Open Access Article
Open Access ArticleCatalytic enantioselective synthesis of α-C chiral sulfones enabled by merging photoactive electron donor–acceptor complexes with nickel catalysis†
Ze-Min
Lai‡
,
Ying
Xie‡
,
Le-Le
Huang
,
Jing
Guo
* and
Gui
Lu
 *
*
Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery, State Key Laboratory of Anti-Infective Drug Development, School of Pharmaceutical Sciences, Sun Yat-sen University, Guangzhou 510006, P. R. China. E-mail: 1043397061@qq.com; lugui@mail.sysu.edu.cn
First published on 23rd January 2025
Abstract
α-C chiral sulfones are privileged building blocks widely found in pharmaceuticals, agrochemicals, natural products, and ligands. Although many nucleophilic or electrophilic protocols have been developed for their construction, radical-based asymmetric catalysis, especially that involving photoactive electron donor–acceptor (EDA) complexes, remains a significant unmet challenge. Herein, we present the first catalytic asymmetric production of α-C chiral sulfones enabled by merging a photoactive EDA complex with a chiral Ni catalyst. With this cooperative asymmetric catalysis system, a wide range of α-C chiral sulfones are achieved in good yields with excellent enantioselectivities (53 examples, up to 99% yield, 99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er). The synthetic utility of this protocol is further demonstrated by the first asymmetric synthesis of the selective MMP-3 (stromelysin-1) inhibitor. Detailed mechanistic and spectroscopic studies suggest that a newly identified type of EDA complex generated from sulfonyl chlorides and Hantzsch esters (HEs) is crucial to the success as a precursor of sulfonyl radicals.
1 er). The synthetic utility of this protocol is further demonstrated by the first asymmetric synthesis of the selective MMP-3 (stromelysin-1) inhibitor. Detailed mechanistic and spectroscopic studies suggest that a newly identified type of EDA complex generated from sulfonyl chlorides and Hantzsch esters (HEs) is crucial to the success as a precursor of sulfonyl radicals.
Introduction
α-C chiral sulfones are essential structural motifs widely found in naturally occurring compounds and constitute a pivotal design element for pharmaceuticals and chiral ligands (Fig. 1A).1–5 For example, dorzolamide (Trusopt, Merck) is the first eye carbonic anhydrase II inhibitor approved by the FDA to treat glaucoma.6 Sulbactam is a competitive, irreversible β-lactamase inhibitor, which is combined with ampicillin to treat bacterial infections.7 The sulfone units are also found in candidate drugs and natural products such as MK-0752 and (−)-agelasidine.8–12 Given their proven significance, great efforts have been devoted to the efficient enantioselective installation of the sulfone groups into various molecules.1,2,13,14 Traditional approaches towards α-C chiral sulfones rely on transition-metal-catalyzed asymmetric hydrogenation, nucleophilic substitution, and hydrothiolation–oxidation.15–22 More recently, organocatalytic synthetic strategies have been developed as alternative protocols to access α-C chiral sulfones.23–27 However, these advances are mainly limited to electrophilic or nucleophilic sulfonyl intermediates. In recent years, radical sulfonylation methodologies have gained considerable interest in the installation of sulfonyl fragments. Various catalytic systems have been developed to generate sulfonyl radicals from sulfonyl chlorides and sulfinate salts, mostly focusing on racemic processes.28–36 Nevertheless, examples of the more appealing enantioselective radical sulfonylation, which can access a much broader range of α-C chiral sulfones, are scarce, and only a few have been revealed with the aid of a photocatalyst (Fig. 1B).37–41 For instance, the groups of Gong37 and Ye38 respectively reported a catalytic asymmetric three-component radical addition reaction, allowing the construction of sulfonyl carbonyl compounds. Meggers39 and Wu40et al. introduced chiral rhodium catalysis to realize comparable asymmetric radical sulfonylation of α,β-unsaturated N-acylpyrazoles. Hong and co-workers pioneered nickel-catalyzed asymmetric hydrosulfonylation of α,β-unsaturated carbonyl compounds and sulfonyl chlorides via a photoinduced halogen-atom transfer (XAT) process.41 Despite these elegant achievements, the development of a more practical and robust pathway to access diverse α-C chiral sulfones through a challenging radical strategy, especially in the absence of a photocatalyst, remains elusive and is highly desirable.In the past decade, the discovery of more generic synthetic paradigms has been a major goal in the field of catalytic asymmetric photocatalysis.42–45 As a result, the EDA complex activation strategy has emerged as a useful tool in synthetic chemistry.46–53 In many cases, this strategy is generally characterized by its mild reaction conditions, operational simplicity, and lower toxicity in the absence of external photocatalysts. Two Li groups respectively employed this approach to racemic sulfonylation reactions, using sulfonyl chlorides as acceptors for alkene sulfonylation.54,55 However, due to the high reactivity of radicals and the inherent significant racemic background reactions, catalytic asymmetric sulfonylation processes facilitated by a photoactive EDA complex remain a tough task. This not only limits the diversity of asymmetric sulfonylation reactions, but also highlights the importance of designing new asymmetric EDA platforms that can facilitate the formation and asymmetric transformations of radicals. Hantzsch esters (HEs) have recently been used as efficient bifunctional electron donors due to their high reduction potential and the hydrogen atom transfer (HAT) nature of the resulting dihydropyridine radical cation.56 Because of their high oxidation potential, commercially available and cheap sulfonyl chlorides (TsCl, Ered = −0.87 V vs. saturated calomel electrode) can produce sulfonyl radicals via single-electron transfer.57,58 Inspired by these observations, we hypothesized that a new class of EDA complexes providing access to sulfonyl radicals in a way that differs from the traditional generation method of photochemistry may be used to stimulate hitherto underexplored asymmetric sulfonylation reactions. Thus, we have designed a new catalytic system in which EDA complexes containing potential sulfonic radicals may be formed via ground-state molecular association between HEs and sulfonyl chlorides in the absence of an external photo-redox catalyst. Under visible light, the EDA complex triggers an intra-complex SET event, resulting in transient sulfonyl radical intermediates that add to alkenes and afford the desired α-C chiral sulfones. A suitable chiral Ni catalyst acts as a Lewis acid, not only activating α,β-unsaturated alkenes but also providing a suitable chiral environment for efficient stereoselective control. By taking advantage of this EDA complex and cooperation with chiral Ni catalysis, we have realized the asymmetric installation of alkyl and aryl sulfone moieties to afford optically enriched α-C chiral sulfones (Fig. 1C). However, this approach requires solving other issues, such as the instability of the bifunctional electron donor in the catalytic cycle, the incompatibility of asymmetric induction with EDA complex activation, and the limited substrate scope.
