Open Access Article
Open Access ArticleReversible redox 19F magnetic resonance imaging nanoprobes for monitoring the redox state in vivo†
Xiaoyao
Xiong
,
Sijia
Li
,
Yumin
Li
,
Suying
Xu
 ,
Chang
Guo
* and
Leyu
Wang
,
Chang
Guo
* and
Leyu
Wang
 *
*
State Key Laboratory of Chemical Resource Engineering, College of Chemistry, Beijing University of Chemical Technology, Beijing, 100029, China. E-mail: lywang@mail.buct.edu.cn; guoc@mail.buct.edu.cn
First published on 20th February 2025
Abstract
Redox processes are indispensable for physiology, and dysregulated redox balance is critical in various metabolic diseases. The development of imaging diagnosis tools for real-time monitoring of the redox state in vivo is of great importance yet highly challenging. Here, we designed trifluoromethyl (–CF3) grafted selenide polymer nanoprobes for reversible redox sensing in vivo. Based on the reversible shift of the 19F-nuclear magnetic resonance (NMR) peak between oxidation and reduction states of the nanoprobes exposed to different redox species, the 19F-magnetic resonance imaging (MRI) signal ratio of SOx/(SOx + SRed) was successfully applied to monitor the redox state in a tumor. These nanoprobes demonstrated good biocompatibility and great potential for exploring physiological and pathological redox processes in deep tissues. We envision that this work will enable the rational design of 19F-MRI nanoprobes with excellent redox response for the real-time monitoring of the redox state at the lesion location.
Introduction
Redox processes play a vital role in many biological and physiological functions.1–3 Dysregulated redox status is associated with many diseases, including cancer4,5 and cardiological6 and neurodegenerative diseases,7–9 but how the redox status impacts diseases is largely unknown. Developing bioimaging probes for dynamic monitoring of redox status in deep tissue is critical for understanding and studying various diseases.10,11Recently, various fluorescent,12 photoacoustic,13,14 and proton magnetic resonance imaging (1H-MRI)15–20 probes have significantly progressed in monitoring redox processes. However, small-molecule fluorescent and photoacoustic probes are only employed to evaluate the redox status within cells or superficial tissues due to the limited penetration depth of light propagation in tissues, and sometimes fluorescence imaging suffers from strong autofluorescence interference of tissue. 1H-MRI allows non-invasive visualization of deep tissues, but the heterogeneous background of tissues makes the interpretation of 1H-MR images more difficult.2119F-MRI is a splendid “hot spot” in vivo imaging technology due to its zero-background in living organisms.22–27 Most redox-responsive 19F-MRI probes28–30 are based on the breakable disulfide bond, which is generally irreversible and unsuitable for monitoring redox cycles. The other interesting strategy31–34 relies on the metal center switched between paramagnetic and diamagnetic states by redox status to reversibly modulate the 19F-signal. Despite great progress, it is still highly challenging to fabricate an ideal imaging probe for in vivo redox sensing with advantages. Specifically, achieving non-invasive and radiation-free imaging with high penetration depth and ultra-low backgrounds is particularly difficult in current techniques. Additionally, ensuring a reversible response for dynamically measuring the redox state and excellent biocompatibility of the probe itself further complicates the development of an ideal imaging probe for redox sensing in vivo.
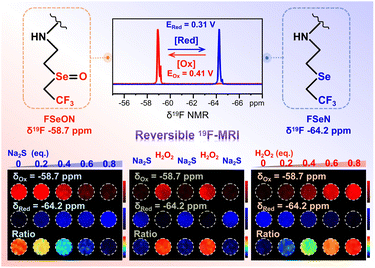
Herein, we designed a PIBAM–FSeN nanoprobe, based on trifluoromethyl (–CF3, as the 19F tag)-grafted selenide (as the redox-recognition site), for reversible redox status 19F-MRI visualization in deep tissue. As depicted in Scheme 1, when exposed to oxidizing species, PIBAM–FSeN was readily oxidized to PIBAM–FSeON; as a result, the 19F-NMR signal at −64.2 ppm decreased and a new 19F-NMR peak emerged at −58.7 ppm. With the increment of the oxidation degree, the signal at −64.2 ppm (SRed) decreased step by step and the signal at −58.7 ppm (SOx) simultaneously increased. This phenomenon would reverse in the presence of reductive species. Therefore, the 19F-MRI signal ratio SOx/(SOx + SRed) could be utilized to monitor reversible redox processes in deep tissue.
