Open Access Article
Open Access ArticleInterfacial engineering of a nanofibrous Ru/Cr2O3 heterojunction for efficient alkaline/acid-universal hydrogen evolution at the ampere level†
Xianqiang
Yu
a,
Mingze
Xia
a,
Ruikai
Qi
a,
Yuezhu
Wang
a,
Mingbin
Gao
*b,
Mengxiao
Zhong
*c and
Xiaofeng
Lu
 *a
*a
aAlan G. MacDiarmid Institute, College of Chemistry, Jilin University, Changchun 130012, P. R. China. E-mail: xflu@jlu.edu.cn
bState Key Laboratory of Physical Chemistry of Solid Surfaces, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China. E-mail: mbgao@xmu.edu.cn
cState Key Laboratory of Integrated Optoelectronics, Key Laboratory of Advanced Gas Sensors, Jilin Province, College of Electronic Science and Engineering, Jilin University, Changchun, 130012, P. R. China. E-mail: zhongmx@jlu.edu.cn
First published on 28th April 2025
Abstract
Interfacial engineering of a heterostructured electrocatalyst is an efficient way to boost hydrogen production, yet it still remains a challenging task to achieve superior performance at ampere-grade current density. Herein, a nanofibrous Ru/Cr2O3 heterojunction is prepared for alkaline/acid-universal hydrogen evolution. Theoretical calculations reveal that the introduction of Cr2O3 modulates the electronic structure of Ru, which is beneficial for *H desorption, resulting in a superior HER performance at ampere-grade current density. Accordingly, the resultant Ru/Cr2O3 catalyst presents an ultra-low overpotential of only 88 mV and a long-term stability of 300 h at 1 A cm−2 in 1 M KOH. Furthermore, it also exhibits a small overpotential of 112 mV and steadily operates for 300 h at 1 A cm−2 in 0.5 M H2SO4. The catalyst outperforms not only the benchmark Pt/C catalyst but also most of the top-performing catalysts reported to date. This study offers a novel conceptual approach for designing highly efficient electrocatalysts that hold significant promise for industrial-scale water splitting applications.
Introduction
Today, environmental pollution and the energy crisis represent the most significant challenges confronting humanity.1 Hydrogen (H2) has the potential to emerge as a key sustainable and green energy source, given its combustion without emitting greenhouse gases, renewable nature and high gravimetric energy density.2,3 As a vital half-reaction in water electrolysis, the hydrogen evolution reaction (HER) is widely recognized as an efficient, viable and promising route for the production of green H2 due to its economic, clean and environmentally friendly attributes.4–6 However, the current limitations of low efficiency, high overpotential, and the excessive reliance on the precious metal platinum (Pt) lead to prohibitively high costs for H2 production. Additionally, many of the high-efficiency non-Pt electrocatalysts developed to date are primarily suited for low-current-density applications (less than 0.1 A cm−2), rather than the high-current-density environments (1 A cm−2 or greater) that are essential for industrial-scale H2 production.7–9 Consequently, it is both challenging and crucial to design and fabricate efficient and cost-effective non-Pt HER electrocatalysts that can meet the demands of industrial H2 production.10–12As a highly promising direction in electrocatalyst research, ruthenium (Ru)-based catalysts have garnered substantial interest due to their cost-effectiveness compared to Pt and iridium (Ir), as well as their similar binding energies to Pt–H bonds.13–15 The electronic structure of a catalyst is pivotal in determining the reaction energy barrier during catalytic processes. To modulate this structure, a variety of strategies such as vacancy engineering, doping, and interface engineering have been employed.16–20 For instance, a hierarchical MoS2/MoP nanorod heterojunction is constructed through a sulfuration and phosphorization process using MoO3 nanorods as the sacrificial template for the HER.21 The distinct hierarchical architecture of the MoS2/MoP heterojunction facilitates electron transfer from S to P, resulting in an optimized H adsorption energy to promote the HER properties. Furthermore, the hierarchical structure provides abundant active sites and facilitates mass transfer, enabling easier H2 release. While the creation of heterostructures significantly boosts the HER performance, it remains a formidable challenge to attain a universally high-performance catalyst that operates efficiently under both alkaline and acidic conditions at industrial-scale current densities for water splitting applications.
In this study, we successfully delineate a reliable electrospinning-calcination-thermal reduction process for constructing robust ruthenium–chromium trioxide (Ru/Cr2O3) heterojunction nanofibers (HNFs) as a highly efficient HER electrocatalyst for ampere-grade-current-density water electrolysis in alkaline and acidic media. The choice of Cr2O3 is due to its ability to modulate the electronic structure of metals and desirable chemical stability.22,23 In addition, the nanofibrous structure offers efficient mass transfer, which is an ideal structural system for electrocatalysis.24–26 The resulting Ru/Cr2O3 HNF catalyst showcases exceptional HER activity with an ultra-low overpotential of only 88 mV and a long-term stability of 300 h at 1 A cm−2 in 1 M KOH. In addition, it also exhibits extremely high HER efficiency and outstanding durability in 0.5 M H2SO4. The excellent alkaline/acid-universal HER performances surpass that of commercial Pt/C and most documented electrocatalysts. Density functional theory (DFT) calculations reveal that the interfacial engineering in Ru/Cr2O3 HNFs modulates the electronic structure of Ru sites, thereby optimizing *H desorption, which yields superior HER properties in both acidic and alkaline environments.
