Open Access Article
Open Access ArticleSilver-mediated formal [4π + 2σ] cycloaddition reactions of bicyclobutanes with nitrile imines: access to 2,3-diazobicyclo[3.1.1]heptenes†
Huijuan
Liao
,
Jianyang
Dong
*,
Xuechen
Zhou
,
Qin
Jiang
,
Zishan
Lv
,
Fang
Lei
and
Dong
Xue
 *
*
Key Laboratory of Applied Surface and Colloid Chemistry, Ministry of Education and School of Chemistry and Chemical Engineering, Shaanxi Normal University, Xi'an, 710062, China. E-mail: jydong@snnu.edu.cn; xuedong_welcome@snnu.edu.cn
First published on 17th February 2025
Abstract
Despite recent advances in the synthesis of aza-bicyclo[3.1.1]heptanes (aza-BCHeps, which have an sp3-hybridized nitrogen atom) and azabicyclo[3.1.1]heptenes (aza-BCHepes, which have an sp2-hybridized nitrogen atom), which are bioisosteres of pyridine, construction of 2,3-diazobicyclo[3.1.1]heptenes (2,3-diazo-BCHepes), which have both sp2- and sp3-hybridized nitrogen atoms, has yet to be achieved. Herein, we disclose a method for silver-enabled formal [4π + 2σ] cycloaddition reactions between bicyclobutanes and nitrile imines (generated from hydrazonyl chlorides) to furnish a diverse array of 2,3-diazo-BCHepes, which feature both sp2- and sp3-hybridized nitrogen atoms embedded in a BCHepe framework. These compounds have the potential to serve as bioisosteres of both pyridines and pyridazines. Owing to the presence of the sp3-hybridized nitrogen, 2,3-diazo-BCHepes can be expected to exhibit geometries similar to those of aza-BCHepes and much better solubility. We demonstrated the synthetic utility of our method by carrying out a scaled-up reaction and diverse postcatalytic transformations.
Introduction
As of 2021, 88% of the drugs approved by the US Food and Drug Administration contained nitrogen heterocycles. In particular, N-heteroarenes such as pyridines and pyridazines are ubiquitous in pharmaceuticals and serve as fundamental building blocks in organic synthesis.1 However, because the presence of planar moieties such as pyridine rings often results in undesirable pharmaceutical properties, medicinal chemists have been prompted to seek bioisosteres.2 In recent years, three-dimensional saturated bridged bicyclic scaffolds such as bicyclo[1.1.1]pentanes,3 bicyclo[2.1.1]hexanes,4 and bicyclo[3.1.1]heptanes (BCHeps)5 have been developed as arene surrogates because these scaffolds can mimic the structural properties of arene rings and because molecules with these scaffolds tend to have better physicochemical properties and pharmacokinetics than the parent drug molecules. Moreover, the Mykhailiuk group reported that 3-aza-BCHeps (Scheme 1A, left), which have an sp3-hybridized nitrogen atom, are promising saturated bioisosteres of pyridines6 and exhibit drug-like solubility, lipophilicity, and metabolic profiles.To meet the increasing demand for 3-aza-BCHeps, the groups of Glorius,7 Li,8 Deng,9 Zhou10 and Feng11 have recently established methods for synthesizing these compounds by means of Lewis acid-catalyzed cycloaddition reactions between bicyclo[1.1.0]butanes (BCBs) and various 1,3-dipoles (Scheme 1B). Because the sp2-hybridized nitrogen atom of pyridines governs their basicity and hydrogen-bonding ability,12 incorporation of an sp2-hybridized imine nitrogen atom into the BCHep core can be expected to result in molecules that are more likely to be good mimics of pyridines; both types of compounds have unconjugated lone pair electrons on the nitrogen atom and π electrons in the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N double bond. In this regard, the Zheng, Li and Wang groups separately reported syntheses of azabicyclo[3.1.1]heptenes (aza-BCHepes), which are regarded as perfect pyridine bioisosteres in terms of 3D conformation and basicity (Scheme 1A, right), from readily accessible vinyl azides and BCBs (Scheme 1B).13
N double bond. In this regard, the Zheng, Li and Wang groups separately reported syntheses of azabicyclo[3.1.1]heptenes (aza-BCHepes), which are regarded as perfect pyridine bioisosteres in terms of 3D conformation and basicity (Scheme 1A, right), from readily accessible vinyl azides and BCBs (Scheme 1B).13
