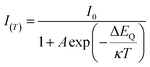Open Access Article
Open Access ArticleAn anti-thermal-quenching organic–inorganic hybrid manganese-based single-crystal scintillator for high-temperature X-ray imaging†
Jing-Hua
Chen
,
Jian-Bin
Luo
,
Zi-Lin
He
,
Qing-Peng
Peng
,
Jun-Hua
Wei
*,
Zhi-Zhong
Zhang
,
Xiu-Xian
Guo
and
Dai-Bin
Kuang
 *
*
Key Laboratory of Bioinorganic and Synthetic Chemistry of Ministry of Education, Lehn Institute of Functional Materials, GBRCE for Functional Molecular Engineering, School of Chemistry, IGCME, Sun Yat-Sen University, Guangzhou 510275, China. E-mail: weijh39@mail.sysu.edu.cn; kuangdb@mail.sysu.edu.cn
First published on 15th April 2025
Abstract
High-temperature X-ray detection holds promising potential in practical applications in the development of industry. Organic–inorganic manganese-based halide (OIMH) scintillators have undergone a research upsurge due to their high X-ray attenuation ability, low preparation cost, outstanding photoluminescence performance, and flexible structures. However, the thermal quenching effects of OIMH materials limit their applications at high temperatures. Herein, we report two zero-dimensional OIMH scintillators, (4-NH3TBP)MnBr4 (4-NH3TBP = 4-aminobutyl triphenylphosphonium) and (4-DMATBP)MnBr4 (4-DMATBP = (4-(dimethylamino)butyl)triphenylphosphonium) single crystals, which were developed through rational molecular design. Their abnormal temperature-dependent luminescence behaviors under ultraviolet lamp excitation and X-ray irradiation are observed. Notably, the (4-DMATBP)MnBr4 single crystal exhibits an anti-thermal-quenching effect under ultraviolet light and X-ray irradiation, which can be attributed to the participation of intrinsic crystal defects, rigid crystal structure and longer Mn–Mn distance. Density functional theory calculations further demonstrate that Br vacancies are responsible for the formation of the trap state. The (4-DMATBP)MnBr4 scintillator exhibits an impressive light yield of 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 photon per MeV at 130 °C, indicating its feasibility for application in high-temperature X-ray detection. The transparent single crystal possesses a robust resolution of 42 lp mm−1 in X-ray imaging and shows high imaging quality at high temperatures.
722 photon per MeV at 130 °C, indicating its feasibility for application in high-temperature X-ray detection. The transparent single crystal possesses a robust resolution of 42 lp mm−1 in X-ray imaging and shows high imaging quality at high temperatures.
Introduction
High-temperature scintillators have been widely used in petroleum exploration, space nuclear exploration, and reactors.1–3 As the core of detectors, scintillators with high light output, excellent thermal stability, and resistance to pressure are required.4–7 Traditional high-temperature scintillators (NaI:Tl+, CsI:Tl+, and LaBr3:Ce3+) and the commercially available scintillators (YAG:Ce3+, SrSi2O2N2:Eu2+, and LuAG:Ce3+) with low thermal quenching (TQ) show negligible emission loss, and have been applied to petroleum exploration.3,8–10 However, the poor scintillation performance under extreme temperatures and high-cost synthesis processes of the above scintillators limit their applications.11 Therefore, materials with excellent scintillation performance at high temperatures that can be prepared through facile low-temperature synthesis routes are needed.Organic–inorganic manganese halides (OIMHs) have attracted extensive research interest due to their low toxicity, flexible structures, and high emission quantum efficiency, and thus show great application prospects in light-emitting diodes (LEDs), X-ray scintillation, and anti-counterfeiting technologies.12–15 OIMHs always possess a low-dimensional structure with highly localized excitons, which promotes radiative recombination and accounts for their high photoluminescence quantum yield (PLQY).16 However, most OIMHs suffer from serious PL quenching as the temperature increases (>100 °C) due to the electron–photon-coupling-induced non-radiative relaxation,17 hampering their application in high-temperature scintillation imaging. Anti-thermal quenching (ATQ) materials with minimal emission loss at elevated temperatures could meet the demands for commercial applications. Many research works towards obtaining ATQ OIMH materials have been carried out.18 Notably, defect engineering and structural modulation are the most common strategies to realize ATQ properties.19–22 Defect engineering can provide extra states that can serve as a carrier storage bank and release carriers to the emissive centers at higher temperatures. Moreover, structure modulation can affect the structural rigidity and symmetric sites through chemical substitution, further regulating the emission behaviors.18
Yang's group synthesized a (TTPhP)2MnCl4 (TTPhP+ = tetraphenylphosphonium cation) material with an ATQ effect through organic cation engineering; the material shows a low thermal quenching effect up to 200 °C under ultraviolet (UV) light (the maximum intensity is about 1.2 times higher than the initial intensity) and a weak TQ property under X-ray irradiation.23 Very recently, Xu and coauthors successfully prepared a 1D Cs5Cu3Cl6I2 scintillator by adjusting the ratio of Cl− and I−; the scintillator exhibits slight emission loss when being heated to 160 °C.24 However, the low optical transmittance and severe light scattering of the above scintillator screens deteriorate their X-ray imaging performance. The X-ray imaging resolution of the (TTPhP)2MnCl4 and Cs5Cu3Cl6I2 scintillator screens can only reach 4 lp mm−1 at 200 °C and nearly 18 lp mm−1 at 150 °C, respectively.23,24 The unsatisfactory X-ray imaging resolution can be ascribed to the TQ properties of the above materials during the heating process under X-ray irradiation. In terms of high-resolution X-ray imaging, single-crystal perovskite scintillators have been praised for their high transparency, strong X-ray attenuation capability, suppressed light scattering, excellent X-ray energy response, and low detection limit.12,25–27 Therefore, an optically transparent single crystal scintillator with weak TQ would be attractive for high-temperature X-ray detection and imaging.
