Open Access Article
Open Access ArticleReticular chemistry guided function customization: a case study of constructing low-polarity channels for efficient C3H6/C2H4 separation†
Jiantang
Li‡
 a,
Zitong
Song‡
a,
Xia
Zhou
a,
Xue
Wang
a,
Meng
Feng
a,
Dongmei
Wang
*a and
Banglin
Chen
a,
Zitong
Song‡
a,
Xia
Zhou
a,
Xue
Wang
a,
Meng
Feng
a,
Dongmei
Wang
*a and
Banglin
Chen
 *ab
*ab
aKey Laboratory of the Ministry of Education for Advanced Catalysis Materials, College of Chemistry and Materials Sciences, Zhejiang Normal University, Jinhua 321004, P. R. China. E-mail: dmwang@zjnu.edu.cn
bFujian Key Laboratory of Polymer Materials, College of Chemistry and Materials Sciences, Fujian Normal University, Fujian, 350007, P. R. China. E-mail: banglin.chen@fjnu.edu.cn
First published on 18th March 2025
Abstract
Guided by the principles of reticular chemistry, we have successfully presented the “bending-bridge” strategy, achieving an extraordinary function-targeted assembly by ingeniously redirecting the coordination direction of traditional SBUs. This led to the synthesis of a novel metal–organic framework (MOF), {[CH3NH3][InTPCA]·2H2O·NMF·DMF} (ZJNU-401). The smart design of bending branches within the ligand effectively transformed the tetrahedrally coordinated mononuclear In(III) into a square-planar configuration, thereby avoiding the introduction of open metal sites (OMSs) commonly associated with traditional ssb networks and creating a low-polarity pore surface environment. ZJNU-401 exhibits an optimal pore system that enhances its efficacy for high uptake of C3H6 and C2H6 over C2H4. The remarkable selectivity ratio of C3H6 to C2H4 reaches up to 15.45, alongside efficient one-step purification of C2H4 (>99.95%) from the mixture of C3H6/C2H4. DFT calculations revealed that multiple O active sites within nonpolar pores provide stronger interactions with both C3H6 and C2H6 compared to those with C2H4.
Introduction
The methanol-to-olefin (MTO) process has become an important sustainable technology that allows non-petroleum resources to be converted into low-carbon olefins like ethylene (C2H4) and propylene (C3H6).1–4 In the chemical industry, these olefins are essential raw materials, especially for the manufacture of polymers. The MTO method produces trace amounts of ethane (C2H6) impurities in addition to around 51 wt% C2H4 and 21 wt% C3H6.5–7 The purification of MTO products is crucial for obtaining high-purity C2H4 and C3H6, which are necessary for the sustainability and effectiveness of the chemical manufacturing industry. The coexistence of these components in combination hampers downstream applications. However, it remains highly challenging to separate these gases due to their similar physical properties. Compared to traditional energy-intensive separation processes, physical adsorption technology based on porous materials offers a safer, more energy-efficient, and environmentally friendly alternative, presenting significant potential for sustainable molecular separations, particularly in the purification of MTO products.8–10The rapid development of reticular chemistry has allowed the integration of various building blocks into extendable frameworks via strong bonds, leading to the emergence of novel porous materials such as metal–organic frameworks (MOFs).11–17 Furthermore, reticular chemistry serves as a powerful strategy for tailoring specific pore environments and structures to achieve targeted separations. For instance, in the context of MTO product purification, the design of low-polarity pore surfaces enables weak interactions with certain molecules, facilitating effective kinetic separations. This approach has proven highly effective for distinguishing molecules with similar physical properties, such as olefins and alkanes.18 Therefore, employing reticular chemistry to design MOFs with precisely tuned pore environments is not only necessary but also a promising pathway to optimize separation efficiency and address the challenges of sustainable chemical manufacturing.