 | ||
| Scheme 1 Schematic illustration of the nanoprobes for 19F-MRI monitoring of reversible redox processes. | ||
Results and discussion
Preparation and characterization of PIBAM–FSeON and PIBAM–FSeN NPs
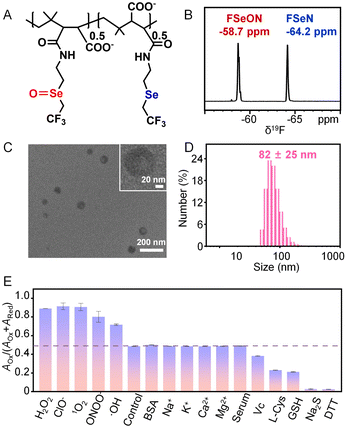
Before the synthesis of reversible 19F-MRI nanoprobes, the precursor, 2-(2,2,2-trifluoroethyl)selanylethan-1-amine (FSeN), was first synthesized and characterized (Fig. S1–S7†). Then, poly(isobutylene-maleic anhydride) (PIBAM) was modified with FSeN to get PIBAM–FSeN through a ring-opening reaction (Fig. S1†). The selenide ether group in PIBAM–FSeN was further oxidized to the selenoxide group, termed PIBAM–FSeON (Fig. S8 and S9†). Notably, compared with most reported fluoropolymers (fluorine content is generally below 5 wt%), both PIBAM–FSeN and PIBAM–FSeON had a high fluorine content of 16 wt% and 15 wt%, respectively (Fig. S10†), which were highly desirable for 19F-MRI benefiting from their high 19F content and thus a strong MRI signal. Next, the PIBAM–FSeN NPs and PIBAM–FSeON NPs were obtained through self-assembly under ultrasonic emulsification (Fig. S11†). The good stability of these NPs in aqueous solution was confirmed by the hardly changed values in the 19F-NMR signal (Fig. S12†) and dynamic light scattering (DLS) size (Fig. S13†) even after 50 days.In vitro detection of redox species by 19F-NMR
Then the redox responsive ability of these NPs was carefully checked. As shown in Fig. 1A, in the presence of Na2S, the 19F-NMR spectrum of PIBAM–FSeON NPs shifted significantly from −58.7 to −64.2 ppm, stemming from the reduction of selenoxide. With the increment of Na2S content, the 19F-NMR signal at −64.2 ppm was enhanced step by step, and a linear relationship between the ratio of AOx/(AOx + ARed) and Na2S content was constructed (Fig. 1B). Here, AOx and ARed are integral areas of the 19F-NMR peak located at −58.7 and −64.2 ppm, respectively, which are proportional to the fluorine atom content. Meanwhile, the reduction state (PIBAM–FSeN) was easily returned to the oxidation state (PIBAM–FSeON) in the presence of H2O2 (Fig. 1C), and the linear relationship between AOx/(AOx + ARed) and H2O2 content was also established (Fig. 1D). The reversibility of the nanoprobes was further verified by alternately exposing nanoprobes to H2O2 and Na2S (Fig. 1E and F), which makes it possible to use them for real-time monitoring of the redox state.Next, the redox selectivity of these nanoprobes was studied via recording the 19F-NMR responses after treatment with various analytes (Fig. S14†). Redox species caused obvious effects on 19F-NMR signal intensity, whereas non-redox analytes had negligible influence. Moreover, the 19F longitudinal (T1) and transverse (T2) relaxation times of PIBAM–FSeN NPs (T1 = 0.96 s, T2 = 0.59 s) and PIBAM–FSeON NPs (T1 = 1.01 s, T2 = 0.45 s) were measured (Table S1†), which is suitable for 19F-MRI with a refocused echo (RARE) sequence.
