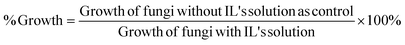Open Access Article
Open Access ArticleSynthesis, characterization, antimicrobial activity and in silico study of pyridinium based ionic liquids†
Md. Jahirul Islama,
Ashutosh Nath *b,
Khadizaa,
Ajoy Kumer*de,
Prattyasha Baruac,
Avra Sarkarc,
Nadia Isratc,
Minhajul Abedinc,
Mohammed Majidul Islamc and
Md. Wahab Khan*ae
*b,
Khadizaa,
Ajoy Kumer*de,
Prattyasha Baruac,
Avra Sarkarc,
Nadia Isratc,
Minhajul Abedinc,
Mohammed Majidul Islamc and
Md. Wahab Khan*ae
aDepartment of Chemistry, Bangladesh University of Engineering and Technology (BUET), Dhaka, Bangladesh
bDepartment of Chemistry, University of Massachusetts Boston, MA 02125, USA. E-mail: ashutosh.nath001@umb.edu
cDepartment of Chemistry, Govt. Hazi Muhammad Mohsin College Chattogram, Chattogram, Bangladesh
dCenter for Global Health Research, Saveetha Medical College and Hospitals, Saveetha Institute of Medical and Technical Sciences, Chennai-602105, Tamil Nadu, India
eDepartment of Chemistry, International University of Business Agriculture and Technology (IUBAT), Dhaka, Bangladesh
First published on 17th July 2025
Abstract
A series of twenty-one pyridinium-based ionic liquids (ILs) was synthesized and thoroughly characterized using FTIR, NMR, UV-Vis spectroscopy, and melting point analysis. The ILs' antibacterial activity was assessed against Aspergillus niger and a panel of bacteria, both Gram-positive and Gram-negative. Notable antibacterial and antifungal properties were demonstrated by IL-10, IL-11, and IL-14 specifically. Several ILs also demonstrated effective Brønsted acidic catalysis for the Mannich process under mild, solvent-free circumstances. The biological activity of IL-19 and IL-21 was found to be correlated with their significant binding affinities to microbial protein targets, as demonstrated by molecular docking experiments. Selected ILs, especially IL-05 and IL-21, were found to be drug-like and biosafe based on ADMET and toxicity predictions. These results highlight the pyridinium-based ILs' multipurpose potential as green catalysts and antibacterial agents for sustainable synthetic applications.
1. Introduction
In recent years, there has been increased interest in the unique and remarkable properties of ionic liquids (ILs), including their strong electrical conductivity, low vapor pressure, non-volatility, non-flammability, and improved thermal stability.1–3 This is attributed to numerous combinations of cations and anions that meet the definition of ILs, leading to adverse suite of behaviors.4 ILs are normally defined as compounds completely composed of ions with melting points below 100 °C.5,6 An ionic liquid (IL) is a liquid containing ions i.e. organic/inorganic cations and anions (Fig. 1). Although in some papers, ionic liquids are referred as ‘molten salts', there is an arbitrary distinction between molten salts and ionic liquids.7,8The first IL (ethyl ammonium nitrate) was reported by Paul Walden in 1914, who at that time never imagined that ILs would become a major scientific area after almost a century. Actually, ILs as innovative fluids have received wide attention only during the past two decades.9,10 Nowadays more and more researchers are engaged in studying this exciting area, with the outcomes being plentiful.11–13 A multidisciplinary study on ILs is emerging, including chemistry, materials science, chemical engineering and environmental science.14,15 More specifically, some important fundamental viewpoints are now different from the original concepts, as insights into the nature of ILs become deeper.16 Besides, it was opened a new window for drug discovery in pharmaceutics and medicine as bioactive molecules of anti-cancer, antimicrobial agents, anti-viral, antifungal and antiparasitic drugs, anti-infective defense, as well as in the pharmaceutical and food industries, agro-chemistry, artificial enzymes biocides and biotechnology.17,18 The environmentally benign nature of many ionic liquids further underscores their appeal, offering sustainable alternatives to conventional solvents laden with environmental and health hazards (Fig. 2).19,20
 | ||
| Fig. 2 Some pyridinium containing ILs, With their low volatility and potential for recycling, ionic liquids present promising opportunities for greener synthesis routes and cleaner processes, aligning with the growing imperative for eco-friendly practices in both academic research and industrial applications.21–28 | ||
In this study, we synthesized a new series of twenty-one pyridinium based ILs and their physicochemical properties were studied by computational approach. Our goal is to identify the ionic liquids by their toxicological analysis, antimicrobial activity and catalytic effects.