Because of the ubiquity of pyridine motifs in drug molecules, exploring novel aza variants of BCHeps is an appealing strategy for drug discovery.14 Mykhailiuk and colleagues showed that three-dimensional heteroatom-containing bioisosteres of arenes have better water solubility, higher metabolic stability, and lower lipophilicity than their arene and all-carbon bicyclic counterparts.15 With the goal of designing a framework with a structure similar to that of aza-BCHepes but with much better solubility, we decided to try incorporating an additional nitrogen atom. We expected that by replacing the methylene group of aza-BCHepes with a nitrogen atom to afford 2,3-diazobicyclo[3.1.1]heptenes (2,3-diazo-BCHepes), we could obtain compounds with geometries similar to those of aza-BCHepes and much better solubility (Scheme 1B). Moreover, 2,3-diazo-BCHepes, which have both sp2- and sp3-hybridized nitrogen atoms, might serve as saturated bioisosteres of pyridazines (1,2-diazines), which have planar structures and have been found to have a wide range of pharmacological activities.16 However, the construction of BCHepe frameworks with both sp2- and sp3-hybridized nitrogen atoms is extremely challenging (Scheme 1B).17
We reasoned that a [4π + 2σ] cycloaddition strategy might offer an appealing, straightforward route to 2,3-diazo-BCHepes. For this purpose, we evaluated nitrile imines, which are generally prepared by treatment of hydrazonyl halides with stoichiometric amounts of base and are versatile CNN 1,3-dipolar building blocks for the synthesis of nitrogen-containing heterocycles.18 However, to date, research on nitrile imines has focused mainly on the construction of six-membered nitrogen heterocycles. Cycloaddition reactions of these compounds to form bridged bicyclic nitrogen heterocycles have not yet been reported.
Herein, we report the first method for silver-catalyzed formal [4π + 2σ] cycloaddition reactions of BCBs with hydrazonyl chloride-derived nitrile imines, providing a platform for the synthesis of previously inaccessible 2,3-diazo-BCHepes, which feature both sp2- and sp3-hybridized nitrogen atoms embedded in a BCHepe framework (Scheme 1C).
Results and discussion
To evaluate the feasibility of the cycloaddition reaction and to optimize the conditions, we used phenyl(3-phenylbicyclo[1.1.0]butan-1-yl)methanone (1a) and hydrazonyl chloride 2a as model substrates (Table 1). First, we screened reactions involving various metal Lewis acids (20 mol%) with K3PO4 as the base in dichloromethane containing 3 Å molecular sieves at room temperature under argon for 16 h (entries 1–4). To our delight, desired product 2,3-diazo-BCHepe 3a was obtained in 15% yield (as indicated by 1H NMR spectroscopy) when AgBF4 was the Lewis acid; the other tested acids gave lower yields. Subsequent variation of the AgBF4 loading revealed that 100 mol% AgBF4 gave a slightly better yield (entries 5 and 6). We also found that replacing K3PO4 with other bases gave lower yields (entries 7–9). Subsequent screening of various solvents for AgBF4-catalyzed reactions revealed that dichloromethane was superior (entries 10–12). Notably, decreasing the reaction temperature to −10 °C increased the yield to 40% (entry 13). Performing the reaction with 3 equiv of 1a at −10 °C gave 3a in 65% yield (entry 14); and, notably, decreasing the reaction time to 1 h gave 3a in 64% yield (entry 15). Control experiments showed that the reaction did not occur in the absence of a Lewis acid (entry 16) and that the yield of 3a was 21% in the absence of a base (entry 17). Moreover, when the 3 Å molecular sieves were omitted, 3a was obtained in only 23% yield (entry 18). Ultimately, the optimal conditions were determined to be as follows: 1a (3.0 equiv), 2a (1.0 equiv), and AgBF4 (100 mol%) in dichloromethane at −10 °C under argon for 1 h (entry 15). The structure of 3a was confirmed by X-ray diffraction analysis of a single crystal (Scheme 2).| Entry | Lewis acid (mol%) | Base | Solvent | Yield (%) |
|---|---|---|---|---|
a Reaction conditions: 1a (0.1 mmol), 2a (0.1 mmol), Lewis acid (20–100 mol%), base (0.15 mmol), solvent (1 mL), 3 Å molecular sieves (50 mg), Ar atmosphere, room temperature (r.t.), 16 h. Yields were determined by 1H NMR spectroscopy with 1,3,5-trimethoxybenzene as an internal standard. DCM, dichloromethane; NR, no reaction.