Results and discussion
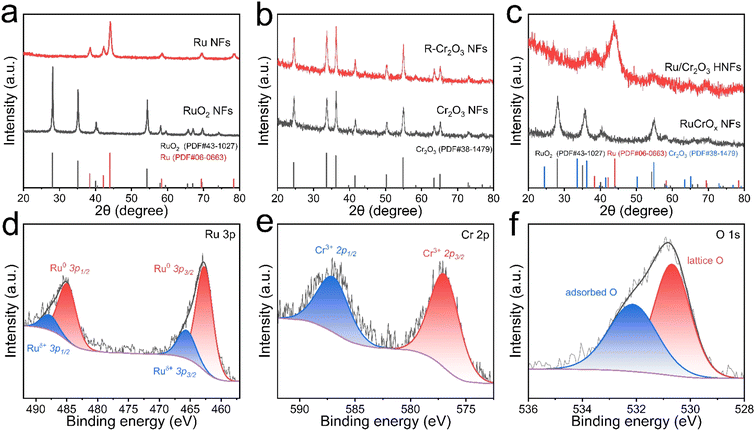
The synthetic pathway for the Ru/Cr2O3 HNFs is depicted in Fig. 1a, encompassing the calcination and reduction steps of the precursor nanofibers (NFs). Field emission scanning electron microscopy (FESEM) is employed to observe and scrutinize the morphological changes of NFs during the preparation process. The precursor NFs consisting of poly(vinylpyrrolidone) (PVP) and Cr3+/Ru3+ are initially fabricated via an electrospinning technique, exhibiting an average diameter of approximately 453.9 nm (Fig. S1, ESI†). Following sequential calcination and reduction treatments, the mean diameter of the obtained Ru/Cr2O3 HNFs is reduced to be around 143.9 nm, which is caused by the thermal decomposition of the polymer within the precursor NFs under high temperature conditions (Fig. 1b and c). Moreover, the FESEM image also presents a rough surface of Ru/Cr2O3 HNFs, a characteristic further corroborated by the transmission electron microscopy (TEM) image (Fig. 1d). Indeed, the Ru/Cr2O3 HNFs are composed of a multitude of nanoparticles. The nanofibrous architecture with a similar rough surface is also observed in Ru/Cr2O3-1, Ru/Cr2O3-2 and Ru/Cr2O3-4 HNFs (Fig. S2, ESI†). For comparison, single metal-component R-Cr2O3 NFs and Ru NFs, as well as RuCrOx composite NFs, are also constructed, all demonstrating the nanofibrous morphologies (Fig. S3, ESI†). The high-resolution TEM (HRTEM) image in Fig. 1e clearly reveals the heterointerface between the (101) plane of Ru and the (110) plane of Cr2O3, corresponding to the lattice fringe spacings of 0.206 nm and 0.247 nm, respectively. The heterojunctions with Cr2O3 and Ru species may be the key factor in the highly efficient active HER, which will be further discussed in the following investigation. The selected area electron diffraction (SAED) pattern in Fig. 1f aligns with the presence of multiple polycrystalline rings, which arises from the (101), (110) and (112) planes of Ru, and the (110), (116) and (300) planes of Cr2O3. Additionally, the energy-dispersive X-ray (EDX) spectroscopy analysis confirms the presence of Ru, Cr and O elements within the Ru/Cr2O3 HNFs (Fig. 1g). It is noted that the elemental contaminants such as C and Cu originate from the carbon-coated copper grid for TEM sample preparation, while Si is attributed to the instrument itself. The elemental mappings of Ru/Cr2O3 HNFs illustrate a uniform distribution of Ru, Cr and O across the entire NF structure (Fig. 1h). Furthermore, N2 adsorption–desorption isotherms are utilized to provide the specific surface area and pore size distribution of the Ru/Cr2O3 HNFs (Fig. S4, ESI†). Herein, the Brunauer–Emmett–Teller (BET) surface area of Ru/Cr2O3 HNFs is evaluated to be 21.26 m2 g−1, and the pore size distribution is mainly dominated by mesopores.X-ray diffraction (XRD) analysis was performed to elucidate the phase structure of the prepared Ru/Cr2O3 HNFs. Fig. 2a reveals that the RuO2 NFs, characterized by the distinctive lattice planes of JCPDS No. 43-1027, are successfully reduced to form Ru NFs (JCPDS No. 06-0663) after the in situ H2/argon thermal reduction process. Conversely, the Cr2O3 NFs retain their original crystal structure, displaying the characteristic lattice planes of Cr2O3 (JCPDS No. 38-1479) even after H2/argon thermal treatment, which demonstrates no reduction occurring (Fig. 2b). In Fig. 2c, the XRD pattern of the as-synthesized RuCrOx NF sample shows the characteristic peaks of both RuO2 and Cr2O3. Subsequent in situ H2/argon reduction yields the Ru/Cr2O3 HNFs, where the XRD pattern indicates the persistence of Cr2O3 diffraction peaks, while the peaks associated with RuO2 have completely vanished (Fig. 2c). In their place, the distinctive peaks of metallic Ru (JCPDS No. 06-0663) emerge, aligning with the HRTEM image findings previously discussed (Fig. 1e). Consistent outcomes are observed in the other comparative HNFs, with all diffraction peaks aligning with those of Ru (JCPDS No. 06-0663) and Cr2O3 (JCPDS No. 38-1479) (Fig. S5, ESI†). X-ray photoelectron spectroscopy (XPS) is further conducted to probe the surface chemical characteristics and electronic configuration of Ru/Cr2O3 HNFs. The survey spectrum presented in Fig. S6, ESI† confirms the presence of Ru, Cr, and O in Ru/Cr2O3 HNFs. Fig. 2d shows the high-resolution Ru 3p spectrum of Ru/Cr2O3 HNFs, which reveals two distinct doublets: the peaks positioned at 462.7 and 484.9 eV are ascribed to Ru0 3p3/2 and Ru0 3p1/2, respectively, while the weak peaks at 465.6 and 487.8 eV correspond to Ruδ+ 3p3/2 and Ruδ+ 3p1/2.27,28 The oxidized Ruδ+ species may originate from exposure to air leading to surface oxidation and their interaction with the Cr2O3 component.29,30 In contrast, the Ru 3p XPS spectrum of RuCrOx NFs shows a pair of strong peaks located at 463.6 and 485.9 eV, attributable to Ru4+ (Fig. S7, ESI†).31 In the Cr 2p spectrum of Ru/Cr2O3 HNFs (Fig. 2e), the prominent characteristic peaks observed at binding energies of 576.9 and 587.0 eV are attributed to Cr3+ 2p3/2 and Cr3+ 2p1/2, respectively.22,32,33 In addition, the narrow-scan O 1s spectrum reveals two distinct components with binding energies at 530.7 and 532.1 eV (Fig. 2f), which correspond to lattice oxygen and adsorbed oxygen species, respectively.34–36 These findings collectively provide compelling evidence that Ru/Cr2O3 HNFs have been successfully synthesized.