2. Experimental
2.1. General method of ILs synthesis
Pyridinium-based ionic liquids (ILs) were produced through the neutralization reaction of pyridine or its derivatives with carboxylic acid in Scheme 1. The initial step involved dissolving the solid base by stirring in ethyl acetate solvent followed by the gradual addition of acid using a pipette. Due to endothermic reaction, after adding acid to control the thermal output of the procedure, an ice bath was utilized, and nitrogen gas was employed to create an inert atmosphere. The stirring remained consistently for a duration of 6–12 hours, and even up to 30 hours, while the reaction was monitored through thin layer chromatography (TLC) using the aluminum sheet coated with silica gel with MeOH![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) CHCl3 (30
CHCl3 (30![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 70) as a mobile phase that shows the formation of expected product spot in UV lamp (254 nm). Additionally, for improving the yield, the reaction mixture was heated for 2–3 hours with paraffin oil bath at 50–55 °C temperature. Then the solvent was removed by rotary evaporator and obtained colored or colorless ILs. For further purification it was kept in vacuum oven at 90 °C for two days. The synthesis of the 21 ionic liquids (ILs) was achieved through the utilization of equimolar quantities of organic acids combined with pyridine or its derivatives.
70) as a mobile phase that shows the formation of expected product spot in UV lamp (254 nm). Additionally, for improving the yield, the reaction mixture was heated for 2–3 hours with paraffin oil bath at 50–55 °C temperature. Then the solvent was removed by rotary evaporator and obtained colored or colorless ILs. For further purification it was kept in vacuum oven at 90 °C for two days. The synthesis of the 21 ionic liquids (ILs) was achieved through the utilization of equimolar quantities of organic acids combined with pyridine or its derivatives.
2.2. Instrumental analysis and characterizations of ILs
The synthesized ILs are characterized by using Spectroscopy Infrared (IR) and Nuclear Magnetic Resonance (NMR). The purity of synthesized ILs were maintained using vacuum oven and desiccators throughout the experimental period. The infrared (FT-IR) spectra of synthesized ILs are obtained on a Shimadzu Fourier-Transform Infrared spectrometer (Shimadzu IR Prestige 21, Shimadzu Corporation). The ILs solid samples were mixed with dry KBr to make a thin plate for IR record. For liquid samples, a blank KBr disc is swapped with a small amount of ILs and put in the hole in a radiation chamber. The FT-IR spectra produced had a wave number range of 600–4000 cm−1. In NMR, 5 to 10 mg of sample was dissolved in 0.7 mL of suitable solvent (CDCl3, CD3OD). The 1H NMR spectra were recorded at room temperature on Bruker Avance 400 MHz spectrometer.![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1556 (C
O); 1556 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1134 (–C–O); 758,722 (C–H). 1H NMR chemical shifts: 7.90 (q, 2H, C-3,5); 8.40 (t, 1H, C-4); 8.87 (d, 2H, C-2,6); 13.96 (s, 1H, NH1).
C); 1134 (–C–O); 758,722 (C–H). 1H NMR chemical shifts: 7.90 (q, 2H, C-3,5); 8.40 (t, 1H, C-4); 8.87 (d, 2H, C-2,6); 13.96 (s, 1H, NH1).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1633 (C
O); 1633 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1186 (–C–O); 860 (C–H). 1H NMR chemical shifts: 1.86 (d, 3H, C-7); 4.38 (q, 1H, C-8); 5.50 (m, 1H, NH1); 8.02 (m, 2H, C-3,5); 8.51 (m, 1H, C-4); 8.86 (d, 2H, C-2,6).
C); 1186 (–C–O); 860 (C–H). 1H NMR chemical shifts: 1.86 (d, 3H, C-7); 4.38 (q, 1H, C-8); 5.50 (m, 1H, NH1); 8.02 (m, 2H, C-3,5); 8.51 (m, 1H, C-4); 8.86 (d, 2H, C-2,6).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1649 (C
O); 1649 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1202 (–CH3); 1188 (–C–O); 1138 (C–F); 860, 718 (C–H). 1H NMR chemical shifts: 3.22 (s, 2H, NC-4); 6.76 (t, 2H, C-3,5); 8.18 (t, 2H, C-2,6); 13.28 (s, 1H, NH1).
C); 1202 (–CH3); 1188 (–C–O); 1138 (C–F); 860, 718 (C–H). 1H NMR chemical shifts: 3.22 (s, 2H, NC-4); 6.76 (t, 2H, C-3,5); 8.18 (t, 2H, C-2,6); 13.28 (s, 1H, NH1).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1648 (C
O); 1648 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1209 (–CH3); 1148 (–C–O); 1138 (C–F); 860, 798 (C–H). 1H NMR chemical shifts: 3.10 (d, 3H, C-8); 4.38 (q, 1H, C-8); 4.70 (s, 6H, NC-4); 6.75 (d, 2H, C-3,5); 7.96 (d, 2H, C-2,6); 13.30 (s, 1H, NH1).