Recent advances in OIMHs have focused on tailoring triphenylphosphine derivatives through strategic functionalization with alkyl chains, rigid phenyl groups, and alkoxy groups to enhance their luminescence properties.28–31 Notably, the introduction of amine functionalities to these systems remains underexplored despite their potential to modulate electronic structures through protonation effects. To address this gap, we designed a novel series of butyltriphenylphosphonium salts incorporating amine cations at the alkyl chain termini. Interestingly, different from the reported monoprotonated structures, the as-synthesized organic salts are diprotonated. This unique protonation state induces enhanced thermal stability, as evidenced by their glass transition temperatures (Tg) exceeding those of reported OIMHs crystals,14,30,32 which is conducive to subsequent scintillation application of the OIMH glass.
In this work, we successfully synthesized two OIMH single crystal scintillators through introducing two methyl (–CH3) groups at the end of the alkyl chain of the organic cation, which adjusted the rigidity and anion–cation interactions. The (4-NH3TBP)MnBr4 single crystal (4-NH3TBP = 4-aminobutyl triphenylphosphonium) has a weak TQ effect, while the (4-DMATBP)MnBr4 single crystal (4-DMATBP = (4-(dimethylamino)butyl) triphenylphosphonium) possesses ATQ properties. Both crystals exhibit green emission under UV light and X-ray radiation, as well as high thermal stability. Notably, the (4-DMATBP)MnBr4 single crystal exhibits an ATQ effect and attains enhancement within the temperature range of 30–200 °C; the maximum intensity is 3.85 times higher than the initial intensity under UV excitation. Moreover, the light yield of the (4-DMATBP)MnBr4 single crystal reaches 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 photon per MeV at 130 °C due to its ATQ properties under X-ray irradiation, which is 3.19 times higher than that at 30 °C. Such a significant radioluminescence (RL) improvement with increasing temperature under X-ray excitation has rarely been reported. The ATQ effect can be mainly attributed to its rigid crystal structure, longer Mn–Mn distance, and the participation of trap states, according to the single-crystal X-ray diffraction (SCXRD), electron paramagnetic resonance (EPR), thermoluminescence (TL), and density functional theory (DFT) results. Additionally, the transparent (4-DMATBP)MnBr4 single crystal shows a high spatial resolution of up to 42 lp mm−1 for X-ray imaging at room temperature and exhibits high-quality imaging at high temperatures.
722 photon per MeV at 130 °C due to its ATQ properties under X-ray irradiation, which is 3.19 times higher than that at 30 °C. Such a significant radioluminescence (RL) improvement with increasing temperature under X-ray excitation has rarely been reported. The ATQ effect can be mainly attributed to its rigid crystal structure, longer Mn–Mn distance, and the participation of trap states, according to the single-crystal X-ray diffraction (SCXRD), electron paramagnetic resonance (EPR), thermoluminescence (TL), and density functional theory (DFT) results. Additionally, the transparent (4-DMATBP)MnBr4 single crystal shows a high spatial resolution of up to 42 lp mm−1 for X-ray imaging at room temperature and exhibits high-quality imaging at high temperatures.
Results and discussion
Synthesis and characterization of OIMH single crystals
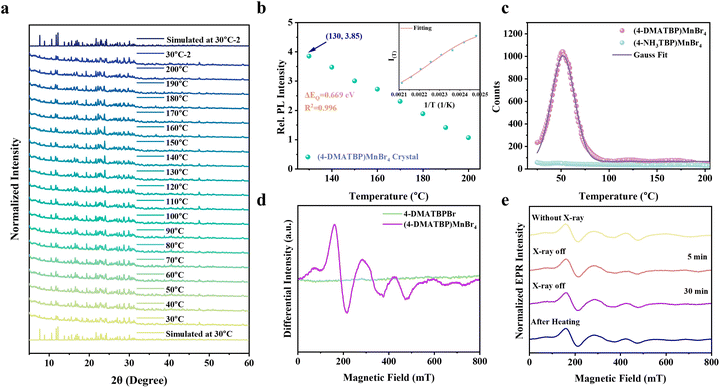
The organic phosphonium salts were synthesized via a facile reflux reaction procedure (ESI Scheme 1, please see the ESI for experimental details†). White crystalline products with blue emission were obtained through recrystallization (Fig. S1a, b, S2a and b†). Attenuated total reflection Fourier-transform infrared (ATR-FTIR) and NMR measurements verified that the target products had been successfully synthesized (Fig. S1c, d, S2c and d†). The difference between 4-NH3TBPBr and 4-DMATBPBr lies in the methyl functional groups attached to the terminal N atom. As shown in Fig. S3a and b,† single-crystal X-ray diffraction measurement (SCXRD) revealed that the 4-NH3TBPBr crystal adopts a triclinic crystal structure, while 4-DMATBPBr crystallizes in a monoclinic space group (Table S1†). The powder X-ray diffraction (PXRD) patterns of the ground powders are consistent with the simulated results based on the SCXRD data (Fig. S3c†), indicating the successful preparation of the functional phosphonium salts in high purity. The thermal properties of 4-NH3TBPBr and 4-DMATBPBr were evaluated through thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC). As shown in Fig. S4 and Table S2,† the melting temperature (Tm) and decomposition temperature (Td) of 4-DMATBPBr were higher than those of 4-NH3TBPBr, indicating that the introduction of the –CH3 groups improved the thermal stability.Mn-based metal halide crystals of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 were grown through the solvent evaporation method (please see the Synthesis section in the ESI†). Intriguingly, the (4-DMATBP)MnBr4 crystals tended to grow as laminar single crystals with a maximum size of 5 mm × 7 mm × 0.5 mm via slow solvent evaporation in a CH3OH and HBr solution (ESI Scheme 2†). The crystal structures of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 were determined via SCXRD measurements, and the detailed crystallographic data are available in Tables S1 and S3.† The (4-NH3TBP)MnBr4 crystal adopts the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) space group, whereas (4-DMATBP)MnBr4 crystallizes in the monoclinic space group P21/n. The crystallographic asymmetric units of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 are shown in Fig. S5,† in which one Mn atom is coordinated by four Br atoms to form a [MnBr4]2− tetrahedral cluster. The [MnBr4]2− tetrahedral clusters are separated by the organic cations, giving rise to zero-dimensional structures for both (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 (Fig. 1a and b). Notably, the shortest Mn–Mn distances in the (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals are 6.608 Å and 7.606 Å, respectively. PXRD measurement proved the high phase purity of the as-synthesized single crystals via comparison to the simulated results (Fig. 1c). Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements were performed to investigate the thermal properties of the crystals. As shown in Fig. S6 and Table S4,† the Td values of the (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals are 288 and 314 °C, while their Tm values are 244 and 262 °C, respectively. The thermal analysis illustrates that the crystals remain in the solid state below 200 °C.
