Open Access Article
Open Access ArticleEnhanced corrosion resistance of zinc phosphate coatings on mild steel through incorporation of nanocrystalline CeO2 and CeO2–CuO nanocomposite†
S. Ayesha Barsanaa,
A. Sultan Nasar b and
M. J. Umapathy
b and
M. J. Umapathy *a
*a
aDepartment of Chemistry, Anna University, College of Engineering, Guindy, Chennai 600025, India. E-mail: mj_umapathy@yahoo.co.in
bDepartment of Polymer Science, University of Madras, Guindy Campus, Chennai 600025, India
First published on 20th June 2025
Abstract
Herein, 5–10 nm-sized rod-like nanocrystalline CeO2 and CeO2–CuO nanocomposite were synthesized and incorporated into zinc phosphate coatings as corrosion inhibition additives. The formulated coatings were studied thoroughly on mild steel in 3.5% NaCl solution and compared with unmodified coating. The concentration of the additive was varied from 0.3 g L−1 to 0.9 g L−1 with three step-ups. X-ray and surface analysis results revealed that both additives favoured the formation of more compact coatings with densely packed zinc phosphate crystals and reduced iron content. Compared with the unmodified coating, the metal oxide incorporated coatings showed low corrosion current density (Icorr) and corrosion rate and high polarisation resistance (Rp) and charge transfer resistance (Rct), confirming that these nanomaterials act as corrosion inhibitors. Among the three concentrations used, nanocrystalline CeO2 performed well at 0.6 g L−1 and CeO2–CuO nanocomposite performed well even at low concentration of 0.3 g L−1, with a more than fifty-one times lower corrosion rate and twenty times higher polarisation resistance (Rp) compared with unmodified coating. Nanocrystalline CeO2 increased the Rct from 114 Ω cm2 to 427.5 Ω cm2 at 0.6 g L−1, whereas the CeO2–CuO nanocomposite increased this value up to 3645 Ω cm2 at 0.3 g L−1. Enhanced corrosion resistance of these coatings was confirmed with an accelerated salt-spray test.
1. Introduction
Low carbon steel, commonly known as mild steel in which the carbon content normally varies from 0.05 to 0.3 weight percentage, is known for its high ductility, machinability and weldability. In addition to these properties, its cost-effectiveness makes it a highly sought-after material for many large and small fabrications, notably automobile parts such as body panels, camshaft, shaft axles, engine cylinders and bolts, gears, brackets, pins and washers. This alloy is more susceptible to rusting when exposed to moisture, which deteriorates the metal's performance, leading to random failures.1 Despite this drawback, mild steel remains an ideal choice for applications where high tensile strength and durability are required.Preventive measures such as using corrosion inhibitors and applying protective coatings can help mitigate these failures.2–5 Coatings are one of the smart ways to control corrosion. Phosphate conversion coatings—particularly zinc phosphate conversion coatings—are more advantageous over other coating methods.6–8 This coating protects the surface of the steel against corrosion by forming a thin, non-conducting layer of insoluble phosphates.9 Zinc phosphate coatings can be applied to a variety of materials, including high carbon steel, medium carbon steel,10 low carbon steel,11 galvanized steel,12 magnesium alloy,13 and aluminum alloy.14 Owing to the ease of application, efficiency and compatibility with various substrates, this coating emerged as a preferred choice for many industrial applications.15
However, the corrosion resistance of the phosphate coatings in adverse environments is often limited due to open pores and cracks through which the electrolytes diffuse and react with the underlying steel surface and induce the conversion of iron into iron oxides and hydroxides.16,17 Many additives improve the coating performance. Careful selection and infusion of these additives are vital for achieving desired coating characteristics, such as improved corrosion resistance, adhesion, hardness, and overall durability of the coated metal surface.
In the recent past, the use of nanomaterials as corrosion inhibitors gained significant attention. The presence of nanomaterials in the coating composition control the electrochemical process that occurs at the interface of the coating and the metal surface. Metal oxides like SiO2,18 ZrO2,19 TiO2,20 and Al2O3 (ref. 21) in the nanoscale, nanocomposites like Al2O3/Mo22 and two-dimensional nanosheets like graphene oxide23,24 and boron nitride25 were studied as additives for zinc phosphate coatings and the results were promising. Their unique properties such as high surface area, water repellent nature, antioxidant properties, and barrier functions against aggressive agents collectively improve the performance of the coatings.26 They also speed-up the phosphate treatment process at room temperature, and thus reduce energy consumption.
Bagal and co-workers found that the corrosion rate significantly reduced from 8 mpy to 3.5 mpy, and the coating porosity decreased by 50% upon incorporation of TiO2 in the phosphate coating.20 In another report, Young Zhou and co-workers explored the effect of the addition of nano CeO2 into zinc phosphate coatings applied on magnesium alloy AZ91D and showed improved adhesion and micro-hardness of the coating at a concentration of 2 g L−1.27 Riyas and co-workers reported Al2O3/Mo composite incorporated zinc phosphate coatings for galvanised steel.22 They found that the Al2O3/Mo particles acted as nucleating agents, forming highly compact and dense phosphate crystals. Moreover, the resulting coating showed high charge-transfer resistance and improved corrosion resistance. Deepa and co-workers formulated zinc coatings with BiVO4/TiO2 composites.28 This coating achieved a water contact angle of 120.2°, which improved its self-cleaning properties. Additionally, it demonstrated a high inhibition efficiency of 91.68% and great corrosion resistance. These recent findings recognise metal oxides as anti-corrosion additives for coatings.
The present study focuses on abundant rare earth metal oxide nanoparticle, i.e., nano CeO2 and its nanocomposite with CuO as additives for zinc phosphate coatings. Generally, this class of metal oxides enhances microstructure formation, refines grain boundaries, and improves overall durability of the coating.29 Rare earth elements inhibit corrosion by forming an insoluble film of oxides and hydroxides, which replaces the natural oxide film on metal surfaces.30 Among the various rare earth elements, cerium with high oxidation state (Ce3+ and Ce4+) forms stable oxides that provide barrier protection against corrosion.31 As far as copper is concerned, it has good thermal stability, mechanical workability and acts as like a noble metal in stringent environmental conditions. A nano copper oxide coating on the same metal formed by anodization was found to increase the corrosion protection efficiency by 86.2% in 3.5% NaCl and 74.5% in 2 mg per L NH3.32 The insoluble nature of copper in water increases the hydrophobicity of the coating.33 We aimed to develop new zinc phosphate conversion coatings using nanocrystalline CeO2 and CeO2–CuO nanocomposite as corrosion inhibition additives. Accordingly, some zinc phosphate conversion coatings were formulated and applied on mild steel. Herein, the synthesis of nanoadditives was reported and the anti-corrosion performance of the coatings was impressive.
