Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Expedient discovery of fluorogenic amino acid-based probes via one-pot palladium-catalysed arylation of tyrosine†
Olivia
Marshall
,
Rochelle
McGrory
,
Sineenard
Songsri
,
Andrew R.
Thomson
 and
Andrew
Sutherland
and
Andrew
Sutherland
 *
*
School of Chemistry, University of Glasgow, Joseph Black Building, University Avenue, Glasgow, G12 8QQ, UK. E-mail: Andrew.Sutherland@glasgow.ac.uk
First published on 23rd January 2025
Abstract
To overcome the limitations of using large extrinsic chromophores for biological imaging, fluorescent unnatural α-amino acids have been widely adopted as intrinsic peptidic probes. Although various classes have been successfully utilised for imaging applications, novel amino acid probes readily prepared through operationally simple synthetic methodology are still required. Here, we report a new approach for the synthesis of unnatural α-amino acids via a one-pot process involving activation and palladium-catalysed arylation of tyrosine. Rapid access to a small library of novel α-amino acids has allowed the discovery of a dimethylaminobiphenyl analogue that displays strong charge transfer-based fluorescent properties and is both solvatochromic and pH sensitive with a significant hypsochromic shift in emission under acidic conditions. The imaging potential of the dimethylaminobiphenyl α-amino acid was demonstrated via its application as a FRET donor in a novel decapeptide substrate for monitoring and evaluating the kinetics of a serine protease.
Introduction
Fluorescent spectroscopy is a key technique for studying biological processes.1 The high detection sensitivity down to single-molecule level and real-time monitoring along with variable time scales have allowed its application for the non-invasive imaging of various events in biomedical and life sciences research.2 However, enabling the full potential of fluorescent spectroscopy requires the development of minimally invasive fluorophores that can report without interrupting the biological process.3 For imaging with proteins and peptides, large chromophores with appropriate photophysical properties are typically attached at the terminus using a chemical spacer.4 However, this can still disrupt the structure and function of the peptide and, positioning of the chromophore at the terminus of a protein limits applications.The issues associated with the use of large extrinsic chromophores for peptide imaging have resulted in the development of fluorescent unnatural α-amino acids as probes for biological imaging.5 Chromophores with specific photophysical properties can be incorporated into the amino acid side chain, and the amino acid probe can be selectively embedded into the protein structure using either solid phase peptide synthesis (SPPS) or genetic encoding. A key approach for the design of novel fluorescent amino acids involves the modification of fluorescent proteinogenic α-amino acids. Although efforts have primarily focused on the development of structural analogues of the most fluorescent proteinogenic α-amino acid, tryptophan,6–8 analogues of phenylalanine (1) have also been reported (Fig. 1a).5 These include a number of polyaromatic analogues such as 4-biphenyl-L-phenylalanine 2, which was incorporated into dihydrofolate reductase (DHFR) via genetic encoding and used in a Förster resonance energy transfer (FRET) experiment to study conformation change following inhibitor binding.9 Other terphenyl α-amino acids such as 3 and prenyl-L-alanine (4) have also been introduced into DHFR and used to investigate protein dynamics.10,11 Acridon-2-yl-L-alanine (5), prepared in five steps from L-tyrosine has been shown to possess a long fluorescent lifetime and emit in the visible region.12 These properties have allowed the use of this fluorescent unnatural α-amino acid for monitoring protein folding and binding interactions using FRET assays with common fluorophores such as coumarins.12b More recently, the Ackermann and Vendrell groups have reported the synthesis of phenylalanine-BODIPY conjugates that have been used to detect Candida infections in human urine samples.13
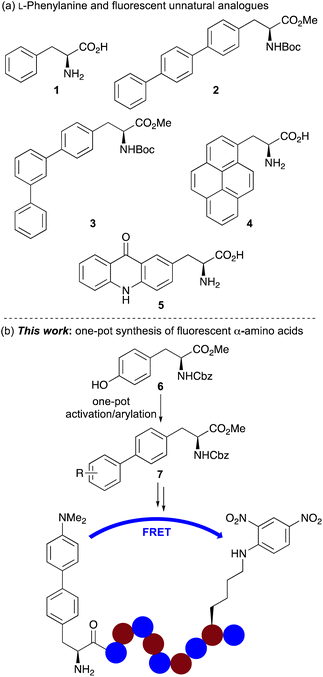 | ||
| Fig. 1 (a) L-Phenylalanine (1) and selected fluorescent analogues. (b) One-pot synthesis of biaryl α-amino acids and application as an internally quenched fluorescent peptide. | ||
Despite the significant progress in identifying fluorescent polyaromatic α-amino acids for biological applications, novel analogues with minimally invasive side chains, which can be readily incorporated into peptides using standard techniques for new imaging experiments are still required. In addition, synthetic methodology that allows rapid access to libraries of novel α-amino acid analogues which can expedite the discovery of new probes is of particular importance. As part of a research programme focused on the development of fluorescent unnatural α-amino acids,8,14 we were interested in exploring new analogues of phenylalanine for the development of a fluorophore that could be used in FRET experiments to monitor protease activity. Here, we report the development of a one-pot synthesis of biaryl α-amino acids by activation of a commercially available tyrosine derivative, followed by palladium-catalysed arylation that allows rapid access to novel fluorescent phenylalanine analogues (Fig. 1b). We also describe how straightforward access to these compounds has resulted in the discovery of several α-amino acids with strong fluorescence, including an environmentally sensitive dimethylaminobiphenyl analogue, that is highly compatible with SPPS and an effective FRET donor for determining the enzyme kinetics of serine proteases.
