Open Access Article
Open Access ArticleA review of construction and sustainable recycling strategies of lithium-ion batteries across electric vehicle platforms
Phuoc-Anh Le *
*
Institute of Chemistry, Vietnam Academy of Science and Technology, Hanoi 100000, Vietnam. E-mail: lephuocanh@ich.vast.vn
First published on 26th September 2025
Abstract
The rapid adoption of electric vehicles (EVs) hinges on addressing two critical challenges of lithium-ion batteries (LIBs): thermal safety risks and end-of-life sustainability. This review provides a systematic comparison of LIB integration across four EV architectures including battery electric (BEV), hybrid (HEV), plug-in hybrid (PHEV), and fuel cell electric vehicles (FCEV), with a dual focus on mitigating thermal runaway and advancing recycling technologies. Through analysis of recent previous studies, we reveal three key findings: (1) battery pack configurations and thermal management systems across platforms; (2) thermal runaway mechanisms and mitigation strategies through case studies of field failures; and (3) emerging recycling methods achieve material recovery with lower energy input, though industrial-scale implementation remains challenging. Our meta-analysis identifies hydrometallurgy as the most viable near-term solution for LIB recycling (80–95% metal recovery), while highlighting promising alternatives like electrochemical relithiation that preserve cathode crystal structure. The work further examines critical infrastructure gaps, demonstrating that renewable-powered charging and localized recycling networks could reduce EV lifecycle emissions by 30–40%. By bridging materials innovation with systems engineering, this review provides a roadmap for developing safer, more sustainable LIB ecosystems from cell design to second-life applications, and prioritizes research directions for next-generation batteries compatible with evolving EV architectures.
1 Introduction
The global reliance on fossil fuels and the environmental degradation caused by internal combustion engine vehicles have spurred intensive research into advanced battery systems for electric and hybrid vehicles.1–3 Lithium-ion batteries (LIBs) have emerged as the dominant energy storage solution, enabling EVs to reduce transportation-related CO2 emissions by up to 70% compared to conventional combustion engine vehicles.4 Beyond greenhouse gas reductions, EVs significantly decrease urban air pollutants such as nitrogen oxides (NOx) and particulate matter (PM2.5), contributing to improved air quality.5–9 Additionally, EVs exhibit a 3–5 dB reduction in traffic noise, mitigating noise pollution in densely populated areas.10,11 However, widespread EV adoption faces critical challenges, including limited energy density,12,13 supply chain vulnerabilities for key materials like lithium and cobalt,14,15 and persistent safety concerns related to thermal runaway.16Secondary batteries, specifically nickel–metal hydride and lithium-ion batteries, are widely recognized as the most technological advancement in rechargeable batteries. They have garnered a lot of attention and are currently the norm for many types of electric and hybrid cars.17,18 The need for next-generation rechargeable batteries with high power, high capacity, fast charging rate, long cycle life, significantly better safety performance, and reasonable cost has become the prevailing focus in the electric and hybrid vehicle industry.19 Additionally, it is anticipated that as electric car sales rise globally over the course of the forecast period, demand for electric vehicles equipped with rechargeable batteries will correspondingly rise.20 The technology for electric vehicles is constantly progressing to meet demands for high performance and power density. In the upcoming years, electric and hybrid cars are expected to extensively utilize lithium-ion batteries.21 The tendency is supported by the rise in fossil fuel prices and public awareness of EVs' benefits, which would ultimately encourage growth within the automotive application category.
Before LIBs—integrated EVs—dominated the commercial market, hybrid electric vehicles often used nickel–metal hydride battery generation to power the electric motor, sharing the load with the use of the internal combustion engine.22 These are, of course, hybrid cars that use both electric and internal combustion engines with the aim of lowering travel costs, lowering emissions, and reducing reliance on charging stations.23 Despite its notable reduction in greenhouse gas emissions, its inherently complex structure plays a role in gasoline engines, producing CO2 during high-load conditions. The widespread commercialization of LIBs starting in the 1990s has led to a significant drop in the cost of these batteries, setting the stage for devices using this generation of batteries to be more widely used.24,25 Until the expansion of hybrid cars using LIBs, the innovation of LIB has created the premise for radically revolutionizing the electric vehicle sector: replacing conventional combustion engines with highly efficient LIB-powered electric motors.26,27 Batteries are currently used in a wide range of applications, primarily consumer electronics. Their advantages also transfer quite well to the stationary energy storage and electric mobility industries, including electric cars, motorcycles, scooters, bicycles, and advanced wheelchairs.28,29 To claim that our lives today would be unimaginable without the use of batteries is not an exaggeration. Batteries are also considered a substitution for gasoline and other energy-related solutions, notably in the present automobile industry transformation.3,30 In the 2030s, battery-powered electric automobiles are anticipated to revolutionize the future of transportation.31
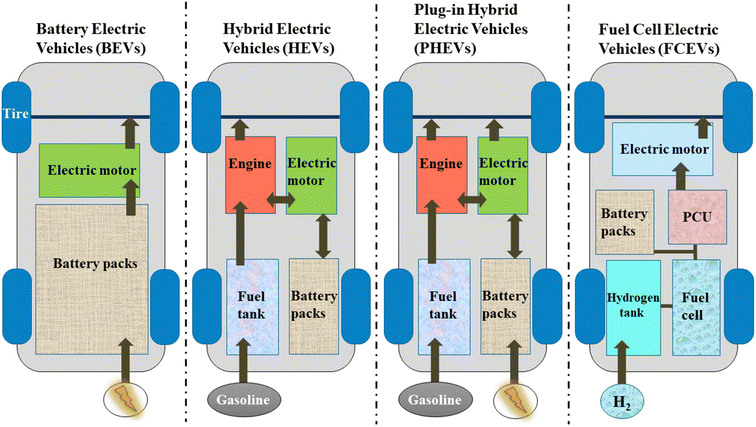
Although electric cars still only account for a small portion of the overall automobile industry, their attractiveness is continuing to grow as automakers adopt modern technology to enhance EVs' performance and characteristics. One of the best zero-emission vehicles today, electric cars come in a variety of unique designs and pricing points. Currently, electric car types have evolved and been able to be customized to different market demands. Different types of EVs, such as full-battery electric vehicles (BEVs) and their commercial variations, including hybrid electric vehicles (HEVs) and plug-in hybrid electric vehicles (PHEVs), in addition to another alternative fuel cell electric car (FCEV), have been extensively developed, appreciably reducing greenhouse gas emissions.16 Compared to internal combustion engines (ICEs), EVs are considered more environmentally friendly, have higher energy efficiency, and have lower maintenance, fuel, and operating costs. How is an electric vehicle operated? The type of electric vehicle determines how it operates. Future developments and a quick discussion on the various types of electric vehicles and their operation will be provided in this review.
This review aims to systematically analyze four commercially prevalent electric vehicle types—BEVs, HEVs, PHEVs, and FCEVs, which focuses on their lithium-ion battery architectures, safety challenges, and sustainability trade-offs. While prior studies have investigated individual EV categories in isolation, such as BEV energy efficiency optimization32–35 and lifecycle cost analyses,36–38 a comprehensive, cross-category evaluation of lithium-ion battery (LIB) integration strategies remains underdeveloped, particularly regarding two critical aspects: (i) comparative thermal runaway mitigation approaches across EV architectures, and (ii) standardized frameworks for end-of-life battery management. This fragmentation hinders systematic progress in EV safety and sustainability. To address this gap, we employ a three-tiered methodology: (1) a meta-analysis of recent experimental data (2015–2023) on LIB performance across EV types, (2) a critical evaluation of thermal management systems and their failure modes, and (3) an assessment of recycling techniques through the lens of scalability and environmental impact. By synthesizing these dimensions, this work provides a roadmap for optimizing EV safety and circular economy practices, while identifying urgent research priorities for next-generation LIBs, including sodium-ion batteries, metal–air batteries, and all solid-state batteries.
2 The discussion for four commercial types of electric vehicles
2.1 Electric vehicle revolution with lithium-ion battery
The lithium-ion battery story began in 1960 with several reports on a new generation of batteries using CuF2/lithium electrodes.39–42 Until the 1970s, the interesting introduction of a TiS2 electrode with high performance provided a promising future for commercialization at that time. However, it was limited by its high cost and hazardous generation of H2S when exposed to ambient air.43,44 A decade later, during the golden decades of the 1980s and 1990s, ground-breaking research, prolific patents, as well as the commercialization of lithium-ion batteries, were established. The introduction of two novel electrode materials, LiCoO2 and graphite, along with the formation of a solid-electrolyte interphase that offers stable operation at high temperatures, increases battery life, and lessens explosive creation, has become a game changer.45,46 The innovation led to the first commercialization of lithium-ion batteries in the 1990s, which fundamentally altered the portable electronics sector.Fig. 1a presents a chronology of the evolution of lithium batteries, highlighting significant events at each stage, from the time it first emerged as an attractive option to the point at which it dominated the market for manufacture, distribution, and consumption. Fig. 1b illustrates the quality and time spent collecting batteries from three large markets in Europe, Australia, and the United States in 2012, when lithium-ion batteries became more popular and appeared in various devices.47 The first generation of dry (low volumes of liquid) battery chemistries, such as lead-acid, zinc-carbon, and nickel–cadmium batteries, dominated much of the market.48–50 Following the success of first-generation batteries, second generations were developed and commercialized with greater storage capacity, longer cycle life, and greater power density. The state-of-the-art has contributed to the electronic technology revolution of the early 20th century. These included numerous elite battery families, such as rechargeable alkaline batteries, including nickel–iron (NiFe) and nickel metal hydride (NiMH).51,52 Nevertheless, rechargeable alkaline batteries have several challenges with energy density, recycling, flammability, thermal safety, and raising other environmental concerns.53 These issues must be resolved by the following generation.
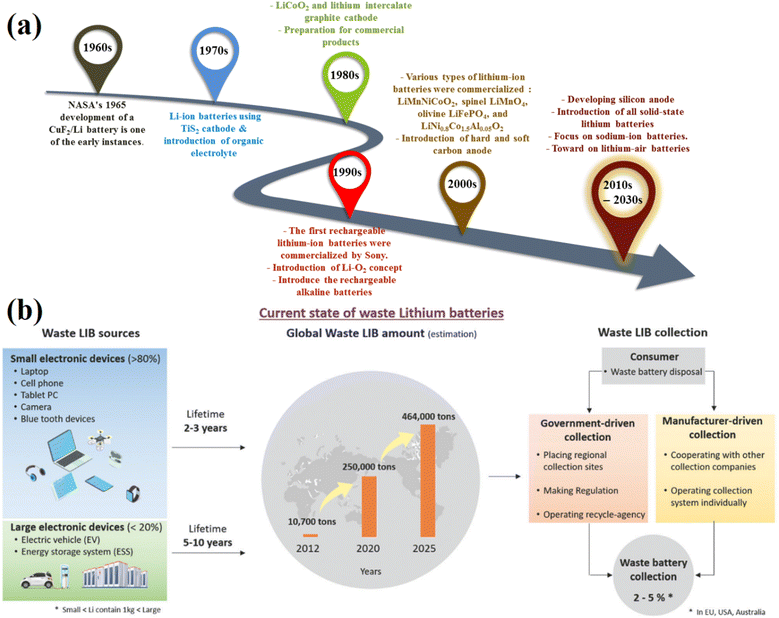 | ||
| Fig. 1 (a) A timeline of lithium-ion batteries following (b) source, quality and spent batteries collection. Figure has been reproduced from ref. 47 with permission from Royal Society of Chemistry, copyright 2021. | ||
Currently on the market, there are many types of lithium-ion batteries obtaining their unique advantages and disadvantages that are utilized for diverse electronic devices. Typical battery cathode and anode chemistry comprises lithium cobalt oxide (LCO), lithium nickel oxide (LNO), lithium manganese oxide (LMO), lithium nickel manganese cobalt oxide (NMC), lithium iron phosphate (LFP), lithium nickel cobalt aluminum oxide (NCA), and, graphite anodes, silicon-based anodes, and lithium titanate (LTO) anodes.54–58 Today, LIBs can be considered a widely commercial product. LIBs have gradually dominated the market, overwhelming the remaining generations owing to their outstanding performance. From tiny smart bracelets to truck-sized electric vehicles, LIBs have infiltrated our daily lives. Thereby, the dominance of LIBs will be long-lasting in the next decade, before the evolution of the next generation. However, this has triggered an unprecedented increase in demand for LIBs in terms of both quantity (number of cells) and quality (energy densities of cells). This leads to concerns regarding safety and the supply chain. Problems pertaining to thermal runaways and overmining critical mineral resources (such as copper, manganese, nickel, aluminum, cobalt, and zinc) are beginning to take center stage and can be arguably highlighted as some of the main hurdles for the research field to overcome. The increasing use and further development of LIBs have generated many consequences (resources and pollution) that need to be overcome and addressed as soon as possible. Thus, the next two parts will illustrate the brief of commercial lithium-ion batteries in electric vehicles in relation to the thermal runaway phenomenon and recycling.
2.2 Lithium-ion battery fabrication and package for electric vehicles
A commercial battery cell consists of the following main components: a cathode, an anode, and an electrolyte. Depending on the application, sub-components such as tabs, separators, current collectors, and housing cases can be varied in number and size. Lithium ions can be stored in cathode and anode layers during the discharging process, while electrolyte acts as a medium for lithium transportation (Fig. 2).59 Typically, in a standard lithium-ion battery, the working mechanism of a lithium-ion battery can be simply explained by the charge and discharge processes. Under the charging voltage, lithium ions are created on the battery's cathode layer and are moved to the anode layer via the electrolyte. In the anode layer, the electrode, mainly made in porous form, is generally composed of different carbon forms or silicon that allow for lithium to be absorbed or alloyed, accommodating a large number of lithium ions within the structure. With more lithium ions stored, the charging capacity rises. In the discharge process and by providing electric current, the anode releases lithium ions to the cathode, creating electron flows from one side to another. That is, each Li ion moves from the anode to the cathode in the battery, and in the external circuit, there is another electron moving from the cathode to the anode, generating current flows. This creates a charge balance between the two electrodes.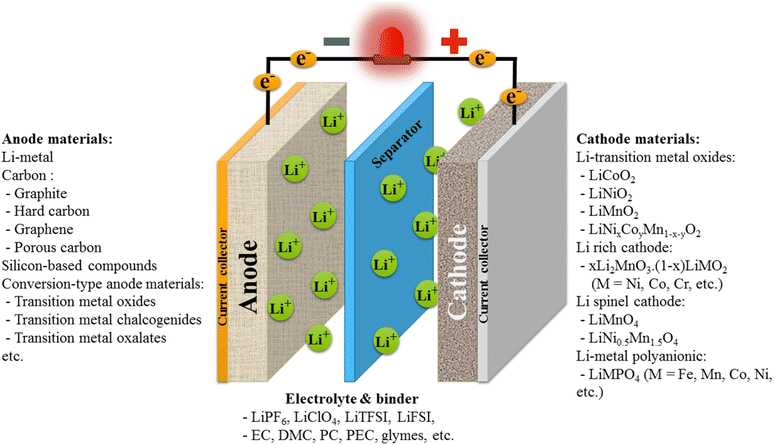 | ||
| Fig. 2 (a) General construction of one lithium-ion battery cell with intensively studied electrode materials. | ||
Different manufacturers utilize different batteries in their electric vehicles. The chemical reaction in each of their batteries varies somewhat, but they are all basically constructed using the same battery cells and modules. Battery modules are constructed from an enormous number of cells, with the nominal voltage falling between 3 and 3.8 volts. These cells are connected in series or parallel to generate the required voltage and current. Currently, the primary lithium-ion battery configurations are cylindrical, prismatic, and pouch cell types, with each geometry exhibiting unique trade-offs in energy density, thermal management, and manufacturability (Fig. 3).60–63 The cylindrical cell batteries are mostly well known due to their low manufacturing costs, robust mechanical structure, streamlining processing, and high packing ratio (jelly roll-to-can ratio). The cylinder's drawbacks, however, are limited in available capacity and heat dissipation. To improve the operating capacity, prismatic cells were widely developed to adapt to applications calling for higher capacity. Each manufacturer develops their own format because they are made in a variety of sizes and have a box-like shape. Prismatic cells, because of their natural flat, rectangular shape, can achieve excellent power while improving thermal dissipation by having a high surface area and an increasing number of tabs for each layer (for stacking design). On the other hand, gas generation can be fatal as it builds up high internal pressure, damaging the housing case and jelly roll structure. As a consequence, severe thermal runaway is the highest priority in designing and developing these high-energy cells. The complex manufacturing process and higher cost per GWh, coming from “dead space” inside the cell, challenge OEMs to optimize. The newly developed type of aluminum-sealed-pouch cell is more flexible for packaging while enhancing the packing ratio (cell-to-module). This offers the advantage of being portable and able to fit into the little space inside the device, but it is particularly vulnerable to external stress and heat generation. Thus, the swelling and flammability that result from prolonged charge–discharge durations are two important issues with pouch cells. In fact, thermal runaway represents a critical safety risk across all lithium-ion battery configurations including cylindrical, prismatic, and pouch cells which though manifestation varies by design.64,65 Cylindrical cells benefit from robust metal casings that may delay propagation, while prismatic cells' compact stacked electrodes and pouch cells' thin, flexible packaging accelerate heat transfer, increasing vulnerability to cascading failures.66–68 Common triggers like internal shorts, overcharging, or mechanical abuse initiate exothermic reactions in any format, but pouch cells exhibit particularly rapid thermal spread due to minimal thermal mass between layers, whereas cylindrical designs may localize damage through built-in venting mechanisms.69–73 These differences underscore that while no form factor is immune, understanding format-specific failure modes is essential for tailored safety solutions in battery systems.
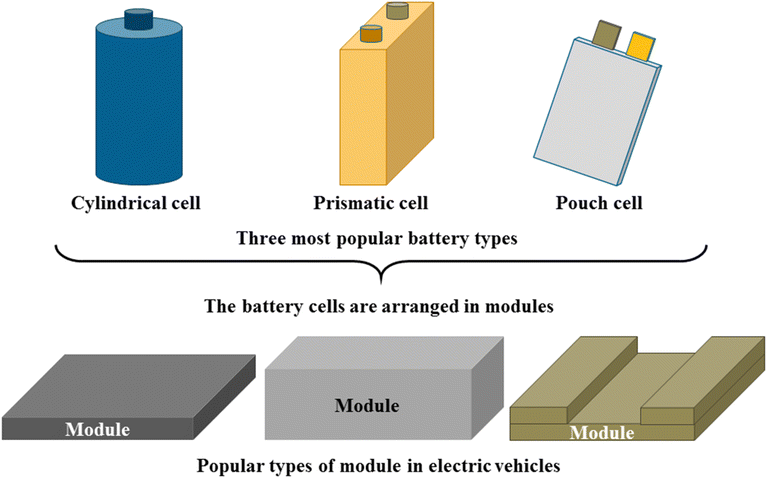 | ||
| Fig. 3 The three most popular electric vehicle battery modules are based on three different cell types. | ||
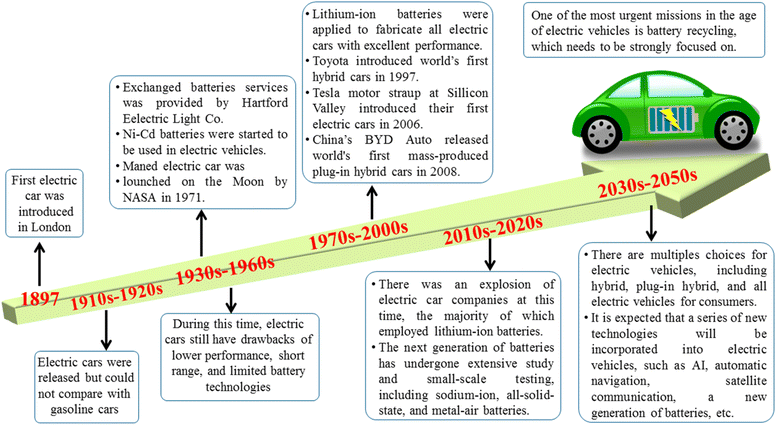
Fig. 4 presents a general timeline of the technological and commercial evolution of EVs from their experimental beginnings in the late 19th century to their projected market dominance in the mid-21st century. The earliest EVs presented in London during the 1890 s, utilizing nickel–cadmium (Ni–Cd) battery technology by Hartford Electric Light Company.74 However, these early efforts were ultimately overshadowed by the rapid development of inexpensive, mass-produced gasoline vehicles, combined with the establishment of extensive petroleum infrastructure, which relegated EVs to niche applications for most of the 20th century. The modern renaissance of electric mobility began with several key milestones: NASA's deployment of the electric Lunar Roving Vehicle during the Apollo 15 mission in 1971 demonstrated the viability of battery-powered transportation in extreme environments.74–76 The 1997 introduction of Toyota's Prius, the first mass-produced hybrid electric vehicle, marked a turning point in consumer acceptance of electrified powertrains. The subsequent decade witnessed critical technological breakthroughs, particularly Tesla Motors' 2006 Roadster, which proved lithium-ion batteries could deliver both performance and range, and BYD Auto's 2008 F3DM, the world's first mass-produced plug-in hybrid electric vehicle. Since 2010, multiple converging factors have driven unprecedented growth in EV adoption. Increasing environmental regulations, volatile fossil fuel prices, and dramatic improvements in lithium-ion battery technology (with energy densities increasing by 5–8% annually while costs decreased by approximately 80% between 2010 and 2020) have transformed the automotive landscape.77–79 Current research focuses on next-generation battery chemistries including sodium-ion, all-solid-state, and metal–air configurations that promise further improvements in safety, energy density, and sustainability. Market projections indicate this growth trajectory will continue, with EVs expected to comprise 20% of global new car sales by 2025, increasing to 40% by 2030. However, complete market penetration faces challenges including the slow turnover of existing vehicle fleets – analysts estimate that even with 100% of new car sales being electric by 2040, approximately half of vehicles on the road may still use conventional powertrains due to the 10–15 years average lifespan of automobiles. This underscores the need for continued advancements in battery technology, expansion of charging infrastructure, and supportive policy frameworks to achieve full transportation electrification.
