Open Access Article
Open Access ArticleZinc(II) ion detection and fluorescence emission properties of a diaminomaleonitrile-derived unsymmetrical Schiff base ligand†
Tesfa Oluma Fufa ,
Hongwei Ma,
Ali R. Ayub,
Karim Y. Nabat,
Yaqoot Khan and
Hui Li
,
Hongwei Ma,
Ali R. Ayub,
Karim Y. Nabat,
Yaqoot Khan and
Hui Li *
*
Key Laboratory of Clusters Science of Ministry of Education, School of Chemistry and Chemical Engineering, Beijing Institute of Technology, 100081, Beijing, P. R. China. E-mail: lihui@bit.edu.cn; Tel: +8613910835773
First published on 10th July 2025
Abstract
A novel Schiff base ligand, 2-amino-3-(((Z)-2,5-dihydroxybenzylidene)amino)maleonitrile hemihydrate (C11H8N4O2·½H2O or H2L), was synthesized by a condensation reaction of diaminomaleonitrile and 2,5-dihydroxybenzaldehyde in ethanol. Its crystal structure, fluorescence (FL) emission and Zn(II)-detection properties were studied. The crystal structure of H2L belongs to a face-centered orthorhombic space group (Fdd2), confirming its crystalline nature. H2L emitted green-colored fluorescence in MeCN, EtOH, and DMF, with varying FL yields of 0.53, 0.68, and 0.95, respectively. This suggests that the solvent environment plays a crucial role in modulating the FL properties of H2L. In DMF, the gradual addition of Zn(II) to the H2L solution resulted in a color change from yellow to orange, accompanied by the appearance of new absorbance bands at 494 nm and two isosbestic points at 300 and 440 nm. These phenomena were not observed either in MeCN or EtOH, indicating that Zn(II)-H2L binding was more pronounced in DMF. Thus, H2L demonstrated sensitive Zn(II) binding, with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding stoichiometry, a high binding constant (3.745 × 107 μM−1), and a low limit of detection (3.455 × 10−6 μM). Overall, the findings highlight the potential of the novel crystalline H2L for efficient FL emission and sensitive Zn(II) detection in DMF, suggesting its potential utilization for fabricating light-emitting organic devices and metal-ion sensors.
1 binding stoichiometry, a high binding constant (3.745 × 107 μM−1), and a low limit of detection (3.455 × 10−6 μM). Overall, the findings highlight the potential of the novel crystalline H2L for efficient FL emission and sensitive Zn(II) detection in DMF, suggesting its potential utilization for fabricating light-emitting organic devices and metal-ion sensors.
1. Introduction
In recent years, Schiff bases have received considerable attention in coordination chemistry and materials science due to their ability to bind metal ions and their interesting optical properties.1–3 In particular, aromatic Schiff bases, such as ortho-hydroxybenzaldehyde groups, have demonstrated strong fluorescence responses through excited-state intramolecular proton transfer (ESIPT).4 ESIPT-active molecules offer distinctive features, such as a large Stokes shift, dual spectra, ultrafast recovery, and sensitivity to the surrounding environment.5 Diaminomaleonitrile-derived Schiff bases have shown remarkable properties in various studies6–10 through their electron-donating and -withdrawing groups, which can modify the electronic properties of the resulting compounds.Zn is an essential trace element in many environmental and biological processes, but an imbalance in Zn(II) ion concentration11 can cause health problems, particularly when Zn is ingested in human nutrition.12 The WHO's13 recommended maximum level of Zn(II) in drinking water is 46 μM. Consequently, several complex analytical detection methods14 have been developed and applied to detect Zn. Thus, the development of novel molecules and simple methods15–18 to detect Zn ions is relevant in both environmental and health/medical fields.19–21 Usually, it is difficult to detect Zn(II) in coordination complexes within the visible range due to the filled 3d orbitals and the weak polarizability of the solvent used during the study. The ligand fields around Zn(II) create highly symmetric environments that can suppress electronic transitions, leading to “spectral silence” in spectroscopic analyses.22,23
To address the aforementioned issues, we developed a novel diaminomaleonitrile-derived unsymmetrical Schiff base, namely, 2-amino-3-(((Z)-2,5-dihydroxybenzylidene)amino)maleonitrile hemihydrate (H2L). The developed H2L is highly polar due to its multiple donor–acceptor functional groups, which contribute to its supramolecular interactions, fluorescence emission, and ionic metal binding. The fluorescence emission and Zn(II) detection potentials of H2L were evaluated in MeCN, EtOH and DMF. The results indicated that H2L emitted green light fluorescence in each solvent, with varying fluorescence yields. In DMF, the gradual addition of Zn(II) into H2L solution resulted in a color change from yellow to orange, accompanied by the appearance of new absorbance bands and isosbestic points, which were not observed either in MeCN or EtOH. This suggests that the solvent environment plays a crucial role in modulating the properties of H2L. This study offers valuable insights into the design of future materials for applications in areas such as fluorescence,24 bio-detection and environmental monitoring,22 and organic electronics and photonic devices.25
2. Experimental section
2.1. Synthesis and recrystallization
H2L was synthesized according to the previous references.9,26 All the chemicals used in the experiments were purchased from Sigma-Aldrich or Merck.In the synthesis, into two beakers each containing EtOH (50 mL), 0.03903 g of 2,5-dihydroxybenzaldehyde (2,5-DHBA) and 0.03055 g of diaminomaleonitrile (DAMN) were added, respectively. The mixtures were stirred on a hot plate for 0.5 h. The first solution was then transferred into a 250 mL round-bottom flask and the second solution was added dropwise followed by 3 mL of AcOH. The mixture was refluxed for 4 h, after which it was allowed to cool to room temperature (RT) for 2 h. The resulting precipitate was filtered by gravity filtration, washed with excess EtOH, and dried at RT for 2 days, giving 0.06 g of H2L (Fig. S1d and S2a†), as obtained by weighing on an analytical balance.
