Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Introduction of amino groups into lignin and lignin model compounds: recent advances
Veronika D. Badazhkova
 a,
Risto Savela
a,
Risto Savela
 bc and
Reko Leino
bc and
Reko Leino
 *a
*a
aJohan Gadolin Process Chemistry Centre, Laboratory of Molecular Science and Engineering, Organic Chemistry Research Group, Åbo Akademi University, FI-20500 Turku, Finland. E-mail: reko.leino@abo.fi
bTurku PET Centre, University of Turku and Turku University Hospital, FI-20520 Turku, Finland
cDepartment of Chemistry, University of Turku, 20500 Turku, Finland
First published on 25th November 2025
Abstract
Research on lignin valorization has attracted considerable attention in recent decades. Lignin, a natural aromatic polymer, is a renewable and readily available source for producing new biobased chemicals and materials. For successful lignin valorization towards value-added products, development of efficient procedures allowing the introduction of new functionalities to its structure becomes essential. One of the approaches for chemical modification of lignin involves the introduction of amino groups. Lignin amination methodologies potentially provide aromatic amines that could be used as building blocks for the synthesis of different compounds, including pharmaceuticals and precursors for bio-based polymeric materials. Due to the complex structure of lignin, however, model compounds are commonly used for studying the reactivity and for developing lignin modification procedures. In several studies, it has been demonstrated that lignin model compounds imitating the most abundant lignin β-O-4 linkage can be converted to nitrogen containing aromatic compounds with various potential applications. However, it has also been observed in several cases that the developed amination procedures only have functioned on the model compounds but not on extracted lignin polymer. Overall, in recent years, several procedures have been developed for amination of lignin model compounds and lignin, allowing materials containing primary, secondary and tertiary amines to be obtained, for example the Mannich reaction, silylation and epoxy functionalization. The chemical modification and analysis of lignin are nevertheless hampered by its complex and inconsistent structure, varying significantly depending on the source and method of isolation. While the bulk of currently available lignin is present mainly in the form of Kraft lignin with condensed structure and low solubility, new efficient methods could facilitate switching to lignin-first approaches, preserving its original structure and improving the processing towards value-added products.
Introduction
Lignin, the second most abundant natural polymer in the world, is a highly promising renewable feedstock for producing new chemicals and materials.1 The industrial production of lignin reaches up to 100 million tons per year. However, a major part of the lignin produced by the paper industry is utilized in combustion for energy production, and only 1–2% of the isolated lignin is used for other applications as value-added products.2The chemical structure of lignin is often presented as a crosslinked network of primarily three phenylpropane units, p-coumaryl, coniferyl, and sinapyl alcohols, also known as monolignols. During lignification – lignin polymerization processes in a plant cell – various inter-unit linkages are formed within the polymeric structure. The most common linkages in lignin structures are β-O-4, β-5, 5-5, 4-O-5, β-β and β-1 linkages (Fig. 1). In native lignin, β-O-4 type is the most abundant linkage.3 The high carbon content and the aromatic structure of lignin then render it as a renewable precursor for producing aromatic compounds.
 | ||
| Fig. 1 Illustration of a representative lignin structure containing the most common linkages formed between the monomeric units.4 | ||
Due to its thermal degradation and mechanical properties, the direct application of lignin is limited, yet possible, for example, as antioxidant, UV stabilizer or additive for plastics.5 The main challenge in lignin valorization is its structural complexity and heterogenity.6 The structure, molecular weight and physical properties of lignin may vary considerably, depending on both the source and the isolation process.7
In recent decades, the research on chemical modification of lignin has rapidly developed, exploring the possibilities of lignin valorization with preservation of its macromolecular nature, as well as the degradation of lignin into smaller molecular components to obtain value-added products.4,8–10
The surface of extracted lignin is rich with phenolic (1.2–2.0 mmol g−1) and aliphatic (4.8–5.9 mmol g−1) hydroxyl groups that can be used for introducing new functionalities to its structure.5,11 In several studies on chemical modification of lignin, it has been shown that various oxidation, degradation, alkylation and amination procedures can be applied to both lignin model compounds and extracted lignin.5
In industry, amines are important building blocks for the synthesis of a wide range of products, including polymers, agrochemicals and pharmaceuticals.12,13 Consequently, development of amination procedures is a key to expand also the product pool of lignin-based chemicals.14 This short review summarizes the recent research on amination of lignin model compounds and extracted lignin by utilizing hydroxyl groups in the lignin structure, with β-O-4 motif bearing aliphatic hydroxyl groups as the major target.
Amination of lignin model compounds
Due to the structural complexity and heterogeneity of lignin, model compounds representing different lignin functionalities are commonly used for studying the reactivity and potential valorization approaches. The formation of low molecular weight compounds is often observed in lignin degradation studies. Thus, the use of model compounds allows to simplify the analytical and procedure development processes, while at the same time potentially providing methods for valorization of lignin degradation products.15Amination of lignin-derived monomers
Amination of monomeric and dimeric compounds obtained by different lignin depolymerization strategies can be considered as valuable strategy for lignin valorization. Depending on the depolymerization approach, various phenolic products can be obtained from lignin and utilized for the synthesis of fine chemicals.15,16 For the transformations of lignin-derived monomers into animated products, various heterogeneous and homogeneous approaches can be used promoting the sustainable conversion and production of nitrogen-containing fine chemicals, including alkyl and aryl amines (Scheme 1).17–21 The possibilities of valorization of lignin-derived monomers towards aminated products have been frequently studied and this topic is sufficiently covered in literature elsewhere.17–24Catalytic amination of β-O-4 model compounds
β-O-4 model compounds, representing the most abundant lignin linkage, are frequently used in lignin valorization research.25 Specifically, 2-phenoxy-1-phenylethanols (1) and 2-phenoxy-1-phenyl-1,3-propanediols (2), bearing methoxy substituents in the aromatic rings to imitate original lignin structure, have been used as model compounds for development of various modification procedures, including amination. Depending on the catalytic system selected, for some reactions, 2-phenoxy-1-phenylethanones (3) and 3-hydroxy-2-phenoxy-1-phenyl-1-propanones (4) obtained from the corresponding alcohols are utilized as β-O-4 model compounds (Fig. 2).Mechanistically, the amination processes generally consist of several subsequent steps. When β-O-4 alcohols are used as the model compounds, oxidation of the benzylic hydroxyl group to ketone is commonly the initial step of the subsequent multistep reaction. The ketone, either formed in the reaction mixture or used as starting material, then undergoes C–O and/or C–C bond cleavage followed by C–N bond formation via different mechanisms, depending on the reaction conditions.
