Open Access Article
Open Access ArticleRecovery of high-quality struvite fertilizer product from swine wastewater using fluidized bed homogeneous crystallization†
The Anh Luua,
Gia Cuong Nguyena,
Manh Tuan Truonga,
Van Giang Le *a and
Xuan Thanh Bui
*a and
Xuan Thanh Bui bc
bc
aCentral Institute for Natural Resources and Environmental Studies, Vietnam National University, Hanoi, 111000, Vietnam. E-mail: levangiangcres@vnu.edu.vn
bKey Laboratory of Advanced Waste Treatment Technology, Ho Chi Minh City University of Technology (HCMUT), Vietnam National University Ho Chi Minh (VNU-HCM), Thu Duc City, Ho Chi Minh City 700000, Vietnam
cFaculty of Environment and Natural Resources, Ho Chi Minh City University of Technology (HCMUT), 268 Ly Thuong Kiet Street, District 10, Ho Chi Minh City 700000, Vietnam
First published on 10th July 2025
Abstract
Recovering phosphorus (PO43−) and nitrogen (NH4+) from swine wastewater is critical because their excess release can trigger eutrophication, severely harming aquatic ecosystems. This study introduces a pioneering fluidized-bed homogeneous crystallization (FBHC) process for single-step co-recovery of PO43− and NH4+. The method ensures compliance with discharge standards and produces a reusable nutrient product. Preliminary jar-test experiments were performed to identify the feasibility of struvite (MgNH4PO4·6H2O) precipitation and to define the appropriate range for key operational parameters. Subsequently, operational parameters including pH, reaction time, up-flow velocity, cross-sectional loading, and bed height were systematically optimized. Under optimal conditions with a working medium at pH 9, reaction time of 24 min, up-flow velocity of 36 m h−1, cross-sectional loading of 0.27 kg m−2 h−1, and bed height of 10 cm, the total removal efficiencies reached 97.42% for PO43− and 86.55% for NH4+. The corresponding crystallization ratios were 95.55% and 83.18%, respectively. The FBHC process exhibited high crystallization efficiency (>95%), contributing to reduced impurity levels and improved energy efficiency compared to conventional fluidized bed and chemical precipitation methods. The recovered product was identified as high-purity struvite (94.3%), predominantly comprising particles smaller than 0.75 mm. These results demonstrate the effectiveness of the FBHC strategy for simultaneous nutrient removal and resource recovery through the production of a value-added fertilizer.
1. Introduction
Untreated pig swine wastewater is rich in nitrogen,1 phosphorus,2 and potassium.3 Releasing this untreated waste poses a significant threat to soil and water quality. Elevated levels of nitrogen and phosphorus, for instance, can lead to eutrophication in water bodies like rivers and lakes. Furthermore, various forms of nitrogen (such as ammonium, nitrate, and nitrite) and phosphorus (including orthophosphate and monophosphate) present in the wastewater can be toxic to aquatic life.4 Global consumption of phosphate rock has been steadily increasing, reaching approximately 44.5 million tonnes in 2016,5 various industrial and human waste streams contain substantial amounts of inorganic phosphate. For instance, dairy wastewater can have 19–445 mg L−1,6,7 swine wastewater 100–453 mg L−1,8,9 urine 20–328 mg L−1,10 and anodized wastewater a striking 3240–9937 mg L−1.11 Struvite, also known as magnesium ammonium phosphate hexahydrate with the chemical formula MgNH4PO4·6H2O, is a valuable fertilizer derived from phosphate removal processes. Its increasing popularity in these systems highlights its role in resource recovery and the circular economy by capturing phosphate from agricultural and livestock wastewater and repurposing it as a fertilizer for agricultural use.12Various recovery methods, such as electrodialysis,13 electrochemical stripping,14 fluidized bed reactors (FBR),15,16 ion exchange adsorbents,17 precipitation, and absorption,18 have been implemented to treat swine wastewater. Current technological efforts are primarily focused on the recovery of nitrogen and phosphorus.19 Among these, conventional chemical precipitation is widely recognized as an effective method for phosphorus reclamation in the form of struvite, owing to its ability to generate valuable by-products with high recovery efficiency and minimal environmental impact.20 Nevertheless, this method produces substantial quantities of sludge, necessitating additional settling units due to its limited efficiency in physically separating the struvite product.21 Moreover, the resulting sludge, being very wet, needs to undergo a drying step afterward. Nitrogen recovery often involves a stripping process. In this method, ammonia gas (NH3) is captured by adsorption onto phosphorous acid. This step prepares the nitrogen for a later precipitation reaction with magnesium, ultimately yielding magnesium potassium phosphate hexahydrate (MgKPO4·6H2O). Nonetheless, this stripping technique is not very efficient when it comes to recovering phosphorus.
The fluidized-bed crystallization (FBC) method enables the recovery of pollutants as solid crystals or granules22,23 (Scheme 1). By introducing magnesium (Mg) or calcium (Ca), phosphorus (P) and nitrogen (N) can be captured as ternary compounds like trimagnesium phosphate 22-hydrate (Mg3(PO4)2·22H2O), magnesium ammonium phosphate hexahydrate (MgNH4PO4·6H2O), and trimagnesium phosphate octahydrate (Mg3(PO4)2·8H2O).24,25 This FBC process enhances precipitation efficiency, using fewer chemicals and minimizing water-rich sludge by forming low-moisture pellets suitable as slow-release fertilizers. However, the process necessitates seeding materials such as silica or aluminum oxide, which can introduce impurities into the final pellets. To overcome this issue of impurity introduction from seed materials in FBC, a seedless fluidized-bed homogeneous crystallization (FBHC) system could be a potential solution. FBHC offers improved separation, purer granules, and faster settling.26,27 Furthermore, regulating the water flow within the FBHC system facilitates the formation of granular pellets and reduces the generation of wet sludge.28 Therefore, the FBHC reactor can generate a stable form of magnesium ammonium phosphate hexahydrate, known as struvite (MgNH4PO4·6H2O), which has a very low solubility product constant (Ksp) of 7.08 × 10−14 at 25 °C.29
Due to its high organic content and the presence of calcium ions and other impurities in swine wastewater, this matrix presents significant challenges in controlling the crystallization process and achieving high recovery efficiency. Therefore, this study was conducted to initially determine the optimal crystallization conditions through jar-test experiments, and subsequently develop and optimize a FBHC process to simultaneously recover nitrogen (NH4+) and phosphorus (PO43−) from swine wastewater. By investigating the effects of operating parameters such as pH, reaction time, up-flow velocity, cross-sectional loading, and particle bed height, the study is expected to identify the optimal conditions for effective struvite crystallization. The outcomes are expected to enhance livestock wastewater treatment technologies while producing high-value, reusable fertilizer products, thereby supporting sustainable agricultural practices and promoting a circular economy.
