Open Access Article
Open Access ArticleEnhancing electroluminescence performance of ultra-deep-blue through-space charge transfer emitters with CIEy ≈ 0.05 via methyl-modification†
Quanwei
Li
a,
Haisong
Zhao
a,
Jinyang
Zhao
a,
Zhongxu
Cao
a,
Chao
Yu
a,
Shouke
Yan
 *ab and
Zhongjie
Ren
*ab and
Zhongjie
Ren
 *a
*a
aState Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China. E-mail: renzj@mail.buct.edu.cn
bDepartment Key Laboratory of Rubber-Plastics Ministry of Education, School of Polymer Science and Engineering, Qingdao University of Science & Technology, Qingdao 266042, China
First published on 10th March 2025
Abstract
Achieving efficient solution-processed ultra-deep-blue OLEDs remains a challenge. Herein, a methyl-modification strategy is proposed to overcome weak intramolecular charge transfer and the large energy gap between the singlet and triplet states (ΔEST) of ultra-deep-blue through-space charge transfer (TSCT)-thermally activated delayed fluorescence (TADF) emitters. In this way, the reverse intersystem crossing (RISC) process is found to be effective from T1 to S1 and can be accelerated with the assistance of T2. As a result, the ultra-deep-blue TSCT emitter 3MeCz-BO exhibits a minimized ΔEST of 0.02 eV, and an enhanced RISC rate of 3.71 × 105 s−1. Additionally, this modification can improve the solubility, enabling the fabrication of solution-processed organic light-emitting diodes (OLEDs). The maximum external quantum efficiency of the 3MeCz-BO-based solution-processed OLED reaches 10.1%, with a Commission Internationale de L'Eclairage (CIE) coordinates of (0.151, 0.051) and a luminance of 1334 cd m−2. This work is the first instance of developing high-performance ultra-deep-blue solution-processed TSCT-TADF OLEDs, which show comparable performance to vacuum-deposited OLEDs. Furthermore, the 3MeCz-BO-based OLED fits well within the standard Red Green Blue (sRGB) of CIE coordinates (0.15, 0.06), and is close to the CIE coordinates (0.131, 0.046) for the Rec. 2020 standard, implying its potential application in colorful display devices.
Introduction
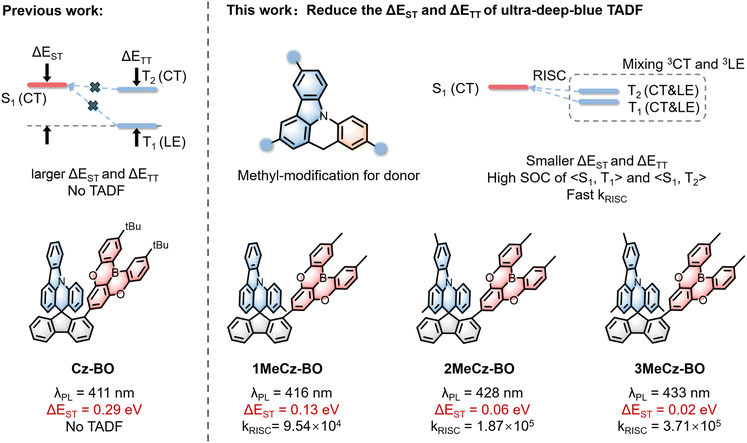
Thermally activated delayed fluorescence (TADF) emitters represent the third generation of light-emitting materials for organic light-emitting diodes (OLEDs), achieving nearly 100% exciton utilization without any precious heavy metals.1–3 Through-space charge transfer (TSCT) allows for the spatial separation of the highest occupied molecular orbital (HOMO) and the lowest unoccupied molecular orbital (LUMO), resulting in a small energy gap between singlet and triplet excited states (ΔEST).4–7 In 2020, π-stacked molecules with space-confined face-to-face donors and acceptors were proposed by Jiang et al. to fabricate high-efficiency TSCT-TADF OLEDs.8In recent years, high-performance TSCT-TADF materials have been widely developed and used in blue,8–17 green,18–27 and red28–31 OLEDs. Although TSCT is advantageous for reducing the ΔEST of TADF materials, achieving a small ΔEST for deep-blue TSCT-TADF materials with CIEy ≤ 0.1 still remains challenging, due to weak intramolecular charge transfer (ICT). Therefore, it is hard to develop high-performance ultra-deep-blue TSCT-TADF materials. Oxygen-bridged boron was first reported for OLED materials32 and has been widely utilized to fabricate deep-blue OLEDs.33–36 Based on this, our group previously developed deep-blue TSCT materials, QAc-BO and Cz-BO, but they possess high ΔEST values of 0.20 and 0.29 eV.13 The high ΔEST limits the performance of the OLEDs. Additionally, Zhao et al. developed violet TSCT materials with extremely low CIEy (<0.03).37 However, the significant ΔEST (≈0.3 eV) makes TADF characteristics nearly unnoticeable. For ultra-deep-blue TADF materials with low CIEy, controlling ΔEST becomes increasingly difficult. Due to weak ICT, these materials generally display a large bandgap, resulting in a high-energy singlet charge transfer state (1CT). The triplet charge transfer state (3CT) is generally positioned near 1CT, with both states residing at high energy levels. In contrast, the triplet local excited state (3LE) is primarily determined by the molecular segment with the lowest energy, which could be the donor, the acceptor, or the bridge connecting them, and this state is typically much lower in energy than 3CT. As a result, the ΔEST of these materials, mainly determined by 1CT and 3LE, tends to increase.38–40 This challenges the reverse intersystem crossing (RISC) process in ultra-deep-blue TSCT-TADF materials.