It has also been demonstrated that several lignin β-O-4 model alcohols 1 and 2 containing benzylic hydroxyl groups can be converted to benzylamines and phenols in the presence of Pd catalysts. Such transformations typically involve dehydrogenation of the benzylic hydroxyl group and hydrogenolysis of the β-O-4 bond in the lignin model alcohol, followed by reductive amination. Such reactions have been applied to several β-O-4 model alcohols with generic structures 1 and 2, using different amines and resulting in the formation of the corresponding benzylamines (5) and phenols (6) (Scheme 2).26,27 Presumably, compounds 1 and 2 undergo dehydrogenations of the benzylic hydroxyl groups, followed by Cβ–Oβ bond cleavage for compound 1 and also Cβ–Cγ for compound 2, with subsequent imine formation and then its reduction to benzylamine. The yields of benzylamines obtained vary significantly, depending on the position of the methoxy substituents in the β-O-4 model alcohols and amines.
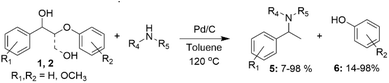 | ||
| Scheme 2 Conversion of β-O-4 lignin model compounds to benzylamines and guaiacol, catalyzed by Pd/C. | ||
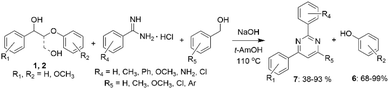
Li et al. have reported a one-pot multi-component cascade reaction for the synthesis of pyrimidines (7) from lignin β-O-4 model alcohols 1 and 2 (Scheme 3).28 According to the proposed mechanism, compound 1 undergoes conversion into acetophenone via dehydrogenation of benzylic hydroxyl group and Cβ–Oβ bond cleavage. Acetophenone then undergoes cross-aldol condensation with arylaldehyde formed via dehydrogenation of the added benzyl alcohol, resulting in formation of chalcone that further reacts with benzamidine hydrochloride to form the final product 7. The reaction does not require the presence of transition metal and can be used for the synthesis of meridianin derivatives, potentially enabling the utilization of biomass for the synthesis of pharmaceutical precursors.
In other work, Li et al. described a one-pot transition-metal-free procedure allowing to obtain heterocyclic compounds from lignin model alcohols 1 and 2 and 1,2-diaminobenzenes (Scheme 3).29 The reaction consists of multiple cascade steps, including dehydrogenation-cleavage towards acetophenone intermediate that reacts with 1,2-diaminobenzene resulting in the formation of quinoxaline derivatives (8), including important biologically active compounds (Scheme 4). When β-O-4 lignin model alcohols with the general structure 1 were used, the yields of quinoxalines ranged from 48 to 73%. However, for the β-O-4 diols 2, the yields of the corresponding quinoxalines reached only 15%, explained by steric hindrance.
Recently, it has been demonstrated that biologically significant nitrogen-containing heterocyclic compounds can be obtained from the lignin β-O-4 model ketones 3 using a chromium-based catalyst. Das et al. synthesized triazine-based Cr-NNN-pincer complexes to catalyze acceptorless dehydrogenative coupling reactions between 2-aminobenzyl alcohol and the lignin β-O-4 model ketones 3 (Scheme 5).30
The reaction subsequently undergoes dehydrogenation of 2-aminoaryl alcohol into corresponding aldehyde followed by aldol condensation between formed aldehyde and compound 3, followed by intramolecular condensation, C–N bond formation and nitrogen-heterocyclic ring construction steps, resulting in the formation of 3-phenoxy-2-phenylquinolines (9). Also, Cu-catalysts have been used in several studies for oxidative amination of lignin β-O-4 model ketones.31–33 Depending on the catalytic system and reaction conditions used, the amination resulted in the formation of different products (Scheme 6). Under air conditions and in the presence of CuCl2, the amination of β-O-4 model ketones 3 with aniline resulted in up to 90% yields of N-phenylbenzamides (10).31 Under oxygen atmosphere, CuI-catalyzed reaction of 2-phenoxyacetophenones (3) with piperidine led to the formation of α-keto amides in up to 88% yields, with no yield of amide reported. However, in the reactions of methoxy-substituted 2-phenoxyacetophenones (3) with piperidine, formation of both α-keto amides (11) as the major product and amides (10) as the minor products were reported.32 In Cu(OAc)2 catalyzed reactions, the amides (10) were found to be the major, while the α-keto amides (11) were minor products. However, the product ratio significantly depended not only on the rection atmosphere, but also on positions of the substituents in the substrate structure and the type of amine used for amination.33 Alternatively, it was shown that amides can be produced from β-O-4 model ketones under mild aqueous conditions using H2O2 instead of Cu-based catalyst in similar yields.34
In other studies, CuI catalyst has been used for the synthesis of substituted imidazo heterocycles (12 and 13) from β-O-4 model ketones 3 (Scheme 7).
Compared to other Cu-catalyzed processes discussed above, C–O bond cleavage does not occur under the applied reaction conditions. This approach allows to obtain heterocyclic products (12, 13) with structures similar to some compounds used in drug design.35
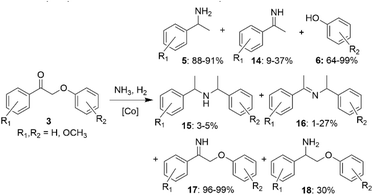
It has also been shown that heterogeneous Co-based catalysts can be used for amination of lignin β-O-4 model ketones.36 In a series of experiments using different Co-based catalysts, the predominant products in the reactions of lignin model ketones 3 with ammonia were benzylamines (5). For one of the catalysts, the reaction was highly selective towards imine aryl ether (17). In some cases, the formation of other imines (15, 16) and amines (14, 18) was also observed (Scheme 8). Selected Co-based catalysts allowed to reach high selectivities towards primary benzylamines that could be used as building blocks for further preparation of value-added products.
New nitrogen-containing products can also be obtained by converting lignin β-O-4 model ketones to oximes for further transformations (Scheme 9). In the presence of MgO, the oxime formed as an intermediate in the reaction mixture, and could be further converted to aromatic nitriles (19) and isoxazoles (20).37 The MgO-catalyzed procedure was applied to dioxasolv birch lignin, resulting in bond cleavage and formation of isoxazole monomers.
Alternatively, the oximes (21) can be preliminary synthesized from the corresponding ketones (3, 4) and then used for subsequent degradation-amination. The reaction of β-O-4 oximes (21) with SOCl2 followed by NaOH addition resulted in a mixture of different products (10, 19, 22, 23) with N-containing functionalities.38
In recent work, Park et al. developed a one-step amination procedure of lignin model diol (2) with diethylenetriamine (Scheme 10).39
The reaction takes place via formation of methylene bridges and allows to convert lignin β-O-4 model compounds to crosslinked polyamines (24, 25). The reaction provides better understanding of crosslinking reactions by one-step amination which can be used for preparation of lignin-based materials.
