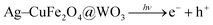Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Environmental applications of magnetic nanohybrid materials
Sidra Pervaiz†
a,
Mohsin Javed†b,
Afzal Shah *b,
Ansa Latifc,
Sidra Nasird and
Iltaf Shah*e
*b,
Ansa Latifc,
Sidra Nasird and
Iltaf Shah*e
aDepartment of Chemistry, Government College University Faisalabad, 38040, Pakistan
bDepartment of Chemistry, Quaid-i-Azam University Islamabad, 45320, Pakistan. E-mail: afzals_qau@yahoo.com
cDepartment of Chemistry, University of Agriculture Faisalabad, 38000, Pakistan
dDepartment of Applied Chemistry, Government College University Faisalabad, 38040, Pakistan
eDepartment of Chemistry, College of Science, United Arab Emirates University, Al Ain P. O. Box 15551, United Arab Emirates. E-mail: altafshah@uaeu.ac.ae
First published on 11th June 2025
Abstract
The distinctive characteristics and versatile functionalities of magnetic nanoparticles (MNPs) position them as promising candidates for numerous environmental applications, attributed to their structural and physiochemical properties. Magnetic nanoparticles can integrate organic polymers, carbon-based materials, and metal oxides to form multifunctional composites that effectively adsorb and degrade pollutants. This review article discusses the magnetic nanomaterials and the techniques employed to functionalize and tailor these materials and their application for wastewater treatment, cleanup of oil spills, and photocatalytic degradation of pollutants of emerging concern. Focusing on their regeneration capabilities, catalytic performance, and adsorption efficiency, the article analyzes how magnetic nanohybrids engage with pollutants remediation. Additionally, it evaluates these materials' stability, implications, and recyclability to assess their practical applicability in real-world environmental scenarios. By presenting a detailed overview of the recent advancements, challenges, and prospects, this work aims to assist researchers in developing and enhancing magnetic nanohybrids as a remediation technology to advance sustainable environmental remediation strategies. The magnetic nanoparticles discussed in this document exhibit remarkable efficacy in contaminant removal due to their magnetic retrievability, facilitating easier recovery and reuse. These advantages not only enhance their practical applications but also align with principles of sustainability and resource efficiency. The innovative integration of advanced magnetic materials with green chemistry principles outlined in this review significantly enhances environmental remediation initiatives. This review adopts a holistic approach by emphasizing the benefits while addressing challenges, including instability, elevated costs, quality and functionality problems, concerns regarding secondary pollution, and issues related to reusability.
1. Introduction
The global population has been increasing consistently with urban areas experiencing more significant population growth, attributed to enhanced healthcare, educational opportunities, and job accessibility, with industrialization playing a key role in this urbanization process.1 As the population increases, the need for goods and services rises, leading to industrial growth and additional factories.2 The need to support larger populations and urban centers drives technological innovations in manufacturing, energy production and transportation. Many industrial and non-industrial sources contribute to water pollution, which harms human health,3 aquatic ecosystems, and biodiversity.4,5 The chemical manufacturing, mining, oil, and textile sectors discharge toxic materials into water bodies through untreated wastewater, unintentional spills, and poor waste disposal.6 These substances include organic pollutants7 like solvents, dyes, hydrocarbons, and heavy metals8 such as lead, mercury, and arsenic. Moreover, agricultural runoff comprising fertilizers, pesticides, and animal manure causes eutrophication, toxic algal blooms, and pathogens9 contamination, in addition to deforestation and soil erosion.10 Furthermore, mining results in metal leaching and acid mine drainage,11 and hydraulic fracturing in oil and gas operations puts groundwater at risk of contamination.12 Marine environments face threats from plastic waste, oil spills, and the atmospheric deposition of pollutants such as nitrogen oxides.13 To protect water quality and ecosystem health, integrated legislation, sustainable practices, and efficient wastewater treatment technologies must be implemented.14 Managing the impact of urbanization and industrialization is crucial for environmental sustainability.In the realm of water and wastewater treatment, nanotechnology stands out as a highly effective and promising avenue for advancement.15 The utilization of magnetic nanoparticles (MNPs) has shown significant benefits in wastewater remediation, owing to their exceptional optical, electrical, and magnetic properties, as well as their high adsorption capacities, mobility, reactivity, and catalytic abilities. Furthermore, the features and applications of MNPs can be tailored through the application of magnetic fields, enhancing their efficacy in treatment processes. MNPs find extensive potential applications in catalysis, biomedicine, tissue targeting, colloidal photonic crystals, MRI, MPI, microfluidics, data storage, environmental remediation, and more.16,17 MNPs of many kinds, including iron oxides (Fe3O4), spinel ferrite (CoFe2O4), bio-metallic, carbon-based, and others are employed for wastewater treatment. With the potential for nearly 100% in situ treatment, MNPs in waste treatment improve efficiency, encourage reuse, and save money, time, and effort.18
Physical and chemical methods are essential for wastewater treatment. Physical methods involve mechanical techniques such as screening, filtration, and sedimentation to eliminate suspended solids and debris. Additionally, flotation and centrifugation are employed to separate materials based on density. Chemical methods utilize various substances to precipitate, neutralize, or oxidize contaminants. Coagulation and flocculation processes introduce chemicals that promote the aggregation of smaller particles into larger ones, improving their removal through sedimentation or filtration.19 Disinfection techniques such as chlorination and ozone treatment eliminate harmful pathogens. Chemical oxidation can break down complex organic pollutants into simpler compounds, often necessitating pH adjustments to optimize these processes. While these methods are effective, they can be costly, require careful handling of hazardous materials, and generate chemical sludge that must be disposed appropriately. Nevertheless, the implementation of both physical and chemical treatments is crucial for the effective removal of diverse pollutants, particularly in industrial wastewater.20 Wastewater treatment utilizing physical and chemical techniques typically involves considerable expenses and substantial energy consumption, especially when dealing with large volumes. Applying these methods often requires the use of chemicals, which can pose environmental and safety risks, in addition to generating chemical sludge that requires proper disposal. Moreover, these methods may not effectively remove certain dissolved pollutants or biodegradable organic materials, leading to the need for further treatment processes.21
Membrane technologies are advanced methods employed in wastewater treatment, leveraging selective barriers to efficiently separate contaminants from water. Commonly utilized membrane processes include nanofiltration, microfiltration, ultrafiltration, and reverse osmosis, each characterized by specific pore sizes and varying effectiveness in pollutant removal. Microfiltration and ultrafiltration are adept at removing suspended solids, bacteria, and larger particles, while nanofiltration and reverse osmosis target smaller dissolved contaminants, such as salts, heavy metals, and organic molecules.22 Nonetheless, membrane technologies can incur significant expenses owing to the energy requirements for sustaining pressure across the membrane and the regular necessity for cleaning or replacing membranes due to fouling. Membrane fouling diminishes efficiency and requires regular cleaning or replacement, leading to increased downtime and operational costs. Furthermore, this technology might only be financially viable for extensive applications with considerable investment.23
Among the advanced oxidation processes (AOPs), photocatalysis stands out due to its effectiveness and versatility in wastewater treatment, surpassing methods like ozone oxidation, Fenton's reagent, and UV/H2O2. A notable advantage of photocatalysis is its ability to operate under ambient conditions, which negates the necessity for high temperatures or pressures, thus streamlining operations and reducing energy costs.24 Unlike chemical methods like Fenton's reagent that rely on iron salts and hydrogen peroxide, photocatalysis does not require potentially harmful chemical additives, resulting in fewer secondary pollutants and a reduced need for subsequent neutralization processes.25 The issue of electron–hole recombination in photocatalysis, which can restrict the efficiency of the process, is currently under investigation. Innovations like heterojunction photocatalysts and plasmonic materials contribute to the reduction of recombination and enhance overall performance.26
Magnetic nanoparticles constitute a distinctive category of nanomaterials utilized in photocatalytic wastewater treatment. They provide specific advantages and functionalities that differentiate them from traditional photocatalysts such as TiO2, ZnO, CdS, and graphene-based materials.27 Magnetic nanoparticles, primarily consisting of iron oxides such as Fe3O4 or γ-Fe2O3, demonstrate significant magnetic properties that facilitate their separation from treated water by applying an external magnetic field. This feature enhances the recovery and reuse of photocatalysts, tackling a considerable challenge of conventional photocatalysts, which often face time-consuming, costly, or inefficient separation from treated effluent. Materials such as TiO2 and ZnO demonstrate high effectiveness; however, their recovery usually necessitates filtration or sedimentation, potentially elevating operational costs. Magnetic nanoparticles can be rapidly and effectively extracted using magnetic fields, enhancing the overall process's economy and scalability, particularly in large-scale wastewater treatment applications.28 MNPs frequently act as a support or core material for other semiconductors, improving their photocatalytic activity. A photocatalyst such as TiO2 or ZnO can be applied to the surface of MNPs, resulting in a composite that utilizes the photocatalytic characteristics of the semiconductor while also providing the benefit of magnetic separation. This hybrid method integrates the advantageous characteristics of both materials: the elevated photocatalytic efficiency of semiconductors and the straightforward recovery of magnetic particles.29 MNPs contribute to the reduction of electron–hole recombination in these systems, a process where electrons and holes recombine, reducing the efficiency of the photocatalyst, as the iron oxide core facilitates electron transfer, enhancing overall photocatalytic performance. This contrasts with conventional semiconductor materials such as TiO2, ZnO, or CdS, where recombination remains a significant challenge restricting reactive species' production. TiO2 is susceptible to electron–hole recombination; however, incorporating magnetic nanoparticles as a support material can enhance charge separation and increase the efficiency of the photocatalyst.30 MNPs display distinctive surface chemistry that can be utilized for additional functionalization. Modification or doping with metals, oxides, or organic molecules can enhance catalytic properties, improve light absorption, or introduce specific functionalities for pollutant binding. Combining Fe3O4 nanoparticles with silver or gold nanoparticles enhances light absorption. It broadens their activity into the visible spectrum, thereby overcoming a significant limitation of conventional photocatalysts such as TiO2, which predominantly function under UV light.31
Magnetic nanoparticles enhance process efficiency by facilitating improved charge separation and easier recovery post-treatment rather than directly influencing photocatalytic reactions. This renders them more complementary, in contrast to traditional photocatalysts designed to degrade pollutants independently.32 The interaction between MNPs and traditional photocatalysts presents a promising approach for enhancing wastewater treatment solutions' efficiency, sustainability, and cost-effectiveness.18 This review explores MNPs, focusing on their structural characteristics and photocatalytic capabilities, while highlighting their unique benefits in addressing different contaminants in wastewater. It examines surface modification and composite strategies employed to enhance the photocatalytic efficiency of magnetic nanoparticles, focusing on prevalent issues such as electron–hole recombination and material stability. This document also evaluates the versatility of these nanoparticles in addressing various pollutants, organic dyes, heavy metals, synthetic drugs, and oil spills across diverse environmental conditions. The review offers insights into recent advancements and applications, aiming to facilitate future innovations using magnetic nanoparticles for scalable and environmentally friendly wastewater treatment technologies. This is the first report that presents the regeneration capabilities, the photocatalytic performance, and adsorption efficiency of magnetic nanoparticles as a remediation technology to advance the renewable and sustainable water treatment strategies. It offers insights into recent advancements and applications, aiming to facilitate future innovations using magnetic nanoparticles for scalable and environmentally friendly wastewater treatment technologies.
2. Synthesis techniques for MNPs
MNPs are synthesized by various methods. In general, the synthesis of MNPs can be broadly classified into three categories (a) physical, (b) chemical, and (c) biological or microbial methods.33 Physical methods are based on the top-down strategy, i.e., synthesis starts from bulk material and depletes to generate NPs. Chemical and biological methods follow a bottom-up or constructive approach, where the atoms/molecules assemble into different sizes of NPs.34 Fig. 1 shows different synthesis methods of MNPs.2.1 Top-down synthesis
This approach employs a destructive method to reduce bulk materials into tiny, nanoscale particles through various physical and chemical procedures. It can be demonstrated by destructive techniques such as physical vapor deposition, grinding, milling, or several others.352.2 Bottom-up synthesis
Bottom-up synthesis, the constructive method, uses chemical and biological processes to construct MNPs from relatively more straightforward substances such as atoms, clusters, and molecules. This process initially creates the nanostructured building blocks of nanoparticles, which are subsequently put together to produce the finished product (MNPs). The final product offers a more uniform shape (physical attributes), size, and chemical composition. Chemical vapor deposition, sol–gel, and pyrolysis are examples of bottom-up synthesis.49![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 790 kPa) using autoclaves. It primarily entails the rapid nucleation and growth of newly created MNPs, resulting in pure particles with controlled morphologies. During this process, MNPs are prepared by hydrolysis and oxidation reactions. The geometry of NPs can be better controlled using this technique.57
790 kPa) using autoclaves. It primarily entails the rapid nucleation and growth of newly created MNPs, resulting in pure particles with controlled morphologies. During this process, MNPs are prepared by hydrolysis and oxidation reactions. The geometry of NPs can be better controlled using this technique.57The synthesis of Fe3O4 nanoparticles (NPs) with a size of 15 nm and a spherical shape is one of the impressive achievements obtained using this technique, which has been effectively applied in tumor MRI applications.54 Modern nanomaterials that are challenging to synthesize through traditional methods are typically prepared thermodynamically in stable and metastable states using the solvothermal approach. Moreover, producing superior crystallized monodispersed nanocrystals is one of the features of solvothermal synthesis.58
2.3 Biological methods
Biological synthesis is a well-established method for synthesizing MNPs using living organisms, including plants and microorganisms such as fungi, viruses, bacteria, and actinomycetes.63 The benefits of this method are its efficiency, eco-friendliness, and clean process. The disadvantage is its poor dispersion of the NPs.64 The synthesis of NPs using plant tissue, extracts, exudates, and other plant parts has become an area of great interest for researchers.65 For instance, particles with an average size of 60 nm ferromagnetic magnetite were reported to be biologically synthesized.66 A biologically synthesized Fe3O4 magnetic material was used as a catalyst in the Suzuki–Miyaura reaction and photocatalysis.672.4 Functionalization of MNPs
The functionalization of magnetic nanoparticles has emerged as a highly effective strategy for environmental remediation, especially in the area of photocatalytic degradation. By modifying the surfaces of these nanoparticles with photocatalytic materials, their ability to decompose organic pollutants in both water and air is significantly improved, leading to more efficient environmental cleanup processes.68 The use of a magnetic core facilitates the efficient recovery and reuse of nanoparticles post-treatment, thereby reducing secondary pollution. When subjected to light, these functionalized nanoparticles produce reactive oxygen species capable of breaking down harmful contaminants, including dyes, pharmaceuticals, and pesticides. This mechanism not only purifies the environment but also presents a sustainable and cost-effective approach to managing industrial and agricultural waste. Additionally, the customization of the nanoparticles' surface chemistry allows for targeted interactions with specific pollutants, enhancing the overall efficiency of the degradation process. Consequently, the functionalization of magnetic nanoparticles represents a significant advancement in utilizing photocatalytic degradation for effective environmental remediation.69The key element in the development of MNP is iron oxide materials, particularly magnetite and maghemite. Various synthesis methods, such as co-precipitation, thermal decomposition, and hydrothermal synthesis, are employed to produce these nanoparticles. Each technique allows for precise control over the size, shape, and magnetic properties of the particles, which in turn affects their performance characteristics. Among these methods, the chemical precipitation of iron salts is the most widely used for creating iron oxide nanoparticles. This process involves the precipitation of iron salts under basic conditions, resulting in the formation of Fe2+ and Fe3+ salts that aggregate into nanoparticles, typically at ambient or slightly elevated temperatures.70 This approach is favored for its simplicity, cost-effectiveness, and scalability, making it suitable for large-scale industrial production. In contrast, thermal decomposition involves the breakdown of organometallic precursors in high-boiling organic solvents, often in the presence of surfactants, allowing for precise control over the nanoparticles' structure and size.71 High-performance materials that are advantageous for various applications require exceptional magnetic properties, making the intricate and expensive single-step decomposition of organometallic precursors in high-boiling organic solvents with surfactants a critical process. Hydrothermal synthesis, which involves chemical reactions at elevated temperatures and pressures within a sealed vessel containing an aqueous solution, offers remarkable control over the formation of crystals and their respective particle sizes. This production technique allows researchers to fabricate nanoparticles with outstanding magnetic saturation and stability, suitable for complex applications.16
MNPs can be effectively utilized in biomedical applications by incorporating organic polymers, which enhance their biocompatibility and stability while improving dispersibility. This integration typically involves coating the nanoparticles with biocompatible polymers such as polyethylene glycol (PEG), polydopamine, or chitosan. The hydrophilic nature of PEG significantly contributes to the nanoparticles' compatibility with biological environments, resulting in prolonged circulation times in biological fluids. For instance, Xu et al., successfully immobilized β-Glucosidase (β-Glu) onto MNPs, creating MNP–β-Glu, which was further modified with PEG to produce MNP–β-Glu–PEG. This modification not only enhances the stability of the nanoparticles in blood serum but also boosts the enzyme's activity.72 PEGylated nanoparticles are designed to avoid detection by the reticuloendothelial system, making them ideal for applications in drug delivery and imaging. These PEG-coated nanoparticles serve as effective drug carriers, enabling the targeted delivery of therapeutic agents to specific tissues or tumors while employing controlled release systems to minimize side effects during treatment. The robust and stable coating of magnetic nanoparticles is derived from polydopamine, a versatile biopolymer inspired by mussel adhesive proteins. This material contains reactive groups that allow healthcare professionals to attach targeting ligands or therapeutic agents. Consequently, polydopamine-coated nanoparticles can fulfill multiple roles, including targeted drug delivery, photothermal therapy, and functioning as biosensors.73 Targeted cancer cell therapy is significantly improved through the precise delivery of cancer cells using polydopamine-coated Fe3O4 nanoparticles in conjunction with antibodies or peptides. These magnetic nanoparticles are enhanced by a biocompatible and biodegradable chitosan coating, a natural polysaccharide that facilitates chemical interactions with drugs, proteins, and nucleic acids. This characteristic makes chitosan an ideal candidate for applications in genetic delivery and tissue engineering. By utilizing chitosan-based coatings on nanoparticles, researchers have established a promising approach for delivering genetic material, which holds potential for advancements in gene therapy and regenerative medicine.74
MNPs demonstrate improved characteristics when combined with carbon-based materials like graphene oxide (GO) and carbon nanotubes (CNTs). This synergy leads to a notable increase in surface area, enhanced electrical conductivity, and greater mechanical strength. Consequently, these hybrid composites prove to be particularly effective for environmental applications, including the removal of pollutants and the treatment of wastewater.75 The oxygen functional groups present on GO derivatives serve as effective binding sites for magnetic nanoparticles, which, along with their adsorption capabilities and stability, define the performance of GO-coated nanoparticles. These advanced materials play a crucial role in extracting heavy metals, dyes, and organic pollutants from water sources.76 Research has demonstrated that Fe3O4/GO composites are effective in removing toxic heavy metals such as Pb2+ and Cd2+ from contaminated water, leveraging their magnetic recovery properties. Additionally, cylindrical CNTs are distinguished by their remarkable electrical, thermal, and mechanical characteristics, making them notable nanostructures in various applications.77 The integration of magnetic nanoparticles with CNTs enhances the performance of the composite system. These hybrid materials are utilized in catalytic pollutant degradation and as electrode components in energy storage applications. The Fe3O4/CNTs composites serve as efficient catalysts for degrading wastewater dyes, attributed to the high surface area and catalytic efficiency of CNTs.78
By functionalizing MNPs with TiO2 or ZnO, their photocatalytic properties are significantly improved, making them suitable for environmental remediation. These composites utilize a magnetic core that facilitates easy separation and reuse while effectively degrading organic pollutants under light irradiation. TiO2 is recognized as a reliable photocatalyst due to its stability when exposed to UV light, and TiO2/MNP composites exhibit strong degradation capabilities for various organic pollutants, including water-soluble dyes, pesticides, and pharmaceutical residues.79 Specifically, Fe3O4/TiO2 composites have proven to be effective in the photodegradation of methylene blue dye under UV light, while also allowing for convenient magnetic recovery for recycling purposes.80 The photocatalytic performance of ZnO is significantly enhanced under UV light, and when ZnO is applied as a coating on MNPs, their photocatalytic efficiency improves, making them suitable for environmental applications. The use of Fe3O4/ZnO composites facilitates water purification through sustainable methods, promoting the degradation of organic pollutants like phenol and bisphenol A. Furthermore, the functionalization of MNPs represents a significant advancement in nanotechnology, providing effective solutions to various application challenges. By integrating MNPs with organic polymers, carbon-based materials, and metal oxides, researchers are addressing specific needs in both medical and environmental fields.81
2.5 Stabilization of nanoparticles
Nanoparticles possess a high surface area-to-volume ratio, which contributes to their inherent instability and susceptibility to aggregation, oxidation, or dissolution over time. This instability is further influenced by factors such as surface charge, ionic strength, pH, temperature, and surface chemistry. Consequently, stabilizing nanoparticles is crucial to maintain their unique properties, including enhanced reactivity, capacity, and optical characteristics. If these properties are compromised, the nanoparticles may become ineffective or behave unpredictably in various applications. Effective stabilization ensures that nanoparticles can be reliably stored, transported, and utilized in fields such as medicine, catalysis, and environmental science.82 When nanoparticles aggregate, they form tightly bound collections that are difficult to separate into individual particles, causing them to behave more like bulk materials and resulting in a loss of their unique qualities, such as high reactivity or catalytic efficiency. By implementing stabilization techniques, aggregation is prevented, allowing the nanoparticles to retain their distinct and active characteristics.83Chemically synthesized nanoparticles are typically stabilized by surface-capping ligands that control their final dimensions. In the absence of these stabilizing agents, van der Waals forces would cause the colloidal particles to aggregate and precipitate. Stabilizers or capping agents counteract this tendency by creating a repulsive force between the particles. For instance, charged capping agents like sodium citrate facilitate electrostatic stabilization, where negatively charged citrate-capped nanoparticles attract positively charged counter-cations from the surrounding medium, resulting in the formation of an electrical double layer that induces coulombic repulsion. Alternatively, steric stabilization is achieved through the use of long-chain thiols or larger molecules such as polymers. Various stabilization methods exist, including the chemical binding of stabilizing molecules to the nanoparticle surface, the introduction of particle charges, or the creation of physical barriers to prevent aggregation.84