2. Materials and methods
2.1 Materials
Analytical grade cerous nitrate hexahydrate (Ce(NO3)3·6H2O, CAS number 10294-41-4), cupric nitrate trihydrate (Cu(NO3)2·3H2O, CAS 10031-43-3), zinc oxide (ZnO, CAS number 1314-13-2), orthophosphoric acid (85% phosphoric acid (H3PO4), CAS number 7664-38-2), sodium hydroxide, sodium carbonate, sodium nitrite and sodium chloride were purchased from Sisco Research Laboratories Pvt. Ltd and used without further purification. Mild steel of grade IS 513/2008 GR4 was purchased from the local market and its chemical composition was given in Table S1 (ESI).† All the experiments were carried out in distilled water.2.2 Synthesis of CeO2 nanoparticle
CeO2 in the form of nanoparticle was prepared from cerium nitrate hexahydrate (Ce(NO3)3·6H2O) and sodium carbonate (Na2CO3) by the precipitation method. A 0.2 M solution of cerium nitrate hexahydrate was prepared by dissolving 4.34 g of Ce(NO3)3·6H2O in 50 mL of distilled water and the solution was subjected to continuous stirring. To this, a 1 M sodium carbonate solution was added dropwise until the pH of the solution reached 10. The whole mixture was stirred for a further 60 minutes, resulting in a pale, yellow coloured liquid–solid suspension. This suspension was centrifuged at 2000 rpm, filtered and washed with deionized water several times to remove the impurities. The obtained precipitate was first dried in a vacuum oven maintained at 80 °C, milled to obtain a fine powder and subsequently calcined at 450 °C in the presence of air for 3 h to obtain CeO2 as a nanopowder.2.3 Preparation of CeO2–CuO nanocomposite
CeO2–CuO nanocomposite was prepared via the co-precipitation method. Twenty-five mL of 0.2 M solutions of Ce(NO3)3·6H2O and Cu(NO3)2·3H2O were mixed together and stirred for 1 h. Next, a 1 M sodium hydroxide solution was added dropwise to the solution until the pH of the solution attained 10. Then, the whole mixture was stirred for 60 min to obtain a pale blue coloured liquid–solid suspension. The rest of the procedure employed to obtain CeO2–CuO nanocomposite was similar to that adopted for the preceding synthesis.2.4 Characterizations of CeO2 nanoparticle and CeO2–CuO nanocomposite
The functional groups of the synthesised metal oxide and composite were analysed using a Thermo iS50 Fourier-transform infra-red spectrophotometer (FT-IR) in the mid IR range of 400–4000 cm−1; samples were prepared using the KBr pellet method. The morphology and elemental distribution of the prepared CeO2 nanoparticle and CeO2–CuO nanocomposite were examined by field emission scanning electron microscopy (FE-SEM) combined with energy dispersive X-ray spectroscopy (EDAX) (TESCAN VEGA3 model and Carl Zeiss Sigma 300 model instruments). The phase and crystalline characteristics of the prepared CeO2 and CeO2–CuO composite were studied using a powder X-ray diffraction (XRD) technique with an Empyrean Series III Diffractometer operated with a monochromatic Cu Kα radiation source (λ = 0.15418 nm). The samples were examined between 2θ of 20° and 90° with a 0.05° step-up and 0.02 s per step. The crystallite size was calculated using the Debye–Scherrer formula as given in eqn (1).
D = Kλ/β![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) cos cos![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ θ
| (1) |
2.5 Preparation of phosphating bath
First, the phosphating bath solution was partially prepared by mixing 7 g of zinc oxide (ZnO) in 14 mL of 85% ortho phosphoric acid (H3PO4). Stirring the obtained paste like mass with 50 mL of distilled water in a magnetic stirrer for 30 min resulted in a clear solution. Then, this solution was diluted to 1 litre with distilled water. The quantity of zinc oxide and orthophosphoric acid was fixed after several trial experiments. Appropriate quantities of the remaining ingredients were added to this partial solution immediately prior to experiments to make a complete formulation. The chemical composition of the zinc phosphating bath is given in Table 1.| Ingredient | Quantity |
|---|---|
| a Either CeO2 or CeO2–CuO was used for a formulation. | |
| ZnO | 7 g L−1 |
| Phosphoric acid | 14 mL L−1 |
| Sodium nitrite | 1 g L−1 |
| Nanocrystalline CeO2a | 0.3 g L−1 (or), 0.6 g L−1 (or), 0.9 g L−1 |
| CeO2–CuO nanocompositea | 0.3 g L−1 (or), 0.6 g L−1 (or), 0.9 g L−1 |
| Total acid | 28.2 (Points) |
| Free acid | 2.9 (Points) |
TA![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) FA FA |
9.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
| pH | 2.8 |
The conventional zinc phosphate coating composition was modified by the addition of synthesized CeO2 nanoparticle and CeO2–CuO nanocomposite into the phosphating bath. Based on the quantity of the additive added (0.3 g, 0.6 g or 0.9 g) into the zinc phosphating bath, the obtained coatings were named as (i) ZnP-0.3CO, ZnP-0.6CO, ZnP-0.9CO, respectively for cerium oxide and (ii) ZnP-0.3COCO, ZnP-0.6COCO, ZnP-0.9COCO, respectively for cerium oxide–copper oxide composite. The zinc phosphate coating formulated without the synthesised nanomaterials as additive is simply represented as ZnP in this study.
2.6 Determination of free acid and total acid
To obtain quality coatings, bath parameters such as total acid value (TA), free acid value (FA) and their ratio should be maintained at appropriate levels. These values were calculated by titration of the bath solution against 0.1 N NaOH. The total acid value was determined by titrating 10 mL of the bath solution against 0.1 N NaOH using phenolphthalein as indicator. The end-point was colour change of the bath solution from colourless to pink (1 mL = 1 point). The free acid value was determined by following the same procedure using methyl orange as indicator. Here, the end-point was a colour change of the bath solution from orange to yellow. In general, the total acid value is maintained between 10 and 25 (points) and the free acid value is maintained between 0.5 and 3 (points) to obtain optimal coatings. The ratio of free acid to total acid gives the acid coefficient of the bath; the higher the acid co-efficient value, the better the coating produced. The total acid value, free acid value and the acid coefficient of the phosphating bath are given in Table 1.2.7 Preparation of substrate and phosphate treatment
Steel panels were fabricated to a dimension of 20 mm × 20 mm × 0.8 mm. The residual rust and scales on the steel surface were removed by abrading them with SiC papers differing in grit number and washed with distilled water. Contaminants like dirt, oil and grease were removed by soaking the panel in 10% NaOH solution at room temperature for 30 min and washed with excess distilled water followed by rinsing with acetone. The cleaned steel substrates were immersed in a zinc phosphating bath at room temperature for 30 min with the composition given in Table 1. After this surface treatment, the panels were dried in air and kept in a desiccator for further studies.2.8 Characterizations of zinc phosphate coatings
 | (2) |
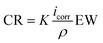 | (3) |
The data obtained from the electrochemical impedance spectroscopy were presented as Nyquist and Bode plots. Bode impedance and the phase angle were plotted as log|Z| vs. log(f) and −phase angle vs. log(f), where |Z| represents the absolute impedance and f indicates the frequency. The obtained data were fitted using ZSimpWin software (Princeton Applied Research, USA) to obtain an appropriate equivalent circuit.
| Corrosion rate = 534W/DAT | (4) |
The percentage inhibition efficiency ‘P’ of the additives was calculated from the following relationship:
| P = 100[CRuninhibited − CRinhibited/CRuninhibited] | (5) |
Other characterizations of the coatings were described in the ESI section,† including the analysis of functional groups, surface morphology, elemental and phase composition, measurements of surface wetting, coating weight, hardness and adhesion tests.
