Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
[4 + 2] Cycloaddition of α-bromotrifluoromethylhydrazone with alkenes: synthesis of trifluoromethyltetrahydropyridazines†
Yanhui Zhaoa,
Hemin Ronga,
Khurshed Bozorov b,
Xueqing Zhang
b,
Xueqing Zhang *a,
Buer Song*a and
Wei Liu
*a,
Buer Song*a and
Wei Liu *a
*a
aXinjiang Key Laboratory of Clean Conversion and High Value Utilization of Biomass Resources, School of Chemistry and Chemical Engineering, Yili Normal University, Xinjiang, Yining 835000, China. E-mail: zhxq0612@163.com; ucasliuwei@126.com; songbuer20211023@163.com
bInstitute of Biochemistry, Samarkand State University, University Blvd. 15, Samarkand, 140104, Uzbekistan
First published on 9th June 2025
Abstract
A catalyst-free [4 + 2] cyclization process between trifluoromethyl-containing 1,2-diazabuta-1,3-diene and simple olefins was developed by in situ generation. Under mild conditions, trifluoromethyl-containing 1,4,5,6-tetrahydropyridazine compounds were obtained, in high yields (up to 96% yields).
1,4,5,6-Tetrahydropyrazines1 are important six-membered nitrogen heterocycles that are widely found in numerous natural products. They also serve as structural subunits in various bioactive molecules and drugs, such as the antihypertensive hydralazine, dihydralazine, and endralazine, as well as the antidepressant drug piperazine.2
In addition, the introduction of trifluoromethyl groups (CF3) into drug molecules can significantly improve the physical and chemical properties, metabolic stability, and drug activity of drug molecules.3 Therefore, CF3 plays an important role in medicine, pesticides, and materials. So far, the direct introduction of trifluoromethylation using trifluoromethylation reagents has been well developed.4 The synthesis of trifluoromethylated organic molecules using trifluoromethylation building blocks is equally attractive and important as another important approach.5
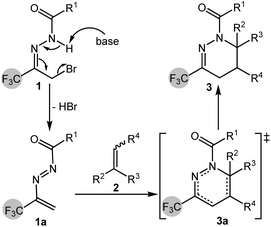
At the same time, by consulting the literature, it was found that α-bromoacylhydrazone can generate 1,2-diazabuta-1,3-diene in situ under the action of alkali, and can undergo [4 + 1]6 cycloaddition, [4 + 2]7 cycloaddition and [4 + 3]8 cycloaddition with dienophiles to prepare biologically active nitrogen heterocyclic compounds. Therefore, in recent years, α-bromoacylhydrazone has been widely used in organic synthesis as a diene precursor. For example, in 2012, Bolm's group reported the asymmetric [4 + 1] cycloaddition of 1,2-diazabuta-1,3-diene in situ generated by α-haloacylhydrazone with a sulfur ylide, catalyzed by copper trifluoromethanesulfonate and Tol-BINAP, a series of dihydropyrazole compounds were obtained in up to 97% yield and 94% enantioselectivity (Scheme 1a).9 In 2015, Luo's group performed the [4 + 2] cycloaddition of α-haloacylhydrazone to 1,2-diazabuta-1,3-diene with simple olefins, especially ethylene, and obtained 1,4,5,6-tetrahydropyridazine compounds in up to 99% yield (Scheme 1b).10 In 2016, Zhao's group obtained 1,2,4,5-oxatriazepines from the [4 + 3] cycloaddition of α-halogenated acylhydrazones with nitrones in the presence of sodium carbonate (Scheme 1c).11
Based on the above research, a catalyst-free [4 + 2] cyclization process between trifluoromethyl-containing 1,2-diazabuta-1,3-diene and simple olefins was developed by in situ generation. Under mild conditions, trifluoromethyl-containing 1,4,5,6-tetrahydropyridazine compounds were obtained (Scheme 1d).