In vitro detection of redox species by 19F-MRI
Phantom studies were conducted to evaluate the 19F-MRI potential of these nanoprobes (Fig. 2). The center frequency in 19F-NMR of PIBAM–FSeON (δ = −58.7 ppm, red channel) and PIBAM–FSeN (δ = −64.2 ppm, blue channel) was chosen for radio frequency (RF) output. As shown in Fig. 2A, with the increment of Na2S content, the 19F-MRI signal of PIBAM–FSeON NPs in the red channel decreased step by step; meanwhile that in the blue channel gradually increased. A good linear relationship between the 19F-MRI signal ratio of SOx/(SOx + SRed) and Na2S content was observed (Fig. 2B). Compared with the signal ratio in the absence of Na2S, it showed a 12.5-fold decrease after exposure to 0.8 eq. of Na2S. Following the treatment with H2O2, the signal ratio of PIBAM–FSeN NPs linearly increased (Fig. 2C and D), and the signal ratio showed a 10.5-fold increase after exposure to 0.8 eq. of H2O2, compared with that without H2O2. The reversibility of PIBAM–FSeN NPs for redox response was also validated using the 19F-MRI results shown in Fig. 2E and F.Preparation and characterization of the semioxidized PIBAM–FSeN NPs
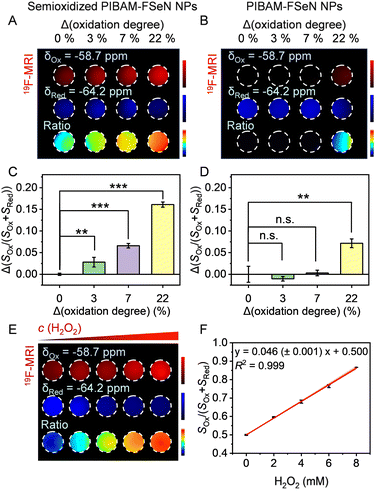
To ensure that the signal of the probes in both channels (−58.7 ppm and −64.2 ppm) is higher than the detection limit of the instrument, achieving the observation of slight changes from the ratio images, we prepared the semioxidized PIBAM–FSeN NPs for further use. Thus, by controlling the oxidation conditions, the semioxidized PIBAM–FSeN polymer (Fig. 3A) was synthesized, where the content ratio of the selenide ether to the selenoxide group was 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Fig. 3B). Thereafter, the semioxidized PIBAM–FSeN NPs with an average size of 82 ± 25 nm were obtained by the same ultrasonic emulsification method for PIBAM–FSeN NPs (Fig. 3C and D). The semioxidized PIBAM–FSeN NPs also exhibited excellent relaxation performance, as evidenced by the longitudinal relaxation time (T1) and transverse relaxation time (T2) values (Table S1†). It was found that no significant variations of T1 and T2 were observed over a concentration range from 20 to 140 mg mL−1, implying that fluorine atoms on the nanoparticles retain excellent relaxation properties (Fig. S15†). The critical aggregation concentration (CAC) of semioxidized PIBAM–FSeN NPs is 4.2 mg L−1 (Fig. S16†), which falls between the CAC of PIBAM–FSeN NPs (1.6 mg L−1) and PIBAM–FSeON NPs (750 mg L−1). Subsequently, their good stability was confirmed by the results of 19F-NMR signals, DLS size distribution tests, and actual photos (Fig. S17–S19 and Table S4†). It is important to note that semioxidized PIBAM–FSeN nanoparticles (NPs) demonstrate enhanced stability under acidic conditions (pH 6.0–6.5) compared to PIBAM–FSeN NPs. This improved stability may be attributed to their lower pKa value of 5.52, in contrast to the pKa of PIBAM–FSeN NPs, which is 6.31 (Fig. S20†).
