Open Access Article
Open Access ArticleSynthesis of 4H-pyrimido[2,1-b]benzimidazoles catalyzed by a Schiff base cobalt(II) complex supported on cobalt ferrite magnetite nanoparticles†
Ahmad Reza Moosavi-Zare *ab,
Zahra Darvishib,
Hamid Goudarziafsharb and
Abedien Zabardastic
*ab,
Zahra Darvishib,
Hamid Goudarziafsharb and
Abedien Zabardastic
aChemistry Department, College of Sciences, Shiraz University, Shiraz 71946-84795, Iran
bDepartment of Chemical Engineering, Hamedan University of Technology, Hamedan, 65155, Iran. E-mail: moosavizare@yahoo.com
cDepartment of Inorganic Chemistry, Faculty of Chemistry, Lorestan University, Khorramabad 68151-44316, Iran
First published on 27th May 2025
Abstract
Nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2] was designed and reported with the Schiff base cobalt(II) complex anchored onto cobalt ferrite magnetite nanoparticles. The resulting nanomagnetite Schiff base cobalt(II) complex was employed as a recoverable catalyst for the condensation reaction of aryl aldehyde, 2-aminobenzimidazole and ethyl acetoacetate at 90 °C to synthesize 4H-pyrimido[2,1-b]benzimidazoles under solvent-free condition. According to the proposed mechanism, the cobalt complex can accelerate the reaction via a structural rearrangement from a tetrahedral to a square planar geometry.
1. Introduction
4H-Pyrimido[2,1-b]benzimidazoles are important heterocycles that have attracted the attention of chemists due to their significant medicinal properties. Antitumor,1 anti-inflammatory,2 antifungal3 and antibacterial activities4 are among the notable biological properties observed in this class of compounds.The multicomponent synthesis of 4H-pyrimido[2,1-b]benzimidazoles through the condensation of a β-keto ester with 2-aminobenzimidazole and various aldehydes is one of the most common protocols for preparing this class of compounds.5 Multicomponent condensation reactions are significant in green chemistry, as they allow the synthesis of the desired product in a single step without the need to isolate reaction intermediates. Savings in raw materials and solvents, compliance with atom economy and reduced waste generation are important advantages of this protocol. Additional benefits include reduced reaction time, increased product yield, fewer synthesis steps and a shortened purification process.6–17
The synthesis of 4H-pyrimido[2,1-b]benzimidazoles has previously been reported via a one-pot, three-component condensation reaction of various aryl aldehydes with a β-keto ester and 2-aminobenzimidazole, using different catalysts such as H3PO4–Al2O3,18 TMGT,19 TiCl2/cellulose,20 GO@PSA-Cu,21 nano-acetic acid,22 [bmim]BF4,23 1,3-disulfonic acid imidazolium trifluoroacetate,24 poly(vinylpyrrolidonium) perchlorate,25 thiamine hydrochloride (VB1),26 TiO2/porous carbon,27 poly(N-bromo-N-ethylbenzene-1,3-disulfonamide),28 nano-[Co–4CSP]Cl2,29 [NSPy]ZnCl3,30 Na+–MMT–[pmim]HSO4,31 Na+-montmorillonite perchloric acid,32 Fe3O4@SiO2@L-glutamine NPs33 and sodium acetate.34 Considering the medicinal importance of these compounds, it is important to introduce new protocols and design efficient catalysts for their synthesis.
In the present work, a nanomagnetic heterogeneous catalyst was designed and introduced for the preparation of 4H-pyrimido[2,1-b]benzimidazoles. Reducing the catalyst size to the nanoscale induced significant qualitative changes in its structure and catalytic performance.35,36 Producing a catalyst at the nanoscale increased its contact surface with the reactants, thereby enhancing the reaction efficiency. One of the most important advantages of heterogeneous catalysts is their ease of separation from the reaction medium.37–40
The use of magnetic nanoparticles in chemical reactions, and the design of catalysts of this size with magnetic properties, such as core–shell and supported catalysts, have received much attention. Designing catalysts with magnetic components facilitates easier recovery and reuse, resulting in significant savings in chemical consumption. In addition to their catalytic ability, magnetic nanoparticles have been widely used in other applications such as drug delivery and removal of inorganic and organic pollutants from the environment.41–45
Building on the above facts and the continued use of Schiff base complexes as catalysts in organic synthesis,46–48 a novel Schiff base cobalt(II) complex was designed and immobilized on cobalt ferrite magnetite nanoparticles to be used as a recoverable catalyst for the three-component synthesis of 4H-pyrimido[2,1-b]benzimidazoles at 90 °C under solvent-free conditions (Scheme 1).