2.3 Introduction of four top commercial electric vehicles
Electric cars have gradually gained the trust of consumers with their affordable price, good performance, and reliable operation based on the recent rapid development of battery manufacturing technology. This is further supported by ground-breaking advancements in information technology, semiconductor technology, and telecommunications technology. In actuality, consumers' willingness to own electric cars is being driven by the expansion of available electric car models and the falling cost of batteries. There are currently four main categories of electric vehicles on the market: battery electric vehicles (BEVs), hybrid electric vehicles (HEVs), plug-in hybrid electric vehicles (PHEVs), and fuel cell electric vehicles (FCEVs) (Fig. 5).80–83EV manufacturers each pursue customized lithium-ion battery solutions balancing cost, performance, and longevity. Current industry efforts focus on reducing production costs while maintaining battery capacity and lifespan. Table 1 compares five commercial EV battery types, highlighting their key properties. Among these, LFP (lithium iron phosphate) batteries offer distinct advantages: (i) superior thermal stability and safety from low resistance, and (ii) lower costs by avoiding expensive cobalt, nickel, and manganese in their phosphate-based cathodes.
| Battery types | LCO | LMO | NMC | LFP | NCA |
|---|---|---|---|---|---|
| Cathode/Anode | LiCoO2/graphite | LiMn2O4/graphite | LiNiMnCoO2/graphite | LiFePO4/graphite | LiNiCoAlO2/graphite |
| Commercial time | 1991 | 1996 | 2008 | 1996 | 1999 |
| Voltage, V | 3.0–4.2 | 3.0–4.2 | 3.0–4.2 | 2.5–3.65 | 3.0–4.2 |
| Energy density, Wh kg−1 | 150–200 | 100–150 | 150–220 | 90–120 | 200–260 |
| Charge (C-rate) | 0.7–1 C. Charge current above 1 C shortens battery life | 0.7–1 C with maximum until 3 C, and charges to 4.2 V | Only from 0.7 to 1 C | 1 C | ∼0.7 C |
| Discharge (C-rate) | 1 C. Discharge current above 1 C shortens battery life | 1 C with maximum until 10 C for special versions | 1 C | 1 C | 1 C typical |
| Cycle life, cycles | 500–1000 | 300–700 | 1000–2000 | 2000 | 500 |
| Thermal runaway, °C | 150 | 250 | 210 | 270 | 150 |
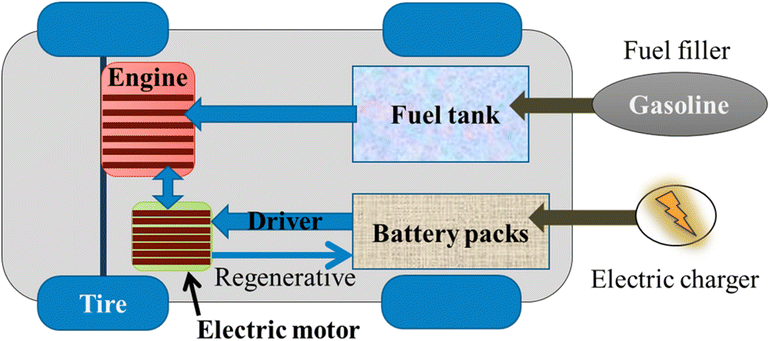
Despite the use of ICE, HEV resembles the same challenge as BEV when the system highlights the complex structure of different temperature control loops to balance temperature operation between the ICE, electric motor, power electronics, batteries, and cabin. The cost of replacements is high, but the lifespan of these batteries is quite long. The goal of eliminating fossil fuels in the future will lead to the replacement of hybrid electric vehicles by all battery electric vehicles, despite the manufacturers constructing newer hybrid cars with battery packs neatly stowed and saving space.
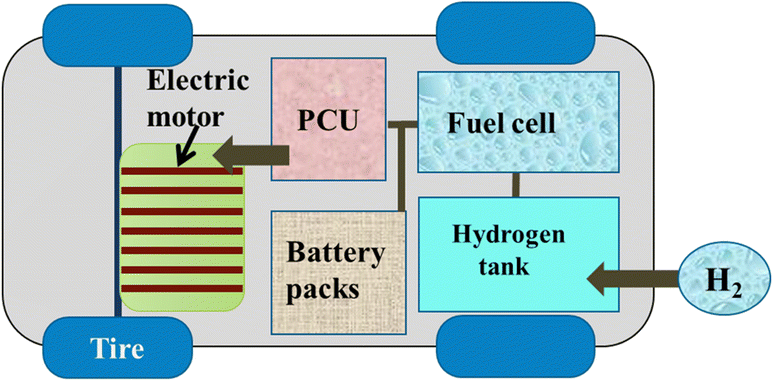
Hydrogen, the energy fuel of FCEVs, can be produced through multiple methods, categorized as fossil fuel-based, such as steam reforming or partial oxidation or renewable-based (electrolysis, biomass conversion).96 While steam methane reforming remains the dominant method (50% of global production), its high CO emissions limit sustainability.96 Renewable methods are increasingly attractive due to lower emissions and abundant feedstock availability. As the hydrogen economy expands, these green production methods will be crucial for enabling sustainable fuel cell electric vehicles and reducing fossil fuel dependence.
 | ||
| Fig. 10 An example of a hybrid thermal management system concept for a battery electric car that combines passive and active cooling systems with an emphasis on the battery pack. Figure has been reproduced from ref. 111 with permission of Elsevier, copyright 2018. | ||
2.4 Battery thermal runaway phenomenon in battery electric vehicles
Safety remains one of the most critical challenges hindering the widespread adoption of LIBs in EVs. As energy densities continue to increase following the increase of battery module, ensuring LIB safety becomes increasingly imperative. The primary safety concern involves thermal runaway – a potentially catastrophic failure mode resulting from sequential, thermally-activated degradation processes of battery components through chain reactions.112 This section examines the underlying chemical mechanisms driving thermal runaway in commercial lithium-ion battery systems, with the aim of providing a comprehensive analysis of this critical safety phenomenon and its mitigation strategies. Following the timeline from 2020 to mid-2025, there have been thousands of electric vehicle fires and explosions worldwide, with the largest number occurring in the two largest electric vehicle markets, China and the United States.113–116 However, the exact number in specific accident situations in the world is difficult to determine due to the confidentiality of electric vehicle manufacturers and political factors. It can be seen that the number of electric vehicle fire and explosion accidents is lower than that of gasoline vehicles, however, with the remarkable growth of electric vehicles in the coming years, the safety of electric vehicles when operating and charging has raised certain concerns from consumers. Next, the thermal runaway phenomenon is discussed as the suspected cause of the explosion of the battery pack, causing the entire electric vehicle to catch fire.Lithium-ion batteries typically maintain a stable structure due to the reversible movement of lithium ions between the cathode and anode during standard charging and discharging cycles. However, in mechatronic systems employing large lithium-ion battery arrays, such as electric vehicles, various operational factors can initiate thermal runaway in the battery system. When the battery cells have a thermal runaway phenomenon, it can be extremely difficult to stop the chain reaction from continuing. This phenomenon occurs when the internal temperature rises to a point of chemical reaction appearance, increasing temperature in the overall cell, which in turn triggers other chemical reactions that release even more heat. The battery cell temperature increases exponentially within milliseconds as stored energy is abruptly released under abusive conditions. This uncontrolled exothermic process generates sequential chain reactions capable of reaching temperatures exceeding 400 °C, with potential escalation to 1000 °C. At these elevated temperatures, the cell typically undergoes violent venting of flammable electrolytes, creating self-sustaining fires that resist conventional suppression methods. Typically, the thermal runaway can be divided into four stages: (i) from 80 to 120 °C: as the temperature increases, electrolyte and solid electrolyte interphase (SEI) components start to decompose. The newly exposed electrode particle area promotes further reactions with electrolyte, losing lithium inventory and creating more SEI that increases the resistance of the battery; (ii) over 140 °C: the exothermic reactions of cathode materials with releasing oxygen; (iii) over 180 °C: the decomposition of both cathode and electrolyte layers; and (iv) the batteries are completely destroyed.117–119 In the actual use of electric vehicles, many factors have been recorded that lead to the phenomenon of thermal runaway. In general, there are three main factors classified as follows: (1) mechanical abuse (physical damage by crash, puncture, penetration, collision, etc.); (2) electrical abuse (overcharge/discharge, internal short circuit, etc.); and (3) thermal abuse (overheating, flame attack, etc.).120–123
What might be the initial cause of thermal runaway? The internal process of thermal runaway is primarily brought on by a series of exothermic chain reactions. In other words, because the heat generated by the side reactions in the previous stage cannot be completely dissipated in time, the battery temperature will increase. As a result, the temperature increase may start a subsequent round of adverse reactions, finally resulting in thermal runaway. Studies have demonstrated the side reactions that occur during the thermal runaway of lithium-ion batteries, such as the SEI decomposition, anode-electrolyte reaction, separator melting, electrolyte decomposition, cathode decomposition (dissolution of transition metal, lithium plating/dendrite causing short circuit, oxygen release), short circuit, and electrolyte burning (Fig. 11a).124,125 For detail, Fig. 11b shows the typical differential scanning calorimetry (DSC) findings of the lithium-ion battery's component materials. The heat release power (Q), the enthalpy (ΔH), which reflects the total heat released, and the distinctive temperatures, such as the onset temperature (Tonset), the peak temperature (Tpeak), and the terminal temperature (Tend), are among the features of the processes.124,126 The heat release for typical battery materials has been outlined, and it has been discovered that the beginning temperature for commercial cathode materials is as follows: LiFePO4 (LFP) > Li4Ti5O12 (LTO) > LiNixCoyMnzO2 (NCM) > LiNixCoyAlzO2 (NCA) > LiCO2 (LCO).127 The energy release diagram also shows where the heat from internal short circuits (ISC) or combustion is released, although the displays for these chemical reactions are different. The separator's collapse temperature and the ISC's onset temperature are closely connected. For the polyethylene (PE) separator, the ISC can happen at around 130 °C, for the polypropylene (PP) or PP/PE/PP separator at 170 °C, and for the ceramic-coated separator around 200 °C.124 Herein, the first breakdown of SEI is thought to be the first side reaction that happens during the entire thermal runaway process. The early disintegration of SEI takes place between 80 and 120 °C, with a peak at about 100 °C.128,129 Once the SEI decomposes at a high temperature, the intercalated lithium-ions in the graphite anode can come into contact with the electrolyte. This leads a greater surface reaction between the lithiated graphite and organic compounds/transition metals/acidic attack, forming a new SEI.129 Thus, due to the nature of electrolytes, a series of undesirable reactions between electrodes and electrolyte are immensely detrimental to thermal runaway. In particular, the electrolytes consist of a blend of different carbonates and are primarily composed of flammable linear carbonates such as combinations of diethyl carbonate (DEC), dimethyl carbonate (DMC), ethyl methyl carbonate (EMC), and ethylene carbonate (EC). Although linear carbonates provide high ionic conductivity, decreasing the electrolyte viscosity corresponds to better ion uptake into the polyolefin-based separator and creating a stable solid electrolyte interface layer on the graphite electrode surface.130 Their low flash points lead to a higher combustion speed and heat release rate. Additionally, the addition of lithium salt does not greatly change the mixture's flash point, even while it lowers the vapor pressure and affects the period of combustion. The exposure of lithium-ion batteries to air during the thermal runaway process can result in dangerous flammability and toxic gas release.
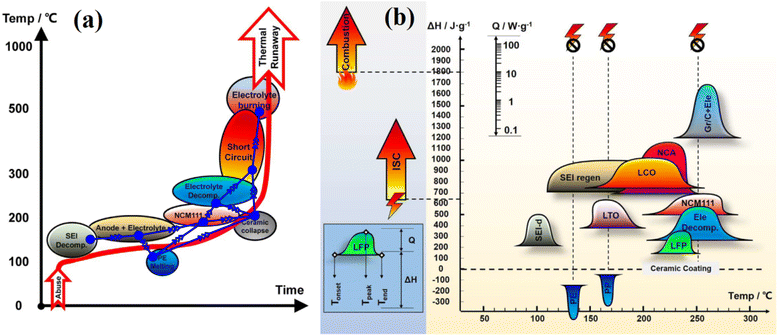 | ||
| Fig. 11 (a) The quantitative chain reactions in thermal runaway, and (b) The typical differential scanning calorimetry (DSC) findings of the lithium-ion battery's component materials. Figure has been reproduced from ref. 124 with permission from Elsevier, copyright 2018. | ||
In detail, the tracking and control of thermal runaway require a deep understanding of the mechanism as well as the physical and chemical processes during the four stages. Recently, X. Feng et al. provided a time sequence map (TSM) to point out the detailed thermal runaway mechanism for LIBs.132 The idea of a thermodynamic system aids in classifying various physical and chemical processes in terms of the places in which they take place. The two-path pattern in Fig. 12 may be used to redraft the TSM since the physical and/or chemical processes can take place both within and outside the battery cell.131 The two-path pattern for deciphering the thermal runaway process of LIBs is shown in Fig. 12a. The OUT path symbolizes the smoke, fire, or explosion that occurs outside the cell, parallel to the IN path, which represents the heat failure caused by chemical reactions inside the cell. Fig. 12b shows more detail of the two-path pattern based on characteristic temperature and thermal-physical temperature. Up to thermal runaway, the IN route depicts the evolution of thermal failure within the cell case. The sequence of vent, smoke, and fire seen outside the cell case is depicted in the OUT route and is explained by the fire triangle. In summary, a time sequence map-based thermal runaway mechanism will show both specific internal triggers and events occurring outside the battery cell. Numerous studies concentrate on early warning of the thermal runaway threat to increase the safety of LIBs. The following categories can be used to categorize the monitoring techniques: (i) abnormal phenomenon monitoring of batteries in the early stage of thermal runaway, such as characterizing gas generation and internal/external force; (ii) early warning based on battery internal electrical characteristics; and (iii) temperature monitoring via the battery management system (BMS).123,133,134 A combination method with external characteristics (voltage, current, temperature, and capacity) and internal mechanisms (electrochemical reaction and degradation of the material) should be developed for evaluating the electrochemical-thermal coupling properties of LIBs during the evolution of the thermal runaway phenomenon in order to establish an accurate and widely applicable thermal runaway prediction and early warning method. Additionally, in order to obtain a quantitative evaluation and forecast of the thermal runaway risk of LIBs, safety limits should be built based on electrochemical characteristics.
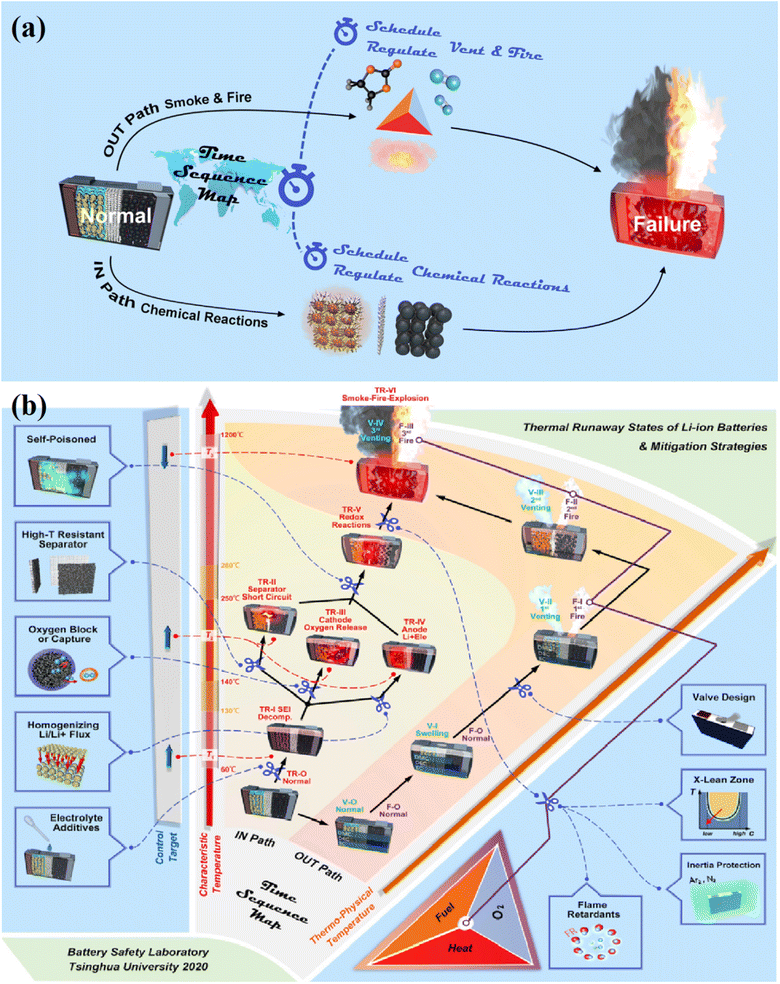 | ||
| Fig. 12 LIB cell thermal runaway states and related mitigation techniques: (a) a description of the thermal runaway phenomena in the batteries and the concept of time sequence regulation, and (b) time sequence map based on characteristics temperature and thermal physical temperature. Figure has been reproduced from ref. 131 with permission from Elsevier, copyright 2020. | ||
Actually, during the thermal runaway phenomenon, the creation and conversion processes between the solid, liquid, and gas phases combine to cause the gas venting of lithium-ion battery cells. This is really the outcome of both chemical reactions and physical transformations. In response to inquiries regarding the reasons behind the jet flow's accompanying droplet and particle emissions during gas venting and the changes that battery materials suffer under thermal abuse, Wang et al. presented a model that demonstrates the transition process and mechanism for different stages during thermal runaway.135 The following three regions, which correspond to different processes, are depicted in Fig. 13a: region 1 is the jelly roll, region 2 is the phase interface and headspace, and region 3 is the ambient fluid.135–140 Moreover, apart from the produced gases and electrolyte vapors, a significant emission component of thermal runaway processes is the solid particle. Fig. 13b reports a schematic picture based on X-ray imaging of the thermal runaway cell, which explains how components of the electrode material are expelled from the jelly roll and are subsequently discharged as particles, which is depicted by four consecutive steps to the entire process.135,139,141,142
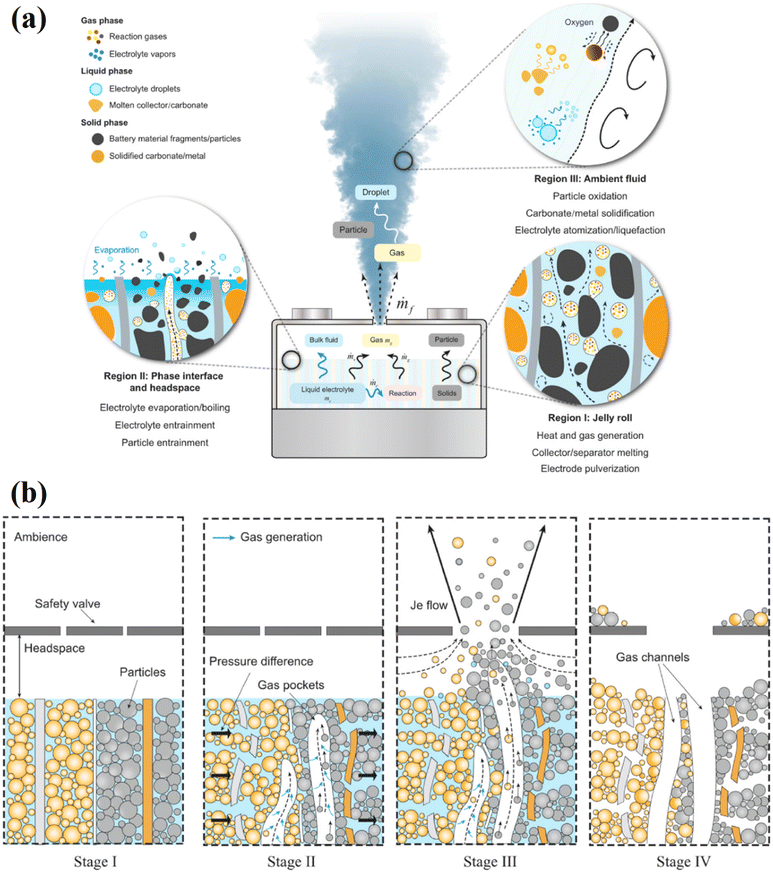 | ||
| Fig. 13 (a) Diagram illustrating the mechanisms and change processes for the three main stages of thermal runaway, and (b) Particle release mechanism from a standard lithium-ion battery cell during thermal runaway. Figure has been reproduced from ref. 135 with permission from Elsevier, copyright 2023. | ||
Recently, J. Kim et al. provided a detailed thermal runaway phenomenon of an 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 lithium-ion battery cell under the temperature evolution process (Fig. 14).143 To investigate the cell venting, internal pressure, and gas-phase dynamics behavior of 18
650 lithium-ion battery cell under the temperature evolution process (Fig. 14).143 To investigate the cell venting, internal pressure, and gas-phase dynamics behavior of 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 Li-ion cells experiencing thermal runaway, a computational model is created. The turbulent flow out of the cells is characterized by a k-ε Reynolds-averaged Navier–Stokes model, while the fluid dynamics inside the cells are described by Darcy–Forchheimer's equation. A single-step lumped reaction model describes the kinetics of gas production and thermal abuse reactions. Then, a sequence of computational fluid dynamics simulations is carried out on a solitary 18
650 Li-ion cells experiencing thermal runaway, a computational model is created. The turbulent flow out of the cells is characterized by a k-ε Reynolds-averaged Navier–Stokes model, while the fluid dynamics inside the cells are described by Darcy–Forchheimer's equation. A single-step lumped reaction model describes the kinetics of gas production and thermal abuse reactions. Then, a sequence of computational fluid dynamics simulations is carried out on a solitary 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 cell at different levels of charge at 100%, 50%, and 25% in order to examine intricate flow and temperature characteristics in relation to the amount of gas produced during cell venting. It has been discovered that the second stage dominates the cell response because thermal runaway produces the majority of the gases. State-of-the-art technology also has a big impact on the tendency for propagation. Due to larger reacting gas masses and concentrations, cells with higher state charges generate more heat and gas during the venting event. As a result, interior cell pressures rise, raising the possibility of sidewall breaching.