Initially, the HER properties of the synthesized Ru/Cr2O3 HNFs are examined in 1 M KOH. Furthermore, to provide a benchmark, the HER activities of commercial 20% Pt/C and other nanofibrous catalysts prepared using varied experimental parameters are assessed for comparison. Fig. 3a and S8, ESI† present the linear sweep voltammetry (LSV) curves along with the corresponding overpotentials required to reach current densities of 0.1 and 1 A cm−2 for Ru/Cr2O3 HNFs, R-Cr2O3 NFs, RuCrOx NFs, Ru NFs and commercial 20% Pt/C. Ru/Cr2O3 HNFs exhibit an overpotential of merely 41 mV at 0.1 A cm−2, significantly outperforming the R-Cr2O3 NFs with negligible HER activity, as well as RuCrOx NFs (135 mV), Ru NFs (88 mV) and 20% Pt/C catalyst (87 mV), respectively. Notably, the prepared Ru-based catalysts exhibit more favorable HER performances compared to the R-Cr2O3 NF sample, which illustrates the key role of Ru species in the heterojunctions. Afterwards, the Ru/Cr2O3 HNF catalyst is optimized by controlling the molar ratio between Ru and Cr in the spinning solution. As illustrated in Fig. S9, ESI,† the optimized Ru/Cr2O3 HNFs with the Ru![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Cr molar ratio of 3
Cr molar ratio of 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 demonstrate superior HER activity compared to the other comparative HNFs. Moreover, the mass activity (MARu) values are calculated to evaluate the practical viability and cost-effectiveness of the catalysts. As anticipated, Ru/Cr2O3 HNFs boast the highest MARu value of 1536.11 A gRu−1 at the overpotential of 100 mV, much better than other control catalysts, including Ru/Cr2O3-1 HNFs (898.62 A gRu−1), Ru/Cr2O3-2 HNFs (1220.54 A gRu−1), Ru/Cr2O3-4 HNFs (812.48 A gRu−1), Ru NFs (129.89 A gRu−1), and RuCrOx NFs (48.73 A gRu−1) (Fig. S10a, ESI†). After that, turnover frequency (TOF) values are calculated to delve into the intrinsic HER activity of catalysts. The highest TOF value of 0.604 s−1 at the overpotential of 100 mV is achieved by Ru/Cr2O3 HNFs, which is also markedly superior to other control catalysts (Fig. S10b, ESI†). It is worth noting that all the prepared HNF samples possess much higher MARu and TOF values than single component Ru NFs and unreduced RuCrOx NF catalysts, further revealing the highly efficient active sites in the heterojunctions of Ru/Cr2O3 during the alkaline HER.
1 demonstrate superior HER activity compared to the other comparative HNFs. Moreover, the mass activity (MARu) values are calculated to evaluate the practical viability and cost-effectiveness of the catalysts. As anticipated, Ru/Cr2O3 HNFs boast the highest MARu value of 1536.11 A gRu−1 at the overpotential of 100 mV, much better than other control catalysts, including Ru/Cr2O3-1 HNFs (898.62 A gRu−1), Ru/Cr2O3-2 HNFs (1220.54 A gRu−1), Ru/Cr2O3-4 HNFs (812.48 A gRu−1), Ru NFs (129.89 A gRu−1), and RuCrOx NFs (48.73 A gRu−1) (Fig. S10a, ESI†). After that, turnover frequency (TOF) values are calculated to delve into the intrinsic HER activity of catalysts. The highest TOF value of 0.604 s−1 at the overpotential of 100 mV is achieved by Ru/Cr2O3 HNFs, which is also markedly superior to other control catalysts (Fig. S10b, ESI†). It is worth noting that all the prepared HNF samples possess much higher MARu and TOF values than single component Ru NFs and unreduced RuCrOx NF catalysts, further revealing the highly efficient active sites in the heterojunctions of Ru/Cr2O3 during the alkaline HER.