3. Results and discussion
3.1 Synthesis of nano CeO2 and CeO2–CuO nanocomposite
Cerium oxide nanoparticles and its composite with copper oxide were synthesized via an economically simple-cum-attractive precipitation method as described in Fig. S1 and S2, respectively (ESI).† For both the synthesis, the respective hydrous forms of metal nitrates were used as precursors with sodium carbonate and sodium hydroxide used as precipitating agents. The reactions were carried out in water medium at pH = 10 and subsequent physical processes of fine grinding and calcination carried out at 450 °C yielded pale yellow CeO2 and greyish black CeO2–CuO nanoparticles. The yield of the compound was quantitative, i.e., >80%. It is worth mentioning that the amount of obtained CeO2 was considerably higher than the expected theoretical value, indicating that this material was in hydrated form.34,353.2 Characterisation of prepared nanoadditives
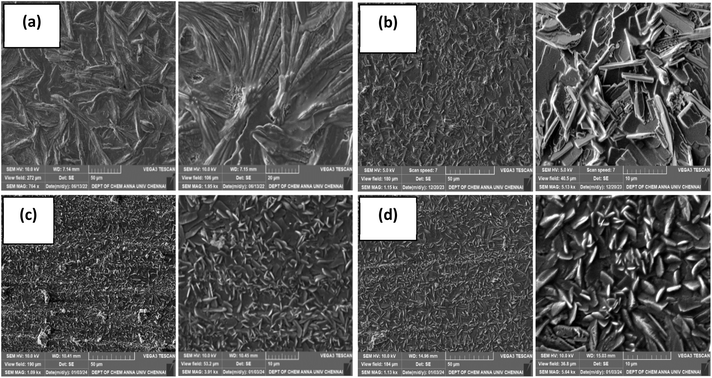
The surface morphology and nano-scale structure of CeO2 and CeO2–CuO composite were examined through FE-SEM and the vivid pictures are shown in Fig. 1. The SEM images reveal that the CeO2 crystals possess small rod-like structures that grew randomly in all directions with an even surface.39 On the other hand, the CeO2–CuO composite grew perpendicularly with an uneven surface and showed a bamboo stem like structure. This well-defined surface morphology is an important requirement that will enhance the electrochemical performance of the nanomaterials due to its high surface-to-volume ratio and large number of active sites.40 This intrinsic characteristic of nanomaterials is particularly beneficial in the context of corrosion protection, where a large surface area leads to improved interactions between the protective layer and the corrosive environment. The EDAX spectra of both nano CeO2 and CeO2–CuO nanocomposite along with elemental composition are shown in Fig. 2. The characteristic peaks corresponding to cerium, copper and oxygen appearing in the spectra with the expected ratios confirm the successful synthesis of these materials.
 | ||
| Fig. 1 SEM micrograph of (a) nanocrystalline CeO2 (scale bar = 5 μm) and (b) CeO2–CuO nanocomposite (scale bar = 200 nm). | ||
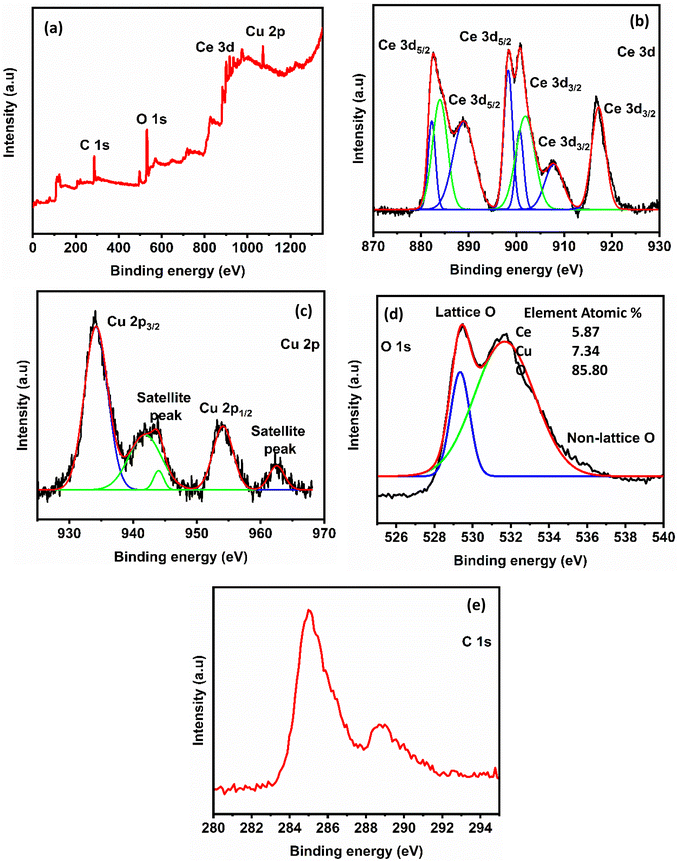
The chemical state and elemental composition of nanocrystalline CeO2 and CeO2–CuO nanocomposite were investigated using XPS and the results were illustrated in Fig. 4 and 5, respectively. The survey scan spectrum of CeO2 identified the presence of C 1s, O 1s, and Ce 3d (Fig. 4a). The XPS spectrum of CeO2–CuO composite was similar to that of CeO2: it showed the presence of Cu 3d in addition to all other elements observed for CeO2 (Fig. 5a). The elemental compositions derived from the XPS survey scans for both CeO2 and CeO2–CuO are shown in the respective figures: the data are consistent with the size of the elements and composition of the materials. In the high-resolution Ce 3d spectrum of both CeO2 and CeO2–CuO composite (Fig. 4b and 5b, respectively), eight peaks were observed corresponding to the spin–orbit splitting of Ce 3d5/2 and Ce 3d3/2. This indicates that CeO2 contains both Ce3+ and Ce4+ oxidation states. The six distinct peaks with binding energy values at 881.8, 888.0, 898.0, 900.4, 906.4, and 916.2 eV are associated with the Ce4+ 3d state, confirming the predominant valence state of Ce as +4. The two weak XPS peaks at the binding energy values of 883.1 and 901.9 eV correspond to the Ce3+ oxidation state.42,43 The observed yellowish colour of CeO2 supports the presence of both Ce4+ and Ce3+ ions in the structure.44 In the CeO2–CuO composite, two significant peaks appeared at 933.9 eV and 953.7 eV: these values are the binding energies of Cu 2p3/2 and Cu 2p1/2, respectively (Fig. 5c). The three satellite peaks in the XPS spectrum of Cu2+ at binding energies of 941.0 eV, 943.6 eV and 962.3 eV indicate a partially filled d-block configuration (3d9) for Cu2+ in the ground state.45,46 The high-resolution XPS spectra of O 1s of both CeO2 and CeO2–CuO composite revealed two peaks: the first peak around 528.8 eV originated from lattice oxygen and the peak of higher binding energy (531.1 eV) is attributed to physically adsorbed oxygen and/or a hydroxyl group. In both of the cases, the C 1s peaks correspond to the carbonate impurities present in the compounds.
