 Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Role of laser ablation synthesis parameters in ORR electrocatalytic performance of MOF-derived hybrid nanocomposites†
Mahshid Mokhtarnejadab,
Erick L. Ribeiro*cd,
Soheil Almasi ab and
Bamin Khomami
ab and
Bamin Khomami *ab
*ab
aDepartment of Chemical & Biomolecular Engineering, University of Tennessee, Knoxville, Tennessee 37996, USA. E-mail: bkhomami@utk.edu
bMaterial Research and Innovation Laboratory (MRAIL), University of Tennessee, Knoxville, Tennessee 37996, USA
cDepartment of Mechanical Engineering, Polytechnic School of the University of São Paulo, São Paulo, SP 05508-030, Brazil. E-mail: erickribeiro@usp.br
dResearch Centre for Greenhouse Gas Innovation (RCGI), University of São Paulo, São Paulo, SP 05508-030, Brazil
First published on 18th July 2025
Abstract
This study presents a rapid, eco-friendly, and scalable method for fabricating non-precious metal electrocatalysts for the oxygen reduction reaction (ORR) using Laser Ablation Synthesis in Solution (LASiS). We demonstrate that by optimizing the laser output power and ablation time, Co-based metal–organic frameworks (MOFs) can be directly synthesized and converted into hybrid nanocomposites composed of Co3O4, CoO, and metallic Co embedded in nitrogen-doped carbon. These materials exhibit high porosity, stable crystalline structures, and enhanced ORR activity, including a four-electron transfer pathway, excellent durability, and performance comparable to commercial Pt/C catalysts. Compared with traditional hydrothermal methods, LASiS provides a template-free and solvent-minimizing alternative that enables precise control over particle size, structure, and composition in a single-step process. This work highlights the potential of LASiS as a powerful tool to develop next-generation sustainable energy materials.
Introduction
Electrochemical energy conversion technologies, particularly fuel cells, have become essential components of the sustainable energy infrastructure because of their high efficiency and low environmental footprint. Various types of fuel cells have been developed to date, including polymer electrolyte membrane fuel cells (PEMFC),1 anion exchange membrane fuel cells (AEMFC),2 alkaline fuel cells,3 solid oxide fuel cells (SOFCs),4 and molten carbonate fuel cells,5 to meet different energy demands. Among the different types of fuel cell technologies, AEMFCs offer notable advantages such as reduced reliance on expensive platinum group metal (PGM) catalysts and extended operational life in alkaline environments. Despite such advantages, the performance of AEMFC systems is hindered by the sluggish kinetics of the oxygen reduction reaction (ORR) at the cathode, which involves a complex four-electron transfer process and requires efficient and cost-effective electrocatalysts to accelerate the reaction kinetics.6To address the limitations of ORR in AEMFCs, significant research has focused on developing high-performance catalysts that can replace conventional platinum-based nanoparticles (NPs). Although platinum-based catalysts exhibit excellent activity, their high cost and poor stability in alkaline environments have led to the search for alternatives, nonprecious metal-based electro-catalysts.7 In this regard, carbon-based hybrid nanocomposites derived from a metal–organic framework (MOF), particularly zeolitic imidazolate frameworks (ZIF), have emerged as promising candidates because of their tunable porosity, high surface area, superior electron conductivity, and ability to host active Co–N–C sites.8–11 Upon pyrolysis, ZIFs, specifically Co/ZIF-67, transform into nitrogen-doped carbon structures embedded with cobalt or cobalt oxide phases, which are recognized for their ORR activity.12 However, optimizing the size, crystallinity, structure, and morphology of these catalysts is essential to maximize the number of accessible active sites and to improve mass transport during electrochemical reactions.13,14 Unfortunately, conventional methods for MOF synthesizing often suffer from long reaction times and limited control over particle size and morphology.15 Furthermore, residual solvents and unreacted precursors can compromise material purity and environmental sustainability.16 Therefore, rapid, green, and morphology-controllable synthesis techniques are needed to fully realize the electrocatalytic potential of MOF-derived hybrid nanocomposites, where precise structural tuning is needed for electrocatalytic applications.