2. Materials and methods
2.1. Materials
Swine wastewater was collected from a pig farm located in Hanoi City, Vietnam and stored at 5.0 ± 1 °C. Before use, actual swine wastewater was filtered (0.45 μm, Whatman) to remove the suspended solids. The properties of the swine wastewater utilized in the experiments are detailed in Table 1.| Parameter | Units | Average values plus standard deviation |
|---|---|---|
| pH | — | 7.14 ± 0.03 |
| COD | mg L−1 | 3800 ± 230 |
| Alkalinity (as Na2CO3) | mg L−1 | 2030 ± 160 |
| TAN (total ammonia nitrogen) | mg L−1 | 549 ± 25 |
| T-P (total phosphorus) | mg L−1 | 149 ± 10.5 |
| PO4–P | mg L−1 | 111 ± 8.0 |
| NH4–N | mg L−1 | 441 ± 21 |
| K | mg L−1 | 312 ± 18 |
| Mg | mg L−1 | 67.4 ± 7.8 |
| Ca | mg L−1 | 47.5 ± 4.5 |
| Cu | mg L−1 | 1.3 ± 0.1 |
| Fe | mg L−1 | 2.2 ± 0.1 |
| Zn | mg L−1 | 0.8 ± 0.1 |
The chemicals used in this study, including calcium chloride dihydrate (CaCl2·2H2O, 96%), potassium chloride (KCl, 99%), magnesium chloride hexahydrate (MgCl2·6H2O, 99%), sodium phosphate monobasic (NaH2PO4·2H2O, 99%), potassium phosphate monobasic (KH2PO4, 99%), and ammonium chloride (NH4Cl, 99.8%), were all sourced from Sinopharm Chemical Reagent Co., Ltd, and used as received without further purification. High-purity standards (Charleston, U.S.A.) provided the ICP-MS standard solutions. A laboratory-grade RO ultrapure water system supplied deionized water for all experiments with a resistance greater than 18.1 MΩ.
2.2. Analytical methods
A portable pH meter (TS-1, Suntex) equipped with a plastic pH electrode (GB-600E, Yeong-Shin Company) was utilized to determine the pH of the samples. This meter was calibrated weekly under ambient conditions employing pH 7 and pH 10 buffer solutions. The concentrations of cations and anions in liquid samples were quantified using an inductively coupled plasma mass spectrometry (ICP-MS, iCAP RQ, Thermofisher Scientific Pte Ltd, Singapore) at specific detection wavelengths (213.857 nm, 214.915 nm, 220.353 nm, 231.604 nm, 267.716 nm, 279.553 nm, 324.754 nm, 422.673 nm, 438.364 nm, and 766.491 nm). The method detection limits (MDLs) for heavy metals were 0.15 μg L−1 for Fe, 1.17 μg L−1 for Zn, and 0.07 μg L−1 for Cu,30 ensuring sufficient sensitivity for detecting trace levels in swine wastewater. Ammonium nitrogen (NH4–N) levels in the influent and effluent of each reactor were measured with a Flow Injection Analyzer (Lachat Instruments 5600) following the SFS-EN ISO 6878 standard method. The crystalline structure of dried precipitates and granules was identified utilizing an X-ray diffractometer (Rigaku RX III, Japan) with CuKα radiation (40 kV, 30 mA), scanning from 10° to 90° in 0.05° steps with a 0.5 s scan time. The elemental composition of solids was analyzed employing an attached Energy Dispersive Spectrometer (LINKS AN10000/85S, Japan). The morphology of the precipitates was visualized utilizing a Scanning Electron Microscope (Hitachi SU8010, Japan) after coating the samples with a thin layer of platinum using a sputter coater (JEOL JFC-1600).2.3. Jar-tests
While various materials like nano zero-valent iron (nZVI), marble dust, and graphene oxide can eliminate phosphorus through adsorption or precipitation, magnesium salts are commonly preferred owing to their widespread availability and lower initial investment. This study investigated phosphorus precipitation in batch experiments by varying pH (±0.2) (7.0, 8.0, 8.5, 9.0, and 9.5, and 10; [N]0 of 314.7 mg L−1, [P]0 of 1660.6 mg L−1), reaction time (0, 5, 10, 15, 20, 25, 30, 45, 60, 75, 90, 120, and 150 min), and the molar ratio of magnesium to phosphorus (1.0, 1.2, 1.3, 1.5, 1.8, and 2.0), all at a consistent [Mg]/[P] ratio, pH, and reaction time respectively. Additionally, the impact of different ammonia concentrations (200, 250, 300, 350, 400, and 450 ppm) was examined. All batch tests were conducted at room temperature (25–28 °C).In jar-test experiments, magnesium chloride and sodium dihydrogen phosphate were dissolved and mixed in deionized water. Swine wastewater was then added to initiate precipitation. The pH was adjusted with sodium hydroxide. The mixture was rapidly stirred, then slowly stirred, and finally allowed to settle. Samples were filtered for analysis. The effect of ammonium concentration on phosphorus and nitrogen removal was also evaluated by adding ammonium chloride. The key experimental factors explored were pH, reaction time, magnesium-to-phosphorus molar ratios, and ammonium concentrations. All jar-test experiments were performed at 25 °C and replicated.