Results and discussion
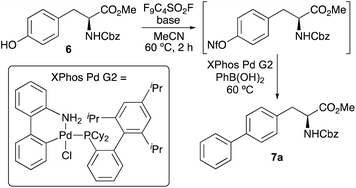
The first objective of this research programme was the development of a one-pot procedure for the preparation of biaryl α-amino acids from tyrosine derivative 6. Although, various methods for the one-pot activation and arylation of simple phenols using transition metal catalysis are known,15 to the best of our knowledge the use of α-amino acid substrates has not been reported. Aryl nonafluorobutylsulfonates (aryl nonaflates, ArONf), which are readily prepared from phenols and commercially available perfluoro-1-butanesulfonyl fluoride are increasingly used for metal-catalysed cross-coupling reactions.16 This is due to the ease of synthesis and purification, as well as their high reactivity compared to other aryl sulfonates. Akai and co-workers have shown these to be useful intermediates during the one-pot conversion of phenols to substituted aryl compounds using Suzuki–Miyaura, Sonogashira and Stille reactions.15a Based on this work, the use of a nonaflate intermediate of tyrosine 6 for subsequent arylation was investigated (Table 1). Under standard conditions, tyrosine 6 was converted to the corresponding nonaflate derivative with complete conversion as shown by 1H NMR spectroscopy. On formation of the nonaflate, Suzuki–Miyaura cross-coupling using phenylboronic acid was initially attempted using the optimised conditions described by Akai and co-workers,15a involving Pd2(dba)3 (1 mol%) and the ligand, SPhos (2 mol%, entry 1). However, this gave biphenyl α-amino acid 7a in only 30% yield. To improve the cross-coupling step, the use of the Buchwald precatalyst XPhos Pd G2 was explored.17 At 1 mol% loading and using Cs2CO3 gave no conversion (entry 2), however, the use of K3PO4 as the base gave 7a in 46% yield (entry 3). Further improvement was achieved with the addition of water as a co-solvent (entry 4), which in combination with the base is known to facilitate transmetallation and reductive elimination.18 This resulted in the isolation of 7a in 66% yield. At this stage, further optimisation studies showed that a reaction time of 20 h and a temperature of 60 °C were necessary for high conversion.19 Further analysis by 1H NMR spectroscopy of the cross-coupling step showed that under standard conditions (entry 4), the reaction was incomplete. Higher catalyst loading (2 mol%) gave a slight increase in yield to 71% (entry 5), however, nonaflate intermediate was still observed in the reaction mixture. To ensure complete conversion during the second step, batch addition of catalyst was investigated. After some optimisation, it was found the addition of three batches of catalyst (3 × 1 mol%) during the reaction resulted in complete conversion of the nonaflate and the isolation of 7a in 91% yield (entry 6).On optimisation of the one-pot activation and Suzuki–Miyaura cross-coupling reaction of tyrosine 6, the scope of the process was explored with a range of aryl boronic acids (Scheme 1). Using polyaromatic or phenyl substituted boronic acids bearing either electron-rich or electron-deficient substituents gave the products in good to excellent yields. The use of ortho-substituted aryl boronic acids was also effective, forming 7e and 7j in 94% and 70% yields, respectively. Heteroaromatic boronic acids were also tolerated with the synthesis of thiophene analogue 7m in 68% yield. Unsuccessful cross-coupling reactions were only observed with sterically hindered ortho-disubstituted boronic acids or very electron-deficient partners such as 2-fluoropyridin-3-yl boronic acid. Previous syntheses of polyaromatic α-amino acids such as 4-biphenyl-L-phenylalanine 2, used for imaging of DHFR,9 have typically been prepared using a four-step approach involving halogenation of phenylalanine using sodium iodate and concentrated sulfuric acid,20 incorporation of protecting groups and then the Suzuki–Miyaura cross-coupling step.9a,21,22 The strategy described here for the synthesis of similar compounds (e.g.7b) avoids both harsh conditions and multi-step routes, with one-pot access from commercially available tyrosine derivative 6.
