Open Access Article
Open Access ArticlePotassium tert-butoxide mediated stereoselective/direct Mannich reaction of α-substituted-γ-lactams with in situ generated aryl N-silyl imines†
Tyler D.
Casselman
a,
Mithun C.
Madhusudhanan
b,
Binh Khanh
Mai
 b,
Peng
Liu‡
b,
Peng
Liu‡
 b and
Brian M.
Stoltz‡
b and
Brian M.
Stoltz‡
 a
a
aWarren and Katherine Schlinger Laboratory for Chemistry and Chemical Engineering, Division of Chemistry and Chemical Engineering, California Institute of Technology, 1200 East California Boulevard, Pasadena, CA 91125, USA. E-mail: stoltz@caltech.edu
bDepartment of Chemistry, University of Pittsburgh, 4200 Fifth Avenue, Pittsburgh, PA 15260, USA
First published on 14th April 2025
Abstract
A potassium tert-butoxide (KOt-Bu)-mediated Mannich reaction between α-substituted-γ-lactams and N-silyl imines is reported. N-silyl imines are generated in situ from readily available aryl nitriles and directly combined with the lactams, without preformation of the lactam enolate, to afford the α-quaternary center-bearing Mannich bases in high yield and with high diastereoselectivity (24 examples). This reaction is shown to be catalytic with respect to KOt-Bu and the catalytic mechanism has been investigated using density functional theory calculations. The computational investigations suggest that the diastereoselectivity is controlled by explicit interactions between a binuclear potassium complex and both the imine nitrogen and the enolate oxygen atoms in the selectivity-determining transition states. The Mannich products are shown to be useful in accessing novel spirocyclic pyrrolidines.
Introduction
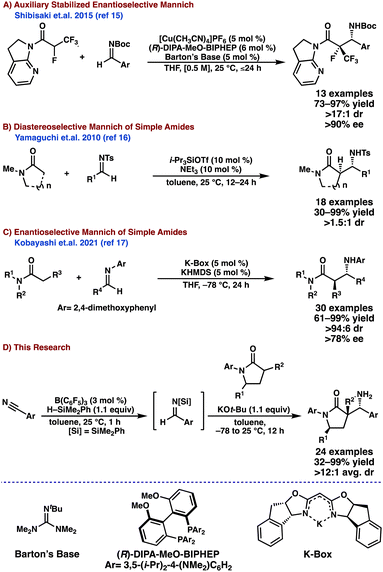
Since the seminal report in 19121 the Mannich reaction has become an important method for C–C bond formation in synthetic organic chemistry.2 The resulting β-amino carbonyl motif produced by this reaction is present in many alkaloid natural products3 and biologically relevant molecules.4 Due to its synthetic utility, the Mannich reaction has received significant attention since the early 1990's,5 particularly toward the development of stereoselective variants.6 Despite the prevalence of quaternary centers in complex synthetic targets,7 the stereoselective Mannich reactions using non-stabilized enolates forming such quaternary centers remains largely underexplored.8 A significant number of these reports rely on the enolate-stabilization (pKa < 30 in DMSO)9 provided by an α-electron-withdrawing10 or an α-aryl11 group to achieve in situ enolization and the desired reactivity. Notable exceptions include those reported by the Barbas group12 and the Trost group,13 utilizing α-substituted aldehydes and ketones. To our knowledge, a stereoselective Mannich reaction using unstabilized, α-substituted lactams as pro-nucleophiles to form quaternary centers has not been reported.Using amides as pro-nucleophiles has been a significant challenge in developing stereoselective Mannich reactions due to their low C–H acidity (pKa ≈ 30–35 in DMSO)9 and the instability of the corresponding metal enolates.14 To overcome these challenges, amide auxiliaries have been critical to promote these stereoselective Mannich reactions (Scheme 1a).15 These auxiliaries have proven to be effective; however, they require additional steps for installation and removal, and are incompatible with cyclic, lactam pro-nucleophiles. A few notable examples using simple, amide pro-nucleophiles without the need for auxiliaries were reported by the Yamaguchi group16 in 2010 and, more recently, the Kobayashi group in 2021.17 To circumvent the isolation of the unstable amide enolates, the Yamaguchi group16 developed a catalytic soft enolization to promote the diastereoselective direct Mannich reaction (Scheme 1b). Similarly, the Kobayashi group17 worked to address enolate stability by designing a chiral potassium salt catalyst to deliver enantioenriched Mannich bases with simple, acyclic amide pro-nucleophiles (Scheme 1c). However, these systems have not been demonstrated to promote the stereoselective, direct Mannich reaction using α-substituted, unactivated amides to generate quaternary centers. Here we report a protocol that enables the diastereoselective direct Mannich reaction of simple, α-branched unactivated γ-lactams in high diastereoselectivity.18 To the best of our knowledge, this is the first report on the utilization of simple, α-substituted amide pro-nucleophiles in a diastereoselective direct Mannich reaction without preformation of the lactam enolate.
Results and discussion
We began investigating the use of unactivated α-substituted-γ-lactam 1 as a Mannich donor with N-acyl imine 3a as the acceptor and employing LiHMDS as the base. We discovered that the unactivated lactam 1 is a competent pro-nucleophile under these conditions, but the diastereoselectivity and yield of the reaction were low (Table 1, entry 1). Increasing the temperature to 25 °C led to increased yield, but the dr remained low (Table 1, entry 2). Changing the acceptor from the N-acyl imine 3a to the N-silyl imine 3b resulted in the desired C–C bond formation in moderate yield, but an unexpected imine condensation adduct 5 was observed and isolated along with the desired Mannich base 4 (Table 1, entry 3). Using KOt-Bu as the base drastically improved the overall yield and minimized the formation of 5. The lability of the N–Si bond allowed for the primary amine 4 to be isolated in 85% yield and a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr after aqueous workup (Table 1, entry 5). Finally, switching from the ortho-methoxy-phenyl (OMP) lactam 1 to the para-methoxy-phenyl (PMP) lactam 2 dramatically improved the dr to 20
1 dr after aqueous workup (Table 1, entry 5). Finally, switching from the ortho-methoxy-phenyl (OMP) lactam 1 to the para-methoxy-phenyl (PMP) lactam 2 dramatically improved the dr to 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 and increased the yield to 90% (Table 1, entry 6). Using these conditions, the reaction can be performed on a 1 mmol scale with similar yield and dr (Table 1, entry 7). Having identified the optimized reaction conditions, we next turned to exploring the generality of the reaction with respect to the α-substitution of the lactam.