To address the issue of large ΔEST in ultra-deep-blue TSCT-TADF materials, this work innovatively modified methyl groups at three positions in the donor; referring to the previously developed fluorescent material Cz-BO, three new TSCT-TADF materials were prepared: 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO. The molecular design concept is shown in Fig. 1. Due to the electron-rich nature of methyl groups, they can enhance ICT and thus reduce the energy levels of 1CT and 3CT, while 3LE remains unaffected. This leads to a reduction in ΔEST. In addition, the energy gap between 3CT and 3LE (ΔE(3CT − 3LE)) was also reduced, leading to the mixing and hybridization of 3CT and 3LE. Therefore, both T1 and T2 have certain LE characteristics. This could enhance the spin–orbit coupling (SOC) values of T1–S1 and T2–S1, allowing RISC to occur from T1 and T2 and to be accelerated with the assistance of T2. In this methyl-modification design, the optimized 3MeCz-BO shows ultra-deep-blue emission at 432 nm, achieving a small ΔEST of 0.02 eV and an enhanced RISC rate of 3.17 × 105 s−1. Additionally, the methyl-modified TSCT-TADF materials show good solubility and are suitable for fabricating OLED devices by solution processing. As a result, the EQEmax of solution-processed 3MeCz-BO-based OLEDs is 10.1%, with a full width at half maximum (FWHM) of 50 nm, and a high luminance of 1334 cd m−2. Furthermore, the notable Commission Internationale de L'Eclairage (CIE) coordinates of 3MeCz-BO (0.151, 0.051) fit well within the standard Red Green Blue (sRGB) of CIE coordinates (0.15, 0.06), and are close to the CIE coordinates (0.131, 0.046) for the Rec. 2020 standard. This suggests its potential application in colorful display devices.
Results and discussion
Synthesis and characterization
The chemical structures of 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO are shown in Fig. 1. The synthetic routes are provided in the ESI (Scheme S1†). The donor units consist of the 4-methylphenyl group (or without methyl group) and dimethyl-carbazole (or carbazole) by nucleophilic substitution reactions. Unlike Cz-BO,13 the tert-butyl groups on the acceptors are replaced with methyl groups, to reduce the steric hindrance caused by tert-butyl groups and generate parallel D–A arrangements. The Suzuki–Miyaura cross-coupling reaction was performed to couple the acceptor and the bridge units to retain the intermediate compound 4. The final emitters are prepared through lithium–bromine exchange of the donor and exchange reaction with compound 4. The emitters show good solubility in organic solvents such as dichloromethane and chlorobenzene, owing to their external methyl groups. The chemical structures of all intermediates and final products were characterized by 1H NMR, 13C NMR, high-resolution mass spectrometry, and elemental analysis (Fig. S1–S19†).To evaluate the thermal stabilities of 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, their thermal decomposition temperatures (Tds) were measured by thermogravimetric analysis (TGA) under a nitrogen atmosphere (Fig. S20†). The Td values for 5% weight loss are 415 °C for 1MeCz-BO, 413 °C for 2MeCz-BO, and 412 °C for 3MeCz-BO. The excellent thermal stability arises from the rigid molecules, which is beneficial for the fabrication of OLEDs. Cyclic voltammetry (CV) measurements were conducted to determine the oxidation–reduction potentials of the materials (Fig. S21a†). The HOMO values of 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO are estimated to be −5.41, −5.29, and −5.23 eV, respectively. 1MeCz-BO shows the deepest HOMO level, while 3MeCz-BO shows the shallowest HOMO level, which results from the number of electronic-rich methyl groups. The LUMO values are measured as −2.85, −2.84, and −2.82 eV for 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, respectively. The similar structure of their acceptors indicates similar LUMO energy levels.
The calculated HOMO–LUMO energy gap (Eg) values are 2.56, 2.45, and 2.41 eV, respectively, suggesting an enhanced ICT and slight redshift emission with the increase in the methyl groups on the donor. Moreover, the pseudo-reversible redox processes are validated by CV measurements over multiple cycles (Fig. S29b–d†), suggesting good device stability when charges are injected repeatedly into the emitter.
Single crystals and theoretical calculations
To unequivocally investigate the molecular spatial structure of the compounds, single crystals of 1MeCz-BO (CCDC 2402467), 2MeCz-BO (CCDC 2402468), and 3MeCz-BO (CCDC 2402469) were obtained by slow evaporation from a mixture of dichloromethane and methanol (v/v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4) at room temperature. The spatial structures were obtained from X-ray diffraction analysis, as shown in Fig. 2a and b from the side and top view. Besides this, X-ray crystallographic data are summarized in Table S1,† the packing modes in the single crystals of the compounds are shown in Fig. S22,† and the ORTEP diagrams of the compounds are shown in Fig. S23–S25.† To distinguish between the donor and acceptor units in different compounds, we designate the donors as D1, D2, and D3, and the acceptors as A1, A2, and A3, in 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, respectively. Variations in distances and angles between the donor and acceptor units in the three compounds arise from the spatial effects of methyl groups. The measured D–A distances are as follows: D1 to A1 is 3.175 Å, D2 to A2 is 3.101 Å, and D3 to A3 is 3.192 Å. The dihedral angles of D1–A1, D2–A2, and D3–A3 are measured as 10.2°, 18.8°, and 14.7°, showing that they all have an aligned and parallel D–A spatial arrangement. This configuration can help enhance the radiative transitions of the compounds. The angle between D1 and the bridge is 84.4°, while those of D2–bridge and D3–bridge are 88.9° and 88.3°, respectively. Both D2 and D3 adopt nearly orthogonal conformations to the bridge, due to the locking effect of spiro-fluorene and the spatial hindrance of the methyl groups near the bridge. In contrast, D1, which has only one methyl group, forms a larger angle with the bridge, making it less conducive to the spatial alignment of D1 and A1. Due to the large steric hindrance imposed on the acceptors by the immobilized donor, the angles of A1, A2, and A3 relative to the bridge are measured to be 74.2°, 77.1°, and 73.6°, respectively. Nevertheless, they can still establish an effective face-to-face parallel arrangement between the donor and acceptor to ensure optimal π-orbital overlap. The observation of intramolecular noncovalent interactions is further corroborated by reduced density gradient (RDG) analysis.41 The RDG iso-surfaces are colored on a blue-green-red scale based on the sign(λ2)ρ, as shown in Fig. 2c. The iso-surfaces colored in green and brown between D and A units illustrate the π–π interactions and steric hindrance. For three compounds, large green and brown iso-surfaces are present between the donor and acceptor, suggesting significant π–π interactions that support the potential occurrence of the TSCT process. Furthermore, the steric hindrance between donor and acceptor units creates considerable rigidity, which restricts molecular vibrations and inhibits non-radiative transitions, leading to a narrow FWHM. In addition, in D2 and D3, the top methyl group introduces steric hindrance with the acceptors, as shown by the red dotted circle in Fig. 2c, which is not found between D1 and A1. This additional steric hindrance further dampen the vibration of the acceptor unit, which helps to further inhibit the process of non-radiative transition. According to the scatter diagrams of the RDG analysis (Fig. 2d), all three compounds show numerous and dense spikes in the green and brown regions, demonstrating the effective π–π interaction between the D and A units.