In this study, we employ the Laser Ablation Synthesis in Solution (LASiS) technique as a rapid, cost-effective, and environmentally friendly method to synthesize a Co MOF, named ZIF-67, with controlled morphology and crystallinity. By tuning key LASiS parameters such as laser output power, ablation time, and postpyrolysis conditions, we aim to precisely control the size, crystallinity, and morphology of these highly porous particles as templates to enhance the electrochemical performance of the MOF-derived HNCs. After pyrolysis, the resulting Co3O4/CoO/Co@C HNCs are investigated as nonprecious metal electrocatalysts for ORR, offering a cost-effective alternative to Pt and other PGM catalysts. The optimal ZIF-67 platform was synthesized with a 10 minutes ablation time and an output power of 330 mJ per pulse, demonstrating the highest electrocatalytic performance after post-treatment at 500 °C. Detailed structural and electrochemical characterizations were used to reveal the correlation between synthesis conditions, nucleation dynamics, and active Co–N–C sites, ultimately providing mechanistic insights into the increased ORR performance of these catalysts. This work highlights the significance of LASiS parameters during MOF synthesis as a scalable pathway for designing high-performance, non-PGM electrocatalysts.
Experiments and methods
The methodology involves the synthesis, structural control, and optimization of ZIF-67 and its derived HNCs as ORR electrocatalysts, achieved by tuning the laser parameters during the LASiS process to tailor their size and morphology.ZIF-67-based HNCs synthesized via optimized laser ablation synthesis in solution (LASiS)
In this study, we synthesized Co-based metal–organic frameworks (Co/ZIF-67) using laser ablation synthesis in solution (LASiS). LASiS involves the laser ablation of a Co-metal target submerged in a liquid medium, forming a laser-induced plasma plume at the solid–liquid interface. In this work, we refer to the product as Co/ZIF-67 to distinguish it from conventionally synthesized ZIF-67, since cobalt ions are introduced by laser ablation of a metallic cobalt pellet rather than from a dissolved salt precursor. This approach enables precise control over composition and purity. The elevated temperature and pressure of the laser-derived plasma facilitate unique chemical reactions, enabling the synthesis of novel material phases by combining elements from the liquid and the metal target. Upon cooling and depressurization, the plasma allows for cluster growth and the formation of nanoparticles (NPs) and nanocrystals. In the context of MOF synthesis, although conventional methodologies, such as hydrothermal and solvothermal approaches, are widely employed, the synthesis of ZIF-67 through these routes presents several notable limitations, including high energy consumption, prolonged reaction times, stringent pressure and temperature requirements, limited scalability, broad particle size distributions, labor-intensive post-synthetic treatments, reliance on hazardous or costly solvents, and environmental concerns associated with solvent waste and inherent inefficiencies of batch processing.17 In contrast, LASiS offers a rapid, environmentally benign, and template-free alternative, allowing precise control over particle size and morphology, positioning itself as a particularly attractive strategy for the scalable and sustainable production of ZIF-67 and derivatives. For more details on the LASiS technique, see the following publications.18–22For the synthesis of Co/ZIF-67, we used an unfocused high-energy pulsed Nd:YAG laser (1064 nm) to ablate a cobalt target (Co) immersed and immobilized in an alkaline solution. We used potassium hydroxide (KOH, >99%) to adjust the solution to a pH of 13.3. Then, we added 1.8 g of 2-methylimidazole (Hmim) to 40 mL of the alkaline solution containing dissolved KOH (3 M) in DI water. A high hydroxyl concentration, provided by initial addition of KOH, is essential, as Hmim deprotonation is not feasible at lower pH values due to its high pKa (pKa(Hmim) = 14.2).23 All the required precursors and materials were obtained from Sigma-Aldrich and cobalt (Co) pallets with 1¼ inch diameter, 1¼ inch height and 99.5% purity, obtained from Kurt J. Lesker. A 1064 nm pulsed Nd-YAG laser with a 10 Hz repetition rate is employed for LASiS. To control the ZIF structures and optimize the laser parameters, ablation was performed at two different laser output powers (110 mJ per pulse and 330 mJ per pulse) for durations of 5, 10, 15, and 20 minutes. To facilitate the crystallization of Co/ZIF-67, given the inherently slow nucleation and crystallization kinetics of MOFs even in alkaline media, the suspensions were allowed to rest at room temperature for a period of 24 hours. Subsequently, the laser-synthesized products were centrifuged at 4700 rpm for 15 minutes, followed by sequential washing with deionized water and methanol multiple times to thoroughly eliminate any residual unreacted precursors. The final product was then treated with a pyrolysis process at various temperatures (500 to 800 °C) under N2 conditions for 2 hours to obtain HNCs based on ZIF Co3O4/CoO/Co@C with optimized structure and morphology for optimal ORR electrocatalyst performance. The LASiS process is schematically illustrated in Fig. 1.