1 and increased the yield to 90% (Table 1, entry 6). Using these conditions, the reaction can be performed on a 1 mmol scale with similar yield and dr (Table 1, entry 7). Having identified the optimized reaction conditions, we next turned to exploring the generality of the reaction with respect to the α-substitution of the lactam.
| Entry | Base | Imine | T (°C) | R 1 | 4d (dr) | 5d |
|---|---|---|---|---|---|---|
| a Reaction conditions: 1 (0.2 mmol), 3 (1.0 equiv.), 3.0 mL toluene at X °C, 8 h; b Lactam 2 was used instead of 1. c When imine 3b is used, product 4 was observed as the primary amine. d Isolated yields. e Reaction was performed with 1.0 mmol 2, 1.5 equiv. 3b, 15.0 mL toluene, −40 to 25 °C, 8 h. ortho-OMe-Ph (OMP), para-OMe-Ph (PMP). | ||||||
| 1 | LiHMDS | 3a | −10 °C | Bz | 20% (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0% |
| 2 | LiHMDS | 3a | 25 °C | Bz | 40% (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0% |
| 3 | LiHMDS | 3b | −10 °C | H(TMS) | 20% (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
40% |
| 4 | LiHMDS | 3b | −40 °C | H(TMS) | 14% (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
36% |
| 5 | KOt-Bu | 3b | −40 °C | H(TMS) | 85% (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5% |
| 6b | KOt-Bu | 3b | −40 °C | H(TMS) | 90% (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
5% |
| 7b,e | KOt-Bu | 3b | −40 °C | H(TMS) | 95% (20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
0% |
Gratifyingly, the reaction tolerates larger alkyl substituents, as lactams 2a–c afforded the desired Mannich products 4a–c (Scheme 2) as the primary amine in high yields and diastereomeric ratios. To our delight, performing the reaction using lactam 2a on a 1 mmol scale delivered the corresponding product 4a in 87% yield and a 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. A major limitation to the scope of our reaction was the need to isolate the water-sensitive N-silyl imine 3b. Our initial approach toward synthesizing the N-silyl imine was through an Aza-Peterson reaction to afford imine 3b.19 Due to the instability of N-silyl imines and challenging isolation,20 we focused our efforts to develop a telescoped hydrosilylation/direct Mannich process.
1 dr. A major limitation to the scope of our reaction was the need to isolate the water-sensitive N-silyl imine 3b. Our initial approach toward synthesizing the N-silyl imine was through an Aza-Peterson reaction to afford imine 3b.19 Due to the instability of N-silyl imines and challenging isolation,20 we focused our efforts to develop a telescoped hydrosilylation/direct Mannich process.
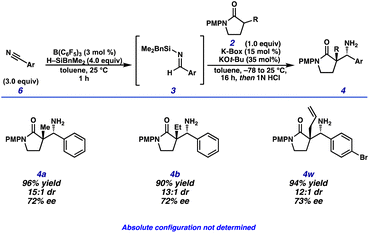
We began our investigation of a telescoped process with a modified catalytic hydrosilylation procedure using H–SiMe2Ph and catalytic B(C6F5)3 in toluene (Scheme 3).21 Following the catalytic hydrosilylation, the reaction mixture was directly added to a solution of KOt-Bu and lactam 2 in toluene to perform the diastereoselective Mannich reaction.
A variety of Mannich donor substrates possessing α-alkyl substitution were subjected to this telescoped reaction sequence (Scheme 4). An excess of the aryl nitrile 6 was shown to be necessary for complete conversion of the lactam Mannich donor due to the formation of the imine transfer adduct between the silyl imine Mannich acceptor 3ca and the Mannich base 4, resulting in products akin to 5. Gratifyingly, this side product can be hydrolyzed upon workup to afford the primary amine 4. Notably, in situ generated imine 3ca (Scheme 3) performed comparably using the telescoped catalytic conditions to those obtained through the two step Aza-Peterson approach. The use of excess aryl nitrile for electron deficient substrates (Scheme 4) was necessary to generate a sufficient concentration of the desired imine as overreduction to the benzyl amine catalysed by B(C6F5)3 was observed in the crude reaction mixture after hydrosilylation.
Allylic and benzylic substitution was generally tolerated at the α-position of the γ-lactam pro-nucleophile (Scheme 4). Gratifyingly, we observed the reaction performed well on modest scale as product 4d was isolated in an 86% yield and 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Sterically congested motifs such as the ortho-Br and ortho-CN benzylic lactams (4f and 4g) gave the desired products in moderate yield and diastereoselectivity, requiring ethereal cosolvents to assist in solubility of the bulkier lactam pro-nucleophiles. Mannich donors bearing β-tertiary carbon centers were also competent, delivering the desired products 4h and 4i in good diastereoselectivity. Notably, β-amino lactam 4i possesses three contiguous stereocenters formed with a 9
1 dr. Sterically congested motifs such as the ortho-Br and ortho-CN benzylic lactams (4f and 4g) gave the desired products in moderate yield and diastereoselectivity, requiring ethereal cosolvents to assist in solubility of the bulkier lactam pro-nucleophiles. Mannich donors bearing β-tertiary carbon centers were also competent, delivering the desired products 4h and 4i in good diastereoselectivity. Notably, β-amino lactam 4i possesses three contiguous stereocenters formed with a 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of the major diastereomer relative to all others, potentially owing to A1,3 strain in the corresponding potassium enolate of 2i.22 Lactam donor 2j, possessing a methyl group at the γ-position, afforded the desired Mannich product 4j in 95% yield and 10
1 ratio of the major diastereomer relative to all others, potentially owing to A1,3 strain in the corresponding potassium enolate of 2i.22 Lactam donor 2j, possessing a methyl group at the γ-position, afforded the desired Mannich product 4j in 95% yield and 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr (see ESI† for details on the tentative assignment of the relative stereochemistry of 4i and 4j).22 This suggests that substitution on the backbone of the γ-lactam can impart facial selectivity for the approach of the N-silyl imine electrophile. For the electrophile scope, electron-neutral as well as electron-deficient arenes are well tolerated in the telescoped reaction sequence. Electron-releasing substituents on the aryl nitrile were not viable pro-electrophiles for the transformation due to the inability to engage in the B(C6F5)3-catalyzed hydrosilylation under our optimized reaction conditions.23 Additionally, we observed the rate of hydrosilylation and the susceptibility of the N-silyl imines to undergo exhaustive hydrosilylation to the corresponding benzylic amine to be dependent on the substitution of the aryl nitrile. Ortho-substitution on the aryl nitriles delivered the desired amines 4l, 4o, and 4u bearing a trifluoromethyl, fluoro and bromo group respectively.