Zhang et al. have demonstrated that lignin model compounds can be subsequently cleaved and aminated by tetrabutylammonium chloride using CeCl3-promoted photocatalysis.40 The benefits of this approach include the commercial availability of CeCl3, the possibility of re-use of the catalytic system over multiple reaction cycles and good reaction control by switching the external light on and off. The reaction was applied to several β-O-4 model alcohols (2, 3) providing aminated products (27) that can be further used for the synthesis of heterocyclic compounds (Scheme 11). The procedure was applied to extracted dioxane pine lignin primary consisting of β-O-4 and β-5 units, resulting in lignin depolymerization towards degradation products, including vanillin and hydrazine products.
Lignin amination
For amination of lignin, various strategies utilizing different lignin functionalities have been used.Due to the structural complexity of lignin and the typical range of functionalities, it is difficult to assess how the modifications take place mechanistically. Application of amination procedures first to lignin model compounds and analyzing the products formed can provide insights on the possible reaction pathways and new structures formed also in extracted lignin after modification. In addition, the data obtained from the analysis and characterization of the model compounds can often be used as reference for lignin characterization.
Different analytical methods and approaches can be used to evaluate the results after lignin amination.41 The combination of NMR spectroscopic techniques can be used for identification of different linkages in the lignin structure and reveal structural changes taking place in the reaction.42 FT-IR spectroscopy allows to determine the functional groups and their transformations.43 Elemental analysis provides information about the total nitrogen content in aminated lignin to reveal the extent of amination.41 X-ray photoelectron spectrometric (XPS) analysis can be performed to analyze the lignin surface and assess the chemical compositions of amino groups introduced.
Amination by Mannich reaction utilizing the phenolic units
One of the most common approaches for introducing amino groups to lignin structures is the Mannich reaction, typically performed using formaldehyde and various amines, including dimethylamine, ethane diamine, diethylenetriamine, ethylenediamine, γ-polyglutamic acid, and other amines.44,45 Mannich reaction, in general, is the simplest and one of the most important fundamental amination reactions in organic synthesis.46 Already in 1956, Mikawa et al. studied the mechanism of Mannich reaction on lignin model compounds.47 In 1988 Brezny et al. reported the application of Mannich reaction to lignin for preparation of ion-exchanging lignin derivatives.48 Over the recent years, lignin pretreated with Mannich reaction has been used for formation of nanoparticles,49 preparation of bio-based fertilizers,50,51 azo-dyes,52 adhesives,53 and composites,54 formation of hydrogels,55 synthesis of polyurethanes,56 and for curing of epoxy resins.57After application of the Mannich reaction to alkali lignin with ethylenediamine, nitrogen contents reaching up to 5.81 wt% and with hexane-diamine up to 8.87 wt% have been obtained.58 To increase the nitrogen content, preliminary phenolation of lignin can be performed to introduce new phenolic units to the lignin structure to be aminated (Scheme 12).59 It has been shown that after the Mannich reaction the nitrogen content in phenolated lignin can reach 4.8 wt% compared to 2.5% in non-phenolated lignin.46 In other work, it was reported that the amount of active sites in phenolated lignin increased up to 8.26 mmol g−1 compared to 2.91 mmol g−1 in non-phenolated alkali lignin. The nitrogen content in phenolated alkali lignin has been reported as 10.13%.50
Despite that Mannich reaction is commonly used for lignin amination, it has several disadvantages. The Mannich reaction predominantly leads to the formation of tertiary amino groups over the primary and secondary ones, and it is difficult to control the actual reaction process. The reaction results in the formation of hindered polyamines with crosslinked structure and low solubility. Nitrogen content in the aminated lignin is typically relatively low, due to the low reactivity of extracted lignin and the limited amount of reactive sites in the phenolic groups that can be functionalized. All these factors restrict the potential application of aminated lignin.41,60 In addition, the reaction is commonly performed with the toxic and carcinogenic formaldehyde.61
Amination by epoxy functionalization utilizing the phenolic hydroxyl groups
To introduce amino groups to lignin structure, the phenolic hydroxyl groups in lignin can also be epoxy functionalized prior to the amination reaction (Scheme 13).41 In different studies it was demonstrated that Kraft lignin, having higher content of phenolic hydroxyl groups than native lignin, can be successfully epoxidized and cured using different amines, allowing to obtain lignin-based epoxy resins with different properties.62–67 After epoxy functionalization followed by amination, nitrogen content in aminated lignin reaching 6.95 wt% has been reported.41 Compared to the Mannich method, this approach allows to obtain lignin containing primary and secondary amino groups that can be used for further crosslinking or polyurethane production. The synthesis of lignin-based epoxy resins, their properties and potential applications are sufficiently covered in the literature elsewhere.68Ethylenediamine pretreatment
In several studies, it has been reported that ethylenediamine (EDA) pretreatment of lignocellulosic biomass is an efficient procedure for deconstructing the lignin–carbohydrate complex structure to enhance the carbohydrate and bioethanol production.69–74 EDA pretreatment allows to enhance the solubility of lignin and improve the lignin removal from the biomass. In general, for this procedure the lignocellulosic biomass is mixed with EDA and heated, followed by cooling of the reaction mixture. After the procedure, the obtained solids can be separated by filtration and subjected to ethanol precipitation to isolate the aminated lignin. The benefit of this approach is that unreacted EDA can be recovered from the filtrate by evaporation and recycled. The amount of added EDA, temperature, residence time and, therefore, the nitrogen content typically varies based on the exact procedure applied.It has been shown that after EDA pretreatment, a major part of the acid-insoluble lignin in lignocellulosic biomass is converted to acid-soluble lignin. As a result of the EDA pretreatment, amination of α-hydroxyl groups in β-O-4 units was detected with 16.70% nitrogen content.73 In other work, EDA pretreatment of alkaline lignin allowed to obtain aminated lignin with the nitrogen content reaching up to 19.6 wt%. Based on the appearance of new groups detected by NMR spectroscopy, the amination pathway involving different lignin units was proposed (Scheme 14).74
In addition, lignin depolymerization and formation of low-molecular weight products was observed. Thus, EDA pretreatment method allows to treat lignocellulosic feedstocks, resulting in the formation of aminated lignin with enhanced solubility and reduced condensation, that can be beneficial for further utilization of lignin in high value-added applications.