The Tafel slope of Ru/Cr2O3 HNFs (5.2 mV dec−1) is also notably lower than those of RuCrOx NFs (44.5 mV dec−1), Ru NFs (11.1 mV dec−1) and the commercial 20% Pt/C catalyst (15.5 mV dec−1), suggesting the Volmer–Tafel mechanism as the HER pathway37,38 and signifying the exceptional catalytic kinetics of Ru/Cr2O3 NFs (Fig. 3b). The ability to achieve high catalytic activity at industrial-grade current density is essential for the practical application of electrocatalysts.39,40 Remarkably, the Ru/Cr2O3 HNFs require an overpotential of only 88 mV to attain a current density of 1 A cm−2 in 1 M KOH, which is also significantly better than the other comparative catalysts (Fig. S8 and S9, ESI†). Owing to the ultra-low overpotential at ampere-grade current density and Tafel slope, Ru/Cr2O3 HNFs exhibit the most outstanding HER activity among the recently reported high-current-density HER catalysts in alkaline environments (Fig. 3c and Table S1, ESI†).6,12,14,41–49 Subsequently, to elucidate the origin of the high catalytic activity of Ru/Cr2O3 HNFs in alkaline environments, electrochemical impedance spectroscopy (EIS) analysis is conducted to assess the interfacial charge transfer capability. The Nyquist plots obtained at the potential of −1.0 V vs. the Hg/HgO electrode, as depicted in Fig. 3d, reveal that the charge transfer resistance (Rct) value of Ru/Cr2O3 HNFs (0.67 Ω) is much lower than those of commercial Pt/C (6.77 Ω), RuCrOx NFs (32.56 Ω), Ru NFs (4.64 Ω), and R-Cr2O3 NFs (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 230 Ω), indicating a substantial enhancement in charge transfer capacity for the heterostructure, which contributes to the enhanced HER efficiency. The equivalent circuit model used for fitting the EIS data is presented in Fig. S11, ESI.†
230 Ω), indicating a substantial enhancement in charge transfer capacity for the heterostructure, which contributes to the enhanced HER efficiency. The equivalent circuit model used for fitting the EIS data is presented in Fig. S11, ESI.†
The double-layer capacitance (Cdl) values for different catalysts are determined from cyclic voltammetry (CV) curves obtained in the non-Faraday potential region, which correlates positively with the electrochemical active surface area (ECSA) (Fig. S12 and S13, ESI†).50 The ECSA values reflect the number of active sites available for the electrocatalytic process.51,52 The Cdl value of the Ru/Cr2O3 HNFs is 88.17 mF cm−2, which is approximately 61.23, 2.98, and 2.93 times as high as that of R-Cr2O3 NFs, RuCrOx NFs and Ru NFs, respectively (Fig. 3e). Evidently, the Ru/Cr2O3 HNFs possess a higher estimated ECSA and roughness factor (RF) values of 198.38 cm2 and 2204, respectively, compared to the other comparative catalysts (Table S2, ESI†). Based on these ECSA values, the ECSA-normalized LSVs are employed to gauge their intrinsic activity (Fig. S14a, ESI†). And as expected, Ru/Cr2O3 HNFs show the lowest overpotential of 91 mV at a current density of 0.5 mA cmECSA−2 compared to that of RuCrOx NFs and Ru NFs (Fig. S14b, ESI†). This demonstrates that the heterostructure not only effectively exposes a great number of HER active sites, but also significantly increases the activity of each site. Fig. 3f shows the six pivotal factors for HER catalysts. It is readily apparent that the Ru/Cr2O3 HNFs encompass the largest area in each category compared to the RuCrOx NFs and Ru NFs, signifying their exceptional electrocatalytic performance in the HER process.
Beyond the exploration into electrocatalytic activity, the stability test is also a crucial factor in evaluating the viability of a catalyst for real-world application. Remarkably, the Ru/Cr2O3 HNFs work stably at a high current density of 1 A cm−2, retaining over 90% of the initial current density even after 300 h of continuous operation in an alkaline medium, as evidenced by the i–t test (Fig. 3g). Obviously, the stability of Ru/Cr2O3 HNFs is considerably better than that of RuCrOx NFs (45% after 15 h) and commercial Pt/C catalyst (30% after 20 h). To shed light on the structural stability of Ru/Cr2O3 HNFs throughout the HER process, a comprehensive suite of characterization studies was conducted after the i–t testing. Fig. S15, ESI† shows the SEM image of the Ru/Cr2O3 HNF catalyst post HER, illustrating its morphological and structural robustness. Moreover, the XRD spectra of the catalyst exhibit no discernible phase transformation, thereby realizing the stability of the crystal structure of the Ru/Cr2O3 HNFs (Fig. S16a, ESI†). The surface chemical valence states of the catalyst following the HER process are further probed in greater detail using XPS spectroscopy (Fig. S16b–d, ESI†). A comparison of the Ru 3p, Cr 2p and O 1s spectra of the Ru/Cr2O3 HNFs before and after the HER test indicates that there are no substantial alterations, underscoring the stability of the catalyst. Furthermore, the electrolyte for Ru/Cr2O3 HNFs after 300 h of continuous HER stability test in 1 M KOH was examined by inductively coupled plasma-optical emission spectrometry (ICP-OES) to estimate the dissolved amount of Ru. The results show that only a very small amount (1.7 wt%) of Ru is dissolved in the electrolyte during the HER stability test, indicating the excellent stability of Ru/Cr2O3 HNFs under alkaline conditions.
The HER performance of these samples in acidic media (0.5 M H2SO4) is also evaluated using a standard three-electrode system. As depicted in Fig. 4a and S17, ESI,† the Ru/Cr2O3 HNFs still retain high catalytic activity, requiring a mere overpotential of 35 mV to reach a current density of 0.1 A cm−2. Notably, this overpotential is not only significantly lower than those of RuCrOx NFs (179 mV) and Ru NFs (176 mV), but also smaller than that of commercial Pt/C (41 mV). Similarly, the Ru/Cr2O3 HNFs also demonstrate the best HER activity in acidic media when compared to the samples obtained under other experimental conditions (Fig. S18, ESI†). In addition, in 0.5 M H2SO4, the Ru/Cr2O3 HNFs achieve the highest MARu value of 900.28 A gRu−1 at an overpotential of 100 mV, far surpassing the performance of the other control catalysts (Fig. S19a, ESI†). The Ru/Cr2O3 HNFs also attain the highest TOF value of 0.354 s−1 at an overpotential of 100 mV in 0.5 M H2SO4, which is significantly superior to that of other control catalysts (Fig. S19b, ESI†). Additionally, the Ru/Cr2O3 HNFs achieve a remarkable current density of 1 A cm−2 with a reduced overpotential of just 112 mV, outperforming the other control catalysts and highlighting their exceptional HER activity for practical application (Fig. S17 and S18b, ESI†). The corresponding Tafel slopes of Ru/Cr2O3 HNFs, commercial Pt/C, RuCrOx NFs, and Ru NFs are 33.4, 38.3, 65.9, and 83.5 mV dec−1, respectively (Fig. 4b). These findings also suggest that the heterostructure of Ru and Cr2O3 can markedly promote the HER reaction kinetics in acidic environments. Fortunately, the robust catalytic activity of Ru/Cr2O3 HNFs in 0.5 M H2SO4 is on par with or even exceeds the performance of recently reported top-tier electrocatalysts (Fig. 4c and Table S3, ESI†).44,53–64 Furthermore, EIS measurement reveals that the Ru/Cr2O3 HNFs exhibit an exceptionally low Rct of merely 2.0 Ω, which is far below than that of the other catalysts (Fig. 4d and S18c, ESI†). This low Rct value suggests an enhanced electrical conductivity and efficient electron transfer capability within the Ru/Cr2O3 HNFs, beneficial for promoting the HER activity.9,65 The ECSA values of these electrocatalysts are estimated by assessing the directly related Cdl values (Fig. S20 and S21, ESI†). As illustrated in Fig. 4e, the calculated Cdl value for Ru/Cr2O3 HNFs is 45.19 mF cm−2, which exceeds that of RuCrOx NFs (34.6 mF cm−2) and is substantially greater than those of the Ru NFs (3.13 mF cm−2) and R-Cr2O3 NFs (0.05 mF cm−2). Clearly, the Ru/Cr2O3 HNFs also possess a higher estimated ECSA and RF values of 101.68 cm2 and 1130, respectively, compared to other catalysts in acidic environments (Table S4, ESI†). In addition, ECSA normalization is also performed to evaluate the intrinsic activity of synthesized catalysts under acidic conditions (Fig. S22, ESI†). The results show that Ru/Cr2O3 HNFs exhibit a low overpotential of only 92 mV at 0.5 mA cmECSA−2, which is much better than that of RuCrOx NFs (249 mV) and Ru NFs (137 mV), indicating that Ru/Cr2O3 HNFs possess both a larger ECSA and higher intrinsic activity.