1 dr (see ESI† for details on the tentative assignment of the relative stereochemistry of 4i and 4j).22 This suggests that substitution on the backbone of the γ-lactam can impart facial selectivity for the approach of the N-silyl imine electrophile. For the electrophile scope, electron-neutral as well as electron-deficient arenes are well tolerated in the telescoped reaction sequence. Electron-releasing substituents on the aryl nitrile were not viable pro-electrophiles for the transformation due to the inability to engage in the B(C6F5)3-catalyzed hydrosilylation under our optimized reaction conditions.23 Additionally, we observed the rate of hydrosilylation and the susceptibility of the N-silyl imines to undergo exhaustive hydrosilylation to the corresponding benzylic amine to be dependent on the substitution of the aryl nitrile. Ortho-substitution on the aryl nitriles delivered the desired amines 4l, 4o, and 4u bearing a trifluoromethyl, fluoro and bromo group respectively.
Notably, the more sterically demanding24 and electron withdrawing25ortho-CF3 group afforded the Mannich base 4l in an excellent 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr, while the ortho-F substituted aryl nitrile delivered the product 4o in a modest 3.5
1 dr, while the ortho-F substituted aryl nitrile delivered the product 4o in a modest 3.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr. Unfortunately, the ortho-Cl and ortho-I substituted aryl nitriles (4r and 4x) did not deliver any desired product due to failures at the hydrosilylation portion of the tandem sequence.26 Gratifyingly, meta- and para-substitution on the arene was well tolerated for electron withdrawing substituents, as the corresponding products were obtained in modest to excellent yield with high diastereoselectivity. The relative stereochemistry was unambiguously confirmed by X-ray diffraction (Fig. 1). By analogy, the relative configuration was adopted for the remaining scope entries.
1 dr. Unfortunately, the ortho-Cl and ortho-I substituted aryl nitriles (4r and 4x) did not deliver any desired product due to failures at the hydrosilylation portion of the tandem sequence.26 Gratifyingly, meta- and para-substitution on the arene was well tolerated for electron withdrawing substituents, as the corresponding products were obtained in modest to excellent yield with high diastereoselectivity. The relative stereochemistry was unambiguously confirmed by X-ray diffraction (Fig. 1). By analogy, the relative configuration was adopted for the remaining scope entries.
The primary amine products provide an excellent functional group handle to allow for further derivatization via N-functionalization and cross-coupling chemistry. Primary amine 4d underwent facile N-Ts and N-Boc protection to afford the corresponding protected amines 7 and 8 in 96% and 95% yields, respectively. Protected amines 7 and 8 cleanly underwent a CAN-promoted N-PMP cleavage to afford the secondary amides 9 and 10, respectively, in excellent yields (Scheme 5).
Functionalization of β-amino lactam 4d with acryloyl chloride delivered acrylamide 12 in an 88% yield (Scheme 6). Subjecting acrylamide 12 to Grubbs' 2nd generation catalyst led to the isolation of the desired spirocyclic ε-lactam 13 in 84% yield.27 Inspired by Wolfe's two-step, one-pot intramolecular carboamination, we subjected amine 4d to the disclosed Pd-catalyzed conditions.28 Gratifyingly, the reaction proceeds with an 82% yield for the bis-arylated spirocyclic pyrrolidine 15a using bromobenzene as the aryl halide electrophile without the need to isolate the intermediate aniline 14a. Using the more electron-rich 4-bromoanisole led to lower yield and diastereoselectivity of the isolated spirocyclic product 15b with significant isolation of the retro-Mannich product 2d.
Gratifyingly, using 3,5-dimethyl bromobenzene as the aryl halide delivered the desired spirocyclic pyrrolidine 15c in good yield and diastereoselectivity. Highly electron-deficient aryl halides such as 3-bromopyridine were not tolerated. Additionally, using the N-Boc protected product 8 as a substrate for the Pd-catalyzed carboamination led to the formation of the N-Boc pyrrolidine 16 using electron-rich aryl halides. However, electron-deficient aryl halides such as 3-bromopyridine were not tolerated. We also identified ortho-Br benzylic product 4f as a suitable candidate for an intramolecular Buchwald–Hartwig-type coupling.29 Subjecting amine 4f to the intramolecular C–N arylation afforded spirocyclic tetrahydroquinoline 17 in an 80% yield, but diminished diastereoselectivity. A minor product 18 assigned as the dihydroquinoline was observed, presumably arising from the oxidation of the major anti diastereomer resulting in lower dr of the isolated tetrahydroquinoline 17.30
During our optimization campaign, we discovered that substoichiometric KOt-Bu can be employed while still delivering the resulting Mannich product in up to 95% yield and 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 dr with base loadings as low as 35 mol% (see Scheme S3† for details).31 As shown in Scheme 7, we hypothesized that formation of potassium enolate 2a′ through the deprotonation of lactam 2a with KOt-Bu dimer 19b would liberate an equivalent of tert-butanol, which could serve to protonate potassium amide 20 formed after C–C bond formation and regenerate the KOt-Bu dimer 19b (Scheme 7).32 The N-silyl amine product 21 would then be protonated upon aqueous work up to deliver the desired product 4a.
