 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
New insights into enhancement of cadmium biosorption from industrial wastewater through Chlorella sorokiniana HMYA based thin-film
Heba M. Youssefa,
Fatma Mohamed bc,
Mohamed S. Abd Elhameeda and
Khaled N. M. Elsayed
bc,
Mohamed S. Abd Elhameeda and
Khaled N. M. Elsayed *ad
*ad
aBotany and Microbiology Department, Faculty of Science, Beni-Suef University, 62511, Egypt. E-mail: k.elsayed@science.bsu.edu.eg
bNano Photonics and ApplicatVions Lab, Faculty of Science, Beni-Suef University, Beni-Suef 62514, Egypt
cMaterials Science Research Laboratory, Chemistry Department, Faculty of Science, Beni-Suef University, Beni-Suef, Egypt
dBiology Department, Faculty of Education and Arts, Sohar University, Sohar 311, Oman. E-mail: kelsayed@su.edu.om
First published on 16th December 2025
Abstract
Cadmium (Cd2+) is a non-essential and highly toxic heavy metal released from industrial and agricultural activities such as electroplating, dyeing, battery manufacturing, fertilizer application, fuel combustion, and cigarette smoke. Its environmental persistence leads to bioaccumulation and food chain transfer, posing severe teratogenic, carcinogenic, and neurotoxic risks to ecosystems and human health, necessitating the development of sustainable remediation strategies. We present a novel biosorption system for industrial wastewater using Chlorella sorokiniana HMYA-C based thin film, demonstrating high biosorption efficiency with strong potential for large-scale applications. This novel thin film outperformed both wet biomass and traditional immobilized beads under optimal conditions (pH 7, 25 °C, 2.3 g biomass/16 ml alginate/50 ml metal solution). The algal thin film successfully removed 100%, 80%, 70%, and 64% of Cd2+ from aqueous solutions at an initial concentration of 10, 20, 50, and 80 ppm. Furthermore, it remarkably eliminated all of the cadmium from real industrial wastewater which containing 0.4 and 2.4 ppm of Cd2+ concentration, highlighting its potential for immediate deployment as a biotechnological tool. After biosorption, the Cd-loaded Chlorella sorokiniana HMYA-C thin film can be safely using mild acidic or chelating agents or converted into biofuels under regulated conditions, while advancing circular bioeconomy principles. Material characterization (FTIR, EDX, SEM, XRD, zeta potential) indicated a porous, heterogeneous surface capable of multilayer adsorption compatible with pseudo-second-order kinetics and Freundlich isotherms. Overall, this innovative microalgae based thin film platform shows great promise for industrial scalability, addressing major economic and environmental concerns while meeting pressing global demands.
1 Introduction
Water is a key resource that is critical to human life and environmental stability.1 Rapid industrialization, population growth, and severe implications of climate change resulted in an extreme water issues.2 According to United Nations forecasts, by 2025, approximately 1.8 billion people will be living in regions with absolute water scarcity.3 Continuous discharge of untreated or inadequately treated industrial wastewater into natural water systems exacerbates the issue.4 Among the diverse contaminants in wastewater, heavy metals are particularly concerning due to their severe toxicity, environmental persistence, and resistance to degrade naturally.5–9 Cadmium is regarded as one of the most dangerous heavy metals due to its higher mobility, prolonged biological half-life, and substantial effects at extremely low doses.10–14 The World Health Organization restricts cadmium levels in drinking water to 3 µg L−1.15,16 Conventional physicochemical treatment technologies, such as chemical precipitation, ion exchange, membrane filtration, and electrochemical methods are widely employed for removing cadmium from wastewater.17–19 However, these technologies are typically associated with high operational expenses, significant energy consumption, lack the selectivity and complex procedures.20–24 These restrictions prompted researchers to look into alternative eco-friendly remediation solutions, with biological techniques appearing particularly promising.25,26 Among biological remediation techniques, phycoremediation, or the use of microalgae to remove toxins, is gaining appeal in biological systems because of its remarkable adaptability, photosynthetic efficiency, rapid development, and metabolic plasticity.27–35 The presence of functional groups (such as carboxyl, hydroxyl, sulfate, and phosphate) in microalgal cell walls promotes metal biosorption detoxification and bioaccumulation; the effectiveness of these processes varies depending on the species, environmental factors, and type of metal.36–39 In particular, carboxyl and hydroxyl groups were primarily involved in ion-exchange and electrostatic interactions, whereas amine and phosphate groups contributed mainly through coordination complexation with Cd2+ ions. Furthermore, their dual role in pollution removal and biomass valorization into high value products strengthens its scalability within circular bioeconomy frameworks.40–43 Despite their potential, traditional harvesting methods (such as centrifugation, filtration, and flocculation) are energy intensive and costly.44 Immobilization techniques offer a scalable alternative that enhances microalgal stability, recyclability, and ease of separation. This study employed sodium alginate and calcium chloride to produce immobilized beads and thin films of the selected microalgae. Table 1 compares different microalgal designs employed in heavy metal bioremediation, highlighting variations in biosorption efficiency arising from differences in the physical structure of the biosorbent. The comparison clearly indicates that the immobilized Chlorella sorokiniana HMY-C thin film developed in this study achieved the highest cadmium removal efficiency (100%), significantly outperforming conventional microalgal designs. This demonstrates the critical role of immobilized thin film structure in enhancing surface area, functional group accessibility, and biosorption performance.| Microalgal strain | Technique | Heavy metal | Removal efficiency % | Ref. |
|---|---|---|---|---|
| Chlorella sorokiniana HMY-C | Immobilized thin film | Cd2+ | 100% | This study |
| C. pyrenoidosa | Wet biomass | Cd2+ | 45.45% | 45 |
| Scenedesmus acutus | 57.14% | |||
| Desmodesmus sp. | Wet biomass | Cu2+ | 95% | 46 |
| Ni2+ | 90% | |||
| Chlorella sp. | Immobilized beads | Pb2+ | > 90% | 47 |
| Chlorella vulgaris | Wet biomass | Zn2+ | 99.4% | 48 |
| Cu2+ | 91.9% | |||
| Chlorophyceae spp. | Wet biomass | As | 88% | |
| Chlorella sorokiniana | Immobilized beads | Cu2+ | 97.10% | 49 |
| Cd2+ | 64.61% | |||
| Tetradesmus obliquus | Immobilized beads | Cd2+ | 99.85% | 50 |
| Dunaliella salina | Wet biomass | Pb2+ | 87.2% | 51 |
| Cd2+ | 72.9% | |||
| Cu2+ | 88.9% | |||
| Chlorella vulgaris | Immobilized beads | Cd2+ | 100% | 52 |
| Pb2+ | 100% | |||
| Turbinaria ornata | Dry biomass | Cd2+ | 94.34% | 53 |
| Immobilized beads | 98.65% | |||
| Chlorella sorokiniana | Dry biomass | Cu2+ | 90.7% | 54 |
| Zn2+ | 87.1% | |||
| Scenedesmus sp. | Dry biomass | Pb2+ | 85% | 55 |
| Cd2+ | 83% |
Alginate, a biodegradable and non-toxic polysaccharide generated from brown seaweed, acts as a protective matrix, enhancing nutrient and light penetration, decreasing cell aggregation, and improving bio-sorption efficiency.56–60 Calcium chloride (CaCl2) is a crosslinking agent that promotes fast gelation by increasing ionic contacts between divalent Ca2+ ions and alginate chains' carboxylate groups, so it acts as a hardening agent for the different shapes of immobilized alginate.61,62 Thin films, in particular, have demonstrated more effective biosorption kinetics and metal absorption efficiency than conventional wet biomass and bead based systems, due to their higher surfaces, long-term stability, better mass transfer, and greater functional group interactions with cadmium ions. Furthermore, they shown increased hydrogen generating capabilities.63 This dual functionality emphasizes the potential of algal thin films as a sustainable platform that enables efficient wastewater remediation while simultaneously generating biomass for biofuels, pharmaceuticals, fertilizers, cosmetics, and other valuable products, Fig. 1.
Although Chlorella sorokiniana HMY-C thin films demonstrated promising cadmium removal capabilities ready-to-deploy potential, a comparison with both conventional and emerging![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif)
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) technologies is still necessary, as shown in Table 2. Even with their effectiveness, emerging techniques like nanomaterials and genetically modified microorganisms have drawbacks in terms of cost, scalability, and safety issues.64–68 Despite growing interest in algal-based remediation, the potential of microalgae thin-film biosorption systems remains unexplored. Conventional microalgal bioremediation employs large open ponds containing suspended cells, exhibit low recyclability, and pose concerns of microbial leakage into natural ecosystems. Immobilized thin-film systems, on the other hand, provide a restricted yet metabolically active environment that boosts biosorption efficiency, operational stability, and reusability. This study fills a significant gap by assessing the biosorptive capacity, scalability, and real-world feasibility of Chlorella sorokiniana HMYA-C thin films for sustainable cadmium removal from industrial wastewater. This study aimed to isolate and purify native green microalgae from Beni-Suef Zoo in Egypt and assess the potential of promised strain for cadmium phycoremediation. A comparative evaluation of wet biomass, immobilized beads, and algal thin film was carried out. The cadmium removal efficiency using algal thin film from real industrial wastewater which containing 0.4 and 2.4 ppm of Cd2+ concentration was determined. The performance and mechanisms of Cd(II) removal from aqueous solutions were examined using batch experiments and sophisticated characterization techniques, such as SEM, EDX, FTIR and zeta potential with isotherm and kinetic modeling.
technologies is still necessary, as shown in Table 2. Even with their effectiveness, emerging techniques like nanomaterials and genetically modified microorganisms have drawbacks in terms of cost, scalability, and safety issues.64–68 Despite growing interest in algal-based remediation, the potential of microalgae thin-film biosorption systems remains unexplored. Conventional microalgal bioremediation employs large open ponds containing suspended cells, exhibit low recyclability, and pose concerns of microbial leakage into natural ecosystems. Immobilized thin-film systems, on the other hand, provide a restricted yet metabolically active environment that boosts biosorption efficiency, operational stability, and reusability. This study fills a significant gap by assessing the biosorptive capacity, scalability, and real-world feasibility of Chlorella sorokiniana HMYA-C thin films for sustainable cadmium removal from industrial wastewater. This study aimed to isolate and purify native green microalgae from Beni-Suef Zoo in Egypt and assess the potential of promised strain for cadmium phycoremediation. A comparative evaluation of wet biomass, immobilized beads, and algal thin film was carried out. The cadmium removal efficiency using algal thin film from real industrial wastewater which containing 0.4 and 2.4 ppm of Cd2+ concentration was determined. The performance and mechanisms of Cd(II) removal from aqueous solutions were examined using batch experiments and sophisticated characterization techniques, such as SEM, EDX, FTIR and zeta potential with isotherm and kinetic modeling.
| Technology | (Microalgal based thin film) | Chemical precipitation | Membrane filtration (MF) |
|---|---|---|---|
| Conceptual mechanism | Immobilized microalgae on thin films facilitate biosorption via evaluation of microalgal biosorptive characteristics | Chemical precipitants, such as CaO and Na2S, are used to transform soluble Cd2+ ions into insoluble compounds69–71 | Membranes segregate contaminants via size exclusion or charge interaction through porous barriers72 |
| Cd2+ removal efficiency % | Removed 100%, 80%, 70%, and 64% of Cd2+ for 10, 20, 50, and 80 ppm (synthetic); 100% removal at 2.4 ppm in real industrial effluent (this study) | Sulfide precipitation process removed 85.6% of Cd2+ (ref. 73). Calcium oxide precipitation removed 99.9% of Cd2+ (ref. 74) | Removal 96% of Cd2+ via micellar-enhanced UF.75 Up to 98% elimination with reverse osmosis.76 97.63% of Cd2+ by clam shell-based membrane |
| Total cost (USD m−3) | (∼$ 0.8–1.11 m−3, this study) | ($ 4 m−3)77 | (∼$ 0.47 m−3, as reported (0.44 €) in 78.) ∼ $ 0.47 m−3, as reported (0.44 €) in ref. 81 |
| Scalability in practice | Still at the pilot and semi-industrial stages; extendable using modular biofilm modules | Highly scalable; often utilized in large-scale wastewater systems79 | Widely used in industrial and municipal wastewater systems70,80–82 |
| Environmental impact | Eco-friendly, reduces CO2 emissions. Biomass might be used for bioenergy | Creates significant amounts of chemical sludge, which must be disposed of or treated83 | Little chemical waste; membrane disposal and cleaning chemicals offer mild environmental problems84 |
| Reusability potential | Fast desorption and several reuses of the biosorbent | No reusability: continual chemical input is required85 | Membranes may be reused after cleaning; however their performance diminishes with fouling and chemical assault86 |
| Operational challenges | Thin film contamination, biofouling | Demands strict control of pH and stoichiometry; sludge processing is labor-intensive87 | Membrane fouling, scaling, and pressure loss necessitate frequent maintenance and pre-treatment procedures17 |
| Activated carbon adsorption | Ion exchange | Nanotechnology | Genetically engineered microorganisms |
|---|---|---|---|
| High surface area (500–1500 m2 g−1) and porous structure allow for physical adsorption of metal ions88 | Charged polymeric resins exchange target metal ions for benign counterions in a reversible, selective process89 | Nanomaterials (e.g., nanotubes, oxides) provide high reactivity and selective adsorption because of designed surface functions | Engineered microorganisms employ metal-binding peptides or improved efflux mechanisms to absorb, sequester, or convert hazardous metals90 |
| Up to 98% of Cd2+ using active carbon prepared from sunflower seed shell. Up to 85% of Cd2+ utilizing rhus pentaphylla sulfuric acid91 | Amberlite IR120H resins removed 96% of Cd.92 Ca(OH)2 and Mg(OH)2 Modified Amberlyst15 remove 99% of Cd93 | Up to 92.5% using nanocellulose (NC).94 Up to 93% using chitosan-grafted poly (carboxymethyl cellulose-Co-acrylamide) nano hydrogel.95 | Engineering bacteria demonstrated a survival rate >70% of Cd2+, whereas wild bacteria's survival rate remained > 50%.96 Up to 80% using engineered Escherichia coli cell factory97 |
| ($ 5–200 m−3,98 as cited in ref. 99) | ($ 0.237 m−3)100 | ($ 6.35 m−3)101 | Currently limited to lab-scale research; no industrial cost estimates are available |
| Scalable, but requires development of cost-effective synthesis methods102 | Effective, but requires well-regulated operating settings103 | Integrated into existing hybrid treatment systems, modular deployment feasible104 | Large-scale optimization is required for full industrial adoption105 |
| Although the usage of bio-carbon is environmentally favorable, waste creation may be an issue106 | Resin production and regeneration. Consume water and create waste streams26 | Engineered surfaces reduce toxicity and, when correctly managed, have a minimal ecological imprint107 | Low environmental load; enhances biodegradability and bioremediation capability108 |
| Adsorbents can be thermally or chemically regenerated several times106 | Depends on resin type, and efficiency decreases with each cycle109 | Nanomaterials can be regenerated; however, aggregation and leaching must be addressed110 | Microbial systems may be regenerated through growth cycles and genetic improvements111 |
| Fouling, and monitoring of the feed stream, as well as factors such as pH and temperature to ensure consistent performance112 | Regular resin replacement; susceptible to clogging and scaling113 | Nanoparticle aggregation, leaching, and recovery all offer engineering issues114 | Maintaining microbial viability, mutation stability, and avoiding gene leaking115 |
2 Materials and methods
2.1 Materials
Wuxal media (universal fertilizer, Wilhelm Haug GmbH and Co. KG, Germany) and deionized water (Botany lab, Beni-Suef university, Egypt). Sodium alginate (MW = 380.000 g mol−1, 0.1 M, Sigma-Aldrich). Cadmium sulfate (3CdSO4·8H2O, 99%, Oxford Laboratory Reagent, INDIA). Calcium chloride (CaCl2, MW = 110.984 g mol−1, 96%, 0.18 M, Piochem). Taq buffer (Ferments, Germany). DNA gel extraction kit from (Sigma-Aldrich, Germany).2.2 Purified microalgal strains preparation
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) by repeated dilution and streak plate methods. A tenfold serial dilution was performed by mixing 1 ml of homogenized samples with 9 ml of sterile culture material in six sterile test tubes. Each dilution step was properly mixed, and the tubes were cultivated for 14 days at 25 °C.118 Aliquots of each dilution were plated onto sterile Petri plates containing 20 ml of solidified nutritional agar in an aseptic laminar flow hood using standard streaking and zigzag inoculation techniques. Frequent streaking was performed to ensure the isolation of pure strains.119 The isolation and purification steps ensure that only pure, viable microalgal strains are selected, which is essential for accurate assessment of biosorption capabilities.
by repeated dilution and streak plate methods. A tenfold serial dilution was performed by mixing 1 ml of homogenized samples with 9 ml of sterile culture material in six sterile test tubes. Each dilution step was properly mixed, and the tubes were cultivated for 14 days at 25 °C.118 Aliquots of each dilution were plated onto sterile Petri plates containing 20 ml of solidified nutritional agar in an aseptic laminar flow hood using standard streaking and zigzag inoculation techniques. Frequent streaking was performed to ensure the isolation of pure strains.119 The isolation and purification steps ensure that only pure, viable microalgal strains are selected, which is essential for accurate assessment of biosorption capabilities.2.2.5.1 Genomic DNA extraction. Genomic DNA was isolated from pure
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) unialgal cultures using the DNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer's directions. DNA content and purity were measured spectrophotometrically at 260 and 280 nm. The extracted DNA's integrity was confirmed using electrophoresis on a 1% agarose gel stained with ethidium bromide. DNA bands were visible during UV transillumination, confirming the existence of intact genetic material.
unialgal cultures using the DNeasy Plant Mini Kit (Qiagen, Germany) according to the manufacturer's directions. DNA content and purity were measured spectrophotometrically at 260 and 280 nm. The extracted DNA's integrity was confirmed using electrophoresis on a 1% agarose gel stained with ethidium bromide. DNA bands were visible during UV transillumination, confirming the existence of intact genetic material.
