Open Access Article
Open Access ArticleCopper(II) chelates of a coumarin-based acyl hydrazone ligand: structural characterization and computational evaluations for prospective applications in antimicrobial, antiviral, antioxidant, and anticancer therapies†
Manar. S. Mahfouz,
Amira A. M. Ali,
Magdy Shebl,
Omima M. I. Adly and
R. Fouad
and
R. Fouad *
*
Department of Chemistry, Faculty of Education, Ain Shams University, Roxy, 11711, Cairo, Egypt. E-mail: Raniaahmed@edu.asu.edu.eg; Fax: +20 (02) 22581243; Tel: +20 01000212207
First published on 4th July 2025
Abstract
This study represents the synthesis and comprehensive characterization of four novel copper(II) chelates (CuR, CuRCT, CuH, and CuHCT) derived from a newly developed coumarin-based acyl hydrazone ligand (HCBH). The chelates were prepared using multidisciplinary synthetic routes, including reflux and hydrothermal methods with or without surfactant assistance, resulting in distinct mono- and binuclear structures with nanoscale morphologies. Structural analyses confirmed that all copper complexes possess tetrahedral geometries, with ligand coordination modes varying between tridentate and tetradentate depending on the synthetic method. TEM imaging revealed unique morphologies for each chelate, and the successful dispersion of the bioactive CuH chelate into silica yielded a porous nanostructured drug delivery system. Biological evaluations revealed promising antimicrobial activity, particularly for CuHCT against E. coli and S. aureus, and for CuRCT against B. subtilis and C. albicans. Antioxidant assays showed that CuRCT and CuHCT exhibited superior activity compared to other complexes and standard ascorbic acid. CuH demonstrated potent cytotoxicity against HepG-2 and MCF-7 cancer cell lines, comparable to cisplatin, while maintaining moderate toxicity toward normal Vero cells (CC50 = 43.34 ± 1.98 μg ml−1). Although the antiviral activity of CuH against HAV was weak, in vitro release studies of CuH–silica composites confirmed controlled release behavior, supporting its potential as a nanodrug delivery system. Density Functional Theory (DFT) and molecular docking analyses corroborated the biological findings, with favorable interactions observed between the compounds and CDK2 kinase. Collectively, these results highlight CuH as a highly promising candidate for further preclinical evaluation, especially in cancer therapy, with silica-based dispersion enhancing its potential as a nanocarrier for targeted drug delivery.
1. Introduction
In recent years, one of the most actively pursued areas of coordination chemistry has been the creation and production of novel nano-chelates with various morphologies, particularly distinctive structures, and significant pharmacological properties for the potential treatment of cancer, viral, and microbial diseases. The pharmacological properties of metal chelates are influenced by several factors, including the type of metal ions used, the selective bioactive chelating agents, the morphology, particle size, surface area, and the method employed to prepare the chelates.1,2 Among bioactive chelating agents, coumarins, known as benzopyran-2-ones, feature a simple and versatile structure, with the benzopyrone ring as their central framework. This core structure enables coumarins to interact with various enzymes and receptors through non-covalent interactions.3 As a result, coumarins exhibit a broad spectrum of biological activities, including anticancer,4,5 anti-inflammatory,6,7 antimicrobial,8,9 and antithrombotic10,11 effects. Copper ions and copper-based metallodrugs have been extensively explored for their biochemical activities within biological systems, primarily due to copper's diverse roles as an essential endogenous trace metal. Copper ions can bind to proteins, including albumin and ceruloplasmin. Moreover, copper is characterized by its high redox activity, wide structural variability, bioavailability, and its capacity to promote the generation of reactive oxygen species (ROS).12,13 Furthermore, the intricate relationship between copper and cancer cell proliferation has spurred investigations into the development of Cu-binding ligands aimed at regulating tumor tissue homeostasis.14 Additionally, copper chelates are thought to be effective alternatives to platinum-based medications and potential antitumor agents, in addition to their activity as chemical nucleases, enzyme inhibitors, or antiviral, antimicrobial, and anti-inflammatory agents. They promote an alternative on-apoptotic mechanism of programmed cell death by inducing oxidative stress towards DNA through the formation of ROS.12 Nanoparticles can be utilized to deliver medications by changing signal transduction or modifying the tumor microenvironment, and they also enable the sustained and regulated delivery of anticancer treatments.15 Particle size, shape, and surface chemistry are examples of physicochemical characteristics of nanoparticles that have been found to significantly impact drug delivery and cellular toxicity.16 Efforts have been undertaken to improve the biological features of nano-chelates by modifying their production process. Nano-chelate synthesis techniques have so far drawn a lot of scientific attention since various synthetic processes can alter the morphology and other biological characteristics of the materials that are generated. Hydrothermal, reflux, microwave, and other synthesis procedures have been introduced to achieve the unique biological features of the nano-chelates. The hydrothermal and reflux procedures in particular are quite alluring among these techniques since they are inexpensive, safe, effective, and have the potential to be environmentally friendly and large-scale manufacturing processes.2 Moreover, the control of crystalline size, distribution, and morphology can be effectively achieved through the addition of counter ions, capping molecules, hydrothermal temperature adjustments, and processing time. The choice of an appropriate stabilizer is crucial in the synthesis process to prevent excessive aggregation of nanoparticles (NPs).17 In this context, surfactants are often preferred over other capping agents due to their ability to form micelles, their excellent solubilization properties, and their ease of removal from the surfaces of NPs. Cetyltrimethylammonium bromide (CTAB) is an amphiphilic compound that possesses both hydrophobic and hydrophilic characteristics, along with a non-toxic nature.18 In light of the aforementioned findings, this study presents a green synthesis of copper(II) nano-chelates (CuR, CuRCT, CuH, CuHCT) from a newly synthesized 3-acetyl coumarin-based ligand (HCBH) using hydrothermal and reflux methods, with or without CTAB. The resulting nano-chelates showed spherical morphologies with diverse nanoscale dimensions and particle distributions, influenced by reaction conditions, and were structurally confirmed via comprehensive characterization. Biologically, the chelates exhibited broad-spectrum antimicrobial and strong antioxidant activities. Notably, CuH showed significant anticancer effects against HepG-2 and MCF-7 cell lines, with moderate cytotoxicity on normal Vero cells. Although its antiviral activity against HAV was limited, embedding CuH in a silica matrix enabled a controlled drug release profile. Molecular docking with CDK2 and DFT calculations supported their anticancer potential, positioning these nano-chelates as promising candidates for further therapeutic development.2. Methodology
2.1. Materials
Reagent-grade chemicals included salicylic acid hydrazide,19 3-acetyl coumarin,20 methyl salicylate (Sigma-Aldrich, 99%), ethyl acetoacetate (Sigma-Aldrich, 99%), salicylaldehyde (Sigma-Aldrich, 99.5%), and hydrazine hydrate (Sigma-Aldrich, 99%). Copper acetate salt, glacial acetic acid, ethylenediamine tetraacetic acid (EDTA) disodium salt, Tris–HCl, 2,2-diphenyl-1-pikryl-hydrazyl (DPPH), ascorbic acid, cetyltrimethylammonium bromide (CTAB), tetraethyl orthosilicate Si(OC2H5)4 (TEOS), ammonium hydroxide, metal indicators, and nitric acid were BDH or Merck. Organic solvents were used as high-quality reagents.2.2. Characterization
Microanalyses of nitrogen, hydrogen, and carbon were conducted at the Micro-analytical Center of Cairo University, Giza, Egypt. The metal content in the chelates was quantified through complexometric analysis. Melting and decomposition points were determined using a Stuart melting point apparatus (England). Molar conductivities were measured using a Corning conductivity meter (model NY 14831) with 10−3 M solutions of the solid nano-chelates prepared in DMF. Thermogravimetric (TGA) and derivative thermogravimetric (DTG) analyses were performed on a Shimadzu thermogravimetric analyzer from room temperature to 800 °C, employing a heating rate of 10 °C per minute. Fourier-transform infrared (FT-IR) spectra of HCBH and its copper chelates, spanning a range of 4000–400 cm−1, were recorded on a Nicolet IS10 FT-IR spectrometer. Proton nuclear magnetic resonance (1H-NMR) spectra at 300 MHz were obtained using a Mercury-300BB spectrometer, with tetramethylsilane (TMS) as an internal standard and dimethyl sulfoxide (DMSO) or DMSO-d6 as solvents. UV-vis absorption spectra for 10−3 M solutions of the solid nano-chelates in DMF were measured using a JASCO V-550 UV-vis spectrophotometer. Magnetic susceptibility measurements were conducted at room temperature using a Sherwood Scientific magnetic susceptibility balance (Cambridge Science Park, England) and the Gouy method. The effective magnetic moments (μeff) were calculated using the relation μeff = 2.828 (cm T)1/2 B.M., where cm denotes the molar susceptibility, corrected using Pascal's constants to account for the diamagnetic contributions of all atoms in the compounds. Electron spin resonance (ESR) spectra were recorded with a Bruker X-band spectrometer (model EMX). X-ray diffraction (XRD) patterns were obtained using a PHILIPS diffractometer with CuKα1 radiation (λ = 1.54056 Å), operating at an emission current of 30 mA and an accelerating voltage of 40 kV. Transmission electron microscopy (TEM) images were captured using a JEOL JEM-2100 TEM instrument at an accelerating voltage of 200 kV.2.3. Synthesis
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with 3-acetyl coumarin in absolute ethanol. The mixture was refluxed for two hours. The reaction mixture was allowed to cool fully to room temperature before separating the yellow crystals. Ethanol recrystallization created fine crystals when the product was filtered away (Scheme 1).