1 dr with base loadings as low as 35 mol% (see Scheme S3† for details).31 As shown in Scheme 7, we hypothesized that formation of potassium enolate 2a′ through the deprotonation of lactam 2a with KOt-Bu dimer 19b would liberate an equivalent of tert-butanol, which could serve to protonate potassium amide 20 formed after C–C bond formation and regenerate the KOt-Bu dimer 19b (Scheme 7).32 The N-silyl amine product 21 would then be protonated upon aqueous work up to deliver the desired product 4a.
The mechanism that controls the diastereoselectivity of the Mannich reaction and the potential role of KOt-Bu17,32 was investigated using density functional theory (DFT) calculations.33,34 Considering the potassium tert-butoxide tetramer can easily dissociate to a dimer,32a and binuclear potassium complexes32,35 have been described in previous reports as the active species in the potassium-catalyzed α-alkylation of benzyl sulfides,36 dimeric potassium tert-butoxide was used as the base in the calculations, in which one toluene solvent molecule was added to bind to each potassium to account for explicit solvent effects. Our DFT calculations indicate that deprotonation of lactam 2a with potassium tert-butoxide dimer to form potassium enolate 2a′ is endergonic by 2.3 kcal mol−1 (see Fig. S3†). After careful conformational search of the transition state (TS) of the reaction between 2a′ and imine 3b, we located the lowest-energy TS conformers, TS1 and TS2, leading to the anti- and syn-products 4a and 4a-epi, respectively (Fig. 2 and S5 in the ESI† for the computed reaction energy profiles). The computed activation free energy for TS1 (ΔG‡ = 14.2 kcal mol−1 with respect to 2a) is 2.3 kcal mol−1 lower than that for TS2 (ΔG‡ = 16.5 kcal mol−1), which is in agreement with the experimentally observed diastereoselectivity of 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. In TS1 and TS2, both potassium atoms bind to the tert-butoxide oxygen and the enolate oxygen, forming a rhombus-shaped geometry that resembles the KOt-Bu dimer. Although this four-atom K2O2 core structure remains similar in TS1 and TS2, when the different prochiral π-faces of the imine are involved in bond formation, different interactions between the imine and potassium are observed.
1. In TS1 and TS2, both potassium atoms bind to the tert-butoxide oxygen and the enolate oxygen, forming a rhombus-shaped geometry that resembles the KOt-Bu dimer. Although this four-atom K2O2 core structure remains similar in TS1 and TS2, when the different prochiral π-faces of the imine are involved in bond formation, different interactions between the imine and potassium are observed.
In the transition state leading to the favoured anti-product (TS1), the imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond is synclinal with the enolate oxygen, enabling a stabilizing interaction (2.69 Å) between the electron-rich imine nitrogen and one of the potassium atoms. The relatively late transition state, evidenced by the shorter forming C–C bond (2.08 Å compared to 2.23 Å in TS2), increases the negative charge on the imine nitrogen (see Fig. S4† for computed NPA charges) and thus further promotes the N–K interaction in TS1. In TS2, the Ph group on the imine, rather than imine C
N bond is synclinal with the enolate oxygen, enabling a stabilizing interaction (2.69 Å) between the electron-rich imine nitrogen and one of the potassium atoms. The relatively late transition state, evidenced by the shorter forming C–C bond (2.08 Å compared to 2.23 Å in TS2), increases the negative charge on the imine nitrogen (see Fig. S4† for computed NPA charges) and thus further promotes the N–K interaction in TS1. In TS2, the Ph group on the imine, rather than imine C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond, points towards the enolate oxygen and the potassium atoms.
N bond, points towards the enolate oxygen and the potassium atoms.
As a result, a cation–π interaction37 (2.95 Å) between a potassium and the Ph group is observed, in place of the N–K interaction. Because the Ph group is less negatively charged and is a worse donor than the imine nitrogen, this cation–π interaction is expected to be weaker than the N–K interaction in TS1. The imine N–K interaction was also observed in a less stable TS conformer (TS3) leading to the minor product 4a-epi. TS3 is less stable than both TS1 and TS2 because this stereoisomeric transition state has a boat geometry rather than the chair geometry in TS1 and TS2. Taken together, the DFT calculations indicate that the stabilizing imine–potassium interaction in the chair-like imine addition transition state (TS1) controls the diastereoselectivity of the Mannich reaction.
We took inspiration from the Kobayashi report17 to render this transformation asymmetric via introduction of a catalytic, chiral potassium salt. Catalytic levels of KOt-Bu were required to suppress the racemic, background Mannich reaction. Gratifyingly, we observed modest levels of stereoselectivity with the introduction of K-Box-1, delivering the desired amine 4b in 60% ee with good yield and dr (Table 2, entry 1).
| Entry | H-SiR3 | X (°C) | 4b (dr) | % ee |
|---|---|---|---|---|
| a K-Box: Dissolve L1 (15 mol%) and KHMDS (15 mol%) in THF (0.75 mL) at 0 °C for 0.5 h Imine 3: nitrile 6 (0.6 mmol), B(C6F5)3, silane (0.8 mmol) in toluene at X °C. 4b: Add K-Box to a solution of 2b (0.2 mmol) in toluene (3.0 mL) at −78 °C, add Imine 3 slowly and held at 25 °C overnight. b L2 was used instead of L1. c No hydrosilylation observed. | ||||
| 1 | PhMe2SiH | 25 °C | 93% (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
60% |
| 2 | PhMe2SiHb | 25 °C | 33% (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3% |
| 3 | Ph2MeSIH | 40 °C | 95% (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
4% |
| 4 | Ph2SiH2 | 40 °C | 85% (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
3% |
| 5 | Ph3SiHc | 100 °C | 0% (n.d.) | n.d |
| 6 | t-BuMe2SiHc | 40 °C | 0% (n.d.) | n.d |
| 7 | Et3SiH | 25 °C | 17% (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
52% |
| 8 | BnMe2SiH | 25 °C | 89% (13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
72% |
Utilizing Indabox precatalyst L2 lacking the acidic methylene C–H bond in precatalyst L1 resulted in suppression of reactivity and stereoselectivity, suggesting that the potassium salt is the active catalyst in this transformation. Significant optimization of solvent and stoichiometry (see Table S2†) resulted in no improvement of the observed enantioselectivity. To improve the enantioselectivity of this transformation, we focused on altering the silane identity to provide a handle to modify the steric and electronic influence around the imine electrophile. Diaryl silanes required elevated temperatures to perform the hydrosilylation (Table 2, entries 3–4); however, the telescoped Mannich reaction proceeded in much lower levels of enantioselectivity. Electronically deficient Ph3Si–H and sterically bulky t-BuMe2Si–H were unable to perform the hydrosilylation, even at elevated temperatures (Table 2, entries 5–6). Triethyl silane promoted the desired telescoped hydrosilylation/Mannich reaction, but in diminished yield and enantioselectivity. Gratifyingly, performing the telescoped process using BnMe2Si–H resulted in the formation of amine 4b in good yield, dr, and an improved enantioselectivity of 72% ee (Table 2, entry 8). Additional optimization of the silane source resulted in no further improvement to date (see Scheme S4†).