space group, whereas (4-DMATBP)MnBr4 crystallizes in the monoclinic space group P21/n. The crystallographic asymmetric units of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 are shown in Fig. S5,† in which one Mn atom is coordinated by four Br atoms to form a [MnBr4]2− tetrahedral cluster. The [MnBr4]2− tetrahedral clusters are separated by the organic cations, giving rise to zero-dimensional structures for both (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 (Fig. 1a and b). Notably, the shortest Mn–Mn distances in the (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals are 6.608 Å and 7.606 Å, respectively. PXRD measurement proved the high phase purity of the as-synthesized single crystals via comparison to the simulated results (Fig. 1c). Thermogravimetric analysis (TGA) and differential scanning calorimetry (DSC) measurements were performed to investigate the thermal properties of the crystals. As shown in Fig. S6 and Table S4,† the Td values of the (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals are 288 and 314 °C, while their Tm values are 244 and 262 °C, respectively. The thermal analysis illustrates that the crystals remain in the solid state below 200 °C.
Optical properties of the OIMH single crystals
The ultraviolet-visible spectra of the OIMH crystals in Fig. S7† show several groups of peaks in the range of 250–500 nm, which originate from the d–d transitions of Mn2+. The bands at 294, 365, 377, 438, 450, and 460 nm correspond to the electronic transitions of Mn2+ from the ground state 6A1 to the excited states 4T1(F), 4E(D), 4T2(D), 4A1/4E(G), 4T2 (G), and 4T1 (G), respectively.33 The photoluminescence excitation (PLE) and PL spectra of the Mn-based crystals were obtained and shown in Fig. S8a and b.† The (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals both exhibit green emission from the 4T1 → 6A1 transition of Mn2+ in a typical tetrahedral coordination geometry (Fig. S8c and d†). Furthermore, the (4-NH3TBP)MnBr4 crystal shows a brighter greenish emission peak at 519 nm with a PLQY of 63.5% and a full width at half maximum (FWHM) of 50 nm. In contrast, the (4-DMATBP)MnBr4 crystal shows an emission peak at 520 nm with a FWHM of 45 nm and a lower PLQY of 26.9% at room temperature (Fig. S8e†). The time-resolved PL (TRPL) spectra of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals indicate average lifetimes of 276 and 125 μs, respectively (Fig. S9†).The temperature-dependent luminescence spectra of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals under 360 nm ultraviolet light and X-ray excitation were collected to investigate the emissive mechanism. As shown in Fig. 1d and S10a,† the PL intensity of the (4-DMATBP)MnBr4 crystal increases with temperature, while (4-NH3TBP)MnBr4 exhibits a decreasing trend as the temperature rises. As displayed in Fig. 1e, the temperature-dependent integrated PL intensity of the (4-DMATBP)MnBr4 crystal reaches a maximum at 130 °C, which is 3.85 times higher than the initial intensity at 30 °C. The temperature-dependent PLQY measurement shown in Fig. S10b† reveals a trend consistent with the temperature-dependent PL intensity, achieving a maximum PLQY value of 98.60% at 130 °C. However, the PLQY of (4-NH3TBP)MnBr4 exhibits a decreasing trend consistent with the weak TQ effect under ultraviolet light excitation. Notably, the temperature-dependent integrated RL intensity under X-ray irradiation suggests that (4-NH3TBP)MnBr4 exhibits a nearly zero-TQ effect up to 200 °C, whereas the (4-DMATBP)MnBr4 crystal shows an ATQ effect (Fig. 1f and S11†). Additionally, the RL intensity of (4-DMATBP)MnBr4 reaches a maximum at 130 °C, which is 1.71 times greater than that at room temperature. Therefore, the prepared Mn-based halide crystals exhibit great potential for application at high temperatures.
Radioluminescence properties of scintillators
To investigate the radioluminescence properties, we further evaluated the performance of the two single-crystal scintillators, including their light yield (LY) and limit of detection (LOD). As displayed in Fig. 2a, the two OIMHs exhibit analogous X-ray absorption coefficients (α) slightly lower than those of the all-inorganic scintillators. The X-ray source used for the test exhibits a characteristic peak and photon energy of 22 keV (Fig. S12†). The attenuation efficiency of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 at an X-ray energy of 22 keV (Fig. 2b) shows that they can absorb nearly 100% of the X-rays at a thickness of 1 mm. To derive the light yield (LY) values of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4, the commercially available LuAG:Ce crystal was selected as a reference scintillator (LY = 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photon per MeV). To minimize morphology-dependent differences in the RL measurement, the samples were pressed into wafers of the same size as the standard scintillator. The light yields of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 were calculated to be 36
000 photon per MeV). To minimize morphology-dependent differences in the RL measurement, the samples were pressed into wafers of the same size as the standard scintillator. The light yields of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 were calculated to be 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 572 photon per MeV and 14
572 photon per MeV and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 663 photon per MeV, respectively (Fig. 2c).
663 photon per MeV, respectively (Fig. 2c).
