Open Access Article
Open Access ArticleNumerical modelling of non-premixed hydrogen blended combustion in a 3D-combustor with jet optimisation
Michael E.
Okolo
 *a,
David S.
Adebayo
*a,
David S.
Adebayo
 b and
Chike F.
Oduoza
b and
Chike F.
Oduoza
 c
c
aWolfson School of Mechanical, Electrical and Manufacturing Engineering, Loughborough University LE11 3TU, UK. E-mail: m.okolo@lboro.ac.uk
bSchool of Engineering and Technology, College of Engineering and Physical Sciences, Aston University, Aston Triangle, Birmingham B4 7ET, UK. E-mail: d.adebayo@aston.ac.uk
cSchool of Engineering, Computing and Mathematical Sciences, Chemical Engineering, University of Wolverhampton, City Campus, WV1 1LY, UK. E-mail: C.F.Oduoza@wlv.ac.uk
First published on 18th February 2025
Abstract
The use of modular combustion systems with multiple jets has gained attention as an efficient and reliable solution for combined heat and power (CHP) applications to enhance the transition to clean and renewable energy for power generation, thereby meeting the United Nations' Sustainable Development Goal (SDG) 7 in addressing climate change challenges. In this study, the performance of a 3D combustor with multiple jets has been modelled numerically and analysed using the computational fluid dynamics (CFD) technique. The efficiency of the combustor is evaluated under varying operational and geometrical conditions such as fuel flow rate, jet number, and hydrogen concentration in the fuel mixture. The investigation results showed that the combustor jet has an optimum operational value for increased efficiency at 16 jets, further indicating the impact of jet number on fuel resident time and mixture in the combustion chamber. In contrast, lower jet numbers resulted in inefficient combustion. The results also revealed that the operation of the combustor should be limited to lower fuel velocities to maintain efficient combustion. The findings of this study provide useful insights for the improvement of modular combustion systems for clean energy production.
1. Introduction
Fossil fuels have been the main source of the world's energy demand and consumption ever since the industrial revolution. In 2019, coal, oil, and natural gas accounted for most of the world's total energy supply and were responsible for 33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 622 Mt of CO2 emissions.1 There are concerns about the sustainability of these fuels with their potential negative impacts on the environment and human health. Specifically, issues such as ozone layer depletion, greenhouse gas emissions, global temperature rise, and environmental pollution have been identified as key areas of concern.2 Hydrogen is becoming increasingly recognised as a promising renewable energy source due to its high energy value, versatility, and environmental benefits. Studies have shown that hydrogen has a higher energy value than other fossil fuels like diesel, gasoline, and natural gas.3 It is reported that it can produce three times more energy per unit of mass than gasoline ignition, making it a highly efficient fuel option.4 Another significant advantage of hydrogen as a fuel source is that it is entirely renewable and sustainable. It is derived from water, which is an abundant natural resource. When combusted, hydrogen condenses back to heated water (H2O), making it an attractive option for the renewable energy transition and a sustainable alternative to fossil fuels.
622 Mt of CO2 emissions.1 There are concerns about the sustainability of these fuels with their potential negative impacts on the environment and human health. Specifically, issues such as ozone layer depletion, greenhouse gas emissions, global temperature rise, and environmental pollution have been identified as key areas of concern.2 Hydrogen is becoming increasingly recognised as a promising renewable energy source due to its high energy value, versatility, and environmental benefits. Studies have shown that hydrogen has a higher energy value than other fossil fuels like diesel, gasoline, and natural gas.3 It is reported that it can produce three times more energy per unit of mass than gasoline ignition, making it a highly efficient fuel option.4 Another significant advantage of hydrogen as a fuel source is that it is entirely renewable and sustainable. It is derived from water, which is an abundant natural resource. When combusted, hydrogen condenses back to heated water (H2O), making it an attractive option for the renewable energy transition and a sustainable alternative to fossil fuels.
Numerical techniques have been employed by many scholars regarding hydrogen combustion analysis in relation to sustainability and clean energy. The study by Shabanian et al.5 used the computational fluid dynamics (CFD) technique to investigate hydrogen–air premixed in a micro-scale combustion chamber. The study showed that the efficiency of combustors is dependent on the feed mass flow rate of reactants, with a lower rate increasing the risk of flame quenching and a higher rate causing flame blowout and inefficient combustion. In a study by Jiaqiang et al.,6 the impact of inlet velocity on combustion characteristics in non-premixed hydrogen/air micro combustors was examined, both with and without catalyst segments. Their study revealed that increasing the inlet velocity increased the maximum temperature and expansion of the high-temperature region in both catalytic and non-catalytic combustors. However, the study was limited in scope, as it only considered the fuel mixture within hydrogen/air and did not investigate the influence of other parameters, such as the fuel/air ratio, on the combustion characteristics of the micro combustor. Eckart et al.7 compared the experimental results with numerical calculations using three different reaction mechanisms. Their numerical results showed good agreement with the experimental data.
A study by Pashchenko8 used the Reynold Average Navier–Stokes (RANS) model to compare the results of 2D and 3D computational domains of a micro cylindrical combustor powered by hydrogen and air. The study showed that the 2D approach was only suitable for preliminary investigations as the result deviates significantly from real data. Furthermore, the flame temperature varied significantly at the combustor outlet, while the temperature of combustion products varied by 25% in the 2D and 3D domains. Ziani et al.9 investigated the effect of hydrogen addition on the combustion of methane at a fixed hydrogen mole fraction, ranging between 0 and 50%, with a 10% step. While their simulation provides valuable insights into the impact of hydrogen addition on the combustion of methane, the number of asymmetric jets in the model was limited to two inlets, further decreasing the efficiency of the mixture before combustion.
Kurdyumov and Matalon10 investigated the self-propagation of flames in long and narrow channels using multi-asymptotic analysis and numerical techniques. The study revealed that the self-acceleration of flames is a function of reactant expansion and the geometry of combustion configurations. In terms of flame transport through channels, the study by Mohan and Matalon11 provided insight into the influence of channel size. The authors found that wide channels allow diffusion flames to pass through the length of the channels without extinguishing, while narrow channels slow the diffusion flame's reaction, resulting in incomplete combustion of the mixture and decreased volumetric and combustion efficiency. Hua, Wu, and Kumar12 employed a numerical method to investigate thermal output and water production as a product using different sizes of inlet diameter to the combustion zone. Their study showed that the largest inlet diameter of 0.4 mm produced the highest temperature, with the flame temperature gradually decreasing as the size decreased.