650 cell at different levels of charge at 100%, 50%, and 25% in order to examine intricate flow and temperature characteristics in relation to the amount of gas produced during cell venting. It has been discovered that the second stage dominates the cell response because thermal runaway produces the majority of the gases. State-of-the-art technology also has a big impact on the tendency for propagation. Due to larger reacting gas masses and concentrations, cells with higher state charges generate more heat and gas during the venting event. As a result, interior cell pressures rise, raising the possibility of sidewall breaching.
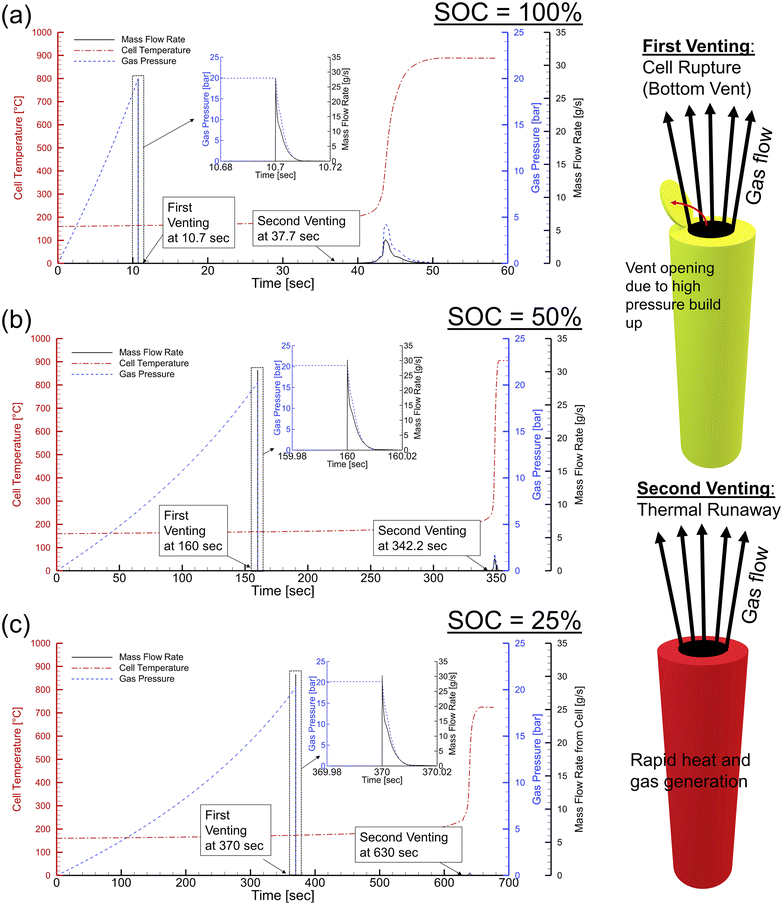 | ||
Fig. 14 The 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650 battery cell under the temperature evolution of thermal abuse leading to gas pressure and mass flow rate at various states of charge (SOC): (a) SOC = 25%, (b) SOC = 50%, and (c) SOC = 75%. Figure has been reproduced from ref. 143 with permission from Elsevier, copyright 2021. 650 battery cell under the temperature evolution of thermal abuse leading to gas pressure and mass flow rate at various states of charge (SOC): (a) SOC = 25%, (b) SOC = 50%, and (c) SOC = 75%. Figure has been reproduced from ref. 143 with permission from Elsevier, copyright 2021. | ||
Recent advancements in thermal runaway early warning have shifted toward monitoring external characteristics that precede catastrophic failure, enabling timely intervention. Novel warning and assessment features, including force, gas, sound, optical signals, and impedance changes which have emerged as critical indicators.144,145 For instance, gas sensors identify volatile organic compounds (e.g., CO, CO2, HF, CH4) released during early-stage thermal runaway.146–148 Acoustic emission techniques capture ultrasonic waves generated by microstructural damage, such as separator cracking or electrode delamination.134,149,150 Optical methods, including infrared thermography and fiber optic sensor, provide real-time temperature and strain mapping with high spatial resolution.151–154 Additionally, impedance spectroscopy tracks abrupt changes in internal resistance due to solid-electrolyte interphase (SEI) breakdown or short-circuit formation.155–158 By integrating these multi-modal signals with machine learning algorithms, modern battery management systems (BMS) can achieve early thermal runaway prediction with over 90% accuracy, significantly improving safety margins for lithium-ion batteries in electric vehicles and energy storage systems.159,160
The rapid growth of EVs has been accompanied by a growing number of high-profile fire incidents, highlighting critical safety challenges in lithium-ion battery systems. Studies of these incidents demonstrate that thermal runaway often initiates at localized hotspots, frequently near cell edges in pouch cells, welding points, or overcharge/discharge within single cells, where microscopic defects (internal short circuits, dendrites, and electrode misalignment) create high-resistance zones.113,121,161 Once triggered, exothermic reactions decompose the electrolyte and cathode materials, accelerating temperatures to 800 °C within seconds. Compounding these risks, thermal runaway from pack-to-pack propagation is spread by insufficient thermal barriers, leading to fire in the battery module. These real failures show a critical gap between standardized lab tests such as nail penetration and actual accident conditions, where multi-factors, including vibration, weather, high SoC, aging, parking space, and geography, interact unpredictably.162–166
2.5 Recycling lithium-ion batteries from electric vehicles
Another critical factor for the widespread adoption of EVs is establishing a circular material economy to ensure that waste from spent lithium-ion batteries does not undermine the environmental benefits. Recycling electric vehicle batteries is not a simple goal and requires managing the entire life cycle, which means coordinating from production and operational management to collection and recycling after use. In fact, lithium-ion batteries used for electric vehicles are very diverse in type, configuration, and generation. Each type of battery has specific requirements and characteristics related to the recycling process, along with strict regulations aimed at maximizing the use of recycled materials and ensuring high standards of environmental protection. In this section, the most general overview is provided to introduce the most realistic and concise view possible to help readers gain certain knowledge and identify current trends. | ||
| Fig. 15 Battery life time prediction based on actually operating data. Figure has been reproduced from ref. 187 with permission Elsevier, copyright 2025. | ||
Accurate state-of-charge (SOC) estimation is crucial in determining the remaining energy capacity of LiBs before they enter the recycling process.188 SOC reflects the available charge relative to the maximum capacity of battery, providing insights into its usability, degradation level, and potential for battery second-life applications.188–190 For recycling decisions, evaluating the SOC helps assess whether a battery has reached its end-of-life or still retains sufficient capacity for repurposing in less demanding applications, such as energy storage systems.191–193 Batteries with high residual capacity may be diverted to secondary markets, reducing waste and improving resource efficiency, while deeply degraded cells with low SOC are more likely to undergo material recovery via recycling process. Thus, SOC estimation serves as a key parameter in the decision-making process, ensuring economically and environmentally optimized battery disposal or reuse. The importance of SOC estimation extends beyond operational performance which can play a pivotal role in sustainable battery lifecycle management. Precise SOC assessment prevents premature recycling of batteries that could still deliver value in secondary applications, thereby extending their service life and reducing environmental impact.194 Additionally, knowing the exact SOC ensures safe handling during recycling, as partially charged batteries may pose thermal runaway risks if improperly dismantled. From a resource recovery perspective, SOC data aids recyclers in optimizing processes such as discharge protocols and material extraction, improving efficiency and cost-effectiveness.195 With the strong growing of EVs, integrating reliable SOC estimation methods into recycling frameworks will be essential for maximizing resource utilization and advancing circular economy principles in the LiB industry.194–197 There are many studies on SOC to provide the most optimal and complete solutions with the ambition to optimize battery performance and help improve durability. To enhance the accuracy and robustness of state-of-charge (SOC) estimation in lithium-ion batteries (LiBs), Shi et al. proposed an Improved Adaptive Square Root Cubature Kalman Filter (IASRCKF) method, which dynamically adjusts the window length of noise statistics based on error innovation sequences.198 The study first identified battery model parameters using an Adaptive Forgetting Factor Bias Compensation Recursive Least Squares (AFFBCRLS) method (Fig. 16a), achieving a voltage estimation RMSE of 0.004 V. The IASRCKF demonstrated superior performance over conventional ASRCKF, reducing SOC estimation errors to 0.18% RMSE and 0.15% MAE while maintaining rapid convergence under varying initial SOC and noise conditions (Fig. 16b). Despite its high accuracy, the method's reliance on empirical parameter tuning and computationally intensive matrix operations may limit its practicality for low-cost hardware. The authors suggest future work on parameter optimization and aging-aware algorithms to improve efficiency and applicability. This work highlights the potential of adaptive filtering techniques for precise SOC estimation, which is critical for battery health assessment prior to recycling decisions.
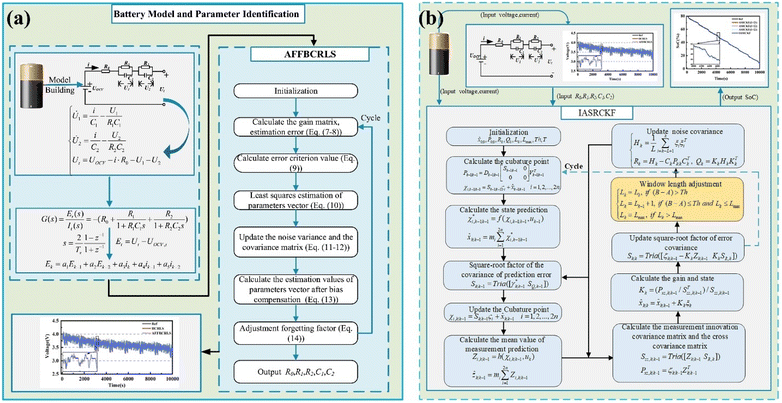 | ||
| Fig. 16 The flowchart of (a) AFFBCRLS approach, and (b) IASRCKF method. Figure has been reproduced from ref. 198 with permission of Elsevier, copyright 2023. | ||
Recently, Wang et al. presented a hybrid Radial Basis Correction-Differential Support Vector Machine (RBC-DSVM) model combined with a Limited Memory Recursive Least Squares (LMRLS) algorithm to achieve high-precision SOC estimation for LiBs in urban electric vehicles.199 The study addresses the challenges of nonlinear SOC dynamics and time-varying battery parameters by integrating three key innovations: (i) an LMRLS-based online parameter identification method for the first-order Thevenin equivalent circuit model (FOT-ECM), which adaptively tracks ohmic resistance (R0), polarization resistance (RP), and capacitance (CP) with a maximum voltage error of 0.064 V—outperforming conventional RLS in dynamic conditions (Fig. 17a); (ii) an adaptive RBC-DSVM model that iteratively corrects SOC errors by fusing Ah integration and AEKF inputs, leveraging a radial basis kernel function to map nonlinear relationships between voltage, current, and SOC (Fig. 17b); (iii) experimental validation under Hybrid Pulse Power Characterization (HPPC) and Dynamic Stress Test (DST) conditions, demonstrating remarkable accuracy with maximum SOC errors of 0.037% (HPPC) and 0.336% (DST), representing 89.78% and 6.15% improvements over AEKF, respectively. The RBC-DSVM's robustness is further evidenced by its stable error convergence under varying initial SOC values and measurement noise. However, the authors note limitations, including computational complexity due to matrix operations, empirical tuning of hyperparameters (e.g., memory length L), and unvalidated performance under real-world noise and temperature fluctuations. Future work aims to optimize computational efficiency and incorporate aging effects. This study advances BMS technologies by bridging model-driven and data-driven approaches, offering a scalable framework for real-time SOC estimation in EV applications.
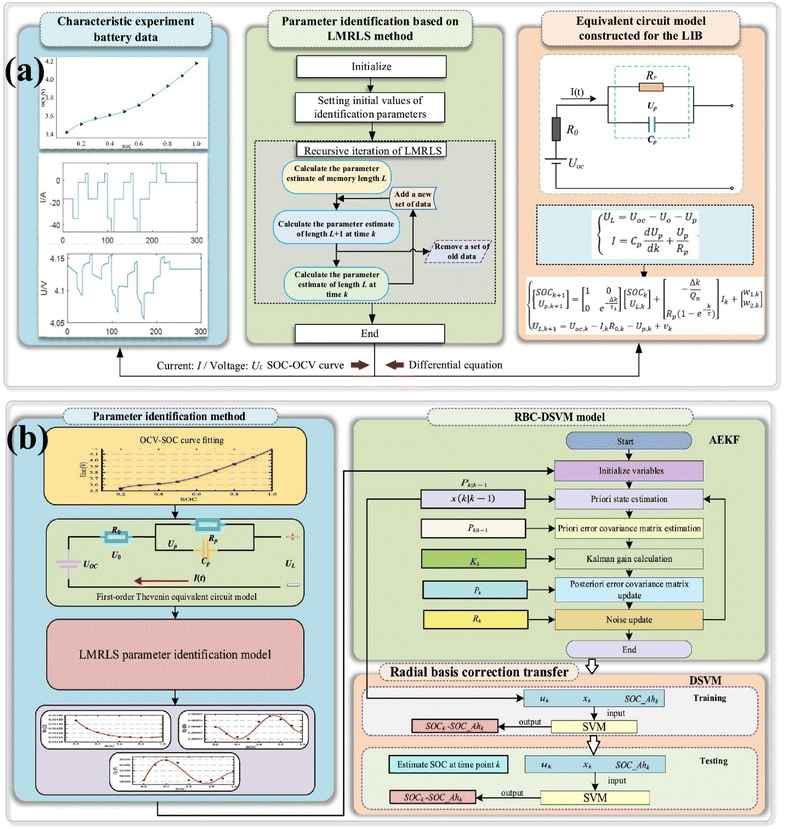 | ||
| Fig. 17 Flowchart of (a) adaptive online parameter identification method and (b) RBC-DSVM model. Figure adapted from Wang et al.199 Copyright 2024 Elsevier. | ||
In summary, the modern SOC estimation methods leverage advanced algorithms, such as adaptive Kalman filters, machine learning models, and hybrid data-driven approaches, to address challenges like nonlinear battery dynamics, noise interference, and real-time computational constraints. However, challenges remain in computational efficiency, real-world noise resilience, and aging-aware modeling. Future advancements aim to integrate these techniques with battery recycling frameworks, ensuring accurate SOC assessment for end-of-life repurposing or material recovery, thereby supporting a sustainable EV ecosystem.
 | ||
| Fig. 18 Production of lithium-ion battery materials worldwide. Fig. 15 adapted from Pantoja et al.200 Copyright 2022 MDPI. | ||
In fact, removing batteries from a device might generate a significant amount of waste for the environment. In addition, batteries contain a lot of dangerous elements, making it unwise to discard them directly. Addressing this, battery recycling is the technique of reusing and processing batteries with the intention of lowering the amount of batteries disposed of as material waste.205 Batteries include a number of toxic compounds and heavy metals, and the pollution of land and water caused by their disposal has raised environmental concerns.206 Currently, in order to guarantee the highest level of safety throughout the transport process, the problem of shipping chemicals for battery manufacture as well as released batteries must meet several stringent and challenging standards. Nowadays, most production materials, including spent cells for recycling as well as commercial cells, are frequently transported by container ships across the ocean due to their low cost, high transport capacity, superior safety compared to airplanes, and ease of movement between continents. Recycling battery is necessary for both environmental and health reasons. Lithium-ion batteries must be disassembled and separated due to their complicated structure and several distinct material types before applying recycling techniques such as pyrometallurgy, hydrometallurgy, direct recycling, and combination procedures (Fig. 19).207–209
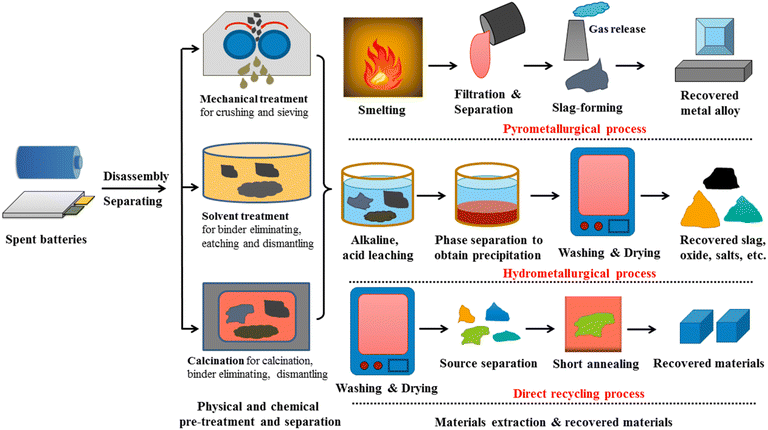 | ||
| Fig. 19 General process to recycle lithium-ion batteries, including pyrometallurgical, hydrometalurgical, and direct recycling methods. | ||
For improved resource recovery and an energy-efficient recycling procedure, particularly in the case of hydrometallurgy and direct recycling pathways, wasted LIBs must be physically and chemically pre-treated and separated.210 The procedure is also necessary for recovering valuable materials like Ni, Co, and Li as well as less valuable elements (also known as impurity elements), such as Al, Fe, Cu, and C.211 Discharging, disassembling, separating, dissolving, and thermal treatment are only a few of the pre-treatment process's sequences, according to numerous phases and procedures that have been classified as part of the pre-treatment process.212 Pre-treatment procedures are often described as those that include classifying and separating different LIB components and elements in order to make future recycling procedures more effective and less impurity-intensive.213,214
Pyrometallurgical technology is a convenient strategy that can convert metal oxides to metal compounds or pure metal by using heating and melting processes.215–217 After pretreatment, the battery materials are heated in reductive roasting (smelting) to change the metal oxides into a mixed metal alloy that may contain cobalt, nickel, copper, iron, and slag containing lithium and aluminum, depending on the battery composition. The crushed wastes are melted in a furnace or molten bath during the pyrometallurgy process to extract polymers. The main drawbacks of pyrometallurgical processes are high investment costs, high energy requirements, and the discharge of hazardous gases into the environment, despite the fact that they are claimed to be economically effective and fully utilize the recovery of a sufficient number of precious metals.218,219
Hydrometallurgical technology generally employs aqueous solutions in order to extract and isolate metals from LIBs.220 In this method, various types of acids such as HCl, HNO3, H2SO4, and organic acids like citric, oxalic, and ascorbic acid are frequently employed, which are used to extract the pretreatment battery components.221,222 Then specific metals are selectively precipitated as salts using pH variation after they have been extracted into solution, or they are extracted using organic solvents that include extractants.