b Reaction temperature, −10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C.
c 3 equiv of 1a.
d Reaction time, 1 h.
e Without 3 Å molecular sieves. °C.
c 3 equiv of 1a.
d Reaction time, 1 h.
e Without 3 Å molecular sieves.
|
||||
| 1 | Sc(OTf)3 (20) | K3PO4 | DCM | Trace |
| 2 | AgOTf (20) | K3PO4 | DCM | 12 |
| 3 | Eu(OTf)3 (20) | K3PO4 | DCM | NR |
| 4 | AgBF4 (20) | K3PO4 | DCM | 15 |
| 5 | AgBF4 (50) | K3PO4 | DCM | 20 |
| 6 | AgBF4 (100) | K3PO4 | DCM | 34 |
| 7 | AgBF4 (100) | Na2CO3 | DCM | 9 |
| 8 | AgBF4 (100) | K2CO3 | DCM | 7 |
| 9 | AgBF4 (100) | Et3N | DCM | NR |
| 10 | AgBF4 (100) | K3PO4 | CH3CN | NR |
| 11 | AgBF4 (100) | K3PO4 | THF | NR |
| 12 | AgBF4 (100) | K3PO4 | DCE | 33 |
| 13b | AgBF4 (100) | K3PO4 | DCM | 40 |
| 14b,c | AgBF4 (100) | K3PO4 | DCM | 65 |
| 15b,c,d | AgBF4 (100) | K3PO4 | DCM | 64 |
| 16b,c,d | — | K3PO4 | DCM | NR |
| 17b,c,d | AgBF4 (100) | — | DCM | 21 |
| 18b,c,d,e | AgBF4 (100) | K3PO4 | DCM | 23 |
 | ||
| Scheme 2 Exploration of substrate scope. Reaction conditions: 1 (0.3 mmol), 2 (0.1 mmol), K3PO4 (0.15 mmol), AgBF4 (0.1 mmol, 100 mol%), 3 Å molecular sieves (50 mg), DCM (1 mL), −10 °C, Ar, 1 h. For details, see the ESI†. | ||
Using the optimized conditions, we evaluated the substrate scope with respect to the BCB (Scheme 2, top panel). Aryl BCB ketones bearing an electron-neutral, electron-donating, or electron-withdrawing substituent on the aryl ring at the bridgehead position (aryl ketone bridgehead substituent) were reactive, delivering the desired products (3a–3i) in 26–53% yields. Specifically, phenyl BCB ketones bearing a para methoxy group, fluorine or chlorine atom, or trifluoromethyl group delivered corresponding products 3b–3e in 29–53% yields. In addition, phenyl BCB ketones with a meta methoxy group or chlorine atom were also suitable substrates, affording 3f and 3g, respectively, in 35% and 26% yields. Notably, an ortho-methyl-substituted substrate afforded desired product 3h in 36% yield. Moreover, a multisubstituted phenyl BCB ketone gave 2,3-diazo-BCHepe 3i in 53% yield. In addition to phenyl BCB ketones, other aryl BCB ketones were compatible with the reaction conditions: 2,3-diazo-BCHepes with a naphthalene (3j), furan (3k), thiophene (3l and 3m), or pyridine (3n) moiety were obtained in 16–59% yields. However, a BCB ketone bearing an acyl pyrazole group failed to produce the desired product (see unsuccessful examples, 3ad), probably because the acyl pyrazole was less reactive toward cycloaddition than the ketones. Finally, substituted aryl rings at the other bridgehead position of the BCB ketone were also well tolerated. Specifically, we obtained moderate yields of 2,3-diazo-BCHepes with a para fluorine or chlorine atom on the aryl ring (3o and 3p). Notably, an ortho-methyl-substituted phenyl BCB ketone also underwent the reaction, affording 3q in 54% yield. However, meta-fluorine and ortho-methoxy-substituted phenyl BCB ketones failed to produce the desired products (see unsuccessful examples, 3ae and 3af).