Results and discussion
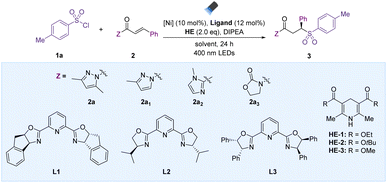
Initially, this asymmetric sulfonylation reaction was performed with 4-methylbenzenesulfonyl chloride (TsCl, 1a) and an α,β-unsaturated carbonyl compound bearing N-acylpyrazole (2a) as model substrates in the presence of 10 mol% NiBr2 and 12 mol% L1 with 2 equiv. of DIPEA in DCM under 400 nm LED irradiation for 24 h without any additional photocatalyst (Table 1). The transformation proceeded smoothly and produced the desired product 3a in NMR yield, with an excellent enantioselectivity profile (98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 er) (see entry 1, Table S1 in the ESI†). Subsequently, a range of bioxazoline ligands were screened, and pyridine–oxazoline ligands were necessary to improve the reaction results (see Table S1 in ESI† for optimization of reaction conditions). A similar yield of 80% and er of 93
2 er) (see entry 1, Table S1 in the ESI†). Subsequently, a range of bioxazoline ligands were screened, and pyridine–oxazoline ligands were necessary to improve the reaction results (see Table S1 in ESI† for optimization of reaction conditions). A similar yield of 80% and er of 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 were obtained using pyridine–oxazoline ligand L2 (entry 2). Increasing the steric hindrance on the 5-position substituent of the oxazoline ring led to a somewhat lower enantioselective ratio of 3a (entry 3). Given the success of NiBr2, alternative Ni(II) catalysts might be used to drive the reaction in good yield with a comparable enantioselective ratio (entries 4–6, Table S1 in the ESI†). Furthermore, the solvent effect was carefully examined, and DCM was proven to be the most suitable medium (entries 7–8, Table S1 in the ESI†). Increasing the reaction time to 36 hours and reducing the number of equivalents of DIPEA improved the result (entries 9–10). Many different electron donors were compared to the best one, HE-1. For example, HE-2 and HE-3 achieved uniformly high enantioselectivities with yields of 68% and 83% (entries 11–12), respectively. Encouraged by this promising result, we further investigated α,β-unsaturated carbonyl substrates bearing different auxiliary groups (Z), and found that N-acylpyrazole (2a) was an appropriate substrate (entries 13–15). When the reaction was conducted in the absence of Ni and ligand, the yield decreased significantly (entry 16), suggesting that Ni/L catalysis was successful in promoting this photochemical process. Essentially, no desired product was detected in the absence of light irradiation or HE, emphasizing the vital role of each of these components in this process (entries 17 and 18).
7 were obtained using pyridine–oxazoline ligand L2 (entry 2). Increasing the steric hindrance on the 5-position substituent of the oxazoline ring led to a somewhat lower enantioselective ratio of 3a (entry 3). Given the success of NiBr2, alternative Ni(II) catalysts might be used to drive the reaction in good yield with a comparable enantioselective ratio (entries 4–6, Table S1 in the ESI†). Furthermore, the solvent effect was carefully examined, and DCM was proven to be the most suitable medium (entries 7–8, Table S1 in the ESI†). Increasing the reaction time to 36 hours and reducing the number of equivalents of DIPEA improved the result (entries 9–10). Many different electron donors were compared to the best one, HE-1. For example, HE-2 and HE-3 achieved uniformly high enantioselectivities with yields of 68% and 83% (entries 11–12), respectively. Encouraged by this promising result, we further investigated α,β-unsaturated carbonyl substrates bearing different auxiliary groups (Z), and found that N-acylpyrazole (2a) was an appropriate substrate (entries 13–15). When the reaction was conducted in the absence of Ni and ligand, the yield decreased significantly (entry 16), suggesting that Ni/L catalysis was successful in promoting this photochemical process. Essentially, no desired product was detected in the absence of light irradiation or HE, emphasizing the vital role of each of these components in this process (entries 17 and 18).