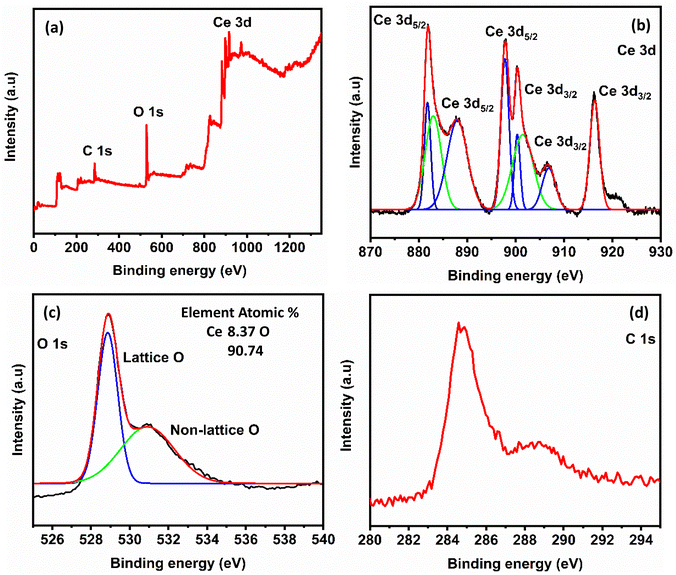 | ||
| Fig. 4 (a) XPS spectrum of nanocrystalline CeO2, (b) high resolution spectrum of Ce 3d, (c) high resolution spectrum of O 1s and (d) high resolution spectrum of C 1s. | ||
3.3 Characterization of the coatings
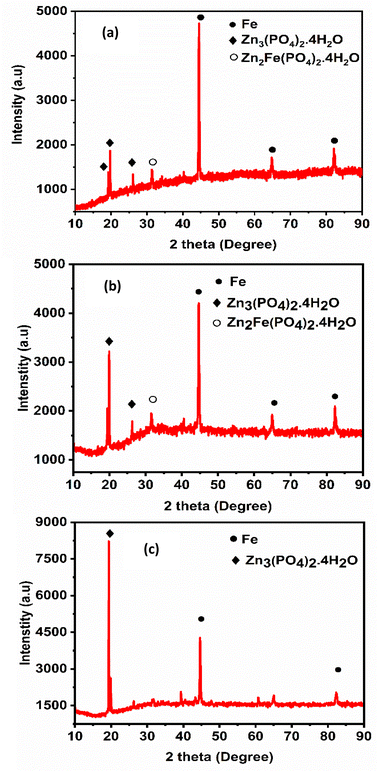
The crystallinity and phase composition of the coatings were studied using XRD and the obtained diffraction patterns are shown in Fig. 6. There are two crystal phases in all the zinc phosphate coatings: the first one is hopeite (Zn3(PO4)2·4H2O, JCPDS file 37-0465), which is a major phase and the other one is phosphophyllite (Zn2Fe(PO4)2·4H2O, JCPDS file 29-1427), which is a minor phase.5 After comparing the XRD patterns of unmodified zinc phosphate coating and those modified with nanocrystalline CeO2 and CeO2–CuO nanocomposite, it was evident that the addition of both of the additives resulted in stronger (high intensity) reflections for the hopeite phase and this was more significant in the case of coatings formulated with CeO2–CuO nanocomposite. The strong reflection observed at 19.5° correspond to the (020) plane of hopeite crystals.22 This increase in intensity indicates that the additives play a vital role in the formation of the phosphate coating layer. The diffraction peak for the phosphophyllite phase appeared at 31.5°, but was weak in the case of coating containing CeO2 and very weak in the CeO2–CuO modified coatings. Further, the XRD patterns reveal a considerable reduction in the intensity of iron upon the addition of nanocrystalline CeO2 and CeO2–CuO nanocomposite, suggesting improved surface coverage and enhanced growth of hopeite.48 The absence of shifts in the 2 theta values of the major components confirms that the addition of these additives did not affect the phase composition of phosphate crystals while influencing its growth. The sizes of the phosphate crystallites were 32.9 ± 0.4 nm, 19.3 ± 0.8 nm and 18.1 ± 0.4 nm for ZnP, ZnP-0.6CO and ZnP-0.3COCO, respectively. These results together with the SEM micrograph showed that both of the nanometal oxides enhanced the nucleation sites, reduced the crystal size and resulted in coatings with more phosphate crystals.
The EDAX mapping spectra of all the zinc phosphate coatings are presented in Fig. S6 and S7 (ESI)† and the elemental composition data obtained thereof are given in Table 2. All the spectra confirm the presence of zinc, phosphorus, iron and oxygen present in all the coatings, whereas the spectra of coatings modified with CeO2 and CeO2–CuO show the presence of cerium and copper in addition to all those elements. This indicates the successful incorporation of the additives into the zinc phosphate matrix which is essential for enhancing the coating's protective properties.
| Coating ID | Nanoadditive added | Elemental composition (in wt%) | |||||
|---|---|---|---|---|---|---|---|
| Zn | Fe | P | O | Ce | Cu | ||
| ZnP | Nil | 21.30 | 15.40 | 24.16 | 38.00 | — | — |
| ZnP-0.3CO | Nano CeO2 | 34.16 | 11.99 | 18.33 | 30.31 | 3.53 | — |
| ZnP-0.6CO | Nano CeO2 | 36.29 | 3.18 | 20.87 | 31.63 | 5.61 | — |
| ZnP-0.9CO | Nano CeO2 | 34.85 | 8.64 | 19.84 | 33.04 | 2.44 | — |
| ZnP-0.3COCO | CeO2–CuO nanocomposite | 39.75 | 1.39 | 18.37 | 24.70 | 1.85 | 12.53 |
| ZnP-0.6COCO | CeO2–CuO nanocomposite | 39.06 | 2.98 | 19.33 | 31.97 | 3.05 | 3.00 |
| ZnP-0.9COCO | CeO2–CuO nanocomposite | 39.40 | 1.64 | 19.69 | 28.33 | 4.76 | 6.21 |
The zinc and iron content play a crucial role in determining the overall performance of the coating. As shown in Table 2, the zinc content is higher in the modified coatings than in the unmodified coatings, indicating a greater precipitation of zinc phosphate crystals. Another key observation is the low iron content in the modified coating: iron comes from the uncovered metal surface due to the porous nature of the coatings.25 The low iron content of the modified zinc phosphate coating compared with the unmodified coating suggests that the nanocrystalline CeO2 and CeO2–CuO nanocomposite improves the surface coverage and integrity of the coatings.24 Within the variations in the additives and their concentrations, the increase in zinc content and decrease in iron content is more pronounced in the coatings formulated with 0.6 g L−1 of CeO2 and 0.3 g L−1 of CeO2–CuO nanocomposites.
| Coating ID | Nanoadditive added | Coating weight (g m−2) | Micro hardness (HV) |
|---|---|---|---|
| ZnP | Nil | 4.8 ± 0.1 | 153.7 ± 2.9 |
| ZnP-0.3CO | Nano CeO2 | 13.8 ± 0.2 | 159.9 ± 1.7 |
| ZnP-0.6CO | Nano CeO2 | 12.0 ± 0.4 | 166.2 ± 5.9 |
| ZnP-0.9CO | Nano CeO2 | 10.8 ± 0.2 | 169.0 ± 3.7 |
| ZnP-0.3COCO | CeO2–CuO nanocomposite | 17.4 ± 0.5 | 158.0 ± 3.1 |
| ZnP-0.6COCO | CeO2–CuO nanocomposite | 20.1 ± 0.8 | 166.6 ± 6.4 |
| ZnP-0.9COCO | CeO2–CuO nanocomposite | 19.4 ± 0.4 | 173.8 ± 5.8 |
The micro hardness of all the coatings was determined and the values were included in Table 3. In both of the series of coatings, the hardness increased with greater concentrations of nanoadditives. The highest hardness was achieved for the coating formulated with 0.9 g L−1 of CeO2–CuO (173.8 ± 5.8 HV), which is 20.1 HV higher than that of unmodified coating. This improved hardness of the zinc phosphate coating modified with nano CeO2 and CeO2–CuO nanocomposite indicate that significant changes occurred in the microstructure of hopeite crystals.
