef CAS PubMed.
- S. Banerjee, T. Hemraj-Benny and S. S. Wong, Adv. Mater., 2005, 17, 17–29 CrossRef CAS.
- F. Hoffmann, M. Cornelius, J. Morell and M. Fröba, Angew. Chem., Int. Ed., 2006, 45, 3216–3251 CrossRef CAS PubMed.
- Z. Wang and S. M. Cohen, J. Am. Chem. Soc., 2007, 129, 12368–12369 CrossRef CAS PubMed.
- J. Liu, Z. Wang, P. Cheng, M. J. Zaworotko, Y. Chen and Z. Zhang, Nat. Rev. Chem., 2022, 6, 339–356 CrossRef PubMed.
- S. Mandal, S. Natarajan, P. Mani and A. Pankajakshan, Adv. Funct. Mater., 2021, 31, 2006291 CrossRef CAS.
- J. L. Segura, S. Royuela and M. Mar Ramos, Chem. Soc. Rev., 2019, 48, 3903–3945 RSC.
- W. Lu, J. P. Sculley, D. Yuan, R. Krishna, Z. Wei and H.-C. Zhou, Angew. Chem., Int. Ed., 2012, 51, 7480–7484 CrossRef CAS PubMed.
- Y. Yang, C. Y. Chuah and T.-H. Bae, Chem. Eng. J., 2019, 358, 1227–1234 CrossRef CAS.
- D. Wang, W. Yang, S. Feng and H. Liu, RSC Adv., 2016, 6, 13749–13756 RSC.
- V. Guillerm, Ł. J. Weseliński, M. Alkordi, M. I. H. Mohideen, Y. Belmabkhout, A. J. Cairns and M. Eddaoudi, Chem. Commun., 2014, 50, 1937–1940 RSC.
- P. J. Milner, R. L. Siegelman, A. C. Forse, M. I. Gonzalez, T. Runčevski, J. D. Martell, J. A. Reimer and J. R. Long, J. Am. Chem. Soc., 2017, 139, 13541–13553 CrossRef CAS PubMed.
- A. A. Uliana, N. T. Bui, J. Kamcev, M. K. Taylor, J. J. Urban and J. R. Long, Science, 2021, 372, 296–299 CrossRef CAS PubMed.
- W. Lu, D. Yuan, J. Sculley, D. Zhao, R. Krishna and H.-C. Zhou, J. Am. Chem. Soc., 2011, 133, 18126–18129 CrossRef CAS PubMed.
- Z. Wang, Y. Zhang, L. Jiang, Q. Han, Q. Wang, J. Jia and G. Zhu, Chem. Synth., 2024, 4, 40 CAS.
- B. Li, Y. Zhang, R. Krishna, K. Yao, Y. Han, Z. Wu, D. Ma, Z. Shi, T. Pham, B. Space, J. Liu, P. K. Thallapally, J. Liu, M. Chrzanowski and S. Ma, J. Am. Chem. Soc., 2014, 136, 8654–8660 CrossRef CAS PubMed.
- Y. Tian, F. Cui, Z. Bian, X. Tao, H. Wang, N. Zhang and G. Zhu, Acc. Chem. Res., 2024, 57, 2130–2143 CrossRef CAS PubMed.
- Y. Jing, C. Wang, B. Zhu, S. Xiao, W. Guan, S. Liu, N. Zhang, S. Wen and G. Zhu, Angew. Chem., Int. Ed., 2024, 63, e202410069 CAS.
- Y. Zhang, B. Li and S. Ma, Chem. Commun., 2014, 50, 8507–8510 RSC.
- P. Zhang, C. Zhang, L. Wang, J. Dong, D. Gai, W. Wang, T. S. Nguyen, C. T. Yavuz, X. Zou and G. Zhu, Adv. Funct. Mater., 2023, 33, 2210091 CrossRef CAS.
- K. Liu, P. Chen, F. Chen, F. Sun, P. Lv, J. Shi and Y.-J. Jiang, Chem.–Eur. J., 2024, 31, e202404128 CrossRef PubMed.
- Z. Wang, H. Jiang, Y. Tian, X. Zou and G. Zhu, Trends Chem., 2023, 5, 446–459 CrossRef CAS.
- Y. Zhai, H. Lei, Y. Li, J. Song, X. Jing, X. Shi, Y. Tian and G. Zhu, J. Mater. Chem. A, 2023, 11, 14119–14125 RSC.
- J. Hu, C. Gu and J. Liu, Micropor. Mesopor. Mater., 2021, 315, 110931 CrossRef CAS.