2.4. Fluidized bed homogeneous crystallization (FBHC)
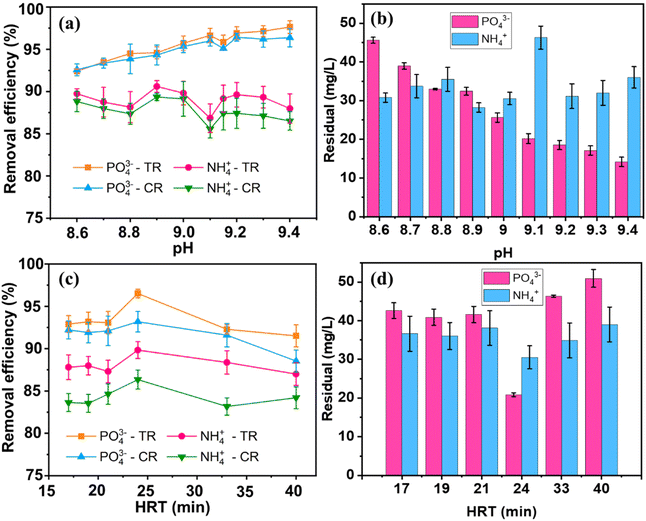
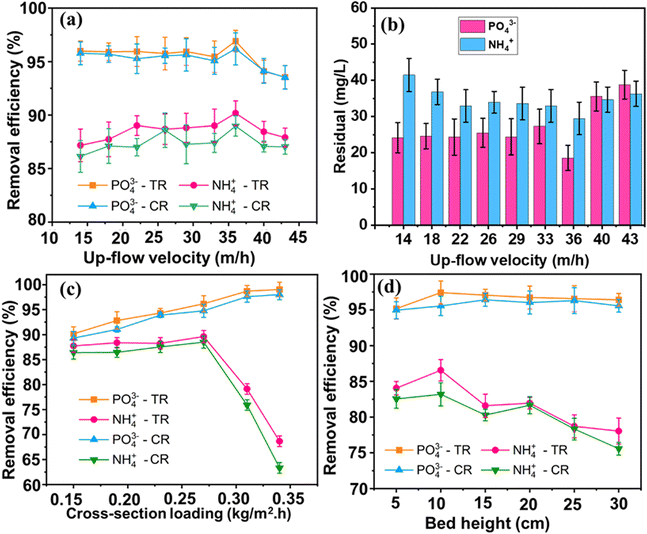
The fluidized-bed reactor, depicted in Scheme 2, consists of a 550 mL cylindrical column and Pyrex glass pipelines. It's constructed with two sections joined by a wider connection. The lower section has a diameter of 2 cm and a height of 20 cm, while the upper section is 4 cm in diameter and 80 cm high. This widening from top to bottom in the FBC column design helps to decrease the water flow rate and prevent excessive loss of fine particles. Two peristaltic pumps are used to introduce swine wastewater chemicals and sodium hydroxide into the reactor. A third peristaltic pump regulates the recirculation flow rate. To support the granulation bed, ensure even distribution of water flow, facilitate bubble formation at the inlets, and prevent blockages, glass beads with a diameter (D) of 0.5 cm are packed to a height of 3.5 cm from the bottom of both columns. The duration that water remained within the system, known as the hydraulic retention time (HRT), was managed by changing the overall incoming water flow (Qt) and the rate at which water was cycled back into the system (Qr).The pH of the precipitant solutions was adjusted employing either NaOH or HNO3. The study investigated struvite recovery under varying conditions: pH levels (±0.2) values (8.6, 8.7, 8.8, 8.9, 9.0, 9.1, 9.15, 9.2, 9.3, and 9.4; [Mg]/[P] ratio of 1.2; up-flow velocity of 33 m h−1), hydraulic retention time (17, 19, 21, 24, 33, and 40; pH 9 ± 0.2; up-flow velocity of 33 m h−1), different up-flow velocity values (14, 18, 22, 26, 29, 33, 36, 40, and 43 m h−1; pH 9.0 ± 0.2; HRT 24 min), cross-sectional loading (L) (0.15, 0.19, 0.23, 0.27, 0.31, and 0.34 kg m−2 h−1; up-flow velocity of 36 m h−1, pH 9 ± 0.2), and bed-height (5, 10, 15, 20, 25, and 30; cross-sectional loading of 0.27 kg m−2 h−1, pH 9 ± 0.2, up-flow velocity 36 m h−1) (Table 2).
| No. | Operational parameters | Jar-tests | FBHC column |
|---|---|---|---|
| 1 | pH (—) | 7.0, 8.0, 8.5, 9.0, 9.5, and 10 | 8.6, 8.7, 8.8, 8.9, 9.0, 9.1, 9.15, 9.2, 9.3, and 9.4 |
| [Mg]/[P] | 1.0 | 1.2 | |
| U (m h−1) | 30 | 33 | |
| Reaction time (min) | 120 | 60 | |
| L (kg m−2 h−1) | 0.23 | 0.23 | |
| Bed height (cm) | 15 | 15 | |
| [NH4+] (mg L−1) | 314 | — | |
| 2 | Reaction time (min) | 0, 5, 10, 15, 20, 25, 30, 45, 60, 75, 90, 120, and 150 | 17, 19, 21, 24, 33, and 40 |
| pH (—) | 9.0 ± 0.2 | 9.0 ± 0.2 | |
| U (m h−1) | 30 | 33 | |
| [Mg]/[P] | 1.0 | 1.2 | |
| L (kg m−2 h−1) | 0.23 | 0.23 | |
| [NH4+] (mg L−1) | 314 | — | |
| Bed height (cm) | 15 | 15 | |
| 3 | [Mg]/[P] | 1.0, 1.2, 1.3, 1.5, 1.8, and 2.0 | 1.2 |
| U (m h−1) | 30 | 33 | |
| pH (—) | 9.0 ± 0.1 | 9.0 ± 0.1 | |
| L (kg m−2 h−1) | 0.23 | 0.23 | |
| Reaction time (min) | 60 | 24 | |
| [NH4+] | 314 | — | |
| Bed height (cm) | 15 | 15 | |
| 4 | [NH4+] | 250, 300, 350, 400, and 450 | — |
| [Mg]/[P] | 1.2 | 1.2 | |
| pH (—) | 9.0 ± 0.1 | 9.0 ± 0.1 | |
| L (kg m−2 h−1) | 0.23 | 0.23 | |
| Reaction time (min) | 60 | 24 | |
| Bed height (cm) | 15 | 15 | |
| U (m h−1) | 30 | 33 | |
| 5 | U (m h−1) | — | 14, 18, 22, 26, 29, 33, 36, 40, and 43 |
| Reaction time (min) | — | 24 | |
| [Mg]/[P] | — | 1.2 | |
| pH (—) | — | 9.0 ± 0.1 | |
| L (kg m−2 h−1) | — | 0.23 | |
| Bed height (cm) | — | 15 | |
| 6 | L (kg m−2 h−1) | — | 0.15, 0.19, 0.23, 0.27, 0.31, and 0.34 |
| Reaction time (min) | — | 24 | |
| pH (—) | — | 9.0 ± 0.2 | |
| Bed height (cm) | — | 15 | |
| U (m h−1) | 36 | ||
| 7 | Bed height (cm) | — | 5, 10, 15, 20, 25, and 30 |
| Reaction time (min) | — | 24 | |
| pH (—) | — | 9.0 ± 0.2 | |
| U (m h−1) | — | 36 | |
| L (kg m−2 h−1) | — | 0.27 |
Scheme 2 visually presents the experimental arrangement for recovering struvite through fluidized bed crystallization. Experiments on the recovery of P and N using the FBHC process were initiated by introducing the feeds of swine wastewater, which was also mixed with NaOH at given flow rates, under room temperature, controlling the ratio of reflux flow (Qr) to the total inflow (Qt = Qswine + QOH). Before operating the FBHC reactor, a 3–5 day initial phase was conducted at a low upward flow speed (15 m h−1) to promote granule formation. Subsequently, the upward velocity (Qup = Qt + Qr) was increased to a superficial velocity of 25 m h−1 by regulating the recirculation flow (Qr). Guided by prior jar-tests results, the reactor was fed with a solution containing magnesium, nitrogen, and phosphorus at a molar ratio of 1.2 (specifically, 402.5 mg-N L−1, 35.2 mg-Mg L−1, and 95.8 mg-P L−1). The FBHC process was run for a duration equivalent to at least nine hydraulic retention times (HRT) to ensure the reaction reached a stable state after any changes to the input conditions.
Effluent samples underwent analysis both with and without filtration utilizing a 0.45 μm filter (GHP Membrane, Pall) to eliminate fine particles. To minimize supersaturation, 1.0 mL of HCl was added to each sample. Soluble nitrogen and phosphorus levels in the filtered samples were used to calculate the overall removal of P and N. Conversely, acidic digestion determined the total P and N content, which was used to assess granulation efficiency.