Notably, the light yield of (4-DMATBP)MnBr4 crystal reaches a maximum of 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 photon per MeV at 130 °C, which is 3.19 times higher than that at room temperature (Fig. 2d). (4-NH3TBP)MnBr4 also demonstrates excellent performance with a high LY value of about 40
722 photon per MeV at 130 °C, which is 3.19 times higher than that at room temperature (Fig. 2d). (4-NH3TBP)MnBr4 also demonstrates excellent performance with a high LY value of about 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 photon per MeV within the temperature range of 30 °C to 200 °C (Fig. 2e). The higher light yields at high temperatures support their application in high-temperature detection scenarios.
000 photon per MeV within the temperature range of 30 °C to 200 °C (Fig. 2e). The higher light yields at high temperatures support their application in high-temperature detection scenarios.
The limit of detection (LOD) is a crucial parameter for scintillators. A photomultiplier tube (PMT) was employed to record the light output under low-dose-rate X-ray irradiation. As shown in Fig. 2f and S13,† the LuAG:Ce standard scintillator and the two OIMH single crystals exhibit a linear dependence between the RL intensity and the X-ray dose rates. The LOD of LuAG:Ce was derived to be 39.01 nGy s−1, while those of the (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals are 12.66 nGy s−1 and 46.15 nGy s−1. All the measured LODs are below the standard for medical imaging (5.5 μGy s−1); such low LOD values are advantageous for obtaining high-quality X-ray images.34
Luminescence properties and crystal structure
In order to clarify the differences in the luminescence properties of the single crystals, we measured the Mn–Mn distance in the OIMHs, since a longer Mn–Mn distance would afford a higher PLQY due to the concentration quenching effect.23,35,36 However, the shortest Mn–Mn distances of the (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals are 6.608 Å and 7.606 Å, while their PLQY values are 63.5% and 26.9%, respectively, indicating that the Mn–Mn distance is not the major factor affecting the PLQY of the two OIMH single crystals.It has been reported that the emission efficiency can be regulated by tuning weak interactions to suppress non-radiative transitions. Therefore, inhibiting the motion of the [MnBr4]2− anions could result in a higher PLQY by decreasing the non-radiative transitions.37,38 As shown in Fig. S14,† SCXRD measurement demonstrated that the [MnBr4]2− anions in the (4-NH3TBP)MnBr4 crystal are fully ordered while those in the (4-DMATBP)MnBr4 crystal are relatively disordered over four positions. Hirshfeld surface calculations were performed using the CrystalExplorer package to shed more light on the weak interactions between [MnBr4]2− and the surrounding cations.39 The 2D fingerprint plots in Fig. S15a and b† show the substantial differences between the Br–H interactions in these two OIMH compounds. The cyan-blue area in the red box clearly indicates the stronger Br⋯H–C(N) interactions between [MnBr4]2− and the organic cations in (4-NH3TBP)MnBr4 compared with those in the (4-DMATBP)MnBr4 crystal, possibly originating from the three hydrogen atoms of the –NH3 group at the end of the cation. Additionally, we measured the Mn⋯H–C (N) bond length between [MnBr4]2− and the organic cations to evaluate the interaction distance between the anions and cations (Tables S5 and 6†). The calculated average Mn⋯H distances for the (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals are 8.19 and 8.64 Å, suggesting a shorter interaction distance and stronger interaction between anions and cations in the (4-NH3TBP)MnBr4 crystal. Overall, the motion of [MnBr4]2− in (4-NH3TBP)MnBr4 is restricted due to the pronounced anion–cation interactions. The more rigid structure of (4-NH3TBP)MnBr4 reduces the thermal vibration of [MnBr4]2− and suppresses the non-radiative transition, affording an improved PLQY.
As (TPPen)2MnBr4 (TPPen = pentyltriphenylphosphonium)40 has a similar structure to (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4, we compared their Br–H interactions. The interaction distance between anions and cations in the (TPPen)2MnBr4 crystal is longer than those of the present two compounds, indicating that the introduction of the N atom and methyl groups shortens the interaction distance and leads to stronger interactions (Fig. S15c†). Moreover, the N atom provides a cationic center, resulting in the longer shortest Mn–Mn distance.35 Thus, the (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 crystals have more rigid structures. As reported, rigid crystal structures can reduce emission loss during the heating process, leading to weak TQ behavior.41
Exploration of the anti-thermal-quenching mechanism of the luminescence of the (4-DMATBP)MnBr4 crystal
To clarify the mechanism of the anti-thermal quenching behavior in the (4-DMATBP)MnBr4 crystal, we performed temperature-dependent XRD, high-temperature SCXRD, TL, EPR, and temperature-dependent TRPL. The temperature-dependent XRD (Fig. 3a) results illustrate that no phase transition is observed when the (4-DMATBP)MnBr4 crystal is heated to 200 °C, in good agreement with the DSC results. Furthermore, single-crystal structures were collected at several temperatures and refined (Table S7†). The crystal structure remained unchanged, suggesting that the ATQ trend in the luminescence intensity is independent of phase transition. Additionally, the shortest Mn–Mn distances and average Mn–Br bond lengths at the corresponding temperatures are listed in Table S8.† It is evident that both the volume and the shortest Mn–Mn distance increase as temperature rises due to lattice expansion. The increasing Mn–Mn distance could suppress the energy transfer process between Mn atoms, thus inhibiting concentration quenching at high temperatures.23 This could be further supported by the fact that the longer shortest Mn–Mn distance in the (4-DMATBP)MnBr4 crystal compared with that in (4-NH3TBP)MnBr4 leads to a weaker TQ effect. Thus, the ATQ property of the (4-DMATBP)MnBr4 crystal could be attributed to the longer Mn–Mn distance.The observed PL intensity of (4-DMATBP)MnBr4 first increases (303–403 K) and then decreases (>403 K) with increasing temperature. To obtain the charge carrier dynamics and the activation energy of thermal quenching, the following equation was applied for fitting:23,42,43
It has been reported that thermally-activated energy transfer from the trap state to the emissive state plays a vital role in abnormal thermally enhanced PL behavior.3,21,42,44 Subsequently, we performed TL measurements to further investigate the trap state. As presented in Fig. 3c, no obvious peak appeared for (4-NH3TBP)MnBr4 while a peak at 52 °C was observed for the (4-DMATBP)MnBr4 crystal. Through fitting the TL curve with a Gaussian function, the trap energy level was estimated to be 0.65 eV via the following equation:
| Etrap = Tmax/500 |
EPR measurement can provide more information about the trap states in crystals; thus, we conducted EPR measurements of the as-synthesized compounds. As shown in Fig. 3d and S17,† the pure phosphonium salts exhibit no signal in their EPR spectra, while the as-prepared OIMH crystals present greatly different spectra. Notably, the spectrum of (4-NH3TBP)MnBr4 demonstrates only one resonance line, whereas the spectrum of (4-DMATBP)MnBr4 is complicated and consists of numerous contributions. The EPR signals in the OIMHs originate from Mn2+, as the pure phosphonium salts are EPR-silent. The EPR spectrum of (4-DMATBP)MnBr4 was fitted using the spin Hamiltonian function (see the EPR measurements section in ESI† for details).45 The fitting result is displayed in Fig. S18,† and matches well with the experimental data. The EPR results indicate that the local surroundings of the Mn2+ in (4-DMATBP)MnBr4 are quite different from those in (4-NH3TBP)MnBr4. Considering the similar structure of these two crystals, this is possibly due to nearby defects. Additionally, we further performed EPR spectroscopy of the crystals after subjecting them to different treatments. As shown in Fig. 3e, the EPR spectra of the crystals after X-ray irradiation and heating exhibit the same profiles as the untreated ones do, confirming that the trap states originate from intrinsic defects in the (4-DMATBP)MnBr4 crystal.