1 with 3-acetyl coumarin in absolute ethanol. The mixture was refluxed for two hours. The reaction mixture was allowed to cool fully to room temperature before separating the yellow crystals. Ethanol recrystallization created fine crystals when the product was filtered away (Scheme 1).
2.3.2.1. Synthesis of CuR nano-chelate. An aqueous solution of copper(II) acetate was added to a methanolic solution of HCBH to produce CuR chelate. Eight hours were spent refluxing the reaction mixture. The resulting precipitate was filtered and rinsed with a 50% (v/v) methanol–water mixture to remove any remaining unreacted starting material residues. Ultimately, anhydrous CaCl2 was used to dry the precipitate overnight in vacuum desiccators.
2.3.2.2. Synthesis of CuRCT nano-chelate. The CuRCT was synthesized by the same previous method of preparation of CuR, in addition to adding CTAB surfactant (1 mM) before the refluxing step.
2.3.2.3. Synthesis of CuH nano-chelate. In a 100 ml Teflon-lined autoclave with 70% filling, a mixture of HCBH and copper(II) acetate was dissolved in water and heated to 120 °C for 24 hours. Deep brown crystals were produced once the reactor gradually cooled to room temperature. After being filtered out, the crystals were cleaned with distilled water and allowed to dry at room temperature over anhydrous CaCl2 in a desiccator.
2.3.2.4. Synthesis of CuHCT nano-chelate. The chelate CuHCT was prepared as the previous method except for adding CTAB (1 mM). Very fine brown crystals were obtained. The chemical formula of the dried copper chelates was collected in Scheme S1.†
2.3.2.5. Synthesis of the CuH nano-chelate silica xerogel nanohybrid. Initially, a silica matrix that contains CuH nano-chelate was obtained by the sol–gel process.21 Then CuH nano-chelate (0.031 g) was dissolved in DMSO (5 ml). Then 0.1 ml of the complex solution was added to 10 ml of the silica sol to obtain a 10−4 M solution of the CuH nano-chelate in silica sol. Then the solution was stirred for 2 h at room temperature. Finally, a transparent sol was obtained after stirring, which lasted for 24 h till the gel formed. The gel is dried at 80 °C for 6 h.
2.4. Investigation of biological activity
2.4.1.1. Agar well diffusion method. The antimicrobial activities of under-investigated compounds were studied using the agar diffusion method22 against three types of bacteria: Staphylococcus aureus and Bacillus subtits (Gram-positive bacteria) and Escherichia coli, (Gram-negative bacteria) using nutrient agar medium. The antifungal activity of the compounds was tested against Candida albicans. Antimicrobial activity was assayed by measuring the diameter of the inhibition zone formed around the well. Ampicillin and Gentamicin were standard drugs for Gram-positive and Gram-negative bacteria, respectively. Nystatin was used as a standard drug for fungi strains.
2.4.1.2. Minimum inhibition concentration (MIC) test. Series concentrations of CuH nano-chelate were tested against E. coli (Gram-negative-bacteria). The MIC value defined as concentration, which causes complete inhibition (no visible microbial growth), was detected.23
2.4.4.1. Mammalian cell line. The Vero cells which were provided from the American Type Culture Collection, were taken from the kidney of an African green monkey (ATCC, Manassas, VA, USA). The Vero cells were grown in Dulbecco's modified Eagle's medium (DMEM), which was enhanced with 50 μg per ml gentamycin, 10% heat-inactivated fetal bovine serum (FBS), 1% L-glutamine, and HEPES buffer. Every cell was incubated twice a week at 37 °C in a humidified environment with 5% CO2.27
2.4.4.2. Virus propagation. Confluent Vero cells were used to propagate and test the cytopathogenic HAV virus.28 The Spearman–Karber method was used to count the infectious viruses by calculating the 50% tissue culture infectious dose (TCID50) with eight wells per dilution and 20 μl of inoculum per well.29
2.4.4.3. Cytotoxicity evaluation. The Vero cell lines were used to assess the cytotoxicity assay. The MTT colorimetric technique was used to determine the cell viability.30 The 50% cytotoxic concentration (CC50), or the concentration required to cause lethal effects in 50% of intact cells, was estimated to use graphical plots of each conc's dose–response curve. A test for cytopathic effect inhibition was used to screen for antivirals. In sensitive mammalian cells, this assay was chosen to demonstrate a cytopathic effect, or particular suppression of a biological function, as determined by the MTT technique. To determine the viral inhibition rate, the formula was [(A − B)/(C − B)] × 100%, where A, B, and C stand for the tested chemicals' absorbance with virus-infected cells, the virus control's absorbance, and the cell control's absorbance, respectively.30
2.5. Theoretical study
3. Results and discussion
3.1. Structural descriptions of HCBH ligand
The condensation of 3-acetyl coumarin with salicylic acid hydrazide results in the formation of a hydrazone ligand in the form of 2-hydroxy-N′-(1-(2-oxo-2H-chromen-3-yl)ethylidene) benzohydrazide (HCBH) as shown in Scheme 1. The displayed chemical formula C18H14N2O4 is consistent with the elemental analysis data. HCBH is air-stable and soluble in organic solvents such as DMSO and DMF. Fig. S1† shows the FT-IR spectrum of HCBH and provides valuable information on the structure of the generated ligand. The HCBH can exhibit keto/enol tautomerism due to its acyclic amide (NH–C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) functional group. The presence of the ν(NH) band at 3260 cm−1 and the ν(C
O) functional group. The presence of the ν(NH) band at 3260 cm−1 and the ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) band at 1640 cm−1 indicates that the HCBH is still in its keto form.40 Moreover, the HCBH exhibits a noticeable band at 1724 cm−1 due to ν(C
O) band at 1640 cm−1 indicates that the HCBH is still in its keto form.40 Moreover, the HCBH exhibits a noticeable band at 1724 cm−1 due to ν(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)lactone stretches.41 Furthermore, the (C
O)lactone stretches.41 Furthermore, the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N) group is responsible for the band at 1572 cm−1.42,43 The ν(OH) group is represented by the broad band, which is visible at 3469 cm−1.44
N) group is responsible for the band at 1572 cm−1.42,43 The ν(OH) group is represented by the broad band, which is visible at 3469 cm−1.44
1H-NMR spectrum analysis verified the production of the HCBH. The 1H-NMR spectrum of HCBH (Fig. S2†) showed singlet signals at δ 14.18, and 11.39 ppm, which could be connected to OHphenolic and NHamide, respectively, and vanished when D2O was introduced (Fig. S2b†).40 Aromatic moieties' protons have been found to exhibit multiplets in the 6.84–8.27 ppm range.40 Also, the signal that appeared at 2.28 ppm has been associated with the methyl group.
