Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Molecular Aharonov–Bohm-type interferometers based on porphyrin nanorings†
Chi Y.
Cheng
 a,
Gil
Harari
a,
Gil
Harari
 b,
Igor
Rončević
b,
Igor
Rončević
 c,
Juan E.
Peralta
c,
Juan E.
Peralta
 d,
Harry L.
Anderson
d,
Harry L.
Anderson
 *c,
Andrew M.
Wibowo-Teale
*c,
Andrew M.
Wibowo-Teale
 *a and
Oded
Hod
*a and
Oded
Hod
 *b
*b
aSchool of Chemistry, University of Nottingham, University Park, Nottingham, NG7 2RD, UK
bSchool of Chemistry, The Sackler Centre for Computational Molecular and Materials Science, Tel Aviv University, Tel Aviv 6997801, Israel
cDepartment of Chemistry, University of Oxford, Chemistry Research Laboratory, Oxford, OX1 3TA, UK
dDepartment of Physics, Central Michigan University, Mount Pleasant, MI 48859, USA
First published on 29th January 2025
Abstract
A goal of molecular electronics and spintronics is to create molecular devices that change their conductance in response to external stimuli. The Aharonov–Bohm (AB) effect implies that an electronic device formed from a quantum ring and metallic leads will exhibit such behavior under external magnetic fields. At first sight, it appears that unrealistically large fields would be required to significantly alter the conductance of a molecular ring. However, the sensitivity of a molecular AB interferometer to magnetic fields can be increased by weakening the coupling between the molecular ring and the metallic leads. An ideal molecular ring for an AB interferometer has a large radius (to encompass a larger fraction of the AB flux quantum), and a small effective mass (high electron mobility) to enhance its response to magnetic fields. Here, we use computational modelling to demonstrate that recently synthesized zinc porphyrin nanorings, with radii of 2–9 nm, could behave as molecular AB interferometers at achievable magnetic field strengths (5–10 T), if weak ring-lead coupling is used. Building on our recently developed semi-empirical approach, which incorporates the effects of finite magnetic fields on the electronic structure, we develop a transport computational platform that allows us to identify sharp Fano resonances in the transmittance probability of porphyrin nanorings that could be exploited to control the current with an applied magnetic field. These resonances are rationalized in terms of a magnetic field-induced delocalization of the molecular orbitals. Our findings indicate that molecular AB interferometry should be feasible with current experimental capabilities.
1 Introduction
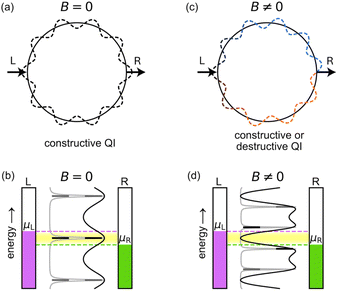
Aharonov and Bohm predicted that the phase of the wavefunction of electrons traversing a metal ring will be affected by the flux of an externally applied magnetic field threading through the ring, even if there is no magnetic field in the vicinity of the electrons.1 This theoretical result, which seemed paradoxical at the time, was fully supported by subsequent experimental and theoretical investigations, demonstrating that the vector potential (rather than the magnetic field) is a fundamental quantity in quantum mechanics.2,3 The term ‘Aharonov–Bohm (AB) effect’, however, is often used more generally to describe the fact that the ring current (or persistent current) flowing round a quantum ring oscillates as a function of the magnetic flux passing through the ring, without necessarily implying that the magnetic field is zero in the vicinity of the ring.2–5 An AB interferometer (Fig. 1) consists of a quantum ring attached to external leads, such that a current can be driven through it and controlled by the application of a magnetic field perpendicular to the ring.4,5 This magnetic control has been used to measure the quantum coherent properties of electrons,5 and it is predicted to enable other unique functionalities, including spin filtering in two-terminal setups,6 and current and spin routing in three-terminal setups.7In the absence of a magnetic field, the upper and lower paths of a quantum ring from the left (L) to the right (R) lead, through a symmetrically connected AB interferometer, are equivalent (Fig. 1a), resulting in constructive quantum interference (QI) and high conductance (Fig. 1b). A magnetic field B perpendicular to the ring plane breaks the symmetry, as the electron accumulates an opposite phase when traversing the ring in a clockwise or counterclockwise path (Fig. 1c). This results in periodically alternating constructive or destructive QI and consequently high or low conductance (Fig. 1d), depending on the magnetic flux Φ, which is given by the product of the perpendicular magnetic field, B, and the cross-sectional area of the ring of radius r,
| Φ(B) = Bπr2. | (1) |
The flux required for a full AB cycle between constructive and destructive interferences is the magnetic flux quantum:8
| Φ0 = 2πℏ / e ≈ 4.1 × 105 T Å2, | (2) |
If the ring is weakly coupled to external metallic leads, the presence of a magnetic field may significantly alter the molecular conductance, even when the magnetic flux through the ring is considerably smaller than a full flux quantum.6,7,13–15 A simple explanation for this effect can be given using the particle-in-a-ring (PIR) model.16,17 The electronic spectrum of an isolated PIR consists of a series of doubly degenerate eigenstates and a non-degenerate ground state (n = 0), with angular momentum quantum numbers ±n and energies,
 | (3) |
 | (4) |
For sufficiently strong ring-lead couplings, the ring state widths become comparable to the width of the Fermi window and conductance variations are only apparent over the entire AB period (black curves in Fig. 1b and d). For weaker ring-lead couplings, conductance variations will occur when the relatively narrow eigenstates approach the edges of the Fermi transport window (gray curves in Fig. 1b and d). As a result, the conductance becomes sensitive to variations in the magnetic field that are much smaller than the flux quantum. This understanding opens the door for achieving AB interferometry in molecular rings and utilizing it for the miniaturization of electronic switching and routing devices. Nonetheless, previous literature considered mainly simplistic model systems of weakly coupled molecular rings, and focused on the phenomenological investigation of the effect, rather than on its practical utilization.6,7,13–15,18,19