After extensive optimization of the reaction conditions, we focused our efforts toward determining if the observed enantioselectivity was general to other aryl nitrile pro-electrophiles and lactam pro-nucleophiles. To our delight, Mannich products 4a, 4b and 4w were synthesized in great yield and diastereoselectivity while maintaining a modest enantioselectivity (72–73% ee) (Scheme 8). Further investigation is ongoing to identify the absolute configuration of the products formed, improve the enantioselectivity and scope of this telescoped transformation.
Conclusion
In conclusion, we have reported a telescoped hydrosilylation/direct Mannich reaction that couples α-substituted-γ-lactams with aryl nitriles to afford β-amino lactam products bearing an all-carbon quaternary stereocenter. This two-step, one-pot process generates the desired Mannich bases in excellent diastereoselectivity and yield. Electron-neutral and electron-poor aryl nitriles were demonstrated to be competent pro-electrophiles, and a wide α-substitution scope of the pro-nucleophiles has been established. The Mannich products have been shown to be valuable building blocks for further exploration, especially toward the synthesis of complex spirocyclic saturated N-heterocycles. The reaction was shown to be promoted by substoichiometric levels of KOt-Bu, as low as 35 mol%, with computational analysis elucidating a potential mechanism for catalysis as well as the transition states involving a binuclear potassium complex promoting the desired C–C bond formation and controlling the diastereoselectivity. Further investigations toward rendering this transformation asymmetric are ongoing in our laboratory and will be disclosed in due course.Data availability
The data supporting this article have been included as part of the ESI.†Author contributions
Conceptualization: T. D. C. and B. M. S. Experimental methodology: T. D. C. and B. M. S. Computational methodology: M. C. M., B. K. M., and P. L. Funding acquisition: B. M. S. and P. L. Project administration: B. M. S. and P. L. Supervision: B. M. S. and P. L. Writing – original draft: T. D. C. Writing – review and editing: T. D. C., M. C. M., B. K. M., P. L. and B. M. S.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The authors thank Dr Scott Virgil and Alex Cusumano for helpful discussions, David VanderVelde for NMR assistance, Dr Michael Takase for XRD assistance, and Dr Mona Shahgholi for mass spectrometry assistance. The authors thank the Beckman Institute for their support of the Caltech XRD facility, as well as the Dow Next Generation Instrument Grant. The authors (BMS and TDC) thank NIH (R35GM145239) and the Heritage Medical Research Institute Investigator Program. Further financial support for this work was provided by NSF (CHE-2247505 to P. L.). Computational studies were performed at the Center for Research Computing at the University of Pittsburgh and the Advanced Cyberinfrastructure Coordination Ecosystem: Services & Support (ACCESS) program supported by NSF. Constructive discussions within the Catalysis Innovation Consortium facilitated this collaborative study.Notes and references
- C. Mannich and W. Krösche, Arch. Pharm., 1912, 250, 647–667 CrossRef CAS.
- For a recent review on the asymmetric Mannich reaction, see: (a) S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626–2704 CrossRef CAS PubMed . For a review on advances in Metal-catalyzed asymmetric Mannich reactions, see:; (b) B. E. Karimi Dieter and E. Jafari, Synthesis, 2013, 45, 2769–2812 CrossRef . For a review on bimetallic Mannich reactions, see:; (c) M. Shibasaki, M. Kanai, S. Matsunaga and N. Kumagai, Acc. Chem. Res., 2009, 42, 1117–1127 CrossRef CAS PubMed . For a microreview on the organocatalytic Mannich reaction, see:; (d) A. Ting and S. E. Schaus, Eur. J. Org Chem., 2007, 2007, 5797–5815 CrossRef . For a review on the direct catalytic Mannich reaction, see:; (e) A. Córdova, Acc. Chem. Res., 2004, 37, 102–112 CrossRef PubMed.
- For a recent review on the use of intramolecular Mannich reactions toward the synthesis of natural products, see: (a) Y. Shi, Q. Wang and S. Gao, Org. Chem. Front., 2018, 5, 1049–1066 RSC . For select examples of intermolecular asymmetric Mannich-type reactions used in the synthesis of natural products, see:; (b) Y. Numajiri, B. P. Pritchett, K. Chiyoda and B. M. Stoltz, J. Am. Chem. Soc., 2015, 137, 1040–1043 CrossRef CAS PubMed; (c) M. Cushman and J. K. Chen, J. Org. Chem., 1987, 52, 1517–1521 CrossRef CAS; (d) B. M. Trost, C.-I. J. Hung and Z. Jiao, J. Am. Chem. Soc., 2019, 141, 16085–16092 CrossRef CAS PubMed; (e) A. E. Cholewczynski, P. C. Williams and J. G. Pierce, Org. Lett., 2020, 22, 714–717 CrossRef CAS PubMed.
- For a review on the use of Mannich reactions in medicinal chemistry and drug design, see: G. Roman, Eur. J. Med. Chem., 2015, 89, 743–816 CrossRef CAS PubMed.
- For a mini review on the history of the Mannich reaction in the 1990’s, see: S. Kobayashi and H. Ishitani, Chem. Rev., 1999, 99, 1069–1094 CrossRef CAS PubMed.
