Open Access Article
Open Access ArticleReductive coupling of azonaphthalenes for the synthesis of BINAMs via a diboron-enabled [5,5]-sigmatropic rearrangement†
Liang-Wen
Qi
,
Emmanuella Bema
Twumasi
,
Xiao-Wei
Li
,
Rui
Li
and
Yixin
Lu
 *
*
Department of Chemistry, National University of Singapore, 3 Science Drive 3, 117543, Singapore. E-mail: chmlyx@nus.edu.sg
First published on 13th December 2024
Abstract
The [5,5]-sigmatropic rearrangement is a less-studied reaction and may be strategically utilized to devise unique synthetic processes. Herein, we document a diboron-enabled [5,5]-sigmatropic rearrangement for practical synthesis of BINAM derivatives. Mechanistically, a concerted activation of azonaphthalenes by diboron creates a unique ten-membered transition state, which subsequently triggers a [5,5]-sigmatropic rearrangement. The reaction occurs under mild conditions, and offers operational simplicity, remarkable chemo- and regioselectivities, and good scalability (>10 grams).
Sigmatropic rearrangements are common processes that find wide application in modern organic synthesis, representing an effective approach to reorganize organic molecules in an atom economic manner.1 Typically, these rearrangements involve the movement of a sigma-bonded atom, flanked by one or more π-electron systems, to a new position with a corresponding rearrangement of the π-electrons.2 A plethora of contributions from different research groups have shed light on this interesting research topic, leading to the development of a variety of sophisticated reactions such as [1,2], [1,5], [2,3], [3,3], [3,5] and [5,5]-sigmatropic rearrangements; many of them are classical named reactions such as Wittig rearrangement and Claisen rearrangements (Fig. 1a).3 Among these reactions, [5,5]-sigmatropic rearrangements are studied to a very limited extent, possibly due to their specific substrate requirements and challenging selectivity issues.4 The earliest reports of [5,5]-sigmatropic rearrangements can be traced back to acid-catalyzed benzidine rearrangement of 1,2-diarylhydrazine to access the para-benzidine, along with [1,3] and [3,3]-sigmatropic rearrangement by-products.5 Similarly, a thermally induced rearrangement of trans-penta-2,4-dienyl-phenyl ether gave a mixture of [3,3] and [5,5]-sigmatropic rearrangement products.6 Very recently, the groups of Hashmi and Li have successfully developed a selective [5,5]-sigmatropic rearrangement in gold catalysis and for the remote diastereoselective functionalization of arynes, respectively.7 Moreover, Peng and co-workers have disclosed a highly robust assembly/deprotonation protocol of aryl sulfoxides with allyl nitriles whereby the chemo- and regioselectivity in the C–C bond formation was well-controlled via [5,5]-sigmatropic rearrangement.8
1,1′-Binaphthyl-2,2′-diamine (BINAM), a privileged atropisomeric biaryl motif,9 has found wide applications in asymmetric catalysis and materials science (Fig. 1b).10 Despite its significant synthetic utility, practical strategies to access this privileged scaffold remain limited. The oxidative coupling of aryl amines is a straightforward method for BINAM synthesis with good atom economy.11 However, the intrinsic electronic nature of β-naphthylamine makes it susceptible to over-oxidation, leading to the formation of various oxidized intermediates, including hydroxylamine, nitroso, azo, and nitro compounds. Since Kocovsky's pioneering work,12 a number of atroposelective strategies for the synthesis of BINAMs via transition metal-promoted oxidative coupling using Cu, Fe, or Rh appeared.13 It is noteworthy that protective groups are often required, and subsequent removal of such groups may be problematic. An alternative approach for the synthesis of this privileged scaffold makes use of acid-catalyzed [3,3]-sigmatropic rearrangement of 1,2-dinaphthylhydrazine, which needs to be prepared by transition metal-promoted C–N coupling of the free arylhydrazine.14,15 By pre-installing the azo as the internal oxidant and activating group and through chiral Lewis acid catalysis, Tan and co-workers developed a redox-neutral cross-coupling of azonaphthalene with β-naphthylamine for the synthesis of BINAM derivatives (Fig. 1c).16 Given the broad applications of BINAM and its derivatives, there still exists a need for the development of an efficient approach for the synthesis of diverse BINAM scaffolds, ideally in a highly economical and sustainable manner.