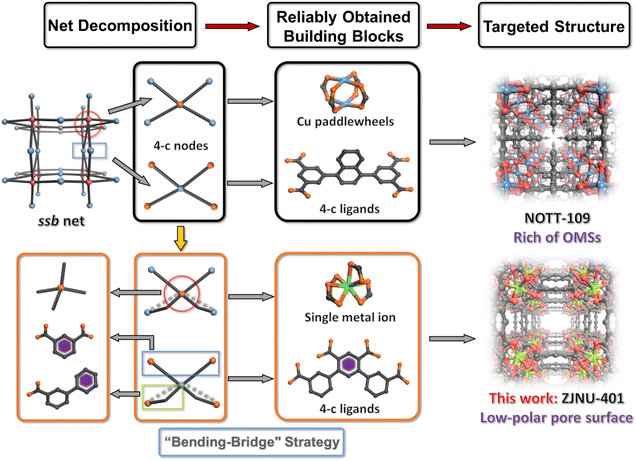
Not all nets are ideal targets for reticular chemistry, which is an important consideration in MOF design. As is known to all, transitivity [pqrs] refers to a structure that has p types of vertexes, q types of edges, r types of faces, and s types of tiles. Edge-transitive nets, which have only one type of edge (q = 1), are quite important.19,20 In 2021, Eddaoudi et al. identified 53 three-dimensional (3D) nets within this category that are particularly suitable for practical applications in reticular chemistry, being considered ideal candidates for the design and construction of new structures.15 One of the most important principles in constructing structures based on edge-transitive nets is building block selection, which includes the design of organic linkers as well as the selection of inorganic building units that are typically aligned with the desired functionality.21–23 For example, the ssb net, which is a 4,4-c net formed by sql layers supported by pillars, has been realized using the Cu-paddlewheel and 4-c bulky organic linkers, as reported in NOTT-109 (ref. 24) and MMPF-1.25 However, such designs pose challenges in creating low-polarity pore environments, as it is well known that Cu-paddlewheel SBUs provide abundant open metal sites (OMSs), which can be disadvantageous in certain separation applications, such as that of C2H6/C2H4.26,27
Developing an effective and controlled design strategy for assembling commonly used secondary building units (SBUs) that satisfies the functional needs while fulfilling the geometric requirements of specific topologies remains one of the most significant challenges in reticular chemistry.28,29 Here, abandoning paddlewheel type SBUs, like Cu or Zn-paddlewheel, complicates the selection of alternative 4-c inorganic SBUs that can achieve square-planar connectivity. Other highly connected polynuclear metal SBUs, although possessing four connections, still do not meet functional requirements due to the presence of OMSs. A straightforward alternative is to use mononuclear metal SBUs based on single metal ions.30,31 For achieving 4-c connectivity with carboxylate groups, In(III) stands out as an optimal choice, forming tetrahedral structures that are representative SBUs in ZMOFs.32,33 These materials highlight the versatility of indium in creating robust frameworks without the complications associated with OMSs.
The next challenge is to transform these tetrahedral nodes into the square-planar 4-c nodes required by ssb nets. This is where the “bending-bridge” strategy in ligand design becomes essential. By introducing a well-planned bend at a specific point in the ligand, the directionality of coordination can be effectively altered. This enables the transformation of tetrahedral 4-c nodes into square-planar 4-c nodes, allowing them to conform to the structural requirements of the ssb network.19,24 This approach offers a flexible and innovative pathway to achieve the desired geometry of the ssb net without compromising the integrity or functionality of the framework. Specifically, considering that the bending of the ligand results in a reduction of effective connection lengths, it is necessary to incorporate redundant space in the design of the bending bridges (Scheme 1). A symmetry-reduced ligand, H4TPCA ([1,1′:3′,1′′]terphenyl-3,4′,6′,3′′-tetracarboxylic acid), has been employed in this system, taking into account two advantages: (1) the bending bridge can achieve large-angle rotations, thus adapting to the demands of the corresponding topology; and (2) the ligand integrates both the isophthalic acid part and the bending bridge part, allowing for a balance between rigidity and flexibility. Ultimately, as we anticipated, we truly succeeded in fabricating a low-polarity pore environmental compound (ZJNU-401) with the ssb network lacking OMSs. This facilitates the preferential selective adsorption of propylene and ethane (C3H6/C2H4: 15.45 and C2H6/C2H4: 1.75), thereby achieving efficient one-step separation and purification of ethylene (>99.95%) in the MTO process.