Subsequently, we evaluated the substrate scope with respect to the hydrazonyl chloride by carrying out reactions with 1a (Scheme 2, bottom panel). Initially, the aryl residue bound to the carbon atom of the hydrazonyl chloride was varied. Hydrazonyl chlorides with an electron-neutral, electron-donating, or electron-withdrawing substituent at the para position of the aryl ring were reactive, delivering the desired products (3r–3u) in 30–61% yields. However, para-nitro-substituted hydrazonyl chloride failed to produce the desired products (see unsuccessful examples, 3ag). Notably, an ortho-fluorophenyl-substituted hydrazonyl chloride afforded desired product 3v in 41% yield. Moreover, a multisubstituted hydrazonyl chloride gave 2,3-diazo-BCHepe 3w in 41% yield. In addition to phenyl hydrazonyl chlorides, other aryl hydrazonyl chlorides were compatible with the reaction conditions: specifically, 2,3-diazo-BCHepes with a naphthalene (3x and 3y) or thiophene (3z) moiety were obtained in 42–54% yields. Finally, substituted aryl rings on the nitrogen atom of the hydrazonyl chloride were amenable to the reaction: we were able to obtain products bearing an electron-donating group methyl group (3aa) or methoxy group (3aa and 3ab) or an electron-withdrawing nitro group (3ac).
According to the Mykhailiuk group's strategy for designing benzene bioisosteres, the similarity between the geometric properties of bioisosteres and the substituted benzene moieties that they are modeled after is of great significance.19 To further explore the potential utility of 2,3-diazo-BCHepes as bioisosteres, we compared their geometric properties with those of structurally related pyridines (Table 2). We compared the d, r and φ exit vectors obtained from X-ray data for 3a with the corresponding vectors for tetrasubstituted pyridine 4 calculated by means of density functional theory. The vectors for 3a were indeed very close to those for 4, indicating that the 2,3-diazo-BCHepe mimicked the pyridine ring well.
| Parameters | X-ray of 3a Xa,b distance a, b, c, d: position of carbon | Values calculated for tetra-substituted pyridine |
|---|---|---|
| d 1 | 4.741.7 Å | 4.951.7 Å |
| r 1 | 2.122.6 Å, 2.293.5 Å | 2.442.6 Å, 2.343.5 Å |
| d 2 | 3.061.4 Å | 3.061.4 Å |
| r 2 | 1.522.3 Å, 1.505.6 Å | 1.412.3 Å, 1.415.6 Å |
| d 3 | 5.634.7 Å | 5.754.7 Å |
| r 3 | 2.653.6 Å, 2.692.5 Å | 2.783.6 Å, 2.762.5 Å |
| φ (8,5,3,4) | 114.0° | 109.0° |
| φ (7,6,2,1) | 121.1° | 115.1° |
To demonstrate the utility of our cycloaddition method, we performed a 5 mmol-scale reaction to generate 2,3-diazo-BCHepe 3j and observed only a slight decrease in the yield (Scheme 3A). Moreover, we carried out several transformations of 3j (Scheme 3B). Specifically, selective reduction of the ketone of 3j afforded alcohol 5 in 59% yield. Additionally, reaction of 3j with allylMgBr or n-butyl lithium produced tertiary alcohol 6 or 7 in 83% and 53% yields, respectively. As shown in Scheme 3C, oxidation of 3ab and subsequent hydrolysis generated alcohol 8 in 56% yield.