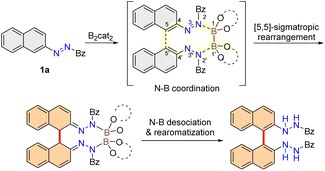
In searching for a viable methodology for practical synthesis of BINAM, we turned our attention to diboron compounds,17 which are tremendously useful in organic synthesis. By exploiting the Lewis acidity and oxidation potential of the diboron reagent, Westcott et al. synthesized racemic vicinal diamines through reductive coupling of imines.18 Recently, Tang and co-workers accomplished enantioselective reductive coupling of isoquinolines19 and imines20 templated by chiral diborons. For the eventual creation of BINAM products, a suitable nitrogen-containing precursor needs to be chosen, and the exploitation of its Lewis base–Lewis acid interaction with diboron is critical for the reaction design. Azonaphthalene was thus selected for our studies. In our working hypothesis, we reasoned that the employment of a diboron reagent may trigger a [5,5]-sigmatropic rearrangement of azonaphthalene, providing a facile synthetic route to access BINAMs. In this reductive homocoupling strategy, the utilization of azonaphthalene as a substrate is crucial; the association of two molecules of azonaphthalenes with diboron is expected to create a ten-membered transition state, enabling the otherwise impossible [5,5]-sigmatropic rearrangement (Fig. 1d). An added advantage of the above design is that the oxidative potential of the substrate may be easily tuned through the installation of different electron-withdrawing groups on the azo moiety.21 We indeed foresee a number of challenges in this design: (1) the direct reduction of unsaturated azo group may occur; (2) the complex regioselectivity issue resulting from other competing sigmatropic rearrangements. Herein, we document a reductive coupling of azonaphthalenes for the synthesis of BINAM derivatives via a diboron-enabled [5,5]-sigmatropic rearrangement.
To start our investigation, the reactions between β-azonaphthalene 1 and different diborons were examined (Table 1). In the presence of bis(catecholato)diboron (B2cat2), the effect of protective groups on the reaction was evaluated. The employment of an ester and a tosyl protecting group led to undesired [3,5]-sigmatropic rearrangement and direct reduction products, respectively (entries 1 and 2). When a benzoyl group was utilized, the desired [5,5]-sigmatropic rearrangement product and the undesired [3,5]-sigmatropic rearrangement product were obtained in a ratio of 1.2 to 1 (entry 3). Given the crucial role that a diboron has played as a template for the rearrangement, a number of other diboron reagents, i.e. B2pin2, B2nep2 and pinB-Bdan, were evaluated. However, none of these diborons provided sufficient activation for the desired [5,5]-sigmatropic rearrangement to take place (entries 4–6). We next conducted a solvent screening, which turned out to be a critical factor for the chemo-selectivity of the reaction (entries 7–10). Gratifyingly, by increasing the amount of B2cat2 to 1.1 equivalence, and running the reaction in 1,2-dimethoxyethane (DME), the desired [5,5]-sigmatropic rearrangement product was obtained in 73% yield, and the formation of other side products was not detected (entry 11). Subsequently, the effects of additives on the reaction were examined. Whereas water was found to be detrimental (entry 12), the addition of Lewis bases such as Cs2CO3 or DMAP improved the reaction efficiency (entries 13 and 14). Finally, 2,6-dibromopyridine (2,6-diBrPy) was found to be best, and the sole [5,5]-sigmatropic rearrangement product was formed in an 89% yield (entry 15).