2.2.5.2 PCR amplification of the 18S gene. PCR amplification of the 18S gene was carried out with species-specific primers: DSs (5′-GCAGGAGAGCTAATAGGA-3′) and DPs (5′-GTAGAGGGTAGGAGAAGT-3′). The reaction mixture (25 µL) includes 0.2 µL Taq DNA polymerase, 1 µL genomic DNA (∼10 ng), 2.5 µL dNTPs, 2.5 µL of each primer (10 pmol µL−1), 5 µL of 10× Taq buffer, and nuclease-free water to the final volume.120 The PCR cycling conditions were as follows: 4 minutes of initial denaturation at 94 °C, 35 denaturation cycles lasting 50 seconds each, 60 seconds of annealing at 58 °C, 1 minute of extension at 72 °C, and 7 minutes of final extension. Agarose gel electrophoresis was used to resolve the amplified products, which were subsequently purified using a commercial DNA gel extraction kit for future use.
2.2.5.3 DNA sequence. Purified PCR products were sequenced bidirectionally using the BigDye® Terminator v3.1 Cycle Sequencing Kit (Applied Biosystems, USA), according to the manufacturer's instructions. Macrogen Inc. (Seoul, South Korea) used an ABI Prism 310 Genetic Analyzer from Applied Biosystems to execute sequencing procedure. The raw sequence data was examined for quality, and any ambiguous base calls were manually curated using Chromas software (Technelysium Pty Ltd).
2.2.5.4 Phylogenetic analysis. The obtained 18S rRNA gene sequences were compared to existing entries in the NCBI GenBank database using the Basic Local Alignment Search Tool (BLAST) to determine their closest taxonomic relatives.121 Multiple sequence alignments were carried out with MUSCLE, which is part of the MEGA 11 software suite. To assess branch support, phylogenetic trees were built using the Maximum Likelihood method and 1000 bootstrap replications. All analyses were carried out using MEGA11 software (Molecular Evolutionary Genetics Analysis version 11).122 Representative sequences were uploaded to GenBank and assigned accession numbers.
2.3 Preparation of cadmium solutions
A primary stock solution of cadmium (1000 mg L−1) was prepared by accurately dissolving cadmium sulfate octahydrate (CdSO4·8H2O(in ultrapure deionized water. A series of working solutions at concentrations of 10, 20, 50, and 80 mg L−1 were prepared before each experimental run.2.4 Collection of natural industrial wastewater samples
Real industrial wastewater samples were collected from two different manufacturing plants in the Kom Abu Rady Industrial Zone, Beni Suef Governorate, Egypt. The sampling area's geographic coordinates are latitude 28°45′ to 29°25′ N and longitude 30°45′ to 31°15′E. All samples were collected in sterile containers, transported on ice, and maintained at 4 °C until further testing to ensure their physicochemical integrity.2.5 Preparation of biosorbent using different immobilization techniques
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) over the filter paper and covered with another filter paper pre-wetted with 5% CaCl2. The assembly was incubated at 4 °C for 15 minutes to solidify. The produced thin film was then transferred to a Petri plate with 5% CaCl2 and allowed for another 15 minutes. Finally, the alginate thin film was rinsed with bi-distilled water. The control films were cut into homogeneous cubes (4 mm × 4 mm × 1 mm) and stored at 4 °C for future use. This approach was implemented and modified in line with Shaaban et al.134
over the filter paper and covered with another filter paper pre-wetted with 5% CaCl2. The assembly was incubated at 4 °C for 15 minutes to solidify. The produced thin film was then transferred to a Petri plate with 5% CaCl2 and allowed for another 15 minutes. Finally, the alginate thin film was rinsed with bi-distilled water. The control films were cut into homogeneous cubes (4 mm × 4 mm × 1 mm) and stored at 4 °C for future use. This approach was implemented and modified in line with Shaaban et al.1342.6 The comparative between different techniques of biosorbent on Cd(II) biosorption
The experiment was carried out by utilizing different biosorbent techniques: wet biomass, alginate immobilized algal beads and novel alginate algal thin film), all different forms of biosorbent contain the same microalgal dose (2.3 gm) in (16 ml) of sodium alginate gel (0.1 M)/50 ml of (10 ppm) concentration of Cd concentration at (25 °C) and (pH 7). Sodium alginate beads and sodium alginate thin films without microalgae strain were employed as controls.2.7 Evaluation of factors affecting Cd(II) biosorption using algal thin films
2.9 Determination of cadmium removal efficiency (R%)
The determination of Cd concentrations in aqueous solution and in real industrial wastewater before and after the biotreatment was measured by using (the Agilent 4200 MP-AES) at Institute of Global Health and Human Ecology, School of Sciences and Engineering, The American University in Cairo. Natural wastewater samples didn't need digestion because they were filtered and diluted 10 times. Calibration was done with standard solution of Cd element and was prepared in 2% nitric acid. The Cd(II) removal percent (R%) was calculated from the eqn (1).| (R%) = (Cb − Ca)/Cb × 100 | (1) |
2.10 Statistical analysis
All experimental measurements were performed in triplicate, and the results are reported as means ± standard error (SE). Statistical significance was assessed using one-way analyses of variance135 and determined significant differences among means at a confidence level of p < 0.05. All statistical computations and graphical representations were conducted using GraphPad Prism software (version 8.0.2, GraphPad Software Inc., USA).2.11 Characterization of thin films (hydrogels)
2.12 Essential analysis of the biosorption mechanism study
(A) The pseudo-first-order model, eqn (2):
Log(qe − qt) = log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe − k × t/2.303 (qt) qe − k × t/2.303 (qt)
| (2) |
Representing the amount adsorbed at time (t),22 as the amount adsorbed at equilibrium (mg g−1), and (k1) as the rate constant for pseudo-first-order adsorption hour−1).134
(B) The pseudo-second-order model, eqn (3):
| t/qt = 1/(k2 qe2) + (1/qe) × t | (3) |
(C) The intraparticle diffusion model, eqn (4):
| qt = kit(0.5) + 1 | (4) |
It is utilized to understand the mechanism and rate-controlling steps within the kinetics of biosorption. It offers essential information regarding the process.136 Where (ki) is the intraparticle diffusion rate constant and (I) is the intercept, the value of ki is determined from the slope of the plot qt vs. t0.5.
(A) The Langmuir isotherm:
It characterizes even adsorption without the movement of adsorbate across a surface with a limited number of adsorption sites, which are for monolayer adsorption. To achieve equilibrium, the linear version of the Langmuir isotherm model was used to analyze the experimental data as, eqn (5).
| Ce/qe = (1/(b × qm)) + (Ce/qm) | (5) |
The adsorbate concentration at equilibrium is denoted as (Ce) in units of mg L−1, while the maximum adsorption capacity is represented by (qm) in units of mg g−1, and the Langmuir constant is denoted as (b)138 eqn (6).
| RL = (1/(1 + b × Ci)) | (6) |
The initial solute concentration is denoted by (Ci), and (b) represents Langmuir's adsorption constant (L mg−1). The (RL) value indicates whether the adsorption is unfavorable (RL > 1), linear (RL = 1), favorable (0 < RL < 1), or irreversible (RL = 0).139,140
(B) Freundlich isotherm model, eqn (7):
Ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KF + (1/n) × ln KF + (1/n) × ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Ce Ce
| (7) |
The Freundlich isotherm serves as an empirical model for heavy metal ion adsorption. Where (ref. 22) is the amount of metal ion adsorbed onto the surface of algal biomass at equilibrium (mg g−1) and (Ce) is the equilibrium concentration of the adsorbate (mg L−1).141,142 The adsorption constant (KF) signifies the adsorption capacity, while (1/n) represents the adsorption intensity, with (n) being dependent on the adsorbate and adsorbent. Any value of (n) is above one favors the adsorption process.
(C) Sips model, eqn (8):
 | (8) |
(D) Dubinin–Radushkevich model, eqn (9):
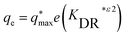 | (9) |
The maximum amount of cadmium sorbed by the microalgae is represented by (qmax) (mg g−1), while (Ce) (mg L−1) stands for the cadmium concentration at equilibrium. The constant related to the isotherm model is denoted as (KDR) with R representing the kinetic gas constant (8.314 J mol−1 K−1and T (K) indicating the temperature of the system.144,145
Linear form of D. R model, eqn (10):
Ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qe = ln qe = ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) qm − β ε2 qm − β ε2
| (10) |
| ε = RT × ln(1 + (1/Ce)) | (11) |
The mean free energy (kJ mol−1) could be calculated using, eqn (12):
| E = 1/√2β | (12) |
The (E) value indicates whether the nature of biosorption process is physical adsorption or chemisorption process.
3 Results and discussion
3.1 Characterization of isolated microalgal strains
| Sample | Submission | Accession number | Strain identification |
|---|---|---|---|
| a SUB: refers to the GenBank submission number; Seq: refers to the sequence code. Accession numbers are officially registered in the NCBI GenBank database. | |||
| B | SUB14206830 Seq1 | PP273965 | Parachlorella kessleri HMYA-B (https://www.ncbi.nlm.nih.gov/nuccore/PP273965) |
| C | SUB14206830 Seq2 | PP273966 | Chlorella sorokiniana HMYA-C (https://www.ncbi.nlm.nih.gov/nuccore/PP273966) |
| D | SUB14206830 Seq3 | PP273967 | Scenedesmus vacuolatus HMYA-D (https://www.ncbi.nlm.nih.gov/nuccore/PP273967) |
| W | SUB14206830 Seq4 | PP273968 | Auxenochlorella pyrenoidosa HMYA-W (https://www.ncbi.nlm.nih.gov/nuccore/PP273968) |
To validate the molecular identification, phylogenetic analysis was performed using MEGA11 software (Molecular Evolutionary Genetics Analysis version 11) and the Maximum Likelihood technique with 1000 bootstrap replicates.121 Phylogenetic trees demonstrated the evolutionary relationships of the isolated strains with their closest known relatives, Fig. 3.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 h light/dark photoperiod) at a controlled pH of 7 and temperature of 25 ± 2 °C, Fig. 4. Suboptimal temperatures (below 16 °C) might inhibit algal growth, whereas temperatures above 35 °C can be lethal to numerous algal species.119 pH plays a pivotal role in metabolic activity and nutritional availability in culture.151–153 Among environmental parameters, light intensity is the most important predictor of microalgal development and biomass accumulation. Continuous white light was shown to stimulate the growth of Haematococcus lacustris more than intermittent blue or red light (12
0 h light/dark photoperiod) at a controlled pH of 7 and temperature of 25 ± 2 °C, Fig. 4. Suboptimal temperatures (below 16 °C) might inhibit algal growth, whereas temperatures above 35 °C can be lethal to numerous algal species.119 pH plays a pivotal role in metabolic activity and nutritional availability in culture.151–153 Among environmental parameters, light intensity is the most important predictor of microalgal development and biomass accumulation. Continuous white light was shown to stimulate the growth of Haematococcus lacustris more than intermittent blue or red light (12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 h cycle).154 Furthermore, spectral quality and light exposure duration have a significant influence on the biochemical profile of algal biomass due to their impact photosynthetic efficiency.155 BG-11 medium, also referred to as Blue green 11 medium, is a widely adopted synthetic medium for a broad range of species and is particularly effective for freshwater strains. It is also frequently employed in lipid synthesis studies due to its well-balanced nutritional profile.156 In comparison, Wuxal is a widely available, broad-spectrum liquid fertilizer (N 8%, P2O5 8%, K2O 6%, Mn 0.012%, Fe 0.02%, B 0.01%, Cu 0.004%, Zn 0.004%) that was used in this work as a cost-effective and simplified nutritional medium.157 All algal strains demonstrated high growth rate on Blue green 11 than Wuxal medium.
12 h cycle).154 Furthermore, spectral quality and light exposure duration have a significant influence on the biochemical profile of algal biomass due to their impact photosynthetic efficiency.155 BG-11 medium, also referred to as Blue green 11 medium, is a widely adopted synthetic medium for a broad range of species and is particularly effective for freshwater strains. It is also frequently employed in lipid synthesis studies due to its well-balanced nutritional profile.156 In comparison, Wuxal is a widely available, broad-spectrum liquid fertilizer (N 8%, P2O5 8%, K2O 6%, Mn 0.012%, Fe 0.02%, B 0.01%, Cu 0.004%, Zn 0.004%) that was used in this work as a cost-effective and simplified nutritional medium.157 All algal strains demonstrated high growth rate on Blue green 11 than Wuxal medium.
Microalgae species demonstrated rapid growth and high nutrient absorption efficiency, indicating their potential for efficient wastewater treatment.158 In this investigation, the development of four algal strains was observed under controlled settings for 18 days. Measurements were taken every two days, in triplicate, using three complementary analytical methods: cell density enumeration using a hemocytometer, based on direct microscopic counting;127 optical density (OD) measurements at 680 nm to estimate chlorophyll-containing biomass;159 and (3) quantification of dry biomass concentration, determined by gravimetric analysis of dried algal biomass per liter of culture, Fig. 5,.160 Cell density measurements were shown to be the most reliable of these approaches, providing a direct and quantitative indication of viable cell populations.161 In contrast, OD measurements may be influenced by the presence of dissolved or suspended compounds in the culture media, resulting in an overestimation or underestimating of biomass.162 Similarly, dry weight measurements are subject to inaccuracies caused by poor biomass recovery or cell lysis during the harvesting process, especially if the approach is not fully optimized. Among the four microalgal strains, Chlorella sorokiniana HMYA-C exhibited superior growth rate, achieving the maximum cell density, optical density, and dry biomass concentration on day 18, as shown in Fig. 5. Unicellular green microalga Chlorella sorokiniana HMYA-C excellent growth performance in nutrient-rich environments shows a higher physiological capability for nutrient absorption, positioning it as a strong candidate for advanced wastewater treatment.
