Open Access Article
Open Access ArticleStructure–activity relationship study of benserazide derivatives as PilB inhibitors†
Joseph E. Quinlan‡
 a,
Ghazal Soleymani‡
a,
Ghazal Soleymani‡ b,
Tori M. Shimozonob,
Zhaomin Yang
b,
Tori M. Shimozonob,
Zhaomin Yang *b and
Webster L. Santos
*b and
Webster L. Santos *a
*a
aDepartment of Chemistry and Virginia Tech Center for Drug Discovery, Virginia Tech, Blacksburg, VA 24060, USA. E-mail: santosw@vt.edu
bDepartment of Biological Sciences Virginia Tech, Blacksburg, VA 24060, USA. E-mail: zmyang@vt.edu
First published on 5th June 2025
Abstract
Antimicrobial resistance is an imminent health threat worldwide. Development of alternative treatments for drug-resistant microbes is of paramount importance. Targeting virulence factors, such as the type IV pilus construction enzyme PilB, is a strategy of treatment. Recently, we reported the discovery of a potent inhibitor of PilB, the FDA approved drug benserazide (IC50 = 3.68 μM). Herein, we report the structure–activity relationship profiling of benserazide analogues and identify key moieties that enable PilB inhibition. We found that bis-hydroxyl groups on the ortho position of the aryl ring, a rigid imine, and exchange of the serine for a thiol have resulted in marked improvement in potency. Our studies identified 11c as a PilB inhibitor with an IC50 of 580 nM and selectivity for PilB over an unrelated ATPase, apyrase. These compounds provide the chemical tools to validate virulence factors as antibacterial mechanisms of action.
Introduction
Infections by antibiotic-resistant pathogens pose a critical public health threat worldwide.1,2 An estimated 4.71 million deaths are associated with antibiotic resistance globally in 2021, with 1.14 million directly caused by drug resistant pathogens.3 By 2050, deaths associated with and due to drug resistant pathogens will nearly double to 8.22 million and 1.91 million, respectively.3 The overuse of antibiotics during the COVID-19 pandemic has exacerbated this problem, particularly with multidrug-resistant pathogens like Acinetobacter baumannii, Klebsiella pneumoniae, and Pseudomonas aeruginosa.1,4 The approval and availability of new antibiotics have inevitably led to the increase in resistance shortly after use in the clinic.5 Repeated exposure of bacteria to antibiotics necessitates the development of drug-resistance mechanisms to enable bacterial cell proliferation. This eventually results in the enrichment and even dominance of resistant bacteria in environments where antibiotics are present. Horizontal Gene Transfer (HTG), a process through which bacteria can transfer drug resistance genes, further accelerates the spread of resistance determinants, presenting a severe threat to public health.6,7 Consequently, it is imperative to develop strategies to combat disease without pressuring bacteria into resistance mechanisms. An emerging strategy is to target virulence factors, the non-vital characteristics of pathogens that enable them to cause disease.8–10Well known virulence factors include two-component systems (TCS), quorum sensing (QS), type III secretion system (T3SS), fimbriae, endotoxin like lipopolysaccharide (LPS), and exotoxin like botulinum.11–14 The notion of targeting virulence factors is not novel and has been shown to be successful. For example, Raxibacumab, Obiltoxaximab, BabyBIG and Botulism Antitoxin Heptavalent are examples of FDA-approved antivirulence biologics.15 There is also some promise for the use of small molecules to target virulence factors. Recently, small molecule inhibitors targeting a response regulator in Staphylococcus aureus, a T3SS, and an inhibitor for biogenesis of a bacterial adhesive pilus have been reported.16–18 However, as of yet, there are no FDA-approved antivirulence small molecules for clinical use.
The bacterial type IV pilus (T4P) is another promising druggable virulence factor.2,19–21 The pilus filament is a flexible and polymeric structure composed of thousands of copies of the pilin protein. These pilins are assembled into the T4P by a hexameric ATPase known as PilB or PilF depending on the bacterial species.20,21 This highly conserved T4P assembly ATPase is present in many high-priority pathogens such as Acinetobacter baumannii, Pseudomonas aeruginosa, and Neisseria meningitidis.22–24 Within pathogens, T4P are dutifully tied to many cell functions, such as bacterial adhesion, biofilm formation, twitching motility and virulence.21,22,24–26 Due to its high conservation and critical role in pathogenesis, T4P has been explored as a possible drug target.2 Some pre-clinical studies demonstrated anti-T4P compounds have therapeutic potential either alone or as an additive to antibiotics.27,28 One study described that phenothazines, T4P targeting small molecules, reduce meningococcal colonization in human vessels, improve humanized mouse survival, and reduce vascular dysfunction and inflammation when used in combination with antibiotics, demonstrating potential benefit beyond antibiotic treatment.
As the T4P assembly ATPase, PilB/PilF is essential for pilus biogenesis. Its absence in bacteria has been shown to eliminate T4P biogenesis, making this ATPase a promising target for the development of antivirulence chemotherapeutics.25,29 A few inhibitors of PilB/F have been reported.19,27,30 A high throughput screening (HTS) based on the inhibition of bacterial attachment to cultured human cells led to the discovery of P4MP4 as a PilB/F inhibitor (Fig. 1).27 It was shown that this compound reduced bacterial adhesion to infected cells by targeting PilF with an IC50 of 175 μM.27 Our previous studies used Chloracidobacterium thermophilum PilB (CtPilB) as a model enzyme in HTS for the discovery of PilB inhibitors. These efforts led to the discovery of quercetin, levodopa and benserazide (Fig. 1), all of which demonstrated to inhibit CtPilB activities in vitro and T4P biogenesis in bacteria.19,30 Among these PilB inhibitors, benserazide was identified as one of the most promising with an IC50 of 3.69 μM against CtPilB.
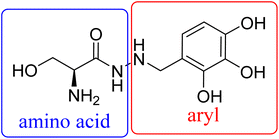
However, little is known about the structure–activity relationship profile of benserazide against PilB. In this study, we performed a medicinal chemistry campaign to develop the pharmacophore and identify key features that promote PilB inhibition. To develop the structure activity relationship, we divided benserazide into two key regions: the amino acid and benzylamine groups linked by a hydrazine and investigated the amino acid composition as well as hydroxy substitution pattern in the aryl ring (Fig. 2). Furthermore, we probed the effect of rigidity by introducing an imine moiety.
Our studies show that changing the substitution pattern in the aryl ring and amino acid composition of benserazide lead to improved activity. In particular, 11c was identified as a compound with significantly improved potency (0.58 μM vs. 3.69 μM) and selectivity against an unrelated ATPase, apyrase.
Results and discussion
Design and synthesis
These compounds were synthesized as shown in Scheme 1. N-Boc-L-serine methyl ester was first protected using 2,2-dimethoxypropane and subsequently reacted with hydrazine to afford intermediates 2 and 3, respectively. Hydrazide 3 was treated with excess hydrochloric acid to afford compound 4 in 99% yield. Alternatively, 3 was condensed with various aldehydes to yield the hydrazone 5, which was isolated via column chromatography. Reduction of the imine was conducted in the presence of sodium borohydride to generate intermediate 6. Subsequent Boc and dimethyl aminal deprotection in acid afforded compounds 7a–g. Alternatively, intermediates 5 can be deprotected under acidic conditions to afford compounds 8a–l with E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratios ranging from 3
Z ratios ranging from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 to 5
7 to 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. For compounds 11a–c, their respective N-Boc amino acid methyl ester 9a–c were treated with hydrazine to provide hydrazide intermediates 9aa–ac and subsequently condensed with 2,6-dihydroxybenzaldehyde to generate intermediates 10a–c after purification by column chromatography. Deprotection using hydrochloric acid afforded final compounds 11a–c with E
1. For compounds 11a–c, their respective N-Boc amino acid methyl ester 9a–c were treated with hydrazine to provide hydrazide intermediates 9aa–ac and subsequently condensed with 2,6-dihydroxybenzaldehyde to generate intermediates 10a–c after purification by column chromatography. Deprotection using hydrochloric acid afforded final compounds 11a–c with E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratios ranging from 3
Z ratios ranging from 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 to 4
2 to 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.
1.