Results and discussion
Synthesis of ZJNU-401
In(NO3)3·4H2O (8 mg, 0.021 mmol), H4TPCA (3 mg, 0.074 mmol), N-methylformamide (NMF) (0.5 mL), N,N-dimethylformamide (DMF) (0.5 mL), deionized water (H2O, 0.5 mL), and HNO3 (500 μL, 2.2 mL of HNO3 in 10 mL NMF) were mixed in a 20 mL vial and then placed in a precision oven for 12 h at 105 °C. After cooling to room temperature, colorless polyhedral crystals were collected and washed frequently with fresh NMF (yield 67.8%, based on H4TPCA). Elemental analysis (%) Calcd for ZJNU-401 {[CH3NH3][InTPCA]·2H2O·NMF·DMF}: C, 46.86; H, 4.46; N, 5.86. Found: C, 47.01; H, 4.27; N, 5.80.Crystal structure
Herein, the solvothermal reaction of a well-designed ligand, H4TPCA, with In(NO3)3·4H2O, and HNO3 in the DMF/NMF/H2O system, enabled the construction of the targeted ssb net by employing the “bending-bridge” strategy. Single-crystal X-ray diffraction structural analysis reveals that ZJNU-401 crystallizes in the rhombohedral crystal system with the Fmm2 space group where its asymmetric unit consists of eight In(III) ions, torsional deprotonated ligand TPCA4− and two [CH3NH3]+ generated from the decomposition of NMF during solvothermal synthesis (Fig. S1†). As shown in Fig. 1a, the bending branch in the H4TPCA ligand features a wider coordination angle compared to the H4TPTA ligand, which can only rotate linearly. Additionally, the isophthalic acid part of the ligand coordinates with the metal In(III), occupying two of the four connectivities. The connections of the bending branches to the metal In(III), similar to a bending bridge, significantly alter the connection angle of the ligand, transforming the expected tetrahedrally coordinated mononuclear In(III) into a square-planar configuration. The asymmetric unit is illustrated by the crystal structure with a thermal ellipsoid diagram in Fig. S2.† This effective design strategy allows for the remarkable assembly of these two SBUs, which initially appear to be incompatible with the ssb topological node configuration, into the network. Moreover, in ZJNU-401, the introduction of open metal sites is effectively avoided, facilitating the formation of low-polarity pore surfaces. As shown in Fig. 1b and c, the bending branches of the ligand result in the straight channels being divided into a series of pockets, with a larger inner diameter of 11 Å and a smaller window size of 5 Å. Compared to the standard ssb network, ZJNU-401 features a more diverse pore channel structure.Thermal and chemical stability
The experimental PXRD results show good agreement with simulation data on crystallographic information (Fig. S3†), verifying phase purity and stability of the synthesized sample. The chemical resistance of ZJNU-401 to water, HCl (aq, pH = 3) and NaOH (aq, pH = 11) was evaluated over a 12-hours period while maintaining an unchanged PXRD pattern. These tests demonstrate the robust chemical stability of the skeleton in both aqueous and harsh environments. Additionally, TGA analysis was conducted on ZJNU-401 revealing an initial weight loss at 160 °C attributed to the removal of solvent molecules. Thermal decomposition commences when the temperature exceeds 300 °C resulting in the formation of corresponding oxides (Fig. S4†). These findings confirm the excellent chemical stability of ZJNU-401.Gas adsorption of ZJNU-401
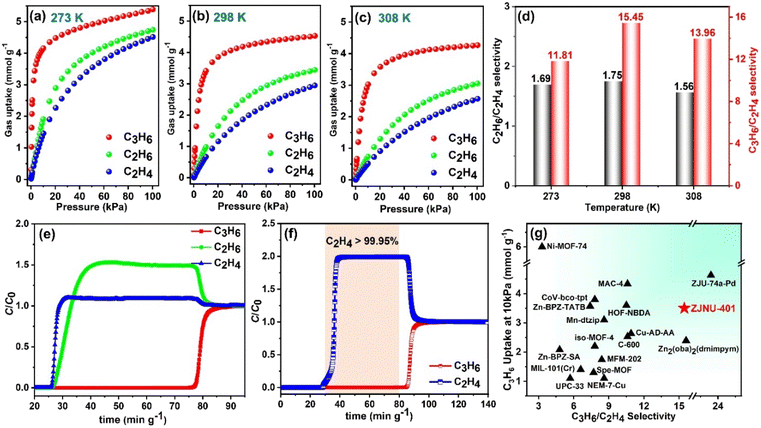