- For the first examples of an asymmetric Mannich reaction, see: (a) E. J. Corey, C. P. Decicco and R. C. Newbold, Tetrahedron Lett., 1991, 32, 5287–5290 CrossRef CAS; (b) K. Ishihara, M. Miyata, K. Hattori, T. Tada and H. Yamamoto, J. Am. Chem. Soc., 1994, 116, 10520–10524 CrossRef CAS; (c) H. Ishitani, M. Ueno and S. Kobayashi, J. Am. Chem. Soc., 1997, 119, 7153–7154 CrossRef; (d) S. Kobayashi, H. Ishitani and M. Ueno, J. Am. Chem. Soc., 1998, 120, 431–432 CrossRef CAS; (e) S. Kobayashi, J. Kobayashi, H. Ishiani and M. Ueno, Chem. - Eur. J., 2002, 8, 4185–4190 CrossRef CAS PubMed; (f) S. Yamasaki, T. Iida and M. Shibasaki, Tetrahedron, 1999, 55, 8857–8867 CrossRef CAS; (g) B. List, P. Pojarliev, W. T. Biller and H. J. Martin, J. Am. Chem. Soc., 2002, 124, 827–833 CrossRef CAS PubMed.
- For reviews on the construction of all-carbon quaternary centers and their significance in natural products, see: (a) J. Christoffers and A. Mann, Angew. Chem., Int. Ed., 2001, 40, 4591–4597 CrossRef CAS; (b) C. J. Douglas and L. E. Overman, Proc. Natl. Acad. Sci. U. S. A., 2004, 101, 5363–5367 CrossRef CAS PubMed; (c) T. Ling and F. Rivas, Tetrahedron, 2016, 72, 6729–6777 CrossRef CAS; (d) Z. Xin, H. Wang, H. He and S. Gao, Tetrahedron Lett., 2021, 71, 153029 CrossRef CAS; (e) E. V. Prusov, Angew. Chem., Int. Ed., 2017, 56, 14356–14358 CrossRef CAS PubMed; (f) C. Li, S. S. Ragab, G. Liu and W. Tang, Nat. Prod. Rep., 2020, 37, 276–292 RSC.
- For an example of an asymmetric Mannich reaction using an α-substituted lactone pro-nucleophile, see: (a) M. Shang, M. Cao, Q. Wang and M. Wasa, Angew. Chem., Int. Ed., 2017, 56, 13338–13341 CrossRef CAS PubMed . For a stereoselective Mannich reaction using a carboxylic acid pro-nucleophile, see:; (b) Y. Morita, T. Yamamoto, H. Nagai, Y. Shimizu and M. Kanai, J. Am. Chem. Soc., 2015, 137, 7075–7078 CrossRef CAS PubMed.
- F. G. Bordwell, Acc. Chem. Res., 1988, 21, 456–463 CrossRef CAS.
- For selected examples of malonate-type Mannich donors, see: (a) M. Hatano, T. Horibe and K. Ishihara, J. Am. Chem. Soc., 2010, 132, 56–57 CrossRef CAS PubMed; (b) T. Poisson, T. Tsubogo, Y. Yamashita and S. Kobayashi, J. Org. Chem., 2010, 75, 963–965 CrossRef CAS PubMed; (c) Y. K. Kang and D. Y. Kim, J. Org. Chem., 2009, 74, 5734–5737 CrossRef CAS PubMed; (d) T. Kano, C. Homma and K. Maruoka, Org. Biomol. Chem., 2017, 15, 4527–4530 RSC; (e) T. Kano, T. Yurino and K. Maruoka, Angew. Chem., Int. Ed., 2013, 52, 11509–11512 CrossRef CAS PubMed; (f) S. Lou, P. Dai and S. E. Schaus, J. Org. Chem., 2007, 72, 9998–10008 CrossRef CAS PubMed; (g) Y.-P. Lou, C.-W. Zheng, R.-M. Pan, Q.-W. Jin, G. Zhao and Z. Li, Org. Lett., 2015, 17, 688–691 CrossRef CAS PubMed; (h) J. H. Lee and D. Y. Kim, Adv. Synth. Catal., 2009, 351, 1779–1782 CrossRef CAS; (i) R. Pan, J. Zhang, C. Zheng, H. Wang, D. Cao, W. Cao and G. Zhao, Tetrahedron, 2017, 73, 2349–2358 CrossRef CAS; (j) Q. Guo and J. C.-G. Zhao, Org. Lett., 2013, 15, 508–511 CrossRef CAS PubMed; (k) X. Jiang, D. Fu, G. Zhang, Y. Cao, L. Liu, J. Song and R. Wang, Chem. Commun., 2010, 46, 4294–4296 RSC.
- For selected examples of indalone Mannich donors, see: (a) S. Shimizu, T. Tsubogo, P. Xu and S. Kobayashi, Org. Lett., 2015, 17, 2006–2009 CrossRef CAS PubMed; (b) R. He, C. Ding and K. Maruoka, Angew. Chem., Int. Ed., 2009, 48, 4559–4561 CrossRef CAS PubMed; (c) X. Tian, K. Jiang, J. Peng, W. Du and Y.-C. Chen, Org. Lett., 2008, 10, 3583–3586 CrossRef CAS PubMed; (d) L. Cheng, L. Liu, H. Jia, D. Wang and Y.-J. Chen, J. Org. Chem., 2009, 74, 4650–4653 CrossRef CAS PubMed; (e) Q. Jin, C. Zheng, G. Zhao and G. Zou, Tetrahedron, 2018, 74, 4134–4144 CrossRef CAS; (f) M. Torii, K. Kato, D. Uraguchi and T. Ooi, Beilstein J. Org. Chem., 2016, 12, 2099–2103 CrossRef CAS PubMed.
- N. S. Chowdari, J. T. Suri and C. F. Barbas, Org. Lett., 2004, 6, 2507–2510 CrossRef CAS PubMed.