 | ||
| Fig. 1 Schematic illustration of LASiS methodology used to synthesize Co/ZIF-67-derived ORR electrocatalysts. | ||
Raman spectroscopy was performed using an NT-MDT NTEGRA spectroscopy apparatus, employing a 532 nm green laser excitation. Thermogravimetric analysis (TGA) was carried out on a TA Instruments Q50 15 GA analyzer under a nitrogen (N2) atmosphere, heating samples from room temperature to 700 °C at a rate of 10 °C min−1. These analyzes were essential to determine the optimal pyrolysis conditions and thermal behavior of the laser-synthesized Co/ZIF-67 and Co3O4/CoO/Co hybrid nanocomposites (HNC). Overall, the characterization results confirmed the controlled synthesis and full crystallization of ZIF-based and hybrid structures suitable for electrocatalytic applications.
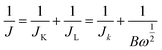 | (1) |
 | (2) |
Results and discussion
Optimization of the LASiS parameters to control the size and structure of Co/ZIF-67
As mentioned earlier, the LASiS process was carried out by ablating a Co pellet immersed in KOH and Hmim solution for durations ranging from 5 to 20 minutes using two different laser output powers: 330 mJ per pulse and 110 mJ per pulse. The laser ablation time and output power play critical roles in determining the structure and morphology of the synthesized Co/ZIF-67 nanomaterials, as shown in Fig. 2. At a high laser output power of 330 mJ per pulse, a short ablation time of 5 minutes is insufficient for the complete formation of the MOF, resulting in small particles that exhibit cubic structures. Increasing the ablation time to 10 minutes leads to well-defined, fully grown hexagonal structures, indicating optimal MOF formation. Extending the ablation time to 15 and 20 minutes leads to significant agglomeration in the ZIF morphology, leading to loss of optimal structure and potentially reducing the final functionality of these MOFs. However, with a lower laser output power of 110 mJ per pulse, the system lacks enough energy to generate adequate concentrations of Co2+ cations through the ablation of the metal target, leading to slow nucleation processes with the formation of defective structures. Although a 15 minutes ablation at this lower power produces smaller particles that begin to grow, extending the ablation time to 20 minutes results in severely aggregated structures with compromised morphology and potential functionality. These findings demonstrate the importance of carefully tuning laser output power and ablation time to achieve highly crystalline Co/ZIF-67 nanomaterials. SEM micrographs of Fig. 2 clearly show the morphological evolution and aggregation tendencies under varying synthesis conditions. Unlike conventional methods such as hydrothermal synthesis, which are time-consuming and solvent-intensive, LASiS offers a rapid, green, and template-free approach with precise control over particle size and morphology, making it especially attractive for scalable and sustainable nanomaterial production. In the context of MOF formation via LASiS, our previous studies18,23 have elucidated a comprehensive mechanistic framework detailing the reaction pathways initiated by high-energy laser ablation that culminate in the assembly of metal–organic structures. Briefly, the laser ablation of a cobalt target in an aqueous medium generates a transient plasma plume characterized by extreme temperatures and pressures, leading to the formation of cobalt nanoparticles (NPs). These NPs undergo rapid oxidation to Co2+ ions driven by redox interactions with solution phase species. Subsequently, the Co2+ ions engage in a sequence of reactions with the organic linker 2-methylimidazole (Hmim), involving coordination, deprotonation, and oligomerization steps, which collectively facilitate the formation of the ZIF-67 framework. | ||
| Fig. 2 SEM micrographs of Co/ZIF-67 nanostructures synthesized using LASiS technique at laser output powers of 110 and 330 mJ per pulse, and ablation times ranging from 5 to 20 minutes. | ||
For structural characterization, XRD and FTIR analysis were performed on the Co/ZIF-67 sample obtained under optimal synthesis conditions (10 minutes ablation time at 330 mJ per pulse). XRD analysis shows the crystalline structure of Co/ZIF-67 by measuring the diffraction patterns produced when X-rays interact with the atomic planes in a crystal lattice. XRD provides information on the crystallinity, phase composition, and lattice parameters of synthesized materials.24 XRD patterns (Fig. 3(a)) of Co/ZIF-67 synthesized under optimized LASiS conditions (330 mJ per pulse, 10 min) show sharp and well-defined peaks matching the simulated ZIF-67, confirming phase purity and crystallinity of the MOFs. Complementing the XRD results, FTIR spectroscopy (Fig. 3(b)) was employed to identify key functional groups and verify the bonding environment