3.2 The comparative between the Cd biosorption efficiency of different form of biosorbent
Chlorella sorokiniana HMYA-C biosorbent was employed in three different configuration (suspended wet biomass, an immobilized beads and a novel immobilized thin film (hydrogel)) for removal of cadmium from aqueous solution with the same condition pH 7, temperature 25 °C, (2.3 gm live concentrated harvested algal biomass)/16 ml sodium alginate gel (0.1 M)/50 ml of 10 ppm cd concentration). Algal biosorbents in the form of thin film)rectangular sheets(outperform typical spherical alginate beads and wet biomass in Cadmium removal efficiency, as shown in Fig. 6a. The physical structure of the biosorbent has a considerable impact on both the biosorption process and efficiency.163 Thin films have more active sites for cadmium adsorption than beads with the same biomass content because of their longer, planar shape, which enhances their effective surface area. Furthermore, the thin film morphology shows rougher and more porous, producing many diffusion paths, promoting deeper cadmium trapping in the matrix and accelerating intraparticle diffusion. The finding is consistent with previous research that has established a direct association between surface roughness, specific surface area.164 In contrast, the immobilized thin film structure utilized in this study significantly increases biosorption efficiency. A one-way ANOVA (F(4, 10) = 2072, p < 0.0001, R2 = 0.9988) show that the treatment groups accounted for virtually all variation in the response. These findings indicate the algal thin-film system's superior efficacy, highlighting its improved functionality and tremendous potential as an effective and sustainable biosorbent for cadmium removal. Compared to suspended-cell cultures, the immobilized design showed significantly better metal uptake, owing to its higher surface roughness, bigger specific surface area, and increased stability. Conventional free-cell systems frequently face issues such as difficult harvesting, decreased metabolic activity under metal stress, and environmental concerns due to potential release into natural ecosystems particularly when genetically modified strains are used.165,166 In contrast, immobilization within a polymeric matrix provides a strong, recyclable platform that sustains cell viability and activity by allowing for nutrition and gas exchange while shielding cells from harsh environmental variations.135 This arrangement reduces biomass washout, improves tolerance to hazardous metals, and reduces contamination hazards. Among immobilized systems, the thin-film design outperformed bead-based matrices by removing internal diffusion constraints, enabling more effective exploitation of active sites, and facilitating improved mass transfer. Furthermore, its planar construction supports even light dispersion and efficient gas exchange, hence maintaining photosynthetic performance and total biosorption efficiency.167 These features emphasize the thin-film immobilization system as a technically advanced, scalable, and safe solution for ongoing industrial bioremediation of heavy metals like cadmium. Recent studies have highlighted the superior performance of thin-film hydrogels as advanced platforms for bioremediation, owing to their high surface-to-volume ratio, enhanced mass transfer, and improved structural stability. For example, immobilization in a thin film shape was significantly more effective than immobilization in beads for Co(II) elimination in bacteria and fungus.134 Collectively, these findings highlight the critical significance of thin-film hydrogel systems as next-generation bioremediation materials. Their unique combination of mechanical stability, reusability, and improved mass transfer makes them an environmentally safe, cost-effective, and highly efficient solution for the continuous removal of hazardous metals from wastewater.3.3 Factors affecting cadmium biosorption
Following characterization and selection of the promising microalgal strain, we evaluated the effects of various operational factors on its cadmium biosorption performance.3.4 Characterization of thin films (hydrogels)
The thin film was generated by the unicellular green microalga Chlorella sorokiniana HMYA-C, which forms a photosynthetic and self-sustaining matrix suitable for metal biosorption.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching (1650–1566 cm−1), sulfone groups (1350–1300 cm−1), and amine C–N stretching (1250–1020 cm−1) as shown in Table 4. The algal thin film FTIR spectra influenced significantly after Cd2+ biosorption, showing active binding of several functional groups. The O–H stretching band migrated to lower wavenumbers (3696.34 cm−1), while a new peak appeared at 3459.21 cm−1, indicating the participation of hydroxyl and amine groups in metal complexation. New bands at 2376.25 and 2309.74 cm−1, ascribed to C
C stretching (1650–1566 cm−1), sulfone groups (1350–1300 cm−1), and amine C–N stretching (1250–1020 cm−1) as shown in Table 4. The algal thin film FTIR spectra influenced significantly after Cd2+ biosorption, showing active binding of several functional groups. The O–H stretching band migrated to lower wavenumbers (3696.34 cm−1), while a new peak appeared at 3459.21 cm−1, indicating the participation of hydroxyl and amine groups in metal complexation. New bands at 2376.25 and 2309.74 cm−1, ascribed to C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C and O
C and O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching modes, respectively, reveal the production of unique chemical bonds via interactions between Cd2+ ions and surface-bound thiocyanate and phosphodiester groups. The ester carbonyl (C
O stretching modes, respectively, reveal the production of unique chemical bonds via interactions between Cd2+ ions and surface-bound thiocyanate and phosphodiester groups. The ester carbonyl (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) band at 1773.00 cm−1 vanished following biosorption, highlighting the importance of carbonyl functionalities in cadmium coordination. Shifts in amide-related regions (1325–1202 cm−1) and phosphate-related peaks (about 1020 cm−1) reveal the role of amide nitrogen and phosphate oxygen in metal binding. Spectral discrepancies between the sulfonyl (S
O) band at 1773.00 cm−1 vanished following biosorption, highlighting the importance of carbonyl functionalities in cadmium coordination. Shifts in amide-related regions (1325–1202 cm−1) and phosphate-related peaks (about 1020 cm−1) reveal the role of amide nitrogen and phosphate oxygen in metal binding. Spectral discrepancies between the sulfonyl (S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) stretching region (1350–1300 cm−1) and C–N stretching vibrations suggest a multidentate binding mechanism. Chemisorption of Cd2+ onto C. sorokiniana thin films mostly occurs via hydroxyl, amine, carbonyl, phosphate, and sulfonyl groups, resulting in spectrum alterations and band emergence/disappearance. Specifically, carboxyl and hydroxyl groups mainly participated through ion exchange and electrostatic interactions, while amine and phosphate groups contributed to coordination complexation with Cd2+ ions. The substantial, and most likely irreversible, chemical interactions between algae functional groups and cadmium ions highlight C. sorokiniana HMY-C thin film superior biosorption efficiency and intriguing potential as an environmentally benign biosorbent for heavy metal removal.
O) stretching region (1350–1300 cm−1) and C–N stretching vibrations suggest a multidentate binding mechanism. Chemisorption of Cd2+ onto C. sorokiniana thin films mostly occurs via hydroxyl, amine, carbonyl, phosphate, and sulfonyl groups, resulting in spectrum alterations and band emergence/disappearance. Specifically, carboxyl and hydroxyl groups mainly participated through ion exchange and electrostatic interactions, while amine and phosphate groups contributed to coordination complexation with Cd2+ ions. The substantial, and most likely irreversible, chemical interactions between algae functional groups and cadmium ions highlight C. sorokiniana HMY-C thin film superior biosorption efficiency and intriguing potential as an environmentally benign biosorbent for heavy metal removal.
| G.F Before biosorption λ (cm−1) | G.F After biosorption λ (cm−1) | C.F Before biosorption λ (cm−1) | C.F After biosorption λ (cm−1) | Bands indicating functional groups |
|---|---|---|---|---|
| a λ (cm−1) represents the wavenumber of FTIR absorption bands indicating the presence of functional groups involved in cadmium biosorption. G.F: Alginate thin film C.F: Chlorella sorokiniana thin films. | ||||
| — | — | 3720.84 | 3696.34 | (O–H alcohol), polyphenols or polysaccharides177,178 |
| 3622.39 | ||||
| 3368.32 | 3363.07 | — | 3459.21 | (O–H/N–H) carbohydrates, proteins, and lipids179 |
| — | 3022.82 | 3023.59 | 3020.11 | (N–H) stretching180 |
| — | 2941.39 | 2948.32 | 2932.36 | Aliphatic (C–H/N–H) presence of lipids,.181,182 |
| 2844.81 | 2829.09 | 2850.68 | ||
| — | 2341.88 | — | 2376.25 | (C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C/O C/O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/–S–C O/–S–C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) N) stretching thiocyanate183,184 N) stretching thiocyanate183,184 |
| — | — | — | 2309.74 | |
| 2170.19 | — | 2125.83 | — | |
| 2018.74 | — | — | 2066.65 | (N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) S/C S/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/P C/P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) isothiocyanate, phytochemicals phosphodiester185,186 O) isothiocyanate, phytochemicals phosphodiester185,186 |
| — | 1991.56 | — | 1994.79 | |
| — | — | 1773.00 | — | (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) esters187 O) esters187 |
| — | — | — | 1596.6 | (–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C/HC = O/R2 C C/HC = O/R2 C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/N–H), cyclic alkene, carbonyl, amines, amides, and some proteins188,189 O/N–H), cyclic alkene, carbonyl, amines, amides, and some proteins188,189 |
| 1602.08 | 1629.65 | 1611.02 | — | |
| 1427.87 | 1430.66 | 1436.78 | 1423.34 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–H bending190 O/C–H bending190 |
| 1310.86 | 1324.1 | 1332.25 | 1325.18 | (–S![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/C–N–C/N–H), sulfone, stretching of amides from proteins191–193 O/C–N–C/N–H), sulfone, stretching of amides from proteins191–193 |
| — | 1211.03 | 1211.04 | 1202.4 | (C–N/ –SO3/P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) aromatic compounds, phosphodiester, polysaccharides)194,195 O) aromatic compounds, phosphodiester, polysaccharides)194,195 |
| 1075.97 | — | — | — | (P![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bonds in (PO4)3/C–O–C/C O bonds in (PO4)3/C–O–C/C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bending, –C–O (alcohol)) polysaccharides (The carbohydrate band spectra)196–198 C bending, –C–O (alcohol)) polysaccharides (The carbohydrate band spectra)196–198 |
| 1015.42 | 1016.12 | 1014.80 | 1020.58 | |
| 934.83 | 917.61 | 922.25 | 904.62 | (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) P/O–H out-of-plane) (the a-(1,4) glycoside bond, polysaccharides)199,200 P/O–H out-of-plane) (the a-(1,4) glycoside bond, polysaccharides)199,200 |
| 815.94 | — | 792.75 | 776.12 | C–H bending/Si–H bending (the carbohydrate band spectra)201,202 |
| 628.9 | 648.49 | — | 660.68 | H2PO4−/PO4–/N–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O/Si–O (C–Cl halo compound) (polysaccharides)203–205 O/Si–O (C–Cl halo compound) (polysaccharides)203–205 |
| — | 608.95 | — | 614.22 | |
| 586.36 | 535.27 | 588.06 | 594.05 | |
| — | — | 510.2 | — | |
3.5 Biosorption kinetics and isotherm modeling
| Kinetic model | Parameter | |||
|---|---|---|---|---|
| a k1: rate constant of the pseudo-first-order model; k2: rate constant of the pseudo-second-order model; qe: equilibrium adsorption capacity (mmol g−1); R2: correlation coefficient. | ||||
| Pseudo-first order | k1 (h−1) = 0.0013 | qe (exp.) = 1.139 | qe (calc.) = 0.593 | R2 = 0.0001 |
| Pseudo-second order | k2 (g (mmol−1 h−1)) = 0.212 | qe (exp.) = 1.139 | qe (calc.) = 1.28 | R2 = 0.984 |
| Intraparticle diffusion | ki (mmol (g−1 h 0.5)) = 0.14 | R2 = 0.980 | ||
The pseudo-first-order model has a low correlation (R2 = 0.0001), resulting in a significant difference between calculated (qe,calc. = 0.593 mg g−1) and experimental uptake (qe,exp. = 1.139 mg g−1), Fig. 10. The regression analysis indicated a non significant slope (p = 0.8369) and too broad confidence ranges (−0.191 to 0.213), indicating that this model does not accurately represent the kinetics of Cd(II) biosorption under the experimental conditions. The pseudo-second-order model accurately predicted experimental data, with a high coefficient of determination (R2 = 0.984) and low standard error (±0.801). The model produced a calculated equilibrium uptake (qe,calc. = 1.28 mg g−1) that was nearly identical to the experimental finding (qe,exp. = 1.139 mg g−1), demonstrating model consistency, as shown Fig. 10. The statistical analysis revealed a significant slope (p = 0.0067) with a narrow 95% confidence range (0.464–0.978) and an intercept p-value at the significance level (p = 0.058), implying that chemisorption is the dominant rate limiting mechanism in the biosorption process. This finding is corroborated by FTIR studies that show distinct interactions between cadmium ions and functional groups such as –OH, –COOH, and –NH. SEM-EDX and XRD imaging demonstrated that cadmium had been effectively deposited on the algal thin film surface. Overall, these data support the idea that the biosorption process involves valence forces or ion exchange, which is consistent with the pseudo-second-order model assumptions. The intraparticle diffusion model is strongly correlated with experimental data (R2 = 0.980), with a corrected R2 value of 0.957 and a low standard error of ±0.035, Fig. 10. The model's slope coefficient was statistically significant (p = 0.0144) with a narrow 95% confidence range (0.074–0.237), indicating that intraparticle diffusion plays a substantial role in the overall biosorption process. The intercept (C = 0.392) was also statistically significant (p = 0.0328), demonstrating that, the biosorption process encompasses many phases, including surface adsorption and internal diffusion. The results of all kinetic models suggest that Cd(II) biosorption onto immobilized algal films is primarily regulated by chemisorption (as depicted by the pseudo-second-order model), with intraparticle diffusion playing a role in a multistage process. The statistical analysis verifies these findings, emphasizing the importance of understanding both surface and interior diffusion mechanisms in biosorption.
| Adsorption isotherm | Parameters | ||
|---|---|---|---|
| a qm: maximum adsorption capacity (mg g−1); b: Langmuir constant related to binding energy (L mg−1); RL: separation factor; Kf and n: Freundlich constants; β: Dubinin–Radushkevich constant (mol2 kJ−2); E: mean adsorption energy (kJ mol−1); Ks and n: Sips model constants; R2: correlation coefficient. | |||
| Langmuir | qm (mg g−1) = 1.38 | b (L mg−1) = 0.13 | R2 = 0.80 |
| RL = 0.085 | |||
| Freundlich | kf (mg g−1) = 0.37 | 1/n = 0.75 | R2 = 0.970 |
| Dubinin–Radushkevich | qm (mg g−1) = 1.02 | B = 4 × 10−6 | R2 = 0.90 |
| E = 354 | |||
| Sips | ks = 0.20 | 1/n = 0.53 | R2 = 0.70 |
The Freundlich isotherm accurately described the experimental data, with the greatest correlation coefficient (R2 = 0.970), as shown in Fig. 11. This provides a viable multilayer adsorption approach for various surfaces, confirmed by the multilayer structure visible in SEM images. The statistical regression analysis verified this, with a low standard error for the intercept (±0.04), a narrow 95% confidence interval (0.80–1.82), and a statistically significant p-value (p = 0.0197). The slope has a standard error of ±1.51 with a 95% confidence interval of −0.35 to 3.36, indicating that the model is robust in predicting adsorption intensity (1/n = 0.75). The Langmuir model accurately predicted qm = 1.38 mg g−1 and b = 0.13 L mg−1, with a R2 value of 0.80, as shown in Fig. 11. However, statistical analysis found wide confidence ranges for the slope (−1.84 to 2.75), intercept (−28.24 to 49.70), and non-significant p-values. These results indicate that the Langmuir model has limited credibility under current conditions, confirming the premise that cadmium biosorption does not occur in monolayers on a homogenous surface. The Dubinin–Radushkevich model has a R2 of 0.90, a theoretical maximum capacity (qm) of 1.02 mg g−1, β = 4 × 10−6 mol2 kJ−2, Fig. 11. The mean free energy (E) value estimated from the model was 354 kJ mol−1, which based on theoretical thresholds, indicated that the biosorption process is driven by particle diffusion and strong chemical interactions,.53,207 The slope had a large standard error (±0.26), a wide confidence interval (−2.97–3.50), and a non-significant p-value (p = 0.492). Even though the Dubinin–Radushkevich (D–R) model showed a non-significant p-value, it was included for internal comparison with other isotherm models and to be consistent with previous microalgal biosorption research, where the D–R model has frequently shown good fit and interpretive value.208–212 Including it here enables comparison with prior studies and indicates how the current system may behave differently in terms of sorption energetics. Also, the Dubinin–Radushkevich (D–R) isotherm model emphasizes the energy aspect of the adsorption process and is commonly used to differentiate between physical and chemical adsorption.213 The Sips model shows a poor correlation (R2 = 0.70), Fig. 11, and large 95% confidence intervals for intercept (−19.42 to 12.83) and slope (−4.82 to 7.79). Non-significant p-values indicate insufficient dependability in characterizing the experimental data. The obtained constants (Ks = 0.20, 1/n = 0.53) show heterogeneous adsorption, however with less statistical support than the Freundlich model.