To take advantage of the AB effect, one needs to maximize the splitting of the energy eigenvalues, En, with the magnetic field. From eqn (1)–(4), this splitting is given by:
 | (5) |
The degenerate orbitals of a conjugated ring system, such as benzene, split under an external field in a way reminiscent of the PIR model, due to their opposite orbital angular momentum. This is illustrated in Fig. 2, where we compare the effect of the magnetic field (B) on the PIR energy eigenvalues obtained viaeqn (4) and on the orbital energies of benzene, calculated using our tight-binding semiempirical GFN1-xTB-M1 method (discussed below in more detail).20 The qualitative agreement between the phenomenological PIR model and the results calculated for the molecular ring allows us to introduce the concept of an effective mass for the π-electrons in the molecular ring, as the mass that provides good agreement between the PIR model and the molecular energy bands. This simple example demonstrates that realistic molecules can present PIR-like behavior. Nevertheless, achieving a full AB cycle in benzene would require an unrealistically large field of ∼80 kT. Clearly, even by reducing the coupling to the leads, the sensitivity of any benzene-based AB interferometric device would be insufficient to respond to a field of around 10 T, and larger molecules are needed.
 | ||
| Fig. 2 Magnetic field dependence of the energy eigenvalues of (a) a particle in a ring (calculated viaeqn (4) with an effective ring radius of 1.39 Å – the radius of the circumcircle of the benzene hexagon used in the GFN1-xTB-M1 calculations, and effective masses of m* = me for all states), and (b) the benzene molecule (calculated using the GFN1-xTB-M1 method and plotted relative to the center of the HOMO–LUMO gap in the absence of a magnetic field). For comparison purposes, in the molecular calculations the same benzene structure (obtained by geometry optimization in the absence of a magnetic field) is used throughout, and only benzene energy eigenvalues of MOs with contributions from the pz atomic orbitals are shown while spin-Zeeman effects are removed (results including spin-Zeeman interactions appear in ESI Section S.1†). | ||
Butadiyne-linked cyclic porphyrin oligomers (c-PN, where N is the number of porphyrin units, Fig. 3a) are promising molecular nanorings for use in AB interferometers because they have been prepared in a variety of different sizes with radii of up to 104 Å (50 porphyrin units)21 and shown to feature extensive electronic delocalization.22 Rings of up to 12 porphyrin units (c-P12, r ≈ 25 Å) were shown experimentally to exhibit global aromatic and anti-aromatic ring currents depending on their oxidation state.23–25 This indicates that their electronic wavefunction is coherently delocalized around the entire ring, with an electronic structure analogous to that of a PIR. In larger rings, structural symmetry breaking, similar to the Peierls distortion, may lead to localization of the molecular orbitals and an increase of the effective electronic mass. However, scanning tunnelling spectroscopy experiments on a butadiyne-linked 40-porphyrin nanoring (c-P40, r ≈ 83 Å) on a silver surface, indicate that its electronic states remain delocalized around the entire ring.24
Edge-fused porphyrin nanobelts (f-PN, Fig. 3b) constitute another molecular ring family, which is predicted to exhibit lower effective electronic masses26 and greater electronic delocalization.27 These properties make them even more interesting in the context of molecular AB interferometry. While f-PN nanobelts have not yet been synthesized, the corresponding linear ribbons have been studied extensively28–31 and are known to mediate coherent ballistic charge transport.30,31
Motivated by the potential applicability of porphyrin nanoring-based AB interferometers, in the present study we explore the conductance properties of realistic molecular rings and possible routes to control their response with experimentally accessible magnetic fields (5–10 T), focusing on macrocycles constructed from zinc porphyrin units. The closed-shell d10 zinc ions do not have a strong influence on the molecular electronic structure, and nanorings containing other closed-shell metal cations are expected to display qualitatively similar behavior.
2 Computational methods
We have developed a nonequilibrium Green's function (NEGF)8,32–34 approach for calculating the electronic current flowing through molecular junctions under finite bias and magnetic field. We implemented this approach within the QUEST code35 that provides a multitude of different electronic structure methods, including current density-functional theory (CDFT),36,37 Hartree–Fock,38,39 and extended tight-binding methods (e.g. GFN1-xTB)40–42 with a non-perturbative treatment of the external magnetic field (GFN1-xTB-M1).When modelling ring currents in c-PN porphyrin nanorings using DFT, it is crucial to include the correct proportion of exact exchange (EE), and this has led to debate about the best choice of density functionals.43–45 Hybrid density functionals with low levels of EE predict too much delocalization, underestimating band gaps and overestimating the magnitude of ring currents.27 As the GFN1-xTB method (and its M1 variant) produce results similar to pure density functionals (0% EE),40 we expect to see an overestimation of AB effect, just as GFN1-xTB underestimates effective masses for infinite linear porphyrin ribbons when compared to hybrid DFT calculations.46
To verify that our GFN1-xTB-M1 approach provides results that are reasonably consistent with hybrid CDFT for the systems considered, we calculated the magnetic field dependence of the orbital energies of the small c-P4 and f-P4 nanorings with both tight-binding and CDFT (PBE0, 25% EE) approaches. The results, presented in ESI Section S.2,† demonstrate good agreement between these two levels of theory. This is promising, as 25% EE was found to be suitable for modelling f-PN systems,27 while for neutral c-PN rings both GFN1-xTB and PBE0 predicted a Peierls-type distortion, which is essential for an accurate account of the electronic structure.43–45 Encouraged by these results, and taking into account the high computational cost of applying CDFT to these large systems, we used the NEGF transport approach in conjunction with the tight-binding GFN1-xTB-M1 method under external magnetic fields, a method that we term NEGF+GFN1-xTB-M1.