- For selected examples, see: (a) B. M. Trost, T. Saget and C.-I. (Joey) Hung, J. Am. Chem. Soc., 2016, 138, 3659–3662 CrossRef CAS PubMed; (b) B. M. Trost, C.-I. J. Hung and E. Gnanamani, ACS Catal., 2019, 9, 1549–1557 CrossRef CAS; (c) B. M. Trost and C.-I. (Joey) Hung, J. Am. Chem. Soc., 2015, 137, 15940–15946 CrossRef CAS PubMed; (d) B. M. Trost, C.-I. J. Hung, G. Mata, Y. Liu, Y. Lu and E. Gnanamani, Org. Lett., 2020, 22, 2437–2441 CrossRef CAS PubMed; (e) B. M. Trost, T. Saget, A. Lerchen and C.-I. (Joey) Hung, Angew. Chem., Int. Ed., 2016, 55, 781–784 CrossRef CAS PubMed; (f) B. M. Trost, T. Saget and C.-I. (Joey) Hung, Angew. Chem., Int. Ed., 2017, 56, 2440–2444 CrossRef CAS PubMed.
- For the formation of N-TMS adducts of amides, see: (a) Y. Nagao, S. Miyamoto, M. Miyamoto, H. Takeshige, K. Hayashi, S. Sano, M. Shiro, K. Yamaguchi and Y. Sei, J. Am. Chem. Soc., 2006, 128, 9722–9729 CrossRef CAS PubMed; (b) S. W. Djuric, J. Org. Chem., 1984, 49, 1311–1312 CrossRef CAS . For preparation of silicon enolates from lithium amide enolates, see:; (c) U. Frick and G. Simchen, Liebigs Ann. Chem., 1987, 1987, 839–845 CrossRef; (d) R. P. Woodbury and M. W. Rathke, J. Org. Chem., 1978, 43, 881–884 CrossRef CAS . For the stability and C–H acidity of amide enolates, see:; (e) J. P. Richard, G. Williams, A. C. O'Donoghu and T. L. Amyes, J. Am. Chem. Soc., 2002, 124, 2957–2968 CrossRef CAS PubMed , and references therein; For a report on the effect of electrostatics on amide enolate stability, see:; (f) P. R. Rablen and K. H. Bentrup, J. Am. Chem. Soc., 2003, 125, 2142–2147 CrossRef CAS PubMed.
- For selected examples of 7-aza-indoline Mannich donors, see: (a) F. A. Arteaga, Z. Liu, L. Brewitz, J. Chen, B. Sun, N. Kumagai and M. Shibasaki, Org. Lett., 2016, 18, 2391–2394 CrossRef CAS PubMed; (b) L. Brewitz, F. A. Arteaga, L. Yin, K. Alagiri, N. Kumagai and M. Shibasaki, J. Am. Chem. Soc., 2015, 137, 15929–15939 CrossRef CAS PubMed; (c) B. Sun, P. V. Balaji, N. Kumagai and M. Shibasaki, J. Am. Chem. Soc., 2017, 139, 8295–8301 CrossRef CAS PubMed; (d) L. Yin, L. Brewitz, N. Kumagai and M. Shibasaki, J. Am. Chem. Soc., 2014, 136, 17958–17961 CrossRef CAS PubMed . For select examples of N-acylpyrazoles, see:; (e) J. Lu, Y. Fan, F. Sha, Q. Li and X.-Y. Wu, Org. Chem. Front., 2019, 6, 2687–2691 RSC.
- S. Kobayashi, H. Kiyohara and M. Yamaguchi, J. Am. Chem. Soc., 2011, 133, 708–711 CrossRef CAS PubMed.
- Y. Yamashita, A. Noguchi, S. Fushimi, M. Hatanaka and S. Kobayashi, J. Am. Chem. Soc., 2021, 143, 5598–5604 CrossRef CAS PubMed.
- For a brief review of pyrrolidines in natural products, see: (a) D. O'Hagan, Nat. Prod. Rep., 2000, 17, 435–446 RSC; (b) C. Bhat and S. G. Tilve, RSC Adv., 2014, 4, 5405–5452 RSC.
- For the synthesis of N-silyl imines via Aza-Peterson olefination, see: (a) G. Cainelli, D. Giacomini, M. Panunzio, G. Martelli and G. Spunta, Tetrahedron Lett., 1987, 28, 5369–5372 CrossRef CAS; (b) D. J. Hart, K. Kanai, D. G. Thomas and T. K. Yang, J. Org. Chem., 1983, 48, 289–294 CrossRef CAS.
- N-TMS aryl imines have a boiling points of >60 °C at <0.5 mmHg and can be very challenging to distill when substitution on the arene is introduced. For examples of the synthesis and direct utilization of N-TMS imines, see: (a) J. M. Fernández-García, M. Á. Fernández-Rodríguez and E. Aguilar, Org. Lett., 2011, 13, 5172–5175 CrossRef PubMed; (b) J. Kikuchi, H. Ye and M. Terada, Org. Lett., 2020, 22, 8957–8961 CrossRef CAS PubMed; (c) P. V. Ramachandran and T. E. Burghardt, Chem.–Eur. J., 2005, 11, 4387–4395 CrossRef CAS PubMed; (d) D. A. Candito and M. Lautens, Angew. Chem., Int. Ed., 2009, 48, 6713–6716 CrossRef CAS PubMed; (e) J. Vidal, S. Damestoy, L. Guy, J.-C. Hannachi, A. Aubry, A. Collet and A. Aubry, Chem. Eur., 1997, 3, 1691–1709 CrossRef CAS; (f) S. E. Denmark, X. Su, Y. Nishigaichi, D. M. Coe, K.-T. Wong, S. B. D. Winter and J. Y. Choi, J. Org. Chem., 1999, 64, 1958–1967 CrossRef CAS PubMed.
- N. Gandhamsetty, J. Jeong, J. Park, S. Park and S. Chang, J. Org. Chem., 2015, 80, 7281–7287 CrossRef CAS PubMed.
- The assignment of Mannich product 4i is tentative based on the computational transition states elucidated for 4a and the diastereoselectivity of 4j & 4w determined via X-ray diffraction (see Schemes S1 and S2† for details). Due to A1,3 strain in the corresponding enolate of lactam 2i enolate, the beta-C-H bond in TS4 is expected to be syn-periplanar with the enolate oxygen in its most stable conformation. This conformation governs the approach of the imine electrophile from the methyl face due to the greater steric interaction present in the opposite facial approach. This selectivity is comparable to the result obtained for Mannich product 4J in which the backbone γ-methyl group favors the imine to approach from the opposite face, resuting in the major diastereomer 4j observed via XRD
 .