4) at room temperature. The spatial structures were obtained from X-ray diffraction analysis, as shown in Fig. 2a and b from the side and top view. Besides this, X-ray crystallographic data are summarized in Table S1,† the packing modes in the single crystals of the compounds are shown in Fig. S22,† and the ORTEP diagrams of the compounds are shown in Fig. S23–S25.† To distinguish between the donor and acceptor units in different compounds, we designate the donors as D1, D2, and D3, and the acceptors as A1, A2, and A3, in 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, respectively. Variations in distances and angles between the donor and acceptor units in the three compounds arise from the spatial effects of methyl groups. The measured D–A distances are as follows: D1 to A1 is 3.175 Å, D2 to A2 is 3.101 Å, and D3 to A3 is 3.192 Å. The dihedral angles of D1–A1, D2–A2, and D3–A3 are measured as 10.2°, 18.8°, and 14.7°, showing that they all have an aligned and parallel D–A spatial arrangement. This configuration can help enhance the radiative transitions of the compounds. The angle between D1 and the bridge is 84.4°, while those of D2–bridge and D3–bridge are 88.9° and 88.3°, respectively. Both D2 and D3 adopt nearly orthogonal conformations to the bridge, due to the locking effect of spiro-fluorene and the spatial hindrance of the methyl groups near the bridge. In contrast, D1, which has only one methyl group, forms a larger angle with the bridge, making it less conducive to the spatial alignment of D1 and A1. Due to the large steric hindrance imposed on the acceptors by the immobilized donor, the angles of A1, A2, and A3 relative to the bridge are measured to be 74.2°, 77.1°, and 73.6°, respectively. Nevertheless, they can still establish an effective face-to-face parallel arrangement between the donor and acceptor to ensure optimal π-orbital overlap. The observation of intramolecular noncovalent interactions is further corroborated by reduced density gradient (RDG) analysis.41 The RDG iso-surfaces are colored on a blue-green-red scale based on the sign(λ2)ρ, as shown in Fig. 2c. The iso-surfaces colored in green and brown between D and A units illustrate the π–π interactions and steric hindrance. For three compounds, large green and brown iso-surfaces are present between the donor and acceptor, suggesting significant π–π interactions that support the potential occurrence of the TSCT process. Furthermore, the steric hindrance between donor and acceptor units creates considerable rigidity, which restricts molecular vibrations and inhibits non-radiative transitions, leading to a narrow FWHM. In addition, in D2 and D3, the top methyl group introduces steric hindrance with the acceptors, as shown by the red dotted circle in Fig. 2c, which is not found between D1 and A1. This additional steric hindrance further dampen the vibration of the acceptor unit, which helps to further inhibit the process of non-radiative transition. According to the scatter diagrams of the RDG analysis (Fig. 2d), all three compounds show numerous and dense spikes in the green and brown regions, demonstrating the effective π–π interaction between the D and A units.
 | ||
| Fig. 2 (a) Side and (b) top views of the single crystal structures for 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO. The distribution of RDG isosurfaces (c) with the scatter plots of RDG versus sign(λ2) (d). | ||
Density functional theory (DFT) and time-dependent density functional theory (TD-DFT) calculations were performed using the B3LYP-D3/6-31G(d) method to optimize the ground state configurations and analyze frontier molecular orbitals (FMOs) along with electron–hole distribution.42 According to DFT simulations (Fig. S26†), we observed that the HOMO and LUMO mainly distribute on the donor and acceptor units, respectively, illustrating the ICT characteristics of the compounds. The HOMO energy levels were determined to be −5.01, −4.95, and −4.87 eV, while the LUMO energy levels were determined to be −1.45, −1.47, and −1.45 eV. Thus, the calculated Eg values of 3.56, 3.48, and 3.42 eV, for 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, respectively, align well with the observed trend in electrochemical measurements. It was further confirmed that the ICTs improve progressively with more methyl groups on the donor. The rigidity of the molecules helps reduce the energy loss that occurs during structural relaxation. Therefore, 2MeCz-BO and 3MeCz-BO present similar radiative decay oscillator strengths (f) (0.0178 for 3MeCz-BO and 0.0168 for 2MeCz-BO), whereas 1MeCz-BO shows an f of 0.0064, which is much lower than that of 3MeCz-BO and 2MeCz-BO owing to its relatively weaker structural rigidity and weaker ICT. Thus, the higher f will provide 3MeCz-BO and 2MeCz-BO with fast radiation transition rates.
The TD-DFT calculations show the excited-state energy levels, natural transition orbital (NTO) distributions, and CT proportion, as shown in Fig. 3. All three compounds exhibit CT-dominated S1 states with 99.6% CT proportions. The energy levels of the S1 states decrease slightly as the number of methyl groups in the donor unit increases, showing values of 2.998, 2.901, and 2.848 eV. This change occurs due to the enhancement of ICT caused by the additional electron-rich methyl groups in the donor. For 1MeCz-BO, the weak ICT results in a large bandgap, leading to high energy levels for both the 1CT and 3CT. However, the 3LE is mainly influenced by the molecular segment with the lowest energy, which is the acceptor unit. Thus, the T1 of 1MeCz-BO shows a 7.6% CT proportion and an energy level of 2.826 eV. Although the value of 〈S1|ĤSOC|T1〉 (0.393 cm−1) is large, the large ΔEST (172 meV) makes the RISC process extremely difficult. In addition, 1MeCz-BO exhibits a significant ΔE(3CT − 3LE) (134 meV), making the RISC process inaccessible with the assistance of T2. This suggests a slow RISC process for 1MeCz-BO. For 2MeCz-BO, the additional methyl group in the donor (compared to 1MeCz-BO) can slightly decrease the energy level of 1CT and 3CT states, resulting in lower S1 and T2 (CT: 70.6%, 2.889 eV) energy levels than those of 1MeCz-BO. However, the additional methyl groups in the donor have minimal impact on the 3LE energy level, resulting in the T1 (CT: 27.6%, 2.818 eV) of 2MeCz-BO similar to that of 1MeCz-BO. Thus the ΔEST of 2MeCz-BO is significantly reduced to 83 meV. Furthermore, because of the distinct LE characteristics of T1, a high value of 〈S1|ĤSOC|T1〉 of 0.330 cm−1 can be achieved, suggesting that an effective RISC process can occur from T1 to S1. In addition, the ΔE(3CT − 3LE) (71 meV) of 2MeCz-BO is also reduced, facilitating the mixing of 3LE and 3CT, as shown in Fig. 3d. Thus, the mixing of T1 and T2 allows the RISC process to be assisted by T2. With the further addition of methyl groups, 3MeCz-BO presents the lowest S1, resulting in a minimized ΔEST of 51 meV. Additionally, the mixing of T1 (CT: 63.8%, 2.797 eV) and T2 (CT: 34.7%, 2.857 eV) is reinforced due to the further reduction of ΔE(3CT − 3LE) (60 meV), suggesting more effective assistance from T2. The smaller ΔEST with the high enough 〈S1|ĤSOC|T1〉 (0.201 cm−1) ensures a faster RISC process of 3MeCz-BO.