Adhesion of a coating with a metal surface plays a crucial role in the coating's protective performance. In corrosive environments, the penetration of corrosive agents into the metal-coating interface weakens the coating's adhesion. The cross-hatch adhesion test images of coated steel substrates were recorded (Fig. S9 and S10, ESI†) and the coating's adhesion strength was assessed according to the ASTM D3359-17 standard. The coating without the additive and the coatings modified with CeO2 demonstrated good adhesion with no detachment or damage at the grid edges and no squares were eliminated in these coatings during removal of the adhesive tape. Thus, these coatings were rated 5B. On the other hand, the coatings formulated with CeO2–CuO composite showed trace removal of the coating within the square grids. However, the edges remained intact and it was rated 4B, indicating less than 5% removal. These adhesion test results suggest that the added nanocrystalline CeO2 and CeO2–CuO nanocomposite did not interfere with the interactions and bonding force acting between the coating and the steel substrate.
| Coating ID | Nano additive added | Electrochemical parameters | |||||
|---|---|---|---|---|---|---|---|
| Icorr (μA cm−2) | Ecorr (V) | βa (V dec−1) | −βc (V dec−1) | Rp (ohm cm2) | Corrosion rate (CR) (mm per year) | ||
| ZnP | Nil | 156.2 | 1.22 | 0.500 | 0.167 | 348 | 1.843 |
| ZnP-0.3CO | Nano CeO2 | 87.44 | 1.16 | 0.155 | 0.333 | 525 | 1.032 |
| ZnP-0.6CO | Nano CeO2 | 44.80 | 1.24 | 0.191 | 0.271 | 1086 | 0.528 |
| ZnP-0.9CO | Nano CeO2 | 65.10 | 1.21 | 0.167 | 0.258 | 676 | 0.768 |
| ZnP-0.3COCO | CeO2–CuO nanocomposite | 3.025 | −0.54 | 0.087 | 0.111 | 7003 | 0.036 |
| ZnP-0.6COCO | CeO2–CuO nanocomposite | 17.50 | −0.59 | 0.106 | 0.500 | 2169 | 0.207 |
| ZnP-0.9COCO | CeO2–CuO nanocomposite | 3.98 | −0.73 | 0.099 | 0.139 | 6321 | 0.047 |
In general, the large diameter of the capacitive loop indicates enhanced corrosion resistance as they reflect greater impedance to ion penetration.55 For nanocrystalline CeO2 modified coatings, the diameter of the first capacitive loop increases successively from that without additive to the inclusion of 0.3 g L−1 and 0.6 g L−1 additive and then decreased. The Bode impedance at the low frequency region corresponds to the barrier properties of the coatings. The higher the impedance modulus, the greater the blocking of corrosive ions by the coating.22 From Fig. 10b and 11b, it is clear that the impedance modulus increased upon the addition of both CeO2 and CeO2–CuO into the coatings. Similarly, these additives increased the phase angle of the coating up to 60°. This indicates that the stability of the modified coatings improved in this impedance measurement condition compared with the unmodified coating.
The impedance curves were fitted to equivalent electrical circuits using ZSimpWin software for further analysis and the generated circuits for unmodified and modified zinc phosphate coatings were given in Fig. 12. From the equivalent circuits, electrochemical parameters including Rs (solution resistance), Rc (coating resistance), Rct (charge transfer resistance), Cc and Qc (coating capacitance), Cdl and Qdl (double layer capacitance) and W (Warburg impedance) were obtained and listed in Table 5. In the circuit, L and R1 are inductive elements. The Rs of all the modified coatings and Rc of all the coatings except the one prepared with 0.9 g L−1 of CeO2–CuO nanocomposite are improved by the addition of metal oxide additives into the coating composition. The low Qc value of coating containing nanocrystalline CeO2 indicates a restriction of the penetration of ions, water molecules and oxygen through the coating. This also means that this additive blocks the pores and reduces the porosity of the coating. Importantly, the Rct component of all the modified coatings are higher than that of the unmodified coating, which indicates that the added nanocrystalline CeO2 and CeO2–CuO nanocomposite successfully protects the substrate. In the case of CeO2 modified coatings, a high Rct (427.5 Ω cm2) was obtained for coating formulated with 0.6 g L−1 of this additive and a remarkable enhancement in this parameter was found in the case of coating added with 0.3 g L−1 of CeO2–CuO nanocomposite for which the Rct value (3645 Ω cm2) was more than thirty-one times higher than the unmodified coating. This parameter represents the resistance towards charge transfer reactions occurring on the electrode. The high Rct indicates that the defects in the coating are reduced due to refinement of hopeite crystals.25,56 The presence of Warburg elements in the CeO2–CuO nanocomposite added coatings shows that the corrosion reaction is diffusion controlled. From these EIS studies, it can be concluded that 0.3 g L−1 addition of CeO2–CuO nanocomposite forms more corrosion resistant coating.