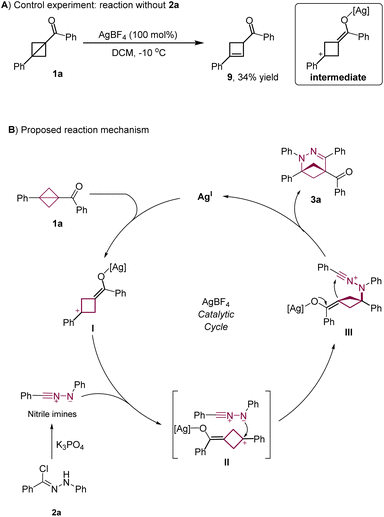
Having explored the reaction's substrate scope and the utility of the products for various transformations, we conducted mechanistic studies to evaluate the validity of the proposed reaction pathway shown in Scheme 1D. When 1a was subjected to the optimized conditions in the absence of 2a, cyclobutene 9 was obtained in 34% yield (Scheme 4A), indicating that direct activation of 1a by AgBF4 to form a cationic intermediate was feasible. On the basis of this result and literature reports,7–11 we propose that the reaction proceeds via the mechanism shown in Scheme 4B. Initially, deprotonation of hydrazonyl chloride 2a and concomitant loss of HCl afford a 1,3-dipolar nitrile imine. Compound 1a is activated by coordination between AgBF4 and the carbonyl group to give cationic intermediate I, which is trapped by the nitrile imine to form δ-carbanionic intermediate IIIvia intermediate II. Subsequent transannular cyclization of III delivers 2,3-diazo-BCHepe 3a and regenerates the AgBF4 catalyst.
Conclusions
In summary, we have established a method for AgBF4-enabled formal [4π + 2σ] cycloaddition reactions between BCBs and nitrile imines derived from hydrazonyl chlorides. This method serves as a versatile platform for de novo construction of highly sought after 2,3-diazo-BCHepes, which have both sp2- and sp3-hybridized nitrogen atoms embedded in a BCHepe framework. This method was used for cycloadditions of a wide range of disubstituted BCBs with hydrazonyl chlorides as nitrile imine precursors, affording access to 2,3-diazo-BCHepes for the first time. The synthetic value of this method was demonstrated through a gram-scale reaction and synthetic transformations of two of the products. 2,3-Diazo-BCHepes have the potential to serve as bioisosteres of both pyridazines and pyridines. With their sp3-hybridized nitrogen atoms, 2,3-diazo-BCHepes can be expected to be more water soluble, more metabolically stable, and less lipophilic than 3-aza-BCHepes. We anticipate that the method reported herein will find applications in the discovery of active pharmaceutical ingredients with nitrogen-containing bicyclic scaffolds.Data availability
The data supporting this article have been included as part of the ESI.† Crystallographic data for 3a (CCDC 2394900) have been deposited at the Cambridge Crystallographic Data Centre.Author contributions
J. Y. D. and D. X. conceived and directed the project. H. J. L. discovered and developed the reaction. H. J. L., X. C. Z., Q. J., Z. S. L. and F. L. performed the experiments and collected the data. All authors discussed and analyzed the data. J. Y. D. and D. X. wrote the manuscript with contribution from other authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was supported by the National Natural Science Foundation of China (22171174 and 22401177), the Fundamental Research Funds for the Central Universities (GK202401008), the Innovation Capability Support Program of Shaanxi (2023-CX-TD-28), the Fundamental Science Research Project of Shaanxi for Chemistry, Biology (22JHZ002), the Young Talent Fund of Association for Science and Technology in Shaanxi, China (20240606), and the Key Research and Development Program of Shaanxi (2024SF2-GJHX32).Notes and references
- P. Bhutani, G. Joshi, N. Raja, N. Bachhav, P. K. Rajanna, H. Bhutani, A. T. Paul and R. Kumar, J. Med. Chem., 2021, 64, 2339–2381 CrossRef CAS PubMed.
- M. A. M. Subbaiah and N. A. Meanwell, J. Med. Chem., 2021, 64, 14046–14128 CrossRef CAS PubMed.
