 Open Access Article
Open Access ArticleLendenfeldaranes W–Y, new 24-homoscalaranes from a marine sponge Lendenfeldia species
Chih-Yin Huangab,
Bo-Rong Pengc,
Yueh-Wen Liud,
You-Ying Chene,
Jui-Hsin Su efg,
Chia-Ching Liaw
efg,
Chia-Ching Liaw hijkl,
Jih-Jung Chen
hijkl,
Jih-Jung Chen j,
Chung-Chih Tsengbm,
Yu-Jen Wun,
Yuan-Bin Chengg,
Lun Kelvin Tsouo,
Mingzi M. Zhang
j,
Chung-Chih Tsengbm,
Yu-Jen Wun,
Yuan-Bin Chengg,
Lun Kelvin Tsouo,
Mingzi M. Zhang p,
Zhi-Hong Weneg,
Li-Guo Zheng
p,
Zhi-Hong Weneg,
Li-Guo Zheng *eq and
Ping-Jyun Sung
*eq and
Ping-Jyun Sung *egirstu
*egirstu
aDepartment of Orthopedics, Kaohsiung Armed Forces General Hospital, Kaohsiung 802301, Taiwan
bInstitute of Medical Science and Technology, National Sun Yat-sen University, Kaohsiung 804201, Taiwan
cResearch Center for Chinese Herbal Medicine, College of Human Ecology, Chang Gung University of Science and Technology, Taoyuan 333324, Taiwan
dDepartment of Cosmetics and Fashion Styling, Chien Shiu University, Kaohsiung 833301, Taiwan
eNational Museum of Marine Biology and Aquarium, Pingtung 944401, Taiwan. E-mail: t0919928409@gmail.com; pjsung@nmmba.gov.tw
fGraduate Institute of Marine Biology, National Dong Hwa University, Pingtung 944401, Taiwan
gDepartment of Marine Biotechnology and Resources, National Sun Yat-sen University, Kaohsiung 804201, Taiwan
hNational Research Institute of Chinese Medicine, MOHW, Taipei 112304, Taiwan
iGraduate Institute of Natural Products, Kaohsiung Medical University, Kaohsiung 807378, Taiwan
jDepartment of Pharmacy, School of Pharmaceutical Sciences, National Yang Ming Chian Tung University, Taipei 112304, Taiwan
kDepartment of Biochemical Science and Technology, National Chiayi University, Chiayi University, Chiayi 600048, Taiwan
lSchool of Chinese Medicine, National Yang Ming Chian Tung University, Taipei 112304, Taiwan
mSchool of Dentistry, College of Oral Medicine, National Defence Medical University, Taipei 114201, Taiwan
nYu Jun Biotechnology Co., Ltd, Donggang, Pingtung 928003, Taiwan
oInstitute of Biotechnology and Pharmaceutical Research, National Health Research Institute, Miaoli 350401, Taiwan
pInstitute of Molecular and Genomics Medicine, National Health Research Institute, Miaoli 350401, Taiwan
qDoctoral Degree Program in Marine Biotechnology, National Sun Yat-sen University, Kaohsiung 804201, Taiwan
rChinese Medicine Research and Development Center, China Medical University Hospital, Taichung 404394, Taiwan
sProgram in Pharmaceutical Biotechnology, Fu Jen Catholic University, New Taipei City 242062, Taiwan
tDepartment of Biochemistry and Molecular Medicine, National Dong Hwa University, Hualien 974301, Taiwan
uSchool of Medicine, Kaohsiung Medical University, Kaohsiung 807378, Taiwan
First published on 18th November 2025
Abstract
Three new scalarane-type sesterterpenoids, lendenfeldaranes W–Y (1–3), along with a known analogue, lendenfeldarane D (4), were isolated from a marine sponge identified as Lendenfeldia species. The structures of all isolates were determined based on spectroscopic methods. Scalarane 1 exhibited significant activity in enhancing alkaline phosphatase (ALP) activity.