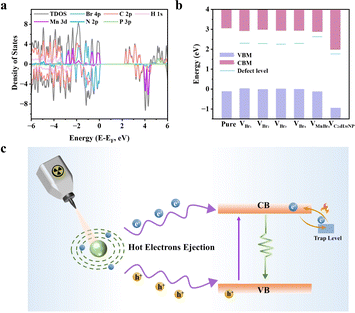
DFT calculations were performed to reveal the defect type, and several possible vacancy structures were proposed. As shown in Fig. S19,† the possible vacancy defect structures include four different Br vacancies, [MnBr4]2− vacancy, and organic (C24H30NP+) vacancy. The density of states (DOS) and projected DOS (PDOS) calculations were plotted to determine the major contributions to the conduction band minimum (CBM) and valence band maximum (VBM). As shown in Fig. 4a, in the perfect (4-DMATBP)MnBr4 crystal, the density states of the VBM are mainly contributed by Mn-d orbitals and C-p orbitals, while the C-p orbitals and P-p orbitals are dominant in the CBM for spin-up states. However, for the spin-down states, the Br-p orbitals and C-p orbitals primarily contribute to the VBM, which is quite different from the case for the spin-up states. The asymmetric DOS diagram indicates the ferromagnetic nature of the (4-DMATBP)MnBr4 crystal.46
Fig. S20† shows the DOS and PDOS plots corresponding to the six possible vacancy defect structures in contrast to the perfect crystal structure. The defect levels corresponding to the different structure defects are shown in Fig. 4b and S21† for the spin-up and spin-down states. Notably, shallow and deep trap levels can be introduced in the different structure defects. For the spin-down states, the ΔEtrap (the energy gap between defect levels and CBM) values for VBr1 to VBr4 were distributed over a wide range of 0.64 to 0.72 eV, while for VMnBr4 and VC24H30NP, they were 0.24 and 0.28 eV, respectively. However, for the spin-up states, the ΔEtrap values for the different structure defects are in the range of 0.09 to 0.28 eV. Considering that halogen defects are widely present in metal halides,21,42 the Br-vacancy structure was regarded as the most probable defect contributing to the trap level. Moreover, the calculated trap state level of VBr is close to the TL result (0.65 eV), further suggesting the possibility of VBr in the crystal structure.
Based on the above analysis, we proposed a possible mechanism for the luminescence under ultraviolet excitation and X-ray irradiation. In the (4-NH3TBP)MnBr4 crystal, the rigid crystal structure inhibits the non-radiative recombination process of the crystal at high temperatures, leading to negligible emission loss as the temperature increases. However, for the (4-DMATBP)MnBr4 crystal, the trap level makes a significant contribution. The rigid crystal structure and the longer shortest Mn–Mn distance of the (4-DMATBP)MnBr4 crystal inhibit concentration quenching at high temperatures, which is beneficial to the ATQ effect. Additionally, carriers will be trapped by the trap states, which can then serve as a carrier bank. As shown in Fig. 4c, the luminescence intensity is enhanced as the temperature increases due to the release of carriers from trap states to the emissive state of Mn2+, resulting in the ATQ effect. Notably, under different excitation light sources, the anti-thermal quenching effect of the emission behavior is not all the same. Under X-ray irradiation, the atoms will be ionized through the photoelectric effect, and the electrons in the inner shell of the atoms will be excited and ejected out of the atoms, forming hot electrons and deep holes. Then, some amount of free excitons will be generated in the conduction and valence bands.47 However, under ultraviolet (UV) light excitation, the valence electrons are excited to the excited state and return to the ground state to generate the green emission. The different relaxation and photophysical processes would result in the different temperature-dependent PL and RL intensities.