| Entry | 2 | [Ni] | Ligand | Solvent | HE | Yieldb (%) | Erc (%) |
|---|---|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.2 mmol), 2 (0.1 mmol), HE (0.2 mmol), Ni catalyst (0.01 mmol), ligand (0.012 mmol), DIPEA (0.2 mmol), solvent (2 mL) under two 24 W 400 nm LEDs at 30 °C, 24 h. b NMR yield using styrene as internal standard. c Determined by HPLC analysis on a chiral stationary phase. d Isolated yield. e 36 h. f DIPEA (0.05 mmol). g No light. | |||||||
| 1 | 2a | NiBr2 | L1 | DCM | HE-1 | 80 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 2 | 2a | NiBr2 | L2 | DCM | HE-1 | 80 | 93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 7 |
| 3 | 2a | NiBr2 | L3 | DCM | HE-1 | 82 | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 95 95 |
| 4 | 2a | Ni(acac)2 | L1 | DCM | HE-1 | 79 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 5 | 2a | Ni(OTf)2 | L1 | DCM | HE-1 | 70 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 6 | 2a | NiCl2 | L1 | DCM | HE-1 | 67 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 7 | 2a | NiBr2 | L1 | THF | HE-1 | 20 | 87![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13 13 |
| 8 | 2a | NiBr2 | L1 | DCE | HE-1 | 81 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 9e | 2a | NiBr2 | L1 | DCM | HE-1 | 85 | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 10e,f | 2a | NiBr2 | L1 | DCM | HE-1 | 93 (90d) | 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
| 11e,f | 2a | NiBr2 | L1 | DCM | HE-2 | 68 | 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 4 |
| 12e,f | 2a | NiBr2 | L1 | DCM | HE-3 | 83 | 97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
| 13e,f | 2a1 | NiBr2 | L1 | DCM | HE-1 | 85 | 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 8 |
| 14e,f | 2a2 | NiBr2 | L1 | DCM | HE-1 | 40 | 50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 50 50 |
| 15e,f | 2a3 | NiBr2 | L1 | DCM | HE-1 | NR | — |
| 16e,f | 2a | — | — | DCM | HE-1 | 17 | — |
| 17e,f | 2a | NiBr2 | L1 | DCM | — | — | — |
| 18e,f,g | 2a | NiBr2 | L1 | DCM | HE-1 | — | — |
Having identified the optimal reaction conditions, we next investigated the substrate scope of this asymmetric sulfonylation reaction with various sulfonyl chlorides and α,β-unsaturated N-acylpyrazoles. As shown in Table 2, a wide range of sulfonyl chlorides underwent this asymmetric radical addition reaction smoothly to achieve the desired products in good yields and excellent enantioselectivities. For the commercially available aryl sulfonyl chlorides, various functional groups (–Me, –OMe, –Et, –F, –Cl, –Br, –I) and even strong electron-withdrawing groups (–CF3) substituted at the para position of the aryl moiety were found to be well tolerated and give the corresponding products 3a–3i in good yields with 94![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6–99
6–99![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 er values. The absolute structure of 3a was unambiguously determined by X-ray single-crystal analysis.59 Other aryl sulfonyl chlorides bearing electron-withdrawing or electron-donating substituents at the meta or the ortho position of the aryl ring also showed good compatibility in this transformation, providing the desired products 3j–3n in 77–91% yields with 91
1 er values. The absolute structure of 3a was unambiguously determined by X-ray single-crystal analysis.59 Other aryl sulfonyl chlorides bearing electron-withdrawing or electron-donating substituents at the meta or the ortho position of the aryl ring also showed good compatibility in this transformation, providing the desired products 3j–3n in 77–91% yields with 91![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9–93
9–93![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 er values. Moreover, fused- and heteroaryl sulfonyl chlorides afforded the related α-C chiral sulfone products 3o–3p in satisfactory yields and enantioselectivities. Remarkably, the introduction of a linear, cyclic, or branched alkyl sulfonyl chloride to deliver the desired products (3q–3t) also proved successful.
7 er values. Moreover, fused- and heteroaryl sulfonyl chlorides afforded the related α-C chiral sulfone products 3o–3p in satisfactory yields and enantioselectivities. Remarkably, the introduction of a linear, cyclic, or branched alkyl sulfonyl chloride to deliver the desired products (3q–3t) also proved successful.