Silylation and amination
Amino groups can also be introduced to lignin structures by silylation of the lignin hydroxyl groups with aminosilanes (Scheme 15).75–77 Silylation of lignin has been investigated in several studies, resulting in successful introduction of silyl groups to lignin structures.60,78,79 Daugaard et al. have demonstrated that amino groups can be introduced to lignin by ring-opening silylation with cyclic aza-silanes for producing lignin-based curing agents for epoxy resins.75 Kim et al. performed one-step silylations of lignin with (3-aminopropyl) triethoxysilane.76 After the reaction, the nitrogen content in the modified lignin reached up to 6.10 wt%. The introduction of aminosilane functionalities to lignin structure led to increase in the molecular weight, enhanced thermal stability and solvent-resistant properties of the obtained modified lignin. In other work, Kim et al. carried out the silylation of lignin with three aminosilanes containing primary, secondary and tertiary amines, producing aminated lignin containing different types of amino groups.77 For primary aminosilane, the tendency to undergo self-condensation was observed, resulting in the formation of silicon network in the lignin studied. When tertiary aminosilane was used, crosslinking processes in the lignin were recorded. The nitrogen content in lignin containing primary amino groups was the highest, reaching 8.29 wt%.Amidation-hydrolysis
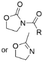
Heterocyclic, nitrogen containing 2-oxazolidinones and oxazolines can also be used for introducing amino groups to lignin structures by two-step amidation-hydrolysis approach (Scheme 16).80,81 Renneckar et al. developed a lignin amination procedure using 2-oxazolidinone both as a solvent and as a reagent. This method allows to obtain lignin containing primary and secondary amino groups with nitrogen contents reaching up to 7.97![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) wt%.80
wt%.80
In recent work, Avérous et al. described a two-step amination procedure to obtain aminated lignin using two heterocyclic compounds, 2-methyl-2-oxazoline and N-acetyl-2-oxazolidinone.81 The procedure introduces primary hydroxyl groups to lignin by utilizing the phenolic hydroxyl groups, providing aminated lignin with low molecular weight and high solubility. The nitrogen content in the aminated lignin reached up to 3.5 mmol g−1 (4.9 wt%).
Discussion and conclusion
In recent years, development of lignin valorization methodologies has emerged as a promising alternative for producing new bio-based materials and chemicals. The number of studies on lignin modification and analysis have expanded significantly, providing better understanding of lignin reactivity and the possible valorization pathways. As an important example, amination of lignin hydroxyl groups provides a highly promising approach towards lignin functionalization and the production of value-added nature-based products, including advanced polymeric materials and fine chemicals, that can be used as building blocks also for the synthesis of biologically active compounds.It has been shown in several studies on lignin model compounds that catalytic amination methodologies allow to obtain a wide range of aromatics, bearing different N-containing functionalities. The reactions commonly undergo several subsequent steps, including dehydrogenation, bond cleavage and reductive amination, resulting in the formation of benzylamines, amides, imines, heterocyclic and other nitrogen containing compounds with a wide range of potential applications. Several catalytic procedures successfully performed on the model compounds have also been applied to extracted lignin for degradation towards monomeric aminated products, demonstrating the significant potential of this valorization pathway. However, only in few studies the developed procedures have been applied to extracted lignin demonstrating applicability towards direct valorization. The most common model compounds used for developing procedures for lignin valorization are β-O-4 ethers imitating the most abundant linkage in native or extracted lignin. Use of the model compounds provides better understanding of lignin reactivity or, more specifically, the reactivity of lignin units that the model compounds represent. However, at the same time, the model compounds lack the structural complexity of lignin that can significantly affect the reactivity. In catalytic reactions the interaction of a catalyst with other lignin functionalities not considered during studies on the model compounds can lead to unknown side reactions and significantly affect the outcome of the catalytic modification. In addition, the impurities in lignin feedstock can cause catalyst poisoning and deactivation.82 Using more complex oligomeric model compounds bearing multiple lignin linkages or mixtures of monomeric and dimeric model compounds could create and simulate reaction conditions that would provide more complete understanding of the reactivity of actual lignin, and applicability of the tested conditions towards real lignin valorization.
Another aspect that should be considered is that in technical lignins obtained after wood biomass processing, the content of β-O-4 units decreases significantly. In lignin directly extracted from Eucalyptus wood the content of β-O-4 units has been reported as 55%, while being 38% in sulfonate lignin, 8% in alkaline lignin, and only 2% in Kraft lignin.83 The decrease of β-O-4 units content could become a limitation for application of catalytic procedures to technical lignins.
For the amination of technical lignins, the approaches targeting phenolic and aliphatic hydroxyl groups can be used. Mannich reaction is a straightforward procedure, successfully applied for amination of different types of industrial lignins. However, the amino groups introduced to lignins by this method are predominantly tertiary amines, restricting the potential application of the obtained product. Lignin amination via preliminary epoxy functionalization, in turn, allows to introduce primary and secondary amino groups to lignin. With this method, it is important to prevent crosslinking reactions between the epoxy groups and the primary/secondary amines leading to formation of tertiary amines. Silylation with aminosilanes, in turn, introduces amino groups with different composition, depending on the exact aminosilanes used for the modification. With this method, lignin bearing multi-layer crosslinked structures containing more amino groups can be formed due to the ability of silanes to undergo self-condensation and to form polysiloxanes. Amidation-hydrolysis allows to obtain aminated lignin containing primary hydroxyl group via a two-step reaction. This method utilizes non-toxic chemicals and results in the formation of highly soluble low molecular weight lignin. EDA pretreatment is another simple method for generation of aminated lignin that can be applied directly to lignocellulosic biomass, extracted lignin and alkaline lignin. This method allows to obtain aminated lignin with high nitrogen content, containing both primary and secondary amino groups. A summary of the lignin amination procedures described in this review are presented in Table 1.
Thus, a wide range of different methodologies can be used for the synthesis of lignin-based aminated products.
Lignin amination can be potentially implemented into the early-stage catalytic conversion of lignin “lignin-first” strategy.84 The amination of model β-O-4 compounds in the presence of several catalytic systems commonly undergoes dehydrogenation and bond cleavage, resulting in the formation of acetophenones that further react with the aminating reagent. Instead of the model β-O-4 compounds, these catalytic systems can be used either for amination of vanillin or other lignin fractionation products or for direct fractionation – amination of organosolv lignin containing β-O-4 units to produce nitrogen containing fine chemicals.
Another approach for lignin valorization involves the amination of ethanosolve lignin under mild conditions, with preservation of its polymeric structure, to produce bio-based polymeric materials.
Technical lignins (Kraft, alkaline, soda) have a higher content of free phenolic hydroxyl groups (3.3–4.3 mmol g−1) that can be targeted for chemical modification and incorporating amino groups into lignin structures.