| Synthesis method | Cost | Size control | Yield | Scalability | Advantages | Disadvantages | Ref. |
|---|---|---|---|---|---|---|---|
| Mechanical ball milling | Low | Good | High | High | Wide particle size distribution, ease of operation, high efficiency | Irregular shape, high energy consumption and less production efficiency | 90 |
| Thermal decomposition | High | Excellent | Low | High | Very narrow size distribution, highly scalable and good shape control | Complicated synthesis, inert atmosphere and high temperature requirement | 54 |
| Laser ablation | High | Good | Low | Low | Narrow size distribution, monodispersity, simple & efficient, high purity & crystallinity | High input energy & uneconomical | 91 |
| Electron beam lithography | High | Fine | Low | Low | Well controlled inter-particle spacing | Expensive & extremely costly machinery needed | 18 |
| Chemical vapour deposition | High | Good | Limited | High | Production of intricate, robust, homogeneous, and highly pure NPs | Production of toxic gases as byproduct, demand for specialized equipment | 18 and 92 |
| Sol gel method | Low | Good | Variable | High | Good control of particle size and microstructure, monodispersity, desirable shape and length of the products, good crystallinity and high purity | Contamination of product with matrix component, weak bonding, low wear resistance, high permeability | 93 and 94 |
| Solvothermal | High | Excellent | Marginal | High | Very narrow size distribution & high crystallinity, precise size & shape control | High reaction temperature & pressure | 95 |
| Spray pyrolysis | Low | Good | Moderate | High | Tunable magnetic properties, homogeneous coatings | High energy consumption, risk of aggregation, low production efficiency | 96 and 97 |
| Coprecipitation | Low | Poor | High | High | Simple, narrow size distribution and high production rate | Poor morphology and non-stoichiometric magnetite | 98 and 99 |
3. Environmental applications of MNPs
3.1 Water purification using MNPs as adsorbents
Freshwater consumption is likely to increase due to the growing population. Over the past century, the demand for water has increased almost sevenfold, while the global population has risen fourfold.100–102 Water availability per capita is expected to decrease by 2050, increasing the likelihood of water stress.103 Despite 2.6 billion people gaining access to quality drinking water since 1990, projections indicate that access to water-stressed areas will be available to 3.9 billion people by 2030.104 Contaminants in water resources, resulting from both natural and human activities, are threatening various sectors, and the quality of these resources is declining significantly. Continued poor results in managing water quality, variations in rainfall, over-exploitation of groundwater sources, and water pollution are equally a problem, as are imbalances in the allocation of resources and the occurrence of droughts. Sewages from industries, urban/peri-urban settlements, poor disposal of wastes, traffic irrigation water, and animal wastes, especially in rural areas, play an excessive role in water pollution.105The iron oxide-flax seed-based hybrid nanocomposites were synthesized by Choudhry et al. and were utilized and examined for the adsorption of malachite green. Experimental outcomes proved that the composite achieved high removal efficiency, as 90% of malachite green was obtained from a 10.0 mg L−1 solution, with 1.0 g L−1 of the composite, within 15 minutes at 30 °C and pH 7. The most time-consuming process in the adsorptive removal of malachite green on the catalyst was intraparticle diffusion, which might occur due to weak electrostatic and hydrogen bonding. In conclusion, this work shows that this magnetic flax seed-based composite is effective for water treatment using adsorption technology.107 Saber et al. synthesized two categories of nanohybrids. First, cobalt iron oxide nanoparticles were combined with Al/Zn nanolayers. The second one used long-chain fatty acids, including stearic acid, to increase the distance of Al/Zn nanolayers and create incorporation pathways for the NPs of cobalt iron oxide. Additionally, the zinc oxide nanohybrids demonstrated a lowering in band gap energy value from 3.20 to 2.75 eV, thus increasing the performance of the cells in the sunlight. This high efficiency of the nanohybrid was illustrated by the total decolorization of Acid Green 1 dye within 10 minutes of exposure to sunlight, unlike the pure and doped ZnO, which took 360–840 minutes. The pseudo-first-order kinetic profile revealed that the ZnO nanohybrid prepared from an inorganic–magnetic–organic composite had higher activity than that prepared from an inorganic–magnetic composite. This approach offers a viable plan for addressing energy and water purification issues using clean, non-harmful power sources.108
Composites of natural polymers have great potential as sorbents for magnetic solid-phase extraction (MSPE). Chitosan, a natural polymer known for its amino and carboxylic groups, offers biodegradability, biocompatibility, and ease of modification.109–111 Recently, magnetic chitosan nanohybrid materials were created by dispersing ferrites throughout the chitosan matrix, forming ternary ferrites chitosan microspheres (TFCM).112 The nanoparticles were prepared using a co-precipitation method, and the chitosan matrix was incorporated to produce an active adsorbent for the degradation of methylene blue dye. Zhou et al. prepared magnetic Fe3O4@SiO2–NH2/F13 hybrid material using a one-step reaction to modify the surface of SiO2 by an amino group and an octyl-perfluorinated chain through a sol–gel procedure. The resulting material (Fe3O4@SiO2–NH2/F13) exhibited tremendous adsorption ability of perfluorinated compounds (PFC) from the aqueous sample, as confirmed by HPLC-MS/MS analysis. 50 mg of the Fe3O4@SiO2–NH2/F13 sorbent in a 500 mL water sample reached the adsorption equilibrium within 30 minutes. Fluorine–fluorine interactions, electrostatic solid attraction, and size exclusion effects are attributed to the high adsorption efficiency. The recovery rates ranged from 90.65% to 106.67%, with low limits of detection (LODs) between 0.029 and 0.099 ng L−1. Therefore, Fe3O4@SiO2–NH2/F13 is an effective adsorbent for measuring the concentration of PFC in large volumes of water samples (Fig. 2).113
 | ||
| Fig. 2 Removal of PFC particles from surface water using Fe3O4@SiO2–NH2/F13 magnetic hybrid composite. This figure has been reproduced from ref. 113 with permission from Elsevier, copyright 2016. | ||
Qin et al. significantly synthesized MNPs, which were coated with mesoporous silica, which was modified using methyl dimethoxy and p-toluenesulfonic acid (PTSA) to prepare Fe3O4/mSiO2–Me–PTSA material. This material was tested for removing polychlorinated biphenyls (PCBs) from wastewater. The material facilitated rapid magnetic separation, with an adsorption time of just 10 minutes for PCBs. The Fe3O4/mSiO2–Me–PTSA showed high enrichment factors for PCBs (119 to 147) and an adsorption efficiency of 46.3 mg g−1. The process demonstrated promising outcomes with recoveries between 85.25% and 118.60% and low LODs ranging from 0.16 to 0.91 ng L−1.114 Wu et al. synthesized a polymorphic graphene-like porous carbon with N-doping/Ni magnetic nanohybrids. The NC@Ni-1 sample demonstrated exceptional long-term cycle stability, exceptional rate performance, and high specific capacity. The NC@Ni-0.75 sample showed a high adsorption capacity for MG dye (889 mg g−1). It also demonstrated a distinctive magnetic separation characteristic, making it suitable for the adsorptive removal of cationic MG dye.115
Choudhry et al. discuss synthesizing magnetic flax seeds (MFS), a unique hybrid nanocomposite based on iron oxide and flax seeds for water treatment. A straightforward co-precipitation technique was used to create the composite. With an equilibrium adsorption capacity of roughly 10 mg g−1 and the highest adsorption capacity (Qmax) of 52.91 mg g−1, the experimental results revealed that MFS successfully absorbed over 90% of malachite green dye (MG) from a ten mg L−1 solution at 30 °C at neutral pH in 15 minutes. Weak electrostatic and hydrogen bonding interactions drove the adsorption process, which followed the Freundlich isotherm, indicating multilayer physisorption. The rate-determining phase was intraparticle diffusion. According to kinetic analyses, the pseudo-second-order model described MG adsorption. While MFS proved effective for water treatment, the study suggests further investigation of desorption and regeneration for future applications.107
Elanchezhiyan et al. effectively synthesized zirconium-imprinted manganese ferrite (ZrMnFe2O4@rGO) nanohybrids based on rGO and assessed their capacity to adsorb PFOA and PFOS. As validated by Raman spectroscopy, appropriate ID/IG ratios demonstrated the intercalation of Zr–MnFe2O4 nanoparticles within rGO. Interactions with the rGO sheets decreased the Zr–MnFe2O4 nanohybrids' saturation magnetization. According to kinetic studies, the highest adsorption efficiency for PFOA and PFOS on Zr–MnFe2O4@rGO was achieved in less than 12 hours. The adsorption course was pH-dependent for both pollutants and adhered to a pseudo-second-order kinetic model. The Langmuir model, with maximum adsorption capacities of 10.1 mg g−1 for PFOA and 12.6 mg g−1 for PFOS, mainly attributable to electrostatic and hydrophobic interactions, was found to have the best fit to the experimental data, according to adsorption isotherm analysis. Notably, the adsorption density of Zr–MnFe2O4@rGO remained unchanged after four consecutive recycling tests, with no observable mass loss of the material.116
Muntean et al. synthesized carbon magnetic nanocomposite and used it as an adsorbent to extract heavy metal ions from aqueous solutions, including copper, lead, and zinc. Working at the natural pH of 5.8 for 240 minutes and utilizing a dose of 1 g L−1 of the nanocomposite were the ideal circumstances for the process, which produced high removal efficiencies of 81.36% for copper, 84.50% for lead and 72.68% for zinc ions. The investigation results showed that these parameters were appropriate for effective heavy metal ion removal operations employing the carbon magnetic nanocomposite material since they struck a good balance within removal rate, practicability, and adherence to ecological and socioeconomic issues.124 The chitosan matrix can promote the growth of MNPs and, at the same time, hinder their aggregation. Tolessa et al. synthesized two μm magnetic chitosan hybrid materials through suspension cross-linking to eliminate Ag NPs. They suspended MNPs in 1% chitosan solution and added toluene. The mixture was stirred at 500 rpm for half an hour; then, the solutions of NaOH and glutaraldehyde were added. The magnetic chitosan composite material was separated from the solution with the help of an external magnet. Alongside inductively coupled plasma-mass spectrometry (ICP-MS), the material provided low LODs for Ag NPs, from 0.016 to 0.023 μg L−1. The high removal efficiency of Ag nanoparticles, between 84.9% and 98.8%, is due to the positive charges on the chitosan's surface, making it an excellent adsorbent. The magnetic chitosan hybrid material remains effective after three recycling uses, with an efficiency of approximately 77.2 ± 2.2%.125
In another study, a core–shell Fe3O4@RF@mTiO2 material was developed and tested as an MSPE sorbent for removing arsenic from highly acidic samples by Zhao et al. The material featured a 130 nm Fe3O4 core, a 50 nm resorcinol–formaldehyde (RF), and a mesoporous TiO2 shell. This design provided quick adsorption, which is 1.16 g mg−1 h−1 and a 139 mg g−1 adsorption capacity. The RF layer offered hydrophobic protection to the Fe3O4 core against acid etching, while the mesoporous TiO2 enabled surface complexation and electrostatic interactions with arsenate. The magnetic properties allowed facile separation and recycling using an external magnetic field. This multi-layer material shows promise for long-term wastewater treatment.126 The adsorption capabilities of magnetic iron oxide nanoparticles for heavy metals, including Pb, Cd, Cu, Zn, and Co, in wastewater treatment procedures are significantly increased by modification with chemicals such as thiosalicylhydrazide. Hybrid magnetic nanohybrid materials have been synthesized to overcome the limits of original magnetic nanoparticles in water remediation and to advance heavy metal removal efficiency by combining magnetic capabilities with other compositions. Magnetic nanohybrid materials with enhanced adsorption capabilities and practicality for environmental remediation applications have been developed due to hybridization operations' new compositions and chemical structure alterations.127
A flower-like magnetic MoS2/Fe2O4 nanohybrid has been successfully synthesized by Wang et al. and used for the removal of Pb(II) and Hg(II) from contaminated wastewater and soil. The nanohybrid was highly efficient in removing heavy metal ions, with rates of 99.56% for Pb(II) and 99.08% for Hg(II). PH, contact time, initial metal ion concentration, and temperature significantly influence the removal efficiency. The nanohybrid also demonstrated excellent reusability after multiple adsorption and desorption cycles, indicating its economic and environmental sustainability. The study concludes that the flower-like magnetic MoS2 nanohybrid is a promising material for efficient remediation of these contaminants, with its high removal efficiency, reusability, and sensitivity to various parameters making it a valuable tool for environmental cleanup processes.128 Using a facile approach, Dai et al. synthesize a ternary adsorbent, PEHA–Phos–GO/MnFe2O4. This material showed improved Pb(II) adsorption properties compared to GO because of the large number of adsorption sites offered by the amino and phosphate groups. Experimental data showed that the adsorption process depended on the pH and involved pseudo-second-order, which pointed to chemisorption as the rate-controlling step. The data obtained from the Langmuir isotherm agreed with the experimental data. The maximum adsorption capacity for Pb(II) was 366.4 mg g−1 at pH 5.5, which was higher than that of pristine GO, 212.1 mg g−1. Recycling tests further supported the higher capacity of regeneration of PEHA–Phos–GO/MnFe2O4, and the work demonstrated the possibility of applying the synthesized material in water treatment processes, as shown in Fig. 3.129
Pirhaji et al. successfully synthesized nano NiFe2O4/HNTs/GQDs as a novel adsorbent for Pb(II) removal (Fig. 4). Batch experiments revealed that its strong adsorption performance is mainly due to complexation interactions between Pb2+ ions and the functional groups on the adsorbent. The Box–Behnken design and response surface methodology determined optimal experimental parameters, achieving a maximum Pb removal efficacy of 97.14%. The Langmuir isotherm model best explained the adsorption behavior, with a Qmax of 42.02 mg g−1 at 25 °C. The Elovich model suggested chemical adsorption and kinetic studies indicated that the process followed a pseudo-second-order model. The thermodynamic analysis confirmed that the adsorption is endothermic and spontaneous, making NiFe2O4/HNTs/GQDs a cost-effective and efficient option for Pb(II) removal.130
 | ||
| Fig. 4 Synthesis of NiFe2O4/HNTs/GQDs and plausible adsorption mechanism of Pb(II) ions onto NiFe2O4/HNTs/GQDs. This figure has been adapted from ref. 130 with permission from Elsevier, copyright 2020. | ||
A new magnetic solid-phase extraction (MSPE) approach using Ni–Al layered double hydroxide/magnetite (Ni–Al LDH/Fe3O4) nano sorbent was synthesized by Abdolmohammad-Zadeh et al. for the enrichment and separation of Mn(VII)/Mn(II) in water samples by flame atomic absorption spectrometry. The nanosorbent selectively adsorbs Mn(VII) because of the electrostatic attraction and intercalation of MnO4− ions into the LDH layers. Mn(II) is quantified indirectly by calculating the difference between the total Mn and Mn(VII) concentrations. The nano-hybrid is paramagnetic, allowing simple separation using an external magnet without filtration or centrifugation. The nanosorbent is non-toxic, recyclable more than 100 times, and offers high sensitivity, accuracy, and selectivity for Mn speciation in water samples.131
In another study, Katubi et al. focused on synthesizing the MnFe2O4/GO nanocomposite using an in situ method for the adsorption of Pb2+ ions in water. The adsorption parameters, including initial concentration, adsorbent dose, pH, and reaction time, were determined to identify the best condition for adsorption. The study revealed that with the increase in the initial concentration of Pb2+ ions, the adsorption capacity ranged between 63 and 625 mg g−1. Also, the extent of removal increased with the increase in the adsorbent dose from 39% to 98.8% as the number of active sites increased. The results also revealed that the adsorption process was most effective at pH 6. The adsorption capacity increased sharply during the first 15 minutes and then progressively at a slower rate as the surface became fully occupied, the maximum contact time being 120 minutes. The study also exhibited that Pb2+ adsorption fitted the Langmuir isotherm model, which supports monolayer adsorption with a maximum 636.94 mg g−1 adsorption capacity. Moreover, reusability tests proved the stability and efficiency of the nanocomposite for at least five cycles, thus making it an efficient and cost-effective adsorbent for Pb2+ ions in water treatment.132 rGO was synthesized utilizing the modified Hummers' method, and ZnFe2O4 nanoparticles were incorporated onto the surface of rGO to fabricate the adsorbent material to remove lead ions. Experimental parameters, including the amount of the adsorbent, initial concentration of the pollutant, and pH, were thus adjusted to maximize the pollutant removal efficiency. The developed rGO nanocomposite had an adsorption capacity of 89.8 mg g−1 for lead ions. Vacancy defects and oxygen-containing functional groups on the rGO surface were identified as the primary mode for pollutant elimination. Adsorption was consistent with the Langmuir isotherm and fitted a pseudo-second-order kinetic model, suggesting that the adsorption process was chemisorptive and through pollutant monolayers. The synthesized rGO nanocomposite was stable and reusable for up to five cycles, which is suitable for application in heavy metals and dye removal in the water treatment process.133
In another work, coprecipitation and Hummer's modified method were combined to synthesize the magnetic Fe3O4–GO nanohybrid composite material. The morphology of the hybrid sample demonstrated that the diffusion of Fe3O4 nanoparticles into the mesoporous GO layers of porous channels limited GO nanosheet restacking and stopped magnetic nanoparticle leaching and agglomeration. The pseudo-second-order kinetic and Langmuir isotherm models suit the results of the kinetic and isotherm analyses conducted to assess the adsorption mechanism quite well. After 60 minutes, the As(V) adsorption efficiency, H, peaked at 99.37%. The GO/Fe3O4 nanohybrid material in an acidic media showed a Qmax of 14.1 mg g−1. Further research was also done on the function and contribution of GO and Fe3O4 nanoparticles in adsorptive capacity and the enhancement of As(V) adsorption efficiency.134
3.2 MNPs for the cleanup of oil spills
NH2-functionalized magnetic materials can be used for extracting oil droplets from water.135 High magnetization and a large surface area are required for nanocomposites to have a strong oil absorption capability.136 Atta et al.137 synthesized a novel class of magnetic sorbents by employing the co-precipitation approach to coat magnetite nanoparticles with rosin amidoximes, which produced a hydrophobic coating on the surface of Fe2O3 nanoparticles. This hydrophobic coating can increase the oil uptake selectivity, stop Fe2O3 nanoparticles from oxidizing, and prevent magnetic nanoparticles from aggregating. By comparing iron oxide coated with RK-AN amidoxime to that coated with R-AN and bare Fe3O4 nanoparticles, oil recovery measurements indicate that the latter is a worse oil sorbent. Fe3O4 nanoparticles coated with RK-AN amidoxime exhibit a higher level of oil removal than those coated with R-AN amidoxime, as shown by their respective magnetization saturation values of 60.9 and 70.8 emu g−1.Using the unique physicochemical characteristics of ZnO-T, PDMS, and Fe2O3-NR, a magnetic nanosorbent was effectively synthesized by Sharma et al. in 2019. With a WCA of 157°, the as-synthesized PZF nanosorbent exhibited superhydrophobic super oleophilic properties. With an oil removal (%) of 96% and an adsorption capacity of 1135 mg g−1, respectively, this magnetic nanosorbent efficiently separates diesel oil from water. The kinetics of oil adsorption showed that diesel oil adsorptive capacity on PZF nanosorbent is high, with equilibrium potentially reached in as little as 50 minutes. The pseudo-first-order kinetics and Langmuir isotherm models matched the oil adsorption process well. The simple synthesis process and the superhydrophobic–superoleophobic and magnetic responsive characteristics of the as-prepared nano sorbent offer an alternate method for treating oily wastewater.138 Shao and Yu successfully made a sepiolite/graphene oxide (Sep/GO) membrane for wastewater treatment. The excellent oil/water selectivity and low oil adherence of the synthetic Sep/GO membranes made them highly successful in separating oil-in-water emulsions. Moreover, the membrane preserved its underwater high stability and superoleophobicity in severe settings. As a result, the Sep/GO membrane works well in processes involving wastewater treatment and oil/water separation.139
Qian and Chen described a TiO2/sulfonated GO/Ag NPs membrane with beneficial photocatalytic and wettability capabilities.140 When solubilized methylene blue (MB) was exposed to UV light, the synthesized membrane demonstrated good photodegradation and an effective oil/water separation performance. As a result, the membrane has interesting uses in wastewater treatment and oil/water separation.140 Zhang et al. synthesized a superoleophilic and oil–superhydrophobic composite nanofibrous membrane (GPNM) for gravity-driven surfactant-stabilized water-in-oil emulsions using a straightforward one-step electrospinning technique. The synthetic composite membrane demonstrated a remarkable separation efficiency of 99.8% for water-in-oil emulsions. These findings revealed that the composite nanofibrous membrane could potentially treat oil-contaminated wastewater.141
In another study, Cheng and Barras effectively created a superoleophilic/superhydrophobic rGO–polydopamine membrane functionalized with 1H,1H,2H, and 2H-perfluorodecanethiol (rGO–PDA–PFDT) using a straightforward two-step procedure. The rGO–PDA–PFDT membrane was utilized to clean water by separating oil. The membrane's superhydrophobicity and superoleophilicity allowed it to extract chloroform from water. Therefore, in oil/water separation operations, the synthesized rGO–PDA–PFDT membrane is exceptionally favorable.142 Shokry et al. synthesized a novel magnetic sorbent material from water hyacinth to remove oil from polluted water. The chemical and thermal treatment established that the root segment of the water hyacinth had a higher oil adsorption capacity than the shoot segment. The comparison of the two segments with nano-activated carbon and NMAC (nano-magnetic activated carbon) showed that NMAC had a more crystalline structure, better pore structure, and thermal stability. It also registered a saturation magnetization of 17.2587 emu g−1. Oil removal efficiency increased with the solution temperature and NMAC dosage increase. The results indicated that the Freundlich isotherm model best described the oil adsorption process rather than the Langmuir isotherm and Dubinin–Radushkevich models, implying monolayer adsorption and surface heterogeneity. The pseudo-second-order and intra-particle diffusion models were utilized to determine adsorption kinetics. The primary process through which oil was adsorbed on NMAC was physical absorption through van der Waals forces.143
Sharma et al. investigated the physicochemical characteristics of ZnO-T, PDMS, and Fe2O3-NR to give large surface area, low surface energy, and super-paramagnetism, respectively, in synthesizing a magnetic nanosorbent. The PZF nanosorbent exhibited superhydrophobic and superoleophilic properties, exhibiting a WCA 157°. With an oil removal percentage of 96% and an adsorption capacity of 1135 mg g−1, respectively, this magnetic nanosorbent efficiently separates diesel oil from water. According to the oil adsorption kinetics, diesel oil can quickly adsorb on PZF nanosorbent, reaching equilibrium in as little as 50 minutes. The Langmuir isotherm and pseudo-first-order kinetics models fit the oil adsorption process well. The easily synthesized nanosorbent's superhydrophobic and superoleophilic properties, combined with its magnetic responsiveness, offer a novel approach to treating oily wastewater.138
3.3 MNPs for photocatalytic degradation of organic dyes
Among the various pollutants, waterway dyes are recognized as a global ecological concern that can impact humans, plants, and animals. Materials that are widely studied for the photocatalytic degradation of organic dyes found in water effluents are magnetic nanohybrids. Organic dye degradation via photocatalysis using magnetic nanohybrid materials follows the mechanism shown in Fig. 5.Yuan et al. prepared Fe3O4@SiO2@meso-TiO2 nanocomposites with a core–shell architecture by a simple and efficient method with increased photocatalytic performance. The magnetic core, SiO2 interlayer, and mesoporous TiO2 outer shell constitute the as-made core–shell architecture. It was produced with TiOSO4 as the titanium source and cetyltrimethylammonium bromide (CTAB) as a pore-forming agent. Fe3O4@SiO2 was made using a sol–gel method. In comparison to Fe3O4@SiO2@solid-TiO2, the Fe3O4@SiO2@meso-TiO2 composite showed better photocatalytic efficiency for the degradation of Rh B in aqueous suspension due to the large BET surface area produced by the mesoporous TiO2 shell.144 Aliyan et al. have explained the immobilization of Fe3O4 to modify the surface of meso-structured silica (SBA-15) to produce a nanocatalyst. Aliyan et al. used a UV lamp as a light source to investigate the catalytic activity of Fe3O4@SBA-15 toward the photodegradation of MG in a photo-catalytic reactor. The impact of several experimental factors on the process's degrading performance was assessed by analyzing catalyst dose, initial dye concentration, and dye solution pH in the presence of Fe3O4@SBA-15 as a photo-catalyst. Even after five MG degradation cycles, the described photocatalyst showed notably high catalytic stability and low activity loss.145 Sahoo et al. investigated the adsorption of methyl orange (MO) on monodispersed magnetic mesoporous manganese ferrite composites with a size of around 200 nm. Sonication and photolysis in the presence of sunshine improved the MO's degrading efficiency. The authors concluded that their catalyst exhibited adsorption, degradation, recovery, and reusability in a single system. The electron transfer activities inside the MnFe2O4 particles cause the potential dye degradation reaction. The following describes the potential method by which Mn2+ ions decompose H2O2.146 Yonghong Ni et al. found that adding Mn2+ to TiO2 nanoparticles increased their photocatalytic activity and that the metal ions, such as Fe2+, actively contributed to the degradation of H2O2 to produce OH radical species.147
| Mn2+ + H2O2 → Mn3+ + HO˙ + OH− |
| H2O2 + HO˙ → H2O + HOO˙ |
| Mn3+ + HOO˙ → Mn2+ + H+ + O2 |
| Fe3+ + HOO˙ → Fe2+ + O2 + H+ |
The organic dye (RH) reacts with the O2 molecule and OH produced above.