Single crystals of H2L were grown based on a previous study.27 In brief, in a 25 mL beaker, 0.0664 g of H2L was dissolved in H2O (3 mL) and MeCN (5 mL), stirred for 0.5 h at 80 °C, filtered by gravity filtration, covered with a needle-punctured para-film and allowed to stand in a cool place without any perturbation. After 10 days, suitable single crystals were obtained (Fig. S2b†).
2.2. Characterization
 | (1) |
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) CH) and 10.33 (l, 1H, OH).
CH) and 10.33 (l, 1H, OH).![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–).
N–).3. Results and discussion
3.1. Synthesis
Following the previous references,9,26 H2L (Scheme 1) was successfully synthesized via a condensation method by refluxing equal moles of diaminomaleonitrile (DAMN) and 2,5-hydroxybenzaldehyde (2,5-DHBA) in EtOH and AcOH. The solution was then cooled at RT, filtered under vacuum, washed with ethanol, dried at room temperature, and finally the product was recrystallized from MeCN/H2O solution.The percentage yield (Y%) of H2L was calculated using the balanced reaction equation, theoretical yield (TY), actual yield (AY), limiting reactant mole (nLR), LR stoichiometric coefficient (kLR) and molar mass of the product (i.e., MH2L = 228.21 g) using eqn (2) and (3).
 | (2) |
 | (3) |
3.2. Crystal structure
The crystal structure of H2L was determined using Olex2 (ref. 28) with the SHELXT29 structure solution program using Intrinsic Phasing and refined with SHELXL30 using least squares minimization. The SC XRD analysis proved that H2L was crystallized as a hemihydrate (C11H8N4O2·½H2O) and belonged to a face-centered orthorhombic space group (Fdd2). Fig. 1a, Tables 1 and S2–S4† present the molecular structure, crystal data and atomic parameters, respectively. The molecule adopted a planar conformation as a result of the strong H-bonding between the H(1) and N(1) with a distance of 1.985 Å and an angle of 138.75°. Also, the flat nature of H2L may be due to the extended π-conjugation to the peripheral nitrile groups. This allowed us to investigate the supramolecular structures, which have been reported to directly impact the fluorescence properties of compounds.44| Parameter | Data |
|---|---|
| CCDC number | 2402446 |
| Molecular formula | C11H8N4O2·½H2O |
| Formula weight (g mol−1) | 237.22 |
| Temperature (K) | 296.15 |
| Crystal system | Orthorhombic |
| Space group | Fdd2(43) |
| Unit cell dimensions (Å) | a = 19.936(7), b = 57.668(19), c = 3.7770(13) |
| Unit cell angles (°) | α = β = γ = 90 |
| Unit cell volume (Å3) | 4342(3) |
| No. of molecules per unit cell, Z | 16 |
| Calculated density, ρcalc (g cm−3) | 1.451 |
| Linear absorption coefficient, μ (mm−1) | 0.108 |
| F (000) | 1968.0 |
| Crystal size (mm3) | 0.35 × 0.3 × 0.25 |
| Radiation | MoKα (λ = 0.71073) |
| 2θ Range for data collection (°) | 2.824 to 52.48 |
| Index ranges | −19 ≤ h ≤ 15, −44 ≤ k ≤ 56, −3 ≤ l ≤ 3 |
| Reflections collected | 2199 |
| Independent reflections | 1037 [Rint = 0.0743, Rsigma = 0.1073] |
| Data/restraints/parameters | 1037/1/165 |
| Goodness-of-fit on F2 | 1.068 |
| Final R indexes [I ≥ 2σ (I)] | R1 = 0.0675, wR2 = 0.1491 |
| Final R indexes [all data] | R1 = 0.1008, wR2 = 0.1769 |
| Largest diff. peak/hole/e Å−3 | 0.27/−0.25 |
| Flack parameter | −8.8(10) |
Notably, the crystal packing exhibited strong H-bonding (1.94–2.49 Å, Fig. 1b) and π–π stacking (3.375 Å, Fig. 1c) between the adjacent H2L molecules, which may contribute to strong fluorescence properties by allowing a greater excitation energy. In rigid structures, molecular vibrations and rotations are restricted, which reduces the non-radiative relaxations where the energy absorbed is dissipated as heat instead of being emitted as fluorescence. By reducing the non-radiative pathways through increasing the rigidity, the fluorescence potentials can be increased. Due to their orthorhombic symmetry, the H2L molecules were arranged in offset parallel along the crystallographic axis in order to minimize the repulsive forces between the electron-dense regions and the voids between the rings (Fig. 1c). Such condensed molecular arrangements are beneficial for efficient electron transfer among orbitals and is recommended for strong fluorescence emission, particularly in polar organic solvents.
The PXRD pattern of H2L showed a series of sharp peaks at 2θ angles corresponding to the interplanar distances (d) according to Bragg's law (nλ = 2d![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ), Fig. 1d. Thus, the PXRD technique proved the crystallinity and phase purity of H2L, which also agreed with the elemental analysis data.
