Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Selective synthesis of gem-dihalopiperidines and 4-halo-1,2,3,6-tetrahydropyridines from halogen substituted homoallylic benzenesulfonamides and aldehydes†
Surjya Kumar Bora and
Anil K. Saikia *
*
Department of Chemistry, Indian Institute of Technology Guwahati, Guwahati-781039, Assam, India. E-mail: asaikia@iitg.ac.in
First published on 23rd June 2025
Abstract
An efficient synthesis of gem-dihalopiperidines and 4-halo-1,2,3,6-tetrahydropyridines via aza-Prins cyclization reaction of homoallylic benzenesulfonamides and aldehydes has been described. The reaction proceeds via aza-Prins followed by base-mediated elimination reaction, giving moderate to good yields. The reaction is highly diastereo- and regio-selective. Furthermore, the gem-dihalopiperidines can be easily converted to 2-substituted-1-tosylpiperidin-4-one and pyridine in good yields. Additionally, 4-halo-1,2,3,6-tetrahydropyridines can be employed to afford their corresponding Sonogashira coupling products in good yield.
Introduction
Six-membered nitrogen heterocycles are key structural units in organic synthesis, as they are found in a wide range of natural products and bioactive compounds.1 Among these, the piperidine ring is highly prevalent in alkaloids,2 and serves as a fundamental scaffold in drug discovery and development,3 e.g., donepezil (A), a widely prescribed medication, is commonly used for the treatment of Alzheimer's disease (AD).4 Naratriptan (B) is utilized for the treatment of migraine headaches,1d while selfotel (C) functions as a competitive N-methyl-D-aspartate (NMDA) receptor agonist.5 Femoxetine (D) belongs to a significant class of serotonin reuptake inhibitors.6 Similarly, tetrahydropyridine compounds E and F show antiproliferative activity in solid tumour cell lines (Fig. 1).7 Due to their broad spectrum of biological activity in both pharmaceuticals and natural products, the synthesis of these compounds continues to be of significant interest to synthetic chemists. As a result, considerable research efforts have been made towards developing novel and efficient synthetic methodologies for their preparation.The aza-Prins cyclization is a well-recognized method for synthesizing nitrogen-containing heterocyclic compounds.8 Similarly, the halo-aza-Prins cyclization, where a halide ion serves as a nucleophile to produce mono-halogenated compounds, is also well documented.9 However, the synthesis of gem-dihalopiperidines and 4-halo-1,2,3,6-tetrahydropyridines has been rarely reported in the literature.
In 2006, Carballo et al. reported the synthesis of tetrahydro-pyridines using iron(III) halides as a reagent (Scheme 1a).10 In another study, Miranda et al. reported a synthetic strategy of oxa- and azacycles through the combination of an iron(III) source with the corresponding trimethylsilyl halide (Scheme 1b).11 Recently, our group introduced a new approach for synthesizing 4,4-dihalopiperidines via halo-aza-Prins cyclization reaction, wherein chlorine acts as nucleophile (Scheme 1c).12 Despite these advancements, the rapid and efficient synthesis of gem-dihalopiperidines and tetrahydropyridine derivatives remains challenging. Therefore, the development of more efficient synthetic strategies is necessary to enhance the practicality of this approach. Herein, we established a new methodology for the synthesis of gem-dihalopiperidines and 4-halo-1,2,3,6-tetrahydropyridines via halo-aza-Prins cyclization reaction of halogen-substituted homoallylic benzenesulfonamide and aldehyde (Scheme 1d). Notably, the position of the double bond of tetrahydropyridines 4 in the present case differs from that of the products reported by Carballo10 and Miranda.11 Also, in gem-dihalopiperidines, Br (axial) acts as a nucleophile, which differs from our previous report where Cl (axial) acted as a nucleophile. Consequently, the two cases give products with different stereochemistry, particularly in the case of 4-bromo-4-chloro derivatives.
Results and discussion
Initially, N-(3-bromobut-3-en-1-yl)-4-methylbenzenesulfon-amide (1a) was reacted with 4-chloro-benzaldehyde (2c) using 1.1 equiv. of boron trifluoride etherate (BF3·OEt2) in dichloromethane (DCM) at 0 °C to room temperature under a nitrogen atmosphere for 1.5 hours (Table 1, entry 1). However, this led to the decomposition of the starting material. When the reagent was changed to boron tribromide (BBr3), it gave 18% of product 3ac with unreacted starting material (Table 1, entry 2).| S. no | Reagents (equiv.) | Base (eq.) (temp2) (time2) | Solvent | Temp1 | Time1/h | 3ac | 4ac![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ac′ 4ac′ |
|---|---|---|---|---|---|---|---|
| % yieldb | % yield (4ac![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4ac′) 4ac′) |
||||||
| a Reaction conditions: all reactions were carried out under a nitrogen atmosphere, 1a (0.6 mmol) and 2c (0.66 mmol), solvent (3.0 mL).b Isolated yield.c Decomposed. NR = no reaction.d Complex mixture. Regioselectivity was determined by 1H NMR spectroscopy. | |||||||
| 1 | BF3·OEt2 (1.1) | — | DCM | 0 °C-rt | 1.5 | —c | — |
| 2 | BBr3 (1.1) | — | DCM | 0 °C | 12 | 18 | — |
| 3 | InBr3 (1.1) | — | DCM | 0 °C-rt | 12 | NR | |
| 4 | BBr3 (1.1) | — | DCM | 40 °C | 2 | —c | — |
| 5 | BBr3 (0.25) + TMSBr (1.2) | — | DCM | 0 °C-rt | 12 | 80 | — |
| 6 | In(OTf)3 (0.25) + TMSBr (1.2) | — | DCM | 0 °C-rt | 12 | NR | — |
| 7 | Sc(OTf)3 (0.25) + TMSBr (1.2) | — | DCM | 0 °C-rt | 12 | NR | — |
| 8 | TMSBr (1.2) | — | DCM | 0 °C-rt | 12 | 9 | — |
| 9 | BBr3 (0.1) + TMSBr (1.2) | — | DCM | 0 °C-rt | 12 | 76 | — |
| 10 | BBr3 (0.3) + TMSBr (1.2) | — | DCM | 0 °C-rt | 12 | 78 | — |
| 11 | BBr3(0.25) + TMSBr (1.2) | — | DCE | 0 °C-rt | 12 | 83 | — |
| 12 | BBr3(0.25) + TMSBr (1.2) | — | Toluene | 0 °C-rt | 12 | 71 | — |
| 13 | BBr3(0.25) + TMSBr (1.2) | — | CH3CN | 0 °C-rt | 12 | NR | — |
| 14 | BBr3 (0.25) + TMSBr (1.2) | DBU (1.0) | DCE | 0 °C-rt | 12 | 51 | 20 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
| (rt) (12 h) | |||||||
| 15 | BBr3 (0.25) + TMSBr (1.2) | KOtBu (1.0) | DCE | 0 °C-rt | 12 | 74 | NR |
| (rt) (12 h) | |||||||
| 16 | BBr3 (0.25) + TMSBr (1.2) | DABCO (1.0) | DCE | 0 °C-rt | 12 | — | —d |
| (rt) (4 h) | |||||||
| 17 | BBr3 (0.25) + TMSBr (1.2) | Piperidine (1.0) | DCE | 0 °C-rt | 12 | — | —d |
| (rt) (4 h) | |||||||
| 18 | BBr3 (0.25) + TMSBr (1.2) | DBU (10.0) | DCE | 0 °C-rt | 12 | — | 68 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) 2) |
| (80) (4 h) | |||||||
| 19 | BBr3 (0.25) + TMSBr (1.2) | DBU (20.0) | DCE | 0 °C-rt | 12 | — | 69 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
| (80) (4 h) | |||||||
| 20 | BBr3 (0.25) + TMSBr (1.2) | DBU (40.0) | DCE | 0 °C-rt | 12 | — | 70 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0) 0) |
| (80) (4 h) | |||||||
Changing BBr3 to indium tribromide (InBr3) did not yield any product (Table 1, entry 3). Therefore, in order to increase the yield, the reaction was performed at 40 °C with BBr3, which led to the decomposition of the product (Table 1, entry 4). Fortunately, when the reaction was performed using 0.25 equiv. of BBr3 with 1.2 equiv. of trimethylsilyl bromide (TMSBr) as an additive, the yield of 3ac increased to 80% (Table 1, entry 5). Other combinations, such as indium triflate (In(OTf)3) and scandium triflate (Sc(OTf)3) with TMSBr, failed to produce 3ac (Table 1, entries 6 and 7). When the reaction was performed using only 1.2 equiv. of TMSBr resulted in a mere 9% yield of the product with unreacted starting material (Table 1, entry 8). Additionally, decreasing the BBr3 loading to 0.1 equiv. or increasing it to 0.3 equiv. did not improve the yield (Table 1, entries 9 and 10). However, changing the solvent to 1,2-dichloroethane (DCE) resulted in an improved yield of 83% (Table 1, entry 11). It may be due to its higher polarity than DCM which dissolves both organic substrates and Lewis acids effectively to promote the transformation. Other solvents, such as toluene and acetonitrile, did not improve the yield of product 3ac (Table 1, entries 12 and 13). Thus, 0.25 equiv. of BBr3 and 1.2 equiv. of TMSBr in DCE at 0 °C to rt were the optimal conditions for the product 3ac.
After confirming the formation of product 3ac by thin layer chromatography (TLC), 1.0 equiv. of 1,8-diazabicyclo-[5.4.0]undec-7-ene (DBU) was added to the reaction mixture at rt and stirred for 12 hours. Interestingly, this resulted in 20% yield of regioisomeric products 4ac and 4ac′ with a regioselectivity 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2, along with unreacted 3ac (Table 1, entry 14). In order to improve the yield and regioselectivity, various reaction conditions were examined (Table 1). However, the use of inorganic base potassium tertiary butoxide (KOtBu) failed to produce 4ac, possibly due to solubility issues (Table 1, entry 15). Other organic bases such as 1,4-diazabicyclo[2.2.2]octane (DABCO) and piperidine led to the formation of a complex mixture (Table 1, entries 16–17). Increasing the temperature to 80 °C and using 10.0 equiv. of DBU improved the yield to 68%, but the regioselectivity remains same (Table 1, entry 18). Notably, increasing the loading of DBU to 20.0 equiv. provided 4ac and 4ac′ with a ratio of 1
2, along with unreacted 3ac (Table 1, entry 14). In order to improve the yield and regioselectivity, various reaction conditions were examined (Table 1). However, the use of inorganic base potassium tertiary butoxide (KOtBu) failed to produce 4ac, possibly due to solubility issues (Table 1, entry 15). Other organic bases such as 1,4-diazabicyclo[2.2.2]octane (DABCO) and piperidine led to the formation of a complex mixture (Table 1, entries 16–17). Increasing the temperature to 80 °C and using 10.0 equiv. of DBU improved the yield to 68%, but the regioselectivity remains same (Table 1, entry 18). Notably, increasing the loading of DBU to 20.0 equiv. provided 4ac and 4ac′ with a ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (Table 1, entry 19). Finally, employing 40.0 equiv. of DBU resulted in the formation of a single regioselective product 4ac (Table 1, entry 20). Therefore, it was concluded that 0.25 equiv. of BBr3 and 1.2 equiv. of TMSBr in DCE at 0 °C to rt for 12 h, followed by treatment with 40.0 equiv. of DBU at 80 °C were the optimal conditions for the product 4ac. The high concentration of DBU may be required to abstract the proton from the less hindered site of the gem-dihalopiperidine effectively as DBU is bulky molecule.
1 (Table 1, entry 19). Finally, employing 40.0 equiv. of DBU resulted in the formation of a single regioselective product 4ac (Table 1, entry 20). Therefore, it was concluded that 0.25 equiv. of BBr3 and 1.2 equiv. of TMSBr in DCE at 0 °C to rt for 12 h, followed by treatment with 40.0 equiv. of DBU at 80 °C were the optimal conditions for the product 4ac. The high concentration of DBU may be required to abstract the proton from the less hindered site of the gem-dihalopiperidine effectively as DBU is bulky molecule.