To perform the transport calculations, the molecular junction is divided into three sections: the left lead (L, source), the right lead (R, drain), and the extended molecule (M), which consists of the molecular ring and adjacent lead sections chosen to be sufficiently large to buffer the leads from the ring. The total current, I, flowing across the molecule can be evaluated using the Landauer formalism, according to which the current is proportional to the transmittance probability of an electron to traverse the system at some energy, E:8,32–34
 | (6) |
| fL/R(E) = [1 + e(E−μL/R)/(kBTL/R)]−1, | (7) |
| Tσ(E) = Tr[ΓLσ(E)GMr,σ(E)ΓRσ(E)GMa,σ(E)], | (8) |
We use a non-self-consistent NEGF approach, which neglects the effects of the bias potential on the electronic structure of the device. This approximation is found to be adequate for our purposes, since we consider relatively low bias voltages and weakly coupled molecular bridges (see ESI Section S.5†). Similar non-self-consistent approaches have been successfully implemented both in the Amsterdam Density Functional (ADF)47–49 and the DFTB+50–52 quantum chemistry package for transport calculations in the absence of a magnetic field.
3 Results and discussion
3.1 Response of molecular orbital energies to a magnetic field
We first examine the effect of an external magnetic field on the energy levels of isolated molecular rings. We consider both the planar butadiyne-linked porphyrin nanorings c-PN (results for non-planar geometries are similar, see ESI Section S.6†), and the edge-fused porphyrin nanobelts f-PN. Using the tight-binding GFN1-xTB-M1 method, we optimized planar c-PN and cylindrical f-PN molecules with 10, 20, 30, and 40 porphyrin monomer units in the absence of a magnetic field (see Fig. 3a and b for optimized c-P10 and f-P10 molecular ring structures), yielding nanorings with radii of 21.1, 42.8, 64.4, and 86.0 Å for the c-PN series and 13.3, 26.7, 40.0, and 53.3 Å for the f-PN series, respectively. The magnetic field strengths corresponding to one flux quantum for these radii are listed in Table 1 (B0, calculated from eqn (1) and (2)).| Nanoring | r/nm | m*/me (HOMO) | m*/me (LUMO) | B 0/T |
|---|---|---|---|---|
| c-P10 | 21.1 | −0.1081 | 0.1055 | 296 |
| c-P20 | 42.8 | −0.1083 | 0.1057 | 72 |
| c-P40 | 86.0 | −0.1084 | 0.1057 | 18 |
| f-P10 | 13.3 | −0.0134 | 0.0129 | 744 |
| f-P20 | 26.7 | −0.0073 | 0.0073 | 185 |
| f-P40 | 53.3 | −0.0059 | 0.0058 | 46 |
As the frontier orbital energies of the c-PN and f-PN nanorings respond to an external magnetic field similarly to their corresponding PIR eigenstates, we can fit their orbital energy functions around B = 0 to a quadratic function and determine the effective mass viaeqn (4). For the LUMOs, we obtain effective masses of ∼0.1me for c-PN nanorings and ∼0.01me for f-PN nanobelts (see Table 1).46 Equivalent calculations for the HOMO yield negative effective masses, in analogy with hole quasiparticles53 in solid-state physics. The smaller m* obtained for the f-PN nanobelts is expected, given the larger coupling between its porphyrin monomer units. Unlike the c-PN series, which shows similar effective masses for all nanoring sizes considered, the f-PN series shows decreasing effective mass with increasing ring size. This is attributed to the fact that orbital overlap between neighboring porphyrin units is large and increases as the rings become larger and less strained. A similar method was used previously to estimate the effective masses of charge carriers in benzene (mHOMO = 1.5me) and in a C576 toroid (mHOMO = 0.3me).54–56 The lower values for c-PN and f-PN imply a greater sensitivity to a perpendicular magnetic field.