| Entry | R | Diboron | Solvent | Additive | Yieldb (%) 2/3/4 |
|---|---|---|---|---|---|
| a Unless otherwise indicated, reactions were performed by employing azonaphthalene 1 (0.1 mmol), diboron (0.055 mmol), and additive (0.01 mmol) in the solvent specified (1.0 mL) at room temperature for 2 h. b Yields were determined by crude 1H NMR analysis using 1,3,5-trimethoxybenzene as an internal standard, and the yield of 3 was calculated based on the use of 0.1 mmol azonaphthalene 1. c 0.11 mmol of B2cat2 was used. n.r., no reaction. | |||||
| 1 | CO2Et | B2cat2 | THF | — | 0/0/87 |
| 2 | Tosyl | B2cat2 | THF | — | 0/95/0 |
| 3 | Bz | B2cat2 | THF | — | 40/0/34 |
| 4 | Bz | B2pin2 | THF | — | n.r. |
| 5 | Bz | B2nep2 | THF | — | n.r. |
| 6 | Bz | pinB-Bdan | THF | — | n.r. |
| 7 | Bz | B2cat2 | 1,4-Dioxane | — | 32/0/35 |
| 8 | Bz | B2cat2 | MTBE | — | 44/0/32 |
| 9 | Bz | B2cat2 | DME | — | 51/0/20 |
| 10 | Bz | B2cat2 | MeOH | — | 0/70/30 |
| 11c | Bz | B2cat2 | DME | — | 73/0/0 |
| 12c | Bz | B2cat2 | DME | H2O | 13/72/0 |
| 13c | Bz | B2cat2 | DME | Cs2CO3 | 81/0/0 |
| 14c | Bz | B2cat2 | DME | DMAP | 81/0/0 |
| 15c | Bz | B2cat2 | DME | 2,6-diBrPy | 89/0/0 |
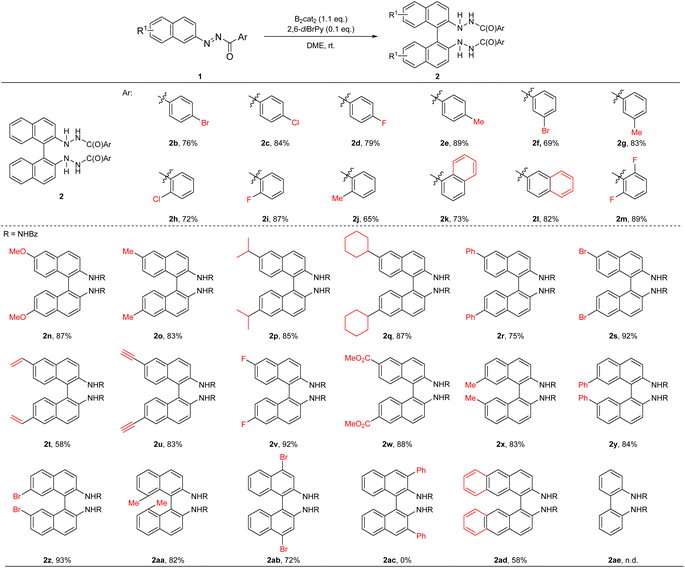
With the optimal conditions in hand, the reaction scope was next examined (Table 2). We first examined the flexibility of the aryl moieties in the benzoyl protective groups on the nitrogen atom of β-azonaphthalenes, and different halogen and methyl-substituted phenyl rings were all found to be suitable, regardless of the substitution patterns (2b–2j). Moreover, both α and β-naphthoyl groups, as well as the difluoro-substituted benzoyl group could be utilized (2k–2m). The reaction scope with regard to the β-azonaphthalenes was next evaluated. Electron-donating substituents such as methoxyl, methyl, isopropyl, and cyclohexyl at the 6-position of naphthalene were well tolerated (2n–2q). When 6-phenyl (2r), bromo (2s), or fluoro-(2v) substituted azonaphthalenes were utilized, the desired rearrangement products were obtained in high yields. Furthermore, naphthalene substrates bearing an alkenyl group (2t), an alkynyl substituent (2u), and an ester moiety (2w) at the 6-position were all found to be suitable. The substituents at the 7-position of azonaphthalenes could also be varied, including methyl (2x), phenyl (2y), and bromo (2z), and consistently high yields of the desired [5,5]-rearranged products were obtained. Azonaphthalene substrates containing a 4-, or 8-substituent were shown to be good substrates as well, forming the desired products in good yields. However, this [5,5]-sigmatropic rearrangement protocol is not applicable to C3-phenyl-substituted azonaphthalene (2ac). Despite extensive efforts, we were unable to obtain the desired product. We reasoned that the steric hindrance introduced