- (a) R. Gianatassio, J. M. Lopchuk, J. Wang, C. M. Pan, L. R. Malins, L. Prieto, T. A. Brandt, M. R. Collins, G. M. Gallego, N. W. Sach, J. E. Spangler, H. C. Zhu and J. J. Zhu, Science, 2016, 351, 241–246 CrossRef CAS PubMed; (b) J. Kanazawa, K. Maeda and M. Uchiyama, J. Am. Chem. Soc., 2017, 139, 17791–17794 CrossRef CAS PubMed; (c) X. H. Zhang, R. T. Smith, C. Le, S. J. McCarver, B. T. Shireman, N. I. Carruthers and D. W. C. MacMillan, Nature, 2020, 580, 220–226 CrossRef CAS PubMed; (d) S. J. Yu, C. C. Jing, A. Noble and V. K. Aggarwal, Angew. Chem., Int. Ed., 2020, 59, 3917–3921 CrossRef CAS PubMed; (e) Y. Y. Yang, J. Tsien, J. M. E. Hughes, B. K. Peters, R. R. Merchant and T. Qin, Nat. Chem., 2021, 13, 950–955 CrossRef CAS PubMed; (f) H. D. Pickford, J. Nugent, B. Owen, J. J. Mousseau and R. C. Smith, J. Am. Chem. Soc., 2021, 143, 9729–9736 CrossRef CAS PubMed; (g) S. Shin, S. Lee, W. Choi, N. Kim and S. Hong, Angew. Chem., Int. Ed., 2021, 60, 7873–7879 CrossRef CAS PubMed; (h) W. Z. Dong, E. Yen-Pon, L. B. Li, A. Bhattacharjee, A. Jolit and G. A. Molander, Nat. Chem., 2022, 14, 1068–1077 CrossRef CAS PubMed; (i) W. C. Huang, S. Keess and G. A. Molander, J. Am. Chem. Soc., 2022, 144, 12961–12969 CrossRef CAS PubMed; (j) S. Livesley, A. J. Sterling, C. M. Robertson, W. R. F. Goundry, J. A. Morris, F. Duarte and C. Aissa, Angew. Chem., Int. Ed., 2022, 61, e202111291 CrossRef CAS PubMed; (k) R. Bychek and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2022, 61, e202205103 CrossRef CAS PubMed; (l) I. F. Yu, J. L. Manske, A. Diéguez-Vázquez, A. Misale, A. E. Pashenko, P. K. Mykhailiuk, S. V. Ryabukhin, D. M. Volochnyuk and J. F. Hartwig, Nat. Chem., 2023, 15, 685–693 CrossRef CAS PubMed.
- (a) A. Denisenko, P. Garbuz, S. V. Shishkina, N. M. Voloshchuk and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2020, 59, 20515–20521 CrossRef CAS PubMed; (b) R. Kleinmans, T. Pinkert, S. Dutta, T. O. Paulisch, H. Keum, G. Daniliuc and F. Glorius, Nature, 2022, 605, 477–482 CrossRef CAS PubMed; (c) R. Y. Guo, Y. C. Chang, L. Herter, C. Salome, S. E. Braley, T. C. Fessard and M. K. Brown, J. Am. Chem. Soc., 2022, 144, 7988–7994 CrossRef CAS PubMed; (d) Y. J. Liang, R. Kleinmans, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2022, 144, 20207–20213 CrossRef CAS PubMed; (e) K. Dhake, K. J. Woelk, J. Becica, A. Un, S. E. Jenny and D. Leitch, Angew. Chem., Int. Ed., 2022, 61, e202204719 CrossRef CAS PubMed; (f) S. Agasti, F. Beltran, E. Pye, N. Kaltsoyannis, M. Crisenza and D. J. Procter, Nat. Chem., 2023, 15, 535–541 CrossRef CAS PubMed; (g) R. Kleinmans, S. Dutta, K. Ozols, H. L. Shao, F. Schäfer, R. E. Thielemann, H. T. Chan, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2023, 145, 12324–12332 CrossRef CAS PubMed; (h) Y. J. Liang, F. Paulus, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2023, 