Under an experimentally accessible field strength of 10 T, a splitting of the low-lying energy eigenvalues of up to ∼20 meV is predicted (see Table 2). Due to their lower charge carrier effective mass, the orbitals of the f-PN rings present a stronger magnetic response than their c-PN counterparts (see Fig. 4). However, the c-PN rings have larger radii (at a given value of N), which somewhat counteract their larger effective mass. The results of these two opposing effects can be observed in Table 2, where below N ≈ 20 the butadiyne-linked c-PN rings exhibit a larger response than the corresponding f-PN rings under a perpendicular magnetic field of 10 T. These results demonstrate that both the chemical nature and the cross-sectional area of molecular rings can be tailored to control their response to externally applied magnetic fields.
| Orbital | c-P10 | c-P20 | c-P30 | c-P40 | f-P10 | f-P20 | f-P30 | f-P40 |
|---|---|---|---|---|---|---|---|---|
| a For all c-P40 orbitals and the f-P30 HOMO−4, the 10 T magnetic field was sufficiently large to induce state crossings. In these cases, we calculate the energy changes by following the orbital through the crossing. | ||||||||
| LUMO+4 | 7.0 | 9.9 | 11.2 | 12.2 | 4.6 | 8.7 | 12.7 | 16.7 |
| LUMO+3 | −5.8 | −8.3 | −8.7 | −8.5 | −3.4 | −7.5 | −11.5 | −15.5 |
| LUMO+2 | 5.2 | 6.1 | 6.7 | 7.4 | 4.6 | 8.6 | 12.6 | 16.5 |
| LUMO+1 | −3.9 | −4.3 | −4.0 | −3.3 | −3.4 | −7.4 | −11.4 | −15.3 |
| LUMO | 0.7 | 1.0 | 1.4 | 2.1 | 0.9 | 2.6 | 5.4 | 8.6 |
| HOMO | 0.5 | 0.2 | −0.3 | −0.9 | 0.3 | −1.4 | −4.2 | −7.5 |
| HOMO−1 | 4.9 | 5.3 | 5.0 | 4.4 | 0.6 | 8.4 | 12.4 | 16.2 |
| HOMO−2 | −3.8 | −4.8 | −5.4 | −6.1 | 2.6 | −7.2 | −11.2 | −15.1 |
| HOMO−3 | 6.7 | 9.2 | 9.7 | 9.4 | −1.4 | 0.5 | 12.3 | 16.3 |
| HOMO−4 | −5.5 | −8.4 | −9.8 | −10.8 | 4.4 | 2.7 | −11.1a | −15.1 |
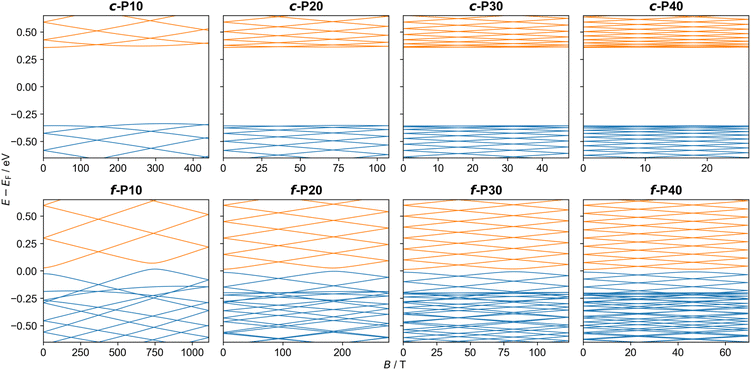 | ||
| Fig. 4 The α-spin orbital energies of planar c-P10, c-P20, c-P30, c-P40 (upper panels) and f-P10, f-P20, f-P30, f-P40 (lower panels) molecular rings as a function of the perpendicular magnetic field strength. Energies are plotted relative to the corresponding field-free HOMO–LUMO gap center. Occupied and unoccupied orbitals are shown in blue and orange, respectively. Results for the β-spin orbital energies show minor differences due to the spin-Zeeman interaction (see ESI Section S.7†). | ||
3.2 Molecular AB interferometry
Having explored the magnetic response of isolated molecular rings, we now evaluate their performance as AB interferometers. We focus on the c-P10 molecular ring, which is experimentally well characterized.23,57–59 The neutral c-P10 nanoring has an electronic structure similar to that of an anti-aromatic compound, with a circuit of 4k (k = 30) π-electrons, non-degenerate HOMO and LUMO, and doubly degenerate orbital pairs below and above (assuming high structural symmetry). Experimental NMR spectroscopy has shown that c-P10 is non-aromatic in its neutral state, globally aromatic in its 6+ oxidation state and globally anti-aromatic in its 8+ oxidation state, following the Hückel rule.23 In the context of molecular interferometry, an important advantage of c-P10 is its ability to assume a planar conformation on surfaces, making it suitable for junction fabrication.57 Furthermore, chemical routes have already been developed for functionalizing its diagonally opposite porphyrin units, which could be extended to add anchoring groups for electrode connection.58–60We performed transport calculations using our NEGF+GFN1-xTB-M1 approach for c-P10 coupled to two polyacene leads via meso-acetylene-linkers positioned in a “para-type” configuration (see Fig. 3c) under finite magnetic fields. The α-spin channel electronic transmittance probability through the c-P10 ring junction as a function of the energy (measured with respect to the center of the HOMO–LUMO gap of the isolated ring at B = 0) is shown in Fig. 5 for three perpendicular magnetic field intensities: B = 0 T (Fig. 5a), B = 5 T (Fig. 5b), and B = 10 T (Fig. 5c).