The permanent porosity of ZJNU-401 was investigated through nitrogen adsorption at 77 K, which exhibited a reversible type-I adsorption isotherm with a maximum saturated uptake capacity and Brunauer–Emmett–Teller (BET) values of 280 cm3 g−1 and 1224 m2 g−1, respectively. Pore size distribution was determined using the density functional theory (DFT) method (Fig. S5†). Notably, JLU-MOF121 with twofold interpenetrated and In-TPTAB with oversized pores did not exhibit any N2 adsorption behavior.34,35 The unique pore environment characteristics of ZJNU-401, including uncoordinated O atoms, a saturated metal coordination mode, and an appropriate pore size, render it a promising candidate for investigating gas adsorption and separation properties.36,37 Consequently, one-component adsorption isotherms for C3H6, C2H4 and C2H6 were evaluated at 273/298/308 K under 1 bar. As illustrated in Fig. 2a–c and S6,†ZJNU-401 demonstrates exceptional adsorption capacities of 5.38/4.53/4.28 mmol g−1 for C3H6 and 4.74/3.43/3.05 mmol g−1 for C2H6, both significantly higher than that of 4.51/2.94/2.57 mmol g−1 for C2H4. These results can be attributed to the smaller molecular size and fewer hydrogen atoms present in C2H4, leading to weaker host–guest interactions compared to the other gases studied.38–40 In Fig. S5,† especially in the low-pressure region, the affinity of ZJNU-401 for C3H6, C2H6 and C2H4 shows a decreasing trend. This is consistent with the results of their respective adsorption enthalpies Qst (C3H6) = 32 kJ mol−1, Qst (C2H6) = 29 kJ mol−1, Qst (C2H4) = 19 kJ mol−1 as depicted in Fig. S7.†IAST selectivity and breakthrough testing of ZJNU-401
Remarkably, the adsorption capacity of ZJNU-401 for C3H6 and C2H6 is significantly higher than that for C2H4, underscoring its suitability as an effective adsorbent material for the purification of C2H4, particularly in MTO processes. Consequently, we employed ideal adsorption solution theory (IAST) to calculate the separation of equimolar two-component gas mixtures at temperatures of 273/298/308 K under 100 kPa. As illustrated in Fig. 2d and S8, S9,†ZJNU-401 exhibited favourable selectivity values of 1.69/1.75/1.56 for C2H6/C2H4. This enhanced selectivity can be attributed to the slightly higher polarizability of C2H6 (44.7 × 1025 cm3) and more hydrogen atoms compared to that of C2H4 (42.52 × 1025 cm3). Furthermore, exceptional selectivity values of 11.81/15.45/13.96 were achieved for C3H6/C2H4, surpassing those reported for many well-known compounds in this field. Moreover, our calculations indicate that ZJNU-401 holds significant promise for efficiently purifying C2H4 from gas mixtures to yield polymer-grade olefin products. A comparison of ZJNU-401's separation results at three different temperatures reveals that 298 K provides optimal purification conditions. Thus, a dynamic breakthrough experiment was conducted at this temperature and pressure using activated ZJNU-401 to separate mixtures of C3H6/C2H6/C2H4 (v/v/v, 1/1/1) and C3H6/C2H4 (v/v, 1/1) (Fig. 2e and f). The carrier gas used was Ar with a total flow rate of 2 mL min−1. Initially, C2H4 elutes from the packed column at around 28 min while no detection of C3H6 occurs until approximately 87 min. As a result, high-purity C2H4 (>99.95%) can be continuously obtained over an extended duration lasting up to 59 min. Furthermore, while good adsorption is desirable, good repeatability is essential for the practical application of porous adsorbents. We performed multiple cycles of adsorption using C2H4 gas and the breakthrough experiment after exposed to air for two months, and performed adsorption tests after six hours of immersion in different acid–base solutions (Fig. S10–S12†). The results indicate that ZJNU-401 remains virtually unchanged, thereby confirming its excellent reproducibility. These findings underscore the potential applicability of ZJNU-401 as an excellent material for industrial MTO product separation. In Fig. 2g, we compare selectivity for both C3H6/C2H4 and C3H6 adsorption capacity at a pressure level of 10 kPa against other reported MOFs at 298 K.41–55 Notably, ZJNU-401 demonstrates considerable advantages in addressing the recognized “trade-off” dilemma.Theoretical simulation