Under the first established optimal conditions, the reaction was screened with different aldehydes, as presented in Scheme 2. Aldehydes bearing moderately electron withdrawing groups at para and meta positions of the aromatic ring such as –F, –Cl and –Br (Scheme 2, 3ab–3ae), as well as those with strongly electron-withdrawing groups, including such as –CO2Me, –CF3 and –NO2 (Scheme 2, 3af–3ah), gave good to excellent yields. Similarly, when the reaction was carried out with aldehydes containing a moderately electron-donating group such as –Me, 3,4-dimethyl or a bulky electron-donating tert-butyl group at the para positions gave the products 3aj, 3am and 3al in 83%, 81% and 65% yields, respectively. However, a highly electron-donating methoxy group on the aromatic ring of aldehyde 2k led to decomposition, similar to many Prins cyclisation reactions.12 Intriguingly, biphenyl aldehyde 2i gave 3ai in 65% yield, and bulky substrate such as 2-naphthaldehyde 2n and sterically-hindered 1-naphthaldehyde 2o provided corresponding products 3an and 3ao in 81% and 73% yields, respectively. Aliphatic aldehydes, including –C2H5, –C3H7, and –C6H13 groups, also furnished their corresponding products in decent yields (3aq–3au). Unfortunately, secondary amide N-(4-bromopent-4-en-2-yl)-4-methylbenzenesulfonamide (1b) and N-(3-bromo-1-(4-chlorophenyl)but-3-en-1-yl)-4-methyl-benzenesulfonamide (1c) did not yield the desired products. Under the same optimized reaction conditions, the reaction also proceeded efficiently with halogen-substituted homoallylic benzenesulfonamides bearing chlorine (Cl) or iodine (I), specifically, N-(3-chlorobut-3-en-1-yl)-4-methylbenzene-sulfonamide (1d) and N-(3-iodobut-3-en-1-yl)-4-methylbenzenesulfonamide (1e), giving products 3dj–3ed in moderate to good yields (Scheme 2). The structure and stereochemistry of all compounds (3aa–3aj, 3al–3au, 3dj, 3ed) were determined by 1H NMR, 13C{1H} NMR, mass spectrometry, and ultimately by X-ray crystallographic analysis of compounds 3ac and 3dj. Likewise, under the second set of optimized reaction conditions, the reaction was explored with different aldehydes as shown in Scheme 3. Substrates with electron-withdrawing groups such as –Cl, –Br on the aromatic ring, provided products 4ac and 4ad in good to moderate yields. Electron-donating group in the aromatic ring of the aldehyde provided a 70% yield of their corresponding product 4aj. Moreover, biphenyl and sterically hindered 1-naphthyl groups were well tolerated under the reaction conditions. The substrate with an aliphatic group gave 62% of their desired product 4aq. The reaction of halogen-substituted homoallylic benzenesulfonamides containing chlorine (Cl) with electron-donating and electron-withdrawing groups at the para and meta positions of the aromatic group of the aldehyde gave their corresponding products 4db, 4de, and 4dj in moderate yields (Scheme 3). The structure of all compounds (4aa–4dj) was determined by 1H NMR, 13C{1H} NMR, and mass spectrometry. The stereochemistry of the compounds was determined by comparison with the reported 1H NMR data.10,11 For example, the olefinic proton of 4aa resonates at 5.92 ppm, whereas the olefinic proton of the corresponding regioisomer of Carballo's group10 resonates at 6.22 ppm. Finally, the structure and stereochemistry of compounds were determined by X-ray crystallographic analysis of compound 4aa.
 | ||
| Scheme 3 Synthesis of 4-halo-1,2,3,6-tetrahydropyridines.a Reaction conditions: 1 (0.6 mmol), 2 (0.66 mmol), BBr3 (0.15 mmol), TMSBr (0.72 mmol), DCE (3 mL), 0 °C-rt, DBU (24.0 mmol), N2 atmosphere. | ||
A plausible mechanism is proposed in Scheme 4. First, BBr3 reacts with TMSBr to generate Me3Si+–BBr4− species.13 In the presence of these species, the halogen-substituted homoallylic benzenesulfonamide 1 reacts with aldehyde 2 to form the iminium ion intermediate (A), which then undergoes aza-Prins cyclisation to produce the carbocation intermediate (B). Further axial attack by bromide ion from BBr4− gives product 3. After the addition of organic base DBU, its abstract proton from compound 3 to give the final product 4. The formation of a single regioisomer may be attributed to the selective abstraction of a proton from C-5 of the piperidine ring. The proton at C-3 is not in a position to be abstracted by bulky DBU as it is sterically hindered due to its cis configuration with the bulky substituent “R” at the C-2 position.
To investigate the utility of this methodology, several reactions were carried out as shown in Schemes 5–7. The gem-dihalogen compounds 3ad, 3ar and 3as were treated with triethylamine and acetic anhydride in DCM/H2O (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1), to give corresponding piperidinone compounds 2-(4-bromophenyl)-1-tosylpiperidin-4-one (5a), 2-propyl-1-tosylpiperidin-4-one (5b) and 2-hexyl-1-tosylpiperidin-4-one (5c) in 80%, 75% and 66% yields, respectively, under a previously reported procedure (Scheme 5).14 Furthermore, treatment with DBU of gem-dihalocompounds 3ad, 3ai and 3aj gave their corresponding pyridine derivatives 6a–6c in 74%, 64% and 70% yields, respectively (Scheme 6).12
1), to give corresponding piperidinone compounds 2-(4-bromophenyl)-1-tosylpiperidin-4-one (5a), 2-propyl-1-tosylpiperidin-4-one (5b) and 2-hexyl-1-tosylpiperidin-4-one (5c) in 80%, 75% and 66% yields, respectively, under a previously reported procedure (Scheme 5).14 Furthermore, treatment with DBU of gem-dihalocompounds 3ad, 3ai and 3aj gave their corresponding pyridine derivatives 6a–6c in 74%, 64% and 70% yields, respectively (Scheme 6).12
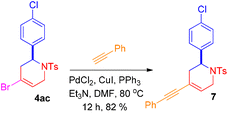
The reaction of 4-halo-1,2,3,6-tetrahydropyridines gives Sonogashira product using literature precedents.15 Thus, the reaction of 4-bromo-2-(4-chlorophenyl)-1-tosyl-1,2,3,6- tetrahydropyridine (4ac) with phenyl acetylene in the presence of PdCl2, CuI, PPh3 and Et3N provided the corresponding Sonogashira product 2-(4-chlorophenyl)-4-(phenylethynyl)-1-tosyl-1,2,3,6-tetrahydropyridine (7) (Scheme 7).
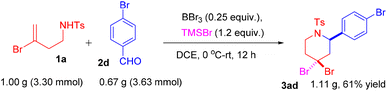
To evaluate the scalability of this methodology, a gram-scale reaction was performed between N-(3-bromobut-3-en-1-yl)-4-methylbenzenesulfonamide 1a (1.00 g, 3.30 mmol) and 4-bromobenzaldehyde 2d (0.67 g, 3.63 mmol), which gave 1.11 g of the product 3ad with 61% yield (Scheme 8).
Conclusions
In conclusion, an efficient methodology has been developed that is useful not only for the synthesis of gem-dihalopiperidine but also for the synthesis of 4-halo-1,2,3,6-tetrahydropyridine derivatives from alkene sulfonamides and aldehydes in good to moderate yields. The selectivity of the reaction is particularly notable, as the first pathway gives a diastereoselective product, while the second provides a regioselective product. Gem-dihalopiperidines can be extended to the synthesis of pyridine and piperidinone derivatives in moderate to good yields, whereas 4-halo-1,2,3,6-tetrahydropyridine can be transformed into its corresponding Sonogashira product. However, the enantioselective or chiral induction strategies for the synthesis of its chiral counterpart are a limitation of the current approach and will be explored in the future. Furthermore, the scalability of the reaction is investigated using a gram-scale experiment, and it shows its potential for large-scale applications with industrial relevance.Experimental
General information
All the reagents were of reagent grade (AR grade) and were used as purchased without further purification. Silica gel (60–120 mesh size) was used for column chromatography. Reactions were monitored by TLC on silica gel GF254 (0.25 mm). Melting points were recorded in an open capillary tube and are uncorrected. Fourier transform-infrared (FT-IR) spectra were recorded as neat liquid or KBr pellets. NMR spectra were recorded in CDCl3 with tetramethylsilane as the internal standard for 1H (600 MHz, 500 MHz and 400 MHz) or 13C (150 MHz, 125 MHz and 100 MHz) NMR. Chemical shifts (δ) are reported in ppm and spin–spin coupling constants (J) are given in Hz. Structural assignments were made with additional information from single-crystal XRD experiments. HRMS spectra were recorded using a Q-TOF mass spectrometer.The starting material N-(3-bromobut-3-en-1-yl)-4-methylbenzenesulfonamide16a (1a), N-(4-bromopent-4-en-2-yl)-4-methylbenzenesulfonamide12 (1b), N-(3-bromo-1-(4-chlorophenyl)but-3-en-1-yl)-4-methylbenzenesulfonamide12 (1c), N-(3-chlorobut-3-en-1-yl)-4-methylbenzenesulfonamide12 (1d) and N-(3-iodobut-3-en-1-yl)-4-methylbenzenesulfonamide16b (1e) was synthesized according to the reported literature. The spectroscopic data of the above compound are in good agreement with the literature. The experimental procedure and the characterization data of all compounds are given as follows.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). Upon completion of the reaction, it was quenched with a saturated sodium bicarbonate solution. A brine solution was then added, and the mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic layer was dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure using a rotary evaporator. The crude product was purified by silica gel column chromatography, employing a mixture of ethyl acetate and hexane (1
9). Upon completion of the reaction, it was quenched with a saturated sodium bicarbonate solution. A brine solution was then added, and the mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic layer was dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure using a rotary evaporator. The crude product was purified by silica gel column chromatography, employing a mixture of ethyl acetate and hexane (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) as the eluent to obtain the final product.
9, v/v) as the eluent to obtain the final product.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.51; mp 136 °C, yield 230 mg, 81%; IR (KBr, neat) ν 2924, 1598, 1495, 1450, 1344, 1158, 1094, 950, 719, 660, 559 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.4 Hz, 2H), 7.24–7.17 (m, 7H), 4.78 (t, J = 5.7 Hz, 1H), 3.84–3.78 (m, 1H), 3.62–3.56 (m, 1H), 3.19 (dd, J = 14.8, 6.4 Hz, 1H), 2.81 (ddd, J = 14.8, 4.8, 1.1 Hz, 1H), 2.66–2.62 (m, 2H), 2.42 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.8, 137.7, 136.6, 129.8, 128.4, 127.8, 127.6, 127.5, 62.4, 59.0, 52.8, 47.7, 43.9, 21.8. HRMS (ESI) calcd for C18H20Br2NO2S (M + H)+ 473.9556, found 473.9571.
1) 0.51; mp 136 °C, yield 230 mg, 81%; IR (KBr, neat) ν 2924, 1598, 1495, 1450, 1344, 1158, 1094, 950, 719, 660, 559 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.50 (d, J = 8.4 Hz, 2H), 7.24–7.17 (m, 7H), 4.78 (t, J = 5.7 Hz, 1H), 3.84–3.78 (m, 1H), 3.62–3.56 (m, 1H), 3.19 (dd, J = 14.8, 6.4 Hz, 1H), 2.81 (ddd, J = 14.8, 4.8, 1.1 Hz, 1H), 2.66–2.62 (m, 2H), 2.42 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.8, 137.7, 136.6, 129.8, 128.4, 127.8, 127.6, 127.5, 62.4, 59.0, 52.8, 47.7, 43.9, 21.8. HRMS (ESI) calcd for C18H20Br2NO2S (M + H)+ 473.9556, found 473.9571.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; mp 143 °C, yield 242 mg, 82%; IR (KBr, neat) ν 2944, 1602, 1510, 1443, 1375, 1162, 1095, 1039, 918, 744, 660, 553 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.48–7.45 (m, 2H), 7.23 (d, J = 8.0 Hz, 2H), 7.15–7.12 (m, 2H), 6.91–6.87 (m, 2H), 4.71 (t, J = 5.7 Hz, 1H), 3.86–3.79 (m, 1H), 3.59–3.53 (m, 1H), 3.15–3.09 (m, 1H), 2.80–2.75 (m, 1H), 2.67–2.62 (m, 2H), 2.42 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 162.4 (d, J = 245.3 Hz), 144.0, 136.6, 133.3 (d, J = 3.2 Hz), 129.8, 129.4 (d, J = 8.1 Hz), 127.6, 115.3 (d, J = 21.5 Hz), 62.2, 58.7, 53.0, 47.7, 44.1, 21.8. 19F NMR (470 MHz, C6F6/CDCl3) δ −114.50. HRMS (ESI) calcd for C18H19Br2FNO2S (M + H)+ 491.9462, found 491.9441.
1) 0.52; mp 143 °C, yield 242 mg, 82%; IR (KBr, neat) ν 2944, 1602, 1510, 1443, 1375, 1162, 1095, 1039, 918, 744, 660, 553 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.48–7.45 (m, 2H), 7.23 (d, J = 8.0 Hz, 2H), 7.15–7.12 (m, 2H), 6.91–6.87 (m, 2H), 4.71 (t, J = 5.7 Hz, 1H), 3.86–3.79 (m, 1H), 3.59–3.53 (m, 1H), 3.15–3.09 (m, 1H), 2.80–2.75 (m, 1H), 2.67–2.62 (m, 2H), 2.42 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 162.4 (d, J = 245.3 Hz), 144.0, 136.6, 133.3 (d, J = 3.2 Hz), 129.8, 129.4 (d, J = 8.1 Hz), 127.6, 115.3 (d, J = 21.5 Hz), 62.2, 58.7, 53.0, 47.7, 44.1, 21.8. 19F NMR (470 MHz, C6F6/CDCl3) δ −114.50. HRMS (ESI) calcd for C18H19Br2FNO2S (M + H)+ 491.9462, found 491.9441.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; mp 159 °C, yield 252 mg, 83%; IR (KBr, neat) ν 2925, 2855, 1597, 1492, 1345, 1162, 1093, 1015, 916, 712, 656, 551 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.48 (d, J = 7.8 Hz, 2H), 7.24 (d, J = 7.9 Hz, 2H), 7.17 (d, J = 8.8 Hz, 2H), 7.10 (d, J = 8.1 Hz, 2H), 4.73 (t, J = 5.6 Hz, 1H), 3.80–3.76 (m, 1H), 3.60–3.55 (m, 1H), 3.12 (dd, J = 14.8, 6.5 Hz, 1H), 2.78 (dd, J = 14.8, 4.7 Hz, 1H), 2.65–2.61 (m, 2H), 2.43 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.1, 136.5, 136.2, 133.7, 129.9, 129.0, 128.5, 127.6, 62.0, 58.5, 52.6, 47.6, 43.8, 21.8. HRMS (ESI) calcd for C18H19Br2ClNO2S (M + H)+ 507.9166, found 507.9174.