θ), Fig. 1d. Thus, the PXRD technique proved the crystallinity and phase purity of H2L, which also agreed with the elemental analysis data.
Furthermore, the structure of H2L was supported by various analytical techniques, including FT-IR, 1H NMR and elemental analysis. The elemental analysis showed the presence of C (57.89%), H (3.53%), N (24.55%) and O (14.02%), consistent with the structure. Also, the calculation of the empirical formula ofH2L based on these aforementioned percentages confirmed its purity. The 1H NMR spectrum (Fig. S3†) of H2L in DMSO-d6 showed a singlet at 10.33 ppm (H(1)) and peaks at 9.76, 8.95, 8.50, and 7.83–6.79 ppm corresponding to H(7), H(2), H(4A & 4B), and H(5, 3 & 2), respectively. The downfield shift at 10.33 ppm was due to H(1), which participated in intramolecular hydrogen bonding. The FT-IR spectrum of H2L in a KBr pellet (Fig. S4†) provided evidence of the mono-imine nature (–HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N– at 1627 cm−1), while the nitriles (–HC
N– at 1627 cm−1), while the nitriles (–HC![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N–) stretching modes were split into two bands at 2228 and 2240 cm−1, which were assigned to the symmetric and asymmetric stretching modes, respectively.49 This was in contrast to the single band observed for the symmetric di-imine nature (at 2210 cm−1).31 Also, the narrow peaks between 3197 and 3466 cm−1 were attributed to the –(H–N–H) and –(O–H) stretching vibrations.26
N–) stretching modes were split into two bands at 2228 and 2240 cm−1, which were assigned to the symmetric and asymmetric stretching modes, respectively.49 This was in contrast to the single band observed for the symmetric di-imine nature (at 2210 cm−1).31 Also, the narrow peaks between 3197 and 3466 cm−1 were attributed to the –(H–N–H) and –(O–H) stretching vibrations.26
To investigate the thermal stability, a sample of H2L was heated from 40 °C to 800 °C at a heating rate of 10 °C min−1 under an inert atmosphere, Fig. S13.† The graph's small decline (40–150 °C) indicated the weight loss percentage of hemi-water (4.43%), which was observed in the single-crystal molecular structure, while the observation of a plateau region (150–250 °C) suggested the thermal stability of the ligand after the loss of hemi-water. The next significant weight loss percentage seen at 250 °C was related to the loss percentage of an amine group (12.32%) while the final weight losses percentage (beyond 250 °C) may be due to the gradual decomposition of the other organic moieties.
3.3. Fluorescence study
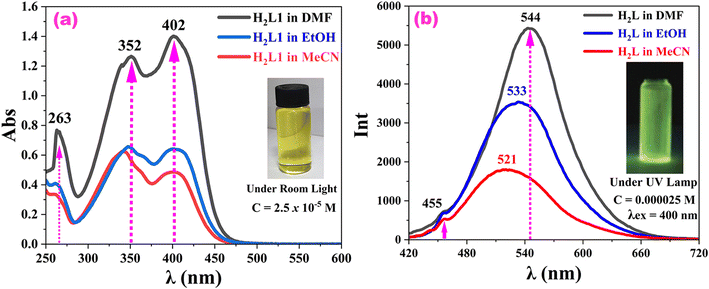
After determining the crystal structure, we investigated the UV-vis absorbance and fluorescence emission spectra of H2L in various polar organic solvents, namely, DMF, EtOH, and MeCN, because it is known that the electronic transitions of Schiff bases are strongly influenced by the nature of the medium.32 In each solution, the color of H2L appeared yellow under room light (see the inset image of Fig. 2a) and green under ultraviolet light (see the inset image of Fig. 2b).First, we studied the absorbance spectra (Fig. 2a) and found that the maximum absorbance wavelength was shifted from 342 nm to 402 nm, which may have been due to the excited-state dipole moment changes, H-bonding strengths (Fig. S6†), and/or due to excited-state protonation.33,34 The influence of the solvents on H2L absorbance increased in order of: MeCN (342 nm) < EtOH (348 nm) < DMF (402 nm). Thus, DMF was the best polar aprotic solvent in this case.35,36 The λmax redshift (402 nm) indicated that DMF could strongly stabilize the H2L excited state. In DMF and between 280 and 600 nm, the absorption spectra of H2L showed two absorption peaks (352 and 402 nm), which were due to the intra-ligand charge transitions (ILCT or n → π*).37,46
Next, we studied the FL emission spectra (Fig. 2b) of H2L in each solvent. Notably, the H2L sample was suspended in each solvent with a concentration of 25 μM, and then excited at 400 nm. The samples exhibited green emission at 544 nm in DMF, 533 nm in EtOH, and 521 nm in MeCN. The inset image in Fig. 1b confirmed this result under UV light (365 nm) irradiation. Further, in each solvent, the FL spectrum of H2L revealed a systematic Stokes's shift (Δλ = λem − λab = 142 nm in DMF, 132 nm in EtOH and 121 nm in MeCN), which reflected the charge-separated nature of the excited states.38,39 Thus, H2L showed the highest charge separation and highest fluorescence in the presence of DMF, whereas the other two solvents were relatively less polar. In each solvent, the weak band around 455 nm was due to some excited enol emission and the strong peaks at 521 nm in MeCN, 533 nm in EtOH and 544 nm in DMF were due to the excited keto emission of H2L. These dual emissions are characteristics of ESIPT-exhibiting molecules.40,41 In DMF solution, the emission band of H2L at 544 nm was further redshifted and more intense compared to in EtOH and in MeCN, indicating a strong preference for the excited keto emission, which may be due to its highest refractive index, highest dielectric constant, most polar nature and high polarizability. Thus, the spectral redshifts observed at λab = 402 nm and at λem = 544 nm suggest that DMF could strongly stabilize H2L (Fig. 2).