1 Introduction
Sponges of the genus Lendenfeldia (phylum Porifera, class Demospongiae, subclass Keratosa, order Dictyoceratida, family Thorectidae, subfamily Phyllospongiinae) are broadly distributed across shallow coral reefs in the Asia–Pacific region. These marine invertebrates have attracted significant scientific attention owing to their rich repertoire of secondary metabolites, many of which display noteworthy pharmacological potential. Among these, sesterterpenoids-particularly 26-carbon homoscalarane and 24-homoscalarane derivatives, represent the dominant chemical constituents of Lendenfeldia species. A variety of biological activities have been reported for these compounds, including anti-inflammatory,1–4 cytotoxic,5–11 antimicrobial,12–14 and anti-osteoporotic effects,15 understanding their promise as valuable leads in drug discovery and biomedical research.In our previous work, we reported the isolation of a series of scalarane-type sesterterpenoids from a Lendenfeldia sponge collected in the coastal waters of Taiwan, together with an evaluation of their biological activities. Building on this research, we have now isolated three new 24-homoscalaranes, designated lendenfeldaranes W–Y (1–3), along with a known analogue, lendenfeldarane D (4) (ref. 9) (Fig. 1). The structures of compounds 1–3 were established through detailed spectroscopic analyses. Furthermore, their anti-osteoporotic potential was assessed by examining their ability to enhance ALP activity in MG63 osteoblast-like cells.
 | ||
| Fig. 1 Structures of lendenfeldaranes W–Y (1–3), lendenfeldarane D (4), felixin B (5), and felixin A (6). | ||
2 Results and discussion
Lendenfeldarane W (1) was obtained as an amorphous solid. The molecular formula was determined to be C28H44O6 from the (+)-HRESIMS ion at m/z 499.30284 [M + Na]+ (calcd for C28H44O6 + Na, 499.30301), corresponding to seven degrees of unsaturation. The IR spectrum showed absorptions at 3419 and 1735 cm−1, indicating the presence of hydroxy and ester carbonyl groups.The 1H NMR data for 1 (Table 1) displayed four tertiary methyl singlets at δH 0.78 (H3-20), 0.84 (H3-23), 0.89 (H3-19), and 1.08 (H3-21); one secondary methyl doublet at δH 1.18 (3H, d, J = 6.6 Hz, H3-26); two olefinic protons at δH 5.98 (1H, dd, J = 10.2, 2.4 Hz, H-15) and 5.71 (1H, dd, J = 10.2, 3.0 Hz, H-16); and three oxymethine protons at δH 5.28 (1H, d, J = 3.0 Hz, H-25), 4.96 (1H, dd, J = 3.0, 3.0 Hz, H-12), and 4.41 (1H, q, J = 6.6 Hz, H-24). In addition, an oxymethylene group was evident from the diastereotopic geminal protons at δH 4.04 (1H, d, J = 12.0 Hz, H-20a) and 3.88 (1H, d, J = 12.0 Hz, H-20b). The 13C NMR and HSQC spectra revealed 28 carbon signals (Table 1), comprising one 1,2-disubstituted double bond (δC 132.2/CH-15; 127.4/CH-16), one acetal carbon (δC 97.8/CH-25), one oxygenated quaternary carbon (δC 80.2/C-17), two oxymethines (δC 85.1/CH-24; 74.1/CH-12), one oxymethylene (δC 62.6/CH2-22), four tertiary methyls (δC 33.9/CH3-19; 21.9/CH3-20; 17.3/CH3-21; 16.6/CH3-23), one secondary methyl (δC 15.7/CH3-26), six aliphatic methylenes (δC 41.7/CH2-3; 41.5/CH2-7; 34.0/CH2-1; 25.1/CH2-11; 18.3/CH2-2; 17.6/CH2-6), four aliphatic methines (δC 62.8/CH-18; 57.0/CH-5; 52.5/CH-9; 52.0/CH-14), four non-oxygenated quaternary carbons (δC 41.7/C-10; 36.7/C-8; 33.0/C-4; 42.1/C-13), and one acetoxy group (δC 21.4/acetate methyl; 170.4/acetate carbonyl).