2. Experimental section
All materials were purchased from Merck and used without further purification, and their purity was checked by thin-layer chromatography (TLC). The melting point of the products were measured using an Electrothermal 9100 instrument. Nuclear magnetic resonance (1H NMR and 13C NMR) spectra were recorded on a Bruker DRX-250 Avance spectrometer with deuterated DMSO as a solvent. Infrared spectra of the products were recorded on a PerkinElmer PE-1600-FTIR instrument.2.1. Synthesis of nano [CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2]
3-Aminopropyltriethoxysilane (1.0 mmol) was added to ortho-vanillin (1.0 mmol) in a round bottom flask connected to a reflux condenser at room temperature, in the absence of solvent, and stirred for 24 h to prepare (OEt)3Si/propyl-1-(o-vanillinaldimine). To metallise the Schiff base ligand in the previous step, (OEt)3Si/propyl-1-(o-vanillinaldimine) (2 mmol) was added to a mixture of CoCl2·6H2O (1 mmol) and toluene (10 mL) in a round bottom-flask connected to a reflux condenser, and the mixture was stirred under reflux for 24 h to afford the cobalt(II) Schiff base complex. Then, the obtained cobalt(II) complex (1 mmol) was added to a round-bottom flask connected to a reflux condenser containing toluene (20 mL) and CoFe2O4@SiO2 nanoparticles (2 g), which were prepared according a previous report,49 and stirred under reflux for 24 h to prepare the nanomagnetite complex as the main catalyst. Finally, for further purification, the catalyst was washed twice with ethanol and dried in a thermal oven at 60 °C.2.2. General procedure for the preparation of 4H-pyrimido[2,1-b]benzimidazole derivatives
An aromatic aldehyde (1 mmol), 2-aminobenzimidazole (1 mmol, 0.133 g), ethyl acetoacetate (1 mmol, 0.13 g) and [CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2] (3 mg) as a catalyst were added to a 25 mL round-bottomed flask connected to a reflux condenser and stirred at 90 °C in the absence of solvent. After completion of the reaction, as monitored by TLC, warm ethanol (10 mL) was added to extract the product, and the catalyst was separated from the reaction mixture using an external magnet. Lastly, the expected product was purified by recrystallization from ethanol (90%).2.3. Spectral data of compounds
3. Results and discussion
In the present work, to design novel cobalt(II) Schiff base complex and immobilize it on a magnetic surface for use as a recoverable catalyst in organic reactions, a Schiff base ligand was first synthesized by reacting ortho-vanillin with 3-aminopropyltriethoxysilane. In the next step, the cobalt(II) Schiff base complex was formed by the metalation of the synthesized Schiff base ligand with cobalt(II) chloride. Finally, the cobalt(II) Schiff base complex was immobilized on CoFe2O4@SiO2 nanoparticles to prepare the magnetite cobalt(II) Schiff base complex as a reusable catalyst (Scheme 2).After the preparation of the supported nanomagnetite cobalt(II) Schiff base complex, FT-IR analysis of nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2] was conducted to identify the key bonds in the structure, in comparison with other components of the catalyst (Fig. 1). The analysis demonstrated that the vibrations at 1095 and 596 cm−1 corresponded to the Si–O–Si and Fe–O bonds, respectively. The vibration at 2925 cm−1 was associated with C–H bond vibration. Additionally, the peak corresponding to the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond of the Schiff base ligand appeared at approximately 1606 cm−1, indicating interaction with cobalt chloride in the prepared complex. This analysis confirmed the presence of the key and desired bonds in the structure of the final catalyst.
N bond of the Schiff base ligand appeared at approximately 1606 cm−1, indicating interaction with cobalt chloride in the prepared complex. This analysis confirmed the presence of the key and desired bonds in the structure of the final catalyst.