In vitro screen of benserazide analogues
With a library of compounds in hand, we surveyed their activity using a malachite green assay to probe the activity of CtPilB. In this assay, PilB activity is measured by the increase in absorbance at 620 nm as a function of phosphate concentration. Thus, a PilB inhibitor would result in decreases in absorbance. Briefly, CtPilB (3.3 nM), in the presence of ATP (1 mM), and putative inhibitor was incubated at 54 °C for 30 minutes in 96 well plates. The reaction was quenched using 30% TCA, with 10 mM malachite green then added, and absorbance at 620 nm was measured using a SpectraMax M5 microplate reader at room temperature. The results are shown in Table 1. In this assay, compounds were assayed at 3 and 30 μM to provide a dose-dependent assessment of inhibition using benserazide as a benchmark. Removal of the aryl ring in 4 led to a drastic loss in activity. Thus, we investigated mono- and di-hydroxy phenyl substitutions. Ortho, meta, and para analogues 7a–c were essentially inactive although 7a had slight activity at 30 μM. Keeping the ortho-hydroxy group constant and adding a second hydroxyl around the ring (7d–g) revealed that the 2,3- and 2,6-dihydroxy substitutions had equal potency at 3 and 30 μM. Our initial investigations indicated the preference for the 2 and 6 positions of the phenyl ring.| Entry | R | % Inhib. 3 μM | % Inhib. 30 μM |
|---|---|---|---|
| a NA = no activity. | |||
| Benserazide | 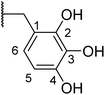 |
34 ± 10 | 80 ± 8 |
| 4 |  |
NA | 27 ± 19 |
| 7a |  |
NA | 32 ± 4 |
| 7b | 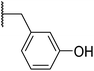 |
NA | NA |
| 7c | 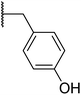 |
NA | NA |
| 7d |  |
19 ± 12 | 78.2 ± 4 |
| 7e | 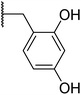 |
NA | 40 ± 2 |
| 7f |  |
NA | 45 ± 6 |
| 7g |  |
32 ± 12 | 74 ± 5 |
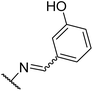
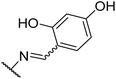
To improve the activity of the benserazide analogues, we investigated the effect of conformational restriction by introduction of a rigid double bond between the hydrazine and benzylic carbon of these derivatives (8a–l, Table 2). Mono-substitutions (8a–c) exhibited a general lack of activity against PilB. Comparing disubstituted compounds 8d–i, an increase in overall inhibitory activity was observed. Among the disubstituted hydroxy analogies, compounds 8f and 8g are more potent than the rest. Due to 8g trending in a more potent direction than 8f, we investigated the effect of the H-donor and steric effect in 8h with a 6-methoxy group; unfortunately, this was less potent. However, the 2-OH substituent appears to be important as a drastic decrease in activity was observed with compound 8i.
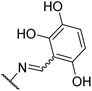
We next investigated the effect of a third hydroxyl group (8j–k). Compound 8j, which is a direct benserazide analogue, exhibited similar inhibitory activity, with 35% and 84% inhibition at 3 and 30 μM, respectively. Exchange of the para hydroxy for a second ortho hydroxy (8k) exhibited high potency against PilB with an inhibition at 3 and 30 μM of 71% and 91%, respectively. To confirm the deleterious effect of methylating the hydroxy moieties, analog 8l was synthesized and essentially had no activity against PilB.
With an understanding of the effect of hydroxy substitutions on the aryl ring, we next investigated the amino acid moiety and synthesized compounds 11a–c (Table 3). Substitution of glycine for serine (11a) had similar activity compared to 8g. Interestingly, addition of a methyl group in alanine had a negative impact on the activity. Exchange of the serine moiety for cysteine, which exchanges an alcohol for a thiol (8g to 11c) caused significant increase in with 90% inhibition at 3 μM.
With the results in hand, we selected the most potent compounds from the series, i.e., 8g, 8k, and 11c for a dose–response curve at concentrations ranging from 0.01–50 μM using benserazide as a control (Fig. 3). Under these conditions, benserazide had an IC50 of 3.68 μM whereas 8g, 8k, and 11c had 1.32, 1.06, and 0.58 μM, respectively. Our results confirm that these analogues are more potent than benserazide in our screen. Indeed, the rank order of potency is 11c > 8k > 8g, with 11c being 6-fold more potent than benserazide.
We next performed an apyrase assay to evaluate the selectivity of our most potent compounds for CtPilB ATPase activity. Each compound was tested at varying concentrations up to 64 μM (approximately 110 fold higher concentration relative to IC50 of 11c) to determine inhibitory effect on apyrase, which serves as a non-specific ATPase control. As shown in Fig. 4, no significant inhibition of apyrase activity at any tested concentration of the compound (p-value > 0.05) was observed. These results indicate that the compounds do not have non-selective ATPase activity, thereby supporting selectivity for CtPilB ATPase.
Each color set represents a biological experiment performed in duplicate. There is no significant difference in apyrase ATPase activity across varying concentrations of (a) benserazide, (b) 8k, and (c) 11c. (p-Values > 0.05 for all comparisons).
Conclusion
This study investigated the underlying structural motifs in benserazide that lead to its potency for PilB inhibition. Our structure–activity relationship study identified key functionalities that led to improved inhibitory activity. In general, we found that trihydroxy > dihydroxy > mono hydroxy substitution in the aryl ring with preference for bis-ortho hydroxy substitutions. In addition, the introduction of a conformationally restricted hydrazone double bond led to significant increase in the activity of compounds. On the amino acid moiety, we found that an exchange of alcohol to a thiol group led to improved potency as exemplified by 11c (IC50 = 0.58 μM). We further demonstrate the lack of activity against an unrelated ATPase apyrase, suggesting selectivity toward PilB. Our studies highlight the potential of targeting PilB and related enzymes as potential anti-virulence factors. These studies are on-going and will be disclosed in due course.Experimental
General chemistry procedures
All chemicals, reagents, and solvents were purchased from commercially available sources as reagent-grade products and used without further purification. Yields reported are for purified products and might not be optimized. Flash silica gel chromatography was performed using SiliaFlash P60 40–63 μm, 60 Å. Thin-layer chromatography was performed to determine the reaction progress utilizing Silicycle aluminum backed silica gel F-254 plates. An Agilent 400 MR 400 MHz or a Varian Inova 400 MHz were used for 1H spectroscopic experiments. A Bruker Avance II 500 MHz was predominately utilized for 13C NMR spectroscopic experiments unless stated otherwise. 1H and 13C NMR spectra are referenced to an internal standard (methanol-d4), and all chemical shifts are reported in δ ppm. NMR spectra characterizations are presented as follows: chemical shift, multiplicity (brs = broad singlet, s = singlet, d = doublet, t = triplet, q = quartet, dd = doublet of doublets, dt = doublet of triplets, dq = doublet of quartets, tt = triplet of triplets, qd = quartet of doublets, and m = multiplet), coupling constants (Hz), and integration. High-resolution mass spectroscopy (HRMS) was performed on a Thermo Electron TSQ triple quadrupole mass spectrometer equipped with an ESI source. Characterization of 8d, 8f, and 8i matches previously reported spectra and E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z ratios of all compounds were determined by analogy to these compounds in 1H NMR.32 All compounds tested are >95% pure by 1H NMR/13C NMR or HPLC, unless otherwise noted.
Z ratios of all compounds were determined by analogy to these compounds in 1H NMR.32 All compounds tested are >95% pure by 1H NMR/13C NMR or HPLC, unless otherwise noted.
ATPase assays and IC50 determination
Two ATPases were used in this study. For the T4P assembly ATPase, the N-terminally truncated CtPilB19,31 was used to analyze the inhibitory activity of newly synthesized compounds in this study. Apyrase,33 an alternative ATPase purchased from Sigma, was used to examine the specificity of PilB inhibitors. The ATPase activity of CtPilB was analyzed as previously described19,31 in a volume of 60 μl with 1 mM of ATP, 0.034% malachite green, 10 mM ammonium molybdate, 1 N HCl, 3.4% ethanol and 3.3 nM of the N-terminally truncated CtPilB hexamer for 30 minutes at 54 °C. The activity of apyrase was analyzed in 20 mM MES (2-(N-morpholino) ethanesulfonic acid), 50 mM NaCl, 5 mM CaCl2, 1 mM DTT (dithiothreitol).33 The reactions were conducted with 6.7 × 10−7 U μl−1 of apyrase and 1 mM of ATP in 60 μl volume at 37 °C for 30 minutes. 30% TCA was used to terminate all enzymatic reactions, which were then followed by the malachite green-based assays as described previously.19,31 To perform this assay, data was analyzed by GraphPad Prism for the calculation of IC50.With the exception of compound 8a, all the compounds in this study were dissolved in DMSO (Thermo fisher) and all enzymatic reactions containing 2% DMSO with or without a test compound. 8a was dissolved in 50% glycerol and all enzymatic reactions with this compound and its controls had a final concentration of glycerol at 2%. Benserazide used in this study was from Tokyo Chemical Industry Co.
Protein expression and purification
The truncated CtPilB variant (without the first 139 residues at the N terminus) were purified as previously described.19,311H NMR (400 MHz, CD3OD) δ 4.15–4.12 (m, 1H), 3.86–3.78 (m, 1H), 3.71–3.63 (M, 1H), 3.42 (brs, 3H), 1.32 (brs, 3H), 1.19 (brs, 6H), 1.08 (brs, 6H). 13C NMR (126 MHz, MeOD) δ 171.7, 151.6, 94.6, 80.2, 65.9, 59.2, 51.5, 27.1, 24.0, 23.1.