From the biorefinery point of view, organosolv process allow to obtain pure lignin with high content of aliphatic hydroxyl groups with low molecular weight, that can be further utilized for chemical modification or fractionation. However, the extraction process involves the use of toxic and flammable organic solvents that can only be recovered at high energy cost. “Lignin-first” strategy, including the reductive catalytic fractionation approach, can be used for production of lignin-based fine chemicals. The reductive conditions of the process allow to prevent lignin condensation and to reach high selectivity and yields. The main disadvantages of this approach are the high cost of the catalysts and their deactivation, and the formation of complex mixtures of products that require separation. Development of robust and efficient catalytic systems, reducing the solvent and energy demands, and integrating these processes into biorefinery environments would facilitate efficient and sustainable lignin utilization.
Depending on the source of lignin and its composition, different methods can be used for valorization towards valuable nitrogen containing products. Lignin-derived monomers or organosolv lignin can be successfully converted into primary, secondary and tertiary amines, including heterocyclic products with potential biological activity. Technical lignins can be converted into various materials with a broad range of potential applications, including fertilizers, bio-based polyurethanes, curing agents for epoxy resins, and nanoparticle formation. Thus, despite the complexity and heterogeneity of lignin as starting material, promising methods for lignin valorization have been developed over the recent years, proving excellent prospectives for successful future valorization towards value-added products.
Author contributions
Veronika Badazhkova: writing – review & editing, writing – original draft. Risto Savela: writing – review & editing, supervision. Reko Leino: writing – review & editing, supervision, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Data availability
No primary research results, software or code have been included and no new data were generated or analysed as part of this review.Acknowledgements
Reko Leino and Veronika D. Badazhkova thank the Finnish Natural Resources Research Foundation (grant #20210053). VDB further thanks the Alfred Kordelin Foundation (Gust. Komppa fund), Fortum and Neste Foundation (grant #20240200) and Åbo Akademi University for financial support. The authors thank Dr Patrik Eklund from Åbo Akademi University for helpful and insightful comments.Notes and references
- J. Ragauskas, G. T. Beckham, M. J. Biddy, R. Chandra, F. Chen, M. F. Davis, B. H. Davison, R. A. Dixon, P. Gilna, M. Keller, P. Langan, A. K. Naskar, J. N. Saddler, T. J. Tschaplinski, G. A. Tuskan and C. E. Wyman, Lignin Valorization: Improving Lignin Processing in the Biorefinery, Science, 2014, 344, 1246843 CrossRef PubMed.
- D. S. Bajwa, G. Pourhashem, A. H. Ullah and S. G. Bajwa, A concise review of current lignin production, applications, products and their environmental impact, Ind. Crops Prod., 2019, 139, 111526 CrossRef CAS.
- A. R. Das, A. Ulbrich, M. Alherech, A. H. Motagamwala, A. Bhalla, L. Da Costa Sousa, V. Balan, J. A. Dumesic, E. L. Hegg, B. E. Dale, J. Ralph, J. J. Coon and S. S. Stahl, Lignin Conversion to Low-Molecular-Weight Aromatics via an Aerobic Oxidation-Hydrolysis Sequence: Comparison of Different Lignin Sources, ACS Sustainable Chem. Eng., 2018, 6, 3367–3374 CrossRef CAS.
- S. Sethupathy, G. Murillo Morales, L. Gao, H. Wang, B. Yang, J. Jiang, J. Sun and D. Zhu, Lignin valorization: Status, challenges and opportunities, Bioresour. Technol., 2022, 347, 126696 CrossRef CAS.
- S. Laurichesse and L. Avérous, Chemical modification of lignins: Towards biobased polymers, Prog. Polym. Sci., 2014, 39, 1266–1290 CrossRef CAS.
- A. Llevot, E. Grau, S. Carlotti, S. Grelier and H. Cramail, From Lignin-derived Aromatic Compounds to Novel Biobased Polymers, Macromol. Rapid Commun., 2016, 37, 9–28 CrossRef CAS PubMed.
- M. N. Collins, M. Nechifor, F. Tanasă, M. Zănoagă, A. McLoughlin, M. A. Stróżyk, M. Culebras and C.-A. Teacă, Valorization of lignin in polymer and composite systems for advanced engineering applications – A review, Int. J. Biol. Macromol., 2019, 131, 828–849 CrossRef CAS PubMed.
- D. Stewart, Lignin as a base material for materials applications: Chemistry, application and economics, Ind. Crops Prod., 2008, 27, 202–207 CrossRef CAS.
- M. V. Tsvetkov and E. A. Salganskii, Lignin: Applications and Ways of Utilization, Russ. J. Appl. Chem., 2018, 91, 1129–1136 CrossRef CAS.
- G. T. Beckham, C. W. Johnson, E. M. Karp, D. Salvachúa and D. R. Vardon, Opportunities and challenges in biological lignin valorization, Curr. Opin. Biotechnol., 2016, 42, 40–53 CrossRef CAS PubMed.
- M. Balakshin and E. Capanema, On the Quantification of Lignin Hydroxyl Groups With31 P and13 C NMR Spectroscopy, J. Wood Chem. Technol., 2015, 35, 220–237 CrossRef CAS.
- A. Lawrence, Amines: synthesis, properties, and applications, Cambridge University Press, Cambridge, 2004 Search PubMed.
- S. Bähn, S. Imm, L. Neubert, M. Zhang, H. Neumann and M. Beller, The Catalytic Amination of Alcohols, ChemCatChem, 2011, 3, 1853–1864 CrossRef.
- X. Chen, S. Song, H. Li, G. Gözaydın and N. Yan, Expanding the Boundary of Biorefinery: Organonitrogen Chemicals from Biomass, Acc. Chem. Res., 2021, 54, 1711–1722 CrossRef CAS PubMed.
- J. Zakzeski, P. C. A. Bruijnincx, A. L. Jongerius and B. M. Weckhuysen, The Catalytic Valorization of Lignin for the Production of Renewable Chemicals, Chem. Rev., 2010, 110, 3552–3599 CrossRef CAS PubMed.
- C. Xu, R. A. D. Arancon, J. Labidi and R. Luque, Lignin depolymerisation strategies: towards valuable chemicals and fuels, Chem. Soc. Rev., 2014, 43, 7485–7500 RSC.
- Z. Chen, H. Zeng, H. Gong, H. Wang and C.-J. Li, Palladium-catalyzed reductive coupling of phenols with anilines and amines: efficient conversion of phenolic lignin model monomers and analogues to cyclohexylamines, Chem. Sci., 2015, 6, 4174–4178 RSC.