1 (Fig. 3B). Thereafter, the semioxidized PIBAM–FSeN NPs with an average size of 82 ± 25 nm were obtained by the same ultrasonic emulsification method for PIBAM–FSeN NPs (Fig. 3C and D). The semioxidized PIBAM–FSeN NPs also exhibited excellent relaxation performance, as evidenced by the longitudinal relaxation time (T1) and transverse relaxation time (T2) values (Table S1†). It was found that no significant variations of T1 and T2 were observed over a concentration range from 20 to 140 mg mL−1, implying that fluorine atoms on the nanoparticles retain excellent relaxation properties (Fig. S15†). The critical aggregation concentration (CAC) of semioxidized PIBAM–FSeN NPs is 4.2 mg L−1 (Fig. S16†), which falls between the CAC of PIBAM–FSeN NPs (1.6 mg L−1) and PIBAM–FSeON NPs (750 mg L−1). Subsequently, their good stability was confirmed by the results of 19F-NMR signals, DLS size distribution tests, and actual photos (Fig. S17–S19 and Table S4†). It is important to note that semioxidized PIBAM–FSeN nanoparticles (NPs) demonstrate enhanced stability under acidic conditions (pH 6.0–6.5) compared to PIBAM–FSeN NPs. This improved stability may be attributed to their lower pKa value of 5.52, in contrast to the pKa of PIBAM–FSeN NPs, which is 6.31 (Fig. S20†).
The influence of some coexisting substances on the 19F-NMR signal ratio of AOx/(AOx + ARed) was further investigated. As shown in Fig. 3E, no change in the signal ratio was observed in the presence of non-redox analytes including BSA, Na+, K+, Ca2+, Mg2+ and serum. However, oxidants such as H2O2, ClO−, 1O2, ONOO− and ·OH would greatly enhance the signal ratio, and conversely reductants including Vc, L-Cys, GSH, Na2S and DTT depressed the signal ratio. The moderate oxidation (0.41 V) and reduction (0.31 V) potentials make it easy to oxidize or reduce the semioxidized PIBAM–FSeN NPs in vivo by the endogenous redox species (Fig. S21 and Tables S2 and S3†). The redox process was further verified by checking the redox state of Se in the nanoprobes through X-ray photoelectron spectroscopy (XPS, Fig. S22†).
In vitro 19F-MRI of semioxidized PIBAM–FSeN NPs
To investigate the imaging benefits of semioxidized nanoprobes for low-concentration analytes, the imaging results of semioxidized PIBAM–FSeN NPs and PIBAM–FSeN NPs were compared. Fig. 4A and C illustrate an increase in the signal at −58.7 ppm and a decrease in the signal at −64.2 ppm as the oxidation degree of semioxidized PIBAM–FSeN NPs deepens. Thus, a significantly elevated signal is obtained in the ratio channel, even though the increasing oxidation degree of the probe is only 3%. However, Fig. 4B and D show that with PIBAM–FSeN NPs, only the signal at −64.2 ppm decreases gradually with an increased oxidation degree. At low oxidation degrees (3% and 7%), the signal at −58.7 ppm is nearly undetectable, but as oxidation levels increase (22%), changes in the signal can be observed due to it reaching the detection limit of the instrument. These results have confirmed the benefits of using semioxidized PIBAM–FSeN NPs.Subsequently, we investigated the 19F-MRI signal response of semioxidized PIBAM–FSeN NPs toward H2O2. As shown in Fig. 4E, the intensity of their 19F-MRI signal ratio gradually enhanced with the increase of the H2O2 concentration. A linear relationship was observed between the ratio and the concentration of H2O2. Furthermore, the 19F-MRI signal ratio of SOx/(SOx + SRed) showed a good linear relationship with the H2O2 concentration with the linear equation: y = 0.046 (±0.001) x + 0.500, R2 = 0.999 (Fig. 4F). The limit of detection (LOD) was detected as 0.18 mM (LOD = 3σ/k, n = 3). Taken together, semioxidized PIBAM–FSeN NPs could be used to quantify H2O2 by 19F-MRI in vitro.