In the solution state, the observed dual peaks in the above spectra (Fig. 1a and b) confirmed that H2L existed in two isomeric forms (i.e., enol tautomeric form, see Fig. S12a,† and the keto tautomeric form, see Fig. S12b†). Following eqn (1), the calculated FL emission yields of H2L were 0.53 in MeCN, 0.68 in EtOH, and 0.95 in DMF.
Both the UV-vis and FL spectral analyses proved that DMF was the strongest electron donor compared to EtOH and MeCN. The amide group in DMF has a highly polarizable oxygen atom with lone pairs, making it a strong Lewis base. This allows DMF to donate electrons effectively to the electron-deficient regions of H2L, stabilizing the Lewis acidic sites in the compound. EtOH was an intermediate electron donor. Although it has polarizable oxygen atom with lone pairs, its primary interactions involve donating protons rather than electrons because it is a protic solvent with an –OH group. This limits its electron-donating capacity compared to DMF. MeCN was the weakest electron donor. The nitrile group in acetonitrile is a weak Lewis base due to the electronegative sp-hybridized nitrogen, which poorly donates electrons. It primarily acts as a polar aprotic solvent with low basicity.
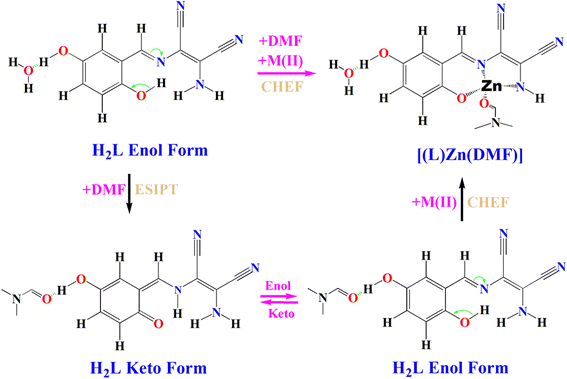
The fluorescence emission mechanisms of H2L are shown in Fig. 3. Upon photo-irradiation, the ground state enol form of H2L (E) relaxes to its excited-state enol form (E*), from where some of the absorbed photons return to the E while emitting E*-FL and the others relax to the excited-state keto form (K*) via an ESPT process due to the redistribution of electronic charges. Then, K* decays to K while emitting K*-FL. Finally, K will turn back to E by a reverse proton transfer (RPT).45 This overall processes are called photo-tautomerism.
Furthermore, based on the former literature,45 in order to understand the dual-fluorescence emission, reactive regions and frontier orbitals of H2L's tautomeric forms, we performed density functional theory (DFT) calculations using the Gaussian 09 software package at the B3LYP/6-31G(d) level. The molecular electrostatic potential of H2L (Fig. S12a and b†) showed the nucleophilic region was near the oxygen atoms and nitrogen atoms and these regions were significant for accepting and donating hydrogen bonds within the supramolecular structure. The blue color in the figures indicates the electron-accepting region and the red-color indicates the electron-donating region. The HOMO was mainly distributed on the benzene ring and C–C double bonds, while the LUMO isosurface was mainly located around the N of the DAMN skeleton. When electrons absorb energy, they move from the HOMO to LUMO. The calculated energy gaps were 3.16 eV for the H2L enol form (see Fig. S12c†) and 3.00 eV for the H2L keto form (see Fig. S12d†), which confirmed the reliability of the aforementioned dual-fluorescence emission mechanisms. As a result, the observed fluorescence property of the H2L supra-molecules were dominated by the keto-fluorescence emission rather than the enol-fluorescence emission, particularly in DMF solution.
3.4. Zn(II) selectivity study
To assess the selectivity of H2L for Zn(II), various experiments were conducted by introducing potential ions such as Cu(II), Ni(II) and [NO3]− into a solution of H2L in 50% DMF/tris–HCl buffer (pH 7.4, 25 °C), following established protocols.51 Spectrophotometric titrations of H2L with Zn(II) ions produced a redshifted absorbance band (at 494 nm) compared to the absorption bands of the competing ions (Fig. S17a, S18c and S19e†). Also, the spectrofluorometric titrations of H2L with Zn(II) induced FL TURN-ON (at λem = 544 nm and λex = 400 nm), attributed to structural-rigidification and non-radiative-decay suppression via a chelation-enhanced fluorescence (CHEF) mechanism. The solution was accompanied by a clear color change from bright-orange to bright-green under a 365 nm UV lamp, Fig. 4b(2). Hence, the emission occurred from a ligand-centered excited state (π → π*) and was more efficient due to the reduced vibrational relaxations.Conversely, the paramagnetic Cu(II) and Ni(II) ions caused FL TURN-OFF (Fig. S17(b) and S18(d)†), via photoinduced electron transfer (PET) and d–d transition-mediated non-radiative decay pathways. Their respective solutions were accompanied by weak-green emission under a 365 nm UV lamp, Fig. S16(e and f).† However, the nitrates did not produce effective spectral (Fig. S19(e and f)) or color (Fig. S16(g)†) responses under the same experimental conditions as they were incapable of coordinating with the ligand's donor sites. This distinct ion-specific behavior demonstrates H2L's potential as a selective FL sensor for Zn(II)(TURN-ON FL). Zn(II) is a borderline Lewis acid, which meant it could bind well with the nearby donor sites of H2L in a highly polar DMF and caused FL TURN-ON. Also, Cu(II) and Ni(II) are borderline acids but caused FL TURN-OFF due to their paramagnetic effects, which promoted intersystem crossing (ISC) from the ligand's singlet excited state (S1) to the metal's triplet state (T1).50 Overall, the selectivity of H2L towards Zn(II) was demonstrated by a redshift in the UV-vis spectra (at λabs = 494 nm), by FL TURN-ON (at λem = 544 nm) and by the color change from right-orange to bright-green under a UV lamp.