Next, we turned our attention to further investigating the scope of various α,β-unsaturated N-acylpyrazoles bearing different substituents. In general, the position and electronic nature had limited effect on the enantioselectivity. α,β-Unsaturated N-acylpyrazoles bearing electron-neutral (–H), electron-withdrawing (–Br, –F, –Cl, –CN, –COOMe, –CF3), and electron-donating (–Me, –OMe) groups on the phenyl ring all worked well to afford the corresponding products 3u–3ab in good yields (77–90%) with high enantioselectivities (86.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 13.5–97
13.5–97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 er). For α,β-unsaturated N-acylpyrazoles with ortho- and meta-substituents on the phenyl ring (3ac–3ag), both electron-deficient and electron-rich arenes were also compatible in this transformation, providing good yields (80–93%) and high stereoselectivities (92
3 er). For α,β-unsaturated N-acylpyrazoles with ortho- and meta-substituents on the phenyl ring (3ac–3ag), both electron-deficient and electron-rich arenes were also compatible in this transformation, providing good yields (80–93%) and high stereoselectivities (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–95
8–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er). The sulfonyl of disubstituted α,β-unsaturated N-acylpyrazole also proceeded smoothly to give the desired products in 85% yield with 95
5 er). The sulfonyl of disubstituted α,β-unsaturated N-acylpyrazole also proceeded smoothly to give the desired products in 85% yield with 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er. Moreover, a wide range of heteroaryl groups, such as furan (3ai) and thiophene (3aj) could be introduced into the α,β-unsaturated N-acylpyrazole, resulting in equally good results (78% yield, 95
5 er. Moreover, a wide range of heteroaryl groups, such as furan (3ai) and thiophene (3aj) could be introduced into the α,β-unsaturated N-acylpyrazole, resulting in equally good results (78% yield, 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5–95.5
5–95.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4.5 er). Given the potential for wide applications, determining whether α,β-unsaturated N-acylpyrazoles with a variety of alkyl groups substituted at the β position would still perform well in this asymmetric C–S bond forming reaction is also important. To our delight, not only acyclic alkyl groups (3ak–3an), especially the branched alkyl group (3ao), but also cycloalkyl groups including three- (3ap), four- (3aq), and six- (3ar–3as) membered rings, were all compatible with regard to yields (70–99%) and enantioselectivities (92
4.5 er). Given the potential for wide applications, determining whether α,β-unsaturated N-acylpyrazoles with a variety of alkyl groups substituted at the β position would still perform well in this asymmetric C–S bond forming reaction is also important. To our delight, not only acyclic alkyl groups (3ak–3an), especially the branched alkyl group (3ao), but also cycloalkyl groups including three- (3ap), four- (3aq), and six- (3ar–3as) membered rings, were all compatible with regard to yields (70–99%) and enantioselectivities (92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8–95
8–95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er). Furthermore, the good functional group compatibility and excellent enantioselectivities of this method inspired us to look into its potential for the functionalization of (−)-10-camphorsulfonyl chloride and diverse pharmaceutical and natural products bearing a Michael acceptor. As shown in Table 2, both (−)-10-camphorsulfonyl chloride (3at) and a series of complex molecules derived from (−)-menthol (3au), diacetone-D-glucose (3av), diacetone-fructose (3aw), estrone (3ax), pregnenolone (3ay), and cholesterol (3az) could be effectively employed as Giese-addition substrates. This allows for the straightforward synthesis of complex drug and natural product derivatives containing chiral α-C sulfones with multiple stereocenters in good yields and excellent diastereoselectivities (>20
5 er). Furthermore, the good functional group compatibility and excellent enantioselectivities of this method inspired us to look into its potential for the functionalization of (−)-10-camphorsulfonyl chloride and diverse pharmaceutical and natural products bearing a Michael acceptor. As shown in Table 2, both (−)-10-camphorsulfonyl chloride (3at) and a series of complex molecules derived from (−)-menthol (3au), diacetone-D-glucose (3av), diacetone-fructose (3aw), estrone (3ax), pregnenolone (3ay), and cholesterol (3az) could be effectively employed as Giese-addition substrates. This allows for the straightforward synthesis of complex drug and natural product derivatives containing chiral α-C sulfones with multiple stereocenters in good yields and excellent diastereoselectivities (>20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr). These results together highlight the practicality and robustness of this transformation, as well as its potential for wide-ranging applications in synthetic and medicinal chemistry.