within the synthesized MOF. FTIR is used to identify the functional groups and chemical bonds present in a material by analyzing the absorption of infrared light at various wavelengths, providing valuable information on molecular vibrations and bonding environments.25 Characteristic peaks observed in FTIR spectra (Fig. 3(b)), particularly those associated with Co–N stretching and imidazole ring vibrations, confirm the successful coordination between cobalt centers and organic ligands, supporting the formation of a well-defined ZIF-67 structure under LASiS conditions. In this case, the FTIR plot displays molecular vibrations characteristic of the 2-methylimidazole (Hmim) linker and metal–ligand coordination. The band at 759 cm−1 corresponds to the out-of-plane bending of the Hmim ring, while the peak at 1420 cm−1 is attributed to its in-plane bending vibrations.26 The prominent absorption bands at 1310 cm−1 and 1585 cm−1 correspond to stretching vibrations of C–N and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N, respectively, which confirms the presence of Hmim as organic linker in the ZIF-67 framework.27,28 Additional peaks between 1300 and 1000 cm−1 further indicate a C–N stretching, whereas bands within the 1600 to 1500 cm−1 range are associated with vibrations of NH bending of the Hmim molecule. A weak peak at 1666 cm−1 arises from the stretching of the Hmim aromatic ring. In particular, the strong peak observed at 421 cm−1 is indicative of metal–nitrogen bonds (in this case, Co–N),29 validating the successful incorporation of metal centers into the ZIF structure. XRD and FTIR characterizations in Fig. 3(a) and (b) confirm the successful synthesis of structurally robust and highly crystalline Co/ZIF-67 frameworks under optimized LASiS conditions.
N, respectively, which confirms the presence of Hmim as organic linker in the ZIF-67 framework.27,28 Additional peaks between 1300 and 1000 cm−1 further indicate a C–N stretching, whereas bands within the 1600 to 1500 cm−1 range are associated with vibrations of NH bending of the Hmim molecule. A weak peak at 1666 cm−1 arises from the stretching of the Hmim aromatic ring. In particular, the strong peak observed at 421 cm−1 is indicative of metal–nitrogen bonds (in this case, Co–N),29 validating the successful incorporation of metal centers into the ZIF structure. XRD and FTIR characterizations in Fig. 3(a) and (b) confirm the successful synthesis of structurally robust and highly crystalline Co/ZIF-67 frameworks under optimized LASiS conditions.
 | ||
| Fig. 3 (a) XRD and (b) FTIR spectra of Co/ZIF-67 synthesized via LASiS under optimized conditions of 10 minutes ablation time and 330 mJ per pulse. | ||
To use these HNCs as ORR electrocatalysts, after the ZIF material undergoes calcination, the crystallinity, stability, and catalytic activity are further improved by enhancing the bonding between cobalt centers and the surrounding nitrogen-doped carbon (N–C) matrix. The strong interaction between Co nodes and the N-doped carbon framework forms Co–N–C active sites with a unique electronic configuration: the wavefunction of sp2 hybridized carbon networks overlaps with the electrons in the orbital d of the Co centers, producing a repulsion that upshifts the Fermi level of the system, resulting in a lower work function and consequently a more favorable charge transfer during the electrocatalytic process. In addition to that, excess valence electrons from N centers increase the local electron density of C–Co systems, leading to a further decrease in the work function of active catalytic sites.18,20 For practical application in electrochemical testing, calcined Co3O4/CoO/Co@C HNCs are dispersed into an ink solution containing carbon black (CB) and ethanol as the solvent, which improves the overall conductivity and improves the dispersion of the catalyst on the electrode surface. The SEM micrographs in Fig. 5(c) and (d) show the HNCs before and after the addition of carbon black. Before CB was added, the pyrolyzed MOF exhibits a somewhat collapsed, saggy carbon structure with visible Co nodes interconnected with nitrogen and carbon. After CB addition, mixing, and rigorous sonication of the electrocatalyst ink material, the carbonaceous material displays improved porosity and particle dispersion, which are critical for facilitating the access of the reactant to active sites during ORR. A schematic illustration in Fig. 4(e) shows the structural arrangement of the final catalyst, showing the Co centers coordinated to nitrogen and carbon within the conductive carbon framework. This well-engineered architecture ensures a good balance between high surface area, efficient electron transport, and abundant active sites, which, together, should enhance ORR activity and durability. This enhancement in ORR performance can be attributed to the hierarchical nanostructure, high surface area, and synergistic interactions between the Co3O4/CoO phases and the nitrogen-doped carbon matrix – observations that are consistent with recent studies on pyrolyzed MOF-derived systems.30