The removal efficiency was evaluated to assess the capability of the FBHC system to precipitate and recover P and N into granular form, based on the residual concentrations in the effluent. Furthermore, the performance of the jar-test and FBHC processes was quantified by calculating the total recovery (TR) and crystallization ratio (CR) for both phosphorus and nitrogen. Granulation efficiency, on the other hand, was evaluated to confirm the formation of granules with low water content and minimal or no sludge production. The percentage recoveries of P and N were determined using the following calculations in eqn (1) and (2):
 | (1) |
 | (2) |
2.5. Purity of struvite pellets
To determine the purity and composition of the heavy metals in struvite pellets, each sample's 1.5 g was dissolved in 0.5% HNO3 solution and then diluted with distilled water to 25 mL.31 Solution pH was adjusted to 4 ± 0.02 by 1.0 M NaOH before analysis. The purity of the struvite was calculated based on the respective N concentrations, calculated by eqn (3):
 | (3) |
To analyze how the struvite particles were sized, samples were collected from the bottom of the experimental setup, dried at 50 °C over an 8 hour period, and subsequently separated into distinct size groups utilizing sieves. These groups corresponded to particle diameters of over 2 mm, between 1.4 and 2 mm, between 0.75 and 1.4 mm, between 0.2 and 0.75 mm, and below 0.2 mm. The mass of particles within each size category was then measured to determine the overall size distribution, and the calculations proceeded (eqn (4)) as follows:
 | (4) |
3. Results and discussion
3.1. Jar-test results
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.0, and a practical application at a larger scale suggests a slightly higher ratio of 1.3
1.0, and a practical application at a larger scale suggests a slightly higher ratio of 1.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.36 However, further increases in the [Mg]/[P] ratio beyond 1.3 (up to 2) result in only a marginal increase or even a plateau in phosphate removal efficiency, suggesting that the optimal threshold for this ratio has been reached. At higher ratios, the ammonium harvesting efficiency tended to decrease slightly, which can be attributed to the consumption of phosphorus in the process of Mg3(PO4)2 formation36 or a smaller fraction of nitrogen was recovered via struvite formation.37 The stability of MgRPO4·nH2O depends on the ionic radius of R.38 Because Na+ and K+ are larger than NH4+, their struvite analogs (Na-struvite and K-struvite) exhibit higher stability than NH4-struvite. Ultimately, although magnesium is required for struvite formation, the optimum [Mg]/[P] ratio for phosphate removal does not exactly coincide with the optimum for ammonium removal, and other factors such as high ammonium initial concentration or crystallization kinetics may be more important in determining ammonium removal efficiency.
1.36 However, further increases in the [Mg]/[P] ratio beyond 1.3 (up to 2) result in only a marginal increase or even a plateau in phosphate removal efficiency, suggesting that the optimal threshold for this ratio has been reached. At higher ratios, the ammonium harvesting efficiency tended to decrease slightly, which can be attributed to the consumption of phosphorus in the process of Mg3(PO4)2 formation36 or a smaller fraction of nitrogen was recovered via struvite formation.37 The stability of MgRPO4·nH2O depends on the ionic radius of R.38 Because Na+ and K+ are larger than NH4+, their struvite analogs (Na-struvite and K-struvite) exhibit higher stability than NH4-struvite. Ultimately, although magnesium is required for struvite formation, the optimum [Mg]/[P] ratio for phosphate removal does not exactly coincide with the optimum for ammonium removal, and other factors such as high ammonium initial concentration or crystallization kinetics may be more important in determining ammonium removal efficiency.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P molar ratio of 1.3, and with ammonium levels varying from 200 to 450 mg L−1 (Fig. 1(d)). An initial observation shows that both the CR and TR values of PO43− and NH4+ increase and reach a threshold of 92–93% when the ammonium concentration is 300 mg L−1. Although the ammonium removal efficiency fluctuated at high concentrations, the results from repeated experiments showed small errors and clear changing trends, indicating that the results were reproducible. However, a contrasting trend is evident at higher ammonium concentrations. Specifically, the CR% and TR% for PO43− reached approximately 97.72 and 99.01%, respectively, while those for NH4+ were significantly lower at only 63.25 and 64.98%. Phosphate removal consistently stayed within the 97–99% range via ammonium struvite precipitation, likely because of the creation of non-crystalline substances like Ca3(PO4)2 and Mg3(PO4)2.41 Additionally, the production of ammonium struvite was sensitive to pH levels, as previously noted.
P molar ratio of 1.3, and with ammonium levels varying from 200 to 450 mg L−1 (Fig. 1(d)). An initial observation shows that both the CR and TR values of PO43− and NH4+ increase and reach a threshold of 92–93% when the ammonium concentration is 300 mg L−1. Although the ammonium removal efficiency fluctuated at high concentrations, the results from repeated experiments showed small errors and clear changing trends, indicating that the results were reproducible. However, a contrasting trend is evident at higher ammonium concentrations. Specifically, the CR% and TR% for PO43− reached approximately 97.72 and 99.01%, respectively, while those for NH4+ were significantly lower at only 63.25 and 64.98%. Phosphate removal consistently stayed within the 97–99% range via ammonium struvite precipitation, likely because of the creation of non-crystalline substances like Ca3(PO4)2 and Mg3(PO4)2.41 Additionally, the production of ammonium struvite was sensitive to pH levels, as previously noted.3.2. Removal of P and N from swine wastewater by FBHC process
| NH4+ + OH− → NH3 + H2O, Kb = 10−4.8 | (5) |
This observation aligns with the work of Çelen and Türker, who similarly found no ammonia loss at pH 7 and a 17.9% loss at pH 9.5.34 In addition, at high pH, Mg3(PO4)2 formation is more favorable,32 struvite crystals begin to dissolve, releasing Mg2+ ions, and Mg(OH)2 precipitation may compete with struvite precipitation.42 Fig. 2(b) illustrates the residual concentrations of phosphate and ammonium, respectively, showing that the residual phosphate concentration decreased as the phosphate removal efficiency increased, while the residual ammonium concentration fluctuated and showed a tendency to rise again at higher pH values, consistent with the trend observed in ammonium removal efficiency. The recovery efficiency values between replicates varied within a narrow range, especially for phosphorus, reflecting the stability and reliability of the FBHC system when operating at different pH values. Overall, the results indicated that a slightly alkaline pH was beneficial for phosphate removal, but excessively high pH levels could negatively impact NH4+ removal. Therefore, a pH of 9 was selected for subsequent experiments.