1) 0.52; mp 159 °C, yield 252 mg, 83%; IR (KBr, neat) ν 2925, 2855, 1597, 1492, 1345, 1162, 1093, 1015, 916, 712, 656, 551 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.48 (d, J = 7.8 Hz, 2H), 7.24 (d, J = 7.9 Hz, 2H), 7.17 (d, J = 8.8 Hz, 2H), 7.10 (d, J = 8.1 Hz, 2H), 4.73 (t, J = 5.6 Hz, 1H), 3.80–3.76 (m, 1H), 3.60–3.55 (m, 1H), 3.12 (dd, J = 14.8, 6.5 Hz, 1H), 2.78 (dd, J = 14.8, 4.7 Hz, 1H), 2.65–2.61 (m, 2H), 2.43 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.1, 136.5, 136.2, 133.7, 129.9, 129.0, 128.5, 127.6, 62.0, 58.5, 52.6, 47.6, 43.8, 21.8. HRMS (ESI) calcd for C18H19Br2ClNO2S (M + H)+ 507.9166, found 507.9174.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; mp 149 °C, yield 274 mg, 83%; IR (KBr, neat) ν 2924, 1597, 1488, 1341, 1162, 1094, 1011, 915, 709, 661, 549 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 8.6 Hz, 2H), 7.24 (d, J = 8.1 Hz, 2H), 7.04 (d, J = 8.3 Hz, 2H), 4.72 (t, J = 5.6 Hz, 1H), 3.81–3.75 (m, 1H), 3.61–3.55 (m, 1H), 3.15–3.09 (m, 1H), 2.78 (dd, J = 14.8, 4.8, 1.2 Hz, 1H), 2.65–2.61 (m, 2H), 2.43 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 144.1, 136.7, 136.5, 131.5, 129.9, 129.3, 127.6, 121.9, 61.9, 58.5, 52.5, 47.6, 43.8, 21.8. HRMS (ESI) calcd for C18H19Br3NO2S (M + H)+ 551.8661, found 551.8637.
1) 0.52; mp 149 °C, yield 274 mg, 83%; IR (KBr, neat) ν 2924, 1597, 1488, 1341, 1162, 1094, 1011, 915, 709, 661, 549 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.48 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 8.6 Hz, 2H), 7.24 (d, J = 8.1 Hz, 2H), 7.04 (d, J = 8.3 Hz, 2H), 4.72 (t, J = 5.6 Hz, 1H), 3.81–3.75 (m, 1H), 3.61–3.55 (m, 1H), 3.15–3.09 (m, 1H), 2.78 (dd, J = 14.8, 4.8, 1.2 Hz, 1H), 2.65–2.61 (m, 2H), 2.43 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 144.1, 136.7, 136.5, 131.5, 129.9, 129.3, 127.6, 121.9, 61.9, 58.5, 52.5, 47.6, 43.8, 21.8. HRMS (ESI) calcd for C18H19Br3NO2S (M + H)+ 551.8661, found 551.8637.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; yield 238 mg, 72%; IR (KBr, neat) ν 2924, 1596, 1475, 1341, 1160, 1091, 1011, 923, 808, 725, 659, 570 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.47 (d, J = 8.1 Hz, 2H), 7.32–7.30 (m, 1H), 7.22 (d, J = 8.0 Hz, 2H), 7.18–7.16 (m, 1H), 7.15–7.10 (m, 2H), 4.76 (t, J = 5.7 Hz, 1H), 3.81–3.77 (m, 1H), 3.63–3.59 (m, 1H), 3.12 (dd, J = 14.7, 6.6 Hz, 1H), 2.80 (ddd, J = 14.8, 4.8, 1.2 Hz, 1H), 2.66–2.60 (m, 2H), 2.42 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 144.2, 139.9, 136.6, 130.9, 130.8, 130.0, 129.9, 127.4, 126.4, 122.5, 61.9, 58.4, 52.5, 47.6, 43.8, 21.8. HRMS (ESI) calcd for C18H19Br3NO2S (M + H)+ 551.8661, found 551.8655.
1) 0.52; yield 238 mg, 72%; IR (KBr, neat) ν 2924, 1596, 1475, 1341, 1160, 1091, 1011, 923, 808, 725, 659, 570 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.47 (d, J = 8.1 Hz, 2H), 7.32–7.30 (m, 1H), 7.22 (d, J = 8.0 Hz, 2H), 7.18–7.16 (m, 1H), 7.15–7.10 (m, 2H), 4.76 (t, J = 5.7 Hz, 1H), 3.81–3.77 (m, 1H), 3.63–3.59 (m, 1H), 3.12 (dd, J = 14.7, 6.6 Hz, 1H), 2.80 (ddd, J = 14.8, 4.8, 1.2 Hz, 1H), 2.66–2.60 (m, 2H), 2.42 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 144.2, 139.9, 136.6, 130.9, 130.8, 130.0, 129.9, 127.4, 126.4, 122.5, 61.9, 58.4, 52.5, 47.6, 43.8, 21.8. HRMS (ESI) calcd for C18H19Br3NO2S (M + H)+ 551.8661, found 551.8655.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.48; mp 169 °C, yield 236 mg, 74%; IR (KBr, neat) ν 2926, 1718, 1611, 1435, 1344, 1278, 1158, 1094, 1017, 950, 917, 868, 771, 662, 550 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 8.5 Hz, 2H), 7.55 (d, J = 8.4 Hz, 2H), 7.28–7.24 (m, 4H), 4.90 (t, J = 5.5 Hz, 1H), 3.91 (s, 3H), 3.77–3.70 (m, 1H), 3.68–3.62 (m, 1H), 3.20 (ddd, J = 14.8, 5.6, 1.1 Hz, 1H), 2.84 (ddd, J = 14.7, 5.2, 0.9 Hz, 1H), 2.65–2.56 (m, 2H), 2.43 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 166.9, 144.3, 143.2, 136.3, 130.0, 129.8, 129.6, 127.6, 127.2, 61.6, 58.3, 52.4, 52.2, 47.4, 43.4, 21.8. HRMS (ESI) calcd for C20H22Br2NO4S (M + H)+ 531.9611, found 531.9610.
1) 0.48; mp 169 °C, yield 236 mg, 74%; IR (KBr, neat) ν 2926, 1718, 1611, 1435, 1344, 1278, 1158, 1094, 1017, 950, 917, 868, 771, 662, 550 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.91 (d, J = 8.5 Hz, 2H), 7.55 (d, J = 8.4 Hz, 2H), 7.28–7.24 (m, 4H), 4.90 (t, J = 5.5 Hz, 1H), 3.91 (s, 3H), 3.77–3.70 (m, 1H), 3.68–3.62 (m, 1H), 3.20 (ddd, J = 14.8, 5.6, 1.1 Hz, 1H), 2.84 (ddd, J = 14.7, 5.2, 0.9 Hz, 1H), 2.65–2.56 (m, 2H), 2.43 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 166.9, 144.3, 143.2, 136.3, 130.0, 129.8, 129.6, 127.6, 127.2, 61.6, 58.3, 52.4, 52.2, 47.4, 43.4, 21.8. HRMS (ESI) calcd for C20H22Br2NO4S (M + H)+ 531.9611, found 531.9610.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.53; yield 201 mg, 62%; IR (KBr, neat) ν 2926, 1620, 1598, 1324, 1160, 1116, 1123, 1069, 1017, 951, 715, 664, 583, 549 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.49–7.45 (m, 4H), 7.30 (d, J = 8.1 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 4.84 (t, J = 5.8 Hz, 1H), 3.81–3.77 (m, 1H), 3.64–3.60 (m, 1H), 3.17 (dd, J = 14.7, 6.4 Hz, 1H), 2.82 (ddd, J = 14.8, 4.9, 1.3 Hz, 1H), 2.68–2.59 (m, 2H), 2.42 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.2, 141.8, 136.5, 130.1 (q, J = 32.2 Hz), 130.0, 127.9, 127.5, 125.3 (q, J = 3.7 Hz), 124.2 (q, J = 270.4 Hz), 61.6, 58.5, 52.4, 47.5, 43.7, 21.8. 19F NMR (470 MHz, CDCl3/C6F6) δ −62.50. HRMS (ESI) calcd for C19H19Br2F3NO2S (M + H)+ 541.9430, found 541.9431.
1) 0.53; yield 201 mg, 62%; IR (KBr, neat) ν 2926, 1620, 1598, 1324, 1160, 1116, 1123, 1069, 1017, 951, 715, 664, 583, 549 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.49–7.45 (m, 4H), 7.30 (d, J = 8.1 Hz, 2H), 7.22 (d, J = 8.0 Hz, 2H), 4.84 (t, J = 5.8 Hz, 1H), 3.81–3.77 (m, 1H), 3.64–3.60 (m, 1H), 3.17 (dd, J = 14.7, 6.4 Hz, 1H), 2.82 (ddd, J = 14.8, 4.9, 1.3 Hz, 1H), 2.68–2.59 (m, 2H), 2.42 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.2, 141.8, 136.5, 130.1 (q, J = 32.2 Hz), 130.0, 127.9, 127.5, 125.3 (q, J = 3.7 Hz), 124.2 (q, J = 270.4 Hz), 61.6, 58.5, 52.4, 47.5, 43.7, 21.8. 19F NMR (470 MHz, CDCl3/C6F6) δ −62.50. HRMS (ESI) calcd for C19H19Br2F3NO2S (M + H)+ 541.9430, found 541.9431.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.46; mp 193 °C, yield 258 mg, 83%; IR (KBr, neat) ν 2927, 1597, 1522, 1349, 1159, 1092, 721, 663, 550 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.11 (d, J = 8.8 Hz, 2H), 7.57–7.56 (m, 2H), 7.40 (d, J = 8.3 Hz, 2H), 7.29 (d, J = 7.9 Hz, 2H), 4.96–4.94 (m, 1H), 3.72–3.64 (m, 2H), 3.18 (dd, J = 15.0, 5.4 Hz, 1H), 2.84 (dd, J = 14.9, 5.3 Hz, 1H), 2.65–2.60 (m, 1H), 2.57–2.52 (m, 1H), 2.44 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 147.4, 145.8, 144.6, 136.0, 130.1, 128.0, 127.5, 123.7, 60.9, 57.9, 52.0, 47.1, 43.3, 21.8. HRMS (ESI) calcd for C18H19Br2N2O4S (M + H)+ 518.9407, found 518.9379.
1) 0.46; mp 193 °C, yield 258 mg, 83%; IR (KBr, neat) ν 2927, 1597, 1522, 1349, 1159, 1092, 721, 663, 550 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.11 (d, J = 8.8 Hz, 2H), 7.57–7.56 (m, 2H), 7.40 (d, J = 8.3 Hz, 2H), 7.29 (d, J = 7.9 Hz, 2H), 4.96–4.94 (m, 1H), 3.72–3.64 (m, 2H), 3.18 (dd, J = 15.0, 5.4 Hz, 1H), 2.84 (dd, J = 14.9, 5.3 Hz, 1H), 2.65–2.60 (m, 1H), 2.57–2.52 (m, 1H), 2.44 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 147.4, 145.8, 144.6, 136.0, 130.1, 128.0, 127.5, 123.7, 60.9, 57.9, 52.0, 47.1, 43.3, 21.8. HRMS (ESI) calcd for C18H19Br2N2O4S (M + H)+ 518.9407, found 518.9379.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.53; mp 152 °C, yield 214 mg, 65%; IR (KBr, neat) ν 3030, 2924, 1598, 1488, 1343, 1262, 1157, 1093, 1008, 949, 866, 758, 728, 697, 550 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.56–7.54 (m, 2H), 7.50–7.421 (m, 6H), 7.37–7.33 (m, 1H), 7.25–7.19 (m, 4H), 4.82–4.79 (m, 1H), 3.91–3.85 (m, 1H), 3.64–3.58 (m, 1H), 3.22 (dd, J = 14.7, 6.9 Hz, 1H), 2.85 (ddd, J = 14.8, 4.6, 1.1 Hz, 1H), 2.70–2.66 (m, 2H), 2.40 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.7, 140.7, 136.8, 136.5, 129.7, 129.0, 128.3, 127.7, 127.6, 127.2, 127.0, 62.6, 59.1, 52.8, 47.8, 44.1, 21.8. HRMS (ESI) calcd for C24H24Br2NO2S (M + H)+ 549.9869, found 549.9863.
1) 0.53; mp 152 °C, yield 214 mg, 65%; IR (KBr, neat) ν 3030, 2924, 1598, 1488, 1343, 1262, 1157, 1093, 1008, 949, 866, 758, 728, 697, 550 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.56–7.54 (m, 2H), 7.50–7.421 (m, 6H), 7.37–7.33 (m, 1H), 7.25–7.19 (m, 4H), 4.82–4.79 (m, 1H), 3.91–3.85 (m, 1H), 3.64–3.58 (m, 1H), 3.22 (dd, J = 14.7, 6.9 Hz, 1H), 2.85 (ddd, J = 14.8, 4.6, 1.1 Hz, 1H), 2.70–2.66 (m, 2H), 2.40 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.7, 140.7, 136.8, 136.5, 129.7, 129.0, 128.3, 127.7, 127.6, 127.2, 127.0, 62.6, 59.1, 52.8, 47.8, 44.1, 21.8. HRMS (ESI) calcd for C24H24Br2NO2S (M + H)+ 549.9869, found 549.9863.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; mp 127 °C, yield 242 mg, 83%; IR (KBr, neat) ν 2923, 1598, 1511, 1343, 1161, 812, 726, 673, 567, 546 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.48 (d, J = 8.0 Hz, 2H), 7.21 (d, J = 8.1 Hz, 2H), 7.05 (d, J = 8.0 Hz, 2H), 7.01 (d, J = 8.0 Hz, 2H), 4.68 (t, J = 5.6 Hz, 1H), 3.85–3.81 (m, 1H), 3.56–3.52 (m, 1H), 3.15 (dd, J = 14.7, 6.8 Hz, 1H), 2.78 (ddd, J = 14.8, 4.6, 1.3 Hz, 1H), 2.68–2.62 (m, 2H), 2.42 (s, 3H), 2.30 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.7, 137.6, 136.6, 134.6, 129.7, 129.0, 127.7, 127.6, 62.7, 59.1, 53.1, 47.8, 44.1, 21.8, 21.3. HRMS (ESI) calcd for C19H22Br2NO2S (M + H)+ 487.9713, found 487.9707.