In another study, to identify the elements present in the nanomagnetite Schiff base complex, energy-dispersive X-ray spectroscopy (EDX) analysis of nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2] was performed. The analysis indicated that the expected elements, including cobalt, iron, nitrogen, oxygen, carbon, silicon and chlorine were detected in the designed complex (Fig. 2).
To determine the distribution of elements in the designed catalyst structure, SEM coupled with EDX (SEM mapping) analysis was conducted. The study revealed that the expected elements, namely, cobalt, iron, nitrogen, oxygen, silicon, carbon and chlorine, were appropriately distributed within the catalyst structure (Fig. 3).
 | ||
| Fig. 3 SEM coupled with EDX (SEM mapping) of nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2]. | ||
To determine the contact surface and particle size of the catalyst, field emission scanning electron microscopy (FESEM) analysis of nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2] was performed. The images obtained showed that the particle size of the designed nanomagnetite cobalt(II) Schiff base complex was less than 100 nm (Fig. 4).
The particle size of the presented nanomagnetite cobalt(II) Schiff base complex was also investigated using transmission electron microscopy (TEM). The TEM images revealed that the particles of the prepared catalyst were smaller than 100 nm (Fig. 5). The creation of nano-sized particles enhanced the catalytic reaction by increasing the contact surface between the catalyst and the starting materials.
Atomic force microscopy (AFM) analysis is a crucial technique for characterizing nanoparticles, as it provides information on surface topography, nanoparticle size and particle dispersion through atomic force measurements. In this study, the nanoparticles were imaged in both two-dimensional and three-dimensional surface topographies, with a size of 2 microns. Considering the mentioned topographies, a nanoparticle size of 75 nm was observed in the surface topographies, similar to the results from another test conducted. It should be noted that the color changes from blue to red indicated an increase in particle size for the obtained particles (Fig. 6).
 | ||
| Fig. 6 Atomic force microscopy (AFM) analysis of nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2]. | ||
To assess the thermal stability of the presented magnetite complex and its thermal capacity for use in various reactions, thermogravimetric analysis (TGA) was performed on the nanomagnetite Schiff base complex. The results obtained from this analysis indicated that the prepared catalyst could be used up to a temperature of approximately 300 °C (Fig. 7). As a result, the TGA analysis indicated that this nanomagnetite complex exhibited acceptable thermal tolerance in various organic transformations.
 | ||
| Fig. 7 Thermogravimetric analysis (TGA) of nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2]. | ||
The magnetic activity of [CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2] was studied using a vibrating sample magnetometer (VSM) at room temperature to evaluate its separation capability under an external magnetic field. The analysis revealed that the saturation magnetization of [CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2] was approximately 12 emu g−1. The saturation magnetization of the prepared nanomagnetite complex is shown in Fig. 8. Based on this analysis, the designed magnetic complex can be effectively used as a recoverable catalyst in various organic transformations.
 | ||
| Fig. 8 Vibrating sample magnetometer (VSM) analysis of nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2]. | ||
The X-ray diffraction (XRD) pattern of nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2] was also investigated in the 2θ range of 20–80° (Fig. 9). As shown in Fig. 9, the XRD patterns of the nanomagnetite heterogeneous catalyst exhibited peaks at 2θ ≈ 29.55°, 35.65°, 43.30°, 51.30°, 53.10°, 57.20°, 62.75°, 74.85° and 76.15°.
After the design and characterization of the nanomagnetite cobalt(II) Schiff base complex through various analyses, its catalytic application was studied in the preparation of several 4H-pyrimido[2,1-b]benzimidazole derivatives. For this purpose, the condensation reaction of 4-nitrobenzaldehyde, 2-aminobenzimidazole and ethyl acetoacetate was selected as a model reaction. Various conditions, including catalyst amount, temperature and solvent types, were tested for the reaction. The mentioned reaction was tested in the presence of the designed catalyst, varying from 1 to 6 mg, and at temperatures ranging from 50 to 110 °C. The best results were obtained using 3 mg of catalyst at 90 °C in the absence of solvent. Furthermore, the reaction was studied in different solvents, including ethyl acetate, CHCl3, CH2Cl2, n-hexane and DMF, and compared with the solvent-free condition. The results were not superior to those obtained under solvent-free conditions (Table 1). Since the catalyst was heterogeneous, the presence of a solvent hindered the contact between the starting materials and the catalyst, leading to a slower reaction and a reduced product yield.