 | ||
| Scheme 1 Three-pot synthesis of α-amino acids 8a–8m. The parentheses underneath each structure show the yield for the one-pot coupling followed by the overall yield for the two-step deprotection. | ||
Following the preparation of a series of analogues, these were deprotected in two steps to the parent α-amino acids (Scheme 1). Ester hydrolysis using caesium carbonate was followed by removal of the Cbz-protecting group under aqueous acidic conditions. Purification by recrystallisation gave α-amino acids 8a–8m in 36–95% yields over the two steps. Overall, the strategy of a general one-pot arylation process using a readily available tyrosine derivative allowed the expedient preparation of a range of novel α-amino acids for photophysical analysis.
The UV/visible absorption and photoluminescence spectra of α-amino acids 8a–8m were then measured (ESI†). As expected, the majority of compounds possessed red-shifted absorption and emission spectra compared to L-phenylalanine (1). In addition, screening revealed that α-amino acids with electron-deficient aryl substituents were found to possess the weakest fluorescent properties with emission from locally excited states (e.g.8e–8i), while those with polyaromatic side chains or electron-rich substituents displayed the brightest emission via internal charge transfer states (Table 2 and Fig. 2a). Amino acids 8b, 8d and 8j were found to have interesting properties with brightness >5000 cm−1 M−1, however, 4-dimethylaminophenyl analogue 8l was found to possess the most impressive overall properties. This included absorption and emission maxima at 300 and 384 nm, respectively, a large Stokes shift, a quantum yield of 0.73 and the strongest brightness of 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460 cm−1 M−1. With absorption and emission properties at longer wavelengths and stronger brightness than 8b, which has previously been used for biological imaging,9 and a higher quantum yield than other rigid, polyaromatic phenylalanines,11b it was proposed that 8l could be developed as a new fluorescent amino acid probe.
460 cm−1 M−1. With absorption and emission properties at longer wavelengths and stronger brightness than 8b, which has previously been used for biological imaging,9 and a higher quantum yield than other rigid, polyaromatic phenylalanines,11b it was proposed that 8l could be developed as a new fluorescent amino acid probe.
| Amino acid | λ Abs (nm) | ε (cm−1 M−1) | λ Em (nm) | Φ F | Brightness (cm−1 M−1) |
|---|---|---|---|---|---|
| a All spectra were recorded at 1–5 μM in MeOH. b Quantum yields (ΦF) were determined in MeOH using L-tryptophan as the standard. | |||||
| L-Phe3a | 258 | 200 | 282 | 0.024 | 5 |
| 8a | 254 | 24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 200 |
314 | 0.12 | 2930 |
| 8b | 284 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
341 | 0.83 | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 740 740 |
| 8c | 282 | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
342 | 0.27 | 2800 |
| 8d | 289 | 30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 900 900 |
356 | 0.18 | 5562 |
| 8h | 262 | 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 300 300 |
312 | 0.15 | 3490 |
| 8j | 288 | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 800 800 |
333 | 0.38 | 5240 |
| 8k | 262 | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100 100 |
328 | 0.24 | 3430 |
| 8l | 300 | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 000 |
384 | 0.73 | 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 460 460 |
To further assess amino acid 8l as an imaging agent, solvatochromic and pH studies were performed. In various solvents, a bathochromic shift in emission was observed on increasing solvent polarity (Fig. 2b). For example, an emission maximum of 370 nm was recorded in ethyl acetate, with a shift to 404 nm in water. This confirms the intramolecular charge-transfer character of the excited state of 8l, which is stabilised in more polar solvents. The solvatochromism of amino acid 8l was further verified by a Lippert–Mataga plot (ESI†), which showed a linear relationship between the Stokes shifts and solvent orientation polarisability.23 The photophysical properties of amino acid 8l were found to be highly sensitive to pH. Acidification from pH 6 to pH 1 resulted in a hypsochromic shift of the main bands