1) 0.52; mp 127 °C, yield 242 mg, 83%; IR (KBr, neat) ν 2923, 1598, 1511, 1343, 1161, 812, 726, 673, 567, 546 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.48 (d, J = 8.0 Hz, 2H), 7.21 (d, J = 8.1 Hz, 2H), 7.05 (d, J = 8.0 Hz, 2H), 7.01 (d, J = 8.0 Hz, 2H), 4.68 (t, J = 5.6 Hz, 1H), 3.85–3.81 (m, 1H), 3.56–3.52 (m, 1H), 3.15 (dd, J = 14.7, 6.8 Hz, 1H), 2.78 (ddd, J = 14.8, 4.6, 1.3 Hz, 1H), 2.68–2.62 (m, 2H), 2.42 (s, 3H), 2.30 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.7, 137.6, 136.6, 134.6, 129.7, 129.0, 127.7, 127.6, 62.7, 59.1, 53.1, 47.8, 44.1, 21.8, 21.3. HRMS (ESI) calcd for C19H22Br2NO2S (M + H)+ 487.9713, found 487.9707.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; mp 137 °C, yield 206 mg, 65%; IR (KBr, neat) ν 2961, 1512, 1494, 1345, 1322, 1155, 1092, 1017, 714, 656, 547 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 8.4 Hz, 2H), 7.16–7.12 (m, 4H), 7.06–7.04 (m, 2H), 4.67 (dd, J = 7.8, 4.1 Hz, 1H), 3.95–3.89 (m, 1H), 3.57–3.51 (m, 1H), 3.16 (dd, J = 14.7, 7.8 Hz, 1H), 2.81–2.76 (m, 1H), 2.70–2.67 (m, 2H), 2.38 (s, 3H), 1.27 (s, 9H). 13C{1H} NMR (125 MHz, CDCl3) δ 150.9, 143.3, 137.2, 133.9, 129.5, 127.9, 127.6, 125.1, 63.2, 59.5, 53.1, 47.9, 44.5, 34.6, 31.5, 21.7. HRMS (ESI) calcd for C22H28Br2NO2S (M + H)+ 530.0182, found 530.0175.
1) 0.52; mp 137 °C, yield 206 mg, 65%; IR (KBr, neat) ν 2961, 1512, 1494, 1345, 1322, 1155, 1092, 1017, 714, 656, 547 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.38 (d, J = 8.4 Hz, 2H), 7.16–7.12 (m, 4H), 7.06–7.04 (m, 2H), 4.67 (dd, J = 7.8, 4.1 Hz, 1H), 3.95–3.89 (m, 1H), 3.57–3.51 (m, 1H), 3.16 (dd, J = 14.7, 7.8 Hz, 1H), 2.81–2.76 (m, 1H), 2.70–2.67 (m, 2H), 2.38 (s, 3H), 1.27 (s, 9H). 13C{1H} NMR (125 MHz, CDCl3) δ 150.9, 143.3, 137.2, 133.9, 129.5, 127.9, 127.6, 125.1, 63.2, 59.5, 53.1, 47.9, 44.5, 34.6, 31.5, 21.7. HRMS (ESI) calcd for C22H28Br2NO2S (M + H)+ 530.0182, found 530.0175.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; yield 243 mg, 81%; IR (KBr, neat) ν 2922, 1597, 1504, 1451, 1342, 1321, 1156, 1092, 1017, 920, 813, 723, 659, 546, 449 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 8.3 Hz, 2H), 7.17 (d, J = 8.0 Hz, 2H), 6.97 (d, J = 7.8 Hz, 1H), 6.92 (dd, J = 7.8, 2.0 Hz, 1H), 6.80–6.79 (m, 1H), 4.65–4.62 (m, 1H), 3.90–3.84 (m, 1H), 3.57–3.51 (m, 1H), 3.15 (dd, J = 14.7, 7.4 Hz, 1H), 2.82–2.76 (m, 1H), 2.69–2.66 (m, 2H), 2.40 (s, 3H), 2.19 (s, 3H), 2.09 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.5, 136.8, 136.3, 136.2, 134.6, 129.5, 129.4, 129.2, 127.6, 125.4, 63.0, 59.3, 53.2, 47.9, 44.3, 21.7, 19.9, 19.6. HRMS (ESI) calcd for C20H24Br2NO2S (M + H)+ 501.9869, found 501.9869.
1) 0.52; yield 243 mg, 81%; IR (KBr, neat) ν 2922, 1597, 1504, 1451, 1342, 1321, 1156, 1092, 1017, 920, 813, 723, 659, 546, 449 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.43 (d, J = 8.3 Hz, 2H), 7.17 (d, J = 8.0 Hz, 2H), 6.97 (d, J = 7.8 Hz, 1H), 6.92 (dd, J = 7.8, 2.0 Hz, 1H), 6.80–6.79 (m, 1H), 4.65–4.62 (m, 1H), 3.90–3.84 (m, 1H), 3.57–3.51 (m, 1H), 3.15 (dd, J = 14.7, 7.4 Hz, 1H), 2.82–2.76 (m, 1H), 2.69–2.66 (m, 2H), 2.40 (s, 3H), 2.19 (s, 3H), 2.09 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.5, 136.8, 136.3, 136.2, 134.6, 129.5, 129.4, 129.2, 127.6, 125.4, 63.0, 59.3, 53.2, 47.9, 44.3, 21.7, 19.9, 19.6. HRMS (ESI) calcd for C20H24Br2NO2S (M + H)+ 501.9869, found 501.9869.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.50; mp 159 °C, yield 254 mg, 81%; IR (KBr, neat) ν 3057, 2925, 1598, 1438, 1343, 1158, 1093, 1016, 816, 662, 559, 478 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.79–7.77 (m, 1H), 7.69 (d, J = 8.6 Hz, 1H), 7.65–7.62 (m, 1H), 7.49–7.42 (m, 5H), 7.33 (dd, J = 8.6, 1.9 Hz, 1H), 7.15–7.04 (m, 2H), 4.91–4.88 (m, 1H), 3.94–3.89 (m, 1H), 3.68–3.62 (m, 1H), 3.30 (dd, J = 14.8, 6.8 Hz, 1H), 2.89 (dd, J = 14.7, 4.6 Hz, 1H), 2.72–2.70 (m, 2H), 2.35 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.9, 136.7, 135.0, 133.2, 133.0, 129.7, 128.1, 128.0, 127.8, 127.6, 126.7, 126.4, 126.3, 125.6, 62.5, 59.5, 53.0, 47.8, 44.1, 21.7. HRMS (ESI) calcd for C22H22Br2NO2S (M + H)+ 523.9713, found 523.9699.
1) 0.50; mp 159 °C, yield 254 mg, 81%; IR (KBr, neat) ν 3057, 2925, 1598, 1438, 1343, 1158, 1093, 1016, 816, 662, 559, 478 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.79–7.77 (m, 1H), 7.69 (d, J = 8.6 Hz, 1H), 7.65–7.62 (m, 1H), 7.49–7.42 (m, 5H), 7.33 (dd, J = 8.6, 1.9 Hz, 1H), 7.15–7.04 (m, 2H), 4.91–4.88 (m, 1H), 3.94–3.89 (m, 1H), 3.68–3.62 (m, 1H), 3.30 (dd, J = 14.8, 6.8 Hz, 1H), 2.89 (dd, J = 14.7, 4.6 Hz, 1H), 2.72–2.70 (m, 2H), 2.35 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.9, 136.7, 135.0, 133.2, 133.0, 129.7, 128.1, 128.0, 127.8, 127.6, 126.7, 126.4, 126.3, 125.6, 62.5, 59.5, 53.0, 47.8, 44.1, 21.7. HRMS (ESI) calcd for C22H22Br2NO2S (M + H)+ 523.9713, found 523.9699.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.51; mp 149 °C, yield 229 mg, 73%; IR (KBr, neat) ν 3051, 1598, 1510, 1494, 1439, 1342, 1316, 1264, 1152, 1088, 1007, 777, 694, 571, 531, 481 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.75–7.73 (m, 1H), 7.66 (d, J = 8.2 Hz, 1H), 7.62–7.58 (m, 1H), 7.53 (d, J = 7.2 Hz, 1H), 7.40–7.31 (m, 3H), 6.88 (d, J = 8.0 Hz, 2H), 6.57 (d, J = 8.0 Hz, 2H), 5.20 (dd, J = 10.9, 2.6 Hz, 1H), 4.34 (dt, J = 14.2, 3.7 Hz, 1H), 3.75 (dd, J = 14.2, 10.8 Hz, 1H), 3.64 (ddd, J = 14.1, 10.9, 2.9 Hz, 1H), 2.98–2.90 (m, 2H), 2.86 (dq, J = 14.6, 2.8 Hz, 1H), 2.10 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 142.4, 135.9, 133.5, 131.7, 131.5, 129.6, 128.7, 128.3, 127.3, 127.0, 126.5, 125.5, 125.0, 123.2, 65.8, 57.5, 51.9, 48.5, 47.0, 21.4. HRMS (ESI) calcd for C22H22Br2NO2S (M + H)+ 523.9713, found 523.9715.
1) 0.51; mp 149 °C, yield 229 mg, 73%; IR (KBr, neat) ν 3051, 1598, 1510, 1494, 1439, 1342, 1316, 1264, 1152, 1088, 1007, 777, 694, 571, 531, 481 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.75–7.73 (m, 1H), 7.66 (d, J = 8.2 Hz, 1H), 7.62–7.58 (m, 1H), 7.53 (d, J = 7.2 Hz, 1H), 7.40–7.31 (m, 3H), 6.88 (d, J = 8.0 Hz, 2H), 6.57 (d, J = 8.0 Hz, 2H), 5.20 (dd, J = 10.9, 2.6 Hz, 1H), 4.34 (dt, J = 14.2, 3.7 Hz, 1H), 3.75 (dd, J = 14.2, 10.8 Hz, 1H), 3.64 (ddd, J = 14.1, 10.9, 2.9 Hz, 1H), 2.98–2.90 (m, 2H), 2.86 (dq, J = 14.6, 2.8 Hz, 1H), 2.10 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 142.4, 135.9, 133.5, 131.7, 131.5, 129.6, 128.7, 128.3, 127.3, 127.0, 126.5, 125.5, 125.0, 123.2, 65.8, 57.5, 51.9, 48.5, 47.0, 21.4. HRMS (ESI) calcd for C22H22Br2NO2S (M + H)+ 523.9713, found 523.9715.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.51; mp 149 °C, yield 131 mg, 55%; IR (KBr, neat) ν 2927, 1598, 1350, 1163, 933, 715, 546 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 8.3 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 3.17 (t, J = 5.3 Hz, 4H), 2.63 (t, J = 5.3 Hz, 4H), 2.44 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.2, 133.6, 130.1, 127.7, 64.6, 47.5, 44.7, 21.8. HRMS (ESI) calcd for C12H16Br2NO2S (M + H)+ 397.9243, found 397.9240.
1) 0.51; mp 149 °C, yield 131 mg, 55%; IR (KBr, neat) ν 2927, 1598, 1350, 1163, 933, 715, 546 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 8.3 Hz, 2H), 7.34 (d, J = 8.0 Hz, 2H), 3.17 (t, J = 5.3 Hz, 4H), 2.63 (t, J = 5.3 Hz, 4H), 2.44 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.2, 133.6, 130.1, 127.7, 64.6, 47.5, 44.7, 21.8. HRMS (ESI) calcd for C12H16Br2NO2S (M + H)+ 397.9243, found 397.9240.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; mp 127 °C, yield 201 mg, 79%; IR (KBr, neat) ν 2970, 2931, 2876, 1597, 1494, 1455, 1341, 1319, 1156, 1092, 1051, 957, 814, 718, 648, 552 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.70 (d, J = 8.3 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H), 3.88–3.84 (m, 1H), 3.68 (dtd, J = 14.8, 3.9, 1.4 Hz, 1H), 3.45–3.41 (m, 1H), 2.81 (dt, J = 15.0, 2.3 Hz, 1H), 2.65 (dd, J = 15.0, 6.3 Hz, 1H), 2.62–2.58 (m, 1H), 2.47–2.44 (m, 1H), 2.43 (s, 3H), 1.89 (p, J = 7.5 Hz, 2H), 0.86 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.9, 138.1, 130.1, 127.3, 62.3, 57.1, 48.5, 48.1, 40.7, 24.6, 21.8, 11.6. HRMS (ESI) calcd for C14H20Br2NO2S (M + H)+ 425.9556, found 425.9557.
1) 0.52; mp 127 °C, yield 201 mg, 79%; IR (KBr, neat) ν 2970, 2931, 2876, 1597, 1494, 1455, 1341, 1319, 1156, 1092, 1051, 957, 814, 718, 648, 552 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.70 (d, J = 8.3 Hz, 2H), 7.31 (d, J = 8.4 Hz, 2H), 3.88–3.84 (m, 1H), 3.68 (dtd, J = 14.8, 3.9, 1.4 Hz, 1H), 3.45–3.41 (m, 1H), 2.81 (dt, J = 15.0, 2.3 Hz, 1H), 2.65 (dd, J = 15.0, 6.3 Hz, 1H), 2.62–2.58 (m, 1H), 2.47–2.44 (m, 1H), 2.43 (s, 3H), 1.89 (p, J = 7.5 Hz, 2H), 0.86 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.9, 138.1, 130.1, 127.3, 62.3, 57.1, 48.5, 48.1, 40.7, 24.6, 21.8, 11.6. HRMS (ESI) calcd for C14H20Br2NO2S (M + H)+ 425.9556, found 425.9557.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; mp 126 °C, yield 211 mg, 80%; IR (KBr, neat) ν 2960, 2931, 2872, 1597, 1494, 1456, 1320, 1157, 1092, 1062, 926, 815, 710, 650, 552 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.70 (d, J = 8.2 Hz, 2H), 7.31 (d, J = 8.0 Hz, 2H), 3.99–3.95 (m, 1H), 3.67 (dtd, J = 14.8, 3.9, 1.4 Hz, 1H), 3.47–3.42 (m, 1H), 2.78 (dt, J = 14.9, 2.2 Hz, 1H), 2.65 (dd, J = 15.0, 6.3 Hz, 1H), 2.61–2.58 (m, 1H), 2.43 (s, 3H), 2.42–2.41 (m, 1H), 1.85–1.81 (m, 2H), 1.32–1.25 (m, 2H), 0.87 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.9, 138.1, 130.1, 127.3, 62.3, 55.3, 48.9, 48.1, 40.7, 33.7, 21.8, 20.2, 13.9. HRMS (ESI) calcd for C15H22Br2NO2S (M + H)+ 439.9713, found 439.9712.