In vivo 19F-MRI of semioxidized PIBAM–FSeN NPs
Encouraged by these promising results, we explored the potential of utilizing these semioxidized PIBAM–FSeN NPs for 19F-MRI visualization of the redox state in vivo. The cytotoxicity and hemolysis tests were first carried out, and the results suggested their favorable biocompatibility and biosafety, making them suitable for in vivo applications (Fig. S23–S27†). A Balb/c mouse model with a 4T1 tumor was constructed to serve as a proof of concept and showcase the ability of the nanoprobes to monitor localized redox state in vivo. As depicted in Fig. 5A and B, the same amounts of semioxidized PIBAM–FSeN NPs colloidal solution were injected into the tumor and healthy tissue of the same mouse, respectively. After injection, 19F-MRI at time points of 0.5 and 4 h post-injection was captured at the center frequencies (−58.7 ppm, red channel) and (−64.2 ppm, blue channel). 1H-MRI was also provided to show the mouse and tumor boundaries in the overlaid 19F-MRI. As shown in Fig. 5C, stronger 19F-MRI signals in the red channel and weaker 19F-MRI signals in the blue channel were observed at the tumor site 4 h post-injection compared to those in normal tissues. The signal ratio was significantly increased (**p < 0.01, n = 3), implying that it is more oxidative in the tumor in this period (Fig. 5D).Moreover, we conducted the 19F-MRI tests to monitor the redox cycle in the tumor of live mice with N-acetyl cysteine (NAC) as the anti-inflammation drug (Fig. 5E). As shown in Fig. 5F and G, the 19F-MRI signal ratio of semioxidized PIBAM–FSeN NPs in the tumor increased at 3 h after intratumoral injection. When pretreated with NAC at 4 h post-injection, the 19F-MRI signal ratio remarkably decreased compared to that without NAC treatment (*p < 0.05, n = 3). These results demonstrated that the nanoprobes could be used for reversible redox sensing in vivo. In addition, the blood biochemical analysis (Fig. S28†) and histological hematoxylin and eosin (H&E) staining (Fig. S29†) further confirmed that no apparent toxicity was induced by the nanoprobes.
Conclusions
In conclusion, we designed selenide polymer nanoprobes with favorable biocompatibility and stability for in situ reversible monitoring of the redox process in vivo. Based on the reversible shift of the 19F-NMR peak between oxidation (FSeON, −58.7 ppm) and reduction (FSeN, −64.2 ppm) states of the nanoprobes exposed to different redox species, the 19F-MRI signal ratio of SOx/(SOx + SRed) was successfully applied to visualize the redox state in a tumor. Importantly, the nanoprobe demonstrates excellent water solubility and good biocompatibility, making it promising for intravenous injection. Considering the wide range of biomolecules, such as proteins, saccharides and aptamers, that can be potentially labeled with the trifluoromethyl-grafted selenide structure, it might be possible to adapt this strategy to redox sensing in other specific regions. This work paves a new way to develop powerful tools for reversible sensing and imaging of redox the state in vivo with high penetration depth.Ethical statement
All experiments involving animals were approved and performed in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) of the China-Japan Friendship Hospital and Beijing University of Chemical Technology.Data availability
Experimental procedures and all relevant data are available in the ESI† and from the authors.Author contributions
The manuscript was written through the contributions of all authors. All authors have approved the final version of the manuscript. L. Y. W. and C. G. supervised the project. L. Y. W., C. G., S. Y. X. and X. Y. X. designed the experiments. X. Y. X., S. J. L. and Y. M. L. conducted the experiments and analyzed the data. L. Y. W., C. G. and X. Y. X. wrote the manuscript. All authors discussed and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was partially supported by the National Natural Science Foundation of China (22334002, 22322402, and 22306010), the Beijing Municipal Natural Science Foundation (Z231100002723006), and the Fundamental Research Funds for the Central Universities (XK2023-19 and JD2308).Notes and references
- B. Faubert, A. Solmonson and R. J. DeBerardinis, Metabolic Reprogramming and Cancer Progression, Science, 2020, 368, eaaw5473 CrossRef CAS PubMed
.