 | ||
| Fig. 5 Transmittance probability T(E) (middle column) of the α-spin channel of the c-P10 molecular AB interferometer (Fig. 3c) under perpendicular magnetic fields of (a) 0, (b) 5, and (c) 10 T. Energies are shown relative to the HOMO–LUMO gap center of the isolated c-P10 molecular ring at 0 T. Left and right columns show a zoom-in on the occupied (blue) and virtual (orange) transmittance peaks, respectively. Transmission probability peaks are labelled with the corresponding isolated c-P10 ring orbital (see ESI Section S.8†). The β-spin channel shows only minor differences due to the spin-Zeeman interaction (see ESI Section S.7†). | ||
In the absence of a magnetic field (Fig. 5a), the doubly degenerate LUMO+1 and LUMO+2 are split by 5 meV due to the lead coupling (see ESI Section S.8†) and only the LUMO+2 conducts current, as manifested by a single broadened Lorentzian-shaped transmittance peak (labelled LUMO+2 in Fig. 5a). At 5 T, another conductance channel opens via the second level, and destructive interference between the neighboring peaks induces an asymmetric shape (peaks labelled LUMO+1 and LUMO+2 in Fig. 5b). Increasing the magnetic field strength to 10 T further increases the inter-peak splitting, resulting in reduced interference and separated, nearly symmetric, Lorentzian-shaped peaks (labelled LUMO+1 and LUMO+2 in Fig. 5c). Similar responses are also seen for the HOMO−1 and HOMO−2 and the HOMO−3 and HOMO−4 pairs as shown in the left-hand side of Fig. 5. Since the peak pairs below the HOMO are considerably broader than those above the LUMO (indicating stronger coupling to the leads), highly asymmetric peaks appear once the additional channels open under a finite magnetic field. We identify these transmittance features as Fano resonances resulting from interference between states localized on the ring and delocalized over the whole device.
In contrast to the degenerate orbital pairs, the LUMO does not conduct current under the considered magnetic field strengths and is not associated with any transmittance peak. The HOMO, on the other hand, conducts current under all magnetic field strengths considered and is associated with a transmittance peak that remains unaffected by the magnetic field, except for a slight energy shift.
The results presented above can be rationalized by analyzing the relevant molecular orbitals in a finite junction model. For example, we can look at the junction orbitals corresponding to the LUMO+2, LUMO+1, and LUMO of the isolated ring. At B = 0, the LUMO+2 of the isolated ring delocalizes over the leads (Fig. 6a) yielding a continuous density across the junction that is manifested as an open channel in the transmittance probability curves. The LUMO+1 and LUMO are fully localized on the ring (Fig. 6c and e), exhibiting no probability density on the acetylene linkers and the adjacent polyacene lead sections, which explains why these orbitals do not carry current. When a 10 T field is applied, the LUMO+2 undergoes a phase shift, but the orbital remains delocalized over the junction (Fig. 6b) and therefore is still conductive. The previously ring-localized LUMO+1 now delocalizes onto the leads (Fig. 6d) with a similar phase shift, yielding a continuous density across the junction that is manifested as a new open channel in the transmittance probability. For the LUMO, no significant changes to the orbital are observed (Fig. 6f), and it remains non-conductive.
The sharp Fano resonances seen in Fig. 5 can be harnessed to modulate the current with accessible magnetic fields. To demonstrate this, we placed 10 meV wide Fermi transport windows around the transmittance peaks marked as HOMO−1 and HOMO−2 (gated to −449 and −439 meV, respectively, as marked by the colored green and red rectangles in Fig. 7a–c) and evaluated the current under various perpendicular magnetic fields using eqn (6). Fig. 7d shows the field dependence of the current, demonstrating that zero-temperature magnetoresistance can be achieved resulting in current variations of up to ∼32–45% at an experimentally accessible magnetic field of 10 T. Notably, by controlling the position of the Fermi transport window using a gate potential one can dictate whether the molecular interferometer will exhibit increased (green) or decreased (red) currents in response to the application of an external perpendicular magnetic field.
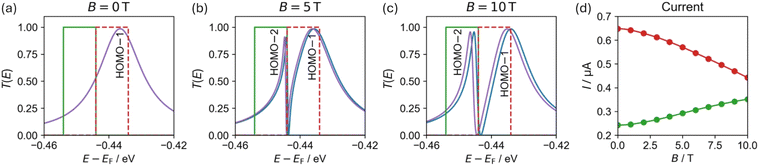 | ||
| Fig. 7 (a–c) Transmittance probability function for α (blue) and β (purple) spin channels in the vicinity of the HOMO−1 and HOMO−2 (see Fig. 5), calculated for a c-P10 junction under a perpendicular magnetic field of (a) 0, (b) 5, and (c) 10 T. (d) Magnetic field dependence of the current, calculated using eqn (6) with 10 meV wide Fermi windows placed around the HOMO−1 and HOMO−2 (red and green), respectively. Transmission probability peaks are labelled with the c-P10 ring orbital they correspond to (see ESI Section S.8†). Results with a 50 meV wide Fermi window show similar qualitative behavior (see ESI Section S.10†). | ||
4 Conclusions