in both absorption and emission spectra. The pKaH of dimethylaminobenzene is 5.06 and thus, the dimethylamino group of 8l is expected to be protonated at pH < 5.24 Thus, under acidic conditions, charge transfer is effectively turned off (8-fold decrease in CT band at 384 nm) and emission occurs from a locally excited state (315 nm, Fig. 2c) in the same manner as unsubstituted biphenyl amino acid 8a. At neutral or basic pH, when the dimethylamino group is not protonated, strong charge transfer fluorescence at 384 nm is retained (ESI†). Studies to investigate the effect of viscosity and aggregation with amino acid 8l were also conducted. Both the absorbance and emission of 8l were independent to changes in viscosity [100% MeOH (η = 0.59 mPa s) to 100% ethylene glycol (η = 13.5 mPa s)],25 while aggregation studies showed increased emission up to 50 μM, before a further increase in concentration (up to 500 μM) resulted in aggregation induced quenching (Fig. 2d). To further probe the charge transfer properties of 8l, which is directly responsible for the polarity and pH sensitivity, DFT calculations were performed. Initially, an energy minimised model of 8l was optimised using the B3LYP/6-31G basis sets,26 which confirmed the expected twisted conformation of the biphenyl side chain (Fig. 3a). Frontier molecular orbital analysis at the same level of theory allowed visualisation of the highest occupied molecular orbital (HOMO) and lowest unoccupied molecular orbital (LUMO) states. The largest contribution of electron density of the HOMO (Fig. 3b) was found localised on the electron-rich 4-dimethylamino-substituent, while the electron density contribution for the LUMO was focused only on the biphenyl ring system (Fig. 3c). This calculated electronic structure is consistent with the emission properties of amino acid 8l. Electron donation from the 4-dimethylamino group through to the biphenyl ring system results in a charge-separated excited state that on de-excitation produces emission at longer wavelengths. In summary, the combination of the photophysical and DFT studies of 8l represents a comprehensive analysis of the properties of this amino acid and confirms its potential as a fluorescent probe for biological applications.
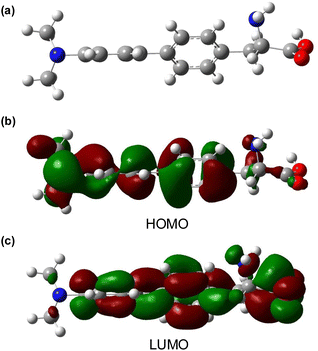 | ||
| Fig. 3 (a) Energy minimised DFT structure of 8l using B3LYP/6-31G basis sets. (b) Calculated HOMO isosurface plot from the DFT model. (c) Calculated LUMO isosurface plot from the DFT model. | ||
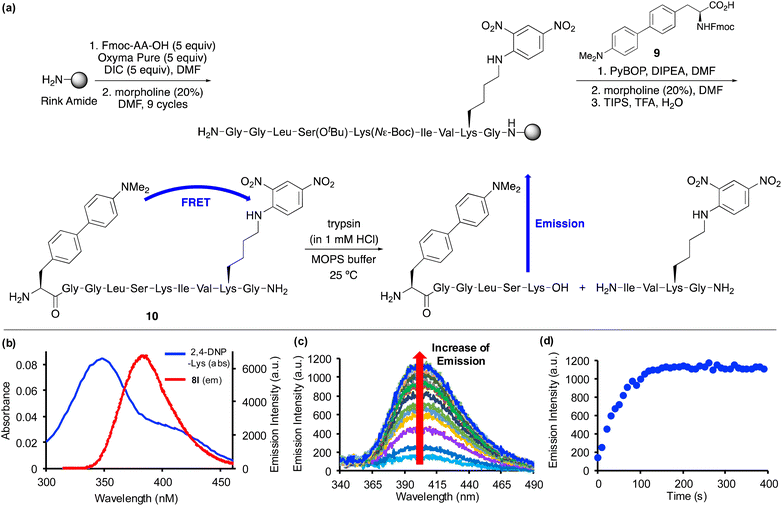
Based on the positive photophysical properties of 8l and in particular the high quantum yield and strong brightness, we were interested in investigating its application as a FRET donor to report on protease activity (Fig. 4). FRET probes contain a fluorophore donor, which when excited, non-radiatively transfer the energy through long range dipole–dipole interactions to another fluorophore (acceptor) in the ground state.27 The emission of intact FRET-based peptides is quenched, but on enzymatic cleavage, fluorescence is turned on. These types of peptide probes are widely used for monitoring protease activity, which are important biomarkers for a range of diseases.28,29 Based on the emission wavelength of amino acid 8l, a scan of potential acceptors revealed significant overlap with the absorption band of 2,4-dinitrophenyl-lysine (Fig. 4b). Thus, in a proof-of-concept experiment, a decapeptide containing both amino acid 8l and 2,4-DNP-lysine was designed to monitor the activity of a protease. For this experiment, the serine protease, trypsin, one of the most widely used digesting enzymes for mass spectrometry-based proteomics and known to cleave substrates after lysine and arginine residues was chosen.30 The decapeptide sequence 10 was based on internally quenched fluorescent peptidic substrates reported by Poreba and co-workers who used a coumarin donor and 2,4-DNP-lysine acceptor pair to investigate the substrate specificity of various proteases.29b Decapeptide 10 was prepared using SPPS methods (Fig. 4a). On coupling of Fmoc-glycine with the polymer support using N,N′-diisopropylcarbodiimide (DIC)/OxymaPure activation, subsequent rounds of morpholine-mediated N-deprotection and coupling with successive amino acids, including Fmoc-2,4-DNP-lysine gave the corresponding nonapeptide. Fmoc-protected amino acid 