| Position | 1 | 2 | 3 | |||
|---|---|---|---|---|---|---|
| δHa (J in Hz) | δCb, Mult.c | δHa (J in Hz) | δCb, Mult.c | δHa (J in Hz) | δCb, Mult.c | |
| a Spectra recorded at 600 MHz in CDCl3.b Spectra recorded at 150 MHz in CDCl3.c Multiplicity was deduced by 13C, HSQC, and HMBC spectra.d Signals overlapped.e — signals were not observed. | ||||||
| 1α | 0.59 dddd (13.2, 13.2, 3.6, 1.2) | 34.0, CH2 | 0.55 dddd (13.8, 13.8, 3.6, 1.2) | 34.6, CH2 | 0.53 dddd (13.2, 13.2, 3.6, 1.2) | 34.5, CH2 |
| β | 2.10 br d (13.2) | 1.99 m | 2.13 m | |||
| 2α | 1.47 ddddd (18.0, 3.6, 3.6, 3.6, 3.6) | 18.3, CH2 | 1.44 md | 18.2, CH2 | 1.45 md | 18.4, CH2 |
| β | 1.56 md | 1.56 m | 1.54 m | |||
| 3α | 1.44 m | 41.7, CH2 | 1.46 m | 41.4, CH2 | 1.44 br d (13.2)d | 41.6, CH2 |
| β | 1.21 dd (13.2, 3.6) | 1.14 m | 1.19 dd (13.2, 4.8) | |||
| 4 | 33.0, C | 33.0, C | 32.9, C | |||
| 5 | 1.01 dd (13.2, 2.4) | 57.0, CH | 1.02 dd (12.0, 1.8) | 57.1, CH | 1.00 dd (12.0, 1.8) | 56.6, CH |
| 6α | 1.56 md | 17.6, CH2 | 1.60 m | 17.7, CH2 | 4.60 m | 68.4, CH |
| β | 1.41 ddd (13.2, 13.2, 3.6) | 1.44 md | ||||
| 7α | 1.06 ddd (13.2, 12.6, 3.6) | 41.5, CH2 | 1.11 ddd (13.2, 13.2, 4.2) | 40.8, CH2 | 1.40 md 2.18 m | 44.3, CH2 |
| β | 1.97 ddd (12.6, 3.6, 3.6) | 1.80 ddd (13.2, 3.6, 3.6) | ||||
| 8 | 36.7, C | 37.2, C | 39.1, C | |||
| 9 | 1.35 br d (12.6) | 52.5, CH | 1.40 dd (13.2, 4.2) | 52.7, CH | 1.40 br d (13.8)d | 53.0, CH |
| 10 | 41.7, C | 40.1, C | —e | |||
| 11α | 1.88 ddd (15.0, 3.0, 2.4) | 25.1, CH2 | 2.07 m | 24.3, CH2 | 1.98 m | 25.0, CH2 |
| β | 2.26 ddd (15.0, 12.6, 3.0) | 2.17 m | ||||
| 12 | 4.96 dd (3.0, 3.0) | 74.1, CH | 5.00 dd (3.0, 2.4) | 76.0, CH | 4.63 dd (3.0, 1.8) | 77.6, CH |
| 13 | 42.1, C | 41.2, C | 39.5, C | |||
| 14 | 2.14 dd (3.0, 2.4) | 52.0, CH | 2.12 dd (14.4, 4.2) | 48.8, CH | 1.57 m | 56.1, CH |
| 15 | 5.98 dd (10.2, 2.4) | 132.2, CH | 2.55 dd (17.4, 4.2)-Hα | 34.9, CH2 | 2.26 m 2.34 md | —e |
| 2.43 dd (17.4, 14.4)-Hβ | ||||||
| 16 | 5.71 dd (10.2, 3.0) | 127.4, CH | 197.4, C | 6.64 dd (3.0, 3.0) | 139.6, CH | |
| 17 | 80.2, C | 136.7, C | —e | |||
| 18 | 2.47 s | 62.8, CH | 7.31 s | 163.5, CH | 1.93 d (17.4)-Hα | 35.2, CH2 |
| 2.27 br d (17.4)-Hβ | ||||||
| 19 | 0.89 s | 33.9, CH3 | 0.89 s | 33.7, CH3 | 0.88 s | 33.8, CH3 |
| 20 | 0.78 s | 21.9, CH3 | 0.84 s | 21.8, CH3 | 0.78 s | 21.9, CH3 |
| 21 | 1.08 s | 17.3, CH3 | 1.01 s | 15.8, CH3 | 1.24 s | 16.9, CH3 |
| 22a | 4.04 d (12.0) | 62.6, CH2 | 4.63 d (12.0) | 64.6, CH2 | 4.05 d (11.4) | 62.9, CH2 |
| b | 3.88 d (12.0) | 4.12 d (12.0) | 3.92 dd (11.4, 1.2) | |||
| 23 | 0.84 s | 16.6, CH3 | 1.17 s | 18.6, CH3 | 0.92 s | 20.8, CH3 |
| 24 | 4.41 q (6.6) | 85.1, CH | 197.8, C | 199.4, C | ||
| 25 | 5.28 d (3.0) | 97.8, CH | 2.44 s | 30.7, CH3 | 2.34 sd | 25.4, CH3 |
| 26 | 1.18 d (6.6) | 15.7, CH3 | ||||
| OAc-12 | 2.15 s | 170.4, C | 2.07 s | 170.3, C 21.2, CH3 | 2.10 s | 170.1, C |
| 21.4, CH3 | 21.4, CH3 | |||||
| OAc-22 | 2.07 s | 170.8, C | ||||
| 21.2, CH3 | ||||||
| OH-17 | 2.31 br s | |||||
| OH-25 | 2.73 br d (3.0) | |||||
Analysis of the NMR data indicated that two degrees of unsaturation were attributed to one acetoxy group and a 1,2-disubstituted olefin, while the remaining five degrees of unsaturation defined a pentacyclic homoscalarane skeleton. This inference was supported by the 1H–1H COSY correlations of 1 (Fig. 2), which established six partial spin systems: H2-1/H2-2/H2-3, H-5/H2-6/H2-7, H-9/H2-11/H-12, H-14/H-15/H-16, H-18/H-25, and H-24/H3-26. Key 2J- and 3J-HMBC correlations from protons to quaternary carbons, such as H2-3, H-5/C-4; H2-7, H-9, H-15/C-8; H-5, H-9/C-10; H-15, H-18, H-25/C-13; and H-15, H-16, H-18, H-24, H-25, H3-26/C-17, confirmed a 6/6/6/6/5 fused pentacyclic 24-homoscalarane framework.