However, XRD patterns of the pyrolyzed Co/ZIF-67 samples showed sharp and well-defined peaks corresponding to Co3O4, CoO and metallic Co, indicating the formation of a multiphase crystalline structure. Raman spectra (Fig. 5(a)) further confirm the dominant Co3O4 spinel phase and show distinct D and G bands, suggesting the presence of graphitized carbon. The coexistence of Co3O4, CoO and metallic Co within a nitrogen-doped carbon matrix, as revealed by Raman and XRD analysis, points to a chemically complex yet stable hybrid network. These phases should originate from thermally driven transformations of Co–N coordination environments during pyrolysis, resulting in electronically and catalytically favorable structures. Post pyrolysis strong vibrational modes A1g and F2g are also observed in Fig. 5(b). The D and G bands (1350 and 1580 cm−1) reflect the presence of disordered and graphitic carbon, respectively.31 Fig. 5(a) also highlights the carbon phases and the presence of Co metal oxides. The D band arises from the vibration mode A1g associated with phonon symmetry, which is indicative of defects and disorder in the carbon structure. In contrast, the G band is attributed to the vibration E2g in the plane of the sp2 hybridized carbon atoms, signifying the presence of graphitic carbon. The intensity ratio of these peaks (ID/IG), also shown in Fig. 5(a), serves as a useful metric to evaluate the degree of disorder versus graphitization within the carbon matrix. A higher ID/IG ratio suggests an increased level of defects or amorphous carbon content, whereas a lower ratio indicates a more ordered graphitic structure. An ID/IG ratio of 0.95 indicates a relatively balanced presence of both defective (amorphous) and graphitic (ordered sp2) carbon phases. In fact, although the electronic conductivity is highly desirable for the HNCs, the presence of dangling bonds arising from defects in the carbon matrix also plays a role in the electrocatalytic properties of the system. Although the mechanisms of these processes are not yet fully understood, other studies have reported that the reactive nature of unsatisfied C bonds can significantly contribute to the ORR electrocatalysis process.32
Taking this into account, the D/G ratio calculated from the Raman spectra ratio suggests that there is a moderate level of structural disorder, with significant graphitization still present,33 representing a desirable balance between an electronic conductor phase and the presence of active electrocatalyst C sites. In addition to the contribution peaks of the carbon D and G bands, the Raman spectra exhibit characteristic peaks corresponding to Co3O4, further confirming the incorporation of the metal oxide phases. The presence of well-defined graphitic carbon and Co3O4 indicates the formation of highly graphitic carbon–metal oxide composites. Thus, Co3O4/CoO/Co@C HNC possesses a well-developed graphitic structure while retaining enough defects to potentially enhance properties such as electrical conductivity and active sites for catalysis, which is especially beneficial when combined with Co3O4 in carbon–metal oxide composites and thus enhances the overall catalytic performance of HNCs. Therefore, the Raman spectrum of the pyrolyzed Co/ZIF-67 shows distinct D and G bands, confirming the formation of a carbonaceous matrix, along with characteristic peaks corresponding to Co3O4. The absence of CoO peaks, in contrast to the XRD data in Fig. 5(b), where both Co3O4 and CoO phases are present, can be attributed to the Raman-inactive nature of CoO due to its highly symmetric cubic structure.34 This suggests that Co3O4 forms Raman-active nodes embedded within a conductive carbon network, while CoO remains undetectable under Raman excitation.
Fig. 5(b) shows the XRD pattern of the material after calcination at 500 °C, which confirms the successful formation of hollow HNCs Co3O4/CoO/Co@C. The diffraction peaks agree excellently with the standard cubic phase of Co3O4/CoO/Co@C, with prominent reflections observed at 2θ values of approximately 19.0°, 31.3°, 36.8°, 44.8°, 59.3°, and 65.2°. These characteristic peaks verify the formation of highly crystalline CoO and Co3O4 following the calcination process. The successful incorporation of CoO and Co3O4 into the ZIF-67-derived carbon matrix results in a composite structure that benefits from the high catalytic activity of CoO and Co3O4 and the conductivity and structural stability provided by the carbon shell. This synergistic combination improves not only the stability and durability of the catalyst but also its overall electrochemical performance.