- P. Hernansanz-Agustín, C. Choya-Foces, S. Carregal-Romero, E. Ramos, T. Oliva, T. Villa-Piña, L. Moreno, A. Izquierdo-alvarez, J. D. Cabrera-García, A. Cortés, A. V. Lechuga-Vieco, P. Jadiya, E. Navarro, E. Parada, A. Palomino-Antolín, D. Tello, R. Acín-Pérez, J. C. Rodríguez-Aguilera, P. Navas, A. Cogolludo, I. López-Montero, A. Martínez-del-Pozo, J. Egea, M. G. López, J. W. Elrod, J. Ruíz-Cabello, A. Bogdanova, J. A. Enríquez and A. Martínez-Ruiz, Na+ Controls Hypoxic Signalling by the Mitochondrial Respiratory Chain, Nature, 2020, 586, 287–291 CrossRef PubMed
.
- M. P. Murphy, H. Bayir, V. Belousov, C. J. Chang, K. J. A. Davies, M. J. Davies, T. P. Dick, T. Finkel, H. J. Forman, Y. Janssen-Heininger, D. Gems, V. E. Kagan, B. Kalyanaraman, N. G. Larsson, G. L. Milne, T. Nyström, H. E. Poulsen, R. Radi, H. Van Remmen, P. T. Schumacker, P. J. Thornalley, S. Toyokuni, C. C. Winterbourn, H. Y. Yin and B. Halliwell, Guidelines for Measuring Reactive Oxygen Species and Oxidative Damage in Cells and in Vivo, Nat. Metab., 2022, 4, 651–662 CrossRef PubMed
.
- H. N. Bell, R. J. Rebernick, J. Goyert, R. Singhal, M. Kuljanin, S. A. Kerk, W. Huang, N. K. Das, A. Andren, S. Solanki, S. L. Miller, P. K. Todd, E. R. Fearon, C. A. Lyssiotis, S. P. Gygi, J. D. Mancias and Y. T. M. Shah, Reuterin in the Healthy Gut Microbiome Suppresses Colorectal Cancer Growth Through Altering Redox Balance, Cancer Cell, 2022, 40, 185–200.e186 CrossRef CAS
.
- H. J. Zhang, Z. Mao, Y. Kang, W. Zhang, L. Mei and X. Y. Ji, Redox Regulation and Its Emerging Roles in Cancer Treatment, Coord. Chem. Rev., 2023, 475, 214897 CrossRef CAS
.
- G. Heusch, I. Andreadou, R. Bell, E. Bertero, H. E. Botker, S. M. Davidson, J. Downey, P. Eaton, P. Ferdinandy, B. J. Gersh, M. Giacca, D. J. Hausenloy, B. Ibanez, T. Krieg, C. Maack, R. Schulz, F. Sellke, A. M. Shah, H. Thiele, D. M. Yellon and F. Di Lisa, Health Position Paper and Redox Perspectives on Reactive Oxygen Species As Signals and Targets of Cardioprotection, Redox Biol., 2023, 67, 102894 CrossRef CAS PubMed
.
- D. Eleftheriadou, D. Kesidou, F. Moura, E. Felli and W. H. Song, Redox-Responsive Nanobiomaterials-Based Therapeutics for Neurodegenerative Diseases, Small, 2020, 16, 1907308 CrossRef CAS PubMed
.
- M. Kim, J. Kang, M. Lee, J. Han, G. Nam, E. Tak, M. S. Kim, H. J. Lee, E. Nam, J. Park, S. J. Oh, J. Y. Lee, J. Y. Lee, M. H. Baik and M. H. Lim, Minimalistic Principles for Designing Small Molecules with Multiple Reactivities Against Pathological Factors in Dementia, J. Am. Chem. Soc., 2020, 142, 8183–8193 CrossRef CAS PubMed
.
- X. Chen, B. Y. Ji, X. X. Hao, X. W. Li, F. Eisele, T. Nyström and D. Petranovic, FMN Reduces Amyloid-β Toxicity in Yeast by Regulating Redox Status and Cellular Metabolism, Nat. Commun., 2020, 11, 867 CrossRef CAS PubMed
.
- H. P. Xiao, M. P. Jedrychowski, D. K. Schweppe, E. L. Huttlin, Q. Yu, D. E. Heppner, J. M. Li, J. N. Long, E. L. Mills, J. Szpyt, Z. X. He, G. Y. Du, R. Garrity, A. Reddy, L. P. Vaites, J. A. Paulo, T. H. Zhang, N. S. Gray, S. P. Gygi and E. T. Chouchani, A Quantitative Tissue-Specific Landscape of Protein Redox Regulation During Aging, Cell, 2020, 180, 968–983 CrossRef CAS PubMed
.