For the electronic spectrum of the chelating agent (HCBH), the absorption band (highest energy) corresponding to π–π* transitions of the aromatic ring system appeared at 274 nm (Fig. S3†).45 An additional band (moderate energy) observed at 331 nm may be associated with n–π* transitions within C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N groups.46
N groups.46
3.2. Structural and thermal descriptions of copper nano-chelates
The CuR and CuRCT chelates are obtained directly by a reflux treatment, whereas the hydrothermal treatment achieved the CuH and CuHCT chelates. Elemental analysis, FT-IR spectroscopy, ESR spectra, molar conductivity, magnetic moment measurement, UV-vis spectroscopy, and thermogravimetric analysis (TGA) were used to investigate the copper chelates. Table S1† lists the physical, analytical, and molar conductance data of the prepared compounds. The prepared nano-chelates (CuR, CuRCT, CuH and CuHCT) are colored, stable, and non-hygroscopic. These chelates are soluble in DMF and DMSO but insoluble in methanol and ethanol. The suggested chemical structure of copper nano-chelates is validated by elemental analysis. Scheme S1† shows that CuR and CuRCT have a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio and the chelates involved copper ions with a coordination number of four. Acetate ion occupies one of the four coordination sites as a monoanionic monodentate group, whereas HCBH occupies the other three sites. Furthermore, the HCBH chelating agent acts as a monobasic tridentate through the O-atom of lactone, the N-atom of azomethine group, and the O-atom of a deprotonated hydroxyl group. While CuH and CuHCT have a 2
1 molar ratio and the chelates involved copper ions with a coordination number of four. Acetate ion occupies one of the four coordination sites as a monoanionic monodentate group, whereas HCBH occupies the other three sites. Furthermore, the HCBH chelating agent acts as a monobasic tridentate through the O-atom of lactone, the N-atom of azomethine group, and the O-atom of a deprotonated hydroxyl group. While CuH and CuHCT have a 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 molar ratio (Cu2+
1 molar ratio (Cu2+![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) HCBH), the chelates involved two copper ions each of them surrounded by four coordination sites. The acetate group occupies two of four coordination sites as monoanionic bidentate group whereas HCBH occupies two other coordination sites. HCBH functions as a bis-bidentate monobasic in CuH and CuHCT chelates through the O-atoms of lactone, N-atom of azomethine group, and oxygen of deprotonated hydroxyl and phenolic groups. Molar conductance values of 10−3 M of CuRCT and CuR nano-chelates (in DMF) are too low and fall within the typical range of non-electrolytes without ionic acetate groups. Under identical conditions, the molar conductance value for CuH and CuHCT nano-chelates fell within the range typical of a 1
HCBH), the chelates involved two copper ions each of them surrounded by four coordination sites. The acetate group occupies two of four coordination sites as monoanionic bidentate group whereas HCBH occupies two other coordination sites. HCBH functions as a bis-bidentate monobasic in CuH and CuHCT chelates through the O-atoms of lactone, N-atom of azomethine group, and oxygen of deprotonated hydroxyl and phenolic groups. Molar conductance values of 10−3 M of CuRCT and CuR nano-chelates (in DMF) are too low and fall within the typical range of non-electrolytes without ionic acetate groups. Under identical conditions, the molar conductance value for CuH and CuHCT nano-chelates fell within the range typical of a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 electrolyte (Table S1†). This confirms the presence of one acetate anion as a counter ion out of the coordination sphere.47–49
1 electrolyte (Table S1†). This confirms the presence of one acetate anion as a counter ion out of the coordination sphere.47–49
To gain insights into the thermal degradation mechanisms of the prepared chelates and to investigate the existence of solvent molecules, the copper chelates were subjected to thermogravimetric analysis (TGA). The scheme of temperature-induced degradation and the steps involved in the thermal degradation of copper nano-chelates are shown in Schemes S2 and S3.† Fig. 1 shows the TGA–DTA curves of copper nano-chelates. The findings agreed with the theoretical formula that the analytical data indicated. In the first stage, CuR nano-chelate loses one coordinated acetic acid molecule at 312.5 °C (weight loss 13.30%; calcd 13.52%). In the second step, the complex loses CH4, HCN, and CO2 molecules at 437.5 °C (weight loss 33.14%; calcd 33.15%). For CuRCT, the half molecule of non-coordinated water was released at 106 °C (weight loss 1.82%; calcd 1.98%). The following stage of CuRCT is related to removing a coordinated acetic acid molecule at 316.5 °C (weight loss 15.35%; calcd 15.24%). The third step corresponds to the loss of CH4, HCN, and CO2 molecules with a weight loss of 34.44% (calcd 34.48%). CuH nano-chelate loses uncoordinated acetic acid at 314 °C (weight loss 9.44%; calcd 9.60%) followed by coordinated two acetic acid molecules at 385 °C (weight loss 28.54%; calcd 28.80%). For CuHCT the uncoordinated acetic acid was removed at a temperature of 240 °C (weight loss 9.31%; calcd 9.60%). Then the two coordinated acetic acid molecules were released at 379 °C (weight loss 28.33%; calcd 28.80%). The isolated copper chelates completed their degradation to reach the final residue CuO: found; (calcd) 17.91% (17.63%), CuO: found; (calcd) 18.10% (17.56%), 2CuO and 6C: found; (calcd) 37.11% (36.94%) and Cu2O: found; (calcd) 22.51% (22.87%) for CuR, CuRCT, CuH and CuHCT respectively.
The thermodynamic parameters governing the decomposition processes of complexes, including the activation energy (ΔE), enthalpy (ΔH), entropy (ΔS), and Gibbs free energy change (ΔG), were systematically evaluated through graphical analysis utilizing the Coats–Redfern equation. This method has proven effective in determining the reaction order (n) and activation parameters for the primary degradation stages of both the ligand and metal complexes.50 The kinetic parameters are listed in Table S2.† The following observations can be made based on the analysis: (i) the degradation processes are endothermic, as indicated by the positive values of enthalpy change (ΔH). (ii) For CuR and CuRCT chelates, the decomposition rate of the second degradation step is faster than the first step, as evidenced by lower activation energy (ΔE) values for the second step compared to the first step. Conversely, for CuH and CuHCT, the activation energy for the second degradation step is higher, suggesting that the second stage of decomposition occurs more slowly51 (iii) the activated chelate formed during the degradation process exhibits a more ordered structure than the reactants, or the reactions are relatively slow, as shown in the negative entropy change (ΔS) values for the complexes.52 Relatively, low positive ΔG values indicate the autocatalytic effect of metal ions on the thermal degradation of the chelates, suggesting that the degradation processes are non-spontaneous.53
3.3. Spectroscopic description of copper nano-chelates
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)amide in all copper nano chelates. (ii) The band of NHamide which appeared at 3260 cm−1 in the HCBH disappeared in all copper nano chelates due to deprotonation of the NH group during the tautomerization process. (iii) Additionally, by deprotonating the NH group, new bands of (C
O)amide in all copper nano chelates. (ii) The band of NHamide which appeared at 3260 cm−1 in the HCBH disappeared in all copper nano chelates due to deprotonation of the NH group during the tautomerization process. (iii) Additionally, by deprotonating the NH group, new bands of (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)enolic in the 1510–1520 cm−1 appeared in copper nano chelates, suggesting the coordination via enol form instead of through keto form. Moreover, the most noticeable alteration of spectral features of HCBH when coordinated to copper ion is the downward shift of the lactonic carbonyl group to the extent of about 30–31 cm−1, which indicates that the metal is coordinated to the oxygen of lactonic carbonyl. Also, new bands corresponding to ν(M–O) bonds (514–585 cm−1) confirm the participation of lactonic carbonyl in coordination.54 Moreover, the (C
N)enolic in the 1510–1520 cm−1 appeared in copper nano chelates, suggesting the coordination via enol form instead of through keto form. Moreover, the most noticeable alteration of spectral features of HCBH when coordinated to copper ion is the downward shift of the lactonic carbonyl group to the extent of about 30–31 cm−1, which indicates that the metal is coordinated to the oxygen of lactonic carbonyl. Also, new bands corresponding to ν(M–O) bonds (514–585 cm−1) confirm the participation of lactonic carbonyl in coordination.54 Moreover, the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N)azomethine group suffered a positive shift of about 12–22 cm−1 in copper nano-chelates, indicating the participation of these groups in complexation. The emergence of non-ligand bands at the 433–454 cm−1 regions, which are assigned (M–N) bonds, subsequently confirms the presence of the azomethine group in the chelating arms.55 The CuR and CuRCT nano-chelates exhibit new bands in the ranges 1486–1487 and 1255–1256 cm−1, which may be due to asymmetric and symmetric vibration modes of the acetate group, respectively. The large difference between the two bands (>200 cm−1) illustrates the monodentate nature of the acetate group.56,57 On the other hand, CuH and CuCTH nano-chelates exhibit new bands in the ranges 1434–1478 and 1301–1313 cm−1, which may be due to asymmetric and symmetric vibration modes of the acetate group, respectively. The relatively small difference between νas and νs bands clearly illustrates the bidentate nature of the acetate group.58
N)azomethine group suffered a positive shift of about 12–22 cm−1 in copper nano-chelates, indicating the participation of these groups in complexation. The emergence of non-ligand bands at the 433–454 cm−1 regions, which are assigned (M–N) bonds, subsequently confirms the presence of the azomethine group in the chelating arms.55 The CuR and CuRCT nano-chelates exhibit new bands in the ranges 1486–1487 and 1255–1256 cm−1, which may be due to asymmetric and symmetric vibration modes of the acetate group, respectively. The large difference between the two bands (>200 cm−1) illustrates the monodentate nature of the acetate group.56,57 On the other hand, CuH and CuCTH nano-chelates exhibit new bands in the ranges 1434–1478 and 1301–1313 cm−1, which may be due to asymmetric and symmetric vibration modes of the acetate group, respectively. The relatively small difference between νas and νs bands clearly illustrates the bidentate nature of the acetate group.583.3.2.1. Stability of compounds. Thermodynamic stability over extended periods is a critical parameter for evaluating potential drugs. All candidate drugs must maintain stability under physiological conditions to effectively reach their target within living organisms. The stability of copper nano-chelates was assessed in a water/DMSO solution (10 μM, 0.1% DMSO) using UV-vis spectroscopy. Time-dependent UV-vis spectra of the CuH chelate, selected as a representative example, were recorded at specific time intervals (0, 2, 6, 12, 24, and 72 hours) in the water/DMSO solution, as illustrated in Fig. S4.† The spectral characteristics and peak absorptions remained consistent over time, indicating the absence of any structural modifications in the chelates. Additionally, the nano-chelates were measured electronically using Nujol mulls and DMF solutions. The minimal impact of DMF on the chelate configuration is demonstrated by the spectra and band positions of all chelates in DMF solutions are roughly identical to those observed as Nujol mulls. Demonstrating that the same species exists in both solid and solution forms further supports the idea that complexes maintain their integrity in solutions.