Direct recycling, one of the most innovative and promising new LIB recycling techniques now under development, has started to be applied commercially.223 In actuality, not every component of a battery needs to be recycled; certain components need to be replaced while others are still functional, such as removing and recycling the cathode while other parts are taken out and utilized again.224–227 Therefore, for the goal of replacing and recycling damaged parts, the direct recycling approach presents a sensible and economical strategy.228 Maintaining the purity of the material waste streams, which necessitates specialized processing for cell packing and component removal, is one of the most crucial aspects of direct recycling.229
Due to the unique benefits and drawbacks of each technique (Table 2), combinations of hydrometallurgical and pyrometallurgical processes are often utilized to produce lithium-ion batteries today. Pyrometallurgical techniques are probably utilized because they give battery materials diversity and because they require fixed investment in already-existing facilities. On the other hand, methods that are still being developed rely more heavily on hydrometallurgy due to its cost-effectiveness. In reality, the classification of the above technologies is only relative when studies have shown that a harmonious combination of the above methods will have the best effect. Therefore, for each type of lithium-ion battery and for each recycling purpose, the above methods will be calculated in detail and combined to form the most optimal process possible.
| Techniques | Direct recycling method | Pyrometallugical method | Hydrometallugical method |
|---|---|---|---|
| Advantages | - Suitable for almost batteries types | - Can recycle a large number of spent batteries | - High selectivity and high recycling efficiency and value-added products |
| - Short recovery path and easy to operate | - Can recovery of the high quality precious metals | - Low working temperature | |
| - Low-cost due to low energy consumption, and high recovery rate | - Small chemical consumption | - High effectiveness at removing other contaminants | |
| - Environmental friendliness | - Short flow and straightforward procedure | - Can recover almost cathodic materials | |
| - Rezo SO2 gas emissions | |||
| Disadvantages | - Take a time for mechanical pretreament and separations | - High investment costs | - Nonenvironmental friendly wastewater after productions |
| - Mixing cathode materials may be reduced the quality of recycled materials | - High energy requirements due to high required temperature | - Incomplete binder and electrolyte recycling | |
| - Recycled materials may not good as virgin materials | - The discharge of hazardous gases into the environment | - Procedure complexity | |
| - Losing lithium materials | - Chemical selectivity |
The recycling of spent EV lithium-ion batteries is being revolutionized by emerging technologies that address the limitations of conventional pyrometallurgical and hydrometallurgical methods. Direct recycling employs low-energy processes such as electrochemical relithiation and solvent-assisted separation to restore degraded cathodes (e.g., NMC, LFP) to their original state, preserving >95% of their structure while reducing energy use by 50–70% compared to smelting (Fig. 20).230–232 Meanwhile, bioleaching leverages bacteria (e.g., Acidophilic microorganisms, Bacillus foraminis, A. ferrooxidans, Gluconobacter oxydans) to selectively extract metals like cobalt and lithium under mild conditions, minimizing toxic chemical waste and operating costs.233–236 For binder and electrolyte removal, supercritical CO2 extraction offers a green alternative to incineration, dissolving PVDF and organic solvents without emissions.237,238 Advanced sorting technologies, including AI-driven robotic disassembly and laser-induced breakdown spectroscopy (LIBS), enable precise separation of battery components by chemistry, critical for handling diverse EV battery designs. However, challenges persist in scaling these technologies such as bioleaching remains slow (days vs. hours for acid leaching), and direct recycling struggles with contaminated or mixed feedstocks. Innovations like plasma-assisted pyrolysis for binder removal and closed-loop solvent recovery systems are under development to improve efficiency.239,240 With the EV market projected to generate more than 11 million tons of battery waste annually by 2030,241 these technologies which backed by policies like the EU Battery Regulation, could transform recycling into a high-yield, low-carbon industry, recovering more than 90% of critical materials while meeting stringent sustainability targets.
 | ||
| Fig. 20 (a) Schematic representation of the cathode regeneration technique, where electrochemical lithium reintegration transforms spent LixCoO2 electrodes into reusable LiCoO2 active materials for new batteries, and thick-electrode regeneration of spent battery materials: (b) schematic overview of the thick-electrode regeneration process for spent lithium-ion batteries, (c) electrochemical relithiation mechanism enabled by thick-electrode architecture, (d) voltage–time profile during constant-current relithiation, (e) compositional analysis (Li/Fe, Li/P, and Fe/P atomic ratios) of regenerated LiFePO4 (LFP) powders, (f) comparative cycling performance (1st vs. 2nd cycle) of regenerated LFP in coin cells. (a) has been reproduced from ref. 230 with permission from American chemical society, copyright 2017. (b–f) has been reproduced from ref. 232 with permission from Elsevier, copyright 2025. | ||
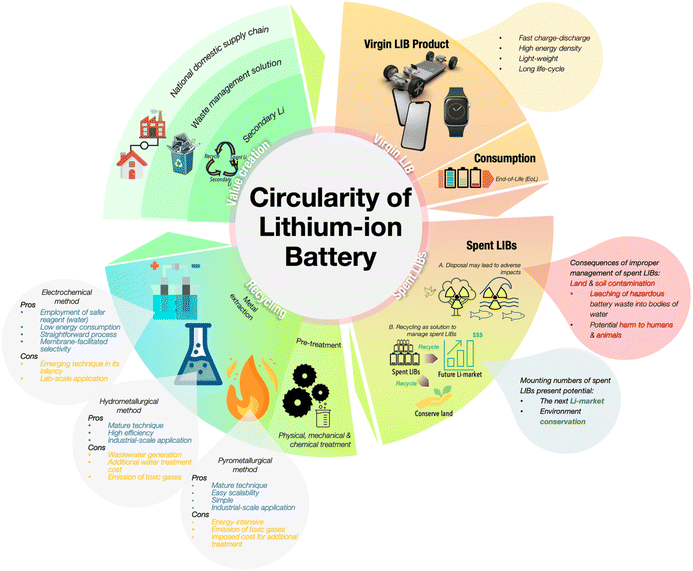 | ||
| Fig. 21 The schematic illustrates a complete circular economic cycle for lithium batteries, starting from their use in electric vehicles to typical end-of-life battery recycling processes and recovery of valuable materials for reuse. Figure 21 has been reproduced from ref. 245 with permission from Royal Chemical Society, copyright 2024. | ||
A circular economy model prioritizes three key phases: (i) reuse of spent battery packs from EVs through second-life applications like stationary energy storage, (ii) building a standard battery collection system for all types of batteries from small electronic devices to electric vehicles, and (iii) recycling for recovering critical materials via pyrometallurgical, hydrometallurgical, or direct recycling methods.245–249 Advanced recycling techniques, such as hydrometallurgy with solvent extraction or electrochemical leaching, can recover high-purity metals, drastically reducing the requirement for virgin mining. However, challenges still persist, including the economic viability of recycling technologies. Recent emerging innovations, such as AI and robotic disassembly systems, promise to enhance recycling efficiency. By closing the loop on battery materials, a circular economy purpose not only mitigates environmental risks and reduces ore mining but also improves the living environment and raises public awareness of environmental protection and sustainable development toward achieving zero tailpipe emissions.
2.6 Future outlook of electric vehicles
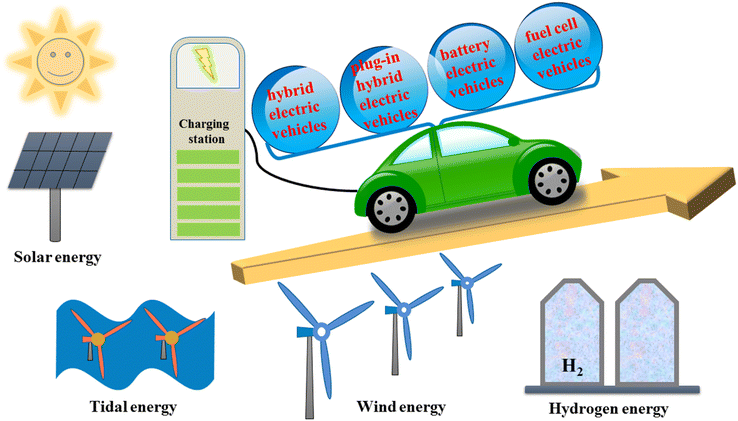
The rapid growth of EVs over the coming decade will undoubtedly place heavy demands on electrical infrastructure. The daily power consumption will be enormous, and the power transmission infrastructure will also need to be improved to supply charging stations that are dispersed over a wide region.251 As a result, the power generation system will be under a lot of strain, raising the important issue of how enough electricity will be generated to satisfy this demand.252,253 The two primary sources of energy used today are hydroelectricity and coal-fired thermal power; however, coal-fired thermal power requires fossil fuels like coal, which contradicts the goal of reducing CO2 emissions. As a result, it is important to develop renewable energy sources like solar and wind power in concert with the existing infrastructure (Fig. 22).
The chance to replace fossil fuels in the transportation industry is provided by electric automobiles. Increased energy efficiency, less air pollution, and a decrease in global warming can all be advantages of electrifying the transportation industry. Yet, there are legitimate worries about how to meet the future energy demand for recharging the batteries of electric vehicles with clean and renewable sources. The concerns associated with the availability of the precursor materials required to make electric car batteries serve as a further reminder of the problem of the long-term viability of EVs. Some of these material resources' exploitation is associated with serious environmental, ethical, and societal problems.
Moreover, one major emergency situation for all types of electrical vehicles is the recycling problem of spent batteries, which are discharged into the environment every year. The requirement to recycle spent electric car batteries can be considered an extremely urgent and important request in the context of increasing pollution of water and air and global warming. As well, a significant number of precursor materials for the manufacturing of electrodes, such as lithium, cobalt, manganese, and nickel, will be required due to the enormous demand for batteries for the electric car revolution in the coming decade. Not only does this endanger the ecosystem, but it also depletes mineral supplies. As a result, better technologies must be created in order to properly recycle spent batteries.
Currently, 50 percent of the world's EV market is now made up of all-electric cars (BEVs). Sales of BEVs are increasing more quickly than those of plug-in hybrid electric vehicles (PHEVs).254 But various markets have very varying preferences for powertrains, which are determined by legislative measures, consumer preferences, and the availability of certain models. Following the various databases of the global market for electric vehicles, a market overview is given by us based on detailed and verifiable statistics from the data collected and presented below.255–259 Fig. 23 shows the global forecast sale of electric vehicles with two biggest markets for electric vehicles in the world, China and North America, continue to offer buyers a wide range of options.260 In the foreseeable future, Europe is starting to emerge as a significant market. Both the further integration of renewable energy and the growing number of electric cars will have a significant impact on the future transformation of the European electrical grid.261 Rest of world, especially India will become the biggest population in the world and is the most attractive market in Asia after China, but it has significant infrastructural challenges that will take 10 to 20 years to resolve.260,262 Japan will be fierce competition from electric vehicle manufacturers due to the rather strict requirements of consumers in these two countries; moreover, the industrial science and infrastructure are very developed. Southeast Asia, although still underdeveloped, has seen strong economic growth in the past two decades and promises to be a dynamic economic region and a fertile ground for electric vehicle manufacturers.263 Overall, China will continue to lead the world in producing electric car batteries over the next ten years, and it will do so with undeniable advantages. In order to jointly develop and build a large number of EVs, many multinational automakers established brand-new joint ventures with local Chinese factories.
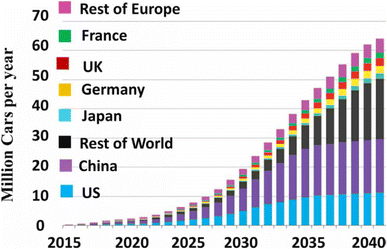 | ||
| Fig. 23 Global EV evolution forecast. Figure has been reproduced from ref. 260 with permission from IGI global scientific publishing, copyright 2019. | ||
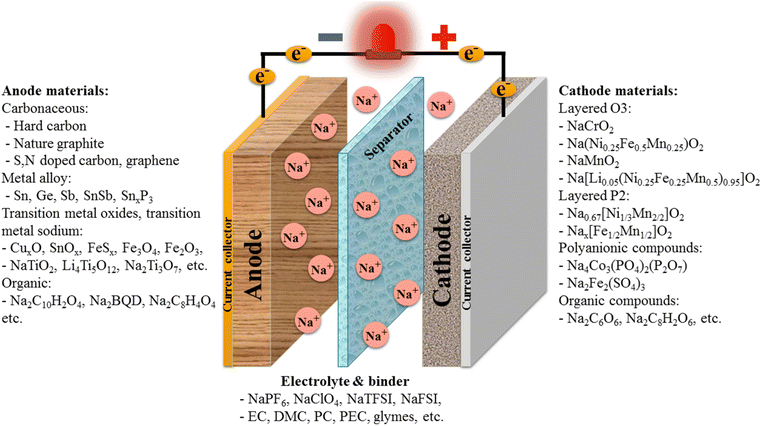
Sodium-ion batteries are a kind of rechargeable battery that works like lithium-ion batteries but uses sodium instead of lithium electrodes as well as a sodium electrolyte (Fig. 24). Sodium-ion batteries could dominate the global battery market in the future due to their low cost and plentiful supply of sodium resources and promising large-scale production, which makes them less expensive per kilowatt-hour.264 Recently, some studies have focused on using hard-carbon anodes for sodium-ion batteries due to their low cost, available sources, and stable structure. Hard-carbon materials have a porous structure that allows sodium ions to easily move and store during charging and discharging, resulting in high energy density and good stability.265 Therefore, hard carbon is considered a highly commercial material for sodium-ion batteries. Sodium-ion batteries leverage abundant, low-cost sodium resources, reducing geopolitical risks associated with lithium, while maintaining similar intercalation chemistry, enabling compatibility with existing manufacturing infrastructure; however, their lower energy density and larger Na+ ionic radius, which slows diffusion kinetics and degrades cycle life, limit widespread adoption.
Metal–air batteries are like lithium-ion batteries, and both are types of rechargeable batteries. As shown in Fig. 25, metal–air batteries use an external cathode (positive electrode) that reacts with oxygen from the air, facilitated by carbon and specific metals, while the anode (negative electrode) can be made from metals such as zinc, aluminum, magnesium, or lithium.266 According to theoretical calculations, metal–air batteries have an energy density many times higher than lithium-ion batteries, which gives hope for a future generation of rechargeable batteries that can be widely used in energy storage industrial systems and opens up great prospects for electric cars.267 Metal–air batteries boast ultrahigh theoretical energy densities, making them ideal for electric vehicles, but suffer from poor rechargeability, electrolyte decomposition, and cathode clogging by discharge products (e.g., Li2O2), necessitating advanced catalysts and stable electrolytes. In the detailed, metal–air batteries have a solid electrolyte interphase (SEI) film is a passivation layer that sits between the metal anode and the electrolyte. It is particularly challenging to prevent the crossover of the air constituents and the degradation of the SEI present on the metal anodes. Batteries also develop dendrites upon metal plating on the anode, which can cause a short circuit and an explosion. Another significant issue is the cathode material's poor resistance to degradation throughout the charge–discharge operation.267–269
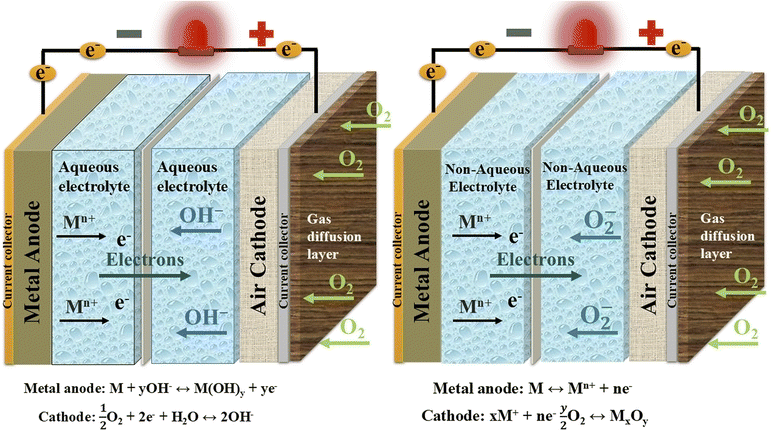 | ||
| Fig. 25 Structure and working principle of aqueous metal–air batteries and non-aqueous metal–air batteries. | ||
All-solid-state lithium batteries (ASSLBs) are more viable alternatives to LIBs, offering higher energy density, improved safety, reliable high-temperature operation, and long-term durability, irrespective of their electrolyte type (solid polymer or inorganic) (Fig. 26).270 The original purpose of solid-state batteries, which have recently attracted a lot of interest, was to enhance the practicality of electric vehicles. Due to their greater energy density and capacity to accommodate larger cells in the same space as lithium-ion batteries, ASSLBs may soon conquer the global market. The ASSLBs are now the subject of considerable study and testing on a very small scale in order to be produced in huge quantities in the future. The high interfacial resistance and lack of stability of solid–solid contact at the electrolyte/electrode interface is one of the obstacles that must be overcome in reality.270–273 The stability and compatibility of the interfacial environment also have a significant impact on the electrochemical performance of batteries.274 In summary, solid-state lithium batteries address safety concerns by replacing flammable liquid electrolytes with solid counterparts, enabling higher energy densities and dendrite suppression, yet face interfacial resistance issues, brittle electrolyte mechanical properties, and high production costs due to complex sintering processes.