1H NMR (400 MHz, CD3OD) δ 4.33–4.21 (m, 1H), 4.18–4.12 (m, 1H), 3.98–3.86 (m, 1H). 1.63 (s, 3H), 1.52–1.38 (m, 12H). 13C NMR (126 MHz, MeOD) δ 172.4, 153.2, 96.1, 81.7, 67.8, 60.3, 28.6, 25.2, 24.6.
1H NMR (400 MHz, DMSO-d6) δ 9.91 (brs, 2H), 8.57 (brs, 3H), 5.63 (brs, 1H), 3.97 (t, J = 4.0 Hz, 1H), 3.86–3.78 (m, 2H). 13C NMR (126 MHz, DMSO) δ 166.3, 60.2, 53.3. HRMS: (ESI) [M + H]+ calc. for C3H10N3O2+ 120.0768, observed 120.0769.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peak denoted with *.
Z mixture of isomers. Major isomer peak denoted with *.1H NMR (400 MHz, CD3OD) δ 8.32* (s, 1H), 8.22 (s, 1H), 7.55–7.48 (m, 1H), 7.45–7.34* (m, 1H), 7.33–7.21* (m, 1H), 6.94–6.83 (m, 2H), 5.21–5.15 (m, 1H), 4.46–4.39 (m, 1H), 4.39–4.32* (m, 1H), 4.25* (t, J = 8.9 Hz, 1H), 4.08–3.95* (m, 1H), 1.66* (brs, 3H), 1.55* (brs, 3H), 1.50* (brs, 3H), 1.40 (brs, 6H). 13C NMR (126 MHz, CD3OD) δ 169.1*, 168.8, 159.4*, 158.4, 153.9*, 153.0*, 151.4, 151.2*,133.0*, 132.9, 132.7, 131.6, 131.5, 129.53, 129.3, 120.8, 120.6, 119.3, 117.7*, 117.2, 96.2*, 95.8, 82.4, 81.9*, 81.4, 67.9*, 67.6, 67.4, 67.3, 60.5*, 59.6, 59.2, 30.7, 28.6*, 26.4, 26.1, 25.7, 25.4, 25.1*, 24.8*, 24.7, 24.6. HRMS: (ESI) [M − H]− calc. for C18H24N3O5− 362.1721, observed 362.1721.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted with *.
Z mixture of isomers. Major isomer peaks denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.06 (s, 1H)*, 7.87 (brs, 1H), 7.34–7.07 (m, 3H)*, 6.88–6.83 (m, 1H)*, 5.33–5.27 (m, 1H), 4.46–4.35 (m, 1H)*, 4.28–4.22 (m, 1H)*, 4.07–3.96 (m, 1H)* 1.68 (brs, 1H)*, 1.67 (brs, 1H), 1.56 (brs, 3H), 1.55 (brs, 3H)*, 1.51 (brs, 3H)*, 1.40 (s, 6H)*. 13C NMR (126 MHz, MeOD) δ 173.5*, 172.9, 169.8, 163.4, 159.0*, 153.9, 153.3*, 153.0, 150.0*, 146.1*, 136.8*, 136.5, 130.9*, 120.7*, 120.1, 119.0, 118.5*, 114.2, 113.7*, 96.2, 96.2*, 95.9, 82.1, 81.8, 81.4*, 67.9, 67.8*, 67.6, 67.5, 61.5, 60.6*, 59.8, 59.4, 28.7, 28.6*, 26.3, 26.1, 25.7, 25.5, 25.2*, 25.1, 24.8, 24.8*. HRMS: (ESI) [M − H]− calc. for C18H24N3O5− 362.1721, observed 362.1717.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 E
2 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer pears denoted with *.
Z mixture of isomers. Major isomer pears denoted with *.1H NMR (400 MHz, (CD3)2CO) δ 8.02 (brs, 1H)*, 7.82 (brs 1H), 7.64–7.47 (m, 2H), 7.52–7.46 (m, 2H), 6.84–6.77 (m, 2H)*, 5.30–5.24 (m, 1H), 4.44–4.30 (m, 1H)*, 4.26–4.19 (m, 1H)*, 4.05–3.94 (m, 1H)*, 1.67–1.64 (m, 3H)*, 1.57–1.52 (m, 3H)*, 1.50 (brs, 3H)*, 1.39 (brs, 6H)*.13C NMR (126 MHz, (CD3)2CO) δ 173.3*, 173.0, 172.8, 169.4*, 161.4, 161.0, 160.9*, 153.9, 153.3*, 153.1, 150.3, 146.4*, 130.6*, 129.9, 126.8, 126.5, 116.6*, 96.2, 96.1*, 95.9, 82.3, 82.1, 81.8, 81.3*, 67.9, 67.8*, 67.7, 61.5, 60.6*, 59.8, 59.4, 28.6*, 26.4, 26.1, 25.8, 25.5, 25.2*, 25.1, 24.8, 24.8*. HRMS: (ESI) [M + Na]+ calc. for C18H26N3O5+ 364.1867, observed 364.1844.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted with *.
Z mixture of isomers. Major isomer peaks denoted with *.1H NMR (400 MHz, (CD3)2CO) δ 8.28 (s, 1H)*, 8.20 (s, 1H), 7.00–6.95 (m, 1H), 6.90–6.82 (m, 2H)*, 6.78–6.72 (m, 1H)*, 5.20–5.14 (m, 1H), 4.46–4.39 (m, 1H), 4.39–4.31 (m, 1H)*, 4.29–4.21 (m, 1H)*, 4.06–3.95 (m, 1H)*, 1.66 (s, 3H)*, 1.54 (s, 3H)*, 1.50 (s, 3H)*, 1.39* (s, 6H)*. 13C NMR (126 MHz, MeOD) δ 171.6, 171.4*, 170.8, 167.7*, 167.4, 152.5, 151.8, 151.6*, 150.3, 150.2*, 146.0*, 145.4*, 145.3, 121.1, 121.0, 119.4, 119.2, 119.1, 117.9, 117.4, 117.4, 116.8, 94.8*, 94.8, 94.5, 94.5, 81.0, 80.5*, 80.1, 66.5*, 66.1, 66.1, 60.1, 59.1*, 58.3, 57.8, 29.3, 27.2*, 25.0, 24.7, 24.3, 24.0, 23.8, 23.7*, 23.4*, 23.3. HRMS: (ESI) [M + H]+ calc. for C18H26N3O6+ 380.1816, observed 380.1824.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted with *.
Z mixture of isomers. Major isomer peaks denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.19 (s, 1H)*, 8.08 (s, 1H), 7.28–7.23 (m, 1H), 7.20–7.13 (m, 1H)*, 6.39–6.29 (m, 2H)*, 5.13–5.08 (m, 1H), 4.43–4.37 (m, 1H), 4.35–4.29 (m, 1H)*, 4.23 (t, J = 8.8 Hz, 1H)*, 4.05–3.95 (m, 1H)*, 1.66 (s, 3H)*, 1.54 (s, 3H)*, 1.50 (s, 3H)*, 1.39 (s, 6H)*. 13C NMR (126 MHz, MeOD) δ 168.7*, 168.5, 162.7*, 162.6, 162.5, 161.4*, 160.4, 153.9, 153.0*, 152.1, 152.0*, 147.7, 133.4, 133.4*, 131.9, 112.2, 111.6*, 109.1, 108.9, 108.9*, 103.8*, 103.6, 96.2, 96.2*, 95.9, 82.4, 81.9*, 81.5, 67.9*, 67.5, 61.5, 60.5*, 59.6, 59.2, 28.7, 28.6, 28.6*, 26.4, 26.1, 25.7, 25.4, 25.2*, 25.1, 24.8*, 24.7, 24.6. HRMS: (ESI) [M + H–C5H9O2]+ calc. for C13H18N3O4+: 280.1292, observed 280.1294. HRMS represents de-BOC structure.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted with *.
Z mixture of isomers. Major isomer peaks denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.25 (s, 1H)*, 8.17 (s, 1H), 7.00–6.96 (m, 1H), 6.88–6.81 (m, 1H)*, 6.79–6.71 (m, 2H)*, 5.22–5.16 (m, 1H), 4.45–4.39 (m, 1H), 4.38–4.32 (m, 1H)*, 4.25 (t, J = 8.5 Hz, 1H)*, 4.06–3.96 (m, 1H)*, 1.66 (s, 3H)*, 1.54 (s, 3H)*, 1.50 (s, 3H)*, 1.40 (s, 6H)*. 13C NMR (126 MHz, MeOD) δ 171.6, 170.9, 167.8*, 167.5, 152.5, 151.9, 151.6, 151.0*, 150.2, 150.0, 149.8*, 149.4, 149.2*, 144.0, 143.8*, 119.6, 119.2*, 119.1, 118.8, 118.7, 118.1, 116.9*, 116.6, 114.8, 114.6*, 112.6, 112.4*, 94.8*, 94.8, 94.5, 94.5, 81.0, 80.8, 80.5*, 80.1, 66.5*, 66.2, 66.1, 66.0, 59.1*, 58.3, 57.9, 29.3, 27.3, 27.2, 27.2*, 24.9, 24.7, 24.3, 24.1, 23.8, 23.7*, 23.4*, 23.3. HRMS: (ESI) [M + H–C5H9O2]+ calc. for C13H18N3O4+: 280.1292, observed 280.1294. HRMS represents de-BOC structure.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted with *.