- E. Blondiaux, J. Bomon, M. Smoleń, N. Kaval, F. Lemière, S. Sergeyev, L. Diels, B. Sels and B. U. W. Maes, Bio-based Aromatic Amines from Lignin-Derived Monomers, ACS Sustainable Chem. Eng., 2019, 7, 6906–6916 CrossRef CAS.
- D. Ruijten, T. Narmon, K. Van Aelst, H. De Weer, R. Van Der Zweep, T. Hendrickx, C. Poleunis, L. Li, K. M. Van Geem, D. P. Debecker and B. F. Sels, Tertiary Amines from RCF Lignin Mono- and Dimers: Catalytic N-Functionalized Antioxidants from Wood, ACS Sustainable Chem. Eng., 2023, 11, 4776–4788 CrossRef CAS.
- J. Ma, D. Le and N. Yan, Single-step conversion of wood lignin into phenolic amines, Chem, 2023, 9, 2869–2880 CAS.
- X. Wu, M. De Bruyn and K. Barta, Primary amines from lignocellulose by direct amination of alcohol intermediates, catalyzed by RANEY® Ni, Catal. Sci. Technol., 2022, 12, 5908–5916 RSC.
- K. Natte, A. Narani, V. Goyal, N. Sarki and R. V. Jagadeesh, Synthesis of Functional Chemicals from Lignin-derived Monomers by Selective Organic Transformations, Adv. Synth. Catal., 2020, 362, 5143–5169 CrossRef CAS.
- A. M. Afanasenko, N. Deak, J. October, R. Sole and K. Barta, Green’ synthesis of amines from renewable resources? A detailed analysis of case studies using the CHEM21 green metrics toolkit, Green Chem., 2025, 27, 5947–5981 RSC.
- Z. Sun, G. Bottari, A. Afanasenko, M. C. A. Stuart, P. J. Deuss, B. Fridrich and K. Barta, Complete lignocellulose conversion with integrated catalyst recycling yielding valuable aromatics and fuels, Nat. Catal., 2018, 1, 82–92 CrossRef CAS.
- W. G. Forsythe, M. D. Garrett, C. Hardacre, M. Nieuwenhuyzen and G. N. Sheldrake, An efficient and flexible synthesis of model lignin oligomers, Green Chem., 2013, 15, 3031 RSC.
- B. Zhang, T. Guo, Y. Liu, F. E. Kühn, C. Wang, Z. K. Zhao, J. Xiao, C. Li and T. Zhang, Sustainable Production of Benzylamines from Lignin, Angew. Chem., Int. Ed., 2021, 60, 20666–20671 CrossRef CAS.
- L. Jia, C.-J. Li and H. Zeng, Cleavage/cross-coupling strategy for converting β-O-4 linkage lignin model compounds into high valued benzyl amines via dual C–O bond cleavage, Chin. Chem. Lett., 2022, 33, 1519–1523 CrossRef CAS.
- B. Zhang, T. Guo, Z. Li, F. E. Kühn, M. Lei, Z. K. Zhao, J. Xiao, J. Zhang, D. Xu, T. Zhang and C. Li, Transition-metal-free synthesis of pyrimidines from lignin β-O-4 segments via a one-pot multi-component reaction, Nat. Commun., 2022, 13, 3365 CrossRef CAS.
- Y. Liu, Q. Luo, Q. Qiang, H. Wang, Y. Ding, C. Wang, J. Xiao, C. Li and T. Zhang, Successive Cleavage and Reconstruction of Lignin β-O-4 Models and Polymer to Access Quinoxalines, ChemSusChem, 2022, 15, e202201401 CrossRef CAS PubMed.
- P. Adhikari, A. Borah and A. Das, Chromium-catalysed selective synthesis of 3-oxo and 3-amino quinolines using β-O-4′ lignin models or α-amino ketones, Org. Chem. Front., 2024, 11, 5454–5461 RSC.
- X. Liu, H. Zhang, C. Wu, Z. Liu, Y. Chen, B. Yu and Z. Liu, Copper-catalyzed synthesis of benzanilides from lignin model substrates 2-phenoxyacetophenones under an air atmosphere, New J. Chem., 2018, 42, 1223–1227 RSC.
- J. Zhang, Y. Liu, S. Chiba and T.-P. Loh, Chemical conversion of β-O-4 lignin linkage models through Cu-catalyzed aerobic amide bond formation, Chem. Commun., 2013, 49, 11439 RSC.
- H. Li, M. Liu, H. Liu, N. Luo, C. Zhang and F. Wang, Amine-Mediated Bond Cleavage in Oxidized Lignin Models, ChemSusChem, 2020, 13, 4660–4665 CrossRef CAS PubMed.
- X. Liu, L. Wang, L. Zhai, C. Wu and H. Xu, H2 O2 -promoted C–C bond oxidative cleavage of β-O-4 lignin models to benzanilides using water as a solvent under metal-free conditions, Green Chem., 2022, 24, 4395–4398 RSC.
- J. Zhang, X. Lu, T. Li, S. Wang and G. Zhong, Copper-Catalyzed Oxidative Cyclization of 2-Amino-azaarenes with Lignin Models: Synthesis of 3-Phenoxy Imidazo Heterocycles, J. Org. Chem., 2017, 82, 5222–5229 CrossRef CAS.
- S. Luan, W. Wu, B. Zheng, Y. Wu, M. Dong, X. Shen, T. Wang, Z. Deng, B. Zhang, B. Chen, X. Xing, H. Wu, H. Liu and B. Han, Atomically dispersed cobalt catalysts for tandem synthesis of primary benzylamines from oxidized β-O-4 segments, Chem. Sci., 2024, 15, 10954–10962 RSC.
- H. Li, M. Wang, H. Liu, N. Luo, J. Lu, C. Zhang and F. Wang, NH2 OH–Mediated Lignin Conversion to Isoxazole and Nitrile, ACS Sustainable Chem. Eng., 2018, 6, 3748–3753 CrossRef CAS.
- Y. Wang, Y. Du, J. He and Y. Zhang, Transformation of lignin model compounds to N -substituted aromatics via Beckmann rearrangement, Green Chem., 2018, 20, 3318–3326 RSC.
- S. Ghahri, E. S. Wibowo and B.-D. Park, Crosslinking reactions of a model aminated lignin compound as a platform for building thermosetting polymers for lignin-based bio adhesives, Bioresour. Technol., 2024, 413, 131500 CrossRef CAS PubMed.
- Y. Wang, J. He and Y. Zhang, CeCl3 -Promoted Simultaneous Photocatalytic Cleavage and Amination of Cα –Cβ Bond in Lignin Model Compounds and Native Lignin, CCS Chem., 2020, 2, 107–117 CrossRef CAS.