The oxymethylene unit (δC 62.6) correlated with the methylene protons at δH 4.04 and 3.88 in the HSQC spectrum, and these protons showed 2J- and 3J-HMBC correlations to C-10 (δC 41.7), C-1 (δC 34.0) and C-9 (δC 52.5), indicating a hydroxymethyl substituent at C-10 (Fig. 2). Further HMBC correlations, H3-19/C-3, C-4, C-5, C-20; H3-20/C-3, C-4, C-5, C-19; H3-21/C-7, C-8, C-9, C-14; H3-23/C-12, C-13, C-14, C-18; and H3-26/C-17, C-24, established the position of Me-19, Me-20, Me-21, Me-23, and Me-26 at C-4, C-4, C-8, C-13, and C-24, respectively.
An acetoxy substituent was placed at C-12, an oxymethine center, based on the chemical shifts of H-12 (δH 4.96, dd, J = 3.0, 3.0 Hz) and C-12 (δC 74.1), which closely matched those reported for felixin D (δH 4.91, dd, J = 3.2, 2.4 Hz; δC 74.6),6 a known 24-homoscalarane analogue possessing an identical functional group. Although no HMBC correlation was observed between H-12 and the acetate carbonyl, the substitution pattern was confirmed by comparison. The hydroxy group at C-25 was deduced from the COSY correlation between the hydroxy proton (δH 2.73, d, J = 3.0 Hz, OH-25) and the acetal proton at δH 5.28 (br d, J = 3.0 Hz, H-25). Formation of a cyclic ether linkage between C-24 and C-25 was evidenced by the HMBC correlation from H-25 (δH 5.28) to the oxymethine carbon at C-24 (δC 85.1). The chemical shift of C-25 (δC 97.8) was consistent with its assignment as an acetal carbon.
Of the six oxygen atoms in the molecular formula, five were accounted for by an acetal (including one hydroxy group), an additional hydroxy group, and an acetoxy substituent. The remaining oxygen atom was assigned as a hydroxy group attached to C-17, supported by the downfield chemical shift of the oxygenated quaternary carbon (δC 80.2).
The stereochemistry of 1 was determined by analysis of NOE correlations in the NOESY spectrum (Fig. 3). Following the established convention for scalarane-type sesterterpenoids, H-5 and the hydroxymethyl group at C-10 were assigned to the α- and β-faces, respectively, based on the absence of an NOE correlation between H-5 and H2-22.16,17 In the NOESY spectrum of 1, H-9 showed correlations with H-5 and H-14, but not with H3-21 and H2-22, indicating that H-9 and H-14 reside on the α face, whereas Me-21 and the C-10 hydroxymethyl group are positioned on the β-face. Correlations of H3-23 with both H3-21 and H-12 established the β-orientations of Me-23 and H-12. H-18 correlated with H-14, but not with H3-23, placing H-18 on the α-face, while H-25 correlated with H-12 and H3-23, supporting its β-orientation. Additionally, a correlation between H-15 and H-16 confirmed the Z-geometry of the C-15/16 double bond. The hydroxy proton at C-25 (OH-25) showed correlation with OH-17, indicating the α-orientation of the hydroxy group at C-17. Taken together, these data established the absolute configuration of 1 as 5S, 8R, 9S, 10R, 12S, 13S, 14S, 17R, 18R, 24S, 25R. Notably, compound 1 represents the first reported example of a 17-hydroxy scalarane derivative.