1) 0.52; mp 126 °C, yield 211 mg, 80%; IR (KBr, neat) ν 2960, 2931, 2872, 1597, 1494, 1456, 1320, 1157, 1092, 1062, 926, 815, 710, 650, 552 cm−1; 1H NMR (600 MHz, CDCl3) δ 7.70 (d, J = 8.2 Hz, 2H), 7.31 (d, J = 8.0 Hz, 2H), 3.99–3.95 (m, 1H), 3.67 (dtd, J = 14.8, 3.9, 1.4 Hz, 1H), 3.47–3.42 (m, 1H), 2.78 (dt, J = 14.9, 2.2 Hz, 1H), 2.65 (dd, J = 15.0, 6.3 Hz, 1H), 2.61–2.58 (m, 1H), 2.43 (s, 3H), 2.42–2.41 (m, 1H), 1.85–1.81 (m, 2H), 1.32–1.25 (m, 2H), 0.87 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.9, 138.1, 130.1, 127.3, 62.3, 55.3, 48.9, 48.1, 40.7, 33.7, 21.8, 20.2, 13.9. HRMS (ESI) calcd for C15H22Br2NO2S (M + H)+ 439.9713, found 439.9712.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.48; yield 184 mg, 64%; IR (KBr, neat) ν 2927, 15
1) 0.48; yield 184 mg, 64%; IR (KBr, neat) ν 2927, 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 967, 1494, 1322, 1157, 812, 651 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 8.3 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 3.96–3.90 (m, 1H), 3.69 (dtd, J = 14.8, 3.9, 1.3 Hz, 1H), 3.47–3.40 (m, 1H), 2.79 (dt, J = 15.0, 2.3 Hz, 1H), 2.67 (dd, J = 15.0, 6.2 Hz, 1H), 2.63–2.58 (m, 1H), 2.49–2.45 (m, 1H), 2.42 (s, 3H), 1.87–1.77 (m, 2H), 1.25–1.17 (m, 8H), 0.86 (t, J = 6.9 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 143.9, 138.1, 130.1, 127.3, 62.4, 55.6, 48.9, 48.2, 40.7, 31.8, 31.5, 29.0, 27.0, 22.8, 21.8, 14.3. HRMS (ESI) calcd for C18H28Br2NO2S (M + H)+ 482.0182, found 482.0181.
967, 1494, 1322, 1157, 812, 651 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 8.3 Hz, 2H), 7.30 (d, J = 8.0 Hz, 2H), 3.96–3.90 (m, 1H), 3.69 (dtd, J = 14.8, 3.9, 1.3 Hz, 1H), 3.47–3.40 (m, 1H), 2.79 (dt, J = 15.0, 2.3 Hz, 1H), 2.67 (dd, J = 15.0, 6.2 Hz, 1H), 2.63–2.58 (m, 1H), 2.49–2.45 (m, 1H), 2.42 (s, 3H), 1.87–1.77 (m, 2H), 1.25–1.17 (m, 8H), 0.86 (t, J = 6.9 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 143.9, 138.1, 130.1, 127.3, 62.4, 55.6, 48.9, 48.2, 40.7, 31.8, 31.5, 29.0, 27.0, 22.8, 21.8, 14.3. HRMS (ESI) calcd for C18H28Br2NO2S (M + H)+ 482.0182, found 482.0181.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.53; mp 136 °C, yield 157 mg, 60%; IR (KBr, neat) ν 2962, 1597, 1493, 1383, 1255, 1155, 1093, 755, 711, 649, 553 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 8.1 Hz, 2H), 7.32 (d, J = 7.9 Hz, 2H), 4.03–3.95 (m, 1H), 3.71 (dt, J = 14.8, 3.6 Hz, 1H), 3.47–3.42 (m, 1H), 3.38 (t, J = 6.2 Hz, 2H), 2.75 (dt, J = 15.1, 2.3 Hz, 1H), 2.65 (dd, J = 15.1, 6.4 Hz, 1H), 2.60–2.54 (m, 1H), 2.43 (s, 3H), 2.40–2.33 (m, 1H), 2.20–2.10 (m, 1H), 1.89–1.81 (m, 2H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.2, 137.7, 130.3, 127.3, 61.6, 54.6, 49.2, 47.7, 40.5, 33.3, 30.4, 30.1, 21.8. HRMS (ESI) calcd for C15H20Br2NO2S (M + H)+ 437.9556, found 437.9544.
1) 0.53; mp 136 °C, yield 157 mg, 60%; IR (KBr, neat) ν 2962, 1597, 1493, 1383, 1255, 1155, 1093, 755, 711, 649, 553 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 8.1 Hz, 2H), 7.32 (d, J = 7.9 Hz, 2H), 4.03–3.95 (m, 1H), 3.71 (dt, J = 14.8, 3.6 Hz, 1H), 3.47–3.42 (m, 1H), 3.38 (t, J = 6.2 Hz, 2H), 2.75 (dt, J = 15.1, 2.3 Hz, 1H), 2.65 (dd, J = 15.1, 6.4 Hz, 1H), 2.60–2.54 (m, 1H), 2.43 (s, 3H), 2.40–2.33 (m, 1H), 2.20–2.10 (m, 1H), 1.89–1.81 (m, 2H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.2, 137.7, 130.3, 127.3, 61.6, 54.6, 49.2, 47.7, 40.5, 33.3, 30.4, 30.1, 21.8. HRMS (ESI) calcd for C15H20Br2NO2S (M + H)+ 437.9556, found 437.9544.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.51; yield 175 mg, 61%; IR (KBr, neat) ν 2926, 1599, 1494, 1342, 1156, 658, 612 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 3.73–3.63 (m, 2H), 3.35–3.28 (m, 1H), 3.03 (dt, J = 15.3, 1.9 Hz, 1H), 2.53–2.44 (m, 2H), 2.43 (s, 3H), 2.37–2.33 (m, 1H), 2.31–2.25 (m, 1H), 1.96–1.91 (m, 1H), 1.79–1.70 (m, 3H), 1.66–1.58 (m, 2H), 1.15–1.07 (m, 2H), 0.86–0.72 (m, 2H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.9, 138.1, 130.1, 127.4, 62.3, 60.6, 47.6, 45.1, 40.4, 36.2, 31.1, 30.3, 26.3, 26.2, 26.0, 21.8. HRMS (ESI) calcd for C18H26Br2NO2S (M + H)+ 480.0026, found 480.0025.
1) 0.51; yield 175 mg, 61%; IR (KBr, neat) ν 2926, 1599, 1494, 1342, 1156, 658, 612 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.71 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 3.73–3.63 (m, 2H), 3.35–3.28 (m, 1H), 3.03 (dt, J = 15.3, 1.9 Hz, 1H), 2.53–2.44 (m, 2H), 2.43 (s, 3H), 2.37–2.33 (m, 1H), 2.31–2.25 (m, 1H), 1.96–1.91 (m, 1H), 1.79–1.70 (m, 3H), 1.66–1.58 (m, 2H), 1.15–1.07 (m, 2H), 0.86–0.72 (m, 2H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.9, 138.1, 130.1, 127.4, 62.3, 60.6, 47.6, 45.1, 40.4, 36.2, 31.1, 30.3, 26.3, 26.2, 26.0, 21.8. HRMS (ESI) calcd for C18H26Br2NO2S (M + H)+ 480.0026, found 480.0025.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (3dj). Colorless solid; Rf (hexane/EtOAc, 9
1) (3dj). Colorless solid; Rf (hexane/EtOAc, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.51; mp 102 °C, yield 204 mg, 77%; IR (KBr, neat) ν 2924, 1597, 1512, 1344, 1154, 734, 660, 556 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 8.3 Hz, 2H, major), 7.46 (d, J = 8.4 Hz, 2H, minor), 7.24 (d, J = 8.0 Hz, 2H), 7.08–7.01 (m, 4H), 4.86 (t, J = 5.3 Hz, 1H, major), 4.67 (dd, J = 7.4, 4.5 Hz, 1H, minor), 3.84–3.77 (m, 1H), 3.67–3.61 (m, 1H), 3.13–3.08 (m, 1H), 2.77–2.72 (m, 1H), 2.56–2.49 (m, 2H), 2.42 (s, 3H), 2.30 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.8, 137.3, 136.9, 134.7, 129.8, 129.1, 127.5, 127.1, 76.0, 74.7, 58.6, 58.1, 51.0, 46.5, 46.4, 43.7, 42.8, 21.8, 21.2. HRMS (ESI) calcd for C19H22BrClNO2S (M + H)+ 442.0238, found 442.0227.
1) 0.51; mp 102 °C, yield 204 mg, 77%; IR (KBr, neat) ν 2924, 1597, 1512, 1344, 1154, 734, 660, 556 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.55 (d, J = 8.3 Hz, 2H, major), 7.46 (d, J = 8.4 Hz, 2H, minor), 7.24 (d, J = 8.0 Hz, 2H), 7.08–7.01 (m, 4H), 4.86 (t, J = 5.3 Hz, 1H, major), 4.67 (dd, J = 7.4, 4.5 Hz, 1H, minor), 3.84–3.77 (m, 1H), 3.67–3.61 (m, 1H), 3.13–3.08 (m, 1H), 2.77–2.72 (m, 1H), 2.56–2.49 (m, 2H), 2.42 (s, 3H), 2.30 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.8, 137.3, 136.9, 134.7, 129.8, 129.1, 127.5, 127.1, 76.0, 74.7, 58.6, 58.1, 51.0, 46.5, 46.4, 43.7, 42.8, 21.8, 21.2. HRMS (ESI) calcd for C19H22BrClNO2S (M + H)+ 442.0238, found 442.0227.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/b_char_2009.gif) 1) (3ed). Colorless gum; Rf (hexane/EtOAc, 9
1) (3ed). Colorless gum; Rf (hexane/EtOAc, 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; yield 202 mg, 61%; IR (KBr, neat) ν 2925, 1597, 1491, 1343, 1161, 712, 666, 551 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 7.6 Hz, 2H, minor), 7.50 (d, J = 8.2 Hz, 2H, minor), 7.41 (d, J = 8.2 Hz, 2H, major), 7.22 (d, J = 8.1 Hz, 2H, major), 7.17 (d, J = 8.6 Hz, 2H), 7.10 (d, J = 8.6 Hz, 2H), 4.60 (dd, J = 5.5, 5.5 Hz, 1H, minor), 4.52 (dd, J = 8.0, 4.1 Hz, 1H, major), 3.66–3.60 (m, 1H), 3.45–3.41 (m, 1H), 3.17 (dd, J = 14.8, 7.9 Hz, 1H), 2.89–2.82 (m, 1H), 2.76–2.71 (m, 1H), 2.65–2.59 (m, 1H), 2.42 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 144.0, 136.0, 135.8, 133.9, 129.7, 129.6, 128.5, 127.7, 59.4, 56.0, 50.3, 50.0, 45.4, 45.1, 29.3, 21.8. HRMS (ESI) calcd for C18H19BrClINO2S (M + H)+ 553.9048, found 553.9027.
1) 0.52; yield 202 mg, 61%; IR (KBr, neat) ν 2925, 1597, 1491, 1343, 1161, 712, 666, 551 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.65 (d, J = 7.6 Hz, 2H, minor), 7.50 (d, J = 8.2 Hz, 2H, minor), 7.41 (d, J = 8.2 Hz, 2H, major), 7.22 (d, J = 8.1 Hz, 2H, major), 7.17 (d, J = 8.6 Hz, 2H), 7.10 (d, J = 8.6 Hz, 2H), 4.60 (dd, J = 5.5, 5.5 Hz, 1H, minor), 4.52 (dd, J = 8.0, 4.1 Hz, 1H, major), 3.66–3.60 (m, 1H), 3.45–3.41 (m, 1H), 3.17 (dd, J = 14.8, 7.9 Hz, 1H), 2.89–2.82 (m, 1H), 2.76–2.71 (m, 1H), 2.65–2.59 (m, 1H), 2.42 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 144.0, 136.0, 135.8, 133.9, 129.7, 129.6, 128.5, 127.7, 59.4, 56.0, 50.3, 50.0, 45.4, 45.1, 29.3, 21.8. HRMS (ESI) calcd for C18H19BrClINO2S (M + H)+ 553.9048, found 553.9027.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). Once the starting material was fully consumed, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (24 mmol, 40.0 equiv.) was added, and the mixture was stirred in an oil bath at 80 °C for 4 to 6 hours. The progress of the reaction was monitored by TLC (ethyl acetate
9). Once the starting material was fully consumed, 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (24 mmol, 40.0 equiv.) was added, and the mixture was stirred in an oil bath at 80 °C for 4 to 6 hours. The progress of the reaction was monitored by TLC (ethyl acetate![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) hexane = 1
hexane = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9). After completion, it was allowed to cool to room temperature, brine was added, and the organic layer was extracted with EtOAc (3 × 10 mL). The combined organic layers were dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure by rotary evaporator and purified by column chromatography over silica gel using hexane/ethyl acetate (9
9). After completion, it was allowed to cool to room temperature, brine was added, and the organic layer was extracted with EtOAc (3 × 10 mL). The combined organic layers were dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure by rotary evaporator and purified by column chromatography over silica gel using hexane/ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent to get the products.