To gain further insights into the host–guest interactions between the framework and the adsorbate, the fixed loading task in the Sorption module based on the metropolis Monte Carlo method in MS 2020 was employed to determine the adsorption sites of acetylene, ethane and ethylene molecules at the MOF of ZJNU-401. Subsequently, binding energy between the adsorbates and framework was calculated for the low energy structure obtained from adsorption site calculation. As illustrated in Fig. 3 and S13,† the optimal adsorption sites for olefins are located near the [In(COO)4]− cluster within the channel, while alkanes tend to be surrounded by nonpolar aromatic rings. In ZJNU-401, we observed that all three gases interact with aromatic carboxylic acid backbones through multiple C–H⋯O/C hydrogen bonds and C–H⋯π interactions. For C3H6, there are six short H⋯C/O distances ranging from 2.608 to 3.095 Å, which is significantly greater than those of C2H6 and C2H4, resulting in a stronger binding affinity. This enhanced interaction can be attributed to the larger polarizability and size of the propylene molecule, which facilitates more extensive interactions with the framework (Fig. S14†). In contrast to C2H4, the saturated hydrocarbon C2H6 has a higher number of hydrogen atoms. Thus, it is more likely to be trapped by low-polarity benzene rings, forming approximately twice as many interactions at distances from 3.189 to 3.538 Å. Moreover, the host–guest binding energies are calculated as ΔE (C3H6) = 57 kJ mol−1, ΔE (C2H6) = 47 kJ mol−1, and ΔE (C2H4) = 40 kJ mol−1, respectively. These results indicate that both the low-polarity pore surface of ZJNU-401 and the intrinsic properties of the gases are conducive to the selective capture of C2H4 from mixtures containing C3H6/C2H4 and C2H6/C2H4. Finally, a comparison of the bond lengths of corresponding parts in the calculated structure and the host-crystal structure of ZJNU-401 was carried out. Since ZJNU-401 is a rigid framework, gas adsorption has a negligible influence on the host-crystal structures, resulting in insignificant bond-length changes (Fig. S15†).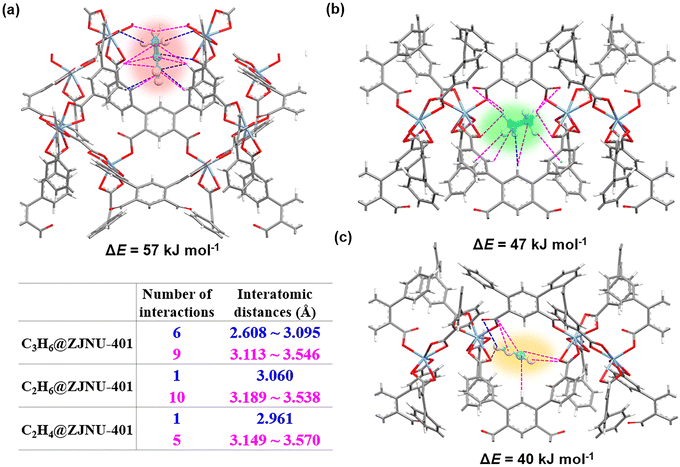 | ||
| Fig. 3 Calculation of the optimized adsorption sites for (a) C3H6 and (b) C2H6 and (c) C2H4 within ZJNU-401, and comparison of the host–guest interactions among them. | ||
Conclusions
In summary, the introduction of a strategically designed bending angle at a specific point in the ligand facilitates the formation of square-planar 4-c nodes, providing a flexible and innovative pathway to achieve the desired geometry of the ssb net. The low polarity pore surfaces with accessible O active sites endow ZJNU-401 with preferential adsorption for C3H6 and C2H6 over C2H4, resulting in significant selectivity of C3H6/C2H4 (15.45) and C2H6/C2H4 (1.75). High-purity C2H4 (>99.95% pure) was produced from C3H6/C2H4 mixtures with a low-energy footprint. Multiple cycle experiments confirm its excellent reproducibility. DFT calculation results show that both the quantity and strength of host–guest interactions synergistically enhance the affinity of ZJNU-401 for C3H6 and C2H6 over C2H4. This work establishes ZJNU-401 as a benchmark MOF for challenging binary separations involving C2H6/C2H4 and C3H6/C2H4, while also providing valuable insights to facilitate the design of ideal target nets based on advanced reticular chemistry for application in C2H4 purification.Data availability
The authors confirm that the data supporting the findings of this study are available within the article [and/or its ESI†].Author contributions
Jiantang Li: writing – original draft, supervision; Zitong Song: preparation and synthesis of materials; Xia Zhou: experimental data analysis; Xue Wang: validation, visualization; Meng Feng: resources, software; Dongmei Wang: writing – review & editing, supervision, data curation; Banglin Chen: writing – review & editing, supervision.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (52102189, 22301154 and W2431013) and the Jinhua City Project (2023-4-019).Notes and references
- L. Hengbo, Z. Yunzhe, C. Cheng, L. Yashuang, L. Zheng, W. Mingyan and H. Maochun, Inorg. Chem., 2024, 63, 21548–21554 Search PubMed.