| Mg2+ + NH4+ + PO43− + 6H2O → MgNH4PO4·6H2O | (6) |
Simultaneously, the intermediate phase like newberyite (MgHPO4·3H2O) which later transform into struvite in the occurrence of PO43− and NH4+ (eqn (7)):
| MgHPO4·3H2O + NH4+ + 3H2O → MgNH4PO4·6H2O + H+ | (7) |
During the initial 24 minute period, the saturation indices (SI) for magnesium sodium phosphate (Na-struvite, MgNaPO4·7H2O) and struvite were calculated as 1.52 and 2.51, respectively. These values were derived utilizing solubility product constants (Ksp) of 10−11.6 for Na-struvite and 10−13.26 for struvite,43 providing preferable thermodynamic conditions for their crystallization. Fig. 2(d) illustrates the residual concentrations of PO43− and NH4+ after treatment. The residual concentration of PO43− decreased to the lowest level of about 20.86 mg L−1 at HRT 24 min, corresponding to the highest removal efficiency. The apparent increase in crystallization yield at HRT = 24 min can be explained by the fact that, at this point, the system reaches a hydraulic and reaction steady state, allowing Mg2+, NH4+, and PO43+ ions to effectively interact and crystallize more efficiently. Shorter hydraulic retention times may not be sufficient for crystal nucleation and growth, while longer retention times may lead to partial re-dissolution or dispersion of the formed crystals, thereby reducing the overall yield. Meanwhile, the residual NH4+ concentration was approximately 1.5 times higher at 30.51 mg L−1, suggesting that the homogeneous crystallization process in the fluidized bed was more effective in removing phosphate than ammonium. In addition, the presence of other multivalent cations in swine wastewater, which have a strong affinity for phosphate, may promote more efficient phosphate precipitation. The difference in removal efficiency between CR and TR suggests that a portion of both phosphate and ammonium is removed through mechanisms other than crystallization, such as adsorption onto the fluidized bed material surface or other chemical reactions within the system.
3.3. Characterization of pellets manufactured via the FBHC process
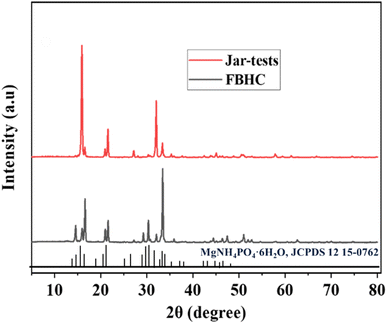
The resulting solid products were analyzed employing XRD and SEM techniques. For the jar-tests (conventional precipitation) method, SEM micrographs, as illustrated in Fig. 4(a) and (b), reveal that the formed precipitates consisted of multiple orthorhombic crystals exhibiting trapezoidal or rectangular morphologies with large precipitate particles (0.10–2 mm), rough surfaces, numerous cracks, and voids. In contrast, the sample in Fig. 4(c) had finer but unevenly distributed and easily agglomerated particles. For the FBHC method, SEM observations revealed that the recovered pellets exhibited a homogeneous structure comprising small particles ranging from 0.05 to 1.2 mm with smooth surfaces. Notably, the particles were predominantly spherical (Fig. 4(d)) or oval-shaped (Fig. 4(f)), with uniform size distribution, making them suitable for application as slow-release fertilizers. The XRD patterns presented in Fig. 5 show characteristic peaks at 2θ = 14.5°, 16°, 16.7°, 21°, 27°, 29.3°, 30.3°, 32°, 33.5°, 36°, 46.4°, and 51°, consistent with the crystal structure of struvite (MgNH4PO4·6H2O, JCPDS 15-0762).50 The obtained struvite product had a purity of 94.3% calculated based on the nitrogen content according to eqn (3). The above results show that the majority of the precipitate mass is pure struvite (MgNH4PO4·6H2O) with very low impurity content, demonstrating that the crystallization process by the FBHC method is effective and suitable for practical application as slow-release fertilizer. It can be affirmed that the FBHC method stands out for its high recovery efficiency, excellent particle quality, and ease of implementation stemming from precise crystallization control and effective impurity removal.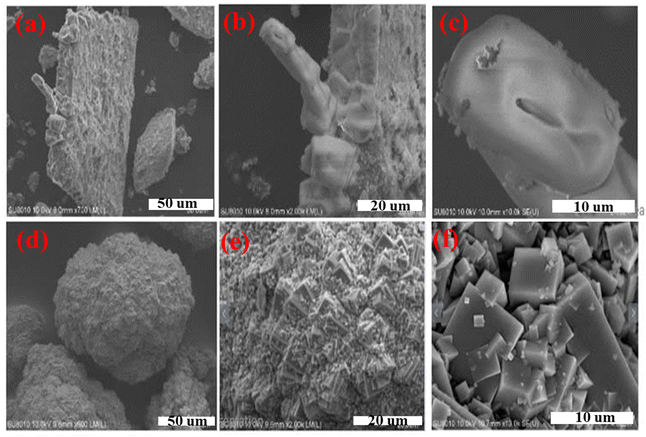 | ||
| Fig. 4 (a–c) SEM images of the conventional precipitate sample (jar-tests) and (d–f) the sample obtained from the FBHC process. | ||
Based on the particle size distribution diagram obtained from the FBHC process (Fig. 6), a substantial portion of the recovered material consists of fine particles smaller than 0.2 mm, accounting for approximately 40.62% of the total mass. This is followed by fine particles (0.2–0.75 mm) at 31.13%, medium particles (0.75–1.4 mm) at 14.23%, coarse particles (1.4–2 mm) at 7.77%, and very coarse particles (>2 mm) accounting for the smallest fraction at about 6.26%. This distribution suggests that the FBHC process, under the specific conditions applied, tends to produce a majority of fine to very fine particles during P and N recovery. Although the FBHC process is designed to minimize the presence of coarse particles, a small fraction is expected to persist under stable operating conditions. This residual coarse material may contribute to abrasion and fragmentation within the upper section of the system during continuous operation. Particle size plays an important role in the effectiveness of slow release fertilizers. Smaller particles tend to dissolve more quickly, allowing plants to absorb nutrients rapidly, but they are also more susceptible to leaching in highly permeable soils. In contrast, larger particles may dissolve at a slower rate, remaining effective over a longer period while potentially limiting their ability to reach deeper soil layers. As a result, the wide size distribution of the crystalline product obtained from the FBHC system offers a dual advantage by supplying nutrients both immediately and over time, while also reducing nutrient losses through leaching.
Fig. 7 depicts SEM images of struvite crystals obtained from the FBHC technique at 250× and 2000× magnifications. The crystals exhibit a well-defined rod-like shape, smooth surfaces, and sharp edges, indicating a high degree of crystallinity. The uniform morphology and absence of abnormal cracks or agglomeration indicate that the precipitation conditions were well controlled, possibly owing to the stability of the fluidized bed and the homogeneous mixing achieved in the FBHC system. The particle sizes are relatively consistent, ranging from 20 μm to 150 μm, which is suitable for applications such as slow-release fertilizers. These morphological characteristics confirm the effectiveness of the FBHC process in producing high-quality struvite crystals.