Further, the radargram presented in Fig. 4f demonstrates that Ru/Cr2O3 HNFs exhibit an outstanding electrocatalytic performance compared to RuCrOx NFs and Ru NFs for the acidic HER, suggesting the exceptional advantage of the heterojunction construction of metallic Ru and Cr2O3 in the acidic HER process. Additionally, Ru/Cr2O3 HNFs also showcase exceptional durability in catalyzing acidic HER. In a continuous i–t test conducted in 0.5 M H2SO4 with a constant applied potential, Ru/Cr2O3 HNFs sustain up to 300 h of HER progression at a high current density of 1 A cm−2, retaining a stable current density (Fig. 4g). In contrast, both RuCrOx NFs and the commercial Pt/C catalyst decay rapidly within 25 h, further confirming the superior long-term stability of the Ru/Cr2O3 HNFs. Finally, we have examined the surface structure and chemical compositions of the Ru/Cr2O3 NFs after the acidic HER stability test. The SEM image (Fig. S23, ESI†) and XRD pattern (Fig. S24a, ESI†) exhibit no significant alterations in either morphology or crystal structure. The surface chemical valence states of the catalyst after the acidic HER process is further investigated using XPS spectroscopy (Fig. S24b–d, ESI†). Compared with the Ru 3p, Cr 2p and O 1s spectra of Ru/Cr2O3 NFs before the HER test, no substantial differences are observed, demonstrating the compositional stability of the catalyst during the acidic HER process.
To delve into the origin of the high activity on Ru/Cr2O3, periodic density functional theory (DFT) calculations are conducted. The structural models are constructed including Ru(101), RuCrOx and Ru/Cr2O3 (Fig. S25, ESI†). Fig. 5a displays the schematic diagram of the adsorption of *H2O and *H on three different models.66–68 The kinetic energy for H2O adsorption (ΔG (*H2O)), hydrogen adsorption (ΔG (*H)), and final H2 release is calculated based on the optimized models for Ru/Cr2O3, Ru(101) and RuCrOx. The adsorption energies of ΔG (*H2O) and ΔG (*H) have been widely accepted as descriptors to evaluate the HER activity of catalysts. In Fig. 5b, the adsorption of H2O occurs almost spontaneously on all three models since the ΔG (*H2O) is less than 0. The value of ΔG (*H) close to 0 eV is considered to be ideal for the HER.7,69 It can be found that the value of ΔG (*H) for Ru/Cr2O3 is |0.25 eV|, which is much closer to 0 eV than the corresponding values for Ru(101) (|−0.64 eV|) and RuCrOx (|−0.84 eV|). This indicates that the interface of the Ru site in proximity to the Cr2O3 possesses the most favorable *H adsorption kinetics. Moreover, the hydrogen evolution process is exergonic on the surface of Ru/Cr2O3 while it is thermodynamically uphill on the surface of the other two models, indicating that the construction of the Ru/Cr2O3 heterojunction interface facilitates *H desorption and thus accelerates the release of H2. The possible reason for this phenomenon is that the Cr2O3 surface effectively modulates the electronic properties of Ru, thereby optimizing the adsorption and desorption of *H (Fig. 5c). This leads to superior HER performance in both alkaline and acidic environments, which can be further substantiated by examining the projected density of states (PDOS). As depicted in Fig. 5d, the d-band centers of Ru in the Ru/Cr2O3, Ru(101) and RuCrOx models relative to the Fermi level are calculated to be −0.98, −0.84 and +0.02 eV, respectively, illustrating that the d-band center is far away from the Fermi level at the heterojunction interface between Ru and Cr2O3. This downshift suggests a weakened interaction between *H and the Ru active site at the heterojunction interface,67 which facilitates the H2 release from the heterojunction interface of Ru/Cr2O3. Furthermore, the differential charge density maps visualize the interaction between *H, *H2O and the surface of catalyst models (Fig. 5a). The robust interactions between *H and Ru in RuCrOx lead to a strong adsorption of *H on RuCrOx, resulting in the higher desorption energy of *H on RuCrOx. Conversely, the weak interactions between *H and Ru in Ru/Cr2O3 facilitate *H desorption, thereby lowering the energy barrier for the HER. The above analysis underscores that the synergistic interaction between Ru and Cr2O3 enhances the electrocatalytic performance of Ru/Cr2O3 HNFs for the HER.