Z mixture of isomers. Major isomer peaks denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.73 (s, 1H)*, 8.57 (s, 1H), 7.08 (t, J = 9.4 Hz, 1H)*, 6.36 (d, J = 8.1 Hz, 2H)*, 5.06–5.02 (m, 1H), 4.44–4.39 (m, 1H), 4.36–4.31 (m, 1H)*, 4.24 (t, J = 8.9 Hz, 1H)*, 4.05–3.95 (m, 1H)*, 1.66 (s, 3H)*, 1.54 (s, 3H)*, 1.49 (brs, 3H)*, 1.40 (s, 6H)*. 13C NMR (126 MHz, MeOD) δ 167.4*, 167.2, 158.7*, 158.3, 152.5, 151.6*, 147.3*, 145.0, 132.6*, 132.4, 106.2*, 105.9, 94.8*, 94.5, 81.0, 80.5*, 66.5*, 66.1, 60.1, 59.1*, 58.2, 57.7, 29.3, 27.2*, 24.7, 24.3, 23.7*, 23.4*. HRMS: (ESI) [M + H]+ calc. for C18H26N3O6+ 380.1816, observed 380.1818.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted with *.
Z mixture of isomers. Major isomer peaks denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.70 (s, 1H)*, 8.54 (s, 1H), 7.26–7.19 (m, 1H)*, 6.55–6.34 (m, 2H)*, 5.06–5.01 (m, 1H), 4.44–4.39 (m, 1H), 4.36–4.30 (m, 1H)*, 4.24 (t, J = 8.4 Hz, 1H)*, 4.05–3.94 (m, 1H)*, 3.84 (s, 3H)*, 1.66 (s, 3H)*, 1.54 (s, 3H)*, 1.49 (s, 3H)*, 1.39 (s, 6H)*. 13C NMR (126 MHz, MeOD) δ 168.8*, 168.6, 160.9, 160.5*, 153.8, 152.9*, 148.1*, 145.8, 134.0*, 133.9, 110.6*, 110.3, 107.9*, 102.7, 102.3*, 96.2*, 95.9, 82.4, 81.9*, 67.8*, 67.5, 60.5*, 56.4*, 28.6*, 26.1, 25.7, 25.1*, 24.8*. HRMS: (ESI) [M + H–C5H9O2]+ calc. for C14H20N3O4+: 294.1448, observed 294.1438. HRMS represents de-BOC structure.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 E
2 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted with *.
Z mixture of isomers. Major isomer peaks denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 7.96 (s, 1H)*, 7.79 (s, 1H), 7.34–7.30 (m, 1H)*, 7.20–7.18 (m, 1H), 7.04–7.00 (m, 1H)*, 6.96–6.93 (m, 1H), 6.83–6.77 (m, 1H)*, 5.31–5.25 (m, 1H), 4.44–4.33 (m, 1H)*, 4.29–4.23 (m, 1H)*, 4.09–3.98 (m, 1H)*, 1.69 (s, 3H)*, 1.68 (s, 3H), 1.58 (s, 3H), 1.57 (s, 3H)*, 1.53 (s, 3H), 1.52 (s, 3H)*, 1.42 (s, 6H)*. 13C NMR (126 MHz, MeOD) δ 173.3*, 172.7, 169.4*, 169.2, 153.9, 153.4*, 153.1, 150.4*, 149.7, 149.3, 149.2, 146.9*, 146.6, 146.6, 127.4*, 127.1, 122.7*, 121.9, 116.2*, 114.0*, 113.5, 96.2, 96.1*, 95.9, 82.3, 82.1, 81.8, 81.4*, 67.9, 67.9*, 67.7, 67.5, 60.5*, 59.8, 59.4, 28.6*, 26.3, 26.1, 25.8, 25.5, 25.2*, 25.1, 24.8, 24.8*. HRMS: (ESI) [M + H–C5H9O2]+ calc. for C13H18N3O4+: 280.1292, observed 280.1279. HRMS represents de-BOC structure.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted with *.
Z mixture of isomers. Major isomer peaks denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.15 (s, 1H)*, 8.04 (s, 1H), 6.74–6.67 (m, 1H)*, 6.43–6.38 (m, 1H)*, 5.13–5.08 (m, 1H), 4.44–4.30 (m, 1H)*, 4.24 (t, J = 8.7 Hz, 1H)*, 4.05–3.95 (m, 1H)*, 1.66 (s, 3H)*, 1.54 (s, 3H)*, 1.50 (s, 3H)*, 1.39 (s, 6H)*. 13C NMR (126 MHz, MeOD) δ 173.0*, 172.3, 168.7*, 153.8, 153.0, 152.7*, 150.2*, 149.0, 148.5*, 147.8, 133.9*, 123.3*, 122.4, 112.5, 112.0*, 108.9, 108.7*, 96.2*, 95.9, 82.4, 81.9*, 67.9*, 67.5, 67.4, 67.2, 61.5, 60.5*, 59.6, 59.1, 30.7, 28.6*, 26.1, 25.7, 25.1*, 24.8*. HRMS: (ESI) [M + H]+ calc. for C18H26N3O7+: 396.1765, observed 396.1763.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted with *.
Z mixture of isomers. Major isomer peaks denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.70 (s, 1H)*, 8.55 (s, 1H), 6.73 (d, J = 8.7 Hz, 1H)*, 6.20 (d, J = 8.6 Hz, 1H)*, 4.34 (t, J = 6.5 Hz, 1H), 4.24 (t, J = 9.4 Hz, 1H)*, 4.06–3.95 (m, 2H)*, 1.55 (brs, 3H)*, 1.50 (brs, 4H)*, 1.45 (brs, 2H), 1.40 (brs, 6H)*. 13C NMR (126 MHz, MeOD) δ 168.8*, 168.6, 153.0, 152.3*, 149.0*, 147.7, 138.7*, 120.1*, 120.0, 107.7, 106.0*, 96.2*, 95.9, 82.4, 81.9*, 67.9*, 67.5, 60.5*, 28.6*, 26.1, 25.7, 25.1*, 24.8*. HRMS: (ESI) [M − H]− calc. for C18H24N3O7+: 394.1620, observed 394.1618.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 E
2 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted with *.
Z mixture of isomers. Major isomer peaks denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.39 (s, 1H)*, 8.19 (s, 1H), 7.82 (m, 1H)*, 7.59 (1H), 6.87 (m, 1H)*, 5.32–5.27 (m, 1H), 4.43–4.33 (m, 1H)*, 4.28–4.24 (m, 1H)*, 4.08–3.83 (m, 11H)*, 1.69 (s, 3H)*, 1.68 (s, 3H), 1.58 (s, 3H), 1.57 (s, 3H)*, 1.53 (s, 3H), 1.52 (s, 3H)*, 1.42 (s, 6H)*. 13C NMR (126 MHz, MeOD) δ 173.4*, 172.8, 169.5*, 169.2, 157.5*, 157.4, 157.1, 157.0, 154.6*, 154.4, 153.9, 153.3, 153.0, 145.7*, 145.6, 143.2, 143.1*, 142.0*, 122.9*, 122.1, 122.0, 121.6, 121.2, 109.5, 109.4*, 96.2, 96.1*, 95.9, 82.3, 82.1, 81.8*, 81.3, 67.9*, 67.8, 67.7, 67.5, 62.4, 62.4, 61.3*, 60.6, 59.8, 59.3, 56.6*, 28.7, 28.6*, 26.3, 26.1, 25.8, 25.5, 25.2, 25.1*, 24.8*. HRMS: (ESI) [M + H]+ calc. for C21H32N3O7+: 438.2235, observed 438.2230.
1H NMR (500 MHz, (CD3)2CO) δ 7.16–7.07 (m, 2H), 6.81–6.73 (m, 2H), 4.31–4.17 (m, 1H), 4.12–4.05 (m, 1H), 4.05–3.94 (m, 1H), 1.60 (brs, 3H), 1.48 (brs, 6H), 1.40 (brs, 3H). 13C NMR (126 MHz, MeOD) δ 171.4, 157.6, 153.2, 131.5, 130.0, 124.2, 120.4, 116.4, 96.1, 81.8, 67.9, 60.2, 52.8, 28.6, 25.2, 24.5. HRMS: (ESI) [M + H]+ calc. for C18H26N3O5−: 364.1878, observed 364.1780.