3.6 Mechanistic perspectives on cadmium biosorption
To gain insight into the underlying mechanisms of cadmium removal, the biosorption process was analyzed in terms of interactions between functional groups and Cd(II) ions, supported by characterization data and modeling results. This finding of cadmium (Cd2+) biosorption by Chlorella sorokiniana HMY-C thin films represent a significant advancement in metal recovery technology. This study uses different analysis such as kinetic modeling, equilibrium isotherms, Fourier-transform infrared spectroscopy (FTIR), scanning electron microscopy-energy dispersive X-ray spectroscopy (SEM-EDX), X-ray diffraction (XRD), and zeta potential measurements to explore the complexities of the biosorption process. In the initial phase, rapid physical adsorption predominated, as demonstrated by a considerable increase in removal efficiency throughout the first three hours. This phase was differentiated by the algal thin film enormous surface area, roughness, and abundance of active binding sites, which allowed cadmium ions to collide and combine via electrostatic interactions. The accessibility of surface regions dominated the first physical adsorption process. As the contact time increased, the biosorption process became slower and more diffusion controlled. The SEM study revealed microcracks and branching holes in the algal films, indicating the emergence of multidimensional diffusion channels. Subsequently, the primary mechanism shifted to chemical binding. Equilibrium data fitting indicated that the Freundlich isotherm, which depicts a heterogeneous surface with multilayer adsorption, was the best model for cadmium biosorption. A kinetic examination confirmed these results, with a pseudo-second-order model indicating that chemisorption, aided by strong chemical interactions, took predominance in the late phases of biosorption. FTIR spectroscopy shows that cadmium biosorption involves interactions between Cd2+ ions and functional groups on algal thin film surfaces. Significant spectrum alterations and the creation of new bands revealed the presence of stable coordination complexes containing hydroxyl, amine, carbonyl, phosphate, and sulfonyl groups. The absence of ester carbonyl bands and the presence of thiocyanate and phosphodiester signals indicate complexation and multi-coordination bonding between Cd2+ ions and the algal thin films surface. XRD examination demonstrated that cadmium was kept inside the structure of the algae without the production of crystalline phases, highlighting the biosorption process's effectiveness in preventing the formation of precipitated cadmium phases. Overall, the results show that cadmium biosorption on Chlorella sorokiniana thin films involves multi-step process, including physical adsorption, intraparticle diffusion, cation exchange with Ca2+, and significant chemisorption through surface functional groups (e.g., –COOH, –OH), Fig. 12. This synergistic, multi-mechanistic technique increases cadmium removal effectiveness, confirming algal thin films viability as a long-term potential for complex heavy metal remediation applications. Finally, the proposed mechanism is therefore strongly supported by the combined evidence obtained from kinetic, isotherm, spectroscopic, microscopic, and electrokinetic analyses.4 Environmental implications and future perspectives
Based on the obtained results, the potential environmental applications and sustainability of the selected microalgal strain for heavy metal remediation are discussed, along with recommendations for future research.4.1 Potential for multi-metal removal
In this study, based on FTIR spectroscopy, the SEM study and the isothermal study, the heterogeneity of the algal thin film surface, which is composed of various functional groups such as carboxyl, hydroxyl, phosphate, and amine groups, provides an excellent platform for binding a wide range of heavy metal ions. The present system's performance with Cd2+ demonstrates potential for large applications. Future research should look at competing adsorption characteristics and compare the selectivity and efficiency of C. sorokiniana thin films to different metal ions. Understanding these interactions will allow for the development of biosorbents that can resist extremely effluent conditions.4.2 Regeneration and reuse of immobilized thin films
Based on the biosorption experiments conducted in this study, cadmium binding is mostly mediated by chemical interactions rather than precipitation, which allows for fast desorption and several reuses of the biosorbent. Immobilized C. sorokiniana thin films may be regenerated with simple desorbing agents, retaining their high biosorption capacity across several cycles. More study is required to assess the long term mechanical integrity and adsorption performance after several regenerations. This contributes to estimating the operating life of films in continuous treatment systems.4.3 Post-use management and environmental safety
Considering the structural and functional properties observed in the present study, although the Chlorella sorokiniana HMY-C thin film is naturally biodegradable, post-use biosorbents contaminated with cadmium must be carefully controlled to avoid subsequent environmental contamination. Chemical desorption using mild acidic or chelating chemicals is the preferred method of eliminating bonded cadmium since the biosorbent may be reused. Following metal removal, the leftover biomass, which is still rich in energy-dense organic material, may be converted into biofuels under controlled conditions. This dual approach enables the safe disposal of hazardous waste while also conforming to circular bioeconomy principles by converting depleted biosorbents into renewable energy resources. To properly explore ecological compromises and scalability, future research should include life cycle assessment (LCA) and investigate the integration of biosorption bioenergy systems into industrial wastewater treatment facilities.4.4 Broader implications for wastewater treatment
This research makes a substantial contribution to the development of sustainable, bio-based industrial wastewater treatment systems. It provides a strong scientific foundation for the use of immobilized algal films by combining kinetic, mechanistic, and surface level research. The system's great efficiency, reusability, and flexibility demonstrate its potential as a practical solution to real world environmental problems.4.5 Scalability and industrial relevance
While the system has shown effective at eliminating cadmium in controlled laboratory settings, commercial use requires validation under dynamic flow and real effluent circumstances. A pilot-scale test with actual industrial effluent containing complicated metal combinations will be required. Furthermore, incorporating these thin films into current modular wastewater treatment frameworks might be a cost-effective and straightforward solution for businesses coping with heavy metal contamination. Furthermore, we propose incorporating this technology into modular treatment units driven by solar energy, which might boost its viability in scattered industrial settings, particularly in rural or resource-constrained locations with limited traditional infrastructure.4.6 Limitations and durability challenges
Despite their promise, immobilized thin films may have limitations owing to biofouling, mechanical deterioration, and structural weaknesses. To address these issues, techniques such as surface modification with antimicrobial nanoparticles and alginate matrix strengthening with crosslinking agents are suggested to improve the films' mechanical and biological durability. Future research should focus on developing biosorption systems that can remove multiple metals at once, incorporating immobilized thin films into existing modular wastewater treatment plants, and improving the mechanical robustness and antifouling properties of biosorbent films to ensure long-term operation in dynamic, real-world environments.4.7 Economic and environmental aspects of algal thin film biosorption
Based on the superior Cd removal efficiency established by the designed Chlorella sorokiniana thin-film system, this approach offers significant environmental and financial advantages for sustainable treatment of wastewater. The production cost of Chlorella sp. In dry wild type is ($ 2.3 to 2.5 kg−1); however, prices can be reduced by (30–40%) by supplementing nutrients with wastewater effluents. In previous study, the growth of microalgae on synthetic media for potential wastewater treatment applications, highlighting the economic and ecological advantages. The utilizing residential wastewater to alter macronutrient levels in the growth environment allowed for the production of biomass with a high protein content (45–57% dry weight).214 Chlorella sorokiniana biomass included 53.25% proteins, 27.95% lipids, 14.25% carbohydrates, and 2.66% pigments, indicating its benefits as a feedstock to produce a variety of bioproducts.215 Microalgae biotechnology as a crucial aspect in achieving sustainable development goals Additionally, treated wastewater has been shown to meet FAO and WHO requirements for agricultural reuse, providing additional economic and environmental advantages.216 Moreover, the thin-film system's treatment cost (USD 0.80–1.11 per m3) is comparable to traditional and emerging technologies like activated carbon (USD 5–200 m−3,98 as cited in 99), chemical precipitation (USD 4 m−3,77 and nanotechnology) USD 6.35 m−3,101 highlighting its economic viability and potential for scalable application. These findings demonstrate the financial and ecological benefits of employing Chlorella sorokiniana based on thin films. In addition to conserving finance, this study promotes the principles of the circular bioeconomy. Future studies should focus on standardized techno-economic modeling, long term field scale validation, and regulatory integration for wastewater reuse in the industrial and agricultural sectors.5 Conclusion
This study was designed to develop and evaluate a sustainable, highly effective biosorption system for removing cadmium from both synthetic and real industrial effluent using Chlorella sorokiniana thin films. All research objectives were achieved, confirming the technology's robustness and potential for scalable wastewater treatment. Under optimal operating conditions, the algal thin film removed 100% of cadmium at low concentrations and maintained high performance up to 80 ppm, exceeding traditional immobilized beads and suspended biosorbents. Kinetic and isotherm modeling revealed a multilayered and heterogeneous biosorption process that corresponded to the pseudo-second-order and Freundlich models. Mechanistic insights confirmed that cadmium uptake occurs in a sequence of physical adsorption, intraparticle diffusion, cation exchange, and strong chemisorption via functional group interactions, with additional structural trapping confirmed by FTIR, SEM-EDX, XRD, and zeta potential analyses all without the formation of crystalline precipitates. The biosorption efficacy of C. sorokiniana thin film was verified under actual industrial wastewater conditions (0.4, 2.4 ppm Cd2+), achieving full removal despite the presence of competing ions. This illustrates the system's remarkable selectivity and adaptability, which is unusual for laboratory-prepared synthetic systems. Demonstrating full-scale efficiency under real-world wastewater circumstances is a significant advance toward industrial implementation since it provides strong evidence of practical viability and environmental significance. Furthermore, immobilizing algal cells within the alginate matrix increased biosorption stability while simultaneously ensuring biosafety. Although changes in microbial community structure were not particularly studied, the immobilized C. sorokiniana HMYA-C thin film system was purposefully intended to limit algal leakage into the surrounding environment. This containment reduces ecological disturbance and guarantees that biosorption occurs without disrupting the natural microbial balance of the treated water. From a sustainability perspective, the suggested thin-film system combines high efficiency, low production costs, and environmental friendliness, making it a suitable platform for large-scale applications. With a focus on long-term performance, regeneration cycles, and sensitivity to several contaminants. Looking forward, shifting from batch to continuous-flow configurations is critical for assessing operational stability and industrial scalability. Additionally, integrating hybrid functionalized biopolymers or nanocomposite matrices may improve film durability, adsorption selectivity, and long-term stability. The use of gene-edited microalgae with high metal binding affinities could be a next-generation approach to precision-engineered biosorbents. Additionally, integrating life-cycle assessment and techno-economic studies is crucial for evaluating this system against standard treatment technologies and assuring its environmental and economic feasibility. Subsequently, this study proposes a comprehensive biosorption technique that not only achieves high cadmium removal efficiency but also respects to circular bioeconomy principles. The closed-loop approach, in which treated biomass is reused for biofuel or biopolymer production, encourages clean water availability, resource recovery, renewable energy generation, and carbon footprint reduction. Overall, these findings highlight Chlorella sorokiniana thin films as a next-generation, environmentally friendly, and economically sustainable biosorbent for real-world wastewater treatment. This work lays a strong platform for enhanced biosorption technologies that combine scientific innovation, practical scalability, and environmental responsibility, thereby accelerating the worldwide transition to a sustainable and circular bioeconomy. This study bridges the gap between laboratory-scale biosorption research and real-world industrial wastewater treatment by developing a scalable, safe, and sustainable algal-based system that challenges the practical limits of bioremediation. Paving way for a cleaner, greener, and more sustainable future.Author contributions
The authors confirm contribution to the paper as follows: Heba M. Youssef was responsible for conceptualization, methodology, experimental work, investigation, data curation, writing – original draft preparation, advanced scientific illustration, writing – review & editing, and final manuscript assembly. Fatma Mohamed provided conceptualization, writing – original draft, continuous guidance throughout the study, writing – review & editing, revising the original draft, validation, supervision, reviewing the results and approved the final version of the manuscript. Mohamed S. Abd Elhameed and Khaled N. M. Elsayed provided conceptualization, supervision, writing – review & editing, validation, reviewing the results and approved the final version of the manuscript. All authors have read and agreed to the published version of the manuscript.Conflicts of interest
The authors affirm that they possess no recognized competing financial interests or personal affiliations that may have seemingly impacted on the research presented in this paper.Data availability
All data supporting the findings of this study are included within the main article. No additional datasets were generated or analyzed during the current study.Acknowledgements
This publication is funded through the United States Agency for International Development USAID. The contents are the responsibility of the Authors and do not necessarily reflect the views of USAID or the United States Government.References
- V. Saxena, Water Quality, Air Pollution, and Climate Change: Investigating the Environmental Impacts of Industrialization and Urbanization, Water, Air, Soil Pollut., 2025, 236(2), 1–40, DOI:10.1007/s11270-024-07702-4
.
- A. P. Onyena and K. Sam, The Blue Revolution: Sustainable Water Management for a Thirsty World, Discov. Sustain., 2025, 6(1), 1–19, DOI:10.1007/s43621-024-00631-6
.
- K. Szálkai, Drinking Water, in The Palgrave Encyclopedia of Global Security Studies, Springer, 2023, pp. 319–326 Search PubMed
.
- T. A. Tella, B. Festus, T. D. Olaoluwa and A. S. Oladapo, Water and Wastewater Treatment in Developed and Developing Countries: Present Experience and Future Plans, in Smart Nanomaterials for Environmental Applications, Elsevier, 2025, pp. 351–385 Search PubMed
.
- A. Saravanan, P. S. Kumar, R. Hemavathy, S. Jeevanantham, P. Harikumar and G. Priyanka, et al., A Comprehensive Review on Sources, Analysis and Toxicity of Environmental Pollutants and Its Removal Methods from Water Environment, Sci. Total Environ., 2022, 812, 152456, DOI:10.1016/j.scitotenv.2021.152456
.
- S. F. Ahmed, P. S. Kumar, M. R. Rozbu, A. T. Chowdhury, S. Nuzhat and N. Rafa, et al., Heavy Metal Toxicity, Sources, and Remediation Techniques for Contaminated Water and Soil, Environ. Technol. Innovation, 2022, 25, 102114, DOI:10.1016/j.eti.2021.102114
.
- M. Sharma, R. Kant, A. Sharma and A. Sharma, Exploring the Impact of Heavy Metals Toxicity in the Aquatic Ecosystem, Int. J. Energy Water Resour., 2024, 1–14, DOI:10.1007/s42108-024-00284-1
.
- A. H. Jagaba, I. M. Lawal, A. H. Birniwa, A. C. Affam, A. K. Usman and U. B. Soja, et al., Sources of Water Contamination by Heavy Metals, in, Membrane Technologies for Heavy Metal Removal from Water, CRC Press, 2024, pp. 3–27 Search PubMed
.
- P. Saravanan, V. Saravanan, R. Rajeshkannan, G. Arnica, M. Rajasimman and B. Gurunathan, et al., Comprehensive Review on Toxic Heavy Metals in the Aquatic System: Sources, Identification, Treatment Strategies, and Health Risk Assessment, Environ. Res., 2024, 119440, DOI:10.1016/j.envres.2024.119440
.