The existence of different phases of cobalt oxide may come from uniformly distributed cobalt ions within an organic framework during rapid energy input and localized heating during LASiS. Upon pyrolysis, the intense thermal decomposition of the organic ligands liberates gaseous byproducts and leaves behind a porous carbon matrix embedded with cobalt species. The cobalt ions, initially stabilized within the MOF architecture, undergo phase transformations depending on the local pyrolysis environment. Specifically, variations in temperature, oxygen partial pressure, and carbonization atmosphere promote the formation of different phases of cobalt oxide. In regions with limited oxygen availability and rapid thermal gradients, CoO tends to form as a result of the partial oxidation of cobalt. In contrast, areas with slightly higher oxygen diffusion or prolonged exposure to elevated temperatures favor the formation of Co3O4, a mixed valence compound. As a result, the pyrolysis of ZIF-67 nanostructures synthesized with LASiS leads to a hybrid material containing CoO and Co3O4 phases, which will enhance electrochemical performance by combining the properties of both oxides.35,36
Structure-controlled ZIF-67 as ORR electrocatalyst
In this section, we present the ORR electrocatalytic performance and long-term stability of HNC derived from LASiS Co3O4/CoO/Co@C.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In contrast, the commercially used C/Pt catalyst showed a significantly greater performance degradation, with its current density decreasing by approximately more than 30% under the same testing conditions. This emphasizes the robust electrochemical durability of LASiS-synthesized Co3O4/CoO/Co@C HNCs, making them promising candidates as ORR electrocatalysts. The presence of catalytically active Co–N–C moieties and interconnected cobalt oxide domains in the HNCs, as evidenced by XRD and FTIR analyses, can be directly correlated with the improved ORR electrocatalytic performance observed in the electrochemical measurements. Nitrogen doping in carbon-based materials, particularly in conjunction with cobalt, plays a pivotal role in modulating the electronic structure and enhancing ORR activity. Nitrogen functions as an electron donor, increasing the local electron density and shifting the Fermi level toward the conduction band, thus improving the charge transfer across the carbon matrix.37 In Co–N–C systems, nitrogen incorporation not only promotes efficient electron transport but also contributes to the formation of catalytically active sites by stabilizing cobalt species, either as atomically dispersed centers or as nanoparticles.38 This synergistic interaction between nitrogen and cobalt facilitates the adsorption and activation of O2, thus accelerating the overall reduction kinetics and accounting for the high catalytic performance. These results support the statement that a 10 minutes ablation under high-power laser pulses (330 mJ per pulse) results in the most effective HNC morphology and electrochemical performance. These outcomes stress the influence of LASiS parameters on structural and functional characteristics of MOF-based electrocatalysts and support the importance of tuning synthesis conditions during LASiS for optimal catalytic behavior.
000 cycles. In contrast, the commercially used C/Pt catalyst showed a significantly greater performance degradation, with its current density decreasing by approximately more than 30% under the same testing conditions. This emphasizes the robust electrochemical durability of LASiS-synthesized Co3O4/CoO/Co@C HNCs, making them promising candidates as ORR electrocatalysts. The presence of catalytically active Co–N–C moieties and interconnected cobalt oxide domains in the HNCs, as evidenced by XRD and FTIR analyses, can be directly correlated with the improved ORR electrocatalytic performance observed in the electrochemical measurements. Nitrogen doping in carbon-based materials, particularly in conjunction with cobalt, plays a pivotal role in modulating the electronic structure and enhancing ORR activity. Nitrogen functions as an electron donor, increasing the local electron density and shifting the Fermi level toward the conduction band, thus improving the charge transfer across the carbon matrix.37 In Co–N–C systems, nitrogen incorporation not only promotes efficient electron transport but also contributes to the formation of catalytically active sites by stabilizing cobalt species, either as atomically dispersed centers or as nanoparticles.38 This synergistic interaction between nitrogen and cobalt facilitates the adsorption and activation of O2, thus accelerating the overall reduction kinetics and accounting for the high catalytic performance. These results support the statement that a 10 minutes ablation under high-power laser pulses (330 mJ per pulse) results in the most effective HNC morphology and electrochemical performance. These outcomes stress the influence of LASiS parameters on structural and functional characteristics of MOF-based electrocatalysts and support the importance of tuning synthesis conditions during LASiS for optimal catalytic behavior.