Molecular interferometry offers a unique route for controlling and manipulating the transport behavior and response properties of quantum electronic and spintronic nanodevices. Here, we have demonstrated that molecular AB interferometers based on synthetically available c-P10 porphyrin nanorings can be realized under realistic (∼10 T) magnetic fields. Using a newly developed semi-empirical computational approach for steady-state electronic transport calculations under finite magnetic fields, we have identified several important principles for designing a molecular AB interferometer: (i) the candidate molecular rings should be sufficiently large to allow for significant magnetic response at feasible field intensities, but not so large as to lose coherent transport; (ii) the chemical nature of the molecule should support a low effective mass of the conducting electrons to increase the state energy response towards the external magnetic field; and (iii) weak ring-lead couplings are required to allow for sharp transmittance features that increase the sensitivity of the current to the applied magnetic field. We calculated the effective mass, m*, of electrons in the molecular rings by fitting the variation in their orbital energies, as a function of magnetic field, to the PIR model. This analysis shows that butadiyne-linked nanorings, c-PN, have larger effective masses than edge-fused porphyrin nanobelts, f-PN. On the other hand, c-PNs have larger radii than f-PNs, for a given value of N, and c-PNs are more synthetically accessible.59,60 For the c-P10 molecular ring, we predict the emergence of Fano resonances at finite fields, resulting from field-induced channel openings. At low temperatures, these sharp transmittance features can be harnessed for sensitive interferometry and current switching. At higher electronic temperatures, the broadened Fermi transport window will result in reduced magneto-sensitivity (see ESI Section S.10†). In the model used here, the nanoring is weakly coupled to covalently attached polyacene leads, via meso-acetylene-linkers. An alternative design strategy for reducing the ring-lead couplings to achieve narrower transmittance peaks and higher magneto-sensitivity, would be to use weakly coupled non-covalent stacking interactions to graphene leads.30 In this study, we have focused on an AB interferometer derived from c-P10 because it is realistic to synthesize this nanoring with suitable substituents for attaching electrodes.59,60 On the other hand, the results in Table 2 imply that other molecular rings, such as c-P40, f-P30 and f-P40 would give even more sensitive interferometers.Most previously reported magnetoresistance devices are based on paramagnetic systems, i.e. spin valves.61–64 The principles formulated here for designing diamagnetic molecular magnetoresistance devices are not limited to the c-PN family and are expected to be manifested in other molecular rings, provided they have large radii and charge carriers with small effective masses. For example, toroidal carbon nanotubes55,56 and charged polycyclic systems65 are promising candidates as AB interferometers. This work opens the door for the exploration of a variety of chemical and physical factors, such as the chemical nature of the molecular ring, the identity and position of the linkers, and the character of the metallic leads, which can be tuned to optimize the magnetic response of the system towards the experimental realization of molecular AB interferometry.
Data availability
The data that support the findings of this study are available in the ESI† of this article.Author contributions
H. L. A. and O. H. initiated the project; C. Y. C. performed most of the computational work, supervised by A. M. W.-T.; geometries of porphyrin nanorings and nanobelts were calculated by I. R.; a draft manuscript was written by O. H., C. Y. C., and I. R.; G. H., O. H., and J. E. P. provided an initial NEGF code and participated in discussions regarding the code development and in the early stages of the calculations. All authors discussed the results and contributed towards refining the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We thank the European Research Council (grant 885606, ARO-MAT) and the UKRI (Horizon Europe Guarantee MSCA Postdoctoral Fellowship ElDelPath, EP/X030075/1) for funding. This work is part of a project that has received funding from the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation programme (Project topDFT, Grant agreement No. 772259). O. H. is grateful for the generous financial support of the United States – Israel Binational Science Foundation under NSF-BSF grant #2023602, the Israel Science Foundation under grant #3645/24, the Center for Nanoscience and Nanotechnology of Tel-Aviv University, and the Heinemann Chair of Physical Chemistry. J. E. P. acknowledges support from NSF award number DMR-2318872.Notes and references
- Y. Aharonov and D. Bohm, Phys. Rev., 1959, 115, 485 CrossRef.
- S. Washburn and R. A. Webb, Adv. Phys., 1986, 35, 375 CrossRef CAS.
- E. Cohen, H. Larocque, F. Bouchard, F. Nejadsattari, Y. Gefen and E. Karimi, Nat. Rev. Phys., 2019, 1, 437 CrossRef.
- D.-I. Chang, G. L. Khym, K. Kang, Y. Chung, H.-J. Lee, M. Seo, M. Heiblum, D. Mahalu and V. Umansky, Nat. Phys., 2008, 4, 205 Search PubMed.
- C. Kreisbeck, T. Kramer, S. S. Buchholz, S. F. Fischer, U. Kunze, D. Reuter and A. D. Wieck, Phys. Rev. B:Condens. Matter Mater. Phys., 2010, 82, 165329 CrossRef.
- G. Cohen, O. Hod and E. Rabani, Phys. Rev. B:Condens. Matter Mater. Phys., 2007, 76, 235120 CrossRef.
- O. Hod, R. Baer and E. Rabani, J. Am. Chem. Soc., 2005, 127, 1648 CrossRef CAS PubMed.
- S. Datta, Electronic Transport in Mesoscopic Systems, Cambridge Studies in Semiconductor Physics and Microelectronic Engineering, Cambridge University Press, 1995 Search PubMed.
- D. K. Ferry, Transport in Semiconductor Mesoscopic Devices, IOP Publishing, 2nd edn, 2020, 2053–2563 Search PubMed.
- A. Bachtold, C. Strunk, J.-P. Salvetat, J.-M. Bonard, L. Forró, T. Nussbaumer and C. Schönenberger, Nature, 1999, 397, 673 CrossRef CAS.
- S. Zaric, G. N. Ostojic, J. Kono, J. Shaver, V. C. Moore, M. S. Strano, R. H. Hauge, R. E. Smalley and X. Wei, Science, 2004, 304, 1129 CrossRef CAS PubMed.
- J. A. Alexander-Webber, C. Faugeras, P. Kossacki, M. Potemski, X. Wang, H. D. Kim, S. D. Stranks, R. A. Taylor and R. J. Nicholas, Nano Lett., 2014, 14, 5194 CrossRef CAS PubMed.