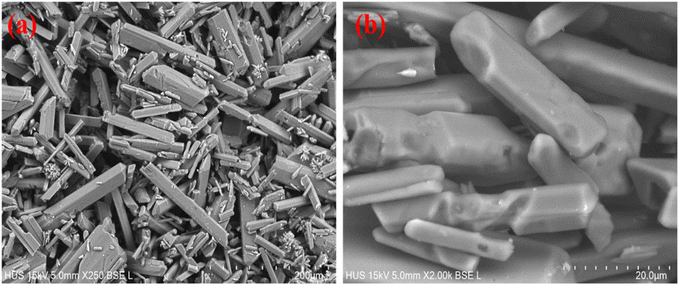 | ||
| Fig. 7 SEM images of FBHC product (a) ×250 magnification, scale bar = 50 μm; (b) ×2000 magnification, scale bar = 20 μm. | ||
As shown in Fig. 8, the EDS analysis was applied to determine the elemental composition of the samples. For the FBHC process results, the EDS spectrum showed peaks related to Mg, O, K, P, and Na, suggesting the potential formation of MgNaPO4·7H2O. Nonetheless, the detection of K reveals that potassium ions became incorporated into the crystallized samples, potentially sourced from the initial components in the swine wastewater. This implies a potential involvement of K in the development of complex phosphate precipitates. The presence of a minor quantity of Na indicates the likely formation of a Na-struvite impurity,51 with the Na+ ions potentially originating from the NaOH utilized for pH adjustment. In addition, the theoretical value of struvite for Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na
Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O is 1
O is 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11. However, the experimental result showed that the atomic ratios of Mg
11. However, the experimental result showed that the atomic ratios of Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Na
Na![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P
P![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O were 1
O were 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.5
0.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.7
1.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 6.5. This deviation may be due to two main reasons. Firstly, during the crystallization of FBHC, it is possible that other precipitates such as Mg3(PO4)2 were formed. These precipitate phases have different stoichiometry ratios than struvite, resulting in a change in the measured elemental ratios. Secondly, phosphate ions may have been adsorbed on the surface of the struvite crystals. A higher uptake of phosphorus relative to magnesium on the material's surface will result in an increased phosphorus-to-magnesium ratio as detected by surface EDS.
6.5. This deviation may be due to two main reasons. Firstly, during the crystallization of FBHC, it is possible that other precipitates such as Mg3(PO4)2 were formed. These precipitate phases have different stoichiometry ratios than struvite, resulting in a change in the measured elemental ratios. Secondly, phosphate ions may have been adsorbed on the surface of the struvite crystals. A higher uptake of phosphorus relative to magnesium on the material's surface will result in an increased phosphorus-to-magnesium ratio as detected by surface EDS.
 | ||
Fig. 8 EDS results of FBHC crystallized pellets after 7 days of operation (struvite pellet formation conditions: pH 9, Mg![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) P of 1.2, U 36 m h−1, L 0.27 kg m−2 h−1, and bed height 10 cm). P of 1.2, U 36 m h−1, L 0.27 kg m−2 h−1, and bed height 10 cm). | ||
The struvite particles obtained using FBHC technology in Fig. 9 exhibit a relatively uniform shape, smooth surface, and bright white color, indicating that the crystallization process was effective and the product is of high purity. In Fig. 9(a), the struvite appears evenly distributed without clumping, whereas Fig. 9(b) clearly reveals a nearly spherical structure with small and uniform particle sizes. These observations suggest that the operating conditions in the FBHC system such as pH, ion concentration, and up-flow rate were well controlled, leading to the formation of stable struvite crystals suitable for PO43− and NH4+ recovery, particularly for use as fertilizer. Although the struvite pellets recovered in this study exhibited high purity, uniform morphology, and an appropriate particle size distribution for use as slow release fertilizers, their actual effectiveness on plant growth has not been verified. To evaluate the practical application of this product in agricultural production, further studies should focus on bioassays conducted in soil or hydroponic systems. A relevant example is the study by Zabaleta et al.,52 in which almond shell biochar was applied in a soilless Eruca sativa cultivation model and can be used as a reference method for assessing the effectiveness of biofertilizers.
4. Conclusions
In summary, the seed-free FBHC technology was successfully developed and applied for the recovery of PO43− and NH4+ from swine wastewater. Key operational parameters influencing recovery efficiency were systematically optimized. Under the optimal conditions at pH 9, reaction time of 24 min, up-flow velocity of 36 m h−1, cross-sectional loading of 0.27 kg m−2 h−1, and 10 cm bed height, the system achieved a TR% of 97.42% for PO43− and 86.55% for NH4+, with corresponding CR% of 95.55 and 83.18%, respectively. Morphological and particle size analyses indicated that the resulting pellets were predominantly spherical and uniform, with 71.75% of particles measuring below 0.75 mm. The formation of crystalline struvite confirmed the effectiveness of the FBHC process in producing high-purity, homogeneous pellets. These characteristics make the recovered product suitable for use as a slow-release fertilizer in agricultural and horticultural applications. With its high recovery efficiency, low chemical demand, and operational simplicity, FBHC offers strong potential for broader applications in nutrient and resource recovery.Data availability
Data for this study, including figures and tables are available at article. The data supporting this article have been included as part of the ESI.†Author contributions
The Anh Luu: methodology, formal analysis, data curation, writing – original draft. Manh Tuan Truong: methodology, writing – review & editing. Gia Cuong Nguyen: writing – review & editing, project administration, formal analysis, data curation. Xuan Thanh Bui: writing – review & editing, methodology. Van Giang Le: writing – review & editing, supervision, project administration, funding acquisition, conceptualization.Conflicts of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.Acknowledgements
This research was funded by the research project CT05/03-2023-3 of Hanoi Department of Science and Technology, Vietnam. We also thank the editors and anonymous reviewers for their helpful comments and suggestions. We would like to thank Dr Minh Thuan Pham (Cheng Shiu University, Taiwan) for his kind support and helpful suggestions.References
- H. Huang, D. Xiao, J. Liu, L. Hou and L. Ding, Recovery and removal of nutrients from swine wastewater by using a novel integrated reactor for struvite decomposition and recycling, Sci. Rep., 2015, 5(1), 10183 CrossRef CAS PubMed.
- Z.-L. Ye, S.-H. Chen, S.-M. Wang, L.-F. Lin, Y.-J. Yan, Z.-J. Zhang and J.-S. Chen, Phosphorus recovery from synthetic swine wastewater by chemical precipitation using response surface methodology, J. Hazard. Mater., 2010, 176(1), 1083–1088 CrossRef CAS PubMed.
- Z.-L. Ye, S.-H. Chen, M. Lu, J.-W. Shi, L.-F. Lin and S.-M. Wang, Recovering phosphorus as struvite from the digested swine wastewater with bittern as a magnesium source, Water Sci. Technol., 2011, 64(2), 334–340 CrossRef CAS PubMed.
- V. G. Le, D. V. N. Vo, C. T. Vu, X. T. Bui, Y. J. Shih and Y. H. Huang, Applying a novel sequential double-column fluidized bed crystallization process to the recovery of nitrogen, phosphorus, and potassium from swine wastewater, ACS ES&T Water, 2020, 1(3), 707–718 Search PubMed.