1H NMR (500 MHz, (CD3OD)) δ 6.75–6.68 (m, 1H), 6.66–6.57 (m, 2H), 4.31–3.75 (m, 5H), 1.61 (brs, 3H), 1.43 (brs, 6H), 1.40 (brs, 6H). 13C NMR (126 MHz, MeOD) δ 171.8*, 158.7*, 154.1, 154.0, 153.5, 153.2*, 140.2*, 130.5*, 121.2, 121.0*, 117.0, 116.7*, 115.5*, 96.1*, 95.7, 94.9, 94.7, 82.3, 81.9, 81.7*, 81.2, 67.9*, 67.3, 66.1*, 65.8, 62.3*, 62.0, 60.2*, 59.8, 56.4*, 56.2, 28.7, 28.6*, 27.8, 27.0, 25.2*, 24.5*, 23.2. HRMS: (ESI) [M + H]+ calc. for C18H26N3O5−: 364.1878, observed 364.1781.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with rotamers.
1 with rotamers.1H NMR (400 MHz, (CD3OD)) δ 7.19 (d, J = 8.1 Hz, 2H), 6.78 (d, J = 8.7 Hz, 2H), 4.33–4.20 (m, 1H), 4.14–4.07 (m, 1H), 3.92–3.75 (m, 3H), 1.67–1.58 (m, 3H), 1.54–1.46 (m, 6H), 1.45–1.37 (m, 6H). 13C NMR (126 MHz, MeOD) δ 171.6*, 158.1*, 153.1*, 131.4*, 129.2*, 116.1*, 96.0*, 95.7, 82.3, 81.7*, 67.9*, 67.3, 60.2*, 56.0*, 55.7, 28.6*, 26.4, 25.3*, 24.5*. HRMS: (ESI) [M + H]+ calc. for C18H28N3O5+: 366.2023, observed 366.2022.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with rotamers.
1 with rotamers.1H NMR (400 MHz, (CD3OD)) δ 6.76–6.68 (m, 1H), 6.67–6.59 (m, 2H), 4.30–4.16 (m, 1H), 4.13–3.90 (m, 3H), 3.91–3.75 (m, 1H), 1.61 (s, 3H), 1.48 (s, 6H), 1.41 (s, 6H). 13C NMR (126 MHz, MeOD) δ 176.3, 175.8, 171.6*, 171.5, 153.9*, 153.7, 153.3, 153.2*, 146.4*, 146.3, 145.9, 145.6*, 145.5, 144.4, 124.4*, 122.0*, 120.4*, 115.9*, 96.1*, 95.8, 95.7, 95.6, 82.3, 81.9, 81.8*, 81.2, 67.9, 67.8*, 67.5, 67.3, 60.2*, 52.9*, 28.6*, 26.4, 26.2, 25.6, 25.3, 25.1, 24.7, 24.5. HRMS: (ESI) [M + H]+ calc. for C18H28N3O6+: 382.1973, observed 382.1974.
1H NMR (400 MHz, (CD3OD)) δ 6.91 (d, J = 8.2 Hz, 1H), 6.30 (d, J = 2,4 Hz, 1H), 6.24 (dd, J = 2.3 Hz, 8.1 Hz, 1H), 4.33–4.18 (m, 1H), 4.15–4.04 (m, 1H), 3.97–3.75 (m, 3H), 1.64–1.56 (m, 3H), 1.51–1.44 (m, 6H), 1.43–1.37 (m, 6H). 13C NMR (126 MHz, MeOD) δ 170.0, 158.1, 157.2, 151.8, 130.9, 113.9, 106.0, 102.3, 94.7, 80.4, 66.5, 58.9, 51.1, 27.2, 23.9, 23.1. HRMS: (ESI) [M + H]+ calc. for C18H28N3O6+: 382.1973, observed 382.1965.
1H NMR (400 MHz, (CD3OD)) δ 6.64–6.60 (m, 2H), 6.58–6.54 (m, 1H), 4.30–4.17 (m, 1H), 4.13–4.07 (m, 1H), 3.98–3.76 (m, 3H), 1.65–1.56 (m, 3H), 1.49 (s, 6H), 1.40 (s, 6H). 13C NMR (126 MHz, MeOD) δ 171.5*, 153.2*, 151.0*, 150.4*, 124.9*, 117.9*, 117.1*, 116.2*, 96.1*, 95.8, 82.4, 81.8*, 67.9*, 67.4, 60.2*, 52.9*, 28.6*, 26.4, 25.3, 25.2*, 24.5*. HRMS: (ESI) [M − H]− calc. for C18H26N3O6−: 380.1827, observed 380.1813.
1H NMR (400 MHz, (CD3OD)) δ 6.92 (t, J = 7.9 Hz, 1H), 6.33 (d, J = 7.9 Hz, 2H), 4.32–4.20 (m, 1H), 4.15–4.05 (m, 3H), 3.94–3.80 (m, 1H), 1.63–1.59 (m, 3H), 1.52–1.47 (m, 6H), 1.44–1.41 (m, 6H). 13C NMR (126 MHz, MeOD) δ 171.0*, 158.5*, 153.2, 129.8*, 110.9*, 107.6*, 96.0*, 95.7, 82.3, 81.8*, 67.8*, 67.3, 60.2*, 45.9*, 28.6*, 26.3, 25.4, 25.2*, 24.6*. HRMS: (ESI) [M + H]+ calc. for C13H20N3O4+: 282.1448, observed 282.1422.
1H NMR (400 MHz, (CD3OD)) δ 7.36–7.27 (m, 2H)*, 6.96–6.87 (m, 2H)*, 4.18 (s, 2H)*, 4.08–4.01 (m, 1H), 4.01–3.94 (m, 1H)*, 3.91–3.82 (m, 1H)*, 3.79–3.72 (m, 1H)*, 3.70–3.62 (m, 1H). 13C NMR (126 MHz, CDCl3) δ 167.1, 157.6, 132.4, 131.7, 131.2, 130.2, 121.2, 120.7, 116.8, 116.4, 61.5, 60.1, 55.1, 52.3. *. HRMS: (ESI) [M + H]+ calc. for C10H16N3O3+: 226.1186, observed 226.1181.
1H NMR (400 MHz, (CD3OD)) δ 7.30 (t, J = 9.0 Hz, 1H), 7.02–6.98 (m, 2H), 6.93–6.89 (m, 1H), 4.40 (dd, J = 12.3, 17.1 Hz, 2H), 4.15–4.12 (m, 1H), 3.96–3.91 (m, 1H), 3.89–3.84 (m, 1H). 13C NMR (126 MHz, MeOD) δ 165.8, 157.9, 130.4, 130.0, 121.3, 117.2, 116.6, 59.8, 54.3, 53.8. HRMS: (ESI) [M + H]+ calc. for C10H16N3O3+: 226.1186, observed 226.1107.
1H NMR (400 MHz, (CD3OD)) δ 7.36–7.30 (m, 2H), 6.86–6.81 (m, 2H), 4.32–4.24 (m, 2H), 4.09–4.05 (m, 1H), 3.89 (d, J = 4.25 Hz, 1H), 3.85 (d, J = 5.8 Hz, 1H).13C NMR (126 MHz, MeOD) δ 167.1, 160.1, 133.3, 121.9, 116.8, 66.9, 61.3, 55.4, 55.1. HRMS: (ESI) [M + H]+ calc. for C10H16N3O3+: 226.1186, observed 226.1188.
1H NMR (400 MHz, (CD3OD)) δ 6.87–6.83 (m, 1H), 6.79–6.75 (m, 1H), 6.74–6.68 (m, 1H), 4.39–4.29 (m, 2H), 4.03–3.98 (m, 1H), 3.87 (d, J = 4.4 Hz, 1H), 3.81 (d, J = 6.2 Hz, 1H). 13C NMR (126 MHz, MeOD) δ 167.0, 146.5, 146.3, 123.2, 120.8, 118.9, 117.5, 61.3, 55.2, 51.8. HRMS: (ESI) [M + H]+ calc. for C10H14N3O4+: 242.1135, observed 242.1135.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with rotamers. Major peak denoted with *.
1 with rotamers. Major peak denoted with *.1H NMR (400 MHz, (CD3OD)) δ 7.01–6.73 (m, 1H), 6.45–6.17 (m, 2H), 4.20–4.06 (m, 1H), 5.02–3.85 (m, 2H), 3.85–3.56 (m, 2H). 13C NMR (126 MHz, MeOD) δ 167.7, 166.9, 161.3, 159.0, 134.0, 120.5, 109.2, 108.1, 103.4, 68.0, 66.9, 61.3, 55.1, 51.7. 13C NMR (126 MHz, MeOD) δ 167.7*, 166.9*, 161.3*, 159.0*, 134.0*, 120.5, 109.2, 108.1*, 103.4*, 68.0*, 66.9, 61.3*, 55.1*, 51.7. HRMS: (ESI) [M + H]+ calc. for C10H16N3O4+: 242.1135, observed 242.1138.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 with rotamers. Major peak denoted with *.
1 with rotamers. Major peak denoted with *.1H NMR (400 MHz, (CD3OD)) δ 6.80–6.74 (m, 3H)*, 4.36 (s, 2H)*, 4.18–4.14 (m, 1H)*, 4.09–4.06 (m, 1H)*, 4.01–3.96 (m, 1H), 3.96–3.89 (m, 1H)*, 3.86–3.81 (m, 1H). 13C NMR (126 MHz, MeOD) δ 167.7, 167.0, 151.5, 150.7, 119.3, 119.0, 117.1, 61.2, 55.2, 52.0. HRMS: (ESI) [M + H]+ calc. for C10H16N3O4+: 242.1135, observed 242.1128.
