Open Access Article
Open Access ArticleHigh performance self-assembled pyrene-based emitter with narrowband emission and excellent luminescence efficiency†
Zhongxing Geng *a,
Zhentan Wanga,
Hailong Liua,
Xiangyi Sua,
Liangyu Hua,
Fengyan Song
*a,
Zhentan Wanga,
Hailong Liua,
Xiangyi Sua,
Liangyu Hua,
Fengyan Song c and
Kun Yao
c and
Kun Yao *b
*b
aSchool of Energy, Materials and Chemical Engineering, Hefei University, Hefei, Anhui 230601, China. E-mail: gzx@hfuu.edu.cn
bSchool of Chemical and Printing-Dyeing Engineering, Henan University of Engineering, Zhengzhou, Henan 450007, China. E-mail: yaokun0820@163.com
cCollege of Chemistry and Life Science, Beijing University of Technology, Beijing 100124, China
First published on 21st July 2025
Abstract
To address the problem of broad emission spectra of organic luminescent materials, a novel and effective molecular design strategy is presented to reduce the full width at half maximum (FWHM) of emission by integrating the multi resonance (MR) B–N moiety into the pyrene-based backbone system, named DtBuCzB-Py. Taking advantages of BN-core in narrowband emission and planar rigid structure, the obtained compound DtBuCzB-Py not only exhibited narrowband emission (FWHM = 25 nm in toluene solution; FWHM = 38 nm in spin-coated film) and high fluorescence quantum yield (ΦF = 77.2% in toluene solution; ΦF = 36.8% in spin-coated film), but also strong self-assembly ability.
Highly efficient organic luminescent materials with narrow-band emission have garnered significant interest in recent years owing to their wide range of potential applications in high-resolution organic optoelectronic display devices.1,2 Unfortunately, because of the vibrational coupling between the ground state and the excited state as well as the structural relaxation in the excited state, the emission spectra of traditional fluorescence emitters exhibit an inherent broadband emission with full width at half-maximum (FWHM), resulting in a relatively low color purity.3,4 Moreover, most organic luminescent materials suffer from an unfavorable aggregation-caused quenching (ACQ) effect, which significantly limits their practical application requirements.5,6 Therefore, it is a great challenge to achieve organic luminescent materials that exhibit both narrow-band emission (less than 50 nm) and high luminescence efficiency.
Pyrene and its derivatives, one of the most important blue-coloured chromophores and supramolecular self-assembly moieties, have been widely applied for various optoelectronic devices due to their conjugated structure, long fluorescence lifetime, and high luminous efficiency.7–9 Due to the planar π-conjugated molecular structure, pyrene is more inclined to form dimers through strong π–π stacking interactions in the aggregated or film state, resulting in corresponding redshift emissions and reducing the fluorescence quantum yield (ΦF).10–12 To overcome the nature of the ACQ effect, one of the most commonly used strategies is to functionalize the pyrene core by introducing chromophores with aggregation-induced luminescence (AIE) characteristics to achieve novel pyrene-based AIE luminogens (AIE gens) with considerable luminous efficiency.13–15 For instance, Tang and his collaborators synthesized an asymmetrical tetraphenylethylene (TPE)-substituted pyrene with AIE characteristics, where blue emission of naked TPE and yellow emission of diethylaminophenyl-substituted TPE units were introduced at the 2- and 7-positions of pyrene, respectively.16 Interestingly, the resulting asymmetric compound not only displays a tunable AIE emission from blue (435 nm) to warm white (dual emission: 436 nm and 538 nm), but also exhibits a high luminous efficiency (ΦF = 22%). However, like most organic luminescent materials, the traditional pyrenes show a broad emission under irradiation. In particular, the pyrene-based AIEgens containing twisted confirmations tend to exhibit bright emissive properties with wider FWHM, due to the diversity of excited-state structural relaxation.4 Thus, developing high-performance pyrene emitters with both narrowband emission and high photoluminescence efficiency is a meaningful but challenging task.