C); 1209 (–CH3); 1148 (–C–O); 1138 (C–F); 860, 798 (C–H). 1H NMR chemical shifts: 3.10 (d, 3H, C-8); 4.38 (q, 1H, C-8); 4.70 (s, 6H, NC-4); 6.75 (d, 2H, C-3,5); 7.96 (d, 2H, C-2,6); 13.30 (s, 1H, NH1).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1568 (C
O); 1568 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1202 (–CH3); 1142 (–C–O); 1096 (C–F); 805, 628 (C–H). 1H NMR chemical shifts: 5.85 (s, 1H, NH1); 6.81 (q, 1H, C-4); 7.28 (s, 2H, NH2); 8.36 (d, 1H, C-3); 8.53 (d, 1H, C-2).
C); 1202 (–CH3); 1142 (–C–O); 1096 (C–F); 805, 628 (C–H). 1H NMR chemical shifts: 5.85 (s, 1H, NH1); 6.81 (q, 1H, C-4); 7.28 (s, 2H, NH2); 8.36 (d, 1H, C-3); 8.53 (d, 1H, C-2).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O); 1568 (C
O); 1568 (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1202 (–CH3); 1142 (–C–O); 1096 (C–F); 805, 628 (C–H). 1H NMR chemical shifts: 1.90 (d, 3H, C-5); 4.42 (m, 1H, C-6); 7.70 (s, 1H, NH1); 6.83 (q, 1H, C-4); 7.88 (s, 2H, NH2); 8.30 (d, 1H, C3); 8.60 (d, 1H, C-2).
C); 1202 (–CH3); 1142 (–C–O); 1096 (C–F); 805, 628 (C–H). 1H NMR chemical shifts: 1.90 (d, 3H, C-5); 4.42 (m, 1H, C-6); 7.70 (s, 1H, NH1); 6.83 (q, 1H, C-4); 7.88 (s, 2H, NH2); 8.30 (d, 1H, C3); 8.60 (d, 1H, C-2).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1207 (–CH2); 1159 (–C–O); 1027 (N–Cl); 748, 628 (C–H). 1H NMR chemical shifts: 6.34 (s, 2H, C-7); 7.36 (t, 3H, C 9–11); 7.69 (t, 2H, C-8,12); 8.02 (t, 2H, C-3,5); 8.38 (t, 1H, C-4); 9.66 (d, 2H, C-2,6).
C); 1207 (–CH2); 1159 (–C–O); 1027 (N–Cl); 748, 628 (C–H). 1H NMR chemical shifts: 6.34 (s, 2H, C-7); 7.36 (t, 3H, C 9–11); 7.69 (t, 2H, C-8,12); 8.02 (t, 2H, C-3,5); 8.38 (t, 1H, C-4); 9.66 (d, 2H, C-2,6).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C); 1207 (–CH2); 1120 (–C–O); 748, 705 (C–H). 1H NMR chemical shifts: 1.90 (m, 4H, C-3,4); 1.74 (m, 2H, C-5); 2.23 (s, 3H, C-10); 2.48 (t, 2H, C-6); 2.84 (t, 1H, C-7); 4.64 (d, 1H, C-8); 4.84 (d, 1H, C-2); 6.44 (m, 3H, C11-13); 6.98 (m, 1H, C9); 7.30 (m, 5H, C14–18).