- G. Genchi, M. S. Sinicropi, G. Lauria, A. Carocci and A. Catalano, The Effects of Cadmium Toxicity, Int. J. Environ. Res. Public Health, 2020, 17(11), 3782, DOI:10.3390/ijerph17113782
.
- H. Sharma, N. Rawal and B. B. Mathew, The Characteristics, Toxicity and Effects of Cadmium, Int. J. Nanosci. Nanotechnol., 2015, 3(10), 1–9 Search PubMed
; doi: https://www.researchgate.net/publication/305778858.
- S. Mohammadi, A. Kosari, H. Eslami, E. F. Moghadam and A. Ghaffarian-Bahraman, Toxic Metal Contamination in Edible Salts and Its Attributed Human Health Risks: A Systematic Review and Meta-Analysis, Environ. Sci. Pollut. Res., 2025, 1–12, DOI:10.1007/s11356-025-35940-4
.
- R. Ankush, S. Lamba and P. R. Deepika, Cadmium in Environment—an Overview, Cadmium Toxicity in Water: Challenges and Solutions, 2024, pp. 3–20, DOI:10.1007/978-3-031-54005-9
.
- G. M. Naja, B. Volesky, X. L. Jin and L. K. Wang, 2 Toxicity and Sources of Pb, Cd. Control of Heavy Metals in the Environment: Recent Advances in Metal Toxicity, Pollution Control, and Remediation Techniques, 2025, p. 29, DOI:10.1201/9781003541615
.
- M. Irfan, X. Liu, K. Hussain, S. Mushtaq, J. Cabrera and P. Zhang, The Global Research Trend on Cadmium in Freshwater: A Bibliometric Review, Environ. Sci. Pollut. Res., 2021, 1–14, DOI:10.1007/s11356-021-13894-7
.
- M. Mahajan, P. K. Gupta, A. Singh, B. Vaish, P. Singh and R. Kothari, et al., A Comprehensive Study on Aquatic Chemistry, Health Risk and Remediation Techniques of Cadmium in Groundwater, Sci. Total Environ., 2022, 818, 151784, DOI:10.1016/j.scitotenv.2021.151784
.
- S. Pathak, A. Kumar and S. U. Khan, An Overview of Physical and Chemical Methods of Heavy Metal Removal from Wastewater, Heavy Metal Contamination in the Environment, 2024, pp. 203–216, DOI:10.1016/j.jenvman.2010.11.011
.
- A. SSa, R. E. A. Mohammad, A. H. Jagaba, H. Musa and A. H. Birniwa, Natural, Synthetic, and Composite Materials for Industrial Effluents Treatment: A Mini Review on Current Practices, Cost-Effectiveness, and Sustainability, Case Stud. Chem. Environ. Eng., 2024, 9, 100570, DOI:10.1016/j.cscee.2023.100570
.
- J. Shamshad and R. U. Rehman, Innovative Approaches to Sustainable Wastewater Treatment: A Comprehensive Exploration of Conventional and Emerging Technologies, Environ. Sci.: Adv., 2025 10.1039/d4va00136b
.
- Y. G. Wibowo, T. Taher, K. Khairurrijal, B. S. Ramadan, H. Safitri and S. Sudibyo, et al., Recent Advances in the Adsorptive Removal of Heavy Metals from Acid Mine Drainage by Conventional and Novel Materials: A Review, Bioresour. Technol. Rep., 2024, 101797, DOI:10.1016/j.ccr.2021.214100
.
- R. B. Senthil, P. Senthil Kumar, S. Sanjay, M. Prem Kumar and G. Rangasamy, Artificial Intelligence Integration in Conventional Wastewater Treatment Techniques: Techno-Economic Evaluation, Recent Progress and Its Future Direction, Int. J. Environ. Sci. Technol., 2025, 22(1), 633–658, DOI:10.1007/s13762-024-05725-2
.
- T. A. Saleh, M. Mustaqeem and M. Khaled, Water Treatment Technologies in Removing Heavy Metal Ions from Wastewater: A Review, J. Environ. Nanotechnol., 2022, 17, 100617, DOI:10.1016/j.enmm.2021.100617
.
- T. Oladimeji, M. Oyedemi, M. Emetere, O. Agboola, J. Adeoye and O. Odunlami, Review on the Impact of Heavy Metals from Industrial Wastewater Effluent and Removal Technologies, Heliyon, 2024, 10, e40370, DOI:10.1016/j.heliyon.2024.e40370
.
- P. Yaashikaa, J. Palanivelu and R. Hemavathy, Sustainable Approaches for Removing Toxic Heavy Metal from Contaminated Water: A Comprehensive Review of Bioremediation and Biosorption Techniques, Chemosphere, 2024, 141933, DOI:10.1016/j.chemosphere.2024.141933
.
- K. Iqbal, S. Yahya, M. Jadoon, E. Yaseen and Z. Nadeem, Strategies for Cadmium Remediation in Nature and Their Manipulation by Molecular Techniques: A Comprehensive Review, Int. J. Environ. Sci. Technol., 2024, 1–18, DOI:10.1007/s13762-024-05690-w
.
- N. Kuppan, M. Padman, M. Mahadeva, S. Srinivasan and R. Devarajan, A Comprehensive Review of Sustainable Bioremediation Techniques: Eco Friendly Solutions for Waste and Pollution Management, Waste Manag. Bull., 2024 DOI:10.1016/j.wmb.2024.07.005
.
- K. G. Coronado-Apodaca, C. García-Gómez, D. A. Buentello-Montoya and M. Martínez-Ruiz, Algal Bioreactors as Strategies for Heavy Metal Phycoremediation, Algal Bioreact., 2025, 695–709, DOI:10.1016/B978-0-443-14058-7.00012-9
.
- S. B. Ummalyma, A. Udayan and N. Sreekumar, Phycoremediation of Emerging Contaminants and Heavy Metals from Industrial Wastewater: A Sustainable Green Approach for Bioeconomy, Bioresour. Technol. Rep., 2024, 101920, DOI:10.1016/j.biteb.2024.101920
.
- R. M. Alharbi, N. Abdel-Raouf, M. S. Mohamed, W. A. Fathy, I. B. M. Ibraheem and W. G. Hozayen, Phycomediation of Cadmium Contaminated Aqueous Solutions Using Chlamydomonas Sp.: Process Optimization and Adsorption Characterization, Front. Bioeng. Biotechnol., 2025, 13, 1558757, DOI:10.3389/fbioe.2025.1558757
.
- A. IHNSS and C. BAJAF, Phytoremediation of Heavy Metals by Microalgae: A Mini, 2025, DOI:10.1016/j.heliyon.2021.e07609
.
- N. Potharaju and M. Aruna, Phycoremediation of heavy metals in contaminated water: Factors and processes, in Strategies and Tools for Pollutant Mitigation – Avenues to a Cleaner Environment, ed. J. Aravind, M. Kamaraj, M. Prashanthi Devi and S. Rajakumar, Springer, 2021, DOI:10.1007/978-3-030-63575-6_13
.
- K. Flores-Zambrano, W. Tapia and P. Castillejo, Microalgae Strains Isolated from Piggery Wastewater in Ecuador: Effective Nitrogen Compound Removal and Growth Potential in Extremophile Conditions, Biotechnol. Rep., 2025, e00883, DOI:10.1016/j.btre.2025.e00883
.
- S. Ethiraj and M. S. Samuel, A Comprehensive Review of the Challenges and Opportunities in Microalgae-Based Wastewater Treatment for Eliminating Organic, Inorganic, and Emerging Pollutants, Biocatal. Agric. Biotechnol., 2024, 103316, DOI:10.1016/j.ese.2022.100205
.
- X. Xiao, W. Li, M. Jin, L. Zhang, L. Qin and W. Geng, Responses and Tolerance Mechanisms of Microalgae to Heavy Metal Stress: A Review, Mar. Environ. Res., 2023, 183, 105805, DOI:10.1016/j.marenvres.2022.105805
.
- K. N. Elsayed, T. A. Kolesnikova, A. Noke and G. Klöck, Imaging the Accumulated Intracellular Microalgal Lipids as a Response to Temperature Stress, 3 Biotech, 2017, 7(1), 41, DOI:10.1007/s13205-017-0677-x
.
- O. Spain, M. Plöhn and C. Funk, The Cell Wall of Green Microalgae and Its Role in Heavy Metal Removal, Physiol. Plant., 2021, 173(2), 526–535, DOI:10.1111/ppl.13405
.
- M. Bilal, T. Rasheed, J. E. Sosa-Hernández, A. Raza, F. Nabeel and H. M. Iqbal, Biosorption: An Interplay between Marine Algae and Potentially Toxic Elements—a Review, Marine drugs, 2018, 16(2), 65, DOI:10.3390/md16020065
.
- M. Danouche, N. El Ghachtouli and H. El Arroussi, Phycoremediation Mechanisms of Heavy Metals Using Living Green Microalgae: Physicochemical and Molecular Approaches for Enhancing Selectivity and Removal Capacity, Heliyon, 2021, 7(7), e0760, DOI:10.1016/j.heliyon.2021.e0760
.
- C. M. Monteiro, P. M. Castro and F. X. Malcata, Metal Uptake by Microalgae: Underlying Mechanisms and Practical Applications, Biotechnol. Prog., 2012, 28(2), 299–311, DOI:10.1002/btpr.1504
.
- S. N. Eladl, A. M. Elnabawy and E. G. Eltanahy, Recent Biotechnological Applications of Value-Added Bioactive Compounds from Microalgae and Seaweeds, Bot. Stud., 2024, 65(1), 28, DOI:10.1186/s40529-024-00434-y
.
- A. K. Singh, R. K. Srivastava, P. Pal, S. Mandal, U. K. Sahoo and A. Prakash, et al., Microalgal Biorefinery as a Sustainable and Cost-Effective Platform for Co-Production of High-Value-Added Products/Metabolites: An Insight into Emerging Trends, Challenges, and Opportunities, Biocatal. Agric. Biotechnol., 2024, 103192, DOI:10.1016/j.bcab.2024.103192
.
- N. K. Sarker and P. Kaparaju, Microalgal Bioeconomy: A Green Economy Approach Towards Achieving Sustainable Development Goals, Sustainability, 2024, 16(24), 11218, DOI:10.3390/su162411218
.
- B. Thevarajah, S. Piyathilleke, A. Sahu, P. Nimarshana, A. Malik and T. U. Ariyadasa, Microalgae-Based Bioproducts and Biomaterials Towards a Sustainable Circular Bioeconomy, in Bioeconomy for Sustainability, Springer, 2024, pp. 125–162 Search PubMed
.
- N. Kumar, C. Banerjee, S. Negi and P. Shukla, Microalgae Harvesting Techniques: Updates and Recent Technological Interventions, Crit. Rev. Biotechnol., 2023, 43(3), 342–368, DOI:10.1080/07388551.2022.2031089
.
- P. Chandrashekharaiah, D. Sanyal, S. Dasgupta and A. Banik, Cadmium Biosorption and Biomass Production by Two Freshwater Microalgae Scenedesmus Acutus and Chlorella Pyrenoidosa: An Integrated Approach, Chemosphere, 2021, 269, 128755, DOI:10.1016/j.chemosphere.2020.128755
.
- L. Rugnini, G. Costa, R. Congestri and L. Bruno, Testing of Two Different Strains of Green Microalgae for Cu and Ni Removal from Aqueous Media, Sci. Total Environ., 2017, 601, 959–967, DOI:10.1016/j.scitotenv.2017.05.222
.
- H. N. Abdulkareem and A. I. Alwared, Performance of Immobilized Chlorella Algae for Removing Pb (Ii) Ions from Aqueous Solution, J. Chem. Pet. Eng., 2019, 20(3), 1–6, DOI:10.31699/IJCPE.2019.3.1
.
- R. Saavedra Concha, R. Muñoz Torre, M. E. Taboada Meneses, MdS. Vega Alegre and S. Bolado Rodríguez, Comparative Uptake Study of Arsenic, Boron, Copper, Manganese and Zinc from Water by Different Green Microalgae, Bioresour. Technol., 2018, 263, 49–57, DOI:10.1016/j.biortech.2018.04.101
.
- A. Petrovič and M. Simonič, Removal of Heavy Metal Ions from Drinking Water by Alginate-Immobilised Chlorella Sorokiniana, Int. J. Environ. Sci. Technol., 2016, 13, 1761–1780, DOI:10.1007/s13762-016-1015-2
.
- H. Li, H. Sun, J. Wang, X. Ma and Q. Wei, Process Optimization of Cd2+ Removal with Tetradesmus Obliquus-Immobilized Algal Beads, Biochem. Eng. J., 2024, 207, 109336, DOI:10.1016/j.bej.2024.109336
.
- M. Gao, N. Ling, H. Tian, C. Guo and Q. Wang, Toxicity, Physiological Response, and Biosorption Mechanism of Dunaliella Salina to Copper, Lead, and Cadmium, Front. Microbiol., 2024, 15, 1374275, DOI:10.3389/fmicb.2024.1374275
.
- M. M. El-Sheekh, M. A. Metwally, N. G. Allam and H. E. Hemdan, Effect of Algal Cell Immobilization Technique on Sequencing Batch Reactors for Sewage Wastewater Treatment, Int. J. Environ. Res., 2017, 11, 603–611, DOI:10.1007/s41742-017-0053-z
.
- M. A. Fawzy, H. Darwish, S. Alharthi, M. I. Al-Zaban, A. Noureldeen and S. H. Hassan, Process Optimization and Modeling of Cd2+ Biosorption onto the Free and Immobilized Turbinaria Ornata Using Box–Behnken Experimental Design, Sci. Rep., 2022, 12(1), 3256 CrossRef CAS
; doi: https://www.nature.com/articles/s41598-022-07288-z.
- T. Oliomogbe, M. Nkollo and J. Emegha, Assessing the Suitability of Green Algae (Chlorella Sorokiana) as Biosorbent for Removal of Cu (Ii) and Zn (Ii) Ions from Synthetic Wastewater in a Batch System, J. Appl. Sci. Environ. Manag., 2024, 28(4), 1159–1164 Search PubMed
; doi: https://www.ajol.info/index.php/jasem/article/view/269326.
- R. Waqar, M. Kaleem, J. Iqbal, L. A. Minhas, M. Haris and W. Chalgham, et al., Kinetic and Equilibrium Studies on the Adsorption of Lead and Cadmium from Aqueous Solution Using Scenedesmus Sp, Sustainability, 2023, 15(7), 6024, DOI:10.3390/su15076024
.
- S. Mostolizadeh, Alginate, Polymer Purified from Seaweed, in Alginate-Applications and Future Perspectives, IntechOpen, 2024 Search PubMed
.
- N. A. Agoun and F. G. Avci, Phycoremediation: A Path Towards Heavy Metal Bioremediation from Wastewater, J. Appl. Chem. Biotechnol., 2025, 100(1), 13–23, DOI:10.1002/jctb.7745
.
- L. Hassan, Microalgal Treatment of Wastewater by Suspended and Immobilized Algal Systems: An Approach Contributing to Circular Bioeconomy, in Green Technologies for Wastewater Treatment and Bioenergy Production, CRC Press, pp. , pp. 110–131 Search PubMed.
- Z. Chen, A. I. Osman, D. W. Rooney, W.-D. Oh and P.-S. Yap, Remediation of Heavy Metals in Polluted Water by Immobilized Algae: Current Applications and Future Perspectives, Sustainability, 2023, 15(6), 5128, DOI:10.3390/su15065128
.
- J. I. Ordóñez, S. Cortés, P. Maluenda and I. Soto, Biosorption of Heavy Metals with Algae: Critical Review of Its Application in Real Effluents, Sustainability, 2023, 15(6), 5521, DOI:10.3390/su15065521
.
- A. Damayanti, A. Kumoro and Z. Bahlawan, Review Calcium Alginate Beads as Immobilizing Matrix of Functional Cells: Extrusion Dripping Method, Characteristics, and Application, in IOP Conference Series: Materials Science and Engineering, vol. 1053, IOP publishing, 2021, 012017 Search PubMed
.