- M. A. de Boer, L. Wolzak and J. C. Slootweg, Phosphorus: Reserves, Production, and Applications, in Phosphorus Recovery and Recycling, ed. H. Ohtake and S. Tsuneda, Springer Singapore, Singapore, 2019, pp. 75–100 Search PubMed.
- C. Numviyimana, J. Warchoł, B. Ligas and K. Chojnacka, Nutrients Recovery from Dairy Wastewater by Struvite Precipitation Combined with Ammonium Sorption on Clinoptilolite, Mater., 2021, 14(19), 5822 CrossRef CAS PubMed.
- W. G. Harris, A. C. Wilkie, X. Cao and R. Sirengo, Bench-scale recovery of phosphorus from flushed dairy manure wastewater, Bioresour. Technol., 2008, 99(8), 3036–3043 CrossRef CAS PubMed.
- D. Cândido, A. C. Bolsan, C. E. Hollas, B. Venturin, D. C. Tápparo, G. Bonassa, F. G. Antes, R. L. R. Steinmetz, M. Bortoli and A. Kunz, Integration of swine manure anaerobic digestion and digestate nutrients removal/recovery under a circular economy concept, J. Environ. Manage., 2022, 301, 113825 CrossRef.
- H.-D. Ryu, D. Y. Lim, S.-J. Kim, U.-I. Baek, E. G. Chung, K. Kim and J. K. Lee, Struvite Precipitation for Sustainable Recovery of Nitrogen and Phosphorus from Anaerobic Digestion Effluents of Swine Manure, Sustainability, 2020, 12(20), 8574 CrossRef CAS.
- A. Sendrowski and T. H. Boyer, Phosphate removal from urine using hybrid anion exchange resin, Desalination, 2013, 322, 104–112 CrossRef CAS.
- J. M. Chimenos, A. I. Fernández, A. Hernández, L. Haurie, F. Espiell and C. Ayora, Optimization of phosphate removal in anodizing aluminium wastewater, Water Res., 2006, 40(1), 137–143 CrossRef CAS PubMed.
- Q. Guan, Y. Li, Y. Zhong, W. Liu, J. Zhang, X. Yu, R. Ou and G. Zeng, A review of struvite crystallization for nutrient source recovery from wastewater, J. Environ. Manage., 2023, 344, 118383 CrossRef CAS PubMed.
- M. E. R. Christiaens, S. Gildemyn, S. Matassa, T. Ysebaert, J. De Vrieze and K. Rabaey, Electrochemical Ammonia Recovery from Source-Separated Urine for Microbial Protein Production, Environ. Sci. Technol., 2017, 51(22), 13143–13150 CrossRef CAS PubMed.
- W. A. Tarpeh, J. M. Barazesh, T. Y. Cath and K. L. Nelson, Electrochemical Stripping to Recover Nitrogen from Source-Separated Urine, Environ. Sci. Technol., 2018, 52(3), 1453–1460 CrossRef CAS PubMed.
- X. Chen, Y. Gao, D. Hou, H. Ma, L. Lu, D. Sun, X. Zhang, P. Liang, X. Huang and Z. J. Ren, The Microbial Electrochemical Current Accelerates Urea Hydrolysis for Recovery of Nutrients from Source-Separated Urine, Environ. Sci. Technol. Lett., 2017, 4(7), 305–310 CrossRef CAS.
- Z. G. Liu, X. B. Min, F. Feng, X. Tang, W. C. Li, C. Peng, T. Y. Gao, X. L. Chai and C. J. Tang, Development and simulation of a struvite crystallization fluidized bed reactor with enhanced external recirculation for phosphorous and ammonium recovery, Sci. Total Environ., 2021, 760, 144311 CrossRef CAS PubMed.
- W. A. Tarpeh, K. M. Udert and K. L. Nelson, Comparing Ion Exchange Adsorbents for Nitrogen Recovery from Source-Separated Urine, Environ. Sci. Technol., 2017, 51(4), 2373–2381 CrossRef CAS PubMed.
- S. K. Pradhan, A. Mikola and R. Vahala, Nitrogen and Phosphorus Harvesting from Human Urine Using a Stripping, Absorption, and Precipitation Process, Environ. Sci. Technol., 2017, 51(9), 5165–5171 CrossRef CAS PubMed.
- V.-G. Le, D.-V. N. Vo, N.-H. Nguyen, Y.-J. Shih, C.-T. Vu, C.-H. Liao and Y.-H. Huang, Struvite recovery from swine wastewater using fluidized-bed homogeneous granulation process, J. Environ. Chem. Eng., 2021, 9(3), 105019 CrossRef CAS.
- Y.-J. Shih, R. R. M. Abarca, M. D. G. de Luna, Y.-H. Huang and M.-C. Lu, Recovery of phosphorus from synthetic wastewaters by struvite crystallization in a fluidized-bed reactor: Effects of pH, phosphate concentration and coexisting ions, Chemosphere, 2017, 173, 466–473 CrossRef CAS PubMed.
- L. Hu, J. Yu, H. Luo, H. Wang, P. Xu and Y. Zhang, Simultaneous recovery of ammonium, potassium and magnesium from produced water by struvite precipitation, Chem. Eng. J., 2020, 382, 123001 CrossRef CAS.
- F. C. Ballesteros, A. F. S. Salcedo, A. C. Vilando, Y.-H. Huang and M.-C. Lu, Removal of nickel by homogeneous granulation in a fluidized-bed reactor, Chemosphere, 2016, 164, 59–67 CrossRef CAS PubMed.
- H. P. R. Guevara, F. C. Ballesteros, A. C. Vilando, M. D. G. de Luna and M.-C. Lu, Recovery of oxalate from bauxite wastewater using fluidized-bed homogeneous granulation process, J. Cleaner Prod., 2017, 154, 130–138 CrossRef CAS.
- W. D. Scott, T. J. Wrigley and K. M. Webb, A computer model of struvite solution chemistry, Talanta, 1991, 38(8), 889–895 CrossRef CAS PubMed.
- M. L. Salutsky and J. M. Sanborn, Magnesium potassium phosphate-containing fertilizer and process, US Pat., US3285731A, 1966 Search PubMed.
- X. Ye, Z.-L. Ye, Y. Lou, S. Pan, X. Wang, M. K. Wang and S. Chen, A comprehensive understanding of saturation index and upflow velocity in a pilot-scale fluidized bed reactor for struvite recovery from swine wastewater, Powder Technol., 2016, 295, 16–26 CrossRef CAS.
- B. B. B. Quedi, F. C. Ballesteros, A. C. Vilando and M.-C. Lu, Recovery of fluoride from wastewater in the form of cryolite granules by fluidized-bed homogeneous crystallization process, J. Water Process Eng., 2024, 66, 106063 CrossRef.