| R–H + HOO˙ → X + H2O + CO2 |
Santos et al. prepared the magnetic CoFe2O4/TiO2 composite through a facile solvothermal route. Compared with pristine TiO2, the CoFe2O4/TiO2 catalyst had a reduced band gap, allowing it to exhibit higher photocatalytic efficiency. The nanohybrid catalyst showed exceptional activity in degrading Erionyl Red A-3G dye, with 69.4% TOC eradication after 120 minutes and 95.39% color removal after 15 minutes of reaction. One possible explanation for the degradation of Erionyl Red A-3G is that when the CoFe2O4/TiO2 composite is exposed to UV light, the TiO2 in the composite absorbs light and produces more charges. As a result, holes (h+) are formed on the valence band (VB) of TiO2 due to an electron transfer (e−) from VB to CB. A successful e−/h+ pair separation is subsequently promoted by the photogenerated electrons (e−) migrating from the CB of TiO2 to that of CoFe2O4, which helps to limit photogenerated charge recombination and enhance photodegradation activity. The e− on the CB of CoFe2O4 reacts with O2 to produce O2˙− radical, which destroys pollutants. While the h+ on the VB of TiO2 directly oxidizes organic molecules and combines with OH− or H2O to produce ˙OH radical, which also functions as an oxidizing species.148
| TiO2 + hν → e− + h+ |
| CoFe2O4 + TiO2 → CoFe2O4 + TiO2 (e−) |
| e− + O2 → O2˙− |
| O2˙− + Erionyl dye → degradation products |
| OH˙ + Erionyl dye → degradation product |
Rehman et al. synthesized Fe3O4@GO by modifying 3-aminopropyl triethoxysilane (APTES) and tetraethyl orthosilicate (TEOS) using a mechanical stirring technique. Under UV light, the synthesized nanocomposite was evaluated as a possible heterogeneous catalyst for methylene blue (MB) degradation. Over time, the catalysts' photocatalytic activity increased progressively. The Fe3O4@GO composite catalyst had the highest MB removal efficiency of 70.06%, which was more excellent than pure Fe3O4 (57.56%).149 Vigneshwaran et al. reported that co-precipitation created the Fe2O3-reinforced chitosan (Fe2O3@CS) nanocomposite. The surface of the chitosan was made available to develop a beneficial nanocomposite. Because of the increased partition efficiency and quicker transfer of the photo-generated charge carriers, these Fe2O3 wrappings on chitosan offer synergistically enhanced characteristics. MO and OG dyes were degraded using this combination. According to the data, the most significant degradations for MO and OG were 89.2% and 94.6%, respectively. The trapping study highlighted how OH radicals contributed to dye degradation over Fe2O3@CS composites. The suggested method used Fe2O3@CS to oxidize anionic MO and OG dyes, as shown in Fig. 6.
| Fe2O3@CS → hν (h+ + e−) Fe2O3@CS |
| (e− + h+) → O2˙− + OH˙ |
| O2˙− + H+ → OH˙ |
| OH˙ + OH˙− → H2O2 |
| H2O2 + e− → OH˙ + OH− |
| h+ + H2O → OH˙ + MO + OG → by-products + HO2 + CO2 |
 | ||
| Fig. 6 Illustration of MO and OG dye molecule oxidation after e−–h+ production on a Fe2O3@CS photocatalyst. | ||
Furthermore, the production of reactive species increases quickly due to the e−–h+ pair's delayed recombination. It has a significant impact on the materials' photocatalytic efficiency.150
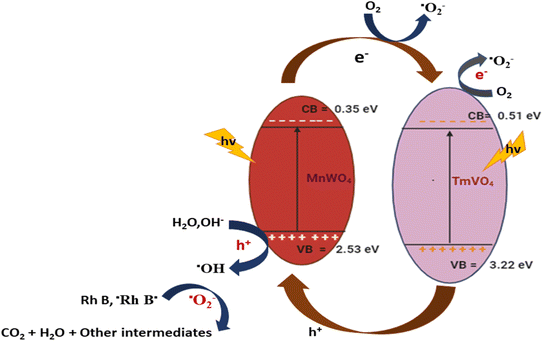
Sobhani-Nasab et al. successfully created a novel MnWO4/TmVO4 ternary nanohybrid using a straightforward sonochemical technique. Under visible light, the degradation of phenol red (Ph R), eosin Y (EY), 2-naphthol, and rhodamine B (Rh B) by MnWO4/TmVO4 was examined. The corresponding photocatalytic efficiencies for Rh B, Na, Ph R, and EY are 99.2%, 93.3%, 88.3%, and 83.1%.151 When MnWO4/TmVO4 is excited by visible light, the photogenerated holes on the VB of TmVO4 will move to MnWO4, while the photoinduced electrons on the CB of MnWO4 will migrate to TmVO4 (depicted in Fig. 7). The heterojunction forms relatively tiny amounts of  and OH˙. The degradation results demonstrate that MnWO4/TmVO4 has a greater photodegradation capacity than pure TmVO4 or MnWO4.152
and OH˙. The degradation results demonstrate that MnWO4/TmVO4 has a greater photodegradation capacity than pure TmVO4 or MnWO4.152
Roostaee et al. investigated the reduction process by sodium citrate, Hummer's method, and hydrothermal approach was used to synthesize Fe3O4@Au core–shell nanoparticles decorated on rGO nanocomposite, respectively. These nanostructures were used to break down crystal violet (CV) by photocatalysis. Under ideal circumstances and visible light irradiation, it was found that the Fe3O4@Au–rGO performed better than Fe3O4@Au and rGO alone. In the presence of 0.008 g of the produced nano-photocatalyst, 100% degradation was obtained after 1 minute, and in the presence of 0.005 g, 100% degradation was obtained after 5 minutes.153 Because rGO can function as an effective electron transporter for separating photo-generated electron–hole pairs through interfacial charge transfer, Fe3O4@Au–rGO demonstrated remarkable photocatalytic activity to destroy crystal violet.154
3.4 MNPs for photocatalytic degradation of emerging contaminants
Emerging contaminants (EC) are new types of pollutants recently introduced into aquatic systems that harm the environment and the health of living things. Personal care products (PPCPs) and medications are prominent examples of emerging pollutants.155 Since PPCPs are more difficult to degrade than other organic contaminants, magnetic nanohybrids are an excellent option for eradication.156 Using the sol–gel technique, Rostami et al. synthesized ZnFe2O4–4 weight percent GR nano-hybrids. It is evident that when exposed to visible light, ZnFe2O4 itself is nearly photo-catalytically inert; however, the addition of graphene significantly improves photocatalytic activity. Under exposure to visible light, the ZnFe2O4–x weight percent GR nano-hybrids demonstrated enhanced charge separation and nano-photocatalytic activity towards PARA decomposition. Fig. 8 is a potential mechanism for increasing nano-photocatalytic activity.157| ZnFe2O4 + hν → ZnFe2O4 (h+ + e−) |
| ZnFe2O4 (e−) + graphene → ZnFe2O4 + graphene (e−) |
| Graphene (e−) + O2 → O2˙− + graphene |
| ZnFe2O4 (h+) + OH− → ZnFe2O4 + OH˙ |
| ZnFe2O4 (h+) + OH˙ + O2˙− + PARA → intermediate products + CO2 + H2O |
 | ||
| Fig. 8 A graphical representation of PARA's photocatalytic degradation over the ZnFe2O4–x wt% GR nano-hybrid. | ||
Tang et al., using a hydrothermal process, prepared rGO–CdS/ZnS heterostructure composites that have been effectively synthesized by building the CdS/ZnS heterostructure nanoparticles on rGO sheets. In contrast, GO is reduced at the same time. With 15% RGO content, the rGO–CdS/ZnS composites, as synthesized, show very active TC photodegradation (about 85%). When RGO is added, the rGO–CdS/ZnS heterostructure photocatalysts show less electron–hole pair recombination than CdS/ZnS, facilitating better charge-carrier migration and photocatalytic activity.158 The charge transfer and photocatalytic activity by RGO–CdS/ZnS composites are schematically illustrated in figure. The production of holes in the VB is caused by the very initial excitation of the electron–hole pairs in CdS, which stimulates the electrons to the conduction band (CB). Because of the band potential, the electrons in the CB of CdS may be immediately transported into the CB of ZnS. The potential of ZnS is around 0.91 eV, which is less negative than the CB level of CdS (about 0.95 V). Additionally, RGO may trap the free photo-generated electrons in the ZnS CB, which are linked to the 2D carbon network. Thus, the visible-light activity was significantly produced by the photo-generated electrons of ZnS and RGO being so active that they participated in the surface reaction to make radicals.159
To degrade amoxicillin (AMX), a new MIL-68(In)-NH2/GO composite was prepared as a visible-light-driven photocatalyst. Compared to pure MIL-68(In)-NH2, the MIL-68(In)-NH2/GO composite demonstrated much greater photocatalytic activity. After 120 and 210 minutes of irradiation, respectively, 93% degradation and 80% TOC removal toward AMX were possible using 0.6 g per L MIL-68(In)-NH2/GO and pH = 5. The modification by employing GO, which worked as a sensitizer to increase visible light absorption in addition to acting as an electron transporter to reduce photogenerated carrier recombination, is responsible for the increased activity for MIL-68(In)-NH2/GO.160 The photocatalytic mechanism for the degradation of AMX is shown in Fig. 9.
 | ||
| Fig. 9 Photocatalytic mechanism of MIL-68(In)-NH2/GO composite. This figure has been adapted from ref. 160 with permission from Elsevier, copyright 2017. | ||
MIL-68(In)-NH2/GO's ECB and EVB have been estimated to be −0.40 and 2.03 eV vs. NHE, respectively. The photogenerated electrons on the CB of MIL-68(In)-NH2 are beneficial for reducing the O2 because the ECB of MIL-68(In)-NH2/GO (−0.40 eV) is more damaging than the O2/˙O2− potential (−0.046 eV vs. NHE).161 Similarly, these electrons can also reduce O2 to H2O2 (O2/H2O2 is 0.915 eV vs. NHE), and by trapping one electron, the resulting H2O2 can be further converted into ˙OH. Due to the lower positive potential of MIL-68(In)-NH2/GO's VB (2.03 eV) compared to the typical ˙OH/OH− (2.38 eV vs. NHE), the photogenerated holes on the VB of MIL-68(In)-NH2/GO can simultaneously oxidize the AMX directly, but not the OH− to form ˙OH.162 Bastami et al. reported that magnetic Fe3O4/Bi2WO6 nanohybrids were created in two steps. A solvothermal technique was used to develop Fe3O4 nanospheres in a polyol medium, and a subsequent hydrothermal process produced Bi2WO6 nanocrystals. Tungstophosphoric acid hydrate (H3PW12O40) was used as an acidic agent in the Bi2WO6 nanoparticle manufacturing. Ibuprofen (IBP) was photo-catalytically degraded from an aqueous solution under sunlight to assess the photocatalyst's activity. Particularly at pH 4.7, the as-prepared nanohybrid demonstrated significant efficiency in the photocatalytic destruction of IBP and was readily recovered by a magnet.163 The OH˙ radical may be produced due to a chain reaction when hydrogen peroxide comes into contact with Fe(III) sites on the surface of the Fe3O4/Bi2WO6 photocatalyst.164 First, the photocatalyst surface produces the Fe(III)H2O2 complex. Then, the generated species is transformed into Fe(II) and  . Additionally, Fe(II) species are produced on the photocatalyst surface. Every type of Fe(II) species produced on the photocatalyst surface has the potential to react with H2O2 and create reactive radicals (
. Additionally, Fe(II) species are produced on the photocatalyst surface. Every type of Fe(II) species produced on the photocatalyst surface has the potential to react with H2O2 and create reactive radicals (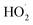 and HO˙) (Fig. 10).
and HO˙) (Fig. 10).
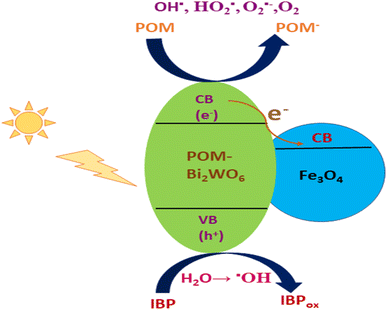
The Fe3O4/Bi2WO6 photocatalyst's polyoxometalate-based materials (POM) are considered to increase photo-efficiency by inhibiting the rapid recombination of a charge pair (h+–e−) on nanohybrids and generating a potent oxidant  .165 When sunlight irradiation excites Fe3O4/Bi2WO6, the electron shifts from its conducting band to the POM's LUMO; following the attack of radicals on organic molecules, the adsorbed O2 and H2O2 can readily trap an electron in the LUMO of the POM anion to produce the oxidizing species OH˙. Consequently, the enhanced photocatalytic activity can slow down the rapid recombination of photo-induced electrons and holes. Sayadi et al. produced a novel Ag–CuFe2O4@WO3 magnetic photocatalytic nanosystem and examined how well it degraded two drug pollutants, gemfibrozil (GEM) and tamoxifen (TAM), when exposed to UV light. At pH 5, a photocatalyst dosage of 0.2 g L−1, a drug concentration of 5 mg L−1, and a contact time of 150 minutes, the highest levels of photodegradation for GEM (81%) and TAM (83%) were attained. Excellent catalytic efficiencies were produced by the slow electron–hole recombination brought about by the photocatalyst's heterostructure. The following reactions can be used to characterize the primary mechanism of the drug models' photodegradation process, and the mechanism can be seen in Fig. 11.166
.165 When sunlight irradiation excites Fe3O4/Bi2WO6, the electron shifts from its conducting band to the POM's LUMO; following the attack of radicals on organic molecules, the adsorbed O2 and H2O2 can readily trap an electron in the LUMO of the POM anion to produce the oxidizing species OH˙. Consequently, the enhanced photocatalytic activity can slow down the rapid recombination of photo-induced electrons and holes. Sayadi et al. produced a novel Ag–CuFe2O4@WO3 magnetic photocatalytic nanosystem and examined how well it degraded two drug pollutants, gemfibrozil (GEM) and tamoxifen (TAM), when exposed to UV light. At pH 5, a photocatalyst dosage of 0.2 g L−1, a drug concentration of 5 mg L−1, and a contact time of 150 minutes, the highest levels of photodegradation for GEM (81%) and TAM (83%) were attained. Excellent catalytic efficiencies were produced by the slow electron–hole recombination brought about by the photocatalyst's heterostructure. The following reactions can be used to characterize the primary mechanism of the drug models' photodegradation process, and the mechanism can be seen in Fig. 11.166
| O2 +e− → O2˙− |
| O2˙− + 2H+ → H2O2 |
| OH− + h+ → OH˙ |
| H2O + h+ → OH˙ + H+ |
| TAM–GEM + O2˙− + H2 + OH + h+ → H2O + CO2 + C3H10NO4 + C6H14O + CH3COOH + C6H10O |
Xie et al. synthesized Fe3O4/BiOI magnetic photocatalyst to break down sulfadiazine sodium, which is used to treat infectious disorders. As mentioned above, the photocatalyst activity was about 80%. It was discovered that adding iron oxide to the nanocomposite enhanced the separation of electrons and holes and gave it magnetic properties that made it easier to separate after drug degradation.167 Kumar et al. reported N–TiO2@SiO2@Fe3O4 magnetic nanocomposite for prescription photodegradation ibuprofen, carbamazepine, and benzophenone-3 (UV filter). The combination exhibited superior degradation against ibuprofen (94%), benzophenone-3 (93%), and carbamazepine (71%). The remarkable photodegradation efficiency has been ascribed to the modification of the electronic band structure caused by nitrogen doping. Following the degradation phenomena, the nanocomposite produced easy separation (95% in 20–25 minutes) under an external magnetic field.168 Khan et al. described the synthesis of a magnetic photocatalyst BiOBr/Fe3O4@SiO2 by solvothermal method for large-scale ibuprofen decontamination. Ibuprofen was nearly wholly (99%) degraded, and the nanocomposite was recycled using an electromagnet in under five minutes, demonstrating the photocatalyst's high stability.169 Additionally, Fe3O4/ZnO has been synthesized to eliminate four distinct antibiotics. All four antibiotics showed photocatalytic efficiencies of greater than 90%. Additionally, it was noted that the composite has comparable photocatalytic capability in subsequent reuse cycles, indicating its stability and reusability.170
Nasseh et al. reported a magnetic FeNi3@SiO2@ZnO nanocomposite to remove tamoxifen from wastewater successfully. ZnO was the primary component of this composite because of its appropriate bandgap. FeNi3 was a magnetic core, and the silica layer shielded the magnetic core from oxidation and light.171 There have been reports of the photocatalytic breakdown of anticancer medications using the magnetically separable nanocomposite Fe3O4/Au. Perhaps because of its magnetic iron core, the nanocomposite, as mentioned above, was easily separated by a simple magnet. However, gold nanoparticles stopped iron oxide from oxidizing and clumping together. After five cycles, the photocatalyst demonstrated extraordinary reusability and outstanding photocatalytic effectiveness for imipenem (84%) and imatinib (82%), both exposed to visible light.172 Ahmadpour et al. synthesized ZnFe2O4@TiO2/Cu using a solvothermal process and applied it against naproxen photodegradation. Comparing the ZnFe2O4@TiO2/Cu (80.73%) nanocomposite to ZnFe2O4@TiO2 (73.81%) and ZnFe2O4 (50.58%), the former demonstrated greater photoactivity. Cu particles have been associated with increasing ZnFe2O4@TiO2/Cu's photocatalytic effectiveness by decreasing electron/hole pair recombination and producing more free radicals.173
Wang et al. described the SnFe2O4/ZnFe2O4 photocatalyst that is active in visible light for the degradation of TC from aqueous media. Because of its ideal magnetic saturation value of 24.8 emu g−1, this magnetic heterojunction photocatalyst demonstrated 93.2% degrading ability and simple separation from aqueous solution. According to the theorized process, the holes in the valence band of SnFe2O4 were neutralized by the photoinduced electrons in ZnFe2O4, and these electron–hole pairs produced potent radicals that degraded tetracycline.174 Xu et al. revealed a Fe3O4/BiVO4/CdS nanocomposite that is visible light active and can photocatalytically break down tetracycline. They found that combining BiVO4 and CdS reduced the rate at which photoinduced charge carriers reacted, improving the nanocomposite's capacity to absorb light and increasing its surface area. On the other hand, Fe3O4 loading enabled its high separation via the magnetic field. These characteristics in one material have been linked with exceptional photocatalytic performance (87%) against tetracycline. Even after five runs, the stability and recyclability were determined to be above 81% in catalytic efficiency.175
Gabelica et al. showed that magnetite-based MNPs were effectively created utilizing a quick microwave-assisted synthesis method. The Fe3O4/SiO2/TiO2 nanocomposite was a potential option for ciprofloxacin degradation. After three consecutive replicates, Fe3O4/SiO2/TiO2 showed a remarkable 94% photodegradation efficiency and remained intact. The loading of Fe3O4 also confirmed the straightforward catalyst recovery using a magnet.176 ZnFe2O4/carbon derivatives (ZnFe2O4@CNTs, ZnFe2O4@GO, and ZnFe2O4@fullerene) have been reported for the photocatalytic degradation of norfloxacin. Carbon moieties enhanced light absorption in the visible spectrum because of the carbon derivatives' conjugated π–π structure and efficient electron trapping. However, ZnFe2O4@carbon nanotubes exhibited the highest degradation efficiency (91.36%) against norfloxacin because of the outstanding charge carrier separation efficiency brought about by the good interfacial connection between ZnFe2O4 and CNTs.177 Another visible light-active nanocomposite that had been synthesized for the efficient breakdown of 94% tetracycline is Fe3O4/g-C3N4/MoO3. The heterojunction creation between g-C3N4 and MoO3 has been attributed to this exceptional efficiency, as it has improved the segregation of charge carriers and increased the production of active species.178 Using a hydrothermal process, a different magnetic composite (g-C3N4/NiO/ZnO/Fe3O4) was synthesized for the photocatalytic degradation of esomeprazole at 95.05%. The synergistic contribution of the individual composite components that provided the structural and optical alterations is the only reason for this efficiency. The creation of light-induced charge carriers, their separation, and decreased recombination—all of which ultimately resulted in exceptional photocatalytic activity are appropriate for these modifications.179 According to the structural analysis, the synthesized AgCuFe2O4@MC/AC catalyst was created on a nanoscale with a crystalline structure, large surface area, appropriate magnetic characteristics, and optical activity. High efficiency in photodegrading TC, which is 90.91% from synthetic solutions and 87.17% from actual wastewater, was demonstrated by the synthesized catalyst under ideal circumstances (neutral pH, starting TC content of 5 mg L−1, nano-photocatalyst dosage of 0.5 g L−1, one and half hour). The results showed that the heterogeneous magnetic nano-photocatalyst AgCuFe2O4@MC/AC may be utilized successfully to clean wastewater from hospitals and pharmaceutical enterprises because of its strong capacity to degrade antibiotics, notably TC.180 Table 2 represents the MNPs used in different environmental applications.