1 dr). These results together highlight the practicality and robustness of this transformation, as well as its potential for wide-ranging applications in synthetic and medicinal chemistry.
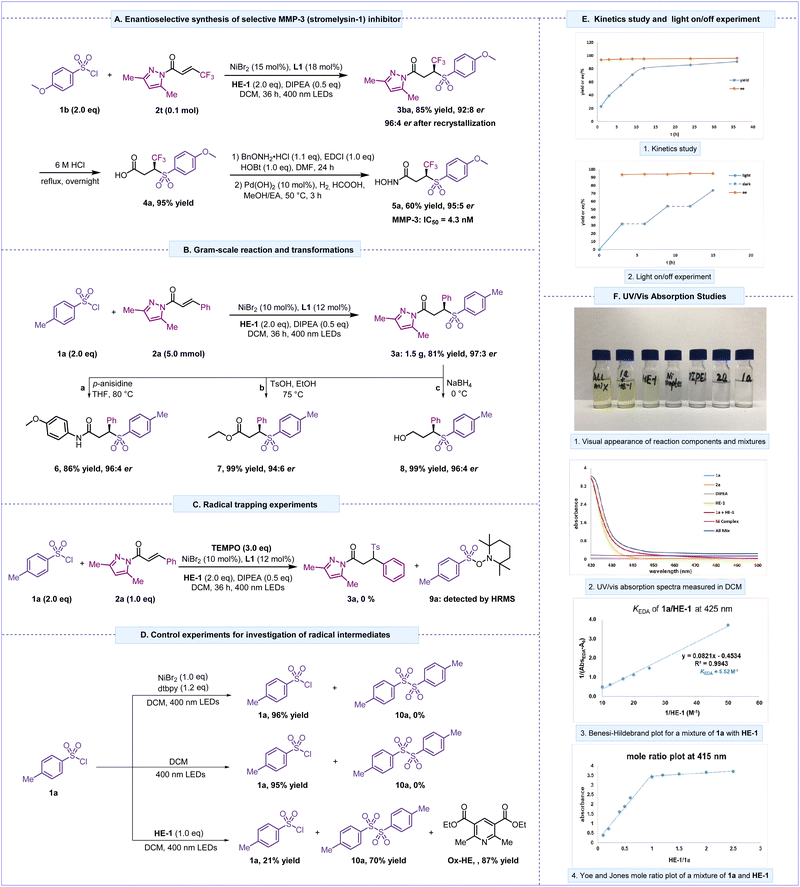
The efficiency and convenience of this asymmetric sulfonylation reaction were further demonstrated in a short synthesis of enantiomerically pure γ-trifluoromethyl γ-sulfone hydroxamate (5a) as a selective MMP-3 (stromelysin-1) inhibitor for the treatment of heart failure and cancer therapy, which was previously synthesized in six steps with 17% overall yield. In this synthesis, chromatographic separation was required to form the key enantiomerically pure intermediate.60,61 Our asymmetric sulfonylation approach employed a commercially available sulfonyl chloride (1b) to produce α-C chiral sulfone intermediate 3ba in 85% yield with 92![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 8 er, and the er could be further improved to 96
8 er, and the er could be further improved to 96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 after recrystallization. Treatment of 3ba with 6 M HCl then provided the corresponding (S)-4,4,4-trifluoro-3-((4-methoxyphenyl)sulfonyl) butanoic acid (4a) in 95% yield. Subsequent amidation and hydrogenation led to the MMP-3 inhibitor 5a in 60% yield with 95
4 after recrystallization. Treatment of 3ba with 6 M HCl then provided the corresponding (S)-4,4,4-trifluoro-3-((4-methoxyphenyl)sulfonyl) butanoic acid (4a) in 95% yield. Subsequent amidation and hydrogenation led to the MMP-3 inhibitor 5a in 60% yield with 95![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 er. This unique four-step route not only achieved the first asymmetric catalytic synthesis of a selective MMP-3 inhibitor, but also represented a significant simplification and improvement to 48% overall yield (Scheme 1A). In addition, a scaled-up experiment was performed using 1a and 2a (Scheme 1B). The reaction proceeded smoothly and generated 3a in a slightly decreased yield (81% yield) but with excellent er (97
5 er. This unique four-step route not only achieved the first asymmetric catalytic synthesis of a selective MMP-3 inhibitor, but also represented a significant simplification and improvement to 48% overall yield (Scheme 1A). In addition, a scaled-up experiment was performed using 1a and 2a (Scheme 1B). The reaction proceeded smoothly and generated 3a in a slightly decreased yield (81% yield) but with excellent er (97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3); 3a can be converted into the corresponding amide (6) or ester (7) by substitution of the pyrazole moiety with an amino or an ethoxy group, and an alcohol derivative (8) was obtained with the aid of NaBH4.
3); 3a can be converted into the corresponding amide (6) or ester (7) by substitution of the pyrazole moiety with an amino or an ethoxy group, and an alcohol derivative (8) was obtained with the aid of NaBH4.
To shed light on the mechanism of this visible light-induced asymmetric sulfonylation reaction, we then performed control experiments. Firstly, a radical-trapping experiment was conducted in the presence of 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO) under standard conditions. The desired product 3a was not observed, but the TEMPO-coupling product 9a was detected by HRMS, which implied possible involvement of sulfonyl radicals in this reaction (Scheme 1C). Next, we carried out control experiments to explain the source of the sulfonyl radicals. When 1a was irradiated by visible light, no reaction was observed, and a large amount of 1a was recycled. A similar outcome occurred when Ni/L was added to catalyze the reaction. In contrast, when 1a and HE-1 were subjected to the reaction conditions without the Ni/L system, both sulfonyl dimer and Ox-HE were detected (Scheme 1D), which unambiguously demonstrated that a new EDA complex might be generated between electron-deficient sulfonyl chloride and electron-rich HE-1, proving the formation of plausible sulfonyl radicals via SET events. Moreover, the reaction progress of 1a, 2a, and HE-1 was probed over time under the standard conditions. While the yield increased gradually over the course of the reaction, the er value remained constant at around 98![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 (Scheme 1E1). An on/off visible light irradiation experiment showed that this reaction did not proceed during the “dark” period (Scheme 1E2). Furthermore, we analyzed the reaction components using UV/vis absorption spectroscopy. The individual absorption spectra of sulfonyl chloride (1a), DIPEA, and α,β-unsaturated N-acylpyrazoles (2a) in DCM were located in the ultraviolet region, whereas HE-1 displayed absorption in the visible light region (Scheme 1F2, orange line). In contrast, a significant bathochromic shift was observed with the mixture of sulfonyl chloride 1a and HE-1 in DCM (Scheme 1F2, red line), which demonstrated that the mixture had formed a new molecular aggregate, that is, a colored EDA complex. Preliminary studies on mixing different concentrations of HE-1 with 1a revealed an association constant of 5.52 M−1 for 1a/HE-1 through the Benesi–Hildebrand method,62 suggesting plausible EDA complex formation prior to homolytic fragmentation (Scheme 1F3 and ESI†). The analysis of this EDA complex via the Yoe and Jones method demonstrated that 1