 | ||
| Fig. 3 Stereo-view of 1 (generated by computer modeling) and calculated distances (Å) between selected protons with key NOESY correlations. | ||
Lendenfeldarane X (2) was assigned a molecular formula of C29H42O6 based on its (+)-HRESIMS ion at m/z 509.28714 (calcd for C29H42O6 + Na, 509.28736), corresponding to nine degrees of unsaturation. Analysis of the 1H and 13C NMR data (Table 1) indicated that 2 belongs to the 24-homoscalarane class, closely resembling the known analogue felixin B (5) (Fig. 1), originally isolated from the Formosan marine sponge Ircinia felix.6 The key structural difference between 2 and 5 lies in the substitution at C-10: in 5, a hydroxymethyl group is present (δH 4.03, 1H, d, J = 11.6 Hz; 3.89, 1H, d, J = 11.6 Hz/δC 63.0, CH2-22; δC 41.8, C-10),6 whereas in 2 this functionality is replaced by an acetoxymethyl group (δH 4.63, 1H, d, J = 12.0 Hz; 4.12, 1H, d, J = 12.0 Hz/δC 64.6, CH2-22; δC 40.1, C-10). Detailed interpretation of the 2D NMR spectroscopic data of 2 corroborated this substitution, thereby establishing its planar structure (Fig. 4).
NOESY correlations of 2 established the configurations of the stereogenic centers in rings A–D, which were consistent with those of 1 and 5 (Fig. 5). The olefinic proton H-18 (δH 7.31) exhibited correlations with H-12 (δH 5.00) and H3-23 (δH 1.17), but not with the acetyl methyl H3-25 (δH 2.44), supporting an s-cis diene configuration for C-18/17/24. Based on these data, the stereogenic carbons were assigned as 5S, 8R, 9S, 10R, 12S, 13S, 14S. Thus, the structure of lendenfeldarane X (2) was established.
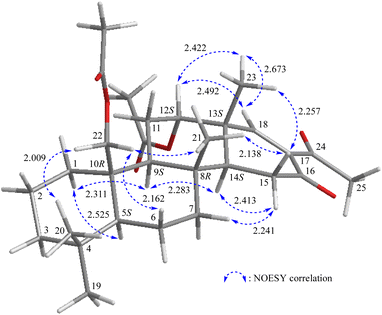 | ||
| Fig. 5 Stereo-view of 2 (generated by computer modeling) and calculated distances (Å) between selected protons with key NOESY correlations. | ||
Lendenfeldarane Y (3) was obtained as an amorphous powder with the molecular formula C27H42O5, established by (+)-HRESIMS at m/z 469.29239 (calcd for C27H42O5 + Na, 469.29245), indicating seven degrees of unsaturation. IR absorptions at 3443, 1731, and 1664 cm−1 revealed hydroxy, ester carbonyl, and α,β-unsaturated ketone groups. NMR data of 3 closely resembled those of felixin A (6)(ref. 6) (Fig. 1), except for an additional oxymethine signal (δC 68.4/δH 4.60, 1H, m, CH-6), consistent with a C-6 hydroxy substitution, further confirmed by 1H–1H COSY crosslations of H-5/H-6/H2-7 (Fig. 6).
In the NOESY spectrum of 3 (Fig. 7), a correlation between H-6 (δH 4.60) and H3-21 (δH 1.24) placed the C-6 hydroxy group on the α-face. H-16 (δH 6.64) showed a correlation with H3-25 (δH 2.34), consistent with an s-trans α,β-unsaturated ketone. The NOESY data of 3 were comparable to those of 2 and 6, indicating the same stereochemical framework, and the stereogenic centers of 3 were assigned as 5S, 6S, 8R, 9S, 10S, 12S, 13R, 14S.
 | ||
| Fig. 7 Stereo-view of 3 (generated by computer modeling) and calculated distances (Å) between selected protons with key NOESY correlations. | ||
Compounds 1, 2, and 4 were evaluated for osteogenic activity in MG63 cells at 10 µM (72 h). Compound 1 enhanced ALP activity (20.04 KU per mgprot) and cell viability (159.90%), whereas compound 2 reduced viability to 30.02%. Compound 4 was the most cytotoxic, lowering viability to 14.64% (Table 2).