Since Hatakeyama et al. pioneered the multi resonance (MR) molecules with narrowband emission to improve the color purity of organic light-emitting diodes (OLEDs) in 2016, MR molecules have received extensive attention for the development of high-performance organic functional materials and devices.17–25 Consequently, combining the advantages of MR molecules with narrowband emission and the pyrene core with tunable planar rigidity structure is the promising candidate strategy to overcome the challenge of pyrene luminescent material with narrowband emission and high photoluminescence efficiency, especially in the film state. In this work, a novel pyrene-based emitter DtBuCzB-Py was successfully synthesized by incorporating a multi-resonance (MR) B–N moiety into the pyrene-based backbone through the Suzuki coupling reaction (Scheme 1). It was found that the obtained DtBuCzB-Py not only exhibited narrowband emission (FWHM = 25 nm in toluene solution; FWHM = 38 nm in spin-coated film) and high fluorescence quantum yield (ΦF = 77.2% in toluene solution; ΦF = 36.8% in spin-coated film), but also possessed strong assembly ability. Notably, the ΦF value of DtBuCzB-Py in both solution and spin-coated film states can be maintained at a relatively high level compared with the reported pyrene-based emitters.9,11,16 This work presents a novel molecular design strategy that can simultaneously achieve narrowband emission, high luminescence efficiency, and strong self-assembly ability of pyrene-based emitters.
The target compound DtBuCzB-Py was synthesized through a Suzuki coupling reaction and the detailed procedures and characterization data of DtBuCzB-Py are listed in the ESI.† The thermal stability of DtBuCzB-Py was evaluated by thermogravimetric analysis (TGA) under a nitrogen atmosphere with a heating rate of 10 °C min−1. As depicted in Fig. S1,† the degradation temperatures (Td) corresponding 5% weight loss exceeds 419.4 °C, indicating good thermal stability. Moreover, DtBuCzB-Py exhibits excellent solubility in common solvents, such as dichloromethane (CH2Cl2), tetrahydrofuran (THF), and toluene, which is conducive to the formation of high-quality films by spin-coating.
The UV-Vis absorption and photoluminescence (PL) emission spectra of DtBuCzB-Py were investigated in both dilute toluene (1 × 10−5 mol L−1) and spin-coated states (SI). As shown in Fig. 1, DtBuCzB-Py exhibits dominant long-wavelength absorption bands at around 369 nm in toluene solution and 381 nm in spin-coated film, respectively, which can be regarded as the n–π* transition in the pyrene system.26,27 Additionally, the strong absorption band of DtBuCzB-Py that appears near 470 nm can be attributed to the BN moiety.20 Moreover, similar PL bands of DtBuCzB-Py can be observed at around 487 nm in toluene solution and 504 nm in spin-coated film, with the CIE coordinates of (0.08, 0.34) and (0.21, 0.61), respectively (Fig. S2†). To our delight, by integrating the B–N moiety into the pyrene-based backbone, the DtBuCzB-Py can exhibit narrow-band emission in both toluene solution and spin-coated film, with FWHM values of 25 nm and 38 nm, respectively. This indicated that the B–N moiety has effectively suppressed the intramolecular interaction and fixed the molecular motion of pyrene core in the film state, resulting in a narrowed FWHM emission. The fluorescence quantum yield (ΦF) of DtBuCzB-Py in THF solution and spin-coated film was 77.2% (fluorescence lifetime: τ = 8.6 ns, Fig. S3a†) and 36.8% (τ = 2.2 ns, Fig. S3b†), respectively, indicating that this novel pyrene-based emitter achieves both narrowband emission and high ΦF, simultaneously. Notably, the fluorescence lifetimes of DtBuCzB-Py in spin-coated films at 77 K and 300 K are close, both at the ns level (Fig. S4†), indicating that it belongs to the fluorescent emission rather than thermally activated delayed fluorescence (TADF). Furthermore, the THF/water mixed solvent PL tests demonstrate that the decrease in the PLQY value of DtBuCzB-Py can be attributed to the ACQ effect caused by the π–π stacking interactions of pyrene emitters in the aggregated or spin-coated film state (Fig. S5†).