- M. S. Rahaman, D. S. Mavinic, A. Meikleham and N. Ellis, Modeling phosphorus removal and recovery from anaerobic digester supernatant through struvite crystallization in a fluidized bed reactor, Water Res., 2014, 51, 1–10 CrossRef CAS PubMed.
- A. W. Taylor, A. W. Frazier and E. L. Gurney, Solubility products of magnesium ammonium and magnesium potassium phosphates, Trans. Faraday Soc., 1963, 59, 1580–1584 RSC.
- N. Da Le, T. T. Ha Hoang, V. P. Phung, T. L. Nguyen, T. T. Duong, L. M. Dinh, T. M. Huong Pham, T. X. Binh Phung, T. D. Nguyen, T. N. Duong, T. M. Hanh Le, P. T. Le and T. P. Quynh Le, Trace Metal Element Analysis in Some Seafood in the Coastal Zone of the Red River (Ba Lat Estuary, Vietnam) by Green Sample Preparation and Inductively Coupled Plasma-Mass Spectrometry (ICP-MS), J. Anal. Methods Chem., 2021, 2021, 6649362 Search PubMed.
- C. T. Vu, C. Lin, C.-C. Shern, G. Yeh, V. G. Le and H. T. Tran, Contamination, ecological risk and source apportionment of heavy metals in sediments and water of a contaminated river in Taiwan, Ecol. Indic., 2017, 82, 32–42 CrossRef CAS.
- Warmadewanthi and J. C. Liu, Selective Precipitation of Phosphate from Semiconductor Wastewater, J. Environ. Eng., 2009, 135(10), 1063–1070 CrossRef CAS.
- V.-G. Le, C.-T. Vu, Y.-J. Shih, X.-T. Bui, C.-H. Liao and Y.-H. Huang, Phosphorus and potassium recovery from human urine using a fluidized bed homogeneous crystallization (FBHC) process, Chem. Eng. J., 2020, 384, 123282 CrossRef CAS.
- I. Çelen and M. Türker, Recovery of Ammonia as Struvite from Anaerobic Digester Effluents, Environ. Technol., 2001, 22(11), 1263–1272 CrossRef PubMed.
- H. Huang, Y. Chen, Y. Jiang and L. Ding, Treatment of swine wastewater combined with MgO-saponification wastewater by struvite precipitation technology, Chem. Eng. J., 2014, 254, 418–425 CrossRef CAS.
- D. Kim, K. J. Min, K. Lee, M. S. Yu and K. Y. Park, Effects of pH, molar ratios and pre-treatment on phosphorus recovery through struvite crystallization from effluent of anaerobically digested swine wastewater, Environ. Eng. Res., 2017, 22(1), 12–18 CrossRef.
- H.-D. Ryu, D. Kim and S.-I. Lee, Application of struvite precipitation in treating ammonium nitrogen from semiconductor wastewater, J. Hazard. Mater., 2008, 156(1), 163–169 CrossRef CAS PubMed.
- E. Banks, R. Chianelli and R. Korenstein, Crystal chemistry of struvite analogs of the type MgMPO4.6H2O (M+ = potassium(1+), rubidium(1+), cesium (1+), thallium(1+), ammonium(1+), Inorg. Chem., 1975, 14(7), 1634–1639 CrossRef CAS.
- J. Coppens, R. Lindeboom, M. Muys, W. Coessens, A. Alloul, K. Meerbergen, B. Lievens, P. Clauwaert, N. Boon and S. E. Vlaeminck, Nitrification and microalgae cultivation for two-stage biological nutrient valorization from source separated urine, Bioresour. Technol., 2016, 211, 41–50 CrossRef CAS PubMed.
- S. Başakçilardan-Kabakci, A. N. İpekoğlu and I. Talinli, Recovery of Ammonia from Human Urine by Stripping and Absorption, Environ. Eng. Sci., 2007, 24(5), 615–624 CrossRef.
- K. S. Le Corre, E. Valsami-Jones, P. Hobbs, B. Jefferson and S. A. Parsons, Agglomeration of struvite crystals, Water Res., 2007, 41(2), 419–425 CrossRef CAS PubMed.
- S. I. Lee, S. Y. Weon, C. W. Lee and B. Koopman, Removal of nitrogen and phosphate from wastewater by addition of bittern, Chemosphere, 2003, 51(4), 265–271 CrossRef CAS PubMed.
- M. Ronteltap, M. Maurer and W. Gujer, Struvite precipitation thermodynamics in source-separated urine, Water Res., 2007, 41(5), 977–984 CrossRef CAS PubMed.
- K.-Y. Chang, N. N. N. Mahasti and Y.-H. Huang, Fluidized-bed homogeneous crystallization of α-Al(OH)3 for continuous aluminum removal from aqueous solution: Parameter optimization and crystallization mechanism, J. Water Process Eng., 2023, 53, 103700 CrossRef.
- K. P. Fattah, D. S. Mavinic and F. A. Koch, Influence of Process Parameters on the Characteristics of Struvite Pellets, J. Environ. Eng., 2012, 138(12), 1200–1209 CrossRef CAS.
- C. Zhang, K.-n. Xu, J.-y. Li, C.-w. Wang and M. Zheng, Recovery of Phosphorus and Potassium from Source-Separated Urine Using a Fluidized Bed Reactor: Optimization Operation and Mechanism Modeling, Ind. Eng. Chem. Res., 2017, 56(11), 3033–3039 CrossRef CAS.
- V. C. T. Costodes and A. E. Lewis, Reactive crystallization of nickel hydroxy-carbonate in fluidized-bed reactor: Fines production and column design, Chem. Eng. Sci., 2006, 61(5), 1377–1385 CrossRef CAS.
- M. M. Bello, A. A. Abdul Raman and M. Purushothaman, Applications of fluidized bed reactors in wastewater treatment – A review of the major design and operational parameters, J. Cleaner Prod., 2017, 141, 1492–1514 CrossRef CAS.
- N. N. N. Mahasti, Y.-J. Shih, X.-T. Vu and Y. H. Huang, Removal of calcium hardness from solution by fluidized-bed homogeneous crystallization (FBHC) process, J. Taiwan Inst. Chem. Eng., 2017, 78, 378–385 CrossRef CAS.
- C. C. Wang, X. D. Hao, G. S. Guo and M. C. M. van Loosdrecht, Formation of pure struvite at neutral pH by electrochemical deposition, Chem. Eng. J., 2010, 159(1), 280–283 CrossRef CAS.
- K. Xu, J. Li, M. Zheng, C. Zhang, T. Xie and C. Wang, The precipitation of magnesium potassium phosphate hexahydrate for P and K recovery from synthetic urine, Water Res., 2015, 80, 71–79 CrossRef CAS PubMed.
- R. Zabaleta, E. Sánchez, P. Fabani, G. Mazza and R. Rodriguez, Almond shell biochar: characterization and application in soilless cultivation of Eruca sativa, Biomass Convers. Biorefin., 2024, 14(15), 18183–18200 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra03370e |
| This journal is © The Royal Society of Chemistry 2025 |