Initially, α-bromotrifluoromethyl acylhydrazone 1a (1.0 equiv.), styrene 2a (3.0 equiv.) and K2CO3 (2.0 equiv.) were reacted in dichloromethane at room temperature to give the target compound 3a in 64% yield (Table 1, entry 1). To further improve the yield of the target product, the reaction conditions were optimized in terms of solvents, bases, and material ratios, and representative results are summarized in Table 1. When we studied the effect of different bases on the reaction, such as Cs2CO3, compound 3a was obtained in 25% yield (Table 1, entry 2). When the base is Na2CO3, compound 3a was obtained in 83% yield (Table 1, entry 3). At the same time, we also investigated the effect of organic base Et3N on the reaction, and obtained compound 3a in 16% yield (Table 1, entry 4). Therefore, we use Na2CO3 as the optimal alkali. Subsequently, we optimized the material ratio of the reaction (Table 1, entry 5–8). The results showed that the appropriate molar ratio of 1a/2a/Na2CO3 was 1/3/2 (Table 1, entry 3). Finally, we optimized the effects of different solvents on the reaction. We explored the effects of THF, CH3CN, and MeOH on the reaction (Table 1, entry 9–11). The results show that the optimal solvent is CH2Cl2.
| Entry | Molar ratio of 1a/2a/base | Base | Solvent | Yieldb (%) |
|---|---|---|---|---|
| a All reactions were carried out by using 0.2 mmol of 1a, 3 eq. of 2a and 2 eq. of base in 3 mL of solvent.b Isolated yields. | ||||
| 1 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
K2CO3 | CH2Cl2 | 64 |
| 2 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Cs2CO3 | CH2Cl2 | 25 |
| 3 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Na2CO3 | CH2Cl2 | 83 |
| 4 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Et3N | CH2Cl2 | 16 |
| 5 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Na2CO3 | CH2Cl2 | 66 |
| 6 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Na2CO3 | CH2Cl2 | 50 |
| 7 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2.5 2.5 |
Na2CO3 | CH2Cl2 | 52 |
| 8 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 1.5 |
Na2CO3 | CH2Cl2 | 66 |
| 9 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Na2CO3 | THF | 19 |
| 10 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Na2CO3 | CH3CN | 28 |
| 11 | 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
Na2CO3 | MeOH | N. R. |
Under the optimized conditions, the substrate suitability of this transformation reaction was further investigated. The results are shown in Scheme 2.
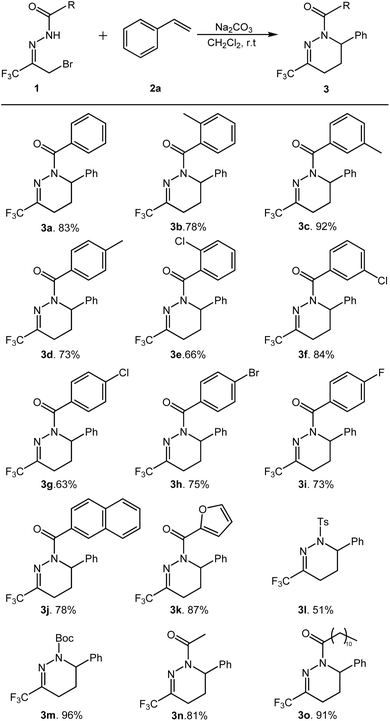 | ||
| Scheme 2 Substrate scope of hydrazonesa,b. aAll reactions were carried out by using 0.2 mmol of 1, 3 eq. of 2a and 2 eq. of Na2CO3 in 3 mL of CH2Cl2. bIsolated yields. | ||
Firstly, the reaction adaptability of substrate 1 was studied, and the effect of R1 substituent on substrate activity was investigated. Various substituted α-bromotrifluoromethyl acylhydrazones can efficiently generate the target products in moderate to good yields (Scheme 2, 3a–3o). The results indicate that the reaction proceeds efficiently when the R1 group is either aromatic or aliphatic, affording the corresponding products in good yields. Notably, for aromatic R1 groups, the electronic characteristics of substituents on the phenyl ring exhibit minimal influence on product yield, whereas the positional isomerism of substituents on the phenyl ring demonstrates a pronounced effect on yield. For example, when R1 was the o-/m-/p-methylphenyl (3b–3d), the m-methylphenyl product (3c), was obtained with the highest yield. When R1 is o-/m-/p-chlorophenyl, m-chlorophenyl gives the target product in the highest yield (3e–3g). When R1 is p-bromophenyl and p-fluorophenyl, the target product was obtained in 75% and 73% yields, respectively (3h–3i). When R1 is a fused ring or a heterocyclic ring, such as 2-naphthyl and 2-furan groups, the corresponding products can also be successfully generated (3j–3k). When the R1 group was Ts-(3l) and Boc-(3m), the yields obtained were 51 and 96%, respectively. In addition, when R1 is aliphatic ethyl and lauryl, the target products can also be obtained in good to excellent yields (3n–3o).