. - We observed the presence of Lewis basic heteroatoms significantly hindered the B(C6F5)3 catalyzed hydrosilylation. Esters, ketones, alkynes and nitro-groups were shown to be tolerant in a Ru-catalyzed hydrosilylation in D. V. Gutsulyak and G. I. Nikonov, Angew. Chem., Int. Ed., 2010, 49, 7553–7556 CrossRef CAS PubMed.
- V. Belot, D. Farran, M. Jean, M. Albalat, N. Vanthuyne and C. Roussel, J. Org. Chem., 2017, 82, 10188–10200 CrossRef CAS PubMed.
- C. Hansch, A. Leo and R. W. Taft, Chem. Rev., 1991, 91, 165–195 CrossRef CAS.
- For a mechanistic insight on the B(C6F5)3-catalyzed hydrosilylation, see: (a) D. J. Parks, J. M. Blackwell and W. E. Piers, J. Org. Chem., 2000, 65, 3090–3098 CrossRef CAS PubMed; (b) D. J. Parks and W. E. Piers, J. Am. Chem. Soc., 1996, 118, 9440–9441 CrossRef CAS; (c) J. Hermeke, M. Mewald and M. Oestreich, J. Am. Chem. Soc., 2013, 135, 17537–17546 CrossRef CAS PubMed; (d) J. M. Blackwell, E. R. Sonmor, T. Scoccitti and W. E. Piers, Org. Lett., 2000, 2, 3921–3923 CrossRef CAS PubMed.
- For examples of an RCM reaction of acrylamides to form an ε-lactam, see: (a) S. Fustero, F. Mojarrad, M. D. P. Carrión, J. F. Sanz-Cervera and J. L. Aceña, Eur. J. Org Chem., 2009, 2009, 5208–5214 CrossRef; (b) B. Sundararaju, T. Sridhar, M. Achard, G. V. M. Sharma and C. Bruneau, Eur. J. Org Chem., 2010, 2010, 6092–6096 CrossRef.
- For a Pd-catalyzed, tandem N-arylation/carboamination sequence, see: (a) Q. Yang, J. E. Ney and J. P. Wolfe, Org. Lett., 2005, 7, 2575–2578 CrossRef CAS PubMed; (b) J. E. Ney and J. P. Wolfe, J. Am. Chem. Soc., 2005, 127, 8644–8651 CrossRef CAS PubMed; (c) G. S. Lemen and J. P. Wolfe, Org. Lett., 2011, 13, 3218–3221 CrossRef CAS PubMed; (d) M. B. Bertrand, M. L. Leathen and J. P. Wolfe, Org. Lett., 2007, 9, 457–460 CrossRef CAS PubMed.
- For intramolecular Buchwald-Hartwig type annulations to form N-heterocycles, see: (a) J. A. Sirvent, F. Foubelo and M. Yus, J. Org. Chem., 2014, 79, 1356–1367 CrossRef CAS PubMed; (b) L. Ding, J. Chen, Y. Hu, J. Xu, X. Gong, D. Xu, B. Zhao and H. Li, Org. Lett., 2014, 16, 720–723 CrossRef CAS PubMed; (c) G. T. Notte and J. L. Leighton, J. Am. Chem. Soc., 2008, 130, 6676–6677 CrossRef CAS PubMed.
- The trans-diastereomer of tetrahydroquinoline 17 was observed to spontaneously oxidize to dihydroquinoline 18 when dissolved in CDCl3.
- Due to the low acidity of the amide α-C–H bond, we believe that lower base loadings result in too low of an initial concentration of potassium enolate 2’ to begin the catalytic cycle.
- For computational studies of KOt-Bu tetramer-mediated reactions, see: (a) W.-B. Liu, D. P. Schuman, Y.-F. Yang, A. A. Toutov, Y. Liang, H. F. T. Klare, N. Nesnas, M. Oestreich, D. G. Blackmond, S. C. Virgil, S. Banerjee, R. N. Zare, R. H. Grubbs, K. N. Houk and B. M. Stoltz, J. Am. Chem. Soc., 2017, 139, 6867–6879 CrossRef CAS PubMed; (b) Y. Lu, R. Zhao, J. Guo, Z. Liu, W. Menberu and Z.-X. Wang, Chem.–Eur. J., 2019, 25, 3939–3949 CrossRef CAS PubMed; (c) I. D. Jenkins and E. H. Krenske, ACS Omega, 2020, 5, 7053–7058 CrossRef CAS PubMed.
- DFT calculations were carried out at the M06-2X/6-311G++(d,p)/SMD(toluene)//M06-2X/6-31G(d) level of theory. Conformational sampling was performed using CREST. See SI for computational details.
- For a recent computational study of stereoselective Mannich reactions, see: M. Feng, I. Mosiagin, D. Kaiser, B. Maryasin and N. Maulide, J. Am. Chem. Soc., 2022, 144, 13044–13049 CrossRef CAS PubMed.
- T. X. Gentner and R. E. Mulvey, Angew. Chem., Int. Ed., 2021, 60, 9247–9262 CrossRef CAS PubMed.
- Y.-F. Liu, L. Zheng, D.-D. Zhai, X.-Y. Zhang and B.-T. Guan, Org. Lett., 2019, 21, 5351–5356 CrossRef CAS PubMed.
- (a) D. A. Dougherty, Acc. Chem. Res., 2013, 46, 885–893 CrossRef CAS PubMed; (b) Z.-W. Qu, H. Zhu, R. Streubel and S. Grimme, ACS Catal., 2023, 13, 1686–1692 CrossRef CAS.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures, spectroscopic data (1H NMR, 13C NMR, IR, HRMS), computational details, Cartesian coordinates, and energies of DFT-computed structures. CCDC 2253010, 2253012 and 2253013. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc06391k |
| ‡ Principal Investigator |
| This journal is © The Royal Society of Chemistry 2025 |