3.4. Morphological description (TEM study)
The TEM image of CuR chelate shows the hybrid morphology of connected nanospheres (an average diameter of 23 nm) and nanorods (an average diameter of 27 nm with several lengths) (Fig. 3). A high separate uniform accumulated spherical particles formed a spherical cluster with an average size of 73 nm appeared in the case of CuRCT chelate (Fig. 3). Also, as represented in the TEM image of CuH Fig. 3, the particles of CuH have uniform and separated spherical shapes with an average size of 8 nm. On the other hand, CuHCT has an aggregated spherical shape with an average diameter of 29 nm. It is clear from Fig. 3 that the treatment methods (reflux and hydrothermal) with or without surfactant (CTAB) during the preparation process might influence the morphology and crystal sizes of copper chelates.TEM image of CuH chelate dispersed into silica exhibited porous sheet morphology with an average pore diameter of 18 nm. In silica pores, CuH chelate is evenly dispersed (Fig. S6†). These findings clarify that the CuH chelate particles were restricted to the silica pores' internal region preventing the development of crystals larger than the pore domain.
CTAB significantly alters morphology. The reason for this is that during the preparation phase, CTAB formed micelles that confined the particles within their micelle chamber. Small spherical CTAB structures lower than CMC (critical micelle concentration) can be used to explain the shape-directing function of CTAB; these sub-micellar aggregates can physically interact with the reactants to generate active entities.61 The following mechanism could be used to explain how aggregated and accumulated spherical nanoparticles of chelate form during the addition of CTAB with hydrothermal or reflux processes. Once CTAB-assisted reflux or hydrothermal process, the organic ligand was solubilized by the surfactant micelles through electrostatically attracting of the positive heads of CTAB and electron-donating groups of organic ligand like C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N and –OH groups.62 In CuRCT chelate, the electrostatic interaction is more prevalent due to the presence of free OH groups. This interaction slows down the ligand–Cu2+ ion reaction rates and further regulates the morphologies of the resulting nano-complex. Copper ions are not attracted to CTAB alone. However, there is a greater chance of interaction between the copper ion and the organic ligand's donating group than there is with the nano-complex that forms. Accordingly, micelles serve as nucleating sites for the development of spherical crystals of nano-chelate (Scheme 2). A portion of the CTAB molecule is released when the nano-chelate grows to particles, while other molecules adsorb on the surface of the chelate particle by hydrogen bonding with the free phenolic –OH group. Thus, the higher accumulated spherical complex particles are formed as seen from TEM analysis. As shown from Scheme S1,† the structure of CuHCT is binuclear without a free phenolic group, but the structure of CuRCT has a free phenolic group which confirms the separately accumulated spherical chelate particles higher than the previous complex (TEM images).
N and –OH groups.62 In CuRCT chelate, the electrostatic interaction is more prevalent due to the presence of free OH groups. This interaction slows down the ligand–Cu2+ ion reaction rates and further regulates the morphologies of the resulting nano-complex. Copper ions are not attracted to CTAB alone. However, there is a greater chance of interaction between the copper ion and the organic ligand's donating group than there is with the nano-complex that forms. Accordingly, micelles serve as nucleating sites for the development of spherical crystals of nano-chelate (Scheme 2). A portion of the CTAB molecule is released when the nano-chelate grows to particles, while other molecules adsorb on the surface of the chelate particle by hydrogen bonding with the free phenolic –OH group. Thus, the higher accumulated spherical complex particles are formed as seen from TEM analysis. As shown from Scheme S1,† the structure of CuHCT is binuclear without a free phenolic group, but the structure of CuRCT has a free phenolic group which confirms the separately accumulated spherical chelate particles higher than the previous complex (TEM images).
3.5. Biological properties descriptions
3.5.1.1. Inhibition zone's diameter investigation. The antibacterial and antifungal properties of HCBH and its synthesized copper nano-chelates were investigated using the agar diffusion method22 in DMSO solvent against various bacterial species, including Gram-positive bacteria (Staphylococcus aureus and Bacilus subtilis) and Gram-negative bacteria (Escherichia coli), using nutrient agar medium. The antifungal activity of the compounds was tested against Candida albicans using Sabouraud dextrose agar medium. Standard drugs for the Gram-positive and Gram-negative bacteria were Ampicillin and Gentamicin, respectively, while nystatin was used for the fungal strains. Table 1 displays the results, which are reported as the inhibition zone's diameter. A good comparability between the studied chelates and common antibacterial agents is also displayed in Table 1. Except for Gram-negative bacteria (Escherichia coli), the chelating agent (HCBH) is ineffective against all other bacteria and fungi. Each of the copper nano-chelates showed a different level of growth inhibition on the tested bacterial and fungal species. However, antimicrobial activity of all nano-chelates varied from high to moderate activity compared with standard antibiotics. CuR < CuH < CuRCT < CuHCT was the order that inhibited E. coli and S. aureus the most, according to the findings in Fig. S7.† Therefore, using the prepared nano-chelates to treat some common disorders caused by S. aureus and E. coli seems reasonable. CuR < CuH < CuHCT < CuRCT is the order in which the nano-chelates inhibit B. subtits. CuRCT also exhibits a discernible antifungal effect against Candida albican. From the above findings, it is observed the coordination enhances the antibacterial activity of the copper chelates, which is superior to that of the HCBH ligand. These findings may be explained according to Overton's concept and chelation theory. The chelates have a great chance of acting as more potent and stronger fungicidal and bactericidal drugs due to the chelation between the ligand and the metal ion, destroying more bacteria and fungus than the ligand as a result. In particular, the presence of a metal's positive charge that is partially shared with the donor group causes the π–electron to delocalize throughout the entire chelating system. According to Overton's theory of cell permeability, a key factor causing antimicrobial action is liposolubility. Chelation makes coordinated molecules more lipophilic, which improves metal chelate penetration into lipid membranes and prevents additional microbial growth. Metal coordinates also impact the respiration process of cells, which prevents protein synthesis and impedes the organism's ability to grow. Additionally, the activity of various complexes against various bacteria varies depending on either the ribosome variations in microbial cells or the impermeability of the bacteria's cells.63,64
| Sample | HCBH | CuR | CuRCT | CuH | CuHCT | Standard antibiotic |
|---|---|---|---|---|---|---|
| Microorganism | ||||||
| a Zone of inhibition is expressed as mean ± standard deviation (mm).b NA: no activity, well diameter 6 mm, 100 μl was tested. | ||||||
| Gram negative bacteria | Gentamicin | |||||
| Escherichia coli (ATCC: 10536) | 7.3 ± 1.0 | 9.3 ± 0.6 | 12.7 ± 0.6 | 11.7 ± 0.6 | 13.7 ± 0.6 | 27 ± 1.0 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Gram positive bacteria | Ampicilin | |||||
| Staphylococcus aureus (ATCC: 13565) | NA | 14.3 ± 0.6 | 19.0 ± 1.0 | 17.3 ± 0.6 | 21.3 ± 0.6 | 21.0 ± 1.0 |
| Bacilus subtits (DSM: 1088) | NA | 11.3 ± 0.6 | 19.3 ± 0.6 | 17.0 ± 1.0 | 18.7 ± 0.6 | 21.3 ± 0.6 |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
||||||
| Fungi | Nystatin | |||||
| Candida albicans (ATCC: 10231) | NA | 9.3 ± 0.6 | 11.7 ± 0.6 | 10.0 ± 1.0 | 9.0 ± 1.0 | 21.0 ± 1.0 |
Furthermore, antimicrobial activity was affected by various factors like geometry, morphological structure, and particle size of under-studied compounds. These factors may be responsible for the variety of antimicrobial activity of the prepared copper nano chelates. Although CuR and CuRCT have the same geometry, the CuRCT recorded higher activity than CuR. Also, CuHCT showed higher activity than CuH. That may be due to the effect of CTAB which leads to an increase in the hydrophobic and lipophilic character of the chelates, consequently, enhancing the permeability to the microbial cells and killing the organisms effectively.
Also, it is shown that CuH has higher antimicrobial than CuR. This could be attributed to the smaller nanoparticle size, which enhances its spreading to microbial cells.
3.5.1.2. MIC investigation. Series concentrations of highly antimicrobial active copper nano-chelates (CuHCT and CuRCT) were tested against B. subtits and S. aureus and (Gram-positive bacterium), E. coli (Gram-negative bacterium) and C. albicans (fungi). The concentration that results in complete inhibition (no discernible microbial growth) is known as the MIC value.23,65,66 CuHCT recorded stronger antimicrobial action against S. aureus with MIC of 31.25 μg ml−1 with respect to CuRCT (MIC of 62.5 μg ml−1) and standard Ampicillin (MIC = 62.5 μg ml−1). Also, the CuHCT and CuRCT show weak activity against E. coli (MIC = 250 μg ml−1), B. subtits (MIC = 250 μg ml−1) and C. ablicans (MIC = 125 μg ml−1).