8l, compound 9 was then manually coupled with the polymer-supported nonapeptide.31 Following a final Fmoc-deprotection step, a TFA cleavage cocktail was used to remove the sidechain protecting groups and release decapeptide 10 from the polymer support. Purification by reverse-phase HPLC allowed isolation of decapeptide 10 in >99% purity. Characterisation of 10 by high resolution electrospray ionisation mass spectrometry verified the preparation of decapeptide 10 and the compatibility of amino acid 8l with SPPS methods. Excitation of decapeptide 10 at 300 nm resulted in near complete suppression of emission with only 10% activity retained, thus, confirming effective energy transfer between the 4-dimethylaminobiphenyl donor and the 2,4-DNP acceptor. The Förster distance (R0), which is the distance when energy transfer between a donor and acceptor is 50% efficient was calculated for the FRET pair (see ESI†). The Förster distance was found to be 36.45 Å, which is comparable to other commonly used FRET pairs.1,29b On reaction with trypsin, decapeptide 10 was confirmed as a substrate with the restoration of fluorescence emission (Fig. 4c). The increasing emission intensity was monitored as a function of time (Fig. 4d) and this data was used to measure the protease activity. Using the observed pseudo-first order kinetics, KM and kcat values were calculated as 0.33 ± 0.073 μM and 0.73 ± 0.057 s−1 (n = 3), respectively. Overall, amino acid 8l was found to be compatible with SPPS, resulting in the straightforward preparation of an internally quenched decapeptide. Using the fluorescent properties of 8l as part of this peptidic substrate allowed measurement of the enzyme kinetics of trypsin-mediated hydrolytic cleavage.
Conclusions
In summary, one-pot nonaflate activation and Suzuki–Miyaura cross-coupling of a tyrosine derivative has led to the expedient synthesis of biaryl α-amino acids, allowing rapid analysis of their photophysical properties and the discovery of several compounds with interesting fluorescent properties. From this library, amino acid 8l bearing a 4-dimethylaminobiphenyl side chain displayed the most red-shifted absorption and emission properties and with a quantum yield of 0.73, exhibited the highest brightness. A solvatochromic study and DFT calculations confirmed the charge-transfer nature of this fluorophore and further studies demonstrated its potential to act as a pH sensor with a major hypsochromic shift of the main emission band on acidification of the dimethylamino group. Due to the enhanced properties of 8l compared to other fluorescent polyaromatic amino acids previously used for measuring enzymatic kinetics, the final aim of this study demonstrated application of this novel fluorophore. An internally quenched fluorescent decapeptide containing amino acid 8l as a donor and 2,4-DNP-lysine as an acceptor was used to monitor and evaluate the kinetics of enzymatic hydrolysis by the serine protease, trypsin. Overall, this work demonstrates a fast and straightforward approach to access functionalised phenylalanine analogues with enhanced photophysical properties that in combination with SPPS techniques, can be incorporated into peptide substrates for biological applications. Current work is underway to further exploit the compatibility of these amino acids with SPPS and the preparation of fluorescent peptide substrates for new applications in biological chemistry.Data availability
All experimental and characterisation data, as well as photophysical and NMR spectra are available in the ESI.†Author contributions
A. R. T., and A. S. conceived the project. R. M. optimised the one-pot activation and cross-coupling reaction. O. M., R. M. and S. S. performed the synthesis of amino acids 8a–8m and conducted photophysical analysis. O. M. conducted full photophysical analysis of 8l, DFT calculations, synthesis of peptide 10, trypsin digestion assay and enzyme kinetic analysis. A. S. wrote the manuscript with comments from all authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Financial support from EPSRC (studentship to O. M., EP/T517895/1), the University of Glasgow (studentship to R. M.) and the Royal Thai Government (studentship to S. S.) is gratefully acknowledgement.Notes and references
- J. R. Lakowicz, Principles of Fluorescence Spectroscopy, Springer, 3rd edn, New York, 2006 Search PubMed.