| MNPs | Contaminant removed | Removal mechanism | Contact time | Removal efficiency | Saturation magnetization (Ms) | Size (nm) | Reusability | Ref. |
|---|---|---|---|---|---|---|---|---|
| Humic acid functionalized Fe3O4 | Carcinogenic malachite green dye | Adsorption | 35 min | 97% | 70.05 emu g−1 | 5–15 nm | Up to five cycles for 85% removal | 181 |
| Combination of Fe3O4 and Fe2O3 | Bromophenol blue (BPB) | Photocatalytic degradation | 60 min | 98% | — | 10–80 nm | Up to three cycles for 95% removal | 182 |
| Superparamagnetic iron-oxide nanoparticles (SPION) and SPION/β-CD | Bisphenol A and malachite green dye | Photocatalytic degradation | 10 min | 82.5% | 68.34 emu g−1 | 10–15 nm | 70% removal for first five cycles | 183 |
| 39.21 emu g−1 | Up to five cycles for 90.13% removal | |||||||
| Epoxy-triazinetrione-functionalized MNPs (Fe3O4-ETT) | Malachite green (MG) | Adsorption | 15 min | 95% | 30.7 emu g−1 | 25–52 nm | Up to six cycles for 61% removal | 184 |
| JC-Fe3O4 and CT-Fe3O4 NPs | Organic dyes | Adsorption | 120 min | 99% | 38.46 emu g−1 | 20–42 nm | — | 185 |
| 34.35 emu g−1 | 26–35 nm | |||||||
| Fe3O4@NiO core shell MNPs | Alizarin red S dye | Adsorption | 60 min | 96.58% | 24.7 emu g−1 | 65 ± 10 nm | Up to six cycles for 43.93% removal | 186 |
| MAPE-mediated iron oxide nanoparticles (MION) | Sunset yellow dye | Adsorption | 180 min | 92% | 44.14 emu g−1 | 20–60 nm | Up to five cycles for 75.4% recovery | 187 |
| Yeast cells immobilized Fe3O4 MNPs | Methyl orange dye | Biosorption | 140 min | 96.52% | — | 87.46–231.4 nm | — | 188 |
| Copper-doped ZrO2 MNPs | Methyl orange dye | Photocatalytic degradation | 100 min | 98% | — | 20–45 nm | Up to four cycles for 90% removal | 189 |
| MCPEI (polyethyleneimine and magnetic nanoparticle) | Black 5 dye | Adsorption | 180 min | 100% | 3.3 emu g−1 (before adsorption) | 1.92 nm (pore size) | Up to five cycles for 48% removal | 190 |
| 3.2 emu g−1 (after adsorption) | ||||||||
| Zeolite/Fe3O4 | Basic violet 16 (BV16) | Adsorption | 45 min | 99% | 18.11 emu g−1 | 1–4 nm | Up to five cycles for 64.17% removal | 191 |
| Fe3O4 MNPs | Methylene blue (MB) | Adsorption | 60 min | 88.8% | 49.48 emu g−1 | 21–32 nm | Up to four cycles for 77% removal | 192 |
| Magnesium oxide modified magnetic nanoparticles (MgO@MNPs) | Eriochrome black T (EBT) dye | Adsorption | 5 min | 98% | 11.70 emu g−1 (before adsorption) | 33.33–50.80 nm | Up to seven cycles for 96% removal | 193 |
| 10.62 emu g−1 (after adsorption) | ||||||||
| Fe3O4 MNPs | Anionic azo dye | Adsorption | 120 min | 99.99% | — | 50 nm | — | 194 |
| Magnetic core–shell Fe3O4@SiO2 nanoparticles | Methyl orange | Adsorption | 30 min | 83% | 72.08 emu g−1 | 120 nm | Reusable up to 5 cycles | 195 |
| Magnesium doped CoFe2O4 NPs | Methylene blue (MB) dye | Adsorption | 30 min | 98.6% | 15.2971 emu g−1 | 7.9 nm | Up to five cycles 82.5% removal for MB and 79.6% removal for RhB | 196 |
| Rhodamine B (RhB) dye | 95.3% | |||||||
| Nanoparticles of titanomagnetite (NTM) | Methylene blue (MB) | Adsorption | 20 min | — | 62 emu g−1 | 35 nm | Reusable for up to three successive cycles | 197 |
| Fe3O4@Ag@MESNa | Hg(II) | Adsorption | 30 s | 100% | — | — | Up to two cycles for 100% removal | 198 |
| Magnetic eggshell membrane (MESM) | Lead | Adsorption | 48 h | 95% | 66 emu g−1 | ∼50 nm | Reusable | 199 |
| Fe3O4@EPS | PO42− | Adsorption | 13 h | 91% | 5.0 emu g−1 | 10–20 nm | — | 200 |
| Magnetic tubular carbon nanofibers (MTCFs) | Cu(II) | Adsorption | 10 min | 99.9 ± 0.1% | 10.65 emu g−1 | 25–110 nm | Up to six cycles for 85% removal | 201 |
| Magnetic nanocomposite activated hydrochar (Fe3O4-ACH) | Cr(VI) | Adsorption | 120 min | 94.1% | 0.110334 emu g−1 | 0.7–1.5 mm | Up to five cycles for 82.7% removal | 202 |
| Cabuya fibers impregnated magnetite nanoparticles (FC–MNPs) and MNPs | Hg(II) | Adsorption | 4 h | 93% | — | 19 nm | — | 203 |
| JC-Fe3O4 and CT-Fe3O4 NPs | Co2+ and Cu2+ | Adsorption | 120 min | 90% | 38.46 emu g−1 | 20–42 nm | — | 185 |
| 34.35 emu g−1 | 26–35 nm | |||||||
| Hyperbranched polyglycerol HPG–MNPs | Ni, Cu and Al | Adsorption | 130 min | 94% | −77 to +77 emu g−1 | 21–30 nm | Up to nine cycles for 90% removal | 204 |
| Chitosan-coated magnetic nanoparticles (cMNPs) | Bio-refinery wastewater containing heavy metals (Cu, Cr, and As) | Adsorption | 90 min | Cu (42.2%), Cr (18.7%), and As (2.44%) | 26.96 emu g−1 | — | Up to five cycles for 20% of initial removal efficiency | 205 |
| Superparamagnetic Fe3O4@SiO2@GLYMO(S)-en | Pb2+ and Cd2+ | Adsorption | 55 min | — | 40 emu g−1 | 16 nm | Up to five cycles for 90% removal | 206 |
| Epoxy-triazinetrione-functionalized MNPs (Fe3O4-ETT) | Pb(II) | Adsorption | 15 min | 95% | 30.7 emu g−1 | 25–52 nm | Up to six cycles for 61% removal | 184 |
| Eucalyptus leaf extracts (EL–MNP@zeolite) | PO42− | Adsorption | 30 min | 99.8% | — | 60 nm | — | 207 |
| NH4+ | 43.3% | |||||||
| MNP@SiO2–PEI–DTPA | Pb2+ | Adsorption | 72 h | >90% | 85 emu g−1 | 25–150 nm | Up to five cycles for 80% removal | 208 |
| Cd2+ | ||||||||
| Nano-chitosan coated MNPs | Pb(II) | Adsorption | 10–30 min | 57.6–95.5% | — | 9.32–20.57 nm | Up to three cycles for 96% removal | 209 |
| Cu(II) | ||||||||
| Cd(II) | ||||||||
| C–CoFe3O4/N, S-BC | Pb2+ | Adsorption | 30 min | 99.1% | — | — | Up to eight cycles with >80% removal efficiency | 210 |
| Fe3O4/CNTs nanocomposites | Pb2+ | Adsorption | 60 min | 91.64% | 8.9 and 16.4 A m2 kg−1 | 2–30 nm | Reusable up to six cycles | 211 |
| Ionic-liquid modified MNPs (Fe3O4@SiO2–[C3C1Im]Cl NPs) | Platinum (Pt) | Adsorption | 15 min | 22–24% | 51 emu g−1 | 65 nm | — | 212 |
| Palladium (Pd) | 1 h | 90–93% | ||||||
| 6 h | ||||||||
| DES/GO–Fe3O4 nanohybrid | Pb(II) | Adsorption | 20 min | 80% | — | 6–30 nm | — | 213 |
| Magnetic nanoparticles coated mixed fungal biomass (MNP–FB) | Cr(VI) | Adsorption | 60 min | 99.4% | — | — | — | 214 |
| AG-g-PAN/Cu Fe2O4 | Pd(II) | Adsorption | 30–120 min | 97.01% | 12 emu g−1 | 37 nm | Up to three cycles for 93.98% removal | 215 |
| Fe3O4 and Fe3O4–chitosan NPs | Pb(II) | Adsorption | 31.2 min | 69.02 and 89.54% | 51.22 and 27.86 emu g−1 | 30 nm | Up to five cycles for 59.43% removal | 216 |
| Fe3O4@DA–DMSA magnetic nanoparticles (FDDMs) | Pb2+ | Adsorption | 45 min | 90.11% | 21.50 emu g−1 | 446 nm | — | 217 |
| Cu2+ | 40.61% | |||||||
| Cd2+ | 13.87% | |||||||
| Core shell magnetic nanoparticles (cMNPs) | As(V) | Adsorption | 10 min | 97.5% | 36.4 emu g−1 | 4.9 nm | Up to five cycles for 80% removal | 218 |
| Fe3O4@ZIF-8 and Fe3O4@SiO2@ZIF-8 | Pb2+ | Adsorption | 15 min | 93.61% | — | 110 ± 10 nm and 80 ± 10 nm | — | 219 |
| 99.2% | ||||||||
| Magnetic hybrid alumina nanoparticles (MHAI-NPsP) | Cu(II) | Adsorption | 60 min | 85% | — | — | — | 220 |
| Gum arabic magnetic nanoparticles (GA–MNPs) | Ciprofloxacin (CIP) | Adsorption | 120 min | 96.30% | — | — | Up to seven cycles for initial removal efficiency of 96.34% | 221 |
| Fe3O4-ACLM | Ciprofloxacin (CIP) | Adsorption | 75 min | 100% | 37.6 emu g−1 | 9.8 ± 1.5 nm | Up to six cycles for 90% removal | 222 |
| C–CoFe3O4/N, S-BC | Ciprofloxacin (CIP) | Adsorption | 30 min | 94.7% | — | — | Up to eight cycles with >80% removal efficiency | 210 |
| Fe3O4@MIL-125-NH2 | (Pesticides) carbaryl | Photocatalytic degradation | 150 min | 91% | — | 24.57 nm | — | 223 |
| Methomyl | 98% | |||||||
| Activated carbon magnetic nanoparticles (AC–MNPs) | Metronidazole (MTZ) | Adsorption | 90 min | 100% | 24.93 emu g−1 | 20–35 nm | Up to five cycles for 90.5% removal | 224 |
| PAC@Fe3O4–MN | Ciprofloxacin (CIP) | Adsorption | 60 min | 100% | 51.5 emu g−1 | <80 nm | Up to eight cycles for 96% removal | 225 |
| MOM-Fe3O4 | Pharmaceutical substance (metformin) | Adsorption | 720 min | 93.9% | — | — | — | 226 |
| Fe3O4/clinoptilolite | Tetracycline | Adsorption | 20 s | 98.6% | 30.0 emu g−1 | 60–80 nm | Up to four cycles for 80.6% removal | 227 |
| Fe3O4@gly@indole@CuNO3 magnetic nanoparticle (FGICu) | Tetracycline | Adsorption | 60 min | 95% | 38.4 emu g−1 | 17.8 nm | Up to five cycles for 70% removal | 228 |
| Magnetic sporopollenin supported magnesium nanoparticles (MSP@MgO) | Tetracycline | Adsorption | 180 min | 98.05% | ∼32 emu | — | Up to 15 cycles for 84.72% removal | 229 |
| Magnetic Ho2MoO6/Fe2O3 | Tetracycline | Adsorption | 90 min | 99.96% | 14.82 emu g−1 | 25–50 nm | Up to five consecutive cycles for 79.1% removal | 230 |
| FeNi3@SiO2@TiO2 | Humic acid (HA) | Photocatalytic degradation | 30 min | 100% | 18.84 emu g−1 | 20–30 nm | — | 231 |
| Nano porous Co2O3/Cu2O3:Al2O3:SiO2 | E. faecalis | Disinfection | 05 min | 100% | 0.157–0.24 emu g−1 | 23–31 nm | — | 232 |
| Chitosan-coated magnetic nanoparticles (cMNPs) | Lignocellulosic biorefinery containing phenol | Adsorption | 90 min | 46.2% | 26.96 emu g−1 | — | Up to five cycles for 20% of initial removal efficiency | 205 |
| Novel MNP–alum conjugate | Natural organic matter | Adsorption cum enhanced coagulation–flocculation | 30 min | 98.7% | — | 20–30 nm | Up to five cycles with ∼91% removal | 233 |
| NFO@SiO2@APTS | Acetaminophen | Adsorption | 30 min | 94% | 22 emu g−1 | 130–150 nm | Up to four cycles for 89% removal | 234 |
| Magnetic mesoporous silica microspheres (MSMS) | Acetaminophen | Adsorption | 30 min | 97.4% | 8.3 emu g−1 | 26 nm | Reusable for up to 14 cycles | 235 |
| Fe3O4/Douglas fir biochar | Acetylsalicylic acid | Adsorption | 5 min | 70% | — | 12.3 ± 7.1 nm | Reusable for up to five cycles | 236 |
| NFO@SiO2@APTS | Ibuprofen | Adsorption | 30 min | 97% | 22 emu g−1 | 130–150 nm | Up to three cycles for 92% removal | 234 |
| Magnetic activated carbon–Fe3O4 (AC–Fe3O4) | Promazine | Adsorption | 06 min | 99.97% | — | 20 nm | Up to five cycles for 99% removal | 237 |
| Magnetic silica-based nanoadsorbents (MMST and MMST-Ph) | Pharmaceutical substance | Adsorption | 200 min | 80% | 16.0 emu g−1 (MMST) | 3.75 nm (pore size) | Up to eight cycles for 100% removal | 238 |
| 18.6 emu g−1 (MMST-Ph) | ||||||||
| MNPs coated with rhamnolipids (Rh-cMNP) | Acetaminophen | Adsorption | 60 min | 96.7% | 16.5 emu g−1 (before adsorption) | — | Up to eight cycles for | 239 |
| 8.2 emu g−1 (after adsorption) | ||||||||
| Fe3O4@GNP | Ketoprofen (KP) | Adsorption | 30 min | For wastewater (65.0–83.6%) | — | 50 nm | Reusable for up to five cycles | 240 |
| Ibuprofen (IP) | For tap water (67.1–90.0%) | |||||||
| Rape straw biomass fiber-β-CD–Fe3O4 (RSBCDF) | Ibuprofen (IP) | Adsorption | 5–150 min | 94.42% | — | 150–250 nm | Up to five cycles for 73.56% | 241 |
| Fe3O4@GNP | Naproxen (NX) | Adsorption | 30 min | For wastewater (65.0–83.6%) | — | 50 nm | Reusable for up to five cycles | 240 |
| Diclofenac sodium salt (DF) | For tap water (67.1–90.0%) | |||||||
| Biochar/MgFe2O4 | Levofloxacin (LFX) | Adsorption | 30 min | Up to 80% | 66.815 emu g−1 | — | Up to four cycles for 74.25% | 242 |
| Fe3O4/Douglas fir biochar | Caffeine | Adsorption | 5 min | 70% | — | 12.3 ± 7.1 nm | Reusable for up to five cycles | 236 |
| 3D-GC/MGO–SO3H | Tetracycline | Adsorption | 1 h | 85% | 55.4 emu g−1 | 80–90 nm | Up to five cycles for 85% removal | 243 |
| Magnetic polydopamine-chitosan modified adsorbent (MPDA-CS) | Diclofenac sodium (DCFS) | Adsorption | 40 min | 96.38% | — | 290.2 nm | Up to three cycles for 94% removal | 244 |
| Fe3O4-gINPs | Levofloxacin (LEV) | Adsorption | 24 h | 86.15% | — | 14.34 nm | Up to four cycles for 80.47% removal | 245 |
| Magnetic molecularly imprinted polymers (MMIP) | Norfloxacin | Adsorption | — | — | — | — | Reusable | 246 |
| Fe–TiO2@Fe3O4 | 2,4-Dichlorophenol | Sono-catalytic degradation | 90 min | 94% | 25 emu g−1 | 15.05–18.12 nm | Up to five cycles for 91% removal | 247 |
| Fe3O4@Phe NPs | Ciprofloxacin (CIP) | Adsorption | 30 min | 62.5% | 60 emu g−1 | 150–270 nm | Up to four cycles for 80% removal | 248 |
| Zn0.5Ni0.5FeCrO4 MNPs | 4-Nitrophenol | Photo-degradation | 180 min and 150 min | 95% and 80% | 7.06 emu g−1 | 25–35 nm | Up to four cycles reusable | 249 |
| Aniline | ||||||||
| Polyvinylidene fluoride (PVDF)/Fe3O4@carboxymethyl cellulose | Oil sludge (oleic acid) | Sorption | 30 min | — | 62–55 emu g−1 | 20 nm | Up to five cycles reusable | 250 |
| Magnetic Janus nanoparticles (M-Janus NPs) | Cooking oil and crude oil | Phase-separation | 15 min | 96% | — | 2 mm | Up to five cycles for 90% removal | 251 |
| Surface-modified MNPs, AU–MNPs, and AP–MNPs | Oil spills (crude oil) | Adsorption | 3–20 min | 98% | 40.23 emu g−1 | 141.8 nm | Up to four cycles for 89% removal | 252 |
| 43.85 emu g−1 | 133.4 nm | |||||||
| VCL/Fe3O4 and VAA/Fe3O4 | Crude oil | Adsorption | 5–20 min | 98% | 54.6 emu g−1 | 117.5 nm | Up to four cycles for 80% removal | 253 |
| 99.3% | 49.4 emu g−1 | 173.6 nm | ||||||
| Fe3O4 MNP | Crude oil | Adsorption | 5–30 min | 94% | — | 3 nm | Up to nine cycles for 64% removal | 254 |
| 83% | 15–20 nm | |||||||
| Fe3O4 MNP | Crude oil | Adsorption | — | 98.6% | 51 ± 0.4 emu g−1 | 5–10 nm | Up to 7 cycles for 90% removal | 255 |
| Nanoparticles of titanomagnetite (NTM) | Crude oil spills | Adsorption | 20 min | — | 62 emu g−1 | 35 nm | Reusable for up to three successive cycles | 197 |
| HAN–Fe3O4 | Crude oil | Adsorption | 8 min | 100% | — | 10 nm | Up to five cycles for reusable | 256 |
| HAT–Fe3O4 | 89% | 30 nm | ||||||
| Magnetic corncob powder (CoFe2O4) | Crude oil (API = 23.3°) | Adsorption | 30 min | — | 32 emu g−1 | 5–50 nm | Up to 10th cycle with 41.98 g per g oil sorption capacity | 257 |
| Crude oil (API = 29.7°) | ||||||||
| Fresh motor oil | ||||||||
| Used motor oil | ||||||||
| Kerosene | ||||||||
| DOTAC-coated MNPs (M3) | Oil spills | Adsorption | 3 min | 94% | ∼26.0 emu g−1 | — | Up to 7 cycles in W/O emulsion with >90% efficiency | 258 |
| MNP–S@CTAB | Oil | Adsorption | 20 min | 100% | — | 15–20 nm | Up to 10th cycles with 19 mg g−1 adsorption efficiency | 259 |
| 50–100 nm | ||||||||
| Silica coated ferroferric oxide (Fe3O4@SiO2) | Emulsified oil | Transformation | 05 min | 98% | 69.1 emu g−1 | 200 nm | Up to five cycles for 95% removal | 260 |
3.5 Scalability challenges
Scalability challenges encompass several factors, including the recovery rates and reusability of nanoparticles, treatment costs, and the economic feasibility of the processes involved, as well as the influence of complex environmental conditions such as high salinity and low temperatures. Among these, the reusability of MNPs stands out as a critical aspect. The economic viability of MNPs is significantly tied to their ability to be reused; however, issues like surface fouling and structural degradation can diminish their adsorption efficiency after multiple cycles.261 For MNPs to be commercially feasible in large-scale applications, it is essential to develop more sustainable and efficient regeneration methods. Enhancing the reusability of MNPs can be achieved by exploring strategies that minimize nanoparticle degradation, such as enzymatic treatments or low-temperature regeneration techniques.18 Metal–organic frameworks and metal-based adsorbents exhibit high adsorption capacities due to the nature of adsorption through metal complexation. However, challenges remain regarding the recovery, stability, recycling, and potential metal leaching associated with these materials.262 Furthermore, the economic feasibility of large-scale applications may be hindered by the energy costs associated with magnetic recovery, necessitating further optimization.263 Recent literature reveals a shift from traditional carbon-based materials to various promising alternatives that are not only more cost-effective for industrial applications but also demonstrate comparable performance to carbon-based adsorbents.264Despite the exploration of advanced adsorbent materials such as ceramics, MOFs, graphene oxide, and composite adsorbents in small-scale studies using simulated hospital wastewater or simplified water sources, their long-term effectiveness in real hospital wastewater (HWW) remains largely unexamined. Currently, only granular and powdered activated carbons have seen widespread application. Challenges such as high operational costs, non-specific adsorption, degradation of material and adsorption capacity due to frequent regeneration, and the management of spent adsorbents pose significant economic and environmental hurdles. To address these issues, the development of innovative adsorbent materials that offer high adsorption capacity, durability, and ease of regeneration is essential for effective full-scale wastewater treatment.265 While MOF-derived magnetic nanocomposites offer numerous advantages, such as significant structural and chemical flexibility, the costs involved in their synthesis still require optimization. This includes expenses related to raw materials, like metallic salts and organic binders, as well as processing methods that often utilize non-recyclable organic solvents and activation techniques. One potential solution is to adopt aqueous synthesis in place of solvothermal methods, which could lead to a cost reduction of approximately US$ 35 for every US$ 13 spent on synthesizing MOFs at a large scale.266 For industrial applications to be economically viable, the cost of these materials should ideally be below $10 per kilogram. Consequently, it is essential to focus on less expensive organic ligands, advocating for the use of shorter and smaller binders rather than more expensive longer or aromatic alternatives.267 Nevertheless, there is a notable gap in the literature regarding cost evaluations, which are essential for assessing economic viability at scale. Research is increasingly focused on analyzing the economic feasibility of pollution control methods that incorporate these nanocomposites, taking into account production, operational costs, waste management, and material regeneration.268
Despite the advantages offered by nanostructured adsorbents, several challenges hinder their broader application, primarily their high production costs and difficulties in scaling.269 The expense associated with manufacturing these materials renders them impractical for large-scale water treatment initiatives. Additionally, while nanostructured adsorbents have demonstrated effectiveness in laboratory settings, translating this success to larger systems remains problematic. Key considerations, such as the efficient distribution and maintenance of these adsorbents in real-world applications, must be thoroughly addressed before they can be adopted on a wider scale.270
In light of these challenges, further research and development are essential to address the existing issues and enhance the potential benefits of nanostructured adsorbents in water treatment applications. These materials have proven effective in various wastewater treatment scenarios, including the removal of organic pollutants, dyes, and heavy metals. For instance, surface-modified iron oxide nanoparticles have successfully eliminated toxic elements such as lead and arsenic from industrial wastewater. Additionally, carbon-based nanostructured materials, including carbon nanotubes and modified graphene, have demonstrated significant efficacy in extracting organic contaminants and dyes due to their high surface area and adsorption capacity. These practical applications underscore the versatility and effectiveness of nanostructured adsorbents in tackling diverse challenges in wastewater treatment.