 | ||
| Fig. 12 Equivalent electrical circuit used to obtain the electrochemical parameters from the Nyquist plots of coatings in 3.5% NaCl. (a) ZnP; (b) ZnP-CO (common for three variations) and (c) ZnP-COCO (common for three variations). Fitted curves are given in Fig. S11, ESI.† | ||
| Coating ID | Nano additive added | Fitting values of equivalent electrical circuit | ||||||
|---|---|---|---|---|---|---|---|---|
| Rs (Ω cm2) | Rc (Ω cm2) | Rct (Ω cm2) | Qc | Cdl × 10−2 F cm−2 | χ2 × 10−3 | |||
| Y × 10−5 (Ω−1 Sn cm−2) | n | |||||||
| ZnP | Nil | 2.98 | 30.82 | 114.3 | 178.9 | 0.63 | 0.629 | 3.44 |
| ZnP-0.3CO | Nano CeO2 | 12.69 | 29.08 | 167.4 | 9.928 | 0.74 | 3.107 | 3.21 |
| ZnP-0.6CO | Nano CeO2 | 8.34 | 35.25 | 427.5 | 5.498 | 0.68 | 0.025 | 0.92 |
| ZnP-0.9CO | Nano CeO2 | 9.62 | 58.47 | 400.2 | 3.510 | 0.73 | 1.337 | 0.75 |
| Coating ID | Nano additive added | Fitting values of equivalent electrical circuit | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Rs (Ω cm2) | Rc (Ω cm2) | Rct (Ω cm2) | Cc × 10−5 F cm−2 | Qdl | W × 10−3 (Ω cm2) | χ2 × 10−3 | |||
| Y × 10−5 (Ω−1 Sn cm−2) | n | ||||||||
| ZnP-0.3COCO | CeO2–CuO nanocomposite | 15.95 | 11.17 | 3645 | 0.10 | 204.8 | 0.73 | 0.53 | 1.40 |
| ZnP-0.6COCO | CeO2–CuO nanocomposite | 25.27 | 43.31 | 2424 | 0.23 | 7.38 | 0.69 | 6.44 | 6.19 |
| ZnP-0.9COCO | CeO2–CuO nanocomposite | 19.35 | 10.73 | 1179 | 0.22 | 12.75 | 0.69 | 4.47 | 3.67 |
 | ||
| Fig. 13 Salt spray test images of coatings formulated without nano additive (top), with nanocrystalline CeO2 (left) and with CeO2–CuO nanocomposite (right). | ||
| Coating ID | Nanoadditive added | Coating weight loss after 48 h (×10−3 g cm−2) | Corrosion rate (CR) (×10−3 mpy) | Inhibition efficiency (%) |
|---|---|---|---|---|
| a Not calculated, since the corrosion rate of the inhibited systems were higher than that of uninhibited systems. | ||||
| ZnP | Nil | 1.32 | 1.86 | No additive |
| ZnP-0.3CO | Nano CeO2 | 1.20 | 1.70 | 8.6 |
| ZnP-0.6CO | Nano CeO2 | 0.81 | 1.14 | 38.7 |
| ZnP-0.9CO | Nano CeO2 | 1.02 | 1.44 | 22.5 |
| ZnP-0.3COCO | CeO2–CuO nanocomposite | 1.19 | 1.68 | 9.6 |
| ZnP-0.6COCO | CeO2–CuO nanocomposite | 2.36 | 3.33 | Not calculateda |
| ZnP-0.9COCO | CeO2–CuO nanocomposite | 2.58 | 3.67 | |
| 3CeO2 + 4H3PO4 → Ce3(PO4)4 + 6H2O | (6) |
| 3CuO + 2H3PO4 → Cu3(PO4)2 + 3H2O | (7) |
The characteristic reflections of these phosphates were not seen in the XRD patterns of the coat. The reason may be (i) the metal oxide additives used were in very low quantity (≈150–600 ppm) and (ii) these phosphates may be in the amorphous phase. Therefore, the cerium and copper phosphates that formed from their oxides have played the role of agents that nucleate the crystallisation and interfere with the crystallization of zinc phosphate into lengthy crystallites by suppressing the diffusion of nutrients for the growth of crystals. This can be supported with FE-SEM results of the coatings in which the formation of zinc phosphate as tiny crystals were evidently seen in large quantities compared with the coating without additive. Obviously, the formation of a dense, compact and uniform crystal layer enhanced the corrosion resistance of the coatings.
4. Conclusions
In this systematic study, nanocrystalline CeO2 and CeO2–CuO nanocomposite were synthesized and their function as additives to enhance corrosion resistance of zinc phosphate coatings on mild steel substrates in 3.5% NaCl solution was evaluated. The XRD patterns of the coatings revealed that the addition of these compounds decreased the iron content (improved the surface coverage) and enhanced the growth of phosphate crystals without affecting the hopeite–phosphophyllite phase composition in the coatings. The XRD pattern and FE-SEM results revealed that these nanometal oxide additives act as nucleation sites and produced compact coating with more phosphate crystals. The SEM micrographs also revealed that both of these additives reduced the cracks that were seen in the coating prepared without the additive. Among the two metal oxides used in three different concentrations, 0.6 g L−1 and 0.3 g L−1 was found to be optimum for nanocrystalline CeO2 and CeO2–CuO nanocomposite, respectively. It was found that (i) the iron content in the coating decreased from 15.4 wt% (ZnP) to 3.18 wt% upon the addition of CeO2 and this value was lower following the addition of CeO2–CuO (1.39 wt%); (ii) the zinc content of the coating increased from 21.3 wt% (ZnP) to 36.29 wt% and 39.75 wt%, respectively following the addition of CeO2 and CeO2–CuO; and (iii) the corrosion rate decreased from 1.843 mm per year (ZnP) to 0.528 mm per year and 0.036 mm per year following the addition of nanocrystalline CeO2 and CeO2–CuO nanocomposite, respectively. Based on the cross-hatch adhesion test, the coating without the additive and the coatings modified with CeO2 were rated as 5B and the coatings formulated with CeO2–CuO composite were rated as 4B. From the results of all the coating characterizations including the accelerated salt-spray test, it was concluded that both of the nanocompounds act as potential corrosion inhibitors, except for the results of the salt-spray test carried out for coatings formulated with higher amounts of CeO2–CuO nanocomposite, and all the results confirmed that the CeO2–CuO nanocomposite showed more potential than nanocrystalline CeO2.Data availability
All the data related to this research work have been included in this article and in the ESI section.†Author contributions
S. Ayesha Barsana: conceptualization, methodology, investigation, visualization, analysis and writing – original draft. M. J. Umapathy: supervision, resources, investigation, methodology, data curation, reviewing and editing. A. Sultan Nasar: methodology, data curation and writing – original draft.Conflicts of interest
The authors declare no conflicts of interest.Acknowledgements
The authors acknowledge the characterisation facilities provided by the Sophisticated Instrumentation Facility, Department of Chemistry, College of Engineering, Guindy, Anna University, India.References
- D. D. Thiruvoth and M. Ananthkumar, Evaluation of cerium oxide nanoparticle coating as corrosion inhibitor for mild steel, Mater. Today: Proc., 2021, 49, 2007–2012 Search PubMed.
- A. Kawsihan, D. M. S. N. Dissanayake, N. P. W. Rathuwadu, H. C. S. Perera, K. E. D. Y. T. Dayananda, K. R. Koswattage, R. Mahadeva, A. Ganguly, G. Das and M. M. M. G. P. G. Mantilaka, Synthesis of an eco-inspired anticorrosive composite for mild steel applications, RSC Adv., 2023, 13, 28852–28860 RSC.
- A. Shukla, B. Sivakumar and S. K. Mishra, Corrosion behavior of Ti-Si-B-C nanocomposite hard coating with different Si contents on 4130 steel, Metall. Mater. Trans. A, 2020, 51, 3576–3586 CrossRef CAS.
- M. Tamilselvi, P. Kamaraj, M. Arthanareeswari and S. Devikala, Nano zinc phosphate coatings for enhanced corrosion resistance of mild steel, Appl. Surf. Sci., 2015, 327, 218–225 CrossRef CAS.
- Y. Xie, M. Chen, D. Xie, L. Zhong and X. Zhang, A fast, low temperature zinc phosphate coating on steel accelerated by graphene oxide, Corros. Sci., 2017, 128, 1–8 CrossRef CAS.
- L. Zhang, X. Tong, J. Lin, Y. Li and C. Wen, Enhanced corrosion resistance via phosphate conversion coating on pure Zn for medical applications, Corros. Sci., 2020, 169, 2–8 Search PubMed.