1H NMR (400 MHz, (CD3OD)) δ 7.08 (t, J = 8.9 Hz, 1H), 6.47–6.34 (m, 2H), 4.59–4.47 (m, 1H), 4.31–4.05 (m, 2H), 3.96–3.82 (m, 2H). 13C NMR (126 MHz, MeOD) δ 167.4, 166.8, 159.0, 156.4, 132.1, 107.8, 107.5, 61.4, 55.1, 54.8. HRMS: (ESI) [M + H]+ calc. for C10H16N3O4+: 242.1135, observed 242.1138.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 E
3 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks designated with *.
Z mixture of isomers. Major isomer peaks designated with *.1H NMR (400 MHz, (CD3OD)) δ 8.45 (s, 1H)*, 8.36 (1H), 7.69–7.65 (m, 1H), 7.46–7.42 (m, 1H)*, 7.33–7.23 (m, 1H)*, 6.93–6.85 (m, 2H)*, 4.74 (q, J = 3.7 Hz, 1H), 4.18–4.09 (m, 1H)*, 4.04–3.92 (m, 2H)*. 13C NMR (126 MHz, MeOD) δ 168.8*, 167.7, 164.7*, 159.4*, 158.4, 152.1*, 146.0, 133.2*, 133.0, 131.6*, 128.4, 120.8, 120.7*, 119.2, 117.6*, 117.1, 61.6*, 61.2, 60.5, 55.6*, 55.4, 55.1. HRMS: (ESI) [M + H]+ calc. for C10H14N3O3+: 224.1030, observed 224.1023.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 E
7 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. E isomer denoted with *.
Z mixture of isomers. E isomer denoted with *.1H NMR (400 MHz, (CD3OD)) δ 8.14 (s, 1H), 7.92 (s, 1H)*, 7.26–7.11 (m, 3H)*, 6.88–6.84 (s, 1H)*, 4.75 (q, J = 3.4 Hz1H)*, 4.15–4.09 (m, 1H), 3.97–3.89 (m, 2H)*. 13C NMR (126 MHz, MeOD) δ 169.1, 167.7*, 159.1*, 151.3, 147.7*, 136.4*, 136.2, 131.0*, 120.8, 120.1*, 119.2, 118.8*, 114.5, 114.2*, 61.6, 61.2*, 60.6, 55.6, 55.5*, 55.1. HRMS: (ESI) [M + H]+ calc. for C10H14N3O3+: 224.1030, observed 224.1033.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 7 E
7 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. E isomer denoted with *.
Z mixture of isomers. E isomer denoted with *.1H NMR (400 MHz, (CD3OD)) δ 8.14 (s, 1H), 7.92 (s, 1H)*, 7.66–7.61 (m, 2H), 7.58–7.53 (m, 2H)*, 6.86–6.80 (m, 2H)*, 4.76 (q, J = 3.6 Hz, 1H)*, 4.18–4.11 (m, 1H), 4.03–3.89 (m, 2H)*. 13C NMR (126 MHz, MeOD) δ 168.8*, 167.7, 165.1, 161.6, 161.3*, 151.5, 147.9*, 130.8, 130.1*, 126.4*, 126.2, 116.7*, 61.7, 61.2, 60.6*, 55.6, 55.5*, 55.1. HRMS: (ESI) [M + H]+ calc. for C10H14N3O3+: 224.1030, observed 224.1033.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 E
3 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer denoted with *. Compound spectra matches previously reported spectra.32
Z mixture of isomers. Major isomer denoted with *. Compound spectra matches previously reported spectra.321H NMR (400 MHz, (CD3OD)) δ 8.42 (s, 1H)*, 8.34 (s, 1H), 7.14–7.11 (m, 1H), 6.93–6.84 (m, 2H)*, 6.79–6.69 (m, 1H)*, 4.76–4.72 (m, 1H)*, 4.66–4.63 (m, 1H), 4.22–4.17 (m, 1H), 4.15–4.09 (m, 1H)*, 4.06–3.78 (m, 3H)*. HRMS: (ESI) [M + H]+ calc. for C10H14N3O3+: 240.0979, observed 240.0983.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peak denoted with *.
Z mixture of isomers. Major isomer peak denoted with *.1H NMR (400 MHz, (CD3OD) δ 8.40 (s, 1H)*, 8.21 (s, 1H), 7.45 (d, J = 8.3 Hz, 1H), 7.33 (d, J = 8.6 Hz, 1H)*, 6.45–6.41-m, 1H)*, 6.38–6.34 (m, 1H)*, 6.33 (d, J = 3.0 Hz, 1H), 4.69 (q, J = 3.3 Hz,1H), 4.19–4.14 (m, 1H), 4.13–4.07 (m, 1H)*, 4.04–3.89 (m, 2H)*. 13C NMR (126 MHz, MeOD) δ 168.3, 167.6*, 164.7*, 164.5, 162.7, 162.4*, 160.2, 160.1, 154.9*, 147.5*, 134.5*, 130.6, 112.5, 110.7, 109.6*, 109.1, 103.7*, 103.4, 61.6*, 61.2, 60.7, 60.4, 55.5*, 55.3, 55.1. HRMS: (ESI) [M + H]+ calc. for C10H14N3O3+: 240.0979, observed 240.0975.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer denoted with *. Spectra matches previously reported spectra.32
Z mixture of isomers. Major isomer denoted with *. Spectra matches previously reported spectra.321H NMR (400 MHz, (CD3OD)) δ 8.33 (s, 1H)*, 8.29 (s, 1H), 7.11–7.08 (m, 1H), 6.91–6.88 (m, 1H)*, 6.81–6.68 (m, 2H)*, 4.74–4.69 (m, 1H), 4.16–3.87 (m, 3H)*. 13C NMR (126 MHz, MeOD) δ 168.8*, 164.7, 152.5*, 151.7, 151.3*, 151.3, 145.6*, 121.2, 120.9*, 120.5*, 119.4, 118.3*, 117.9, 115.9*, 113.0, 61.5*, 61.2, 60.5, 55.6*, 55.4, 55.1. HRMS: (ESI) [M + H]+ calc. for C10H14N3O3+: 240.0979, observed 240.0966.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5 E
1.5 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer denoted with *.
Z mixture of isomers. Major isomer denoted with *.1H NMR (400 MHz, (CD3OD)) δ 8.83 (s, 1H)*, 8.63 (s, 1H), 7.12 (t, J = 8.5 Hz, 1H)*, 6.38–6.34 (m, 2H)*, 4.64–4.59 (m, 1H), 4.20–4.15 (m, 1H), 4.14–4.09 (m, 1H)*, 4.05–3.92 (m, 2H)*. 13C NMR (126 MHz, MeOD) δ 167.9, 167.6*, 164.4*, 162.4, 160.2*, 159.7, 149.9*, 147.7, 134.4*, 133.9, 107.0*, 61.6*, 61.2, 60.6, 60.2, 56.0, 55.6, 55.6*, 55.1. HRMS: (ESI) [M + H]+ calc. for C10H14N3O3+: 240.0979, observed 240.0965.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer denoted with *.
Z mixture of isomers. Major isomer denoted with *.1H NMR (400 MHz, (CD3OD)) δ 8.78 (s, 1H)*, 8.59 (s, 1H), 7.28–7.21 (m, 1H)*, 6.55–6.46 (m, 2H)*, 4.64–4.60 (m, 1H), 4.12–4.07 (m, 1H)*, 4.06–3.91 (m, 2H)*, 3.88–3.83 (m, 3H). 13C NMR (126 MHz, MeOD) δ 168.0, 167.8*, 164.4*, 161.0, 160.6*, 148.9*, 146.9, 134.2*, 110.6*, 110.3, 108.0, 107.8*, 102.9, 102.4*, 61.6*, 61.5, 60.2, 56.4*, 55.6*, 55.1. HRMS: (ESI) [M + H]+ calc. for C11H16N3O4+: 254.1135, observed 254.1132.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. E isomer denoted with *. Spectra matches previously reported spectra.32
Z mixture of isomers. E isomer denoted with *. Spectra matches previously reported spectra.321H NMR (400 MHz, (CD3OD)) δ 8.10 (1H)*, 7.86 (1H), 7.28 (d, J = 1.9 Hz, 1H)*, 7.22 (d, J = 2.2 Hz, 1H), 7.04 (dd, J = 2.4, 8.6 Hz, 1H)*, 6.98 (dd, J = 2.3, 8.1 Hz, 1H), 6.80 (d, J = 3.0 Hz, 1H)*, 6.79 (d, J = 2.9 Hz, 1H), 4.78–4.74 (m, 1H), 4.17–4.10 (m, 1H)*, 4.08–3.91 (m, 3H)*. HRMS: (ESI) [M + H]+ calc. for C10H14N3O3+: 240.0979, observed 240.0979.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer denoted with *. Spectra matches previously reported spectra.32
Z mixture of isomers. Major isomer denoted with *. Spectra matches previously reported spectra.321H NMR (400 MHz, (CD3OD)) δ 8.27 (s, 1H)*, 8.15 (s, 1H), 6.89 (d, J = 9.2 Hz, 1H), 6.73 (d, J = 8.6 Hz, 1H)*, 6.44 (d, J = 9.2 Hz, 1H)*, 4.70–4.67 (m, 1H), 4.13–4.08 (m, 1H)*, 4.06–3.91 (m, 2H)*. HRMS: (ESI) [M + H]+ calc. for C10H14N3O5+: 256.0928, observed 256.0935.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer peaks denoted by *.