It is well known that pyrene-based luminescent molecules usually have good assembly ability due to their rigid planar molecular structure.28–32 To deeply study the self-assembly ability of the DtBuCzB-Py, its morphology under different conditions was characterized by scanning electron microscopy (SEM). As presented in Fig. 2, at film concentration of 10 mg mL−1, 0.1 mg mL−1, and 1 × 10−3 mg mL−1, DtBuCzB-Py forms a sheet-like morphology. Interestingly, the morphology of DtBuCzB-Py in the aggregated state (1 × 10−3 mg mL−1, THF/H2O = 80/20, v/v) changes from twisted sheet-like to regular fibrous structure, indicating that it has good self-assembly ability in both the film and the aggregated states. The regular fibrous morphology of DtBuCzB-Py possibly originates from the planar rigid molecular of pyrene-core, which can form ordered molecular arrangements driven by π–π stacking interactions.
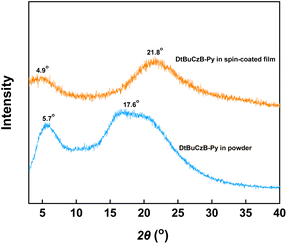
X-ray diffraction (XRD) patterns and 2D grazing incidence wide-angle X-ray scattering (2D-GIWAXS) were employed to clarify the possible molecular packing mode of DtBuCzB-Py. As depicted in Fig. 3, DtBuCzB-Py shows the broad characteristic diffraction peaks of pyrene-based emitters in both powder and spin-coated films. It should be noted that the powder and spin-coated film of DtBuCzB-Py exhibited similar diffraction peaks, and the two main diffraction peaks were centered at 2θ = 5.7°/17.6° and 4.9°/21.8°. Moreover, their corresponding distances were calculated to be 1.55/0.50 nm and 1.80/0.40 nm according to the Bragg's equation. Among them, DtBuCzB-Py spin-coated film with a d-spacing of 0.40 nm could be attributed to the ordered π–π stacking.33,34 Meanwhile, the 2D GIWAXS pattern of DtBuCzB-Py spin-coated film consists of two rings at qxy = 0.49 and 1.44 Å−1 (Fig. 4a and b), corresponding to d-spacings of 12.8 and 4.4 Å, respectively, which matches well with the XRD pattern. The results of XRD and 2D GIWAXS confirm that π–π stacking interactions are the driving force for the ordered assembly of DtBuCzB-Py. Furthermore, the morphology of spin-coated film of DtBuCzB-Py was also studied by atomic force microscopy (AFM). As shown in Fig. 4c, the root mean square (RMS) roughness value of DtBuCzB-Py film was 0.93 nm, which further confirmed the existence of regular and ordered self-assembly, and also indicate the good film-forming ability.
 | ||
| Fig. 4 (a) 2D GIWAXS pattern of DtBuCzB-Py spin-coated film; (b) 1D GIWAXS curve of DtBuCzB-Py spin-coated film; (c) AFM top and 3D images (5 × 5 μm2) of DtBuCzB-Py in the spin-coated film. | ||
The optimized molecular geometry and the electronic properties of DtBuCzB-Py in the ground state were performed by density functional theory (DFT) calculations at the B3LYP-D3BJ/6-31G (d) level using the Gaussian 16 program. As displayed in Fig. 5, the highest occupied molecular orbital (HOMO), the lowest unoccupied molecular orbital (LUMO), and the energy gap levels (Eg) of DtBuCzB-Py were calculated to be −5.00 eV, −1.8 eV, and 3.20 eV, respectively. Notably, the electron density of the HOMO is localized on the BN-moiety, while the electron density of the LUMO is mainly located on the pyrene-core. The clear separation between the HOMO and LUMO levels is beneficial for efficient carrier injection and transport. The DFT calculation of DtBuCzB-Py in the excited state has also been performed (Fig. 5b). The calculated S1, T1, and ΔEST energy levels of DtBuCzB-Py are 3.97, 2.84, and 1.13 eV, respectively. Moreover, we have also collected the fluorescence and phosphorescence spectra of DtBuCzB-Py in spin-coated films under liquid nitrogen (77 K). As shown in Fig. S6,† the measured S1, T1 and ΔEST energy levels are 2.72 eV, 2.28 eV, and 0.44 eV, respectively. The large ΔEST confirm that DtBuCzB-Py is a common fluorescent emission dye.35
 | ||
| Fig. 5 (a) Optimized structures and calculated HOMO–LUMO spatial distributions of DtBuCzB-Py; (b) the S1, T1, and ΔEST energy levels of DtBuCzB-Py in the excited state. | ||
In summary, we propose a simple yet efficient design strategy to narrow the pyrene-based emitters with high luminescence efficiency by integrating the multi resonance (MR) B–N moiety into the pyrene-based backbone. Interestingly, the obtained compound DtBuCzB-Py exhibited narrowband emission (FWHM = 25 nm in toluene solution; FWHM = 38 nm in spin-coated film) and high fluorescence quantum yield (ΦF = 77.2% in toluene solution; ΦF = 36.8% in spin-coated film). Moreover, due to the assembly ability of pyrene core and the planar rigidity of BN-moiety, DtBuCzB-Py can self-assemble into a regular fibrous structure. This work proposes a novel molecular design strategy to achieve narrowband emission, high luminescence efficiency, and strong self-assembly ability of pyrene-based emitters, simultaneously.