- (a) A. B. Chinen, C. M. Guan, J. R. Ferrer, S. N. Barnaby, T. J. Merkel and C. A. Mirkin, Chem. Rev., 2015, 115, 10530 CrossRef CAS PubMed; (b) X. Chen, F. Wang, J. Y. Hyun, T. Wei, J. Qiang, X. Ren, I. Shin and J. Yoon, Chem. Soc. Rev., 2016, 45, 2976 RSC; (c) S. Benson, F. de Moliner, W. Tipping and M. Vendrell, Angew. Chem., Int. Ed., 2022, 61, e202204788 CrossRef CAS PubMed.
- (a) R. W. Sinkeldam, N. J. Greco and Y. Tor, Chem. Rev., 2010, 110, 2579 CrossRef CAS PubMed; (b) L.-Y. Niu, Y.-Z. Chen, H.-R. Zheng, L.-Z. Wu, C.-H. Tung and Q.-Z. Yang, Chem. Soc. Rev., 2015, 44, 6143 RSC; (c) H. Singh, K. Tiwari, R. Tiwari, S. K. Pramanik and A. Das, Chem. Rev., 2019, 119, 11718 CrossRef CAS PubMed.
- (a) R. Y. Tsien, Annu. Rev. Biochem., 1998, 67, 509 CrossRef CAS PubMed; (b) R. Heim, A. B. Cubitt and R. Y. Tsien, Nature, 1995, 373, 663 CrossRef CAS PubMed; (c) E. Pazos, O. Vázquez, J. L. Mascareñas and M. E. Vázquez, Chem. Soc. Rev., 2009, 38, 3348 RSC; (d) M. Vendrell, D. Zhai, J. C. Er and Y.-T. Chang, Chem. Rev., 2012, 112, 4391 CrossRef CAS PubMed; (e) R. Kubota and I. Hamachi, Chem. Soc. Rev., 2015, 44, 4454 RSC.
- (a) A. T. Krueger and B. Imperiali, ChemBioChem, 2013, 14, 788 CrossRef CAS PubMed; (b) A. H. Harkiss and A. Sutherland, Org. Biomol. Chem., 2016, 14, 8911 RSC; (c) Z. Cheng, E. Kuru, A. Sachdeva and M. Vendrell, Nat. Rev., 2020, 4, 275 CAS.
- (a) L. Mendive-Tapia, C. Zhao, A. R. Akram, S. Preciado, F. Alberico, M. Lee, A. Serrels, N. Kielland, N. D. Read, R. Lavilla and M. Vendrell, Nat. Commun., 2016, 7, 10940 CrossRef CAS PubMed; (b) R. Subiros-Funosas, L. Mendive-Tapia, J. Sot, J. D. Pound, N. Barth, Y. Varela, F. M. Goñi, M. Paterson, C. D. Gregory, F. Albericio, I. Dransfield, R. Lavilla and M. Vendrell, Chem. Commun., 2017, 53, 945 RSC; (c) R. Subiros-Funosas, V. C. L. Ho, N. D. Barth, L. Medive-Tapia, M. Pappalardo, X. Barril, R. Ma, C.-B. Zhang, B.-Z. Qian, M. Sintes, O. Ghashghaei, R. Lavilla and M. Vendrell, Chem. Sci., 2020, 11, 1368 RSC; (d) N. Kaplaneris, J. Son, L. Mendiva-Tapia, A. Kopp, N. D. Barth, I. Maksso, M. Vendrell and L. Ackermann, Nat. Commun., 2021, 12, 3389 CrossRef CAS PubMed; (e) N. D. Barth, L. Mendive-Tapia, R. Subrios-Funosas, O. Ghashghaei, R. Lavilla, L. Maiorino, X.-Y. He, I. Dransfield, M. Egeblad and M. Vendrell, Angew. Chem., Int. Ed., 2022, 61, e202113020 CrossRef CAS PubMed.
- (a) Y. S. Moroz, W. Binder, P. Nygren, G. A. Caputo and I. V. Korendovych, Chem. Commun., 2013, 49, 490 RSC; (b) T. J. Williams, A. J. Reay, A. C. Whitwood and I. J. S. Fairlamb, Chem. Commun., 2014, 50, 3052 RSC; (c) M. R. Hilaire, I. A. Ahmed, C.-W. Lin, H. Jo, W. F. DeGrado and F. Gai, Proc. Natl. Acad. Sci. U. S. A., 2017, 114, 6005 CrossRef CAS PubMed; (d) K. Zhang, I. A. Ahmed, H. T. Kratochvil, W. F. DeGrado, F. Gai and H. Jo, Chem. Commun., 2019, 55, 5095 RSC.