- P. Tian, Y. Wei, M. Ye and Z. Liu, ACS Catal., 2015, 5, 1922–1938 CAS.
- M. Yang, D. Fan, Y. Wei, P. Tian and Z. Liu, Adv. Mater., 2019, 31, 1902181 CAS.
- G.-D. Wang, Y.-Z. Li, W.-J. Shi, L. Hou, Y.-Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2022, 61, e202205427 CAS.
- C. Wang, L. Yang, M. Gao, X. Shao, W. Dai, G. Wu, N. Guan, Z. Xu, M. Ye and L. Li, J. Am. Chem. Soc., 2022, 144, 21408–21416 CAS.
- A. Cesarini, S. Mitchell, G. Zichittella, M. Agrachev, S. P. Schmid, G. Jeschke, Z. Pan, A. Bodi, P. Hemberger and J. Pérez-Ramírez, Nat. Catal., 2022, 5, 605–614 CAS.
- Z. Belohlav, P. Zamostny and T. Herink, Chem. Eng. Process., 2003, 42, 461–473 CAS.
- G. Zhen, Y. Liu, Y. Zhou, Z. Ji, H. Li, S. Zou, W. Zhang, Y. Li, Y. Liu, C. Chen and M. Wu, ACS Appl. Mater. Interfaces, 2024, 16, 1179–1186 CAS.
- R. Qin, Z. Zhao, N. Shi, G. Chen, M. Yang, Q. Ren, H. Ji and K. Chai, Microporous Mesoporous Mater., 2024, 373, 113134 CAS.
- Z. Di, Z. Ji, C. Chen, R. Krishna, D. Yuan, M. Hong and M. Wu, Chem. Eng. J., 2024, 493, 152442 CrossRef CAS.
- O. M. Yaghi, M. O'Keeffe, N. W. Ockwig, H. K. Chae, M. Eddaoudi and J. Kim, Nature, 2003, 423, 705–714 CrossRef CAS PubMed.
- R. Freund, S. Canossa, S. M. Cohen, W. Yan, H. Deng, V. Guillerm, M. Eddaoudi, D. G. Madden, D. Fairen-Jimenez, H. Lyu, L. K. Macreadie, Z. Ji, Y. Zhang, B. Wang, F. Haase, C. Wöll, O. Zaremba, J. Andreo, S. Wuttke and C. S. Diercks, Angew. Chem., Int. Ed., 2021, 60, 23946–23974 CrossRef CAS PubMed.
- W. Xu, B. Tu, Q. Liu, Y. Shu, C.-C. Liang, C. S. Diercks, O. M. Yaghi, Y.-B. Zhang, H. Deng and Q. Li, Nat. Rev. Mater., 2020, 5, 764–779 CrossRef CAS.
- Z. Chen, K. O. Kirlikovali, P. Li and O. K. Farha, Acc. Chem. Res., 2022, 55, 579–591 CrossRef CAS PubMed.
- V. Guillerm and M. Eddaoudi, Acc. Chem. Res., 2021, 54, 3298–3312 CrossRef CAS PubMed.
- H. Jiang, D. Alezi and M. Eddaoudi, Nat. Rev. Mater., 2021, 6, 466–487 CrossRef CAS.