A thorough research into the use of nanostructured adsorbents for the continuous treatment of sewage, industrial effluents, and polluted water bodies is essential. While surface-functionalized nanostructured adsorbents have demonstrated effectiveness in laboratory settings, their application in industrial environments is limited due to high costs and complex modification processes. To promote advancements in wastewater treatment technologies, it is crucial to focus on developing cost-effective preparation methods, selectively modifying surface functional groups, and further investigating pollutant removal mechanisms, recovery of valuable elements from wastewater, and the recycling of adsorbents.271 Scalability challenges such as high salinity in wastewater and low temperatures can significantly hinder the effectiveness of MNPs in large-scale environmental remediation efforts. These conditions can diminish treatment efficiency, compromise material stability, and reduce adsorption capabilities. The presence of elevated salt concentrations, commonly found in oilfield brines, desalination processes, and various industrial effluents, poses a particular obstacle to the scalability of magnetic nanohybrid materials. Salts like NaCl, MgCl2, and CaSO4 interfere with the electrostatic interactions between contaminants and nanomaterials, disrupting surface chemistry and leading to decreased adsorption effectiveness due to competition for active sites.272 Furthermore, chloride ions can promote the leaching of iron ions from magnetite, thereby destabilizing metal oxide nanoparticles and affecting their durability and magnetic separability. Additionally, elevated ionic strength can lead to the aggregation of nanoparticles, further reducing their reactivity and active surface area.273
The effectiveness of MNPs in environmental remediation can be significantly influenced by low ambient temperatures. Key processes such as diffusion rates, redox reactions, and the kinetics of pollutant adsorption, which are vital for nanohybrid-based remediation, are all temperature-dependent. For instance, semiconductor–metal nanohybrid-mediated photocatalytic degradation is notably less efficient at lower temperatures due to reduced activation energy and slower electron transfer rates. Additionally, colder temperatures increase the viscosity of wastewater, hindering the mobility of nanoparticles and their capacity to engage with contaminants. These thermal constraints pose challenges to the sustainability and cost-effectiveness of large-scale applications, making the deployment of MNPs in temperate or cold regions less feasible without additional energy support.274
The large-scale application of MNPs in real-world settings faces significant challenges due to the interplay of low temperatures and high salinity. These conditions can slow down reaction kinetics and promote the aggregation of nanoparticles, thereby reducing their effectiveness in pollutant removal. To address these issues, it is essential to develop more robust nanohybrids that maintain their dispersibility and catalytic performance across diverse environmental conditions, such as surface-functionalized or polymer-stabilized MNPs. Furthermore, this situation underscores the importance of conducting pilot studies and implementing site-specific adaptive designs before embarking on large-scale remediation efforts.275 Scalability is essential for enhancing productivity and transitioning laboratory-scale synthesis to large-scale operations, particularly in both batch and continuous processes. A key engineering challenge in designing modular reactors for large-scale water treatment lies in achieving uniform flow dynamics and treatment efficacy across all modules. Typically arranged in parallel or series within decentralized systems, these modular reactors face theoretical difficulties in ensuring consistent flow distribution and hydraulic retention time. Such variations can lead to overloaded or underutilized reactor zones, ultimately diminishing pollutant removal efficiency. Additionally, non-uniform utilization of reactive media, such as MNPs, can hinder effective magnetic separation and result in unequal pollutant exposure, especially in high-flow industrial or municipal environments.276 While the application of nanoparticles in real-world scenarios is increasingly viable, challenges remain regarding their production and broader implementation. Most research is still conducted at the bench level, necessitating pilot-scale studies to validate findings. The scaling up of green nanoparticle production is promising, as larger-scale operations can provide more accurate data on cost-effectiveness, repeatability, and efficiency.
3.6 Environmental impact assessment
The increasing interest in magnetic nanohybrid materials highlights their unique characteristics and wide-ranging applications, especially in environmental remediation. While extensive research has been conducted on the efficacy and uses of MNPs, it is crucial to also assess their potential environmental impacts. An effective environmental impact assessment for MNPs should concentrate on three primary aspects: the release of harmful substances during their application, their influence on ecosystems, and the strategies for ensuring safe ecological remediation. A unified scientific framework is essential for researchers to evaluate sustainable practices associated with MNP applications. It is vital to investigate the potentially harmful substances that may be released during MNP usage, as their production involves various chemical compounds, some of which could be toxic. Furthermore, the degradation of MNPs can result in the release of nanoparticles, metal ions, or other byproducts that may endanger human health and the environment. For instance, a study by Osman et al. focused on synthesizing a highly active magnetic sorbent composite from mixed plastic and biomass waste, employing Life Cycle Assessment (LCA) to evaluate the environmental impacts of producing the magnetic char composite adsorbent, adhering to the methodologies and standards set forth in ISO 14040:2006 and ISO 14044:2006.277,278Ferric iron is primarily transported in the body by transferrin, which interacts with transferrin receptors on cell surfaces, while its storage occurs mainly in ferritin proteins within cells. Initially, MNPs were considered non-toxic due to their ability to release ferric iron into normal iron metabolism. However, their diminutive size enables them to accumulate in high concentrations within cells, complicating their elimination from the body.279 This accumulation raises significant concerns, particularly because excess free iron can produce harmful free radicals that threaten sensitive tissues, such as the brain. Research by Bucak et al. has shown that toxicity can emerge when serum proteins adhere to the surfaces of MNPs, thereby modifying the cellular environment. To mitigate this risk, coating MNPs with biocompatible materials can effectively reduce protein binding and surface interactions, ultimately lowering their toxicity.280
A comprehensive examination of the effects of MNPs on ecosystems is crucial due to their distinctive magnetic properties and large surface area, which allow them to interact with various environmental components such as water, soil, air, and living organisms. Although MNPs can effectively break down water pollutants, their degradation products may pose risks to aquatic ecosystems and disrupt food webs. Furthermore, the application of MNPs in soil remediation can modify microbial communities, potentially hindering plant growth. To assess these impacts, thorough ecotoxicological evaluations are essential. Establishing best practices and guidelines for the safe use of MNPs in environmental remediation is vital, emphasizing the principles of green chemistry and sustainable engineering in their synthesis and design. This approach should focus on utilizing sustainable chemical materials that foster non-toxic interactions and incorporate recyclable, eco-friendly processes to reduce harmful byproducts. Techniques such as coating and functionalization can enhance the stability and biocompatibility of MNPs, thereby lowering their toxicity. The integration of biocompatible polymers and organic ligands can improve the distribution of MNPs in environmental media and prevent aggregation, which in turn minimizes their chemical reactivity and potential negative effects.
The effective and safe integration of MNPs necessitates collaboration among researchers, industry stakeholders, and regulatory bodies to ensure sustainable practices. It is crucial to disseminate research findings on the environmental impacts of MNPs through scientific journals, conferences, and user-friendly online platforms. Industry stakeholders are encouraged to adopt best practices for the synthesis, management, and disposal of MNPs, adhering to established standards and guidelines. Regulatory agencies must create clear regulations that outline the proper control of MNPs during environmental impact assessments at the company level. Additionally, public education and community engagement are vital for raising awareness about both the beneficial and harmful aspects of MNPs, empowering individuals to make informed decisions.
Environmental assessments of magnetic nanohybrid materials are essential for their sustainable application in environmental remediation. By carefully evaluating the potential release of toxic substances and their ecological effects, we can reduce the environmental risks linked to magnetic nanoparticles through the implementation of appropriate safety measures. This thorough evaluation framework not only bolsters the safety and security of these materials but also establishes a scientific foundation for their responsible and sustainable utilization. Continued collaboration in research and regulatory initiatives is vital to enhance our understanding of the environmental consequences of magnetic nanoparticles, facilitating the creation of safe and sustainable practices for their ecological use. Fig. 12 illustrates the environmental impact and future challenges associated with these materials.
4. Conclusions and future directions
Magnetic nanohybrid materials (MNMs) have gained recognition as effective agents for environmental remediation due to their distinctive properties, including high surface reactivity, magnetic separability, and customizable functionalities for pollutant extraction. Their versatility allows for applications in water purification, soil decontamination, and air filtration, as they can efficiently adsorb, catalyze, or degrade various contaminants. Nevertheless, challenges such as agglomeration, long-term stability in environmental settings, scalability of production, and cost-effectiveness pose significant barriers to their broader implementation. To overcome these issues, future research should focus on innovative material designs and the integration of advanced technologies. A particularly promising avenue is the creation of stimuli-responsive MNMs, which can adjust to changes in environmental conditions, thereby enhancing pollutant capture and regeneration. Furthermore, the application of artificial intelligence and machine learning holds the potential to transform the optimization of nanomaterials by accurately predicting synthesis parameters, surface modifications, and interactions with pollutants, ultimately facilitating closed-loop systems for real-time performance improvements.A vital area of focus is the enhancement of MNMs' durability and reusability through advanced stabilization methods, such as atomic-layer deposition and bio-inspired coatings like polydopamine and cellulose derivatives, which help reduce aging and aggregation. Additionally, employing core–shell structures with protective layers can further bolster their chemical and magnetic stability in challenging environments. For broader application, it is essential to investigate green synthesis techniques that utilize plant extracts or microbial processes, thereby minimizing the use of hazardous chemicals and decreasing production costs. Moreover, the integration of MNMs into hybrid photocatalytic systems presents an opportunity for solar-driven degradation of persistent pollutants, facilitating easy magnetic recovery and effectively linking photocatalysis with practical remediation efforts.
In conclusion, while MNMs hold immense promise for addressing environmental pollution, their transition from laboratory innovations to practical applications, a multidisciplinary approach is essential. Emphasizing smart material design, AI-driven optimization, sustainable manufacturing, and hybrid catalytic systems will enable researchers to address current challenges and fully harness the capabilities of these nanomaterials. Collaborative efforts among chemists, engineers, and data scientists are crucial for refining synthesis methods, enhancing stability, and ensuring economic feasibility. With focused advancements in these domains, MNMs have the potential to significantly contribute to sustainable environmental remediation, fostering cleaner ecosystems and supporting a circular economy.
Data availability
Data are available within the manuscript in the form of figures and tables. No primary research results, software or code have been included and no new data were generated or analyzed as part of this review.Conflicts of interest
The authors declare no conflict of interest regarding the publication of this manuscript.Acknowledgements
We acknowledge the support of Quaid-i-Azam University and UAE University for supporting this work.References
- D. Lagakos, A. M. Mobarak and M. E. Waugh, The welfare effects of encouraging rural–urban migration, Econometrica, 2023, 91(3), 803–837 CrossRef.
- M. H. Brenner, Industrialization and economic growth: Estimates of their effects on the health of populations, in Assessing Contributions/h, Routledge, 2019, pp. 65–115 Search PubMed.
- P. Chowdhary, R. N. Bharagava, S. Mishra and N. Khan, Role of industries in water scarcity and its adverse effects on environment and human health, Environmental Concerns and Sustainable Development: Volume 1: Air, Water and Energy Resources, 2020, pp. 235–256 Search PubMed.
- S. Garg, Z. Z. Chowdhury, A. N. M. Faisal, N. P. Rumjit and P. Thomas, Impact of industrial wastewater on environment and human health, Advanced Industrial Wastewater Treatment and Reclamation of Water: Comparative Study of Water Pollution Index during Pre-industrial, Industrial Period and Prospect of Wastewater Treatment for Water Resource Conservation 2022, pp. 197–209 Search PubMed.
- X. Zhang, M. Yan, P. Chen, J. Li, Y. Li, H. Li, X. Liu, Z. Chen, H. Yang, S. Wang, J. Wang, Z. Tang, Q. Huang, J. Lei, T. Hayat, Z. Liu, L. Mao, T. Duan and X. Wang, Emerging MOFs, COFs, and their derivatives for energy and environmental applications, Innovation, 2025, 6(2), 100778–100798 CAS.
- Y. Wang, Q. Geng, J. Yang, Y. Liu and C. Liu, Hybrid system of flocculation–photocatalysis for the decolorization of crystal violet, reactive red X-3B, and acid orange II dye, ACS Omega, 2020, 5(48), 31137–31145 CrossRef CAS PubMed.
- R. Sharma, A. Rana and D. Panthari, Wastewater pollution induced detrimental impacts on aquatic biodiversity: A review, Advances in Environmental Pollution Management: Wastewater Impacts and Treatment Technologies, Agro Environ Media, Haridwar, Uttarakhand, India, 2020, pp. 113–127 Search PubMed.
- S. Dutta, S. Adhikary, S. Bhattacharya, D. Roy, S. Chatterjee, A. Chakraborty, D. Banerjee, A. Ganguly, S. Nanda and P. Rajak, Contamination of textile dyes in aquatic environment: Adverse impacts on aquatic ecosystem and human health, and its management using bioremediation, J. Environ. Manage., 2024, 353, 120103 Search PubMed.
- V. M. Pathak, V. K. Verma, B. S. Rawat, B. Kaur, N. Babu, A. Sharma, S. Dewali, M. Yadav, R. Kumari and S. Singh, Current status of pesticide effects on environment, human health and it's eco-friendly management as bioremediation: A comprehensive review, Front. Microbiol., 2022, 13, 962619 CrossRef PubMed.
- J. A. Silva, Wastewater treatment and reuse for sustainable water resources management: a systematic literature review, Sustainability, 2023, 15(14), 10940 Search PubMed.
- A. Singh, A. Sharma, R. K. Verma, R. L. Chopade, P. P. Pandit, V. Nagar, V. Aseri, S. K. Choudhary, G. Awasthi and K. K. Awasthi, Heavy metal contamination of water and their toxic effect on living organisms, in The Toxicity of Environmental Pollutants, IntechOpen, 2022 Search PubMed.
- W. U. Rehman, S. Y. Barykin, A. V. Sharkova, K. M. J. Iqbal, M. I. Khan, K. S. Alpysbayev and L. N. Danilova, A Novel Approach to Waste Load Management and Environmental Compliance for Marine Pollution Based on Econometric Modeling, J. Leg. Ethical Regul. Issues, 2021, 24(Pt. 2), 1 Search PubMed.
- E. Lusweti, E. K. Kanda, J. Obando and M. Makokha, Effects of oil exploration on surface water quality–a review, Water Pract. Technol., 2022, 17(10), 2171–2185 CrossRef.
- A. K. Sharma, M. Gupta, S. A. Khan, A. Neem, H. Kumar, A. Bhati and U. Patel, in Effects of Air Pollution on Surface Water Contamination, IOP Conference Series: Earth and Environmental Science, IOP Publishing, 2024, p. 012036 Search PubMed.
- X. Pan, J. Ji, N. Zhang and M. Xing, Research progress of graphene-based nanomaterials for the environmental remediation, Chin. Chem. Lett., 2020, 31(6), 1462–1473 Search PubMed.
- A. Ali, T. Shah, R. Ullah, P. Zhou, M. Guo, M. Ovais, Z. Tan and Y. Rui, Review on recent progress in magnetic nanoparticles: Synthesis, characterization, and diverse applications, Front. Chem., 2021, 9, 629054 CrossRef CAS PubMed.
- C. Verma, D. K. Verma, E. Berdimurodov, I. Barsoum, A. Alfantazi and C. M. Hussain, Green magnetic nanoparticles: a comprehensive review of recent progress in biomedical and environmental applications, J. Mater. Sci., 2024, 59(2), 325–358 Search PubMed.
- S. Shukla, R. Khan and A. Daverey, Synthesis and characterization of magnetic nanoparticles, and their applications in wastewater treatment: A review, Environ. Technol. Innovation, 2021, 24, 101924 CrossRef CAS.
- E. Mutegoa, Efficient techniques and practices for wastewater treatment: an update, Discover Water, 2024, 4(1), 69 CrossRef CAS.
- P. Kumari and A. Kumar, Advanced oxidation process: a remediation technique for organic and non-biodegradable pollutant, Results Surf. Interfaces, 2023, 11, 100122 CrossRef.
- S. Kato and Y. Kansha, Comprehensive review of industrial wastewater treatment techniques, Environ. Sci. Pollut. Res., 2024, 31(39), 51064–51097 CrossRef PubMed.
- A. H. Birniwa, S. Habibu, S. S. a. Abdullahi, R. E. A. Mohammad, A. Hussaini, H. Magaji, B. N. S. Al-dhawi, A. Noor and A. H. Jagaba, Membrane technologies for heavy metals removal from water and wastewater: A mini review, Case Stud. Chem. Environ. Eng., 2024, 9, 100538 CrossRef CAS.
- F. Saffarimiandoab, B. Y. Gul, R. S. Tasdemir, S. E. Ilter, S. Unal, B. Tunaboylu, Y. Z. Menceloglu and I. Koyuncu, A review on membrane fouling: Membrane modification, Desalin. Water Treat., 2021, 216, 47–70 CrossRef CAS.
- E. M. Cuerda-Correa, M. F. Alexandre-Franco and C. Fernández-González, Advanced oxidation processes for the removal of antibiotics from water. An overview, Water, 2019, 12(1), 102 CrossRef.
- X. Yang and D. Wang, Photocatalysis: from fundamental principles to materials and applications, ACS Appl. Energy Mater., 2018, 1(12), 6657–6693 CrossRef CAS.
- S. K. Lakhera, K. P. Kangeyan, C. Yazhini S, S. Golda A and N. Bernaurdshaw, Advances in hybrid strategies for enhanced photocatalytic water splitting: Bridging conventional and emerging methods, Appl. Phys. Rev., 2024, 11(4), 041305 CAS.
- C. Zuo, X. Tai, Z. Jiang, M. Liu, J. Jiang, Q. Su and X. Yan, S-Scheme 2D/2D Heterojunction of ZnTiO3 Nanosheets/Bi2WO6 Nanosheets with Enhanced Photoelectrocatalytic Activity for Phenol Wastewater under Visible Light, Molecules, 2023, 28(8), 3495 CrossRef CAS PubMed.
- F. Tanos, A. Razzouk, G. Lesage, M. Cretin and M. Bechelany, A Comprehensive Review on Modification of Titanium Dioxide-Based Catalysts in Advanced Oxidation Processes for Water Treatment, ChemSusChem, 2024, 17(6), e202301139 CrossRef CAS PubMed.
- P. Tiwari, M. Verma, Ambika, H. Chutani, P. P. Singh, S. Kanodia and T. Verma, Titanium dioxide-based nanoparticles and their applications in water remediation, J. Environ. Eng. Sci., 2023, 19(1), 46–54 Search PubMed.
- J. Radić, G. Žerjav, L. Jurko, P. Bošković, L. Fras Zemljič, A. Vesel, A. Mavrič, M. Gudelj and O. Plohl, First Utilization of Magnetically-Assisted Photocatalytic Iron Oxide-TiO2 Nanocomposites for the Degradation of the Problematic Antibiotic Ciprofloxacin in an Aqueous Environment, Magnetochemistry, 2024, 10(9), 66 CrossRef.
- L. F. O. Maia, M. S. Santos, T. G. Andrade, R. de Carvalho Hott, M. C. da Silva Faria, L. C. A. Oliveira, M. C. Pereira and J. L. Rodrigues, Removal of mercury (II) from contaminated water by gold-functionalised Fe3O4 magnetic nanoparticles, Environ. Technol., 2020, 41, 959–970 CrossRef CAS PubMed.
- S. K. Panigrahy, A. Nandha, M. Chaturvedi and P. K. Mishra, Novel nanocomposites with advanced materials and their role in waste water treatment, Next Sustainability, 2024, 4, 100042 CrossRef.
- J. Kudr, Y. Haddad, L. Richtera, Z. Heger, M. Cernak, V. Adam and O. Zitka, Magnetic nanoparticles: From design and synthesis to real world applications, Nanomaterials, 2017, 7(9), 243 CrossRef PubMed.
- N. Baig, I. Kammakakam and W. Falath, Nanomaterials: A review of synthesis methods, properties, recent progress, and challenges, Mater. Adv., 2021, 2(6), 1821–1871 Search PubMed.
- S. Kumari, S. Raturi, S. Kulshrestha, K. Chauhan, S. Dhingra, K. András, K. Thu, R. Khargotra and T. Singh, A comprehensive review on various techniques used for synthesizing nanoparticles, J. Mater. Res. Technol., 2023, 27, 1739–1763 CrossRef CAS.
- M. S. El-Eskandarany, A. Al-Hazza, L. A. Al-Hajji, N. Ali, A. A. Al-Duweesh, M. Banyan and F. Al-Ajmi, Mechanical milling: a superior nanotechnological tool for fabrication of nanocrystalline and nanocomposite materials, Nanomaterials, 2021, 11(10), 2484 CrossRef CAS PubMed.
- M. Bououdina, T. Alwqyan, L. Khezami, B. Al-Najar, M. Shaikh, R. Gill, A. Modwi, K. K. Taha and O. Lemine, Fabrication and characterization of nanostructured MgO· Fe2O3 composite by mechanical milling as efficient adsorbent of heavy metals, J. Alloys Compd., 2019, 772, 1030–1039 CrossRef CAS.
- Y. Li, A. R. Zimmerman, F. He, J. Chen, L. Han, H. Chen, X. Hu and B. Gao, Solvent-free synthesis of magnetic biochar and activated carbon through ball-mill extrusion with Fe3O4 nanoparticles for enhancing adsorption of methylene blue, Sci. Total Environ., 2020, 722, 137972 CrossRef CAS PubMed.
- A. A. Demessie, Y. Park, P. Singh, A. S. Moses, T. Korzun, F. Y. Sabei, H. A. Albarqi, L. Campos, C. R. Wyatt and K. Farsad, An advanced thermal decomposition method to produce magnetic nanoparticles with ultrahigh heating efficiency for systemic magnetic hyperthermia, Small Methods, 2022, 6(12), 2200916 CrossRef CAS PubMed.
- J. Park, K. An, Y. Hwang, J.-G. Park, H.-J. Noh, J.-Y. Kim, J.-H. Park, N.-M. Hwang and T. Hyeon, Ultra-large-scale syntheses of monodisperse nanocrystals, Nat. Mater., 2004, 3(12), 891–895 CrossRef CAS PubMed.
- F. B. Effenberger, R. A. Couto, P. K. Kiyohara, G. Machado, S. H. Masunaga, R. F. Jardim and L. M. Rossi, Economically attractive route for the preparation of high quality magnetic nanoparticles by the thermal decomposition of iron (III) acetylacetonate, Nanotechnology, 2017, 28(11), 115603 CrossRef PubMed.
- K. Khan, S. Rehman, H. U. Rahman and Q. Khan, Synthesis and application of magnetic nanoparticles, Nanomagnetism, J. M. Gonzalez Estevez, One Central Press (OCP), UK, 2014 Search PubMed.
- A. Balachandran, S. P. Sreenilayam, K. Madanan, S. Thomas and D. Brabazon, Nanoparticle production via laser ablation synthesis in solution method and printed electronic application-A brief review, Results Eng., 2022, 16, 100646 CrossRef CAS.
- M. Kim, S. Osone, T. Kim, H. Higashi and T. Seto, Synthesis of nanoparticles by laser ablation: A review, KONA Powder Part. J., 2017, 34, 80–90 CrossRef CAS.
- K. Sakiyama, K. Koga, T. Seto, M. Hirasawa and T. Orii, Formation of size-selected Ni/NiO core− shell particles by pulsed laser ablation, J. Phys. Chem. B, 2004, 108(2), 523–529 CrossRef CAS.