- H. Pan, G. Sun and T. Zhao, Synthesis and characterization of aminated lignin, Int. J. Biol. Macromol., 2013, 59, 221–226 CrossRef CAS.
- J. C. Del Río, J. Rencoret, P. Prinsen, Á. T. Martínez, J. Ralph and A. Gutiérrez, Structural Characterization of Wheat Straw Lignin as Revealed by Analytical Pyrolysis, 2D-NMR, and Reductive Cleavage Methods, J. Agric. Food Chem., 2012, 60, 5922–5935 CrossRef.
- Z. Shi, G. Xu, J. Deng, M. Dong, V. Murugadoss, C. Liu, Q. Shao, S. Wu and Z. Guo, Structural characterization of lignin from D. sinicus by FTIR and NMR techniques, Green Chem. Lett. Rev., 2019, 12, 235–243 CrossRef CAS.
- T. Jayaramudu, K. Varaprasad, G. Adamus, J. Amber Jennings and J. D. Bumgardner, Mannich Reaction: Review of Amine-Functionalized Lignin Derivatives and Their Applications, ChemistrySelect, 2023, 8, e202204451 CrossRef CAS.
- X. Meng, S. Zhang, B. Scheidemantle, Y. Wang, Y. Pu, C. E. Wyman, C. M. Cai and A. J. Ragauskas, Preparation and characterization of aminated co-solvent enhanced lignocellulosic fractionation lignin as a renewable building block for the synthesis of non-isocyanate polyurethanes, Ind. Crops Prod., 2022, 178, 114579 CrossRef CAS.
- X. Du, J. Li and M. E. Lindström, Modification of industrial softwood kraft lignin using Mannich reaction with and without phenolation pretreatment, Ind. Crops Prod., 2014, 52, 729–735 CrossRef CAS.
- H. Mikawa, K. Sato, C. Takasaki and K. Ebisawa, Studies on the Cooking Mechanism of Wood. XV. Mannich Reaction on Lignin Model Compounds and the Estimation of the Amount of the Simple Guaiacyl Nucleus in Thiolignin, Bull. Chem. Soc. Jpn., 1956, 29, 259–265 CrossRef CAS.
- R. Brežny, L. Paszner, M. M. Micko and D. Uhrín, The Ion-Exchanging Lignin Derivatives Prepared by Mannich Reaction with Amino Acids, Holzforschung, 1988, 42, 369–373 CrossRef.
- Y. Pang, Z. Chen, R. Zhao, C. Yi, X. Qiu, Y. Qian and H. Lou, Facile synthesis of easily separated and reusable silver nanoparticles/aminated alkaline lignin composite and its catalytic ability, J. Colloid Interface Sci., 2021, 587, 334–346 CrossRef CAS.
- G.-J. Jiao, P. Peng, S.-L. Sun, Z.-C. Geng and D. She, Amination of biorefinery technical lignin by Mannich reaction for preparing highly efficient nitrogen fertilizer, Int. J. Biol. Macromol., 2019, 127, 544–554 CrossRef CAS PubMed.
- Z. Wang, A. Abbas, H. Sun, H. Jin, T. Jia, J. Liu and D. She, Amination-modified lignin recovery of aqueous phosphate for use as binary slow-release fertilizer, Int. J. Biol. Macromol., 2023, 242, 124862 CrossRef CAS PubMed.
- X. Wang, Y. Zhang, C. Hao, X. Dai, Z. Zhou and N. Si, Ultrasonic-assisted synthesis of aminated lignin by a Mannich reaction and its decolorizing properties for anionic azo-dyes, RSC Adv., 2014, 4, 28156 RSC.
- S. Ghahri and B.-D. Park, Amination and crosslinking of acetone-fractionated hardwood kraft lignin using different amines and aldehydes for sustainable bio-based wood adhesives, Bioresour. Technol., 2024, 399, 130645 CrossRef CAS PubMed.
- X. Yue, F. Chen and X. Zhou, Improved interfacial bonding of PVC/wood-flour composites by lignin amine modification, BioResources, 2011, 6, 2022–2034 CAS.
- T. Song, H. He, X. Li, X. Liu, Y. Lv, Y. Tao, J. Lu, J. Du, J. Hu and H. Wang, A green strategy to realize the high value utilization of lignin for hydrogel formation, Sci. Total Environ., 2024, 955, 176760 CrossRef CAS PubMed.
- X. Meng, S. Zhang, B. Scheidemantle, Y. Wang, Y. Pu, C. E. Wyman, C. M. Cai and A. J. Ragauskas, Preparation and characterization of aminated co-solvent enhanced lignocellulosic fractionation lignin as a renewable building block for the synthesis of non-isocyanate polyurethanes, Ind. Crops Prod., 2022, 178, 114579 CrossRef CAS.
- M. W. Ott, C. Dietz, S. Trosien, S. Mehlhase, M. J. Bitsch, M. Nau, T. Meckel, A. Geissler, G. Siegert, J. Huong, B. Hertel, R. W. Stark and M. Biesalski, Co-curing of epoxy resins with aminated lignins: insights into the role of lignin homo crosslinking during lignin amination on the elastic properties, Holzforschung, 2021, 75, 390–398 CrossRef CAS.
- Z. Liu, J. Zeng, Z. Dong, Y. Chen, H. Yang and H. Chen, Insight into the mechanism of lignin amination pretreatment on lignin structure and its pyrolysis property for lignin valorization, Chem. Eng. J., 2024, 499, 156386 CrossRef CAS.
- N. Ahadyani and M. Abdollahi, Phenolation, amination and cross-linking of lignin: synthesis and characterization of functionalized lignin, Polym. Bull., 2024, 81, 8643–8661 CrossRef CAS.
- P. Buono, A. Duval, P. Verge, L. Averous and Y. Habibi, New Insights on the Chemical Modification of Lignin: Acetylation versus Silylation, ACS Sustainable Chem. Eng., 2016, 4, 5212–5222 CrossRef CAS.
- M. Siahkamari, S. Emmanuel, D. B. Hodge and M. Nejad, Lignin-Glyoxal: A Fully Biobased Formaldehyde-Free Wood Adhesive for Interior Engineered Wood Products, ACS Sustainable Chem. Eng., 2022, 10, 3430–3441 CrossRef CAS.
- J. R. Gouveia, G. E. S. Garcia, L. D. Antonino, L. B. Tavares and D. J. Dos Santos, Epoxidation of Kraft Lignin as a Tool for Improving the Mechanical Properties of Epoxy Adhesive, Molecules, 2020, 25, 2513 CrossRef CAS.