- J. Liu, B. Zhang, W. H. Qi, Y. G. Deng and R. D. K. Misra, Corrosion response of zinc phosphate conversion coating on steel fibers for concrete applications, J. Mater. Res. Technol., 2020, 9, 5912–5921 CrossRef CAS.
- C. V. Geethanjali, L. Elias, B. I. Bijimol and S. M. A. Shibli, Strategic enhancement of antimicrobial activity of zinc phosphate conversion coatings through effective integration of P-doped MoS2 nanoparticles for marine applications, J. Environ. Chem. Eng., 2024, 12, 114155 CrossRef CAS.
- C. Jiang, X. Zhang, D. Wang, L. Zhang and X. Cheng, Phosphate conversion coatings on 35CrMnSi steels subjected to different heat treatments, Electrochem. Commun., 2020, 110, 106636 CrossRef CAS.
- C. Jiang, G. Xiao, X. Zhang and R. Zhu, Formation and corrosion resistance of a phosphate chemical conversion coating on medium carbon low alloy steel, New J. Chem., 2016, 1347–1353 RSC.
- K. Abdalla, H. Zuhailawati, A. Rahmat and A. Azizan, Characteristics of zinc phosphate coating activated by different concentrations of nickel acetate solution, Metall. Mater. Trans. A, 2017, 48, 771–779 CrossRef CAS.
- H. Y. Su and C. S. Lin, Effect of additives on the properties of phosphate conversion coating on electrogalvanized steel sheet, Corros. Sci., 2014, 83, 137–146 CrossRef CAS.
- N. Van Phuong, K. H. Lee, D. Chang and S. Moon, Effects of Zn2+ concentration and pH on the zinc phosphate conversion coatings on AZ31 magnesium alloy, Corros. Sci., 2013, 74, 314–322 CrossRef.
- B. Herbáth, K. Kovács, M. Jakab and É. Makó, Crystal structure and properties of zinc phosphate layers on aluminum and steel alloy surfaces, Crystals, 2023, 13, 1–15 CrossRef.
- M. Golabadi, M. Aliofkhazraei, M. Toorani and A. S. Rouhaghdam, Corrosion and cathodic disbondment resistance of epoxy coating on zinc phosphate conversion coating containing Ni2+ and Co2+, J. Ind. Eng. Chem., 2017, 47, 154–168 CrossRef CAS.
- J. S. Daubert, G. T. Hill, H. N. Gotsch, A. P. Gremaud, J. S. Ovental, P. S. Williams, C. J. Oldham and G. N. Parsons, Corrosion protection of copper using Al2O3, TiO2, ZnO, HfO2, and ZrO2 atomic layer deposition, ACS Appl. Mater. Interfaces, 2017, 9, 4192–4201 CrossRef CAS PubMed.
- V. Dias, H. Maciel, M. Fraga, A. O. Lobo, R. Pessoa and F. R. Marciano, Atomic layer deposited TiO2 and Al2O3 thin films as coatings for aluminum food packaging application, Materials, 2019, 12, 682 CrossRef CAS PubMed.
- M. Tamilselvi, P. Kamaraj, M. Arthanareeswari, S. Devikala and J. A. Selvi, Development of nano SiO2 incorporated nano zinc phosphate coatings on mild steel, Appl. Surf. Sci., 2015, 332, 12–21 CrossRef CAS.
- M. Tamilselvi, P. Kamaraj, M. Arthanareeswari, S. Devikala and J. Arockiaselvi, Effect of nano ZrO2 on nano zinc phosphating of mild steel, Mater. Today: Proc., 2018, 5, 8880–8888 CAS.
- N. S. Bagal, V. S. Kathavate and P. P. Deshpande, Nano-TiO2 phosphate conversion coatings – A chemical approach, Electrochem. Energy Technol., 2018, 4, 47–54 CAS.
- M. Eskandari, A. Shanaghi, M. Kamani and M. A. Niari, Effect of nano-metal oxides (ZnO, Al2O3, CuO, and TiO2) on the corrosion behavior of a nano-metal oxide/epoxy coating applied on the copper substrate in the acidic environment, Appl. Nanosci., 2021, 11, 1605–1615 CrossRef CAS.
- A. H. Riyas, C. V. Geethanjali, S. Arathy, A. Anil and S. M. A. Shibli, Exploration and tuning of Al2O3/Mo composite for enhancement of anti-corrosion and tribological characteristics in zinc phosphate conversion coatings, Appl. Surf. Sci., 2022, 593, 153370 CrossRef CAS.
- V. A. Bautin, I. V. Bardin, A. R. Kvaratskheliya, S. V. Yashchuk and E. V. Hristoforou, Effect of graphene oxide addition on the anticorrosion properties of the phosphate coatings in neutral and acidic aqueous media, Materials, 2022, 15, 6588 CrossRef CAS PubMed.
- E. Soroush, A. Davarpanah, M. Keramatinia, N. Nouri and B. Ramezanzadeh, Synergistic impact of the functionalized graphene oxide (fGO) nano-sheets and Mn2+-doped zinc phosphate conversion film on the polyester coating corrosion protection properties, Colloids Surf., A, 2023, 678, 132510 CrossRef CAS.
- H. Huang, H. Wang, Y. Xie, D. Dong, X. Jiang and X. Zhang, Incorporation of boron nitride nanosheets in zinc phosphate coatings on mild steel to enhance corrosion resistance, Surf. Coat. Technol., 2019, 374, 935–943 CrossRef CAS.
- P. Jain, B. Patidar and J. Bhawsar, Potential of nanoparticles as a corrosion inhibitor: A review, J. Bio Tribo-Corros., 2020, 6, 43 CrossRef.
- Y. Zhou, J. Xiong and F. Yan, The preparation and characterization of a nano-CeO2/phosphate composite coating on magnesium alloy AZ91D, Surf. Coat. Technol., 2017, 328, 335–343 CrossRef CAS.
- M. J. Deepa, S. R. Arunima and S. M. A. Shibli, Hydrophobic and corrosion-resistant composite (BiVO4/TiO2) hot-dip zinc coating with enhanced self-cleaning ability, J. Alloys Compd., 2022, 924, 166522 CrossRef CAS.
- J. Antonio, C. Mendez, Y. Meas, J. De Jesús and P. Bueno, Cerium and other rare earth salts as corrosion inhibitors – A review, Prot. Met. Phys. Chem. Surf., 2022, 58, 801–810 CrossRef.
- S. Zhang, L. Liu, Q. Lei, T. Zhang, J. Bing and J. Dong, A nano-CeO2/Zn–Mn composite conversion coatings on AZ91D magnesium alloy surface of corrosion resistance research, Coatings, 2023, 13, 929 CrossRef CAS.
- M. Gobara, A. Baraka, R. Akid and M. Zorainy, Corrosion protection mechanism of Ce4+/organic inhibitor for AA2024 in 3.5% NaCl, RSC Adv., 2020, 10, 2227–2240 RSC.
- M. H. Mahmood, S. Suryanto, M. H. F. Al Hazza and F. I. Haidera, Developing of corrosion resistance nano copper oxide coating on copper using anodization in oxalate solution, Int. J. Eng., Trans. B, 2018, 31, 450–455 CAS.