![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N), lactonic oxygen (C
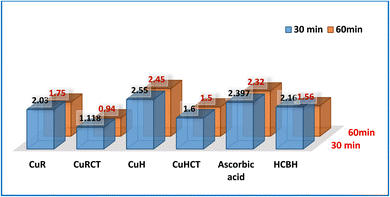
N), lactonic oxygen (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O), and phenolic group (OH) where the reactivity of free radical can be neutralized by the donation of an electron or hydrogen.67 The HCBH records a significantly high antioxidant activity due to the presence of various free functional groups. Among the copper nano-chelates that were investigated, the strongest antioxidant activity was shown by the CuRCT (IC50 = 1.11 μM). Similar to the CuRCT, the CuHCT nano-chelate exhibited strong free radical scavenging activity (IC50 = 1.60 μM). Copper chelates with CTAB (CuRCT and CuHCT) show more antioxidant activity than pure copper chelates (CuR and CuH), although the chemical structures of chelates are comparable. It is assumed that the positively charged CTAB headgroups attract copper chelate stabilizing of oxidized products intermediate i.e., antioxidants in the free radical forms, and this process leads to the accumulation of the copper chelate anion in the interfacial region.68,69
O), and phenolic group (OH) where the reactivity of free radical can be neutralized by the donation of an electron or hydrogen.67 The HCBH records a significantly high antioxidant activity due to the presence of various free functional groups. Among the copper nano-chelates that were investigated, the strongest antioxidant activity was shown by the CuRCT (IC50 = 1.11 μM). Similar to the CuRCT, the CuHCT nano-chelate exhibited strong free radical scavenging activity (IC50 = 1.60 μM). Copper chelates with CTAB (CuRCT and CuHCT) show more antioxidant activity than pure copper chelates (CuR and CuH), although the chemical structures of chelates are comparable. It is assumed that the positively charged CTAB headgroups attract copper chelate stabilizing of oxidized products intermediate i.e., antioxidants in the free radical forms, and this process leads to the accumulation of the copper chelate anion in the interfacial region.68,69
3.6. Theoretical study
CuR/CuRCT appears to be less reactive based on these characteristics as well as its higher electronegativity (χ) (7.40 eV) and chemical hardness (η) (1.42 eV). On the contrary, CuH is more polarizable and expected to interact with biological targets more easily due to its lower IP and higher softness (σ = 0.38). The greater reactivity and polarizability of CuH/CuHCT that promote binding interactions with cellular targets, support the higher antitumor activity (lower IC50 values) compared to CuR/CuRCT (Fig. S8†). These findings demonstrate how CuH's electronic characteristics increase its chemical reactivity and suitability for improved interactions with cancer cell targets, which is consistent with its stronger anticancer efficacy. The relationship between electronic structure and biological efficacy appears to have a mechanistic basis since the electronic configuration of CuR/CuRCT chelates seems to restrict its biological reactivity when compared to CuH/CuHCT. Table S4† collects the calculated EHOMO, ELUMO, energy gap (ΔE), ionization potential (IP), electron affinity (EA), electronegativity (χ), chemical potentials (μ), chemical hardness (η), softness (σ), and electrophilicity index (ω), by eV unit, of HCBH and its copper chelates.
Accurately predicting interactions in molecular docking studies requires an understanding of the distribution of partial charges on proteins and substrates. Maps of molecular electrostatic potential (MEP) are a crucial three-dimensional tool that graphically depicts molecular structure and charge distribution. Red denotes areas of negative charge (nucleophilic sites) and blue denotes areas of positive charge (electrophilic sites) in these maps.78,79 For docking, researchers can rapidly identify important electrostatic characteristics and possible interaction positions on the protein and substrate owing to this color-coded arrangement.80 The MEP map of the investigated compounds in Fig. 7 shows red regions surrounding electron-rich heteroatoms, indicating positions appropriate for electrophilic interactions, and blue regions which are usually seen around hydrogen atoms, observing the positions that could easily engage in intermolecular interactions with proteins. Our knowledge of chemical reactivity and interaction potential inside various molecular areas is enhanced by this imaging, which also provides guidance for docking and molecular recognition procedures.
Firstly, N-(1-[cis-3-(acetylamino)cyclobutyl]-1H-imidazol-4-yl)-2-(4-methoxyphenyl)acetamide, the native ligand that was initially co-crystallized with the 3IG7 active site, was used in a re-docking and superimposition procedure to thoroughly validate the docking methodology. This co-crystallized ligand's binding pose was faithfully replicated during the validation, guaranteeing the dependability of the docking procedure. A high degree of alignment was seen when the re-docked ligand was superimposed with the native ligand, indicating that the docking process could accurately replicate the interactions of the original ligand within the active site. The durability and accuracy of the docking methodology are demonstrated by this successful validation, which is shown in Fig. S12.† As a result, it can be used to predict the binding modes of other drugs. To assess the binding interactions of the investigated drugs (HCBH and its copper nano-chelates), molecular docking was performed against the crystal structure of CDK-5 inhibitors, specifically the inhibitor EFP bound to CDK-2 (PDB ID: 3IG7). As shown in Fig. 8, the docking simulations identified the drugs' ideal binding modes. These docked patterns reveal information about the chemicals' interactions with the protein's active site. Table S5† provides a summary of the specific interactions with important amino acid residues. According to the molecular docking data, the HCBH and its copper nano-chelates with the target protein (PDB ID: 3IG7) exhibit different interaction profiles with different amino acid residues, which contribute to their binding energies and biological activities. The HCBH forms a hydrogen bond with HIS84 at a distance of 2.75 Å, and a binding energy of −6.70 kcal mol−1, As well as Pi–anion and Pi–alkyl interactions with ASP145 and LYS89, respectively. However, the binding of HCBH is relatively weaker compared to the nano-chelates. In contrast, CuR/CuRCT displays various strong interactions, including hydrogen bonds with LYS33, ASN132, and ASP145, with distances ranging from 2.54 Å to 3.00 Å, and a notable Pi–anion interaction with ASP86. These interactions lead to a stronger binding energy of −8.2 kcal mol−1, indicating enhanced affinity toward the protein. For CuH/CuHCT exhibits the highest binding energy of −8.40 kcal mol−1, driven by hydrogen bonds with LEU32 and GLY11, along with Pi–Pi interactions with PHE80 and multiple Pi–alkyl interactions, particularly with VAL18, demonstrating its strong interaction network. A pattern becomes apparent when comparing this molecular docking data with the in vitro cytotoxicity data. Poor antitumor effectiveness is indicated by the HCBH ligand's highest IC50 value (71 μM) and weakest binding energy (−6.70 kcal mol−1). On the other hand, CuR/CuRCT exhibits considerably better cytotoxicity and a stronger binding (−8.20 kcal mol−1). CuH/CuHCT has the strongest binding (−8.40 kcal mol−1) and the most varied interactions with the target protein. Its greater antitumor activity is reflected in its lowest IC50 value. According to this relationship between binding energy and IC50 values, these drugs' anticancer activity is probably going to be increased by greater interactions with the target protein.
 | ||
| Fig. 8 3D representation of the hydrogen bonding between the studied compounds and the amino acid residues of the target protein (pdb ID: 3IG7). | ||
4. Conclusion
This study developed eco-friendly copper(II) nano-chelates (CuR, CuRCT, CuH, CuHCT) from a coumarin-based ligand (HCBH) using hydrothermal and reflux methods, with or without CTAB. Structural analysis confirmed tetrahedral geometries and coordination through ONO or ONOO donor sites. TEM showed morphology varied with synthesis conditions. Biologically, CuHCT had strong antibacterial effects (against E. coli and S. aureus), while CuRCT was effective against B. subtilis and Candida. Both CuRCT and CuHCT showed excellent antioxidant activity (IC50: 1.11–1.60 μM). CuH displayed potent anticancer effects on HepG-2 and MCF-7 cells (comparable to cisplatin), with moderate toxicity on normal cells. Although CuH showed weak antiviral activity, encapsulation in silica improved its release profile. Computational studies (DFT, docking) supported its bioactivity. Overall, CuH is a strong antitumor candidate, and its silica nanohybrid offers a promising cancer drug delivery platform.Data availability
Data are available on request from the authors.Conflicts of interest
There are no conflicts to declare.Acknowledgements
This paper is based on work supported by Science, Technology & Innovation Funding Authority (STDF) under grant number 48871.References
- P. J. Dyson and G. Sava, Metal-based antitumor drugs in the post genomic era, Dalton Trans., 2006, 16, 1929–1933 Search PubMed.
- M. Saif, H. F. El-Shafiy, M. M. Mashaly, M. F. Eid, A. I. Nabeel and R. Fouad, Hydrothermal preparation and physicochemical studies of new copper nano-complexes for antitumor application, J. Mol. Struct., 2018, 1155, 765–775 CrossRef CAS.
- L. Gao, F. Wang, Y. Chen, F. Li, B. Han and D. Liu, The antithrombotic activity of natural and synthetic coumarins, Fitoterapia, 2021, 154, 104947 CrossRef CAS.