| Entry | Solvent | Catalyst amount (mg) | Temp. (°C) | Time (min) | Yielda (%) |
|---|---|---|---|---|---|
| a Isolated yield. | |||||
| 1 | — | — | 90 | 120 | 27 |
| 2 | — | 1 | 90 | 20 | 38 |
| 3 | — | 2 | 90 | 20 | 54 |
| 4 | — | 3 | 90 | 20 | 95 |
| 5 | — | 6 | 90 | 20 | 95 |
| 6 | — | 3 | 50 | 20 | 30 |
| 7 | — | 3 | 70 | 20 | 72 |
| 8 | — | 3 | 110 | 20 | 90 |
| 9 | Ethyl acetate | 3 | Reflux | 20 | 60 |
| 10 | CHCl3 | 3 | Reflux | 20 | 87 |
| 11 | EtOH | 3 | Reflux | 20 | 59 |
| 12 | CH2Cl2 | 3 | Reflux | 20 | 75 |
| 13 | n-Hexane | 3 | Reflux | 20 | 39 |
| 14 | DMF | 3 | Reflux | 20 | 48 |
To compare the catalytic activity of the designed nanomagnetite Schiff base complex with its constituent components, the selected reaction was investigated in the presence of each catalyst component individually. The results of this investigation revealed that the catalytic activity of these components was not as effective as that of nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2] (Table 2).
After determining the optimal reaction conditions in the previous study, different aromatic aldehydes, including those with electron-withdrawing groups, electron-donating groups and halogens on their aromatic rings, were used in the reaction with ethyl acetoacetate and 2-aminobenzimidazole to prepare 4H-pyrimido[2,1-b]benzimidazole derivatives. The corresponding products were obtained with acceptable results (Scheme 3). All reactions were carried out by reacting various aromatic aldehydes with ethyl acetoacetate and 2-aminobenzimidazole in the presence of 3 mg of the nanomagnetite catalyst at 90 °C under solvent-free conditions.
 | ||
| Scheme 3 Synthesis of 4H-pyrimido[2,1-b]benzimidazoles. All reactions were carried out using 3 mg of nanomagnetite catalyst at 90 °C under solvent-free conditions. | ||
Based on the structure of the prepared complex, the presence of bulky groups on the nitrogen atoms in the cobalt(II) Schiff base ligand structure can lead to a reduction in repulsion. As a result, the cobalt complex may undergo a structural rearrangement, shifting from a tetrahedral form to a square-planar configuration. Based on the calculations, the energy difference between these two structures is not significant, with the tetrahedral arrangement being only 18 kcal more stable than the square-planar arrangement. Therefore, these two structures can exist in equilibrium with each other. The complex adopting a square-planar arrangement could serve as a precursor for the cobalt to achieve a coordination number of six, enabling it to interact with the starting materials and catalyze the reaction (Scheme 4).
3.1. Computational methods
Calculations were performed using the Gaussian 09 system50 with the B3LYP/6-31G(d) method and basis set.51,52 Frequency calculations were performed at the same computational level to confirm that the obtained structures corresponded to energy minima.The internal energies of the tetrahedral and square-planar structures were −3917.848479 and −3917.819183 hartree, respectively. Based on the calculated internal energies in the gas phase, the tetrahedral geometry was slightly more stable (by about −18 kcal mol−1) than the square-planar structure. It appeared that the two geometries were in equilibrium, with a slight preference for the tetrahedral geometry (Fig. 10).