- O. Hod, R. Baer and E. Rabani, J. Phys.: Condens. Matter, 2008, 20, 383201 CrossRef PubMed.
- O. Hod, R. Baer and E. Rabani, J. Phys. Chem. B, 2004, 108, 14807 CrossRef CAS.
- O. Hod, E. Rabani and R. Baer, Acc. Chem. Res., 2006, 39, 109 CrossRef CAS PubMed.
- J. W. Linnett, Wave Mechanics and Valency, Methuen, London, 1960 Search PubMed.
- M. Solà and F. M. Bickelhaupt, J. Chem. Educ., 2022, 99, 3497 CrossRef PubMed.
- D. Rai, O. Hod and A. Nitzan, J. Phys. Chem. C, 2010, 114, 20583 CrossRef CAS.
- D. Rai, O. Hod and A. Nitzan, Phys. Rev. B:Condens. Matter Mater. Phys., 2012, 85, 155440 CrossRef.
- GFN1-xTB is a semi-empirical tight-binding method originally developed by the Grimme group, see ref. 40 and 41. The GFN1-xTB belongs to the family of GFN methods, which includes the GFN-FF and GFN2-xTB methods. GFN1-xTB is an acronym for Geometry, Frequency, Noncovalent, eXtended Tight-Binding. GFN1-xTB-M1 is the GFN1-xTB method with the M1 extension, which includes the interaction with an arbitrary strength uniform magnetic field, for more details see ref. 42.
- D. V. Kondratuk, L. M. A. Perdigão, A. M. S. Esmail, J. N. O'Shea, P. H. Beton and H. L. Anderson, Nat. Chem., 2015, 7, 317 CrossRef CAS PubMed.
- Jirásek, H. L. Anderson and M. D. Peeks, Acc. Chem. Res., 2021, 54, 3241 CrossRef PubMed.
- M. Rickhaus, M. Jirasek, L. Tejerina, H. Gotfredsen, M. D. Peeks, R. Haver, H.-W. Jiang, T. D. W. Claridge and H. L. Anderson, Nat. Chem., 2020, 12, 236 CrossRef CAS PubMed.
- C. J. Judd, A. S. Nizovtsev, R. Plougmann, D. V. Kondratuk, H. L. Anderson, E. Besley and A. Saywell, Phys. Rev. Lett., 2020, 125, 206803 CrossRef CAS PubMed.
- M. Jirásek, M. Rickhaus, L. Tejerina and H. L. Anderson, J. Am. Chem. Soc., 2021, 143, 2403 CrossRef PubMed.
- V. Posligua, A. Aziz, R. Haver, M. D. Peeks, H. L. Anderson and R. Grau-Crespo, J. Phys. Chem. C, 2018, 122, 23790 CrossRef CAS.
- M. Vitek, J.-R. Deng, H. L. Anderson and I. Rončević, ACS Nano, 2025, 19, 1405 CrossRef CAS PubMed.
- A. Tsuda and A. Osuka, Science, 2001, 293, 79 CrossRef CAS PubMed.
- T. Tanaka and A. Osuka, Chem. Soc. Rev., 2015, 44, 943 RSC.
- Z. Chen, J.-R. Deng, S. Hou, X. Bian, J. L. Swett, Q. Wu, J. Baugh, G. A. D. Briggs, J. A. Mol, C. J. Lambert, H. L. Anderson and J. O. Thomas, J. Am. Chem. Soc., 2023, 145, 15265 CrossRef CAS PubMed.
- J.-R. Deng, M. T. González, H. Zhu, H. L. Anderson and E. Leary, J. Am. Chem. Soc., 2024, 146, 3651 CrossRef CAS PubMed.
- J. C. Cuevas and E. Scheer, Molecular Electronics: An Introduction to Theory and Experiment. World Scientific, 1st edn, 2010 Search PubMed.
- M. Di Ventra, Electrical Transport in Nanoscale Systems, Cambridge University Press, 2008 Search PubMed.
- G. Cuniberti, K. Richter and G. Fagas, Introducing Molecular Electronics, Lecture Notes in Physics, Springer, 2005, vol. 680 Search PubMed.
- QUEST, A rapid development platform for QUantum Electronic Structure Techniques, https://quest.codes/.
- E. I. Tellgren, A. M. Teale, J. W. Furness, K. K. Lange, U. Ekström and T. Helgaker, J. Chem. Phys., 2014, 140, 034101 CrossRef CAS PubMed.
- J. W. Furness, J. Verbeke, E. I. Tellgren, S. Stopkowicz, U. Ekström, T. Helgaker and A. M. Teale, J. Chem. Theory Comput., 2015, 11, 4169 CrossRef CAS PubMed.
- E. I. Tellgren, A. Soncini and T. Helgaker, J. Chem. Phys., 2008, 129, 154114 CrossRef PubMed.
- E. I. Tellgren, T. Helgaker and A. Soncini, Phys. Chem. Chem. Phys., 2009, 11, 5489 RSC.
- S. Grimme, C. Bannwarth and P. Shushkov, J. Chem. Theory Comput., 2017, 13, 1989 CrossRef CAS PubMed.
- C. Bannwarth, E. Caldeweyher, S. Ehlert, A. Hansen, P. Pracht, J. Seibert, S. Spicher and S. Grimme, Wiley Interdiscip. Rev.: Comput. Mol. Sci., 2021, 11, e1493 CAS.
- C. Y. Cheng and A. M. Wibowo-Teale, J. Chem. Theory Comput., 2023, 19, 6226 CrossRef CAS PubMed.