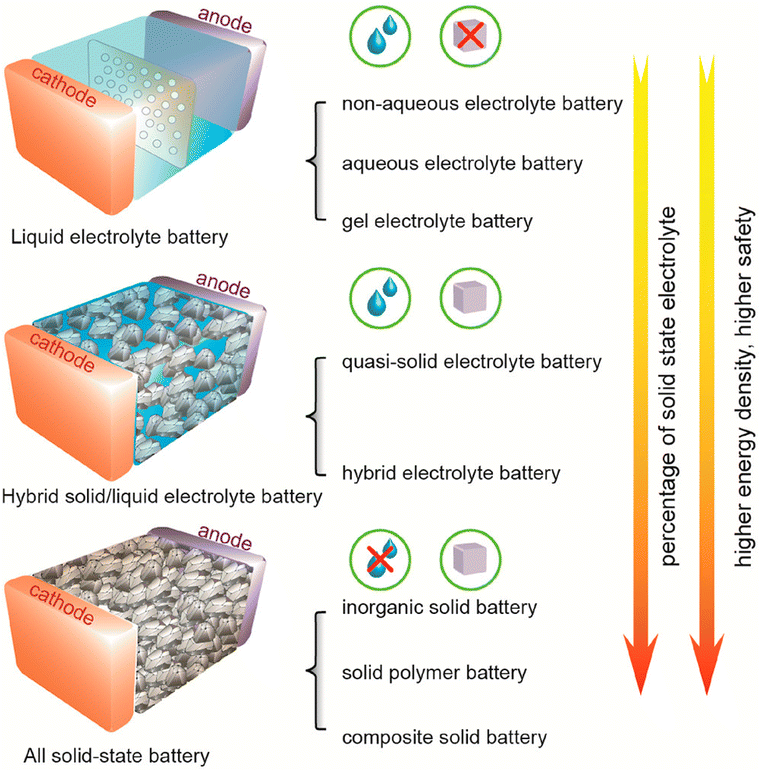 | ||
| Fig. 26 Typical lithium-ion battery categories are shown schematically. Based on the volume of liquid and percentage of SSE in the built batteries, three different types of batteries may be identified. Figure has been reproduced from ref. 270 with permission of America Chemical Society, copyright 2020. | ||
The recycling of next-generation batteries presents significant challenges due to their diverse chemistries and structural complexities. Sodium-ion batteries, while sharing similar properties with lithium-ion systems, suffer from lower material value, making traditional pyrometallurgical or hydrometallurgical recycling economically unviable without government assistance.275,276 Additionally, the prevalence of aluminum current collectors in both electrodes complicates separation processes. Metal–air batteries have some limitations of their open cathode structures, which absorb atmospheric CO2 and moisture, leading to carbonate formation that contaminates recyclable components.277–279 Solid-state lithium batteries require new recycling strategies, as their ceramic- or sulfide-based solid electrolytes are often brittle and intermixed with electrode layers, necessitating energy-intensive mechanical separation, and their stable inorganic components resist traditional leaching processes.280–282 Furthermore, the lack of standardized designs across these emerging technologies hampers the development of universal recycling protocols, increasing costly and specific recycling strategies for each battery type. Overcoming these challenges requires advances in direct recycling methods, automated disassembly, and policy frameworks to incentivize recovery of low-value materials like sodium or complex solid electrolytes.
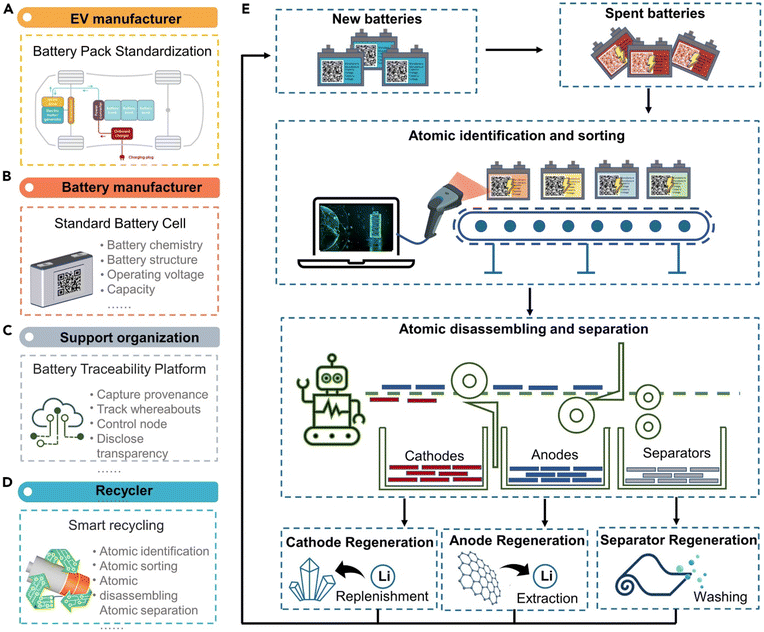 | ||
| Fig. 27 The ideas toward a circularity for electric vehicle battery packs: (A and B) electric vehicle battery manufacturing and assembly, (C) settings to support and manage battery packs in real time during use, and (D and E) smart and closed-loop recycling processes using AI and robots. Figure has been reproduced from ref. 286 with permission from Elsevier, copyright 2023. | ||
The direction of battery recycling technology in the near future is to incorporate AI and robots into the diagnostic phases of pre-recycling as well as the entire process of disassembly, separation, and refinement to recover the most precious metals possible in the form of metal salts, lithium salts, and pure foil. These new technologies will reduce the hazards of gas venting and chemical emissions during the disassembly and recycling process, as well as the explosion incidents and toxic exposures to humans. In addition, breakthrough technological improvements will also be applied to improve traditional recycling methods such as hydrometallurgy and pyrometallurgy to reduce process operating costs and increase output product quality.
3 Conclusions
This review comprehensively examines four commercial EV types (BEVs, HEVs, PHEVs, and FCEVs) with a focus on their LIB architectures and safety challenges, particularly thermal runaway risks. By synthesizing recent experimental studies, we demonstrate that thermal management systems (e.g., liquid cooling, phase-change materials) and battery design modifications (e.g., ceramic-coated separators, silicon anodes) significantly enhance safety but require further optimization for extreme conditions. Our analysis also reveals that while current recycling methods—pyrometallurgy (80–95% metal recovery), hydrometallurgy (high purity but energy-intensive), and direct recycling (cost-effective but scalability-limited)—address LIB waste, none yet offer a perfect solution for the impending surge of end-of-life EV batteries.The transition to EVs is inevitable, driven by climate policies and technological advances. However, critical barriers persist, including: (1) charging infrastructure scalability, (2) renewable energy integration to ensure true decarbonization, and (3) sustainable, closed-loop battery recycling systems. Innovations like solid-state batteries and sodium-ion alternatives show promise but demand rigorous validation. This work not only consolidates current knowledge but also provides actionable insights for policymakers and researchers to accelerate EV adoption while mitigating environmental trade-offs. Future efforts must prioritize interdisciplinary collaboration to address these challenges holistically, ensuring EVs fulfill their potential as a sustainable alternative to ICE vehicles.
Author contributions
Phuoc-Anh Le: writing – review & editing, writing – original draft, validation, methodology, investigation, formal analysis, data curation, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships.Data availability
No new data were generated or analyzed in this study. All data supporting the findings of this review are available within the manuscript and the references cited.Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.References
- M. A. Hannan, M. S. H. Lipu, A. Hussain and A. Mohamed, A review of lithium-ion battery state of charge estimation and management system in electric vehicle applications: Challenges and recommendations, Renewable Sustainable Energy Rev., 2017, 78, 834–854, DOI:10.1016/j.rser.2017.05.001.
- M. B. Anwar, M. Muratori, P. Jadun, E. Hale, B. Bush, P. Denholm, O. Mab and K. Podkaminerb, Assessing the value of electric vehicle managed charging: A review of methodologies and results, Energy Environ. Sci., 2022, 15, 466–498, 10.1039/d1ee02206g.
- J. Dunn, M. Slattery, A. Kendall, H. Ambrose and S. Shen, Circularity of Lithium-Ion Battery Materials in Electric Vehicles, Environ. Sci. Technol., 2021, 55(8), 5189, DOI:10.1021/acs.est.0c07030.
- J. Buberger, A. Kersten, M. Kuder, R. Eckerle, T. Weyh and T. Thiringer, Total CO2-equivalent life-cycle emissions from commercially available passenger cars, Renewable Sustainable Energy Rev., 2022, 159, 112158, DOI:10.1016/j.rser.2022.112158.
- W. J. Requia, M. Mohamed, C. D. Higgins, A. Arain, and M. Ferguson, ‘How clean are electric vehicles? Evidence-based review of the effects of electric mobility on air pollutants, greenhouse gas emissions and human health’, 2018, DOI:10.1016/j.atmosenv.2018.04.040.
- S. Pan, A. Roy, Y. Choi, E. Eslami, S. Thomas, X. Jiang and H. O. Gao, Potential impacts of electric vehicles on air quality and health endpoints in the Greater Houston Area in 2040, Atmos. Environ., 2019, 207, 38–51, DOI:10.1016/j.atmosenv.2019.03.022.
- T. Ercan, N. C. Onat, N. Keya, O. Tatari, N. Eluru and M. Kucukvar, Autonomous electric vehicles can reduce carbon emissions and air pollution in cities, Transp. Res. D Trans. Environ., 2022, 112, 103472, DOI:10.1016/j.trd.2022.103472.
- S. Y. Chang, J. Huang, M. R. Chaveste, F. W. Lurmann, D. S. Eisinger, A. D. Mukherjee, G. B. Erdakos, M. Alexander and E. Knipping, Electric vehicle fleet penetration helps address inequalities in air quality and improves environmental justice, Commun. Earth Environ., 2023, 4(1), 135, DOI:10.1038/s43247-023-00799-1.
- C. Deng, et al., Are electric vehicles really the optimal option for the transportation sector in China to approach pollution reduction and carbon neutrality goals?, J. Environ. Manage., 2024, 356, 120648, DOI:10.1016/j.jenvman.2024.120648.
- F. G. Pratico, P. G. Briante, and G. Speranza, ‘Acoustic Impact of Electric Vehicles’, in 20th IEEE Mediterranean Electrotechnical Conference, MELECON 2020 - Proceedings, 2020, DOI:10.1109/MELECON48756.2020.9140669.
- K. H. Tsoi, B. P. Y. Loo, X. Li and K. Zhang, The co-benefits of electric mobility in reducing traffic noise and chemical air pollution: Insights from a transit-oriented city, Environ. Int., 2023, 178, 108116, DOI:10.1016/j.envint.2023.108116.
- M. Şen, M. Özcan and Y. R. Eker, A review on the lithium-ion battery problems used in electric vehicles, Next sustain., 2024, 3, 100036, DOI:10.1016/j.nxsust.2024.100036.
- S. S. Rawat, R. Kumar and K. Das, Lithium-ion battery progress in surface transportation: status, challenges, and future directions, Multim. Tools Appl., 2024, 84, 32927–32958, DOI:10.1007/s11042-024-20505-3.
- A. L. Cheng, E. R. H. Fuchs, V. J. Karplus and J. J. Michalek, Electric vehicle battery chemistry affects supply chain disruption vulnerabilities, Nat. Commun., 2024, 15(1), 2143, DOI:10.1038/s41467-024-46418-1.
- M. Dixit, et al., Insights into the Critical Materials Supply Chain of the Battery Market for Enhanced Energy Security, ACS Energy Lett., 2024, 9(8), 3780–3789, DOI:10.1021/acsenergylett.4c01300.
- P. A. Le, D. T. Vuong, J. Natsuki, and T. Natsuki, ‘Overview of the Thermal Runaway in Lithium-Ion Batteries with Application in Electric Vehicles: Working Principles, Early Warning, and Future Outlooks’, 2023, DOI:10.1021/acs.energyfuels.3c02548.
- J. M. Tarascon and M. Armand, ‘Issues and challenges facing rechargeable lithium batteries’, 2001, DOI:10.1038/35104644.
- W. Liu, T. Placke, and K. T. Chau, ‘Overview of batteries and battery management for electric vehicles’, 2022, DOI:10.1016/j.egyr.2022.03.016.
- R. Chen, R. Luo, Y. Huang, F. Wu, and L. Li, ‘Advanced High Energy Density Secondary Batteries with Multi-Electron Reaction Materials’, 2016, DOI:10.1002/advs.201600051.
- M. S. Koroma, D. Costa, M. Philippot, G. Cardellini, M. S. Hosen, T. Coosemans and M. Messagie, Life cycle assessment of battery electric vehicles: implications of future electricity mix and different battery end-of-life management, Sci. Total Environ., 2022, 831, 154859, DOI:10.1016/j.scitotenv.2022.154859.
- G. Zhao, X. Wang, and M. Negnevitsky, ‘Connecting battery technologies for electric vehicles from battery materials to management’, 2022, DOI:10.1016/j.isci.2022.103744.
- S. R. Ovshinsky, M. A. Fetcenko and J. Ross, A Nickel Metal Hydride Battery for Electric Vehicles, Science, 1979, 260(5105), 176–181, DOI:10.1126/science.260.5105.176.
- M. Fetcenko, J. Koch, and M. Zelinsky, ‘6 – Nickel–metal hydride and nickel–zinc batteries for hybrid electric vehicles and battery electric vehicles’, in Advances in Battery Technologies for Electric Vehicles, ed. B. Scrosati, J. Garche, and W. Tillmetz, Woodhead Publishing, 2015, pp. 103–126, DOI:10.1016/B978-1-78242-377-5.00006-6.
- A. J. Crawford, Q. Huang, M. C. W. K. Meyer, J. G. Zhang, D. M. Reed, V. L. Sprenkle, V. V. Viswanathan and D. Choi, Lifecycle comparison of selected Li-ion battery chemistries under grid and electric vehicle duty cycle combinations, J. Power Sources, 2018, 380, 185–193, DOI:10.1016/j.jpowsour.2018.01.080.
- B. Cox, C. L. Mutel, C. Bauer, A. Mendoza Beltran and D. P. Van Vuuren, Uncertain Environmental Footprint of Current and Future Battery Electric Vehicles, Environ. Sci. Technol., 2018, 52(8), 4989–4995, DOI:10.1021/acs.est.8b00261.
- M. S. Hossain, L. Kumar, M. El Haj Assad and R. Alayi, Advancements and Future Prospects of Electric Vehicle Technologies, A Comprehensive Review, Complexity, 2022 DOI:10.1155/2022/3304796.
- A. Eftekhari, Lithium Batteries for Electric Vehicles: From Economy to Research Strategy, ACS Sustain. Chem. Eng., 2019, 7(6), 5602–5613, DOI:10.1021/acssuschemeng.8b01494.
- D. Choi, N. Shamim, A. Crawford, Q. Huang, C. K. Vartanian, V. V. Viswanathan, M. D. Paiss, M. J. E. Alam, D. M. Reed and V. L. Sprenkle, Li-ion battery technology for grid application, J. Power Sources, 2021, 511 DOI:10.1016/j.jpowsour.2021.230419.
- M. S. H. Lipu, et al., Battery Management, Key Technologies, Methods, Issues, and Future Trends of Electric Vehicles: A Pathway toward Achieving Sustainable Development Goals, Batteries, 2022, 8(9), 119, DOI:10.3390/batteries8090119.
- L. Peiseler, V. Wood and T. S. Schmidt, Reducing the carbon footprint of lithium-ion batteries, what’s next?, Next Energy, 2023, 1(2), 100017, DOI:10.1016/j.nxener.2023.100017.
- M. Fichtner, Recent Research and Progress in Batteries for Electric Vehicles, Batteries Supercaps, 2022, 5(2), e202100224, DOI:10.1002/batt.202100224.
- J. Zhao, Z. Rao, Y. Huo, X. Liu and Y. Li, Thermal management of cylindrical power battery module for extending the life of new energy electric vehicles, Appl. Therm. Eng., 2015, 85, 33–43, DOI:10.1016/j.applthermaleng.2015.04.012.
- Y. Xu, H. Zhang, Y. Yang, J. Zhang, F. Yang, D. Yan, H. Yang and Y. Wang, Optimization of energy management strategy for extended range electric vehicles using multi-island genetic algorithm, J. Energy Storage, 2023, 61, 106802, DOI:10.1016/j.est.2023.106802.
- X. Sun and J. Fu, Many-objective optimization of BEV design parameters based on gradient boosting decision tree models and the NSGA-III algorithm considering the ambient temperature, Energy, 2024, 288, 129840, DOI:10.1016/j.energy.2023.129840.
- M. Q. Elahi, M. Elsaadany, H. Rehman, and S. Mukhopadhyay, ‘Battery Energy Consumption Optimization for the EV Traction System’, in 2022 IEEE 16th International Conference on Compatibility, Power Electronics, and Power Engineering, CPE-POWERENG 2022, 2022, DOI:10.1109/CPE-POWERENG54966.2022.9880859.
- A. Bocca and D. Baek, ‘Optimal life-cycle costs of batteries for different electric cars’, in 2020 AEIT International Conference of Electrical and Electronic Technologies for Automotive, AEIT AUTOMOTIVE 2020, 2020, DOI:10.23919/aeitautomotive50086.2020.9307426.
- J. Furch, V. Konečný and Z. Krobot, Modelling of life cycle cost of conventional and alternative vehicles, Sci. Rep., 2022, 12(1), 10661, DOI:10.1038/s41598-022-14715-8.
- D. Baek, A. Bocca and A. Macii, A cost of ownership analysis of batteries in all-electric and plug-in hybrid vehicles, Energy. Ecol. Environ., 2022, 7(6), 604–613, DOI:10.1007/s40974-022-00256-3.
- H. F. Bauman, ‘Development of the copper fluoride–lithium couple for limited-cycle secondary battery’, in Proceedings of the 18th Annual Power Sources Conference, PSC Publications, 1964, pp. 89–91 Search PubMed.
- R. W. Adler, W. E. Elliott, J. R. Huff, J. L. Jamrozy and W. L. Towle, A program to develop a high-energy density primary battery with a minimum of 200 W hours per pound of total battery weight, NTRS - NASA Technical Reports Server, 1965 Search PubMed.
- S. G. Abens, W. C. Merz and C. R. Walk, Development of high energy density primary batteries, NTRS - NASA Technical Reports Server, 1965 Search PubMed.
- H. F. Bauman, ‘Development of lithium–cupric fluoride batteries’, in Proceedings of the 20th Annual Power Sources Conference, PSC Publications, 1966, pp. 73–76 Search PubMed.
- R. R. Haering and J. A. R. Stiles, ‘US Patent Electrical Storage Device 1980’, no. US4233377A, 1980, accessed: Mar. 31, 2025, https://patents.google.com/patent/US4233377 Search PubMed.
- M. S. Whittingham, Electrical Energy Storage and Intercalation Chemistry, Science, 1979, 192(4244), 1126–1127, DOI:10.1126/science.192.4244.1126.
- K. Mizushima, P. C. Jones, P. J. Wiseman and J. B. Goodenough, LixCoO2 (0<x<−1): a new cathode material for batteries of high energy density, Mater. Res. Bull., 1980, 15(6), 171–174, DOI:10.1016/0025-5408(80)90012-4.
- R. Yazami and Ph. Touzain, A reversible graphite-lithium negative electrode for electrochemical generators, J. Power Sources, 1983, 9(3), 365–371, DOI:10.1016/0378-7753(83)87040-2.
- H. Bae and Y. Kim, Technologies of lithium recycling from waste lithium ion batteries: A review’, Mater. Adv., 2021, 2, 3234–3250, 10.1039/d1ma00216c.
- J. Yang, C. Hu, H. Wang, K. Yang, J. B. Liu and H. Yan, Review on the research of failure modes and mechanism for lead–acid batteries, Int. J. Energy Res., 2017, 41, 336–352 CrossRef.
- J. N. Liu, C. X. Zhao, J. Wang, D. Ren, B. Q. Li, and Q. Zhang, ‘A brief history of zinc-air batteries: 140 years of epic adventures’, 2022, 10.1039/d2ee02440c.
- P. J. Tsai and S. L. I. Chan, ‘Nickel-based batteries: materials and chemistry’, in Electricity Transmission, Distribution and Storage Systems, Elsevier Ltd, 2013, pp. 309–397, DOI:10.1533/9780857097378.3.309.
- Y. Morioka, S. Narukawa and T. Itou, State-of-the-art of alkaline rechargeable batteries, J. Power Sources, 2001, 100(1–2), 107–116, DOI:10.1016/S0378-7753(01)00888-6.
- S. Arya and S. Verma, ‘Nickel–Metal Hydride (Ni-MH) Batteries’, in Rechargeable Batteries, 2020, pp. 131–175, DOI:10.1002/9781119714774.ch8.
- C. Jeyaseelan, A. Jain, P. Khurana, D. Kumar, and S. Thatai, ‘Ni–Cd Batteries’, in Rechargeable Batteries, 2020, pp. 177–194, DOI:10.1002/9781119714774.ch9.
- N. Nitta, F. Wu, J. T. Lee, and G. Yushin, ‘Li-ion battery materials: Present and future’, 2015, DOI:10.1016/j.mattod.2014.10.040.
- T. Kim, W. Song, D. Y. Son, L. K. Ono, and Y. Qi, ‘Lithium-ion batteries: outlook on present, future, and hybridized technologies’, 2019, 10.1039/C8TA10513H.
- X. Shen et al., ‘Advanced Electrode Materials in Lithium Batteries: Retrospect and Prospect’, 2021, DOI:10.34133/2021/1205324.
- M. Weiss et al., ‘Fast Charging of Lithium-Ion Batteries: A Review of Materials Aspects’, 2021, DOI:10.1002/aenm.202101126.
- Y. X. Yao, C. Yan and Q. Zhang, Emerging interfacial chemistry of graphite anodes in lithium-ion batteries, Chem. Commun., 2020, 56(93), 14570–14584, 10.1039/d0cc05084a.