In the proposed mechanism, the cobalt complex in its tetrahedral form rearranged to a square-planar structure, and aldehyde and ethyl acetoacetate in their enol form were added to this complex, allowing cobalt to achieve six coordination. According to the calculations, the transition from tetrahedral to square-planar arrangement required minimum energy. In the next step of the reaction, the nucleophilic attack of ethyl acetoacetate in its enol form occurred on the activated aldehyde, forming intermediate (I). Additionally, the chloride anion generated from the cobalt complex served as a base, reacting with the acidic hydrogen in the structure of ethyl acetoacetate to facilitate the Knoevenagel reaction. Subsequently, 2-aminobenzimidazole could replace the aldehyde in the empty coordination site of the cobalt complex and react with intermediate (I) as a Michael acceptor, forming intermediate (II), which then underwent tautomerization to yield the keto form (III). Through the intramolecular nucleophilic attack of the imine group on the carbonyl group of intermediate (III), which was activated by the cobalt complex, intermediate (IV) was formed. Finally, by the removal of one molecule of H2O, a process activated by the cobalt complex and accelerated by the chloride anion generated from the cobalt complex, the main product was obtained (Scheme 5). According to the proposed mechanism, the rearrangement of the cobalt complex from a tetrahedral to a square-planar form increased the coordination number of cobalt. This rearrangement activated the cobalt complex, allowing the starting materials to bind as ligands and position themselves in close proximity, facilitating their reaction in the correct orientation.
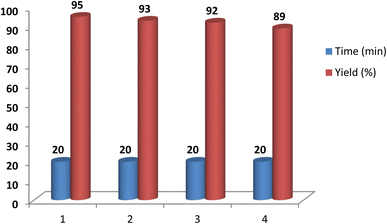
Placing the Schiff base cobalt(II) complex on the nanomagnetite substrate allowed for the easy separation of the catalyst from the reaction mixture. To assess this, the recovery of the catalyst was investigated in the reaction of 4-nitrobenzaldehyde, 2-aminobenzimidazole and ethyl acetoacetate. At the end of the reaction, the mixture was extracted with warm ethanol, and the catalyst was separated from the reaction mixture using an external magnet. The isolated catalyst was recovered and used in subsequent reactions after being washed with acetone. Catalyst recovery was performed three times without a notable decrease in reaction yield or any change in reaction time. The results of this study are presented in Fig. 11. The recovery and reuse of the catalyst not only save raw materials but also help prevent environmental pollution, which is one of the important advantages of using the designed catalyst.
To demonstrate the accuracy, the structure of the reused catalyst was investigated by FT-IR analysis and compared with that of the fresh catalyst (Fig. 12). According to Fig. 12, the FT-IR spectrum of the reused catalyst matched that of the fresh catalyst. The bond vibrations for various bonds in the reused catalyst were compared with those in the fresh catalyst and are presented in Table 3.
| Reused catalyst | Fresh catalyst | ||
|---|---|---|---|
| Bond | Wavenumber (cm−1) | Bond | Wavenumber (cm−1) |
| Co–O | 450 | Co–O | 468 |
| Fe–O | 595 | Fe–O | 596 |
| Si–O–Si | 1095 | Si–O–Si | 1095 |
C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
1703 | C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N N |
1606 |
| C–H | 2928 | C–H | 2925 |
| O–H | 3409 | O–H | 3373 |
To investigate the reason for the decrease in product yield with the reused catalyst, the leaching of cobalt from the surface of the catalyst was investigated using atomic absorption spectroscopy. The investigation revealed that the fresh catalyst contained 12.89% cobalt, while the recovered catalyst, after being used four times, contained 11.01% cobalt. As a result, leaching of cobalt from the magnetically supported cobalt(II) Schiff base complex can lead to a reduction in the product yield.
4. Conclusions
In summary, we have synthesized and fully characterized nano-[CoFe2O4@SiO2/propyl-1-(o-vanillinaldimine)][CoCl2] through various analyses, introducing it as a magnetically supported Schiff base cobalt(II) complex. This complex served as an effective catalyst for the three-component preparation of 4H-pyrimido[2,1-b]benzimidazoles, via the reaction of 2-aminobenzimidazole with ethyl acetoacetate and various aromatic aldehydes, yielding high product yields and suitable reaction times at 90 °C under solvent-free conditions.Data availability
The datasets supporting this article have been uploaded as part of the ESI.†Conflicts of interest
There are no conflicts to declare.References
- M. T. Gabr, N. S. El-Gohary, E. R. El-Bendary and M. M. El-Kerdawy, Eur. J. Med. Chem., 2014, 85, 576–592 Search PubMed.
- S. Nalawade, V. Deshmukh and S. Chaudhari, J. Pharma Res., 2013, 7, 433–438 CrossRef CAS.