C); 1207 (–CH2); 1120 (–C–O); 748, 705 (C–H). 1H NMR chemical shifts: 1.90 (m, 4H, C-3,4); 1.74 (m, 2H, C-5); 2.23 (s, 3H, C-10); 2.48 (t, 2H, C-6); 2.84 (t, 1H, C-7); 4.64 (d, 1H, C-8); 4.84 (d, 1H, C-2); 6.44 (m, 3H, C11-13); 6.98 (m, 1H, C9); 7.30 (m, 5H, C14–18).2.3. Computational procedure
2.4. Biological properties of ILs
Pyridinium-based ionic liquids (ILs) were evaluated for their antibacterial and antifungal properties using standard microbiological techniques. ILs were prepared in concentrations ranging from 1000 mM to 50 mM via serial dilution. Antibacterial activity was assessed against six human pathogens (Gram-positive and Gram-negative) using the well diffusion method on nutrient agar. Zones of inhibition were measured, and minimal inhibitory concentrations (MICs) were determined for active compounds. Antifungal activity was tested against Aspergillus niger and Rhizopus azzahra using potato dextrose broth medium and well diffusion method. The influence of IL concentration, anionic structure, and functional groups on antimicrobial activity was systematically analyzed. More details of antibacterial working procedure in ESI.†2.5. Investigation of catalytic properties of ILs
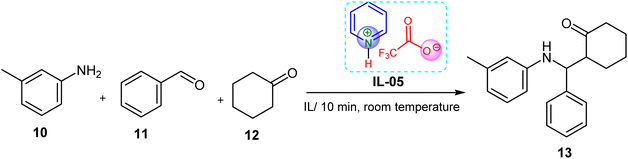
In a typical reaction, a mixture of benzaldehyde (1 mmol), 3-methylaniline (1 mmol), cyclohexanone (1.1 mmol), and synthesized ionic liquid (0.20–0.25 g) was stirred at room temperature (25 °C) in a round-bottom flask. Two drops of water (∼0.06 g) were added to facilitate homogenization. The reaction mixture gradually became viscous and solidified. The resulting solid was isolated by filtration, washed, and recrystallized from 98% ethanol. The final product was dried under reduced pressure using a Hoover pump for 5 hours. Reaction yields and times were summarized in Table 1. The products were characterized by 1H NMR and FT-IR spectroscopy.3. Result and discussion
3.1. Catalytic properties of ILs
In a standard reaction, a solution containing 1 mmol of benzaldehyde 11, 1 mmol of 3-methylaniline 10, 1.1 mmol of cyclohexanone 12, and 18.69 wt% (20 g, based on cyclohexanone) of ionic liquids as catalysts and solvent was prepared by mixing them in a round-bottomed flask at the ambient temperature of 25 °C. The mixture of the reaction became viscous and solidified. The solid was segregated through the process of filtration, and subsequently, the resulting product 13 underwent recrystallization from 98% ethanol and was subjected to hoover drying for a duration of 5 hours. Table 1 is presents a summary of the results obtained from the Mannich reaction involving aldehydes, ketones, and amines. This reaction takes place under the influence of Brønsted acidic ionic liquids and is typically carried out at ambient temperature. Room temperature, a small amount of ionic liquid can facilitate this single-step reaction involving aldehydes, amines, and ketones, known as the Mannich type reaction. The identification of the compound was accomplished by employing 1H NMR and as well as FT-IR.In comparison, IL-11 required a longer reaction time of 30 minutes and resulted in a lower yield (85%), suggesting lower catalytic activity. IL-17 performed moderately, offering a 93% yield in 20 minutes with 23.36 wt% of catalyst. These findings highlight IL-05 (21.49 wt%) as the most effective and rapid catalyst, capable of significantly enhancing product 96.7% yield in minimal time, thereby confirming its promising application in green and efficient Mannich-type transformations under mild conditions.
In contrast, 4-dimethylaminopyridinium trifluoroacetate (IL-11) showed comparatively lower activity, providing a yield of 85% over 30 minutes, despite being used at the same concentration. The reduced efficiency of IL-11 may be attributed to the electron-donating dimethylamino substituent, which likely decreases the Brønsted acidity of the ionic liquid, thereby diminishing its catalytic performance.
The products obtained were characterized and confirmed by 1H NMR and FT-IR spectroscopy. These results collectively demonstrate that the catalytic efficiency of pyridinium-based ILs is significantly influenced by their electronic structure, with IL-05 emerging as the more effective catalyst for Mannich-type transformations under mild, solvent-assisted conditions.