- M. Shebl, T. E. Ali and M. A. Assiri, Novel mononuclear Cu(II), Ni(II), and Co(II) complexes of coumarinyl-pyrazolyl-thiazole thiosemicarbazone: Synthesis, characterization, and biological evaluation, Appl. Organomet. Chem., 2025, 39, e7948 CrossRef CAS.
- A. Benazzouz-Touami, k. Ighilahriz, M. Makhloufi-Chebli, N. Hadhoum, J. -Bernard Behr and A. Chouh, Synthesis, biological evaluation, ADMET studies, and molecular docking of novel coumarin–isoxazole derivatives as dual inhibitors of Hsp90 protein and acetylcholinesterase enzyme, J. Mol. Struct., 2025, 1333, 141776 CrossRef CAS.
- Y. Gong, X. Li, H. Tang, Y. Liu, S. Wang and Y. Geng, Synthesis, crystal structure, spectroscopic characterization, DFT calculations, Hirshfeld surface analysis, biological activity studies, molecular docking investigation, and ADMET properties evaluation of a novel 3-substituted coumarin derivative, J. Mol. Struct., 2025, 1323, 140739 CrossRef CAS.
- A.-S. Badran, M. A. Ibrahim and A. Ahmed, Nucleophilic reactions with the novel condensation product derived from 3-formylchromone and 4-hydroxycoumarin, Syn. Comm., 2021, 51, 1868–1881 CrossRef CAS.
- G. Yang, L. Shi, Z. Pan, L. Wu, L. Fan, C. Wang, C. Xu and J. Liang, The synthesis of coumarin thiazoles containing a trifluoromethyl group and their antifungal activities, Arabian J. Chem., 2021, 14, 102880 CrossRef CAS.
- M. S. Ebaid, M. Korycka-Machala, M. A. Shaldam, M. G. Thabit, M. Kawka, B. Dziadek, M. Kuziola, H. A. A. Ibrahim, X. Lei, A. I. Elshamy, J. Dziadek and A. Sabt, Exploring antitubercular activity of new coumarin derivatives targeting enoyl acyl carrier protein reductase (InhA): Synthesis, biological evaluation and computational studies, J. Mol. Struct., 2025, 1336, 142074 CrossRef CAS.
- K. Kasperkiewicz, M. B. Ponczek, J. Owczarek, P. Guga and E. Budzisz, Antagonists of vitamin K-popular Coumarin drugs and new synthetic and natural Coumarin derivatives, Molecules, 2020, 25, 1465 CrossRef CAS.
- P. Karan, B. Shit, P. Panja, A. Khatun, M. Bera, J. Pal, S. Patra, S. Chakrabarti, S. Pal, A. Ghosh and M. Hossain, Synthesis of some new arylazo-coumarin derivatives and their comparative biological activity studies: Cytotoxicity, gene expression, DNA binding, BSAbinding, and in silico analysis, J. Mol. Liq., 2025, 420, 126813 CrossRef CAS.
- D. Ganci, L. D. Anna, G. Abruscato, M. L. Chevalier, O. Quideau, S. Cataldo, A. Pettignano, S. Rubino, R. Chiarelli, G. Barone, C. Luparello and R. Bonsignore, Harnessing redox reactions for anticancer effects: A copper(II) Schiff base complex induces apoptosis in HepG2 liver cancer cells via ROS generation, J. Inorg. Biochem., 2025, 270, 112938 CrossRef CAS.
- A. Kellett, Z. Molphy, V. McKee and C. Slator, Recent Advances in Anticancer Copper Compounds: Metal-Based Anticancer Agents, The Royal Society of Chemistry, 2019, pp. 91–119 Search PubMed.
- J. O. Pinho, I. V. Silva, J. D. Amaral, C. M. P. Rodrigues, A. Casini b, G. Soveral and M. M. Gaspar, circulating liposomes for colon cancer management, Int. J. Pharm., 2021, 599, 120463 CrossRef CAS.
- D. Chang, Y. Gao, L. Wang, G. Liu, Y. Chen, T. Wang, W. Tao, L. Mei, L. Huang and X. Zeng, Polydopamine-based surface modification of mesoporous silica nanoparticles as pH-sensitive drug delivery vehicles for cancer therapy, J. Colloid Interface Sci., 2016, 463, 279–287 CrossRef CAS.
- W. Hongdi, F. Jialing, L. Guijin, C. Baoqiong, J. Yanbin and X. Qiuling, In vitro and in vivo anti-tumor efficacy of 10-hydroxycamptothecin polymorphic nanoparticle dispersions: shape- and polymorph-dependent cytotoxicity and delivery of 10-hydroxycamptothecin to cancer cells, Nanomedicine, 2016, 12, 881–891 CrossRef.
- M. Mohammadikish and H. Hajisadeghi, Hierarchical crystal growth of sheaf-like CdS by microemulsion/hydrothermal route, J. Mater. Sci.:Mater. Electron., 2017, 28, 1455–1462 CrossRef CAS.
- I. M. A. Mohamed, Y. A. Khalifa, A. M. Shaker, L. Abdel-Mohsen and E. Nassr, Insights into the impact of surfactants on the kinetics of selenium nanoparticles formation via lemon juice-assisted methodology, followed by their antibacterial and antioxidant assessment, J. Mol. Liq., 2024, 398, 124241, DOI:10.1016/j.molliq.2024.124241.
- B. S. Furniss, A. J. Hannaford, P. W. G. Smith and A. R. Patchel, Vogel's Textbook of Organic Chemistry, Pearson Education Pvt. Ltd, Singapore, 5th edn, 1996, p.1269 Search PubMed.
- E. Knoevengel, Über den Chemismus der condensierenden Wirkung des Ammoniaks und organischer Amine bei Reactionen zwischen Aldehyden und Acetessigester, Ber. Dtsch. Chem. Ges., 1898, 31, 732–748 Search PubMed.
- D. Bokov, A. Turki Jalil, S. Chupradit, W. Suksatan, M. J. Ansari, I. H. Shewael and G. H. Valiev, 8 and Ehsan Kianfar, Nanomaterial by Sol-Gel Method: Synthesis and Application, Adv. Mater. Sci. Eng., 2021, 1–21 Search PubMed.
- A. C. Scott, J. G. Collee, J. P. Duguid, A. G. Fraser and B. P. Marmion, in Mackie & McCartney-Practical Medical Microbiology, Churchill Livingstone, 13th edn, 1989, pp. 161–181 Search PubMed.
- I. Wiegand, K. Hilpert and E. W. H. Robert, Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances, Nat. Protoc., 2008, 3(2), 163–175 CrossRef CAS.
- T. Takao, N. Watanabe, I. Yagi and K. Sakata, A Simple Screening Method for Antioxidants and Isolation of Several Antioxidants Produced by Marine Bacteria from Fish and Shellfish, Biosci. Biotechnol. Biochem., 1994, 58, 1781–1783, DOI:10.1271/bbb.58.1780.
- S. M. Gomha, S. M. Riyadh, E. A. Mahmmoud and M. M. Elaasser, Synthesis and Anticancer Activities of Thiazoles, 1,3-Thiazines, and Thiazolidine Using Chitosan-Grafted-Poly(vinylpyridine) as Basic Catalyst, Heterocycles, 2015, 91(2015), 1227–1243 Search PubMed.
- H. K. Mahmoud, H. A. Abdelhady, M. M. Elaasser, D. Z. H. Hassain and S. M. Gomha, Microwave-Assisted One-Pot Three Component Synthesis of Some Thiazolyl (Hydrazonoethyl) Thiazoles as Potential Anti-Breast Cancer Agents, Polycyclic Aromat. Compd., 2022, 42, 7232–7246 Search PubMed.
- P. Vijayan, C. Raghu, G. Ashok, S. A. Dhanaraj and B. Suresh, Antiviral activity of medicinal plants of Nilgiris, Indian J. Med. Res., 2004, 120, 24–29 CAS.
- W. Randazzo, J. Piqueras, J. Rodrıguez-Diaz, R. Aznar and G. Sanchez, Improving efficiency of viability-qPCR for selective detection of infectious HAV in food and water samples, J. Appl. Microbiol., 2018, 124, 958–964 CrossRef CAS.
- R. M. Pinto, J. M. Diez and A. Bosch, Use of the colonic carcinoma cell line CaCo-2 for in vivo amplification and detection of enteric viruses, J. Med. Virol., 1994, 44, 310–315 CrossRef CAS.
- T. Mosmann, Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays, J. Immunol. Methods, 1983, 65, 55–63 CrossRef CAS.
- A. D. Becke, Density-functional exchange-energy approximation with correct asymptotic behavior, Phys. Rev. A: At., Mol., Opt. Phys., 1988, 38, 3098 Search PubMed.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision A.02, Gaussian Inc., Wallingford, CT. 2009 Search PubMed.
- R. Krishnan, J. S. Binkley, R. Seeger and J. A. Pople, Self-consistent molecular orbital methods. XX. A basis set for correlated wave functions, J. Chem. Phys., 1980, 72, 650–654 Search PubMed.
- P. J. Hay and W. R. Wadt, Ab initio effective core potentials for molecular calculations. Potentials for the transition metal atoms Sc to Hg, J. Chem. Phys., 1985, 82, 270–283 Search PubMed.