2 (Scheme 1E1). An on/off visible light irradiation experiment showed that this reaction did not proceed during the “dark” period (Scheme 1E2). Furthermore, we analyzed the reaction components using UV/vis absorption spectroscopy. The individual absorption spectra of sulfonyl chloride (1a), DIPEA, and α,β-unsaturated N-acylpyrazoles (2a) in DCM were located in the ultraviolet region, whereas HE-1 displayed absorption in the visible light region (Scheme 1F2, orange line). In contrast, a significant bathochromic shift was observed with the mixture of sulfonyl chloride 1a and HE-1 in DCM (Scheme 1F2, red line), which demonstrated that the mixture had formed a new molecular aggregate, that is, a colored EDA complex. Preliminary studies on mixing different concentrations of HE-1 with 1a revealed an association constant of 5.52 M−1 for 1a/HE-1 through the Benesi–Hildebrand method,62 suggesting plausible EDA complex formation prior to homolytic fragmentation (Scheme 1F3 and ESI†). The analysis of this EDA complex via the Yoe and Jones method demonstrated that 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry was the most effective absorption ratio (Scheme 1F4).63–65
1 stoichiometry was the most effective absorption ratio (Scheme 1F4).63–65
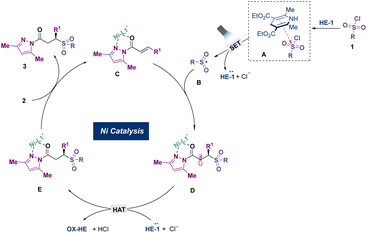
Based on the abovementioned experiments, we envisioned a possible mechanism for this visible-light-induced asymmetric sulfonylation (Scheme 2). The EDA complex was initially formed with the electron-deficient sulfonyl chloride and the electron-rich HE-1via π–π stacking. Under visible light irradiation, an intracomplex SET process from HE-1 to sulfonyl chloride was triggered to produce a dihydropyridine radical cation, chlorine anion, and sulfonyl radical. At the same time, the chiral nickel catalyst underwent ligand exchange with α,β-unsaturated N-acylpyrazole to obtain an intermediate complex, which reacted with the sulfonyl radical via asymmetric radical Giese addition. Subsequently, the resulting radical intermediate was quenched by the dihydropyridine radical cation through a hydrogen atom transfer (HAT) process to yield final product 3.
Conclusions
In summary, we have developed the first enantioselective addition of sulfonyl radicals to α,β-unsaturated N-acylpyrazoles via cooperative visible-light-induced EDA complexes and a chiral Ni catalyst. The current protocol provides facile access to a range of pharmaceutically and biologically important α-C chiral sulfones in excellent yield and er. This work develops a new type of EDA complex, generated from sulfonyl chloride and HEs, and opens an unexplored mode of sulfonyl free radicals in organic synthesis chemistry. Compared with former advanced work, this method not only addresses the challenges caused by the difficulty related to the stereocontrol of radicals but also tolerates a wide range of sulfonyl chlorides and α,β-unsaturated N-acylpyrazoles in the absence of any external photocatalyst. Additionally, the method features redox neutrality, simple starting materials, mild reaction conditions, and good functional group tolerance. Furthermore, the merit of this method was demonstrated through expedited syntheses of a selective MMP-3 inhibitor. We believe that this unprecedented synergistic EDA/Ni catalytic system will inspire the further development of new methods of asymmetric radical reaction.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
G. Lu and J. Guo conceived and directed the project. Z.-M. Lai, Y. Xie and L.-L. Huang performed experiments and prepared the ESI.† G. Lu and J. Guo prepared the manuscript. All authors discussed the results and commented on the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 81972824), Guangdong Basic and Applied Basic Research Foundation (No. 2020A1515011513) and Guangdong Provincial Key Laboratory of Construction Foundation (No. 2023B1212060022).Notes and references
- C. Zhu, Y. Cai and H. Jiang, Org. Chem. Front., 2021, 8, 5574–5589 RSC
.
- Y. Huang, J. Li, H. Chen, Z. He and Q. Zeng, Chem. Rec., 2021, 21, 1216–1239 CrossRef CAS PubMed
.
- M. Yamada, T. Ichikawa, M. Ii, K. Itoh, N. Tamura and T. Kita-zaki, Bioorg. Med. Chem., 2008, 16, 3941–3958 CrossRef CAS PubMed
.
- M. Feng, B. Tang, S. H. Liang and X. Jiang, Curr. Top. Med. Chem., 2016, 16, 1200–1216 CrossRef CAS PubMed
.
- K. A. Scott and J. T. Njardarson, Top. Curr. Chem., 2018, 376, 5 CrossRef PubMed
.
- J. A. Balfour and M. I. Wilde, Drugs Aging, 1997, 10, 384–403 CrossRef CAS PubMed
.
- J. D. Williams, Clin. Infect. Dis., 1997, 24, 494–497 CrossRef CAS PubMed
.
- I. Krop, T. Demuth, T. Guthrie, P. Y. Wen, W. P. Mason, P. Chinnaiyan, N. Butowski, M. D. Groves, S. Kesari, S. J. Freed-man, S. Blackman, J. Watters, A. Loboda, A. Podtelezhnikov, J. Lunceford, C. Chen, M. Giannotti, J. Hing, R. Beckman and P. LoRusso, J. Clin. Oncol., 2012, 30, 2307–2313 CrossRef CAS PubMed
.
- M. Fouladi, C. F. Stewart, J. Olson, L. M. Wagner, A. Onar-Thomas, M. Kocak, R. J. Packer, S. Goldman, S. Gururangan, A. Gajjar, T. Demuth, L. E. Kun, J. M. Boyett and R. J. Gilbertson, J. Clin. Oncol., 2011, 29, 3529–3534 CrossRef CAS PubMed
.