- J. B. Goodenough and K. S. Park, ‘The Li-ion rechargeable battery: A perspective’, 2013, DOI:10.1021/ja3091438.
- S. Stock, J. Hagemeister, S. Grabmann, J. Kriegler, J. Keilhofer, M. Ank, J. L. S. Dickmanns, M. Schreiber, F. Konwitschny, N. Wassiliadis, M. Lienkamp and R. Daub, Cell teardown and characterization of an automotive prismatic LFP battery, Electrochim. Acta, 2023, 471, 143341, DOI:10.1016/j.electacta.2023.143341.
- M. Ank, A. Sommer, K. A. Gamra, J. Schöberl, M. Leeb, J. Schachtl, N. Streidel, S. Stock, M. Schreiber, P. Bilfinger, C. Allgäuer, P. Rosner, J. Hagemeister, M. Rößle, R. Daub and M. Lienkamp, Lithium-Ion Cells in Automotive Applications: Tesla 4680 Cylindrical Cell Teardown and Characterization, J. Electrochem. Soc., 2023, 170(12), 120536, DOI:10.1149/1945-7111/ad14d0.
- H. Pegel, A. Grimm, C. Frey, V. Seefeldt, S. Baazouzi and D. U. Sauer, Manufacturing of tabless cylindrical lithium-ion cells: Quantifying the influence of cell dimensions and housing material via process-based cost modeling, J. Energy Storage, 2024, 98, 112863, DOI:10.1016/j.est.2024.112863.
- J. Gorsch, et al., Contrasting a BYD Blade prismatic cell and Tesla 4680 cylindrical cell with a teardown analysis of design and performance, Cell Rep. Phys. Sci., 2025, 6(3), 102453, DOI:10.1016/j.xcrp.2025.102453.
- S. Shahid and M. Agelin-Chaab, ‘A review of thermal runaway prevention and mitigation strategies for lithium-ion batteries’, 2022, DOI:10.1016/j.ecmx.2022.100310.
- H. T. Reid, et al., Key considerations for cell selection in electric vertical take off and landing vehicles: a perspective, EES Batteries, 2025, 1(2), 227–241, 10.1039/D4EB00024B.
- H. Niu, et al., Thermal-Runaway Propagation over a Linear Cylindrical Battery Module, Fire Technol., 2020, 56(6), 2491–2507, DOI:10.1007/s10694-020-00976-0.
- Y. Jia, J. Darst, A. Surelia, D. Delafuente, D. Finegan and J. Xu, Deformation and fracture behaviors of cylindrical battery shell during thermal runaway, J. Power Sources, 2022, 539, 231607, DOI:10.1016/j.jpowsour.2022.231607.
- W. Li, et al., Comparison on Thermal Runaway and Critical Characteristics of Cylindrical Lithium-Ion Batteries: A Review, ACS Chem. Health Saf., 2025, 32(2), 133–156, DOI:10.1021/acs.chas.4c00121.
- L. Torres-Castro, A. Kurzawski, J. Hewson and J. Lamb, Passive Mitigation of Cascading Propagation in Multi-Cell Lithium Ion Batteries, J. Electrochem. Soc., 2020, 167(9), 090515, DOI:10.1149/1945-7111/ab84fa.
- M. P. Macdonald, S. Chandrasekaran, S. Garimella and T. F. Fuller, Thermal runaway in a prismatic lithium ion cell triggered by a short circuit, J. Energy Storage, 2021, 40, 102737, DOI:10.1016/j.est.2021.102737.
- L. Gagnon, D. Zeng, B. Ditch and Y. Wang, Cell-level thermal runaway behavior of large-format Li-ion pouch cells, Fire Saf. J., 2023, 140, 103904, DOI:10.1016/j.firesaf.2023.103904.
- C. C. Xu, Z. Fan, M. Zhang, P. Wang, H. Wang, C. Jin, Y. Peng, F. Jiang, X. Feng and M. Ouyang, A comparative study of the venting gas of lithium-ion batteries during thermal runaway triggered by various methods, Cell Rep. Phys. Sci., 2023, 4(12), 101705, DOI:10.1016/j.xcrp.2023.101705.
- S. K. Lee, H. K. Kim and K. J. Lee, Investigation of the risk of thermal runaway in a pouch-type lithium-ion battery with an internal short based on a multiphysics simulation, Appl. Therm. Eng., 2024, 236, 121582, DOI:10.1016/j.applthermaleng.2023.121582.
- D. C. Fessler, ‘The History of Electric Vehicles’, in The Energy Disruption Triangle: Three Sectors that Will Change How We Generate, Use, and Store Energy, David C. Fessler., John Wiley & Sons, 2019, pp. 95–117, DOI:10.1002/9781119347101.ch6.
- X. Zhang, C. Zhu, and H. Song, ‘Introduction’, in Wireless Power Transfer Technologies for Electric Vehicles, ed. X. Zhang, C. Zhu, and H. Song, Singapore, Springer Nature Singapore, 2022, pp. 1–26., DOI:10.1007/978-981-16-8348-0_1.
- M. Haghani, F. Sprei, K. Kazemzadeh, Z. Shahhoseini and J. Aghaei, Trends in electric vehicles research, Transp. Res. D Trans. Environ., 2023, 123, 103881, DOI:10.1016/j.trd.2023.103881.
- T. Nogueira, E. Sousa and G. R. Alves, Electric vehicles growth until 2030: impact on the distribution network power, Energy Rep., 2022, 8, 145–152, DOI:10.1016/j.egyr.2022.01.106.
- C. Domarchi and E. Cherchi, Electric vehicle forecasts: a review of models and methods including diffusion and substitution effects, Transp. Rev., 2023, 43(6), 1118–1143, DOI:10.1080/01441647.2023.2195687.
- B. Jones, V. Nguyen-Tien and R. J. R. Elliott, The electric vehicle revolution: Critical material supply chains, trade and development, World Economy, 2023, 46(1), 2–26, DOI:10.1111/twec.13345.
- M. Guarnieri, ‘Looking back to electric cars’, in 3rd Region-8 IEEE HISTory of Electro - Technology CONference: the Origins of Electrotechnologies, HISTELCON 2012 - Conference Proceedings, 2012, DOI:10.1109/HISTELCON.2012.6487583.
- X. Su, K. Meng, and D. Liu, ‘Research on chinese new energy vehicles based on ecological view’, in E3S Web of Conferences, 2020, DOI:10.1051/e3sconf/202017901026.
- B. G. Pollet, I. Staffell, and J. L. Shang, ‘Current status of hybrid, battery and fuel cell electric vehicles: From electrochemistry to market prospects’, 2012, DOI:10.1016/j.electacta.2012.03.172.
- J. A. Sanguesa, V. Torres-Sanz, P. Garrido, F. J. Martinez, and J. M. Marquez-Barja, ‘A review on electric vehicles: Technologies and challenges’, 2021, DOI:10.3390/smartcities4010022.
- J. B. Dunn, L. Gaines, J. C. Kelly, C. James and K. G. Gallagher, The significance of Li-ion batteries in electric vehicle life-cycle energy and emissions and recycling’s role in its reduction, Energy Environ. Sci., 2015, 8(1), 158–168, 10.1039/c4ee03029j.
- P. A. Le, ‘A general introduction to lithium-ion batteries: From the first concept to the top six commercials and beyond’, 2023, DOI:10.5599/jese.1544.
- K. Sasaki, M. Yokota, H. Nagayoshi and K. Kamisako, Evaluation of electric motor and gasoline engine hybrid car using solar cells, Sol. Energy Mater. Sol. Cells, 1997, 47(1), 259–263, DOI:10.1016/S0927-0248(97)00047-0.
- F. V Conte, Battery and battery management for hybrid electric vehicles: a review, Elektrotech. Inf., 2006, 123(10), 424–431, DOI:10.1007/s00502-006-0383-6.
- W. Enang and C. Bannister, Modelling and control of hybrid electric vehicles (A comprehensive review), Renewable Sustainable Energy Rev., 2017, 74, 1210–1239, DOI:10.1016/j.rser.2017.01.075.
- K. V. Singh, H. O. Bansal, and D. Singh, ‘A comprehensive review on hybrid electric vehicles: architectures and components’, 2019, DOI:10.1007/s40534-019-0184-3.
- A. K. Jha, A. K. Gupta, S. Singh, and S. K. Singh, ‘A Review on Hybrid Electric Vehicles and Power Sources’, in 2022 2nd International Conference on Emerging Frontiers in Electrical and Electronic Technologies (ICEFEET), 2022, pp. 1–6, DOI:10.1109/ICEFEET51821.2022.9848278.
- S. Zhang and R. Xiong, Adaptive energy management of a plug-in hybrid electric vehicle based on driving pattern recognition and dynamic programming, Appl. Energy, 2015, 155, 68–78, DOI:10.1016/j.apenergy.2015.06.003.
- H. S. Das, C. W. Tan and A. H. M. Yatim, Fuel cell hybrid electric vehicles: A review on power conditioning units and topologies, Renewable Sustainable Energy Rev., 2017, 76, 268–291, DOI:10.1016/j.rser.2017.03.056.
- T. Selmi, A. Khadhraoui, and A. Cherif, ‘Fuel cell–based electric vehicles technologies and challenges’, 2022, DOI:10.1007/s11356-022-23171-w.
- M. Genovese and P. Fragiacomo, Hydrogen refueling station: Overview of the technological status and research enhancement, J. Energy Storage, 2023, 61, 106758, DOI:10.1016/j.est.2023.106758.
- H. Togun, et al., Development and comparative analysis between battery electric vehicles (BEV) and fuel cell electric vehicles (FCEV), Appl. Energy, 2025, 388, 125726, DOI:10.1016/j.apenergy.2025.125726.
- J. O. Abe, A. P. I. Popoola, E. Ajenifuja, and O. M. Popoola, ‘Hydrogen energy, economy and storage: Review and recommendation’, 2019, DOI:10.1016/j.ijhydene.2019.04.068.
- F. S. Hwang, et al., Review of battery thermal management systems in electric vehicles, Renewable Sustainable Energy Rev., 2024, 192, 114171, DOI:10.1016/j.rser.2023.114171.
- S. Koundal, S. L. Sharma and A. Debbarma, Battery thermal management systems for electric vehicles: an overview of cooling techniques and performance optimization, J. Therm. Anal. Calorim., 2025, 150(8), 5855–5882, DOI:10.1007/s10973-025-14170-3.
- K. Murashko, H. Wu, J. Pyrhonen, and L. Laurila, ‘Modelling of the battery pack thermal management system for Hybrid Electric Vehicles’, in 2014 16th European Conference on Power Electronics and Applications, EPE-ECCE Europe 2014, 2014, DOI:10.1109/EPE.2014.6910774.
- Y. Zhang, J. Huang, L. He, D. Zhao and Y. Zhao, A study of a thermal management system for passenger compartment comfort and battery heating in hybrid vehicles considering drive mode switching, Therm. Sci. Eng. Prog., 2024, 53, 102735, DOI:10.1016/j.tsep.2024.102735.
- X. Zhang, X. Wang, Z. Dang, P. Yuan, H. Tian and G. Shu, An integrated hybrid electric vehicle central thermal management system, Cell Rep. Phys. Sci., 2025, 6(1), 102353, DOI:10.1016/j.xcrp.2024.102353.
- J. L. Sanz, C. O. Martinez, J. Á. Flórez, M. M. Eguilaz, R. R. Mansilla and J. Kalmus, Thermal Management in Plug-In Hybrid Electric Vehicles: A Real-Time Nonlinear Model Predictive Control Implementation, IEEE Trans. Veh. Technol., 2017, 66(9), 7751–7760, DOI:10.1109/TVT.2017.2678921.
- Y. Masoudi and N. L. Azad, ‘MPC-based battery thermal management controller for Plug-in hybrid electric vehicles’, in Proceedings of the American Control Conference, 2017, DOI:10.23919/ACC.2017.7963627.
- L. Engbroks, D. Görke, S. Schmiedler, T. Gödecke, B. Beyfuss, and B. Geringer, ‘Combined energy and thermal management for plug-in hybrid electric vehicles -analyses based on optimal control theory’, in IFAC-PapersOnLine, 2019, DOI:10.1016/j.ifacol.2019.09.097.
- D. Song, D. Bi, X. Zeng and S. Wang, Energy Management Strategy of Plug-In Hybrid Electric Vehicles Considering Thermal Characteristics, Int. J. Automot. Technol., 2023, 24(3), 655–668, DOI:10.1007/s12239-023-0055-0.
- F. Zhu, Y. Liu, C. Lu, Q. Huang and C. Wang, Research on thermal energy management for PHEV based on NSGA-II optimization algorithm, Case Stud. Therm. Eng., 2024, 54, 104046, DOI:10.1016/j.csite.2024.104046.
- P. G. Anselma, S. Luciani and A. Tonoli, Dynamic Programming for Thermal Management of Automotive Fuel Cell Systems: Investigating Hydrogen Saving Potential, IEEE Access, 2023, 11, 48080–48098, DOI:10.1109/ACCESS.2023.3272830.
- A. Broatch, P. Olmeda, X. Margot and S. Aceros, Different strategies in an integrated thermal management system of a fuel cell electric bus under real driving cycles in winter, Energy Convers. Manage., 2023, 288, 117137, DOI:10.1016/j.enconman.2023.117137.
- M. S. F. A and K. C. S. Thampatty, ‘Design of an Effective Thermal Management System for Fuel Cell Electric Vehicles (FCEV) Using Ansys/CFD’, in 2024 International Conference on Futuristic Technologies in Control Systems & Renewable Energy (ICFCR), 2024, pp. 1–7, DOI:10.1109/ICFCR64128.2024.10763267.
- A.-U.-H. Najmi, A. Wahab, R. Prakash, O. Schopen, T. Esch and B. Shabani, Thermal management of fuel cell-battery electric vehicles: Challenges and solutions, Appl. Energy, 2025, 387, 125635, DOI:10.1016/j.apenergy.2025.125635.
- A. Gharehghani, et al., Progress in battery thermal management systems technologies for electric vehicles, Renewable Sustainable Energy Rev., 2024, 202, 114654, DOI:10.1016/j.rser.2024.114654.
- L. He, Z. Gu, Y. Zhang, H. Jing, and P. Li, ‘Review on Thermal Management of Lithium-Ion Batteries for Electric Vehicles: Advances, Challenges, and Outlook’, 2023, DOI:10.1021/acs.energyfuels.2c04243.
- P. Sun, R. Bisschop, H. Niu and X. Huang, A Review of Battery Fires in Electric Vehicles, Fire Technol., 2020, 56(4), 1361–1410, DOI:10.1007/s10694-019-00944-3.
- Y. Cui, B. Cong, J. Liu, M. Qiu and X. Han, Characteristics and Hazards of Plug-In Hybrid Electric Vehicle Fires Caused by Lithium-Ion Battery Packs With Thermal Runaway, Front. Energy Res., 2022, 10–2022, 878035, DOI:10.3389/fenrg.2022.878035.
- Y. Cui, J. Liu, B. Cong, X. Han and S. Yin, Characterization and assessment of fire evolution process of electric vehicles placed in parallel, Process Saf. Environ. Prot., 2022, 166, 524–534, DOI:10.1016/j.psep.2022.08.055.
- S. Lee, et al., Assessing fire dynamics and suppression techniques in electric vehicles at different states of charge: Implications for maritime safety, Case Stud. Therm. Eng., 2024, 64, 105474, DOI:10.1016/j.csite.2024.105474.
- J. Belt and A. Sorensen, Thermal and Electrochemical Analysis of Thermal Runaway Propagation of Samsung Cylindrical Cells in Lithium-ion Battery Modules, J. Electrochem. Soc., 2023, 170(1), 010515, DOI:10.1149/1945-7111/aca939.
- L. Kong, Y. Li, and W. Feng, ‘Strategies to Solve Lithium Battery Thermal Runaway: From Mechanism to Modification’, 2021, DOI:10.1007/s41918-021-00109-3.
- M. K. Tran, A. Mevawalla, A. Aziz, S. Panchal, Y. Xie and M. Fowler, A Review of Lithium-Ion Battery Thermal Runaway Modeling and Diagnosis Approaches, Processes, 2022, 10(6), 1192, DOI:10.3390/pr10061192.
- D. Ren, X. Feng, L. Lu, M. Ouyang, S. Zheng, J. Li and X. He, An electrochemical-thermal coupled overcharge-to-thermal-runaway model for lithium ion battery, J. Power Sources, 2017, 364, 328–340, DOI:10.1016/j.jpowsour.2017.08.035.
- Z. Wang, T. He, H. Bian, F. Jiang and Y. Yang, Characteristics of and factors influencing thermal runaway propagation in lithium-ion battery packs, J. Energy Storage, 2021, 41, 102956, DOI:10.1016/j.est.2021.102956.
- C. Un and K. Aydın, Thermal Runaway and Fire Suppression Applications for Different Types of Lithium Ion Batteries, Vehicles, 2021, 3(3), 480–497, DOI:10.3390/vehicles3030029.
- X. Zhang, S. Chen, J. Zhu, and Y. Gao, ‘A Critical Review of Thermal Runaway Prediction and Early-Warning Methods for Lithium-Ion Batteries’, 2023, DOI:10.34133/energymatadv.0008.
- X. Feng, M. Ouyang, X. Liu, L. Lu, Y. Xia and X. He, Thermal runaway mechanism of lithium ion battery for electric vehicles: A review, Energy Storage Mater., 2018, 10, 246–267, DOI:10.1016/j.ensm.2017.05.013.
- C. Zhao, J. Sun and Q. Wang, Thermal runaway hazards investigation on 18650 lithium-ion battery using extended volume accelerating rate calorimeter, J. Energy Storage, 2020, 28, 101232, DOI:10.1016/j.est.2020.101232.
- S. Zheng, L. Wang, X. Feng and X. He, Probing the heat sources during thermal runaway process by thermal analysis of different battery chemistries, J. Power Sources, 2018, 378, 527–536, DOI:10.1016/j.jpowsour.2017.12.050.
- Y. Chen, Y. Kang, Y. Zhao, L. Wang, J. Liu, Y. Li, Z. Liang, X. He, X. Li, N. Tavajohi and B. Li, A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards, J. Energy Chem., 2021, 59, pp. 83–89, DOI:10.1016/j.jechem.2020.10.017.
- M. N. Richard and J. R. Dahn, Accelerating Rate Calorimetry Study on the Thermal Stability of Lithium Intercalated Graphite in Electrolyte. I. Experimental, J. Electrochem. Soc., 1999, 146(6), 2068, DOI:10.1149/1.1391893.
- R. Spotnitz and J. Franklin, Abuse behavior of high-power, lithium-ion cells, J. Power Sources, 2003, 113(1), 81–100, DOI:10.1016/S0378-7753(02)00488-3.
- S. S. Zhang, T. R. Jow, K. Amine and G. L. Henriksen, LiPF6-EC-EMC electrolyte for Li-ion battery, J. Power Sources, 2002, 107(1), 18–23, DOI:10.1016/S0378-7753(01)00968-5.
- X. Feng, D. Ren, X. He, and M. Ouyang, Mitigating Thermal Runaway of Lithium-Ion Batteries, Joule, 2020, 4, pp. 743–770, DOI:10.1016/j.joule.2020.02.010.
- X. Feng, S. Zheng, X. He, L. Wang, Y. Wang, D. Ren and M. Ouyang, Time Sequence Map for Interpreting the Thermal Runaway Mechanism of Lithium-Ion Batteries With LiNixCoyMnzO2 Cathode, Front. Energy Res., 2018, 6, 126, DOI:10.3389/fenrg.2018.00126.
- M. Nascimento, M. S. Ferreira and J. L. Pinto, Temperature fiber sensing of Li-ion batteries under different environmental and operating conditions, Appl. Therm. Eng., 2019, 149, 1236–1243, DOI:10.1016/j.applthermaleng.2018.12.135.
- M. C. Appleberry, J. A. Kowalski, S. A. Africk, J. Mitchell, T. C. Ferree, V. Chang, V. Parekh, Z. Xu, Z. Ye, J. F. Whitacre and S. D. Murphy, Avoiding thermal runaway in lithium-ion batteries using ultrasound detection of early failure mechanisms, J. Power Sources, 2022, 535, 231423, DOI:10.1016/j.jpowsour.2022.231423.
- G. Wang, D. Kong, P. Ping, J. Wen, X. He, H. Zhao, X. He, R. Peng, Y. Zhang and X. Dai, Revealing particle venting of lithium-ion batteries during thermal runaway: a multi-scale model toward multiphase process, eTransportation, 2023, 16, 100237, DOI:10.1016/j.etran.2023.100237.