Fu et al.13 investigated the effects of adding hydrogen to a traditional counter-flow combustor fuelled by methane to improve the applicability to gas turbines. The results show that a scheme with primary air of 50%, secondary air of 20%, and dilution air of 30% leads to an ideal temperature distribution at the exit. The addition of hydrogen decreases the outlet temperature and controls the emission of NOx within an ultra-low range, with the emission decreasing as the hydrogen content increases up to 20%. Additionally, the study found that when the hydrogen content exceeded 20%, it had a significant impact on the field synergy in the combustor, which is beneficial for achieving a uniform temperature distribution at the exit and relatively low flow resistance.
Mardani and Tabejamaat14 conducted a study on the effect of hydrogen under non-premixed flame conditions. The authors found that the presence of hydrogen in the fuel mixture improved the reaction magnitude and high temperature released from the burning mixture under varying conditions ranging from 5% to 20% mass of hydrogen and 3% to 9% for oxygen. A lower proportion of hydrogen in the mixture reduced turbulent kinetic energy and flame entrainment, indicating the mixture's sensitivity to the addition of hydrogen. Karyeyen15 used a numerical study to investigate the combustion of non-premixed methane under distributed conditions using the non-premixed model and standard k–ε turbulence model with probability density function (PDF). The study investigated the concentration of oxygen in five cases ranging from 15% to 21% in volume at temperatures ranging from 300 K to 600 K. The model predicted uniform thermal output at 300 K under 18% and 15% of oxygen volume, with 15% producing zero NOx and CO emissions. However, the study by Boussetla, Mameri and Hadef16 on the analysis of NO emission from MILD combustion of biogas–syngas mixtures reveals that the production of NO is dominated by different mechanisms depending on the operating conditions. The NNH route, which is one of the three mechanisms for the formation of NOx under mild combustion, dominated the NO production for low methane volumes in the fuel mixture, whereas the prompt route mechanism is preferred for high methane content. The prompt route can also be enhanced by both low and high hydrogen volumes, but at high hydrogen volumes, it dominates NO production. At high oxygen contents, the thermal route mechanism governs NO production, irrespective of the volume of methane or hydrogen. As a result, the thermal route mechanism (Zeldovich mechanism) has been used in this study to analyse the impact of hydrogen and methane ratio on NOx pollutant formation. The route was selected as it predicts NOx irrespective of volume fractions of the fuel. The study by Eckart et al.7 revealed that NOx formation increases with residence time with a logarithmic increase for the considered range of cases.
Notably, most of the existing scientific literature predominantly concentrated on the analysis of two-dimensional (2D) combustion systems, with limited research on the investigation of three-dimensional (3D) combustors involving non-pre-mixed combustion. While the investigations on the 2D phenomenon have provided valuable insights into fundamental combustion processes and the development of combustion models, their applicability to real world scenarios involving 3D complexities, such as multiple micro combustors operating in a non-premixed combustion environment, remains largely unexplored. The study conducted by Pashchenko8 represents one of the few research endeavours that have attempted the analysis of 3D combustors. Even with this study, there remains a significant research gap regarding the comprehensive investigation of multiple micro combustors within a 3D model, particularly in non-premixed combustion. Therefore, this current study presents a unique opportunity to expand the understanding of complex combustion dynamics in 3D environments with non-premixed combustion. Such investigations can advance scientific knowledge of combustion processes and their practical applications, ranging from industrial combustion systems to energy conversion technologies.
This current study employed the CFD technique using the RNG k–ε turbulence model and a streamlined chemical mechanism. Specifically, the study investigated and analysed the combustion of a mixture of hydrogen, methane, and air, focusing on how various boundary and geometric conditions impact the flame temperature. In addition, the study examined the effects of the number of jets, the proportion of hydrogen in the fuel mixture, and the fuel velocity on combustion efficiency under various conditions using a non-premixed model. Furthermore, the velocity, species distribution, and static temperature within the combustion tube are investigated.
2. Computation domain and flow conditions
2.1 Geometry
A three-dimensional (3D) numerical model is used to model the flow, mixing, and heat transfer in three different combustion tubes, each with 24, 16, and 8 symmetrical jets of 2.5 mm diameter. The geometrical CAD model used to study the 3D combustor with multiple jets designed for application in tubular combined heat and power (CHP) models using hydrogen and methane fuels is shown in Fig. 1. | ||
| Fig. 1 Geometric and computational domain of the 3D combustor. (a) 2D view; (b) Y–Z axial view; (c) X–Y axial view; (d) X–Y–Z axial view. | ||
2.2 Boundary conditions
The internal flow within the combustor is modelled as fully enclosed using the commercial CFD software ANSYS Fluent version 2023 R2. The boundary conditions used for the initial and final simulations were defined based on similar studies by Pashchenko8 and Yilmaz et al.17 whose geometries were limited to micro cylindrical combustors in three dimensions and two dimensions, respectively, to provide a means of validation for the model. The reactant characteristics used for the optimisation study are shown in Table 1.| Test cases | Fuel | Equivalence ratio | Oxidizer | Fuel inlet velocity | Oxidizer inlet velocity | Inlet temperature |
|---|---|---|---|---|---|---|
| 24 Jets | 70% CH4 + 30% H2 | 1.0 | 23.3% O2 + 76.7% N2 | 5 m s−1 | 15 m s−1 | 300 K |
| 16 Jets | 70% CH4 + 30% H2 | 1.0 | 23.3% O2 + 76.7% N2 | 5 m s−1 | 15 m s−1 | 300 K |
| 8 Jets | 70% CH4 + 30% H2 | 1.0 | 23.3% O2 + 76.7% N2 | 5 m s−1 | 15 m s−1 | 300 K |
At the methane inlet channel, a speed of 5 m s−1 was assigned to facilitate combustion from the mixing chamber, while the flow at the oxygen and hydrogen inlets was assigned a speed of 15 m s−1 with a temperature of 300 K. The outlet boundary condition at the bottom of the tube was defined as atmospheric pressure similar to previous simulations in the literature.
The non-premixed combustion model has been used in this study to capture the mixture of hydrogen and oxygen prior to complete combustion with methane in the combustion chamber because this modelling approach solves more than one conserved scalar quantities with different mixture fractions. It conforms well with the model as fuel inputs have been designed to mix in the mixing chamber before entry into the combustion tube. Under the non-premixed combustion model, the mass fractions of each species were set at 0.7 and 0.3 for methane and hydrogen, respectively. These boundary conditions have been utilised to perform an optimisation of 24, 16, and 8 jets respectively.