- J. E. Pero, J. M. Matthews, D. J. Behm, E. J. Brnardic, C. Brooks, B. W. Budzik, M. H. Costell, C. A. Donatelli, S. H. Eisennagel, K. Erhard, M. C. Fischer, D. A. Holt, L. J. Jolivette, H. Li, P. Li, J. J. McAtee, B. W. McCleland, I. Pendrak, L. M. Posobiec, K. L. K. Rivera, R. A. Rivero, T. J. Roethke, M. R. Sender, A. Shu, L. R. Terrell, K. Vaidya, X. Xu and B. G. Lawhorn, J. Med. Chem., 2018, 61, 11209–11220 CrossRef CAS PubMed
.
- I.-T. Lu, S.-C. Lin, Y.-C. Chu, Y. Wen, Y.-C. Lin, W.-C. Cheng, J.-H. Sheu and C.-C. Lin, Mar. Drugs, 2022, 20, 109 CrossRef CAS PubMed
.
- J. J. Morales and A. D. Rodriguez, J. Nat. Prod., 1992, 55, 389–394 CrossRef CAS PubMed
.
- J. Zhang, P. Wang, Y. Li and J. Wu, Chem. Commun., 2023, 59, 3821–3826 RSC
.
- M. Nielsen, C. B. Jacobsen, N. Holub, M. W. Paixão and K. A. Jørgensen, Angew. Chem., Int. Ed., 2010, 49, 2668–2679 CrossRef CAS PubMed
.
- M. Ueda and J. F. Hartwig, Org. Lett., 2010, 12, 92–94 CrossRef CAS PubMed
.
- J. Long, L. Shi, X. Li, H. Lv and X. Zhang, Angew. Chem., Int. Ed., 2018, 57, 13248–13251 CrossRef CAS PubMed
.
- W. Li, T. Wagener, L. Hellmann, C. G. Daniliuc, C. Muck-Lichtenfeld, J. Neugebauer and F. Glorius, J. Am. Chem. Soc., 2020, 142, 7100–7107 CrossRef CAS PubMed
.
- G. Liu, C. Yin, X. Yang, A. Li, M. Wang, X. Zhang and X. Q. Dong, Org. Lett., 2021, 23, 19–24 CrossRef CAS PubMed
.
- J. Choi, P. Martin-Gago and G. C. Fu, J. Am. Chem. Soc., 2014, 136, 12161–12165 CrossRef CAS PubMed
.
- J. E. Gomez, A. Cristofol and A. W. Kleij, Angew. Chem., Int. Ed., 2019, 58, 3903–3907 CrossRef CAS PubMed
.
- X. S. Wu, Y. Chen, M. B. Li, M. G. Zhou and S. K. Tian, J. Am. Chem. Soc., 2012, 134, 14694–14697 CrossRef CAS PubMed
.
- A. B. Pritzius and B. Breit, Angew. Chem., Int. Ed., 2015, 54, 3121–3125 CrossRef CAS PubMed
.
- Z. Ding, J. Yang, T. Wang, Z. Shen and Y. Zhang, Chem. Commun., 2009, 571–573 RSC
.
- K. Bera and I. N. Namboothiri, Chem. Commun., 2013, 49, 10632–10634 RSC
.
- L. Li, Y. Liu, Y. Peng, L. Yu, X. Wu and H. Yan, Angew. Chem., Int. Ed., 2016, 55, 331–335 CrossRef CAS PubMed
.
- P. Chen, K. Wang, W. Guo, X. Liu, Y. Liu and C. Li, Angew. Chem., Int. Ed., 2017, 56, 3689–3693 CrossRef CAS PubMed
.
- R. Deng, S. Wu, C. Mou, J. Liu, P. Zheng, X. Zhang and Y. R. Chi, J. Am. Chem. Soc., 2022, 144, 5441–5449 CrossRef CAS PubMed
.
- S. M. Hell, C. F. Meyer, A. Misale, J. B. I. Sap, K. E. Christensen, M. C. Willis, A. A. Trabanco and V. Gouverneur, Angew. Chem., Int. Ed., 2020, 59, 11620–11626 CrossRef CAS PubMed
.
- S. M. Hell, C. F. Meyer, G. Laudadio, A. Misale, M. C. Willis, T. Noel, A. A. Trabanco and V. Gouverneur, J. Am. Chem. Soc., 2020, 142, 720–725 CrossRef CAS PubMed
.
- L. Quebatte, K. Thommes and K. Severin, J. Am. Chem. Soc., 2006, 128, 7440–7441 CrossRef CAS PubMed
.
- X. J. Tang and W. R. J. Dolbier, Angew. Chem., Int. Ed., 2015, 54, 4246–4249 CrossRef CAS PubMed
.
- A. Hossain, S. Engl, E. Lutsker and O. Reiser, ACS Catal., 2018, 9, 1103–1109 CrossRef
.
- J. J. Wang and W. Yu, Org. Lett., 2019, 21, 9236–9240 CrossRef CAS PubMed
.
- G. Qiu, K. Zhou and J. Wu, Chem. Commun., 2018, 54, 12561–12569 RSC
.
- S. Ye, M. Yang and J. Wu, Chem. Commun., 2020, 56, 4145–4155 RSC
.