3.2. Biological properties of ILs
| Ionic liquids | Bacillus cereus (+) | Staphylococcus aureus (+) | Sarcina lutea (+) | Salmonella typhi (−) | Escherichia coli (−) | Pseudomonas aeruginosa (−) |
|---|---|---|---|---|---|---|
| IL-01 | 0 | 0 | 19 ± 1 | 0 | 32 ± 1 | 30 ± 1 |
| IL-02 | 0 | 0 | 24 ± 1 | 0 | 0 | 22 ± 1 |
| IL-03 | 0 | 19 ± 1 | 20 ± 1 | 0 | 0 | 28 ± 1 |
| IL-04 | 0 | 0 | 0 | 0 | 27 ± 1 | 0 |
| IL-05 | 0 | 0 | 0 | 0 | 25 ± 1 | 0 |
| IL-06 | 18 ± 1 | 16 ± 1 | 22 ± 1 | 15 ± 1 | 27 ± 1 | 21 ± 1 |
| IL-07 | 20 ± 1 | 19 ± 1 | 20 ± 1 | 28 ± 1 | 30 ± 1 | 26 ± 1 |
| IL-08 | 16 ± 1 | 22 ± 1 | 21 ± 1 | 24 ± 1 | 25 ± 1 | 21 ± 1 |
| IL-09 | 22 ± 1 | 20 ± 1 | 18 ± 1 | 24 ± 1 | 20 ± 1 | 25 ± 1 |
| IL-10 | 30 ± 1 | 35 ± 1 | 31 ± 1 | 36 ± 1 | 33 ± 1 | 31 ± 1 |
| IL-11 | 28 ± 1 | 26 ± 1 | 31 ± 1 | 30 ± 1 | 32 ± 1 | 26 ± 1 |
| IL-12 | 24 ± 1 | 22 ± 1 | 20 ± 1 | 28 ± 1 | 28 ± 1 | 25 ± 1 |
| IL-13 | 18 ± 1 | 19 ± 1 | 21 ± 1 | 24 ± 1 | 28 ± 1 | 25 ± 1 |
| IL-14 | 23 ± 1 | 17 ± 1 | 16 ± 1 | 24 ± 1 | 25 ± 1 | 28 ± 1 |
| IL-15 | 18 ± 1 | 16 ± 1 | 17 ± 1 | 18 ± 1 | 23 ± 1 | 21 ± 1 |
| IL-16 | 19 ± 1 | 15 ± 1 | 14 ± 1 | 16 ± 1 | 15 ± 1 | 21 ± 1 |
| IL-17 | 20 ± 1 | 24 ± 1 | 22 ± 1 | 24 ± 1 | 23 ± 1 | 26 ± 1 |
| IL-18 | 22 ± 1 | 21 ± 1 | 24 ± 1 | 18 ± 1 | 25 ± 1 | 23 ± 1 |
| IL-19 | 09 ± 1 | 18 ± 1 | 19 ± 1 | 24 ± 1 | 23 ± 1 | 24 ± 1 |
| IL-20 | 18 ± 1 | 19 ± 1 | 17 ± 1 | 23 ± 1 | 18 ± 1 | 20 ± 1 |
| IL-21 | 20 ± 1 | 18 ± 1 | 17 ± 1 | 35 ± 1 | 30 ± 1 | 28 ± 1 |
 | ||
| Fig. 3 Six human pathogens Gram positive and Gram negative which were killed by different types of pyridinium based ionic liquids. | ||
The antimicrobial screening of 21 from Table 2 synthesized ILs against Gram-positive (Bacillus cereus, Staphylococcus aureus, Sarcina lutea) and Gram-negative (Salmonella typhi, Escherichia coli, Pseudomonas aeruginosa) bacteria showed significant variation in activity depending on IL structure and bacterial strain. IL-10, IL-11, and IL-21 exhibited strong, broad-spectrum activity with inhibition zones of 26–36 ± 1 mm across all tested bacteria. In contrast, IL-01, IL-02, IL-04, and IL-05 showed limited activity against Gram-positive strains but moderate to high efficacy against E. coli and P. aeruginosa. IL-06 through IL-09 and IL-12 through IL-20 demonstrated moderate antimicrobial effects (15–28 ± 1 mm), with some showing greater potency against Gram-negative bacteria, likely due to differences in membrane permeability and ionic interactions. The enhanced activity of ILs such as IL-10 and IL-11 may be linked to structural features like electron-donating substituents, aromatic rings, or polar functional groups that facilitate membrane disruption or ionic transport, highlighting important structure–activity relationships.
Activity of antifungal can be considered as a term inversely with growth percentage called inhibition percentage. Subtracting the growth percentage from control, inhibition percentage can be calculated.
From the listed value of inhibition percentage in Table 3, it is evaluated that the effect of the compound is clearly visible. ILs containing 2-amino-3-nitro perform higher antifungal activity. Though all the compounds showed antifungal activity, the rate is dependent on different groups of compounds. Among all, 2-amino-3-nitro pyridinium based ILs perform comparatively higher antifungal activity. IL-14 has the lowest growth zone that indicates the highest inhibition percentage and the lowest antifungal activity for the fungi.