Photophysical properties
To investigate the photophysical properties of these emitters, UV-Vis absorption spectra, fluorescence spectra in dilute toluene solution (10−5 mol L−1), and photoluminescence spectra in doped films were measured at 298 K, as shown in Fig. 4a. In the films, emitters (20 wt%) are doped in 10 wt% poly(N-vinylcarbazole) (PVK, Mw ≥ 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) and 70 wt% 1,3-bis(N-carbazolyl)benzene (mCP). As seen in the UV-Vis absorption spectra, all three emitters show two strong absorption bands at 280–320 nm and 340–410 nm. The band at 280–320 nm is attributed to the local π–π* transitions, and the band at 340–410 nm includes the characteristic absorption band of oxygen-bridged boron acceptor and ICT between D and A. The optical band-gap values are calculated from the onset of the absorption spectra, which are 3.00, 2.99, and 2.98 eV for 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, respectively. The PL emission peaks in toluene solution are located at 416, 428, and 432 nm for 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, with narrow FWHM values of 42, 41, and 39 nm, respectively. Compared to the PL emission in the toluene solution, the doped films exhibit more redshifted emission (423, 434, and 441 nm), and larger FWHM values (52, 51, and 49 nm). As expected, as the number of methyl groups in the donor increases, the emitters exhibit more redshifted emission, due to the electron-rich nature of the methyl groups. The fluorescence and phosphorescence spectra at 77 K in toluene were recorded to calculate the energy levels of S1 and T1 states, shown in Fig. 4b. The energy levels of S1 states are determined to be 3.08, 3.01, and 2.95 eV, while the energy levels of T1 states are determined to be 2.95, 2.95, 2.93 eV, for 1MeCz-BO, 2MeCz-BO, 3MeCz-BO, respectively. The increase in the number of methyl groups in the donor is beneficial for improving the electron-donating ability of the donor, which in turn enhances the ICT process and reduces the energy level of the S1 states with CT characteristics. By contrast, these three emitters have similar T1 energy levels, since T1 states are mainly distributed on the same acceptor units. In particular, because the T1 state of 3MeCz-BO exhibits more CT characteristics, it shows a relatively low T1 energy level. Therefore, the corresponding ΔESTs of 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO are calculated to be 0.13, 0.06, and 0.02 eV, respectively. 3MeCz-BO shows extremely small ΔEST, leading to the most effective RISC process. Fig. 4c illustrates the PL spectra of three emitters in different solvents at 298 K, showing obvious solvatochromism and highlighting the distinct CT characteristics of the S1 states.
000) and 70 wt% 1,3-bis(N-carbazolyl)benzene (mCP). As seen in the UV-Vis absorption spectra, all three emitters show two strong absorption bands at 280–320 nm and 340–410 nm. The band at 280–320 nm is attributed to the local π–π* transitions, and the band at 340–410 nm includes the characteristic absorption band of oxygen-bridged boron acceptor and ICT between D and A. The optical band-gap values are calculated from the onset of the absorption spectra, which are 3.00, 2.99, and 2.98 eV for 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, respectively. The PL emission peaks in toluene solution are located at 416, 428, and 432 nm for 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, with narrow FWHM values of 42, 41, and 39 nm, respectively. Compared to the PL emission in the toluene solution, the doped films exhibit more redshifted emission (423, 434, and 441 nm), and larger FWHM values (52, 51, and 49 nm). As expected, as the number of methyl groups in the donor increases, the emitters exhibit more redshifted emission, due to the electron-rich nature of the methyl groups. The fluorescence and phosphorescence spectra at 77 K in toluene were recorded to calculate the energy levels of S1 and T1 states, shown in Fig. 4b. The energy levels of S1 states are determined to be 3.08, 3.01, and 2.95 eV, while the energy levels of T1 states are determined to be 2.95, 2.95, 2.93 eV, for 1MeCz-BO, 2MeCz-BO, 3MeCz-BO, respectively. The increase in the number of methyl groups in the donor is beneficial for improving the electron-donating ability of the donor, which in turn enhances the ICT process and reduces the energy level of the S1 states with CT characteristics. By contrast, these three emitters have similar T1 energy levels, since T1 states are mainly distributed on the same acceptor units. In particular, because the T1 state of 3MeCz-BO exhibits more CT characteristics, it shows a relatively low T1 energy level. Therefore, the corresponding ΔESTs of 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO are calculated to be 0.13, 0.06, and 0.02 eV, respectively. 3MeCz-BO shows extremely small ΔEST, leading to the most effective RISC process. Fig. 4c illustrates the PL spectra of three emitters in different solvents at 298 K, showing obvious solvatochromism and highlighting the distinct CT characteristics of the S1 states.