1, v/v) as eluent to get the products.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; mp 116 °C, yield 157 mg, 67%; IR (KBr, neat) ν 2926, 2851, 1663, 1597, 1493, 1441, 1343, 1160, 1093, 1011, 912, 811, 731, 571, 492, 444 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.70 (d, J = 8.0 Hz, 2H), 7.32–7.28 (m, 7H), 5.92 (s, 1H), 5.30 (d, J = 5.9 Hz, 1H), 4.17–4.12 (m, 1H), 3.34 (dt, J = 18.5, 2.1 Hz, 1H), 2.74–2.68 (m, 2H), 2.44 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.9, 138.0, 137.5, 130.0, 128.9, 128.2, 127.4, 127.2, 125.1, 118.5, 54.8, 42.6, 35.9, 21.8. HRMS (ESI) calcd for C18H19BrNO2S (M + H)+ 392.0315, found 392.0307.
1) 0.52; mp 116 °C, yield 157 mg, 67%; IR (KBr, neat) ν 2926, 2851, 1663, 1597, 1493, 1441, 1343, 1160, 1093, 1011, 912, 811, 731, 571, 492, 444 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.70 (d, J = 8.0 Hz, 2H), 7.32–7.28 (m, 7H), 5.92 (s, 1H), 5.30 (d, J = 5.9 Hz, 1H), 4.17–4.12 (m, 1H), 3.34 (dt, J = 18.5, 2.1 Hz, 1H), 2.74–2.68 (m, 2H), 2.44 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.9, 138.0, 137.5, 130.0, 128.9, 128.2, 127.4, 127.2, 125.1, 118.5, 54.8, 42.6, 35.9, 21.8. HRMS (ESI) calcd for C18H19BrNO2S (M + H)+ 392.0315, found 392.0307.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.54; yield 176 mg, 69%; IR (KBr, neat) ν 2925, 2852, 1660, 1597, 1493, 1443, 1346, 1160, 1093, 1015, 911, 814, 733, 711, 654, 573, 491, 444 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 8.0 Hz, 2H), 7.31–7.28 (m, 4H), 7.25–7.24 (m, 2H), 5.93–5.92 (m, 1H), 5.26 (t, J = 3.9 Hz, 1H), 4.14 (dd, J = 18.6, 4.7 Hz, 1H), 3.32 (dq, J = 18.4, 3.0 Hz, 1H), 2.70–2.68 (m, 2H), 2.44 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 144.1, 137.3, 136.5, 134.1, 130.1, 129.1, 128.9, 127.2, 125.2, 118.1, 54.2, 42.6, 35.8, 21.8. HRMS (ESI) calcd for C18H18BrClNO2S (M + H)+ 425.9925, found 425.9899.
1) 0.54; yield 176 mg, 69%; IR (KBr, neat) ν 2925, 2852, 1660, 1597, 1493, 1443, 1346, 1160, 1093, 1015, 911, 814, 733, 711, 654, 573, 491, 444 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 8.0 Hz, 2H), 7.31–7.28 (m, 4H), 7.25–7.24 (m, 2H), 5.93–5.92 (m, 1H), 5.26 (t, J = 3.9 Hz, 1H), 4.14 (dd, J = 18.6, 4.7 Hz, 1H), 3.32 (dq, J = 18.4, 3.0 Hz, 1H), 2.70–2.68 (m, 2H), 2.44 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 144.1, 137.3, 136.5, 134.1, 130.1, 129.1, 128.9, 127.2, 125.2, 118.1, 54.2, 42.6, 35.8, 21.8. HRMS (ESI) calcd for C18H18BrClNO2S (M + H)+ 425.9925, found 425.9899.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.54; mp 119 °C, yield 198 mg, 70%; IR (KBr, neat) ν 2922, 2851, 1660, 1596, 1489, 1442, 1344, 1159, 1095, 909, 814, 708, 696, 544, 481, 410 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.71–7.68 (m, 2H), 7.45–7.42 (m, 2H), 7.30 (d, J = 8.0 Hz, 2H), 7.18 (d, J = 8.4 Hz, 2H), 5.93–5.91 (m, 1H), 5.24 (t, J = 3.9 Hz, 1H), 4.17–4.11 (m, 1H), 3.35–3.28 (m, 1H), 2.70–2.68 (m, 2H), 2.44 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 144.1, 137.3, 137.0, 132.0, 130.1, 129.2, 127.2, 125.2, 122.3, 118.1, 54.2, 42.6, 35.7, 21.8. HRMS (ESI) calcd for C18H18Br2NO2S (M + H)+ 471.9400, found 471.9387.
1) 0.54; mp 119 °C, yield 198 mg, 70%; IR (KBr, neat) ν 2922, 2851, 1660, 1596, 1489, 1442, 1344, 1159, 1095, 909, 814, 708, 696, 544, 481, 410 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.71–7.68 (m, 2H), 7.45–7.42 (m, 2H), 7.30 (d, J = 8.0 Hz, 2H), 7.18 (d, J = 8.4 Hz, 2H), 5.93–5.91 (m, 1H), 5.24 (t, J = 3.9 Hz, 1H), 4.17–4.11 (m, 1H), 3.35–3.28 (m, 1H), 2.70–2.68 (m, 2H), 2.44 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 144.1, 137.3, 137.0, 132.0, 130.1, 129.2, 127.2, 125.2, 122.3, 118.1, 54.2, 42.6, 35.7, 21.8. HRMS (ESI) calcd for C18H18Br2NO2S (M + H)+ 471.9400, found 471.9387.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.55; mp 114 °C, yield 154 mg, 55%; IR (KBr, neat) ν 3030, 2922, 2851, 1658, 1598, 1442, 1344, 1159, 1094, 1008, 909, 814, 766, 720, 658, 568, 543 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 8.3 Hz, 2H), 7.58–7.54 (m, 4H), 7.46–7.42 (m, 2H), 7.40–7.35 (m, 3H), 7.30 (d, J = 8.1 Hz, 2H), 5.96–5.95 (m, 1H), 5.35–5.33 (m, 1H), 4.20–4.14 (m, 1H), 3.44–3.38 (m, 1H), 2.83–2.70 (m, 2H), 2.44 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 143.9, 141.1, 140.6, 137.5, 137.0, 130.0, 129.1, 127.9, 127.7, 127.6, 127.3, 127.2, 125.2, 118.4, 54.6, 42.7, 35.9, 21.8. HRMS (ESI) calcd for C24H23BrNO2S (M + H)+ 468.0628, found 468.0603.
1) 0.55; mp 114 °C, yield 154 mg, 55%; IR (KBr, neat) ν 3030, 2922, 2851, 1658, 1598, 1442, 1344, 1159, 1094, 1008, 909, 814, 766, 720, 658, 568, 543 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.73 (d, J = 8.3 Hz, 2H), 7.58–7.54 (m, 4H), 7.46–7.42 (m, 2H), 7.40–7.35 (m, 3H), 7.30 (d, J = 8.1 Hz, 2H), 5.96–5.95 (m, 1H), 5.35–5.33 (m, 1H), 4.20–4.14 (m, 1H), 3.44–3.38 (m, 1H), 2.83–2.70 (m, 2H), 2.44 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 143.9, 141.1, 140.6, 137.5, 137.0, 130.0, 129.1, 127.9, 127.7, 127.6, 127.3, 127.2, 125.2, 118.4, 54.6, 42.7, 35.9, 21.8. HRMS (ESI) calcd for C24H23BrNO2S (M + H)+ 468.0628, found 468.0603.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.54; mp 144 °C, yield 170 mg, 70%; IR (KBr, neat) ν 2922, 1659, 1597, 1514, 1345, 1159, 1095, 909, 813, 725, 657, 575, 550 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 8.3 Hz, 2H), 7.29 (d, J = 8.1 Hz, 2H), 7.20 (d, J = 8.2 Hz, 2H), 7.12 (d, J = 8.0 Hz, 2H), 5.92–5.89 (m, 1H), 5.26 (d, J = 5.7 Hz, 1H), 4.16–4.09 (m, 1H), 3.37–3.30 (m, 1H), 2.72–2.66 (m, 2H), 2.44 (s, 3H), 2.32 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 143.8, 138.0, 137.6, 134.9, 130.0, 129.5, 127.3, 127.2, 125.1, 118.6, 54.5, 42.5, 35.9, 21.8, 21.3. HRMS (ESI) calcd for C18H21BrNO2S (M + H)+ 406.0471, found 406.0470.
1) 0.54; mp 144 °C, yield 170 mg, 70%; IR (KBr, neat) ν 2922, 1659, 1597, 1514, 1345, 1159, 1095, 909, 813, 725, 657, 575, 550 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 8.3 Hz, 2H), 7.29 (d, J = 8.1 Hz, 2H), 7.20 (d, J = 8.2 Hz, 2H), 7.12 (d, J = 8.0 Hz, 2H), 5.92–5.89 (m, 1H), 5.26 (d, J = 5.7 Hz, 1H), 4.16–4.09 (m, 1H), 3.37–3.30 (m, 1H), 2.72–2.66 (m, 2H), 2.44 (s, 3H), 2.32 (s, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 143.8, 138.0, 137.6, 134.9, 130.0, 129.5, 127.3, 127.2, 125.1, 118.6, 54.5, 42.5, 35.9, 21.8, 21.3. HRMS (ESI) calcd for C18H21BrNO2S (M + H)+ 406.0471, found 406.0470.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.52; mp 146 °C, yield, 164 mg, 62%; IR (KBr, neat) ν 3051, 2924, 1656, 1598, 1511, 1439, 1340, 1317, 1159, 1092, 1049, 779, 717, 664, 572, 542 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.53 (d, J = 8.6 Hz, 1H), 7.79 (d, J = 8.2 Hz, 1H), 7.76–7.74 (m, 1H), 7.69 (d, J = 7.9 Hz, 2H), 7.55 (t, J = 7.8 Hz, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.32–7.29 (m, 2H), 7.20 (d, J = 8.1 Hz, 2H), 6.03 (d, J = 7.2 Hz, 1H), 5.88 (s, 1H), 3.93 (dd, J = 17.5, 4.3 Hz, 1H), 3.13 (d, J = 19.3 Hz, 1H), 2.90–2.84 (m, 1H), 2.70 (d, J = 18.4 Hz, 1H), 2.36 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.2, 136.8, 134.3, 133.3, 131.7, 129.9, 129.8, 128.9, 127.9, 127.1, 126.3, 125.4, 124.9, 124.5, 124.4, 119.5, 52.0, 42.8, 36.5, 21.8. HRMS (ESI) calcd for C22H21BrNO2S (M + H)+ 442.0471, found 442.0446.
1) 0.52; mp 146 °C, yield, 164 mg, 62%; IR (KBr, neat) ν 3051, 2924, 1656, 1598, 1511, 1439, 1340, 1317, 1159, 1092, 1049, 779, 717, 664, 572, 542 cm−1; 1H NMR (500 MHz, CDCl3) δ 8.53 (d, J = 8.6 Hz, 1H), 7.79 (d, J = 8.2 Hz, 1H), 7.76–7.74 (m, 1H), 7.69 (d, J = 7.9 Hz, 2H), 7.55 (t, J = 7.8 Hz, 1H), 7.45 (t, J = 7.6 Hz, 1H), 7.32–7.29 (m, 2H), 7.20 (d, J = 8.1 Hz, 2H), 6.03 (d, J = 7.2 Hz, 1H), 5.88 (s, 1H), 3.93 (dd, J = 17.5, 4.3 Hz, 1H), 3.13 (d, J = 19.3 Hz, 1H), 2.90–2.84 (m, 1H), 2.70 (d, J = 18.4 Hz, 1H), 2.36 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.2, 136.8, 134.3, 133.3, 131.7, 129.9, 129.8, 128.9, 127.9, 127.1, 126.3, 125.4, 124.9, 124.5, 124.4, 119.5, 52.0, 42.8, 36.5, 21.8. HRMS (ESI) calcd for C22H21BrNO2S (M + H)+ 442.0471, found 442.0446.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.54; yield 128 mg, 62%; IR (KBr, neat) ν 2968, 2933, 2876, 1654, 1598, 1494, 1454, 1335, 1266, 1155, 1092, 1042, 951, 882, 814, 717, 648, 540 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 8.3 Hz, 2H), 7.21 (d, J = 8.1 Hz, 2H), 5.96–5.94 (m, 1H), 4.22–4.17 (m, 1H), 3.80–3.75 (m, 1H), 3.20–3.12 (m, 1H), 2.35 (s, 3H), 2.09–1.96 (m, 2H), 1.59–1.51 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.7, 138.1, 129.9, 129.2, 127.1, 119.5, 57.3, 39.7, 32.9, 27.9, 21.7, 10.9. HRMS (ESI) calcd for C14H19BrNO2S (M + H)+ 344.0315, found 344.0316.