| Chemicals tested | Zone of growth | Percent of growth |
|---|---|---|
| Control | 28 mm | 100% |
| IL-01 | 20.5 ± 1 | 78.57% |
| IL-02 | 19.0 ± 1 | 67.80% |
| IL-03 | 25.0 ± 1 | 89.28% |
| IL-04 | 14.5 ± 1 | 51.78% |
| IL-05 | 12.5 ± 1 | 46.67% |
| IL-06 | 25.5 ± 1 | 91.07% |
| IL-07 | 22.0 ± 1 | 78.85% |
| IL-08 | 18.0 ± 1 | 64.28% |
| IL-09 | 17.0 ± 1 | 60.71% |
| IL-10 | 23.0 ± 1 | 82.14% |
| IL-11 | 18.5 ± 1 | 64.28% |
| IL-12 | 27.0 ± 1 | 96.62% |
| IL-13 | 25.0 ± 1 | 89.28% |
| IL-14 | 5.0 ± 1 | 17.85% |
| IL-15 | 12.7 ± 1 | 45.53% |
| IL-16 | 14.8 ± 1 | 52.75% |
| IL-17 | 22.7 ± 1 | 81.25% |
| IL-18 | 26.5 ± 1 | 92.85% |
| IL-19 | 15.5 ± 1 | 53.35% |
| IL-20 | 18.5 ± 1 | 66.07% |
| IL-21 | 26.2 ± 1 | 93.75% |
The antimicrobial efficacy of the synthesized ILs was quantitatively assessed by measuring the zone of growth inhibition and calculating the corresponding percent growth relative to the control. The control showed a zone of growth of 28 ± 1 mm, representing 100% bacterial growth. Among the ILs, IL-12 (27.0 ± 1 mm, 96.62%), IL-18 (26.5 ± 1 mm, 92.85%), and IL-21 (26.2 ± 1 mm, 93.75%) showed the least inhibition of bacterial growth, indicating relatively low antimicrobial activity. Conversely, IL-14 demonstrated the strongest inhibitory effect with only 5.0 ± 1 mm zone of growth and 17.85% growth, suggesting high antimicrobial potency. Other ILs such as IL-05 (12.5 ± 1 mm, 46.67%), IL-04 (14.5 ± 1 mm, 51.78%), and IL-15 (12.7 ± 1 mm, 45.53%) also exhibited significant bacterial growth suppression. Moderate inhibitory effects were observed in IL-01, IL-02, IL-08, and IL-11 with zones of growth between 18.0–20.5 ± 1 mm, corresponding to 64–79% growth. The variation in antimicrobial performance among ILs suggests that their chemical structure influences their ability to inhibit bacterial proliferation. ILs with lower percent growth values demonstrate stronger antimicrobial action, which could be due to enhanced membrane disruption or interference with bacterial metabolism.
3.3. In silico study of ILs
| Ionic liquids | Binding affinity (kcal mol−1) | ||||||
|---|---|---|---|---|---|---|---|
| Aspergillus niger (2BJH) | Bacillus cereus (+) (1QS1) | Escherichia coli (−) (1JG0) | Pseudomonas aeruginosa (−) (4ESH) | Salmonella typhi (−) (1 A5A) | Sarcina lutea (+) (1GWF) | Staphylococcus aureus (+) (4CJN) | |
| IL-06 | −3.98 | −4.28 | −4.15 | −4.54 | −4.08 | −4.31 | −3.80 |
| IL-09 | −4.86 | −5.08 | −4.61 | −4.99 | −4.56 | −5.15 | −4.03 |
| IL-10 | −4.82 | −5.08 | −4.61 | −4.99 | −4.56 | −5.12 | −4.15 |
| IL-15 | −5.39 | −6.14 | −5.32 | −5.92 | −5.39 | −5.50 | −4.85 |
| IL-16 | −5.39 | −6.14 | −5.33 | −5.91 | −5.42 | −5.46 | −4.85 |
| IL-19 | −6.09 | −7.14 | −5.51 | −6.81 | −6.42 | −7.29 | −5.79 |
| IL-21 | −6.06 | −7.01 | −5.53 | −6.72 | −6.41 | −6.32 | −5.09 |
IL-06 topped among the pyridinium groups whereas IL-09 and IL-10 showed the highest scores in the 4-dimethyl amino pyridinium group. On the other hand, IL-16 had the best result in the 2-Amino-3-nitro pyridinium group. Lastly, IL-19 and IL-21 had the highest binding affinity in the benzyl pyridinium group as well as among the total twenty-one ionic liquids (IL). All of the ILs' docking scores are shown in Table S5.† The parameters (Grid box value) for all seven proteins (PDB) are mentioned in Table S5A.†
| b [Based on the criterion of US EPA, the acute oral toxicity is classified in four categories based on LD50 values. Category I = Highly toxic; Category II = Moderately toxic; Category III = Slightly toxic; Category IV = Practically non-toxic]. |
|---|
 |
According to toxicity data, all ILs are non-carcinogenic. ADMET toxicity was accomplished and IL-13 to IL-20 tested positive. Based on In vivo antibacterial activity and estimated LD50 levels in rat acute toxicity studies, the drugs appear to be safe (1.6339–2.7181 mol kg−1).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) LOG
LOG![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P and TPSA.37 The white yolk of the egg demonstrates a significant likelihood of being passively absorbed by the gastrointestinal tract, whereas the yellow region (yolk) exhibits a high probability of penetrating the brain. Fig. 4 shows that IL-05, IL-11 and IL-21 are in the yellow region while IL-01, IL-10, IL-14 and IL-17 are in the white part (Fig. 5).