- H. Sheibani and M. Babaie, Russ. Chem. Bull., 2013, 62, 2202–2208 Search PubMed.
- P. H. Tran, T.-P. T. Bui, X.-Q. B and Lam and X.-T. T. Nguyen, RSC Adv., 2018, 8, 36392–36399 RSC.
- R. Talaei and A. Olyaei, Iran. J. Catal., 2016, 6, 339–343 Search PubMed.
- M. Farajpour, S. M. Vahdat, S. M. Baghbanian and M. Hatami, Chem. Methodol., 2023, 7, 540–551 Search PubMed.
- R. M. Muhiebes, L. Fatolahi and S. Sajjadifar, Asian J. Green Chem., 2023, 7, 121–131 CAS.
- Y. Ghalandarzehi and H. Kord-Tamandani, Asian J. Green Chem., 2023, 7, 70–84 CAS.
- M. A. Amiri, H. Younesi, H. K. Aqmashhadi, G. F. Pasha, S. Asghari and M. Tajbakhsh, Chem. Methodol., 2024, 8, 1–22 CAS.
- R. M. Mhaibes, Z. Arzehgar, M. M. Heydari and L. Fatolahi, Asian J. Green Chem., 2023, 7, 1–8 CAS.
- H. Ghafuri, M. Zargari and A. Emami, Asian J. Green Chem., 2023, 7, 54–69 CAS.
- M. kidwai, P. Dwivedi and A. Jahan, J. Appl. Organomet. Chem., 2023, 3, 156–168 CAS.
- B. Baghernejad and M. Fiuzat, J. Med. Nanomater. Chem., 2023, 5, 235–242 Search PubMed.
- A. R. Moosavi-Zare, M. A. Zolfigol, E. Noroozizadeh, O. Khaledian and B. S. Shaghasemi, Res. Chem. Intermed., 2016, 42, 4759–4772 CrossRef CAS.
- A. R. Moosavi-Zare, M. A. Zolfigol, E. Noroozizadeh, M. Zarei, R. Karamian and M. Asadbegy, J. Mol. Catal. A:Chem., 2016, 425, 217–228 CrossRef CAS.
- M. A. Zolfigol, S. Baghery, A. R. Moosavi-Zare and S. M. Vahdat, J. Mol. Catal. A: Chem., 2015, 409, 216–226 CrossRef CAS.
- A. R. Moosavi-Zare, M. A. Zolfigol and A. Mousavi-Tashar, Res. Chem. Intermed., 2016, 42, 7305–7312 CrossRef CAS.
- H. R. Shaterian, N. Fahimi and K. Azizi, Res. Chem. Intermed., 2014, 40, 1879–1898 CrossRef CAS.
- A. Shaabani, A. Rahmati and S. Naderi, Bioorg. Med. Chem. Lett., 2005, 15, 5553–5557 CrossRef CAS PubMed.
- S. Azad and B. F. Mirjalili, Res. Chem. Intermed., 2017, 43, 1723–1734 CrossRef CAS.
- N. Shekarlab, R. Ghorbani-Vaghei and S. Alavinia, Appl. Organomet. Chem., 2020, e5918 CrossRef CAS.
- P. K. Sahu, P. K. Sahu, Y. Sharma, D. D. Agarwal and J. Heterocyclic, Chem, 2014, 51, 1193–1198 CAS.
- C. Yao, S. Lei, C. Wang, T. Li, C. Yu, X. Wang and S. Tu, J. Heterocycl. Chem., 2010, 47, 26–32 CrossRef CAS.
- N. Basirat, S. S. Sajadikhah and A. Zare, Res. Chem. Intermed., 2020, 46, 3263–3275 CrossRef CAS.
- M. Abedini, F. Shirini, M. Mousapour and O. Goli-Jolodar, Res. Chem. Intermed., 2016, 42, 6221–6229 Search PubMed.
- J. Liu, M. Lei and L. Hu, Green Chem., 2012, 14, 840–846 RSC.
- Z. Jalilian, A. R. Moosavi-Zare, M. Ghadermazi and H. Goudarziafshar, RSC Adv., 2023, 13, 10642–10649 RSC.