Further, to assess the selectivity of H2L for Zn(II), fluorometric competitive binding experiments were conducted by introducing the potential interferent ions Cu(II), Ni(II) and [NO3]− into a solution containing H2L and Zn(II) (Fig. S20†). Upon adding 60 μL of Cu(II) to the H2L-Zn(II) mixture, the fluorescence intensity dropped sharply to 613 due to Cu(II)'s competitive binding and paramagnetic quenching. For Ni(II), the fluorescence decreased moderately to 2105, reflecting the partial Zn(II) displacement and weaker quenching. In contrast, [NO3]−caused no significant change (2970 vs. 3000), as it could not coordinate with H2L. Thus, the interference order was: [NO3]− ≪ Ni(II) < Cu(II).
3.5. Zn(II) binding study
Although the single-crystal structure determination of the Zn(II)-H2L complex was unsuccessful despite repeated efforts, UV-vis titration experiments confirmed the coordination interaction between H2L and Zn(II). Upon the gradual addition of Zn(II) to H2L in DMF (Fig. 4a), EtOH (Fig. S14†), and MeCN (Fig. S15†), the yellow solution turned orange in each solvent. In DMF, the absorbance bands of H2L (402 and 352 nm) decreased, accompanied by the appearance of two isosbestic points (440 and 300 nm) and a new band at 494 nm. These spectral changes indicated Zn(II) binding to the three donor sites of H2L, facilitated by the removal of the acidic proton H(1) and the basic proton H(4A) (Fig. 5). The presence of isosbestic points suggests the direct formation of a stable complex without intermediates. The new band at 494 nm likely arose from ligand-to-metal charge transfer (LMCT),24 where electrons shifted from the donor atoms (O/N) to Zn(II) (3d10 4s0) or from Zn(II) (3d10) to H2L, enhanced by the two strong electron-withdrawing –CN groups.To determine the H2L-Zn(II) binding stoichiometry, Job's plot analysis was performed using the continuous variation method. As shown in Fig. 4c, the absorbance measured at 494 nm exhibited a maximum at a Zn(II) mole fraction (χZn(II)) of 0.5, confirming a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 binding ratio. The data were analyzed according to eqn (4) below.47
1 binding ratio. The data were analyzed according to eqn (4) below.47
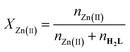 | (4) |
The binding constant (3.745 × μM−1) of Zn(II) with H2L was calculated using a Benesi–Hildebrand plot (Fig. 4d) or (eqn (5)).15
 | (5) |
The detection limit (DL) of Zn(II) by H2L was calculated as 3.455 × 10−6 μM using the standard deviation (σ) of the UV-vis absorbance of H2L (see Table S1, eqn (S1) and (S2)†) and the slope (m) of the linear regression curve (Fig. 4d), and following eqn (6):47
 | (6) |
The determined limit of detection of H2L for Zn(II) was significantly lower than that of the existing ref. 13. This shows the sensitivity of the UV-vis spectroscopy approach and the selectivity of H2L in DMF solution.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 L-Zn(II)–DMF complex forms via coordination to the imine-N, the deprotonated hydroxyl-O and amine-N sites, as shown by the following eqn (7):
1 L-Zn(II)–DMF complex forms via coordination to the imine-N, the deprotonated hydroxyl-O and amine-N sites, as shown by the following eqn (7):
 | (7) |
The complexation was evidenced by a new absorbance band at 494 nm, clear isosbestic points at 300 and 440 nm, a strong emission band at 544 nm, and a 50 nm Stokes shift (494 → 544 nm). The coordination rigidified the molecular structure, leading to a suppression of ESIPT, reduction of the non-radiative decay pathways and the emergence of strong FL at 544 nm through the CHEF effect. The TURN-ON response and visible color changes confirmed this as a characteristic Schiff base-Zn(II) chemo-sensing system, where metal chelation-enhanced emission by restricting molecular motions and extending π-conjugation. The proposed binding mechanism (Fig. 5) for H2L with Zn(II) in DMF is presented below in Fig. 5.
4. Conclusion
A highly crystalline, fluorescent and Zn(II)-detection novel ligand system H2L was synthesized by a condensation method and was characterized by various techniques (SC XRD, PXRD, UV-vis, FL, FT-IR, 1H NMR and TGA). H2L is highly polar due to its multi donor–acceptor functional groups, which contribute to supramolecular interactions, FL emissions, and metal-ion binding. H2L is a yellow solid that is insoluble in water, but highly soluble and stable in polar organic solvents.In solution, the H2L existed as an enol form and keto form. H2L was found to be thermally stable up to 250 °C. The FL emission and Zn(II) ion-binding abilities of H2L were studied in MeCN, EtOH and DMF, separately. Notably in each solvent, H2L emitted green light with varying FL emission yields of 0.9491 in DMF, 0.68 in EtOH and 0.53 in MeCN. The UV-vis and FL spectral analyses prove that DMF was the strongest electron donor compared to EtOH and MeCN due to its highly polarizable oxygen atoms, which can stabilize H2L.