P and TPSA.37 The white yolk of the egg demonstrates a significant likelihood of being passively absorbed by the gastrointestinal tract, whereas the yellow region (yolk) exhibits a high probability of penetrating the brain. Fig. 4 shows that IL-05, IL-11 and IL-21 are in the yellow region while IL-01, IL-10, IL-14 and IL-17 are in the white part (Fig. 5).
The bioavailability radar visualizes the drug-likeness and pharmacokinetic properties of an ionic liquid. The lipophilicity (Log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P) indicates the solubility of an IL in fats vs. water. Bioavailability radar of twenty-one ILs is shown in Table S9.†
P) indicates the solubility of an IL in fats vs. water. Bioavailability radar of twenty-one ILs is shown in Table S9.†
4. Conclusion
This study used FTIR, NMR, and UV-Vis techniques to successfully synthesize and analyze 21 pyridinium-based ionic liquids (ILs). The synthesized ionic liquids (ILs) were successfully prepared with high purity and yields (91–97%) and characterized by FT-IR and 1H NMR, confirming their structural integrity. UV-Vis data supported aromatic features, and defined melting points indicated reproducible synthesis. These ILs hold promises for further applications due to their distinct structural and electronic properties. In mild conditions, several ILs also functioned as efficient catalysts in the Mannich process. The study shows that several synthesized ILs exhibit notable antimicrobial activity. IL-10, IL-11, and IL-21 are effective broad-spectrum agents, while IL-01 and IL-05 selectively target Gram-negative bacteria. Some ILs like IL-14, IL-05, and IL-04 strongly inhibit bacterial growth, whereas IL-12, IL-18, and IL-21 are less effective. These results highlight the role of structural features in antimicrobial efficacy and support further research into their mechanisms and therapeutic potential. Their antibacterial potential was supported by molecular docking, which showed that IL-19 and IL-21 had favorable binding affinities against bacterial and fungal proteins. For important ILs like IL-05 and IL-21, ADMET and toxicology tests verified good drug-like qualities and minimal toxicity. These results imply that pyridinium-based ILs hold promise for use in green catalysis and medication development.Data availability
The datasets generated and analyzed in this current study are available from the corresponding author upon reasonable request.Author contributions
JI and AN writing – original draft, review & editing, visualization, software, formal analysis, data curation, software, resources. WK and AK: writing – review & editing, resources, methodology, investigation, supervision. PB, AS, NI, MA, and M: software, methodology, review & editing.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
We are grateful to the Bangladesh University of Engineering and Technology (BUET), Dhaka, Dhaka-1000, Bangladesh for all kinds of experimental and financial supports.References
- M. J. Earle, J. M. Esperança, M. A. Gilea, J. N. Canongia Lopes, L. P. Rebelo, J. W. Magee, K. R. Seddon and J. A. Widegren, Nature, 2006, 439, 831–834 CrossRef CAS PubMed.
- J.-Z. Yang and J.-S. Gui, Acta Chim. Sin., 2005, 63, 577 CAS.
- S. Ahrens, A. Peritz and T. Strassner, Angew. Chem., Int. Ed. Engl., 2009, 48, 7908–7910 CrossRef CAS PubMed.
- A. Mehrkesh and A. T. Karunanithi, Fluid Phase Equilib., 2016, 427, 498–503 CrossRef CAS.
- M. Ahrenberg, M. Beck, C. Neise, O. Keßler, U. Kragl, S. P. Verevkin and C. Schick, Phys. Chem. Chem. Phys., 2016, 18, 21381–21390 RSC.
- T. Welton, Biophys. Rev., 2018, 10, 691–706 CrossRef CAS PubMed.
- M. M. Santos, C. Alves, J. Silva, C. Florindo, A. Costa, Ž. Petrovski, I. M. Marrucho, R. Pedrosa and L. C. Branco, Pharmaceutics, 2020, 12, 694 CrossRef CAS PubMed.
- J. Claus, F. O. Sommer and U. Kragl, Solid State Ionics, 2018, 314, 119–128 CrossRef CAS.
- Z. Zhang, J. Song and B. Han, Chem. Rev., 2017, 117, 6834–6880 CrossRef CAS PubMed.
- T. Welton, Chem. Rev., 1999, 99, 2071–2084 CrossRef CAS PubMed.