Data availability
All data generated or analysed during this study are included in this published article and its ESI.†Author contributions
Zhongxing Geng: conceptualization, methodology, investigation, and writing – original draft. Zhentan Wang, Hailong Liu, and Xiangyi Su: investigation and data analysis. Liangyu Hu and Fengyan Song: formal analysis, and writing – review & editing. Kun Yao: supervision, validation, and funding acquisition.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the Anhui Provincial Natural Science Foundation (2308085QB59), National Natural Science Foundation of China (22305059 and 22303028), and the University Natural Science Research Project of Anhui Province (2024AH051501).Notes and references
- G. Hong, X. Gan, C. Leonhardt, Z. Zhang, J. Seibert, J. M. Busch and S. Bräse, Adv. Mater., 2021, 33, 2005630 CrossRef CAS PubMed.
- Y. Huang, M. Jia, C. Li, Y. Yang, Y. He, Y. J. Luo, Y. Huang, L. Zhou and Z. Lu, Chem. Commun., 2024, 60, 3194 RSC.
- M. H. Jung, H. H. Seon, P. Ambika, J. E. Jeong and H. Y. Woo, NPG Asia Mater., 2021, 13, 53 CrossRef.
- X. Feng, X. Wang, C. Redshaw and B. Z. Tang, Chem. Soc. Rev., 2023, 52, 6715–6753 RSC.
- Y. Wang, L. Cui, Y. Wang, F. Li, Y. Li and Q. Meng, Chem. Eur. J., 2023, 29, e202302373 CrossRef CAS PubMed.
- Q. Meng, L. Cui, Q. Liao, J. Xu and Y. Wang, Chem. Commun., 2022, 58, 10384–10387 RSC.
- K. Takaishi, S. Murakami, K. Iwachido and T. Ema, Chem. Sci., 2021, 12, 14570–14576 RSC.
- O. A. Krasheninina, D. S. Novopashina, E. K. Apartsin and A. G. Venyaminova, Molecules, 2017, 22, 2108 CrossRef PubMed.
- W. Liu, S. Li, Z. Xie, K. Huang, K. Yan, Y. Zhao, C. Redshaw, X. Feng and B. Z. Tang, Adv. Opt. Mater., 2024, 12, 2400301 CrossRef CAS.
- D. Niu, Y. Jiang, L. Ji, G. Ouyang and M. Liu, Angew. Chem., Int. Ed., 2019, 58, 5946–5950 CrossRef CAS PubMed.
- A. K. Swain, K. Kolanji, C. Stapper and P. Ravat, Org. Lett., 2021, 23, 1339–1343 CrossRef CAS PubMed.
- Q. Cheng, A. Hao and P. Xing, ACS Nano, 2024, 18, 5766–5777 CAS.
- M. M. Islam, Z. Hu, Q. Wang, C. Redshaw and X. Feng, Mater. Chem. Front., 2019, 3, 762–781 RSC.
- J. Y. Cao, G. Yang, Z. M. Xue, W. X. Zhao, S. H. Chen, H. T. Lin, T. Yamato, C. Redshaw, D. Y. Gu and C. Z. Wang, Dyes and Pigm., 2023, 220, 111758 CrossRef CAS.