To evaluate the characteristics of thermal output and velocity, the proportion of fuel mixture with varying hydrogen concentration at 300 K inlet temperature shown in Table 2 is explored.
| S/N | Fuel mixture | Equivalence ratio | Oxidizer | Fuel inlet velocity | Oxidizer inlet velocity |
|---|---|---|---|---|---|
| 1 | 90% CH4 + 10% H2 | 1.0 | 23.3% O2 + 76.7% N2 | 5 m s−1 | 15 m s−1 |
| 2 | 80% CH4 + 20% H2 | 1.0 | 23.3% O2 + 76.7% N2 | 5 m s−1 | 15 m s−1 |
| 3 | 70% CH4 + 30% H2 | 1.0 | 23.3% O2 + 76.7% N2 | 5 m s−1 | 15 m s−1 |
| 4 | 60% CH4 + 40% H2 | 1.0 | 23.3% O2 + 76.7% N2 | 5 m s−1 | 15 m s−1 |
| 5 | 50% CH4 + 50% H2 | 1.0 | 23.3% O2 + 76.7% N2 | 5 m s−1 | 15 m s−1 |
| 6 | 40% CH4 + 60% H2 | 1.0 | 23.3% O2 + 76.7% N2 | 5 m s−1 | 15 m s−1 |
2.3 Numerical model
All the CFD simulations in this study assume isothermal, viscous and incompressible fluid.18 The incompressible Reynolds Averaged Navier–Stokes (RANS) equations for the conservation of mass, momentum and energy governed the flow in this study. The governing equations for fluid flow, turbulence, and species have been solved using the defined model and conditions at a steady state.The Navier–Stokes equations for a steady incompressible fluid flow for conservation of mass and momentum are usually represented by eqn (1) and (2).
| ∇·u = 0 | (1) |
 | (2) |
The energy equation is extensively utilised in the study of practical applications such as heat transfer, combustion, and energy systems. It plays a crucial role in understanding the thermal behaviour of fluids and obtaining solutions for temperature distribution and heat transfer rates. The general form of the energy equation, considering combustion effects, is expressed by eqn (3).
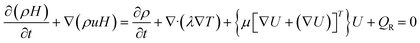 | (3) |
Additionally, eqn (3) incorporates combustion effects through the term QR, which represents the rate of heat release resulting from chemical reactions.
The total enthalpy H here is defined by eqn (4).
 | (4) |
2.4 Chemical reaction of species
The chemical composition of the different combustion reactions can be calculated using the stoichiometric ratio of air/fuel. In the case of methane combustion, methane reacts with oxygen and nitrogen present in air according to eqn (5).| CH4 + 2(O2 + 3.76N2) → CO2 + 2H2O + 7.52N2 | (5) |
| CH4 + H2 + 1.5(O2 + 3.76N2) → CO + 3H2O + 5.64N2 | (6a) |
| CH4 + H2 + 2.5(O2 + 3.76N2) → CO2 + 3H2O + 9.4N2 | (6b) |
| CH4 + H2 + 2.5O2 → CO2 + 3H2O | (7) |
2.5 Turbulence modelling
In this study, the RNG k–ε turbulence model represented by eqn (8) and a simplified chemical mechanism in ANSYS Fluent has been employed to model the flow physics and combustion process in the combustor. The RNG k–ε turbulence model was used in this study due to its versatility and it has been largely found to be accurate when predicting velocity and temperature in the combustor.19 Additionally, the RNG model accurately accounts for the effects of swirl and motion of smaller scales, which contribute to the turbulent diffusion.20 This will effectively enhance the accuracy of the recirculation exhibited by the mixture of gas in the mixing chamber during pre-ignition as well as its exit into the combustion tube for final combustion, thus improving combustion stability and the correctness of turbulence flow parameters. The Reynolds Average Navier–Stokes (RANS) equation has been used to obtain the precise transport equations for the turbulence kinetic energy, κ, and its rate of dissipation, ε, to further express the molecular mixing properties in relation to the characteristics of the combustion process.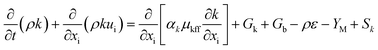 | (8a) |
 | (8b) |
 | (9) |
| η ≡ Sk/ε, η0 = 4.38, β = 0.012, Cμ = 0.0845 |
The solution method for the pressure–velocity coupling is coupled scheme with the second-order upwind discretization scheme for modelling the momentum, turbulent kinetic energy, turbulent dissipation rate, energy, mean mixture fraction, and mixture fraction variance. Furthermore, the convergence of the solution was monitored through the residuals for the relevant equations. When the value of each residual is between three to four orders of magnitude below its initial value, the solution is said to achieve convergence to an acceptable level. In this study, the solution is taken as converged when the momentum and other equations' residuals reduce to 10−4 of their initial value.
2.6 Grid independence test
The computational domain of the 3D model in Fig. 1 was discretized using tetrahedral cell grids as illustrated in Fig. 2(a). The computational domain was subjected to a grid independence test (GIT) to determine the optimal number of elements required for precise model prediction. In this study, five different mesh types shown in Table 3 were explored. The element size of the mesh type ranges from 3.2 million cells to 7.4 million cells. All the five mesh types were subjected to identical flow conditions during the simulation and the GIT was performed using a parametric analysis. Temperature profiles across axial distances at 50 mm were used as monitoring parameters to establish the grid independence of the predictions. As shown in Table 3, the percentage difference of the predicted temperature between mesh types 1, 2, 3, 4, and 5 is quite close with a maximum variation of 2.7%. Since no significant change was observed in the results, mesh type 2 was selected in this study as it provides sufficient grid-independent predictions, given the available computational resources. Additionally, mesh type 2 has a peak temperature close to the theoretical adiabatic temperature estimated by Shabanian et al.5Fig. 2(a) shows the unstructured tetrahedral mesh of the intermediate mesh density (type 2) used for this study. | ||
| Fig. 2 (a) Tetrahedral computational mesh structure; (b) axial distances across sections of the combustion tube. | ||
| Mesh type size | Number of elements | Predicted adiabatic temperature | % Difference |
|---|---|---|---|
| Mesh type 1: 2.0 mm | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 208 208![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 575 575 |
2281 K | |
| Mesh type 2: 1.8 mm | 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 694 694![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 222 222 |
2343 K | 2.7% |
| Mesh type 3: 1.6 mm | 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 434 434![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 982 982 |
2348 K | 0.2% |
| Mesh type 4: 1.4 mm | 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 521 521![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 927 927 |
2297 K | 2.2% |
| Mesh type 5: 1.2 mm | 7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 406 406![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 317 317 |
2343 K | 2% |
Fig. 3 shows the validation of the numerical results of thermal output and combustion products against the experimental results reported by Reyes et al.21 The numerical and experimental results are reasonably in good agreement, demonstrating the validity of the model and grid size used in this study as the major numerical parameters fall within the acceptable limits. These results allow for full investigation into the effects of various boundary conditions within the 3D combustor.