To investigate the TADF properties of these emitters, the transient photoluminescence decay curves of the doped films in air and in a vacuum were measured, as shown in Fig. 4d (for 3MeCz-BO) and Fig. S27† (for 1MeCz-BO and 2MeCz-BO). They show noticeable delayed fluorescence and display a clear difference between the decay curves in a vacuum and in air, confirming that triplet excitons are involved in delayed fluorescence processes. Fig. S28† shows the decay curves over a larger range. Then the temperature-dependent transient PL decay spectra of the three emitters were measured in the doped films, as shown in Fig. 4e (for 3MeCz-BO) and Fig. S29† (for 1MeCz-BO and 2MeCz-BO). The enhanced lifetimes and proportions of delayed fluorescence as the temperature increases from 100 to 300 K further confirm the thermally activated mechanism of the three emitters. It can be seen that temperature has the greatest effect on the decay curve of 1MeCz-BO, followed by 2MeCz-BO with the least impact observed for 3MeCz-BO. This phenomenon is closely related to their ΔESTvalues. For 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO films, the prompt lifetimes (τp) are measured as 22.9, 22.5, and 20.2 ns, while the delayed lifetime (τd) are measured as 14.1, 7.32, and 4.73 μs, respectively. Furthermore, the photoluminescence quantum yields (PLQYs) of doped films were measured in a vacuum (by converting the PLQYs of doped films in air, using the integral ratio of the steady-state emissions of the corresponding films in a vacuum to those in air). 1MeCz-BO, 2MeCz-BO and 3MeCz-BO display PLQYs of 83%, 86%, and 88%, respectively. All three compounds exhibit the highest PLQY at a doping ratio of 20 wt%, which may be attributed to the formation of rigid π-stacked structures17 (Fig. S30†). To quantitatively compare the exciton kinetics of the emitters, the rate constants of radiation (kr), non-radiation (knr), and RISC (kRISC) (Fig. 4f) were precisely calculated according to a previously reported method.43 The kr values for 1MeCz-BO, 2MeCz-BO and 3MeCz-BO are 2.75 × 107, 2.81 × 107, and 2.92 × 107 s−1, respectively. High kr values demonstrate the face-to-face D–A arrangements and short D–A distances. Meanwhile, the kRISC values of 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO are measured to be 9.5 × 104, 1.87 × 105, and 3.17 × 105 s−1, respectively. With the modification of methyl groups, the RISC rate gradually increases, which is consistent with the results obtained by the NTO analysis. Moreover, the values of knr have been calculated to be 5.75 × 106, 4.38 × 106, and 3.87 × 106 s−1 for 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, respectively. It has been demonstrated that the increased molecular rigidity from the methyl groups can inhibit non-radiative transitions. The photophysical data of three emitters are given in Tables 1 and S5.†
| Compound | λ PL [nm] | FWHMb [nm] | S1/T1c [eV] | ΔESTd [eV] | HOMO/LUMOe [eV] | E g [eV] | PLQYg [%] | k r [107 s−1] | k RISC [105 s−1] |
|---|---|---|---|---|---|---|---|---|---|
| a The photoluminescence spectra in toluene (10−5 M) solution and doped films at 298 K. b The full-width-at-half maximum of PL spectra in toluene solution and doped films. c Determined from the onset of fluorescence and phosphorescence spectra in toluene solution at 77 K. d ΔEST = S1 − T1. e Estimated from the oxidation and redox potential in cyclic voltammetry. f The energy gap (Eg) values calculated from the HOMO–LUMO levels. g The photoluminescence quantum yields of doped films in a vacuum. h The radiative rate constants. i The rate constants of reverse intersystem crossing. | |||||||||
| 1MeCz-BO | 416, 423 | 42, 52 | 3.08/2.95 | 0.13 | −5.41/−2.85 | 2.56 | 83 | 2.75 | 0.95 |
| 2MeCz-BO | 428, 434 | 41, 51 | 3.01/2.95 | 0.06 | −5.29/−2.84 | 2.45 | 86 | 2.81 | 1.87 |
| 3MeCz-BO | 432, 441 | 39, 49 | 2.95/2.93 | 0.02 | −5.23/−2.82 | 2.41 | 88 | 2.92 | 3.17 |
Electroluminescence properties
Promised by the minimized ΔEST and enhanced RISC process of the ultra-deep-blue emitter 3MeCz-BO, the corresponding OLED devices were fabricated to investigate the electroluminescence (EL) performances of the emitters with the following structure: ITO/PEDOT:PSS (30 nm)/PVK (10 nm)/mCP: 20 wt% emitters and 10 wt% PVK (40 nm)/DPEPO (5 nm)/TmPyPB (35 nm)/LiF (0.8 nm)/Al (100 nm). Poly(3,4-ethylenedioxythiophene):poly(styrenesulfonate) (PEDOT:PSS) was used as the hole-injection and hole-transporting layer, lithium fluoride (LiF) was used as the electron-injection layer, and 1,3,5-tri[(3-pyridyl)-phen-3-yl] benzene (TmPyPB) was used as the electron-transporting layer. PVK and bis[2-(diphenylphosphino)phenyl]ether oxide (DPEPO) were used as the electron- and hole-blocking layers, respectively, while mCP was used as the host material. “nMeCz-BO” refers to the series of these emitters: 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO. The energy level diagram of the materials used in OLEDs is shown in Fig. 5a, and the EL properties are summarized in Table 2. The EL properties at other doping ratios are shown in Fig. S31–S33 and Tables S6–S8.†| Materials | λ EL [nm] | EQEmaxb [%] | EQE100c [%] | EQE500d [%] | CEmaxe [cd A−1] | PEmaxf [lm W−1] | L max [cd m−2] | FWHMh [nm] | CIEi [x, y] |
|---|---|---|---|---|---|---|---|---|---|
| a The peak value of electroluminescence. b Maximum external quantum efficiency. c External quantum efficiency at 100 cd m−2. d External quantum efficiency at 500 cd m−2. e Maximum current efficiency. f Maximum power efficiency. g Maximum luminance. h Full width at half-maximum of the electroluminescence peak. i Coordinates of Commission Internationale de L'Eclairage. | |||||||||
| 1MeCz-BO | 430 | 6.1 | 5.5 | 4.2 | 11.7 | 10.2 | 1024 | 49 | 0.162, 0.048 |
| 2MeCz-BO | 436 | 8.6 | 7.5 | 6.0 | 17.1 | 14.9 | 1277 | 49 | 0.160, 0.050 |
| 3MeCz-BO | 442 | 10.1 | 9.0 | 7.1 | 19.6 | 16.9 | 1334 | 50 | 0.151, 0.051 |
For three emitters, the highest efficiency of devices can be obtained at a doped ratio of 20 wt%. Devices based on 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO display EL peaks (λEL) at 430, 436, and 442 nm with FWHM values of 49, 49, and 50 nm, respectively (Fig. 5b). The corresponding CIE coordinates show ultra-deep-blue colors of (0.162, 0.048), (0.160, 0.050), and (0.151, 0.051) for 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, respectively. The emissions from these devices show a slight redshift as the number of methyl groups in the donor increases, which is consistent with the results of molecular simulations and photophysics. All three solution-processed devices exhibit narrow emission, which aligns with the measured FWHM values in photophysics. The rigid molecular structures effectively reduce vibrational relaxation, leading to narrow emissions. This is advantageous for the color purity of ultra-deep-blue OLEDs.