Conclusion
In summary, we have successfully fabricated a nanofibrous Ru/Cr2O3 heterojunction through an electrospinning-calcination-thermal reduction procedure for efficient alkaline/acid-universal electrocatalytic properties at industrial-grade current density. The obtained Ru/Cr2O3 HNF catalyst achieves an exceptional HER activity and long-term stability at a high current density of 1 A cm−2, significantly outperforming commercial Pt/C and many recently reported top-tier electrocatalysts. DFT calculations reveal that the construction of the heterojunction of Ru/Cr2O3 optimizes the *H desorption capability during the HER process. In addition, the strong interactions between Ru and Cr2O3 enable more charge carriers participating in the electrocatalytic reactions, resulting in a promoted HER performance. This approach of significantly enhancing the alkaline/acid-universal HER properties at the ampere level can be broadened as a novel design paradigm for other catalyst systems, thereby advancing the evolution and practical utilization for high-efficiency H2 production.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
X. Lu, M. Zhong and M. Gao supervised this project. X. Yu performed the experiments and characterized the catalysts. X. Yu, M. Xia, R. Qi, Y. Wang and M. Zhong analyzed the data. M. Gao carried out the theoretical calculation. X. Yu and M. Gao wrote the manuscript. X. Lu and M. Zhong revised the manuscript. All authors have approved the final version of the manuscript.Conflicts of interest
The authors declare no conflict of interest.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (52273056 and 22208337) and the Jilin Province Science and Technology Development Program (YDZJ202501ZYTS305).References
- S. Chu and A. Majumdar, Nature, 2012, 488, 294–303 CrossRef CAS PubMed.
- M. D. Allendorf, V. Stavila, J. L. Snider, M. Witman, M. E. Bowden, K. Brooks, B. L. Tran and T. Autrey, Nat. Chem., 2022, 14, 1214–1223 CrossRef CAS PubMed.
- R.-T. Liu, Z.-L. Xu, F.-M. Li, F.-Y. Chen, J.-Y. Yu, Y. Yan, Y. Chen and B. Y. Xia, Chem. Soc. Rev., 2023, 52, 5652–5683 RSC.
- Y. Jiang, Z. Liang, H. Fu, M. Sun, S. Wang, B. Huang and Y. Du, J. Am. Chem. Soc., 2024, 146, 9012–9025 CrossRef CAS PubMed.
- Y.-C. Zhang, M. Zhao, J. Wu, Y. Wang, L. Zheng, F. Gu, J.-J. Zou, J. Gao and X.-D. Zhu, ACS Catal., 2024, 14, 7867–7876 CrossRef CAS.
- J. Liu, J. Ren, Y. Du, X. Chen, M. Wang, Y. Liu and L. Wang, Adv. Funct. Mater., 2024, 34, 2315773 CrossRef CAS.
- L. Zhang, H. Hu, C. Sun, D. Xiao, H.-T. Wang, Y. Xiao, S. Zhao, K. H. Chen, W.-X. Lin, Y.-C. Shao, X. Wang, C.-W. Pao and L. Han, Nat. Commun., 2024, 15, 7179 CrossRef CAS PubMed.
- Q. Hu, J. Cao, S. Qi, N. Meng, J. Zhao, T. Huang, J. You, T. Liang, C. Shang, J. Yu, H. Yang and C. He, Angew. Chem., Int. Ed., 2024, e202416402 Search PubMed.
- J.-B. Chen, J. Ying, Y.-X. Xiao, G. Tian, Y. Dong, L. Shen, S. I. Córdoba de Torresi, M. D. Symes, C. Janiak and X.-Y. Yang, ACS Catal., 2023, 13, 14802–14812 CrossRef CAS.
- W. Li, C. Wang and X. Lu, J. Mater. Chem. A, 2021, 9, 3786–3827 RSC.
- L. Zhang, W. Li, S. Ren, W. Song, C. Wang and X. Lu, Adv. Energy Mater., 2024, 2403136 Search PubMed.
- W. Li, W. Gou, L. Zhang, M. Zhong, S. Ren, G. Yu, C. Wang, W. Chen and X. Lu, Chem. Sci., 2024, 15, 11890–11901 RSC.
- D. Wu, K. Kusada, S. Yoshioka, T. Yamamoto, T. Toriyama, S. Matsumura, Y. Chen, O. Seo, J. Kim, C. Song, S. Hiroi, O. Sakata, T. Ina, S. Kawaguchi, Y. Kubota, H. Kobayashi and H. Kitagawa, Nat. Commun., 2021, 12, 1145 CrossRef CAS PubMed.
- W. Li, R. Liu, G. Yu, X. Chen, S. Yan, S. Ren, J. Chen, W. Chen, C. Wang and X. Lu, Small, 2024, 20, 2307164 CrossRef CAS PubMed.
- M. Li, H. Wang, W. Zhu, W. Li, C. Wang and X. Lu, Adv. Sci., 2020, 7, 1901833 CrossRef CAS PubMed.
- C. W. Lee, C. Cazorla, S. Zhou, D. Zhang, H. Xu, W. Zhong, M. Zhang, D. Chu, Z. Han and R. Amal, Adv. Energy Mater., 2024, 2402786 Search PubMed.
- B. Zhang, J. Wang, G. Liu, C. M. Weiss, D. Liu, Y. Chen, L. Xia, P. Zhou, M. Gao, Y. Liu, J. Chen, Y. Yan, M. Shao, H. Pan and W. Sun, Nat. Catal., 2024, 7, 441–451 CrossRef CAS.
- J. Li, X. Meng, X. Song, J. Qi, F. Liu, X. Xiao, Y. Du, G. Xu, Z. Jiang, S. Ye, S. Huang and J. Qiu, Adv. Funct. Mater., 2024, 34, 2316718 CrossRef CAS.
- S. Zuo, Z.-P. Wu, D. Xu, R. Ahmad, L. Zheng, J. Zhang, L. Zhao, W. Huang, H. Al Qahtani, Y. Han, L. Cavallo and H. Zhang, Nat. Commun., 2024, 15, 9514 CrossRef CAS PubMed.
- M. Li, X. Wang, K. Liu, H. Sun, D. Sun, K. Huang, Y. Tang, W. Xing, H. Li and G. Fu, Adv. Mater., 2023, 35, 2302462 CrossRef CAS PubMed.
- Q. Liu, Z. Xue, B. Jia, Q. Liu, K. Liu, Y. Lin, M. Liu, Y. Li and G. Li, Small, 2020, 16, 2002482 CrossRef CAS PubMed.