- (a) J. D. Bell, A. H. Harkiss, D. Nobis, E. Malcolm, A. Knuhtsen, C. R. Wellaway, A. G. Jamieson, S. W. Magennis and A. Sutherland, Chem. Commun., 2020, 56, 1887 RSC; (b) A. C. Dodds, H. G. Sansom, S. W. Magennis and A. Sutherland, Org. Lett., 2023, 25, 8942 CrossRef CAS PubMed; (c) R. Clarke, L. Zeng, B. C. Atkinson, M. Kadodwala, A. R. Thomson and A. Sutherland, Chem. Sci., 2024, 15, 5944 RSC.
- (a) S. Chen, N. E. Fahmi, L. Wang, C. Bhattacharya, S. J. Benkovic and S. M. Hecht, J. Am. Chem. Soc., 2013, 135, 12924 CrossRef CAS PubMed; (b) J. S. Lampkowski, D. M. Uthappa and D. D. Young, Bioorg. Med. Chem. Lett., 2015, 25, 5277 CrossRef CAS PubMed.
- S. Chen, N. E. Fahmi, C. Bhattacharya, L. Wang, Y. Jin, S. J. Benkovic and S. M. Hecht, Biochemistry, 2013, 52, 8580 CrossRef CAS PubMed.
- (a) S. Chen, L. Wang, N. E. Fahmi, S. J. Benkovic and S. M. Hecht, J. Am. Chem. Soc., 2012, 134, 18883 CrossRef CAS PubMed; (b) P. M. T. Ferreira, L. S. Monteiro, G. Pereira, E. M. S. Castanheira and C. G. Frost, Eur. J. Org Chem., 2013, 550 CrossRef CAS.
- (a) J. M. Goldberg, L. C. Speight, M. W. Fegley and E. J. Petersson, J. Am. Chem. Soc., 2012, 134, 6088 CrossRef CAS PubMed; (b) L. C. Speight, A. K. Muthusamy, J. M. Goldberg, J. B. Warner, R. F. Wissner, T. S. Willi, B. F. Woodman, R. A. Mehl and E. J. Petersson, J. Am. Chem. Soc., 2013, 135, 18806 CrossRef CAS PubMed.
- L. Mendive-Tapia, D. Mendive-Tapia, C. Zhao, D. Gordon, S. Benson, M. J. Bromley, W. Wang, J. Wu, A. Kopp, L. Ackermann and M. Vendrell, Angew. Chem., Int. Ed., 2022, 61, e202117218 CrossRef CAS PubMed.
- (a) R. McGrory, R. Clarke, O. Marshall and A. Sutherland, Org. Biomol. Chem., 2023, 21, 6932 RSC; (b) S. Songsri, A. H. Harkiss and A. Sutherland, J. Org. Chem., 2023, 88, 13214 CrossRef CAS PubMed; (c) L. M. Riley, O. Marshall, A. H. Harkiss, H. M. Senn and A. Sutherland, Org. Lett., 2024, 26, 5391 CrossRef PubMed.
- (a) T. Ikawa, K. Saito and S. Akai, Synlett, 2012, 23, 2241 CrossRef CAS; (b) L. Chen, H. Lang, L. Fang, M. Zhu, J. Liu, J. Yu and L. Wang, Eur. J. Org Chem., 2014, 4953 CrossRef CAS; (c) N. Iranpoor, F. Panahi, F. Jamedi and J. Organometallic, Chem, 2015, 781, 6 CAS; (d) H. Wang, S. Zhang, M. Xü and G. Zou, Molecules, 2023, 28, 636 CrossRef CAS PubMed.
- For example, see: (a) M. Rottländer and P. Knochel, J. Org. Chem., 1998, 63, 203 CrossRef PubMed; (b) S. E. Denmark and R. F. Sweis, Org. Lett., 2002, 4, 3771 CrossRef CAS PubMed; (c) R. E. Tundel, K. W. Anderson and S. L. Buchwald, J. Org. Chem., 2006, 71, 430 CrossRef CAS PubMed; (d) J. Barluenga, L. Florentino, F. Aznar and C. Valdés, Org. Lett., 2011, 13, 510 CrossRef CAS PubMed; (e) C. Eidamshaus, P. Hommes and H.-U. Reissig, Synlett, 2012, 23, 1670 CrossRef CAS; (f) J. Wannberg, C. Wallinder, M. Ünlüsoy, C. Sköld and M. Larhed, J. Org. Chem., 2013, 78, 4184 CrossRef CAS PubMed; (g) Y. An, H. Xia and J. Wu, Org. Biomol. Chem., 2016, 14, 1665 RSC; (h) K.-F. Zhang, F. Christoffel and O. Baudoin, Angew. Chem., Int. Ed., 2018, 57, 1982 CrossRef CAS PubMed; (i) H. McErlain, L. M. Riley and A. Sutherland, J. Org. Chem., 2021, 86, 17036 CrossRef CAS PubMed.