Conclusion
This study demonstrates the successful application of LASiS in the engineering of Co-based HNCs derived from MOFs as high-performance nonprecious metal electrocatalysts for ORR. By precisely tuning the laser output power and ablation time, we achieved fine control over the size and crystallinity of the Co/ZIF-67 precursors, resulting in pyrolyzed HNCs composed of a mixed phase Co3O4/CoO/Co embedded in a conductive carbon matrix, where CoO dominates the Raman response due to its stable crystalline structure, while CoO remains undetected due to its symmetric cubic phase, despite both being visible in XRD. The optimized LASiS conditions led to highly porous crystalline structures that exhibited a four-electron ORR pathway, excellent durability, and performance comparable to those of commercial Pt/C catalysts. Compared with pyrolyzed hydrothermal MOFs, LASiS-derived hybrid nanocomposites offer superior structural purity, optimized nanoscale morphology, and enhanced ORR activity with excellent durability, demonstrating the potential of this scalable method for next-generation electrocatalyst developments.Data availability
The datasets generated and/or analyzed during the current study are not publicly available due to ongoing data protection and confidentiality considerations, but are available from the corresponding author upon reasonable request. The data can be made available to reviewers for the purpose of peer review.Author contributions
MM and BM conceived the experiments, MM conducted the experiments, and MM, SA, ER, and BB analyzed the results. MM, ER, and BK contributed to the writing of the manuscript.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The HP-TEM and elemental mapping measurements were performed at the Vanderbilt Institute of Nanoscale Science and Engineering (VINSE), located at Vanderbilt University, Nashville. SEM and XRD have been performed at the Institute for Advanced Materials & Manufacturing (IAMM) Diffraction Facility and Electron Microscopy. TEM analysis was performed at the Advanced Microscopy and Imaging Center (AMIC) located at the University of Tennessee, Knoxville.Notes and references
- K. A. Page and B. W. Rowe, Polymers for Energy Storage and Delivery: Polyelectrolytes for Batteries and Fuel Cells, 2012, pp. 147–164 Search PubMed.
- G. Das, J.-H. Choi, P. K. T. Nguyen, D.-J. Kim and Y. S. Yoon, Polymers, 2022, 14, 1197 CrossRef CAS PubMed.
- T. B. Ferriday and P. H. Middleton, Int. J. Hydrogen Energy, 2021, 46, 18489–18510 CrossRef CAS.
- J.-H. Kim and A. Manthiram, J. Mater. Chem. A, 2015, 3, 24195–24210 RSC.
- C. Cui, S. Li, J. Gong, K. Wei, X. Hou, C. Jiang, Y. Yao and J. Ma, Materials for Renewable and Sustainable Energy, 2021, 10, 1–24 CrossRef.
- D. R. Dekel, J. Power Sources, 2018, 375, 158–169 CrossRef CAS.
- H. Li, H. Zhao, B. Tao, G. Xu, S. Gu, G. Wang and H. Chang, Nanomaterials, 2022, 12, 4173 CrossRef CAS PubMed.
- J.-D. Qu, Y. Wang, T.-T. Sun, X.-Y. Chu, Y.-X. Jiang, N.-N. Zhang, Z.-H. Zhao, H. Dong, Y.-Q. Lan and F.-M. Zhang, Angew. Chem., Int. Ed., 2025, e202502821 CAS.
- X. Wang, J. Zhou, H. Fu, W. Li, X. Fan, G. Xin, J. Zheng and X. Li, J. Mater. Chem. A, 2014, 2, 14064–14070 RSC.
- W. Huang, X. Zhu, H. Zhu, Z. Wang, H. Yu, Y. Shao, Q. Liu and S. Lan, Carbon Neutralization, 2025, 4, e189 CrossRef CAS.
- Y. Zhao, J. Li, Y. Tan, C. Zhu and Y. Chen, Carbon Neutralization, 2024, 3, 32–63 CrossRef CAS.
- K. Zhang, W. Guo, Z. Liang and R. Zou, Sci. China: Chem., 2019, 62, 417–429 CrossRef CAS.
- Z. Liang, R. Zhao, T. Qiu, R. Zou and Q. Xu, EnergyChem, 2019, 1, 100001 CrossRef.
- W. Xia, A. Mahmood, R. Zou and Q. Xu, Energy Environ. Sci., 2015, 8, 1837–1866 RSC.
- H. Furukawa, K. E. Cordova, M. O'Keeffe and O. M. Yaghi, Science, 2013, 341, 1230444 CrossRef PubMed.