by the bulky diboron and the substituent at the 3-position of naphthalene prevents the concerted [5,5]-sigmatropic rearrangement from taking place. Finally, azoanthracene and azobenzene were subjected to our standard reaction conditions; whereas the anthracene diamine was obtained in moderate yield (2ad), the formation of the biphenyl diamine derivative was not observed (2ae) as phenyl hydrazine was the dominant product. The structure of 2z was unambiguously confirmed by X-ray crystallographic analysis.22
| a Reaction conditions: 1 (0.2 mmol), B2cat2 (0.22 mmol) and 2,6-dibromopyridine (0.02 mmol) in DME (2 mL) at room temperature for 2 h; n.d.: not detected. |
|---|
|
Synthetic applications of this [5,5]-sigmatropic rearrangement protocol are depicted in Scheme 1. A scale-up experiment was carried out, and the desired rearranged product 2a was obtained in 91% yield (13.7 g). The N–N bond was readily cleaved when treated with Raney-Ni, furnishing various BINAM structures in excellent yields (Scheme 1a). The operational simplicity of the protocol is worth highlighting; the reaction is typically completed within one hour, and purification requires only simple washing with methanol, and laborious chromatography is not needed. As an illustration, BINAM 3a was readily converted to an atropisomeric primary amine thiourea catalyst 4a (Scheme 1b).10a Given the importance of azo molecules in liquid crystal structures,232a was oxidized to give azo-BINAM 5a in excellent yield (Scheme 1c).
To gain insight into the reaction mechanism, we performed a number of control experiments for the reductive coupling of azonaphthalenes. The addition of 2,2,6,6-tetramethylpiperidinyloxy (TEMPO) as a radical scavenger reduced both the rate and yield of the reaction. However, 2a was isolated in a comparable yield when either diphenylethylene or BHT was introduced as a radical scavenger. These experiments suggested that the reaction does not proceed through a radical pathway. On the basis of the above experiments and related literature precedents on diboron-promoted reductive coupling,19,20 a plausible mechanism is proposed. The concerted activation of two molecules of azonaphthalenes by B2cat2via a Lewis base–Lewis acid interaction initiates a ten-membered transition state. Although the beneficial effects of adding 2,6-diBrPy is not entirely clear, we reason that it may serve as a base to facilitate the proton transfer during the rearomatization process. This proposal is similar to Tang's proposal on the concerted diboron activation of imines19 and isoquinolines.20 The subsequent [5,5]-sigmatropic rearrangement leads to the remote construction of the biaryl axis, forming a dearomatization intermediate. A rearomatization step then takes place to yield the desired thermodynamically stable BINAM derivative (Fig. 2).