- D. Szopa, M. Mielczarek, D. Skrzypczak, G. Izydorczyk, K. Mikula and K. Chojnacka, et al., Encapsulation Efficiency and Survival of Plant Growth-Promoting Microorganisms in an Alginate-Based Matrix–a Systematic Review and Protocol for a Practical Approach, Ind. Crop. Prod., 2022, 181, 114846, DOI:10.1016/j.indcrop.2022.114846
.
- N. Khedr, K. N. Elsayed, I. B. Ibraheem and F. Mohamed, New Insights into Enhancement of Bio-Hydrogen Production through Encapsulated Microalgae with Alginate under Visible Light Irradiation, Int. J. Biol. Macromol., 2023, 253, 127270, DOI:10.1016/j.ijbiomac.2023.127270
.
- S. J. Ukkund, B. Alke and U. T. Syed, Bioremediation of Various Industrial Effluents Utilizing Microorganism-Assisted Nanotechnology within a Circular Economy, in Nano-Microbiology for Sustainable Development, Springer, 2025, pp. 373–399 Search PubMed
.
- N. Yadav, V. K. Garg, A. K. Chhillar and J. S. Rana, Detection and Remediation of Pollutants to Maintain Ecosustainability Employing Nanotechnology: A Review, Chemosphere, 2021, 280, 130792, DOI:10.1016/j.chemosphere.2021.130792
.
- R. Naaz, S. Ahmad and W. A. Siddiqi, Insights of Nanobiotechnology as Bio-Adsorbents for Wastewater Remediation, in Applications of Nanotechnology in Microbiology, Springer, 2024, pp. 421–437 Search PubMed
.
- S. Malik and D. Kumar, Perspectives of Nanomaterials in Microbial Remediation of Heavy Metals and Their Environmental Consequences: A Review, Biotechnol. Genet. Eng. Rev., 2024, 40(1), 154–201, DOI:10.1080/02648725.2023.2182546
.
- P. Sharma, R. Sirohi, Y. W. Tong, S. H. Kim and A. Pandey, Metal and Metal (Loids) Removal Efficiency Using Genetically Engineered Microbes: Applications and Challenges, J. Hazard. Mater., 2021, 416, 125855, DOI:10.1016/j.jhazmat.2021.125855
.
- Y. Nthwane, B. Fouda-Mbanga, M. Thwala and K. Pillay, A Comprehensive Review of Heavy Metals (Pb2+, Cd2+, Ni2+) Removal from Wastewater Using Low-Cost Adsorbents and Possible Revalorisation of Spent Adsorbents in Blood Fingerprint Application, Environ. Technol., 2025, 46(3), 414–430, DOI:10.1080/09593330.2024.2358450
.
- M. C. Benalia, L. Youcef, M. G. Bouaziz, S. Achour and H. Menasra, Removal of Heavy Metals from Industrial Wastewater by Chemical Precipitation: Mechanisms and Sludge Characterization, Arabian J. Sci. Eng., 2022, 47(5), 5587–5599, DOI:10.1007/s13369-021-05525-7
.
- N. A. Qasem, R. H. Mohammed and D. U. Lawal, Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review, Npj Clean Water, 2021, 4(1), 1–15, DOI:10.13140/RG.2.2.35367.36003
.
- Y. H. Teow, M. K. H. K. Ko, W. J. Chan, J. Y. Sum and P. V. Chai, Advances in Membrane Technology for Industrial Applications, in Advances in Separation Sciences. Elsevier, 2025, pp. 281–299 Search PubMed
.
- S. Kouzbour, B. Gourich, F. Gros, C. Vial and Y. Stiriba, A Novel Approach for Removing Cadmium from Synthetic Wet Phosphoric Acid Using Sulfide Precipitation Process Operating in Batch and Continuous Modes, Miner. Eng., 2022, 187, 107809, DOI:10.1016/j.mineng.2022.107809
.
- I. Loughlaımı, Z. Bakher and A. Zouhri, Enhanced Heavy Metal Removal from Wastewater Produced by Chemical Analysis Laboratory Using Calcium Oxide Precipitation: Ph Improvement and Characterization of Precipitated Phases, J. Turk. Chem. Soc. Sect. Chem., 2024, 11(1), 83–92, DOI:10.18596/jotcsa.1321183
.
- Q. Yang, Y. Xie, B. Zhu, Y. Zeng, H. Zhou and P. Ai, et al., Positively Charged Pvc Ultrafiltration Membrane Via Micellar Enhanced Ultrafiltration for Removing Trace Heavy Metal Cations, J. Water Proc. Eng., 2022, 46, 102552, DOI:10.1016/j.jwpe.2021.102552
.
- D. J. Ennigrou, L. Gzara, M. R. B. Romdhane and M. Dhahbi, Cadmium Removal from Aqueous Solutions by Polyelectrolyte Enhanced Ultrafiltration, Desalination, 2009, 246(1–3), 363–369, DOI:10.1080/10934528709375382
.
- N. Das, H. Rajput, A. Aly Hassan and S. Kumar, Application of Different Coagulants and Cost Evaluation for the Treatment of Oil and Gas Produced Water, Water, 2023, 15(3), 464, DOI:10.3390/w15030464
.
- F. K. Katrivesis, V. Sygouni, C. A. Paraskeva and V. G. Papadakis, A Performance Comparison of Pilot-Scale Sand Filtration and Membrane Filtration of Glafkos River Water, J. Mar. Sci. Eng., 2021, 9(2), 203, DOI:10.3390/jmse9020203
.
- Y. Dong, H. Wu, F. Yang and S. Gray, Cost and Efficiency Perspectives of Ceramic Membranes for Water Treatment, Water Res., 2022, 220, 118629, DOI:10.1016/j.watres.2022.118629
.
- S. Kato and Y. Kansha, Comprehensive Review of Industrial Wastewater Treatment Techniques, Environ. Sci. Pollut. Res., 2024, 31(39), 51064–51097, DOI:10.1007/s11356-024-34584-0
.
- N. P. Nandakumar, M. Naveenkumar and K. Senthilkumar, Heavy Metal Harmony: Sustainable Solutions for Industrial Effluent Treatment, in Metal Value Recovery from Industrial Waste Using Advanced Physicochemical Treatment Technologies, Elsevier, 2025. p. 55–69 Search PubMed
.
- K. Sathya, K. Nagarajan, G. Carlin Geor Malar, S. Rajalakshmi and P. Raja Lakshmi, A Comprehensive Review on Comparison among Effluent Treatment Methods and Modern Methods of Treatment of Industrial Wastewater Effluent from Different Sources, Appl. Water Sci., 2022, 12(4), 70, DOI:10.1007/s13201-022-01594-7
.
- N. P. Nandakumar, V. Jaikumar, M. Naveenkumar and K. Senthilkumar, Approach of Microfiltration, Ultrafiltration, and Membrane Reactors in the Recovery of Essential Metals from Industrial Wastewater, in Metal Value Recovery from Industrial Waste Using Advanced Physicochemical Treatment Technologies, Elsevier, 2025, pp. 17–34 Search PubMed
.
- M. Al-Mutair, R. Kumar, B. A. Al-Mur, O. A. Mohamed and M. Barakat, An Overview of Metals Extraction and Recovery from Industrial Wastewater Sludge, Can. J. Chem. Eng., 2024, 102(8), 2892–2908, DOI:10.1002/cjce.25230
.
- A. I. Osman, Z. Chen, A. M. Elgarahy, M. Farghali, I. M. Mohamed and A. Priya, et al., Membrane Technology for Energy Saving: Principles, Techniques, Applications, Challenges, and Prospects, Advanced Energy and Sustainability Research, 2024, 5(5), 2400011, DOI:10.1002/aesr.202400011
.
- L. Lupa and L. Cocheci, Heavy Metals Removal from Water and Wastewater, Heavy Metals-Recent Advances, 2023, https://www.intechopen.com/chapters/86109 Search PubMed
.
- H. Xiang, X. Min, C.-J. Tang, M. Sillanpää and F. Zhao, Recent Advances in Membrane Filtration for Heavy Metal Removal from Wastewater: A Mini Review, J. Water Proc. Eng., 2022, 49, 103023, DOI:10.1016/j.jwpe.2022.103023
.
- C. P. Sharma, Z. Zhu and A. Ronen, Membrane Filtration for Wastewater Treatment–Fouling Mitigation, 2024, https://www.intechopen.com/chapters/1179011 Search PubMed
.
- D. Singh, Insights into Adsorbents: Activated Carbon for Effective Adsorption, Jabirian J. Biointerface Res. Pharmaceut. Appl. Chem., 2024, 1(2), 11–21, DOI:10.55559/jjbrpac.v1i2.233
.
- A. Q. Jasim and S. K. Ajjam, Removal of Heavy Metal Ions from Wastewater Using Ion Exchange Resin in a Batch Process with Kinetic Isotherm, BioMed Res. Int., 2024, 49(1), 43–54, DOI:10.10520/ejc-chemeng-v49-n1-a4
.
- S. K. Misra, A. Kumar, K. Pathak, G. Kumar and T. Virmani, Role of Genetically Modified Microorganisms for Effective Elimination of Heavy Metals, BioMed Res. Int., 2024, 2024(1), 9582237, DOI:10.1155/2024/9582237
.
- I. A. Mawlood, W. M. Saod, A. S. Al-Rawi, A. M. Aljumialy and N. Hilal, Characterization and Use of Activated Carbon Synthesized from Sunflower Seed Shell in the Removal of Pb (Ii), Cd (Ii), and Cr (Iii) Ions from Aqueous Solution, Environ. Monit. Assess., 2024, 196(4), 364, DOI:10.1007/s10661-024-12525-1
.
- M. Ghazoui, O. Boudouch, Y. Miyah, M. Benjelloun, A. Sidigh Sylla and K. Moulakhnif, et al., Assessment of Rhus Pentaphylla-Sulfuric Acid Activated Carbon Performance for Cadmium Ions Adsorption: Mechanism, Response Surface Methodology Optimisation, and Cost Estimation, Indian Chem. Eng., 2025, 1–22, DOI:10.1080/00194506.2025.2462953
.
- E. El Naili and S. H. Almabrok, Cadmium Ion (Cd) Removal from Produced Water Using Ion Technique: Amberlite Ir-120h and Modified Amberlite Ira-400 Cl-Resins, Int. J. Sci. Res. Arch., 2024, 13(2), 1331–1338, DOI:10.30574/ijsra.2024.13.2.2253
.
- M. Batool, A. Mehmood, S. Arif, M. Jalal, F. Ahmad and M. Waseem, Novel and Recyclable Ca (Oh) 2 and Mg (Oh) 2 Modified Amberlyst-15 for Selective Removal of Heavy Metal Ions, Process Saf. Environ. Prot., 2025, 107246, DOI:10.1016/j.psep.2025.107246
.
- B. M. Al-Dabash and M. S. H. Al-Kahali, Nanocellulose from Banana Pseudostem for Pb2+ and Cd2+ Ions Removal from Aqueous Solutions: Banana Pseudostem Nanocellulose for Pb2+ and Cd2+ Ions Removal, Bioresour. Technol., 2025, 3(1), 14–36, DOI:10.24191/bioenv.v3i1.90
.
- Q. K. M. Alshamusi, K. A. A. Hameed, A. M. Taher, M. Batool and L. S. Jasim, Efficiency of Chitosan-Grafted Poly (Carboxymethyl Cellulose-Co-Acrylamide) Nano Hydrogel for Cadmium (Ii) Removal: Batch Adsorption Study, J. Nanostruct., 2024, 14(4), 1122–1133, DOI:10.22052/JNS.2024.04.013
.
- I. Ali, M. Asim and T. A. Khan, Low Cost Adsorbents for the Removal of Organic Pollutants from Wastewater, J. Environ. Manage., 2012, 113, 170–183, DOI:10.1016/j.jenvman.2012.08.028
.
- Y. GadelHak, M. El-Azazy, M. F. Shibl and R. K. Mahmoud, Cost Estimation of Synthesis and Utilization of Nano-Adsorbents on the Laboratory and Industrial Scales: A Detailed Review, Sci. Total Environ., 2023, 875, 162629, DOI:10.1016/j.scitotenv.2023.162629
.
- V. V. Akula, G. Ramalingam, A. K. Verma, Z. Ronen, Y. Oren and J. Gilron, et al., Performance Evaluation of Pilot Scale Ion Exchange Membrane Bioreactor for Nitrate Removal from Secondary Effluent, J. Clean. Prod., 2024, 442, 141087, DOI:10.1016/j.jclepro.2024.141087
.
- N. Mpongwana and S. Rathilal, A Review of the Techno-Economic Feasibility of Nanoparticle Application for Wastewater Treatment, Water, 2022, 14(10), 1550, DOI:10.3390/w14101550
.
- I. Pet, M. N. Sanad, M. Farouz, M. M. ElFaham, A. El-Hussein and M. A. El-Sadek, et al., Recent Developments in the Implementation of Activated Carbon as Heavy Metal Removal Management, Water Conserv. Sci. Eng., 2024, 9(2), 62, DOI:10.1007/s41101-024-00287-3
.
- C. Firincă, L.-G. Zamfir, M. Constantin, I. Răut, M.-L. Jecu and M. Doni, et al., Innovative Approaches and Evolving Strategies in Heavy Metal Bioremediation: Current Limitations and Future Opportunities, J. Xenobiot., 2025, 15(3), 63, DOI:10.3390/jox15030063
.
- O. A. Adeleke, M. R. Saphira, Z. Daud, N. Ismail, A. Ahsan and N. A. Ab Aziz, et al., Locally Derived Activated Carbon from Domestic, Agricultural and Industrial Wastes for the Treatment of Palm Oil Mill Effluent, in Nanotechnology in Water and Wastewater Treatment, Elsevier, 2019, pp. 35–62 Search PubMed
.
- A. Q. Jasim and S. K. A. Ajjam, Heavy Metal Removal Technologies with Details of Ion Exchange: Review Study, J. Univ. Babylon Eng. Sci., 2024, 32(4), 116–132 Search PubMed
.
- Y. Devarajan, Nanomaterials-Based Wastewater Treatment: Addressing Challenges and Advancing Sustainable Solutions, BioNanoScience, 2025, 15(1), 1–14, DOI:10.1007/s12668-024-01780-8
.
- M. Drupitha, M. Misra and A. K. Mohanty, Recent Advances on Value-Added Biocarbon Preparation by the Pyrolysis of Renewable and Waste Biomass, Their Structure and Properties: A Move toward an Ecofriendly Alternative to Carbon Black, Environ. Sci.: Adv., 2023, 2(10), 1282–1301 Search PubMed
; doi: https://pubs.rsc.org/en/content/articlehtml/2023/va/d3va00107e.
- M. K. Barman, A. Bhattarai and B. Saha, Applications of Ion Exchange Resins in Environmental Remediation, Vietnam J. Chem., 2023, 61(5), 533–550, DOI:10.1002/vjch.202300027
.
- A. Saravanan, P. S. Kumar, B. Ramesh and S. Srinivasan, Removal of Toxic Heavy Metals Using Genetically Engineered Microbes: Molecular Tools, Risk Assessment and Management Strategies, Chemosphere, 2022, 298, 134341, DOI:10.1016/j.chemosphere.2022.134341
.
- A. Maazouzi, H. Aguedal, A. Driouch, D. R. Merouani, K. Singh and G. Goel, et al., Performance Study of Paracetamol Sequestration from Hospital Wastewater by Thermo-Chemical Activated Sandstone Clay: Understanding of the Removal Mechanism, Int. J. Environ. Anal. Chem., 2024, 104(20), 9723–9745, DOI:10.1080/03067319.2023.2243833
.
- M. Caluori, Ion Exchange Equilibrium: Selectivity Coefficient and Ion Exchange Capacity, Heavy Metals Removal, and Mathematical Modelling, 2020 Search PubMed
.