To further expand the substrate scope, other simple olefins were tested next. As seen from Scheme 3, styrenes bearing either electron-donating or -withdrawing moieties can both be used equally well for this reaction, with slightly higher yields for the former (4a–4f). The yield of 2,3-dimethyl-2-butene was 42% (4g).
 | ||
| Scheme 3 Substrate scope for simple alkenesa,b. aAll reactions were carried out by using 0.2 mmol of 1a, 3 eq. of 2 and 2 eq. of Na2CO3 in 3 mL of CH2Cl2. bIsolated yields. | ||
With α-bromo trifluoromethyl N-Boc acylhydrazone compounds, we also examined different olefins, and the results are shown in Scheme 4. Olefins bearing either electron-donating (5a–5b) or electron-withdrawing groups (5c–5d) could be applied to give good yields.
 | ||
| Scheme 4 Substrate scope for hydrazone 1m with substituted styrenesa,b. aAll reactions were carried out by using 0.2 mmol of 1m, 3 eq. of 2 and 2 eq. of Na2CO3 in 3 mL of CH2Cl2. bIsolated yields. | ||
A plausible reaction mechanism was proposed based on a review of literature and reaction outcomes, as illustrated in Scheme 5. In the presence of a base, α-bromotrifluoromethyl acylhydrazone 1 undergoes dehydrohalogenation to eliminate one equivalent of HBr, generating the 1,2-diazabuta-1,3-diene intermediate 1a. This intermediate then participates in a Diels–Alder reaction with substituted olefin 2, forming the cyclic transition state 3a. The transition state subsequently evolves into the final product molecule 3, wherein the cleavage of preexisting bonds and the formation of new bonds occur in a concerted manner during a single mechanistic step.
Conclusions
In summary, we reported here in a mild and catalyst-free [4 + 2] cycloaddition between in situ generated trifluoromethyl 1,2-diazabuta-1,3-diene with simple olefins. This protocol provides facile and atom economic access to trifluoromethyltetrahydropyridazine with moderate to excellent yields.Data availability
The authors confirm that the data supporting the findings of this study are available within the article and its ESI.†Author contributions
Yanhui Zhao: investigation, data curation, and methodology. Hemin Rong: investigation and data curation. Khurshed Bozorov: investigation and data curation. Buer Song: writing – original draft. Wei Liu: supervision and writing – review & editing. Xueqing Zhang: writing – review & editing, supervision, funding acquisition, and conceptualization.Conflicts of interest
There are no conflicts to declare.Acknowledgements
The work was supported by the Xinjiang Key Laboratory of Clean Conversion and High Value Utilization of Biomass Resources (No. XJSWZ202430).References
- (a) S. Xu, R. Chen, Z. Qin, G. Wu and Z. He, Org. Lett., 2012, 14, 996–999 CrossRef CAS PubMed; (b) R. Na, C. Jing, Q. Xu, H. Jiang, X. Wu, J. Shi, J. Zhong, M. Wang, D. Benitez, E. Tkatchouk, W. A. Goddard, H. Guo and O. Kwon, J. Am. Chem. Soc., 2011, 133, 13337–13348 CrossRef CAS; (c) L. Shen, L. Sun and S. Ye, J. Am. Chem. Soc., 2011, 133, 15894–15897 CrossRef CAS PubMed; (d) Q. Zhang, L. Yang and X. Tong, J. Am. Chem. Soc., 2010, 132, 2550–2551 CrossRef CAS PubMed; (e) A. J. Oelke, D. J. France, T. Hofmann, G. Wuitschik and S. V. Ley, Angew. Chem., 2010, 122, 6275–6278 (Angew. Chem., Int. Ed., 2010, 49, 6139–6142) CrossRef.
- C. G. Wermuth, G. Schlewer, J. J. Bourguignon, G. Maghioros, M.-J. Bouchet, C. Moire, J.-P. Kan, P. Worms and K. Biziere, J. Med. Chem., 1989, 32, 528–537 Search PubMed.