- S. Shinde, S. Kulkarni, A. Banpurkar, R. Nawathey-Dixit, S. Date and S. Ogale, Magnetic properties of nanosized powders of magnetic oxides synthesized by pulsed laser ablation, J. Appl. Phys., 2000, 88(3), 1566–1575 CrossRef CAS.
- Y. Wang, J. A. Pan, H. Wu and D. V. Talapin, Direct Wavelength-Selective Optical and Electron-Beam Lithography of Functional Inorganic Nanomaterials, ACS Nano, 2019, 13(12), 13917–13931 CrossRef CAS PubMed.
- T. A. Shifa, F. Wang, Z. Cheng, P. He, Y. Liu, C. Jiang, Z. Wang and J. He, High Crystal Quality 2D Manganese Phosphorus Trichalcogenide Nanosheets and their Photocatalytic Activity, Adv. Funct. Mater., 2018, 28, 1800548 CrossRef.
- S. Kumar, P. Bhushan and S. Bhattacharya, Fabrication of nanostructures with bottom-up approach and their utility in diagnostics, therapeutics, and others, Environmental, Chemical and Medical Sensors, 2018, pp. 167–198 Search PubMed.
- A. A. Dinata, A. M. Rosyadi, S. Hamid and R. Zainul, A Review Chemical Vapor Deposition: Process And Application, 2018 Search PubMed.
- H. Niu, Y. Yang, W. Zhao, H. Lv, H. Zhang and Y. Cai, Single-crystalline Fe7S8/Fe3O4 coated zero-valent iron synthesized with vacuum chemical vapor deposition technique: Enhanced reductive, oxidative and photocatalytic activity for water purification, J. Hazard. Mater., 2021, 401, 123442 CrossRef CAS PubMed.
- H. Tavakol, S. Salimpour and W. Salvenmoser, The synthesis of sulfur-doped carbon nanofibers using chemical vapor deposition on the nickel-ferrite catalyst and the gold decoration of the product for morphine sensing, SN Appl. Sci., 2020, 2(12), 2018 CrossRef CAS.
- D. Bokov, A. Turki Jalil, S. Chupradit, W. Suksatan, M. Javed Ansari, I. H. Shewael, G. H. Valiev and E. Kianfar, Nanomaterial by sol-gel method: synthesis and application, Adv. Mater. Sci. Eng., 2021, 2021(1), 5102014 CrossRef.
- G. F. Stiufiuc and R. I. Stiufiuc, Magnetic nanoparticles: synthesis, characterization, and their use in biomedical field, Appl. Sci., 2024, 14(4), 1623 CrossRef CAS.
- M. K. Mohammed, Sol-gel synthesis of Au-doped TiO2 supported SWCNT nanohybrid with visible-light-driven photocatalytic for high degradation performance toward methylene blue dye, Optik, 2020, 223, 165607 CrossRef CAS.
- J. Zia and U. Riaz, Studies on the spectral, morphological and magnetic properties of PCz-PPy copolymer encapsulated BaFe2O4 nanohybrids, J. Mater. Sci.: Mater. Electron., 2020, 31(24), 22856–22865 CrossRef CAS.
- E. M. Materón, C. M. Miyazaki, O. Carr, N. Joshi, P. H. Picciani, C. J. Dalmaschio, F. Davis and F. M. Shimizu, Magnetic nanoparticles in biomedical applications: A review, Appl. Surf. Sci. Adv., 2021, 6, 100163 CrossRef.
- J. Li, Q. Wu and J. Wu, Synthesis of Nanoparticles via Solvothermal and Hydrothermal Methods, in Handbook of Nanoparticles, ed. M. Aliofkhazraei, Springer, Cham, 2015, DOI:10.1007/978-3-319-13188-7_17-1.
- M. U. Zahid, E. Pervaiz, A. Hussain, M. I. Shahzad and M. B. K. Niazi, Synthesis of carbon nanomaterials from different pyrolysis techniques: a review, Mater. Res. Express, 2018, 5(5), 052002 CrossRef.
- D. Nugroho, R. Benchawattananon, J. Janshongsawang, N. Pimsin, P. Porrawatkul, R. Pimsen, P. Nuengmatcha, P. Nueangmatcha and S. Chanthai, Ultra-trace analysis of chromium ions (Cr3+/Cr6+) in water sample using selective fluorescence turn-off sensor with natural carbon dots mixed graphene quantum dots nanohybrid composite synthesis by pyrolysis, Arabian J. Chem., 2024, 17(1), 105443 CrossRef CAS.
- M. Tsompanoglou, Fe3C/Fe Magnetic nanohybrids: Structural, magnetic features & biomedical applicability, Doctoral dissertation, Aristotle University Of Thessaloniki, 2023.
- M. A. Albalah, Y. A. Alsabah and D. E. Mustafa, Characteristics of co-precipitation synthesized cobalt nanoferrites and their potential in industrial wastewater treatment, SN Appl. Sci., 2020, 2(5), 804 CrossRef CAS.
- R. Verma, S. Pathak, A. K. Srivastava, S. Prawer and S. Tomljenovic-Hanic, ZnO nanomaterials: Green synthesis, toxicity evaluation and new insights in biomedical applications, J. Alloys Compd., 2021, 876, 160175 CrossRef CAS.
- A. Komeili, Molecular mechanisms of compartmentalization and biomineralization in magnetotactic bacteria, FEMS Microbiol. Rev., 2012, 36(1), 232–255 CrossRef CAS PubMed.
- S. Gul, S. B. Khan, I. U. Rehman, M. A. Khan and M. Khan, A comprehensive review of magnetic nanomaterials modern day theranostics, Frontiers in Materials, 2019, 6, 179 CrossRef.
- J. J. Lenders, C. L. Altan, P. H. Bomans, A. Arakaki, S. Bucak, G. de With and N. A. Sommerdijk, A bioinspired coprecipitation method for the controlled synthesis of magnetite nanoparticles, Cryst. Growth Des., 2014, 14(11), 5561–5568 CrossRef CAS.
- M. Duan, J. G. Shapter, W. Qi, S. Yang and G. Gao, Recent progress in magnetic nanoparticles: synthesis, properties, and applications, Nanotechnology, 2018, 29(45), 452001 CrossRef PubMed.
- U. Qumar, J. Z. Hassan, R. A. Bhatti, A. Raza, G. Nazir, W. Nabgan and M. Ikram, Photocatalysis vs adsorption by metal oxide nanoparticles, J. Mater. Sci. Technol., 2022, 131, 122–166 CrossRef CAS.
- N. S. Omar, L. I. Abd Ali, A. F. Qader and R. A. Omer, Use of magnetic nanoparticles for the removal of organic and inorganic pollution in wastewater treatment-a review, Rev. Inorg. Chem., 2025 DOI:10.1515/revic-2024-0117.
- N. Ajinkya, X. Yu, P. Kaithal, H. Luo, P. Somani and S. Ramakrishna, Magnetic iron oxide nanoparticle (IONP) synthesis to applications: present and future, Materials, 2020, 13(20), 4644 CrossRef CAS PubMed.
- K. Hachem, M. J. Ansari, R. O. Saleh, H. H. Kzar, M. E. Al-Gazally, U. S. Altimari, S. A. Hussein, H. T. Mohammed, A. T. Hammid and E. Kianfar, Methods of chemical synthesis in the synthesis of nanomaterial and nanoparticles by the chemical deposition method: a review, BioNanoScience, 2022, 12(3), 1032–1057 CrossRef.
- Q. Xu, J. Hou, J. Rao, G.-H. Li, Y.-L. Liu and J. Zhou, PEG modification enhances the in vivo stability of bioactive proteins immobilized on magnetic nanoparticles, Biotechnol. Lett., 2020, 42(8), 1407–1418 CrossRef CAS PubMed.
- V. F. Cardoso, A. Francesko, C. Ribeiro, M. Bañobre-López, P. Martins and S. Lanceros-Mendez, Advances in magnetic nanoparticles for biomedical applications, Adv. Healthcare Mater., 2018, 7(5), 1700845 CrossRef PubMed.
- M. I. Anik, M. K. Hossain, I. Hossain, A. Mahfuz, M. T. Rahman and I. Ahmed, Recent progress of magnetic nanoparticles in biomedical applications: A review, Nano Sel., 2021, 2(6), 1146–1186 Search PubMed.
- J. Guo, H. Jiang, Y. Teng, Y. Xiong, Z. Chen, L. You and D. Xiao, Recent advances in magnetic carbon nanotubes: synthesis, challenges and highlighted applications, J. Mater. Chem. B, 2021, 9(44), 9076–9099 RSC.
- M. Selvaraj, A. Hai, F. Banat and M. A. Haija, Application and prospects of carbon nanostructured materials in water treatment: A review, J. Water Proc. Eng., 2020, 33, 100996 CrossRef.
- M. Lu, X. J. Wu, C. X. Wan, Q. P. Gong, J. X. Li, S. S. Liao, Y. A. Wang and S. H. Yuan, Evaluation of Fe3O4-MnO2@ RGO magnetic nanocomposite as an effective persulfate activator and metal adsorbent in aqueous solution, Environ. Sci. Pollut. Res., 2023, 30(17), 51125–51142 Search PubMed.
- A. Karnwal and T. Malik, Nano-revolution in heavy metal removal: engineered nanomaterials for cleaner water, Front. Environ. Sci., 2024, 12, 1393694 CrossRef.
- C. Donga, S. B. Mishra, A. S. Abd-El-Aziz and A. K. Mishra, Advances in graphene-based magnetic and graphene-based/TiO2 nanoparticles in the removal of heavy metals and organic pollutants from industrial wastewater, J. Inorg. Organomet. Polym. Mater., 2021, 31(2), 463–480 CrossRef CAS.
- S. Dhadda, N. Jangir, P. Sihag, S. K. Bagaria and D. K. Jangid, The designing of magnetic nanomaterials synthesis and applications in dye degradation: a review, Environ. Sci. Pollut. Res., 2024, 1–23 Search PubMed.
- S. Yueyu, The synergistic degradation of pollutants in water by photocatalysis and PMS activation, Water Environ. Res., 2023, 95(10), e10927 CrossRef PubMed.
- G. M. Nair, T. Sajini and B. Mathew, Advanced green approaches for metal and metal oxide nanoparticles synthesis and their environmental applications, Talanta Open, 2022, 5, 100080 Search PubMed.
- E. M. Hotze, T. Phenrat and G. V. Lowry, Nanoparticle aggregation: challenges to understanding transport and reactivity in the environment, J. Environ. Qual., 2010, 39(6), 1909–1924 Search PubMed.
- A. Heuer-Jungemann, N. Feliu, I. Bakaimi, M. Hamaly, A. Alkilany, I. Chakraborty, A. Masood, M. F. Casula, A. Kostopoulou and E. Oh, The role of ligands in the chemical synthesis and applications of inorganic nanoparticles, Chem. Rev., 2019, 119(8), 4819–4880 Search PubMed.
- J. Hang, L. Shi, X. Feng and L. Xiao, Electrostatic and electrosteric stabilization of aqueous suspensions of barite nanoparticles, Powder Technol., 2009, 192(2), 166–170 Search PubMed.
- T. L. Doane, C.-H. Chuang, R. J. Hill and C. Burda, Nanoparticle ζ-potentials, Acc. Chem. Res., 2012, 45(3), 317–326 CrossRef CAS PubMed.
- P. May, U. Khan, J. M. Hughes and J. N. Coleman, Role of solubility parameters in understanding the steric stabilization of exfoliated two-dimensional nanosheets by adsorbed polymers, J. Phys. Chem. C, 2012, 116(20), 11393–11400 Search PubMed.
- C. Vasilescu, M. Latikka, K. D. Knudsen, V. M. Garamus, V. Socoliuc, R. Turcu, E. Tombácz, D. Susan-Resiga, R. Ras and L. Vékás, High concentration aqueous magnetic fluids: structure, colloidal stability, magnetic and flow properties, Soft Matter, 2018, 14(32), 6648–6666 RSC.
- J. Hierrezuelo, A. Sadeghpour, I. Szilagyi, A. Vaccaro and M. Borkovec, Electrostatic stabilization of charged colloidal particles with adsorbed polyelectrolytes of opposite charge, Langmuir, 2010, 26(19), 15109–15111 CrossRef CAS PubMed.
- J. Alonso, J. M. Barandiarán, L. Fernández Barquín and A. García-Arribas, Chapter 1 - Magnetic Nanoparticles, Synthesis, Properties, and Applications, in Magnetic Nanostructured Materials, ed. A. A. El-Gendy, J. M. Barandiarán and R. L. Hadimani, Elsevier, 2018, pp. 1–40 Search PubMed.
- S. Wang, D. Chen, Q. Hong, Y. Gui, Y. Cao, G. Ren and Z. Liang, Surface functionalization of metal and metal oxide nanoparticles for dispersion and tribological applications – A review, J. Mol. Liq., 2023, 389, 122821 CrossRef CAS.
- C. Pechyen, B. Tangnorawich, S. Toommee, R. Marks and Y. Parcharoen, Green synthesis of metal nanoparticles, characterization, and biosensing applications, Sens. Int., 2024, 5, 100287 Search PubMed.
- J. I. Orege, J. Wei, Q. Ge and J. Sun, Spinel-structured nanocatalysts: New opportunities for CO2 hydrogenation to value-added chemicals, Nano Today, 2023, 51, 101914 Search PubMed.
- N. Dudchenko, S. Pawar, I. Perelshtein and D. Fixler, Magnetite nanoparticles: Synthesis and applications in optics and nanophotonics, Materials, 2022, 15(7), 2601 CrossRef CAS PubMed.
- P. Kumar, S. Pathak, K. Jain, A. Singh, G. A. Basheed and R. P. Pant, Low-temperature large-scale hydrothermal synthesis of optically active PEG-200 capped single domain MnFe2O4 nanoparticles, J. Alloys Compd., 2022, 904, 163992 CrossRef CAS.
- M. A. Dahamni, M. Ghamnia, S. E. Naceri, C. Fauquet, D. Tonneau, J.-J. Pireaux and A. Bouadi, Spray Pyrolysis Synthesis of Pure and Mg-Doped Manganese Oxide Thin Films, Coatings, 2021, 11(5), 598 CrossRef CAS.
- M. Marć, W. Wolak, A. Drzewiński, S. Mudry, I. Shtablavyi and M. R. Dudek, The Concept of Using 2D Self-Assembly of Magnetic Nanoparticles for Bioassays, Appl. Sci., 2024, 14(5), 1906 CrossRef.
- H. Al-Madhagi, V. Yazbik, W. Abdelwahed and L. Alchab, Magnetite nanoparticle Co-precipitation synthesis, characterization, and applications: mini review, Bionanoscience, 2023, 13(2), 853–859 CrossRef.
- N. Dudchenko, S. Pawar, I. Perelshtein and D. Fixler, Magnetite Nanoparticles: Synthesis and Applications in Optics and Nanophotonics, Materials, 2022, 15, 2601 CrossRef CAS PubMed.
- R. R. Karri, J. Sahu and B. Meikap, Improving efficacy of Cr (VI) adsorption process on sustainable adsorbent derived from waste biomass (sugarcane bagasse) with help of ant colony optimization, Ind. Crops Prod., 2020, 143, 111927 CrossRef CAS.
- H. Rasoulzadeh, M. H. Dehghani, A. S. Mohammadi, R. R. Karri, R. Nabizadeh, S. Nazmara, K.-H. Kim and J. Sahu, Parametric modelling of Pb (II) adsorption onto chitosan-coated Fe3O4 particles through RSM and DE hybrid evolutionary optimization framework, J. Mol. Liq., 2020, 297, 111893 CrossRef CAS.
- M. Ruthiraan, N. Mubarak, E. Abdullah, M. Khalid, S. Nizamuddin, R. Walvekar and R. R. Karri, An overview of magnetic material: preparation and adsorption removal of heavy metals from wastewater, Magnetic Nanostructures: Environmental and Agricultural Applications, 2019, pp. 131–159 Search PubMed.
- M. Bora and D. C. Goswami, Water quality assessment in terms of water quality index (WQI): case study of the Kolong River, Assam, India, Appl. Water Sci., 2017, 7, 3125–3135 CrossRef.
- R. L. Droste and R. L. Gehr, Theory and Practice of Water and Wastewater Treatment, John Wiley & Sons, 2018 Search PubMed.
- J. Machell, K. Prior, R. Allan and J. M. Andresen, The water energy food nexus–challenges and emerging solutions, Environ. Sci.:Water Res. Technol., 2015, 1(1), 15–16 RSC.
- A. Nasiri, M. R. Heidari, N. Javid and G. Yazdanpanah, New efficient and recyclable magnetic nanohybrid adsorbent for the metronidazole removal from simulated wastewater, J. Mater. Sci.: Mater. Electron., 2022, 33(33), 25103–25126 CrossRef CAS.
- A. Choudhry, A. Sharma, T. A. Khan and S. A. Chaudhry, Flax seeds based magnetic hybrid nanocomposite: An advance and sustainable material for water cleansing, J. Water Proc. Eng., 2021, 42, 102150 Search PubMed.
- O. Saber, M. Osama, N. M. Shaalan, A. Osama, A. Alshoaibi and D. Osama, Designing novel strategy to produce active nanohybrids in sunlight for purification of water based on inorganic nanolayers, magnetic nanocomposites and organic species, Molecules, 2022, 27(12), 3673 CrossRef CAS PubMed.
- A. Khan, N. Ali, S. Malik, M. Bilal, H. Munir, L. F. R. Ferreira and H. M. Iqbal, Chitosan-based green sorbents for toxic cations removal, in Sorbents Materials for Controlling Environmental Pollution, Elsevier, 2021, pp. 323–352 Search PubMed.
- A. Aziz, N. Ali, A. Khan, M. Bilal, S. Malik, N. Ali and H. Khan, Chitosan-zinc sulfide nanoparticles, characterization and their photocatalytic degradation efficiency for azo dyes, Int. J. Biol. Macromol., 2020, 153, 502–512 CrossRef CAS PubMed.
- N. Ali, M. Bilal, A. Khan, F. Ali, H. Khan, H. A. Khan, K. Rasool and H. M. Iqbal, Understanding the hierarchical assemblies and oil/water separation applications of metal-organic frameworks, J. Mol. Liq., 2020, 318, 114273 CrossRef CAS.
- A. Nawaz, A. Khan, N. Ali, N. Ali and M. Bilal, Fabrication and characterization of new ternary ferrites-chitosan nanocomposite for solar-light driven photocatalytic degradation of a model textile dye, Environ. Technol. Innovation, 2020, 20, 101079 CrossRef CAS.
- Y. Zhou, Z. He, Y. Tao, Y. Xiao, T. Zhou, T. Jing, Y. Zhou and S. Mei, Preparation of a functional silica membrane coated on Fe3O4 nanoparticle for rapid and selective removal of perfluorinated compounds from surface water sample, Chem. Eng. J., 2016, 303, 156–166 CrossRef CAS.
- S. B. Qin, Y. H. Fan, X. X. Mou, X. S. Li and S. H. Qi, Preparation of phenyl-modified magnetic silica as a selective magnetic solid-phase extraction adsorbent for polycyclic aromatic hydrocarbons in soils, J. Chromatogr. A, 2018, 1568, 29–37 CrossRef CAS PubMed.
- P. Wu, S. Yu, M. Feng, H. Liu, S. Liu and J. Fu, Controllable synthesis of the polymorphic porous carbon with N-doping/Ni magnetic nanohybrids for high performance supercapacitor and environment applications, Appl. Surf. Sci., 2021, 567, 150875 CrossRef CAS.
- S. S. Elanchezhiyan, S. M. Prabhu, J. Han, Y. M. Kim, Y. Yoon and C. M. Park, Synthesis and characterization of novel magnetic Zr-MnFe2O4@ rGO nanohybrid for efficient removal of PFOA and PFOS from aqueous solutions, Appl. Surf. Sci., 2020, 528, 146579 CrossRef CAS.
- I. Ali, The quest for active carbon adsorbent substitutes: inexpensive adsorbents for toxic metal ions removal from wastewater, Sep. Purif. Rev., 2010, 39(3–4), 95–171 CrossRef CAS.
- R. R. Karri and J. Sahu, Process optimization and adsorption modeling using activated carbon derived from palm oil kernel shell for Zn (II) disposal from the aqueous environment using differential evolution embedded neural network, J. Mol. Liq., 2018, 265, 592–602 CrossRef CAS.
- B. Samiey, C. H. Cheng and J. Wu, Organic-inorganic hybrid polymers as adsorbents for removal of heavy metal ions from solutions: a review, Materials, 2014, 7(2), 673–726 Search PubMed.
- L. Järup, Hazards of heavy metal contamination, Br. Med. Bull., 2003, 68(1), 167–182 CrossRef PubMed.
- L. P. Lingamdinne, J. R. Koduru, Y.-Y. Chang and R. R. Karri, Process optimization and adsorption modeling of Pb (II) on nickel ferrite-reduced graphene oxide nano-composite, J. Mol. Liq., 2018, 250, 202–211 CrossRef CAS.
- K. F. Amin, F. Gulshan, F. Asrafuzzaman, H. Das, R. Rashid and S. M. Hoque, Synthesis of mesoporous silica and chitosan-coated magnetite nanoparticles for heavy metal adsorption from wastewater, Environ. Nanotechnol., Monit. Manage., 2023, 20, 100801 CAS.
- S. Bosu, N. Rajamohan and M. Rajasimman, Enhanced remediation of lead (II) and cadmium (II) ions from aqueous media using porous magnetic nanocomposites-A comprehensive review on applications and mechanism, Environ. Res., 2022, 213, 113720 CrossRef CAS PubMed.
- S. G. Muntean, L. Halip, M. A. Nistor and C. Păcurariu, Removal of metal ions via adsorption using carbon magnetic nanocomposites: optimization through response surface methodology, kinetic and thermodynamic studies, Magnetochemistry, 2023, 9(7), 163 Search PubMed.
- T. Tolessa, X.-X. Zhou, M. Amde and J.-F. Liu, Development of reusable magnetic chitosan microspheres adsorbent for selective extraction of trace level silver nanoparticles in environmental waters prior to ICP-MS analysis, Talanta, 2017, 169, 91–97 CrossRef CAS PubMed.
- Y. Zhao, C. Wang, S. Wang, C. Wang, Y. Liu, A. A. Al-Khalaf, W. N. Hozzein, L. Duan, W. Li and D. Zhao, Magnetic mesoporous TiO 2 microspheres for sustainable arsenate removal from acidic environments, Inorg. Chem. Front., 2018, 5(9), 2132–2139 RSC.
- Y. Pang, J. Yu, L. Tang, G. Zeng, C. Zhu and X. Wei, Magnetic nanohybrid materials for water-pollutant removal, in Nanohybrid and Nanoporous Materials for Aquatic Pollution Control, Elsevier, 2019, pp. 1–30 Search PubMed.
- Z. Wang, J. Zhang, T. Wen, X. Liu, Y. Wang, H. Yang, J. Sun, J. Feng, S. Dong and J. Sun, Highly effective remediation of Pb (II) and Hg (II) contaminated wastewater and soil by flower-like magnetic MoS2 nanohybrid, Sci. Total Environ., 2020, 699, 134341 CrossRef CAS PubMed.