- N. Dhingra and N. Rani, Innovations in Wastewater Treatment: A Nanotechnology Perspective, Environ. Sci., 2025, 4(1), 122, DOI:10.5281/ZENODO.14878483
.
- R. Pant, A. Gupta, K. Sah, B. Negi and P. N. Sheetal, Genetic Engineering for Cadmium Removal from Wastewater, in Cadmium Toxicity: Challenges and Solutions, Springer, 2024, pp. 379–395 Search PubMed
.
- L. Jaber, S. N. Backer, T. Laoui, F. Abumadi, M. M. S. Koujan and K. A. Khalil, et al., Recent Trends in Surface Impregnation Techniques on Activated Carbon for Efficient Pollutant Removal from Wastewater, Desalin. Water Treat., 2024, 100562, DOI:10.1016/j.dwt.2024.100562
.
- N. Hisano, Y. Yamashita and Y. Nitanai, Metal Removal Technology and Pretreatment Method of Ion Exchange Resin, in Advances in Patterning Materials and Processes XLI, SPIE, 2024, vol. 12957, pp. 266–268 Search PubMed
.
- A. Anwar and S. Baker, Environmental Applications of Nanoparticles: Water Purification and Pollution Control, Nanoscale Rep., 2025, 8(2), 1–4, DOI:10.26524/nr.8.4
.
- N. Akoijam and S. Joshi, Genome Editing and Genetically Engineered Bacteria for Bioremediation of Heavy Metals, in Genome Editing in Bacteria (Part 2), Bentham Science Publishers, 2024, pp. 184–221 Search PubMed
.
- S. Fernandez-Valenzuela, F. Chávez-Ruvalcaba, J. C. Beltran-Rocha, P. M. San Claudio and R. Reyna-Martínez, Isolation and Culturing Axenic Microalgae: Mini–Review, Open Microbiol. J., 2021, 15(1), 1–11, DOI:10.2174/1874285802115010111
.
- C. H. T. Vu, H.-G. Lee, Y. K. Chang and H.-M. Oh, Axenic Cultures for Microalgal Biotechnology: Establishment, Assessment, Maintenance, and Applications, Biotechnol. Adv., 2018, 36(2), 380–396, DOI:10.1016/j.biotechadv.2017.12.018
.
- E. T. Sero, N. Siziba, T. Bunhu and R. Shoko, Isolation and Screening of Microalgal Species, Native to Zimbabwe, with Potential Use in Biodiesel Production, All Life, 2021, 14(1), 256–264, DOI:10.1080/26895293.2021.1911862
.
- M. A. Alam, G. Muhammad, A. Rehman, M. Russel, M. Shah and Z. Wang. Standard Techniques and Methods for Isolating, Selecting and Monitoring the Growth of Microalgal Strain, Microalgae Biotechnology for Development of Biofuel and Wastewater Treatment, 2019, pp. 75–93, DOI:10.1007/978-981-13-2264-8_4
.
- T. J. White, T. Bruns, S. Lee and J. Taylor. Amplification and Direct Sequencing of Fungal Ribosomal Rna Genes for Phylogenetics, PCR protocols: a guide to methods and applications, 1990, vol. 18, 1, pp. 315–322, https://www.researchgate.net/publication/262687766 Search PubMed
.
- Y. Xu, A. Lu, X. Chen, D. Su, W. Wang and Y. Zhang, Isolation, Identification and Growth Optimisation of Freshwater Microalgae, Turk. J. Fish. Aquat. Sci., 2018, 18(11), 1293–1302, DOI:10.4194/1303-2712-v18_11_06
.
- S. Kumar, G. Stecher, M. Li, C. Knyaz, K. Tamura and X. Mega, Molecular Evolutionary Genetics Analysis across Computing Platforms, Mol. Biol. Evol., 2018, 35(6), 1547–1549, DOI:10.1093/molbev/msy096
.
- D. M. Pinho, R. S. Oliveira, V. Md Santos, W. F. Marques, A. C. Pinto and M. J. Rezende, et al., Evaluating the Potential of Biodiesel Production through Microalgae Farming in Photobioreactor and High Rate Ponds from Wastewater Treatment, J. Braz. Chem. Soc., 2017, 28(12), 2429–2437, DOI:10.21577/0103-5053.20170097
.
- A. Mukherjee and G. D. Bhowmick, Sources of Heavy Metal Contamination in Surface Waters of Indian Freshwater Ecosystems and Their Effect on Aquatic Biota: A State of the Art Review, J. Hazard. Toxic Radioact. Waste, 2024, 28(2), 03123005, DOI:10.1061/JHTRBP.HZENG-1292
.
- D. Winckelmann, F. Bleeke, P. Bergmann and G. Klöck, Growth of Cyanobacterium Aponinum Influenced by Increasing Salt Concentrations and Temperature, 3 Biotech., 2015, 5(3), 253–260, DOI:10.1007/s13205-014-0224-y
.
- J. S. Tan, S. Y. Lee, K. W. Chew, M. K. Lam, J. W. Lim and S.-H. Ho, et al., A Review on Microalgae Cultivation and Harvesting, and Their Biomass Extraction Processing Using Ionic Liquids, Bioengineered, 2020, 11(1), 116–129, DOI:10.1080/21655979.2020.1711626
.
- A. S. Japar, N. M. Azis, M. S. Takriff and N. H. M. Yasin, Application of Different Techniques to Harvest Microalgae, Trans. Sci. Technol., 2017, 4(2), 98–108 Search PubMed
; doi: https://tost.unise.org/pdfs/vol4/no2/4x2x98x108.pdf.
- L. H. R. Rodrigues, A. Arenzon, M. T. RayaRodrigues and N. F. Fontoura, Algal Density Assessed by Spectrophotometry: A Calibration Curve for the Unicellular Algae Pseudokirchneriella Subcapitata, J. Environ. Chem. Ecotoxicol., 2011, 3(8), 225–228, DOI:10.5897/JECE2011.025
.
- R. M. Rahulkumar Maurya, C. P. Chetan Paliwal, T. G. Tonmoy Ghosh, I. P. Imran Pancha, K. C. Kaumeel Chokshi and M. M. Madhusree Mitra, , Applications of De-Oiled Microalgal Biomass Towards Development of Sustainable Biorefineret al.y, 2016, DOI:10.1016/j.biortech.2016.04.115
.
- R. Maurya, C. Paliwal, T. Ghosh, I. Pancha, K. Chokshi and M. Mitra, et al., Applications of De-Oiled Microalgal Biomass Towards Development of Sustainable Biorefinery, Bioresour. Technol., 2016, 214, 787–796, DOI:10.1016/j.biortech.2016.04.115
.
- H. Omar, A. Abdel-Razek and M. Sayed, Biosorption of Cesium-134 from Aqueous Solutions Using Immobilized Marine Algae: Equilibrium and Kinetics, J. Nat. Sci., 2010, 8(11), 214–221 Search PubMed
; doi: https://www.sciepub.com/reference/135046.
- M. T. Shaaban, A. S. Abdel-Razek, S. A. Mahmoud and E. M. Kandeel, Comparative Studies to Remove Cobalt Ions from Hazardous Waste Solutions by Immobilized Microbial Species Using Several Techniques: Beads and Thin Film, Desalin. Water Treat., 2020, 206, 215–228 CrossRef CAS
.
- G. S. Caldwell, P. Na, R. Hart, E. Sharp, A. Stefanova and M. Pickersgill, et al., Immobilising Microalgae and Cyanobacteria as Biocomposites: New Opportunities to Intensify Algae Biotechnology and Bioprocessing, Energies, 2021, 14(9), 2566 CrossRef CAS
.
- S. Ramola and V. Rajput, Adsorption Isotherm and Kinetic Study of Pb Removal by Spent Tea Waste, https://www.researchgate.net/profile/Sudipta-Ramola/publication/320280455 Search PubMed.
- M. Wassel, A. Swelam, A. Salem and A. El-Feky, Kinetic and Thermodynamic Studies of Co (Ii), Ni (Ii) and Mn (Ii) Adsorption Using Resinex, https://www.curresweb.com/mejas/mejas/2014/288-299.pdf Search PubMed.
- G. W. Kajjumba, S. Emik, A. Öngen, H. K. Özcan and S. Aydın, Modelling of Adsorption Kinetic Processes—Errors, Theory and Application, Advanced sorption process applications, 2018, pp. 1–19, DOI:10.5772/intechopen.80495
.
- M. M. Majd, V. Kordzadeh-Kermani, V. Ghalandari, A. Askari and M. Sillanpää, Adsorption Isotherm Models: A Comprehensive and Systematic Review (2010−2020), Sci. Total Environ., 2022, 812, 151334, DOI:10.1016/j.scitotenv.2021.151334
.
- X. Chen, M. F. Hossain, C. Duan, J. Lu, Y. F. Tsang and M. S. Islam, et al., Isotherm Models for Adsorption of Heavy Metals from Water-a Review, Chemosphere, 2022, 307, 135545, DOI:10.1016/j.chemosphere.2022.135545
.
- H. Swenson and N. P. Stadie, Langmuir's Theory of Adsorption: A Centennial Review, Langmuir, 2019, 35(16), 5409–5426, DOI:10.1021/acs.langmuir.9b00154
.
- E. Heraldy, Y. Hidayat and M. Firdaus, The Langmuir Isotherm Adsorption Equation: The Monolayer Approach, in IOP Conference Series: Materials science and engineering, IOP Publishing; 2016, vol. 107, p. 012067 Search PubMed
.
- Z. Chen, W. Ma and M. Han, Biosorption of Nickel and Copper onto Treated Alga (Undaria Pinnatifida): Application of Isotherm and Kinetic Models, J. Hazard. Mater., 2008, 155(1–2), 327–333, DOI:10.1016/j.jhazmat.2007.11.064
.
- A. Kalra, P. Hadi, H. R. Mackey, T. Al Ansari and G. McKay, Sorption of Heavy Metal Ions onto E-Waste-Derived Ion-Exchange Material–Selecting the Optimum Isotherm, Desalin. Water Treat., 2018, 126, 196–207, DOI:10.5004/dwt.2018.23038
.
- A. Khalid, M. A. Kazmi, M. Habib and K. Shahzad, Kinetic & Equilibrium Modelling of Copper Biosorption, J. Eng. Technol., 2015, 22(1), 131–145 Search PubMed
; doi: http://journals.pu.edu.pk/journals/index.php/jfet/article/view/527.
- G. Sandoval-Flores, S. Alvarado-Reyna, L. Elvir-Padilla, D. Mendoza-Castillo, H. Reynel-Avila and A. Bonilla-Petriciolet, Kinetics, Thermodynamics, and Competitive Adsorption of Heavy Metals from Water Using
Orange Biomass, Water Environ. Res., 2018, 90(12), 2114–2125, DOI:10.2175/106143017X15131012188321
.
- R. Sips, On the Structure of a Catalyst Surface, J. Chem. Phys., 1948, 16(5), 490–495, DOI:10.1063/1.1746922
.
- K. Vijayaraghavan, T. Padmesh, K. Palanivelu and M. Velan, Biosorption of Nickel (Ii) Ions onto Sargassum Wightii: Application of Two-Parameter and Three-Parameter Isotherm Models, J. Hazard. Mater., 2006, 133(1–3), 304–308, DOI:10.1016/j.jhazmat.2005.10.016
.
- D. T. Mekonnen, in Low-Cost Adsorptive Technologies: Batch Reactor and Fixed-Bed Column Experiments for the Removal of Phosphate from Wastewater, Dissertation, Rostock, Universität Rostock, 2022 Search PubMed
.
- A. Khelfaoui and N. Chaouch, Equilibrium, Kinetics, and Thermodynamics Study of Phenol Adsorption onto Phoenix Dactylifera Leaf Adsorbents, Desalin. Water Treat., 2023, 310, 167–180, DOI:10.5004/dwt.2023.29954
.
- K. Tamura, G. Stecher and S. Kumar, Mega11: Molecular Evolutionary Genetics Analysis Version 11, Mol. Biol. Evol., 2021, 38(7), 3022–3027, DOI:10.1093/molbev/msab120
.
- Z. Yang, Phylogenetic Analysis Using Parsimony and Likelihood Methods, J. Mol. Evol., 1996, 42, 294–307, DOI:10.1007/bf02198856
.
- D. Bhattacharya An Introduction to Algal Phylogeny and Phylogenetic Methods. Springer; 1997 Search PubMed
.
- B. Elisabeth, F. Rayen and T. Behnam, Microalgae Culture Quality Indicators: A Review, Crit. Rev. Biotechnol., 2021, 41(4), 457–473, DOI:10.1080/07388551.2020.1854672
.
- D. Mahlangu, K. Mphahlele, F. De Paola and N. H. Mthombeni, Microalgae-Mediated Biosorption for Effective Heavy Metals Removal from Wastewater: A Review, Water, 2024, 16(5), 718, DOI:10.3390/w16050718
.
- Y. Wong, Effects of Light Intensity, Illumination Cycles on Microalgae Haematococcus Pluvialis for Production of Astaxanthin, 2016, pp. 1–6, DOI:10.15436/2381-0750.16.1083
.
- T. Chrismadha and M. A. Borowitzka, Effect of Cell Density and Irradiance on Growth, Proximate Composition and Eicosapentaenoic Acid Production of Phaeodactylum Tricornutum Grown in a Tubular Photobioreactor, J. Appl. Phycol., 1994, 6, 67–74, DOI:10.1007/BF02185906
.
- S. Pandey, I. Narayanan, R. Vinayagam, R. Selvaraj, T. Varadavenkatesan and A. Pugazhendhi, A Review on the Effect of Blue Green 11 Medium and Its Constituents on Microalgal Growth and Lipid Production, J. Environ. Chem. Eng., 2023, 11(3), 109984, DOI:10.1016/j.jece.2023.109984
.
- J. Shi, B. Podola and M. Melkonian, Removal of Nitrogen and Phosphorus from Wastewater Using Microalgae Immobilized on Twin Layers: An Experimental Study, J. Appl. Phycol., 2007, 19, 417–423 Search PubMed
.
- V. A. Thiviyanathan, P. J. Ker, S. G. H. Tang, E. P. Amin, W. Yee and M. Hannan, et al., Microalgae Biomass and Biomolecule Quantification: Optical Techniques, Challenges and Prospects, Renew. Sustain. Energy Rev., 2024, 189, 113926, DOI:10.1016/j.rser.2023.113926
.
- M. H. Sarrafzadeh, H.-J. La, S.-H. Seo, H. Asgharnejad and H.-M. Oh, Evaluation of Various Techniques for Microalgal Biomass Quantification, J. Biotechnol., 2015, 216, 90–97, DOI:10.1016/j.jbiotec.2015.10.010
.
- E. A. Suyono, W. Haryadi, M. Zusron, M. Nuhamunada, S. Rahayu and A. P. Nugroho, The Effect of Salinity on Growth, Dry Weight and Lipid Content of the Mixed Microalgae Culture Isolated from Glagah as Biodiesel Substrate, J. Life Sci., 2015, 9, 229–233 CAS
; doi: https://pdfs.semanticscholar.org/79ac/56c2ac3035a430da7638a646cd04e092df39.pdf.
- M. M. Ghoneim, H. S. El-Desoky, K. M. El-Moselhy, A. Amer, E. H. Abou El-Naga and L. I. Mohamedein, et al., Removal of Cadmium from Aqueous Solution Using Marine Green Algae, Ulva Lactuca, Egypt. J. Aquat. Res., 2014, 40(3), 235–242, DOI:10.1016/j.ejar.2014.08.005
.
- S. Gu and C. Q. Lan, Mechanism of Heavy Metal Ion Biosorption by Microalgal Cells: A Mathematic Approach, J. Hazard. Mater., 2024, 463, 132875, DOI:10.1016/j.jhazmat.2023.132875
.
- R. G. Bai, S. Chandrasekharan Nair, L. Joller-Vahter and T. Kikas, Microalgae in Mitigating Industrial Pollution: Bioremediation Strategies and Biomagnification Potential, Biomass, 2025, 5(4), 61 CrossRef
.