- M. M. Khalaf, H. M. A. El-Lateef, M. Gouda, A. A. Amer, A. A. Abdelhamid, M. F. A. Taleb, A. Alfarsi, T. M. A. Ibrahim, H. El-Shamy and A. Abdou, Synthesis, characterization, DFT, biological activity evaluation, and molecular docking analysis of new 8-[(2-hydroxynaphthalen-1-yl)diazenyl]naphthalene-1,3-disulfonic acid based complexes, J. Mol. Struct., 2024, 1300, 137175 CrossRef CAS.
- O. Trott and A. J. Olson, AutoDock Vina: improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading, J. Comput. Chem., 2010, 31, 455–461 Search PubMed.
- Z. Hussain, M. A. Ibrahim, N. M. Hassanin and A.-S. Badran, Synthetic approaches for novel annulated pyrido [2, 3-d] pyrimidines: Design, Structural Characterization, Fukui functions, DFT Calculations, Molecular docking and Anticancer efficiency, J. Mol. Struct., 2024, 1318, 139335 Search PubMed.
- A. Zahirović, S. Fetahović, M. Feizi-Dehnayebi, R. Bešta-Gajević, M. Dizdar, J. Ostojić and S. Roca, Substituent effect in salicylaldehyde 2-furoic acid hydrazones: Theoretical and experimental insights into DNA/BSA affinity modulation, antimicrobial and antioxidant activity, J. Mol. Struct., 2024, 1312, 138628 Search PubMed.
- R. K. Mohapatra, A. Mahal, A. Ansari, M. Kumar, J. P. Guru, A. K. Sarangi, A. Abdou, S. Mishra, M. Aljeldah and B. M. AlShehail, Comparison of the binding energies of approved mpox drugs and phytochemicals through molecular docking, molecular dynamics simulation, and ADMET studies: An in silico approach, Int. J. Biosaf. Biosecur., 2023, 5, 118–132 CrossRef CAS.
- (a) M. Saif, H. F. El-Shafiy, M. M. Mashaly, M. F. Eid, A. I. Nabeel and R. Fouad, Synthesis, characterization, and antioxidant/cytotoxic activity of new chromone Schiff base nano-complexes of Zn(II), Cu(II), Ni(II) and Co(II), J. Mol. Struct., 2016, 1118, 75–82 Search PubMed; (b) R. Fouad, M. A. N. Mahdi and O. M. I. Adly, Selective Metal-Coordinated Nanodrugs Based on Thiazine Moiety for Anticancer Therapeutics: Spectroscopic, Theoretical, and Molecular Docking Studies, Appl. Organomet. Chem., 2025, 39, e7840 CrossRef.
- D. S. Ranade, A. M. Bapat, S. N. Ramteke, B. N. Joshi, P. Roussel, A. Tomas, P. Deschamps and P. P. Kulkarni, Thiosemicarbazone modification of 3-acetyl coumarin inhibits Ab peptide aggregation and protect against Ab-induced cytotoxicity, Eur. J. Med. Chem., 2016, 121, 803–809 CrossRef CAS.
- N. Salah, A. A. A. Emara, O. M. I. Adly, A. Taha, A. I. Nabeel, M. A. Aziz and M. A. Ibrahim, Novel NO2 semicarbazone ligand and its metal complexes as VEGFR-2 inhibitors: Synthesis, spectral characterization, density functional theory calculations, molecular docking, and antimicrobial and antitumor evaluation, Appl. Organomet. Chem., 2022, 36, e6845 Search PubMed.
- (a) O. M. I. Adly and H. F. El-Shafiy, Synthesis, spectroscopic studies, DFT calculations, antimicrobial and antitumor activity of tridentate NNO Schiff base metal complexes based on 5-acetyl-4-hydroxy-2H-1,3-thiazine-2,6(3H)-dione, J. Coord. Chem., 2019, 72, 218 CrossRef CAS; (b) F. Samy and M. Shebl, Co(II), Ni(II), and Cu(II) complexes of 4,6-bis(2-hydroxynaphthalen-1-yl)methyl-ene)hydrazono)ethyl) benzene-1,3-diol: Synthesis, spectroscopic, biological, and theoretical studies, Appl. Organomet. Chem., 2022, 36, e6650 CrossRef CAS.
- R. Fouad, M. Saif, M. M. Mashaly and M. Zekrallah, New Mononuclear Metal Complexes Based on 3- Acetyl- 4- Hydroxy Coumarin as Potential Antitumor Agents: Full Structural Elucidation, Theoretical Calculations, and Biological Activity Studies, Appl. Organomet. Chem., 2025, 39, e7924 CrossRef CAS.
- M. Ayad, P. Schollhammer, Y. L. Mest, L. Wojcik, F. Y. Pétillon, N. L. Poul and D. Mandon, Mononuclear copper(II) complexes containing a macrocyclic ditopic ligand: Synthesis, structures and properties, Inorg. Chim. Acta, 2019, 497, 119081 CrossRef CAS.
- A. A. A. Emara, A. A. Saleh and O. M. I. Adly, Spectroscopic investigations of new binuclear transition metal complexes of Schiff bases derived from 4,6-diacetylresorcinol and 3-amino-1-propanol or 1,3-diamino-propane, Spectrochim. Acta, Part A, 2007, 68, 592 CrossRef.
- H. G. Sogukomerogullari, E. Basaran, R. A. Kepekçi, B. Türkmenoglu, A. O. Sarıoglu and M. Kose, Novel europium(III), terbium(III), and gadolinium(III) Schiff base complexes: Synthesis, structural, photoluminescence, antimicrobial, antioxidant, and molecular docking studies, Polyhedron, 2025, 265, 117275 CrossRef CAS.
- S. Y. Ebrahimipour, F. K. Nejad, M. Khosravan, J. Castro, J. M. White, P. Mohammadi, G. Mohamadi-Nejad and M. Mohamadi, Studies on a novel oxo-vanadium(V) complex of a tridentate ONO Schiff base ligand: Synthesis, characterization, X-ray crystal structure, Hirshfeld surface analysis, biological and catalytic activity, J. Mol. Struct., 2025, 1321, 139923 Search PubMed.
- W. J. Geary, The Use of Conductivity Measurements in Organic Solvents for the Characterization of Coordination Compounds, Coord. Chem. Rev., 1971, 7, 81 CrossRef CAS.
- A. W. Coats and J. P. Redfern, Kinetic Parameters from Thermogravimetric Data, Nature, 1964, 201, 68 Search PubMed.
- A. H. M. Siddaligaiah and S. G. Naik, Spectroscopic and thermogravimetric studies on Ni(II), Cu(II) and Zn(II) complexes of di(2,6-dichlorophenyl)carbazone, J. Mol. Struct., 2002, 582, 129 CrossRef.
- C. R. Vinodkumar, M. K. Muraleedharan Nair and P. K. Radhakrishnan, Thermal Studies on Lanthanide Nitrate Complexes of 4-n-(2′-furfurylidene)aminoantipyrine, J. Therm. Anal. Calorim., 2000, 61, 143 Search PubMed.
- M. A. Ibrahim, A. A. A. Emara, A. Taha, O. M. I. Adly, A. I. Nabeel, M. A. Aziz and N. Salah, Synthesis, characterization, TD-DFT, molecular docking, biological applications, and solvatochromic studies of some new metal complexes derived from semicarbazone of pyrano[3,2-c]quinoline-3-carboxaldehyde, Appl. Organomet. Chem., 2023, e7169 CrossRef CAS.
- M. Saif, M. M. Mashaly, M. F. Eid and R. Fouad, Synthesis, characterization and thermal studies of binary and/or mixed ligand complexes of Cd(II), Cu(II), Ni(II) and Co(III) based on 2-(Hydroxybenzylidene) thiosemicarbazone: DNA binding affinity of binary Cu(II) complex, Spectrochim. Acta, Part A, 2012, 92, 347–356 Search PubMed.
- M. M. Mashaly, Z. H. Abd El-Wahab and A. A. Faheim, Preparation, spectral characterization and antimicrobial activities of Schiff base complexes derived from 4-aminoantipyrine. Mixed ligand complexes with 2-aminopyridine, 8-hydroxyquinoline and oxalic acid and their pyrolytical products, J. Chin. Chem. Soc., 2004, 51, 901–915 CrossRef CAS.
- (a) K. Nakamoto, Infrared Spectra of Inorganic and Coordination Compounds, John Willey & Sons, New York, 5th edn, 1997 Search PubMed; (b) E. M. Abdelrhman, B. A. El-Shetary, M. Shebl and O. M. I. Adly, Coordinating behavior of hydrazone ligand bearing chromone moiety towards Cu(II) ions: Synthesis, spectral, density functional theory (DFT) calculations, antitumor, and docking studies, Appl. Organomet. Chem., 2021, 35, e6183 Search PubMed.