62, e202305043 CrossRef CAS PubMed; (i) D. S. Ni, S. Hu, X. Tan, Y. Yu, Z. H. Li and L. Deng, Angew. Chem., Int. Ed., 2023, 62, e202308606 CrossRef CAS PubMed; (j) N. Radhoff, C. G. Daniliuc and A. Studer, Angew. Chem., Int. Ed., 2023, 62, e202304771 CrossRef CAS PubMed; (k) L. Tang, Y. J. Xiao, F. Wu, J. L. Zhou, T. T. Xu and J. J. Feng, Angew. Chem., Int. Ed., 2023, 27, e202310066 Search PubMed; (l) Y. Liu, S. Lin, Y. Li, J. H. Xue, Q. J. Li and H. G. Wang, ACS Catal., 2023, 13, 5096–5103 CrossRef CAS; (m) Q. Q. Fu, S. S. Cao, J. H. Wang, X. X. Lv, H. Wang, X. W. Zhao and Z. Y. Jiang, J. Am. Chem. Soc., 2024, 146, 8372–8380 CrossRef CAS PubMed; (n) S. Dutta, D. Lee, K. Ozols, C. G. Daniliuc, R. Shintani and F. Glorius, J. Am. Chem. Soc., 2024, 146, 2789–2797 CrossRef CAS PubMed; (o) J. L. Tyler, F. Schäfer, H. L. Shao, C. Stein, A. Wong, C. G. Daniliuc, K. N. Houk and F. Glorius, J. Am. Chem. Soc., 2024, 146, 16237–16247 CrossRef CAS PubMed; (p) Q. Q. Hu, L. Y. Wang, X. H. Chen, Z. X. Geng, J. Chen and L. Zhou, Angew. Chem., Int. Ed., 2024, e202405781 CAS; (q) J. J. Wang, L. Tang, Y. J. Xiao, W. B. Wu, G. Q. Wang and J. J. Feng, Angew. Chem., Int. Ed., 2024, 63, e202405222 CrossRef CAS PubMed; (r) Y. H. Liu, Z. X. Wu, J. R. Shan, H. P. Yan, E. J. Hao and L. Shi, Nat. Commun., 2024, 15, 4374–4382 CrossRef CAS PubMed.
- J. Y. Dong, H. J. Liao and D. Xue, Synthesis, 2024, 56, A–J CrossRef.
- D. Dibchak, M. Snisarenko, A. Mishuk, O. Shablykin, L. Bortnichuk, O. Klymenko-Ulianov, Y. Kheylik, I. V. Sadkova, H. S. Rzepa and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2023, 62, e202304246 CrossRef CAS PubMed.
- (a) Y. J. Liang, R. Nematswerani, C. G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2024, 63, e202402730 CrossRef CAS PubMed; (b) P. Bellotti and F. Glorius, J. Am. Chem. Soc., 2023, 145, 20716–20732 CrossRef CAS PubMed.
- X. Wang, R. Gao and X. Li, J. Am. Chem. Soc., 2024, 146, 21069–21077 CrossRef CAS PubMed.
- J. Zhang, J. Y. Su, H. L. Zheng, H. Li and W. P. Deng, Angew. Chem., Int. Ed., 2024, e202318476 CAS.
- X. G. Zhang, Z. Y. Zhou, J. X. Li, J. J. Chen and Q. L. Zhou, J. Am. Chem. Soc., 2024, 146, 27274–27281 CrossRef CAS PubMed.
- W. Wu, B. Xu, X. Yang, F. Wu, H. He, X. Zhang and J. Feng, Nat. Commun., 2024, 15, 8005 CrossRef CAS PubMed.
- K. K. Rajbongshi, B. Dam and B. K. Patel, Pyridines dihydropyridines and piperidines: an outline on synthesis and biological activities, in N-Heterocycles: Synthesis and Biological Evaluation, ed. K. L. Ameta, R. Kant, A. Penoni, A. Maspero and L. Scapinello, Springer Nature Singapore, 2022, pp. 1–49 Search PubMed.
- (a) Z. R. Lin, H. S. Ren, X. B. Lin, X. H. Yu and J. Zheng, J. Am. Chem. Soc., 2024, 146, 18565–18575 CrossRef CAS PubMed; (b) Y. Liu, S. Lin, Z. Ding, Y. Li, Y. Tang, J. Xue, Q. Li, P. Li and H. Wang, Chem, 2024, 10, 3699–3708 CrossRef CAS.