1) 0.54; yield 128 mg, 62%; IR (KBr, neat) ν 2968, 2933, 2876, 1654, 1598, 1494, 1454, 1335, 1266, 1155, 1092, 1042, 951, 882, 814, 717, 648, 540 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.62 (d, J = 8.3 Hz, 2H), 7.21 (d, J = 8.1 Hz, 2H), 5.96–5.94 (m, 1H), 4.22–4.17 (m, 1H), 3.80–3.75 (m, 1H), 3.20–3.12 (m, 1H), 2.35 (s, 3H), 2.09–1.96 (m, 2H), 1.59–1.51 (m, 2H), 0.91 (t, J = 7.4 Hz, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.7, 138.1, 129.9, 129.2, 127.1, 119.5, 57.3, 39.7, 32.9, 27.9, 21.7, 10.9. HRMS (ESI) calcd for C14H19BrNO2S (M + H)+ 344.0315, found 344.0316.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.55; mp 77 °C, yield, 151 mg, 69%; IR (KBr, neat) ν 2962, 1603, 1510, 1343, 1159, 1484, 1442, 1093, 740, 661, 484, 403 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 8.4 Hz, 2H), 7.31–7.27 (m, 4H), 7.02–6.98 (m, 2H), 5.72–5.70 (m, 1H), 5.31 (d, J = 6.1 Hz, 1H), 4.22–4.16 (m, 1H), 3.37–3.31 (m, 1H), 2.67–2.56 (m, 2H), 2.43 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 162.5 (d, J = 245.7 Hz), 144.0, 137.4, 133.8 (d, J = 3.2 Hz), 130.1, 129.2 (d, J = 8.2 Hz), 129.0, 127.2, 121.1, 115.7 (d, J = 21.2 Hz), 53.4, 41.4, 33.9, 21.8. 19F NMR (470 MHz, CDCl3/C6F6) δ −114.01. HRMS (ESI) calcd for C18H18ClFNO2S (M + H)+ 366.0726, found 366.0708.
1) 0.55; mp 77 °C, yield, 151 mg, 69%; IR (KBr, neat) ν 2962, 1603, 1510, 1343, 1159, 1484, 1442, 1093, 740, 661, 484, 403 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.69 (d, J = 8.4 Hz, 2H), 7.31–7.27 (m, 4H), 7.02–6.98 (m, 2H), 5.72–5.70 (m, 1H), 5.31 (d, J = 6.1 Hz, 1H), 4.22–4.16 (m, 1H), 3.37–3.31 (m, 1H), 2.67–2.56 (m, 2H), 2.43 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 162.5 (d, J = 245.7 Hz), 144.0, 137.4, 133.8 (d, J = 3.2 Hz), 130.1, 129.2 (d, J = 8.2 Hz), 129.0, 127.2, 121.1, 115.7 (d, J = 21.2 Hz), 53.4, 41.4, 33.9, 21.8. 19F NMR (470 MHz, CDCl3/C6F6) δ −114.01. HRMS (ESI) calcd for C18H18ClFNO2S (M + H)+ 366.0726, found 366.0708.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.54; mp 118 °C, yield 156 mg, 61%; IR (KBr, neat) ν 2924, 1659, 1596, 1488, 1441, 1344, 1159, 1093, 906, 814, 707, 481, 409 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 7.8 Hz, 2H), 7.40 (d, J = 7.9 Hz, 1H), 7.35 (s, 1H), 7.30 (d, J = 7.9 Hz, 2H), 7.24 (d, J = 7.8 Hz, 1H), 7.19 (t, J = 7.8 Hz, 1H), 5.73 (s, 1H), 5.29 (d, J = 6.4 Hz, 1H), 4.23 (d, J = 18.2 Hz, 1H), 3.41–3.36 (m, 1H), 2.67–2.61 (m, 1H), 2.56 (d, J = 17.7 Hz, 1H), 2.44 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.1, 140.4, 137.3, 131.4, 130.6, 130.5, 130.1, 128.8, 127.2, 125.9, 123.0, 121.0, 53.7, 41.6, 33.9, 21.8. HRMS (ESI) calcd for C18H18BrClNO2S (M + H)+ 425.9925, found 425.9904.
1) 0.54; mp 118 °C, yield 156 mg, 61%; IR (KBr, neat) ν 2924, 1659, 1596, 1488, 1441, 1344, 1159, 1093, 906, 814, 707, 481, 409 cm−1; 1H NMR (500 MHz, CDCl3) δ 7.69 (d, J = 7.8 Hz, 2H), 7.40 (d, J = 7.9 Hz, 1H), 7.35 (s, 1H), 7.30 (d, J = 7.9 Hz, 2H), 7.24 (d, J = 7.8 Hz, 1H), 7.19 (t, J = 7.8 Hz, 1H), 5.73 (s, 1H), 5.29 (d, J = 6.4 Hz, 1H), 4.23 (d, J = 18.2 Hz, 1H), 3.41–3.36 (m, 1H), 2.67–2.61 (m, 1H), 2.56 (d, J = 17.7 Hz, 1H), 2.44 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 144.1, 140.4, 137.3, 131.4, 130.6, 130.5, 130.1, 128.8, 127.2, 125.9, 123.0, 121.0, 53.7, 41.6, 33.9, 21.8. HRMS (ESI) calcd for C18H18BrClNO2S (M + H)+ 425.9925, found 425.9904.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.53; mp 130 °C, yield 152 mg, 70%; IR (KBr, neat) ν 2923, 1658, 1597, 1514, 1342, 1159, 1095, 906, 811, 575, 551 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 8.3 Hz, 2H), 7.28 (d, J = 7.9 Hz, 2H), 7.19 (d, J = 8.2 Hz, 2H), 7.11 (d, J = 8.0 Hz, 2H), 5.69–5.67 (m, 1H), 5.31–5.29 (m, 1H), 4.20–4.14 (m, 1H), 3.40–3.33 (m, 1H), 2.61–2.58 (m, 2H), 2.43 (s, 3H), 2.32 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.8, 137.9, 137.6, 135.0, 130.0, 129.5, 129.3, 127.3, 127.2, 121.0, 53.8, 41.5, 33.9, 21.8, 21.3. HRMS (ESI) calcd for C19H21ClNO2S (M + H)+ 362.0977, found 362.0953.
1) 0.53; mp 130 °C, yield 152 mg, 70%; IR (KBr, neat) ν 2923, 1658, 1597, 1514, 1342, 1159, 1095, 906, 811, 575, 551 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.70 (d, J = 8.3 Hz, 2H), 7.28 (d, J = 7.9 Hz, 2H), 7.19 (d, J = 8.2 Hz, 2H), 7.11 (d, J = 8.0 Hz, 2H), 5.69–5.67 (m, 1H), 5.31–5.29 (m, 1H), 4.20–4.14 (m, 1H), 3.40–3.33 (m, 1H), 2.61–2.58 (m, 2H), 2.43 (s, 3H), 2.32 (s, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 143.8, 137.9, 137.6, 135.0, 130.0, 129.5, 129.3, 127.3, 127.2, 121.0, 53.8, 41.5, 33.9, 21.8, 21.3. HRMS (ESI) calcd for C19H21ClNO2S (M + H)+ 362.0977, found 362.0953.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent to get the products.
1, v/v) as eluent to get the products.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.48; mp 113 °C, yield 72 mg, 80%; IR (KBr, neat) ν 2924, 1721, 1596, 1339, 1153, 1091, 1009, 930, 815, 709, 548 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.81 (d, J = 8.2 Hz, 2H), 7.42 (d, J = 8.5 Hz, 2H), 7.35 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 8.3 Hz, 2H), 5.56 (d, J = 7.0 Hz, 1H), 4.04–3.98 (m, 1H), 3.16–3.08 (m, 1H), 2.88–2.84 (m, 1H), 2.68 (dd, J = 15.3, 7.0 Hz, 1H), 2.45 (s, 3H), 2.42–2.38 (m, 1H), 2.26–2.21 (m, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 206.2, 144.5, 137.6, 137.4, 132.2, 130.4, 129.3, 127.3, 122.5, 56.2, 43.4, 40.6, 40.4, 21.8. HRMS (ESI) calcd for C18H19BrNO3S (M + H)+ 408.0264, found 408.0235.
1) 0.48; mp 113 °C, yield 72 mg, 80%; IR (KBr, neat) ν 2924, 1721, 1596, 1339, 1153, 1091, 1009, 930, 815, 709, 548 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.81 (d, J = 8.2 Hz, 2H), 7.42 (d, J = 8.5 Hz, 2H), 7.35 (d, J = 8.1 Hz, 2H), 7.12 (d, J = 8.3 Hz, 2H), 5.56 (d, J = 7.0 Hz, 1H), 4.04–3.98 (m, 1H), 3.16–3.08 (m, 1H), 2.88–2.84 (m, 1H), 2.68 (dd, J = 15.3, 7.0 Hz, 1H), 2.45 (s, 3H), 2.42–2.38 (m, 1H), 2.26–2.21 (m, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 206.2, 144.5, 137.6, 137.4, 132.2, 130.4, 129.3, 127.3, 122.5, 56.2, 43.4, 40.6, 40.4, 21.8. HRMS (ESI) calcd for C18H19BrNO3S (M + H)+ 408.0264, found 408.0235.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.47; yield 49 mg, 75%; 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 7.8 Hz, 2H), 7.32 (d, J = 7.8 Hz, 2H), 4.42–4.37 (m, 1H), 4.15–4.10 (m, 1H), 3.30–3.23 (m, 1H), 2.55–2.51 (m, 1H), 2.43 (s, 3H), 2.41–2.36 (m, 1H), 2.22 (d, J = 14.3 Hz, 2H), 1.39–1.32 (m, 2H), 1.26–1.22 (m, 2H), 0.85 (q, J = 7.2, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 206.9, 144.1, 137.8, 130.2, 127.3, 54.6, 45.6, 40.5, 40.3, 34.6, 21.8, 19.3, 13.7.
1) 0.47; yield 49 mg, 75%; 1H NMR (500 MHz, CDCl3) δ 7.77 (d, J = 7.8 Hz, 2H), 7.32 (d, J = 7.8 Hz, 2H), 4.42–4.37 (m, 1H), 4.15–4.10 (m, 1H), 3.30–3.23 (m, 1H), 2.55–2.51 (m, 1H), 2.43 (s, 3H), 2.41–2.36 (m, 1H), 2.22 (d, J = 14.3 Hz, 2H), 1.39–1.32 (m, 2H), 1.26–1.22 (m, 2H), 0.85 (q, J = 7.2, 3H). 13C{1H} NMR (125 MHz, CDCl3) δ 206.9, 144.1, 137.8, 130.2, 127.3, 54.6, 45.6, 40.5, 40.3, 34.6, 21.8, 19.3, 13.7.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.46; yield 49 mg, 66%; 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 4.39–4.33 (m, 1H), 4.18–4.12 (m, 1H), 3.29–3.22 (m, 1H), 2.54 (dd, J = 14.3, 6.5 Hz, 1H), 2.43 (s, 3H), 2.41–2.36 (m, 1H), 2.25–2.21 (m, 2H), 1.41–1.33 (m, 3H), 1.22–1.15 (m, 7H), 0.85 (t, J = 7.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 207.0, 144.0, 137.8, 130.2, 127.3, 54.9, 45.7, 40.6, 40.2, 32.4, 31.8, 28.9, 26.0, 22.7, 21.8, 14.3.
1) 0.46; yield 49 mg, 66%; 1H NMR (400 MHz, CDCl3) δ 7.77 (d, J = 8.3 Hz, 2H), 7.32 (d, J = 8.0 Hz, 2H), 4.39–4.33 (m, 1H), 4.18–4.12 (m, 1H), 3.29–3.22 (m, 1H), 2.54 (dd, J = 14.3, 6.5 Hz, 1H), 2.43 (s, 3H), 2.41–2.36 (m, 1H), 2.25–2.21 (m, 2H), 1.41–1.33 (m, 3H), 1.22–1.15 (m, 7H), 0.85 (t, J = 7.0 Hz, 3H). 13C{1H} NMR (100 MHz, CDCl3) δ 207.0, 144.0, 137.8, 130.2, 127.3, 54.9, 45.7, 40.6, 40.2, 32.4, 31.8, 28.9, 26.0, 22.7, 21.8, 14.3.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as eluent to get the products.
1, v/v) as eluent to get the products.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.53; yield 38 mg, 74%; IR (KBr, neat) ν 3052, 3008, 2924, 1586, 1463, 1432, 1393, 1153, 1070, 1006, 839, 771 cm−1; 1H NMR (600 MHz, CDCl3) δ 8.72 (d, J = 4.8 Hz, 1H), 7.90 (d, J = 8.3 Hz, 2H), 7.81–7.78 (m, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.63 (d, J = 8.2 Hz, 2H), 7.30–7.28 (m, 1H). 13C{1H} NMR (150 MHz, CDCl3) δ 156.5, 149.9, 138.4, 137.2, 132.2, 128.7, 123.8, 122.7, 120.6. HRMS (ESI) calcd for C11H9BrN (M + H)+ 233.9913, found 233.9909.
1) 0.53; yield 38 mg, 74%; IR (KBr, neat) ν 3052, 3008, 2924, 1586, 1463, 1432, 1393, 1153, 1070, 1006, 839, 771 cm−1; 1H NMR (600 MHz, CDCl3) δ 8.72 (d, J = 4.8 Hz, 1H), 7.90 (d, J = 8.3 Hz, 2H), 7.81–7.78 (m, 1H), 7.73 (d, J = 8.0 Hz, 1H), 7.63 (d, J = 8.2 Hz, 2H), 7.30–7.28 (m, 1H). 13C{1H} NMR (150 MHz, CDCl3) δ 156.5, 149.9, 138.4, 137.2, 132.2, 128.7, 123.8, 122.7, 120.6. HRMS (ESI) calcd for C11H9BrN (M + H)+ 233.9913, found 233.9909.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.53; mp 133 °C, yield 33 mg, 64%; 1H NMR (400 MHz, CDCl3) δ 8.72 (dt, J = 4.8, 1.5 Hz, 1H), 8.10–8.06 (m, 2H), 7.79–7.77 (m, 2H), 7.73–7.71 (m, 2H), 7.67–7.65 (m, 2H), 7.49–7.45 (m, 2H), 7.39–7.35 (m, 1H), 7.26–7.24 (m, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 157.2, 149.8, 142.0, 140.8, 138.3, 137.2, 129.1, 127.8, 127.7, 127.6, 127.3, 122.4, 120.8.