- K. Dai, G. Liu, W. Xu, Z. Deng, Y. Wu, C. Zhao and Z. Zhang, Judicious fabrication of bifunctionalized graphene oxide/MnFe2O4 magnetic nanohybrids for enhanced removal of Pb (II) from water, J. Colloid Interface Sci., 2020, 579, 815–822 CrossRef CAS PubMed.
- J. Z. Pirhaji, F. Moeinpour, A. M. Dehabadi and S. A. Y. Ardakani, Synthesis and characterization of halloysite/graphene quantum dots magnetic nanocomposite as a new adsorbent for Pb (II) removal from water, J. Mol. Liq., 2020, 300, 112345 CrossRef.
- H. Abdolmohammad-Zadeh, Z. Ayazi and K. Nezami, Magnetic solid-phase extraction based on Ni–Al layered double hydroxide/magnetite nano-hybrid for speciation of Mn (vii)/Mn (ii) in water samples by FAAS, Anal. Methods, 2019, 11(4), 462–471 RSC.
- K. M. M. Katubi, N. S. Alsaiari, F. M. Alzahrani, S. M. Siddeeg and M. A. Tahoon, Synthesis of manganese ferrite/graphene oxide magnetic nanocomposite for pollutants removal from water, Processes, 2021, 9(4), 589 CrossRef CAS.
- M. A. Ahmed, M. A. Ahmed and A. A. Mohamed, Facile adsorptive removal of dyes and heavy metals from wastewaters using magnetic nanocomposite of zinc ferrite@ reduced graphene oxide, Inorg. Chem. Commun., 2022, 144, 109912 CrossRef CAS.
- M. D. Dung, T. T. V. Nga, N. T. Lan and N. K. Thanh, Adsorption behavior and mechanism of As (V) on magnetic Fe3O4–graphene oxide (GO) nanohybrid composite material, Anal. Sci., 2022, 38(2), 427–436 Search PubMed.
- S. Ko, E. S. Kim, S. Park, H. Daigle, T. E. Milner, C. Huh, M. V. Bennetzen and G. A. Geremia, RETRACTED ARTICLE: Amine functionalized magnetic nanoparticles for removal of oil droplets from produced water and accelerated magnetic separation, J. Nanopart. Res., 2017, 19, 1–14 CrossRef CAS.
- O. Saber, N. Mohamed and S. Arafat, Conversion of iron oxide nanosheets to advanced magnetic nanocomposites for oil spill removal, RSC Adv., 2015, 5(89), 72863–72871 Search PubMed.
- A. M. Atta, H. A. Al-Lohedan and S. A. Al-Hussain, Functionalization of magnetite nanoparticles as oil spill collector, Int. J. Mol. Sci., 2015, 16(4), 6911–6931 CrossRef CAS PubMed.
- M. Sharma, M. Joshi, S. Nigam, D. K. Avasthi, R. Adelung, S. K. Srivastava and Y. K. Mishra, Efficient oil removal from wastewater based on polymer coated superhydrophobic tetrapodal magnetic nanocomposite adsorbent, Appl. Mater. Today, 2019, 17, 130–141 CrossRef.
- L. Shao, Z. Yu, X. Li, H. Zeng and Y. Liu, One-step preparation of sepiolite/graphene oxide membrane for multifunctional oil-in-water emulsions separation, Appl. Clay Sci., 2019, 181, 105208 CrossRef.
- D. Qian, D. Chen, N. Li, Q. Xu, H. Li, J. He and J. Lu, TiO2/sulfonated graphene oxide/Ag nanoparticle membrane: In situ separation and photodegradation of oil/water emulsions, J. Membr. Sci., 2018, 554, 16–25 Search PubMed.
- T. Zhang, C. Xiao, J. Zhao, X. Liu, D. Ji and H. Zhang, One-step facile fabrication of PVDF/graphene composite nanofibrous membrane with enhanced oil affinity for highly efficient gravity-driven emulsified oil/water separation and selective oil absorption, Sep. Purif. Technol., 2021, 254, 117576 CrossRef CAS.
- Y. Cheng, A. Barras, S. Lu, W. Xu, S. Szunerits and R. Boukherroub, Fabrication of superhydrophobic/superoleophilic functionalized reduced graphene oxide/polydopamine/PFDT membrane for efficient oil/water separation, Sep. Purif. Technol., 2020, 236, 116240 CrossRef CAS.
- H. Shokry, M. Elkady and E. Salama, Eco-friendly magnetic activated carbon nano-hybrid for facile oil spills separation, Sci. Rep., 2020, 10(1), 10265 Search PubMed.
- Q. Yuan, N. Li, W. Geng, Y. Chi and X. Li, Preparation of magnetically recoverable Fe3O4@ SiO2@ meso-TiO2 nanocomposites with enhanced photocatalytic ability, Mater. Res. Bull., 2012, 47(9), 2396–2402 CrossRef CAS.
- H. Aliyan, R. Fazaeli and R. Jalilian, Fe3O4@ mesoporous SBA-15: A magnetically recoverable catalyst for photodegradation of malachite green, Appl. Surf. Sci., 2013, 276, 147–153 Search PubMed.
- B. Sahoo, S. K. Sahu, S. Nayak, D. Dhara and P. Pramanik, Fabrication of magnetic mesoporous manganese ferrite nanocomposites as efficient catalyst for degradation of dye pollutants, Catal. Sci. Technol., 2012, 2(7), 1367–1374 Search PubMed.
- Y. Ni, Y. Zhu and X. Ma, A simple solution combustion route for the preparation of metal-doped TiO 2 nanoparticles and their photocatalytic degradation properties, Dalton Trans., 2011, 40(14), 3689–3694 RSC.
- S. B. F. Santos, L. R. Hollanda, Y. Vieira, G. L. Dotto, E. L. Foletto and O. Chiavone-Filho, Enhanced UV-light driven photocatalytic performance of magnetic CoFe2O4/TiO2 nanohybrid for environmental applications, Environ. Sci. Pollut. Res., 2023, 30(30), 75078–75088 CrossRef CAS PubMed.
- G. U. Rehman, A. Ismail, P. Goh, M. R.-D. Arzhandi and N. Ismail, Aptes and teos modified binary recyclable hybrid Fe3O4@ GO nanocomposite for photocatalytic dye removal, Jurnal Teknologi, 2018, 80(4), 157–164 Search PubMed.
- S. Vigneshwaran, P. Sirajudheen, C. P. Nabeena, V. P. Sajna and S. Meenakshi, Photocatalytic performance of chitosan tethered magnetic Fe2O3-like (3D/2D) hybrid for the dynamic removal of anionic dyes: degradation and mechanistic pathways, Int. J. Biol. Macromol., 2021, 183, 2088–2099 CrossRef CAS PubMed.
- M. Ghiyasiyan-Arani, M. Salavati-Niasari and S. Naseh, Enhanced photodegradation of dye in waste water using iron vanadate nanocomposite; ultrasound-assisted preparation and characterization, Ultrason. Sonochem., 2017, 39, 494–503 CrossRef CAS PubMed.
- A. Sobhani-Nasab, S. Pourmasoud, F. Ahmadi, M. Wysokowski, T. Jesionowski, H. Ehrlich and M. Rahimi-Nasrabadi, Synthesis and characterization of MnWO4/TmVO4 ternary nano-hybrids by an ultrasonic method for enhanced photocatalytic activity in the degradation of organic dyes, Mater. Lett., 2019, 238, 159–162 CrossRef CAS.
- M. Roostaee and I. Sheikhshoaie, Low-temperature synthesis of hetero-structures of magnetically separable iron oxide@ Au-rGO nanocomposite for efficient degradation of organic dye under visible light irradiation, Environ. Res., 2022, 205, 112510 Search PubMed.
- L. Yu, L. Wang, X. Sun and D. Ye, Enhanced photocatalytic activity of rGO/TiO2 for the decomposition of formaldehyde under visible light irradiation, J. Environ. Sci., 2018, 73, 138–146 CrossRef CAS PubMed.
- J. Wilkinson, P. S. Hooda, J. Barker, S. Barton and J. Swinden, Occurrence, fate and transformation of emerging contaminants in water: An overarching review of the field, Environ. Pollut., 2017, 231, 954–970 CrossRef CAS PubMed.
- I. A. Al-Baldawi, A. A. Mohammed, Z. H. Mutar, S. R. S. Abdullah, S. S. Jasim and A. F. Almansoory, Application of phytotechnology in alleviating pharmaceuticals and personal care products (PPCPs) in wastewater: Source, impacts, treatment, mechanisms, fate, and SWOT analysis, J. Cleaner Prod., 2021, 319, 128584 CrossRef CAS.
- M. Rostami, R. M. Zamani, K. M. Aghajanzadeh and H. Danafar, Sol–gel synthesis and characterization of zinc ferrite–graphene nano-hybrids for photo-catalytic degradation of the paracetamol, J. Pharm. Invest., 2018, 48, 657–664 CrossRef CAS.
- Y. Tang, X. Liu, C. Ma, M. Zhou, P. Huo, L. Yu, J. Pan, W. Shi and Y. Yan, Enhanced photocatalytic degradation of tetracycline antibiotics by reduced graphene oxide–CdS/ZnS heterostructure photocatalysts, New J. Chem., 2015, 39(7), 5150–5160 RSC.
- C. Cui, Y. Wang, D. Liang, W. Cui, H. Hu, B. Lu, S. Xu, X. Li, C. Wang and Y. Yang, Photo-assisted synthesis of Ag3PO4/reduced graphene oxide/Ag heterostructure photocatalyst with enhanced photocatalytic activity and stability under visible light, Appl. Catal., B, 2014, 158, 150–160 CrossRef.
- C. Yang, X. You, J. Cheng, H. Zheng and Y. Chen, A novel visible-light-driven In-based MOF/graphene oxide composite photocatalyst with enhanced photocatalytic activity toward the degradation of amoxicillin, Appl. Catal., B, 2017, 200, 673–680 CrossRef CAS.
- L. Ye, J. Liu, Z. Jiang, T. Peng and L. Zan, Facets coupling of BiOBr-g-C3N4 composite photocatalyst for enhanced visible-light-driven photocatalytic activity, Appl. Catal., B, 2013, 142, 1–7 Search PubMed.
- H. Cheng, B. Huang, Y. Dai, X. Qin and X. Zhang, One-step synthesis of the nanostructured AgI/BiOI composites with highly enhanced visible-light photocatalytic performances, Langmuir, 2010, 26(9), 6618–6624 CrossRef CAS PubMed.
- T. R. Bastami, A. Ahmadpour and F. A. Hekmatikar, Synthesis of Fe3O4/Bi2WO6 nanohybrid for the photocatalytic degradation of pharmaceutical ibuprofen under solar light, J. Ind. Eng. Chem., 2017, 51, 244–254 CrossRef.
- T. R. Bastami and M. H. Entezari, Activated carbon from carrot dross combined with magnetite nanoparticles for the efficient removal of p-nitrophenol from aqueous solution, Chem. Eng. J., 2012, 210, 510–519 CrossRef CAS.
- A. Dolbecq, P. Mialane, B. Keita and L. Nadjo, Polyoxometalate-based materials for efficient solar and visible light harvesting: application to the photocatalytic degradation of azo dyes, J. Mater. Chem., 2012, 22(47), 24509–24521 RSC.
- M. H. Sayadi, N. Ahmadpour and S. Homaeigohar, Photocatalytic and antibacterial properties of Ag-CuFe2O4@ WO3 magnetic nanocomposite, Nanomaterials, 2021, 11(2), 298 CrossRef CAS PubMed.
- X. Xie, Y. Liu, X. Dong, C. Lin, X. Wen and Q. Yan, Synthesis and characterization of Fe3O4/BiOI n-p heterojunction magnetic photocatalysts, Appl. Surf. Sci., 2018, 455, 742–747 Search PubMed.
- A. Kumar, M. Khan, L. Fang and I. M. Lo, Visible-light-driven N-TiO2@ SiO2@ Fe3O4 magnetic nanophotocatalysts: synthesis, characterization, and photocatalytic degradation of PPCPs, J. Hazard. Mater., 2019, 370, 108–116 CrossRef CAS PubMed.
- M. Khan, C. S. Fung, A. Kumar and I. M. Lo, Magnetically separable BiOBr/Fe3O4@ SiO2 for visible-light-driven photocatalytic degradation of ibuprofen: mechanistic investigation and prototype development, J. Hazard. Mater., 2019, 365, 733–743 CrossRef CAS PubMed.
- L. Fernández, M. Gamallo, M. González-Gómez, C. Vázquez-Vázquez, J. Rivas, M. Pintado and M. Moreira, Insight into antibiotics removal: Exploring the photocatalytic performance of a Fe3O4/ZnO nanocomposite in a novel magnetic sequential batch reactor, J. Environ. Manage., 2019, 237, 595–608 CrossRef PubMed.
- N. Nasseh, T. J. Al-Musawi, M. R. Miri, S. Rodriguez-Couto and A. H. Panahi, A comprehensive study on the application of FeNi3@ SiO2@ ZnO magnetic nanocomposites as a novel photo-catalyst for degradation of tamoxifen in the presence of simulated sunlight, Environ. Pollut., 2020, 261, 114127 Search PubMed.
- S. Mirsadeghi, H. Zandavar, M. Yousefi, H. R. Rajabi and S. M. Pourmortazavi, Green-photodegradation of model pharmaceutical contaminations over biogenic Fe3O4/Au nanocomposite and antimicrobial activity, J. Environ. Manage., 2020, 270, 110831 Search PubMed.
- N. Ahmadpour, M. H. Sayadi, S. Sobhani and M. Hajiani, A potential natural solar light active photocatalyst using magnetic ZnFe2O4@ TiO2/Cu nanocomposite as a high performance and recyclable platform for degradation of naproxen from aqueous solution, J. Cleaner Prod., 2020, 268, 122023 Search PubMed.
- J. Wang, Q. Zhang, F. Deng, X. Luo and D. D. Dionysiou, Rapid toxicity elimination of organic pollutants by the photocatalysis of environment-friendly and magnetically recoverable step-scheme SnFe2O4/ZnFe2O4 nano-heterojunctions, Chem. Eng. J., 2020, 379, 122264 Search PubMed.
- G. Xu, M. Du, T. Li, Y. Guan and C. Guo, Facile synthesis of magnetically retrievable Fe3O4/BiVO4/CdS heterojunction composite for enhanced photocatalytic degradation of tetracycline under visible light, Sep. Purif. Technol., 2021, 275, 119157 CrossRef CAS.
- I. Gabelica, L. Ćurković, V. Mandić, I. Panžić, D. Ljubas and K. Zadro, Rapid microwave-assisted synthesis of Fe3O4/SiO2/TiO2 core-2-layer-shell nanocomposite for photocatalytic degradation of ciprofloxacin, Catalysts, 2021, 11(10), 1136 CrossRef CAS.
- A. Behera, S. Mansingh, K. K. Das and K. Parida, Synergistic ZnFe2O4-carbon allotropes nanocomposite photocatalyst for norfloxacin degradation and Cr (VI) reduction, J. Colloid Interface Sci., 2019, 544, 96–111 CrossRef CAS PubMed.
- T. He, Y. Wu, C. Jiang, Z. Chen, Y. Wang, G. Liu, Z. Xu, G. Ning, X. Chen and Y. Zhao, Novel magnetic Fe3O4/g-C3N4/MoO3 nanocomposites with highly enhanced photocatalytic activities: visible-light-driven degradation of tetracycline from aqueous environment, PLoS One, 2020, 15(8), e0237389 CrossRef CAS PubMed.
- S. Raha and M. Ahmaruzzaman, Enhanced performance of a novel superparamagnetic g-C3N4/NiO/ZnO/Fe3O4 nanohybrid photocatalyst for removal of esomeprazole: Effects of reaction parameters, co-existing substances and water matrices, Chem. Eng. J., 2020, 395, 124969 CrossRef CAS.
- M. Pourshaban-Mazandarani and A. Nasiri, Photocatalytic degradation of tetracycline in wastewater with bio-based matrix magnetic heterogeneous nanocatalyst: performance and mechanism study, J. Polym. Environ., 2024, 1–25 Search PubMed.
- R. K. Gautam and I. Tiwari, Humic acid functionalized magnetic nanomaterials for remediation of dye wastewater under ultrasonication: Application in real water samples, recycling and reuse of nanosorbents, Chemosphere, 2020, 245, 125553 Search PubMed.
- I. Fatimah, E. Z. Pratiwi and W. P. Wicaksono, Synthesis of magnetic nanoparticles using Parkia speciosa Hassk pod extract and photocatalytic activity for Bromophenol blue degradation, Egypt. J. Aquat. Res., 2020, 46(1), 35–40 CrossRef.
- A. Kumar, G. Sharma, M. Naushad and S. Thakur, SPION/β-cyclodextrin core–shell nanostructures for oil spill remediation and organic pollutant removal from waste water, Chem. Eng. J., 2015, 280, 175–187 CrossRef CAS.
- S. Bakhshi Nejad and A. Mohammadi, Epoxy-triazinetrione-functionalized magnetic nanoparticles as an efficient magnetic nanoadsorbent for the removal of malachite green and Pb (II) from aqueous solutions, J. Chem. Eng. Data, 2020, 65(5), 2731–2742 CrossRef CAS.
- C. Das, S. Sen, T. Singh, T. Ghosh, S. S. Paul, T. W. Kim, S. Jeon, D. K. Maiti, J. Im and G. Biswas, Green synthesis, characterization and application of natural product coated magnetite nanoparticles for wastewater treatment, Nanomaterials, 2020, 10(8), 1615 CrossRef CAS PubMed.
- R. Nodehi, H. Shayesteh and A. Rahbar-Kelishami, Fe3O4@NiO core–shell magnetic nanoparticle for highly efficient removal of Alizarin red S anionic dye, Int. J. Environ. Sci. Technol., 2022, 19(4), 2899–2912 CrossRef CAS.
- M. N. Begam, K. Muthukumaran and P. Thamarai, Application of Musa acuminata mediated iron oxide nanoparticles in the removal of sunset yellow dye, Desalin. Water Treat., 2024, 317, 100022 CrossRef CAS.
- R. A. Azeez and F. K. I. Al-Zuhairi, Biosorption of dye by immobilized yeast cells on the surface of magnetic nanoparticles, Alexandria Eng. J., 2022, 61(7), 5213–5222 CrossRef.
- C. V. Reddy, I. N. Reddy, K. Ravindranadh, K. R. Reddy, N. P. Shetti, D. Kim, J. Shim and T. M. Aminabhavi, Copper-doped ZrO2 nanoparticles as high-performance catalysts for efficient removal of toxic organic pollutants and stable solar water oxidation, J. Environ. Manage., 2020, 260, 110088 Search PubMed.
- A. H. Nordin, S. Wong, N. Ngadi, M. M. Zainol, N. A. F. Abd Latif and W. Nabgan, Surface functionalization of cellulose with polyethyleneimine and magnetic nanoparticles for efficient removal of anionic dye in wastewater, J. Environ. Chem. Eng., 2021, 9(1), 104639 CrossRef CAS.
- A. Peyghami, A. Moharrami, Y. Rashtbari, S. Afshin, M. Vosuoghi and A. Dargahi, Evaluation of the efficiency of magnetized clinoptilolite zeolite with Fe3O4 nanoparticles on the removal of basic violet 16 (BV16) dye from aqueous solutions, J. Dispersion Sci. Technol., 2023, 44(2), 278–287 Search PubMed.
- E. Ghoohestani, F. Samari, A. Homaei and S. Yosuefinejad, A facile strategy for preparation of Fe3O4 magnetic nanoparticles using Cordia myxa leaf extract and investigating its adsorption activity in dye removal, Sci. Rep., 2024, 14(1), 84 Search PubMed.
- A. N. Tiwari, K. Tapadia and C. Thakur, A Facile Synthesis of MgO Decorated Spinel Magnetic Nanoparticles Using Terminalia catappa Leaf Extract: Its Application in EBT Dye Removal, Chem. Afr., 2024, 1–22 Search PubMed.
- N. S. Ali, E. H. Khader, M. A. Abdulrahman, I. K. Salih and T. M. Albayati, Removal of anionic azo dye from wastewater using Fe3O4 magnetic nanoparticles adsorbents in a batch system, Desalin. Water Treat., 2024, 317, 100033 CrossRef CAS.
- H. Li, H. Jin, R. Li, J. Hua, Z. Zhang and R. Li, Magnetic Fe3O4@ SiO2 study on adsorption of methyl orange on nanoparticles, Sci. Rep., 2024, 14(1), 1217 CrossRef CAS PubMed.
- S. J. Salih, L. I. Abd Ali and W. M. Hamad, Novel synthesis and characterization of magnesium-doped CoFe2O4 nanoparticles–SiO2–3-aminopropylethoxysilane–gallic acid magnetic nanocomposite for effective removal of cationic dyes, Arabian J. Chem., 2024, 17(3), 105647 CrossRef CAS.
- A. Hethnawi, O. Kashif, R. Jeong, F. Sagala, K. Hashlamoun, A. D. Manasrah and N. N. Nassar, Green synthesis of novel titanomagnetite nanoparticles for oil spill cleanup, Colloids Surf., A, 2023, 664, 131191 CrossRef CAS.
- Y. Vicente-Martínez, M. Caravaca and A. Soto-Meca, Total removal of Hg (II) from wastewater using magnetic nanoparticles coated with nanometric Ag and functionalized with sodium 2-mercaptoethane sulfonate, Environ. Chem. Lett., 2020, 18(3), 975–981 CrossRef.
- A. Peigneux, J. D. Puentes-Pardo, A. B. Rodríguez-Navarro, M. T. Hincke and C. Jimenez-Lopez, Development and characterization of magnetic eggshell membranes for lead removal from wastewater, Ecotoxicol. Environ. Saf., 2020, 192, 110307 CrossRef CAS PubMed.
- M. Govarthanan, C. H. Jeon, Y. H. Jeon, J. H. Kwon, H. Bae and W. Kim, Non-toxic nano approach for wastewater treatment using Chlorella vulgaris exopolysaccharides immobilized in iron-magnetic nanoparticles, Int. J. Biol. Macromol., 2020, 162, 1241–1249 CrossRef CAS PubMed.
- M. Ahmad, J. Wang, J. Xu, Q. Zhang and B. Zhang, Magnetic tubular carbon nanofibers as efficient Cu(II) ion adsorbent from wastewater, J. Cleaner Prod., 2020, 252, 119825 Search PubMed.
- I. K. Rind, N. Memon, A. Sarı, M. Y. Khuhawar, M. Tuzen, S. N. ul Hasan, A. A. Memon, W. A. Soomro, R. O. Z. Brohi and T. A. Saleh, Magnetic nanoparticles loaded hydrochar for effective Cr (VI) removal from water: Batch and column studies, Mater. Chem. Phys., 2024, 318, 129077 CrossRef CAS.
- H. Sánchez-Moreno, L. García-Rodríguez and C. Recalde-Moreno, Natural cellulose fibers (Agave Americana L. ASPARAGACEAE) impregnated with magnetite nanoparticles as a novel adsorbent of mercury (Hg) in aqueous solutions, Adsorption, 2025, 31(1), 1–22 Search PubMed.
- F. Almomani, R. Bhosale, M. Khraisheh, A. kumar and T. Almomani, Heavy metal ions removal from industrial wastewater using magnetic nanoparticles (MNP), Appl. Surf. Sci., 2020, 506, 144924 Search PubMed.