- E. V. Varghese, C. Y. Yao and C. H. Chen, Chem. – Asian J., 2024, 19, e202300910 CrossRef CAS PubMed.
- X. Feng, C. Qi, H. T. Feng, Z. Zhao, H. H. Y. Sung, I. D. Williams, R. T. K. Kwok, J. W. Y. Lam, A. Qin and B. Z. Tang, Chem. Sci., 2018, 9, 5679–5687 RSC.
- T. Hatakeyama, K. Shiren, K. Nakajima, S. Nomura, S. Nakatsuka, K. Kinoshita, J. Ni, Y. Ono and T. Ikuta, Adv. Mater., 2016, 28, 2777 CrossRef CAS PubMed.
- Y. Xu, Q. Wang, X. Cai, C. Li and Y. Wang, Adv. Mater., 2021, 33, 2100652 CrossRef CAS.
- W. Luo, T. Wang, Z. Huang, H. Huang, N. Li and C. Yang, Adv. Funct. Mater., 2024, 34, 2310042 CrossRef CAS.
- K. K. Tan, W. C. Guo, L. Zhao, W, M. Li and C. F. Chen, Angew. Chem., Int. Ed., 2024, 63, e202412283 CAS.
- C. H. Guo, Y. Zhang, W. L. Zhao, K. K. Tan, L. Feng, L. Duan, C. F. Chen and M. Li, Adv. Mater., 2024, 36, 2406550 CrossRef CAS PubMed.
- Y. Huo, H. Qi, S. He, J. Li, S. Song, J. Lv, Y. Liu, L. Peng, S. Ying and S. Yan, Aggregate, 2023, 4, e391 CrossRef CAS.
- Y. Wang, Z. Ma, J. Pu, D. Guo, G. Li, Z. Chen, S. J. Su, H. Deng, J. Zhao and Z. Chi, Aggregate, 2024, 5, e585 CrossRef CAS.
- X. F. Luo, S. Q. Song, X. Wu, C. F. Yip, S. Cai and Y. X. Zheng, Aggregate, 2024, 5, e445 CrossRef CAS.
- J. Wang, H. Hafeez, S. Tang, T. Matulaitis, L. Edman, I. D. Samuel and E. Zysman-Colman, Aggregate, 2024, 5, e571 CrossRef CAS.
- S. Zhang, Y. Sheng, G. Wei, Y. Quan, Y. Cheng and C. Zhu, Polym. Chem., 2015, 6, 2416–2422 RSC.
- Y. Zhang, Z. Geng, Y. Zhang, Z. Xu, H. Li, Y. Cheng and Y. Quan, J. Phys. Chem. Lett., 2021, 12, 3767–3772 CrossRef CAS PubMed.
- T. M. Figueira-Duarte and K. Müllen, Chem. Rev., 2011, 111, 7260–7314 CrossRef CAS PubMed.
- Y. Zhang, H. Li, Z. Geng, W. Zheng, Y. Quan and Y. Cheng, Nat. Commun., 2022, 13, 4905 CrossRef CAS.
- H. Shigemitsu, K. Kawakami, Y. Nagata, R. Kajiwara, S. Yamada, T. Mori and T. Kida, Angew. Chem., Int. Ed., 2022, 61, e202114700 CrossRef CAS.
- S. Yang, F. Li, Q. Li, J. Han and Y. Cheng, ACS Appl. Opt. Mater., 2023, 1, 1492–1500 CrossRef CAS.
- S. Yang, F. Hu, T. Xu, F. Lin, J. Han and F. Li, Chem. Eur. J., 2024, 30, e202402012 CrossRef CAS.
- P. Qin, Z. Wu, P. Li, D. Niu, M. Liu and M. Yin, ACS Appl. Mater. Interfaces, 2021, 13, 18047–18055 CrossRef CAS PubMed.
- M. Ma, A. Hao and P. Xing, Nanoscale, 2022, 14, 8163–8171 RSC.
- D. Wan, J. Zhou, Y. Yang, G. Meng, D. Zhang, L. Duan and J. Ding, Adv. Mater., 2024, 36, 2409706 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra03596a |
| This journal is © The Royal Society of Chemistry 2025 |