 | ||
| Fig. 3 Comparison of numerical and experimental results: (a) CO mass fraction, (b) CO2 mass fraction, (c) temperature and (d) NOx mass fraction. | ||
3. Results and discussion
The results of the CFD simulations presented in this section provide a comprehensive understanding of the combustion properties within the combustion tube which could be used to optimise the combustor for improved performance. These results have been processed and analysed to obtain important metrics such as velocity, species distribution, and static temperature of the system. These results were analysed using various tools available in ANSYS Fluent software version 2023 R2. The combustor's efficiency was evaluated under varying operational and geometrical conditions, such as fuel flow rate, jet number, and hydrogen concentration in the fuel mixture for each test case shown in Table 2.3.1 Combustor jet optimisation: thermal output and efficiency
A comprehensive analysis of temperature across axial planes of the model is shown in Fig. 4. The data for the analysis and profiles of Fig. 4 is obtained from the axial positions illustrated in Fig. 2(b). Overall, the 24 jets and 16 jets configurations exhibited the most elevated temperature outputs across all axial positions examined. Notably, in Fig. 4(a), the 24 jets configuration exhibited an enhanced thermal output of 2263 K at 30 mm axial position. This is closely followed by the 16 jets model which exhibited a wide range of thermal output at the centre of the combustion tube before it gradually falls below the 24 jets configuration at the end of the wall. | ||
| Fig. 4 Comparison of flame temperature across different numbers of jets (24, 16, and 8 jets) in the combustor: (a) 30 mm axial distance, (b) 50 mm axial distance, and (c) 70 mm axial distance for the three model cases shown in Table 1. | ||
In contrast, the thermal output at 50 mm axial position, shown in Fig. 4(b), and at 70 mm, shown in Fig. 4(c), shows high thermal performance, yielding higher combustion temperatures in the 16 jets model, indicating complete combustion of reactants in the combustion tube. The 8 jets configuration consistently recorded the lowest temperatures across all axial positions of the combustion chamber making it the least efficient model to be considered for optimisation.
These results show that the combustor jet operates optimally at 24 and 16 jets. However, the 16 jets model maintained a significantly higher thermal output leading to an increase in efficiency. It is noted that the higher number of jets could decrease the residence time for hydrogen, oxygen and methane mixing within the combustion chamber under a non-premixed model, consequently leading to incomplete combustion with temperature reduction expected to occur. This observation suggests that operating with the baseline of 16 jets would offer a more stable temperature and significantly high temperature gradient compared to the 24 jets model.
The velocity profiles for the different jet configurations are shown in Fig. 5, which illustrates the velocity magnitude generated in the combustor head for the 8, 16, and 24 jets. The velocity profiles in Fig. 5 show peak-to-peak symmetrical output on the boundaries of the combustor with axial positions indicating the distance of the flow into the tube with a decreasing effect in increasing order. This feature may be due to the design of the combustor as shown in Fig. 1(c).
 | ||
| Fig. 5 Velocity profile for jets in the combustor at 24, 16 and 8 jets at (a) 30 mm axial distance, (b) 50 mm axial distance, and (c) 70 mm axial distance. | ||
The velocity output across all axial sections for the 24 jets model is shown to be the lowest followed closely by the 16 jets and the 8 jets producing the highest magnitude.
The velocity magnitudes observed from the 24 jets model resulted from the influence of the accompanying jets which increased the total outlet area of the gas mixture from the mixing chamber creating a widely distributed exit through the jets. This caused the overall velocity magnitude to be lower in the combustion tube. The 16 jets, however, showed a different feature from the 24 jets, with higher velocity peaks on either side of the combustor indicating the impact of reduction in the number of outlets on all the axial sections with increasing intensity down the combustion tube. The 8 jets model, however, produced the highest velocity magnitude, further buttressing the effects of outlet jet numbers on all the axial positions. The low velocity observed for all the axial positions in the range of 0.47–0.54 m of the combustor in Fig. 5 can be attributed to the lateral entry of the flow as well as the low inlet velocity of the methane gas at the centre hindering further agitation of the mixture.
The results of Fig. 5 show that a larger jet number with multiple outlets reduces the velocity at the exit of the jets, while fewer outlets increase the exit velocity from the jets.
The axial temperature contours of the three combustor types are presented in Fig. 6. In all cases, the hottest zone in the combustors is located on the right side of the combustion tube. This phenomenon suggests that a larger volume of gas mixture exits the mixing chamber through the aligned jets on the right side of the combustion head. Fig. 6 also indicates that flame quenching occurs more prominently in the combustor with the lowest number of jets as observed in Fig. 6(c), while the quenching effect is minimised in the combustor with the highest number of jets as observed in Fig. 6(a). Consequently, extremely low and high numbers of jets could render the combustion model inefficient and lead to unstable combustion output. The results indicate that the mixture residence time is influenced by the number of jets, with the maximum temperature aligning to the right wall of the combustion chamber due to an increased exit velocity of the mixture in that specific zone. Additionally, Fig. 6 further indicates that the 24 and 16 jets models exhibited more stable and well-defined flame formation within the combustion chamber, which align with the lower velocity magnitude observed in Fig. 5. These findings align with the study conducted by Li et al.,22 which also highlighted the impact of combustor size and geometry on flame temperature in micro combustors. Specifically, micro combustors with 1 mm diameter under increased velocities were found to produce higher flame temperatures compared to larger ones. Additionally, the work of Hua, Wu, and Kumar12 is in close agreement with the current findings, although their study was based on a hydrogen and air mixture.