Conclusion
In conclusion, we have successfully developed a reductive coupling of azonaphthalene for the synthesis of structurally diverse BINAMs. The key to the above process is the discovery of a diboron-enabled [5,5]-sigmatropic rearrangement, which concertedly activates two molecules of azonaphthalenes and creates the crucial axial bond of the BINAM derivatives in a single step reaction. The reported protocol is operationally simple, and offers practical synthesis of a class of important axial molecules. The novel [5,5]-sigmatropic rearrangement process we introduced offers new insight into the reaction development through structural reorganization of molecular architectures at remote sites.Data availability
All experimental procedures, characterization, and copies of NMR spectra for all new compounds related to this article can be found in the ESI.†Author contributions
L.-W. Q. and E. B. T. contributed equally to this work.Conflicts of interest
There are no conflicts to declare.Acknowledgements
Y. L. thanks the National University of Singapore (A-8001466-00-00), and the Ministry of Education (MOE) of Singapore (A-0008481-00-00) for generous financial support.Notes and references
- (a) T. Newhouse, P. S. Baran and R. W. Hoffmann, The economies of synthesis, Chem. Soc. Rev., 2009, 38, 3010–3021 RSC; (b) A. C. Jones, J. A. May, R. Sarpong and B. M. Stoltz, Toward a symphony of reactivity: cascades involving catalysis and sigmatropic rearrangements, Angew. Chem., Int. Ed., 2014, 53, 2556–2591 CrossRef CAS PubMed.
- R. Hoffmann and R. B. Woodward, Conservation of orbital symmetry, Acc. Chem. Res., 1968, 1, 17–22 CrossRef CAS.
- (a) C. W. Spangler, Thermal [1,j] sigmatropic rearrangements, Chem. Rev., 1976, 76, 187–217 CrossRef CAS; (b) T. Nakai and K. Mikami, [2,3]-Wittig sigmatropic rearrangements in organic synthesis, Chem. Rev., 1986, 86, 885–902 CrossRef CAS; (c) A. M. Martín Castro, Claisen rearrangement over the past nine decades, Chem. Rev., 2004, 104, 2939–3002 CrossRef PubMed; (d) E. A. Ilardi, C. E. Stivala and A. Zakarian, [3,3]-Sigmatropic rearrangements: recent applications in the total synthesis of natural products, Chem. Soc. Rev., 2009, 38, 3133–3148 RSC; (e) Y. Zhang and J. Wang, Catalytic [2,3]-sigmatropic rearrangement of sulfur ylide derived from metal carbene, Coord. Chem. Rev., 2010, 254, 941–953 CrossRef CAS; (f) X. Zhang, Y. Tong, G. Li, H. Zhao, G. Chen, H. Yao and R. Tong, 1,5-Allyl shift by a sequential achmatowicz/oxonia-cope/retro-achmatowicz rearrangement, Angew. Chem., Int. Ed., 2022, 61, e202205919 CrossRef CAS PubMed; (g) C. Yang, X. Zhou, L. Shen, Z. Ke, H. Jiang and W. Zeng, Mn(I)-catalyzed sigmatropic rearrangement of β,γ-unsaturated alcohols, Nat. Commun., 2023, 14, 1862 CrossRef CAS PubMed.
- B. Dinda, in Essentials of Pericyclic and Photochemical Reactions, ed. B. Dinda, Springer International Publishing, Cham, 2017, pp. 107–160 Search PubMed.
- (a) D. V. Banthorpe, E. D. Hughes, C. Ingold and J. Roy, 652. Mechanism of the benzidine and semidine rearrangements. Part VIII. Some acidity functions in aqueous dioxan. Kinetics of rearrangement of hydrazobenzene at high acidities, J. Chem. Soc., 1962, 3294–3299 RSC; (b) H. J. Shine, H. Zmuda, K. H. Park, H. Kwart, A. G. Horgan, C. Collins and B. E. Maxwell, Mechanism of the benzidine rearrangement. Kinetic isotope effects and transition states. Evidence for concerted rearrangement, J. Am. Chem. Soc., 1981, 103, 955–956 CrossRef CAS; (c) H. J. Shine, H. Zmuda, K. H. Park, H. Kwart, A. G. Horgan and M. Brechbiel, Benzidine rearrangements. 16. The use of heavy-atom kinetic isotope effects in solving the mechanism of the acid-catalyzed rearrangement of hydrazobenzene. The concerted pathway to benzidine and the nonconcerted pathway to diphenyline, J. Am. Chem. Soc., 1982, 104, 2501–2509 CrossRef CAS.