- O. M. Yaghi, Nano Lett., 2020, 20, 8432–8434 CrossRef CAS PubMed.
- R.-B. Lin, H. Wu, L. Li, X.-L. Tang, Z. Li, J. Gao, H. Cui, W. Zhou and B. Chen, J. Am. Chem. Soc., 2018, 140, 12940–12946 CrossRef CAS PubMed.
- M. Li, D. Li, M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2014, 114, 1343–1370 CrossRef CAS PubMed.
- Z. Chen, H. Jiang, M. O'Keeffe and M. Eddaoudi, Faraday Discuss., 2017, 201, 127–143 RSC.
- L. Liu and S. G. Telfer, J. Am. Chem. Soc., 2015, 137, 3901–3909 CrossRef CAS PubMed.
- B. Zheng, J. Bai, J. Duan, L. Wojtas and M. J. Zaworotko, J. Am. Chem. Soc., 2011, 133, 748–751 CrossRef CAS PubMed.
- J. Li, P. M. Bhatt, J. Li, M. Eddaoudi and Y. Liu, Adv. Mater., 2020, 32, 2002563 CrossRef CAS PubMed.
- X. Lin, I. Telepeni, A. J. Blake, A. Dailly, C. M. Brown, J. M. Simmons, M. Zoppi, G. S. Walker, K. M. Thomas, T. J. Mays, P. Hubberstey, N. R. Champness and M. Schröder, J. Am. Chem. Soc., 2009, 131, 2159–2171 CrossRef CAS PubMed.
- X.-S. Wang, L. Meng, Q. Cheng, C. Kim, L. Wojtas, M. Chrzanowski, Y.-S. Chen, X. P. Zhang and S. Ma, J. Am. Chem. Soc., 2011, 133, 16322–16325 CrossRef CAS PubMed.
- B. Li, Y. Zhang, R. Krishna, K. Yao, Y. Han, Z. Wu, D. Ma, Z. Shi, T. Pham, B. Space, J. Liu, P. K. Thallapally, J. Liu, M. Chrzanowski and S. Ma, J. Am. Chem. Soc., 2014, 136, 8654–8660 CrossRef CAS PubMed.
- L. Zhang, L. Li, E. Hu, L. Yang, K. Shao, L. Yao, K. Jiang, Y. Cui, Y. Yang, B. Li, B. Chen and G. Qian, Adv. Sci., 2020, 7, 1901918 CrossRef CAS PubMed.
- M. O'Keeffe and O. M. Yaghi, Chem. Rev., 2012, 112, 675–702 CrossRef PubMed.
- M. J. Kalmutzki, N. Hanikel and O. M. Yaghi, Sci. Adv., 2018, 4, 9180 CrossRef PubMed.
- V. Kumar Maka, P. Tamuly, S. Jindal and J. Narasimha Moorthy, Appl. Mater. Today, 2020, 19, 100613 CrossRef.
- Y.-Q. Zhang, L. Liu, W.-Z. Li, B.-H. Wu, C.-N. Li, J.-Q. Chu and Z.-B. Han, Inorg. Chem., 2024, 63, 7705–7713 CrossRef CAS PubMed.
- M. Eddaoudi, D. F. Sava, J. F. Eubank, K. Adil and V. Guillerm, Chem. Soc. Rev., 2015, 44, 228–249 RSC.
- M. H. Alkordi, J. A. Brant, L. Wojtas, V. C. Kravtsov, A. J. Cairns and M. Eddaoudi, J. Am. Chem. Soc., 2009, 131, 17753–17755 CrossRef CAS PubMed.
- Y. Zhu, J. Cai, L. Xu, G. Li and Y. Liu, Inorg. Chem., 2022, 61, 10957–10964 CrossRef CAS PubMed.
- M. Feng, L. Wu, X. Wang, J. Wang, D. Wang and C. Li, Appl. Organomet. Chem., 2022, 36, e6546 CrossRef CAS.
- F. Meng, L. Jiantang, W. Xirong, W. Jingyu, W. Dongmei and C. Banglin, Inorg. Chem. Front., 2024, 11, 7947–7954 RSC.
- Q. Ding, Z. Zhang, Y. Liu, K. Chai, R. Krishna and S. Zhang, Angew. Chem., Int. Ed., 2022, 61, e202208134 CrossRef CAS PubMed.