- S. Chen, Z. Wang and W. Yan, Identification and characteristic analysis of powder ejected from a lithium ion battery during thermal runaway at elevated temperatures, J. Hazard. Mater., 2020, 400, 123169, DOI:10.1016/j.jhazmat.2020.123169.
- Q. Wang, B. Mao, S. I. Stoliarov, and J. Sun, ‘A review of lithium ion battery failure mechanisms and fire prevention strategies’, 2019, DOI:10.1016/j.pecs.2019.03.002.
- Q. Wang, P. Ping, X. Zhao, G. Chu, J. Sun, and C. Chen, ‘Thermal runaway caused fire and explosion of lithium ion battery’, 2012, DOI:10.1016/j.jpowsour.2012.02.038.
- D. P. Finegan, M. Scheel, J. B. Robinson, B. Tjaden, I. Hunt, T. J. Mason, J. Millichamp, M. D. Michiel, G. J. Offer, G. Hinds, D. J. L. Brett and P. R. Shearing, In-operando high-speed tomography of lithium-ion batteries during thermal runaway, Nat. Commun., 2015, 6, 6924, DOI:10.1038/ncomms7924.
- M. Ouallal, S. Leyer, and S. Gupta, ‘Literature survey of droplet entrainment from water pools’, 2021, DOI:10.1016/j.nucengdes.2021.111188.
- D. P. Finegan, E. Darcy, M. Keyser, B. Tjaden, T. M. M. Heenan, R. Jervis, J. J. Bailey, N. T. Vo, O. V. Magdysyuk, M. Drakopoulos, M. D. Michiel, A. Rack, G. Hinds, D. J. L. Brett and P. R. Shearing, Identifying the Cause of Rupture of Li-Ion Batteries during Thermal Runaway, Adv. Sci., 2018, 5(1), 1700369, DOI:10.1002/advs.201700369.
- L. Lin, J. T. Sears and C. Y. Wen, Elutriation and attrition of char form a large fluidized bed, Powder Technol., 1980, 27(1), 105, DOI:10.1016/0032-5910(80)85045-5.
- J. Kim, A. Mallarapu, D. P. Finegan and S. Santhanagopalan, Modeling cell venting and gas-phase reactions in 18650 lithium ion batteries during thermal runaway, J. Power Sources, 2021, 489, 229496, DOI:10.1016/j.jpowsour.2021.229496.
- D. Kong, H. Lv, P. Ping and G. Wang, A review of early warning methods of thermal runaway oflithium ion batteries, J. Energy Storage, 2023, 64, 107073, DOI:10.1016/j.est.2023.107073.
- D. Han, J. Wang, C. Yin and Y. Zhao, Advances in Early Warning of Thermal Runaway in Lithium-Ion Battery Energy Storage Systems, Adv. Sens. Res., 2025, 4(5), 2400165, DOI:10.1002/adsr.202400165.
- Z. Wang, L. Zhu, J. Liu, J. Wang and W. Yan, Gas Sensing Technology for the Detection and Early Warning of Battery Thermal Runaway: A Review, Energy Fuels, 2022, 36(12), 6038–6057, DOI:10.1021/acs.energyfuels.2c01121.
- J. Li, Y. Li, and W. Zeng, ‘Gas Sensing Technology as the Key to Safety Warning of Lithium-Ion Battery: Recent Advance’, 2024, DOI:10.1016/j.sna.2023.114890.
- X. Shao, et al., Recent advances in semiconductor gas sensors for thermal runaway early-warning monitoring of lithium-ion batteries, Coord. Chem. Rev., 2025, 535, 216624, DOI:10.1016/j.ccr.2025.216624.
- Y. Shen, B. Zou, Z. Zhang, M. Xu, S. Wang, Q. Li, H. Li, M. Zhou, K. Jiang and K. Wang, In situ detection of lithium-ion batteries by ultrasonic technologies, Energy Stor. Mater., 2023, 62, 102915, DOI:10.1016/j.ensm.2023.102915.
- W. Xu, L. Li, F. Shi and Q. Chen, Ultrasonic spectroscopy for in situ early detection and dynamic monitoring of lithium plating in lithium-ion batteries, Cell Rep. Phys. Sci., 2025, 6(4), p.102507, DOI:10.1016/j.xcrp.2025.102507.
- D. B. Sulas, S. Johnston, N. Seitzman, H. Platt, M. Al-Jassim and H. Guthrey, Defect Detection in Solid-State Battery Electrolytes Using Lock-In Thermal Imaging, J. Electrochem. Soc., 2018, 165(13), A3205, DOI:10.1149/2.0131814jes.
- M. S. Md Said and M. Z. Mohd Tohir, Visual and thermal imaging of lithium-ion battery thermal runaway induced by mechanical impact, J. Loss Prev. Process Ind., 2022, 79, 104854, DOI:10.1016/j.jlp.2022.104854.
- W. Mei, Z. Liu, C. Wang, C. Wu, Y. Liu, P. Liu, X. Xia, X. Xue, X. Han, J. Sun, G. Xiao, H. Tam, J. Albert, Q. Wang and T. Guo, Operando monitoring of thermal runaway in commercial lithium-ion cells via advanced lab-on-fiber technologies, Nat. Commun., 2023, 14(1), 5251, DOI:10.1038/s41467-023-40995-3.
- W. Jeong, S.-O. Kim, H. Lim and K. Lee, High-resolution thermal monitoring of lithium-ion batteries using Brillouin scattering based fiber optic sensor with flexible spatial arrangement of sensing points, J. Energy Storage, 2024, 104, 114558, DOI:10.1016/j.est.2024.114558.
- P. Dong, Z. Liu, P. Wu, Z. Li, Z. Wang and J. Zhang, Reliable and Early Warning of Lithium-Ion Battery Thermal Runaway Based on Electrochemical Impedance Spectrum, J. Electrochem. Soc., 2021, 168(9), 090529, DOI:10.1149/1945-7111/ac239b.
- Y. Li, et al., Early warning method for thermal runaway of lithium-ion batteries under thermal abuse condition based on online electrochemical impedance monitoring, J. Energy Chem., 2024, 92, 74–86, DOI:10.1016/j.jechem.2023.12.049.
- J. Schöberl, J. Schumacher, R. Urban and M. Lienkamp, Impedance-based thermal runaway detection and temperature estimation for single and parallel connected large-format automotive lithium-ion batteries, eTransportation, 2025, 100424, DOI:10.1016/j.etran.2025.100424.
- X. Du, J. Meng, Z. Xue, Y. Amirat, F. Gao and M. Benbouzid, Revolutionizing Battery Safety: Real-Time Insights with Dynamic Electrochemical Impedance Spectroscopy, ACS Energy Lett., 2025, 10(5), 2292–2304, DOI:10.1021/acsenergylett.5c00484.
- Q. Yu, et al., Unsupervised learning for lithium-ion batteries fault diagnosis and thermal runaway early warning in real-world electric vehicles, J. Energy Storage, 2025, 109, 115194, DOI:10.1016/j.est.2024.115194.
- M. Naguib, J. Chen, P. Kollmeyer and A. Emadi, Thermal fault detection of lithium-ion battery packs through an integrated physics and deep neural network based model, Comms. Eng., 2025, 4(1), 79, DOI:10.1038/s44172-025-00409-2.
- C. F. Lopez, J. A. Jeevarajan and P. P. Mukherjee, Experimental Analysis of Thermal Runaway and Propagation in Lithium-Ion Battery Modules, J. Electrochem. Soc., 2015, 162(9), A1905–A1915, DOI:10.1149/2.0921509jes.
- B. Truchot, F. Fouillen and S. Collet, An experimental evaluation of toxic gas emissions from vehicle fires, Fire Saf. J., 2018, 97, 111–118, DOI:10.1016/j.firesaf.2017.12.002.
- P. Sturm, et al., Fire tests with lithium-ion battery electric vehicles in road tunnels, Fire Saf. J., 2022, 134, 103695, DOI:10.1016/j.firesaf.2022.103695.
- M. Quant, O. Willstrand, T. Mallin and J. Hynynen, Ecotoxicity Evaluation of Fire-Extinguishing Water from Large-Scale Battery and Battery Electric Vehicle Fire Tests, Environ. Sci. Technol., 2023, 57(12), 4821–4830, DOI:10.1021/acs.est.2c08581.
- E. Funk, K. W. Flecknoe-Brown, T. Wijesekere, B. P. Husted and B. Andres, Fire extinguishment tests of electric vehicles in an open sided enclosure, Fire Saf. J., 2023, 141, 103920, DOI:10.1016/j.firesaf.2023.103920.
- S. Kang, M. Kwon, J. Y. Choi and S. Choi, Full-Scale Fire Testing to Assess the Risk of Battery Electric Vehicle Fires in Underground Car Parks, Fire Technol., 2025 DOI:10.1007/s10694-024-01694-7.
- J. Zhang, H. Huang, G. Zhang, Z. Dai, Y. Wen and L. Jiang, Cycle life studies of lithium-ion power batteries for electric vehicles: A review, J. Energy Storage, 2024, 93, 112231, DOI:10.1016/j.est.2024.112231.
- J. Zhu, W. Xu, M. Knapp, M. S. D. Darma, L. Mereacre, P. Su, W. Hua, X. L. Théato, H. Dai, X. Wei and H. Ehrenberg, A method to prolong lithium-ion battery life during the full life cycle, Cell Rep. Phys. Sci., 2023, 4(7), 101464, DOI:10.1016/j.xcrp.2023.101464.
- T. R. Jow, S. A. Delp, J. L. Allen, J.-P. Jones and M. C. Smart, Factors Limiting Li + Charge Transfer Kinetics in Li-Ion Batteries, J. Electrochem. Soc., 2018, 165(2), A361, DOI:10.1149/2.1221802jes.
- J. S. Edge et al., ‘Lithium ion battery degradation: what you need to know’, 2021, 10.1039/d1cp00359c.
- Y. Li, et al., Evolution of aging mechanisms and performance degradation of lithium-ion battery from moderate to severe capacity loss scenarios, Chem. Eng. J., 2024, 498, 155588, DOI:10.1016/j.cej.2024.155588.
- Y. Makimura, T. Sasaki, T. Nonaka, Y F. Nishimura, T. Uyama, C. Okuda, Y. Itoua and Y. Takeuchi, Factors affecting cycling life of LiNi0.8Co0.15Al0.05O2 for lithium-ion batteries, J. Mater. Chem. A, 2016, 4(21), 8350–8358, 10.1039/c6ta01251e.
- J. R. Belt, C. D. Ho, T. J. Miller, M. A. Habib and T. Q. Duong, The effect of temperature on capacity and power in cycled lithium ion batteries, J. Power Sources, 2005, 142(1–2), 354–360, DOI:10.1016/j.jpowsour.2004.10.029.
- C. Liu, K. Qian, D. Lei, B. Li, F. Kang and Y. B. He, Deterioration mechanism of LiNi0.8Co0.15Al0.05O2/graphite-SiO:X power batteries under high temperature and discharge cycling conditions, J. Mater. Chem. A, 2017, 6(1), 65–72, 10.1039/c7ta08703a.
- T. Waldmann, M. Wilka, M. Kasper, M. Fleischhammer and M. Wohlfahrt-Mehrens, Temperature dependent ageing mechanisms in Lithium-ion batteries - A Post-Mortem study, J. Power Sources, 2014, 262, 129–135, DOI:10.1016/j.jpowsour.2014.03.112.
- Y. Zhao, S. Y. Choe and J. Kee, Modeling of degradation effects and its integration into electrochemical reduced order model for Li(MnNiCo)O2/Graphite polymer battery for real time applications, Electrochim. Acta, 2018, 270, 440–452, DOI:10.1016/j.electacta.2018.02.086.
- W. Wu, W. Wu, X. Qiu and S. Wang, Low-temperature reversible capacity loss and aging mechanism in lithium-ion batteries for different discharge profiles, Int. J. Energy Res., 2019, 43(1), 1–11, DOI:10.1002/er.4257.
- Z. Cai, S. Mendoza, J. Goodman, J. McGann, B. Han, H. Sanchez and R. Spray, The Influence of Cycling, Temperature, and Electrode Gapping on the Safety of Prismatic Lithium-Ion Batteries, J. Electrochem. Soc., 2020, 167(16), 160515, DOI:10.1149/1945-7111/abcabc.
- Y. Gao, J. Jiang, C. Zhang, W. Zhang, Z. Ma and Y. Jiang, Lithium-ion battery aging mechanisms and life model under different charging stresses, J. Power Sources, 2017, 356, 103–114, DOI:10.1016/j.jpowsour.2017.04.084.
- S. Barcellona and L. Piegari, Effect of current on cycle aging of lithium ion batteries, J. Energy Storage, 2020, 29, 101310, DOI:10.1016/j.est.2020.101310.
- W. Wang, B. Yuan, Q. Sun and R. Wennersten, Analysis and Modeling of Calendar Aging and Cycle Aging of LiCoO2/Graphite Cells, J. Therm. Sci., 2024, 33(3), 1109–1118, DOI:10.1007/s11630-024-1918-z.
- E. Peled, D. Golodnitsky and G. Ardel, Advanced Model for Solid Electrolyte Interphase Electrodes in Liquid and Polymer Electrolytes, J. Electrochem. Soc., 1997, 144(8), L208, DOI:10.1149/1.1837858.
- Y. Li, W. Huang, Y. Li, A. Pei, D. T. Boyle and Y. Cui, Correlating Structure and Function of Battery Interphases at Atomic Resolution Using Cryoelectron Microscopy, Joule, 2018, 2(10), 2167–2177, DOI:10.1016/j.joule.2018.08.004.
- A. Maraschky and R. Akolkar, Mechanism Explaining the Onset Time of Dendritic Lithium Electrodeposition via Considerations of the Li+ Transport within the Solid Electrolyte Interphase, J. Electrochem. Soc., 2018, 165(14), D696, DOI:10.1149/2.0601814jes.
- B. Han, et al., Poor Stability of Li2CO3 in the Solid Electrolyte Interphase of a Lithium-Metal Anode Revealed by Cryo-Electron Microscopy, Adv. Mater., 2021, 33(22), 2100404, DOI:10.1002/adma.202100404.
- J. P. Pender et al., ‘Electrode Degradation in Lithium-Ion Batteries’, 2020, DOI:10.1021/acsnano.9b04365.
- S. Wang, Y. Wang and Y.-Y. Soo, Evaluation and prediction of lithium-ion battery pack inconsistency in electric vehicles based on actual operating data, Energy, 2025, 319, 134879, DOI:10.1016/j.energy.2025.134879.
- D. N. T. How, M. A. Hannan, M. S. Hossain Lipu and P. J. Ker, State of Charge Estimation for Lithium-Ion Batteries Using Model-Based and Data-Driven Methods: A Review, 2019, vol. 7, pp. 136116–136136, DOI:10.1109/ACCESS.2019.2942213.
- M. A. Hannan, et al., Toward Enhanced State of Charge Estimation of Lithium-ion Batteries Using Optimized Machine Learning Techniques, Sci. Rep., 2020, 10(1), 4687, DOI:10.1038/s41598-020-61464-7.
- X. Li, H. Jiang, S. Guo, J. Xu, M. Li, X. Liu and X. Zhang, SOC Estimation of Lithium-Ion Battery for Electric Vehicle Based on Deep Multilayer Perceptron, Comput. Intell. Neurosci., 2022, 2022, 12, DOI:10.1155/2022/3920317.
- N. Fallah and C. Fitzpatrick, Exploring the state of health of electric vehicle batteries at end of use; hierarchical waste flow analysis to determine the recycling and reuse potential, Journal of Remanufacturing, 2024, 14(1), 155, DOI:10.1007/s13243-024-00137-4.
- S. Tao, et al., Rapid and sustainable battery health diagnosis for recycling pretreatment using fast pulse test and random forest machine learning, J. Power Sources, 2024, 597, 234156, DOI:10.1016/j.jpowsour.2024.234156.
- S. Tao, R. Ma, Z. Zhao, G. Ma, L. Su, H. Chang, Y. Chen, H. Liu, Z. Liang, T. Cao, H. Ji, Z. Han, M. Lu, H. Yang, Z. Wen, J. Yao, R. Yu, G. Wei, Y. Li, X. Zhang, T. Xu and G. Zhou, Generative learning assisted state-of-health estimation for sustainable battery recycling with random retirement conditions, Nat. Commun., 2024, 15(1), 10154, DOI:10.1038/s41467-024-54454-0.
- E. Kim, M. Kim, J. Kim, J. Kim, J. H. Park, K. T. Kim, J. H. Park, T. Kim and K. Min, Data-Driven Methods for Predicting the State of Health, State of Charge, and Remaining Useful Life of Li-Ion Batteries, A Comprehensive Review, 2023, vol. 24, pp. 1281–1304, DOI:10.1007/s12541-023-00832-5.
- S. Wang, S. Zhang, S. Wen and C. Fernandez, An accurate state-of-charge estimation of lithium-ion batteries based on improved particle swarm optimization-adaptive square root cubature kalman filter, J. Power Sources, 2024, 624, 235594, DOI:10.1016/j.jpowsour.2024.235594.
- V. Selvaraj and I. Vairavasundaram, ‘A comprehensive review of state of charge estimation in lithium-ion batteries used in electric vehicles’, 2023, DOI:10.1016/j.est.2023.108777.
- S. B. Sarmah et al., ‘A Review of State of Health Estimation of Energy Storage Systems: Challenges and Possible Solutions for Futuristic Applications of Li-Ion Battery Packs in Electric Vehicles’, 2019, DOI:10.1115/1.4042987.
- Z. Shi, et al., An improved adaptive square root cubature Kalman filter method for estimating state-of-charge of lithium-ion batteries, J. Energy Storage, 2023, 72, 108245, DOI:10.1016/j.est.2023.108245.
- S. Wang, C. Wang, P. Takyi-Aninakwa, S. Jin, C. Fernandez and Q. Huang, An improved parameter identification and radial basis correction-differential support vector machine strategies for state-of-charge estimation of urban-transportation-electric-vehicle lithium-ion batteries, J. Energy Storage, 2024, 80, 110222, DOI:10.1016/j.est.2023.110222.
- W. Pantoja, J. A. Perez-Taborda, and A. Avila, ‘Tug-of-War in the Selection of Materials for Battery Technologies’, 2022, DOI:10.3390/batteries8090105.
- C. P. Makwarimba et al., ‘Assessment of recycling methods and processes for lithium-ion batteries’, 2022, DOI:10.1016/j.isci.2022.104321.
- G. Zhao and J. Baker, Effects on environmental impacts of introducing electric vehicle batteries as storage - a case study of the United Kingdom, Energy Strategy Rev., 2022, 40, 100819, DOI:10.1016/j.esr.2022.100819.
- G. A. Heath, D. Ravikumar, B. Hansen, and E. Kupets, ‘A critical review of the circular economy for lithium-ion batteries and photovoltaic modules–status, challenges, and opportunities’, 2022, DOI:10.1080/10962247.2022.2068878.
- Z. Yang, H. Huang and F. Lin, Sustainable Electric Vehicle Batteries for a Sustainable World: Perspectives on Battery Cathodes, Environment, Supply Chain, Manufacturing, Life Cycle, and Policy, Adv. Energy Mater., 2022, 12(26), 2200383, DOI:10.1002/aenm.202200383.
- L. Gaines, Lithium-ion battery recycling processes: Research towards a sustainable course, Sustain. Mater. Technol., 2018, 17, e00068, DOI:10.1016/j.susmat.2018.e00068.
- A. Pražanová, V. Knap, and D. I. Stroe, ‘Literature Review, Recycling of Lithium-Ion Batteries from Electric Vehicles, Part II: Environmental and Economic Perspective’, 2022, DOI:10.3390/en15197356.
- H. Mahandra and A. Ghahreman, A sustainable process for selective recovery of lithium as lithium phosphate from spent LiFePO4 batteries, Resour. Conserv. Recycl., 2021, 175, 105883, DOI:10.1016/j.resconrec.2021.105883.
- S. Wu, et al., A novel approach for lithium recovery from waste lithium-containing aluminum electrolyte by a roasting-leaching process, Waste Manage., 2021, 134, 89, DOI:10.1016/j.wasman.2021.08.011.