- A. K. R. Kumar, K. Saikia, G. Neeraj, H. Cabana and V. V. Kumar, Remediation of bio-refinery wastewater containing organic and inorganic toxic pollutants by adsorption onto chitosan-based magnetic nanosorbent, Water Qual. Res. J., 2020, 55(1), 36–51 Search PubMed.
- A. Masjedi, E. Askarizadeh and S. Baniyaghoob, Magnetic nanoparticles surface-modified with tridentate ligands for removal of heavy metal ions from water, Mater. Chem. Phys., 2020, 249, 122917 Search PubMed.
- Q. Xu, W. Li, L. Ma, D. Cao, G. Owens and Z. Chen, Simultaneous removal of ammonia and phosphate using green synthesized iron oxide nanoparticles dispersed onto zeolite, Sci. Total Environ., 2020, 703, 135002 CrossRef CAS PubMed.
- N. Zhu, B. Zhang and Q. Yu, Genetic engineering-facilitated coassembly of synthetic bacterial cells and magnetic nanoparticles for efficient heavy metal removal, ACS Appl. Mater. Interfaces, 2020, 12(20), 22948–22957 CrossRef CAS PubMed.
- A. N. Hosain, A. El Nemr, A. El Sikaily, M. E. Mahmoud and M. F. Amira, Surface modifications of nanochitosan coated magnetic nanoparticles and their applications in Pb (II), Cu (II) and Cd (II) removal, J. Environ. Chem. Eng., 2020, 8(5), 104316 Search PubMed.
- Y. Wang, S. Yu, H. Yuan and L. Zhang, Constructing N, S co-doped network biochar confined CoFe2O4 magnetic nanoparticles adsorbent: Insights into the synergistic and competitive adsorption of Pb2+ and ciprofloxacin, Environ. Pollut., 2024, 343, 123178 CrossRef CAS PubMed.
- F. H. Alkallas, S. M. Alghamdi, O. Albeydani, E. A. Mwafy, A. B. G. Trabelsi, W. B. Elsharkawy, E. Alsubhe and A. M. Mostafa, Effect of ablation time for loading amounts of magnetic nanoparticles on CNTs for removal of Pb (II) ions from aqueous solution, Appl. Phys. A:Mater. Sci. Process., 2024, 130(2), 128 Search PubMed.
- J. C. Almeida, C. E. Cardoso, M. C. Neves, T. Trindade, M. G. Freire and E. Pereira, Extraction of platinum and palladium from aqueous solutions using ionic-liquid-modified magnetic nanoparticles, J. Water Proc. Eng., 2024, 63, 105510 CrossRef.
- N. Mehrabi, U. F. A. Haq, M. T. Reza and N. Aich, Application of deep eutectic solvent for conjugation of magnetic nanoparticles onto graphene oxide for lead (II) and methylene blue removal, J. Environ. Chem. Eng., 2020, 8(5), 104222 CrossRef CAS.
- A. Saravanan, P. S. Kumar, M. Govarthanan, C. S. George, S. Vaishnavi, B. Moulishwaran, S. P. Kumar, S. Jeevanantham and P. R. Yaashikaa, Adsorption characteristics of magnetic nanoparticles coated mixed fungal biomass for toxic Cr(VI) ions in aquatic environment, Chemosphere, 2021, 267, 129226 CrossRef CAS PubMed.
- F. Hassanzadeh-Afruzi, G. Heidari and A. Maleki, Magnetic nanocomposite hydrogel based on Arabic gum for remediation of lead (II) from contaminated water, Mater. Chem. Horiz., 2022, 1(2), 107–122 Search PubMed.
- N. Karami, A. Mohammadpour, M. R. Samaei, A. M. Amani, M. Dehghani, R. S. Varma and J. N. Sahu, Green synthesis of sustainable magnetic nanoparticles Fe3O4 and Fe3O4-chitosan derived from Prosopis farcta biomass extract and their performance in the sorption of lead(II), Int. J. Biol. Macromol., 2024, 254, 127663 CrossRef CAS PubMed.
- T. Lei, X. Jiang, Y. Zhou, H. Chen, H. Bai, S. Wang and X. Yang, A multifunctional adsorbent based on 2,3-dimercaptosuccinic acid/dopamine-modified magnetic iron oxide nanoparticles for the removal of heavy-metal ions, J. Colloid Interface Sci., 2023, 636, 153–166 Search PubMed.
- H. Zeng, L. Zhai, J. Zhang and D. Li, As(V) adsorption by a novel core-shell magnetic nanoparticles prepared with Iron-containing water treatment residuals, Sci. Total Environ., 2021, 753, 142002 CrossRef CAS PubMed.
- N. Rafie, M. Khodadadi, M. Zamani, A. Zarepour and A. Zarrabi, Magnetic silica nanoparticles adorned with a metal-organic framework; a novel nanosorbent for elimination of aqueous Pb ions contaminant, Environ. Res., 2023, 226, 115694 Search PubMed.
- A. H. Ragab, M. F. Mubarak, H. A. El-Sabban, J. Kang, A. El Shahawy, H. A. Alshwyeh and M. Hemdan, Exploring the sustainable synthesis pathway and comprehensive characterization of magnetic hybrid alumina nanoparticles phase (MHAl-NPsP) as highly efficient adsorbents and selective copper ions removal, Environ. Technol. Innovation, 2024, 34, 103628 Search PubMed.
- A. H. Birniwa, R. E. A. Mohammad, M. Ali, M. F. Rehman, S. S. a. Abdullahi, S. M. Eldin, S. Mamman, A. C. Sadiq and A. H. Jagaba, Synthesis of gum Arabic magnetic nanoparticles for adsorptive removal of ciprofloxacin: equilibrium, kinetic, thermodynamics studies, and optimization by response surface methodology, Separations, 2022, 9(10), 322 CrossRef CAS.
- M. Yilmaz, T. J. Al-Musawi, M. k. Saloot, A. D. Khatibi, M. Baniasadi and D. Balarak, Synthesis of activated carbon from Lemna minor plant and magnetized with iron (III) oxide magnetic nanoparticles and its application in removal of Ciprofloxacin, Biomass Convers. Biorefin., 2022, 1–14 Search PubMed.
- R. M. Abdelhameed, O. M. Darwesh and M. El-Shahat, Titanium-based metal-organic framework capsulated with magnetic nanoparticles: Antimicrobial and photocatalytic degradation of pesticides, Microporous Mesoporous Mater., 2023, 354, 112543 CrossRef CAS.
- A. Sheikhmohammadi, E. Asgari and J. Yeganeh, Application of Fe3O4@ activated carbon magnetic nanoparticles for the adsorption of metronidazole from wastewater: optimization, kinetics, thermodynamics and equilibrium studies, Desalin. Water Treat., 2021, 222, 354–365 Search PubMed.
- T. J. Al-Musawi, A. H. Mahvi, A. D. Khatibi and D. Balarak, Effective adsorption of ciprofloxacin antibiotic using powdered activated carbon magnetized by iron(III) oxide magnetic nanoparticles, J. Porous Mater., 2021, 28(3), 835–852 CrossRef CAS.
- L. F. Cusioli, H. B. Quesada, A. L. d. B. P. Castro, R. G. Gomes and R. Bergamasco, Development of a new low-cost adsorbent functionalized with iron nanoparticles for removal of metformin from contaminated water, Chemosphere, 2020, 247, 125852 Search PubMed.
- M. Rouhani, S. D. Ashrafi, K. Taghavi, M. N. Joubani and J. Jaafari, Evaluation of tetracycline removal by adsorption method using magnetic iron oxide nanoparticles (Fe3O4) and clinoptilolite from aqueous solutions, J. Mol. Liq., 2022, 356, 119040 Search PubMed.
- M. Moghaddam-Manesh, R. Darvishi, A. Barati, A. Moshkriz and M. Seyedsharifi, Synthesis of novel Fe3O4@ gly@ Indole@ CuNO3 magnetic nanoparticle as high-performance antibiotic absorbent, antimicrobial agent, and reusable magnetic Nano catalyst, J. Environ. Chem. Eng., 2025, 13(1), 114970 Search PubMed.
- J. S. Algethami, K. K. Yadav, A. Gacem, I. H. Ali, S. Rezania, M. S. O. Alhar, A. Mezni, B.-H. Jeon and S. Chaiprapat, Magnetic sporopollenin supported magnesium nanoparticles for removal of tetracycline as an emerging contaminant from water, Environ. Sci. Pollut. Res., 2024, 31(28), 40257–40268 CrossRef CAS PubMed.
- F. Ghadami, M. Valian, F. Atoof, E. A. Dawi, M. B. Miranzadeh, M. A. Mahdi and M. Salavati-Niasari, Response surface methodology for optimization of operational parameters to remove tetracycline from contaminated water by new magnetic Ho2MoO6/Fe2O3 nano adsorbent, Results Eng., 2024, 21, 101746 CrossRef CAS.
- M. Khodadadi, T. J. Al-Musawi, H. Kamani, M. F. Silva and A. H. Panahi, The practical utility of the synthesis FeNi3@ SiO2@ TiO2 magnetic nanoparticles as an efficient photocatalyst for the humic acid degradation, Chemosphere, 2020, 239, 124723 CrossRef CAS PubMed.
- A. B. Abou Hammad, A. M. El Nahwary, B. A. Hemdan and A. L. K. Abia, Nanoceramics and novel functionalized silicate-based magnetic nanocomposites as substitutional disinfectants for water and wastewater purification, Environ. Sci. Pollut. Res., 2020, 27, 26668–26680 CrossRef CAS PubMed.
- M. Kumari and S. K. Gupta, A novel process of adsorption cum enhanced coagulation-flocculation spiked with magnetic nanoadsorbents for the removal of aromatic and hydrophobic fraction of natural organic matter along with turbidity from drinking water, J. Cleaner Prod., 2020, 244, 118899 CrossRef CAS.
- S. C. Kollarahithlu and R. M. Balakrishnan, Adsorption of pharmaceuticals pollutants, Ibuprofen, Acetaminophen, and Streptomycin from the aqueous phase using amine functionalized superparamagnetic silica nanocomposite, J. Cleaner Prod., 2021, 294, 126155 CrossRef.
- R. Natarajan, S. Venkataraman, D. S. Rajendran, B. Tamilselvam, H. Zaveri, N. Jeyachandran, H. Prashar and V. K. Vaidyanathan, Adsorption performance of magnetic mesoporous silica microsphere support toward the remediation of acetaminophen from aqueous solution, J. Water Proc. Eng., 2022, 48, 102835 CrossRef.
- A. S. Liyanage, S. Canaday, C. U. Pittman Jr and T. Mlsna, Rapid remediation of pharmaceuticals from wastewater using magnetic Fe3O4/Douglas fir biochar adsorbents, Chemosphere, 2020, 258, 127336 CrossRef CAS PubMed.
- B. D'Cruz, M. Madkour, M. O. Amin and E. Al-Hetlani, Efficient and recoverable magnetic AC-Fe3O4 nanocomposite for rapid removal of promazine from wastewater, Mater. Chem. Phys., 2020, 240, 122109 Search PubMed.
- M. E. Peralta, D. O. Mártire, M. S. Moreno, M. E. Parolo and L. Carlos, Versatile nanoadsorbents based on magnetic mesostructured silica nanoparticles with tailored surface properties for organic pollutants removal, J. Environ. Chem. Eng., 2021, 9(1), 104841 CrossRef CAS.
- R. Natarajan, M. Anil Kumar and V. K. Vaidyanathan, Synthesis and characterization of rhamnolipid based chitosan magnetic nanosorbents for the removal of acetaminophen from aqueous solution, Chemosphere, 2022, 288, 132532 CrossRef CAS PubMed.
- L. A. Al-Khateeb, W. Hakami, M. A. Salam, J. A. Sanari, R. El-Shaheny and M. El-Maghrabey, Solid phase-fabrication of magnetically separable Fe3O4@ graphene nanoplatelets nanocomposite for efficient removal of NSAIDs from wastewater. Perception of adsorption kinetics, thermodynamics, and extra-thermodynamics, Anal. Chim. Acta, 2022, 1223, 340158 CrossRef CAS PubMed.
- G. Wu, Q. Liu, J. Wang, S. Xia, H. Wu, J. Zong, J. Han and W. Xing, Facile fabrication of rape straw biomass fiber/β-CD/Fe3O4 as adsorbent for effective removal of ibuprofen, Ind. Crops Prod., 2021, 173, 114150 CrossRef CAS.
- B. Yao, Z. Luo, S. Du, J. Yang, D. Zhi and Y. Zhou, Sustainable biochar/MgFe2O4 adsorbent for levofloxacin removal: Adsorption performances and mechanisms, Bioresour. Technol., 2021, 340, 125698 CrossRef CAS PubMed.
- Y. Liu, R. Liu, M. Li, F. Yu and C. He, Removal of pharmaceuticals by novel magnetic genipin-crosslinked chitosan/graphene oxide-SO3H composite, Carbohydr. Polym., 2019, 220, 141–148 CrossRef CAS PubMed.
- M. Sun, Q. Sun, C. Zhao, Y. Huang, J. Jiang, W. Ding and H. Zheng, Degradation of diclofenac sodium with low concentration from aqueous milieu through polydopamine-chitosan modified magnetic adsorbent-assisted photo-Fenton process, Sep. Purif. Technol., 2022, 289, 120771 Search PubMed.
- S. Altaf, R. Zafar, W. Q. Zaman, S. Ahmad, K. Yaqoob, A. Syed, A. J. Khan, M. Bilal and M. Arshad, Removal of levofloxacin from aqueous solution by green synthesized magnetite (Fe3O4) nanoparticles using Moringa olifera: Kinetics and reaction mechanism analysis, Ecotoxicol. Environ. Saf., 2021, 226, 112826 CrossRef CAS PubMed.
- M. M. Gaho, G. Z. Memon, J. u. R. Memon, J. B. Arain, A. J. Arain, A. Shah and M. Q. Samejo, Synthesis of novel magnetic molecularly imprinted polymers by solid-phase extraction method for removal of norfloxacin, Chin. J. Anal. Chem., 2022, 50(6), 100079 Search PubMed.
- E. Norabadi, A. Jahantiq and H. Kamani, Synthesis of Fe-TiO2@ Fe3O4 magnetic nanoparticles as a recyclable sonocatalyst for the degradation of 2, 4-dichlorophenol, Environ. Sci. Pollut. Res., 2023, 30(11), 31446–31460 Search PubMed.
- X. Fu, S. Sarker, W. Ma, W. Zhao, Y. Rong and Q. Liu, Novel phenylalanine-modified magnetic ferroferric oxide nanoparticles for ciprofloxacin removal from aqueous solution, J. Colloid Interface Sci., 2023, 632, 345–356 CrossRef CAS PubMed.
- S. Taghavi Fardood, F. Moradnia and T. M. Aminabhavi, Green synthesis of novel Zn0.5Ni0.5FeCrO4 spinel magnetic nanoparticles: Photodegradation of 4-nitrophenol and aniline under visible light irradiation, Environ. Pollut., 2024, 358, 124534 CrossRef CAS PubMed.
- C. Anushree, F. A. Rahim, S. C. Vanithakumari, C. Thinaharan and J. Philip, Electrospun superparamagnetic fibrous composite nanofiber films for enhanced oil spill recovery: Effect of capping and magnetic nanoparticle loading on oil sorption efficiency, Composites, Part A, 2023, 171, 107591 CrossRef CAS.
- X. He, Q. Liu and Z. Xu, Treatment of oily wastewaters using magnetic Janus nanoparticles of asymmetric surface wettability, J. Colloid Interface Sci., 2020, 568, 207–220 CrossRef CAS PubMed.
- M. M. Abdullah, M. S. Ali and H. A. Al-Lohedan, Oil spill cleanup employing surface modified magnetite nanoparticles using two new polyamines, J. Chem., 2023, 2023(1), 7515345 Search PubMed.
- M. M. Abdullah, H. A. Al-Lohedan, A. Al-Khwlani and B. M. Al-Maswari, Efficient oil spill uptake using surface-modified magnetite nanoparticles with pet waste derivatives, ACS Omega, 2023, 8(46), 43955–43963 CrossRef CAS PubMed.
- R. Malhas, J. H. El Achkar, B. Misbah and S. Al Radhwan, Optimizing Oil Removal from Oil-Water Emulsions Using Novel Iron Oxide Magnetic Nanoparticles, Water, Air, Soil Pollut., 2023, 234(9), 564 Search PubMed.
- W. F. Elmobarak and F. Almomani, Application of magnetic nanoparticles for the removal of oil from oil-in-water emulsion: Regeneration/reuse of spent particles, J. Pet. Sci. Eng., 2021, 203, 108591 Search PubMed.
- M. Abdullah and H. A. Al-Lohedan, Facile fabrication of magnetite nanoparticles with new hydrophobic amides and their application in oil spill remediation, Environ. Sci. Pollut. Res., 2024, 31(25), 36986–36994 CrossRef CAS PubMed.
- F. Rezaei and S. Hassanajili, Facile fabrication of superhydrophobic magnetic bio-waste for oil spill cleanup, Ind. Crops Prod., 2023, 201, 116848 CrossRef CAS.
- T. Lü, X. Zhang, R. Ma, D. Qi, Y. Sun, D. Zhang, J. Huang and H. Zhao, Quaternary ammonium siloxane-decorated magnetic nanoparticles for emulsified oil-water separation, Sep. Purif. Technol., 2023, 309, 123097 Search PubMed.
- H. Hamedi, S. Zendehboudi, N. Rezaei, A. Azizi and F. Shahhoseini, Application of functionalized Fe3O4 magnetic nanoparticles using CTAB and SDS for oil separation from oil-in-water nanoemulsions, Langmuir, 2023, 39(23), 7995–8007 Search PubMed.
- T. Lü, D. Qi, D. Zhang, K. Fu, Y. Li and H. Zhao, Fabrication of recyclable multi-responsive magnetic nanoparticles for emulsified oil-water separation, J. Cleaner Prod., 2020, 255, 120293 CrossRef.
- M. Zhang, K. Lian, L. Ai, W. Kang and T. Zhao, Simultaneous determination of 11 antiseptic ingredients in surface water based on polypyrrole decorated magnetic nanoparticles, RSC Adv., 2020, 10(61), 37473–37481 Search PubMed.
- C. Du, Z. Zhang, G. Yu, H. Wu, H. Chen, L. Zhou, Y. Zhang, Y. Su, S. Tan and L. Yang, A review of metal organic framework (MOFs)-based materials for antibiotics removal via adsorption and photocatalysis, Chemosphere, 2021, 272, 129501 CrossRef CAS PubMed.
- C. K. Gadupudi, L. Rice, L. Xiao and K. Kantamaneni, Endocrine disrupting compounds removal methods from wastewater in the United Kingdom: a review, Sci, 2021, 3(1), 11 CrossRef.
- G. G. Hacıosmanoğlu, C. Mejías, J. Martín, J. L. Santos, I. Aparicio and E. Alonso, Antibiotic adsorption by natural and modified clay minerals as designer adsorbents for wastewater treatment: A comprehensive review, J. Environ. Manage., 2022, 317, 115397 CrossRef PubMed.
- M. W. Nugraha, S. Kim, F. Roddick, Z. Xie and L. Fan, A review of the recent advancements in adsorption technology for removing antibiotics from hospital wastewater, J. Water Proc. Eng., 2025, 70, 106960 CrossRef.
- S. Ramu, I. Kainthla, L. Chandrappa, J. M. Shivanna, B. Kumaran and R. G. Balakrishna, Recent advances in metal organic frameworks–based magnetic nanomaterials for waste water treatment, Environ. Sci. Pollut. Res., 2024, 31(1), 167–190 CrossRef CAS PubMed.
- N. Shehata, D. Egirani, A. Olabi, A. Inayat, M. A. Abdelkareem, K.-J. Chae and E. T. Sayed, Membrane-based water and wastewater treatment technologies: Issues, current trends, challenges, and role in achieving sustainable development goals, and circular economy, Chemosphere, 2023, 320, 137993 CrossRef CAS PubMed.
- Y. Miyah, N. El Messaoudi, M. Benjelloun, J. Georgin, D. S. P. Franco, Y. Acikbas, H. S. Kusuma and M. Sillanpää, MOF-derived magnetic nanocomposites as potential formulations for the efficient removal of organic pollutants from water via adsorption and advanced oxidation processes: A review, Mater. Today Sustain., 2024, 100985 Search PubMed.
- Y. GadelHak, M. El-Azazy, M. F. Shibl and R. K. Mahmoud, Cost estimation of synthesis and utilization of nano-adsorbents on the laboratory and industrial scales: A detailed review, Sci. Total Environ., 2023, 875, 162629 CrossRef CAS PubMed.
- Z. Deng, S. Cheng, N. Xu, X. Zhang and B. Pan, Pilot-scale field demonstration of environmental nanotechnology for groundwater defluoridation, ACS ES&T Eng., 2022, 3(2), 226–235 Search PubMed.
- X. Zhang, K. Zhang, Y. Shi, H. Xiang, W. Yang and F. Zhao, Surface engineering of multifunctional nanostructured adsorbents for enhanced wastewater treatment: A review, Sci. Total Environ., 2024, 920, 170951 Search PubMed.
- M. Arienzo and L. Ferrara, Environmental fate of metal nanoparticles in estuarine environments, Water, 2022, 14(8), 1297 Search PubMed.
- S. Ethaib, S. Al-Qutaifia, N. Al-Ansari and S. L. Zubaidi, Function of nanomaterials in removing heavy metals for water and wastewater remediation: A review, Environments, 2022, 9(10), 123 CrossRef.
- M. A. Mudassir, M. Farhan, S. Kousar and S. Akram, Role of magnetic hybrid/composite nanomaterials for removal of metal ions from aqueous matrices, in Functionalized Magnetic Nanohybrids, Elsevier, 2025, pp. 361–388 Search PubMed.
- J. Govan, Recent advances in magnetic nanoparticles and nanocomposites for the remediation of water resources, Magnetochemistry, 2020, 6(4), 49 Search PubMed.
- B. García-Merino, E. Bringas and I. Ortiz, Synthesis and applications of surface-modified magnetic nanoparticles: Progress and future prospects, Rev. Chem. Eng., 2022, 38(7), 821–842 CrossRef.
- Standard I., ISO Environmental Management—Life Cycle Assessment: Principles and Framework, ISO Standard 2006, p. 14040 Search PubMed.
- Standard I., Environmental Management-Life Cycle Assessment-Requirements and Guidelines, ISO, London, 2006 Search PubMed.
- I. Lynch, T. Cedervall, M. Lundqvist, C. Cabaleiro-Lago, S. Linse and K. A. Dawson, The nanoparticle–protein complex as a biological entity; a complex fluids and surface science challenge for the 21st century, Adv. Colloid Interface Sci., 2007, 134, 167–174 CrossRef PubMed.
- S. Bucak, B. Yavuztürk and A. D. Sezer, Magnetic nanoparticles: synthesis, surface modifications and application in drug delivery, Recent Adv. Novel Drug Carrier Syst., 2012, 2, 165–200 Search PubMed.
Footnote |
| † Equal contributors: Sidra Pervaiz & Mohsin Javed. |
| This journal is © The Royal Society of Chemistry 2025 |