| Compounds | ALP activity (king unit per mgprot) | MTT (% control) |
|---|---|---|
| a Data are expressed with the mean standard error of the mean (SEM) (n = 3). The significance was determined with Student's t-test.b p < 0.01 and comparison with untreated cells. | ||
| 1 | 20.04 ± 3.67b | 159.90 ± 2.28 |
| 2 | 3.81 ± 1.91 | 30.02 ± 1.75 |
| 4 | −5.65 ± 0.79 | 14.64 ± 0.34 |
| Alendronate sodium | 21.45 ±± 5.21b | 95.14 ± 12.24 |
| Control | 2.53 ± 0.63 | 100.03 ± 2.28 |
3 Conclusions
Marine sponges of the genus Lendenfeldia are well known as rich sources of scalarane-type sesterterpenoids with diverse structures and notable biological activities.16,17 In this study, three new 24-homoscalaranes, lendenfeldaranes W–Y (1–3), along with a known analogue, lendenfeldarane D (4),9 were isolated from Lendenfeldia sp. In MG63 osteoblast-like cells, compound 1 significantly enhanced ALP activity and cell viability, showing effects comparable to or exceeding those of alendronate sodium, whereas compound 4 was cytotoxic and suppressed osteogenic differentiation. These results underscore the osteogenic potential of scalarane derivatives and support further investigation of sponge-derived sesterterpenoids as candidates for bone regenerative agents.4 Experimental
4.1 General experimental procedures
Optical rotations were measured on a JASCO P-1010 digital polarimeter using the sodium D line (589 nm) with a 10 mm cell. IR spectra were obtained with a Thermo Scientific Nicolet iS5 FT-IR spectrophotometer. NMR spectra were recorded on a 600 MHz Jeol ECZ NMR spectrometer using the residual CHCl3 (δH 7.26 ppm) and CDCl3 (δC 77.0 ppm) as internal standards for 1H and 13C NMR, respectively; coupling constants (J) are presented in Hertz (Hz). ESIMS and HRESIMS were acquired on a Thermo Fisher Orbitrap Exploris 120 (positive SI). Crude extracts were fractionated by silica gel CC (230–400 mesh, Merck). TLC was performed on silica gel 60F254 (0.20 mm, Macherey-Nagel) and RP-18 F254s (0.16–0.20 mm, Merck) plates, visualized under UV and with 10% H2SO4/heat. Final purification used RP-HPLC (Luna C18(2), 5 µm, 100 Å, 250 × 21.2 mm) on a Hitachi L-7110 pump with L-2400 PDA detector.4.2 Animal material
A specimen of the genus Lendenfeldia was collected by SCUBA diving along the southern coast of Taiwan in April 2019. The material was preserved as a voucher (specimen no. 2019-04-SP) at the National Museum of Marine Biology & Aquarium (NMMBA), Taiwan. Species-level identification was confirmed by Professor Yusheng M. Huang (National Penghu University of Science and Technology).4.3 Extraction and isolation
The freeze-dried sponge (wet/dry: 2900/213 g) was extracted with MeOH/CH2Cl2 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) at room temperature. The crude extract (33.7 g) was partitioned between EtOAc and water, and the EtOAc layer (7.93 g) was fractionated by silical gel CC (n-hexane → n-hexane/EtOAC) to give 14 fractions (A–N). Fraction G was further purified by silica gel CC (n-hexane/acetone, 8
1, v/v) at room temperature. The crude extract (33.7 g) was partitioned between EtOAc and water, and the EtOAc layer (7.93 g) was fractionated by silical gel CC (n-hexane → n-hexane/EtOAC) to give 14 fractions (A–N). Fraction G was further purified by silica gel CC (n-hexane/acetone, 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 → acetone) to yield subfractions G1–G15, and G10 was subjected to RP-HPLC (C18, MeOH/H2O 80
1 → acetone) to yield subfractions G1–G15, and G10 was subjected to RP-HPLC (C18, MeOH/H2O 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 20, 5.0 mL min−1) to afford 3 (0.7 mg, Rt = 20.9 min), 2 (1.6 mg, Rt = 39.1 min), 4 (1.0 mg, Rt = 47.4 min), and 1 (1.2 mg, Rt = 53.4 min), respectively.
20, 5.0 mL min−1) to afford 3 (0.7 mg, Rt = 20.9 min), 2 (1.6 mg, Rt = 39.1 min), 4 (1.0 mg, Rt = 47.4 min), and 1 (1.2 mg, Rt = 53.4 min), respectively.