- G. Fráter and H. Schmid, Thermische Umwandlung von Penta-2, 4-dienyl-phenyläthern in 4-(penta-2, 4-dienyl)-phenole; [5s,5s]-sigmatropische Umlagerungen, Helv. Chim. Acta, 1970, 53, 269–290 CrossRef.
- (a) C. Hu, K. Farshadfar, M. C. Dietl, A. Cervantes-Reyes, T. Wang, T. Adak, M. Rudolph, F. Rominger, J. Li, A. Ariafard and A. S. K. Hashmi, Gold-catalyzed [5,5]-rearrangement, ACS Catal., 2021, 11, 6510–6518 CrossRef CAS; (b) Z. Chen, M. Tan, C. Shan, X. Yuan, L. Chen, J. Shi, Y. Lan and Y. Li, Aryne 1,4-disubstitution and remote diastereoselective 1,2,4-trisubstitution via a nucleophilic annulation-[5,5]-sigmatropic rearrangement process, Angew. Chem., Int. Ed., 2022, 61, e202212160 CrossRef CAS PubMed.
- (a) L. Zhang, J.-N. He, Y. Liang, M. Hu, L. Shang, X. Huang, L. Kong, Z.-X. Wang and B. Peng, Selective [5,5]-sigmatropic rearrangement by assembly of aryl sulfoxides with allyl nitriles, Angew. Chem., Int. Ed., 2019, 58, 5316–5320 CrossRef CAS PubMed; (b) M. Hu, Y. Liu, Y. Liang, T. Dong, L. Kong, M. Bao, Z.-X. Wang and B. Peng, Dearomative di- and trifunctionalization of aryl sulfoxides via [5,5]-rearrangement, Nat. Commun., 2022, 13, 4719 CrossRef CAS PubMed; (c) L. Zhang, M. Hu and B. Peng, [3,3]- and [5,5]-Sigmatropic rearrangements of aryl sulfoxides using an ‘assembly/deprotonation’ technology, Synlett, 2019, 30, 2203–2208 CrossRef CAS.
- (a) Y.-B. Wang and B. Tan, Construction of axially chiral compounds via asymmetric organocatalysis, Acc. Chem. Res., 2018, 51, 534–547 CrossRef CAS PubMed; (b) J. K. Cheng, S.-H. Xiang, S. Li, L. Ye and B. Tan, Recent advances in catalytic asymmetric construction of atropisomers, Chem. Rev., 2021, 121, 4805–4902 CrossRef CAS PubMed.
- (a) P. Galzerano, G. Bencivenni, F. Pesciaioli, A. Mazzanti, B. Giannichi, L. Sambri, G. Bartoli and P. Melchiorre, Asymmetric iminium ion catalysis with a novel bifunctional primary amine thiourea: controlling adjacent quaternary and tertiary stereocenters, Chem.–Eur. J., 2009, 15, 7846–7849 CrossRef CAS PubMed; (b) S. G. Telfer and R. Kuroda, 1,1′-Binaphthyl-2,2′-diol and 2,2′-diamino-1,1′-binaphthyl: versatile frameworks for chiral ligands in coordination and metallosupramolecular chemistry, Coord. Chem. Rev., 2003, 242, 33–46 CrossRef CAS; (c) L. Liu, L. Yu, X. Chen, J. Yue, A. M. Asiri, H. M. Marwani, D. Huang and S. Wang, Synthesis and characterization of binaphthalene-2,2′-diamine-functionalized gold nanoparticles, J. Nanoparticle Res., 2017, 19, 344 CrossRef; (d) N. Ritter, I. Senkovska, S. Kaskel and J. Weber, Towards chiral microporous soluble polymers--binaphthalene-based polyimides, Macromol. Rapid Commun., 2011, 32, 438–443 CrossRef CAS PubMed; (e) A. D. Averin, O. K. Grigorova, A. S. Malysheva, A. V. Shaferov and I. P. Beletskaya, Pd(0)-catalyzed amination in the synthesis of chiral derivatives of BINAM and their evaluation as fluorescent enantioselective detectors, Pure Appl. Chem., 2020, 92, 1367–1386 CrossRef CAS; (f) A. V. Shaferov, A. S. Malysheva, A. D. Averin, O. A. Maloshitskaya and I. P. Beletskaya, Synthesis and evaluation of the (S)-BINAM derivatives as fluorescent enantioselective detectors, Sensors, 2020, 20, 3234 CrossRef CAS PubMed; (g) V. Štrukil, M. D. Igrc, M. Eckert-Maksić and T. Friščić, Click mechanochemistry: quantitative synthesis of “ready to use” chiral organocatalysts by efficient two-fold thiourea coupling to vicinal diamines, Chem.–Eur. J., 2012, 18, 8464–8473 CrossRef PubMed.