Furthermore, the CIE coordinates of 1MeCz-BO and 2MeCz-BO are (0.162, 0.048) and (0.160, 0.050), respectively, exhibiting more violet emission. While the CIE coordinates of 3MeCz-BO (0.151, 0.051) fit well within the sRGB of CIE (0.15, 0.06), and are close to the CIE (0.131, 0.046) for the Rec. 2020 standard. This suggests its potential application in colorful display devices. As shown in Fig. 5c, the current efficiencies (CEs) of 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO-based OLEDs are 11.7, 17.1, and 19.6 cd A−1, respectively. Their power efficiencies (PEs) are 10.2, 14.9, and 16.9 lm W−1. This suggests that the enhanced electrical properties of the emitters are due to the methyl-modification. In terms of device performance, 3MeCz-BO exhibits a maximum EQE of 10.1%, which is higher than that of 2MeCz-BO (8.6%) and 1MeCz-BO (6.1%). The EQEs at 100 cd m−2 are 5.5%, 7.5%, and 9.0%, and the EQEs at 500 cd m−2 are 4.2%, 6.0%, and 7.1% for 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO, respectively, showing the suppressed efficiency roll-offs. Additionally, the maximum luminance of the devices is measured as 1024, 1277, and 1334 cd m−2, indicating that these devices exhibit high brightness as ultra-deep-blue solution-processing OLEDs. The criss-crossed stacking structures (Fig. S22†) prevent triplet–triplet annihilation of the materials, leading to the highest EQE at relatively high doping ratios (20 wt%). The high concentration of emitters provides higher brightness for the OLEDs, which is particularly challenging for ultra-deep-blue or deep-blue solution-processed OLEDs. These results indicate that the methyl groups on the donor suppress the non-radiative transitions and the enhanced ITC accelerates the RISC, thereby significantly enhancing the device performance of ultra-deep-blue TSCT materials. A summary of device data for face-to-face TSCT-TADF emitters (CIEy < 0.5) is shown in Fig. 5f.8–27 It can be seen that this work fills the gap in the violet to deep blue region of TSCT materials. For comparison, thermal evaporated OLEDs based on 3MeCz-BO were also tested, as shown in Fig S34 and Table S9.† As a solution-processed device, it does not demonstrate significant disadvantages compared to the thermally evaporated device. Additionally, the limited broadening in the EL spectrum (compared to the PL in doped films) indicates the potential use of these materials in narrowband ultra-deep-blue OLEDs.
Conclusions
In summary, we proposed a strategy of methyl-modification in the donor to enhance the ICT and accelerate the RISC process of ultra-deep-blue TSCT-TADF emitters, solving the challenging issue of large ΔEST values and low kRISC values in these materials. Due to the electron-rich nature of methyl groups, the ICT was enhanced and the energy levels of the 1CT and 3CT states were reduced, while the 3LE remains unaffected by the methyl group. In this way, the ΔEST and ΔE(3CT − 3LE) of the materials can be minimized. This enables effective RISC processes from T1 to S1, which can be accelerated with the assistance of T2. The prepared emitters 1MeCz-BO, 2MeCz-BO, and 3MeCz-BO display kRISC values of 9.5 × 104, 1.87 × 105, and 3.17 × 105 s−1, respectively. In addition, ultra-deep-blue solution-processed OLED devices based on these materials exhibit good overall performance. The EQEmax of 3MeCz-BO is 10.1%, with CIE coordinates of (0.151, 0.051) and a high luminance of 1334 cd m−2. This work developed the first high-performance ultra-deep-blue solution-processed TSCT-TADF OLEDs. Additionally, their colour fits well within the sRGB of CIE coordinates of (0.15, 0.06), and is close to the CIE coordinates of (0.131, 0.046) for the Rec. 2020 standard.Data availability
The experimental procedures, characterization data, crystals data and device data generated in this study are provided in the ESI.†Author contributions
Z. Ren and Q. Li designed the experiments. Q. Li synthesized the molecules, carried out the photophysical measurements and theoretical calculations, fabricated OLEDs and wrote the manuscript draft. H. Zhao and J. Zhao helped fabricate the OLEDs. Z. Cao and C. Yu helped synthesize the molecules. S. Yan and Z. Ren wrote the manuscript and supervised this study.Conflicts of interest
There is no conflict of interest to report.Acknowledgements
The authors acknowledge the financial support from the National Natural Science Foundation of China (No. 52273164) and the Shandong Provincial Natural Science Foundation (ZR2022ZD37).Notes and references
- H. Uoyama, K. Goushi, K. Shizu, H. Nomura and C. Adachi, Highly efficient organic light-emitting diodes from delayed fluorescence, Nature, 2012, 492, 234–238 Search PubMed.
- Y. Liu, C. Li, Z. Ren, S. Yan and M. R. Bryce, All-organic thermally activated delayed fluorescence materials for organic light-emitting diodes, Nat. Rev. Mater., 2018, 3, 18020 Search PubMed.
- G. Hong, X. Gan, C. Leonhardt, Z. Zhang, J. Seibert, J. M. Busch and S. Brase, A brief history of oleds-emitter development and industry milestones, Adv. Mater., 2021, 33, e2005630 Search PubMed.
- S. Shao, J. Hu, X. Wang, L. Wang, X. Jing and F. Wang, Blue thermally activated delayed fluorescence polymers with nonconjugated backbone and through-space charge transfer effect, J. Am. Chem. Soc., 2017, 139, 17739–17742 Search PubMed.
- H. Tsujimoto, D.-G. Ha, G. Markopoulos, H. S. Chae, M. A. Baldo and T. M. Swager, Thermally activated delayed fluorescence and aggregation induced emission with through-space charge transfer, J. Am. Chem. Soc., 2017, 139, 4894–4900 Search PubMed.