Z mixture of isomers. Major isomer peaks denoted by *.1H NMR (400 MHz, (CD3OD)) δ 8.76 (s, 1H)*, 8.61 (s, 1H), 6.76–6.71 (m, 1H)*, 6.24 (d, J = 9.0 Hz, 1H), 6.21 (d, J = 8.5 Hz, 1H)*, 4.64–4.59 (m, 1H), 4.05–4.01 (m, 1H)*, 4.00–3.90 (m, 2H)*. 13C NMR (126 MHz, MeOD) δ 168.0, 164.3*, 152.4*, 152.2, 149.7*, 147.9, 147.8*, 138.7*, 120.4*, 107.7, 107.6*, 106.3, 106.0*, 61.9, 61.6*, 60.6, 60.2, 55.6*, 55.5, 55.2. HRMS: (ESI) [M + H]+ calc. for C10H14N3O5+: 256.0928, observed 256.0924.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 E
2 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. E isomer is denoted with *.
Z mixture of isomers. E isomer is denoted with *.1H NMR (400 MHz, (CD3OD)) δ 8.46 (s, 1H)* 8.23 (s, 1H), 7.76, (d, J = 9.62, 1H)*, 7.61 (d, J = 9.4 Hz, 1H), 6.85 (d, J = 9.6 Hz, 1H)*, 4.77–4.73 (m, 1H), 4.14–3.80 (m, 13H). 13C NMR (126 MHz, MeOD) δ 168.9*, 165.2*, 157.7*, 157.4, 154.8, 154.6*, 146.9*, 143.5*, 143.2, 122.9, 122.2*, 121.2*, 121.0, 109.5*, 62.5, 62.4*, 61.6, 61.6*, 61.3*, 60.6, 56.6, 55.6*, 55.5*, 55.2. HRMS: (ESI) [M + H]+ calc. for C13H20N3O5+: 298.1397, observed 298.1396.
1H NMR (400 MHz, (CD3OD)) δ 3.68 (s, 2H), 1.44 (s, 9H). 13C NMR (126 MHz, MeOD) δ 170.4, 157.0, 79.3, 41.9, 27.3. HRMS: (ESI) [M + Na]+ calc. for C7H15N3NaO5+: 212.1011, observed 212.1009.
1H NMR (400 MHz, (CD3OD)) δ 4.06 (q, J = 7.0 Hz, 1H), 1.43 (s, 9H), 1.27 (d, J = 7.5 Hz, 3H). 13C NMR (126 MHz, MeOD) δ 175.0*, 157.5*, 152.9, 80.5*, 50.3*, 28.7*, 24.6, 18.5*, 15.5. HRMS: (ESI) [M + Na]+ calc. for C8H17N3NaO5+: 226.1168, observed 226.1161.
1H NMR (400 MHz, (CD3OD)) δ 4.14 (s, 1H), 2.86–2.68 (m, 2H), 1.44 (s, 9H). 13C NMR (126 MHz, MeOD) δ 170.8, 156.2, 79.5, 55.8, 27.3, 25.8. HRMS: (ESI) [M − H]− calc. for C8H16N3O3S: 234.0918, observed 234.0913.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer denoted with *.
Z mixture of isomers. Major isomer denoted with *.1H NMR (400 MHz, (CD3OD)) δ 8.75 (s, 1H)*, 8.58 (s, 1H), 7.11–7.06 (m, 1H)*, 6.39–6.32 (m, 2H)*, 4.17 (s, 2H), 3.84 (s, 2H)*, 1.48 (s, 9H). 13C NMR (126 MHz, MeOD) δ 171.6, 168.2*, 160.1*, 159.7, 158.5, 148.5*, 146.1, 133.7*, 107.6*, 80.8*, 61.5*, 43.4*, 42.6, 28.7*. HRMS: (ESI) [M + H]+ calc. for C14H20N3O5+: 310.1397, observed 310.1395.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer denoted with *.
Z mixture of isomers. Major isomer denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.77 (s, 1H)*, 8.61 (s, 1H), 7.08 (t, J = 8.3 Hz, 1H)*, 6.36 (d, J = 8.9 Hz, 2H), 4.18 (q, J = 6.9 Hz, 1H)*, 1.46 (s, 9H)*, 1.40 (d, J = 7.6 Hz, 3H)*. 13C NMR (126 MHz, MeOD) δ 173.0, 171.7*, 161.1, 160.0*, 159.7, 157.7, 148.7*, 146.4, 135.3, 133.8*, 107.6*, 80.7*, 61.5*, 50.6*, 28.7*, 20.9, 18.4*, 17.2, 14.5*. HRMS: (ESI) [M + H]+ calc. for C15H22N3O5+: 324.1554, observed 324.1553.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer denoted with *.
Z mixture of isomers. Major isomer denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.77 (s, 1H)*, 8.59 (s, 1H), 7.06 (t, J = 7.6 Hz, 1H)*, 6.34 (d, J = 8.0 Hz, 2H)*, 4.26 (s, 1H)*, 2.93–2.76 (m, 2H)*, 1.44 (s, 9H)*. 13C NMR (126 MHz, MeOD) δ 173.0, 168.8*, 160.0*, 157.6, 149.2*, 133.9*, 107.6*, 107.3, 81.0*, 61.5*, 57.4*, 28.6*, 27.0, 20.9*, 14.4*. *. HRMS: (ESI) [M − H]− calc. for C15H20N3O5S−: 354.1129, observed 354.1124.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer denoted with *.
Z mixture of isomers. Major isomer denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.74 (s, 1H)*, 8.62 (s, 1H), 7.10 (t, J = 8.5 Hz, 1H)*, 6.38–6.32 (m, 2H)*, 4.16* (s, 1H)*, 3.83 (s, 2H)*. 13C NMR (126 MHz, MeOD) δ 167.5*, 163.4*, 160.2*, 159.8, 149.2*, 147.3, 134.3*, 134.1*, 107.6*, 40.9, 40.7*. HRMS: (ESI) [M + H]+ calc. for C9H12N3O3+: 210.0873, observed 210.0870.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major isomer denoted with *.
Z mixture of isomers. Major isomer denoted with *.1H NMR (500 MHz, (CD3)2CO) δ 8.80 (s, 1H)*, 8.64 (s, 1H), 7.13–7.06 (m, 1H)*, 6.41–6.31 (m, 2H)*, 4.11–4.01 (m, 1H)*, 1.63–1.54 (m, 3H)*. 13C NMR (126 MHz, MeOD) δ 170.7*, 170.0, 167.0*, 160.1*, 159.8, 149.6*, 147.6, 134.3*, 134.2, 107.6*, 107.1, 17.6, 16.1*. *. HRMS: (ESI) [M + H]+ calc. for C10H14N3O3+: 224.1030, observed 224.1030.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 E
1 E![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Z mixture of isomers. Major peaks denoted with *.
Z mixture of isomers. Major peaks denoted with *.1H NMR (400 MHz, (CD3OD)) δ 9.92–9.76 (m, 1H)*, 7.15–7.02 (m, 1H)*, 6.97–6.87 (m, 1H), 6.40–6.26 (m, 2H)*, 4.74–4.68 (m, 1H), 4.55–4.50 (m, 1H), 4.12–4.06 (m, 1H)*, 3.23–3.08 (m, 1H)*, 3.08–2.95 (m, 1H)*. 13C NMR (126 MHz, MeOD) δ 164.4, 160.2, 149.9, 134.3, 107.7, 107.1, 55.6, 26.2 HRMS: (ESI) [M + H]+ calc. for C10H14N3O3S+: 256.0750, observed 256.0744.
Data availability
All data generated or analyzed during this study are included in this published article and its ESI.†Conflicts of interest
There are no conflicts to declare.Acknowledgements
This research was partially supported by the National Science Foundation grants MCB-1919455 (ZY) and EFMA-2318093 (ZY) as well as the CHRB award 208-07-23 (ZY and WLS) and a pilot grant from the Virginia Tech Center for Emerging, Zoonotic and Arthropod-borne Pathogens.References
- F. Akram, M. Imtiaz and I. U. Haq, Emergent crisis of antibiotic resistance: a silent pandemic threat to 21(st) century, Microb. Pathog., 2023, 174, 105923 CrossRef CAS PubMed.