4.4 ALP activity assay and cell viability assays
The osteogenic activity of compounds 1, 2, and 4 was evaluated in MG63 human osteoblast-like cells obtained from the Bioresource Collection and Research Center (BCRC, Hsinchu, Taiwan; BCRC 60279). ALP activity was measured following treatment with test compounds according to establish protocols with minor modifications.18 Cell viability was assessed by MTT assay: MG63 cells (1 × 103 per well) were seeded in 96-well plates, incubated 24 h, and treated with alendronate (0.01 µM) or compounds (10 µM) for 72 h. MTT solution (10 µL, 5 mg mL−1) and medium (90 µL) were added for 4 h, and formazan crystals were dissolved in 100 µL DMSO. Absorbance at 570 nm was measured as an indicator of viability.19Author contributions
C.-Y. Huang, B.-R. Peng, Y.-W. Liu, Y.-Y. Chen, J.-H. Su, C.-C. Liaw, J.-J. Chen, C.-C. Tseng, Y.-J. Wu, Y.-B. Cheng, L. K. Tsou, M. M. Zhang, Z.-H. Wen: methodology, analysis, investigation, data curation, and draft preparation. L.-G. Zheng and P.-J. Sung: conceptualization, resources, supervision, project administration, visualization, draft review & editing, and funding acquisition.Conflicts of interest
The authors declare no conflicts of interest.Data availability
The datasets supporting this article have been uploaded as part of the supplementary information (SI). Supplementary information: HRESI-MS, 1D, and 2D-NMR spectra of 1–3. See DOI: https://doi.org/10.1039/d5ra07083j.Acknowledgements
We thank Ms. Hsiao-Ching Yu and Ms. Chao-Lien Ho (High Valued Instrument Center, National Sun Yat-sen University) for assistance with MS (MS 006500) and NMR (NMR 001100) data acquisition under NSTC 113-2740-M-110-002. This work was partially supported by the National Museum of Marine Biology & Aquarium and NSTC, Taiwan, (grants NSTC 112-2320-B-291-002-MY3 and 114-2320-B-291-001), awarded to P.-J. Sung.Notes and references
- B.-R. Peng, K.-H. Lai, Y.-C. Chang, Y.-Y. Chen, J.-H. Su, Y. M. Huang, P.-J. Chen, S. S.-F. Yu, C.-Y. Duh and P.-J. Sung, Sponge-derived 24-homoscalaranes as potent anti-inflammatory agents, Mar. Drugs, 2020, 18, 434 CrossRef CAS.
- P. Francis and K. Chakraborty, Anti-inflammatory scalarane-type sesterterpenes, erectascalaranes A–B, from the marine sponge Hyrtios erectus attenuate pro-inflammatory cyclooxygenase-2 and 5-lipoxygenase, Med. Chem. Res., 2021, 30, 886–896 Search PubMed.
- B.-R. Peng, K.-H. Lai, G.-H. Lee, S. S.-F. Yu, C.-Y. Duh, J.-H. Su, L.-G. Zheng, T.-L. Hwang and P.-J. Sung, Scalarane-type sesterterpenoids from the marine sponge Lendenfeldia sp. alleviate inflammation in human neutrophils, Mar. Drugs, 2021, 19, 561 CrossRef CAS PubMed.
- B.-R. Peng, L.-G. Zheng, L.-Y. Chen, M. El-Shazly, T.-L. Hwang, J.-H. Su, M.-H. Lee, K.-H. Lai and P.-J. Sung, Nor-24-homoscalaranes, neutrophilic inflammatory mediators from the marine sponge Lendenfeldia sp, Pharmaceuticals, 2023, 16, 1258 CrossRef CAS PubMed.
- F. Cao, Z.-H. Wu, C.-L. Shao, S. Pang, X.-Y. Liang, N. J. de Voogd and C.-Y. Wang, Cytotoxic scalarane sesterterpenoids from the South China Sea sponge Carteriospongia foliascens, Org. Biomol. Chem., 2015, 13, 4016–4024 RSC.
- Y.-Y. Lai, M.-C. Lu, L.-H. Wang, J.-J. Chen, L.-S. Fang, Y.-C. Wu and P.-J. Sung, New scalarane sesterterpenoids from the Formosan sponge Ircinia felix, Mar. Drugs, 2015, 13, 4296–4309 CrossRef CAS.
- Y.-Y. Lai, L.-C. Chen, C.-F. Wu, M.-C. Lu, Z.-H. Wen, T.-Y. Wu, L.-S. Fang, L.-H. Wang, Y.-C. Wu and P.-J. Sung, New cytotoxic 24-homoscalarane sesterterpenoids from the sponge Ircinia felix, Int. J. Mol. Sci., 2015, 16, 21950–21958 CrossRef CAS.