- W. Gang-Ding, C. Jing, L. Yong-Zhi, H. Lei, W. Yao-Yu and Z. Zhonghua, Chem. Eng. J., 2021, 433, 133786 Search PubMed.
- Y. Ye, Y. Xie, Y. Shi, L. Gong, J. Phipps, A. M. Al-Enizi, A. Nafady, B. Chen and S. Ma, Angew. Chem., Int. Ed., 2023, 62, e202302564 CrossRef CAS PubMed.
- Z. Wang, Y. Zhang, E. Lin, S. Geng, M. Wang, J. Liu, Y. Chen, P. Cheng and Z. Zhang, J. Am. Chem. Soc., 2023, 145, 21483–21490 CrossRef CAS PubMed.
- W. Jia-Xin, Z. Teng-Fei, P. Jiyan, L. Di, W. Yu-Bo, G. Xiao-Wen, Q. Guodong and L. Bin, Chem Bio Eng., 2024, 1, 952–959 CrossRef PubMed.
- Y.-Z. Li, G.-D. Wang, R. Krishna, Q. Yin, D. Zhao, J. Qi, Y. Sui and L. Hou, Chem. Eng. J., 2023, 466, 143056 CAS.
- Y. Zhou, C. Chen, Z. Ji, R. Krishna, Z. Di, D. Yuan and M. Wu, ACS Mater. Lett., 2024, 6, 1388–1395 CrossRef CAS.
- H. Lyu, J. Zhu, B. Zhou, H. Cao, J. Duan, L. Chen, W. Jin and Q. Xu, Carbon, 2018, 139, 740–749 CrossRef CAS.
- G.-D. Wang, Y.-Z. Li, R. Krishna, W.-Y. Zhang, L. Hou, Y.-Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2024, 63, e202319978 CrossRef CAS PubMed.
- L. Zhang, L.-N. Ma, G.-D. Wang, L. Hou, Z. Zhu and Y.-Y. Wang, J. Mater. Chem. A, 2023, 11, 2343–2348 RSC.
- S. Gao, C. G. Morris, Z. Lu, Y. Yan, H. G. W. Godfrey, C. Murray, C. C. Tang, K. M. Thomas, S. Yang and M. Schröder, Chem. Mater., 2016, 28, 2331–2340 CrossRef CAS.
- X. Liu, C. Hao, J. Li, Y. Wang, Y. Hou, X. Li, L. Zhao, H. Zhu and W. Guo, Inorg. Chem. Front., 2018, 5, 2898–2905 RSC.
- Y. Xiao, A. N. Hong, Y. Chen, H. Yang, Y. Wang, X. Bu and P. Feng, Small, 2023, 19, 2205119 CAS.
- G.-D. Wang, Y.-Z. Li, W.-J. Shi, L. Hou, Y.-Y. Wang and Z. Zhu, Angew. Chem., Int. Ed., 2023, 62, e202311654 CrossRef CAS PubMed.
- W. Fan, X. Wang, X. Zhang, X. Liu, Y. Wang, Z. Kang, F. Dai, B. Xu, R. Wang and D. Sun, ACS Cent. Sci., 2019, 5, 1261–1268 CAS.
- H. Fang, B. Zheng, Z.-H. Zhang, H.-X. Li, D.-X. Xue and J. Bai, Angew. Chem., Int. Ed., 2021, 60, 16521–16528 CrossRef CAS PubMed.
- G. Chang, M. Huang, Y. Su, H. Xing, B. Su, Z. Zhang, Q. Yang, Y. Yang, Q. Ren, Z. Bao and B. Chen, Chem. Commun., 2015, 51, 2859–2862 RSC.
- W. Fan, Y. Wang, Q. Zhang, A. Kirchon, Z. Xiao, L. Zhang, F. Dai, R. Wang and D. Sun, Chem. –Eur. J., 2018, 24, 2137–2143 CAS.
- G.-D. Wang, R. Krishna, Y.-Z. Li, Y.-Y. Ma, L. Hou, Y.-Y. Wang and Z. Zhu, ACS Mater. Lett., 2023, 5, 1091–1099 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 2365428. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc07959k |
| ‡ These authors contributed equally to this work. |
| This journal is © The Royal Society of Chemistry 2025 |