 | ||
| Fig. 6 Temperature contour across the central axis of the combustion chamber with (a) 24 jets; (b) 16 jets and (c) 8 jets. | ||
Fig. 7(a) and (b) show the thermal output and NOx emission for the different jet configurations. The flame temperature recorded in Fig. 7(a) shows the 16 jets model consistently producing the highest thermal output at all H2 concentrations and attaining a maximum of 2270 K at 60% H2. This is closely followed by the 24 jets model, with the highest thermal output of 2250 K at 30% H2. The lowest temperature is recorded by the 8 jets model at all H2 concentrations. The results shown in Fig. 7(b) indicate that NOx emission produced at all H2 concentrations for the 16 jets model was higher than those obtained for the 24 jets model. The 8 jets model exhibited almost zero thermal output at all H2 concentrations. It is observed that the thermal output is dependent on the number of jets, with a high number of jets enhancing the combustion rates due to quicker evacuation of the hydrogen and air mixture into the combustion chamber. However, having too many combustors could impact the stability and formation of the flame causing high swirl intensity leading to lower combustion efficiency.23
3.2 Combustion efficiency
The combustion efficiency is defined by eqn (10). | (10) |
| S/N | Jets number | H2 (%) | Density kg m−3 | Velocity (m s−1) | Y H2 | Y CH4 | ṁ H2(in) (kg s−1) | ṁ CH4 (kg s−1) | Max temperature (K) | Wall temperature (K) | Combustion efficiency (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| CASE 1 | 24 Jets | 10 | 0.1611271 | 10.26124 | 0.00673618 | 0.1553851 | 0.044835834 | 0.044835835 | 2214 | 1153 | 91.8 |
| 24 Jets | 20 | 0.1760827 | 9.692895 | 0.00769841 | 0.1248406 | 0.045500423 | 0.045500423 | 2227 | 1152 | 93.2 | |
| 24 Jets | 30 | 0.1719111 | 9.175376 | 0.009845339 | 0.09345882 | 0.002216361 | 0.041155324 | 2260 | 1190 | 89.7 | |
| CASE 2 | 16 Jets | 10 | 0.1574601 | 16.61133 | 0.01409046 | 0.08201706 | 0.007192062 | 0.064728562 | 2245 | 1394 | 90.4 |
| 16 Jets | 20 | 0.2308633 | 13.51803 | 0.00465202 | 0.0820075 | 0.002613634 | 0.083198126 | 2138 | 1241 | 91.3 | |
| 16 Jets | 30 | 0.1523634 | 16.77484 | 0.007546662 | 0.007546662 | 0.001932396 | 0.066686401 | 2260 | 1214 | 98.5 | |
| CASE 3 | 8 Jets | 10 | 0.3661719 | 22.56384 | 0.003891429 | 0.1146012 | 0.003128265 | 0.224055105 | 2107 | 687 | 88.2 |
| 8 Jets | 20 | 0.3573231 | 25.98152 | 0.00566238 | 0.1114094 | 0.007775031 | 0.24749754 | 2151 | 640 | 88.3 | |
| 8 Jets | 30 | 0.3388034 | 23.79767 | 0.007705436 | 0.09102151 | 0.011329086 | 0.210368366 | 2117 | 702 | 90.1 |
3.3 Effect of methane fuel velocity on thermal distribution
The objective of the combustor system is to convert fuel's chemical energy into thermal energy through combustion. Having concluded that the use of the 24 jets model could reduce the residence time for the fuel mixture under a non-premixed model, the impact of varying methane velocity on combustion characteristics of the 16 jets model is analysed and discussed in this section. Fig. 8 presents the variation of the temperature profile within the combustion tube, which is influenced by the methane inlet velocity into the tube. In all cases, the hydrogen and air velocities are kept constant at 15 m s−1, and the ambient temperature before combustion is set to 300 K. The methane inlet velocity is independently controlled and three different values of 5 m s−1, 10 m s−1, and 15 m s−1 were explored. The temperature profiles were taken along the axial distance as illustrated in Fig. 2(b).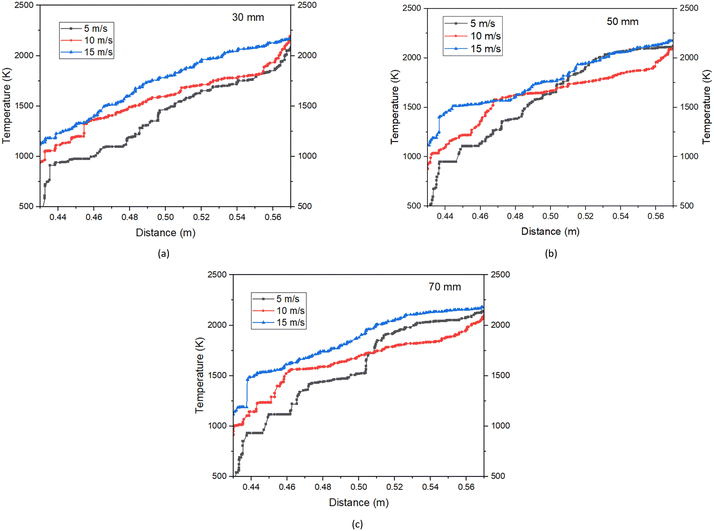 | ||
| Fig. 8 Temperature profile with methane inlet velocity at (a) axial distance of 30 mm, (b) axial distance of 50 mm, and (c) axial distance of 70 mm for the 16-jets model. | ||
Fig. 8(a) shows the temperature profile at an axial distance of 30 mm. The result shows that methane velocities of 5 m s−1 and 15 m s−1 attained a temperature of 2157 K and 2168 K, respectively. Meanwhile, fuel velocity at 10 m s−1 achieved a peak temperature of 2198 K representing the highest temperature at this axial position. At the axial distance of 50 mm in Fig. 8(b), a fuel velocity of 5 m s−1 peaked at a temperature of 2141 K, while 15 m s−1 maintained a temperature of 2189 K in the combustion tube. Similarly in Fig. 8(b), 10 m s−1 velocity achieved the lowest temperature of 2136 K at this axial position. The thermal output in Fig. 8(c) for an axial distance of 70 mm showed that 15 m s−1 fuel flow speed produced 2184 K in the combustion tube, 10 m s−1 recorded a drop in temperature to 2168 K, and 5 m s−1 rising to 2169 K. Overall, the thermal output across these axial positions favours the lowest and highest fuel velocities (5 m s−1 and 15 m s−1) as maximum temperatures were recorded on these locations.