- K. Park, et al., Direct Cathode Recycling of End-Of-Life Li-Ion Batteries Enabled by Redox Mediation, ACS Sustain. Chem. Eng., 2021, 9(24), 8214, DOI:10.1021/acssuschemeng.1c02133.
- S. Kim et al., ‘A comprehensive review on the pretreatment process in lithium-ion battery recycling’, 2021, DOI:10.1016/j.jclepro.2021.126329.
- D. Yu, Z. Huang, B. Makuza, X. Guo, and Q. Tian, ‘Pretreatment options for the recycling of spent lithium-ion batteries: A comprehensive review’, 2021 DOI:10.1016/j.mineng.2021.107218.
- H. Qiu, C. Peschel, M. Winter, S. Nowak, J. Köthe and D. Goldmann, Recovery of Graphite and Cathode Active Materials from Spent Lithium-Ion Batteries by Applying Two Pretreatment Methods and Flotation Combined with a Rapid Analysis Technique, Metals, 2022, 12(4), 677, DOI:10.3390/met12040677.
- L. Li, P. Zheng, T. Yang, R. Sturges, M. W. Ellis and Z. Li, Disassembly Automation for Recycling End-of-Life Lithium-Ion Pouch Cells, JOM, 2019, 71(12), 4457, DOI:10.1007/s11837-019-03778-0.
- L. Wuschke, H. G. Jäckel, T. Leißner and U. A. Peuker, Crushing of large Li-ion battery cells, Waste Manage., 2019, 85, 317, DOI:10.1016/j.wasman.2018.12.042.
- H. Dang, et al., Recycled Lithium from Simulated Pyrometallurgical Slag by Chlorination Roasting, ACS Sustain. Chem. Eng., 2018, 6(10), 13160, DOI:10.1021/acssuschemeng.8b02713.
- X. Zheng, et al., Spent lithium-ion battery recycling – Reductive ammonia leaching of metals from cathode scrap by sodium sulphite, Waste Manage., 2017, 60, 680, DOI:10.1016/j.wasman.2016.12.007.
- M. Zhou, B. Li, J. Li, and Z. Xu, ‘Pyrometallurgical Technology in the Recycling of a Spent Lithium Ion Battery: Evolution and the Challenge’, 2021, DOI:10.1021/acsestengg.1c00067.
- L. D. Hylander and R. B. Herbert, Global emission and production of mercury during the pyrometallurgical extraction of nonferrous sulfide ores, Environ. Sci. Technol., 2008, 42(16), 5971, DOI:10.1021/es800495g.
- Z. Huang, F. Liu, B. Makuza, D. Yu, X. Guo and Q. Tian, Metal Reclamation from Spent Lithium-Ion Battery Cathode Materials: Directional Conversion of Metals Based on Hydrogen Reduction, ACS Sustain. Chem. Eng., 2022, 10(2), 756, DOI:10.1021/acssuschemeng.1c05721.
- E. Fan, et al., Leaching Mechanisms of Recycling Valuable Metals from Spent Lithium-Ion Batteries by a Malonic Acid-Based Leaching System, ACS Appl. Energy Mater., 2020, 3(9), 8532, DOI:10.1021/acsaem.0c01166.
- B. Liu, et al., Direct Recycling of Spent LiNi0.5Co0.2Mn0.3O2 Cathodes Based on Single Oxalic Acid Leaching and Regeneration under Mild Conditions Assisted by Lithium Acetate, Energy Fuels, 2022, 36(12), 6552, DOI:10.1021/acs.energyfuels.2c00974.
- Q. Yan, A. Ding, M. Li, C. Liu and C. Xiao, Green Leaching of Lithium-Ion Battery Cathodes by Ascorbic Acid Modified Guanidine-Based Deep Eutectic Solvents, Energy Fuels, 2023, 37(2), 1216, DOI:10.1021/acs.energyfuels.2c03699.
- L. Gaines, Q. Dai, J. T. Vaughey and S. Gillard, Direct recycling R&D at the recell center, Recycling, 2021, 6(2), 31, DOI:10.3390/recycling6020031.
- Y. Shi, G. Chen and Z. Chen, Effective regeneration of LiCoO2 from spent lithium-ion batteries: A direct approach towards high-performance active particles, Green Chem., 2018, 20(4), 851, 10.1039/c7gc02831h.
- J. Li, Y. Lu, T. Yang, D. Ge, D. L. Wood and Z. Li, Water-Based Electrode Manufacturing and Direct Recycling of Lithium-Ion Battery Electrodes—A Green and Sustainable Manufacturing System, iScience, 2020, 23(5), 101081, DOI:10.1016/j.isci.2020.101081.
- P. Xu, et al., Design and Optimization of the Direct Recycling of Spent Li-Ion Battery Cathode Materials, ACS Sustain. Chem. Eng., 2021, 9(12), 4543, DOI:10.1021/acssuschemeng.0c09017.
- H. Gao, et al., Efficient Direct Recycling of Degraded LiMn2O4 Cathodes by One-Step Hydrothermal Relithiation, ACS Appl. Mater. Interfaces, 2020, 12(46), 51546–51554, DOI:10.1021/acsami.0c15704.
- A. T. Montoya, et al., Direct Recycling of Lithium-Ion Battery Cathodes: A Multi-Stage Annealing Process to Recover the Pristine Structure and Performance, ACS Sustain. Chem. Eng., 2022, 10(40), 13319, DOI:10.1021/acssuschemeng.2c02643.
- Z. J. Baum, R. E. Bird, X. Yu and J. Ma, Lithium-Ion Battery Recycling─Overview of Techniques and Trends, ACS Energy Lett., 2022, 7(2), 712, DOI:10.1021/acsenergylett.1c02602.
- L. Zhang, Z. Xu and Z. He, Electrochemical Relithiation for Direct Regeneration of LiCoO2 Materials from Spent Lithium-Ion Battery Electrodes, ACS Sustain. Chem. Eng., 2020, 8(31), 11596, DOI:10.1021/acssuschemeng.0c02854.
- S. Chen, et al., Electrochemical Relithiation in Spent LiFePO4 Slurry for Regeneration of Lithium-Ion Battery Cathode, Inorg. Chem., 2024, 63(37), 17166–17175, DOI:10.1021/acs.inorgchem.4c02844.
- Y. Gao, et al., Thick electrodes for electrochemical relithiation to regenerate spent battery powder, Energy Stor. Mater., 2025, 78, 104269, DOI:10.1016/j.ensm.2025.104269.
- X. Zhang et al., ‘Advances in bioleaching of waste lithium batteries under metal ion stress’, 2023, DOI:10.1186/s40643-023-00636-5.
- N. Sadeghi, F. Vakilchap, Z. Ilkhani and S. M. Mousavi, Assessment of the visible light effect on one-step bacterial leaching of metals from spent lithium-ion batteries cathode pretreated by a bio-chemical lixiviant, J. Cleaner Prod., 2024, 436, 140432, DOI:10.1016/j.jclepro.2023.140432.
- J. Kim, H. H. Nwe and C. S. Yoon, Enhanced bioleaching of spent Li-ion batteries using A. ferrooxidans by application of external magnetic field, J. Environ. Manage., 2024, 367, 122012, DOI:10.1016/j.jenvman.2024.122012.
- A. Mukhopadhyay, et al., Electrochemically Assisted (Bio)leaching of End-of-Life Lithium-Ion Batteries for Critical Metals Recovery, ACS Sustain. Chem. Eng., 2024, 12(37), 14119–14127, DOI:10.1021/acssuschemeng.4c06090.
- N. Zachmann and B. Ebin, Supercritical CO2 extraction behavior of electrolyte solvents from Li-ion battery black mass, J. CO2 Util., 2025, 99, 103145, DOI:10.1016/j.jcou.2025.103145.
- N. Zachmann, R. V Fox, M. Petranikova and B. Ebin, Implementation of a sub-and supercritical carbon dioxide process for the selective recycling of the electrolyte from spent Li-ion battery, J. CO2 Util., 2024, 81, 102703, DOI:10.1016/j.jcou.2024.102703.
- E. V Beletskii and V. Romanovski, Direct plasma solution recycling of cathode materials for lithium-ion batteries with simultaneous removal of contaminants and relithiation, J. Power Sources, 2024, 624, 235576, DOI:10.1016/j.jpowsour.2024.235576.
- Y. Fan, C. Qian, J. Yang, J. Zhu and Y. Cai, In-situ plasma assisted lithium-ion battery cathodes to catalytically pyrolyze lignin for H2-rich syngas production, Energy Convers. Manage., 2024, 307, 118359, DOI:10.1016/j.enconman.2024.118359.
- M. Jacoby, It's time to recycle lithium-ion batteries, C&EN Global Enterprise, 2019, 97(28), 29–32, DOI:10.1021/cen-09728-cover.
- J. Kirchherr, D. Reike and M. Hekkert, Conceptualizing the circular economy: an analysis of 114 definitions, Resour. Conserv. Recycl., 2017, 127, 221–232, DOI:10.1016/j.resconrec.2017.09.005.
- J. Korhonen, C. Nuur, A. Feldmann and S. E. Birkie, Circular economy as an essentially contested concept, J. Cleaner Prod., 2018, 175, 544–552, DOI:10.1016/j.jclepro.2017.12.111.
- F. Figge, A. S. Thorpe and M. Gutberlet, Definitions of the circular economy: circularity matters, Ecol. Econ., 2023, 208, 107823, DOI:10.1016/j.ecolecon.2023.107823.
- M. A. Kasri, et al., Addressing preliminary challenges in upscaling the recovery of lithium from spent lithium ion batteries by the electrochemical method: a review, RSC Adv., 2024, 14(22), 15515–15541, 10.1039/D4RA00972J.
- M. Goyal, K. Singh and N. Bhatnagar, Circular economy conceptualization for lithium-ion batteries- material procurement and disposal process, Chem. Eng. Sci., 2023, 281, 119080, DOI:10.1016/j.ces.2023.119080.
- A. Thomas and U. Mishra, Industry 4.0 and circular economy model for a sustainable electric vehicle battery with controllable wastewater and carbon emission, Energy Rep., 2024, 11, 4044–4066, DOI:10.1016/j.egyr.2024.03.054.
- M. Rezaei, et al., A review of lithium-ion battery recycling for enabling a circular economy, J. Power Sources, 2025, 630, 236157, DOI:10.1016/j.jpowsour.2024.236157.
- J. Feng, W. Liu and F. Chen, Moving towards a circular economy: A systematic review of barriers to electric vehicle battery recycling, Sustain. Prod. Consum., 2025, 54, 241–260, DOI:10.1016/j.spc.2025.01.006.
- J. L. Holechek, H. M. E. Geli, M. N. Sawalhah and R. Valdez, A Global Assessment: Can Renewable Energy Replace Fossil Fuels by 2050?, Sustainability, 2022, 14(8), 4792, DOI:10.3390/su14084792.
- T. Mihalj, et al., Road Infrastructure Challenges Faced by Automated Driving: A Review, Appl. Sci., 2022, 12(7), 3477, DOI:10.3390/app12073477.
- R. Pagany, L. Ramirez Camargo, and W. Dorner, ‘A review of spatial localization methodologies for the electric vehicle charging infrastructure’, 2019, DOI:10.1080/15568318.2018.1481243.
- K. Anastasiadou, N. Gavanas, M. Pitsiava-latinopoulou and E. Bekiaris, Infrastructure planning for autonomous electric vehicles, integrating safety and sustainability aspects: A multi-criteria analysis approach, Energies, 2021, 14(17), 5269, DOI:10.3390/en14175269.
- S. Li, L. Tong, J. Xing and Y. Zhou, The market for electric vehicles: Indirect network effects and policy design, J. Assoc. Environ. Resour. Econ., 2017, 4(1), 89, DOI:10.1086/689702.
- S. Li, X. Zhu, Y. Ma, F. Zhang, and H. Zhou, ‘The Role of Government in the Market for Electric Vehicles Evidence from China’, 20208, accessed, Apr. 01, 2025, Online, available, http://www.worldbank.org/prwp Search PubMed.
- S. Li, B. Wang, M. Yang, and F. Zhang, ‘The Global Diffusion of Electric Vehicles Lessons from the First Decade’, 2021, accessed, Apr. 01, 2025, Online, available, http://www.worldbank.org/prwp Search PubMed.
- ‘ElEctric mobility in india Energy and Extractives Global Practice Group South Asia Region (SAR)’, 2021, accessed, Apr. 01, 2025, Online, available, https://hdl.handle.net/10986/35655 Search PubMed.
- C. Briceno-Garmendia, W. Qiao, and V. Foster, ‘ELECTRIC VEHICLES for Passenger Transportation’, 2023, accessed, Apr. 01, 2025, Online, available, https://openknowledge.worldbank.org/handle/10986/39513 Search PubMed.
- I. – International Energy Agency, ‘Global EV Outlook 2023: Catching up with climate ambitions’, 2023 Search PubMed.
- M. M. Hussain, M. M. S. Beg, M. S. Alam and S. H. Laskar, Big data analytics platforms for electric vehicle integration in transport oriented smart cities: Computing platforms for platforms for electric vehicle integration in smart cities, Int. J. Digit. Crime Forensics., 2019, 11(3), 883, DOI:10.4018/IJDCF.2019070102.
- G. Blumberg, R. Broll and C. Weber, The impact of electric vehicles on the future European electricity system – A scenario analysis, Energy Policy, 2022, 161, 112751, DOI:10.1016/j.enpol.2021.112751.
- R. Dhairiyasamy, D. Gabiriel, W. Bunpheng and C. C. Kit, A comprehensive analysis of India's electric vehicle battery supply chain: barriers and solutions, Discov. Sustain., 2024, 5(1), 361, DOI:10.1007/s43621-024-00595-7.
- Y. Li, C. Guan, A. J. Purwanto, B. Chen and Y. Zhao, Distribution of the ASEAN battery electric vehicle production network: Mapping the interplay of endowments, policies, and global integration, Energy Sustain. Dev., 2025, 85, 101649, DOI:10.1016/j.esd.2024.101649.
- Q. Wu, T. Zhang, J. Geng, S. Gao, H. Ma and F. Li, Anionic Redox Chemistry for Sodium-Ion Batteries: Mechanisms, Advances, and Challenges, Energy Fuels, 2022, 36(15), 8081, DOI:10.1021/acs.energyfuels.2c01601.
- G. Huang, et al., Overview of hard carbon anode for sodium-ion batteries: Influencing factors and strategies to extend slope and plateau regions, Carbon N Y, 2024, 228, 119354, DOI:10.1016/j.carbon.2024.119354.
- K. A. J. Dilshad and M. K. Rabinal, ‘Review on Molecularly Controlled Design of Electrodes for Metal–Air Batteries: Fundamental Concepts and Future Directions’, 2023, DOI:10.1021/acs.energyfuels.2c04147.
- P. Tan et al., ‘Flexible Zn- and Li-air batteries: Recent advances, challenges, and future perspectives’, 2017, 10.1039/c7ee01913k.
- H. F. Wang and Q. Xu, ‘Materials Design for Rechargeable Metal–Air Batteries’, 2019, DOI:10.1016/j.matt.2019.05.008.
- M. T. Tsehaye, et al., Membranes for zinc-air batteries: Recent progress, challenges and perspectives, J. Power Sources, 2020, 475, 228689, DOI:10.1016/j.jpowsour.2020.228689.
- R. Chen, Q. Li, X. Yu, L. Chen, and H. Li, ‘Approaching Practically Accessible Solid-State Batteries: Stability Issues Related to Solid Electrolytes and Interfaces’, 2020, DOI:10.1021/acs.chemrev.9b00268.
- Z. Ding, J. Li, J. Li and C. An, Review—Interfaces: Key Issue to Be Solved for All Solid-State Lithium Battery Technologies, J. Electrochem. Soc., 2020, 167(7), 070541, DOI:10.1149/1945-7111/ab7f84.
- L. Wang, D. Liu, T. Huang, Z. Geng and A. Yu, Reducing interfacial resistance of a Li1.5Al0.5Ge1.5(PO4)3 solid electrolyte/electrode interface by polymer interlayer protection, RSC Adv., 2020, 10(17), 10038, 10.1039/d0ra00829j.
- S. Kobayashi, et al., Drastic Reduction of the Solid Electrolyte–Electrode Interface Resistance via Annealing in Battery Form, ACS Appl. Mater. Interfaces, 2022, 14(2), 2703, DOI:10.1021/acsami.1c17945.
- Y. Xiao, Y. Wang, S. H. Bo, J. C. Kim, L. J. Miara, and G. Ceder, ‘Understanding interface stability in solid-state batteries’, 2020, DOI:10.1038/s41578-019-0157-5.
- Y. Zhao, et al., Recycling of sodium-ion batteries, Nat. Rev. Mater., 2023, 8(9), 623–634, DOI:10.1038/s41578-023-00574-w.
- H. Rostami, J. Valio, P. Suominen, P. Tynjälä and U. Lassi, Advancements in cathode technology, recycling strategies, and market dynamics: a comprehensive review of sodium ion batteries, Chem. Eng. J., 2024, 495, 153471, DOI:10.1016/j.cej.2024.153471.
- P. Jakes, G. Cohn, Y. Ein-Eli, F. Scheiba, H. Ehrenberg and R.-A. Eichel, Limitation of Discharge Capacity and Mechanisms of Air-Electrode Deactivation in Silicon–Air Batteries, ChemSusChem, 2012, 5(11), 2278–2285, DOI:10.1002/cssc.201200199.
- G. Wang, et al., Understanding Moisture and Carbon Dioxide Involved Interfacial Reactions on Electrochemical Performance of Lithium–Air Batteries Catalyzed by Gold/Manganese-Dioxide, ACS Appl. Mater. Interfaces, 2015, 7(43), 23876–23884, DOI:10.1021/acsami.5b05250.
- C. Lin, et al., High-voltage asymmetric metal–air batteries based on polymeric single-Zn2+-ion conductor, Matter, 2021, 4(4), 1287–1304, DOI:10.1016/j.matt.2021.01.004.
- L. Azhari, S. Bong, X. Ma and Y. Wang, Recycling for All Solid-State Lithium-Ion Batteries, Matter, 2020, 3(6), 1845–1861, DOI:10.1016/j.matt.2020.10.027.
- M. Ahuis, S. Doose, D. Vogt, P. Michalowski, S. Zellmer and A. Kwade, Recycling of solid-state batteries, Nat. Energy, 2024, 9(4), 373–385, DOI:10.1038/s41560-024-01463-4.
- M. Jacob, K. Wissel and O. Clemens, Recycling of solid-state batteries—challenge and opportunity for a circular economy?, Mater. futures, 2024, 3(1), 012101, DOI:10.1088/2752-5724/acfb28.
- Z. Shao, R. Zhao, S. Yuan, M. Ding and Y. Wang, Tracing the evolution of AI in the past decade and forecasting the emerging trends, Expert Syst. Appl., 2022, 209, 118221, DOI:10.1016/j.eswa.2022.118221.
- V. V Krishna, A I and contemporary challenges: The good, bad and the scary, Journal of Open Innovation: Technology, Market, and Complexity, 2024, 10(1), 100178, DOI:10.1016/j.joitmc.2023.100178.
- Z. Bin Akhtar, Correction: Unveiling the evolution of generative AI (GAI): a comprehensive and investigative analysis toward LLM models (2021–2024) and beyond, J. Electr. Syst. Inf. Technol., 2025, 12(1), 5, DOI:10.1186/s43067-025-00196-y.
- G. Wei, et al., Direct recycling of spent Li-ion batteries: Challenges and opportunities toward practical applications, iScience, 2023, 26(9), 107676, DOI:10.1016/j.isci.2023.107676.
- Y. Zang, et al., Robotic disassembly of electric vehicle batteries: Technologies and opportunities, Comput. Ind. Eng., 2024, 198, 110727, DOI:10.1016/j.cie.2024.110727.
- A. Rastegarpanah, C. A. Contreras, M. Ahmeid, M. E. Asif, E. Villagrossi and R. Stolkin, Advanced robotics for automated EV battery testing using electrochemical impedance spectroscopy, Front. Robot. AI, 2025, 11, 1493869, DOI:10.3389/frobt.2024.1493869.
- J. Hathaway, A. Shaarawy, C. Akdeniz, A. Aflakian, R. Stolkin and A. Rastegarpanah, ‘Towards reuse and recycling of lithium-ion batteries: tele-robotics for disassembly of electric vehicle batteries’, Front. Robot AI, 2023, 10–2023, DOI:10.3389/frobt.2023.1179296.
| This journal is © The Royal Society of Chemistry 2025 |