- A. C. Cole, J. L. Jensen, I. Ntai, K. L. T. Tran, K. J. Weaver, D. C. Forbes and J. H. Davis, J. Am. Chem. Soc., 2002, 124, 5962–5963 CrossRef CAS PubMed.
- R. D. Rogers and K. R. Seddon, Science, 2003, 302, 792–793 CrossRef PubMed.
- P. G. Jessop, Faraday Discuss., 2018, 206, 587–601 RSC.
- Z. Lei, B. Chen, Y.-M. Koo and D. R. MacFarlane, Chem. Rev., 2017, 117, 6633–6635 CrossRef PubMed.
- R. Ferraz, L. C. Branco, C. Prudêncio, J. P. Noronha and Ž. Petrovski, ChemMedChem, 2011, 6, 975–985 CrossRef CAS PubMed.
- R. A. Sheldon, Green Chem., 2007, 9, 1273–1283 RSC.
- M. I. Hossain and A. Kumer, Asian J. Chem. Sci., 2017, 3(4), 1–10 Search PubMed.
- W. L. Hough, M. Smiglak, H. Rodríguez, R. P. Swatloski, S. K. Spear, D. T. Daly, J. Pernak, J. E. Grisel, R. D. Carliss and M. D. Soutullo, New J. Chem., 2007, 31, 1429–1436 RSC.
- A. Hospido and H. Rodríguez, in Encyclopedia of Ionic Liquids, Springer, 2023, pp. 813–821 Search PubMed.
- R. F. Frade and C. A. Afonso, Hum. Exp. Toxicol., 2010, 29, 1038–1054 CrossRef CAS PubMed.
- J. R. Harjani, R. D. Singer, M. T. Garcia and P. J. Scammells, Green Chem., 2009, 11, 83–90 RSC.
- M. Suk and K. Kümmerer, Green Chem., 2023, 25, 365–374 RSC.
- M. Trush, L. Metelytsia, I. Semenyuta, L. Kalashnikova, O. Papeykin, I. Venger, O. Tarasyuk, L. Bodachivska, V. Blagodatnyi and S. Rogalsky, Environ. Sci. Pollut. Res., 2019, 26, 4878–4889 CrossRef CAS PubMed.
- K. Aghmih, H. Wakrim, A. Boukhriss, M. El Bouchti, S. Majid and S. Gmouh, Polym. Bull., 2022, 1–13 Search PubMed.
- A. R. Hajipour and M. Seddighi, Synth. Commun., 2012, 42, 227–235 CrossRef CAS.
- F. Hajjaji, R. Salim, M. Taleb, F. Benhiba, N. Rezki, D. Chauhan and M. Quraishi, J. Taiwan Inst. Chem. Eng., 2021, 123, 346–362 CrossRef CAS.
- A. H. M. Fauzi and N. A. S. Amin, Renewable Sustainable Energy Rev., 2012, 16, 5770–5786 CrossRef.
- J. L. Anderson, D. W. Armstrong and G.-T. Wei, Anal. Chem., 2006, 78, 2892–2902 CrossRef PubMed.
- S. Sharma, A. Sharma and U. Gupta, Annals of Antivirals and Antiretrovirals, 2021, 5, 028–032 Search PubMed.
- J. Eberhardt, D. Santos-Martins, A. F. Tillack and S. Forli, J. Chem. Inf. Model., 2021, 61, 3891–3898 CrossRef CAS PubMed.
- M. Bugnon, U. F. Röhrig, M. Goullieux, M. A. Perez, A. Daina, O. Michielin and V. Zoete, Nucleic Acids Res., 2024, 52, W324–W332 CrossRef PubMed.
- M. Rani, A. Nath and A. Kumer, J. Biomol. Struct. Dyn., 2023, 41, 8392–8401 CrossRef CAS PubMed.
- A. Daina, O. Michielin and V. Zoete, Sci. Rep., 2017, 7, 42717 CrossRef PubMed.
- U. A. Çevik, A. Işik and A. Karakaya, Computational Methods for Rational Drug Design, 2025, pp. 123–151 Search PubMed.
- J. A. Pradeepkiran, S. Sainath and K. Shrikanya, in Brucella Melitensis, Elsevier, 2021, pp. 133–176 Search PubMed.
- M. Marzi, M. K. Vakil, M. Bahmanyar and E. Zarenezhad, BioMed Res. Int., 2022, 2022, 7341493 CrossRef PubMed.
- A. Daina and V. Zoete, ChemMedChem, 2016, 11, 1117–1121 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra03648h |
| This journal is © The Royal Society of Chemistry 2025 |