X-ray imaging of single-crystal scintillators
Due to their excellent scintillation performance and high optical transparency, the prepared OIMH single crystals can be employed as high-quality X-ray imaging materials. The crystals were also ground and pressed as polycrystalline wafers with a thickness similar to that of the single crystal. However, the preparation of a transparent (4-NH3TBP)MnBr4 single crystal with large size was difficult, and thus its X-ray imaging is not discussed. As shown in Fig. S22,† the polycrystalline wafers show low transmittance owing to the pronounced light scattering;26,48 the transmittance of the (4-DMATBP)MnBr4 wafer is approximately 50% in the range of 500–800 nm and that of the (4-NH3TBP)MnBr4 wafer reaches only 40%. The (4-DMATBP)MnBr4 wafer with a spatial resolution of 8 line pair per millimeter (lp mm−1) shows higher X-ray imaging quality than the (4-NH3TBP)MnBr4 wafer (Fig. S23†). The X-ray imaging resolution of the (4-DMATBP)MnBr4 single crystal (∼0.5 mm thick) was measured to be 30 lp mm−1, which is much higher than that of the wafer (Fig. 5a). Furthermore, the copper mesh with a rib pitch of 12 μm (namely, 42 lp mm−1) can be clearly distinguished, highlighting its application potential in high-quality X-ray imaging (Fig. 5b). Moreover, as shown in Fig. 5c, the obvious gray value change proves the high imaging quality, indicating that the X-ray imaging resolution can reach up to 42 lp mm−1.Based on its ATQ behavior under X-ray irradiation, the as-synthesized (4-DMATBP)MnBr4 single crystal was applied for high-temperature X-ray imaging. As demonstrated in Fig. 5d, the greenish emission intensity under 365 nm ultraviolet excitation first gradually becomes stronger and then decreases slightly with increasing temperature. Moreover, the single crystal maintains its high transmittance, as evidenced by the sharp visibility of the letter “K”. Subsequently, the focused images of a copper mesh at different temperatures under X-ray irradiation are shown in Fig. 5e. The self-built X-ray imaging system is demonstrated in Fig. 5f. Benefitting from the high optical transparency of up to 95% (Fig. 5g), the X-ray images exhibit high contrast during the heating process (Fig. 5h). Notably, the single-crystal scintillator could give clear resolution, and there is no obvious reduction in the contrast of images in the range of 30–200 °C (Fig. S24†). To further investigate the X-ray imaging quality at high temperature, we selected two miniature electronic components as the imaging objects and performed X-ray imaging experiments (Fig. S25†). The structures of electronic components at variable temperatures can be recognized, demonstrating the (4-DMATBP)MnBr4 single crystal to be an ideal candidate for high-temperature X-ray imaging with high quality. Notably, the wires connecting the pins, indicated by the white arrows, are clearly distinguishable during the heating process. For comparison, we systematically evaluated the commercial LuAG:Ce scintillator under the same temperatures. LuAG:Ce exhibits a spatial resolution approaching nearly 25 lp mm−1 at room temperature (Fig. S26a†). Moreover, similar to the (4-DMATBP)MnBr4 single crystal, the LuAG:Ce scintillator demonstrates excellent X-ray imaging performance at elevated temperatures (Fig. S26b–e†). When observing the images of the copper mesh at various temperatures under X-ray irradiation using LuAG:Ce, there are no significant alterations, and the decrease in image contrast is hardly noticeable (Fig. S26b–d†). Likewise, Fig. S26e† presents the internal structure of electronic component B within the temperature range of 30–200 °C. It can be seen that no remarkable changes are observed. However, it should be noted that the wires indicated by the white arrows become indistinguishable. Consequently, the (4-DMATBP)MnBr4 single crystal was proven to be an excellent high-temperature scintillator with great application potential.
To further investigate the irradiation stability of the (4-DMATBP)MnBr4 single crystal under X-ray excitation, we performed a long-term irradiation stability test. As displayed in Fig. S27,† after exposure to X-rays with a dose rate of 14.77 mGy s−1 for 300 minutes (total X-ray dosage of 133 Gy), the RL intensity of the (4-DMATBP)MnBr4 single crystal remained at 91.6% of its initial value. The slight decrease in RL intensity demonstrates the robust stability of the (4-DMATBP)MnBr4 single crystal in X-ray scintillation applications.
Conclusions
In summary, we successfully synthesized single crystals of (4-NH3TBP)MnBr4 and (4-DMATBP)MnBr4 through a solvent evaporation method; the crystals show abnormal weak-TQ or ATQ behaviors originating from their rigid structure and suppressed non-radiative recombination. Notably, (4-DMATBP)MnBr4 exhibits stronger ATQ ability than (4-NH3TBP)MnBr4. Temperature-dependent SCXRD revealed that the Mn–Mn distance of (4-DMATBP)MnBr4 increases as the temperature rises, inhibiting the Mn–Mn energy-transfer-induced emission quenching and anticipating their ATQ behavior. More importantly, TL and EPR spectra demonstrate that intrinsic defects serve as a carrier bank that can release carriers to the excited state as temperature increases and thus afford the ATQ properties of (4-DMATBP)MnBr4. Further DFT calculations revealed that the defect could be ascribed to a Br-vacancy structure defect. Subsequently, we evaluated the X-ray detection performance and found that the (4-DMATBP)MnBr4 single crystal exhibits a high LY of 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 722 photon per MeV at 130 °C, showing its potential for application in high-temperature detection. Benefiting from its high optical transparency and outstanding scintillation performance, the as-prepared single crystal (5 mm × 7 mm × 0.5 mm) was directly adopted as a high-temperature X-ray imaging screen. (4-DMATBP)MnBr4 crystal exhibits an impressive spatial resolution of 42 lp mm−1 at room temperature and maintains its excellent imaging quality at high temperatures. These results suggest that the prepared OIMH single crystals are promising candidates for high-temperature X-ray detection, which could promote the development of high-temperature scintillators.
722 photon per MeV at 130 °C, showing its potential for application in high-temperature detection. Benefiting from its high optical transparency and outstanding scintillation performance, the as-prepared single crystal (5 mm × 7 mm × 0.5 mm) was directly adopted as a high-temperature X-ray imaging screen. (4-DMATBP)MnBr4 crystal exhibits an impressive spatial resolution of 42 lp mm−1 at room temperature and maintains its excellent imaging quality at high temperatures. These results suggest that the prepared OIMH single crystals are promising candidates for high-temperature X-ray detection, which could promote the development of high-temperature scintillators.
Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 4-NH3TBPBr, 4-DMATBPBr and (4-NH3TBP)MnBr4 at 150 K, and (4-DMATBP)MnBr4 at different temperatures (150 K, 303 K, 403 K, 473 K, and 303 K upon cooling after thermal treatment) have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under [2379301–2379308] and can be obtained from https://www.ccdc.cam.ac.uk/structures/.Author contributions
D.-B. K. and J.-H. C. conceived and designed the experiments. J.-H. C. carried out material synthesis, measurements, and data analysis. J.-B. L., Z.-L. H., Q.-P. P., J.-H. W., Z.-Z. Z. and X.-X. G. assisted with the X-ray imaging measurements and data analysis. J.-B. L. aided in density functional theory calculations and data analysis. All authors participated in discussing the data and writing the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors acknowledge the financial supports from the National Natural Science Foundation of China (22375220, U2001214).Notes and references
- L. A. Boatner, J. S. Neal, J. A. Kolopus, J. O. Ramey and H. Akkurt, Nucl. Instrum. Methods Phys. Res., Sect. A, 2013, 709, 95 CrossRef CAS.
- A. Bala, J. R. Brown, D. G. Jenkins and P. Joshi, Nucl. Instrum. Methods Phys. Res., Sect. A, 2021, 997, 165161 CrossRef CAS.
- T. Wang, S. Hu, T. Ji, X. Zhu, G. Zeng, L. Huang, A. N. Yakovlev, J. Qiu, X. Xu and X. Yu, Laser Photon. Rev., 2024, 18, 2300892 CrossRef CAS.
- K. Murase, Phys. Rev. Lett., 2009, 103, 081102 Search PubMed.
- G. Di Sciascio, Nucl. Part. Phys., Proc., 2016, 279, 166–173 CrossRef.
- L. A. Anchordoqui, Phys. Rep., 2019, 801, 1 CrossRef CAS.
- J. Asfahani, K. Samuding, R. Mostapa and O. Othman, Appl. Radiat. Isot., 2021, 167, 109296 CrossRef CAS PubMed.
- J. Qin, C. Hu, B. Lei, J. Li, Y. Liu, S. Ye and M. Pan, J. Mater. Sci. Technol., 2014, 30, 290 CrossRef CAS.
- M. Nikl and A. Yoshikawa, Adv. Opt. Mater., 2015, 3, 463 CrossRef CAS.
- E. V. D. van Loef, P. Dorenbos, C. W. E. van Eijk, K. W. Krämer and H. U. Güdel, Nucl. Instrum. Methods Phys. Res., Sect. A, 2002, 486, 254 CrossRef CAS.
- Y. H. Kim, P. Arunkumar, B. Y. Kim, S. Unithrattil, E. Kim, S.-H. Moon, J. Y. Hyun, K. H. Kim, D. Lee, J.-S. Lee and W. B. Im, Nat. Mater., 2017, 16, 543 CrossRef CAS PubMed.
- F. Zhou, Z. Li, W. Lan, Q. Wang, L. Ding and Z. Jin, Small Methods, 2020, 4, 2000506 CrossRef CAS.
- Z.-L. He, J.-H. Wei, J.-B. Luo, Z.-Z. Zhang, J.-H. Chen, X.-X. Guo and D.-B. Kuang, Laser Photon. Rev., 2024, 18, 2301249 CrossRef CAS.
- J.-B. Luo, J.-H. Wei, Z.-Z. Zhang, Z.-L. He and D.-B. Kuang, Angew. Chem., Int. Ed., 2022, 62, e202216504 CrossRef PubMed.
- Z.-Z. Zhang, Z.-L. He, J.-B. Luo, J.-H. Wei, X.-X. Guo, J.-H. Chen and D.-B. Kuang, Adv. Opt. Mater., 2024, 12, 2302434 CrossRef CAS.
- D. Liang, H. Xiao, W. Cai, S. Lu, S. Zhao, Z. Zang and L. Xie, Adv. Opt. Mater., 2023, 11, 2202997 CrossRef CAS.
- W. Zhang, P. Sui, W. Zheng, L. Li, S. Wang, P. Huang, W. Zhang, Q. Zhang, Y. Yu and X. Chen, Angew. Chem., Int. Ed., 2023, 135, e202309230 Search PubMed.
- P. Dang, W. Wang, H. Lian, G. Li and J. Lin, Adv. Opt. Mater., 2022, 10, 2102287 Search PubMed.
- S. Wang, Y. Xu, T. Chen, W. Jiang, J. Liu, X. Zhang, W. Jiang and L. Wang, Chem. Eng. J., 2021, 404, 125912 Search PubMed.
- Y. Wei, H. Yang, Z. Gao, X. Yun, G. Xing, C. Zhou and G. Li, Laser Photon. Rev., 2021, 15, 2000048 CrossRef CAS.
- B.-B. Zhang, J.-K. Chen, J.-P. Ma, X.-F. Jia, Q. Zhao, S.-Q. Guo, Y.-M. Chen, Q. Liu, Y. Kuroiwa, C. Moriyoshi, J. Zhang and H.-T. Sun, J. Phys. Chem. Lett., 2020, 11, 2902–2909 Search PubMed.
- Y. Wei, H. Yang, Z. Gao, Y. Liu, G. Xing, P. Dang, A. A. A. Kheraif, G. Li, J. Lin and R. S. Liu, Adv. Sci., 2020, 7, 1903060 CrossRef CAS PubMed.
- Y. Wu, J. Chen, D. Zheng, X. Xia, S. Yang, Y. Yang, J. Chen, T. Pullerits, K. Han and B. Yang, J. Phys. Chem. Lett., 2022, 13, 5794–5800 CrossRef CAS PubMed.
- T.-C. Wang, S.-Y. Yao, S.-P. Yan, J. Yu, Z.-Y. Deng, A. N Yakovlev, B. Meng, J.-B. Qiu and X.-H. Xu, ACS Appl. Mater. Interfaces, 2023, 15, 23421–23428 CrossRef CAS PubMed.