- H. Q. Fu, J. Liu, N. M. Bedford, Y. Wang, J. W. Sun, Y. Zou, M. Dong, J. Wright, H. Diao, P. Liu, H. G. Yang and H. Zhao, Adv. Mater., 2022, 34, 2202854 CrossRef CAS PubMed.
- M. Gong, W. Zhou, M. J. Kenney, R. Kapusta, S. Cowley, Y. Wu, B. Lu, M.-C. Lin, D.-Y. Wang, J. Yang, B.-J. Hwang and H. Dai, Angew. Chem., Int. Ed., 2015, 54, 11989–11993 CrossRef CAS PubMed.
- W. Li, L. Zhang, L. Ma, J. Wang, R. Qi, Y. Pang, M. Xu, C. Zhao, C. Wang, M. Gao and X. Lu, Nano Lett., 2025, 25, 443–452 CrossRef CAS PubMed.
- Y. Wang, X. Yu, J. Xu, M. Zhong, J. Hao, M. Gao and X. Lu, Chem. Eng. J., 2025, 505, 159561 CrossRef CAS.
- S. Liu, K. Ma, H. Teng, W. Miao, X. Zhou, X. Cui, X. Zhou, L. Jiang and S. Guo, Adv. Mater., 2025, 37, 2411148 CrossRef CAS PubMed.
- H. Yao, X. Wang, K. Li, C. Li, C. Zhang, J. Zhou, Z. Cao, H. Wang, M. Gu, M. Huang and H. Jiang, Appl. Catal., B, 2022, 312, 121378 CrossRef CAS.
- Q. He, Y. Zhou, H. Shou, X. Wang, P. Zhang, W. Xu, S. Qiao, C. Wu, H. Liu, D. Liu, S. Chen, R. Long, Z. Qi, X. Wu and L. Song, Adv. Mater., 2022, 34, 2110604 CrossRef CAS PubMed.
- F. Yang, Y. Zhao, Y. Du, Y. Chen, G. Cheng, S. Chen and W. Luo, Adv. Energy Mater., 2018, 8, 1703489 CrossRef.
- C. Hu, E. Song, M. Wang, W. Chen, F. Huang, Z. Feng, J. Liu and J. Wang, Adv. Sci., 2021, 8, 2001881 CrossRef CAS PubMed.
- X. Miao, Z. Peng, L. Shi and S. Zhou, ACS Catal., 2023, 13, 3983–3989 CrossRef CAS.
- J. Guo, Y. Zheng, Z. Hu, C. Zheng, J. Mao, K. Du, M. Jaroniec, S.-Z. Qiao and T. Ling, Nat. Energy, 2023, 8, 264–272 CAS.
- S. Li, T. Liu, W. Zhang, M. Wang, H. Zhang, C. Qin, L. Zhang, Y. Chen, S. Jiang, D. Liu, X. Liu, H. Wang, Q. Luo, T. Ding and T. Yao, Nat. Commun., 2024, 15, 3416 CrossRef CAS PubMed.
- L. Wang, Y. Zhu, Y. Wen, S. Li, C. Cui, F. Ni, Y. Liu, H. Lin, Y. Li, H. Peng and B. Zhang, Angew. Chem., Int. Ed., 2021, 60, 10577–10582 CrossRef CAS PubMed.
- J. Zheng, D. Meng, J. Guo, X. Liu, L. Zhou and Z. Wang, Adv. Mater., 2024, 36, 2405129 CrossRef CAS PubMed.
- P. Xue, M. Qiao, J. Miao, Y. Tang, D. Zhu and C. Guo, Chem. Commun., 2024, 60, 6423–6426 RSC.
- J. Mahmood, F. Li, S.-M. Jung, M. S. Okyay, I. Ahmad, S.-J. Kim, N. Park, H. Y. Jeong and J.-B. Baek, Nat. Nanotechnol., 2017, 12, 441–446 CrossRef CAS PubMed.
- P. Su, W. Pei, X. Wang, Y. Ma, Q. Jiang, J. Liang, S. Zhou, J. Zhao, J. Liu and G. Q. Lu, Angew. Chem., Int. Ed., 2021, 60, 16044–16050 CrossRef CAS PubMed.
- Y. Luo, Z. Zhang, M. Chhowalla and B. Liu, Adv. Mater., 2022, 34, 2108133 CrossRef CAS PubMed.
- A. A. Feidenhans'l, Y. N. Regmi, C. Wei, D. Xia, J. Kibsgaard and L. A. King, Chem. Rev., 2024, 124, 5617–5667 CrossRef PubMed.
- B. Wang, H. Sun, M. Chen, T. Zhou, H. Zheng, M. Zhang, B. Xiao, J. Zhao, Y. Zhang, J. Zhang and Q. Liu, Chem. Eng. J., 2024, 479, 147500 CrossRef CAS.
- Y.-N. Zhou, F.-L. Wang, J. Nan, B. Dong, H.-Y. Zhao, F.-G. Wang, N. Yu, R.-N. Luan, D.-P. Liu and Y.-M. Chai, Appl. Catal., B, 2022, 304, 120917 CrossRef CAS.
- Y. Zhang, K. E. Arpino, Q. Yang, N. Kikugawa, D. A. Sokolov, C. W. Hicks, J. Liu, C. Felser and G. Li, Nat. Commun., 2022, 13, 7784 CrossRef CAS PubMed.
- G. Zhang, A. Wang, L. Niu, W. Gao, W. Hu, Z. Liu, R. Wang and J. Chen, Adv. Energy Mater., 2022, 12, 2103511 CrossRef CAS.
- L. Gao, F. Bao, X. Tan, M. Li, Z. Shen, X. Chen, Z. Tang, W. Lai, Y. Lu, P. Huang, C. Ma, S. C. Smith, Z. Ye, Z. Hu and H. Huang, Energy Environ. Sci., 2023, 16, 285–294 RSC.