Conversely, the flame entrainment in the combustion tube and the non-uniform distribution of hot zones in the combustion tube potentially created the differences obtained on the axial positions. However, the study conducted by Huang et al.24 revealed that subjecting the model to higher velocities can result in a notable pressure loss and a significant drop in thermal efficiency. Consequently, lowering the velocity ensures a complete thermal reaction without significant losses. Based on these observations, it is evident that the operation of the combustor should be limited to lower fuel velocities to ensure flame sustainability and increase the thermal output of the combustion setup.12 However, if the fuel flow rate is too low, the flame may be extinguished due to a low heat generation rate and high heat loss to the surroundings. Therefore, by carefully selecting and controlling the methane inlet velocity, the combustion process can be optimised towards achieving higher efficiency and stable thermal distributions from start-up to full-power conditions.
Fig. 9 displays the temperature distribution contours within the combustor at different methane inlet velocities of 5 m s−1, 10 m s−1, and 15 m s−1 for the 16 jets model. The contour plots provide valuable insights into the behaviour of the flame temperature within the combustion tube under varying conditions.
 | ||
| Fig. 9 Temperature contour across the central axis (Y–Z plane) of the combustion chamber with varying methane inlet velocity: (a) 5 m s−1, (b) 10 m s−1, and (c) 15 m s−1. | ||
Based on the analysis shown in Fig. 9, it is evident that the flame temperature in the combustion tube increases as the methane inlet velocity increases from 5 m s−1 to 10 m s−1. While the maximum temperature value at a methane inlet velocity of 5 m s−1 is 2330 K, the temperature consequently reaches its maximum value of 2334 K at a methane velocity of 10 m s−1.
However, at a higher velocity of 15 m s−1, there is a drop in temperature to 2290 K. The increase in temperature at lower methane velocities may be due to the non-uniform distribution of the flame as observed from the temperature contour plots at these velocities. This non-uniformity is particularly evident in zones of the combustion tube where quenching occurs, indicating incomplete combustion. Conversely, at the highest methane velocity of 15 m s−1, a more uniform combustion is achieved throughout the combustion tube, which might be responsible for the drop in the maximum temperature in the combustor tube.
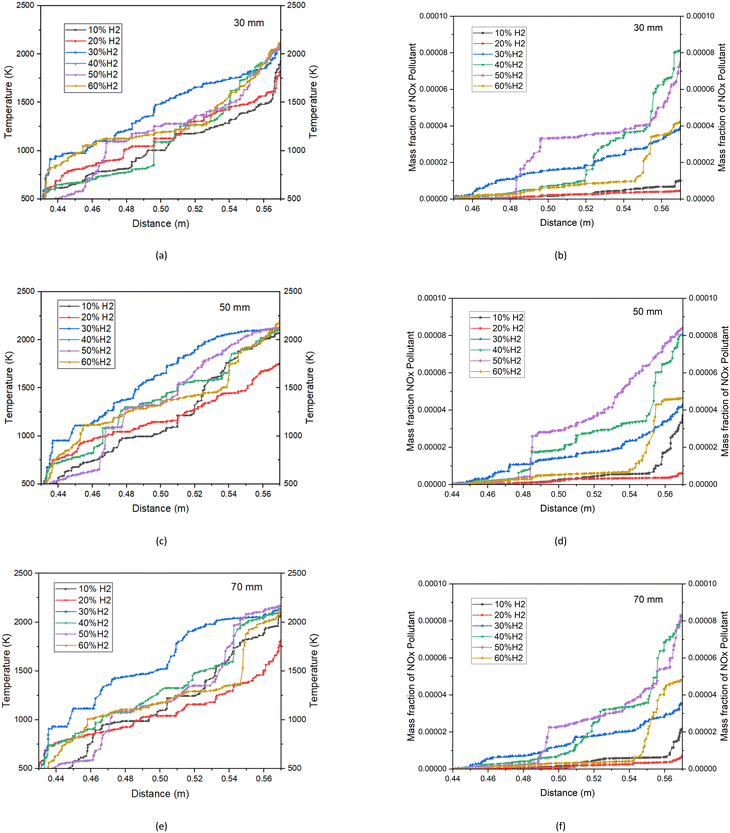
3.4 Effect of hydrogen composition on the temperature profile and NOx pollutant
The impact of hydrogen composition in the fuel was explored for the 16 jets model when the investigation was carried out under the same boundary conditions as shown in Table 2. The methane velocity was set at 5 m s−1, and the feed flow rate of hydrogen and air was maintained at 15 m s−1. To evaluate the effect of hydrogen on thermal output, a range of hydrogen mass fractions was considered, varying from 10% to 60%, as indicated in Table 2. Consequently, six distinct cases were simulated. The contour plots from these simulations are presented in Fig. 10. These contours are represented on the Y–Z axial planes which provides insights into the combustion process. A well-shaped and distributed flame pattern, with flames extending towards the chamber wall, results in a more uniform combustion in the chamber for 10% H2 as observed in Fig. 10(a). This is depicted by the temperature contour attaining a maximum temperature of 2279 K. However, for hydrogen mixtures of 30% and 60%, the temperature contour in Fig. 10(c) and (f), respectively, show reduced flame circulation, with the hot zones appearing skewed negatively towards the chamber wall. These compositions achieve maximum temperatures of 2300 K and 2394 K, respectively. This observation, however, agrees with the findings of Gheshlaghi and Tahsini,25 who reported that an increase in hydrogen composition reduces the recirculation potential of the reaction zone due to its impact on the flow structure of fuel molecules. Overall, the temperature contours indicate a gradual increase in thermal output with increasing hydrogen addition.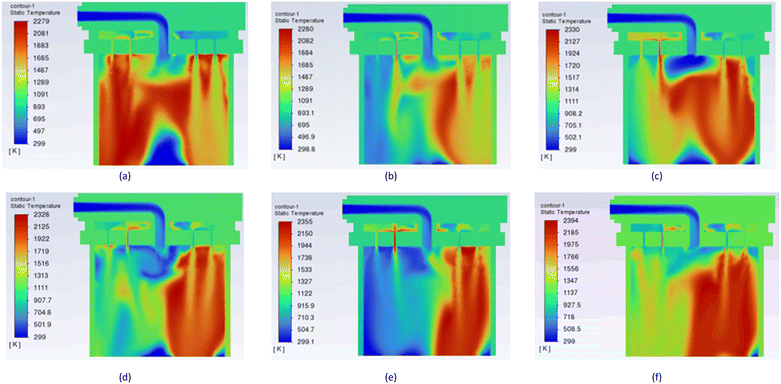 | ||
| Fig. 10 Temperature contour across the central axis (Y–Z plane) of the combustion chamber with varying hydrogen concentration: (a) 10%, (b) 20%, (c) 30%, (d) 40%, (e) 50%, and (f) 60%. | ||
Further evaluation on the impact of hydrogen concentrations is shown in Fig. 11, which shows the velocity vectors of the flow and mixing patterns in the mixing chamber and combustion tube. The velocity vectors have been presented to provide an overview of the effect of varying hydrogen composition in the fuel mix at a constant velocity. The exit of the jets presents the points of high-velocity magnitude with a vertical spray pattern in the combustion tube effectively mixing with the fuel input from the center of the combustor. Across the models, it is observed that the fuel recirculation was well demonstrated in the mixing chamber and combustor. This recirculation is shown in the form of rotational vortex patterns dominant at the center of the combustor. The internal recirculation zones (IRZ) formed are projected from the center of the combustor due to the entry of methane on the central axis while a smaller IRZ is formed from the swirl generated by the flow at the wall. The swirl number of the flow is characterised by eqn (11) with U and W representing the tangential and axial velocity, respectively.