- Q. Xue and G. Xie, Thermally activated delayed fluorescence beyond through-bond charge transfer for high-performance OLEDs, Adv. Opt. Mater., 2021, 9, 2002204 Search PubMed.
- T. Zhang, Y. Xiao, H. Wang, S. Kong, R. Huang, V. Ka-Man Au, T. Yu and W. Huang, Highly twisted thermally activated delayed fluorescence (TADF) molecules and their applications in organic light-emitting diodes (OLEDs), Angew. Chem., 2023, 62, e202301896 Search PubMed.
- X. Tang, L. S. Cui, H. C. Li, A. J. Gillett, F. Auras, Y. K. Qu, C. Zhong, S. T. E. Jones, Z. Q. Jiang, R. H. Friend and L. S. Liao, Highly efficient luminescence from space-confined charge-transfer emitters, Nat. Mater., 2020, 19, 1332–1338 Search PubMed.
- Y. Wada, H. Nakagawa, S. Matsumoto, Y. Wakisaka and H. Kaji, Organic light emitters exhibiting very fast reverse intersystem crossing, Nat. Photonics, 2020, 14, 643–649 Search PubMed.
- S.-Y. Yang, Q.-S. Tian, Y.-J. Yu, S.-N. Zou, H.-C. Li, A. Khan, Q.-H. Wu, Z.-Q. Jiang and L.-S. Liao, Sky-blue thermally activated delayed fluorescence with intramolecular spatial charge transfer based on a dibenzothiophene sulfone emitter, J. Org. Chem., 2020, 85, 10628–10637 Search PubMed.
- T.-T. Wang, G. Xie, H.-C. Li, S.-Y. Yang, H. Li, Y.-L. Xiao, C. Zhong, K. Sarvendra, A. Khan, Z.-Q. Jiang and L.-S. Liao, π-stacked thermally activated delayed fluorescence emitters with alkyl chain modulation, CCS Chem., 2021, 3, 1757–1763 Search PubMed.
- T. Huang, Q. Wang, G. Meng, L. Duan and D. Zhang, Accelerating radiative decay in blue through-space charge transfer emitters by minimizing the face-to-face donor-acceptor distances, Angew. Chem., 2022, 61, e202200059 Search PubMed.
- Z. Zhao, C. Zeng, X. Peng, Y. Liu, H. Zhao, L. Hua, S. J. Su, S. Yan and Z. Ren, Tuning intramolecular stacking of rigid heteroaromatic compounds for high-efficiency deep-blue through-space charge-transfer emission, Angew. Chem., Int. Ed., 2022, 61, e202210864 Search PubMed.
- Q. Li, Z. Zhao, H. Zhao, Y. Guo, X. Tong, S. Yan and Z. Ren, Enhancing light-emitting efficiency of blue through-space charge transfer emitters via fixing configuration induced by intramolecular hydrogen bonding, ACS Appl. Mater. Interfaces, 2024, 16, 22238–22247 Search PubMed.
- Y. K. Qu, D. Y. Zhou, Q. Zheng, P. Zuo, Z. L. Che, L. S. Liao and Z. Q. Jiang, Linearly arranged multi-π-stacked structure for efficient through-space charge-transfer emitters, Angew. Chem., Int. Ed., 2024, 63, e202408712 Search PubMed.
- P. Zuo, Y. J. Yang, F. M. Liu, J. R. Wu, Q. Zheng, H. T. Yuan, L. S. Liao, D. Y. Zhou and Z. Q. Jiang, Achieving deep-blue through-space charge-transfer emitter by designing the donor-π-donor-σ-acceptor molecular structure, Adv. Opt. Mater., 2024, 12, 2400860 Search PubMed.
- S. Y. Yang, Z. Q. Feng, Z. Fu, K. Zhang, S. Chen, Y. J. Yu, B. Zou, K. Wang, L. S. Liao and Z. Q. Jiang, Highly efficient sky-blue π-stacked thermally activated delayed fluorescence emitter with multi-stimulus response properties, Angew. Chem., Int. Ed., 2022, 61, e202206861 Search PubMed.
- C. C. Peng, S. Y. Yang, H. C. Li, G. H. Xie, L. S. Cui, S. N. Zou, C. Poriel, Z. Q. Jiang and L. S. Liao, Highly efficient thermally activated delayed fluorescence via an unconjugated donor-acceptor system realizing EQE of over 30%, Adv. Mater., 2020, 32, e2003885 Search PubMed.
- S. Y. Yang, Y. K. Wang, C. C. Peng, Z. G. Wu, S. Yuan, Y. J. Yu, H. Li, T. T. Wang, H. C. Li, Y. X. Zheng, Z. Q. Jiang and L. S. Liao, Circularly polarized thermally activated delayed fluorescence emitters in through-space charge transfer on asymmetric spiro skeletons, J. Am. Chem. Soc., 2020, 142, 17756–17765 Search PubMed.
- Y. Song, M. Tian, R. Yu and L. He, Through-space charge-transfer emitters developed by fixing the acceptor for high-efficiency thermally activated delayed fluorescence, ACS Appl. Mater. Interfaces, 2021, 13, 60269–60278 Search PubMed.
- X. Q. Wang, S. Y. Yang, Q. S. Tian, C. Zhong, Y. K. Qu, Y. J. Yu, Z. Q. Jiang and L. S. Liao, Multi-layer π-stacked molecules as efficient thermally activated delayed fluorescence emitters, Angew. Chem., Int. Ed., 2021, 60, 5213–5219 Search PubMed.
- Z. Q. Feng, S. Y. Yang, F. C. Kong, Y. K. Qu, X. Y. Meng, Y. J. Yu, D. Y. Zhou, Z. Q. Jiang and L. S. Liao, Indirect control of donor/acceptor interactions for highly efficient space-confined thermally activated delayed fluorescence emitters, Adv. Funct. Mater., 2022, 33, 2209708 Search PubMed.
- F. M. Xie, H. Z. Li, K. Zhang, Y. Shen, X. Zhao, Y. Q. Li and J. X. Tang, A dislocated twin-locking acceptor-donor-acceptor configuration for efficient delayed fluorescence with multiple through-space charge transfer, Angew. Chem., Int. Ed., 2022, 61, e202213823 Search PubMed.