- C. Gioia, M. Colonna, A. Tagami, L. Medina, O. Sevastyanova, L. A. Berglund and M. Lawoko, Lignin-Based Epoxy Resins: Unravelling the Relationship between Structure and Material Properties, Biomacromolecules, 2020, 21, 1920–1928 CrossRef CAS PubMed.
- F. Ferdosian, Z. Yuan, M. Anderson and C. Xu, Sustainable lignin-based epoxy resins cured with aromatic and aliphatic amine curing agents: Curing kinetics and thermal properties, Thermochim. Acta, 2015, 618, 48–55 CrossRef CAS.
- P. Ortiz, R. Vendamme and W. Eevers, Fully Biobased Epoxy Resins from Fatty Acids and Lignin, Molecules, 2020, 25, 1158 CrossRef CAS.
- A. Truncali, T. Laxminarayan, N. Rajagopalan, C. E. Weinell, S. Kiil and M. Johansson, Epoxidized technical Kraft lignin as a particulate resin component for high-performance anticorrosive coatings, J. Coat. Technol. Res., 2024, 21, 1875–1891 CrossRef CAS.
- M. W. Ott, C. Dietz, S. Trosien, S. Mehlhase, M. J. Bitsch, M. Nau, T. Meckel, A. Geissler, G. Siegert, J. Huong, B. Hertel, R. W. Stark and M. Biesalski, Co-curing of epoxy resins with aminated lignins: insights into the role of lignin homo crosslinking during lignin amination on the elastic properties, Holzforschung, 2021, 75, 390–398 CrossRef CAS.
- X. Lu and X. Gu, A review on lignin-based epoxy resins: Lignin effects on their synthesis and properties, Int. J. Biol. Macromol., 2023, 229, 778–790 CrossRef CAS PubMed.
- L. Qin, X. Li, J.-Q. Zhu, W.-C. Li, H. Xu, Q.-M. Guan, M.-T. Zhang, B.-Z. Li and Y.-J. Yuan, Optimization of ethylenediamine pretreatment and enzymatic hydrolysis to produce fermentable sugars from corn stover, Ind. Crops Prod., 2017, 102, 51–57 CrossRef CAS.
- L. Qin, W.-C. Li, J.-Q. Zhu, J.-N. Liang, B.-Z. Li and Y.-J. Yuan, Ethylenediamine pretreatment changes cellulose allomorph and lignin structure of lignocellulose at ambient pressure, Biotechnol. Biofuels, 2015, 8, 174 CrossRef PubMed.
- J.-Q. Zhu, X.-L. Wu, W.-C. Li, L. Qin, S. Chen, T. Xu, H. Liu, X. Zhou, X. Li, C. Zhong, B.-Z. Li and Y.-J. Yuan, Ethylenediamine pretreatment of corn stover facilitates high gravity fermentation with low enzyme loading, Bioresour. Technol., 2018, 267, 227–234 CrossRef CAS PubMed.
- S.-C. Liu, T. Shi, Z.-J. He, K. Chen, Z.-H. Liu, B.-Z. Li and Y.-J. Yuan, Ethylenediamine pretreatment simultaneously improved carbohydrate hydrolysis and lignin valorization, Bioresour. Technol., 2023, 386, 129552 CrossRef CAS PubMed.
- L. Xu, J. Zhang, Q.-J. Zong, L. Wang, T. Xu, J. Gong, Z.-H. Liu, B.-Z. Li and Y.-J. Yuan, High-solid ethylenediamine pretreatment to fractionate new lignin streams from lignocellulosic biomass, Chem. Eng. J., 2022, 427, 130962 CrossRef CAS.
- T. Shi, L. Xu, Y.-N. Wang, S.-C. Liu, Z.-H. Liu, G.-J. Zhao, B.-Z. Li and Y.-J. Yuan, Aminated and amidated structures introduced by ethylenediamine pretreatment endow lignin with bright fluorescence, Green Chem., 2022, 24, 9040–9054 RSC.
- H. Silau, A. Melas, K. Dam-Johansen and A. E. Daugaard, Bio-based amine curing agents prepared from lignin by ring-opening functionalization with cyclic aza-silanes and their curing kinetics investigated for aliphatic and aromatic epoxy species, Prog. Org. Coat., 2023, 184, 107822 CrossRef CAS.
- L. An, C. Si, J. H. Bae, H. Jeong and Y. S. Kim, One-step silanization and amination of lignin and its adsorption of Congo red and Cu(II) ions in aqueous solution, Int. J. Biol. Macromol., 2020, 159, 222–230 CrossRef CAS PubMed.
- J. W. Heo, L. An, J. Chen, J. H. Bae and Y. S. Kim, Preparation of amine-functionalized lignins for the selective adsorption of Methylene blue and Congo red, Chemosphere, 2022, 295, 133815 Search PubMed.
- R. Brežný, J. Schraml, M. Kvíčalová, J. Zelený and V. Chvalovský, Silicon-29 NMR Spectroscopy in Lignin Chemistry - Application to Trimethylsilylated Spruce Dioxane Lignin and Related Model Compounds, Holzforschung, 1985, 39, 297–303 CrossRef.
- R. Shorey, A. Gupta and T. H. Mekonnen, Hydrophobic modification of lignin for rubber composites, Ind. Crops Prod., 2021, 174, 114189 CrossRef CAS.
- L. Liu, X. Wan, S. Chen, P. Boonthamrongkit, M. Sipponen and S. Renneckar, Solventless Amination of Lignin and Natural Phenolics using 2-Oxazolidinone, ChemSusChem, 2023, 16, e202300276 Search PubMed.
- N. Wybo, A. Duval and L. Avérous, Benign and Selective Amination of Lignins towards Aromatic Biobased Building Blocks with Primary Amines, Angew. Chem., Int. Ed., 2024, 63, e202403806 CrossRef CAS.
- Y. Wang, S. Sun, F. Li, X. Cao and R. Sun, Production of vanillin from lignin: The relationship between β-O-4 linkages and vanillin yield, Ind. Crops Prod., 2018, 116, 116–121 CrossRef CAS.
- R. Rinaldi, R. Jastrzebski, M. T. Clough, J. Ralph, M. Kennema, P. C. A. Bruijnincx and B. M. Weckhuysen, Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis, Angew. Chem., Int. Ed., 2016, 55, 8164–8215 CrossRef CAS PubMed.
- M. M. Abu-Omar, K. Barta, G. T. Beckham, J. S. Luterbacher, J. Ralph, R. Rinaldi, Y. Román-Leshkov, J. S. M. Samec, B. F. Sels and F. Wang, Guidelines for performing lignin-first biorefining, Energy Environ. Sci., 2021, 14, 262–292 RSC.
| This journal is © The Royal Society of Chemistry 2025 |