- A. M. Alahdal, H. Z. Asfour, S. A. Ahmed, A. O. Noor, A. M. Al-Abd, M. A. Elfaky and S. S. Elhady, Anti-Helicobacter, antitubercular and cytotoxic activities of scalaranes from the Red Sea sponge Hyrtios erectus, Molecules, 2018, 23, 978 CrossRef PubMed.
- B.-R. Peng, K.-H. Lai, Y.-Y. Chen, J.-H. Su, Y. M. Huang, Y.-H. Chen, M.-C. Lu, S. S.-F. Yu, C.-Y. Duh and P.-J. Sung, Probing anti-proliferative 24-homoscalaranes from a sponge Lendenfeldia sp, Mar. Drugs, 2020, 18, 76 CrossRef CAS PubMed.
- O.-S. Kwon, D. Kim, C.-K. Kim, J. Sun, C. J. Sim, D.-C. Oh, S. K. Lee, K.-B. Oh and J. Shin, Cytotoxic scalarane sesterterpenes from the sponge Hyrtios erectus, Mar. Drugs, 2020, 18, 253 Search PubMed.
- L.-G. Zheng, Y.-Y. Chen, C.-C. Liaw, Y.-C. Lin, S.-Y. Chien, Y.-H. Lo, Y.-C. Tsai, H.-C. Hu, Y.-Y. Chen, T.-L. Hwang, T.-M. Ou and P.-J. Sung, Isolation of 24-homoscalarane sesterterpenoids from a sponge of the genus Lendenfeldia, Phytochem. Lett., 2024, 61, 177–181 CrossRef CAS.
- H. N. Kamel, Y. B. Kim, J. M. Rimoldi, F. R. Fronczek, D. Ferreira and M. Slattery, Scalarane sesterterpenoids: semisynthesis and biological activity, J. Nat. Prod., 2009, 72, 1492–1496 CrossRef CAS.
- C. Hertzer, S. Kehraus, N. Böhringer, F. Kaligis, R. Bara, D. Erpenbeck, G. Wörheide, T. F. Schäberle, H. Wägele and G. M. König, Antibacterial scalarane from Doriprismatica stellata nudibranchs (Gastropoda, Nudibranchia), egg ribbons, and their dietary sponge Spongia cf. agaricina (Demospongiae, Dictyoceratida), Beilstein J. Org. Chem., 2020, 16, 1596–1605 CrossRef CAS PubMed.
- A.-Y. Shin, H.-S. Lee and J. Lee, Isolation of scalimides A–L: β-alanine-bearing scalarane analogs from the marine sponge Spongia sp, Mar. Drugs, 2022, 20, 726 CrossRef.
- A. H. El-Desoky, H. Kato, I. Kagiyama, Y. Hitora, F. Losung, R. E. P. Mangindaan, N. J. de Voogd and S. Tsukamoto, Ceylonins A–F, spongian diterpene derivatives that inhibit RANKL-induced formation of multinuclear osteoclasts, from the marine sponge Spongia ceylonensis, J. Nat. Prod., 2017, 80, 90–95 CrossRef.
- M. A. González, Scalarane sesterterpenoids, Curr. Bioact. Compd., 2010, 6, 178–206 CrossRef.
- H.-B. Yu, H.-Y. Chen, S. Duan, Y.-P. Zhu, B. Hu, Y. He, S.-T. Cheng, B.-H. Jiao and X.-Y. Liu, Bioactive scalarane-type sesterterpenoids from marine sources, Chem. Biodivers., 2022, 19, e202200049 CrossRef PubMed.
- Y. Wang, B. Kong, X. Chen, R. Liu, Y. Zhao, Z. Gu and Q. Jiang, BMSC exosome-enriched acellular fish scale scaffolds promote bone regeneration, J. Nanobiotechnol., 2022, 20, 444 CrossRef PubMed.
- H.-M. Kuo, C.-C. Tseng, N.-F. Chen, M.-H. Tai, H.-C. Hung, C.-W. Feng, S.-Y. Cheng, S.-Y. Huang, Y.-H. Jean and Z.-H. Wen, MSP-4, an antimicrobial peptide, induces apoptosis via activation of extrinsic Fas/FasL- and intrinsic mitochondria-mediated pathways in one osteosarcoma cell line, Mar. Drugs, 2018, 16, 8 CrossRef.
| This journal is © The Royal Society of Chemistry 2025 |