- (a) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef; (b) Y. Zhou, J. Wang, Z. Gu, S. Wang, W. Zhu, J. L. Aceña, V. A. Soloshonok, K. Izawa and H. Liu, Chem. Rev., 2016, 116, 422–518 CrossRef CAS PubMed; (c) W. K. Hagmann, J. Med. Chem., 2008, 51, 4359–4369 CrossRef.
- (a) C. Zhang, Org. Biomol. Chem., 2014, 12, 6580–6589 RSC; (b) L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513–1522 CrossRef; (c) X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683–730 CrossRef PubMed; (d) J. Charpentier, N. Früh and A. Togni, Chem. Rev., 2015, 115, 650–682 CrossRef PubMed.
- (a) J. H. Sim, J. H. Park, P. Maity and C. E. Song, Org. Lett., 2019, 21, 6715–6719 CrossRef PubMed; (b) Z. Chen, N. Ren, X. Ma, J. Nie, F.-G. Zhang and J.-A. Ma, ACS Catal., 2019, 9, 4600–4608 CrossRef; (c) L. Trulli, F. Sciubba and S. Fioravanti, Tetrahedron, 2018, 74, 572–577 CrossRef; (d) L. Trulli, V. Raglione and S. Fioravanti, Eur. J. Org Chem., 2018, 3743–3749 CrossRef; (e) W. N. Mszar, M. S. Mikus, S. Torker, F. Haeffner and A. H Hoveyda, Angew. Chem., Int. Ed., 2017, 56, 8736–8741 CrossRef; (f) M. Miyagawa, M. Yoshida, Y. Kiyota and T. Akiyama, Chem.–Eur. J., 2019, 25, 5677–5681 CrossRef CAS PubMed.
- (a) O. A. Attanasi, L. De Crescentini, G. Favi, F. Mantellini, S. Mantenuto and S. Nicolini, J. Org. Chem., 2014, 79, 8331–8338 CrossRef CAS PubMed; (b) O. A. Attanasi, P. Filippone, B. Guidi, T. Hippe, F. Mantellini and L. F. Tietze, Tetrahedron Lett., 1999, 40, 9277–9280 CrossRef CAS.
- (a) A. M. Shelke and G. Suryavanshi, Org. Lett., 2016, 18, 3968–3971 CrossRef CAS; (b) R. Huang, X. Chang, J. Li and C. J. Wang, J. Am. Chem. Soc., 2016, 138, 3998–4001 CrossRef CAS; (c) S. M. M. Lopes, M. S. C. Henriques, J. A. Paixao and T. M. V. D. P. E. Melo, Eur. J. Org Chem., 2015, 6146–6151 CrossRef CAS; (d) J. Li, R. Huang, Y. K. Xing, G. Qiu, H. Y. Tao and C. J. Wang, J. Am. Chem. Soc., 2015, 137, 10124–10127 CrossRef PubMed; (e) M. C. Tong, X. Chen, J. Li, R. Huang, H. Tao and C. J. Wang, Angew. Chem., Int. Ed. Engl., 2014, 53, 4680–4684 CrossRef PubMed.
- (a) W. Yang, C. Yuan, Y. Liu, B. Mao, Z. Sun and H. Guo, J. Org. Chem., 2016, 81, 7597–7603 CrossRef PubMed; (b) C. Guo, B. Sahoo, C. G. Daniliuc and F. Glorius, J. Am. Chem. Soc., 2014, 136, 17402–17405 CrossRef CAS PubMed.
- J.-R. Chen, W.-R. Dong, M. Candy, F.-F. Pan, M. Jörres and C. Bolm, J. Am. Chem. Soc., 2012, 134, 6924–6927 CrossRef CAS PubMed.
- X. Zhong, J. Lv and S. Luo, Org. Lett., 2015, 17, 1561–1564 CrossRef CAS PubMed.
- H.-W. Zhao, H.-L. Pang, T. Tian, B. Li, X.-Q. Chen, X.-Q. Song, W. Meng, Z. Yang, Y.-Y. Liu and Y.-D. Zhao, Adv. Synth. Catal., 2016, 358, 1826–1832 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, analytical data for products, and NMR spectra of products. See DOI: https://doi.org/10.1039/d5ra03000e |
| This journal is © The Royal Society of Chemistry 2025 |