1) 0.53; mp 133 °C, yield 33 mg, 64%; 1H NMR (400 MHz, CDCl3) δ 8.72 (dt, J = 4.8, 1.5 Hz, 1H), 8.10–8.06 (m, 2H), 7.79–7.77 (m, 2H), 7.73–7.71 (m, 2H), 7.67–7.65 (m, 2H), 7.49–7.45 (m, 2H), 7.39–7.35 (m, 1H), 7.26–7.24 (m, 1H). 13C{1H} NMR (100 MHz, CDCl3) δ 157.2, 149.8, 142.0, 140.8, 138.3, 137.2, 129.1, 127.8, 127.7, 127.6, 127.3, 122.4, 120.8.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.53; yield 26 mg, 70%; 1H NMR (500 MHz, CDCl3) δ 8.68 (t, J = 4.1 Hz, 1H), 7.90–7.88 (m, 2H), 7.74–7.70 (m, 2H), 7.30–7.26 (m, 2H), 7.22–7.20 (m, 1H), 2.41 (s, 3H). 13C {1H} NMR (125 MHz, CDCl3) δ 157.7, 149.7, 139.3, 137.0, 136.7, 129.7, 127.0, 122.1, 120.6, 21.5.
1) 0.53; yield 26 mg, 70%; 1H NMR (500 MHz, CDCl3) δ 8.68 (t, J = 4.1 Hz, 1H), 7.90–7.88 (m, 2H), 7.74–7.70 (m, 2H), 7.30–7.26 (m, 2H), 7.22–7.20 (m, 1H), 2.41 (s, 3H). 13C {1H} NMR (125 MHz, CDCl3) δ 157.7, 149.7, 139.3, 137.0, 136.7, 129.7, 127.0, 122.1, 120.6, 21.5.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) as the eluent to yield the product.
9, v/v) as the eluent to yield the product.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 0.32; yield 88 mg, 82%; IR (KBr, neat) ν 2921, 2851, 2278, 1659, 1597, 1493, 1441, 1344, 1160, 1093, 1012, 911, 812, 733, 491 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.63–7.61 (m, 2H), 7.35–7.32 (m, 2H), 7.24–7.19 (m, 9H), 5.92–5.90 (m, 1H), 5.24 (dd, J = 5.8, 2.0 Hz, 1H), 4.20–4.13 (m, 1H), 3.43–3.36 (m, 1H), 2.54–2.46 (m, 2H), 2.34 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.8, 137.5, 137.1, 133.8, 131.7, 130.0, 129.5, 129.0, 128.9, 128.7, 128.6, 127.1, 122.9, 118.2, 89.6, 88.8, 52.4, 41.4, 30.5, 21.8. HRMS (ESI) calcd for C26H22ClNNaO2S (M + Na)+ 470.0952, found 470.0925.
1) 0.32; yield 88 mg, 82%; IR (KBr, neat) ν 2921, 2851, 2278, 1659, 1597, 1493, 1441, 1344, 1160, 1093, 1012, 911, 812, 733, 491 cm−1; 1H NMR (400 MHz, CDCl3) δ 7.63–7.61 (m, 2H), 7.35–7.32 (m, 2H), 7.24–7.19 (m, 9H), 5.92–5.90 (m, 1H), 5.24 (dd, J = 5.8, 2.0 Hz, 1H), 4.20–4.13 (m, 1H), 3.43–3.36 (m, 1H), 2.54–2.46 (m, 2H), 2.34 (s, 3H). 13C{1H} NMR (150 MHz, CDCl3) δ 143.8, 137.5, 137.1, 133.8, 131.7, 130.0, 129.5, 129.0, 128.9, 128.7, 128.6, 127.1, 122.9, 118.2, 89.6, 88.8, 52.4, 41.4, 30.5, 21.8. HRMS (ESI) calcd for C26H22ClNNaO2S (M + Na)+ 470.0952, found 470.0925.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, v/v) as the eluent. After completion of the reaction, it was quenched with a saturated sodium bicarbonate solution. A brine solution was then added, and the mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic layers were dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure by rotary evaporator. The desired product 3ad was obtained (1.11 g, colorless solid) in 61% yield by silica gel column chromatography using hexane/ethyl acetate (9
9, v/v) as the eluent. After completion of the reaction, it was quenched with a saturated sodium bicarbonate solution. A brine solution was then added, and the mixture was extracted with ethyl acetate (3 × 10 mL). The combined organic layers were dried over anhydrous sodium sulfate (Na2SO4), filtered, and concentrated under reduced pressure by rotary evaporator. The desired product 3ad was obtained (1.11 g, colorless solid) in 61% yield by silica gel column chromatography using hexane/ethyl acetate (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) as the eluent.
1, v/v) as the eluent.Data availability
The data supporting the findings of this study are available within the article and/or its ESI.† Supporting data for this article are provided in the ESI,† which includes copies of the 1H NMR and 13C{1H} NMR spectra for all newly synthesized compounds, along with single-crystal X-ray data for compounds 3ac (CCDC 2429152), 3dj (CCDC 2431627), and 4aa (CCDC 2429151).Conflicts of interest
There are no conflicts to declare.Acknowledgements
SKB is grateful to the MHRD, Govt of India, for the fellowship. The authors are grateful DST-FIST program (Grant No. SR/FST/CS-II/2017/23C) and the Central Instrument Facility (CIF), Indian Institute of Technology Guwahati, for the analytical facility.References
- (a) V. Baliah, R. Jeyarama and L. Chandrasekaran, Synthesis of 2, 6-disubstituted piperidines, oxanes, and thianes, Chem. Rev., 1983, 83, 379–423 CrossRef CAS; (b) M. G. P. Buffat, Synthesis of piperidines, Tetrahedron, 2004, 60, 1701–1729 CrossRef CAS; (c) H. Tsukamoto and Y. Kondo, Palladium (0)-catalyzed alkynyl and allenyl iminium ion cyclizations leading to 1,4-disubstituted 1,2,3,6-tetrahydropyridines, Angew. Chem., Int. Ed., 2008, 47, 4851–4854 CrossRef CAS PubMed; (d) N. A. Frolov and A. N. Vereshchagin, Piperidine derivatives: recent advances in synthesis and pharmacological applications, Int. J. Mol. Sci., 2023, 24, 2937 CrossRef CAS PubMed.
- G. B. Fodor and B. Colasanti, Alkaloids: Chemical and Biological Perspectives, Pelletier, S. W., ed. Wiley, New York, 1985, vol. 23, pp 1–90 Search PubMed.
- (a) P. S. Watson, B. Jiang and B. Scott, A diastereoselective synthesis of 2,4-disubstituted piperidines: Scaffolds for drug discovery, Org. Lett., 2000, 2, 3679–3681 CrossRef CAS PubMed; (b) D. A. Horton, G. T. Bourne and M. L. Smythe, The combinatorial synthesis of bicyclic privileged structures or privileged substructures, Chem. Rev., 2003, 103, 893–930 CrossRef CAS PubMed.
- B. Seltzer, Donepezil: a review, Expert Opin. Drug Metab. Toxicol., 2005, 1, 527–536 CrossRef CAS PubMed.
- J. L. Wright, T. F. Gregory, S. R. Kesten, P. A. Boxer, K. A. Serpa, L. T. Meltzer and L. D. Wise, Subtype-Selective N-Methyl-d-Aspartate Receptor Antagonists: Synthesis and Biological Evaluation of 1-(Heteroarylalkynyl)-4-benzylpiperidines, J. Med. Chem., 2000, 43, 3408 CrossRef CAS PubMed.
- (a) P. N. Reebye, C. Yiptong, J. Samsoon, F. Schulsinger and J. Fabricius, A controlled double-blind study of femoxetine and amitriptyline in patients with endogenous depression, Pharmacopsychiatry, 1982, 15, 164–169 CrossRef CAS PubMed; (b) C. Boerup, I. M. Peterson, P. F. Honore and L. Wetterberg, Reduction of whole blood serotonin in depressed patients treated with a new, selective serotonin-uptake inhibitor, femoxetine, Psychopharmacology, 1979, 63, 241–243 CrossRef CAS PubMed.
- L. G. Leon, R. M. Carballo, M. C. Vega-Hernandez, V. S. Martin, J. I. Padron and J. M. Padron, Antiproliferative activity of 2-alkyl-4-halopiperidines and 2-alkyl-4-halo-1,2,5,6-tetrahydropyridines in solid tumor cell lines, Bioorg. Med. Chem. Lett., 2007, 17, 2681–2684 CrossRef CAS PubMed.
- (a) S. Abdul-Rashed, C. Holt and A. J. Frontier, Alkynyl Prins and Alkynyl Aza-Prins Annulations: Scope and Synthetic Applications, Synthesis, 2020, 52, 1991–2007 CrossRef CAS; (b) N. Gouault, M. Roydor, J. Renault, S. Banik, B. V. S. Reddy and C. Lalli, One-Pot Double Cyclizations Involving an Aza-Prins Process, Eur. J. Org Chem., 2024, 27, e202400617 CrossRef CAS; (c) S. Biswas, B. Porashar, P. J. Arandhara and A. K. Saikia, Synthesis of pyrimido[2,1-a]isoindolone and isoindolo[2,1-a]quinazolinone via intramolecular aza-Prins type reaction, Chem. Commun., 2021, 57, 11701–11704 RSC; (d) V. Sinka, I. Fernández and J. I. Padrón, Synthesis of Tetrahydroazepines through Silyl Aza-Prins Cyclization Mediated by Iron(III) Salts, J. Org. Chem., 2022, 87, 11735–11742 CrossRef CAS PubMed.
- (a) G.-Q. Liu, B. Cui, R. Xu and Y.-M. Li, Preparation of trans-2-Substituted-4-halopiperidines and cis-2-Substituted-4-halotetrahydropyrans via AlCl3-Catalyzed Prins Reaction, J. Org. Chem., 2016, 81, 5144–5161 CrossRef CAS PubMed; (b) G. Alachouzos and A. J. Frontier, Cationic Cascade for Building Complex Polycyclic Molecules from Simple Precursors: Diastereoselective Installation of Three Contiguous Stereogenic Centers in a One-Pot Process, J. Am. Chem. Soc., 2019, 141, 118–122 CrossRef CAS PubMed; (c) J. J. Hernandez, A. P. Lawrie and A. J. Frontier, Alkynyl Halo-Aza-Prins Annulative Couplings, J. Org. Chem., 2023, 88, 16065–16075 CrossRef CAS PubMed; (d) W. F. Cheng, S. Ma, Y. T. Lai, Y. T. Cheung, K. Akkarasereenon, Y. Zhou and R. Tong, BiBr3-Mediated Intramolecular Aza-Prins Cyclization of Aza-Achmatowicz Rearrangement Products: Asymmetric Total Synthesis of Suaveoline and Sarpagine Alkaloids, Angew. Chem., Int. Ed., 2023, 62, e202311671 CrossRef CAS PubMed; (e) A. Milosavljevic, C. Holt and A. J. Frontier, Nitrogen-interrupted halo-Prins/halo-Nazarov fragment coupling cascade for the synthesis of indolines, Chem. Sci., 2023, 14, 5431–5437 RSC.
- R. M. Carballo, M. A. Ramirez, M. L. Rodriguez, V. S. Martin and J. I. Padron, Iron (III)-promoted aza-Prins-cyclization: direct synthesis of six-membered azacycles, Org. Lett., 2006, 8, 3837–3840 CrossRef CAS PubMed.
- P. O. Miranda, R. M. Carballo, V. S. Martin and J. I. Padron, A new catalytic Prins cyclization leading to Oxa-and Azacycles, Org. Lett., 2009, 11, 357–360 CrossRef CAS PubMed.
- S. K. Bora, S. Biswas, B. K. Behera and A. K. Saikia, Stereoselective synthesis of gem-dihalopiperidines via the halo-aza-Prins cyclization reaction: access to piperidin-4-ones and pyridines, Org. Biomol. Chem., 2024, 22, 3893–3903 RSC.
- M. Goldstein, L. I. B. Haines and J. A. G. Hemmings, Kinetics of the Halogen-exchange Reaction Between Alkyl Halides (RY) and Boron Trihalides (BX,). Evidence for Formation of Adducts RY,BX3, J. Chem. Soc., Dalton Trans., 1972, 2260–2263 RSC.
- R. Kimura, Y. Sawayama, A. Nakazaki, K. Miyamoto, M. Uchiyama and T. Nishikawa, Unexpected Metal-Free Transformation of gem-Dibromomethylenes to Ketones under Acetylation Conditions, Chem.–Asian J., 2015, 10, 1035–1041 CrossRef CAS PubMed.
- A. K. Saikia, P. Ghosh and A. K. Kautarya, Synthesis of 4-trifluoromethanesulfonate substituted 3,6-dihydropyrans and their application in various C–C coupling reactions, RSC Adv., 2016, 6, 44774 RSC.
- (a) C. D. Campbell, R. L. Greenaway, O. T. Holton, P. R. Walker, H. A. Chapman, C. A. Russell, G. Carr, A. L. Thomson and E. A. Anderson, Ynamide Carbopalladation: A Flexible Route to Mono-, Bi-and Tricyclic Azacycles, Chem.–Eur. J., 2015, 21, 12627–12639 CrossRef CAS PubMed; (b) K. Takasu, H. Ohsato, J. Kuroyanagi and M. Ihara, Novel intramolecular [4+1] and [4+2] annulation reactions employing cascade radical cyclizations, J. Org. Chem., 2002, 67, 6001–6007 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2429152, 2431627 and 2429151. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra03630e |
| This journal is © The Royal Society of Chemistry 2025 |