- Y. Huang, X. Zhang, L. Li, M. Humayun, H. Zhang, X. Xu, S. P. Anthony, Z. Chen, J. Zeng, D. V. Shtansky, K. Huo, H. Song, C. Wang and W. Zhang, Adv. Funct. Mater., 2024, 2401011 CrossRef.
- S. Li, Y. Liu, K. Feng, C. Li, J. Xu, C. Lu, H. Lin, Y. Feng, D. Ma and J. Zhong, Angew. Chem., Int. Ed., 2023, 62, e202308670 CrossRef CAS PubMed.
- X. Zhang, Y. Yang, Y. Liu, Z. Jia, Q. Wang, L. Sun, L.-C. Zhang, J. J. Kruzic, J. Lu and B. Shen, Adv. Mater., 2023, 35, 2303439 CrossRef CAS PubMed.
- Y. Zou, S. A. Kazemi, G. Shi, J. Liu, Y. Yang, N. M. Bedford, K. Fan, Y. Xu, H. Fu, M. Dong, M. Al-Mamun, Y. L. Zhong, H. Yin, Y. Wang, P. Liu and H. Zhao, EcoMat, 2023, 5, e12274 CrossRef CAS.
- Y. Chen, Q. Li, Y. Lin, J. Liu, J. Pan, J. Hu and X. Xu, Nat. Commun., 2024, 15, 7278 CrossRef CAS PubMed.
- M. Zhong, M. Xu, S. Ren, W. Li, C. Wang, M. Gao and X. Lu, Energy Environ. Sci., 2024, 17, 1984–1996 RSC.
- Z. Li, H. Sheng, Y. Lin, H. Hu, H. Sun, Y. Dong, X. Chen, L. Wei, Z. Tian, Q. Chen, J. Su and L. Chen, Adv. Funct. Mater., 2024, 34, 2409714 CrossRef CAS.
- S. Venkateswarlu, S. Kim, M. Balamurugan, Y. Son, M. Yoon, K. T. Nam, S. S. Han and M. J. Kim, Appl. Catal., B, 2024, 345, 123609 CrossRef CAS.
- H. Chen, J. Yu, L. Liu, R.-T. Gao, Z. Gao, Y. Yang, Z. Chen, S. Zhan, X. Liu, X. Zhang, H. Dong, L. Wu and L. Wang, Adv. Energy Mater., 2024, 14, 2303635 CrossRef CAS.
- J. Yang, A. R. Mohmad, Y. Wang, R. Fullon, X. Song, F. Zhao, I. Bozkurt, M. Augustin, E. J. G. Santos, H. S. Shin, W. Zhang, D. Voiry, H. Y. Jeong and M. Chhowalla, Nat. Mater., 2019, 18, 1309–1314 CrossRef CAS PubMed.
- G. Chen, W. Chen, R. Lu, C. Ma, Z. Zhang, Z. Huang, J. Weng, Z. Wang, Y. Han and W. Huang, J. Am. Chem. Soc., 2023, 145, 22069–22078 CrossRef CAS PubMed.
- Z. Zheng, L. Yu, M. Gao, X. Chen, W. Zhou, C. Ma, L. Wu, J. Zhu, X. Meng, J. Hu, Y. Tu, S. Wu, J. Mao, Z. Tian and D. Deng, Nat. Commun., 2020, 11, 3315 CrossRef CAS PubMed.
- J. Chen, J. Huang, Y. Zhao, L. Cao, K. Kajiyoshi, Y. Liu, Z. Li and Y. Feng, Chem. Eng. J., 2022, 450, 138026 CrossRef CAS.
- C. Zhang, Y. Luo, J. Tan, Q. Yu, F. Yang, Z. Zhang, L. Yang, H.-M. Cheng and B. Liu, Nat. Commun., 2020, 11, 3724 CrossRef CAS PubMed.
- R. Liu, Z. Gong, J. Liu, J. Dong, J. Liao, H. Liu, H. Huang, J. Liu, M. Yan, K. Huang, H. Gong, J. Zhu, C. Cui, G. Ye and H. Fei, Adv. Mater., 2021, 33, 2103533 CrossRef CAS PubMed.
- D. Zhang, Z. Wang, X. Wu, Y. Shi, N. Nie, H. Zhao, H. Miao, X. Chen, S. Li, J. Lai and L. Wang, Small, 2022, 18, 2104559 CrossRef CAS PubMed.
- M. Cui, F. Wang, W. Zhao, D. Zhang, R. Liang, Q. Ou and S. Zhang, Chem. Eng. J., 2023, 460, 141676 CrossRef CAS.
- S. Parvin, A. Kumar, A. Ghosh and S. Bhattacharyya, Chem. Sci., 2020, 11, 3893–3902 RSC.
- Q. Yu, Z. Zhang, S. Qiu, Y. Luo, Z. Liu, F. Yang, H. Liu, S. Ge, X. Zou, B. Ding, W. Ren, H.-M. Cheng, C. Sun and B. Liu, Nat. Commun., 2021, 12, 6051 CrossRef CAS PubMed.
- C. Wang and L. Qi, Angew. Chem., Int. Ed., 2020, 59, 17219–17224 CrossRef CAS PubMed.
- R. Zhang, Y. Li, X. Zhou, A. Yu, Q. Huang, T. Xu, L. Zhu, P. Peng, S. Song, L. Echegoyen and F.-F. Li, Nat. Commun., 2023, 14, 2460 CrossRef CAS PubMed.
- L. Wang, Y. Liu, Z. Chen, Q. Dai, C.-L. Dong, B. Yang, Z. Li, X. Hu, L. Lei and Y. Hou, Nano Energy, 2023, 115, 108694 CrossRef CAS.
- J. Duan, S. Chen, C. A. Ortíz-Ledón, M. Jaroniec and S.-Z. Qiao, Angew. Chem., Int. Ed., 2020, 59, 8181–8186 CrossRef CAS PubMed.
- X. Xu, K. Guo, J. Sun, X. Yu, X. Miao, W. Lu and L. Jiao, Adv. Funct. Mater., 2024, 34, 2400397 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5sc00248f |
| This journal is © The Royal Society of Chemistry 2025 |