- G. Duménil, Type IV Pili as a Therapeutic Target, Trends Microbiol., 2019, 27(8), 658–661 CrossRef PubMed.
- M. Naghavi, E. V. Stein, K. S. Ikuta, L. R. Swetschinski, A. P. Gray, E. E. Wool, G. R. Aguilar, T. Mestrovic, G. Smith and C. Han, Global burden of bacterial antimicrobial resistance 1990–2021: a systematic analysis with forecasts to 2050, Lancet, 2024, 404(10459), 1199–1226 CrossRef PubMed.
- D. Sibi, C. Sethi Das, V. G. Jibin and C. D. Silvanose, Emerging antibiotic resistance in post-COVID-19 co-infections, J. Clin. Med. Case Rep., 2023, 8(1), 01–08 Search PubMed.
- C. L. Ventola, The antibiotic resistance crisis: part 1: causes and threats, P T, 2015, 40(4), 277–283 Search PubMed.
- N. A. Lerminiaux and A. D. S. Cameron, Horizontal transfer of antibiotic resistance genes in clinical environments, Can. J. Microbiol., 2019, 65(1), 34–44 CrossRef CAS PubMed.
- M. A. Salam, M. Y. Al-Amin, M. T. Salam, J. S. Pawar, N. Akhter, A. A. Rabaan and M. A. A. Alqumber, Antimicrobial Resistance: A Growing Serious Threat for Global Public Health, Healthcare, 2023, 11(13), 1946 CrossRef PubMed.
- R. Dehbanipour and Z. Ghalavand, Anti-virulence therapeutic strategies against bacterial infections: recent advances, Germs, 2022, 12(2), 262–275 CrossRef CAS PubMed.
- H. Ogawara, Possible drugs for the treatment of bacterial infections in the future: anti-virulence drugs, J. Antibiot., 2021, 74(1), 24–41 CrossRef PubMed.
- W. Y. V. Lau, P. K. Taylor, F. S. L. Brinkman and A. H. Y. Lee, Pathogen-associated gene discovery workflows for novel antivirulence therapeutic development, EBioMedicine, 2023, 88, 104429 CrossRef CAS PubMed.
- L. Cegelski, G. R. Marshall, G. R. Eldridge and S. J. Hultgren, The biology and future prospects of antivirulence therapies, Nat. Rev. Microbiol., 2008, 6(1), 17–27 CrossRef CAS PubMed.
- M. O. Gaytan, V. I. Martinez-Santos, E. Soto and B. Gonzalez-Pedrajo, Type Three Secretion System in Attaching and Effacing Pathogens, Front. Cell. Infect. Microbiol., 2016, 6, 129 Search PubMed.
- A. W. Lo, K. Moonens and H. Remaut, Chemical attenuation of pilus function and assembly in Gram-negative bacteria, Curr. Opin. Microbiol., 2013, 16(1), 85–92 CrossRef CAS PubMed.
- J. W. Peterson, Bacterial pathogenesis, in Medical Microbiology, Univ of Texas Medical Branch, 1996 Search PubMed.
- G. Rampioni, P. Visca, L. Leoni and F. Imperi, Drug repurposing for antivirulence therapy against opportunistic bacterial pathogens, Emerging Top. Life Sci., 2017, 1(1), 13–22 CrossRef CAS PubMed.
- O. M. El-Halfawy, T. L. Czarny, R. S. Flannagan, J. Day, J. C. Bozelli Jr, R. C. Kuiack, A. Salim, P. Eckert, R. M. Epand and M. J. McGavin, et al., Discovery of an antivirulence compound that reverses beta-lactam resistance in MRSA, Nat. Chem. Biol., 2020, 16(2), 143–149 CrossRef CAS PubMed.
- A. M. Kauppi, R. Nordfelth, H. Uvell, H. Wolf-Watz and M. Elofsson, Targeting bacterial virulence: inhibitors of type III secretion in Yersinia, Chem. Biol., 2003, 10(3), 241–249 CrossRef CAS PubMed.
- J. S. Pinkner, H. Remaut, F. Buelens, E. Miller, V. Aberg, N. Pemberton, M. Hedenstrom, A. Larsson, P. Seed and G. Waksman, et al., Rationally designed small compounds inhibit pilus biogenesis in uropathogenic bacteria, Proc. Natl. Acad. Sci. U. S. A., 2006, 103(47), 17897–17902 CrossRef CAS PubMed.
- K. J. Dye, N. J. Vogelaar, M. O'Hara, P. Sobrado, W. Santos, P. R. Carlier and Z. Yang, Discovery of Two Inhibitors of the Type IV Pilus Assembly ATPase PilB as Potential Antivirulence Compounds, Microbiol. Spectrum, 2022, 10(6), e0387722 CrossRef PubMed.
- M. McCallum, L. L. Burrows and P. L. Howell, The Dynamic Structures of the Type IV Pilus, Microbiol. Spectrum, 2019, 7(2), 1–12 Search PubMed.
- C. K. Ellison, G. B. Whitfield and Y. V. Brun, Type IV Pili: dynamic bacterial nanomachines, FEMS Microbiol. Rev., 2022, 46(2), 1–14 CrossRef PubMed.
- A. Siryaporn, S. L. Kuchma, G. A. O'Toole and Z. Gitai, Surface attachment induces Pseudomonas aeruginosa virulence, Proc. Natl. Acad. Sci. U. S. A., 2014, 111(47), 16860–16865 CrossRef CAS PubMed.
- K. H. Piepenbrink, E. Lillehoj, C. M. Harding, J. W. Labonte, X. Zuo, C. A. Rapp, R. S. Munson Jr, S. E. Goldblum, M. F. Feldman and J. J. Gray, et al., Structural Diversity in the Type IV Pili of Multidrug-resistant Acinetobacter, J. Biol. Chem., 2016, 291(44), 22924–22935 CrossRef CAS PubMed.
- J. P. Barnier, D. Euphrasie, O. Join-Lambert, M. Audry, S. Schonherr-Hellec, T. Schmitt, S. Bourdoulous, M. Coureuil, X. Nassif and M. El Behi, Type IV pilus retraction enables sustained bacteremia and plays a key role in the outcome of meningococcal sepsis in a humanized mouse model, PLoS Pathog., 2021, 17(2), e1009299 CrossRef CAS PubMed.
- I. Dos Santos Souza, N. Maissa, J. Ziveri, P. C. Morand, M. Coureuil, X. Nassif and S. Bourdoulous, Meningococcal disease: a paradigm of type-IV pilus dependent pathogenesis, Cell. Microbiol., 2020, 22(4), e13185 CrossRef CAS PubMed.
- H. C. Winther-Larsen, F. T. Hegge, M. Wolfgang, S. F. Hayes, J. P. van Putten and M. Koomey, Neisseria gonorrhoeae PilV, a type IV pilus-associated protein essential to human epithelial cell adherence, Proc. Natl. Acad. Sci. U. S. A., 2001, 98(26), 15276–15281 CrossRef CAS PubMed.
- F. Aubey, J. P. Corre, Y. Kong, X. Xu, D. Obino, S. Goussard, C. Lapeyrere, J. Souphron, C. Couturier and S. Renard, et al., Inhibitors of the Neisseria meningitidis PilF ATPase provoke type IV pilus disassembly, Proc. Natl. Acad. Sci. U. S. A., 2019, 116(17), 8481–8486 CrossRef CAS PubMed.
- K. Denis, M. Le Bris, L. Le Guennec, J. P. Barnier, C. Faure, A. Gouge, H. Bouzinba-Segard, A. Jamet, D. Euphrasie and B. Durel, et al., Targeting Type IV pili as an antivirulence strategy against invasive meningococcal disease, Nat. Microbiol., 2019, 4(6), 972–984 CrossRef CAS PubMed.
- D. Nunn, S. Bergman and S. Lory, Products of three accessory genes, pilB, pilC, and pilD, are required for biogenesis of Pseudomonas aeruginosa pili, J. Bacteriol., 1990, 172(6), 2911–2919 CrossRef CAS PubMed.
- K. J. Dye, N. J. Vogelaar, P. Sobrado and Z. Yang, High-Throughput Screen for Inhibitors of the Type IV Pilus Assembly ATPase PilB, mSphere, 2021, 6(2), 1–12 CrossRef PubMed.
- K. J. Dye and Z. Yang, Cyclic-di-GMP and ADP bind to separate domains of PilB as mutual allosteric effectors, Biochem. J., 2020, 477(1), 213–226 CrossRef CAS PubMed.
- A. Deb, M. E. Jung, X. Chen, S. Li, Y. Xing and H. Ding, Small Molecule Inhibitors of ENPP1. US Pat. WO 2022056068A1, 2022 Search PubMed.
- Apyrase, https://www.neb.com/en-us/products/m0398-apyrase.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: https://doi.org/10.1039/d5ra02702k |
| ‡ Equal contributions. |
| This journal is © The Royal Society of Chemistry 2025 |