- J. He, G. Chen, B. Zhang, Y. Li, J.-R. Chen, W.-J. Xiao, F. Liu and C. Li, Chem, 2020, 6, 1149–1159 CAS
.
- S. Cao, W. Hong, Z. Ye and L. Gong, Nat. Commun., 2021, 12, 2377 CrossRef CAS PubMed
.
- F. S. He, C. Zhang, M. Jiang, L. Lou, J. Wu and S. Ye, Chem. Sci., 2022, 13, 8834–8839 RSC
.
- X. Huang, S. Luo, O. Burghaus, R. D. Webster, K. Harms and E. Meggers, Chem. Sci., 2017, 8, 7126–7131 RSC
.
- Y. Kuang, K. Wang, X. Shi, X. Huang, E. Meggers and J. Wu, Angew. Chem., Int. Ed., 2019, 58, 16859–16863 CrossRef CAS PubMed
.
- S. Cao, D. Kim, W. Lee and S. Hong, Angew. Chem., Int. Ed., 2023, 62, e202312780 CrossRef CAS PubMed
.
- M. J. Genzink, J. B. Kidd, W. B. Swords and T. P. Yoon, Chem. Rev., 2022, 122, 1654–1716 CrossRef CAS PubMed
.
- T. Rigotti and J. Aleman, Chem. Commun., 2020, 56, 11169–11190 RSC
.
- R. Brimioulle, D. Lenhart, M. M. Maturi and T. Bach, Angew. Chem., Int. Ed., 2015, 54, 3872–3890 CrossRef CAS PubMed
.
- W. Yao, E. A. B. Bergamino and M. Y. Ngai, ChemCatChem, 2022, 14, e202101292 CrossRef CAS PubMed
.
- B. Saxena, R. I. Patel and A. Sharma, Adv. Synth. Catal., 2023, 365, 1538–1564 CrossRef CAS
.
- A. K. Wortman and C. R. J. Stephenson, Chem, 2023, 9, 2390–2415 CAS
.
- E. Arceo, I. D. Jurberg, A. Alvarez-Fernández and P. Melchiorre, Nat. Chem., 2013, 5, 750–756 CrossRef CAS PubMed
.
- G. E. M. Crisenza, D. Mazzarella and P. Melchiorre, J. Am. Chem. Soc., 2020, 142, 5461–5476 CrossRef CAS PubMed
.
- E. Arceo, A. Bahamonde, G. Bergonzini and P. Melchiorre, Chem. Sci., 2014, 5, 2438–2442 RSC
.
- Z. Y. Cao, T. Ghosh and P. Melchiorre, Nat. Commun., 2018, 9, 3274 CrossRef PubMed
.
- Ł. Woźniak, J. J. Murphy and P. Melchiorre, J. Am. Chem. Soc., 2015, 137, 5678–5681 CrossRef PubMed
.
- J. Y. Kim, Y. S. Lee and D. H. Ryu, ACS Catal., 2021, 11, 14811–14818 CrossRef CAS
.
- L. Lin, Z. Yang, J. Liu, J. Wang, J. Zheng, J.-L. Li, X. Zhang, X.-W. Liu, H. Jiang and J. Li, Green Chem., 2021, 23, 5467–5473 RSC
.
- J. D. Lasso, D. J. Castillo-Pazos, J. M. Salgado, C. Ruchlin, L. Lefebvre, D. Farajat, D. F. Perepichka and C.-J. Li, J. Am. Chem. Soc., 2024, 146, 2583–2592 CrossRef CAS PubMed
.
- J. Guo, Y. Xie, Z.-M. Lai, J. Weng, A. S. C. Chan and G. Lu, ACS Catal., 2022, 12, 13065–13074 CrossRef CAS
.
- A. Paul, S. Banerjee and S. Yadav, Asian J. Org. Chem., 2022, 11, e202100629 CrossRef CAS
.
- J.-M. Li, Y.-H. Wang, Y. Yu, R.-B. Wu, J. Weng and G. Lu, ACS Catal., 2017, 7, 2661–2667 CrossRef CAS
.
- Crystallographic data for 3a is available free of charge from the Cambridge Crystallographic Data Centre, accession number CCDC 2237566†.
- M. Sani, G. Candiani, F. Pecker, L. Malpezzi and M. Zanda, Tetrahedron Lett., 2005, 46, 2393–2396 CrossRef CAS
.
- L. M. Coussens, B. Fingleton and L. M. Matrisian, Science, 2002, 295, 2387–2392 CrossRef CAS PubMed
.
- H. A. Benesi and J. H. Hildebrand, J. Am. Chem. Soc., 2002, 71, 2703–2707 CrossRef
.
- J. H. Yoe and A. L. Jones, Ind. Eng. Chem., Anal. Ed., 1944, 16, 111–115 CrossRef CAS
.
- A. S. Meyer and G. H. Ayres, J. Am. Chem. Soc., 1957, 79, 49–53 CrossRef CAS
.
- A. Monga, S. Bagchi, R. K. Soni and A. Sharma, Adv. Synth. Catal., 2020, 362, 2232–2237 CrossRef
.
Footnotes |
| † Electronic supplementary information (ESI) available: Procedures, optimization, characterization data, HPLC and NMR spectra. CCDC 2237566. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc07264b |
| ‡ Authors with equal contribution. |
| This journal is © The Royal Society of Chemistry 2025 |