- I. Casademont-Reig, R. Guerrero-Avilés, E. Ramos-Cordoba, M. Torrent-Sucarrat and E. Matito, Angew. Chem., Int. Ed., 2021, 60, 24080 CrossRef CAS PubMed.
- J.-R. Deng, D. Bradley, M. Jirásek, H. L. Anderson and M. D. Peeks, Angew. Chem., Int. Ed., 2022, 61, e202201231 CrossRef CAS PubMed.
- I. Casademont-Reig, L. Soriano-Agueda, E. Ramos-Cordoba, M. Torrent-Sucarrat and E. Matito, Angew. Chem., Int. Ed., 2022, 61, e202206836 CrossRef CAS PubMed.
- The effective masses for electrons in the conduction band of infinite linear porphyrin ribbons, analogous to f-PN, are 0.0054me for Zn-metallated porphyrins and 0.042me for Ni-metallated porphyrins, calculated using the GFN1-xTB method with DFTB+ (see ESI, Section S.11†). These values fit with the trend of decreasing m* with increasing ring size (Table 1). However, comparison with previously published values of m* for the conduction band of the infinite linear ribbon, with Ni cations instead of Zn, indicates that GFN1-xTB underestimates effective masses; see: (a) H. Zhu, Q. Chen, I. Rončević, K. E. Christensen and H. L. Anderson, Angew. Chem., Int. Ed., 2023, 62, e202307035 CrossRef PubMed; (b) Q. Chen, A. Lodi, H. Zhang, A. Gee, H. I. Wang, F. Kong, M. Clarke, M. Edmondson, J. Hart, J. N. O'Shea, W. Stawski, J. Baugh, A. Narita, A. Saywell, M. Bonn, K. Müllen, L. Bogani and H. L. Anderson, Nat. Chem., 2024, 16, 1133–1140 CrossRef CAS PubMed.
- G. te Velde, F. M. Bickelhaupt, E. J. Baerends, C. Fonseca Guerra, S. J. A. van Gisbergen, J. G. Snijders and T. Ziegler, J. Comput. Chem., 2001, 22, 931 CrossRef CAS.
- ADF 2023.1, SCM, Theoretical Chemistry, Vrije Universiteit, Amsterdam, The Netherlands, 2023, http://www.scm.com.
- J. S. Seldenthuis, Electrical and Mechanical Effects in Single-Molecule Junctions, PhD thesis, Delft University of Technology, 2011.
- A. Pecchia and A. Di Carlo, Rep. Prog. Phys., 2004, 67, 1497 CrossRef CAS.
- A. Pecchia, A. Di Carlo, A. Gagliardi, S. Sanna, Th. Fraunheim and R. Gutierrez, Nano Lett., 2004, 4, 2109 CrossRef CAS.
- A. Di Carlo, M. Gheorghe, P. Lugli, M. Stenberg, G. Seifert and Th. Frauenheim, Physica B, 2002, 314, 86 CrossRef CAS.
- N. W. Ashcroft and N. D. Mermin, Solid State Physics, Saunders Collage Publishing, 1976 Search PubMed.
- F. London, J. Phys. Radium, 1937, 8, 397 CrossRef CAS.
- R. C. Haddon, Nature, 1997, 388, 32 CrossRef.
- M. Orozco-Ic, M. Dimitrova, J. Barroso, D. Sundholm and G. Merino, J. Phys. Chem. A, 2021, 125, 5753 CrossRef CAS PubMed.
- A. Summerfield, M. Baldoni, D. V. Kondratuk, H. L. Anderson, S. Whitelam, J. P. Garrahan, E. Besley and P. H. Beton, Nat. Commun., 2019, 10, 2932 CrossRef PubMed.
- S. Richert, J. Cremers, H. L. Anderson and C. R. Timmel, Chem. Sci., 2016, 7, 6952 RSC.
- J. Cremers, S. Richert, D. V. Kondratuk, T. D. W. Claridge, C. R. Timmel and H. L. Anderson, Chem. Sci., 2016, 7, 6961 RSC.
- C. Roche, Q. Luo, G. Gil-Ramírez, H.-W. Jiang, D. R. Kohn, Y. Xiong, A. L. Thompson and H. L. Anderson, J. Org. Chem., 2017, 82, 7446 CrossRef CAS PubMed.
- L. Guo, S. Hu, X. Gu, R. Zhang, K. Wang and W. Yan, Adv. Mater., 2024, 36, 2301854 CrossRef CAS PubMed.
- T. Endo, S. Tsuruoka, Y. Tadano, S. Kaneta-Takada, Y. Seki, M. Kobayashi, L. D. Anh, M. Seki, H. Tabata, M. Tanaka and S. Ohya, Adv. Mater., 2023, 35, 2300110 CrossRef CAS PubMed.
- H. Tahir, N. Eedugurala, S.-N. Hsu, P. Mahalingavelar, B. M. Savoie, B. W. Boudouris and J. D. Azoulay, Adv. Mater., 2024, 36, 2306389 CrossRef CAS PubMed.
- Y. H. Lee, Science, 2023, 382, eadl0823 CrossRef PubMed.
- Q. Jiang, Yi. Han, X. Hou, S. Wu, T. Xu, M. Orozco-Ic, G. Merino and C. Chi, Angew. Chem., Int. Ed., 2025, 64, e202416833 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d4sc07992b |
| This journal is © The Royal Society of Chemistry 2025 |