- K. Matsumoto, K. Dougomori, S. Tachikawa, T. Ishii and M. Shindo, Aerobic oxidative homocoupling of aryl amines using heterogeneous rhodium catalysts, Org. Lett., 2014, 16, 4754–4757 CrossRef CAS PubMed.
- M. Smrcina, M. Lorenc, V. Hanus, P. Sedmera and P. Kocovsky, Synthesis of enantiomerically pure 2,2'-dihydroxy-1,1'-binaphthyl, 2,2'-diamino-1,1'-binaphthyl, and 2-amino-2'-hydroxy-1,1'-binaphthyl. Comparison of processes operating as diastereoselective crystallization and as second order asymmetric transformation, J. Org. Chem., 1992, 57, 1917–1920 CrossRef CAS.
- (a) Y. Yusa, I. Kaito, K. Akiyama and K. Mikami, Asymmetric catalysis of homo-coupling of 3-substituted naphthylamine and hetero-coupling with 3-substituted naphthol leading to 3,3′-dimethyl-2,2′-diaminobinaphthyl and -2-amino-2′-hydroxybinaphthyl, Chirality, 2010, 22, 224–228 CrossRef CAS PubMed; (b) X.-L. Li, J.-H. Huang and L.-M. Yang, Iron(III)-promoted oxidative coupling of naphthylamines: synthetic and mechanistic investigations, Org. Lett., 2011, 13, 4950–4953 CrossRef CAS PubMed; (c) R. F. Fritsche, T. Schuh, O. Kataeva and H.-J. Knölker, Atroposelective synthesis of 2,2′-bis(arylamino)-1,1′-biaryls by oxidative iron(III)- and phosphoric acid-catalyzed C−C coupling of diarylamines**, Chem.–Eur. J., 2023, 29, e202203269 CrossRef CAS PubMed.
- (a) C. K. De, F. Pesciaioli and B. List, Catalytic asymmetric benzidine rearrangement, Angew. Chem., Int. Ed., 2013, 52, 9293–9295 CrossRef CAS PubMed; (b) G.-Q. Li, H. Gao, C. Keene, M. Devonas, D. H. Ess and L. Kürti, Organocatalytic aryl–aryl bond formation: an atroposelective [3,3]-rearrangement approach to BINAM derivatives, J. Am. Chem. Soc., 2013, 135, 7414–7417 CrossRef CAS PubMed.
- (a) S.-E. Suh, I.-K. Park, B.-Y. Lim and C.-G. Cho, Acid-catalyzed [3,3] sigmatropic rearrangement of N-Cbz-diaryl hydrazide for the synthesis of mono-N-Cbz-1,1′-biaryl-2,2′-diamine, Eur. J. Org Chem., 2011, 2011, 455–457 CrossRef; (b) B. Li, S. Zhang and W. Chen, An efficient and practical synthesis of BINAM derivatives by diastereoselective [3,3]-rearrangement, Tetrahedron:Asymmetry, 2014, 25, 1002–1007 CrossRef CAS.
- L.-W. Qi, S. Li, S.-H. Xiang, J. Wang and B. Tan, Asymmetric construction of atropisomeric biaryls via a redox neutral cross-coupling strategy, Nat. Catal., 2019, 2, 314–323 CrossRef CAS.