- T. Kinzel, Y. Zhang and S. L. Buchwald, J. Am. Chem. Soc., 2010, 132, 14073 CrossRef CAS PubMed.
- C. Amatore, G. Le Duc and A. Jutand, Chem.–Eur. J., 2013, 19, 10082 CrossRef CAS PubMed.
- Using lower temperatures for the Suzuki-Miyaura step resulted in lower conversion and isolated yields of 7a. For example, the use of 40 °C gave 18% yield of 7a.
- (a) H. Lei, M. S. Stoakes, K. P. B. Herath, J. Lee and A. W. Schwabacher, J. Org. Chem., 1994, 59, 4206 CrossRef CAS; (b) M. Ousmer, V. Boucard, N. Lubin-Germain, J. Uziel and J. Augé, Eur. J. Org Chem., 2006, 1216 CrossRef CAS.
- (a) S. Kotha and K. Lahiri, Bioorg. Med. Chem. Lett., 2001, 11, 2887 CrossRef CAS PubMed; (b) M. Vilaró, G. Arsequell, G. Valencia, A. Ballestereos and J. Barluenga, Org. Lett., 2008, 10, 3243 CrossRef PubMed; (c) J. M. Chalker, C. S. C. Wood and B. G. Davis, J. Am. Chem. Soc., 2009, 131, 16346 CrossRef CAS PubMed; (d) T. Willemse, K. Van Imp, R. J. M. Goss, H. W. T. Van Vlijmen, W. Schepens, B. U. V. Maes and S. Ballet, ChemCatChem, 2015, 7, 2055 CrossRef CAS; (e) J. Maity, D. Honcharenko and R. Strömberg, Tetrahedron Lett., 2015, 56, 4780 CrossRef CAS.
- Suzuki-Miyaura reactions have also been performed using triflate-activated tyrosines: W.-C. Shieh and J. A. Carlson, J. Org. Chem., 1992, 57, 379 CrossRef CAS.
- (a) E. Lippert, Z. Naturforsch., 1955, 10a, 541 CrossRef CAS; (b) N. Mataga, Y. Kaifu and M. Koizumi, Bull. Chem. Soc. Jpn., 1955, 28, 690 CrossRef CAS.
- E. A. Braude and F. C. Nachod, Determination of Organic Structures by Physical Methods, Academic Press, New York, 1955 Search PubMed.
- For viscosity and polarity values, see: J. A. Dean, Lange's Handbook of Chemistry, McGraw-Hill, New York, 1992 Search PubMed.
- W. J. Hehre, R. Ditchfield and J. A. Pople, J. Chem. Phys., 1972, 56, 2257 CrossRef CAS.
- (a) B. Wallace and P. J. Atzberger, PLoS One, 2017, 12, e0177122 CrossRef PubMed; (b) J. Zhang, X. Chai, X.-P. He, H.-J. Kim, J. Yoon and H. Tian, Chem. Soc. Rev., 2019, 48, 683 RSC.
- M. Rodriguez-Rios, A. Megia-Fernandez, D. J. Norman and M. Bradley, Chem. Soc. Rev., 2022, 51, 2081 RSC.
- For example, see: (a) H. Sun, R. C. Panicker and S. Q. Yao, Pept. Sci., 2007, 88, 141 CrossRef CAS PubMed; (b) M. Poreba, A. Szalek, W. Rut, P. Kasperkiewicz, I. Rutkowska-Wlodarczyk, S. J. Snipas, Y. Itoh, D. Turk, B. Turk, C. M. Overall, L. Kaczmarek, G. S. Salvesen and M. Drag, Sci. Rep., 2017, 7, 43135 CrossRef CAS PubMed; (c) S. Bernegger, C. Brunner, M. Vizovišek, M. Fonovic, G. Cuciniello, F. Giordano, V. Stanojlovic, M. Jarzab, P. Simister, S. M. Feller, G. Obermeyer, G. Posselt, B. Turk, C. Cabrele, G. Schneider and S. Wessler, Sci. Rep., 2020, 10, 10563 CrossRef CAS PubMed.
- J. V. Olsen, S.-E. Ong and M. Mann, Mol. Cell. Proteomics, 2004, 3, 608 CrossRef CAS PubMed.
- See ESI† for synthesis of Fmoc-protected amino acid 9 and characterization data of decapeptide 10.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterisation data for compounds, photophysical data for amino acids, computation data, peptide synthesis, enzyme assay procedure, enzyme kinetic analysis, and NMR spectra for all compounds. See DOI: https://doi.org/10.1039/d5sc00020c |
| This journal is © The Royal Society of Chemistry 2025 |