Furthermore, the binding of H2L with Zn(II) was studied by UV-vis spectroscopy in the presence of DMF, EtOH and MeCN, separately. Obviously, there were no spectral changes for EtOH or MeCN in the visible range upon Zn(II) titration with H2L. However, upon Zn(II) titration with H2L in DMF, a new spectral band appeared at 494 nm along with two isosbestic points at 300 and 440 nm. Therefore, for the L-Zn(II)-DMF, a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometric ratio, a strong binding constant (3.745 × 107 μM−1), and a significantly low detection limit (3.455 × 10−6 μM) were calculated. Overall, these findings suggest that the developed H2L could be utilized for fabricating green light-emitting organic diodes in the materials science field and for detecting metal ions in the environmental and biological sciences fields.
1 stoichiometric ratio, a strong binding constant (3.745 × 107 μM−1), and a significantly low detection limit (3.455 × 10−6 μM) were calculated. Overall, these findings suggest that the developed H2L could be utilized for fabricating green light-emitting organic diodes in the materials science field and for detecting metal ions in the environmental and biological sciences fields.
Data availability
Additional data of this article are provided in the ESI.† Crystallographic data of H2L can also be obtained from the CCDC using the 2402446.Authors contributions
“Tesfa Oluma Fufa” was the primary author responsible for the experimental work, manuscript drafting, data analysis and interpretation. “Hui Li” was a corresponding author who provided continuous supervision and aided successful completion of the research. “Hongwei Ma” was the 2nd author and made significant contributions to the crystallographic work and manuscript revisions. Also, the 3rd, 4th & 5th authors actively participated in the data collection and manuscript revisions.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This work was supported by the National Natural Science Foundation of China (No. 21071018, 21271026, 21471017). Therefore, for this work, our heart gratitude is to the Chinese Scholarship Council and to the Analysis and Testing Centre of the Beijing Institute of Technology.References
- M. Zhao, B. Li, H. Zhang and F. Zhang, Activatable fluorescence sensors for in vivo bio-detection in the second near-infrared window, Chem. Sci., 2021, 12, 3448–3459 RSC.
- K. Rurack, Flipping the light switch ‘ON’–the design of sensor molecules that show cation-induced fluorescence enhancement with heavy and transition metal ions, Spectrochim. Acta, Part A, 2001, 57, 2161–2195 CrossRef CAS PubMed.
- A. P. De Silva, H. N. Gunaratne, T. Gunnlaugsson, A. J. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Signaling recognition events with fluorescent sensors and switches, Chem. Rev., 1997, 97, 1515–1566 CrossRef CAS PubMed.
- L. Chen, C. Yan, M. Pan, H.-P. Wang, Y.-N. Fan and C.-Y. Su, Multi-Mode White Light Emission in a ZnII Coordination Polymer from Excited-State Intramolecular Proton Transfer (ESIPT) Ligands, Eur. J. Inorg. Chem., 2016, 2016, 2676–2680 CrossRef CAS.
- J. Seo, S. Kim and S. Y. Park, Strong solvatochromic fluorescence from the intramolecular charge-transfer state created by excited-state intramolecular proton transfer, J. Am. Chem. Soc., 2004, 126, 11154–11155 CrossRef CAS PubMed.
- S. Paul and P. Barman, Exploring diaminomaleonitrile-derived Schiff base ligand and its complexes: Synthesis, characterization, computational insights, biological assessment, and molecular docking, J. Mol. Struct., 2024, 1296, 136941 CrossRef CAS.
- Q. Meng, P. Zhou, F. Song, Y. Wang, G. Liu and H. Li, Controlled fluorescent properties of Zn (II) salen-type complex based on ligand design, CrystEngComm, 2013, 15, 2786–2790 RSC.
- C. Justin Dhanaraj and M. Sivasankaran Nair, Synthesis, characterization, and antimicrobial studies of some Schiff-base metal (II) complexes, J. Coord. Chem., 2009, 62, 4018–4028 CrossRef.
- M. J. MacLachlan, M. K. Park and L. K. Thompson, Coordination compounds of Schiff-base ligands derived from diaminomaleonitrile (DMN): mononuclear, dinuclear, and macrocyclic derivatives, Inorg. Chem., 1996, 35, 5492–5499 CrossRef CAS PubMed.
- D. Wöhrle, H. Bohlen and H.-W. Rothkopf, Polymeric schiff's base chelates and their precursors, 4. Syntheses of schiff's base chelates from diaminomaleonitrile and investigation of their activity for the valence isomerisation of quadricyclane to norbornadien, Macromol. Chem. Phys., 1983, 184, 763–778 CrossRef.
- R. K. Sharma and M. Agrawal, Biological effects of heavy metals: an overview, J. Environ. Biol., 2005, 26, 301–313 CAS.
- H. Liu, S. Ding, Q. Lu, Y. Jian, G. Wei and Z. Yuan, A versatile Schiff Base Chemosensor for the determination of trace Co2+, Ni2+, Cu2+, and Zn2+ in the water and its bioimaging applications, ACS Omega, 2022, 7, 7585–7594 CrossRef CAS PubMed.