- (a) J. Takagi, K. Takahashi, T. Ishiyama and N. Miyaura, Palladium-catalyzed cross-coupling reaction of bis(pinacolato)diboron with 1-alkenyl halides or triflates: convenient synthesis of unsymmetrical 1,3-dienes via the borylation-coupling sequence, J. Am. Chem. Soc., 2002, 124, 8001–8006 CrossRef CAS PubMed; (b) C. Kleeberg, L. Dang, Z. Lin and T. B. Marder, A facile route to aryl boronates: room-temperature, copper-catalyzed borylation of aryl halides with alkoxy diboron reagents, Angew. Chem., Int. Ed., 2009, 48, 5350–5354 CrossRef CAS PubMed; (c) G. Zhang, Y. Xie, Z. Wang, Y. Liu and H. Huang, Diboron as a reductant for nickel-catalyzed reductive coupling: rational design and mechanistic studies, Chem. Commun., 2015, 51, 1850–1853 RSC; (d) E. C. Neeve, S. J. Geier, I. A. I. Mkhalid, S. A. Westcott and T. B. Marder, Diboron(4) compounds: from structural curiosity to synthetic workhorse, Chem. Rev., 2016, 116, 9091–9161 CrossRef CAS PubMed; (e) F. W. Friese and A. Studer, New avenues for C–B bond formation via radical intermediates, Chem. Sci., 2019, 10, 8503–8518 RSC; (f) H. Lyu, I. Kevlishvili, X. Yu, P. Liu and G. Dong, Boron insertion into alkyl ether bonds via zinc/nickel tandem catalysis, Science, 2021, 372, 175–182 CrossRef CAS PubMed; (g) J. Fan, A. P. Koh, J. Zhou, Z. F. Zhang, C. S. Wu, R. D. Webster, M. D. Su and C. W. So, Tetrakis(N-heterocyclic carbene)-diboron(0): double single-electron-transfer reactivity, J. Am. Chem. Soc., 2023, 145, 11669–11677 CrossRef CAS PubMed.
- C. A. G. Carter, K. D. John, G. Mann, R. L. Martin, T. M. Cameron, R. T. Baker, K. L. Bishop, R. D. Broene and S. A. Westcott, Group 13 chemistry, Am. Chem. Soc., 2002, 822, 70–87 CAS.
- D. Chen, G. Xu, Q. Zhou, L. W. Chung and W. Tang, Practical and asymmetric reductive coupling of isoquinolines templated by chiral diborons, J. Am. Chem. Soc., 2017, 139, 9767–9770 CrossRef CAS PubMed.
- (a) M. Zhou, K. Li, D. Chen, R. Xu, G. Xu and W. Tang, Enantioselective reductive coupling of imines templated by chiral diboron, J. Am. Chem. Soc., 2020, 142, 10337–10342 CrossRef CAS PubMed; (b) M. Zhou, Y. Lin, X.-X. Chen, G. Xu, L. W. Chung and W. Tang, Asymmetric synthesis of vicinal tetrasubstituted diamines via reductive coupling of ketimines templated by chiral diborons, Angew. Chem., Int. Ed., 2023, 62, e202300334 CrossRef CAS PubMed.
- M. H. Kim and J. Kim, Aerobic oxidation of alkyl 2-phenylhydrazinecarboxylates catalyzed by CuCl and DMAP, J. Org. Chem., 2018, 83, 1673–1679 CrossRef CAS PubMed.
- Deposition number 2294982 (for 2z) contains the supplementary crystallographic data for this paper..
- Q. Li, L. Green, N. Venkataraman, I. Shiyanovskaya, A. Khan, A. Urbas and J. W. Doane, Reversible photoswitchable axially chiral dopants with high helical twisting power, J. Am. Chem. Soc., 2007, 129, 12908–12909 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2294982. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d4sc06273f |
| This journal is © The Royal Society of Chemistry 2025 |