- (a) M. Baumann and I. R. Baxendale, Beilstein J. Org. Chem., 2013, 9, 2265–2319 CrossRef PubMed; (b) E. Vitaku, D. T. Smith and J. T. Njardarson, J. Med. Chem., 2014, 57, 10257–10274 CrossRef CAS PubMed; (c) S. De, A. Kumar S K, S. K. Shah, S. Kazi, N. Sarkar, S. Banerjee and S. Dey, RSC Adv., 2022, 12, 15385–15406 RSC.
- (a) V. V. Levterov, Y. Panasyuk, V. O. Pivnytska and P. K. Mykhailiuk, Angew. Chem., Int. Ed., 2020, 59, 7161–7167 CrossRef CAS PubMed; (b) A. Denisenko, P. Garbuz, N. M. Voloshchuk, Y. Holota, G. Al-Maali, P. Borysko and P. K. Mykhailiuk, Nat. Chem., 2023, 15, 1155–1163 CrossRef CAS PubMed; (c) V. V. Levterov, Y. Panasiuk, K. Sahun, O. Stashkevych, V. Badlo, O. Shablykin, I. Sadkova, L. Bortnichuk, O. Klymenko-Ulianov, Y. Holota, L. Lachmann, P. Borysko, K. Horbatok, I. Bodenchuk, Y. Bas, D. Dudenko and P. K. Mykhailiuk, Nat. Commun., 2023, 14, 5608–5607 CrossRef CAS PubMed.
- (a) C. G. Wermuth, Med. Chem. Commun., 2011, 2, 935–941 Search PubMed; (b) A. M. Subbaiah and N. A. Meanwell, J. Med. Chem., 2021, 64, 14046–14128 Search PubMed; (c) N. A. Meanwell and A. Heterocycl, Chem, 2017, 123, 245–361 Search PubMed.
- J. Zhang, J. Su, H. Zheng, H. Li and W. Deng, ACS Catal., 2024, 14, 17837–17849 CrossRef CAS.
- For selected examples of cycloaddition reactions of nitrile imines, see: (a) L. K. Garve, M. Petzold, P. G. Jones and D. B. Werz, Org. Lett., 2016, 18, 564–567 CrossRef CAS PubMed; (b) S. M. Gomha, T. A. Farghaly, A. R. Sayed, M. M. Abdalla and J. Heterocycl, Chem, 2016, 53, 1505–1511 CAS; (c) H. Gazzeh, S. Boudriga, M. Askri, A. Khatyr, M. Knorr, C. Strohmann, C. Golz, Y. Rousselin and M. M. Kubicki, RSC Adv., 2016, 6, 49868–49875 Search PubMed; (d) A. Alizadeh and L. Moafi, Chim. Acta., 2016, 99, 457–461 Search PubMed; (e) Y. Miura and N. Yoshioka, Chem. Phys. Lett., 2015, 626, 11–14 CrossRef CAS; (f) M. Yıldırım and Y. Dürüst, Tetrahedron, 2011, 67, 3209–3215 CrossRef; (g) A. L. Gerten, M. C. Slade, K. M. Pugh and L. M. Stanley, Org. Biomol. Chem., 2013, 11, 7834–7837 RSC; (h) C. X. Guo, W. Z. Zhang, N. Zhang and X. B. Lu, J. Org. Chem., 2017, 82, 7637–7642 CrossRef CAS PubMed; (i) M. P. Sibi, L. M. Stanley and C. P. Jasperse, J. Am. Chem. Soc., 2005, 127, 8276–8277 CrossRef CAS PubMed; (j) A. Singh, A. L. Loomer and G. P. Roth, Org. Lett., 2012, 14, 5266–5269 CrossRef CAS PubMed; (k) G. Wang, X. H. Liu, T. Y. Huang, Y. L. Kuang, L. L. Lin and X. M. Feng, Org. Lett., 2013, 15, 76–79 CrossRef CAS PubMed.
- P. K. Mykhailiuk, Org. Biomol. Chem., 2019, 17, 2839–2849 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: General information, experimental procedures and characterization data of the synthesized compounds. CCDC 2394900. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc08280j |
| This journal is © The Royal Society of Chemistry 2025 |