- T. L. Nguyen, T. C. Cheng, J. Y. Yang, C. J. Pan and T. H. Lin, A zinc–manganese composite phosphate conversion coating for corrosion protection of AZ91D alloy: growth and characteristics, J. Mater. Res. Technol., 2022, 19, 2965–2980 CrossRef CAS.
- B. Djuričić and S. Pickering, Nanostructured cerium oxide: Preparation and properties of weakly-agglomerated powders, J. Eur. Ceram. Soc., 1999, 19, 1925–1934 CrossRef.
- K. Castkova, A. Matousek, E. Bartonickova, J. Cihlar, P. Vanysek and J. Cihlar, Sintering of Ce, Sm, and Pr oxide nanorods, J. Am. Ceram. Soc., 2016, 99, 1155–1163 CrossRef CAS.
- A. Nefedova, K. Rausalu, E. Zusinaite, A. Vanetsev, M. Rosenberg, K. Koppel, S. Lilla, M. Visnapuu, K. Smits, V. Kisand, T. Tätte and A. Ivask, Antiviral efficacy of cerium oxide nanoparticles, Sci. Rep., 2022, 12, 1–16 CrossRef PubMed.
- J. Calvache-Muñoz, F. A. Prado and J. E. Rodríguez-Páez, Cerium oxide nanoparticles: Synthesis, characterization and tentative mechanism of particle formation, Colloids Surf., A, 2017, 529, 146–159 CrossRef.
- J. Saranya, B. S. Sreeja, G. Padmalaya, S. Radha and T. Manikandan, Ultrasonic assisted cerium oxide/graphene oxide hybrid: Preparation, anti-proliferative, apoptotic induction and G2/M cell cycle arrest in HeLa cell lines, J. Inorg. Organomet. Polym. Mater., 2020, 30, 2666–2676 CrossRef CAS.
- L. Tan, Q. Tao, H. Gao, J. Li, D. Jia and M. Yang, Preparation and catalytic performance of mesoporous ceria-base composites CuO/CeO2, Fe2O3/CeO2 and La2O3/CeO2, J. Porous Mater., 2017, 24, 795–803 CrossRef CAS.
- N. Rezaee, M. M. Attar and B. Ramezanzadeh, Studying corrosion performance, microstructure and adhesion properties of a room temperature zinc phosphate conversion coating containing Mn2+ on mild steel, Surf. Coat. Technol., 2013, 236, 361–367 CrossRef CAS.
- M. P. Rao, P. Sathishkumar, R. V. Mangalaraja, A. M. Asiri, P. Sivashanmugam and S. Anandan, Simple and low-cost synthesis of CuO nanosheets for visible-light-driven photocatalytic degradation of textile dyes, J. Environ. Chem. Eng., 2018, 6, 2003–2010 CrossRef CAS.
- R. Bortamuly, G. Konwar, P. K. Boruah, M. R. Das, D. Mahanta and P. Saikia, CeO2-PANI-HCl and CeO2-PANI-PTSA composites: synthesis, characterization, and utilization as supercapacitor electrode materials, Ionics, 2020, 26, 5747–5756 CrossRef CAS.
- J. Gong, F. Meng, X. Yang, Z. Fan and H. Li, Controlled hydrothermal synthesis of triangular CeO2 nanosheets and their formation mechanism and optical properties, J. Alloys Compd., 2016, 689, 606–616 CrossRef CAS.
- D. H. Youn, N. M. Tran, B. J. Kim, Y. Kim, J. P. Jeon and H. Yoo, Shape effect of cerium oxide nanoparticles on mild traumatic brain injury, Sci. Rep., 2021, 11, 1–8 CrossRef PubMed.
- C. Wu, M. Yin, S. O. Brien and J. T. Koberstein, Quantitative analysis of copper oxide nanoparticle, Chem. Mater., 2006, 18, 6054–6058 CrossRef CAS.
- J. Mim, M. S. Sultana, P. K. Dhar, M. K. Hasan and S. K. Dutta, Green mediated synthesis of cerium oxide nanoparticles by using Oroxylum indicum for evaluation of catalytic and biomedical activity, RSC Adv., 2024, 14, 25409–25424 RSC.
- A. Adhilakshmi, K. Ravichandran and T. S. N. Sankara Narayanan, Cathodic electrodeposition of zinc-zinc phosphate-calcium phosphate composite coatings on pure iron for biodegradable implant applications, New J. Chem., 2020, 44, 6475–6489 RSC.
- R. Amini, H. Vakili and B. Ramezanzadeh, Studying the effects of poly (vinyl) alcohol on the morphology and anti-corrosion performance of phosphate coating applied on steel surface, J. Taiwan Inst. Chem. Eng., 2016, 58, 542–551 CrossRef CAS.
- R. Zeng, Z. Lan, L. Kong, Y. Huang and H. Cui, Characterization of calcium-modified zinc phosphate conversion coatings and their influences on corrosion resistance of AZ31 alloy, Surf. Coat. Technol., 2011, 205, 3347–3355 CrossRef CAS.
- S. Hu, Y. Fan, M. Muhammad, M. Wang, R. Ma, A. Du, X. Zhao and X. Cao, Corrosion resistance performance of nano-MoS2-containing zinc phosphate coating on Q235 steel, Mater. Lett., 2020, 265, 6–9 Search PubMed.
- T. S. N. S. Narayanan, Surface pretreatment by phosphate conversion coatings – A review, Rev. Adv. Mater. Sci., 2005, 9, 130–177 CAS.
- Y. Zhang and H. Wang, Novel phosphate conversion coating with superior corrosion resistance on the Mg-Al-RE alloy based on the introduction of silane, ACS Omega, 2023, 8, 29374–29387 CrossRef CAS PubMed.
- B. P. Charitha and P. Rao, Pullulan as a potent green inhibitor for corrosion mitigation of aluminum composite: Electrochemical and surface studies, Int. J. Biol. Macromol., 2018, 112, 461–472 CrossRef CAS PubMed.
- G. Lou, P. Jia, X. Teng, J. Zhang, Z. Ye, H. Wu and J. Leng, Nano ZnO-assisted formation of zinc phosphate conversion coating for improving corrosion protection of AZ91D magnesium alloy, Mater. Res. Express, 2019, 6, 086405 CrossRef CAS.
- J. Shang, S. Sun and S. Liu, Corrosion resistance and mechanism of (100), (110) and (111) preferred orientation of single crystal copper in NaCl solution, Int. J. Electrochem. Sci., 2023, 18, 100378 CrossRef.
- Y. Tian, W. Li, Q. Yang, Y. Gong, W. Yue, Y. Ou, C. Li and X. Sheng, PDA@α-ZrP/SiO2 incorporated phosphate coating with enhanced corrosion resistance and friction resistance, RSC Adv., 2024, 14, 24661–24670 RSC.
- M. Akbari, B. S. Boroujeny and M. Raeissi, Cerium oxide nanoparticles as an accelerating agent for zinc phosphate coatings with enhanced corrosion resistance, J. Adv. Mater. Process., 2021, 9, 3–16 Search PubMed.
- X. Sun, D. Susac, R. Li, K. C. Wong, T. Foster and K. A. R. Mitchell, Some observations for effects of copper on zinc phosphate conversion coatings on aluminum surfaces, Surf. Coat. Technol., 2002, 155, 46–50 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02800k |
| This journal is © The Royal Society of Chemistry 2025 |