- A. A. Najim, A. Y. Radeef, I. al-Doori and Z. H. Jabbar, Immobilization: The Promising Technique to Protect and Increase the Efficiency of Microorganisms to Remove Contaminants, J. Appl. Chem. Biotechnol., 2024, 99(8), 1707–1733 Search PubMed
.
- S. C. Moreno-Rivas, M. J. Ibarra-Gutiérrez, D. Fernández-Quiroz, A. Lucero-Acuña, A. J. Burgara-Estrella and P. Zavala-Rivera, Ph-Responsive Alginate/Chitosan Gel Films: An Alternative for Removing Cadmium and Lead from Water, Gels, 2024, 10(10), 669 CrossRef CAS
.
- N. R. Moheimani, M. A. Borowitzka, A. Isdepsky and S. F. Sing, Standard Methods for Measuring Growth of Algae and Their Composition, in Algae for Biofuels and Energy, Springer, 2012, pp. 265–284 Search PubMed
.
- M. J. Griffiths, C. Garcin, R. P. van Hille and S. T. Harrison, Interference by Pigment in the Estimation of Microalgal Biomass Concentration by Optical Density, J. Microbiol. Methods, 2011, 85(2), 119–123, DOI:10.1016/j.mimet.2011.02.005
.
- A. A. Mohammed, A. A. Najim, T. J. Al-Musawi and A. I. Alwared, Adsorptive Performance of a Mixture of Three Nonliving Algae Classes for Nickel Remediation in Synthesized Wastewater, J. Environ. Health Sci. Eng., 2019, 17, 529–538, DOI:10.1007/s40201-019-00367-w
.
- O. Nateras-Ramírez, M. Martínez-Macias, D. Sánchez-Machado, J. López-Cervantes and R. Aguilar-Ruiz, An Overview of Microalgae for Cd2+ and Pb2+ Biosorption from Wastewater, Bioresour. Technol. Rep., 2022, 17, 100932, DOI:10.1016/j.biteb.2021.100932
.
- S. Permadi, A. Bayu, A. Sari, D. Yogaswara and F. Budiyanto, The Effect of Ph and Salinity on the Capability of Marine Microalgae Biomass for Removing Cd and Pb, in IOP Conference Series: Earth and Environmental Science, IOP Publishing, vol. 462, 2020, p. 012051 Search PubMed
.
- M. El-Sheekh, S. S. El, G. Abou El-Souod and A. Elbeltagy, Biosorption of Cadmium from Aqueous Solution by Free and Immobilized Dry Biomass of Chlorella Vulgaris, Int. J. Environ. Res., 2019, 13, 511–521, DOI:10.1007/s41742-019-00190-z
.
- V. J. Vilar, C. M. Botelho and R. A. Boaventura, Influence of Ph, Ionic Strength and Temperature on Lead Biosorption by Gelidium and Agar Extraction Algal Waste, Process Biochem., 2005, 40(10), 3267–3275, DOI:10.1016/j.procbio.2005.03.023
.
- S. Ahmad, R. Kothari, R. Shankarayan and V. Tyagi, Temperature Dependent Morphological Changes on Algal Growth and Cell Surface with Dairy Industry Wastewater: An Experimental Investigation, 3 Biotech., 2020, 10(1), 24, DOI:10.1007/s13205-019-2008-x
.
- A. Mohammadi and F. Mahmoudnia, Biological Treatment of Heavy Metals with Algae, in Heavy Metals-Recent Advances, IntechOpen, 2023 Search PubMed
.
- Y. Ammar, D. Swailes, B. Bridgens and J. Chen, Influence of Surface Roughness on the Initial Formation of Biofilm, Surf. Coat. Technol., 2015, 284, 410–416, DOI:10.1016/j.surfcoat.2015.07.062
.
- C. Tong and C. Derek, Membrane Surface Roughness Promotes Rapid Initial Cell Adhesion and Long Term Microalgal Biofilm Stability, Environ. Res., 2022, 206, 112602, DOI:10.1016/j.envres.2021.112602
.
- K. Sudhakar and M. Premalatha, Characterization of Micro Algal Biomass through Ftir/Tga/Chn Analysis: Application to Scenedesmus Sp, Energy Sources, Part A, 2015, 37(21), 2330–2337, DOI:10.1080/15567036.2013.825661
.
- Y. Li, S. Song, L. Xia, H. Yin, J. V. G. Meza and W. Ju, Enhanced Pb (Ii) Removal by Algal-Based Biosorbent Cultivated in High-Phosphorus Cultures, Chem. Eng. J., 2019, 361, 167–179, DOI:10.1016/j.cej.2018.12.070
.
- H. El-Rafie, M. El-Rafie and M. Zahran, Green Synthesis of Silver Nanoparticles Using Polysaccharides Extracted from Marine Macro Algae, Carbohydr. Polym., 2013, 96(2), 403–410, DOI:10.1016/j.carbpol.2013.03.071
.
- I. Michalak, M. Mironiuk and K. Marycz, A Comprehensive Analysis of Biosorption of Metal Ions by Macroalgae Using Icp-Oes, Sem-Edx and Ftir Techniques, PLoS One, 2018, 13(10), e0205590, DOI:10.1371/journal.pone.0205590
.
- J. Khattar, D. Singh, N. Jindal, N. Kaur, Y. Singh and P. Rahi, et al., Isolation and Characterization of Exopolysaccharides Produced by the Cyanobacterium Limnothrix Redekei Pupccc 116, Appl. Biochem. Biotechnol., 2010, 162, 1327–1338, DOI:10.1007/s12010-010-8922-3
.
- A. Šimonovičová, A. Takáčová, I. Šimkovic and S. Nosalj, Experimental Treatment of Hazardous Ash Waste by Microbial Consortium Aspergillus Niger and Chlorella Sp.: Decrease of the Ni Content and Identification of Adsorption Sites by Fourier-Transform Infrared Spectroscopy, Front. Microbiol., 2021, 12, 792987, DOI:10.3389/fmicb.2021.792987
.
- D. S. Alobaidi and A. I. Alwared, Role of Immobilised Chlorophyta Algae in Form of Calcium Alginate Beads for the Removal of Phenol: Isotherm, Kinetic and Thermodynamic Study, Heliyon, 2023, 9(4) CrossRef CAS
); doi: https://www.cell.com/heliyon/fulltext/S2405-8440(23)02058-3.
- P. Nieuwenhuizen, J. Reedijk, M. Van Duin and W. McGill, Thiuram-and Dithiocarbamate-Accelerated Sulfur Vulcanization from the Chemist's Perspective; Methods, Materials and Mechanisms Reviewed, Rubber Chem. Technol., 1997, 70(3), 368–429, DOI:10.5254/1.3538436
.
- K. C. Wong, Review of Spectrometric Identification of Organic Compounds, ACS Publications, 2015 Search PubMed
.
- N. E.-A. El-Naggar, M. H. Hussein, S. A. Shaaban-Dessuuki and S. R. Dalal, Production, Extraction and Characterization of Chlorella Vulgaris Soluble Polysaccharides and Their Applications in Agnps Biosynthesis and Biostimulation of Plant Growth, Sci. Rep., 2020, 10(1), 3011 CrossRef CAS
; doi: https://www.nature.com/articles/s41598-020-59945-w.
- N. Sultana, S. Z. Hossain, M. E. Mohammed, M. Irfan, B. Haq and M. Faruque, et al., Experimental Study and Parameters Optimization of Microalgae Based Heavy Metals Removal Process Using a Hybrid Response Surface Methodology-Crow Search Algorithm, Sci. Rep., 2020, 10(1), 15068, DOI:10.1038/s41598-020-72236-8
.
- J. Oomens and J. D. Steill, Free Carboxylate Stretching Modes, J. Phys. Chem. A, 2008, 112(15), 3281–3283, DOI:10.1021/jp801806e
.
- A. Petrovič and M. Simonič, The Effect of Carbon Source on Nitrate and Ammonium Removal from Drinking Water by Immobilised Chlorella Sorokiniana, Int. J. Environ. Sci. Technol., 2015, 12, 3175–3188 CrossRef
.
- M. N. Abbas, S. M. M. Al-Hermizy, Z. N. Abudi and T. A. Ibrahim, Phenol Biosorption from Polluted Aqueous Solutions by Ulva Lactuca Alga Using Batch Mode Unit, J. Ecol. Eng., 2019, 20(6), 225–235 CrossRef
.
- M. Mudasir, K. Karelius, N. H. Aprilita and E. T. Wahyuni, Adsorption of Mercury (Ii) on Dithizone-Immobilized Natural Zeolite, J. Environ. Chem. Eng., 2016, 4(2), 1839–1849 CrossRef CAS
.
- G. Racić, I. Vukelić, B. Kordić, D. Radić, M. Lazović and L. Nešić, et al., Screening of Native Trichoderma Species for Nickel and Copper Bioremediation Potential Determined by Ftir and Xrf, Microorganisms, 2023, 11(3), 815, DOI:10.3390/microorganisms11030815
.
- S. Husien, A. Labena, E. El-Belely, H. M. Mahmoud and A. S. Hamouda, Absorption of Hexavalent Chromium by Green Micro Algae Chlorella Sorokiniana: Live Planktonic Cells, Water Pract. Technol., 2019, 14(3), 515–529, DOI:10.2166/wpt.2019.034
.
- R. N. Oliveira, M. C. Mancini, F. C. Sd Oliveira, T. M. Passos, B. Quilty and R. MdS. M. Thiré, et al., Ftir Analysis and Quantification of Phenols and Flavonoids of Five Commercially Available Plants Extracts Used in Wound Healing, Matéria, 2016, 21(03), 767–779, DOI:10.1590/S1517-707620160003.0072
.
- N. Yee, L. G. Benning, V. R. Phoenix and F. G. Ferris, Characterization of Metal− Cyanobacteria Sorption Reactions: A Combined Macroscopic and Infrared Spectroscopic
Investigation, Environ. Sci. Technol., 2004, 38(3), 775–782 CrossRef CAS
.
- L. M. Laurens and E. J. Wolfrum, Feasibility of Spectroscopic Characterization of Algal Lipids: Chemometric Correlation of Nir and Ftir Spectra with Exogenous Lipids in Algal Biomass, BioEnergy Res., 2011, 4, 22–35 CrossRef
.
- A. R. Fajardo, M. B. Silva, L. C. Lopes, J. F. Piai, A. F. Rubira and E. C. Muniz, Hydrogel Based on an Alginate–Ca 2+/Chondroitin Sulfate Matrix as a Potential Colon-Specific Drug Delivery System, Rsc Adv., 2012, 2(29), 11095–11103, 10.1039/C2RA20785K
.
- L. D'Souza, P. Devi, M. Divya Shridhar and C. G. Naik, Use of Fourier Transform Infrared (Ftir) Spectroscopy to Study Cadmium-Induced Changes in Padina Tetrastromatica (Hauck), Anal. Chem. Insights, 2008, 3, 117739010800300001, DOI:10.4137/117739010800300001
.
- N. E.-A. El-Naggar, R. A. Hamouda, I. E. Mousa, M. S. Abdel-Hamid and N. H. Rabei, Statistical Optimization for Cadmium Removal Using Ulva Fasciata Biomass: Characterization, Immobilization and Application for Almost-Complete Cadmium Removal from Aqueous Solutions, Sci. Rep., 2018, 8(1), 12456 CrossRef
.
- M. Gomaa, M. A. Fawzy, A. F. Hifney and K. M. Abdel-Gawad, Use of the Brown Seaweed Sargassum Latifolium in the Design of Alginate-Fucoidan Based Films with Natural Antioxidant Properties and Kinetic Modeling of Moisture Sorption and Polyphenolic Release, Food Hydrocolloids, 2018, 82, 64–72 CrossRef CAS
.
- D. Radhika and A. Mohaideen, Fourier Transform Infrared Analysis of Ulva Lactuca and Gracilaria Corticata and Their Effect on Antibacterial Activity, Asian J. Pharm. Clin. Res., 2015, 8(2), 209–212 CAS
.
- S. V. Shah, B. Y. Lamba, A. K. Tiwari and R. Sharma, Self-Flocculation Behaviour of Cellulose-Based Bioflocculant Synthesized from Sewage Water Grown Chlorella Sorokiniana and Scenedesmus Abundans, Bioprocess Biosyst. Eng., 2024, 47(5), 725–736 CrossRef CAS PubMed
.
- A. Kumar and P. Lingfa, Sodium Bentonite and Kaolin Clays: Comparative Study on Their Ft-Ir, Xrf, and Xrd, Mater. Today: Proc., 2020, 22, 737–742 CAS
.
- Z. Wang, J. Chen, J. Tan, Z. Lu, X. Wang and J. Li, The Bio-Immobilization of Pb (Ii) Induced by the Chlorella–Montmorillonite Composite in the Ca (Ii) Environment, Front.Environ. Sci., 2022, 10, 983430, DOI:10.3389/fenvs.2022.983430
.
- A. Dąbrowski, Adsorption—from Theory to Practice, Adv. Colloid Interface Sci., 2001, 93(1–3), 135–224 CrossRef
.
- A. Ahmad, A. Bhat and A. Buang, Biosorption of Transition Metals by Freely Suspended and Ca-Alginate Immobilised with Chlorella Vulgaris: Kinetic and Equilibrium Modeling, J. Clean. Prod., 2018, 171, 1361–1375, DOI:10.1016/j.jclepro.2017.09.252
.
- J. T. Phiri and S. Oh, Biosorption of Cd (Ii), Co (Ii), and Cu (Ii) onto Microalgae under Acidic and Neutral Conditions, Sustainability, 2024, 16(15), 6342, DOI:10.3390/su16156342
.
- M. Plöhn, C. Escudero-Onate and C. Funk, Biosorption of Cd (Ii) by Nordic Microalgae: Tolerance, Kinetics and Equilibrium Studies, Algal Res., 2021, 59, 102471, DOI:10.1016/j.algal.2021.102471
.
- M. S. Abdelkarim, M. H. Ali and D. A. Kassem, Ecofriendly Remediation of Cadmium, Lead, and Zinc Using Dead Cells of Microcystis Aeruginosa, Sci. Rep., 2025, 15(1), 3677, DOI:10.1038/s41598-025-86884-1
.
- M. A. Fawzy, S. Abdelsalam, W. A. Hafez and A. A. Fathi, Bioremediation Potential of Microalgae for Copper Ion from Wastewater and Its Impact on Growth and Biochemical Contents: Equilibrium Isotherm Studies, Mater. Res. Express, 2024, 11(5), 055404, DOI:10.1088/2053-1591/ad495a
.
- Q. Hu and Z. Zhang, Application of Dubinin–Radushkevich Isotherm Model at the Solid/Solution Interface: A Theoretical Analysis, J. Mol. Liq., 2019, 277, 646–648, DOI:10.1016/j.jprot.2019.01.005
.
- S. S. Bulynina, E. E. Ziganshina and A. M. Ziganshin, Growth Efficiency of Chlorella Sorokiniana in Synthetic Media and Unsterilized Domestic Wastewater, BioTech, 2023, 12(3), 53, DOI:10.3390/biotech12030053
.
- M. Yang, C. Xue, L. Li, Z. Gao, Q. Liu and P. Qian, et al., Design and Performance of a Low-Cost Microalgae Culturing System for Growing Chlorella Sorokiniana on Cooking Cocoon Wastewater, Algal Res., 2022, 62, 102607, DOI:10.1016/j.algal.2021.102607
.
- C. Y. B. Oliveira, A. Jacob, C. Nader, C. D. L. Oliveira, Â. P. Matos and E. S. Araújo, et al., An Overview on Microalgae as Renewable Resources for Meeting Sustainable Development Goals, J. Environ. Manage., 2022, 320, 115897, DOI:10.1016/j.jenvman.2022.115897
.
| This journal is © The Royal Society of Chemistry 2025 |