- (a) M. Shebl, Mononuclear, homo- and hetero-binuclear complexes of 1-(5-(1-(2-aminophenylimino)ethyl)-2,4-dihydroxyphenyl)ethanone: synthesis, magnetic, spectral, antimicrobial, antioxidant, and antitumor studies, J. Coord. Chem., 2016, 69(2), 199–214 CrossRef CAS; (b) O. M. I. Adly, M. Shebl, E. M. Abdelrhman and B. A. El-Shetary, Synthesis, spectroscopic, X-ray diffraction, antimicrobial and antitumor studies of Ni(II) and Co(II) complexes derived from 4- acetyl-5,6-diphenyl-3(2H)-pyridazinone and ethylenediamine, J. Mol. Struct., 2020, 1219, 128607 CrossRef CAS.
- O. M. I. Adly, M. Shebl, H. F. El-Shafiy, S. M. E. Khalil, A. Taha and M. A. N. Mahdi, Synthesis, spectroscopic characterization, antimicrobial and antitumor studies of mono-, bi- and tri-nuclear metal complexes of a new Schiff base ligand derived from o-acetoacetylphenol, J. Mol. Struct., 2017, 1150, 507–522 CrossRef CAS.
- F. A. Cotton and G. Wilkinson, Advanced Inorganic Chemistry, John Wiley, 3rd edn, 1978, p. 541 Search PubMed.
- A. A. M. Ali and R. Fouad, Novel thiosemicarbazone complexes as anticancer agents: Synthesis, characterization, cytotoxicity, docking, and density functional theory studies, Appl. Organomet. Chem., 2024, 38, e7430 CrossRef CAS.
- S. A. Al-Thabaiti, A. Y. Obaid, S. Hussain and Z. Khan, Shape-directing role of cetyltrimethylammonium bromide on the morphology of extracellular synthesis of silver nanoparticles, Arabian J. Chem., 2015, 8, 538–544 CrossRef CAS.
- J. Gong, W. Zeng and H. Zhang, Hydrothermal synthesis of controlled morphologies of MoO3 nanobelts and hierarchical structures, Mater. Lett., 2015, 154, 170–172 CrossRef CAS.
- R. S. Joseyphus and M. S. Nair, Antibacterial and Antifungal Studies on Some Schiff Base Complexes of Zinc (II), Mycobiology, 2008, 36, 93–98 CrossRef CAS.
- H. F. El-Shafiy, M. F. Eid, M. Saif, M. M. Mashaly, S. Abdel Halim, A. I. Nabeel and R. Fouad, New nano-complexes of Zn(II), Cu(II), Ni(II) and Co(II) ions; spectroscopy, thermal, structural analysis, DFT calculations and antimicrobial activity application, J. Mol. Struct., 2017, 1147, 452–461 CrossRef CAS.
- E. Chudáčková, T. Bergerová, K. Fajfrlík, D. Červená, P. Urbášková, J. Empel, M. Gniadkowski and J. Hrabák, Carbapenem-nonsusceptible strains of Klebsiella pneumoniae producing SHV-5 and/or DHA-1 b-lactamases in a Czech hospital, FEMS Microbiol. Lett., 2010, 309, 62–70 Search PubMed.
- European Committee for Antimicrobial Susceptibility Testing (EUCAST) of the European Society of Clinical Microbiology and Infectious Diseases (ESCMID), Determination of minimum inhibitory concentrations (MICs) of antibacterial agents by broth dilution, Clin. Microbiol. Infect., 2003, 9, 11–25 Search PubMed.
- H. E. Miller, F. Rigelhof, L. Marquart, A. Prakash and M. Kanter, Antioxidant content of whole grain breakfast cereals, fruits and vegetables, J. Am. Coll. Nutr., 2000, 312S–319S CrossRef CAS.
- M. Szymula and J. Narkiewicz-Michalek, Atmospheric and electrochemical oxidation of ascorbic acid in anionic, nonionic and cationic surfactant systems, J. Colloid Polym. Sci., 2003, 281, 1142–1148 CrossRef CAS.
- D. Kwasniewska and J. Kiewlicz, Study of interaction between cationic surfactant (CTAB) and ascorbic acid/ascorbic acids derivatives by tensiometric and spectroscopic methods, J. Mol. Liq., 2022, 354, 118917 CrossRef CAS.
- Q. Xue, R. Kang, D. J. Klionsky, D. Tang, J. Liu and X. Chen, Copper metabolism in cell death and autophagy, Autophagy, 2003, 19, 2175–2195 CrossRef.
- Y. Luo, X. Luo, Y. Ru, X. Zhou, D. Liu, Q. Huang, M. Linghu, Y. Wu, Z. Lv, M. Chen, Y. Ma, Y. Huang and J. Wang, Copper (II)-based nano-regulator correlates cuproptosis burst and sequential immunogenic cell death for synergistic cancer immunotherapy, Biomater. Res., 2004, 28, 0039 CrossRef.
- P. Huang, G. Wu, M. Huang, Y. Deng, X. Chen, G. Ye, X. Yu, H. Wang, H. Wen and Y. Zhou, Copper-coordinated nanomedicine for the concurrent treatment of lung cancer through the induction of cuproptosis and apoptosis, Eur. J. Pharm. Sci., 2025, 204, 106942 CrossRef CAS.
- R. Budzynska, D. Nevozhay, U. Kanska, M. Jagiello, A. Opolski, J. Wietrzyk and J. Boratynski, Antitumor activity of mannan-methotrexate conjugate in vitro and in vivo, Oncol. Res., 2007, 16, 415–421 CrossRef CAS.
- B. Singh, V. Sharma, G. Singh, R. Kumar, S. Arora and M. P. S. Ishar, Synthesis and In Vitro Cytotoxic Activity of Chromenopyridones, Int. J. Med. Chem., 2013, 2013, 984329 Search PubMed.
- R. K. Mohapatra, A. Mahal, A. Ansari, M. Kumar, J. P. Guru, A. K. Sarangi, A. Abdou, S. Mishra, M. Aljeldah, B. M. AlShehail, M. Alissa, M. Garout, A. Alsayyah, A. A. Alshehri, A. Saif, A. Alqahtani, F. A. Alshehri, A. A. Alamri and A. A. Rabaan, Comparison of the binding energies of approved mpox drugs and phytochemicals through molecular docking, molecular dynamics simulation, and ADMET studies: An in silico approach, J. Biosaf. Biosecur., 2023, 5, 118–132 CrossRef CAS.
- H. M. Abd El-Lateef, M. M. Khalaf, F. El-Taib Heakal and A. Abdou, Fe(III), Ni(II), and Cu(II)-moxifloxacin-tri-substituted imidazole mixed ligand complexes: Synthesis, structural, DFT, biological, and protein-binding analysis, Inorg. Chem. Commun., 2023, 158, 111486 CrossRef CAS.
- H. M. Abd El-Lateef, M. M. Khalaf, A. A. Amer, A. A. Abdelhamid and A. Abdou, Antibacterial, antifungal, anti-inflammatory evaluation, molecular docking, and density functional theory exploration of 2-(1H-benzimidazol-2-yl)guanidine mixed-ligand complexes: Synthesis and characterization, Appl. Organomet. Chem., 2024, 38, e7299 CrossRef CAS.
- H. M. Abd El-Lateef, M. M. Khalaf, F. E. T. Heakal and A. Abdou, Fe (III), Ni (II), and Cu (II)-moxifloxacin-tri-substituted imidazole mixed ligand complexes: Synthesis, structural, DFT, biological, and protein-binding analysis, Inorg. Chem. Commun., 2023, 158, 111486 CrossRef CAS.
- W. Derafa, N. A. Elkanzi, A. M. Ali and A. Abdou, Three Co (II), Ni (II) and Cu (II) Schiff base complexes incorporating 2-[(4-{[(4-methylphenyl) sulfonothioyl] oxy} phenyl) methylene] amino} benzoic acid: Synthesis, structural, dft, biological and molecular docking investigation, Bull. Chem. Soc. Ethiop., 2024, 38, 325–346 CrossRef CAS.
- A. Abdou, H. Mostafa and A.-M. Abdel-Mawgoud, New Fe(III) and Ni(II) Azocoumarin Based Complexes: Synthesis, Characterization, DFT, Antimicrobial, Anti-inflammatory Activity, and Molecular Docking Analysis, Russ. J. Gen. Chem., 2023, 93, 3006–3019 CrossRef CAS.
- T. Lanez, M. Feizi-Dehnayebi and E. Lanez, Assessment of the electrostatic binding of ferrocenylmethyl-nitroaniline derivatives to DNA: A combined experimental and theoretical study, J. Mol. Struct., 2024, 1308, 138386 CrossRef CAS.
- S. Cherukupalli, B. Chandrasekaran, R. R. Aleti, N. Sayyad, G. A. Hampannavar, S. R. Merugu, H. R. Rachamalla, R. Banerjee and R. Karpoormath, Synthesis of 4,6-disubstituted pyrazolo[3,4-d]pyrimidine analogues: Cyclin-dependent kinase 2 (CDK2) inhibition, molecular docking and anticancer evaluation, J. Mol. Struct., 2019, 1176, 538–551 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra03317a |
| This journal is © The Royal Society of Chemistry 2025 |