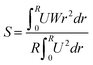 | (11) |
 | (12) |
 | ||
| Fig. 11 Velocity vector with varying hydrogen concentration: (a) 10% H2, (b) 20% H2, (c) 30% H2, (d) 40% H2, (e) 50% H2 and (f) 60% H2. | ||
Fig. 12 shows the temperature variation and NOx production across the sectional axis of the combustion tube. At the axial distance of 30 mm in Fig. 12(a), the highest thermal output from the combustion is revealed at 60% H2 mass fraction, closely followed by 50%, 40%, and 30% H2 concentration with the lowest temperature shown at 20% concentration of H2. Fig. 12(b) shows the NOx production resulting from increased hydrogen concentration at 30 mm axial distance. The lowest NOx produced is observed at 20% and 10% hydrogen concentration respectively, while the higher NOx emissions were observed with the incremental addition of hydrogen to the fuel mixture except 60% H2 which indicated a drop in emissions.
It was observed in Fig. 12(c), at 50 mm axial distance, that the highest thermal output also resulted from 60% hydrogen concentration, followed closely by 50%, 30%, 20%, and 10% hydrogen concentration. However, the corresponding NOx produced in Fig. 12(d) shows that hydrogen concentrations of 20% and 10% produced the lowest NOx emission in agreement with the study by Fu et al.13 However, 30% hydrogen also produced a low NOx emission, this is mostly due to consistent distribution of temperature across the combustor which could have increased with uneven temperature distribution under the thermal NOx mechanism where uneven temperature output increases the NOx emission for fuel mixtures.
The increased NOx emissions in 40% and 50% hydrogen concentrations can be attributed to the maximum flame temperatures and increased turbulence introduced by hydrogen in the fuel mix leading to less effective mixing between the fuel and oxidant. These conditions favour the rapid formation of NOx through temperature-dependent reactions, making NOx control a critical consideration in hydrogen-enriched combustion systems.
At 30 mm axial position (Fig. 12b), it was noticed that 60% hydrogen mass fraction produced a close NOx emission as with other axial distances and hydrogen concentration resulting from the fuel mixture gradient which produced a skewed flame. In Fig. 12(e), the temperature profile of 70 mm axial distance produced similar results when compared with the 50 mm section with a H2 concentration of 30% attaining a consistently high thermal output at the centre of the combustion tube indicating an effective fuel mixture ratio at the point.
Overall, the effect of hydrogen composition showed distinct differences between low and high concentrations of H2, suggesting concentrations within the range of 10% to 30% are significant in reducing NOx emissions, and controlling the thermal output within the combustion chamber. This highlights the potential efficacy of H2 in controlling NOx emission through variation of the fuel mixture, with particular emphasis on the 10% to 30% H2 concentration range. This observation aligns with the findings by Liu et al.,27 who investigated jets in MILD combustors suggesting that controlling emissions should involve limiting the amount of hydrogen in the mixture. Similar studies by Wang et al.28 and Ziani et al.9 have also observed this phenomenon in their analytical simulations of methane/hydrogen gas mixtures.
4. Conclusion
This study discussed and analysed the performance of a 3D combustor with multiple jets using numerical methods. The paper focused on evaluating the efficiency of the combustor under varying operational and geometrical conditions such as fuel flow rate, jet number, and hydrogen concentration in the fuel mixture. From the findings of this study, the following conclusions were drawn.Fuel mixture resident time is dependent on the number of jets, with the maximum temperature aligning to the right wall of the combustion tube due to the increased exit velocity of the mixture in the tube.
16 jets have been identified as the optimal operational value for increased efficiency, while further study would be required to investigate a range of 18–22 jets as the maximum number of jets to anticipate a decline. However, higher jet numbers could reduce the residence time for the hydrogen and methane mixture in the combustion chamber, which could result in a reduction in temperature. Very low and high numbers of jets will result in inefficient combustion leading to flame quenching.
As observed in this study with a limited velocity range, increasing methane velocity will create a corresponding rise in flame temperature due to the resulting mass flow rate of reactants entering the combustion tube.
Additionally, the operation of the combustor should be limited to lower fuel velocities to maintain even distribution in the combustion tube and increase combustion efficiency. However, further study is required to investigate parameters beyond the scope of this current paper, including the use of different fuel mixtures, an extended number of jets, a wider range of flow parameters, and an investigation of the impact of NOx mechanisms on the model. Furthermore, a wider range of velocities will be investigated in the future to study the behaviour of the reactions and products of the combustion.
Data availability
All data used in this study have been generated from ANSYS Fluent Software for modelling the combustion process in the designed model.Author contributions
The project was conceptualised and originally drafted by M. E. O. with help from C. F. O. and D. S. A. Investigation, methodology, validation, and visualisation were done by M. E. O., while supervision, visualisation, review, and editing were done by D. S. A. and C. F. O.Conflicts of interest
There are no conflicts to declare.Acknowledgements
We would like to thank the University of Wolverhampton's Faculty of Engineering, School of Engineering, Computing and Mathematical Sciences and the Centre for Engineering Innovation and Research (CEIR) for providing the computing resources for the study. M. E. O. acknowledges support from Commonwealth Scholarship Commission (CSC) under the Commonwealth Shared Scholarship (NGSS-2021-507).References
- IEA, https://www.iea.org/data-and-statistics/data-product/world-energy-balances, 2022, World Energy Balances - Data product.