- W. Morris, C. J. Doonan, H. Furukawa, R. Banerjee and O. M. Yaghi, J. Am. Chem. Soc., 2008, 130, 12626–12627 CrossRef CAS PubMed.
- H. Kudo, R. Hayashi, K. Mitani, T. Yokozawa, N. C. Kasuga and T. Nishikubo, Angew. Chem., Int. Ed., 2006, 45, 7948–7952 CrossRef CAS PubMed.
- H. Ren, T. Ben, F. Sun, M. Guo, X. Jing, H. Ma, K. Cai, S. Qiu and G. Zhu, J. Mater. Chem., 2011, 21, 10348–10353 RSC.
- Y. Yuan, D. Cao, F. Cui, Y. Yang, C. Zhang, Y. Song, Y. Zheng, J. Cao, S. Chen, Y. Song, F. Wang and G. Zhu, Nat. Water, 2025, 3, 89–98 CrossRef CAS.
- Z. Zhao, K. G. Kulkarni and G. K. Murphy, Adv. Synth. Catal., 2017, 359, 2222 CrossRef CAS.
- M. Deng, J. Guo, X. Ma, Y. Fu, H. Du, D. Hao and Q. Wang, Sep. Purif. Technol., 2023, 326, 124786 CrossRef CAS.
- F. Li, D. Wang, Q.-J. Xing, G. Zhou, S.-S. Liu, Y. Li, L.-L. Zheng, P. Ye and J.-P. Zou, Appl. Catal., B, 2019, 243, 621–628 CrossRef CAS.
- M.-L. Xu, M. Lu, G.-Y. Qin, X.-M. Wu, T. Yu, L.-N. Zhang, K. Li, X. Cheng and Y.-Q. Lan, Angew. Chem., Int. Ed., 2022, 61, e202210700 CrossRef CAS PubMed.
- S. Bhattacharya, R. Sarkar and B. Chakraborty, ACS Sens., 2017, 2, 1215–1224 CrossRef CAS PubMed.
- L. Wang, J. Guo, X. Xiang, Y. Sang and J. Huang, Chem. Eng. J., 2020, 387, 124070 CrossRef CAS.
- D. Lee, C. Zhang and H. Gao, Macromol. Chem. Phys., 2015, 216, 489–494 CrossRef CAS.
- D. Thirion, V. Rozyyev, J. Park, J. Byun, Y. Jung, M. Atilhan and C. T. Yavuz, Phys. Chem. Chem. Phys., 2016, 18, 14177–14181 RSC.
- L. Wang, Q. Xiao, D. Zhang, W. Kuang, J. Huang and Y.-N. Liu, ACS Appl. Mater. Interfaces, 2020, 12, 36652–36659 CrossRef CAS PubMed.
- X. Wang, Y. Liu, X. Ma, S. K. Das, M. Ostwal, I. Gadwal, K. Yao, X. Dong, Y. Han, I. Pinnau, K.-W. Huang and Z. Lai, Adv. Mater., 2017, 29, 1605826 CrossRef PubMed.
- H. Ouyang, K. Song, J. Du, Z. Zhan and B. Tan, Chem. Eng. J., 2022, 431, 134326 CrossRef CAS.
- T. İslamoğlu, M. Gulam Rabbani and H. M. El-Kaderi, J. Mater. Chem. A, 2013, 1, 10259–10266 RSC.
- X. Kong, S. Li, M. Strømme and C. Xu, Nanomaterials, 2019, 9, 1020 CrossRef CAS PubMed.
- G. Jiangfei, W. Lizhi, D. Zhang and J. Huang, Energy Fuels, 2020, 34, 9771–9778 CrossRef CAS.
- V. Rozyyev, M. S. Yavuz, D. Thirion, T. S. Nguyen, T. P. N. Nguyen, A.-H. Emwas and C. T. Yavuz, Micropor. Mesopor. Mater., 2021, 328, 111450 CrossRef CAS.
- P. Jorayev, I. Tashov, V. Rozyyev, T. S. Nguyen, N. A. Dogan and C. T. Yavuz, ChemSusChem, 2020, 13, 6433–6441 CrossRef CAS PubMed.
- Y. Li, L. Yang, X. Zhu, J. Hu and H. Liu, Int. J. Coal Sci. Technol., 2017, 4, 50–59 CrossRef CAS.
- J. Yu and S. S. C. Chuang, Energy Fuels, 2016, 30, 7579–7587 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc00355e |
| ‡ Q. Wang and L. Lin contributed equally to this paper. |
| This journal is © The Royal Society of Chemistry 2025 |