- R. Ghorbani-Vaghei, Z. Toghraei-Semiromi, R. Karimi-Nami and Z. Salimi, Helv. Chim. Acta, 2014, 97, 979–984 Search PubMed.
- A. R. Moosavi-Zare, H. Goudarziafshar and P. Fashi, Res. Chem. Intermed., 2020, 46, 5567–5582 CrossRef CAS.
- H. Goudarziafshar, N. Taheriazod and A. R. Moosavi-Zare, Appl. Catal. O: Open, 2024, 193, 206970 CAS.
- M. Makhsous, F. Shirini, M. Seddighi and M. Mazloumi, Polycyclic Aromat. Compd., 2020, 40, 494–501 CrossRef CAS.
- M. Mashhadinezhad, F. Shirini, M. Mamaghani and M. Rassa, Polycyclic Aromat. Compd., 2020, 40, 1417–1433 CrossRef CAS.
- A. Thongni, R. Nongkhlaw, C. Pandya, A. Sivaramakrishna, P. M. Gannon and W. Kaminsky, J. Heterocycl. Chem., 2024, 61, 581–599 CrossRef CAS.
- R. Alajarin, J. J. Vaquero, J. Alvarez-Builla, M. F. d. Casa-Juana, C. Sunkel, J. G. Priego, P. Gomez-Salcpd and R. Torres, Bioorg. Med. Chem., 1994, 2, 323–329 CrossRef CAS PubMed.
- A. R. Moosavi-Zare, M. A. Zolfigol, V. Khakyzadeh, C. Böttcher, M. H. Beyzavi, A. Zare, A. Hasaninejad and R. Luque, J. Mater. Chem. A, 2014, 2, 770–777 RSC.
- A. R. Moosavi-Zare, M. A. Zolfigol, F. Derakhshan-Panah and S. Balalaie, Mol. Catal., 2018, 449, 142–151 Search PubMed.
- L. V. Chopda and P. N. Dave, Results Chem., 2021, 3, 100169 CrossRef CAS.
- L. V. Chopda and P. N. Dave, Arabian J. Chem., 2020, 13, 5911–5921 Search PubMed.
- L. V. Chopda and P. N. Dave, Chemistryselect, 2020, 5, 5552–5572 CrossRef CAS.
- L. V. Chopda and P. N. Dave, Chemistryselect, 2020, 2395–2400 CrossRef CAS.
- A. R. Moosavi-Zare, R. Najafi and H. Goudarziafshar, RSC Adv., 2024, 27, 19167–19173 RSC.
- A. Gorji, S. Esmaili, A. R. Moosavi-Zare and A. Khazaei, J. Mol. Struct., 2024, 1314, 138797 CrossRef CAS.
- H. Goudarziafshar, M. Zafari and A. R. Moosavi-Zare, RSC Adv., 2024, 14, 27565–27574 Search PubMed.
- H. Goudarziafshar, M. Zafari and A. R. Moosavi-Zare, Chem. Methodol., 2024, 8, 820–832 CAS.
- Z. Jalilian, A. R. Moosavi-Zare and M. Ghadermazi, Chem. Methodol., 2025, 9, 326–342 Search PubMed.
- A. R. Moosavi-Zare, H. Goudarziafshar and Z. Jalilian, Appl. Organomet. Chem., 2019, 33, e4584 CrossRef.
- A. R. Moosavi-Zare, H. Goudarziafshar and Z. Bahrami, Res. Chem. Intermed., 2023, 49, 507–523 CrossRef CAS.
- A. R. Moosavi-Zare, H. Goudarziafshar and K. Saki, Appl. Organomet. Chem., 2018, 32, e3968 CrossRef.
- M. Aliavazi, M. H. Ardakani and A. Naeimi, Inorg. Chem. Commun., 2024, 160, 111895 CrossRef CAS.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr, J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski, and D. J. Fox ,Gaussian 09, Revision B.01, Gaussian Inc., Wallingford, 2010 Search PubMed.
- P. J. Hay and W. R. Wad, J. Chem. Phys., 1985, 82, 299–310 CrossRef CAS.
- A. E. Reed, R. B. Weinstock and F. Winhold, Natural population analysis, J. Chem. Phys., 1985, 83, 735–746 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02906f |
| This journal is © The Royal Society of Chemistry 2025 |