- J. Liu, Z. Feng, C. Peng, Y. Yu, S. Yang, Z. Jiang and L. Liao, Acceptor modulation for improving thermally activated delayed fluorescence emitter in through-space charge transfer on spiroskeletons, Chin. Chem. Lett., 2023, 34, 107634 Search PubMed.
- Y. Song, K. Zhang, P. Wang, Y. Cao and L. He, Simultaneously improving radiative decay and reverse intersystem crossing in space-confined through-space, Adv. Funct. Mater., 2024, 2411957 Search PubMed.
- X. Lan, J. Zeng, J. Chen, T. Yang, X. Dong, B. Z. Tang and Z. Zhao, Robust sandwich-structured thermally activated delayed fluorescence molecules utilizing 11,12-dihydroindolo[2,3-a]carbazole as bridge, Angew. Chem., Int. Ed., 2024, e202414488 Search PubMed.
- H.-T. Yuan, Z.-H. Qu, P. Zuo, Y.-J. Yang, Q. Zheng, F.-M. Liu, M.-T. Li, L.-S. Liao and Z.-Q. Jiang, Comparative study on two kinds of spiro systems for constructing through-space charge-transfer emitters, J. Phys. Chem. C, 2024, 128, 14538–14545 Search PubMed.
- C. Jiang, J. Miao, D. Zhang, Z. Wen, C. Yang and K. Li, Acceptor-donor-acceptor π-stacking boosts intramolecular through-space charge transfer towards efficient red TADF and high-performance OLEDs, Research, 2022, 2022, 9892802 Search PubMed.
- X. F. Song, C. Jiang, N. Li, J. Miao, K. Li and C. Yang, Simultaneously enhancing the planarity and electron-donating capability of donors for through-space charge transfer TADF towards deep-red emission, Chem. Sci., 2023, 14, 12246–12254 Search PubMed.
- Y.-J. Yu, M. Song, X.-Y. Meng, Y.-K. Qu, X.-Q. Wang, L. Chen, S.-Y. Yang, D.-Y. Zhou, Z.-Q. Jiang and L.-S. Liao, Design and synthesis of red through-space charge transfer thermally activated delayed fluorescence emitters with donor/acceptor/donor stacking, Org. Lett., 2023, 25, 6024–6028 Search PubMed.
- P. Chen, C. Jiang, N. Li, X.-F. Song, X. Wan, H. Liu, J.-G. Yang and K. Li, Sandwich-type thermally activated delayed fluorescence molecules with through-space charge transfer excited state for red OLEDs, Org. Electron., 2024, 133, 107114 Search PubMed.
- H. Hirai, K. Nakajima, S. Nakatsuka, K. Shiren, J. Ni, S. Nomura, T. Ikuta and T. Hatakeyama, One-step borylation of 1,3-diaryloxybenzenes towards efficient materials for organic light-emitting diodes, Angew. Chem., Int. Ed., 2015, 54, 13581–13585 Search PubMed.
- D. H. Ahn, S. W. Kim, H. Lee, I. J. Ko, D. Karthik, J. Y. Lee and J. H. Kwon, Highly efficient blue thermally activated delayed fluorescence emitters based on symmetrical and rigid oxygen-bridged boron acceptors, Nat. Photonics, 2019, 13, 540–546 Search PubMed.
- T. Hua, X. Cao, J. Miao, X. Yin, Z. Chen, Z. Huang and C. Yang, Deep-blue organic light-emitting diodes for ultrahigh-definition displays, Nat. Photonics, 2024, 18, 1161–1169 Search PubMed.
- J. Ochi, Y. Yamasaki, K. Tanaka, Y. Kondo, K. Isayama, S. Oda, M. Kondo and T. Hatakeyama, Highly efficient multi-resonance thermally activated delayed fluorescence material toward a BT.2020 deep-blue emitter, Nat. Commun., 2024, 15, 2361 Search PubMed.
- R. W. Weerasinghe, S. Madayanad Suresh, D. Hall, T. Matulaitis, A. M. Z. Slawin, S. Warriner, Y. T. Lee, C. Y. Chan, Y. Tsuchiya, E. Zysman-Colman and C. Adachi, A boron, nitrogen, and oxygen doped π-extended helical pure blue multiresonant thermally activated delayed fluorescent emitter for organic light emitting diodes that shows fast kRISC without the use of heavy atoms, Adv. Mater., 2024, 36, e2402289 Search PubMed.
- T. Yang, N. Qiu, X. Lan, X. Dong, B. Z. Tang and Z. Zhao, Efficient folded molecules with intramolecular through-space charge transfer for near-ultraviolet organic light-emitting diodes, J. Phys. Chem. C, 2024, 128, 16085–16092 Search PubMed.
- Q. Zhang, J. Li, K. Shizu, S. Huang, S. Hirata, H. Miyazaki and C. Adachi, Design of efficient thermally activated delayed fluorescence materials for pure blue organic light emitting diodes, J. Am. Chem. Soc., 2012, 134, 14706–14709 Search PubMed.
- Q. Zhang, B. Li, S. Huang, H. Nomura, H. Tanaka and C. Adachi, Efficient blue organic light-emitting diodes employing thermally activated delayed fluorescence, Nat. Photonics, 2014, 8, 326–332 Search PubMed.
- X. Cai and S. J. Su, Marching toward highly efficient, pure-blue, and stable thermally activated delayed fluorescent organic light-emitting diodes, Adv. Funct. Mater., 2018, 28, 1802558 Search PubMed.
- T. Lu and F. Chen, Multiwfn: a multifunctional wavefunction analyzer, J. Comput. Chem., 2012, 33, 580–592 Search PubMed.
- S. Grimme, S. Ehrlich and L. Goerigk, Effect of the damping function in dispersion corrected density functional theory, J. Comput. Chem., 2011, 32, 1456–1465 Search PubMed.
- K. Masui, H. Nakanotani and C. Adachi, Analysis of exciton annihilation in high-efficiency sky-blue organic light-emitting diodes with thermally activated delayed fluorescence, Org. Electron., 2013, 14, 2721–2726 Search PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2402467–2402469 contain the crystallographic data for 1MeCz-BO, 2MeCz-BO and 3MeCz-BO. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc08094g |
| This journal is © The Royal Society of Chemistry 2025 |