- F. Edition, Guidelines for Drinking-Water Quality, WHO chronicle, 2011, vol. 38, pp. 104–108 Search PubMed.
- A. Gonzales, M. Firmino, C. S. Nomura, F. R. P. Rocha, P. de Oliveira and I. Gaubeur, Peat as a natural solid-phase for copper preconcentration and determination in a multicommuted flow system coupled to flame atomic absorption spectrometry, Anal. Chim. Acta, 2009, 636, 198–204 CrossRef CAS PubMed.
- S. Bhunia, S. Halder, K. Naskar, B. Dutta, D. Sahoo, K. Jana and C. Sinha, Spectrophotometric determination of trace amount of total FeII/FeIII and live cell imaging of a carboxylato Zn (II) coordination polymer, Inorg. Chem., 2022, 61, 19790–19799 CrossRef CAS PubMed.
- S. Mukherjee, A. Ghati and G. Paul, An ultraviolet–visible spectrophotometric approach to establish a method for determining the presence of rhodamine B in food articles, ACS Food Sci. Technol., 2021, 1, 1615–1622 CrossRef CAS.
- M. Soylak, Y. E. Unsal, E. Yilmaz and M. Tuzen, Determination of rhodamine B in soft drink, waste water and lipstick samples after solid phase extraction, Food Chem. Toxicol., 2011, 49, 1796–1799 CrossRef CAS PubMed.
- Y. E. Unsal, M. Soylak and M. Tuzen, Dispersive liquid–liquid microextraction–spectrophotometry combination for determination of rhodamine B in food, water, and environmental samples, Desalin. Water Treat., 2015, 55, 2103–2108 CrossRef CAS.
- P. Pallavi, V. Kumar, M. W. Hussain and A. Patra, Excited-state intramolecular proton transfer-based multifunctional solid-state emitter: a fluorescent platform with “write-erase-write” function, ACS Appl. Mater. Interfaces, 2018, 10, 44696–44705 CrossRef CAS PubMed.
- T.-B. Ren, W. Xu, W. Zhang, X.-X. Zhang, Z.-Y. Wang, Z. Xiang, L. Yuan and X.-B. Zhang, A general method to increase stokes shift by introducing alternating vibronic structures, J. Am. Chem. Soc., 2018, 140, 7716–7722 CrossRef CAS PubMed.
- S. Park, O.-H. Kwon, S. Kim, S. Park, M.-G. Choi, M. Cha, S. Y. Park and D.-J. Jang, Imidazole-based excited-state intramolecular proton-transfer materials: synthesis and amplified spontaneous emission from a large single crystal, J. Am. Chem. Soc., 2005, 127, 10070–10074 CrossRef CAS PubMed.
- O. McCubbin Stepanic, J. Ward, J. E. Penner-Hahn, A. Deb, U. Bergmann and S. DeBeer, Probing a silent metal: a combined X-ray absorption and emission spectroscopic study of biologically relevant zinc complexes, Inorg. Chem., 2020, 59, 13551–13560 CrossRef PubMed.
- J. E. Penner-Hahn, Characterization of “spectroscopically quiet” metals in biology, Coord. Chem. Rev., 2005, 249, 161–177 CrossRef CAS.
- K. Sarkar, K. Dhara, M. Nandi, P. Roy, A. Bhaumik and P. Banerjee, Selective zinc (II)-ion fluorescence sensing by a functionalized mesoporous material covalently grafted with a fluorescent chromophore and consequent biological applications, Adv. Funct. Mater., 2009, 19, 223–234 CrossRef CAS.
- S. P. Anthony, Organic solid-state fluorescence: strategies for generating switchable and tunable fluorescent materials, ChemPlusChem, 2012, 77, 518–531 CrossRef CAS.
- J. P. Costes, J. F. Lamere, C. Lepetit, P. G. Lacroix, F. Dahan and K. Nakatani, Synthesis, crystal structures, and nonlinear optical (NLO) properties of new Schiff-base nickel (II) complexes. Toward a new type of molecular switch?, Inorg. Chem., 2005, 44, 1973–1982 CrossRef CAS PubMed.
- K. Aich, S. Goswami, S. Das, C. D. Mukhopadhyay, C. K. Quah and H.-K. Fun, Cd2+ triggered the FRET “ON”: a new molecular switch for the ratiometric detection of Cd2+ with live-cell imaging and bound X-ray structure, Inorg. Chem., 2015, 54, 7309–7315 CrossRef CAS PubMed.
- O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard and H. Puschmann, OLEX2: a complete structure solution, refinement and analysis program, J. Appl. Crystallogr., 2009, 42, 339–341 CrossRef CAS.
- G. M. Sheldrick, SHELXT–Integrated space-group and crystal-structure determination, Acta Crystallogr., Sect. A:Found. Adv., 2015, 71, 3–8 CrossRef PubMed.
- G. M. Sheldrick, Crystal structure refinement with SHELXL, Acta Crystallogr., Sect. C:Struct. Chem., 2015, 71, 3–8 Search PubMed.
- P. G. Lacroix, S. Di Bella and I. Ledoux, Synthesis and second-order nonlinear optical properties of new copper (II), nickel (II), and zinc (II) Schiff-base complexes. Toward a role of inorganic chromophores for second harmonic generation, Chem. Mater., 1996, 8, 541–545 CrossRef CAS.
- X. Chen, J. Zhang, H. Zhang, Z. Jiang, G. Shi, Y. Li and Y. Song, Preparation and nonlinear optical studies of a novel thermal stable polymer containing azo chromophores in the side chain, Dyes Pigm., 2008, 77, 223–228 CrossRef CAS.