- S. Emmert, G. Quargnali, S. Thallmair and P. Rivera-Fuentes, A Locally Activatable Sensor for Robust Quantification of Organellar Glutathione, Nat. Chem., 2023, 15, 1415–1421 CrossRef CAS PubMed
.
- D. Song, C. C. Li, M. T. Zhu, S. Y. Chi and Z. H. Liu, Tracking Hepatic Ischemia-Reperfusion Injury in Real Time with a Reversible NIR-IIb Fluorescent Redox Probe, Angew. Chem., Int. Ed., 2022, 61, e202212721 CrossRef CAS PubMed
.
- J. D. Zheng, Q. Zeng, R. J. Zhang, D. Xing and T. Zhang, Dynamic-Reversible Photoacoustic Probe for Continuous Ratiometric Sensing and Imaging of Redox Status in Vivo, J. Am. Chem. Soc., 2019, 141, 19226–19230 CrossRef CAS
.
- M. Y. Lucero and J. Chan, Photoacoustic Imaging of Elevated Glutathione in Models of Lung Cancer for Companion Diagnostic Applications, Nat. Chem., 2021, 13, 1248–1256 CrossRef CAS PubMed
.
- C. Zhang, L. Xu, B. Nan, C. Lu, H. Y. Liu, L. L. Lei, R. Y. Yue, G. Q. Guan, M. He, X. B. Zhang and G. S. Song, Dynamic-Reversible MRI Nanoprobe for Continuous Imaging Redox Homeostasis in Hepatic Ischemia-Reperfusion Injury, ACS Nano, 2023, 17, 9529–9542 CrossRef CAS PubMed
.
- Y. C. Liu, L. L. Teng, X. F. Lou, X. B. Zhang and G. S. Song, “Four-In-One” Design of a Hemicyanine-Based Modular Scaffold for High-Contrast Activatable Molecular Afterglow Imaging, J. Am. Chem. Soc., 2023, 145, 5134–5144 CrossRef CAS
.
- G. Q. Guan, C. Zhang, H. Y. Liu, Y. J. Wang, Z. Dong, C. Lu, B. Nan, R. Y. Yue, X. Yin, X. B. Zhang and G. S. Song, Ternary Alloy PtWMn As a Mn Nanoreservoir for High-Field MRI Monitoring and Highly Selective Ferroptosis Therapy, Angew. Chem., Int. Ed., 2022, 61, e202117229 CrossRef CAS
.
- C. K. Fu, S. Herbst, C. Zhang and A. K. Whittaker, Polymeric 19F MRI Agents Responsive to Reactive Oxygen Species, Polym. Chem., 2017, 8, 4585–4595 RSC
.
- J. S. Enriquez, M. Yu, B. S. Bouley, D. Xie and E. L. Que, Copper (II) Complexes for Cysteine Detection Using 19F Magnetic Resonance, Dalton Trans., 2018, 47, 15024–15030 RSC
.
- Y. Y. Zhang, Q. Ma, Y. H. Yan, C. Guo, S. Y. Xu and L. Y. Wang, Intratumoral Glutathione Activatable Nanoprobes for Fluorescence and 19F Magnetic Resonance Turn-On Imaging, Anal. Chem., 2020, 92, 15679–15684 CrossRef CAS PubMed
.
- G. Angelovski, B. J. Tickner and G. J. Wang, Opportunities and Challenges with Hyperpolarized Bioresponsive Probes for Functional Imaging Using Magnetic Resonance, Nat. Chem., 2023, 15, 755–763 CrossRef CAS PubMed
.
- J. Cui, R. Jiang, C. Guo, X. Bai, S. Y. Xu and L. Y. Wang, Fluorine Grafted Cu7S4–Au Heterodimers for Multimodal Imaging Guided Photothermal Therapy with High Penetration Depth, J. Am. Chem. Soc., 2018, 140, 5890–5894 CrossRef CAS PubMed
.