- A. G. Chmielewski, Environmental effects of fossil fuel combustion, Interactions, Energy/Environment, 1999, pp. 56–74 Search PubMed.
- D. Pashchenko, Hydrogen-rich fuel combustion in a swirling flame: CFD-modeling with experimental verification, Int. J. Hydrogen Energy, 2020, 45(38), 19996–20003 CrossRef CAS.
- S. Sorrell, Reducing energy demand: A review of issues, challenges and approaches, Renewable Sustainable Energy Rev., 2015, 47, 74–82 CrossRef.
- S. R. Shabanian, M. Rahimi, A. Khoshhal and A. A. Ammar, CFD study on hydrogen-air premixed combustion in a micro scale chamber, Iranian Journal of Chemistry and Chemical Engineering, 2010, 29(4), 161–172 CAS.
- E. Jiaqiang, L. Cai, J. Li, J. Ding, J. Chen and B. Luo, Effects analysis on the catalytic combustion and heat transfer performance enhancement of a non-premixed hydrogen/air micro combustor, Fuel, 2022, 1, 309 Search PubMed.
- S. Eckart, F. Z. Rong, C. Hasse, H. Krause and A. Scholtissek, Combined experimental and numerical study on the extinction limits of non-premixed H2/CH4 counterflow flames with varying oxidizer composition, Int. J. Hydrogen Energy, 2023, 48(37), 14068–14078 CrossRef CAS.
- D. Pashchenko, Comparative analysis of hydrogen/air combustion CFD-modeling for 3D and 2D computational domain of micro-cylindrical combustor, Int. J. Hydrogen Energy, 2017, 42(49), 29545–29556 CrossRef CAS.
- L. Ziani, A. Chaker, K. Chetehouna, A. Malek and B. Mahmah, Numerical simulations of non-premixed turbulent combustion of CH 4-H2 mixtures using the PDF approach, Int. J. Hydrogen Energy, 2013, 8597–8603 CrossRef CAS.
- V. N. Kurdyumov and M. Matalon, Self-accelerating flames in long narrow open channels, Proc. Combust. Inst., 2015, 35(1), 921–928 CrossRef CAS.
- S. Mohan and M. Matalon, Diffusion flames and diffusion flame-streets in three dimensional micro-channels, Combust. Flame, 2017, 177, 155–170 CrossRef CAS.
- J. Hua, M. Wu and K. Kumar, Numerical simulation of the combustion of hydrogen-air mixture in micro-scaled chambers. Part I: Fundamental study, Chem. Eng. Sci., 2005, 60(13), 3497–3506 CrossRef CAS.
- Z. Fu, L. Sui, J. Lu, J. Liu, P. Weng and Z. Zeng, et al., Investigation on effects of hydrogen addition to the thermal performance of a traditional counter-flow combustor, Energy, 2023, 262, 125465 CrossRef CAS.
- A. Mardani and S. Tabejamaat, Effect of hydrogen on hydrogen-methane turbulent non-premixed flame under MILD condition, Int. J. Hydrogen Energy, 2010, 35(20), 11324–11331 CrossRef CAS.
- S. Karyeyen, Combustion characteristics of a non-premixed methane flame in a generated burner under distributed combustion conditions: A numerical study, Fuel, 2018, 230, 163–171 CrossRef CAS.
- S. Boussetla, A. Mameri and A. Hadef, NO emission from non-premixed MILD combustion of biogas-syngas mixtures in opposed jet configuration, Int. J. Hydrogen Energy, 2021, 46(75), 37641–37655 CrossRef CAS.
- H. Yilmaz, O. Cam, S. Tangoz and I. Yilmaz, Effect of different turbulence models on combustion and emission characteristics of hydrogen/air flames, Int. J. Hydrogen Energy, 2017, 42(40), 25744–25755 CrossRef CAS.
- C. E. Baukal Jr., Oxygen-enhanced combustion, CRC press, 2010 Search PubMed.
- A. Escue and J. Cui, Comparison of turbulence models in simulating swirling pipe flows, Appl. Math. Model., 2010, 34(10), 2840–2849 CrossRef.
- D. S. Adebayo and A. Rona, The Three-Dimensional Velocity Distribution of Wide Gap Taylor-Couette Flow Modelled by CFD, Int. J. Rotating Mach., 2016, 2016(1), 8584067 Search PubMed.
- M. Reyes, R. Sastre, B. Giménez and C. Sesma, Experimental, Kinetic Modeling and Morphologic Study of the Premixed Combustion of Hydrogen/Methane Mixtures, Energies, 2022, 15(10), 3722 CrossRef CAS.
- J. Li, S. K. Chou, W. M. Yang and Z. W. Li, A numerical study on premixed micro-combustion of CH4-air mixture: Effects of combustor size, geometry and boundary conditions on flame temperature, Chem. Eng. J., 2009, 150(1), 213–222 CrossRef CAS.
- H. Zhou and L. Hu, Mitigation of Combustion Instability and NOx Emissions by Microjets in Lean Premixed Flames with Different Swirl Numbers, J. Therm. Sci., 2023, 32(4), 1697–1709 CrossRef CAS.
- M. Huang, Z. Zhang, W. Shao, Y. Xiong, Y. Liu and F. Lei, et al., Effect of air preheat temperature on the MILD combustion of syngas, Energy Convers. Manage., 2014, 86, 356–364 CrossRef CAS.
- M. K. G. Gheshlaghi and A. M. Tahsini, Numerical investigation of hydrogen addition effects to a methane-fueled high-pressure combustion chamber, Int. J. Hydrogen Energy, 2023, 48(86), 33732–33745 CrossRef.
- A. Hoda, T. M. R. Rahman, W. Asrar and S. A. Khan, A Comparative Study of Natural Gas and Biogas Combustion in A Swirling Flow Gas Turbine Combustor, Combust. Sci. Technol., 2022, 194(13), 2613–2640 CrossRef CAS.
- Z. Liu, Y. Xiong, Z. Zhu, Z. Zhang and Y. Liu, Effects of hydrogen addition on combustion characteristics of a methane fueled MILD model combustor, Int. J. Hydrogen Energy, 2022, 47(36), 16309–16320 CrossRef CAS.
- F. Wang, J. Mi, P. Li and C. Zheng, Diffusion flame of a CH4/H2 jet in hot low-oxygen coflow, Int. J. Hydrogen Energy, 2011, 36(15), 9267–9277 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2025 |