- C. Anitha, C. Sheela, P. Tharmaraj and R. Shanmugakala, Studies on Synthesis and Spectral Characterization of Some Transition Metal Complexes of Azo-Azomethine Derivative of Diaminomaleonitrile, Int. J. Inorg. Chem., 2013, 2013, 436275 Search PubMed.
- A. R. Katritzky, D. C. Fara, H. Yang, K. Tämm, T. Tamm and M. Karelson, Quantitative measures of solvent polarity, Chem. Rev., 2004, 104, 175–198 CrossRef CAS PubMed.
- P. Muthukumar, M. Surya, M. Pannipara, A. G. Al-Sehemi, D. Moon and S. Philip Anthony, Easily Accessible Schiff Base ESIPT Molecules with Tunable Solid State Fluorescence: Mechanofluorochromism and Highly Selective Co2+ Fluorescence Sensing, ChemistrySelect, 2020, 5, 3295–3302 CrossRef CAS.
- A. Bhattacharyya, S. K. Mandal and N. Guchhait, Imine–amine tautomerism vs. keto–enol tautomerism: Acceptor basicity dominates over acceptor electronegativity in the ESIPT process through a six-membered intramolecular H-bonded network, J. Phys. Chem. A, 2019, 123, 10246–10253 CrossRef CAS PubMed.
- S. Sinha, B. Chowdhury and P. Ghosh, A highly sensitive ESIPT-based ratiometric fluorescence sensor for selective detection of Al3+, Inorg. Chem., 2016, 55, 9212–9220 CrossRef CAS PubMed.
- J. Junyong, L. H. Yong, L. Wenjun, O. András, C. Chun-Hsing and L. Dongwhan, Reactivity-Based Detection of Copper (II) Ion in Water: Oxidative Cyclization of Azoaromatics as Fluorescence Turn-On Signaling Mechanism.
- J. Lakowicz, Principles of fluorescence spectroscopy, Springer, NY, 3rd edn, 2006, pp. 205–235 Search PubMed.
- F. A. Chipem and G. Krishnamoorthy, Temperature effect on dual fluorescence of 2-(2′-Hydroxyphenyl) benzimidazole and its nitrogen substituted analogues, J. Phys. Chem. B, 2013, 117, 14079–14088 CrossRef CAS PubMed.
- A. Heller and D. L. Williams, Intramolecular proton transfer reactions in excited fluorescent compounds, J. Phys. Chem., 1970, 74, 4473–4480 CrossRef CAS.
- R. F. Kubin and A. N. Fletcher, Fluorescence quantum yields of some rhodamine dyes, J. Lumin., 1982, 27, 455–462 CrossRef.
- G. A. Crosby and J. N. Demas, Measurement of photoluminescence quantum yields. Review, J. Phys. Chem., 1971, 75, 991–1024 CrossRef CAS.
- H.-T. Feng, J. Zeng, P.-A. Yin, X.-D. Wang, Q. Peng, Z. Zhao, J. W. Lam and B. Z. Tang, Tuning molecular emission of organic emitters from fluorescence to phosphorescence through push-pull electronic effects, Nat. Commun., 2020, 11, 2617 CrossRef CAS PubMed.
- J.-B. Li, H.-W. Zheng, M. Wu, Q.-F. Liang, D.-D. Yang, X.-J. Zheng and H.-W. Tan, Multistimulus Response of Two Tautomeric Zn (II) Complexes and Their White-Light Emission Based on Different Mechanisms, Inorg. Chem., 2021, 60, 17677–17686 CrossRef CAS PubMed.
- W. Kemp, Organic Spectroscopy, Bloomsbury Publishing, 2017 Search PubMed.
- S. Luangphai, J. Fongsiang, P. Thuptimdang, S. Buddhiranon and K. Chanawanno, Colorimetric Cu2+ Detection of (1 E, 2 E)-1, 2-Bis ((1 H-pyrrol-2-yl) methylene) hydrazine Using a Custom-Built Colorimeter, ACS Omega, 2022, 7, 44448–44457 CrossRef CAS PubMed.
- L. Zou, B. Yan, D. Pan, Z. Tan and X. Bao, A colorimetric and absorption ratiometric anion sensor based on indole & hydrazide binding units, Spectrochim. Acta, Part A, 2015, 148, 78–84 CrossRef CAS PubMed.
- V. P. Gupta and T. Poonam, Conformational and vibrational studies of isomeric hydrogen cyanide tetramers by quantum chemical methods, Spectrochim. Acta, Part A, 2012, 89, 55–66 CrossRef CAS PubMed.
- A. El Majzoub, C. Cadiou, I. Déchamps-Olivier, B. Tinant and F. Chuburu, Cyclam-methylbenzimidazole: a selective OFF-ON fluorescent sensor for Zinc, Inorg. Chem., 2011, 50(9), 4029–4038 CrossRef CAS PubMed.
- R. Bawa, S. Negi, B. Singh, B. Pani and R. Kumar, A pyridine dicarboxylate based hydrazone Schiff base for reversible colorimetric recognition of Ni 2+ and PPi, RSC Adv., 2023, 13(23), 15391–15400 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. CCDC 2402446. For ESI and crystallographic data in CIF or other electronic format see DOI: https://doi.org/10.1039/d5ra03383g |
| This journal is © The Royal Society of Chemistry 2025 |