- H. Zhu, X. J. Yin, Y. Zhou, S. Y. Xu, T. D. James and L. Y. Wang, Nanoplatforms with Synergistic Redox Cycles and Rich Defects for Activatable Image-Guided Tumor-Specific Therapy, Chem, 2022, 8, 2498–2513 CAS
.
- F. Liu, C. Guo, X. Li, Y. Li, S. Y. Xu, T. D. James and L. Y. Wang, A Versatile Nano-Transformer for Efficient Localization-Specific Imaging and Synergistic Therapy of Bladder Cancer, Nano Today, 2024, 54, 102116 CrossRef CAS
.
- Q. Y. Wang, Y. Yu, Y. X. Chang, X. Xu, M. Wu, G. R. Ediriweera, H. Peng, X. Zhen, X. Q. Jiang, D. J. Searles, C. K. Fu and A. K. Whittaker, Fluoropolymer-MOF Hybrids with Switchable Hydrophilicity for 19F MRI-Monitored Cancer Therapy, ACS Nano, 2023, 17, 8483–8498 CrossRef CAS
.
- C. Guo, X. Xiong, X. Zhao, Y. Li, S. Li, S. Xu, T. D. James and L. Wang, Superhydrophilic Fluorinated Polymer Probe for Zero-Background 19F MRI with Adaptable Targeting Ability, ACS Appl. Mater. Interfaces, 2024, 16(47), 65319–65327 CrossRef CAS PubMed
.
- F. Liu, X. Li, Y. Li, S. Xu, C. Guo and L. Wang, Visualization of drug release in a chemo-immunotherapy nanoplatform via ratiometric 19F magnetic resonance imaging, Chem. Sci., 2024, 15, 17397–17406 RSC
.
- C. K. Fu, J. Tang, A. D. Pye, T. Q. Liu, C. Zhang, X. Tan, F. Han, H. Peng and A. K. Whittaker, Fluorinated Glycopolymers as Reduction-Responsive 19F MRI Agents for Targeted Imaging of Cancer, Biomacromolecules, 2019, 20, 2043–2050 CrossRef CAS PubMed
.
- T. Nakamura, H. Matsushita, F. Sugihara, Y. Yoshioka, S. Mizukami and K. Kikuchi, Activatable 19F MRI Nanoparticle Probes for the Detection of Reducing Environments, Angew. Chem., Int. Ed., 2015, 54, 1007–1010 CrossRef CAS PubMed
.
- M. Zheng, Y. Wang, H. Shi, Y. Hu, L. Feng, Z. Luo, M. Zhou, J. He, Z. Zhou, Y. Zhang and D. Ye, Redox-Mediated Disassembly to Build Activatable Trimodal Probe for Molecular Imaging of Biothiols, ACS Nano, 2016, 10, 10075–10085 CrossRef CAS
.
- R. T. Kadakia, R. T. Ryan, D. J. Cooke and E. L. Que, An Fe Complex for 19F Magnetic Resonance-Based Reversible Redox Sensing and Multicolor Imaging, Chem. Sci., 2023, 14, 5099–5105 RSC
.
- M. Yu, B. S. Bouley, D. Xie, J. S. Enriquez and E. L. Que,
19F PARASHIFT Probes for Magnetic Resonance Detection of H2O2 and Peroxidase Activity, J. Am. Chem. Soc., 2018, 140, 10546–10552 CrossRef CAS
.
- D. Xie, T. L. King, A. Banerjee, V. Kohli and E. L. Que, Exploiting Copper Redox for 19F Magnetic Resonance-Based Detection of Cellular Hypoxia, J. Am. Chem. Soc., 2016, 138, 2937–2940 CrossRef CAS
.
- R. T. Kadakia, D. Xie, D. Martinez, M. Yu and E. L. Que, A Dual-Responsive Probe for Detecting Cellular Hypoxia Using 19F Magnetic Resonance and Fluorescence, Chem. Commun., 2019, 55, 8860–8863 RSC
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc08297d |
| This journal is © The Royal Society of Chemistry 2025 |