- Y. Wu, J. Feng, Z. Yang, Y. Liu and S. Liu, Adv. Sci., 2023, 10, 2205536 CrossRef CAS PubMed.
- Z.-Z. Zhang, J.-H. Wei, J.-B. Luo, X.-D. Wang, Z.-L. He and D.-B. Kuang, ACS Appl. Mater. Interfaces, 2022, 14, 47913 CrossRef CAS PubMed.
- Z.-L. He, J.-H. Wei, Z.-Z. Zhang, J.-B. Luo and D.-B. Kuang, Adv. Opt. Mater., 2023, 11, 2300449 CrossRef CAS.
- J.-B. Luo, J.-H. Wei, Z.-L. He, J.-H. Chen, Q.-P. Peng, Z.-Z. Zhang and D.-B. Kuang, Chem. Sci., 2024, 15, 16338 RSC.
- H. Meng, W. Zhu, Z. Zhou, R. Zhou, D. Yan, Q. Zhao and S. Liu, J. Mater. Chem. C, 2022, 10, 12286 RSC.
- Q. Qiu, G. Zhang, J. Chen, Y. Di, L. Wu, H. Chen, S. C. Chen and M. J. Lin, Small, 2024, 21, 2407346 CrossRef PubMed.
- H. Cui, W. Zhu, Y. Deng, T. Jiang, A. Yu, H. Chen, S. Liu and Q. Zhao, Aggregate, 2023, 5, e454 CrossRef.
- B. Li, J. Jin, M. Yin, K. Han, Y. Zhang, X. Zhang, A. Zhang, Z. Xia and Y. Xu, Chem. Sci., 2023, 14, 12238 RSC.
- H. Zheng, A. Ghosh, M. J. Swamynadhan, G. Wang, Q. Zhang, X. Wu, I. Abdelwahab, W. P. D. Wong, Q.-H. Xu, S. Ghosh, J. Chen, B. J. Campbell, A. Stroppa, J. Lin, R. Mahendiran and K. P. Loh, J. Am. Chem. Soc., 2023, 145, 18549 CrossRef CAS PubMed.
- Q. Chen, J. Wu, X. Ou, B. Huang, J. Almutlaq, A. A. Zhumekenov, X. Guan, S. Han, L. Liang and Z. Yi, Nature, 2018, 561, 88 CrossRef CAS PubMed.
- L. Mao, P. Guo, S. Wang, A. K. Cheetham and R. Seshadri, J. Am. Chem. Soc., 2020, 142, 13582 CrossRef CAS PubMed.
- G. Zhou, Z. Liu, J. Huang, M. S. Molokeev, Z. Xiao, C. Ma and Z. Xia, J. Phys. Chem. Lett., 2020, 11, 5956 CrossRef CAS PubMed.
- M.-S. Kwon, Y. Yu, C. Coburn, A.-W. Phillips, K. Chung, A. Shanker, J. Jung, G. Kim, K. Pipe, S.-R. Forrest, J.-H. Youk, J. Gierschner and J. Kim, Nat. Commun., 2015, 6, 8947 CrossRef PubMed.
- L.-K. Gong, Q.-Q. Hu, F.-Q. Huang, Z.-Z. Zhang, N.-N. Shen, B. Hu, Y. Song, Z.-P. Wang, K.-Z. Du and X.-Y. Huang, Chem. Commun., 2019, 55, 7303 RSC.
- P.-R. Spackman, M.-J. Turner, J.-J. McKinnon, S.-K. Wolff, D.-J. Grimwood, D. Jayatilaka and M.-A. Spackman, J. Appl. Crystallogr., 2021, 54, 1006–1011 CrossRef CAS PubMed.
- J. Jin, K. Han, Y. Hu and Z. Xia, Adv. Opt. Mater., 2023, 11, 2300330 CrossRef CAS.
- S. He, F. Xu, T. Han, Z. Lu, W. Wang, J. Peng, F. Du, F. Yang and X. Ye, Chem. Eng. J., 2020, 392, 123657 CrossRef CAS.
- Z. Tang, R. Liu, J. Chen, D. Zheng, P. Zhou, S. Liu, T. Bai, K. Zheng, K. Han and B. Yang, Angew. Chem., Int. Ed., 2022, 134, e202210975 CrossRef.
- N. Yang, J. Li, Z. Zhang, D. Wen, Q. Liang, J. Zhou, J. Yan and J. Shi, Chem. Mater., 2020, 32, 6958 CrossRef CAS.
- L. Wu, S. Sun, Y. Bai, Z. Xia, L. Wu, H. Chen, L. Zheng, H. Yi, T. Sun, Y. Kong, Y. Zhang and J. Xu, Adv. Opt. Mater., 2021, 9, 2100870 CrossRef CAS.
- W. Li, Y. Li, Y. Wang, Z. Zhou, C. Wang, Y. Sun, J. Sheng, J. Xiao, Q. Wang, S. Kurosawa, M. Buryi, D. John, K. Paurová, M. Nikl, X. Ouyang and Y. Wu, Laser Photonics Rev., 2024, 18, 2300860 CrossRef CAS.
- M.-S. Pervez, M.-F. Hossain and M.-A.-I. Nahid, Mater. Sci. Semicond. Process., 2022, 137, 106179 CrossRef CAS.
- W. Zhang, W. Zheng, L.-Y. Li, P. Huang, J. Xu, W. Zhang, Z.-Q. Shao and X.-Y. Chen, Adv. Mater., 2024, 36, 2408777 CrossRef CAS PubMed.
- W. Shao, G. Zhu, X. Wang, Z. Zhang, H. Lv, W. Deng, X. Zhang and H. Liang, ACS Appl. Mater. Interfaces, 2023, 15, 932–941 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2379301–2379308. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc08499c |
| This journal is © The Royal Society of Chemistry 2025 |