- J. Zhou and B. Wang, Chem. Soc. Rev., 2017, 46, 6927–6945 RSC.
- C. Duan, Y. Yu and H. Hu, Green Energy Environ., 2022, 7, 3–15 CrossRef CAS.
- E. L. Ribeiro, E. M. Davis, M. Mokhtarnejad, S. Hu, D. Mukherjee and B. Khomami, Catal. Sci. Technol., 2021, 11, 3002–3013 RSC.
- M. Mokhtarnejad, E. L. Ribeiro, D. Mukherjee and B. Khomami, RSC Adv., 2022, 12, 17321–17329 RSC.
- M. Mokhtarnejad, E. L. Ribeiro, D. Mukherjee and B. Khomami, ACS Appl. Nano Mater., 2023, 6, 13698–13707 CrossRef.
- M. Mokhtarnejad, N. Mokhtarinori, E. L. Ribeiro, S. Kamali, S. Dai, D. Mukherjee and B. Khomami, Adv. Mater. Technol., 2024, 9, 2470093 CrossRef.
- M. Mokhtarnejad, Engineering of Functional Hybrid Nanocomposites for Renewable Energy Applications via Laser Ablation, PhD thesis, University of Tennessee, 2024, https://trace.tennessee.edu/utk_graddiss/10143.
- E. L. Ribeiro, S. A. Davari, S. Hu, D. Mukherjee and B. Khomami, Mater. Chem. Front., 2019, 3, 1302–1309 RSC.
- A. Ali, Y. W. Chiang and R. M. Santos, Minerals, 2022, 12, 205 CrossRef CAS.
- F. Faghihzadeh, N. M. Anaya, L. A. Schifman and V. Oyanedel-Craver, Nanotechnol. Environ. Eng., 2016, 1, 1–16 CrossRef.
- M. Adnan, K. Li, L. Xu and Y. Yan, Catalysts, 2018, 8, 96 CrossRef.
- D. N. Ta, H. K. Nguyen, B. X. Trinh, Q. T. Le, H. N. Ta and H. T. Nguyen, Can. J. Chem. Eng., 2018, 96, 1518–1531 CrossRef CAS.
- D. Huang, Q. Xin, Y. Ni, Y. Shuai, S. Wang, Y. Li, H. Ye, L. Lin, X. Ding and Y. Zhang, RSC Adv., 2018, 8, 6099–6109 RSC.
- S. Roy, A. Mathur and A. Bihari Pati, Electroanalysis, 2023, 35, e202200203 CrossRef CAS.
- J.-Z. Xiao, Z.-H. Zhao, N.-N. Zhang, H.-T. Che, X. Qiao, G.-Y. Zhang, X. Chu, Y. Wang, H. Dong and F.-M. Zhang, Chin. J. Catal., 2025, 69, 219–229 CrossRef.
- R. Saito, M. Hofmann, G. Dresselhaus, A. Jorio and M. Dresselhaus, Adv. Phys., 2011, 60, 413–550 CrossRef CAS.
- Y. Jiang, L. Yang, T. Sun, J. Zhao, Z. Lyu, O. Zhuo, X. Wang, Q. Wu, J. Ma and Z. Hu, ACS Catal., 2015, 5, 6707–6712 CrossRef CAS.
- J. Schwan, S. Ulrich, V. Batori, H. Ehrhardt and S. Silva, J. Appl. Phys., 1996, 80, 440–447 CrossRef CAS.
- S. Farhadi, M. Javanmard and G. Nadri, Acta Chim. Slov., 2016, 63, 335–343 CrossRef CAS PubMed.
- I. S. Imaduddin, S. R. Majid, S. B. Aziz, I. Brevik, S. N. F. Yusuf, M. Brza, S. R. Saeed and M. F. Z. A. Kadir, Materials, 2021, 14, 573 CrossRef CAS PubMed.
- Y. Bai, J. Dong, Y. Hou, Y. Guo, Y. Liu, Y. Li, X. Han and Z. Huang, Chem. Eng. J., 2019, 361, 703–712 CrossRef CAS.
- Q. Li, J. Lei, X. Zhao, Q. Geng, H. Xie, Y. Li and Y. Cao, Fuel, 2025, 380, 133153 CrossRef CAS.
- A. Bahr, G.-h. Moon and H. Tuysuz, ACS Appl. Energy Mater., 2019, 2, 6672–6680 CrossRef.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra04056f |
| This journal is © The Royal Society of Chemistry 2025 |



